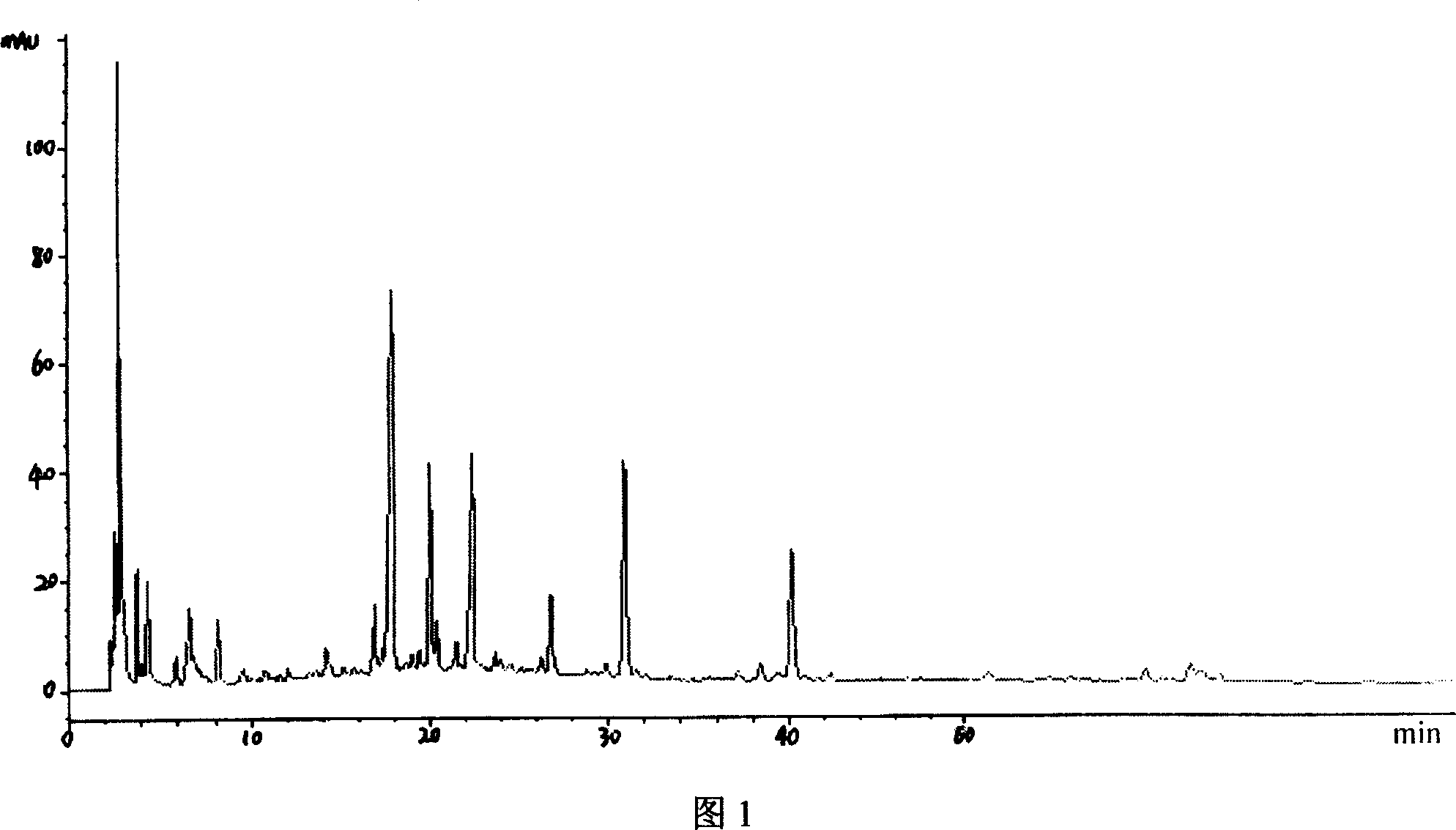
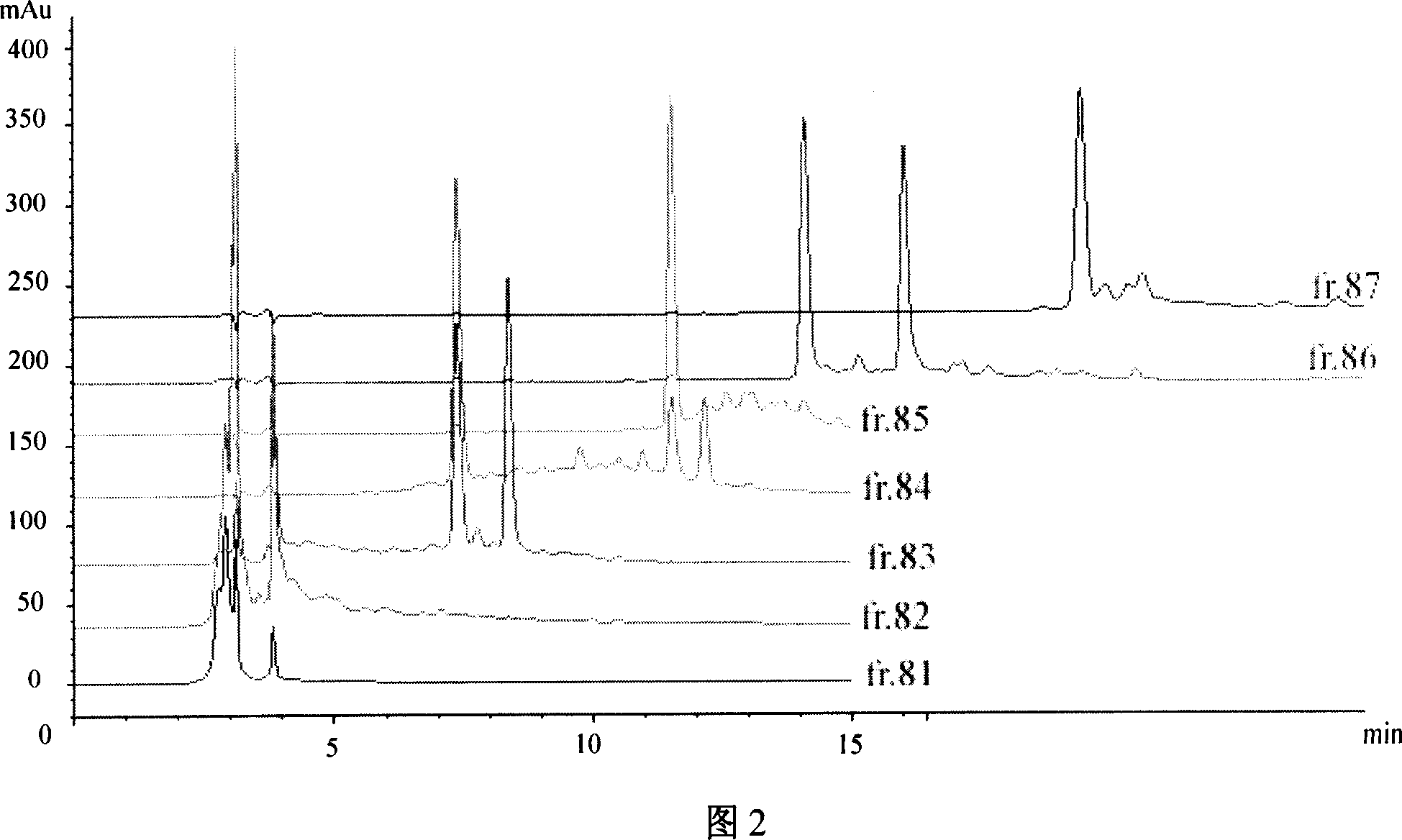
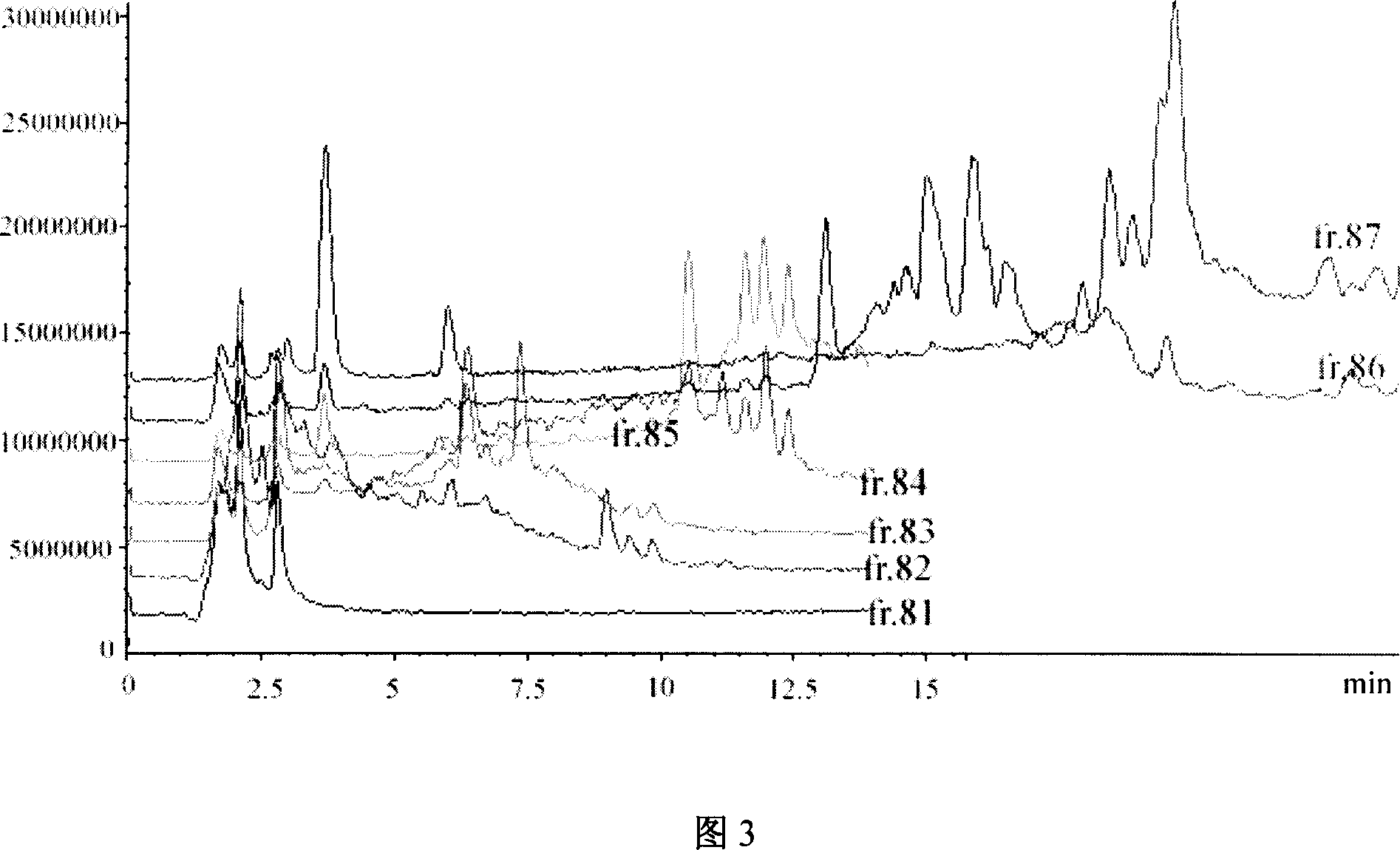
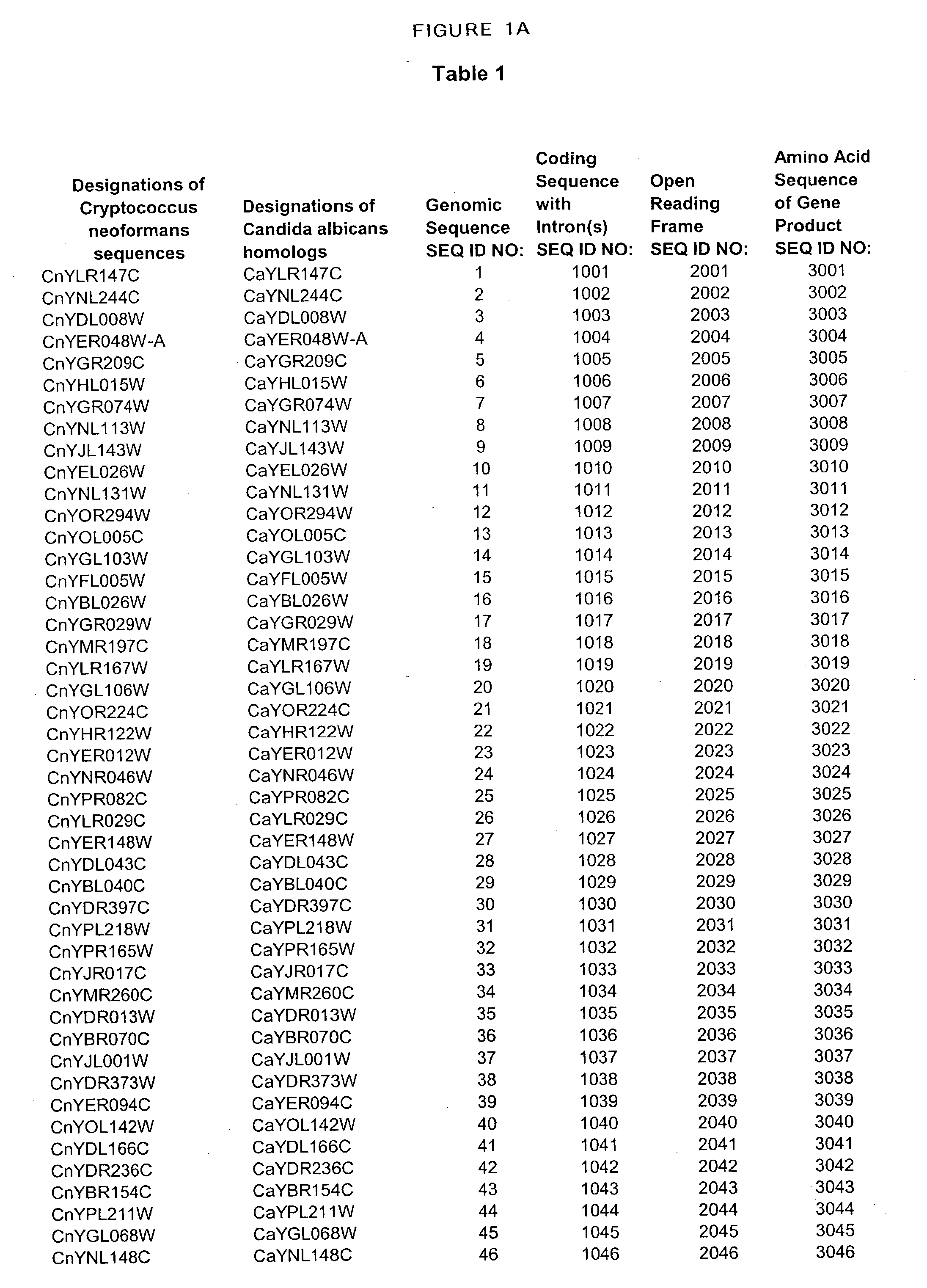
Patents
Literature
1144 results about "New medications" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
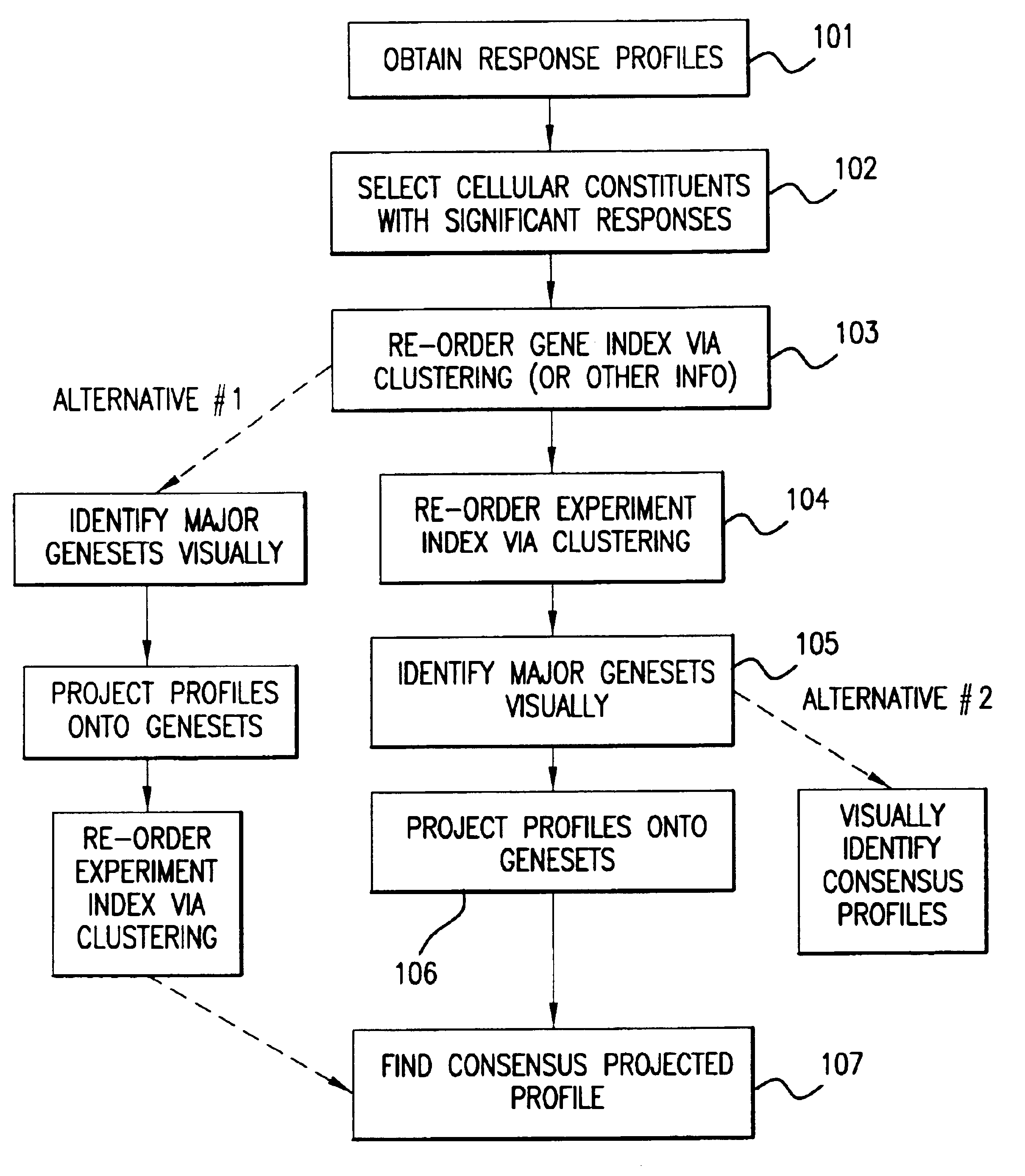
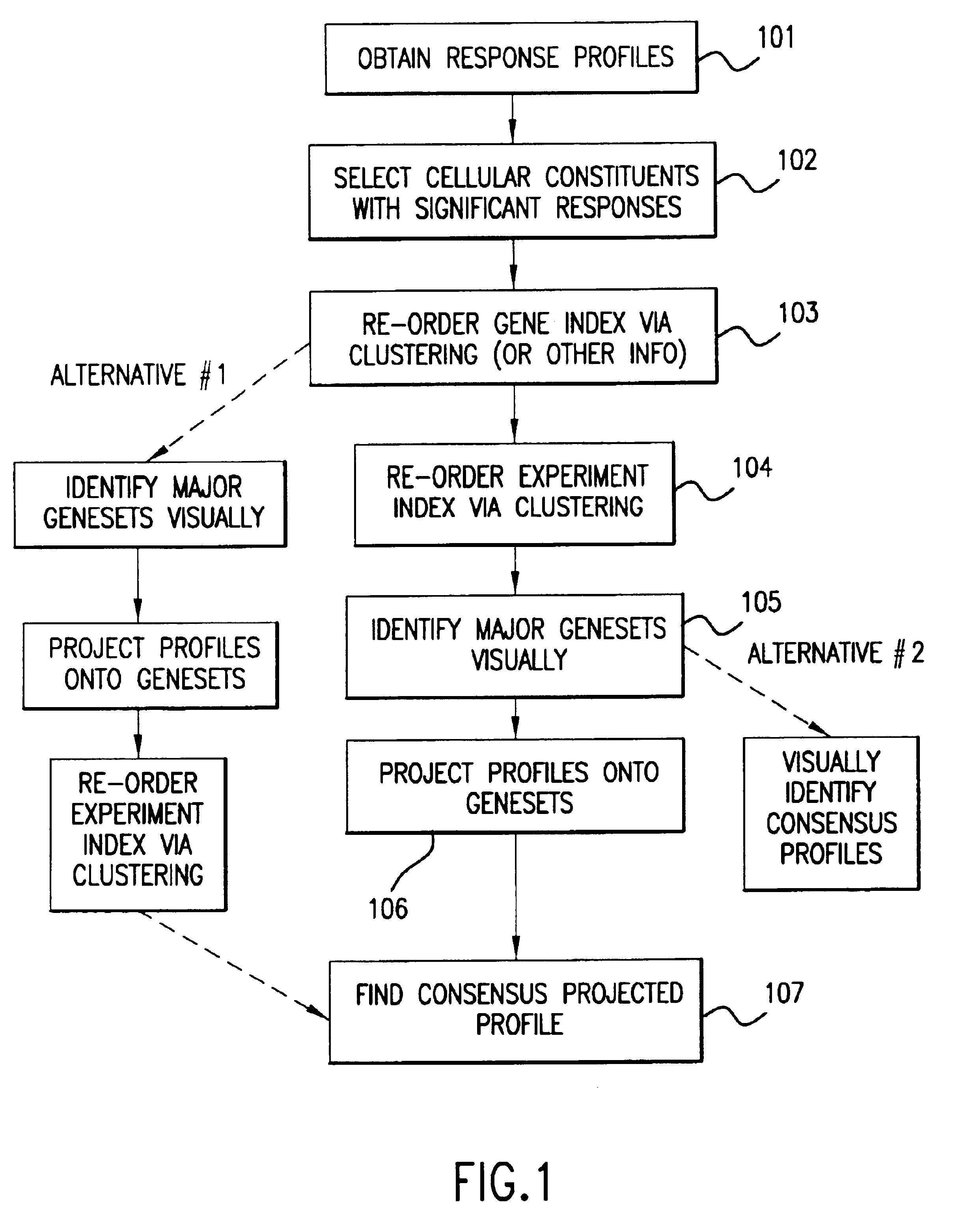
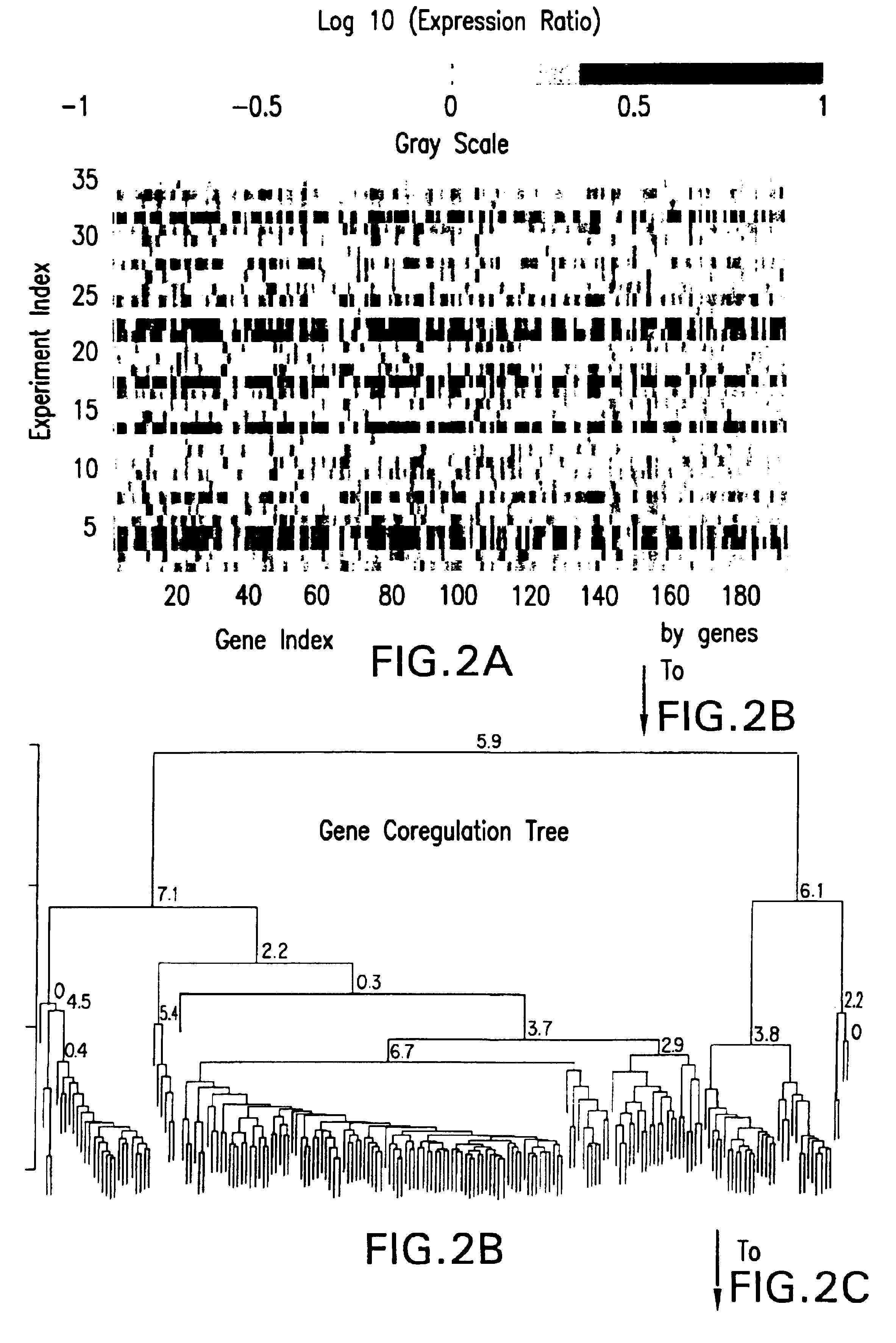
Methods of characterizing drug activities using consensus profiles
InactiveUS6801859B1Good detection and classification and comparisonSimple structureMicrobiological testing/measurementAnalogue computers for chemical processesNew medicationsPharmaceutical drug
The present invention provides methods for enhanced detection of biological response profiles. In particular, the methods of this invention allow for the detection of biological response patterns, such as gene expression patterns, in response to different drug treatments. The methods of the invention also allow the determination of a "consensus profile" which describes a particular class or type of biological response. In certain embodiments the consensus profile may describe the biological response of a particular group or class of drugs. In other embodiments, the consensus profile may describe an "ideal" biological response such as one associated with a desired therapeutic effect. The methods of the present invention also allow for the comparison of different biological responses. Thus, the methods of the invention may be used, e.g., to identify and / or study new drugs.
Owner:MICROSOFT TECH LICENSING LLC
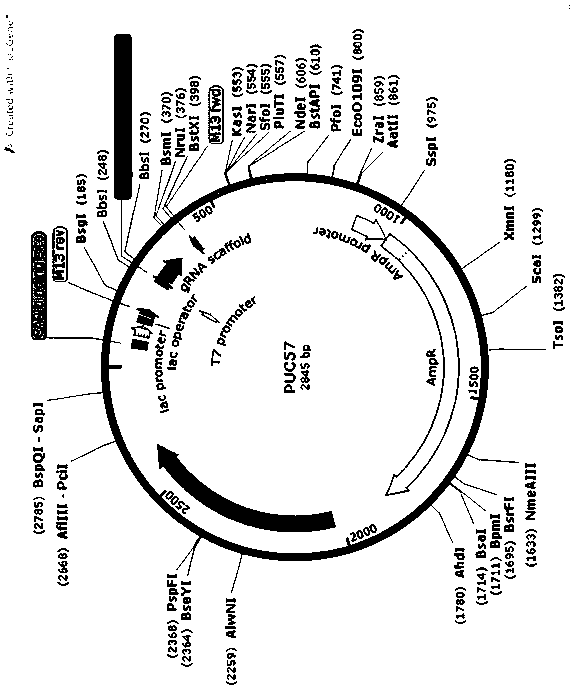
Method for establishing Gadd45a knockout rabbit model by adopting knockout technology
InactiveCN107630043APredictive effectReduce the risk of research and developmentStable introduction of DNAAnimal husbandryRabbit modelPlasmid dna
The invention relates to a method for establishing a Gadd45a knockout rabbit model by adopting a knockout technology and belongs to the technical field of biotechnology. The invention aims to establish a rabbit model by knocking out GADD45a gene by utilizing a Grispr / cas9 technology and to provide the method for establishing the Gadd45a knockout rabbit model by adopting the knockout technology andfor researching the influence of the gene on animal liver. The method provided by the invention comprises the following steps: establishing sgRNA; synthesizing double-stranded DNA; linearizing p UC-57 carrier; linking p UC-57 carrier with double-stranded DNA; converting; performing monoclonal picking and plasmid DNA extraction; identifying plasmid sequence; expressing plasmid with CAS9; and performing digestion linearization for in vitro transcription. Through related detection, the invention successfully acquires the Gadd45a knockout rabbit model; the model is established for simulating theclinic pathological processes of the liver diseases, such as, fatty liver, liver cirrhosis and liver cancer after giving the corresponding alcohol stimulation, is capable of effectively forecasting the clinic application effects of new vaccine, new drugs and new diagnostic reagents, and meanwhile, and is capable of greatly reducing the risk in researching and developing the new drugs; and a basismodel is supplied for the clinical research.
Owner:JILIN UNIV
Method for separating and extracting Milkvetch Root
InactiveCN101073592AOrganic compounds purification/separation/stabilisationSugar derivativesChemical compositionMedicine
The invention is concerned with the standard extraction and isolation method of Astragalus root. It can form a medicinal materials component store according to different polarity levels of the Astragalus root divide into several groups, which each group contents few types of compound. It can use to discover new medicine by conducting drug screening from the component store. The method can reduce the period of medicine extraction and isolation, and reduce the consumption of manpower and material resources, and it suits for most types of medicine.
Owner:TIANJIN TASLY PHARMA CO LTD
Identification of essential genes of cryptococcus neoformans and methods of use
InactiveUS20040014955A1Simple and reasonable designSugar derivativesMicrobiological testing/measurementBiotechnologyVirulent characteristics
The present invention provides C. neoformans genes that are essential and are potential targets for drug screening. The nucleotide sequence of the target genes can be used for various drug discovery purposes, such as expression of the recombinant protein, hybridization assay and construction of nucleic acid arrays. The uses of proteins encoded by the essential genes, and genetically engineered cells comprising modified alleles of essential genes in various screening methods are also encompassed by the invention. The present invention also provides methods and compositions that enable the experimental determination as to whether any gene in the genome of Cryptococcus neoformans is essential, and whether that gene is required for virulence or pathogenicity. The identification of essential genes and those genes critical to the development of virulent infections, provides a basis for the development of screens for new drugs against C. neoformans.
Owner:MERCK & CO INC
Anticancer prior-medicine and method for preparing the same and use thereof
ActiveCN101134109AGood biocompatibilityHas lymphatic targeting propertiesPowder deliveryPharmaceutical non-active ingredientsEster bondChemical technology
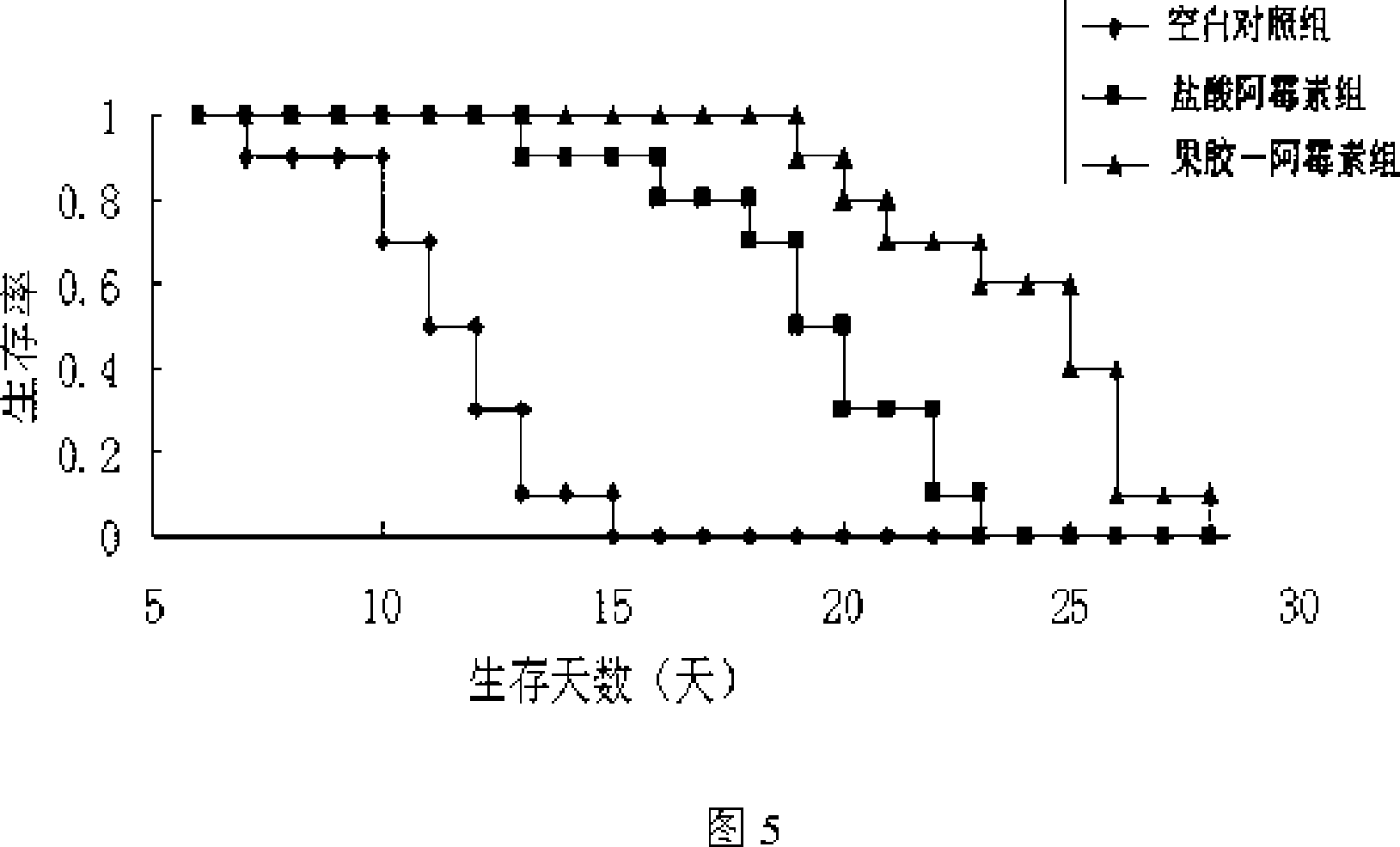
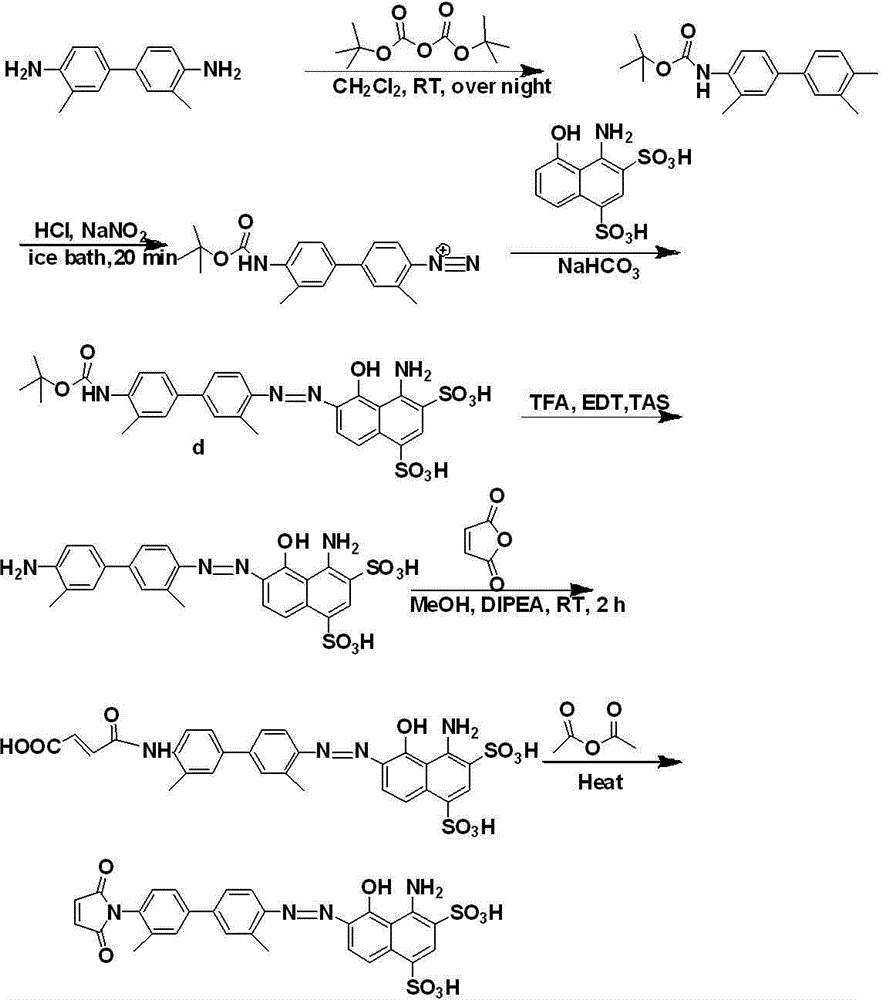
The present invention is one kind of anticancer prodrug and its preparation process and use, and belongs to the field of medicine and chemical technology. The anticancer prodrug is macromolecular conjugate formed with lower ester pectin and anticarcinogen containing amino group or hydroxyl group and through amido bond or ester bond coupling. The present invention provides one new way for designing and developing new anticarcinogen and is scientifically significant for applying anticancer prodrug in treating tumor. The macromolecular conjugate is hopeful in developing lymph targeting anticancer prodrug, liver targeting anticancer prodrug and lung targeting anticancer prodrug.
Owner:SICHUAN YINGRUI PHARMA TECH CO
Exendin-4 modified by Evans blue or derivatives of Evans blue and preparation method and application of Exendin-4
ActiveCN104650217AProlong half-life in vivoEasy to manufactureHormone peptidesPeptide/protein ingredientsDiseaseHalf-life
The invention relates to a preparation method and application of Exendin-4 biologically located and modified by Evans blue (Evans Blue) or derivatives of Evans blue. The product generated after the C terminal of Exendin-4 is specifically modified with Evans blue or derivatives of Evans blue has biological activity similar to Exendin-4 which is not modified but has longer in vivo half-life than the Exendin-4 which is not modified. Based on the significance of the Exendin-4 in treatment of diseases, the invention also discloses application of the Exendin-4 modified by Evans blue or derivatives of Evans blue in preparation of medicines for treating type-II diabetes and myocardial infarction. The Exendin-4 has the advantages of simplicity and convenience in preparation, obvious curative effect, long-lasting and stable drug effect, convenience in storage and the like and has significance in research and development of new drugs for promoting anti-diabetic and anti-infarction high-efficiency treatment.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
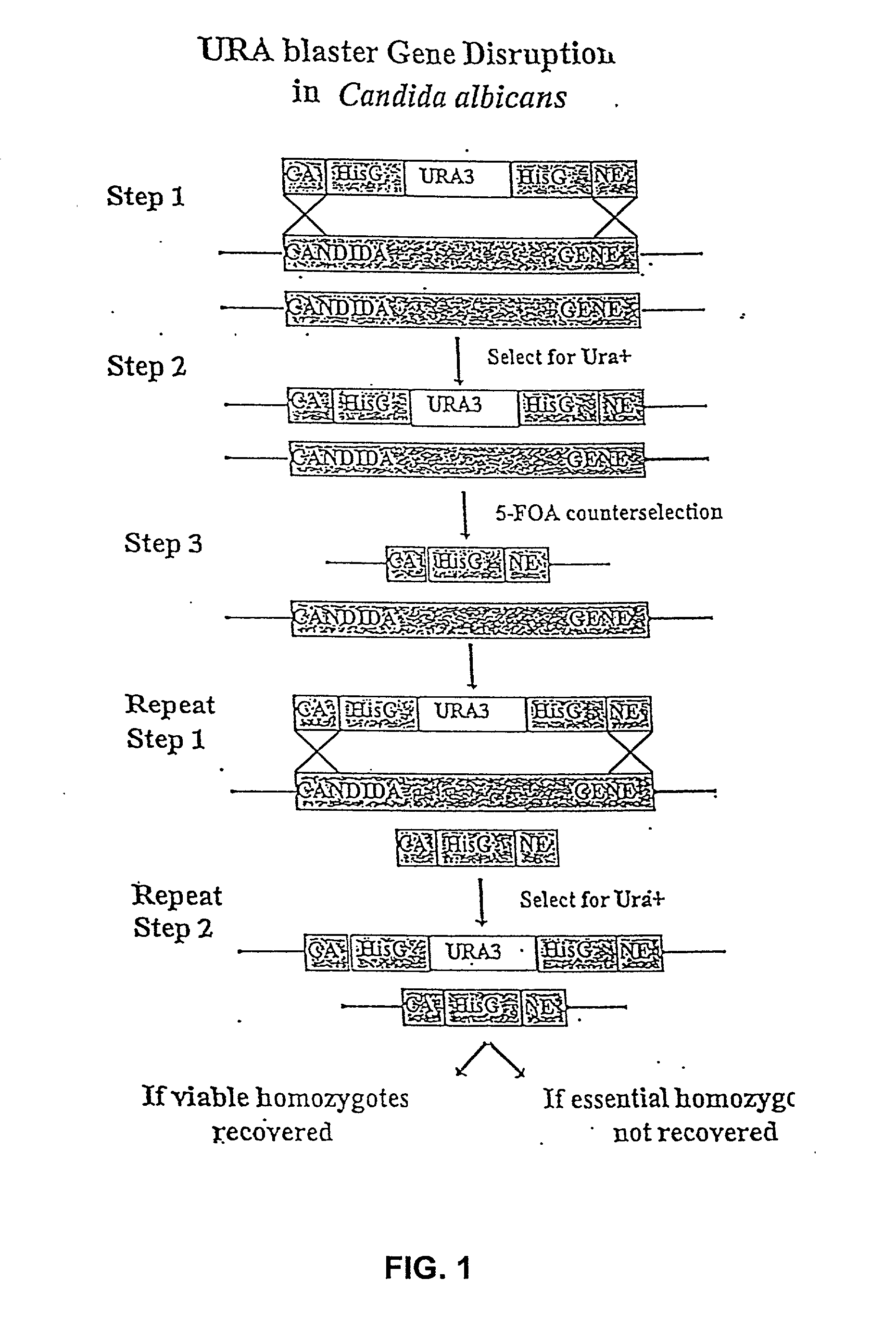
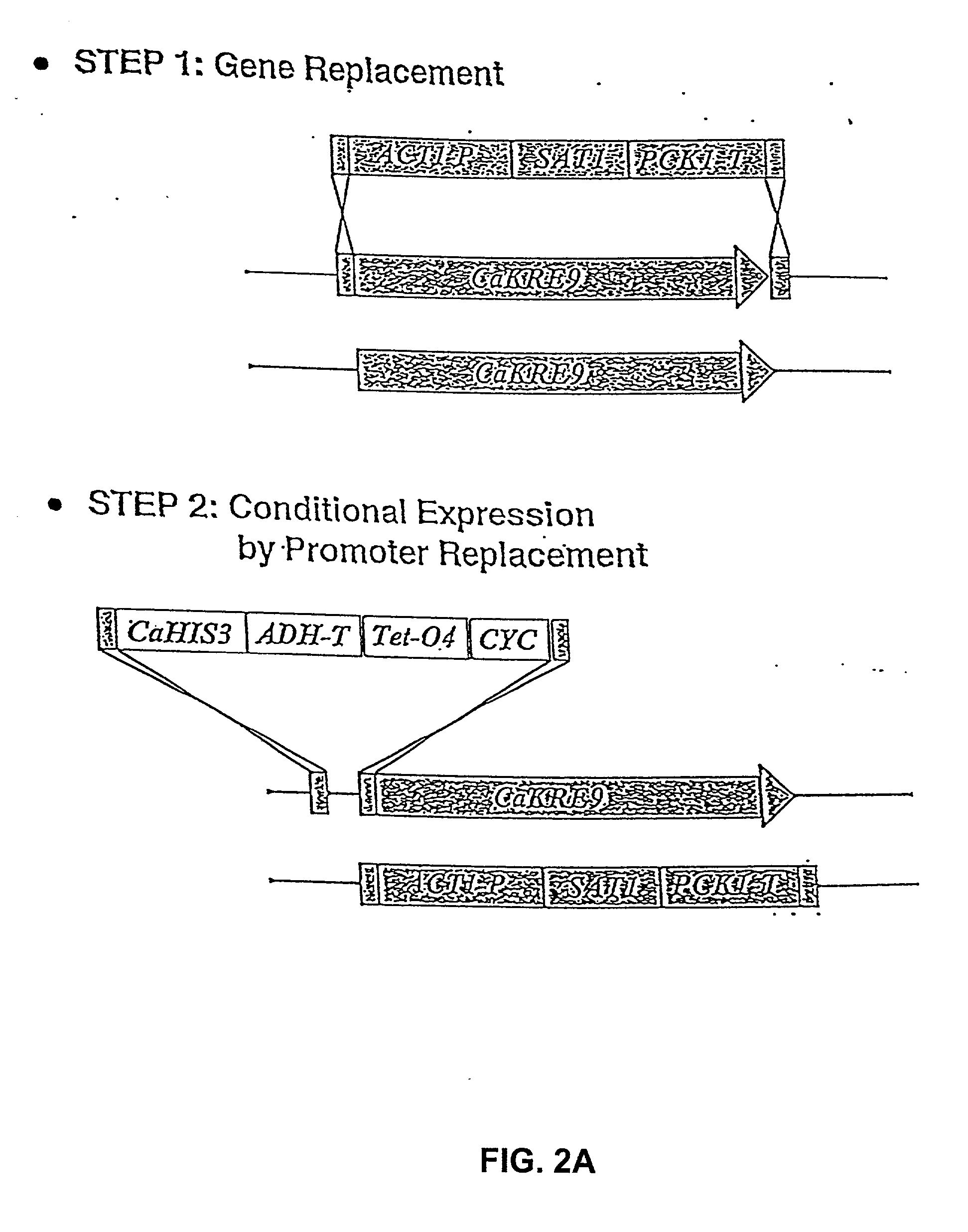
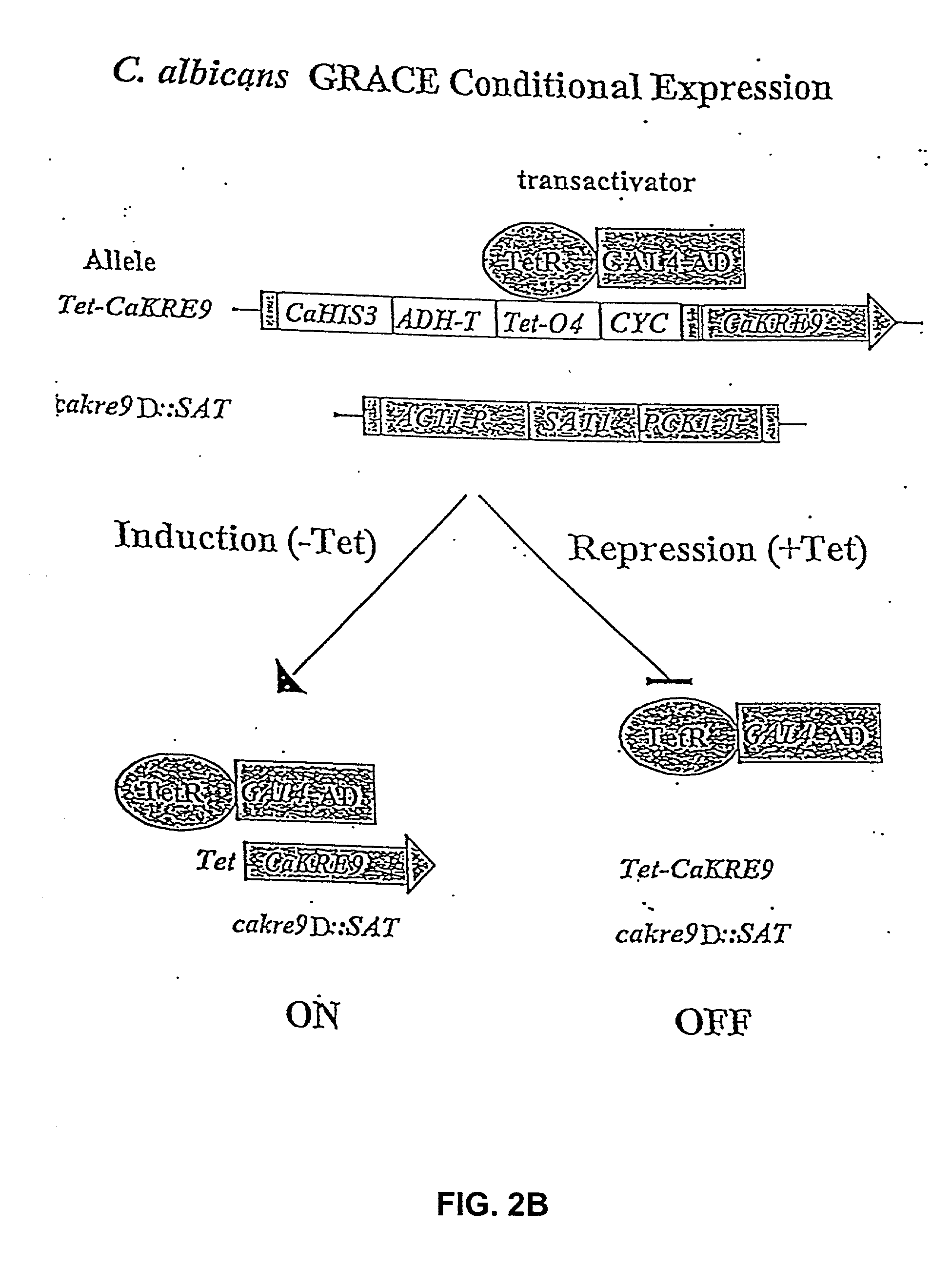
Gene disruption methodologies for drug target discovery
The present invention provides methods and compositions that enable the experimental determination as to whether any gene in the genome of a diploid pathogenic organism is essential, and whether it is required for virulence or pathogenicity. The methods involve the construction of genetic mutants in which one allele of a specific gene is inactivated while the other allele of the gene is placed under conditional expression. The identification of essential genes and those genes critical to the development of virulent infections, provides a basis for the development of screens for new drugs against such pathogenic organisms. The present invention further provides Candida albicans genes that are demonstrated to be essential and are potential targets for drug screening. The nucleotide sequence of the target genes can be used for various drug discovery purposes, such as expression of the recombinant protein, hybridization assay and construction of nucleic acid arrays. The uses of proteins encoded by the essential genes, and genetically engineered cells comprising modified alleles of essential genes in various screening methods are also encompassed by the invention.
Owner:MERCK & CO INC
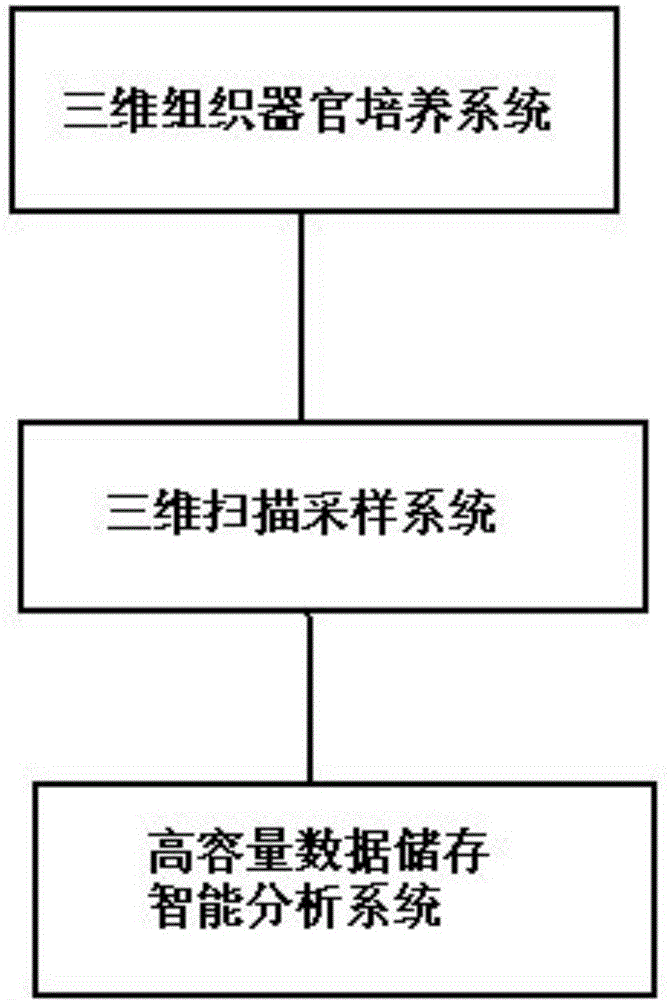
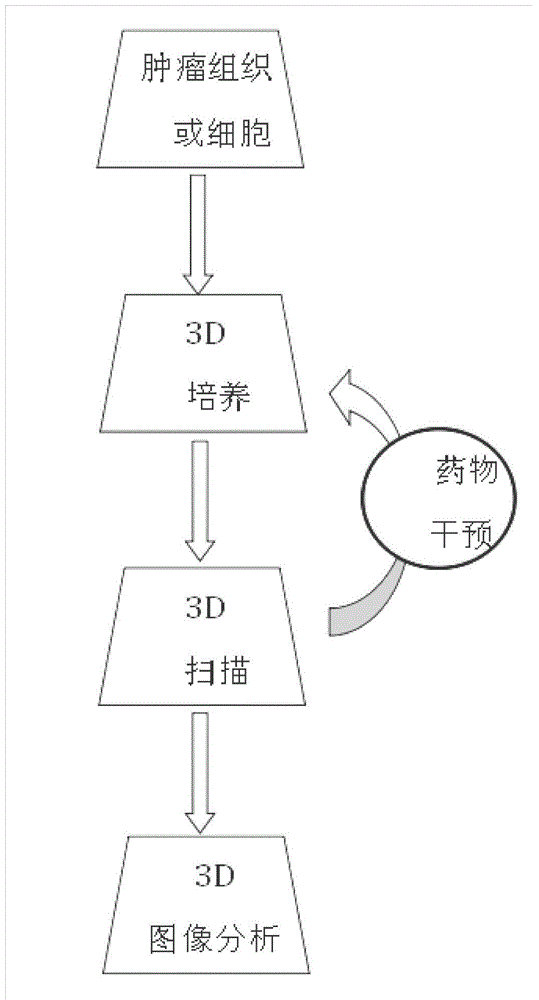
Three dimensional tissue and organ culture model, high throughput automatic stereo image analyzing platform and applications thereof
ActiveCN104403923APrecise screeningHigh speedBioreactor/fermenter combinationsBiological substance pretreatmentsBiological macromoleculeComputer analysis
The invention discloses a three dimensional tissue and organ culture model, a high throughput automatic stereo image analyzing platform thereof, and a method utilizing the provided platform to screen antitumor drugs. The platform can be applied to the culture system of three dimensional tissue and organ / organ-like substance, three dimensional scanning and sampling system, and high-volume data storage and computer analysis system. The provided platform can simultaneously process a large amount of clinical samples and screen a plurality of antitumor drugs, is capable of greatly reducing the cost and maximally reducing the detection time, thus is widely applied to the drug selection schemes and dosage optimization in clinic, novel drug development, and basic researches on interactions among tissue, organ, biological macromolecules, and other micromolecules.
Owner:NANJING KDRB BIOTECH INC LTD
Self-assembly short peptides constructed by D type amino acid, use for nano-biomedicine
InactiveCN101337985AIncrease success rateIncrease typeCosmetic preparationsPeptide/protein ingredients3D cell cultureIn vivo
A self-assembling peptide constituted by D-amino acid is named as d-RAD16 and has an amino acid sequence represented by SEQ ID NO.1 in a sequence list. Test shows that hydrogel formed by the self-assembling peptide can be used for preparing a moisture keeping and water locking agent, and has the function of stopping bleeding rapidly for wound, so that the hydrogel can be used for preparing a hemostatic drug suiting clinical application by adding a pharmaceutically acceptable carrier or excipient. Nanofiber scaffold formed by the self-assembling peptide supports the growth of various cells and can be used as the substrate material for 3D cell culture. The substrate material for 3D cell culture can simulate the in-vivo environment to support the 3D growth of cells in the substrate, thereby providing a cell 3D culture drug screening mode capable of simulating the in-vivo environment and improving the success rate for the development of new drugs.
Owner:成都瑞恩生物技术有限公司
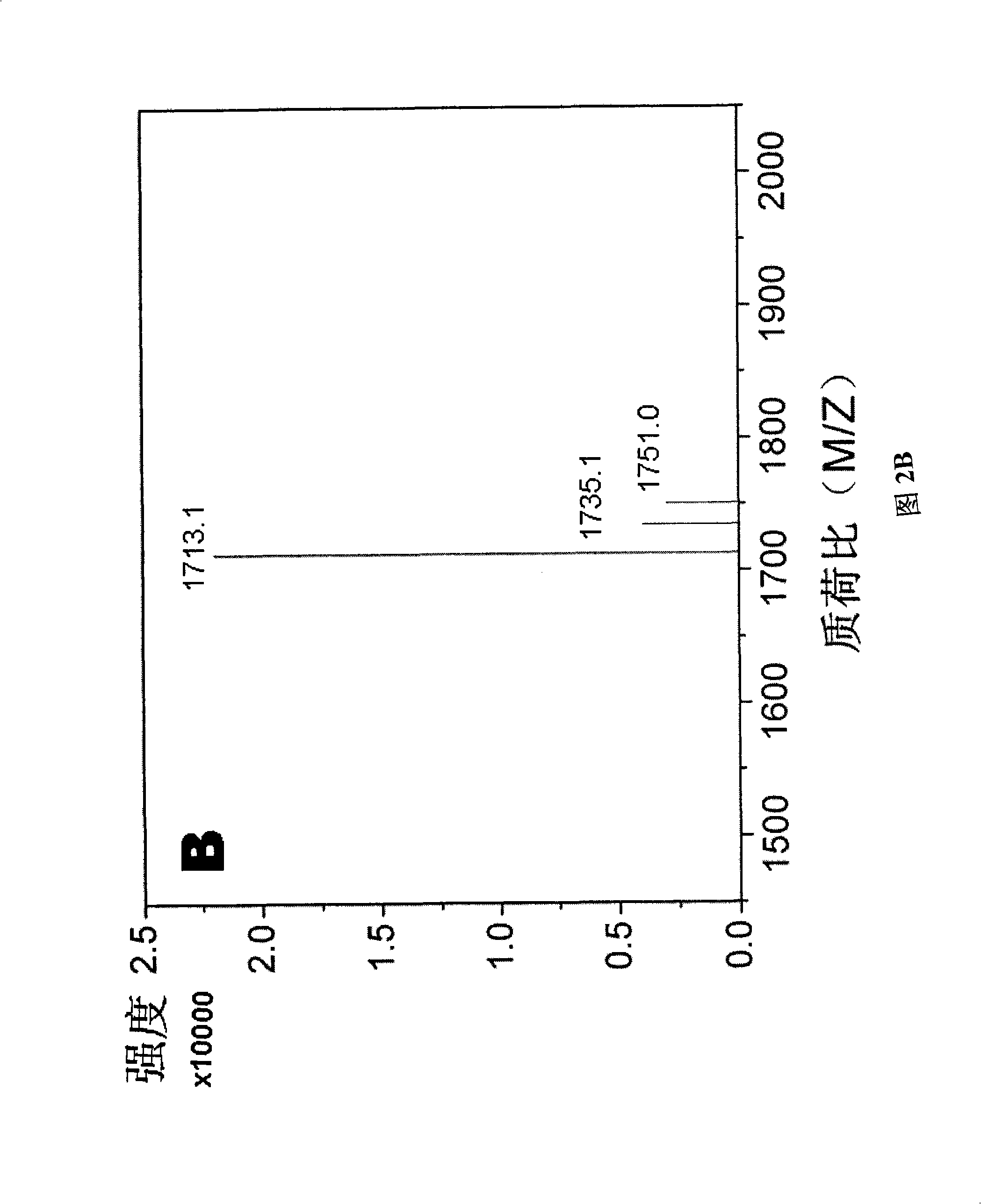
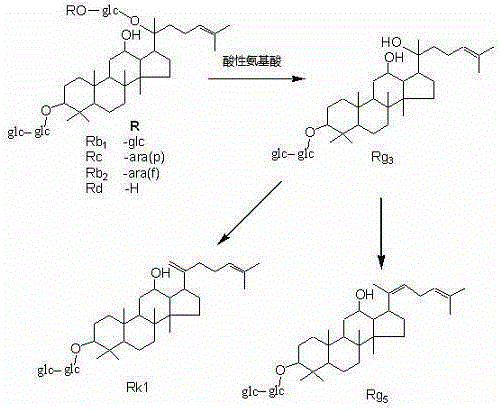
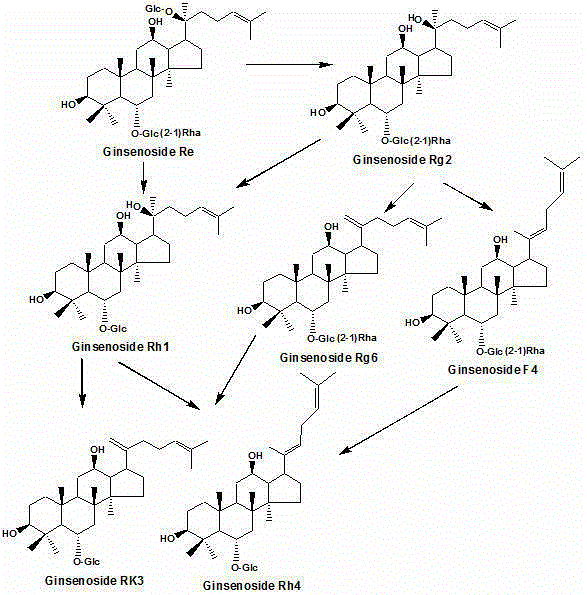
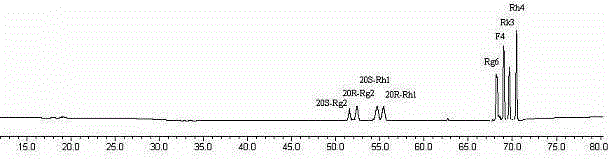
Method for preparing rare ginsenoside by hydrolyzing ginsenoside with acidic amino acid
ActiveCN105273032AOvercoming poor hydrolysis specificityLow overcoming rateSteroidsNeutral Amino AcidsNew medications
The invention discloses a method for preparing rare ginsenoside by hydrolyzing ginsenoside with acidic amino acid. Panoxadiol saponins are converted to generate 20R-Rg3, 20S-Rg3, Rg5 and Rk1, and panaxatriol saponins are converted to generate Rg6, F4, Rk3 and Rh4. Compared with an existing preparation process, the hydrolysis capacity of acidic amino acid is more than 10 times of that of neutral amino acid and that of basic amino acid and can better meet requirements of industrial preparation of rare ginsenoside, the defects that the hydrolysis specifity of ginsenoside is poor, the yield is low, the corrosion is high, environment pollution is caused, multiple byproducts are produced and the like due to the fact that a large amount of strong acid and strong base are used are overcome, ginseng resources are fully used, rare ginsenoside is extracted and enriched to the largest extent, the purpose of simple, quick, environment-friendly and low-cost enrichment of rare ginsenoside is achieved, and the method guarantees industrial production and preparation of new medicines.
Owner:JILIN YATAI PHARM CO LTD
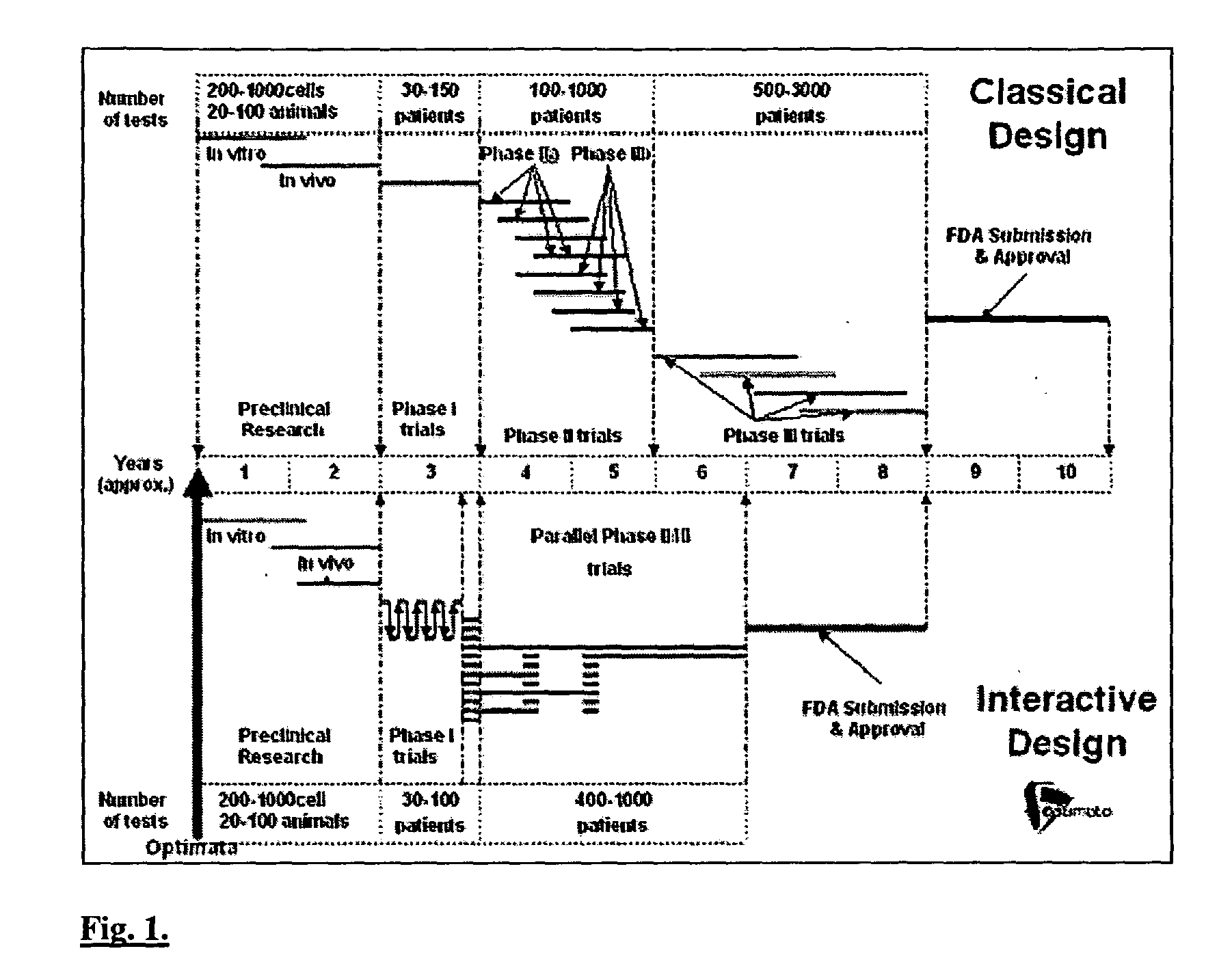
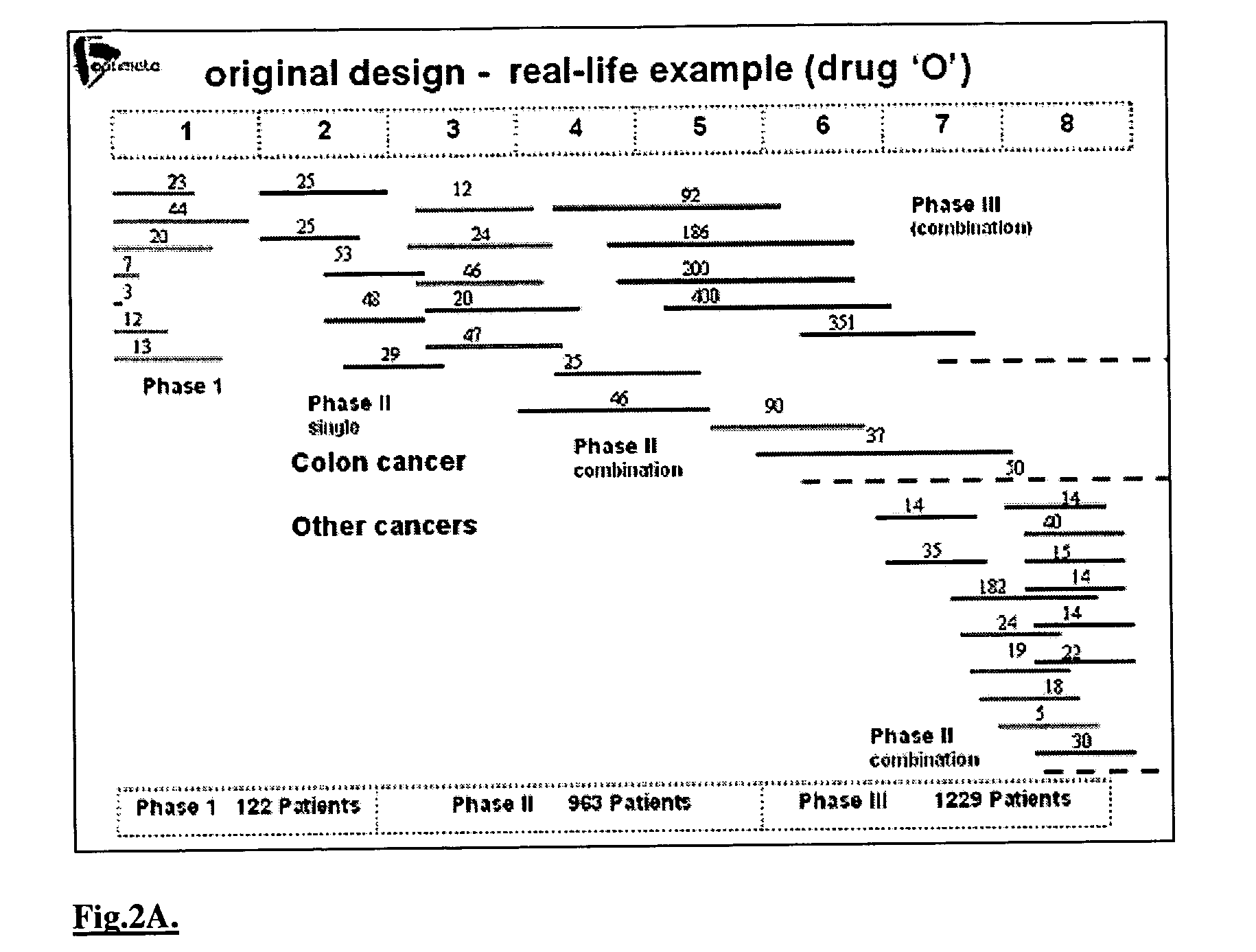
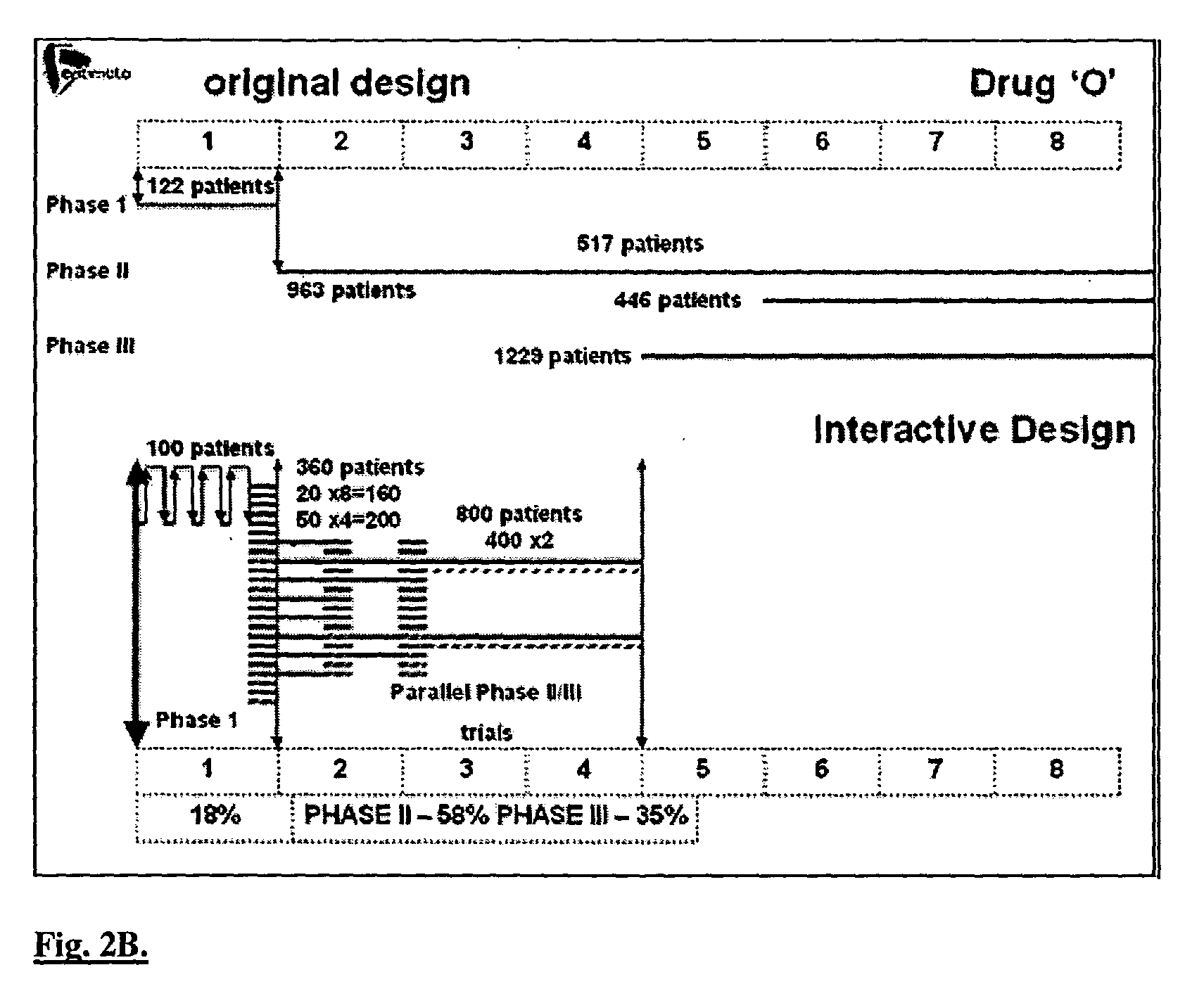
Interactive technique for optimizing drug development from the pre-clinical phases through phase-IV
InactiveUS20040107084A1Chemical property predictionMedical simulationPatient populationPhases of clinical research
A method of performing interactive clinical trials for testing a new drug comprising performing a pre-clinical phase in which a computer model for pharmacokinetics and pharmacodynamics of the drug is created and adjusted based on in vitro studies and in vivo studies in animals. A phase I clinical research is performed in which a clinical trial on at least a single dose is performed in parallel with performing computer simulation studies using the computer model. The computer model is adjusted based on comparison of the results of the clinical research and the computer simulation. A maximal tolerated dose, minimum effective dose, and a recommended dose is determined based on the phase I clinical research in conjunction with the computer simulations. The drug is checked for cumulative effects and providing this information to the computer model. Multiple simulations are performed using the computer model with different doses and dosing intervals. An optimal protocol is determined for the most responsive patient populations and indications for a phase II clinical trial. Phase II clinical trial is performed where a number of small scale clinical trials are performed in parallel based on results of the above. The interim results are analyzed to choose the most promising regimens for continued clinical trials. Phase III clinical research is performed for chosen indications by chosen protocols. Phase IV studies are performed for post-marketing subpopulation analysis and long term product safety assessment.
Owner:OPTIMATA
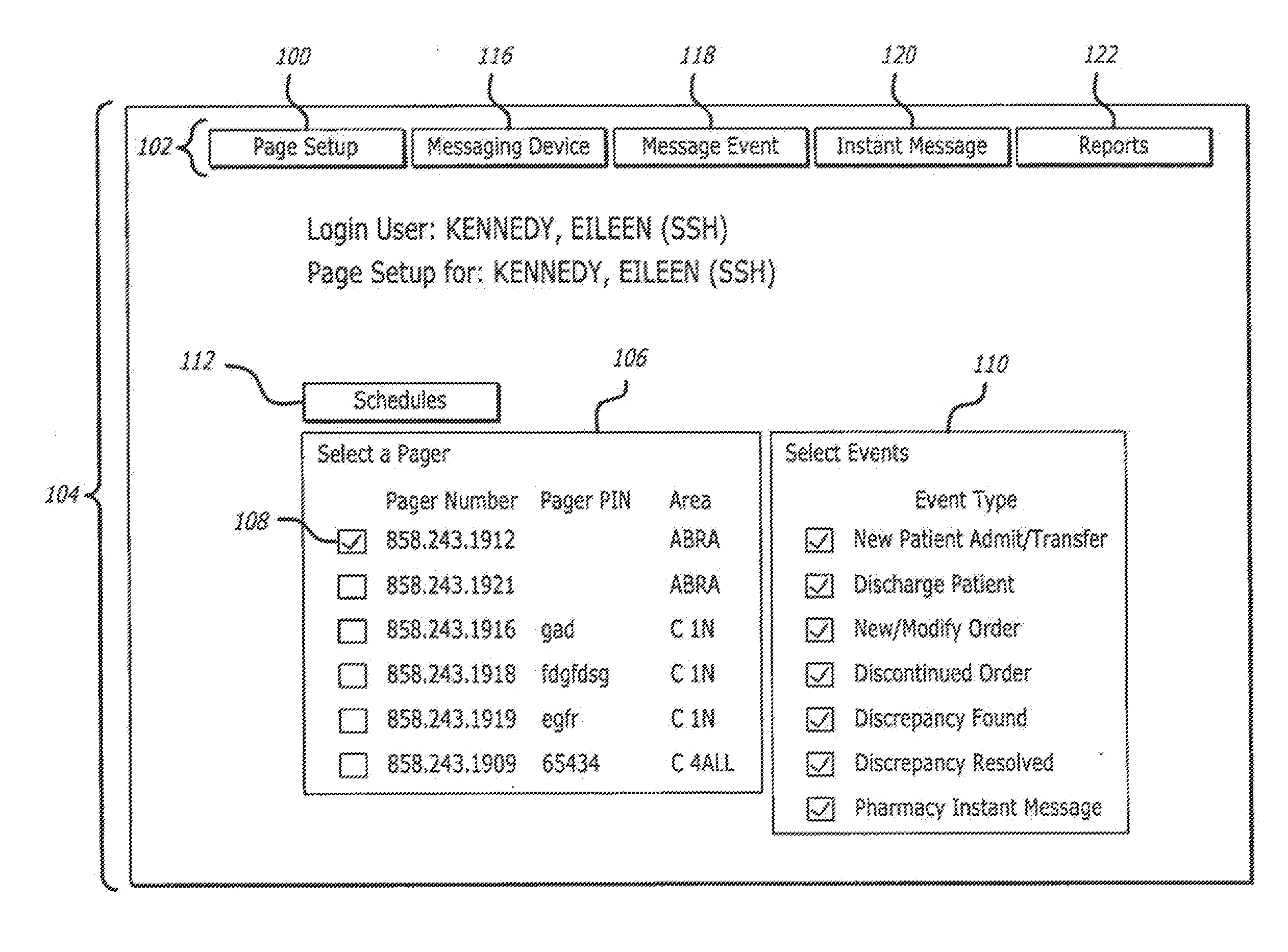
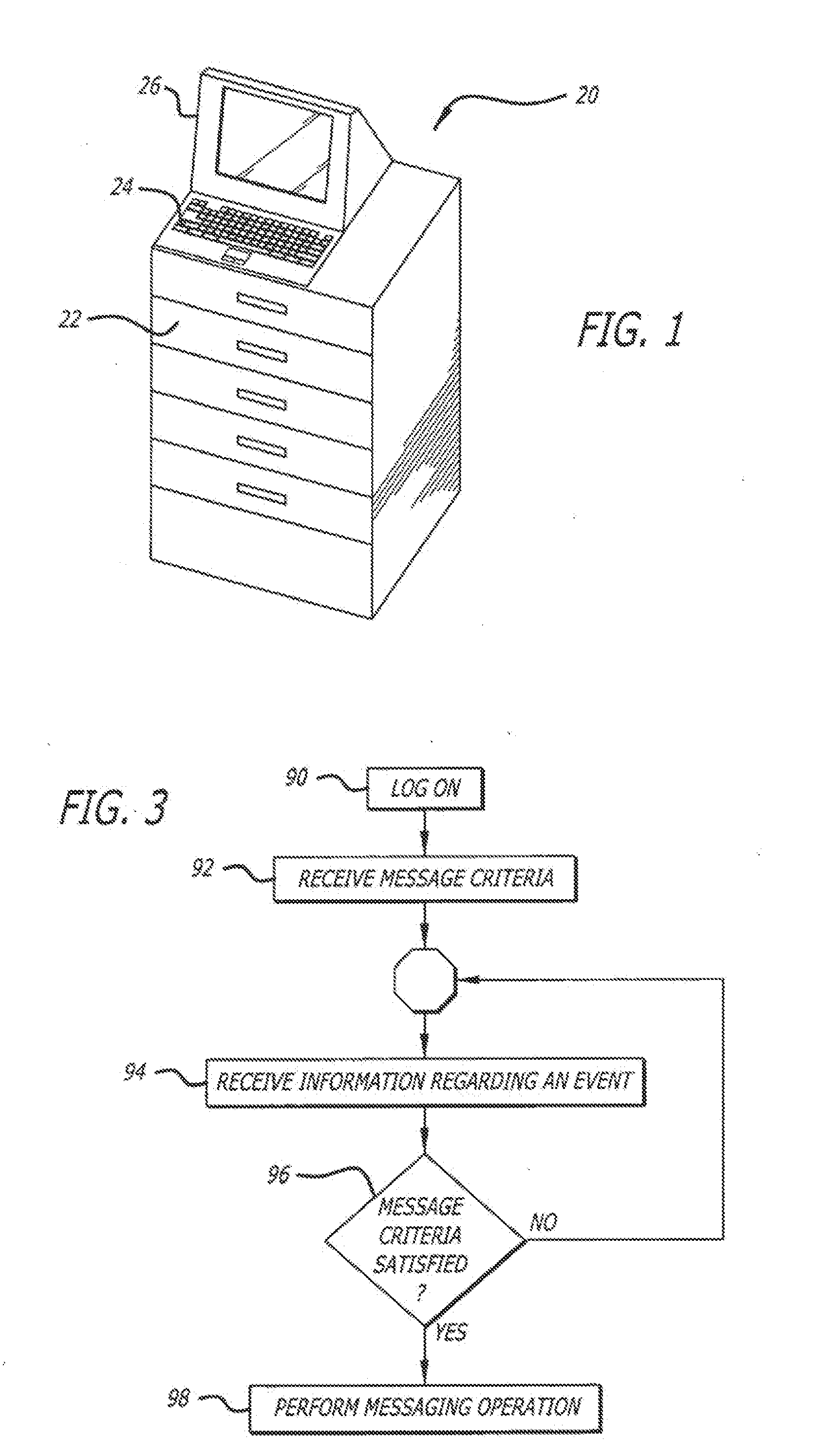
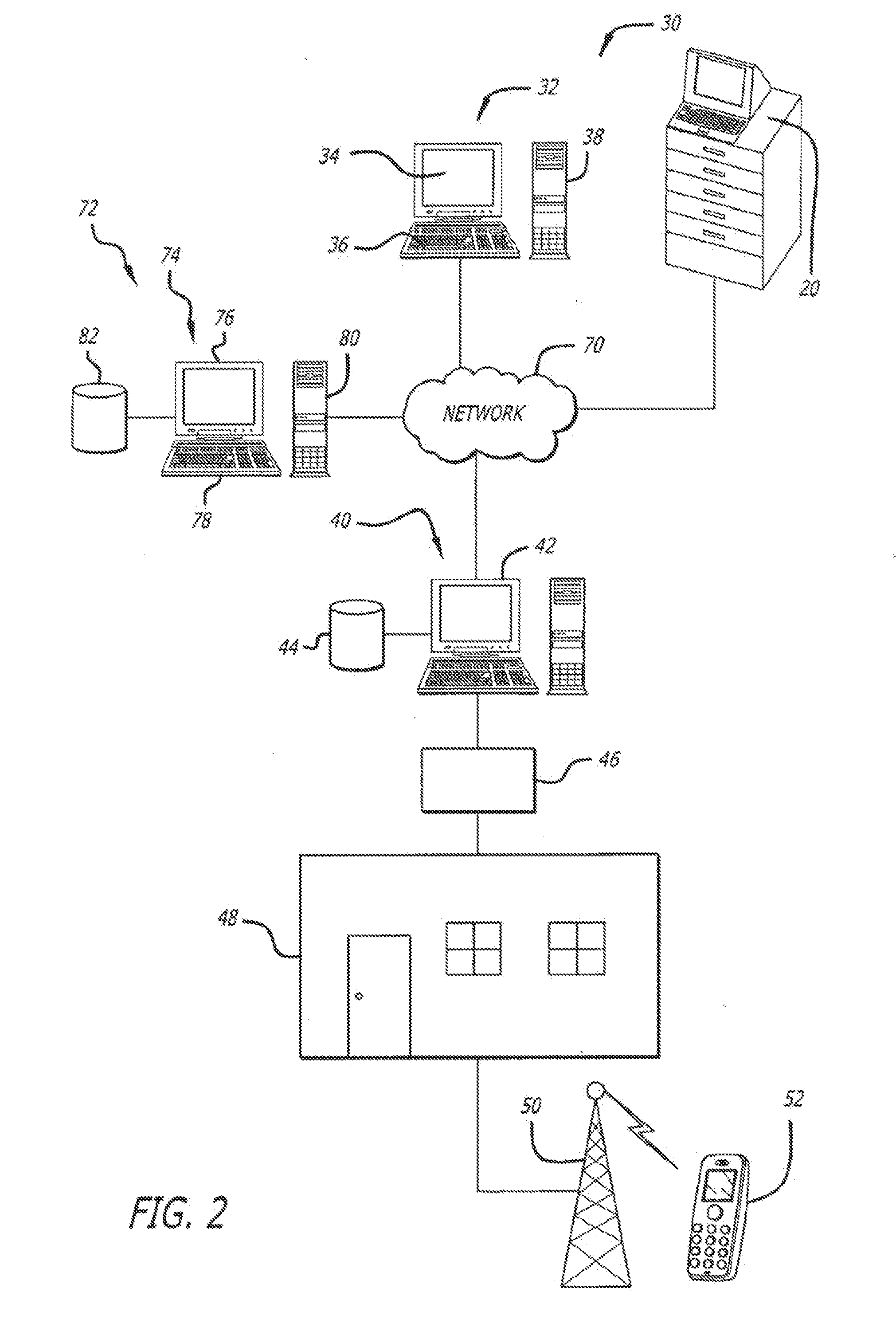
System and method for managing patient care through automated messaging
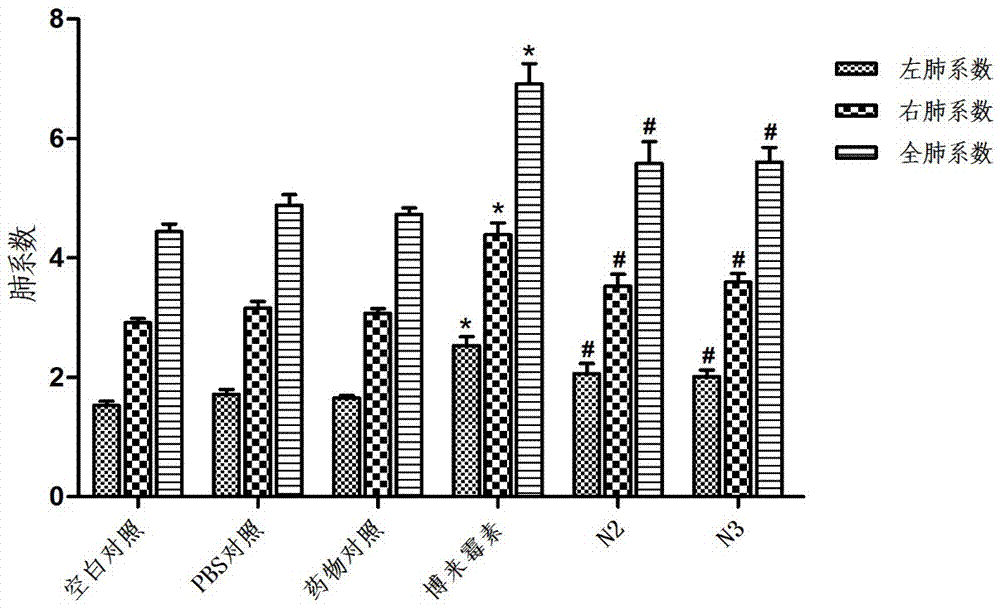
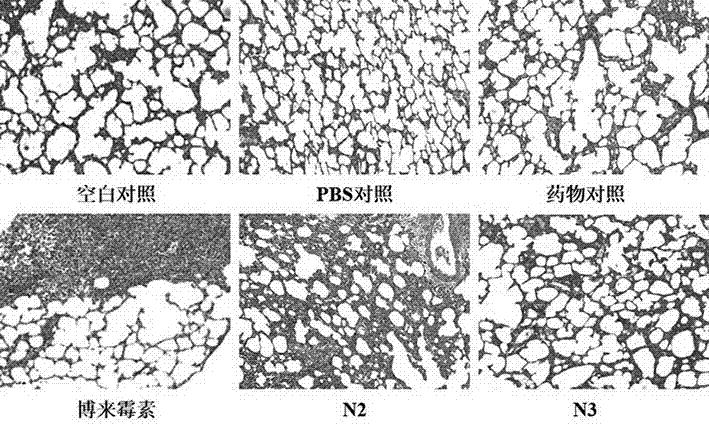
ActiveUS20120116796A1Efficient managementOptimize workflowData processing applicationsDrug and medicationsMedication DispenserNew medications
A system and method are disclosed that allow a user to select when messages related to medication events are sent and to select the content parameters of those messages. In one aspect, a medication dispenser is configured to notify a messaging processor when an identified medication that was not previously loaded in the medication dispenser is newly loaded into the medication dispenser, and the messaging processor is configured to send a message to a user through a communication link upon receipt of this notification. In another aspect, the medication is identified during a review of a new medication order issued by a doctor.
Owner:CAREFUSION 303 INC
Application of polypeptide and polypeptide derivative in prevention and treatment of fibrotic diseases
The invention belongs to the technical field of medicines, relates to the field of prevention and treatment of fibrotic diseases and discloses application of a polypeptide fragment, a polypeptide fragment derivative and a polypeptide derivative in preparation of medicines for preventing and treating the fibrotic diseases. The invention relates to a product obtained by carrying out amino acid deletion, addition and substitution on the polypeptide and a product obtained through various chemical modification, a composition of the polypeptide fragment, the polypeptide fragment derivative and the polypeptide derivative, a pharmaceutically acceptable carrier and dose form and application of the polypeptide fragment, the polypeptide fragment derivative and the polypeptide derivative in preparation of medicines for preventing and treating the fibrotic diseases. By utilizing the polypeptide fragment, the polypeptide fragment derivative and the polypeptide derivative, the inflammation and fibrotic development of tissues and organs can be effectively inhibited, and the polypeptide fragment, the polypeptide fragment derivative and the polypeptide derivative have ideal new medicine development values.
Owner:CHENGDU HUITAI BIOMEDICINE CO LTD
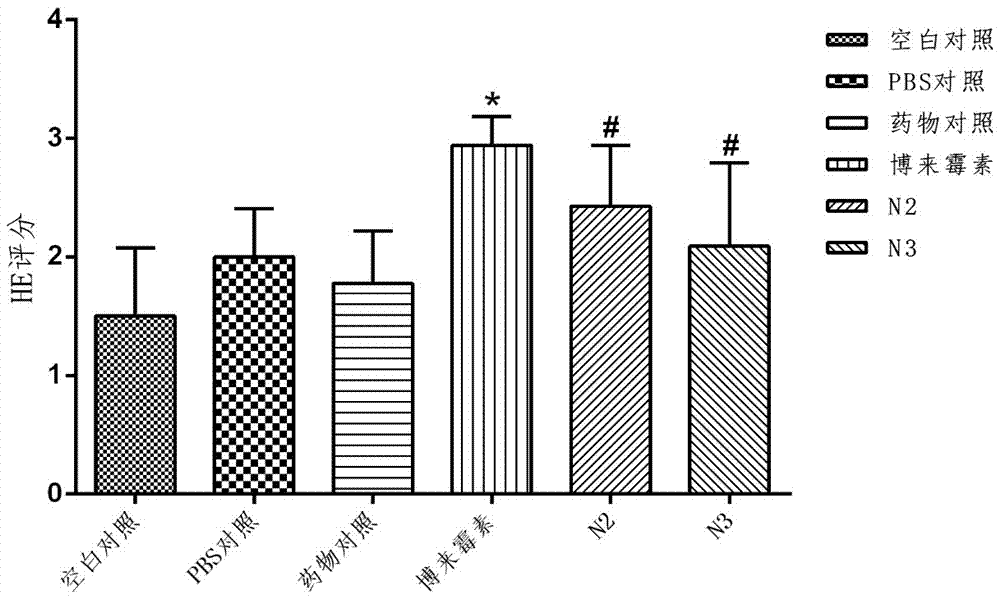
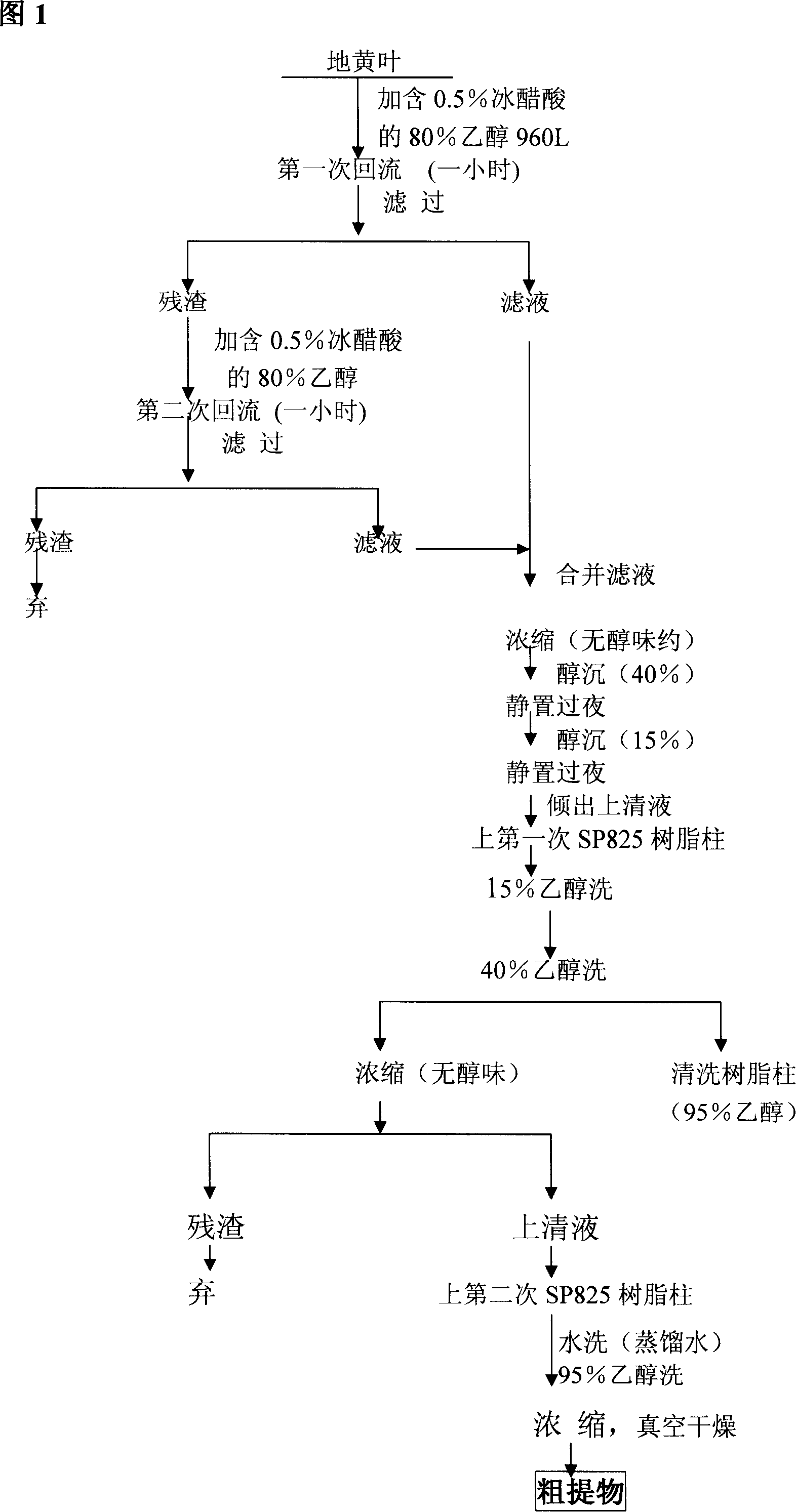
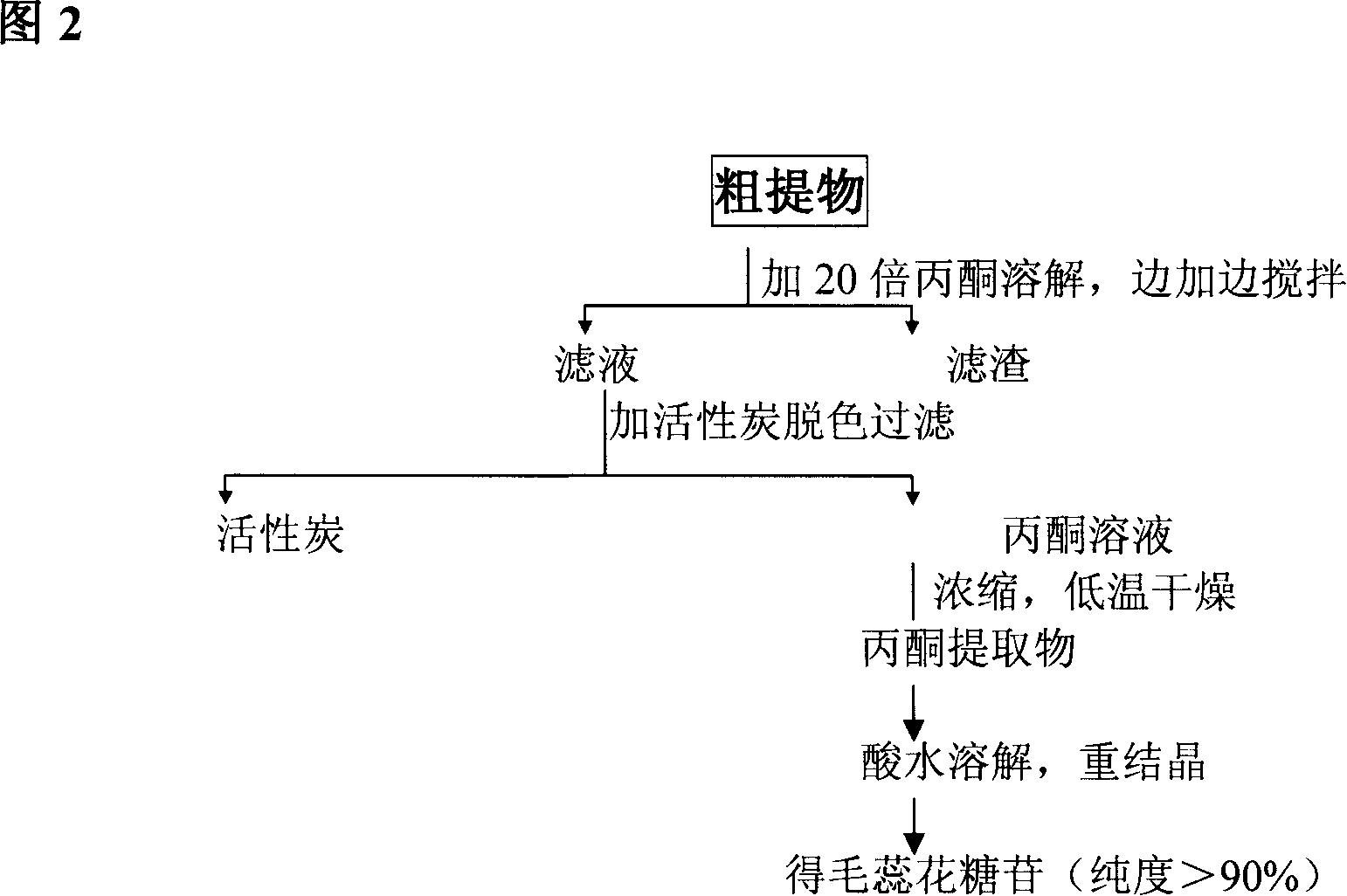
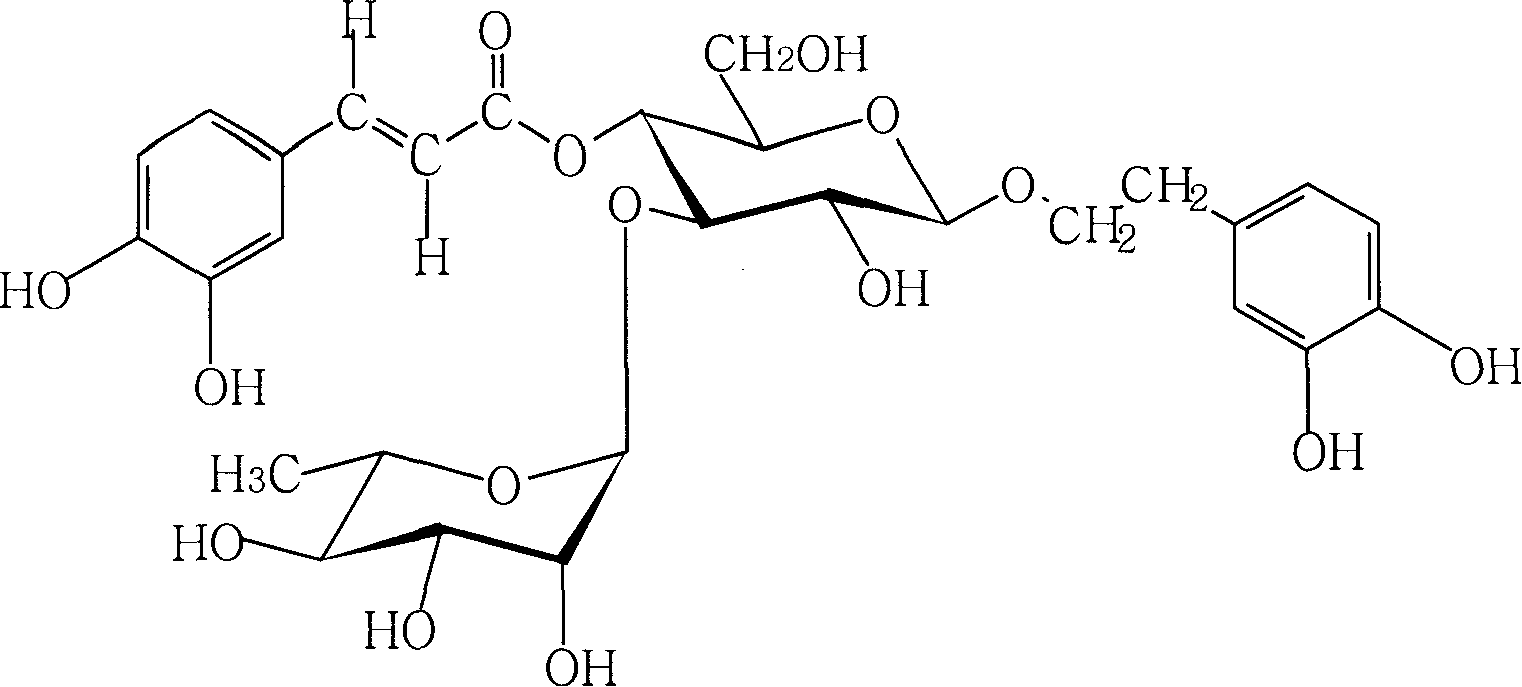
Technique for preparing verbascoside with function of curing chronic glomerulonephritis in glutinous rehmannia leaf
InactiveCN101121740ANon-toxic and safeConducive to extensive clinical applicationEsterified saccharide compoundsSugar derivativesAcute toxicity testingDigitalis
The invention belongs to the technological field of the Chinese medicine, particularly relating to an extraction and separation processes of the active ingredients with the active function of treating the chronic glomerulonephritis. The mullein indican is the active compound researched and developed for many years in our research institute; the mullein indican is extracted, separated and refined from the digitalis leaf and is an active compound with the function of treating the chronic glomerulonephritis. The invention is the research result of many years held and developed by the Chinese Medicine Research Department of the Chinese Medicine Research Institute of China and is supported by the special fund of the Central Institute of the National Science and Technology Department; now the compound has been approved by the State Food and Drug Administration Bureau as the novel drug and the mechanism research of the treatment of the chronic nephritis has been completed; the pre-clinical research work includes the main pharmacodynamic experiment, the production technology experiment (including the industrialized extraction and separation processes), the quality control standard, the experiment of the affecting factors of the quality, the experiment of the long-term stability, the acute toxicity of the rat, the experiment of the long-term toxicity, the teratogenic and mutagenic experiment, the general pharmacological experiment, the pharmacokinetics experiment, and so on. The digitalis leaf is mainly produced in the Huaiqing area of the Henan province, and is also grown wild and cultivated in the Shaanxi, Hebei and other places of China.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
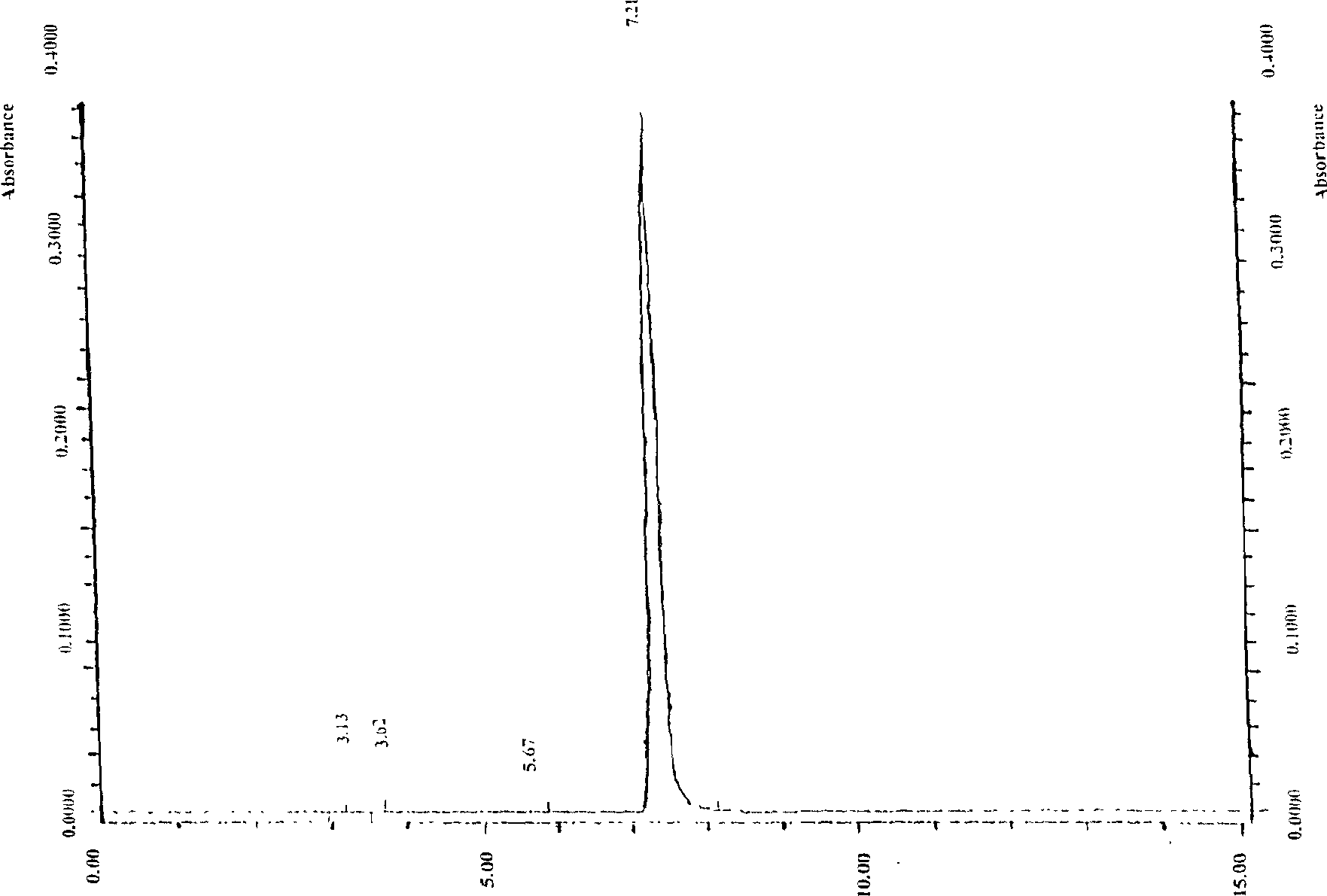
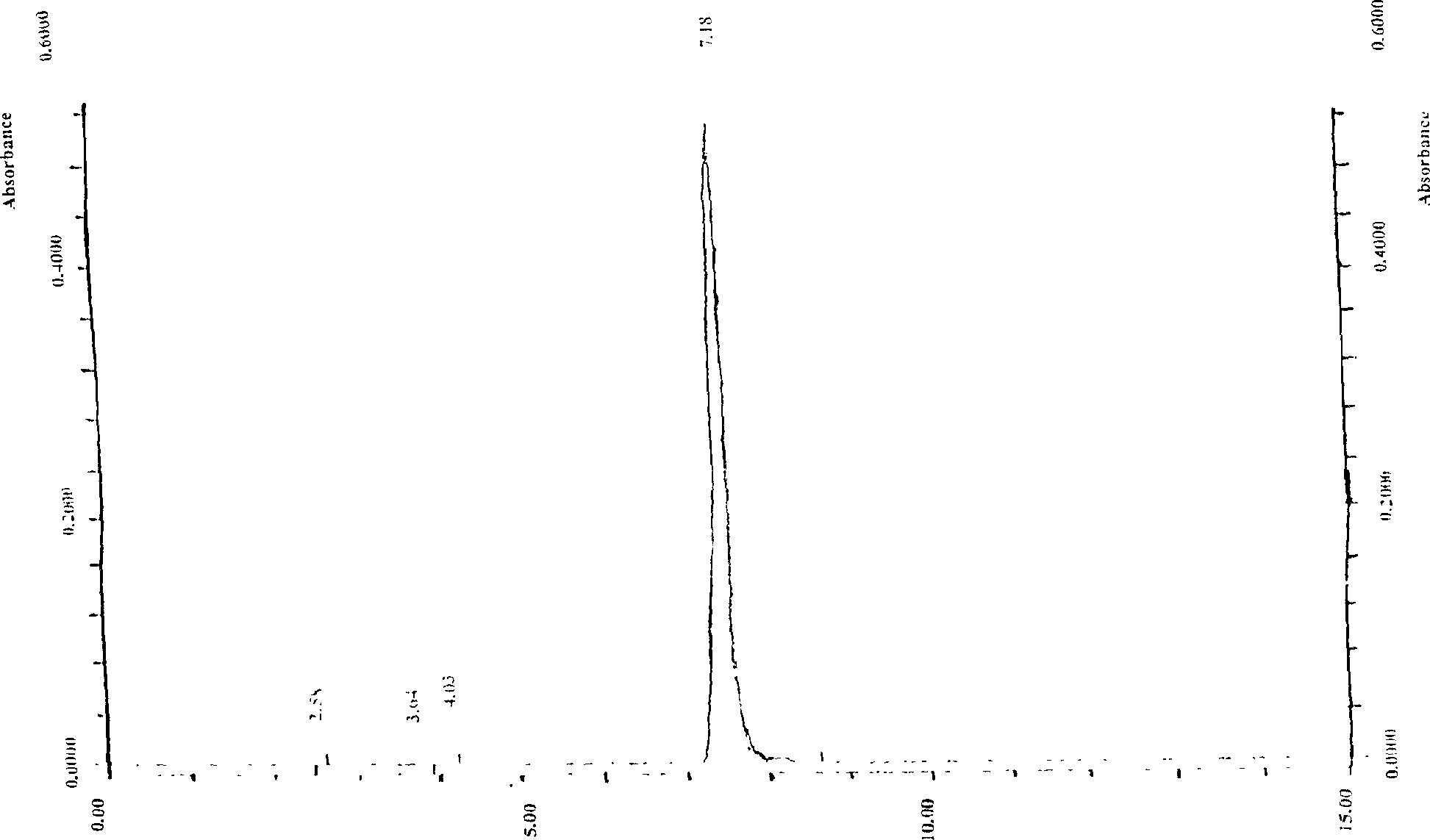
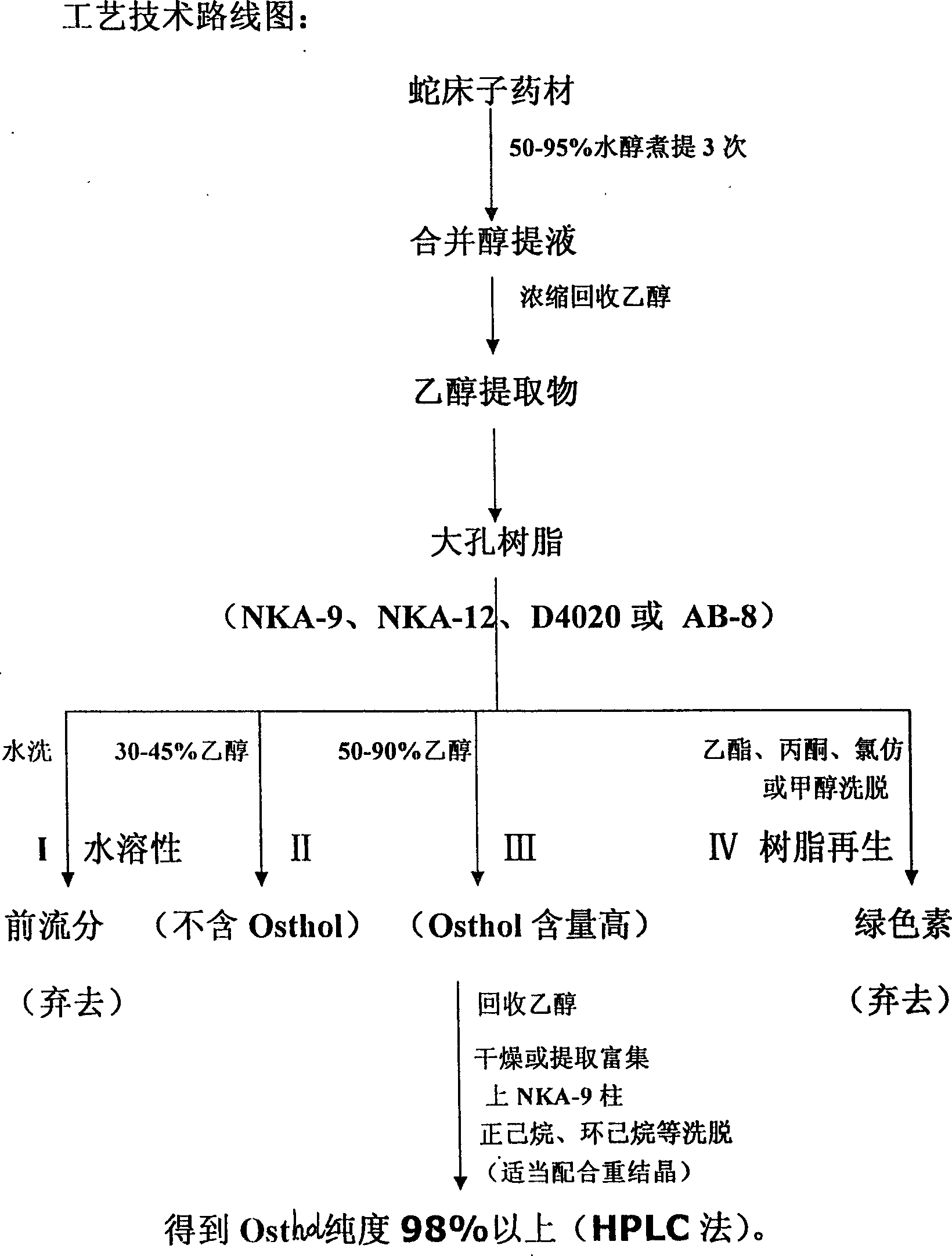
High purity cnidicin and its preparation method and medicinal composition using said compound as active component
InactiveCN1724529AOptimizing the Extraction and Separation ProcessGood reproducibilityOrganic active ingredientsOrganic chemistryStability studyAcute toxicity testing
The invention discloses a high- purity of osthol, structure proof with spectral analysis, method for preparation, stability study, acute toxicity study, the drug compositions containing osthol, and new drug utilities of the natural product. By the technique steps of extracted Chinese pharmacy conidium fruit with alcohol, decolorizing and enriching by large-hole adsorption resin, and separating and purifying, it can prepare osthol with a purity of above 98%. The study expresses that the osthol has a clear utility of anti-cancer, anti-auto lymphoma, and so on.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
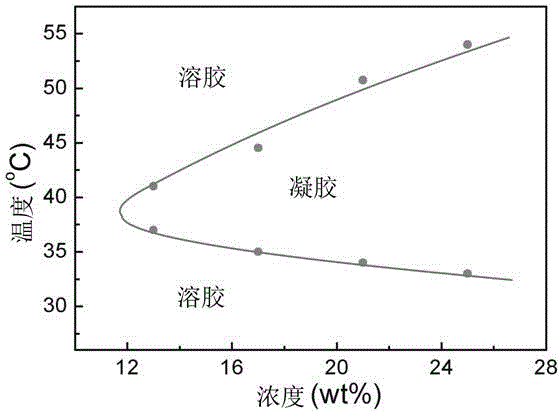
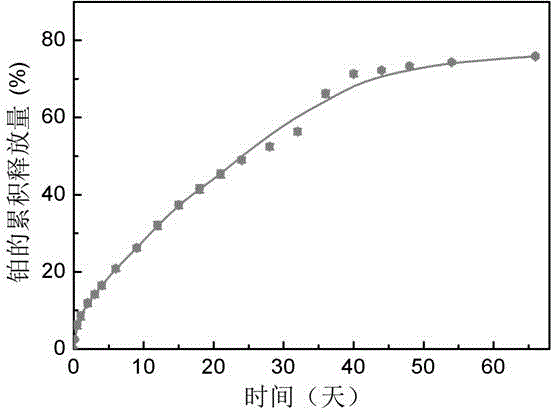
Thermal gel controlled-release injection of platinum-containing antitumor drug and preparation method of thermal gel controlled-release injection
InactiveCN104353083AReversible thermal gelation propertiesImprove liquidityAerosol deliveryOintment deliveryGel preparationPolyester
The invention belongs to the field of new chemically synthesized drugs and medical preparations thereof, particularly discloses a thermal gel controlled-release injection of a platinum-containing antitumor drug and a preparation method of the thermal gel controlled-release injection. The thermal gel controlled-release injection consists of Pt(IV)-containing amphipathic segmented copolymer and a solvent, wherein the water system can generate phase transformation of thermal gelation with temperature rise to spontaneously form physical hydrogel; the bonded Pt(IV) complex is easily reduced into Pt(IV); the amphipathic segmented copolymer consists of a hydrophilic block and a hydrophobic block which can be degraded into polyester, wherein a functional group is connected at the terminal of the block copolymer. The controlled-release gel preparation disclosed by the invention can be used for prolonging the release period of the platinum antitumor drugs. By virtue of an injection way, the preparation is dosed in tumor, around tumor or in a postoperative tumor cavity; after the preparation is gelled in situ in the body, the bonded platinum drugs can be slowly released from the gel, so that the dosing frequency and the whole body toxic and side effects are lowered.
Owner:FUDAN UNIV
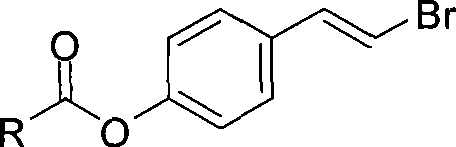
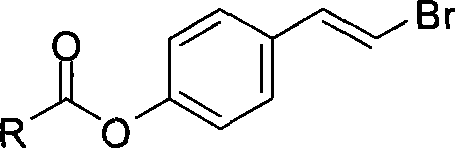
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
The invention belongs to the technical field of new drug synthesis, and in particular relates to an IDO inhibitor containing (E)-4-(beta-bromovinyl)phenoxy acyl structure, as well as a preparation method thereof. The preparation method comprises the following steps: solvent benzene, (E)-4-(beta-bromovinyl) phenol, carboxylic acid and p-dimethylaminopyridine are added to a flask respectively and magnetically stirred for 5 to 20 minutes at room temperature; then N, N'-dicyclohexylcarbodiimide is added and reacts for 2 to 24 hours at room temperature; after the reaction is completed, the solvent benzene is steamed off after decompression; residue is subjected to column chromatography separation and purification by taking ethyl acetate / petroleum ether as leacheate, and then a needed product can be obtained, wherein the molar ratio of the solvent benzene to the (E)-4-(beta-bromovinyl) phenol is 50-200:1; the molar ratio of the carboxylic acid to the (E)-4-(beta-bromovinyl) phenol is 1-1.5:1; the molar ratio of the p-dimethylaminopyridine to the (E)-4-(beta-bromovinyl) phenol is 0.1-1.5:1; and the molar ratio of the N, N'-dicyclohexylcarbodiimide to the (E)-4-(beta-bromovinyl) phenol is 1-1.2:1. The method takes the (E)-4-(beta-bromovinyl) phenol as a synthesis building block and obtains a series of the novel IDO inhibitors containing the (E)-4-(beta-bromovinyl)phenoxy acyl structure through esterification reaction, and the IDO inhibitors can be used for treating the diseases with the pathological features of IDO mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Method of detecting mononucleotide pleomorphism of CYP2D6 gene ninth exon
InactiveCN101109018ASignificant practical valueSugar derivativesMicrobiological testing/measurementNucleotideExon
The invention relates to a testing method of CYP2D6 gene exon 9's single nucleotide polymorphism, and meanwhile relates to a separation nucleic acid and an allel-specific nucleic acid primer. The method comprises steps as described below: firstly the confirmation of the 1332 nucleic acid showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9, then test of the existence of the single nucleotide polymorphism, specifically a separation nucleic acid with the SEQ ID NO: 1 and the 1322 position is A, an allel-specific nucleic acid primer with the length of 15 to 50bp and specifically hybridizes and amplifies the amplified products of the 1322 nucleic acid polymorphism showed in the SEQ ID No: 1 in the human CYP2D6 gene exon 9. The invention can be used to research the relation between CYP2D6 gene polymorphism in Chinese people and the clinical drug safety, and provide guidance to the development of new drugs.
Owner:SHANGHAI JIAO TONG UNIV
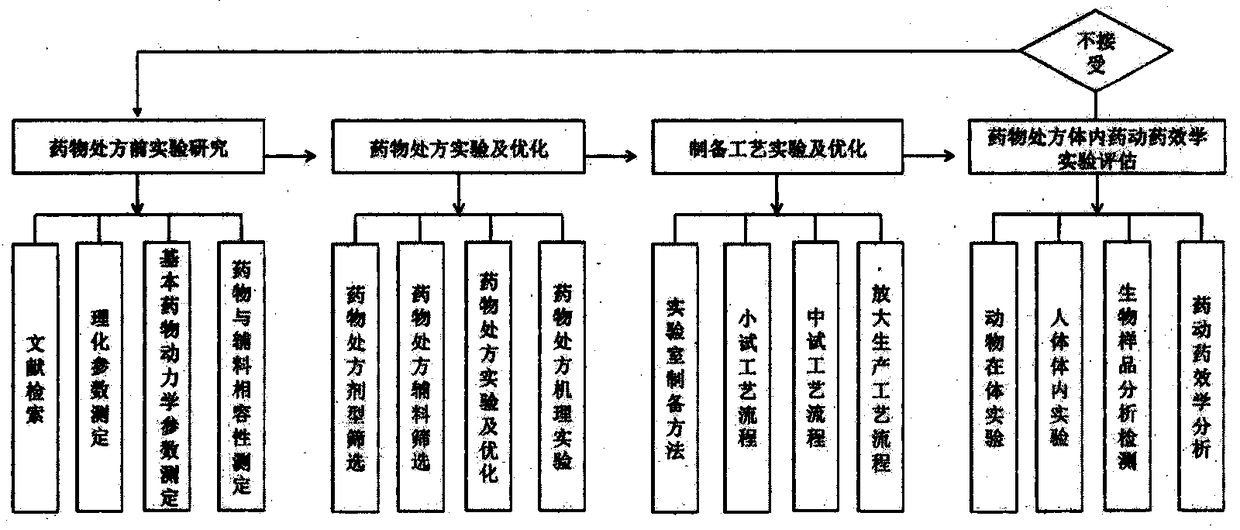
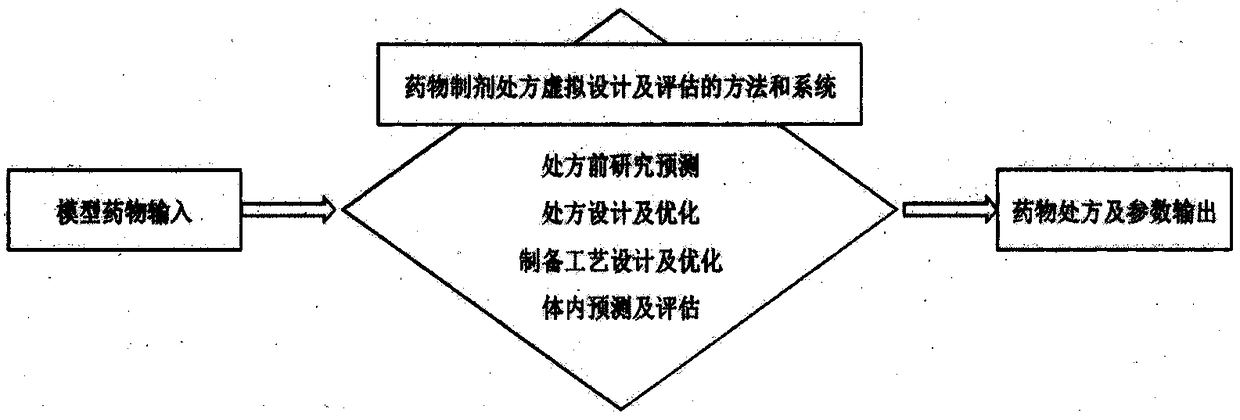
A method and a system for virtual design and evaluation of pharmaceutical formulation
InactiveCN108984811AObtain basic physical and chemical parametersGain stabilityComputing modelsDesign optimisation/simulationPre formulationIn vivo
A method and a system for virtual design and evaluation of pharmaceutical formulation are provided. The invention discloses the method and the system for virtual design and evaluation of pharmaceutical preparation prescriptions, and the system mainly comprises a pre-prescription research module, a pharmaceutical prescription design and optimization module, a pharmaceutical preparation process design and optimization module, and a pharmaceutical prescription in-vivo prediction and evaluation module. The method and the system for virtual design and evaluation of pharmaceutical formulation predict the whole process of pharmaceutical formulation from four aspects of pharmaceutical pre-formulation research, formulation design, preparation process design and pharmacokinetics prediction in vivo.Compare with a conventional empirical drug prescription screening and preparation method in a traditional laboratory, the method and the system can accelerate the development of drug product, do not consume any laboratory equipment, complete the virtual design and evaluation of drug formulation prescription through a computer platform, shorten the drug development cycle and save the development cost of new drugs.
Owner:欧阳德方
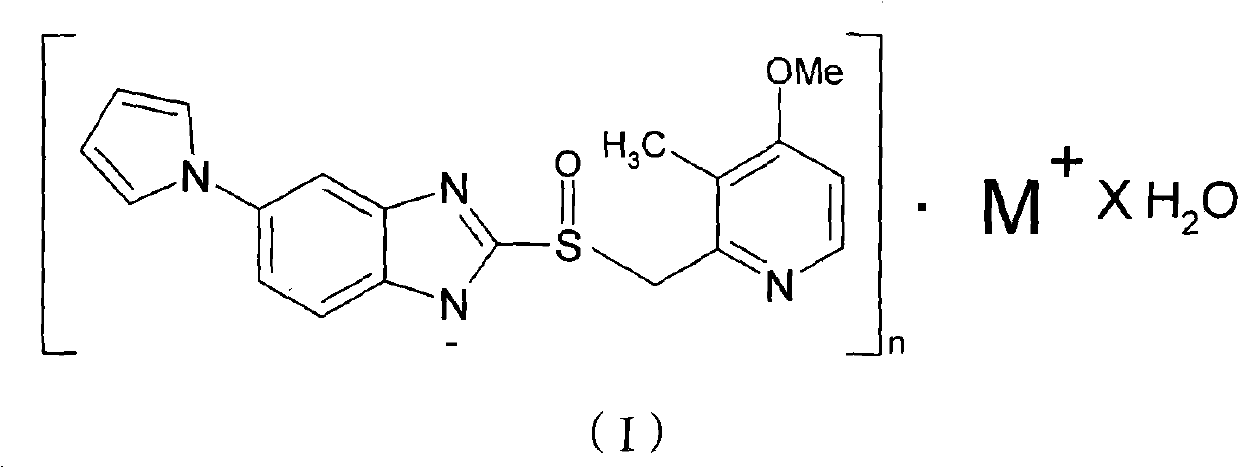
Hydrate of ilaprazole salt, preparation method thereof and application thereof
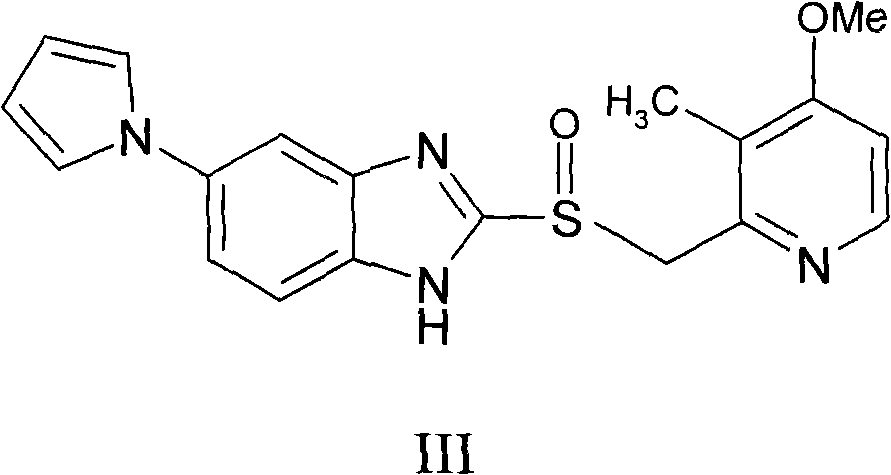
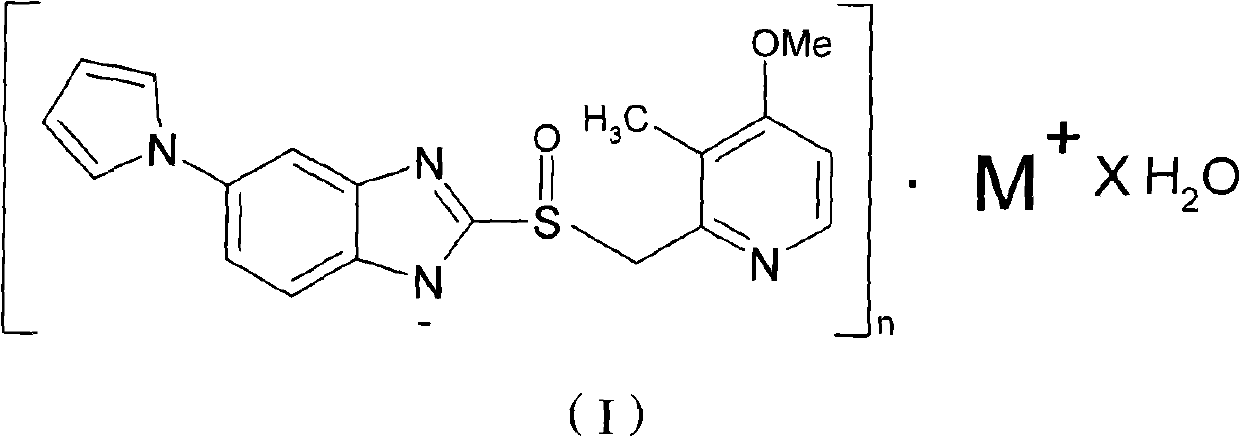
ActiveCN102140092AGood treatment effectLittle side effectsOrganic active ingredientsOrganic chemistrySide effectIlaprazole
The invention provides a hydrate of an ilaprazole salt and a preparation method thereof. The hydrate is shown by a formula (I), wherein M is Li, Na or K, n is equal to 1, and x is equal to 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 4.5 or M is Ca, Mg or Zn, n is equal to 2 and x is equal to 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 4.5. The invention also provides application of the hydrate of the ilaprazole salt. Compared with ilaprazole and a salt thereof, the hydrate of the ilaprazole salt can be used as a proton pump inhibitor for treating diseases relevant with disorder in gastric acid secretion, has low side effect, and can be developed to form new clinically-acceptable medicaments. Simultaneously, the preparation method is simple and easy, and repeated experiments prove that the hydrate of the ilaprazole salt has a stable process and can be used for industrial production.
Owner:LIVZON PHARM GRP INC
Method for detecting esculin, aesculetin, fraxin and fraxetin in cortex fraxini or extract thereof
InactiveCN102091132AReduce pollutionPromote resultsAntibacterial agentsSenses disorderCortex FraxiniCapillary electrophoresis
The invention discloses a high performance capillary electrophoresis (HPCE) quick quantitative method for four active components of fraxin, esculin, fraxetin and aesculetin in cortex fraxini or extract thereof. By the method provided by the invention, four cortex fraxini coumarins are well separated and determined in 10min, and simultaneously all the components have good linear relations and highrecovery rates. The method provided by the invention has good relativity of determination results compared with a high performance liquid chromatography (HPLC) method, greatly shortens the analyzing time, obviously reduces environmental pollution, notably decreases the analyzing and operating cost, provides a quick efficient analyzing means for researching the distribution of the active components of cortex fraxini and the variation rule of content in different harvest phases, and also can be used for researching the inherent quality of the cortex fraxini extract and for referencing in development research of new drugs.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Tacrolimus ophthalmic in-situ gel preparation and preparation method thereof
InactiveCN101810563AImprove bioavailabilityDosing is easy and accurateOrganic active ingredientsSenses disorderSolubilityKeratoconjunctivitis
The invention provides a tacrolimus ophthalmic in-situ gel preparation and a preparation method thereof, which micronizes medicaments or adopts the solid dispersion technology, the cyclodextrin inclusion technology or the like to obviously improve the solubility and then to prepare into an ophthalmic preparation which is in the liquid state in vitro and is in the gel state when being dropped into eyes. The invention is applicable to the treatment for preventing and curing ophthalmic corneal transplantation rejection, keratoconjunctivitis (KC), ocular allergic disorders and the like. The invention prepares tacrolimus into the ophthalmic in-situ gel for the first time. The ophthalmic gel is in the liquid state in vitro, the dosage can be easily and accurately controlled, the operation is convenient, and the ophthalmic gel can be uniformly spread when being dropped into eyes to form the gel. The ophthalmic gel can stay in eyes for long, can not be easily diluted by tears, can maintain the concentration of the effective medicaments, enhances the treatment effect, and has the advantages of less stimulation and good biocompatibility, thereby being an effective new ophthalmic medicine, being applicable to the clinic treatment and having extensive development prospect.
Owner:宋洪涛
Method, system, apparatus and device for discovering and preparing chemical compounds for medical and other uses.
InactiveUS20040115726A1Intervention of moreModification of moreCompound screeningApoptosis detectionProtein targetUser interface
Disclosed in this invention are methods, systems, databases, user-interfaces, software, media, and services useful for evaluating interactions between chemical compounds and proteins and for utilizing the information resulting from such evaluation for the purpose of discovering chemical compounds for medical and other fields. An approach termed "reverse proteomics" is disclosed. This invention generates an enormously large pool of new target proteins for drug discovery, novel methods for designing of new drugs, and a previously unthinkable pool of virtually synthesized small molecules for therapeutic uses. This invention is also applicable, for example, to discovery of substitutes for environmentally hazardous chemicals, more effective agrochemicals, and healthier food additives.
Owner:REVERSE PROTEOMICS RES INST
Alkaloid compound in purslane herb and extraction and separation method thereof
ActiveCN106946766AHigh purityEasy to operateNervous disorderOrganic chemistryAdditive ingredientUltraviolet
The invention relates to the field of extraction and separation of traditional Chinese medicines, and in particular relates to a novel alkaloid compound extracted, separated and identified from purslane herb and an extraction and separation method thereof. The molecular formula of the novel alkaloid compound is C18H17NO4, and the name is oleraciamide D. The invention further provides the extraction and separation method for the novel alkaloid compound, which sequentially adopts water decoction for extraction, extraction in ethyl acetate, silica gel column chromatography, ODS (octadecylsilyl) medium-pressure column, purification by Sephadex LH-20 and liquid phase separation for preparation. The structure is determined as a novel alkaloid compound by adopting UV (ultraviolet), <1>H, NMR (nuclear magnetic resonance), <13>C NMR and two-dimensional NMR spectrum analysis methods. The compound has potential anti-inflammatory and neuroprotection activity and the like, moreover, the preparation method is provided, and a primer and a theoretical basis are provided for the development of new medicines and new ingredients.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Medicine preparations for curing dermatosis and preparation method thereof
InactiveCN101181395AWith expelling wind and dampnessClearing away heat and cooling bloodImmunological disordersDermatological disorderDiseaseSide effect
The invention provides a pharmaceutical preparation for treating skin diseases and a preparation method thereof. It is mainly prepared from the raw materials of S. Some or all of the Kochia scoparia medicinal materials are used in combination. Compared with the prior art, the formula provided by the present invention can be prepared into oral preparations or external preparations according to the conventional preparation process. Sexual skin diseases have a good curative effect, the total effective rate can reach more than 95%, and it is safe to use, has no toxic and side effects, and does not produce drug resistance. It is a safe, effective and quality-controllable treatment for itchy skin diseases. New medicine of traditional Chinese medicine.
Owner:贵阳春科药业技术研发有限公司
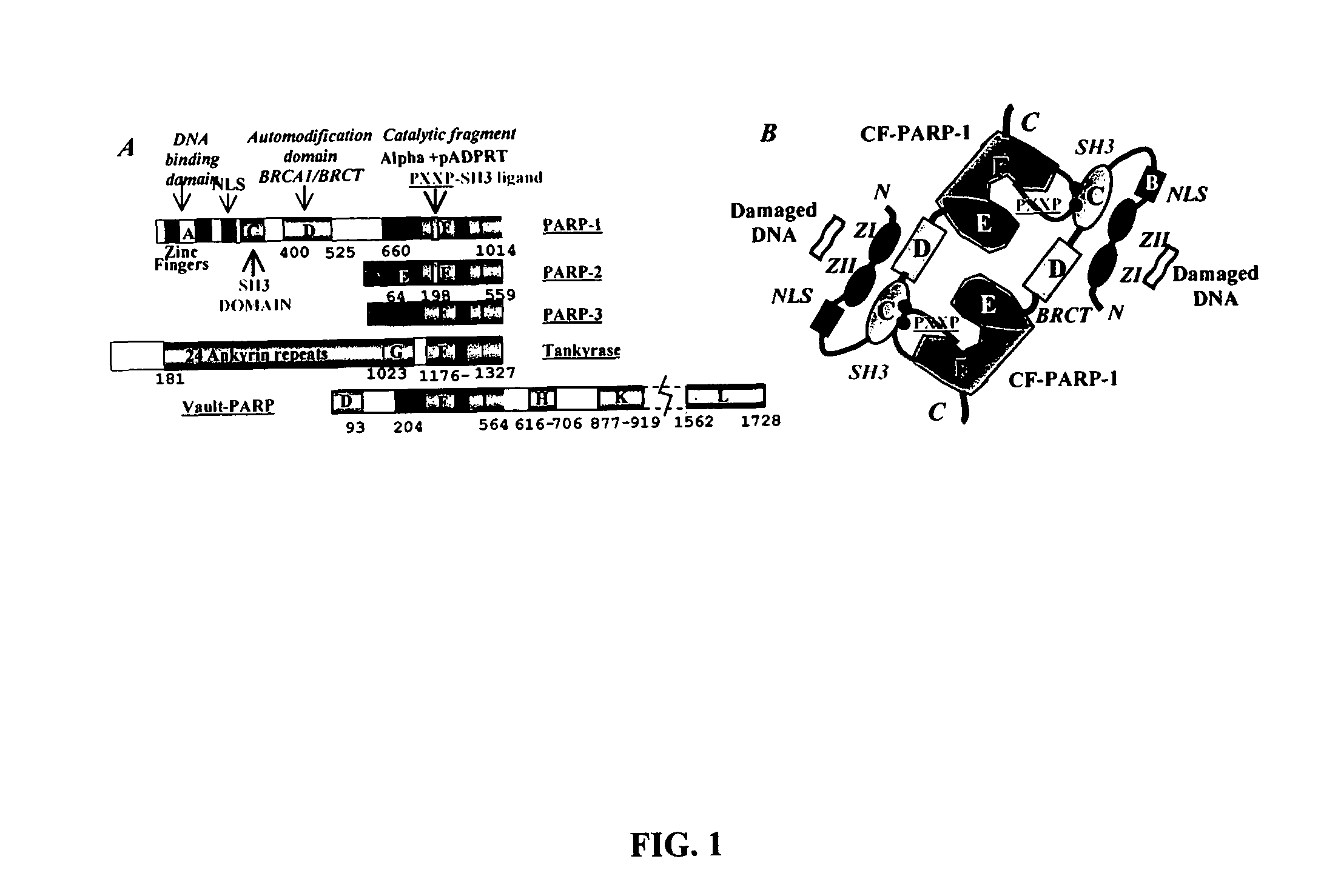
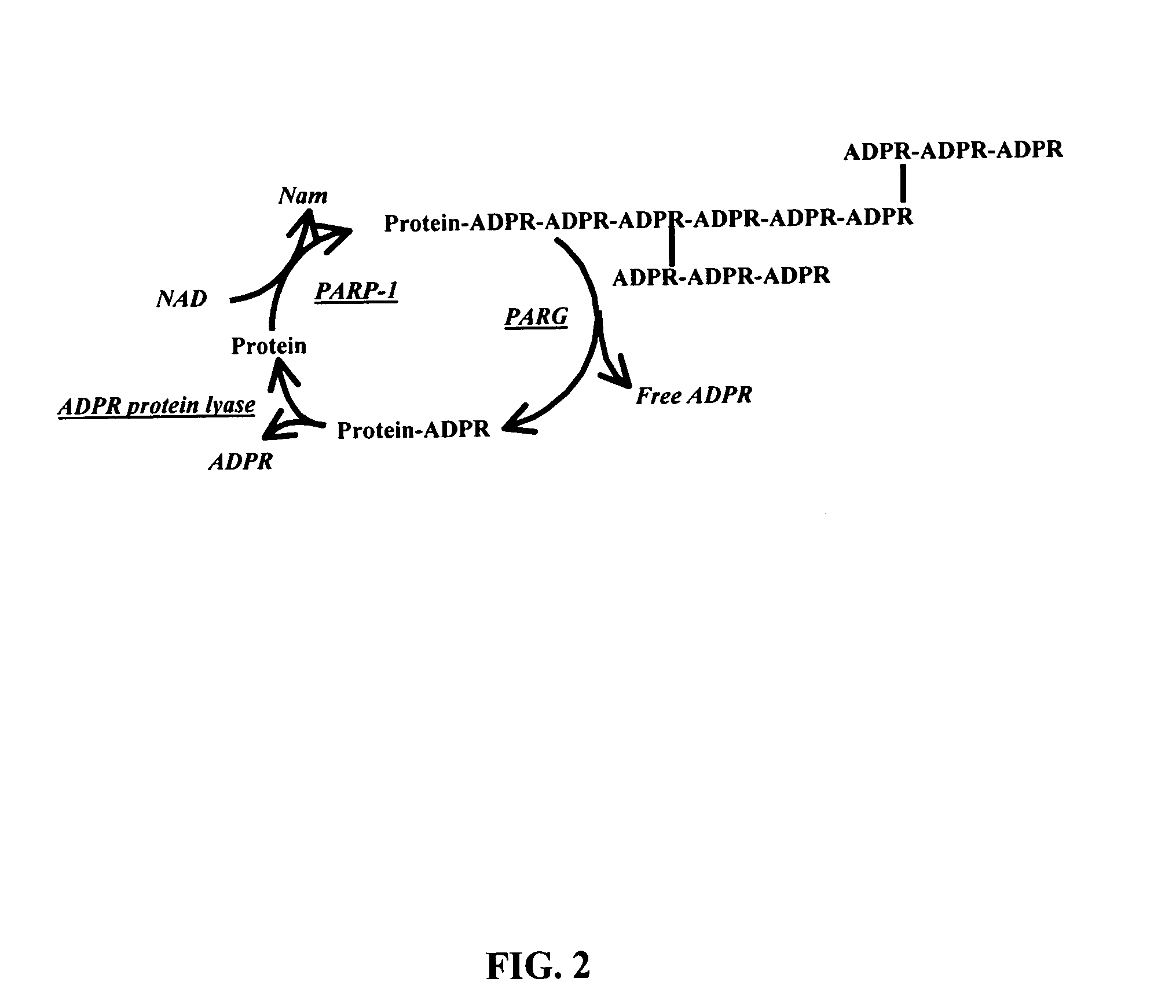
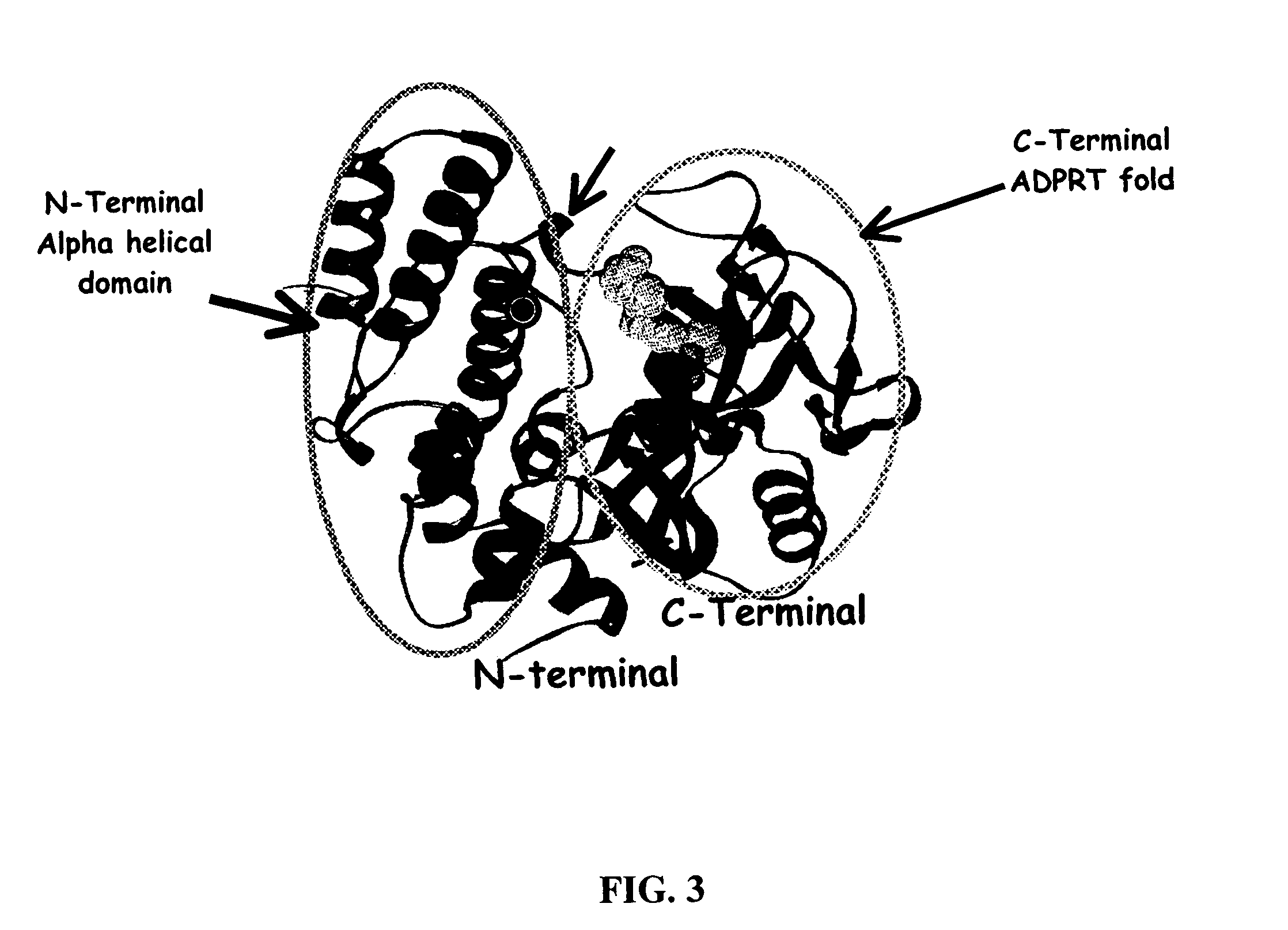
Selective PARP-1 targeting for designing chemo/radio sensitizing agents
InactiveUS7072771B2Affect activityMicrobiological testing/measurementBiological testingBiological activationDrug target
Poly(ADP-ribose) polymerase-1 (PARP-1) is a central signaling enzyme in a cell nucleus. PARP-1 is a target for the development of radio and chemo sensitizing agents in cancer treatment as well as providing protection from stroke. An SH3 domain and an SH3 ligand domain have now been discovered on the PARP-1 protein. These domains are involved in PARP-1 activation. This discovery makes possible the use of bioinformatics tools for the design of new drugs and strategies for drug target selection, specifically targeting the PARP-1 enzyme.
Owner:UNIV OF KENTUCKY RES FOUND
Method for screening traditional Chinese medicine composition with network targeting effect from molecular level
PendingCN113808759AThe therapeutic target is clearClear mechanismChemical property predictionProteomicsDiseaseNew medications
Although the compatibility principle of monarch, minister, assistant and guide formed under the traditional Chinese medicine theory is ordered, the action mechanism and scientific essence of the medicine cannot be revealed from essence, and the clinical effective rate of the traditional Chinese medicine is low. Therefore, the invention provides a method for screening the traditional Chinese medicine composition with the network targeting effect from the molecular level, the correlation between the disease symptom and the traditional Chinese medicine symptom is established from the molecular level through gene data analysis and traditional Chinese medicine data analysis, so that a traditional Chinese medicine action network with clear targeting and clear mechanism is obtained, and finally a network targeting formula is formed. A new thought is provided for research and development and action mechanism research of new traditional Chinese medicines, and modernization and internationalization of traditional Chinese medicines are assisted.
Owner:华子昂
Method for quantitatively evaluating medicament toxicity by using metabonomic technology
InactiveCN101813680AReduce deviationImprove objectivityComponent separationNMR - Nuclear magnetic resonanceMathematical model
The invention discloses a method for quantitatively evaluating medicament toxicity by using metabonomic technology, which is characterized by comprising the following steps: comprehensively and quantitatively measuring small molecule compounds in a biological sample by using the measuring technology based on mass spectrum, nuclear magnetic resonance and the like, then establishing a multi-dimensional spatial mathematical model by adopting a multi-variable data processing method, calculating a relative distance between an administration group and a control group and taking the relative distance as a quantitative index for evaluating the medicament toxicity so as to solve the difficult problem that the medicament toxicity evaluation lacks the quantitative evaluating index. Compared with the conventional toxicity evaluating method, the method has the advantages of wide application, sensitivity, simple and convenient sampling, no harm to the body, and capability of reflecting the toxicity function more comprehensively, and providing a comprehensive and reliable quantitative evaluating method for toxicity evaluation in new medicament research and development and pharmacologic research.
Owner:CHINA PHARM UNIV
Application of polypeptide, polypeptide fragments and derivatives thereof in the prevention and treatment of fibrotic diseases
ActiveCN109384830AInhibition of transductionReduce infiltrationPeptide/protein ingredientsAntipyreticDiseaseFibrosis
The invention belongs to the technical field of a medicine and relates to the field of prevention and treatment of fibrotic diseases, and discloses polypeptide, polypeptide fragments and derivatives thereof, and an application of polypeptide, polypeptide fragments and derivatives in preparing medicines for preventing and treating fibrotic diseases. The present invention relates to a product afteramino acid deletion, addition, and replacement of the polypeptide, and relates to the application of a chemically modified product, a composition of the polypeptide, the polypeptide fragment and the derivative thereof, pharmaceutically available carriers and dosage forms, and drugs for prevention and treatment of fibrotic diseases. The polypeptide, the polypeptide fragment and the derivative thereof can effectively inhibit the development of tissue and organ inflammation and fibrosis, and have an ideal new drug development value.
Owner:CHENGDU HUITAI BIOMEDICINE CO LTD
Methods for identifying new drug leads and new therapeutic uses for known drugs
InactiveUS20060094059A1Large scaleWide applicabilityPeptide librariesLibrary screeningCellular pathwaysHuman tumor
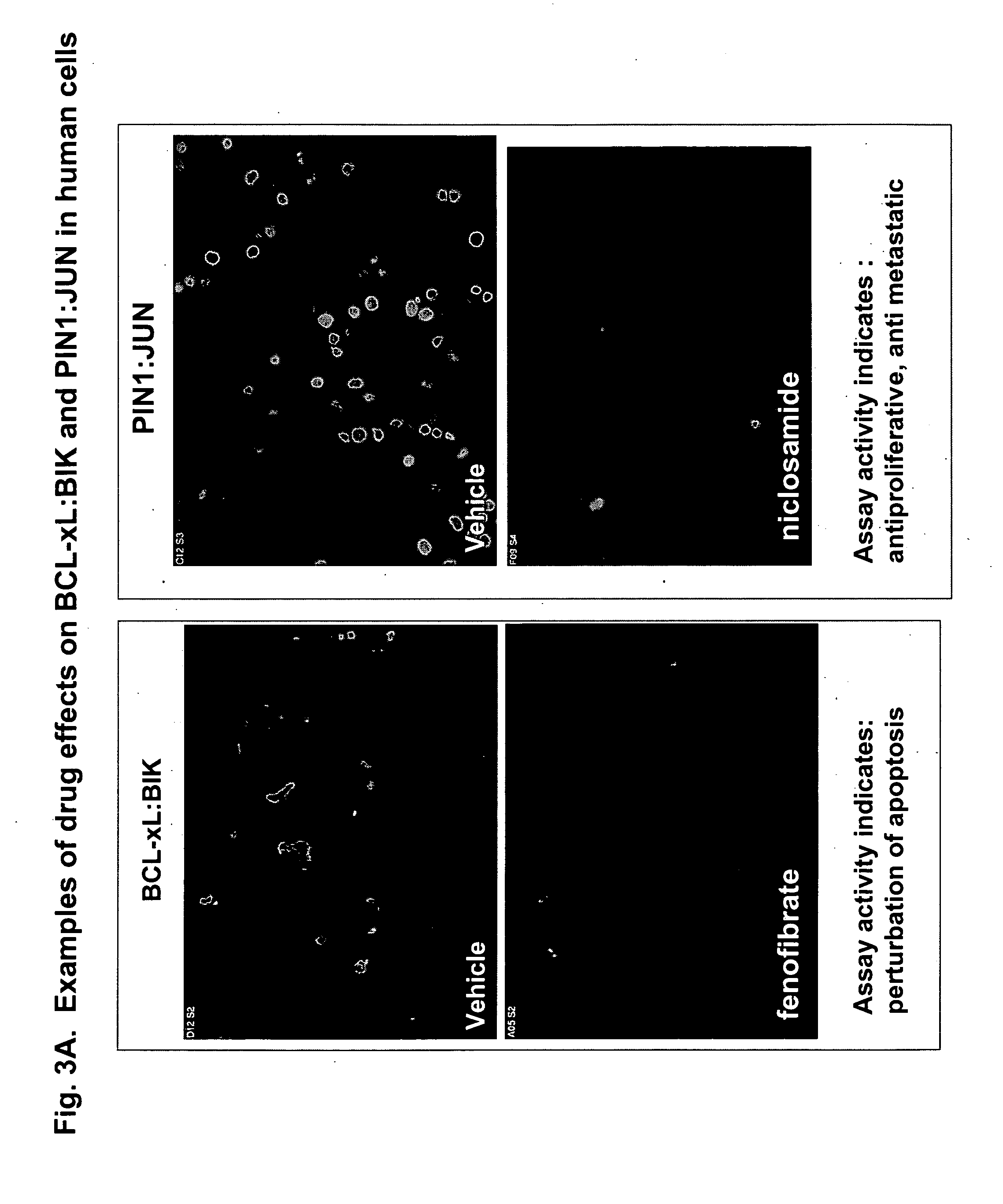
The screening system utilizes dynamic measurements of pathway activity to detect the activities of drugs within cellular pathways. The methods of the invention can be used to identify previously unknown drug activities and therapeutic uses, even for drugs that have been well characterized with standard biochemical assays. We demonstrated the utility of the invention by screening a portion of the known pharmacopeia. We identified dozens of drugs, previously or currently marked for a variety of indications, with surprising and previously-unsuspected activity against ‘hallmark’ cancer pathways. We also showed that over 20 of these drugs indeed have anti-proliferative activity in human tumor cells, underscoring the utility and predictability of the screening system. The methodology will extend the utility of the current pharmacopeia and provide the basis for de novo discovery of drugs with a broad range of therapeutic indications.
Owner:ODYSSEY THERA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com