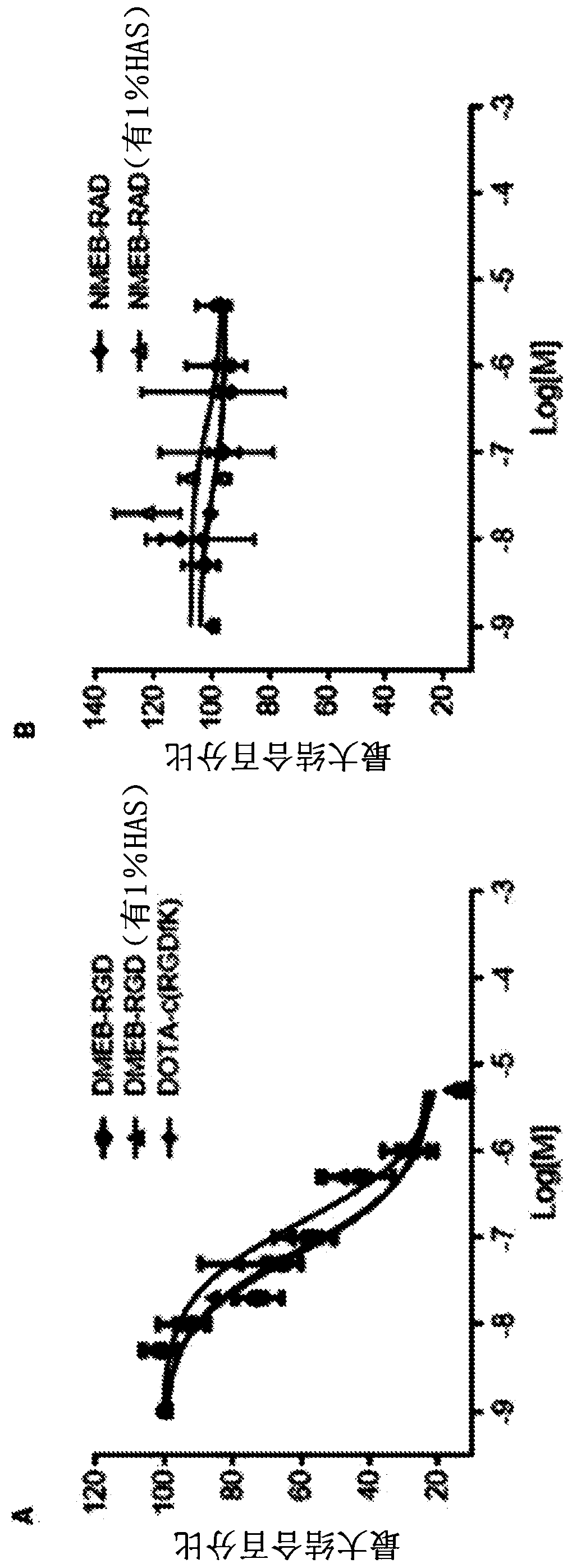
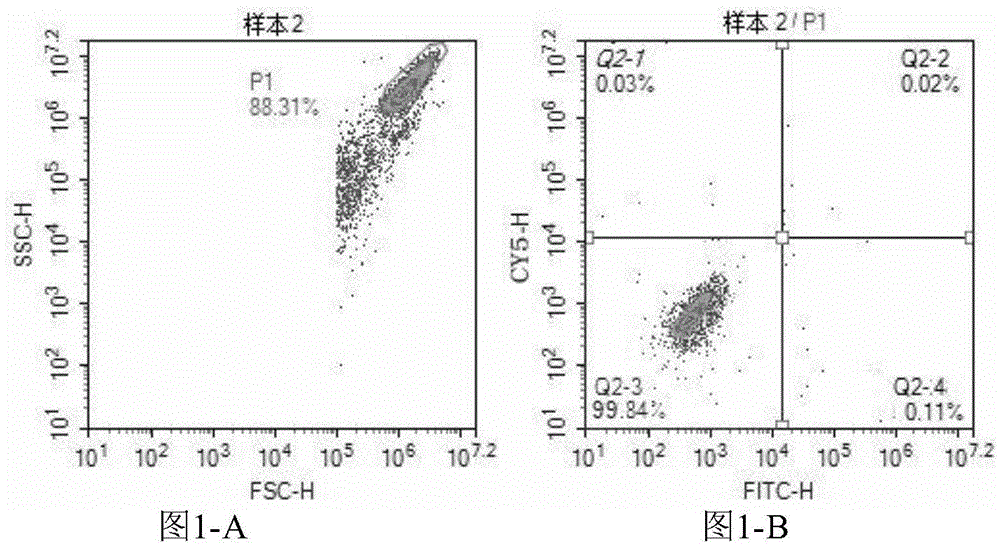
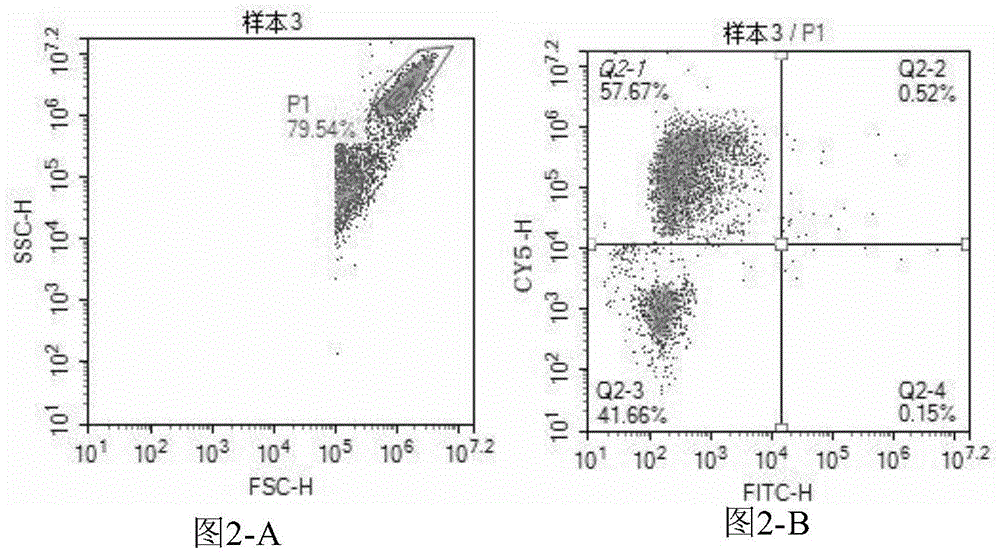
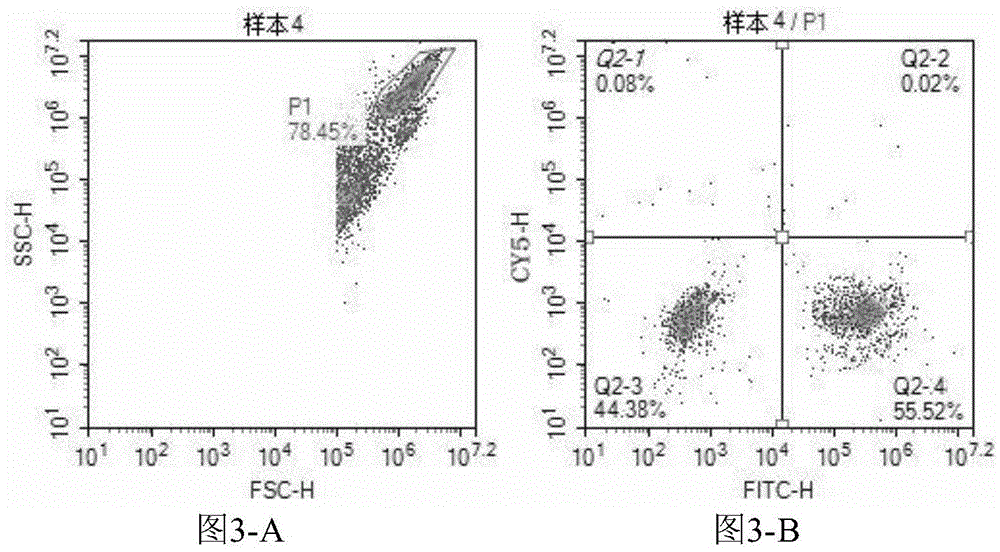
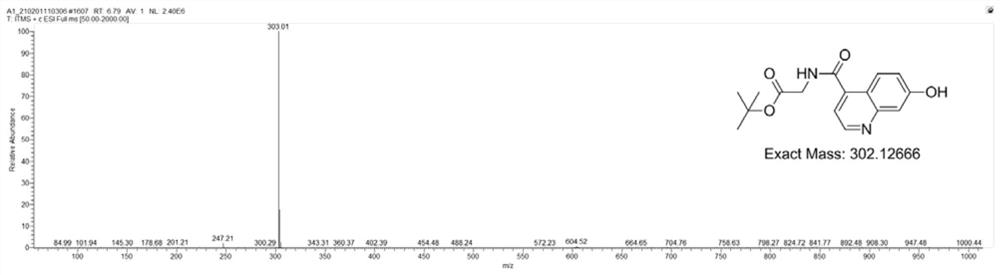
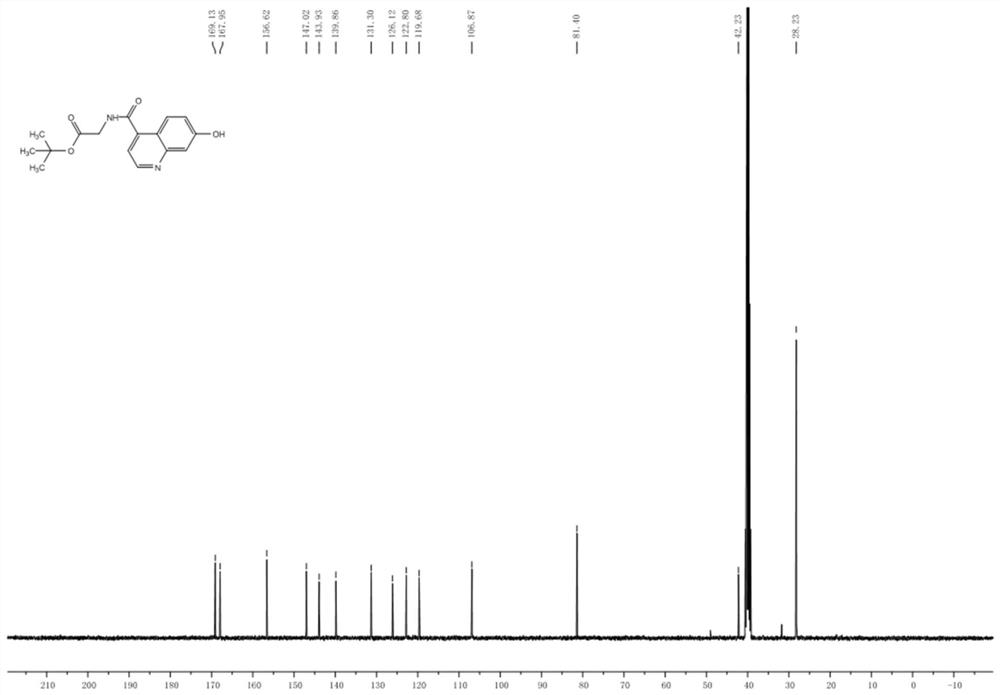
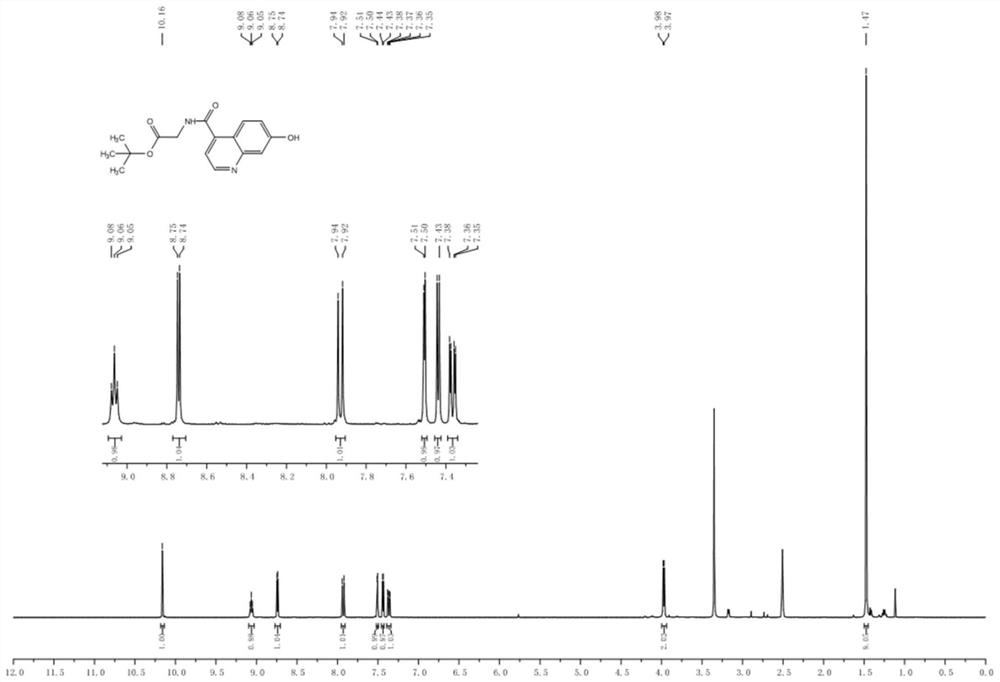
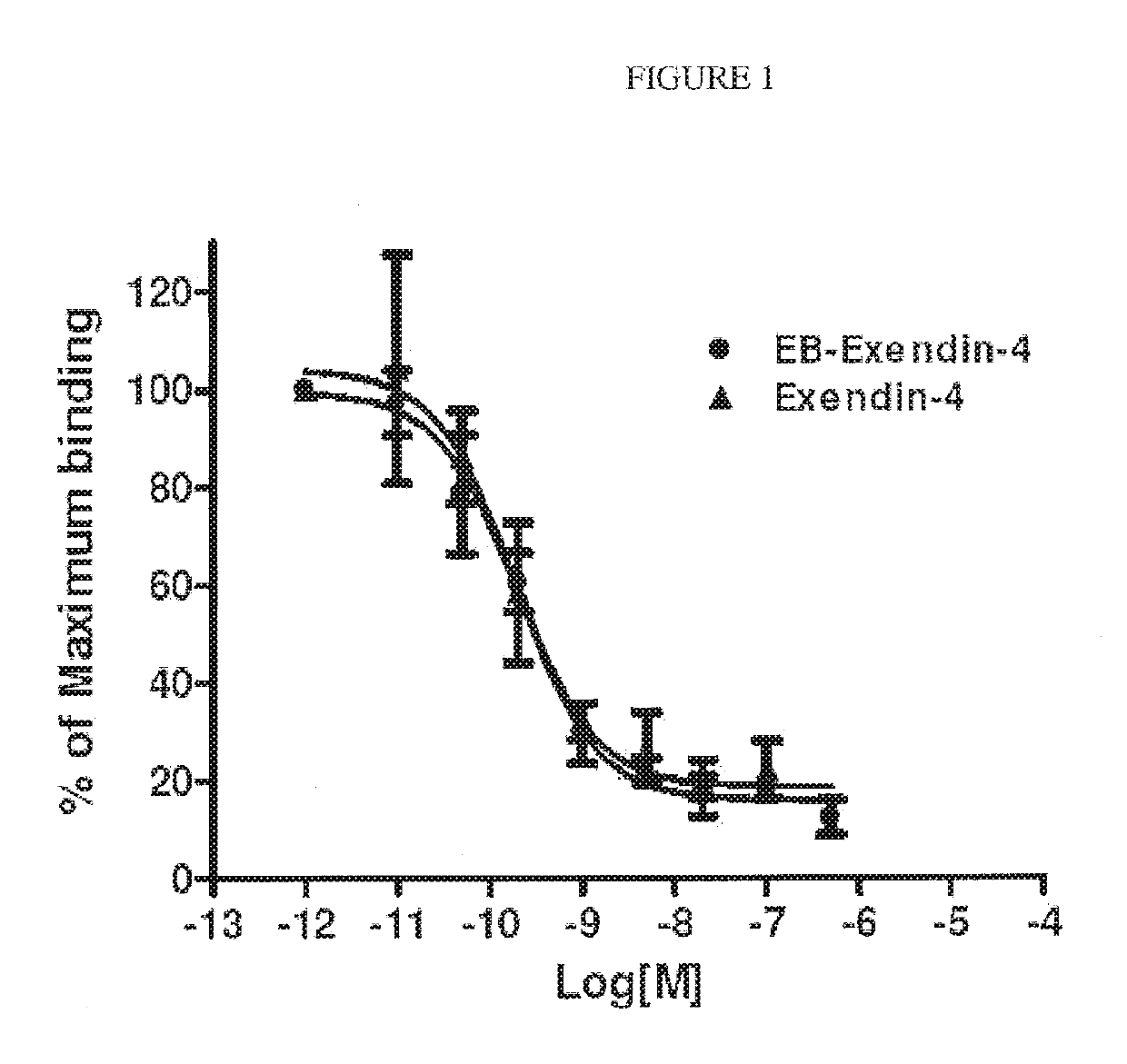
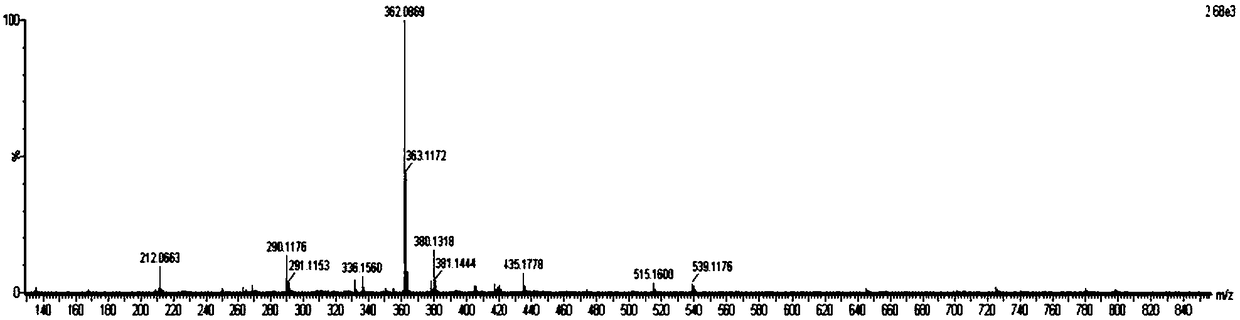
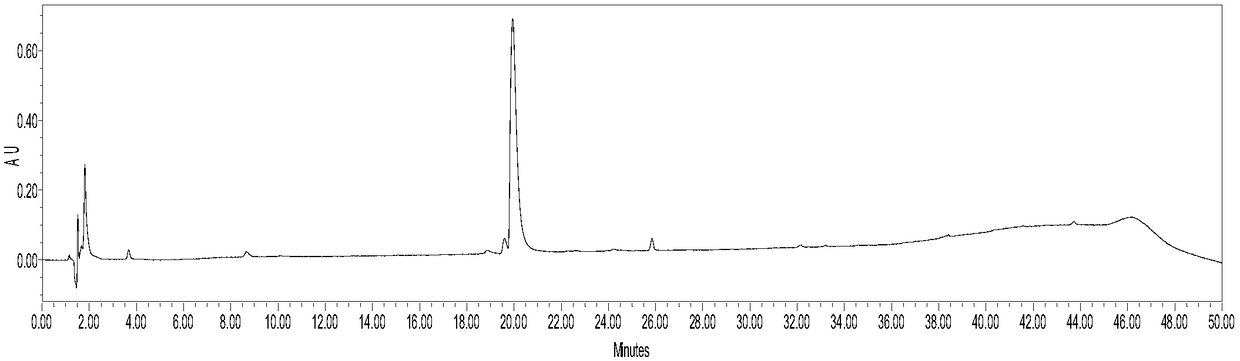
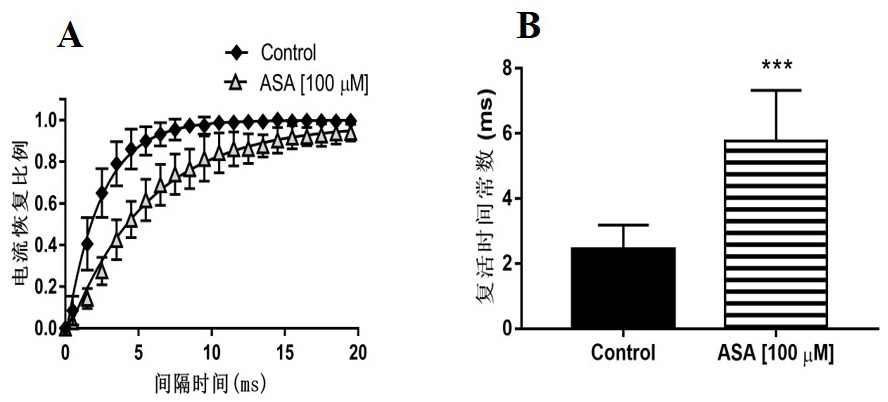
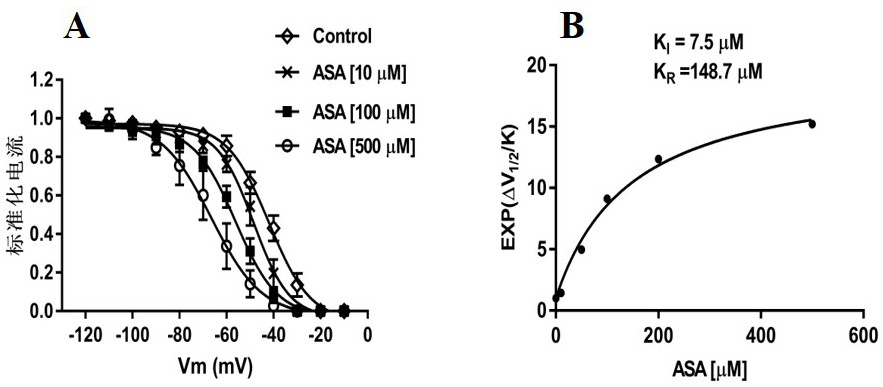
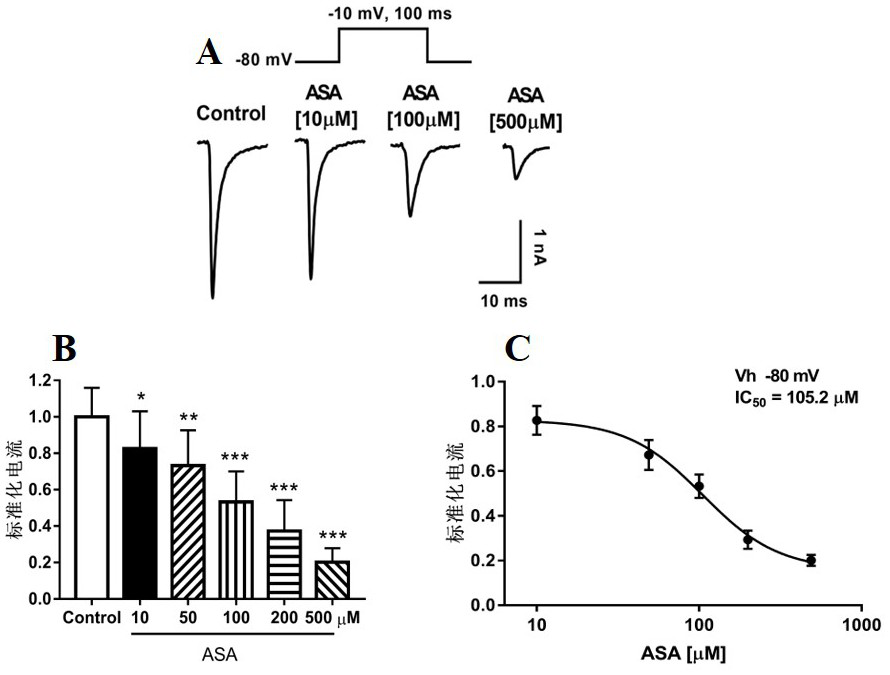
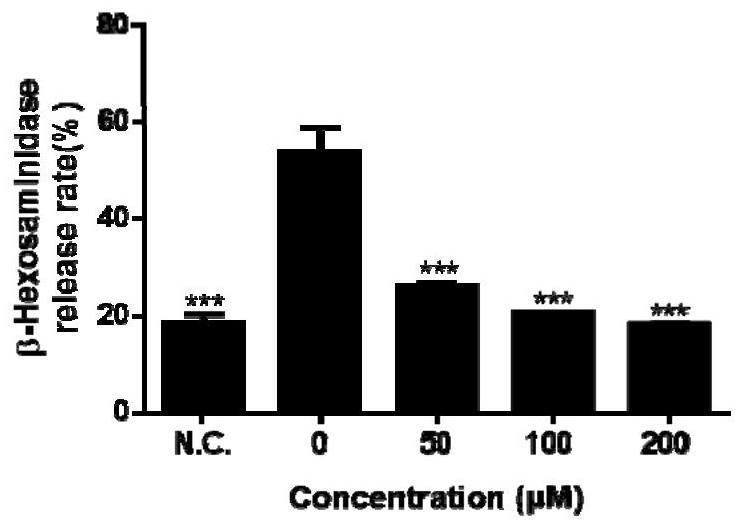
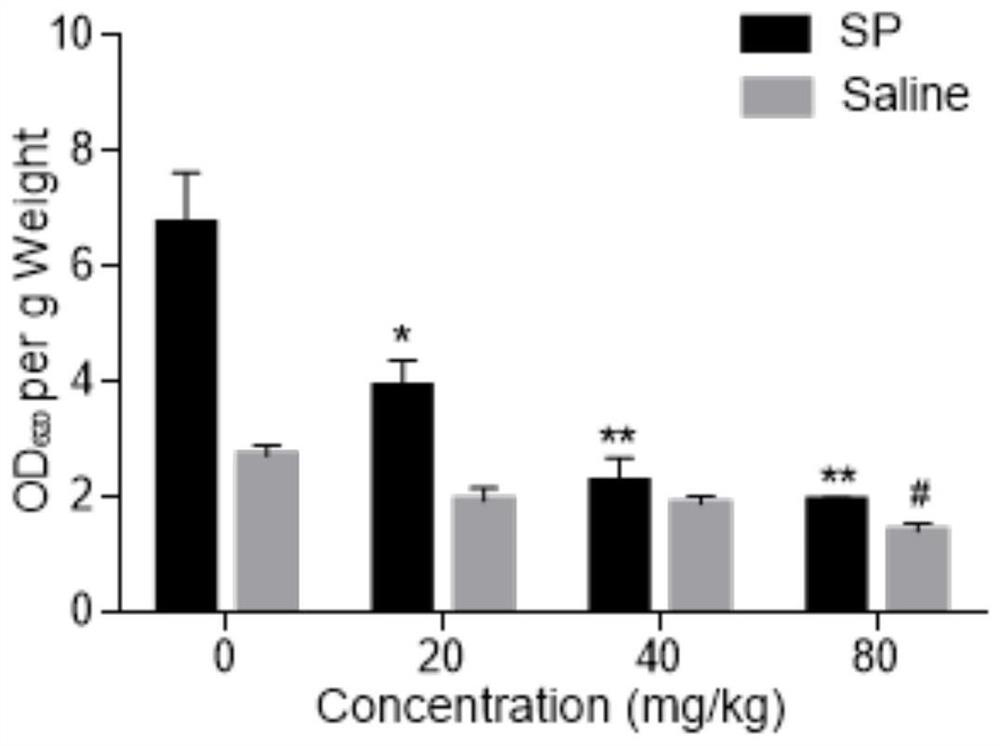
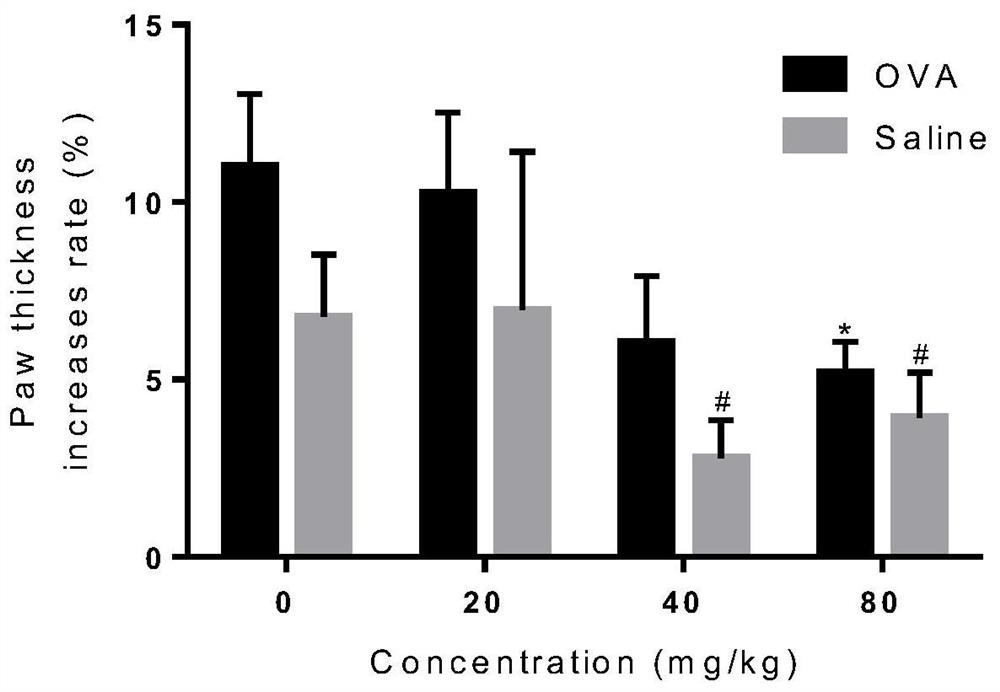
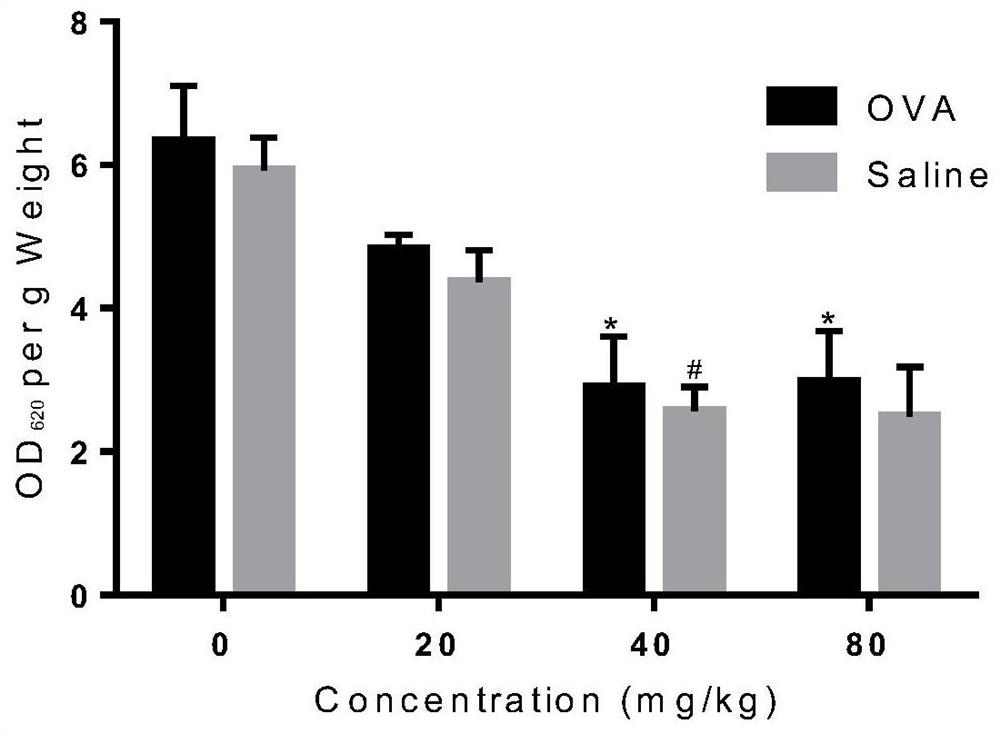
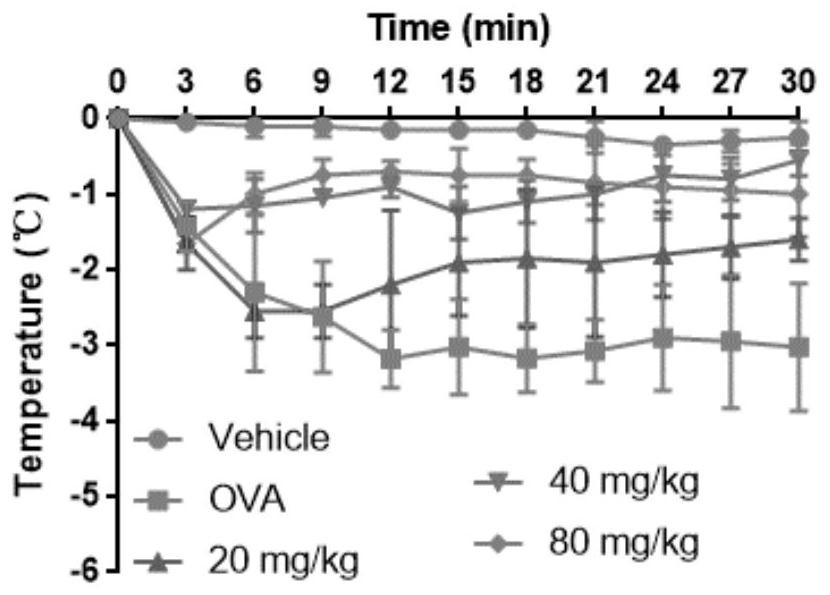
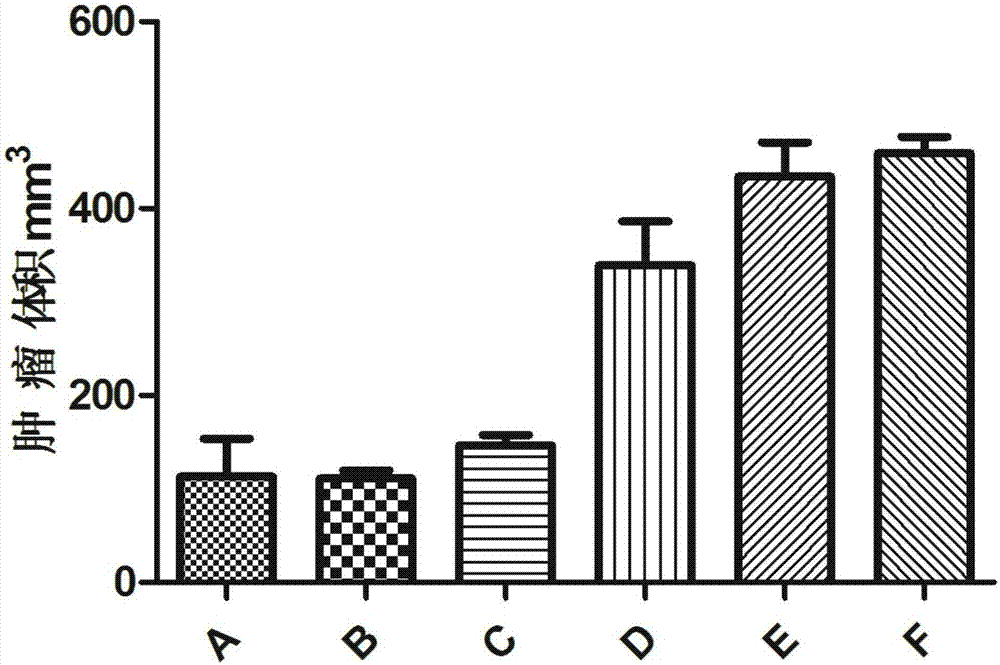Patents
Literature
41 results about "Evans Blue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
T-1824 or Evans blue, often incorrectly rendered as Evan's blue, is an azo dye that has a very high affinity for serum albumin. Because of this, it can be useful in physiology in estimating the proportion of body water contained in blood plasma. It fluoresces with excitation peaks at 470 and 540 nm and an emission peak at 680 nm.
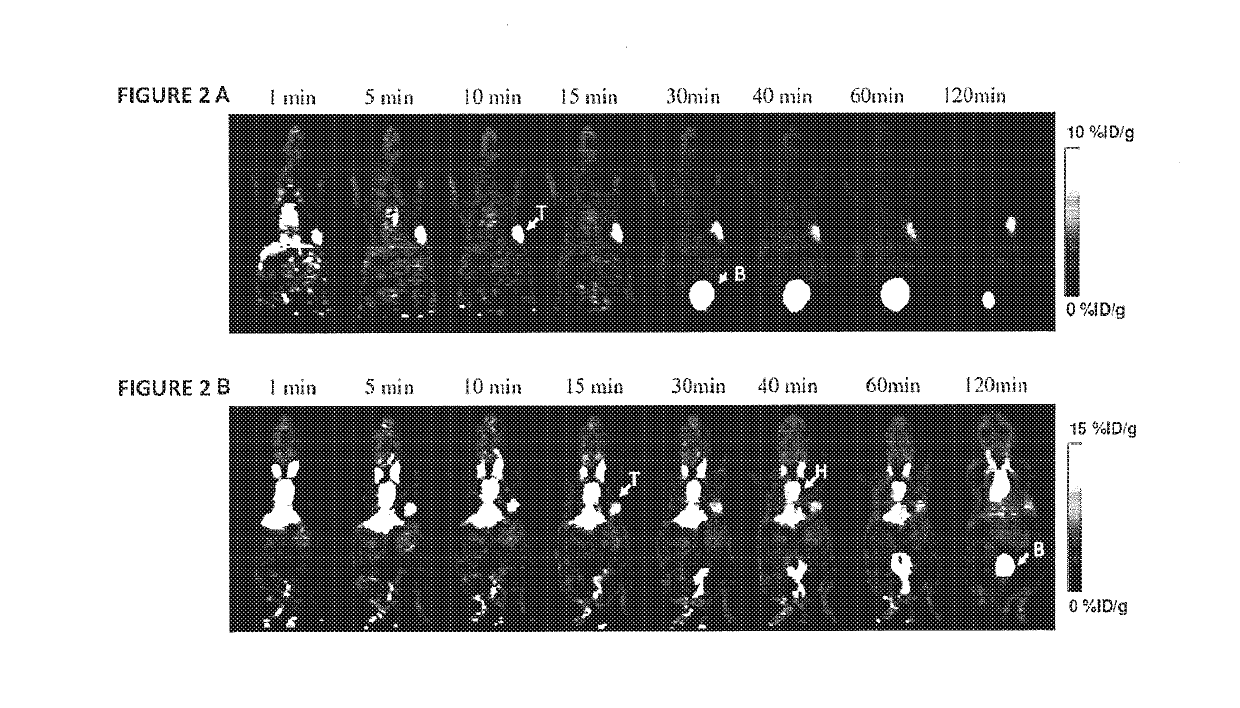
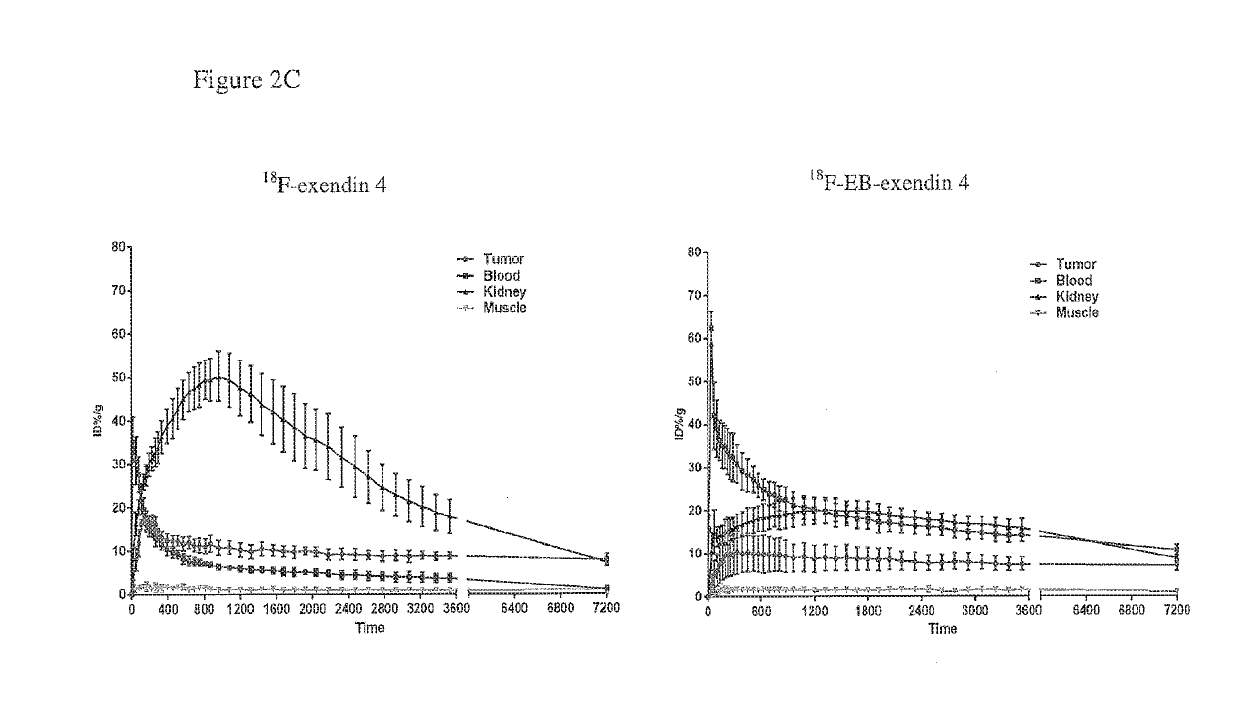
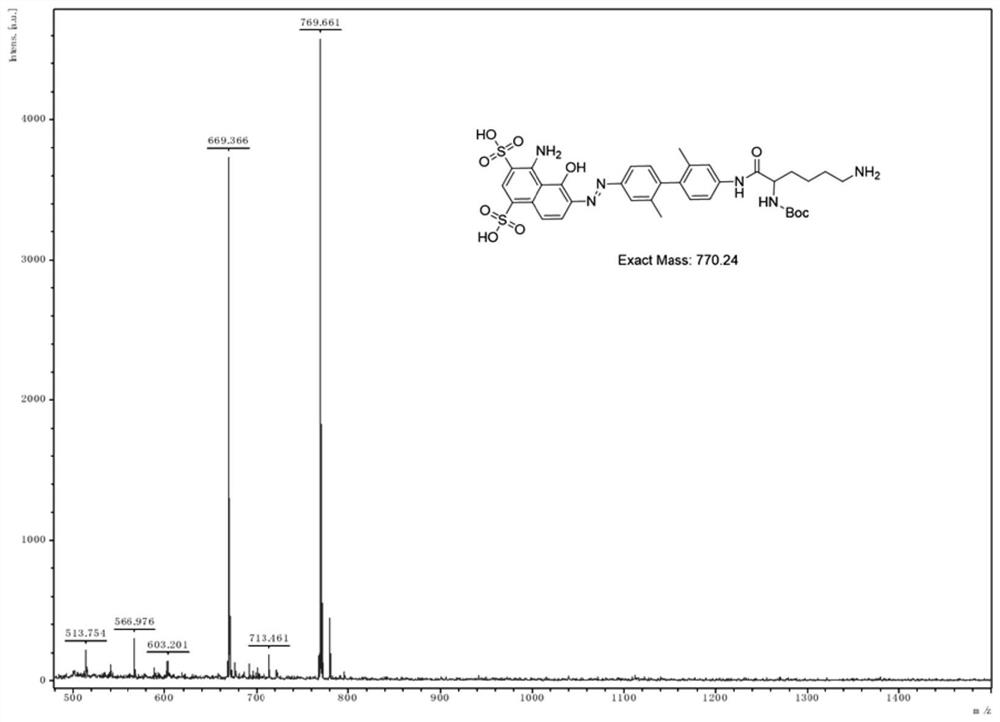
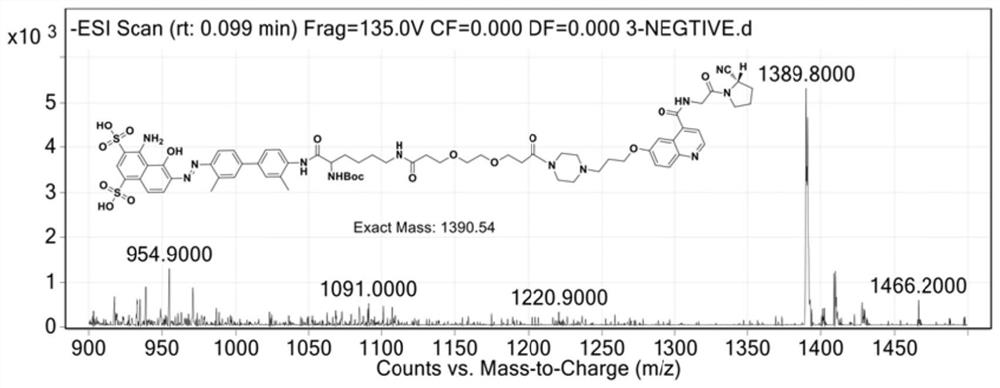
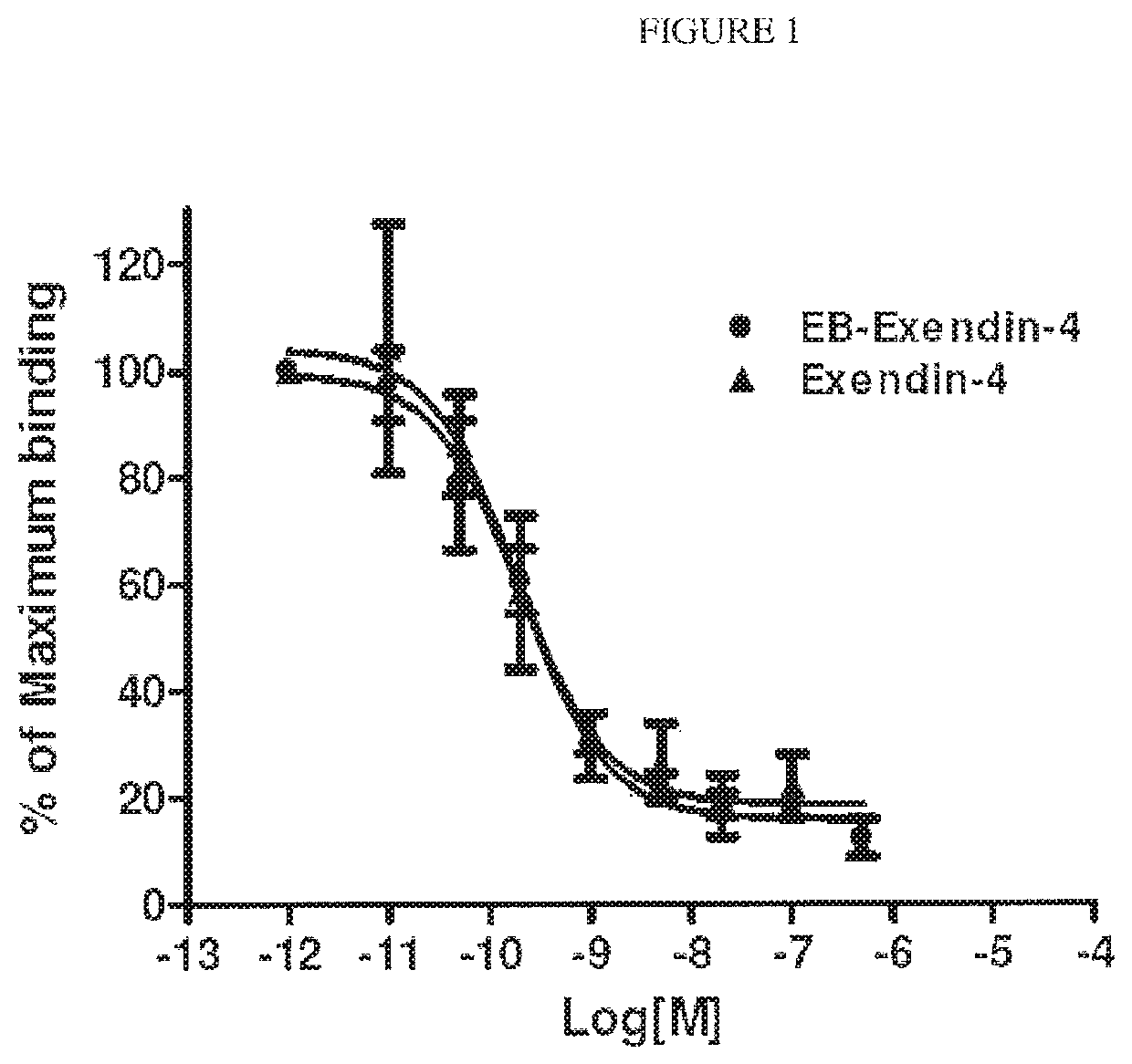
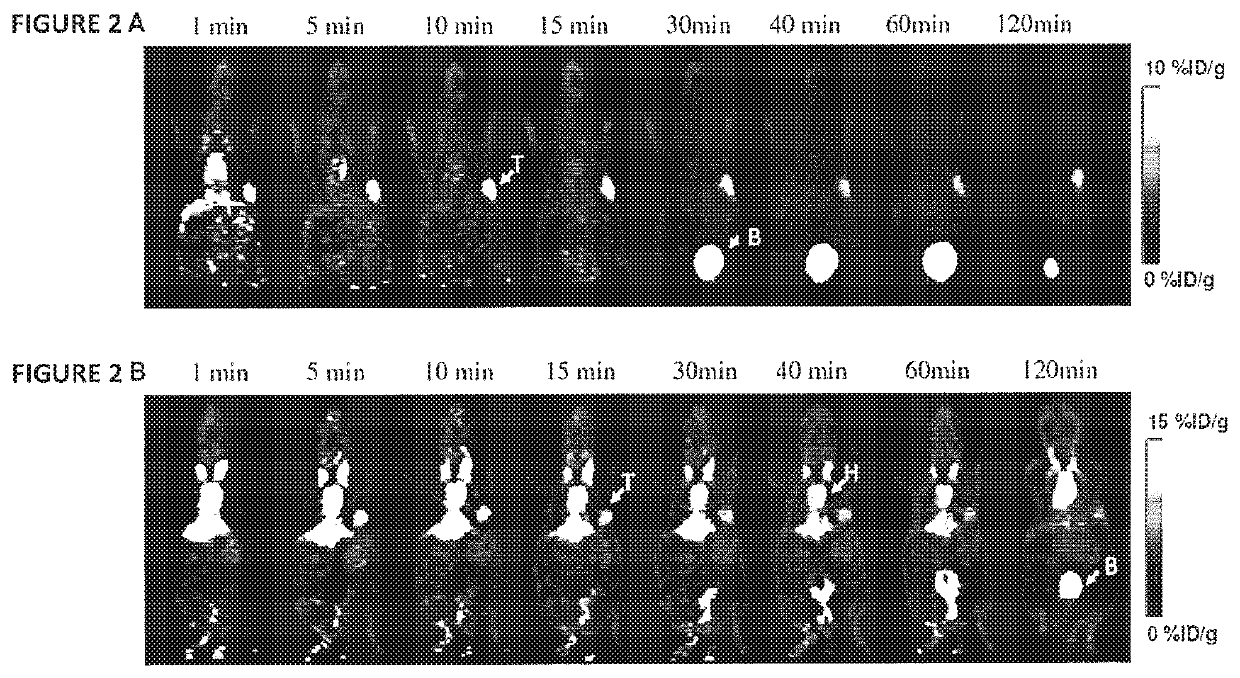
Exendin-4 modified by Evans blue or derivatives of Evans blue and preparation method and application of Exendin-4
ActiveCN104650217AProlong half-life in vivoEasy to manufactureHormone peptidesPeptide/protein ingredientsDiseaseHalf-life
The invention relates to a preparation method and application of Exendin-4 biologically located and modified by Evans blue (Evans Blue) or derivatives of Evans blue. The product generated after the C terminal of Exendin-4 is specifically modified with Evans blue or derivatives of Evans blue has biological activity similar to Exendin-4 which is not modified but has longer in vivo half-life than the Exendin-4 which is not modified. Based on the significance of the Exendin-4 in treatment of diseases, the invention also discloses application of the Exendin-4 modified by Evans blue or derivatives of Evans blue in preparation of medicines for treating type-II diabetes and myocardial infarction. The Exendin-4 has the advantages of simplicity and convenience in preparation, obvious curative effect, long-lasting and stable drug effect, convenience in storage and the like and has significance in research and development of new drugs for promoting anti-diabetic and anti-infarction high-efficiency treatment.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
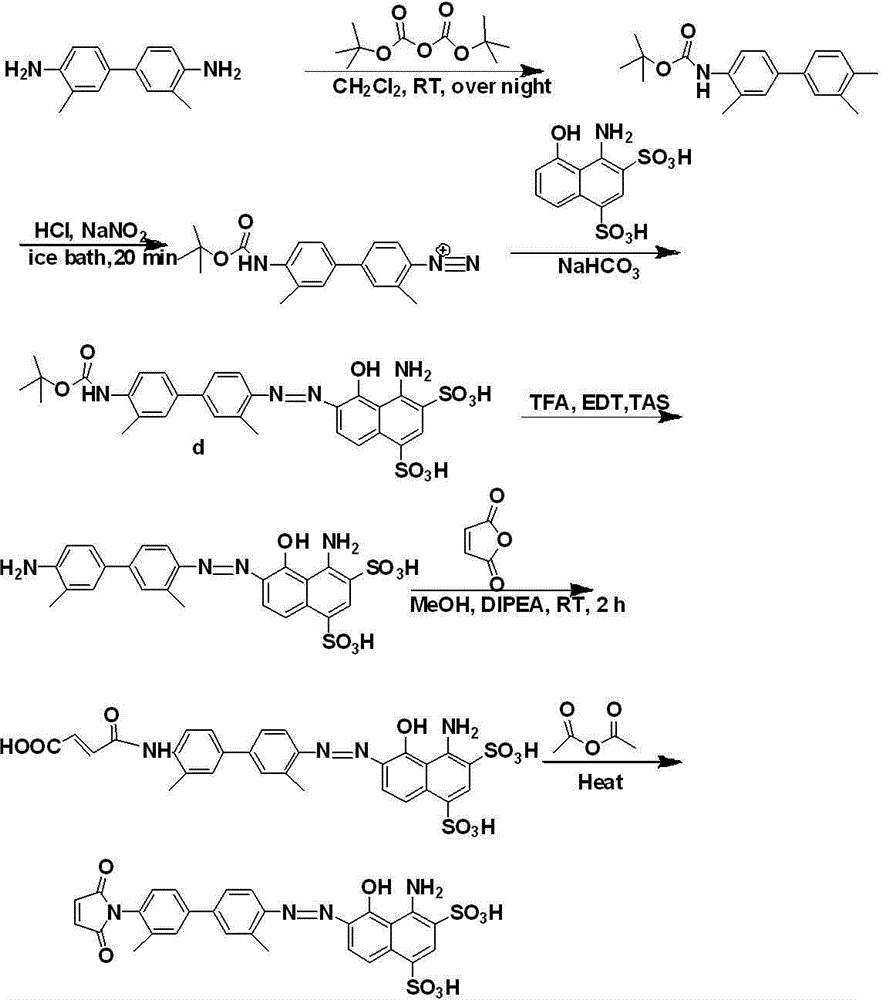
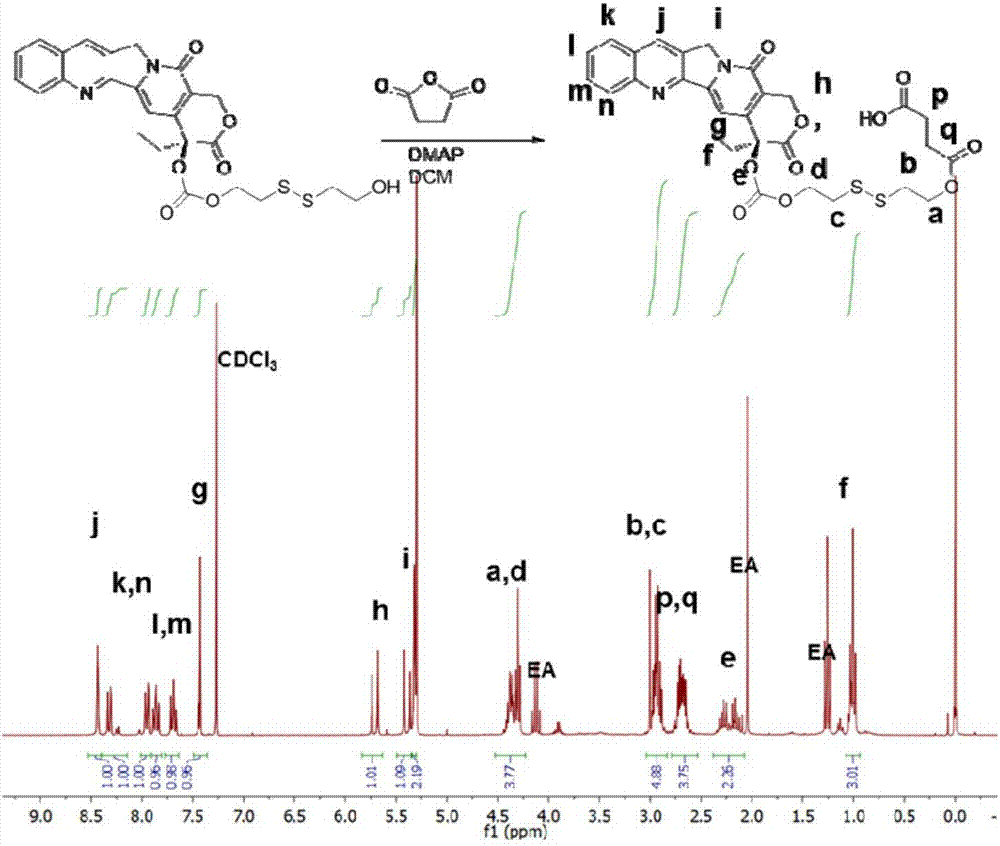
Camptothecin prodrug, and preparation method and application thereof
ActiveCN106946899AEasy to makeGood water dispersibilityMaterial nanotechnologyOrganic chemistryCancer cellStructural formula
The invention provides a camptothecin prodrug. The camptothecin prodrug is a prodrug formed bycamptothecin or a derivative thereof and Evans blue, the structural formula of the prodrug is represented by formula (I); and in the formula, R<1> is camptothecin or a derivative group thereof, R<2> is one of -CH2- and -O-, R<3> is one of -CH2-, -O-CO- and -O-CO-NH-, X is one of S and -CH2-, and n1 and n2 are the repeating unit number, and are integers in a range of 0-10. The camptothecin prodrug has an excellent tumor cell uptake effect in vivo and in vitro, and a part of the prodrug has a substantial cancer cell inhibition effect. The invention also provides a preparation method of the camptothecin prodrug and an application of the camptothecin prodrug in the preparation of cancer treatment drugs.
Owner:YANTAI LANNACHENG BIOTECHNOLOGY CO LTD
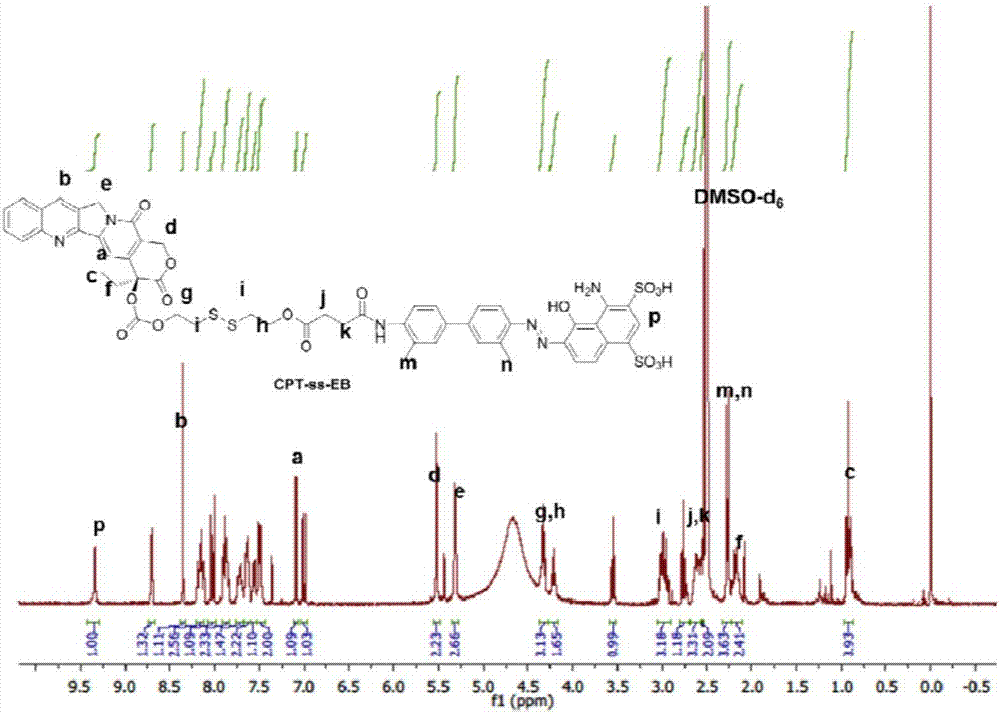
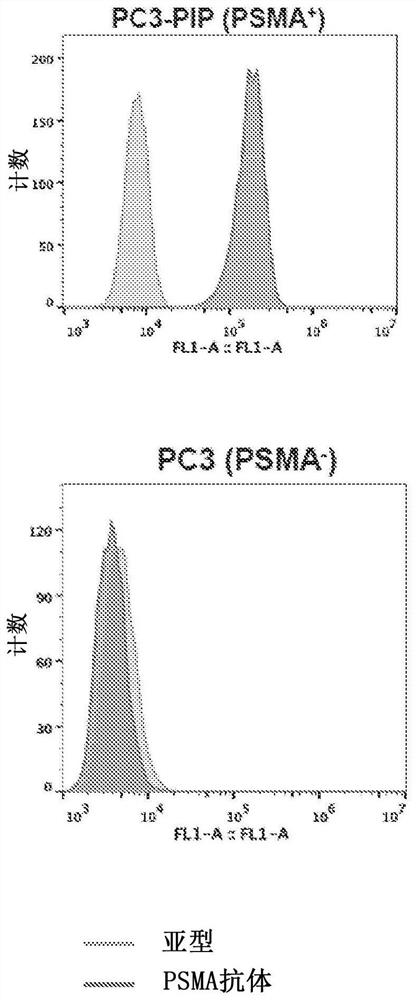
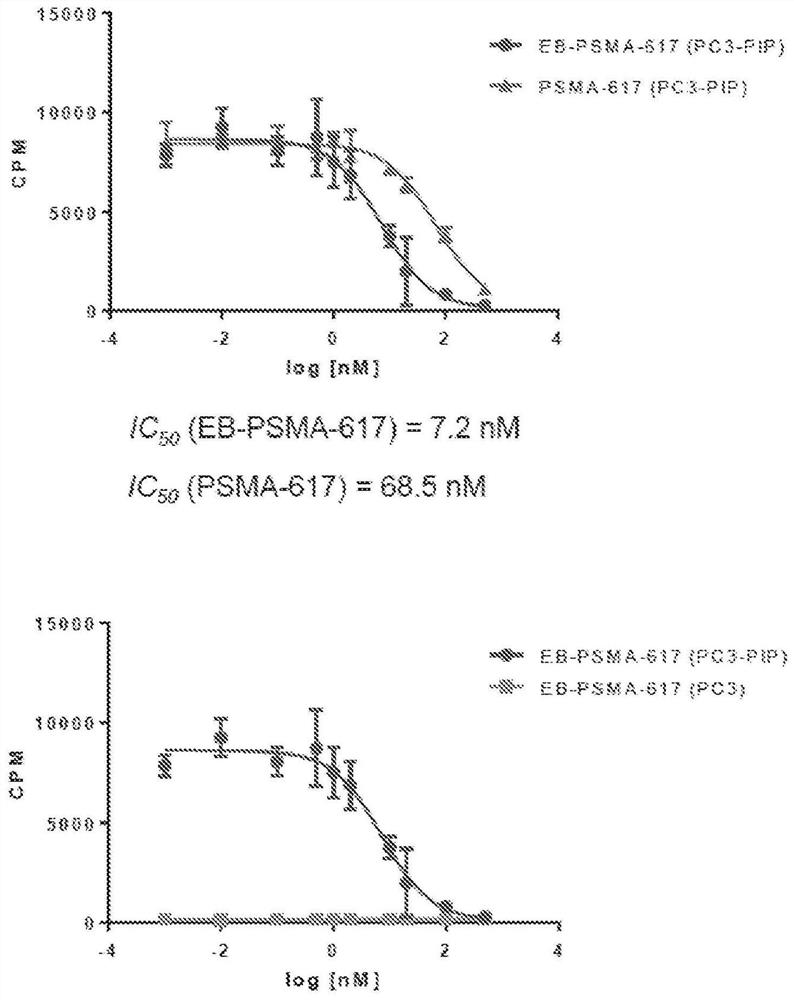
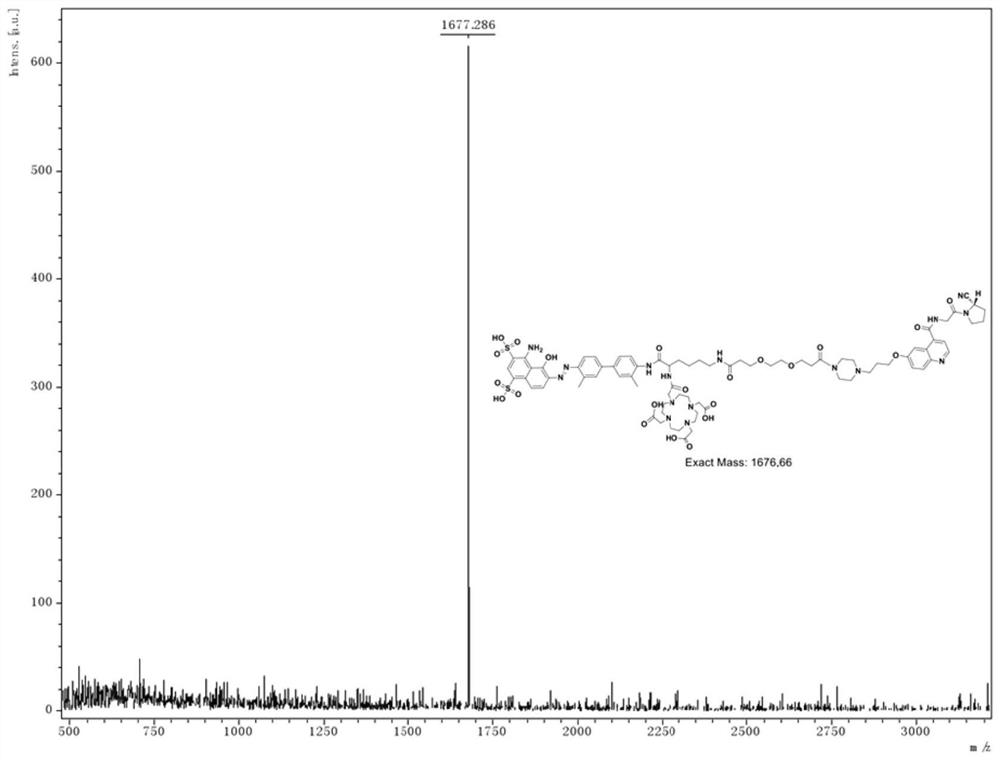
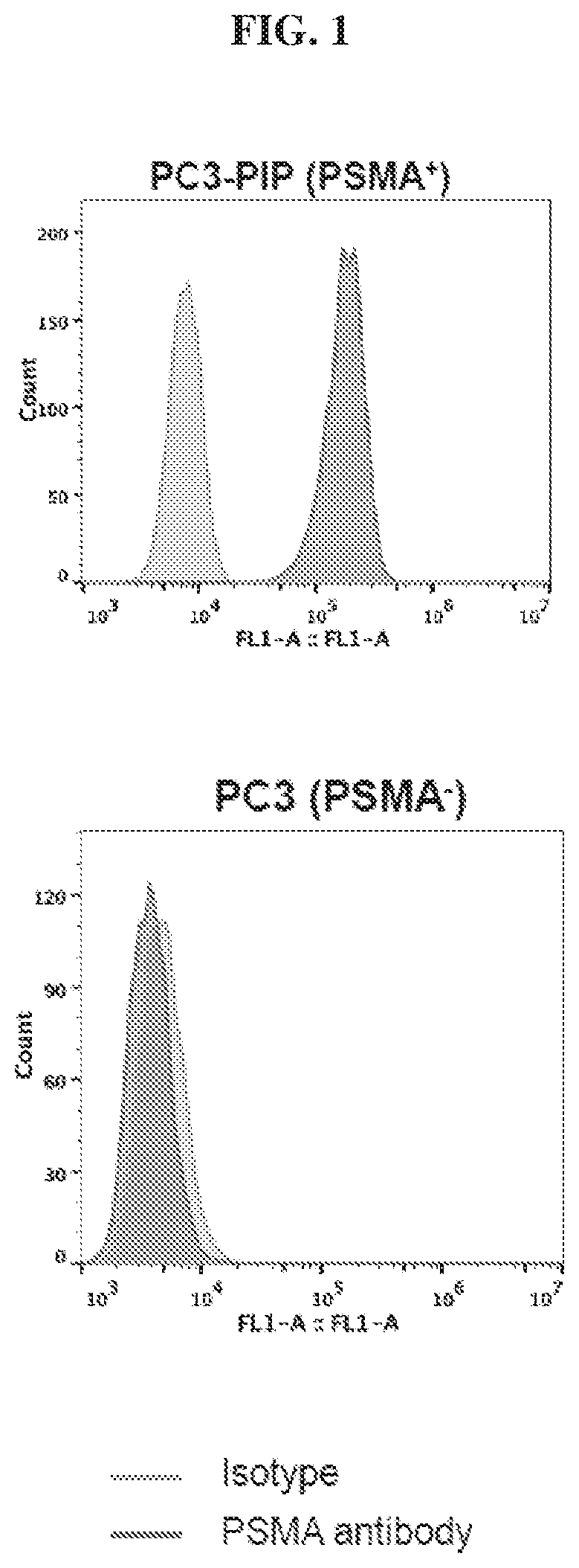
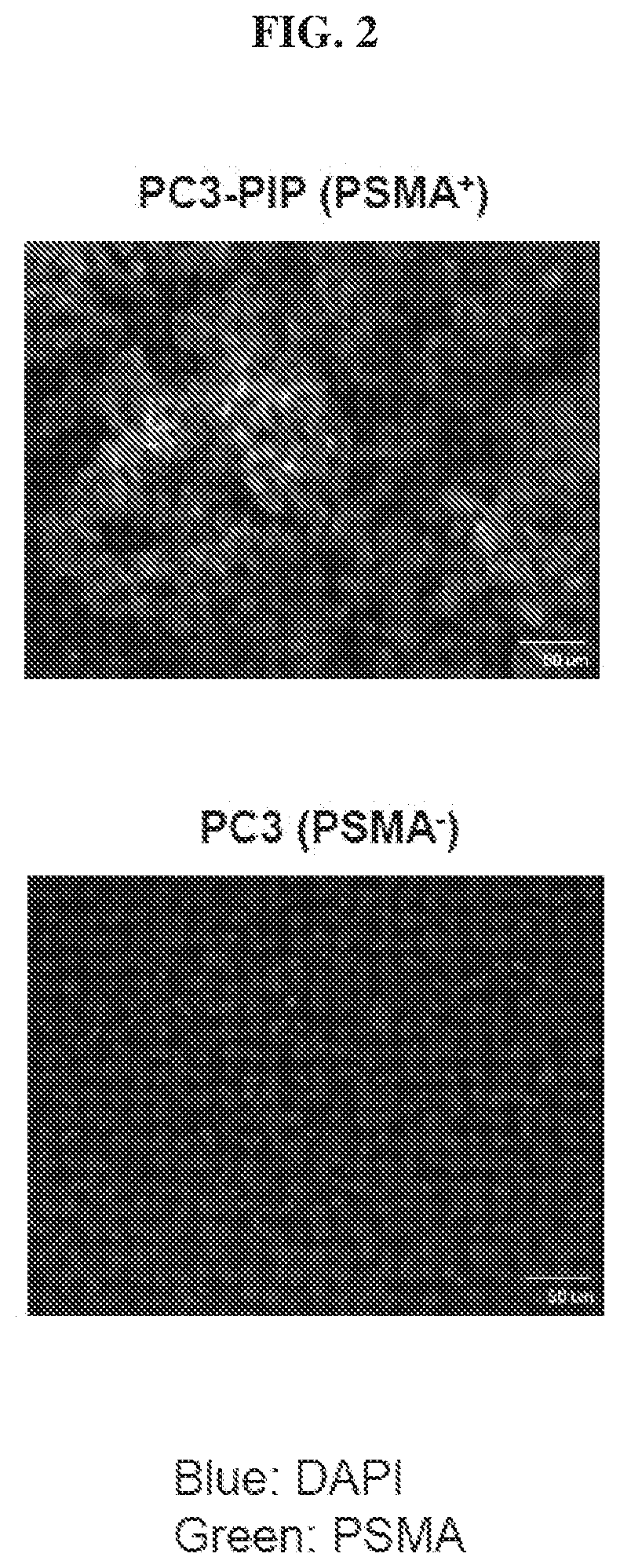
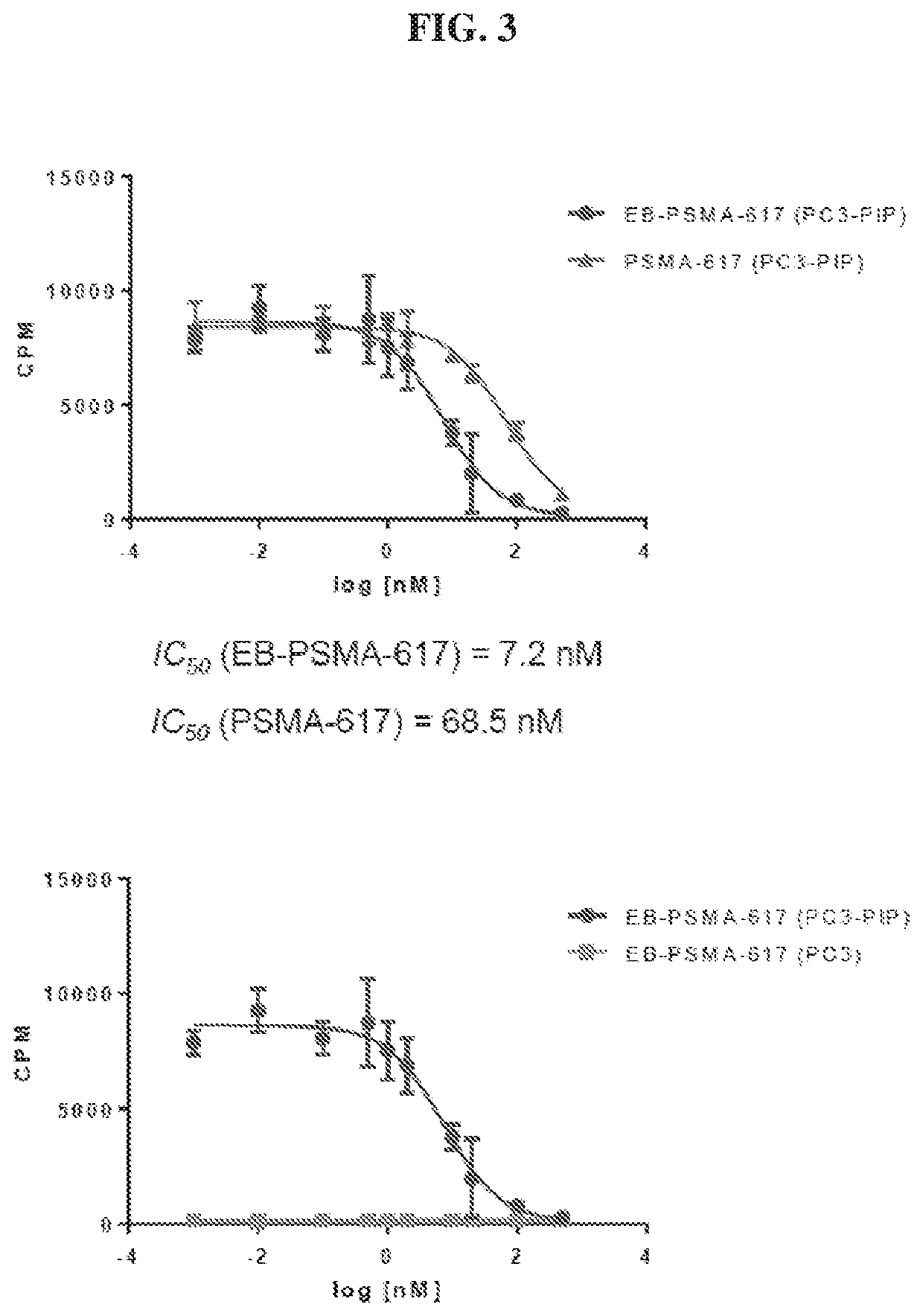
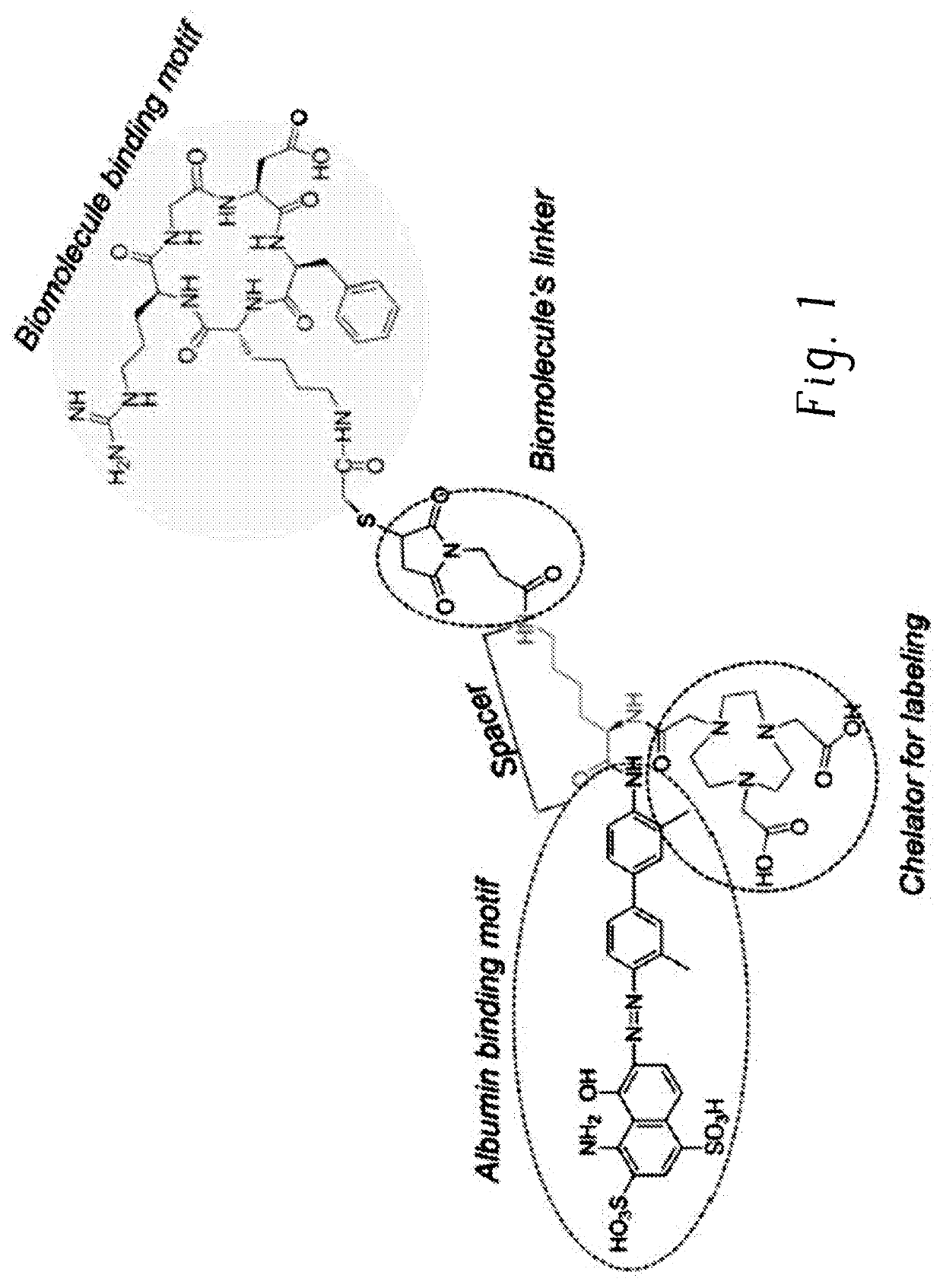
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents for targeting prostate cancer
PendingCN111741751AEasy to addAdjustment quantityOrganic active ingredientsMonoazo dyesAntigenImaging agent
A compound of Formula (I) or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of such an ester or amide or a solvate of such an ester amide or salt. Definitions of R1-R13 and L1-L4 are provided in the disclosure, and R14 is a group capable of binding to prostate-specific membrane antigen (PSMA).
Owner:UNITED STATES OF AMERICA
Combination technique of organic blue dye for labeling the sentinel node and fluorescence imaging
InactiveCN101380226ARelieve painAvoid Radiation HazardsLuminescence/biological staining preparationDiagnostics using lightOrganic dyeLength wave
The invention relates to a combined technology for marking an organic blue dye of a sentinel lymph node and fluorescence imaging. The fluorescence given out by the organic blue dye is displayed by a fluorescence imaging device by the illumination of excitation light of the fluorescence imaging device with a wavelength not less than 570nm; wherein, the organic blue dye is one from methylene blue, Evans blue, isosulfan blue and patent blue or a compound thereof. The combined technology has the advantages of: overcoming the defect of needing to cut a larger opening on a skin for finding out a blue stain lymph node when using an organic dye to dye and position in the normal method for carrying out location of the sentinel lymph node of a tumor in clinical practice, and the sentinel lymph node can be located without an operation, thus reducing the pain of a patient. The combined technology has non-radioactivity, is convenient, fast and accurate and is a method which has excellent clinical application value.
Owner:TONGJI UNIV
Efficient separation, transformation and regeneration system for tobacco protoplast
InactiveCN104789518AHigh purityPlant tissue cultureHorticulture methodsHydrolysateTransformation efficiency
The invention relates to an efficient separation, transformation and regeneration system for tobacco protoplast. The regeneration system comprises special separation, transformation and regeneration reagent combination and method for the tobacco protoplast. The reagent combination comprises a 1 / 2 MS (Murashige and Skoog) medium, an MS medium, enzymatic hydrolysate, a medium C, a W5 washing liquid, the Evans Blue dye, a transformation liquid, a polyethylene glycol solution, a C:B medium, a medium A and an MS regeneration medium. The transformation method comprises steps of culture of aseptic seedlings, separation and purification of the protoplast, transformation of the protoplast, culture and regeneration of the protoplast and the like. The method has the advantages of simple steps, normative operation, low pollution rate, high transformation efficiency and the like.
Owner:BEIJING GENOVO BIOTECH CO LTD
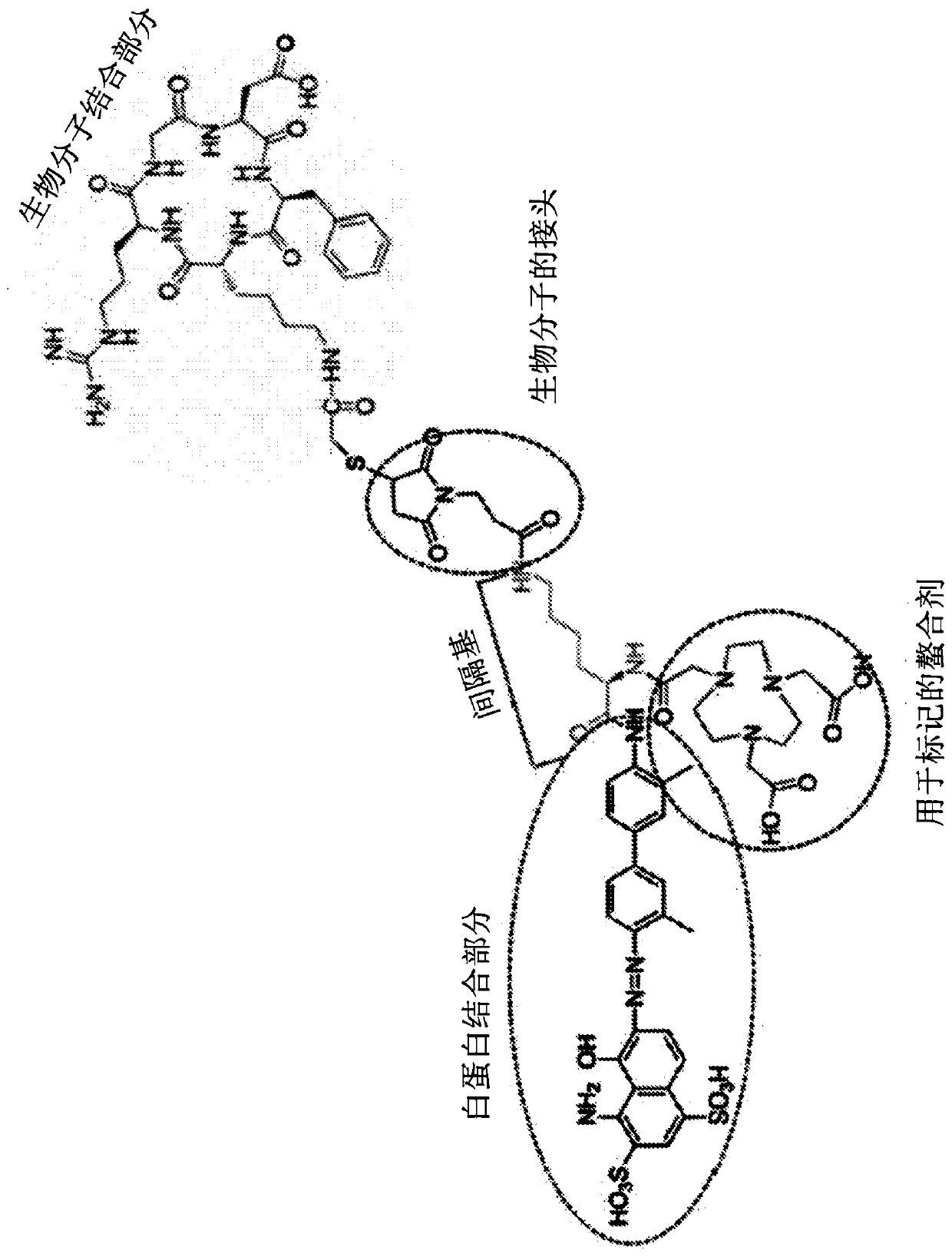
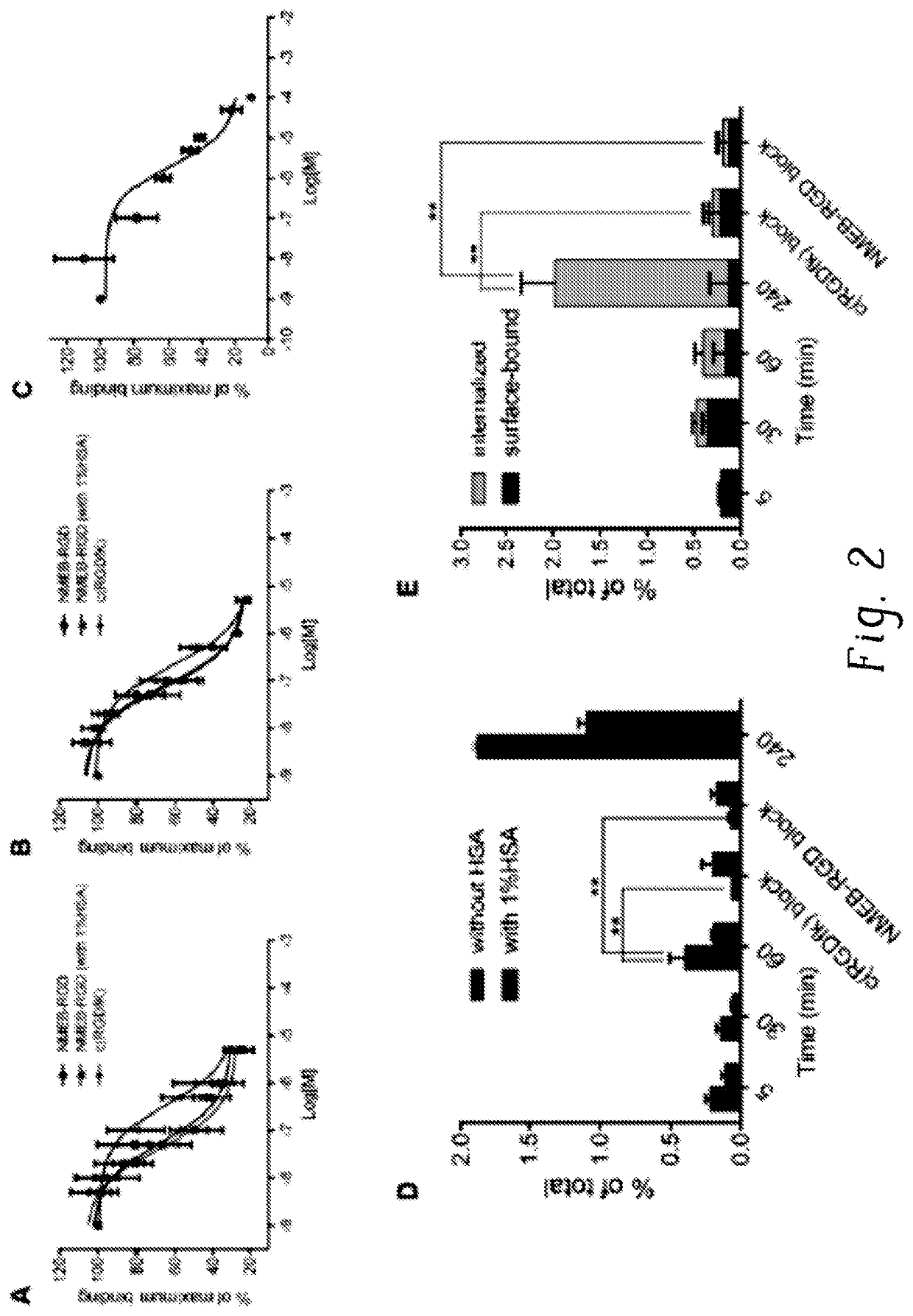
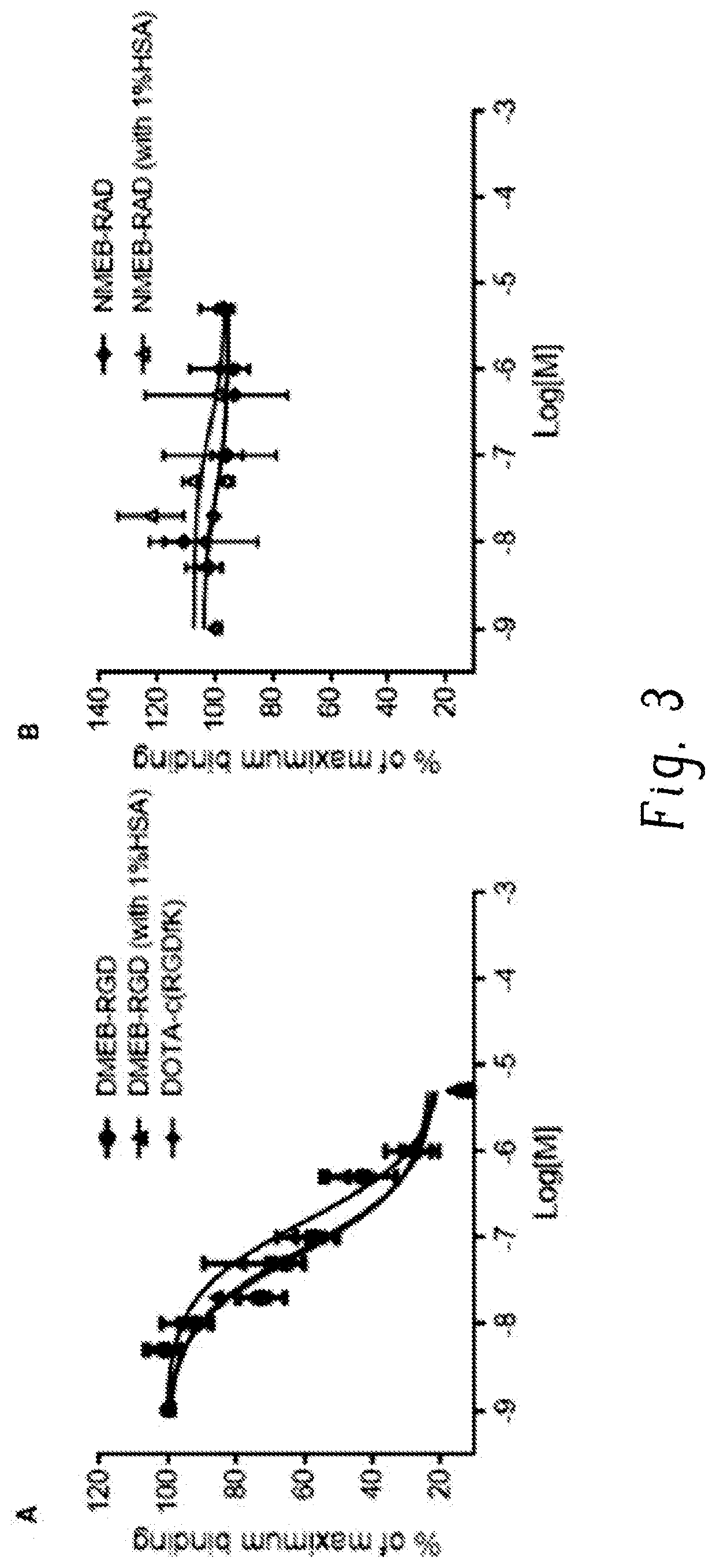
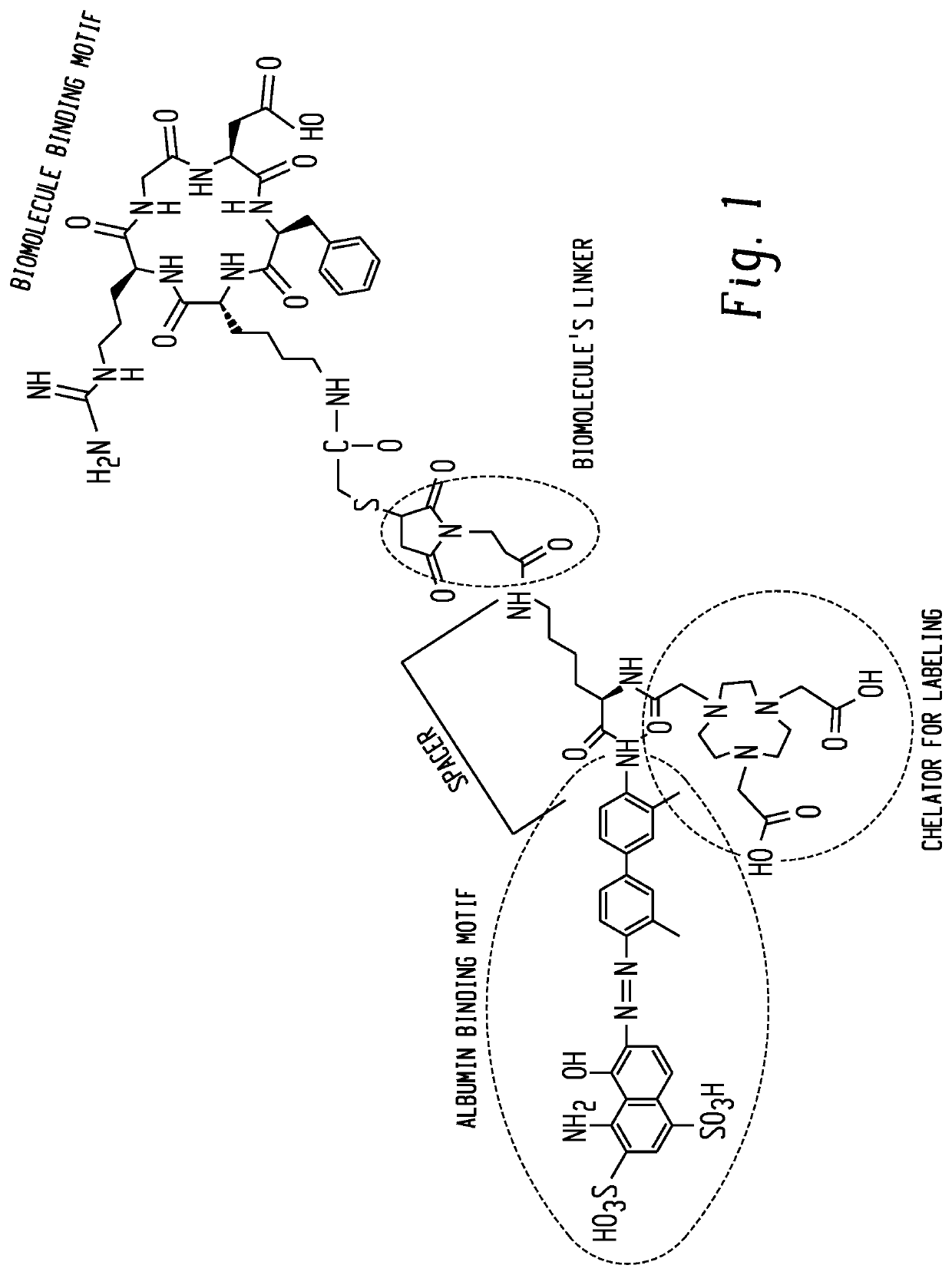
Evans blue complex as well as preparation method and application thereof
The invention provides an Evans blue complex which is of formula (I) as shown in the specification. The Evans blue complex structurally comprises two serum albumin combined groups and one macrocyclicpolyamine chelated group NOTA. The invention further provides a marking complex prepared from the Evans blue complex after radionuclide marking. The marked complex provided by the invention is simpleto mark and biological property; biological test results show that the complex has a property of distinguishing sentinel nodes from secondary lymphatic glands. The invention further relates to a preparation method and application of the complex in preparing organ and tissue photographic developers of human beings or animals.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents
ActiveCN111542518AEasy to addReduced likelihood of biological functionOrganic active ingredientsIsotope introduction to heterocyclic compoundsDiseaseImaging agent
The present invention is directed to a compound of Formula III or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of the an ester or amide or a solvate of the an esteramide or salt,wherein the definitions of R1-R12 and L1-L4 are provided in the disclosure, R13 is a chelating group comprising 177Lu, and wherein R14 is a peptide. The compounds of Formula I may be covalently bonded to a peptide via a linker to provide a compound of Formula III and thereby extend the half-life of the therapeutic compound. The invention is also directed to pharmaceutical compositions of the disclosed compounds, as well as their use in the diagnosis or treatment of diseases.
Owner:UNITED STATES OF AMERICA
Kit for directly detecting 71 type enterovirus and A16 type coxsackievirus and application thereof
InactiveCN105527430AQuick checkSensitive detectionBiological material analysisReference sampleFluorescence
The invention provides a kit for directly detecting 71 type enterovirus and A16 type coxsackievirus and an application thereof. The kit comprises an antibody, a cell fixing solution, a PBS (phosphate buffer solution), a PBST (phosphate buffer solution twen-20), 1% Triton-X100, a cell reference sample, a preservative of sodium azide, an antibody stabilizer of BSA (bovine serum albumin), a fluorescent dye of Evans blue, a mounting medium, a slide carrier and a quality control slide, wherein the antibody contains a fluorescence marker. The kit has the advantages that the 71 type enterovirus and A16 type coxsackievirus in the sample cell can be directly detected; the advantages of flow cytometry and direct immunofluorescent technique on automation and high flux are realized, the virus can be quickly, sensitively and efficiently detected, the important significance is realized for the quick and accurate diagnosis of clinics, and different requirements of large hospitals and base hospitals can be met.
Owner:广州锐达生物科技有限公司
Truncated Evans blue modified fibroblast activating protein inhibitor as well as preparation method and application thereof
ActiveCN114369084AExtended retention timeProlong blood circulation half-lifeRadioactive preparation carriersPeptide preparation methodsFibroblastChemical compound
The invention provides a truncated Evans blue modified fibroblast activating protein inhibitor compound, which is formed by connecting truncated Evans blue, a fibroblast activating protein inhibitor and a nuclide chelating group together by linking groups L1, L2, L3, L4 and X. The structure of the truncated Evans blue modified fibroblast activating protein inhibitor compound is as shown in formula (I), r1 is a fibroblast activating protein inhibitor; l1 is lysine, glutamic acid or a derivative structure thereof; l2 is-(CH2) n-, where n is an integer from 0 to 30, where each CH2 may be substituted individually with-O-,-NH (CO)-or-(CO)-NH-; l3 is-(CH2) m-, where m is an integer from 0 to 30, where each CH2 may be replaced individually with-O-, or-(CO)-; l4 is-(CH2) p-, where p is an integer from 0 to 30, where each CH2 may be substituted individually with-O-,-NH (CO)-or-(CO)-NH-; x is selected from N, C, O, S or R2 is a nuclide chelating group. The invention further provides a radioactive marker based on the structure of the compound, and the compound and the radioactive marker have remarkably prolonged blood circulation half-life period and enhanced tumor uptake enrichment and retention time, and are suitable for nuclide treatment and imaging of FAP protein high-expression tumors.
Owner:YANTAI LANNACHENG BIOTECHNOLOGY CO LTD
Chemical conjugates of evans blue derivatives and their use in the production of long-acting therapeutics
The present invention is directed to a compound of Formula I or Formula II or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of such an ester or amide or a solvate of such an ester amide or salt: Formula I Formula II The compounds of Formula I may be covalently bonded to a therapeutic compound or fragment thereof to provide a compound of Formula II and thereby extend the half-life of the therapeutic compound. The invention is also directed to pharmaceutical compositions of the disclosed compounds, as well as their use in the diagnosis or treatment of diseases.
Owner:UNITED STATES OF AMERICA
Therapeutic drug targeting fibroblast activating protein and preparation method thereof
PendingCN113621021ARadioactive preparation carriersPeptide preparation methodsFibroblastChemical compound
The invention provides a therapeutic prodrug compound targeting fibroblast activating protein. The structure of the therapeutic prodrug compound is shown as a formula (I). The invention also provides a therapeutic drug targeting the fibroblast activating protein, the therapeutic drug is a complex obtained by taking the compound as shown in the formula (I) as a ligand and labeling with therapeutic nuclide, and the structure of the therapeutic drug is shown in the formula (II). The invention also provides a preparation method of the prodrug compound and the therapeutic drug, and an application of the prodrug compound and the therapeutic drug in preparation of the therapeutic drug for FAP protein high-expression tumors. According to the therapeutic drug targeting the fibroblast activation protein, the circulating half-life period of the fibroblast activation protein inhibitor modified by truncated Evans blue can be remarkably prolonged, and tumor uptake enrichment and retention time can be prolonged.
Owner:YANTAI LANNACHENG BIOTECHNOLOGY CO LTD
Method for detecting dengue virus in mosquito by immunofluorescence technique
InactiveCN101109751AControl spreadOmit isolation cultureMaterial analysis by optical meansAgainst vector-borne diseasesFluorescenceSurgical knife
The invention provides a method for detecting dengue virus in mosquitoes by using an immunity fluorescent technology. DEN-2 virus from artificially infected mosquito is taken, detection is carried out respectively by cell culturing inoculating and tablet immunity fluorescent way at the head of the mosquito, and the body of the mosquito is detected to carry or not virus by tablet immunity fluorescent way at the head of the mosquito. In the invention, a single blood-fully infected aedes albopictus is frozen under -20 centigrade, the head of the aedes albopictus is cut down under a dissecting microscope by a surgical knife, and the head is placed on a slide and extruded by a dissecting needle, so that the substance in the head flows out and is evenly coated on the slide; the slide is aired dry and fixed by pre-cooled acetone for 10 min.; mouse anti DEN-2 virus IgG diluted by PBS is dripped on the slide; the slide is then placed in a wet box, incubated for 30 min under 37 centigrade; then the slide is washed 3 times by PBGS, aired dry; goat anti-mouse IgG-FITC that is diluted by Evans blue PBS to operation concentration is dripped on the slide; the slide is then incubated for 30 min under 37 centigrade, washed by PBS; the dyed result is observed under a fluorescent microscope. The invention allows earlier diagnosis or timely discovering of positive mosquito media, so as to control the spreading of dengue.
Owner:山东省寄生虫病防治研究所
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents for targeting prostate cancer
PendingUS20210008232A1Easy to modifyReduce the possibilityOrganic active ingredientsMonoazo dyesAntigenImaging agent
A compound of Formula (I) or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of such an ester or amide or a solvate of such an ester amide or salt wherein the definitions of R1-R13 and L1-L4 are provided in the disclosure, and wherein R14 is a group capable of binding to prostate-specific membrane antigen (PSMA).
Owner:UNITED STATES OF AMERICA
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents
ActiveUS20200231543A1Easy to modifyReduce the possibilityOrganic active ingredientsIsotope introduction to heterocyclic compoundsDiseaseImaging agent
The present invention is directed to a compound of Formula III or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of such an ester or amide or a solvate of such an ester amide or salt: Formula III wherein the definitions of R1-R12 and L1-L4 are provided in the disclosure, wherein R13 is a chelating group comprising 177Lu, and wherein R14 is a peptide. The compounds of Formula I may be covalently bonded to a peptide via a linker to provide a compound of Formula III and thereby extend the half-life of the therapeutic compound. The invention is also directed to pharmaceutical compositions of the disclosed compounds, as well as their use in the diagnosis or treatment of diseases.
Owner:UNITED STATES OF AMERICA
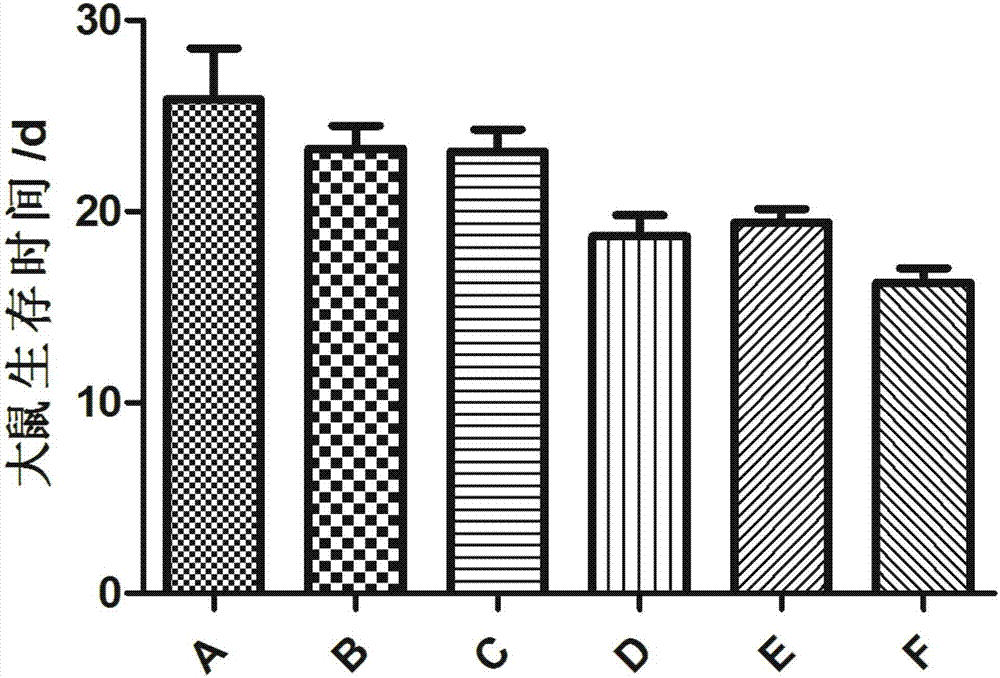
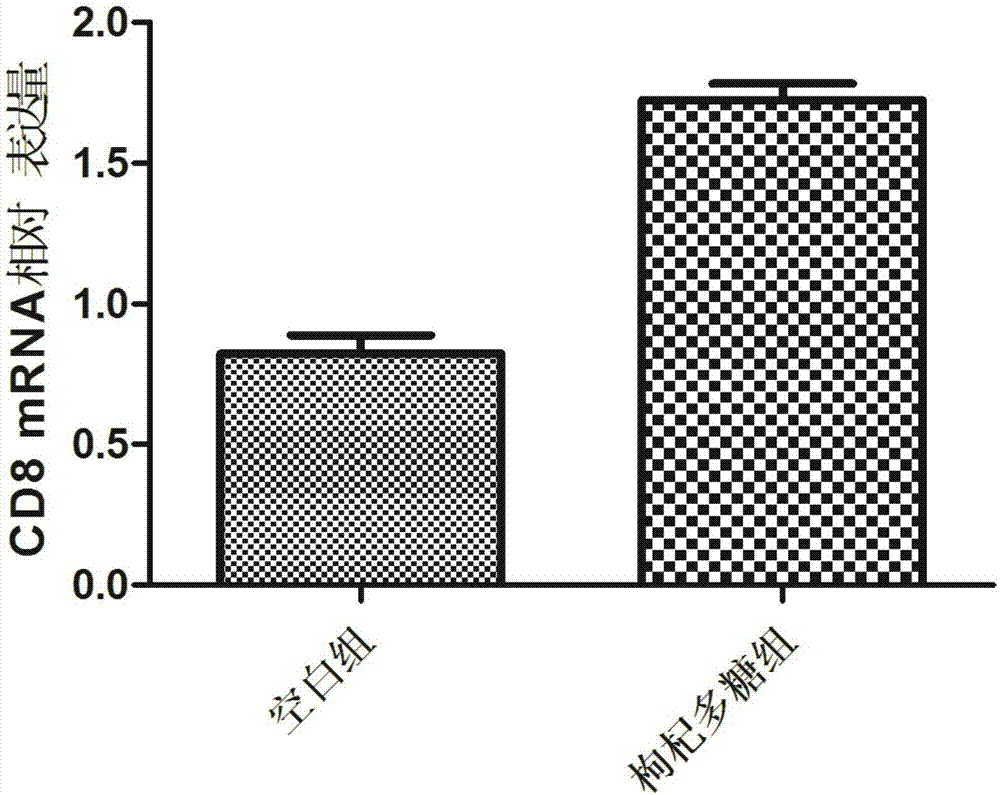
Experimental method of lycium barbarum polysaccharide in inhibition of glioma growth by regulating blood brain barrier
InactiveCN107385029AGrowth inhibitionReduce the family burdenMicrobiological testing/measurementBiological testingWilms' tumorDisease cause
The invention provides an experimental method of lycium barbarum polysaccharide in inhibition of glioma growth by regulating a blood brain barrier and belongs to the field of traditional Chinese medicine. The experimental method comprises the following steps: taking a male SD rat, feeding for 3-4 weeks with lycium barbarum polysaccharide solution; then constructing a rat glioma model; molding, continuing feeding with the lycium barbarum polysaccharide solution, and detecting, namely firstly detecting survival time, survival rate and median survival time of rats in each group; secondly taking brain tissues of the rats in each group, observing and measuring tumor size; thirdly judging permeability change of the blood brain barrier by carrying out femoral vein Evans blue solution on the rats in each group, taking the same amount of brain tissues and detecting Evans blue content; fourthly observing ultrastructure change of the blood brain barrier by virtue of an electron microscope; fifthly carrying out cardiac apex injection on the rats in each group with paraformaldehyde solution, taking the brain tissues, slicing and then carrying out an immunohistochemical test; and sixthly directly cutting off heads of the rats in each group, taking brains, extracting RNA and proteins and carrying out RT-PCR and Western blotting experiments. The experimental method provided by the invention provides a new treatment approach for treatment of multiple blood brain barrier diseases.
Owner:沈冰
Chemical conjugates of Evans Blue derivatives and their use in the production of long-acting therapeutics
The present invention is directed to a compound of Formula I or Formula II or a pharmaceutically acceptable ester, amide, solvate, or salt thereof, or a salt of such an ester or amide or a solvate of such an ester amide or salt: Formula I Formula II The compounds of Formula I may be covalently bonded to a therapeutic compound or fragment thereof to provide a compound of Formula II and thereby extend the half-life of the therapeutic compound. The invention is also directed to pharmaceutical compositions of the disclosed compounds, as well as their use in the diagnosis or treatment of diseases.
Owner:UNITED STATES OF AMERICA
Saprolegnia Evans Blue intravital staining method
InactiveCN104988204AKnow vitalityShorten the timeMicrobiological testing/measurementMicroorganism based processesStainingBiology
The invention discloses a saprolegnia Evans Blue intravital staining method. The method comprises the following steps of separating Saprolegnia strains, purifying and culturing the Saprolegnia strains, obtaining Saprolegnia spores, preparing Evans Blue dye liquor, dyeing and the like. The activity of water mildew mycelia can be quickly judged by detecting whether the dyed water mildew mycelia and the spores become blue or not under a microscope; and the percentage of living cells and dead cells can be simply calculated according to the percentage of colored cells in total cells, so as to rapidly judge the vitality of Saprolegnia mycelia. The method has the characteristics of simplicity, feasibility, intuition and the like, and is suitable for scientific research institutions, schools, technique popularization stations and aquaculture companies.
Owner:HANGZHOU ENJOY ENVIRONMENTAL PROTECTION TECH
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents
ActiveUS10696631B2Easy to modifyReduce the possibilityMonoazo dyesIsotope introduction to heterocyclic compoundsDiseaseImaging agent
Owner:UNITED STATES OF AMERICA
Non-invasive method of diagnosing dysphagia in patients having a tracheostomy
The invention provides a save and non-invasive alternative to the traditional modified Evans blue dye test for dysphagia in intubated patients. The method employs direct application of dye to the tongue, in combination with a tracheal tube that provides suction in the subglottal region above the cuff. By keeping the cuff inflated, the aspiration of food and liquid, as used in the traditional test, is avoided entirely.
Owner:FLOSURE TECH LLC
Nanovesicle drug formed by self-assembly and its preparation method and application
ActiveCN113476404BHigh drug loadingHigh drug loading rateOrganic active ingredientsNanomedicineOncologyDrug loading dose
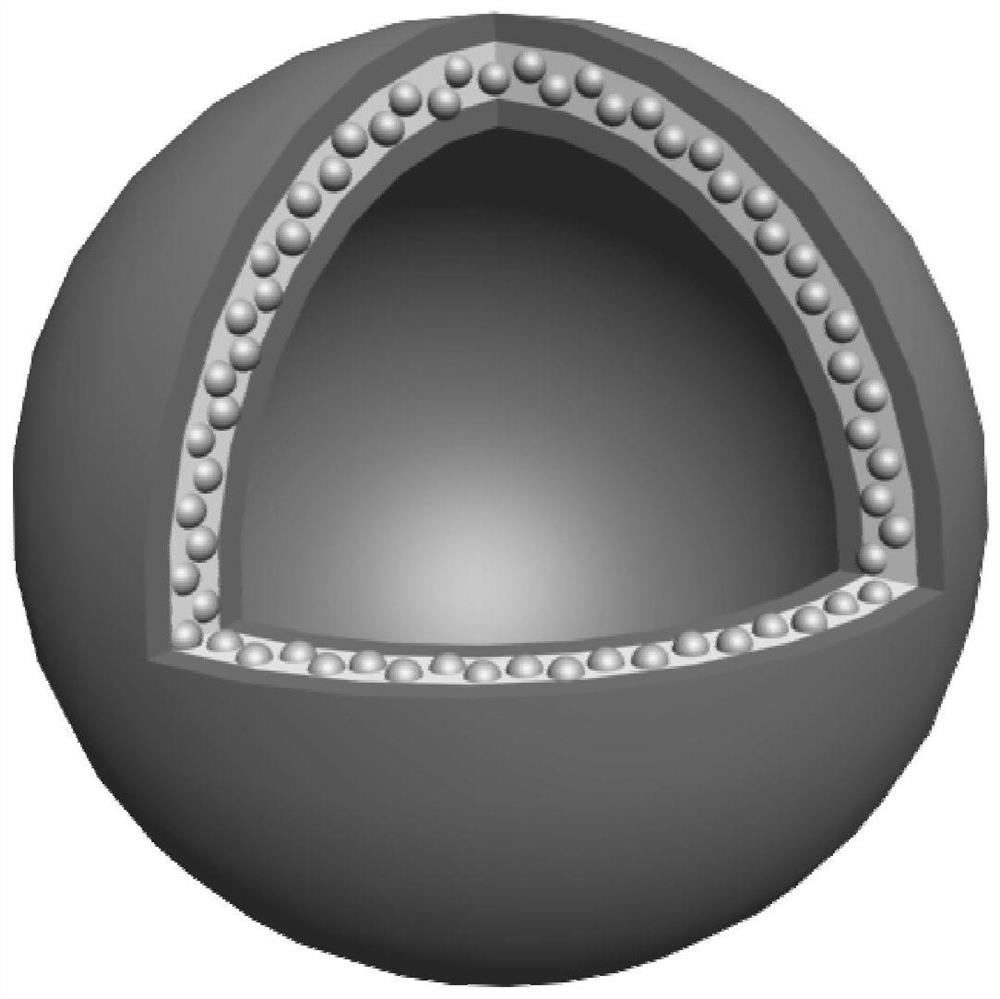
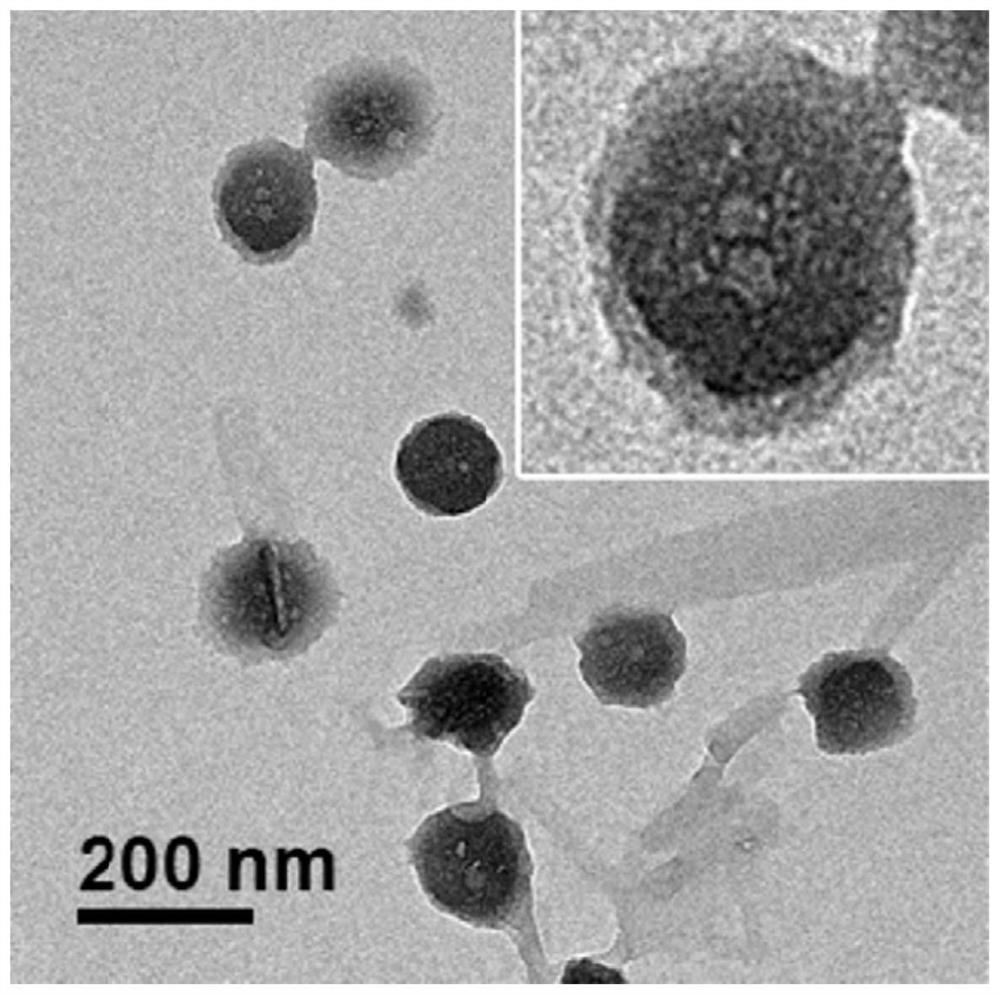
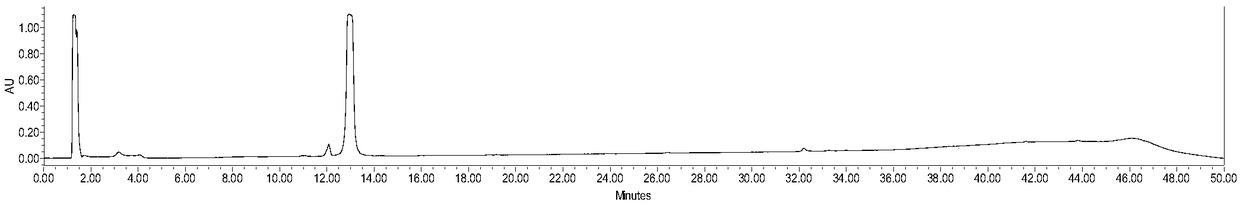
The present invention provides a nanovesicle drug, containing camptothecin (EB-CPT) and paclitaxel coupled with Evans blue; the inside of the nanovesicle has a cavity, and its wall structure is determined by the EB-CPT CPT physically wraps the paclitaxel; the nanovesicle drug is spherical, the diameter of any cross-section is not greater than 150nm, and the size ratio of any two dimensions in the three-dimensional dimension is not greater than 2:1, and the thickness of the capsule wall is between 2 and 1. 20nm; The EB-CPT structure is shown in the following formula (I), wherein, R1 is camptothecin or its derivative group from the structure shown in formula (II) or formula (III); R2 is -CH 2 ‑, or one of ‑O‑; X is S or ‑CH 2 One of ‑; n1, n2 are the number of repeating units, which are all integers of 0‑10. The nanovesicle drug of the present invention has the advantage of being more suitable for clinical application, and simultaneously has high drug loading capacity and drug loading rate. The present invention also provides a preparation method of the nanovesicle drug and its application in the preparation of cancer treatment drugs.
Owner:YANTAI LANNACHENG BIOTECHNOLOGY CO LTD
A kind of polypeptide compound with high affinity to glp-1 receptor and its preparation method and application
ActiveCN107365375BEasy to prepareEasy to manufacturePeptide/protein ingredientsMetabolism disorderDiseaseReceptor
The present invention provides a polypeptide compound having high affinity for GLP-1 receptors, the molecular structure thereof being as shown in the following formula (I), wherein R represents Evans blue or a derivative group thereof. The polypeptide compound of the present invention has the advantages of stable structure, simple preparation, significant therapeutic effect, long-term drug efficacy, convenient storage, etc. The present invention further provides a preparation method for the polypeptide compound and use thereof in preparing a medicament for diagnosing, preventing or treating GLP-1 / GLP-1 receptor pathway-related diseases.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
Process of Evaluating Blood-Brain Barrier Permeability of Stroke Rat by Using Fluorescent Substance
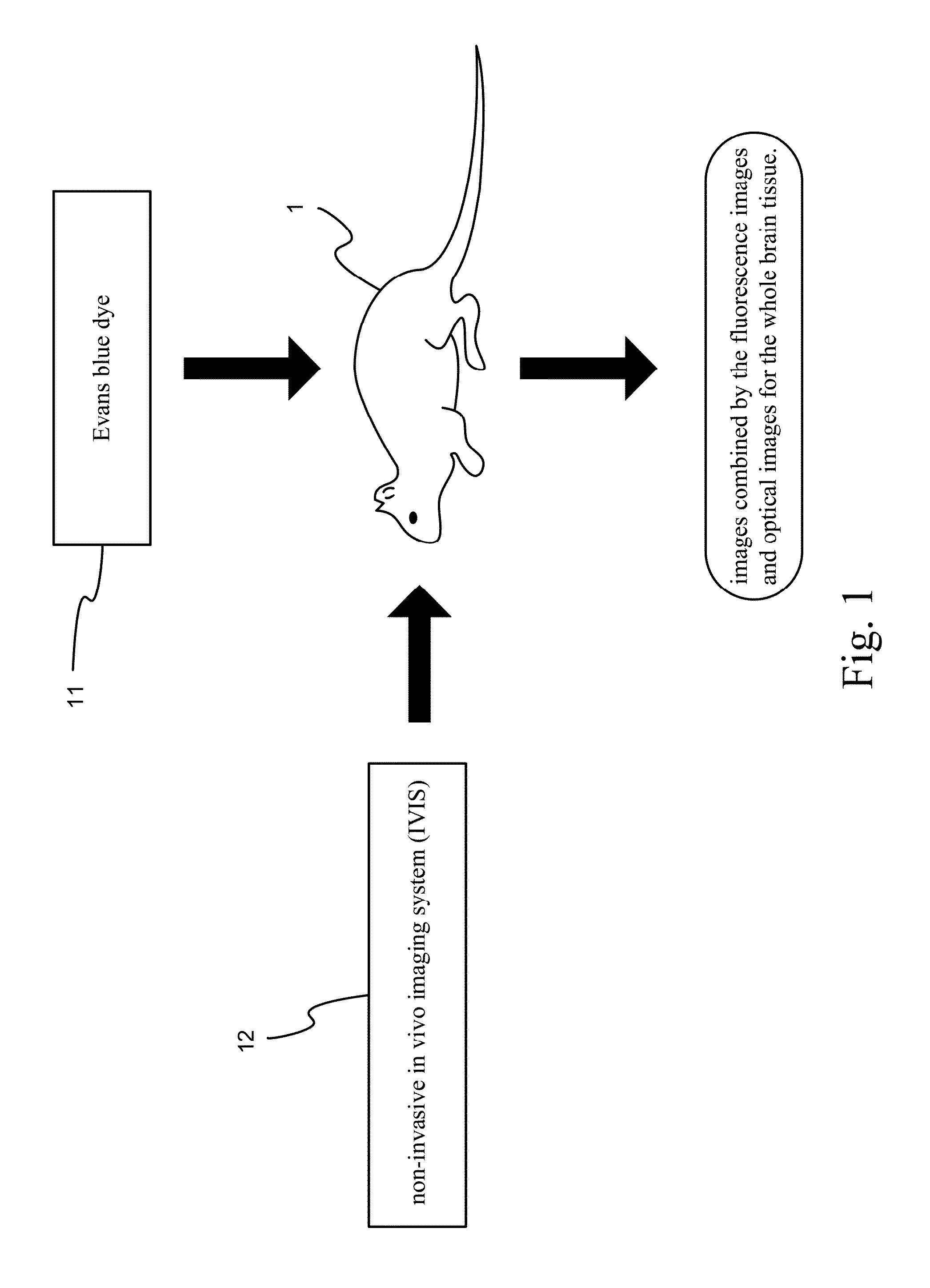
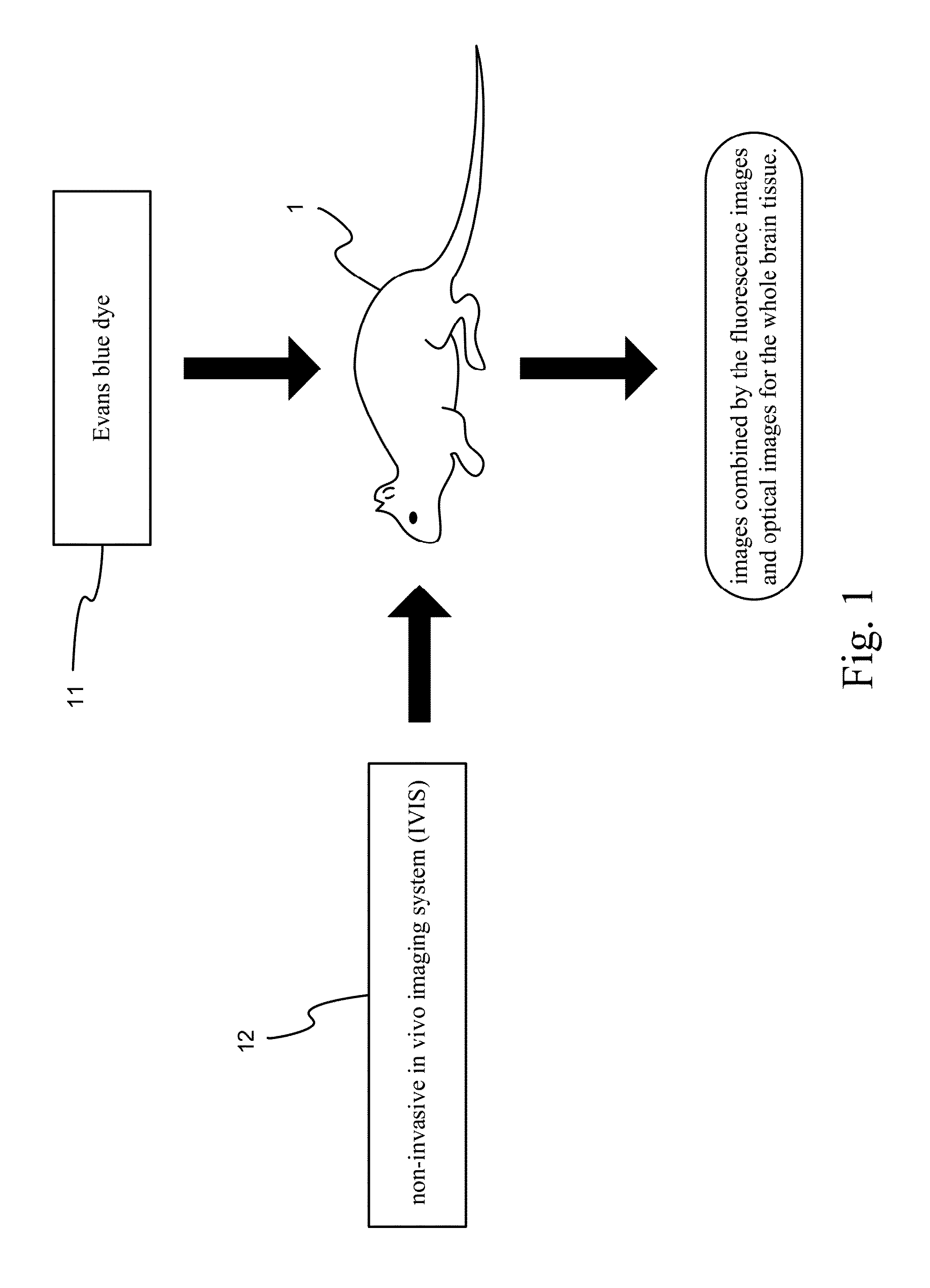
A process of evaluating blood-brain barrier permeability of a stroke rat by using fluorescent substance is disclosed. This process uses an Evans blue dye having spontaneous fluorescence properties, in combination with the use of a new non-invasive in vivo imaging system (IVIS), to obtain fluorescent signals so as to assess the change in the blood-brain barrier permeability of rodents after a cerebral artery stroke model surgery. In operation, an Evans blue dye is injected into a stroke rat of middle cerebral artery occlusion model. A non-invasive in vivo imaging system is used to detect the fluorescence distribution of the whole brain, and obtain images combined by the fluorescence images and optical images for the whole brain tissue. Thereby, the change in the blood-brain barrier permeability of the stroke rat can be completely realized.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Non-invasive method of diagnosing dysphagia in patients having a tracheostomy
InactiveUS20170188935A1Highly accurateHighly well-toleratedCompounds screening/testingTracheal tubesNostrilTracheal tube
The invention provides a save and non-invasive alternative to the traditional modified Evans blue dye test for dysphagia in intubated patients. The method employs direct application of dye to the tongue, in combination with a tracheal tube that provides suction in the subglottal region above the cuff. By keeping the cuff inflated, the aspiration of food and liquid, as used in the traditional test, is avoided entirely.
Owner:FLOSURE TECH LLC
Application of asarone in preparation of medicine for treating or preventing cerebral infarction hemorrhage transformation
PendingCN114010621AReduce volumeExtended respawn timeNervous disorderEther/acetal active ingredientsBrain edemaIonic Channels
The invention relates to the technical field of medicines, in particular to a novel application of alpha-asarone in preparation of a medicine for treating or preventing cerebral infarction hemorrhage transformation, and particularly relates to a novel application of alpha-asarone in preparation of a medicine for treating or preventing cerebral infarction hemorrhage transformation caused by thrombolytic therapy. The alpha-asarone can inhibit the current of the voltage-gated sodium ion channel, and can selectively act on the inactivated sodium ion channel and prolong the reactivation time of the sodium ion channel. Accordingly, it is found that alpha-asarone can significantly improve the neurological function score of a cerebral infarction hemorrhage transformation model rat, reduce the cerebral infarction volume, relieve encephaledema and improve blood-brain barrier permeability (reduce the content of Evans blue in brain tissue), and therefore nerve injury caused by cerebral infarction hemorrhage transformation is relieved. The clinical use of alpha-asarone is expected to be helpful for improving the prognosis of a cerebral apoplexy patient and prolonging the thrombolysis treatment time window of the cerebral apoplexy patient; the nerve injury of cerebral apoplexy patients is relieved, the death rate is reduced, and the life quality is improved.
Owner:SICHUAN UNIV
Application of vitamin k3 in the preparation of antiallergic drugs
ActiveCN111110665BReduced degranulationImprove allergic symptomsOrganic active ingredientsImmunological disordersDiseaseVitamin K3
The invention discloses the application of vitamin K3 in the preparation of anti-allergic drugs. Vitamin K3 has a clear therapeutic effect on improving anaphylactoid symptoms. By comparing the degranulation of KU812 cells, the degree of swelling of the toe, and the degree of Evans blue exudation of the toe , Serum histamine release evaluation test drug group, normal saline control group and normal control group, to evaluate the therapeutic effect of drugs on allergic diseases, to determine that vitamin K3 can be used in the preparation of anti-allergic drugs. Vitamin K3 can effectively alleviate the symptoms of local and systemic anaphylaxis in mice; reduce mast cell degranulation and significantly improve the symptoms caused by anaphylaxis.
Owner:XI AN JIAOTONG UNIV
Application of licochalcone A in preparation of anti-anaphylactoid drugs
PendingCN113304130AReduced degranulationImprove allergic symptomsKetone active ingredientsImmunological disordersAnaphylactoid reactionsDisease
The invention discloses an application of licochalcone A in preparation of an anti-anaphylactoid drug. KU812 cell degranulation, toe swelling degree, toe Evans blue exudation degree and serum TNF-alpha release amount are compared to evaluate a to-be-detected medicine group, a normal saline control group and a normal control group, so that the treatment effect of the medicine on anaphylactoid diseases is evaluated, and the invention determines that the licochalcone A can be used for preparing the anti-anaphylactoid drug. The licochalcone A can effectively relieve local and systemic anaphylactoid reaction symptoms of mice; and the licochalcone A can reduce mast cell degranulation, and remarkably improve symptoms caused by anaphylactoid.
Owner:XI AN JIAOTONG UNIV
Application of Licochalcone A in preparation of antiallergic drug
InactiveCN113440501AReduced degranulationImprove allergic symptomsKetone active ingredientsImmunological disordersDiseaseLicochalcone A
The invention discloses application of Licochalcone A in preparation of an antiallergic drug. A to-be-detected drug group, a normal saline control group and a normal control group are evaluated by comparing a mastocyte degranulation degree, a mouse toe swelling degree, a toe Evans blue exudation degree and a body temperature lowering degree, so that the treatment effect of the drug for allergic diseases is evaluated; a result determines that the Licochalcone A can be used for preparing the antiallergic drug. The Licochalcone A can effectively relieve local and systemic anaphylactic reaction symptoms of mice. Mastocyte degranulation is reduced, and symptoms caused by allergy are remarkably eliminated.
Owner:XIAN HONGHUI HOSPITAL
Application of TIMP-1 in preparation of medicine for preventing or treating traumatic brain injury
PendingCN112451683AImprove experiment latencyImprove exercise balanceNervous disorderPeptide/protein ingredientsInjury brainCell membrane
The invention discloses application of TIMP-1 in preparation of a medicine for preventing or treating traumatic brain injury. The invention discloses application of TIMP-1 in preparation of a medicinefor treating blood-brain barrier dysfunction of traumatic brain injury. The medicine can be in the form of injection powder, water aqua, lipidosome and nano materials. The protein TIMP-1 can prolongthe incubation period of a mouse with cerebral trauma in a rotarod test; the motion balance capability of the mouse with cerebral trauma on a balance beam is improved; and the Evans blue permeabilityof brain tissues is reduced. In brain microvascular endothelial cells HBMEC, the TIMP-1 can participate in maintaining intercellular tight junction integrity, up-regulating tight junction complex expression, reducing luciferase leakage and increasing intercellular resistance. The invention also provides application of the protein TIMP-1 as a CD63-Integrin[beta]1 ligand in preparation of a medicinefor treating central nervous system diseases caused by blood-brain barrier dysfunction. The TIMP-1 plays a role by combining with a cell membrane complex CD63-Integrin[beta]1 to regulate and controlthe assembly of important molecules F-actin of cytoskeleton so as to maintain the intercellular tight junction integrity. The protein TIMP-1 has a good application prospect in treating the blood-brainbarrier dysfunction of thetraumatic brain injury.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Process of evaluating blood-brain barrier permeability of stroke rat by using fluorescent substance
A process of evaluating blood-brain barrier permeability of a stroke rat by using fluorescent substance is disclosed. This process uses an Evans blue dye having spontaneous fluorescence properties, in combination with the use of a new non-invasive in vivo imaging system (IVIS), to obtain fluorescent signals so as to assess the change in the blood-brain barrier permeability of rodents after a cerebral artery stroke model surgery. In operation, an Evans blue dye is injected into a stroke rat of middle cerebral artery occlusion model. A non-invasive in vivo imaging system is used to detect the fluorescence distribution of the whole brain, and obtain images combined by the fluorescence images and optical images for the whole brain tissue. Thereby, the change in the blood-brain barrier permeability of the stroke rat can be completely realized.
Owner:INST NUCLEAR ENERGY RES ROCAEC
A kind of polypeptide prodrug modified by Evans blue and its preparation and application
ActiveCN108690119BEasy to makeSmall toxicityOrganic active ingredientsTetrapeptide ingredientsPeptide drugCancer cell
The present invention provides a polypeptide drug prodrug, which is a prodrug formed by monomethylauristat or maytansine and Evans blue, and its structural formula is as formula (I); wherein, R 1 for or ‑CH 2 One of ‑; R 2 It is one of the new structures of monomethyl auristatin or metan, a polypeptide drug; m and n are the number of repeating units, and both are integers of 0-4. The polypeptide drug prodrugs of the present invention have excellent tumor cell uptake both in vivo and in vitro, and some prodrugs also show significant cancer cell inhibitory effect. The present invention also provides the preparation method of the prodrug and the application of the prodrug in the preparation of cancer treatment drugs.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com