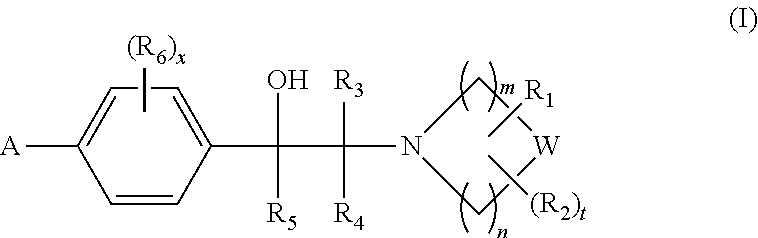
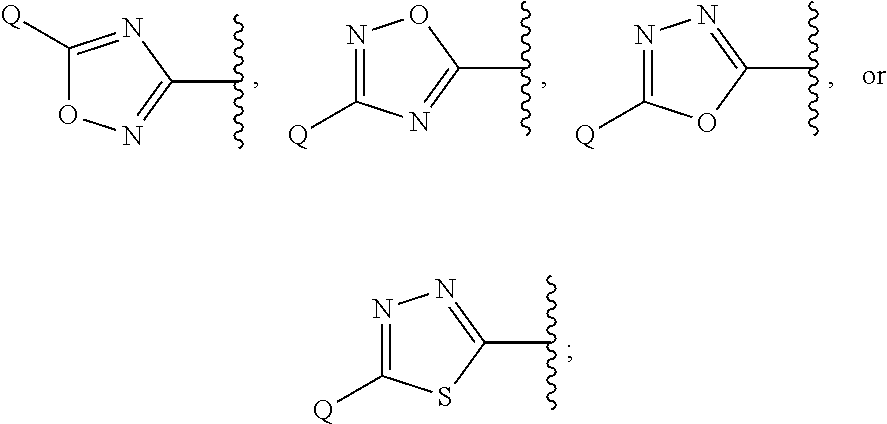
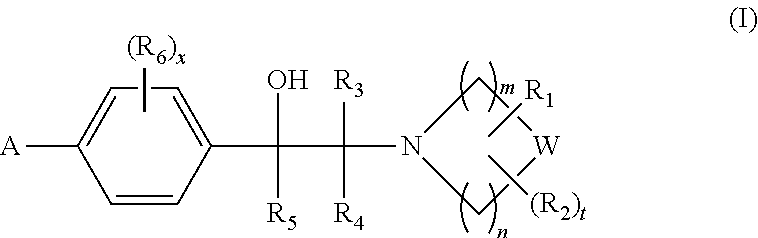
Heterocyclic compounds
a technology of heterocyclic compounds and compounds, applied in the field of heterocyclic compounds, can solve problems such as bradycardia and hypertension, undesirable cardiovascular effects, and transient bradycardia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(2-Hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)azetidine-3-carboxylic acid
[0331]To tert-butyl 1-(2-hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)azetidine-3-carboxylate (8 mg, 0.015 mmol) was added DCM (2 mL) and TFA (2.000 mL). The reaction mixture was stirred 2 hours. Next, the solvent was removed and the remaining contents were freeze dried from MeCN to yield 8 mg of 1-(2-hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)azetidine-3-carboxylic acid as a TFA salt. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.19 (2H, d, J=8.35 Hz), 7.83 (2H, dd, J=8.02, 1.65 Hz), 7.58-7.73 (5H, m), 4.32-4.83 (3H, m), 3.43-4.20 (6H, m), 2.95-3.05 (2H, m), 1.65-1.75 (2H, m), 0.97 (3H, t, J=7.36 Hz). MS (m+1)=475. HPLC Peak RT=3.38 minutes (Analytical Method A). Purity=90%.
example 2
1-(2-Hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)piperidine-2-carboxylic acid
[0332]
[0333]To a mixture of 2-bromo-1-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethanol, Preparation 1C (30 mg, 0.066 mmol) and piperidine-2-carboxylic acid (25.6 mg, 0.198 mmol) in DMSO (2 mL) was added DBU (0.030 mL, 0.198 mmol). The reaction mixture was heated at 80° C. for 2 hours. The reaction mixture was filtered and purified by HPLC. HPLC conditions: PHENOMENEX® Luna C18 5 micron column (250×30mm); 25-100% CH3CN / water (0.1% TFA); 25 minute gradient; 30 mL / min. Isolated fractions with correct mass were freeze-dried overnight to yield 19 mg of 1-(2-hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)piperidine-2-carboxylic acid as a TFA salt. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.10 (2H, dd, J=8.35, 2.20 Hz), 7.77 (2H, dd, J=7.91, 1.54 Hz), 7.52-7.64 (5H, m), 5.20 (1H, d, J=18.24 Hz), 3.67 (2H, br. s.), 2.87-3.01...
example 3
1-(2-Hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)piperidine-3-carboxylic acid
[0334]
[0335]To a mixture of 2-bromo-1-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethanol, Preparation 1C (30 mg, 0.066 mmol) and piperidine-3-carboxylic acid (25.6 mg, 0.198 mmol) in DMSO (2 mL) was added DBU (0.030 mL, 0.198 mmol). The reaction mixture was heated at 80° C. for 2 hours. The reaction mixture was filtered and purified by HPLC. HPLC conditions: PHENOMENEX® Luna C18 5 micron column (250×30mm); 25-100% CH3CN / water (0.1% TFA); 25 minute gradient; 30 mL / min. Isolated fractions with correct mass were freeze-dried overnight to yield 18 mg 1-(2-hydroxy-2-(4-(5-(5-phenyl-4-propylisoxazol-3-yl)-1,2,4-oxadiazol-3-yl)phenyl)ethyl)piperidine-3-carboxylic acid as a TFA salt. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.16 (2H, dd, J=8.35, 1.98 Hz), 7.83 (2H, dd, J=7.91, 1.54 Hz), 7.56-7.75 (5H, m), 5.13-5.39 (1H, m), 4.11-4.31 (2H, m), 3.21-3.41 (3H, m), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com