Exendin-4 modified by Evans blue or derivatives of Evans blue and preparation method and application of Exendin-4
A technology of Evans blue and its derivatives, applied in the field of pharmaceutical preparations and biomedicine, can solve the problems of weakening the binding force between Exendin-4 and receptors, reducing the biological activity of Exendin-4, limiting the application of Exendin-4, etc., to achieve drug efficacy Long-lasting, easy to prepare, and easy to store effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
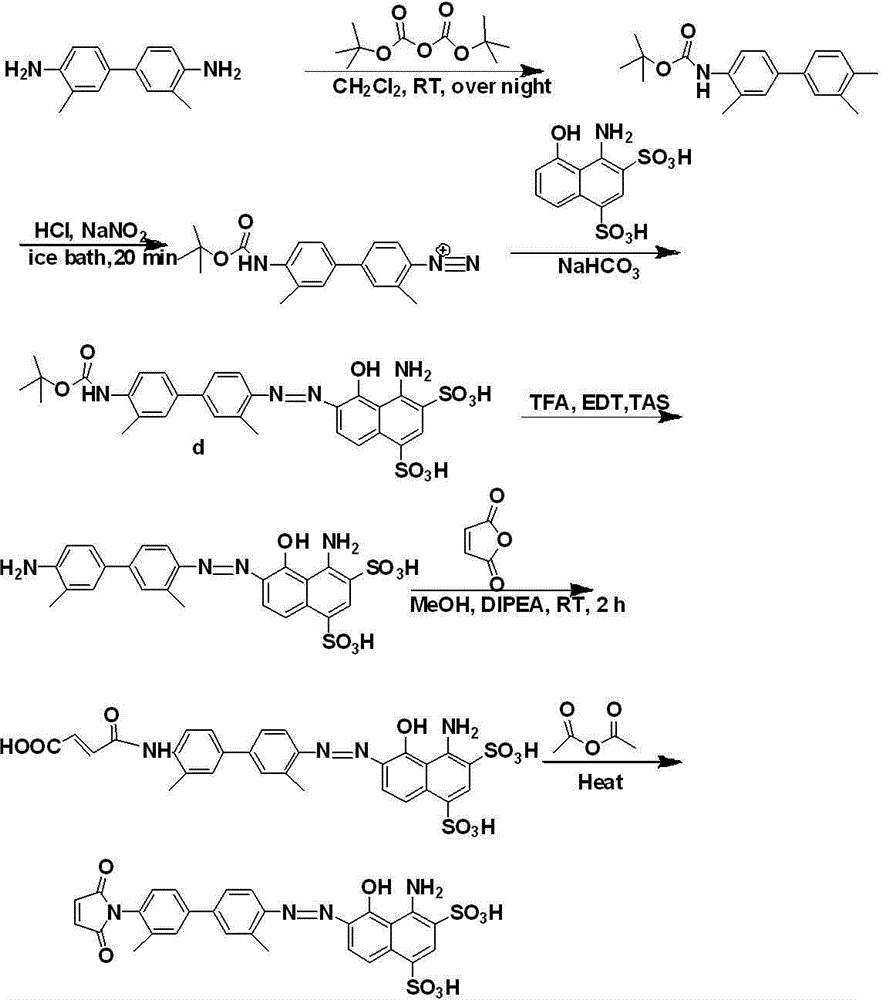
[0115] Preparation of Maleimide Evans Blue Derivatives
[0116] a.Boc-benzidine
[0117] Dissolve 4.3g of benzidine (dimethyl diaminobiphenyl) in 40ml of dichloromethane, place it in a 100ml round bottom flask, add 4.4g (4.6mL) of di-tert-butyl dicarbonate to the solution . Stir overnight at room temperature. Monitor the reaction process with thin-layer chromatography, climb the plate in the developer of n-hexane:ethyl acetate=7:3, three points (benzidine, Boc-benzidine, two-dicarbonic acid ditert-tert) appear butyl ester). After column separation, 3.2 g of the target product was obtained.
[0118] b.Boc-EB
[0119] 0.46 g of Boc-benzidine was dissolved in 10 ml of chloroform, and 15 ml of water (4.5 mmol of HCl in it) was added to the solution under ice cooling. Dissolve 0.31g NaNO2 in 5ml water under ice bath, and add this solution dropwise to the above solution, and stir for 20min under ice bath. At this point, the solution turned positive yellow indicating the forma...
Embodiment 2
[0136] Add 0.5mL Exendin-4 (1mg / mL in 50mM sodium acetate, pH 5.5) to Evans blue (0.76μmol in 50mM sodium acetate, pH 5.5) with aldehyde group, and then add 20mM NaC-NBH 3 as a reducing agent. The molar ratio of Evans blue to Exendin-4 is 1:1. Evan's blue and Exenatide-4 were reacted at 4°C for 2 hours under dark conditions. The reaction was terminated with 0.1% aqueous trifluoroacetic acid (TFA) to obtain Evans blue-modified Exendin-4.
Embodiment 3
[0138] Add 0.5 mL of Exendin-4 (1 mg / mL in PBS, pH 7.5) to Evans blue (0.76 μmol in PBS, pH 7.5) with a pyridyldithiol group, followed by 20 mM NaC -NBH 3 as a reducing agent. The molar ratio of Evans blue to Exendin-4 is 1:1. Evan's blue and Exenatide-4 were reacted at 4°C for 2 hours under dark conditions. The reaction was terminated with 0.1% aqueous trifluoroacetic acid (TFA) to obtain Evans blue-modified Exendin-4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com