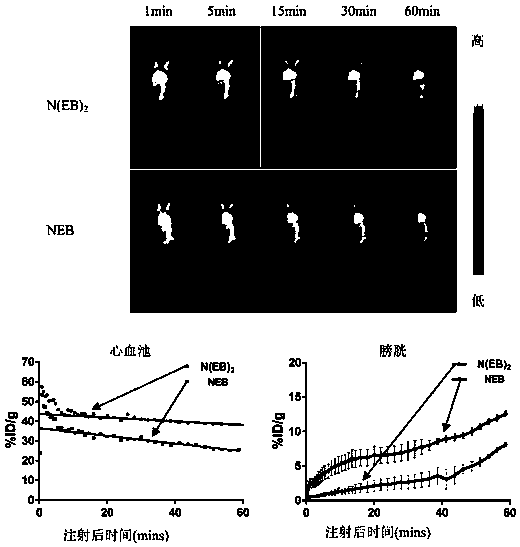
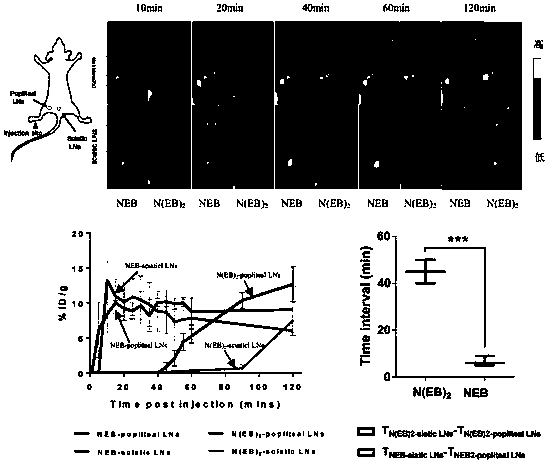
Evans blue complex as well as preparation method and application thereof
A technology of Evans blue and complexes, applied in copper organic compounds, chemical instruments and methods, fluorescence/phosphorescence, etc., can solve the problems of increasing surgeons to distinguish sentinel lymph nodes, signal interference, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1. Preparation of Evans blue derivative ligand (compound 13)
[0073] Synthesis of Compound 2:
[0074] Compound 1 (3,3'-dimethylbenzidine, 4.25 g, 20.0 mmol) and 50 mL of dry dichloromethane were respectively put into a 250 mL flask to obtain a pale yellow solution. 20 mL of di-tert-butyl dicarbonate (4.36 g, 20.0 mmol) in dichloromethane was slowly dropped into the flask. After stirring at room temperature for 24 h, the solvent was spin-dried to obtain a yellow solid. The residue was purified by silica gel column (petroleum ether / ethyl acetate=1:3) to obtain light yellow compound 2 with a yield of 50%. 1 H NMR (300MHz, CDCl 3)δ7.79(d, J=8.0Hz, 1H), 7.35(d, J=8.5Hz, 1H), 7.31(s, 1H), 7.24(d, J=3.7Hz, 1H), 6.71(d, J=7.9Hz,1H),6.27(s,1H),3.63(s,2H),2.28(s,3H),2.21(s,3H),1.53(s,9H).
[0075] Synthesis of compound 3:
[0076] Under ice bath condition, 15 mL of iced 2.0 M HCl was added dropwise to 40 mL of compound 2 (3.12 g, 10.0 mmol) in acetonitrile. St...
Embodiment 2~20
[0096] The N(EB) of embodiment 2-20 2 The compound structure is shown in the following formula (I), wherein, R can be a linking group shown in the following formula (II), the selection of each part of the group in the formula (II) is listed in Table 1 below, and their preparation method All can refer to embodiment 1:
[0097]
[0098] Table 1
[0099]
[0100]
Embodiment 21
[0101] Example 21. Radioactivity 18 Preparation of F-labeled Evan's blue complex
[0102] 1. Radioactivity 18 Preparation of F-labeled freeze-dried kit (take the preparation of 100 as an example)
[0103] Weigh 10 mg of compound 13 prepared in Example 1 and dissolve it in 10 mL of 0.5 mol / L acetic acid-sodium acetate buffer solution (pH=4), then add 0.1 mg of aluminum chloride (AlCl 3 ) was dissolved in 10 mL of 0.5 mol / L acetic acid-sodium acetate buffer solution (pH=4), and the two were evenly mixed. After sterile filtration, it is divided into 100 controlled antibiotic vials, and then placed in a freeze dryer for 24 hours to freeze-dry, and then sealed with a stopper to obtain a freeze-dried medicine box. According to the output of the kit and the requirements for the content of the components in each kit, the amount of compound 13 and aluminum chloride can be adjusted so that the mass ratio is in the range of 20-100:1.
[0104] 2. 18 Preparation of F-labeled Evan's bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com