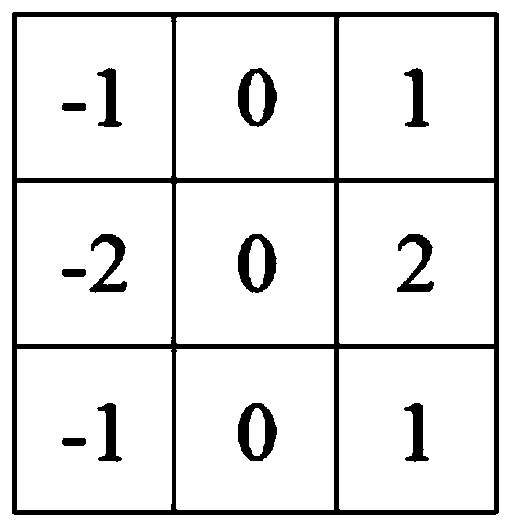
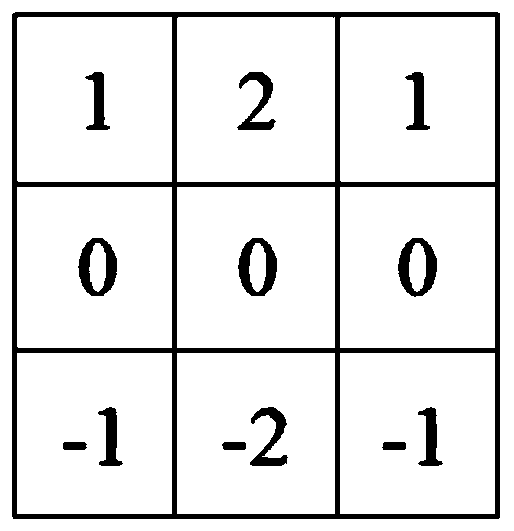
Patents
Literature
48 results about "Tumor margin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Margin: This refers to the area of normal-seeming tissue surrounding a tumor that has been removed during a biopsy or other surgery. This area may contain microscopic cancer cells, so, a pathologist examines it during a comprehensive margin evaluation. If the margins are narrow (one or two millimeters), malignant tumors are more likely to recur.
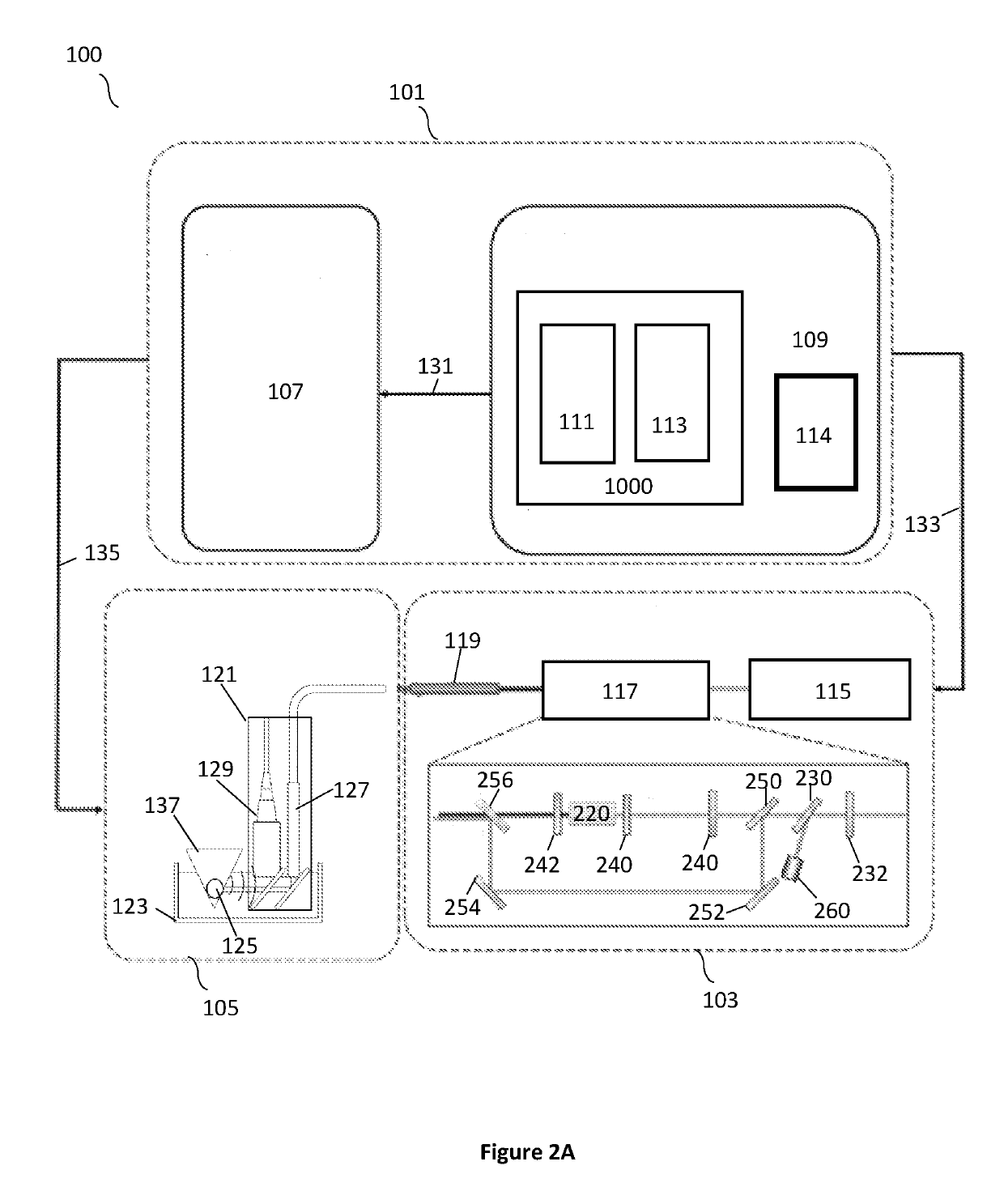
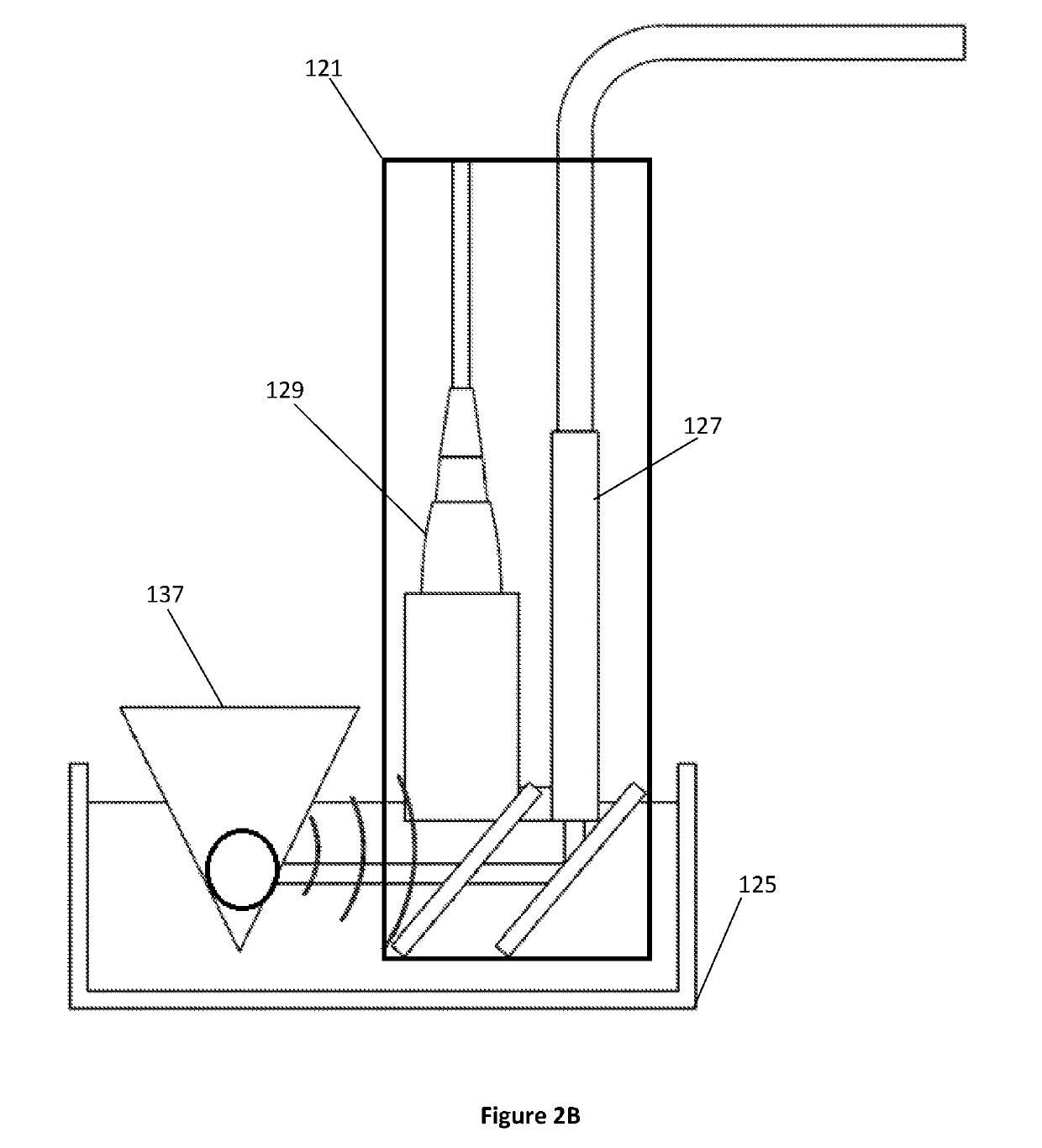
Selective modulation of intracellular effects of cells using pulsed electric fields
ActiveUS10471254B2Optimization mechanismGood effectElectrotherapyDiagnosticsAbnormal tissue growthDisease
Owner:VIRGINIA TECH INTPROP INC
Selective modulation of intracellular effects of cells using pulsed electric fields
ActiveUS20170266438A1Optimization mechanismMinimize impactElectrotherapyDiagnosticsDiseaseCancer cell
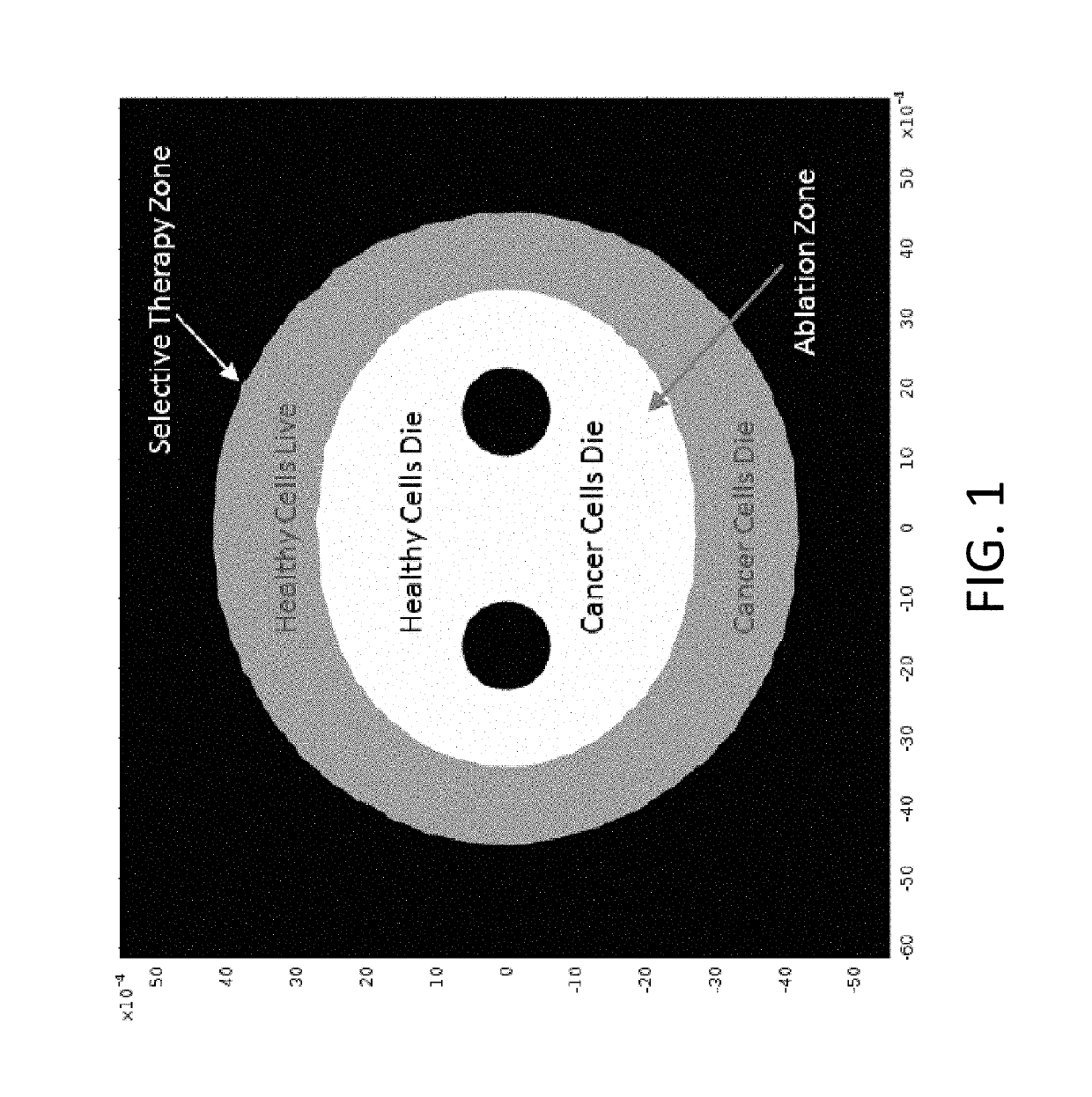
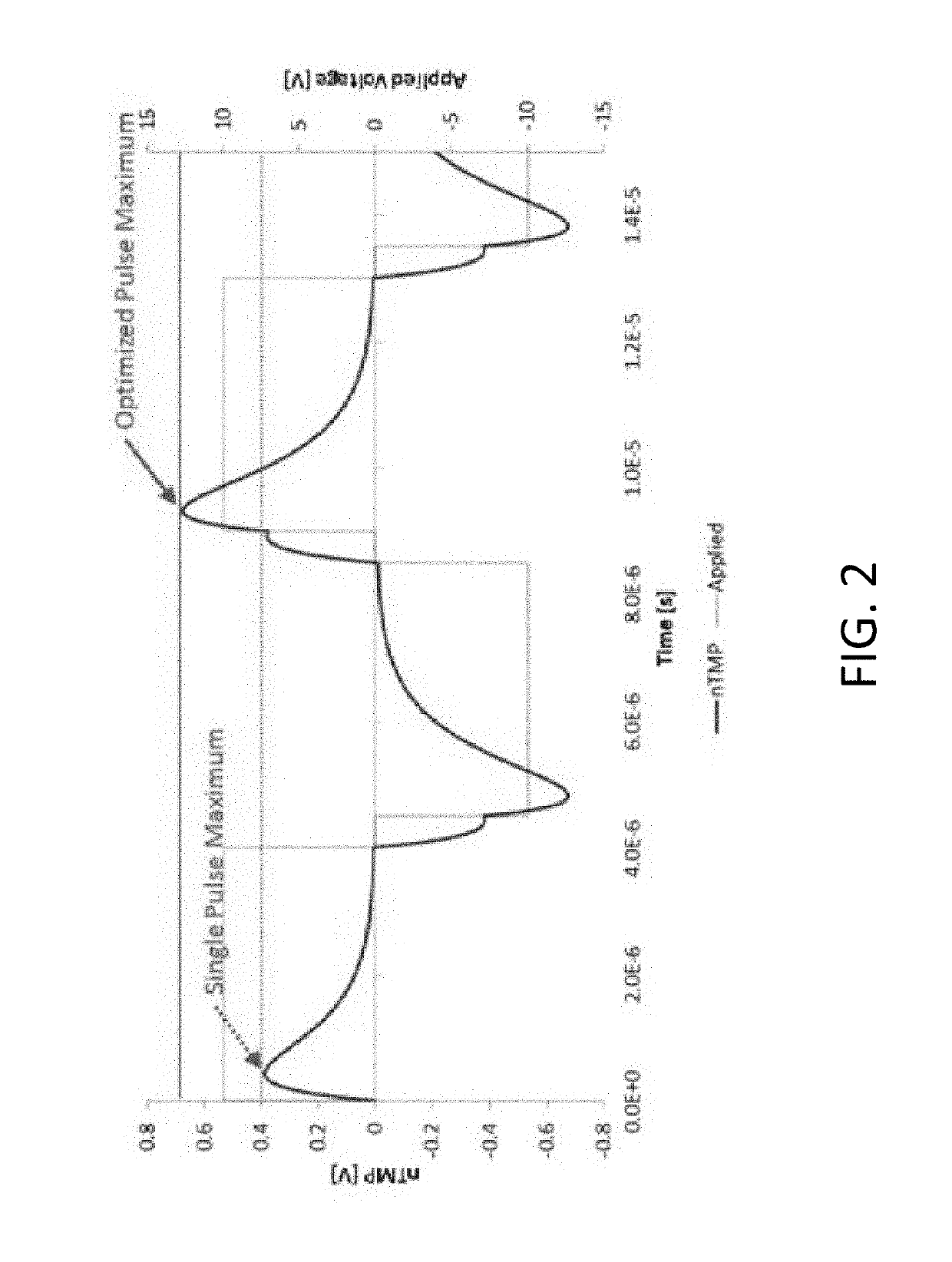
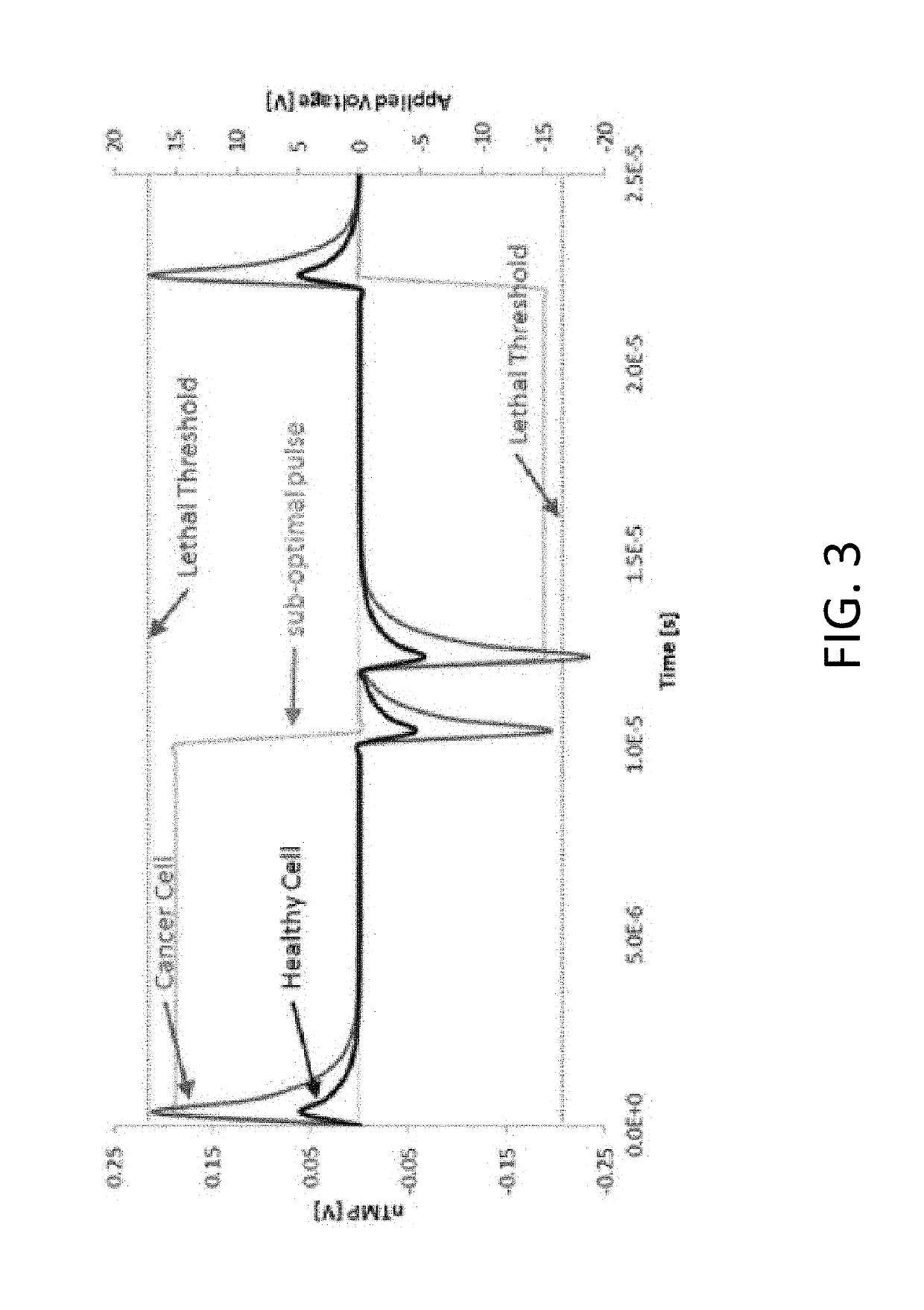
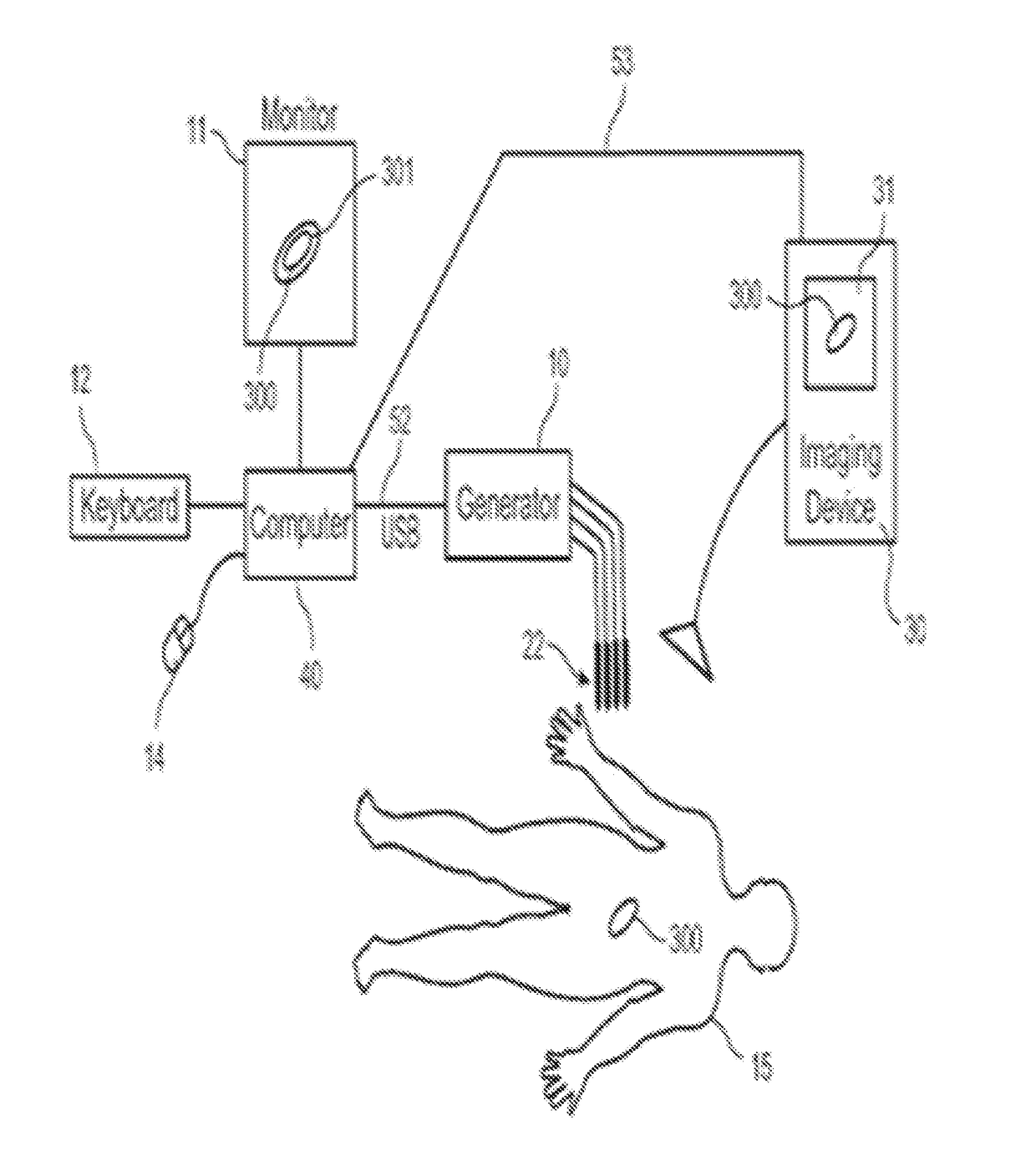
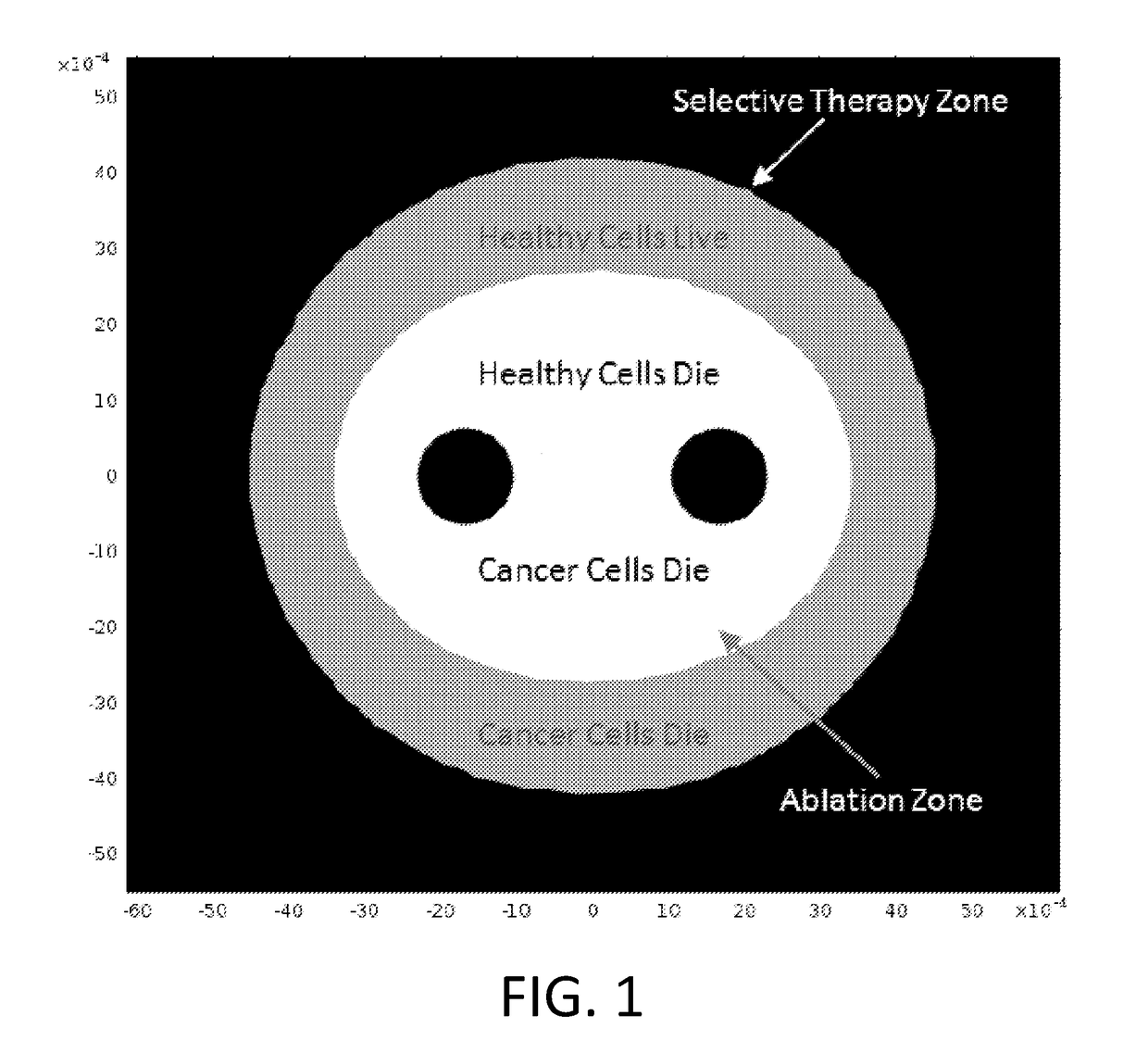
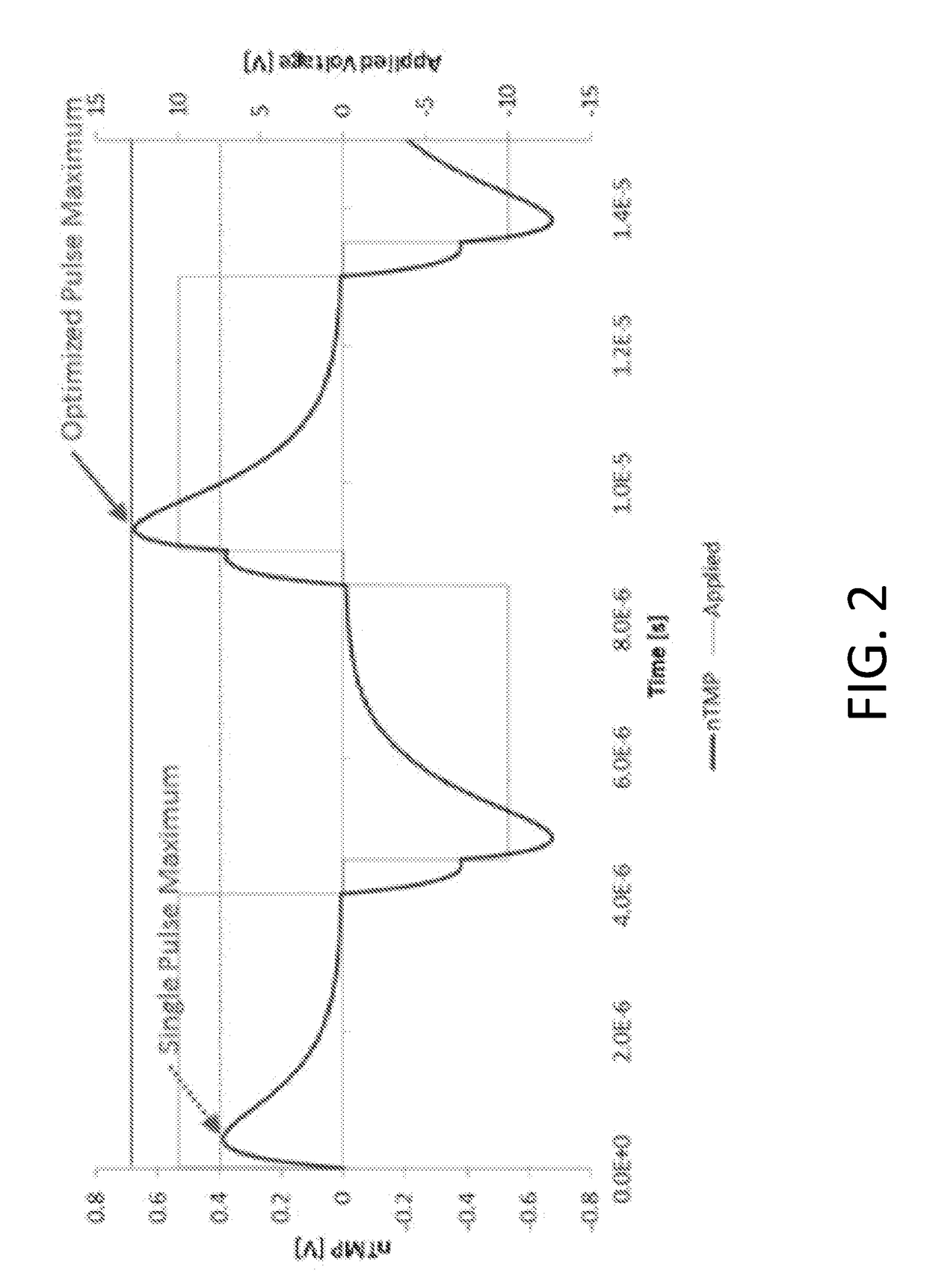
A system and method for selectively treating aberrant cells such as cancer cells through administration of a train of electrical pulses is described. The pulse length and delay between successive pulses is optimized to produce effects on intracellular membrane potentials. Therapies based on the system and method produce two treatment zones: an ablation zone surrounding the electrodes within which aberrant cells are non-selectively killed and a selective treatment zone surrounding the ablation zone within which target cells are selectively killed through effects on intracellular membrane potentials. As a result, infiltrating tumor cells within a tumor margin can be effectively treated while sparing healthy tissue. The system and method are useful for treating various cancers in which solid tumors form and have a chance of recurrence from microscopic disease surrounding the tumor.
Owner:VIRGINIA TECH INTPROP INC
Multimodal imaging for the detection of tissue structure and composition
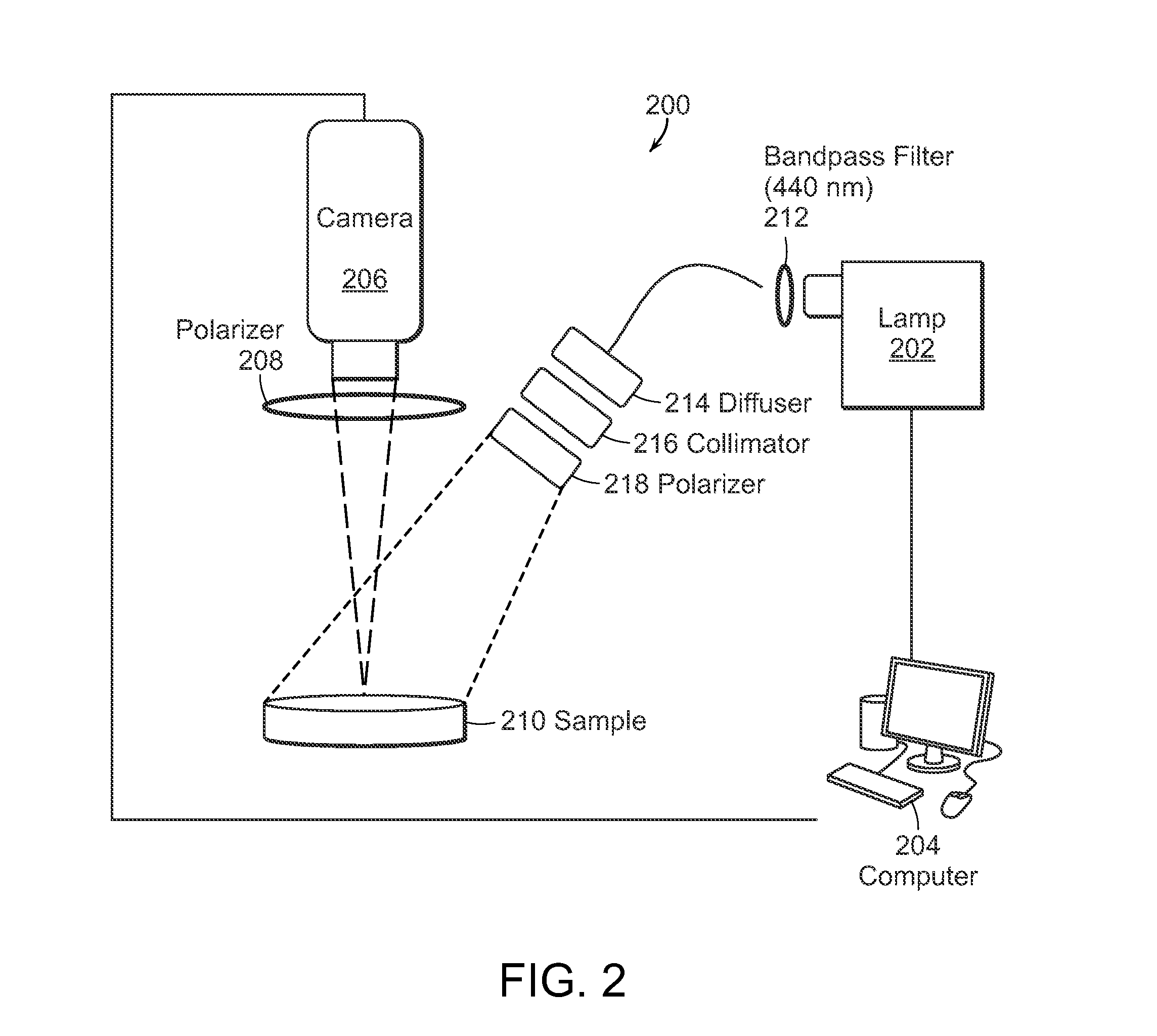
InactiveUS20150164327A1Accurately presentedSolution value is not highMedical imagingPolarisation-affecting propertiesTumor marginLight delivery
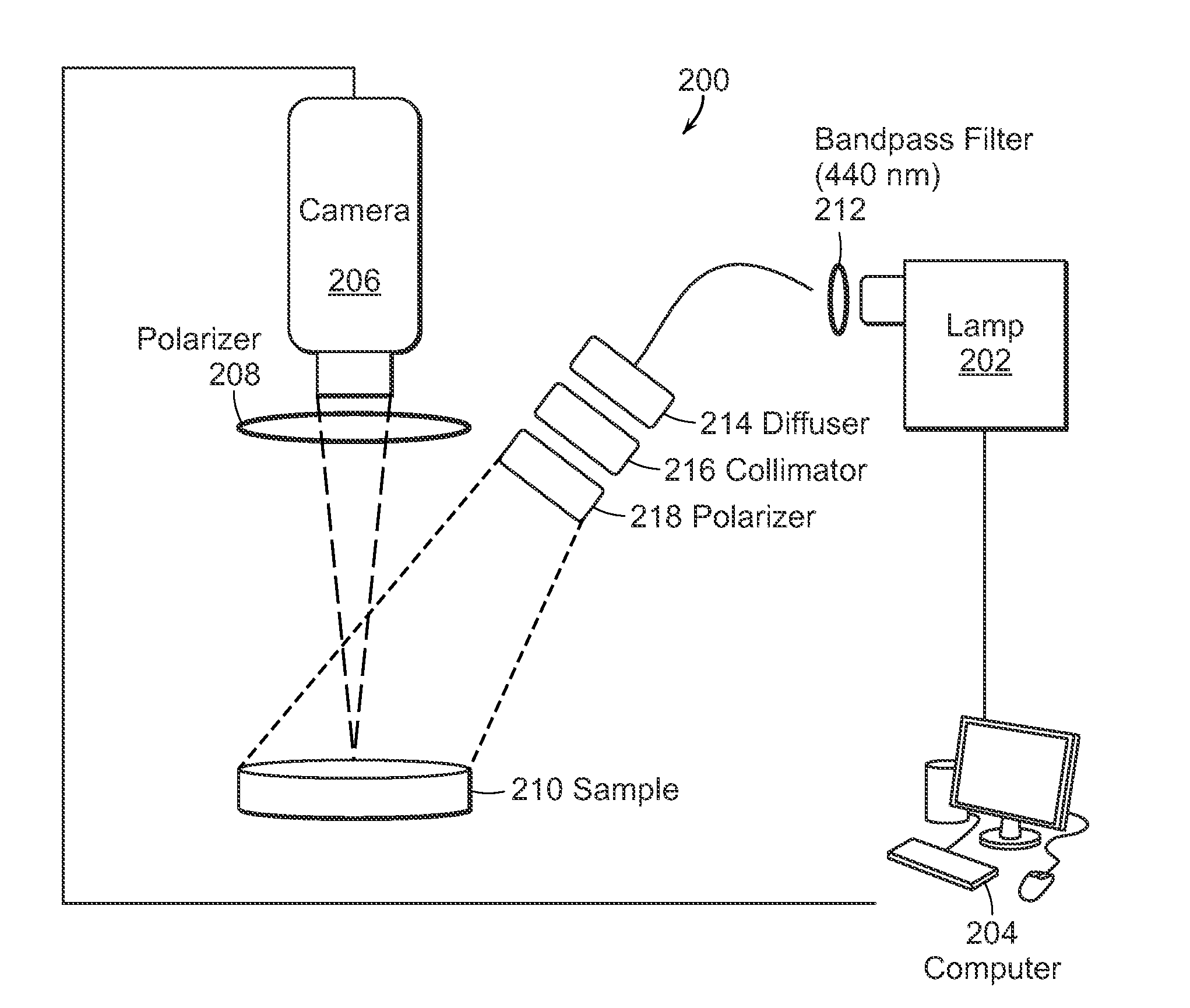
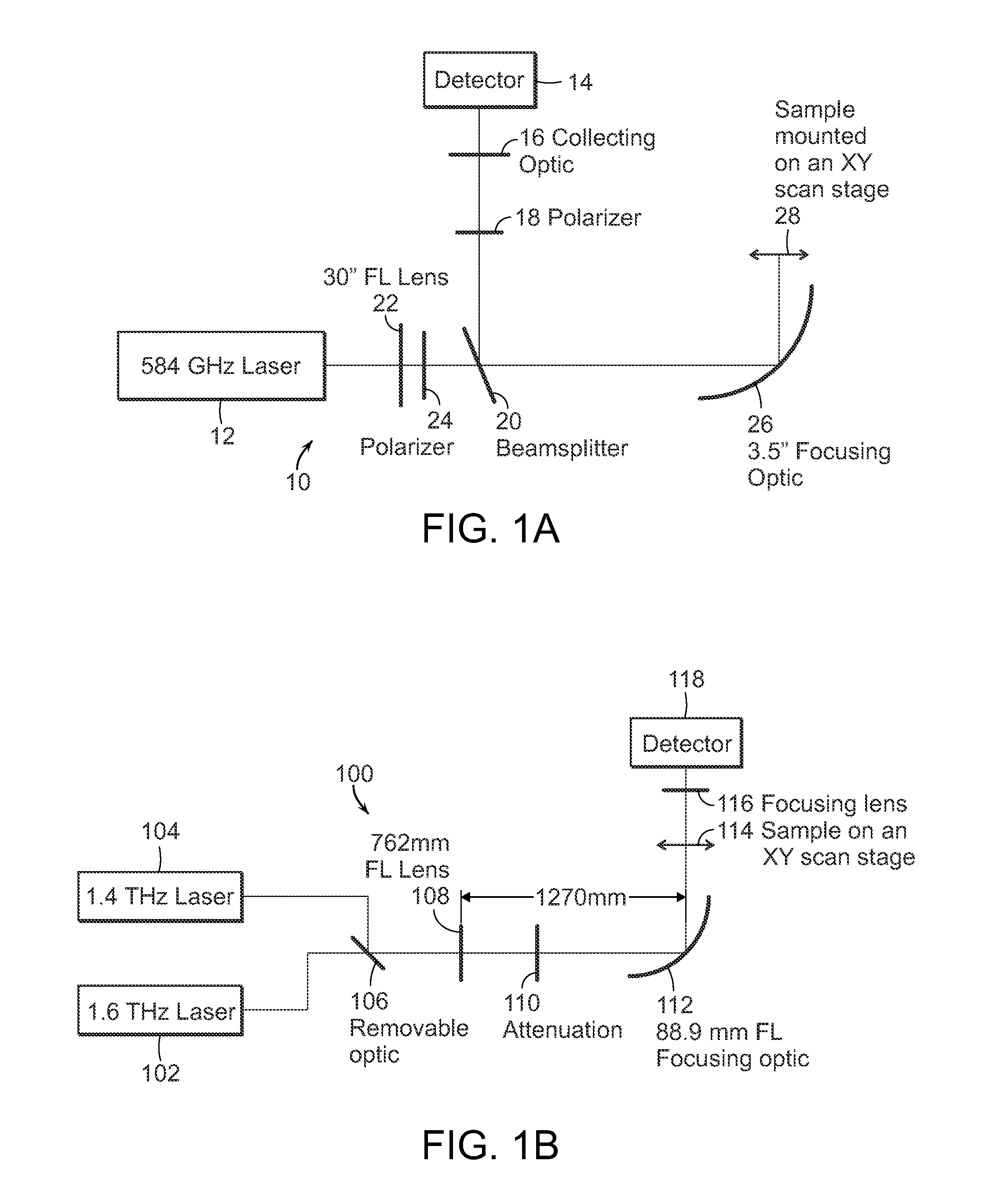
The present invention relates to the use of optical and terahertz imaging of tissue for measuring characteristics to assist in diagnosis. A light delivery and collection system is used that can aid in the detection of tumor margins, for example. A data processor processes the image data to determine characteristics of a region of tissue.
Owner:UNIV OF MASSACHUSETTS
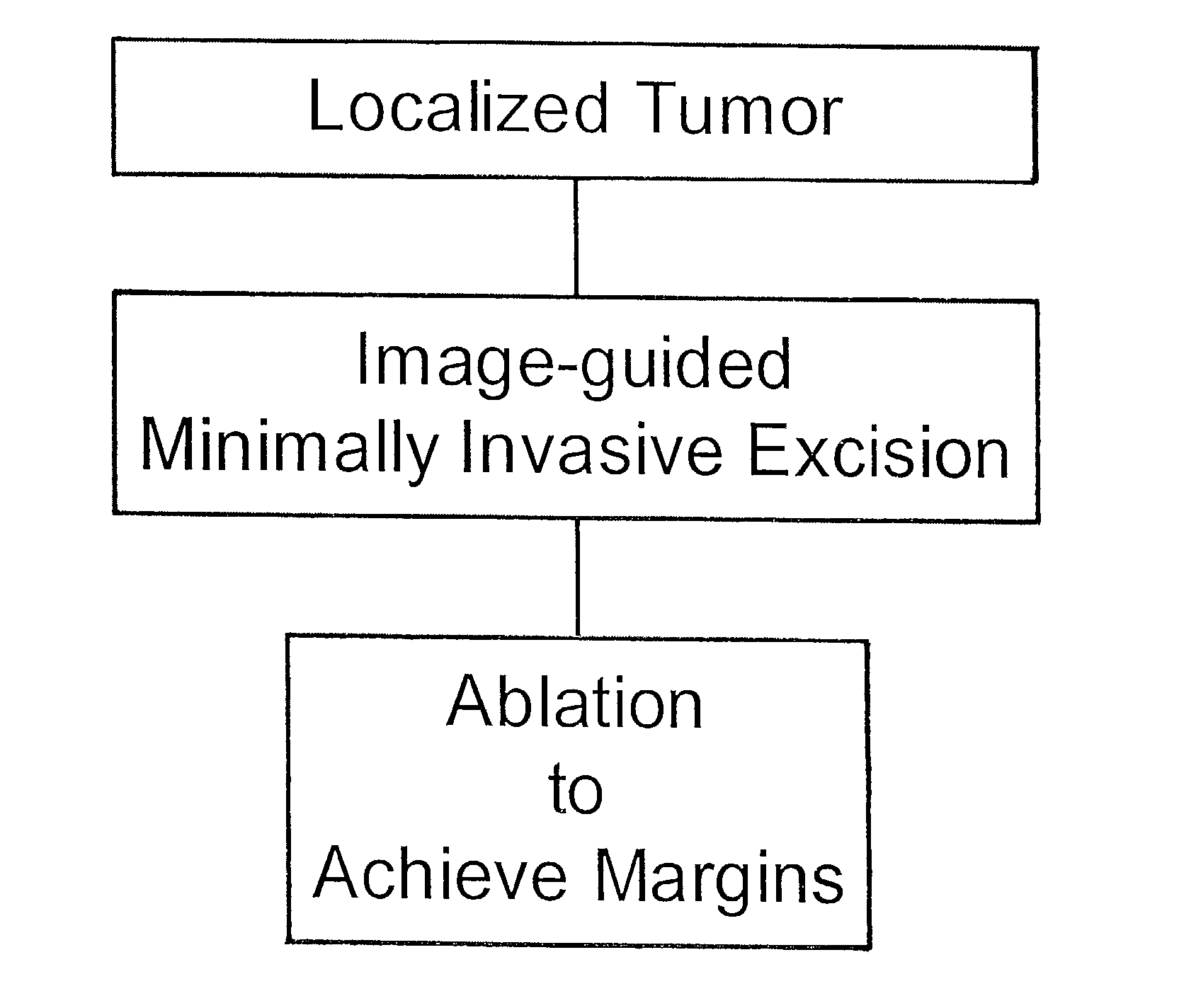
Minimally invasive treatment for breast cancer
InactiveUS6978788B2Improve beauty effectShorten recovery timeSurgical needlesVaccination/ovulation diagnosticsDiseaseTumor removal
The present invention provides a minimally invasive, comprehensive same-day diagnosis and treatment method to remove tumor and ablate margins in breast cancer patients, comprising disease diagnosis by MRI and touch preparation cytology, followed by tumor removal and ablation of tumor margins.
Owner:INNOBLATIVE DESIGNS INC +1
Instant, in-situ, nondestructive material differentiation apparatus and method
InactiveUS20140358447A1Improve abilitiesMaximize transmissivityUltrasonic/sonic/infrasonic diagnosticsProcessing detected response signalCell phenotypePrincipal component analysis
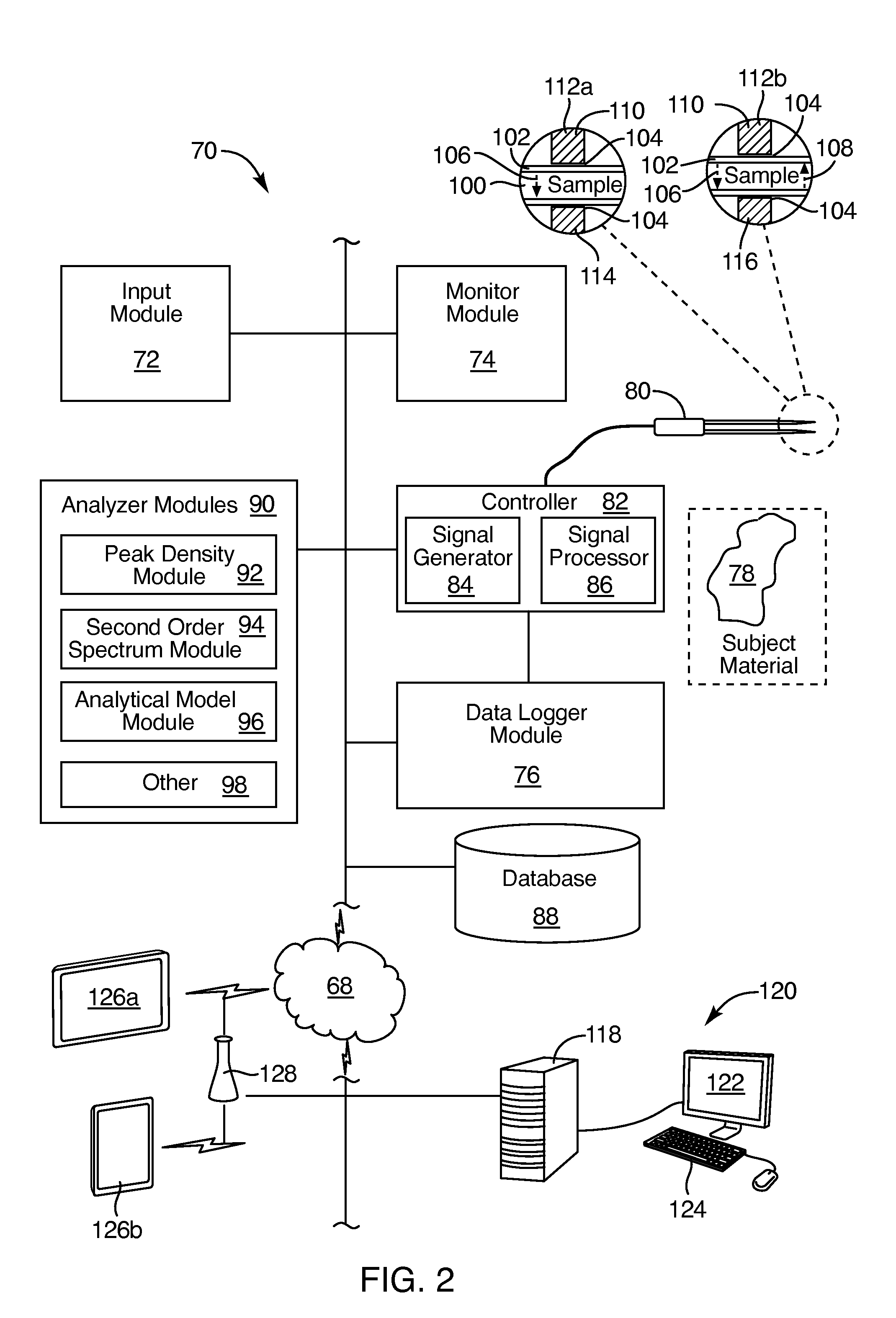
Specular, ultrasonic, piezoelectric, detection devices provide real-time, analytical, edge finding in tissues during tumor surgery. Piezoelectric probe sensors at high frequencies (e.g., 10 to 100 MHz) characterize microstructure of cells and tissues. Through-transmission or specular reflection enables nondestructive testing in real time. Peak density analysis in power spectra, second-order spectrum analysis measuring the slope of the Fourier transform of the power spectrum, artificial intelligence pattern recognition, and modeling interpret the results. Model-based data analysis may compare experimental data with a computer simulation. Such comparisons may be based upon pattern classifications, including principal component analysis (PCA). Combining the above detection devices and analytical methods provides speed, accuracy, simplicity, and nondestructive mechanisms that militate for reliable, real-time diagnosis of tumor margins, tissue pathology, cell phenotypes, and molecular subtypes.
Owner:UTAH VALLEY UNIVERSITY
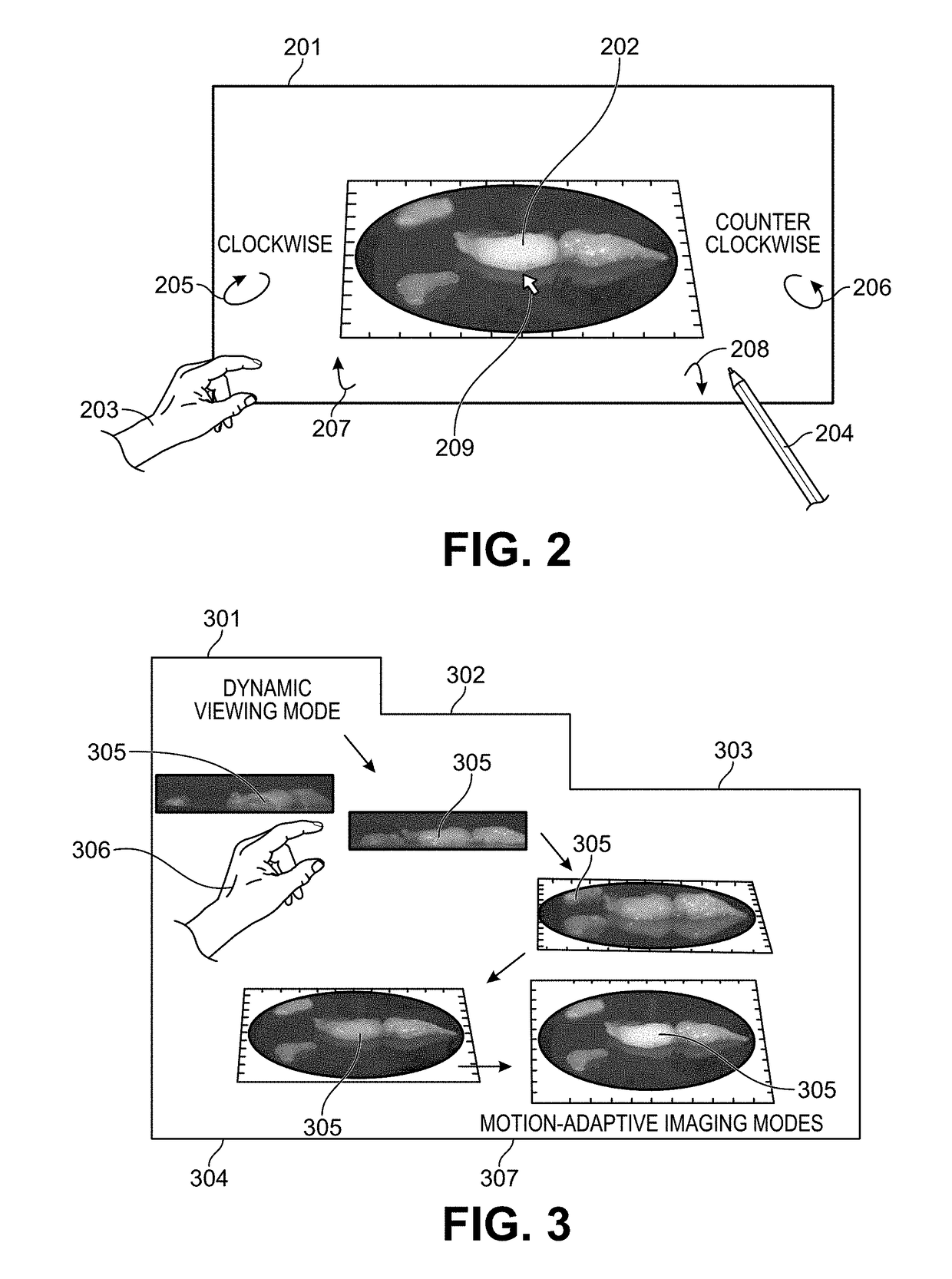
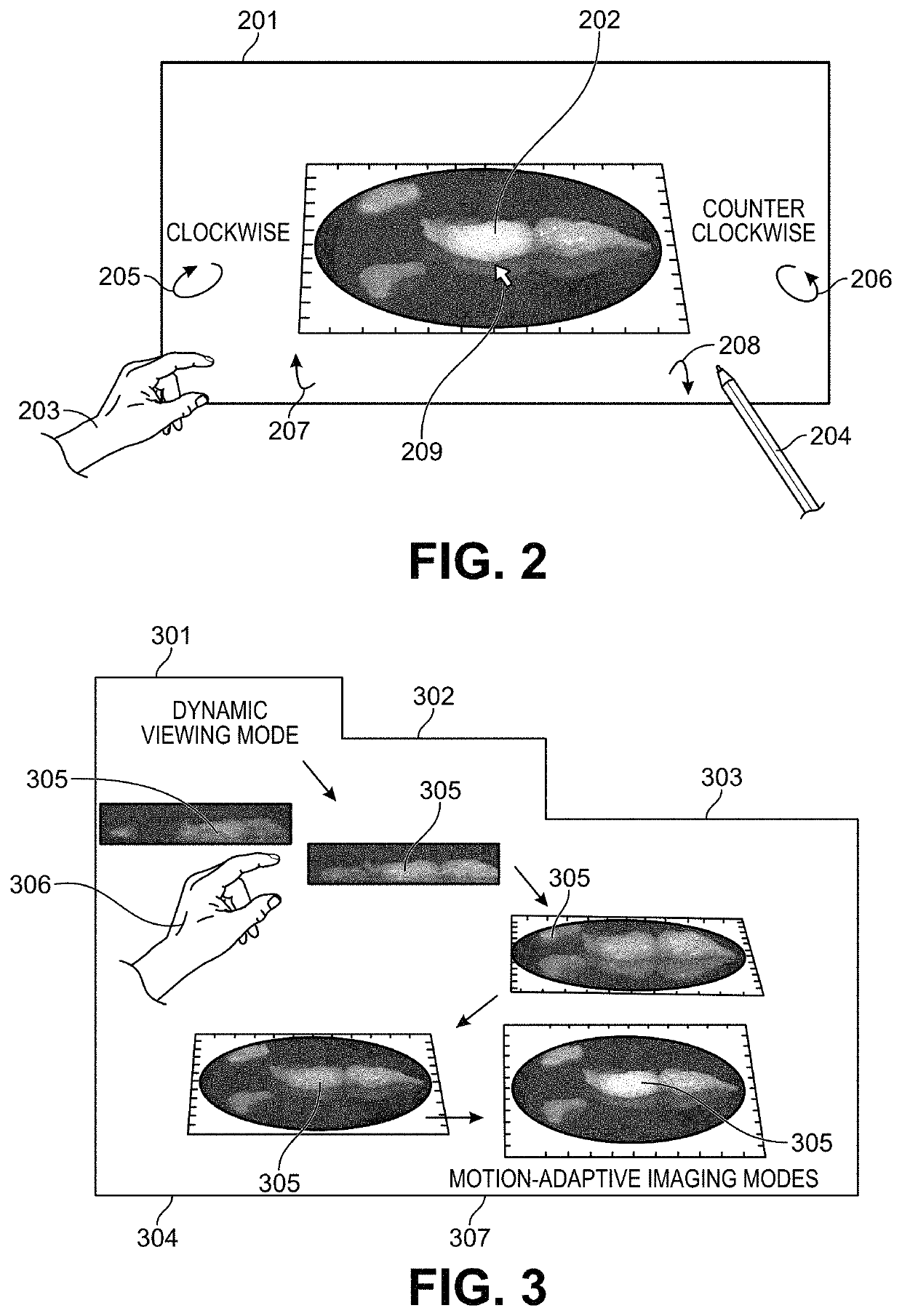
Motion-Adaptive Interactive Imaging Method
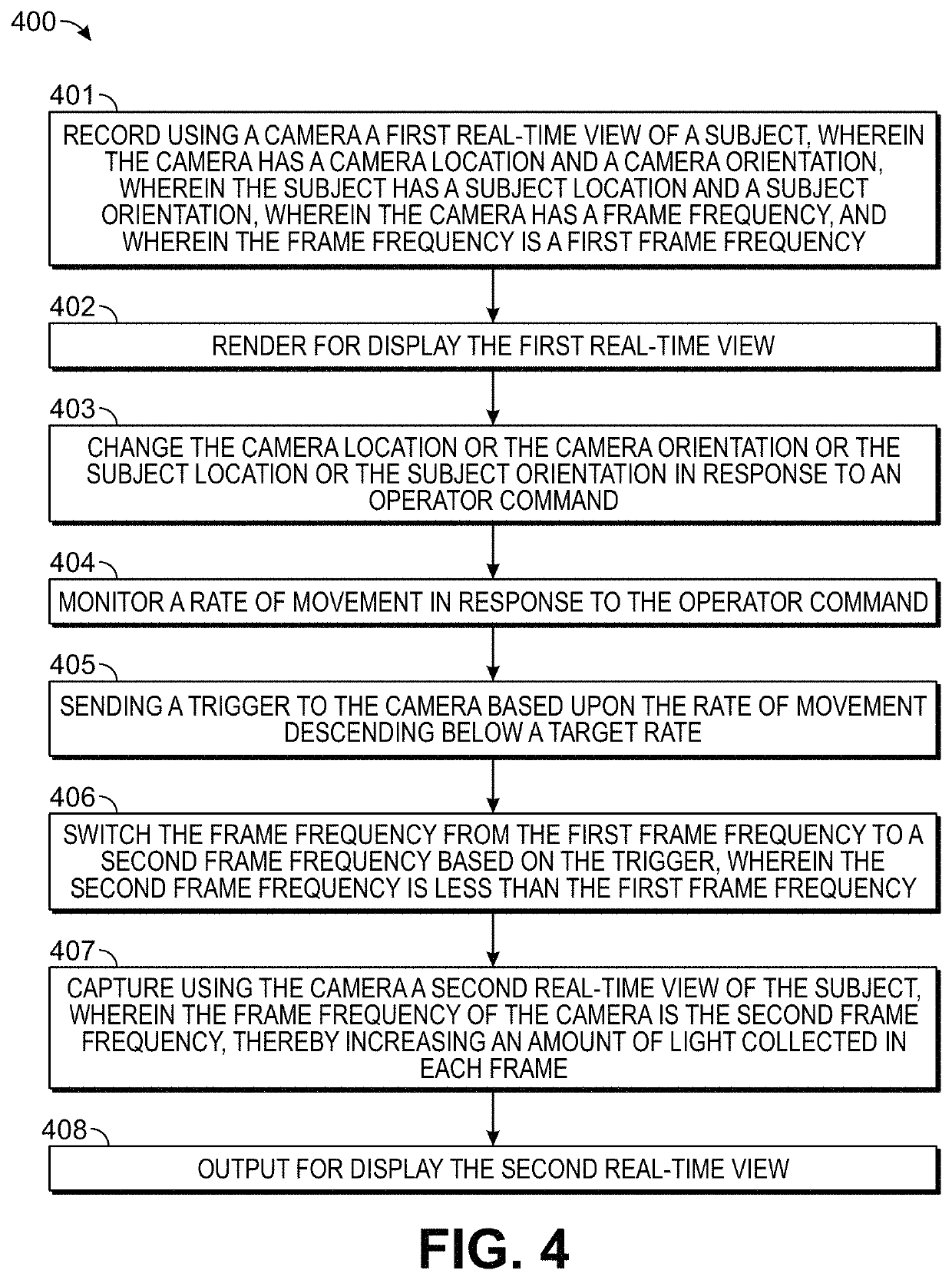
ActiveUS20180140197A1Long integration timeHigh sensitivityTelevision system detailsRadiation diagnostic clinical applicationsHigh frame rateTumor margin
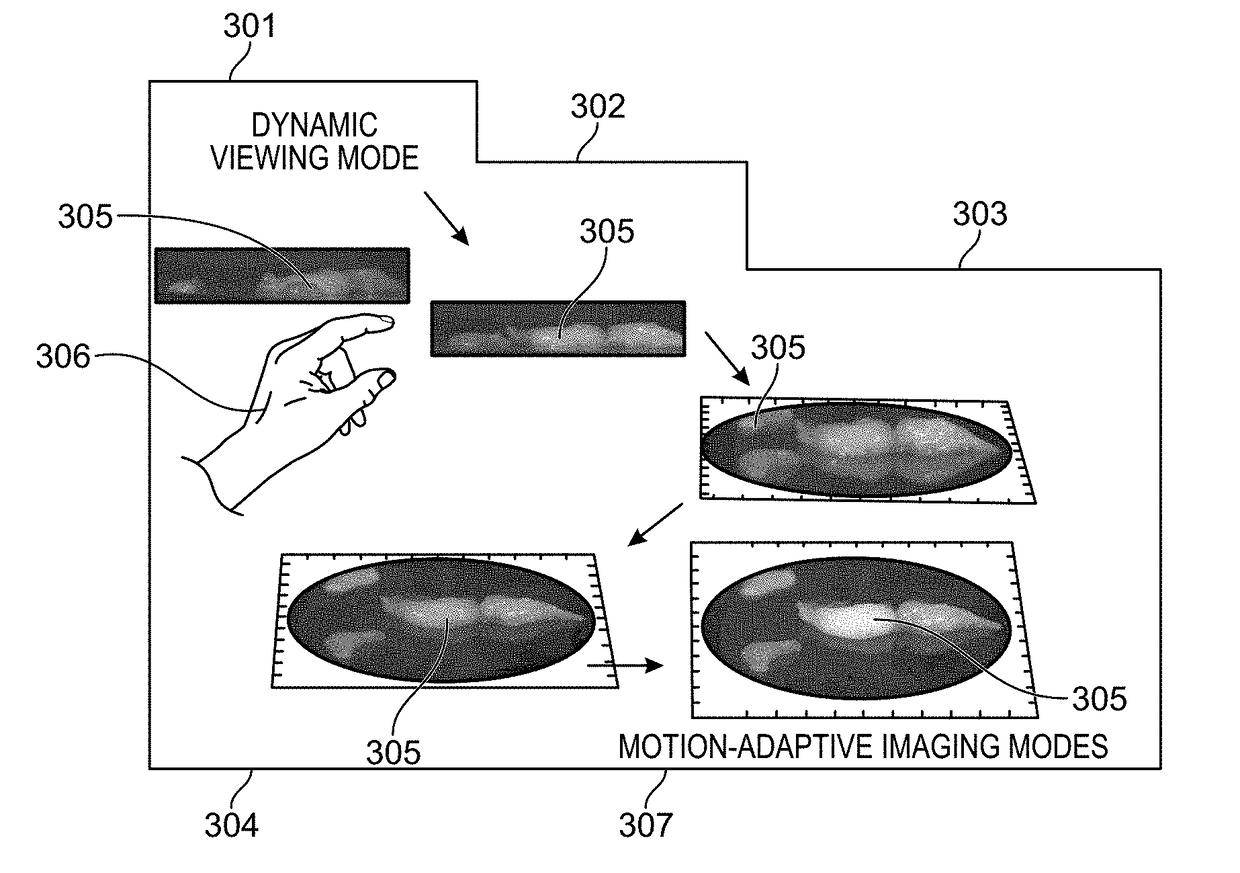
Methods and systems are provided for the detecting and presenting of faint signals from a real-time interactive imager. A real-time view of a subject is controlled by a user by entering commands that move the subject or a camera used to record the real-time view. The rate of this movement is monitored, and when the rate descends below a preset value, a trigger is sent to the camera. In response to the trigger, the camera switches to a lower frame frequency for capturing the real-time view of the subject, thereby increasing the amount of light collected in each frame, and increasing the sensitivity of the camera to faint signals. The methods and systems are particularly relevant for surgical or biopsy imaging, in which both a higher frame rate preview image of a subject for 3D visualization, and a higher sensitivity fluorescence image of the subject for tumor margin assessment, are desired.
Owner:LI COR
Tumor margin imaging agents
InactiveUS20100143258A1Ultrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsTumor marginFluorescence
In one aspect, the present invention provides a functionalized monodisperse polylysine comprising a linear monodisperse polylysine chain comprising constituent lysine monomer residues containing appended C4-C24 polyethylene glycol groups and at least one appended fluorescent dye moiety is provided.
Owner:GENERAL ELECTRIC CO
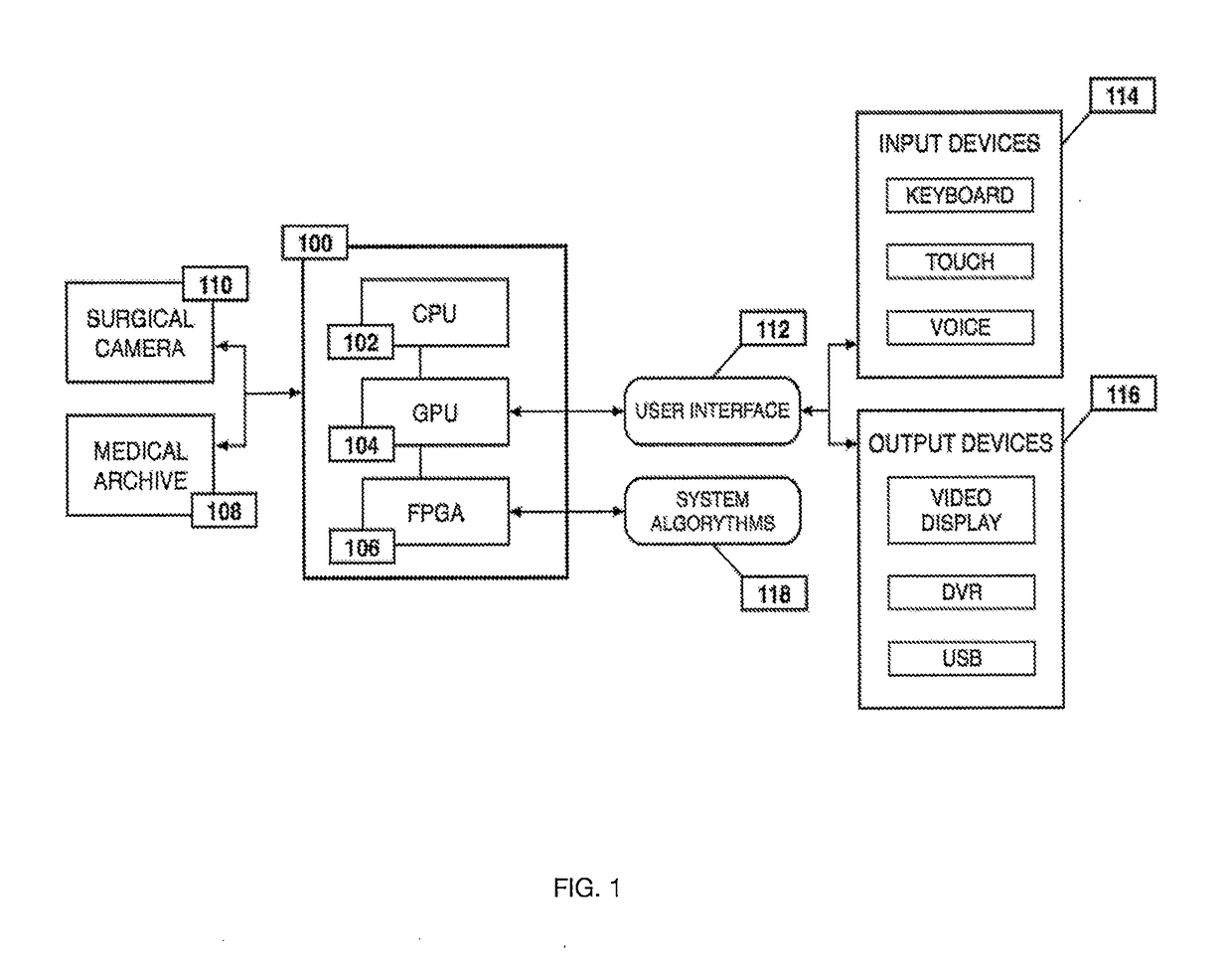
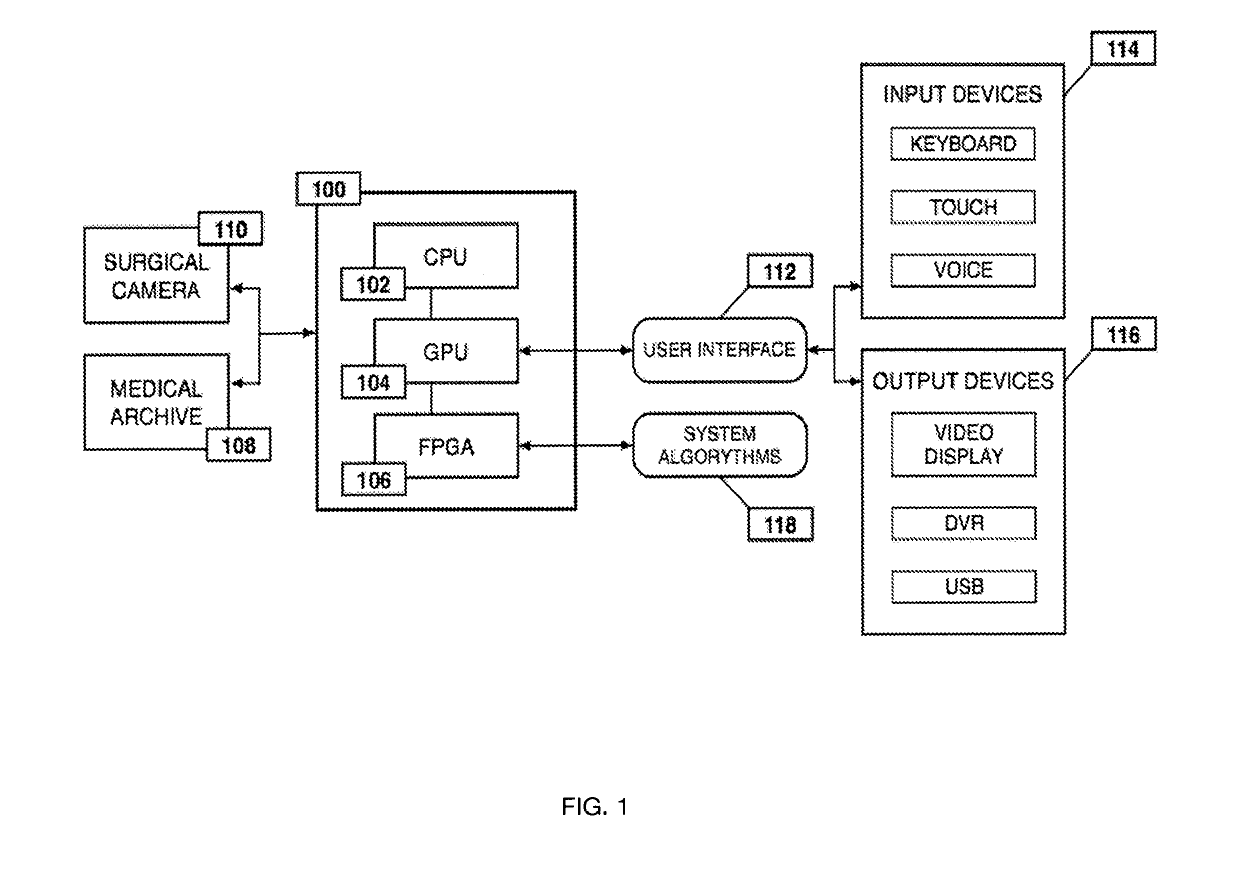
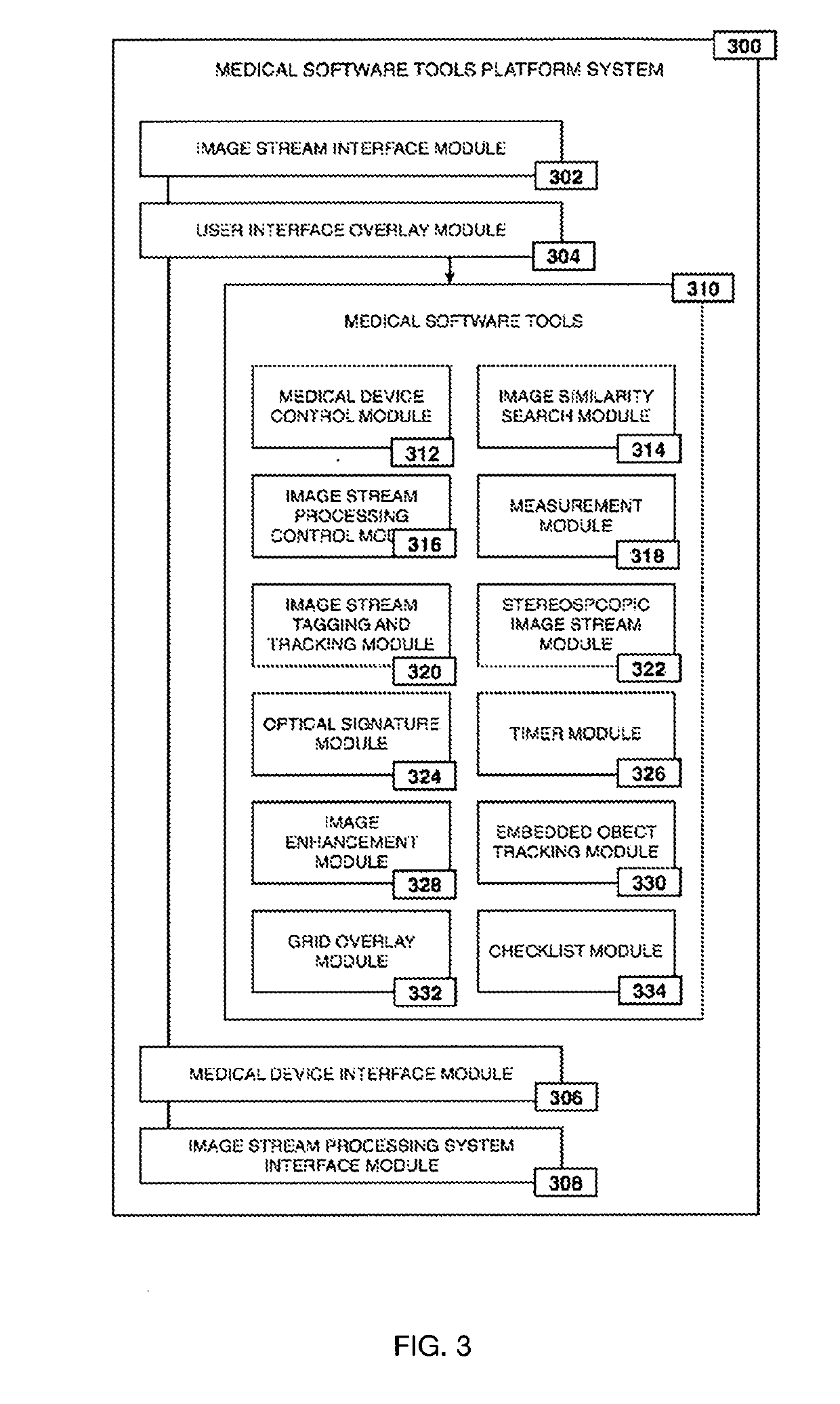
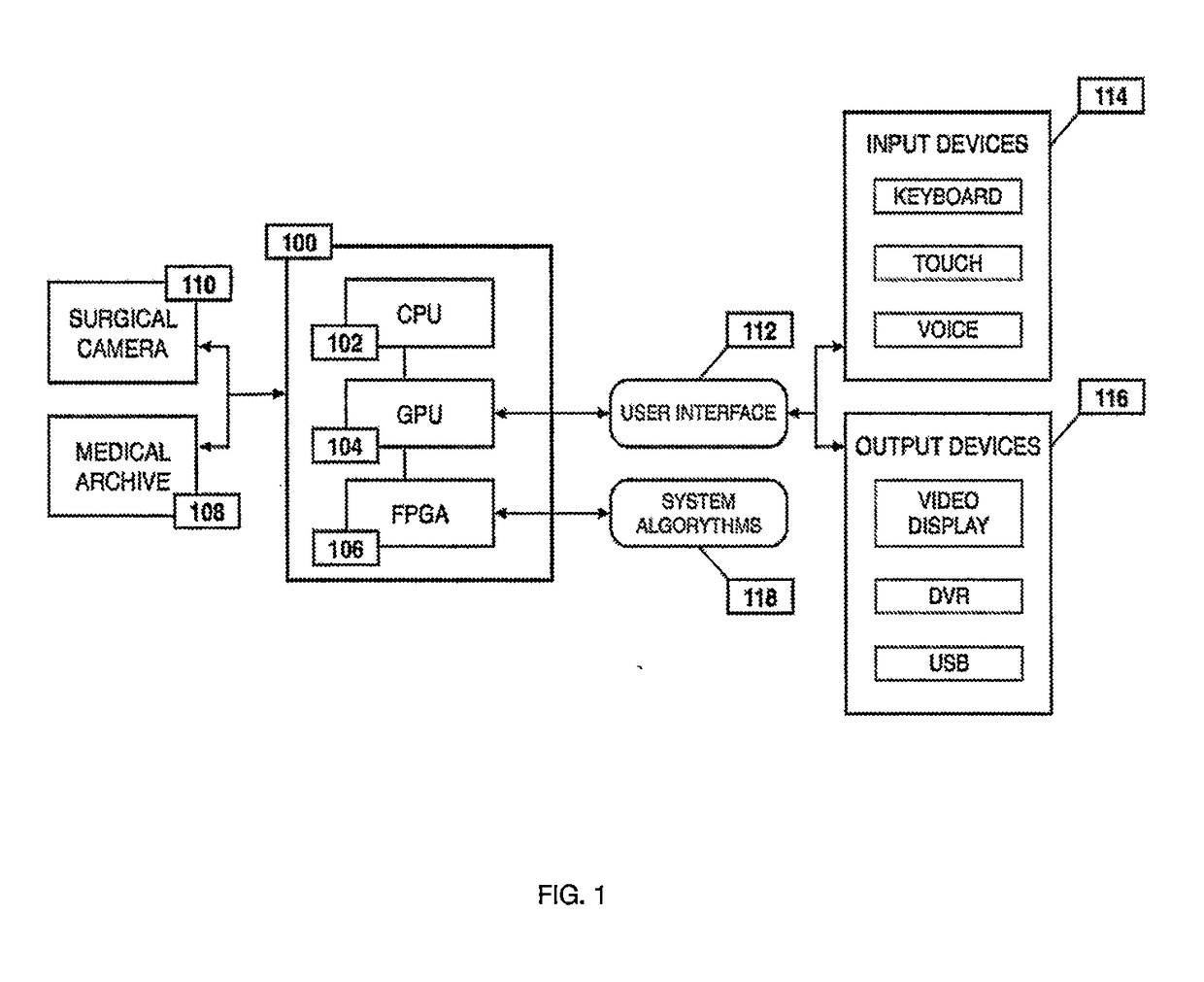
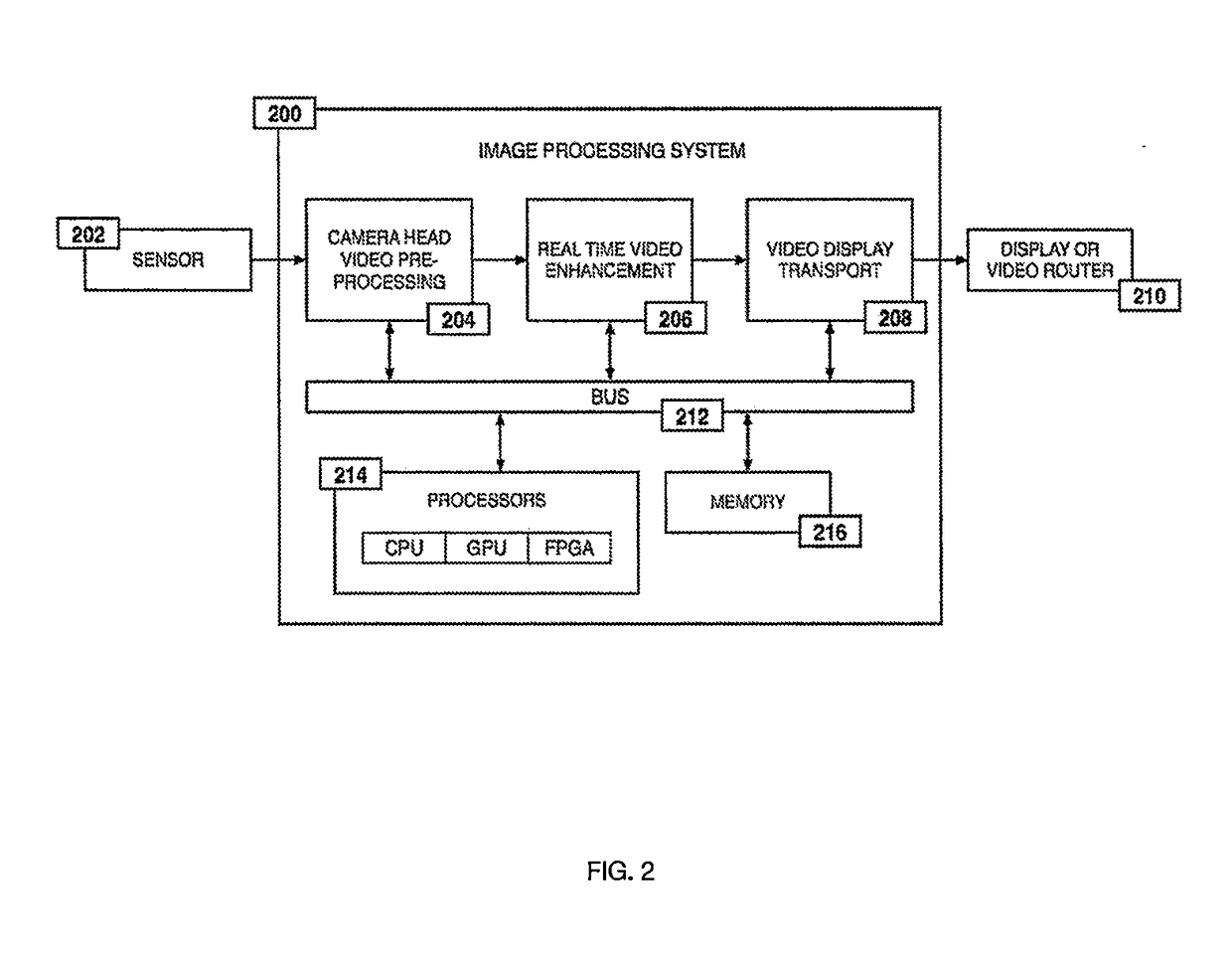
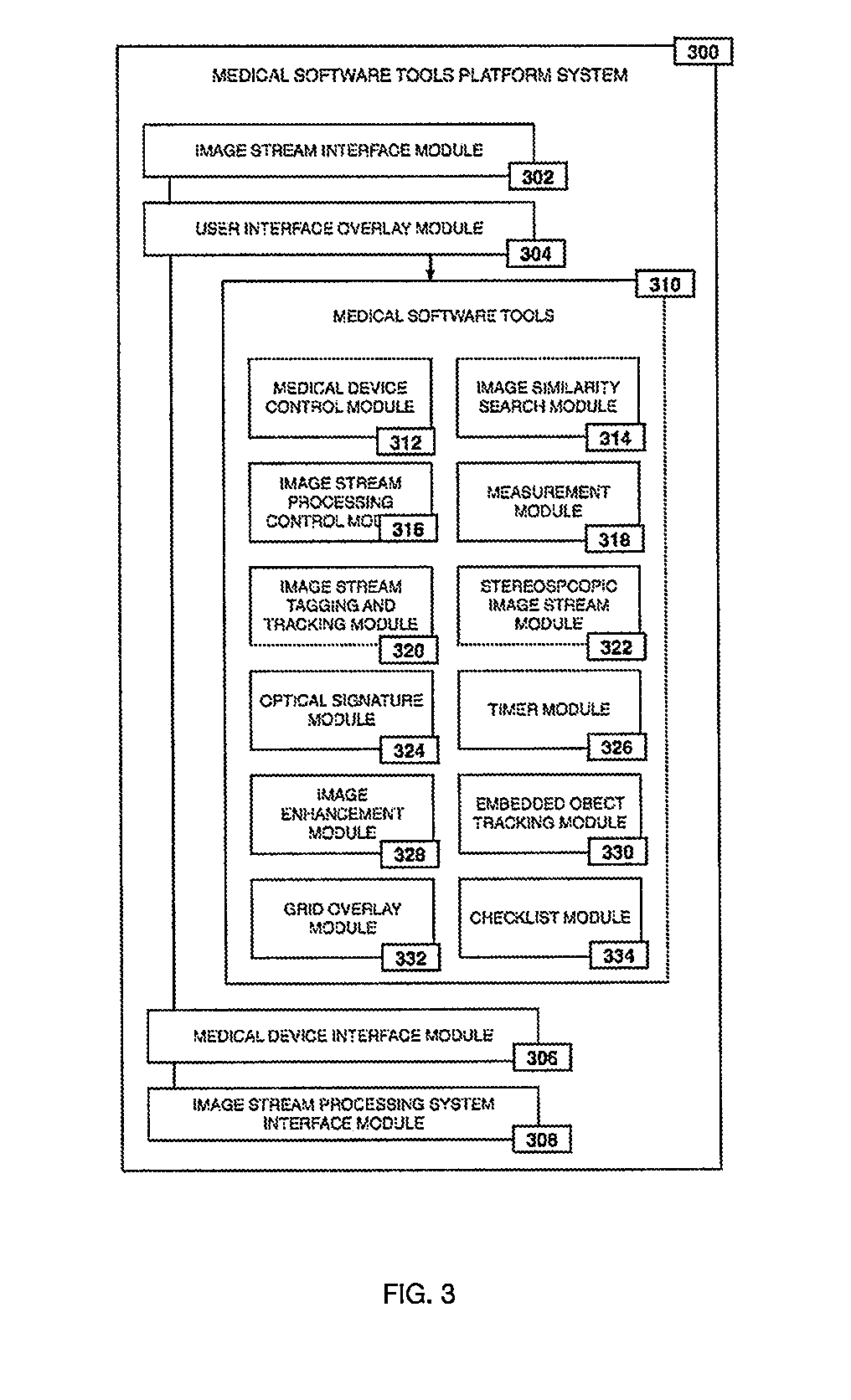
Method for enhanced data analysis with specialized video enabled software tools for medical environments
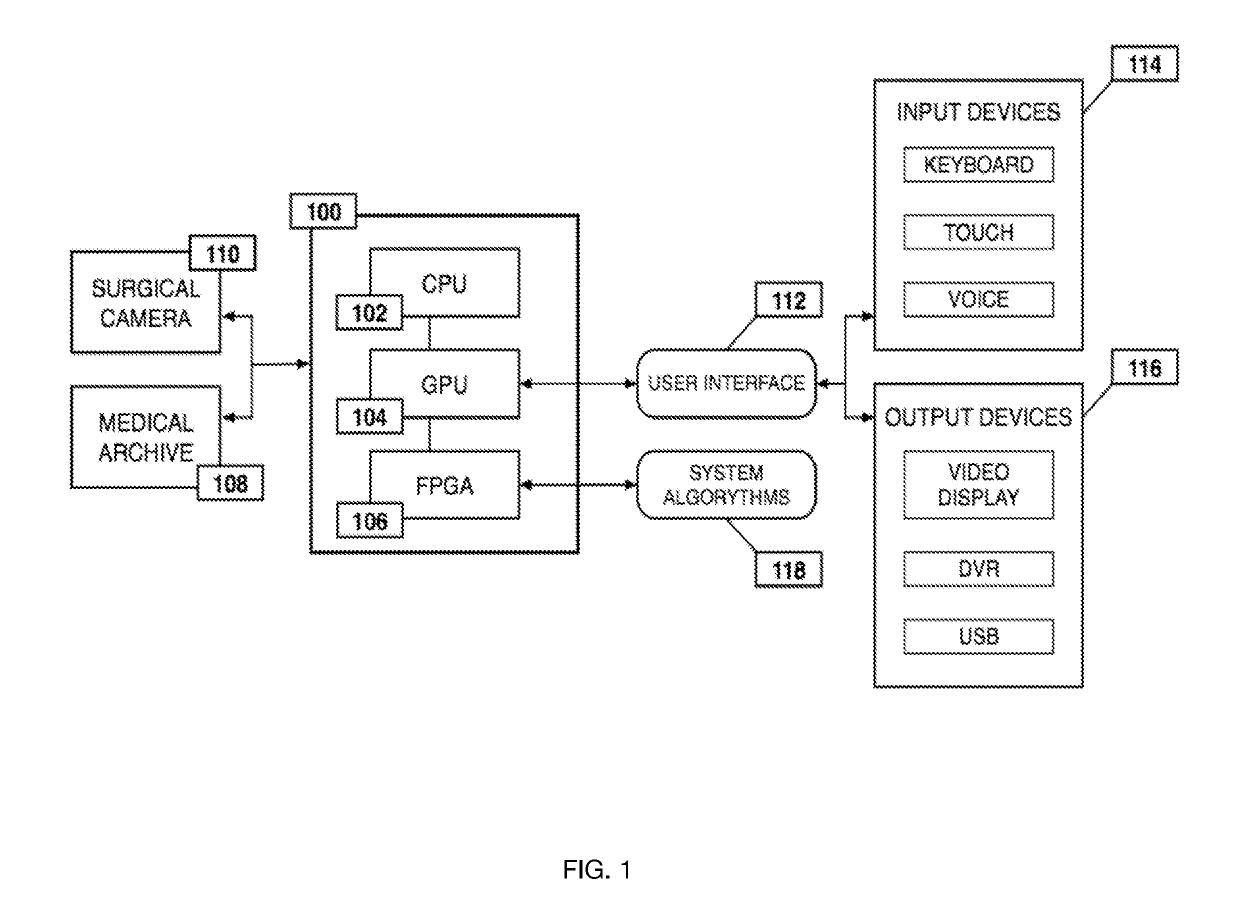
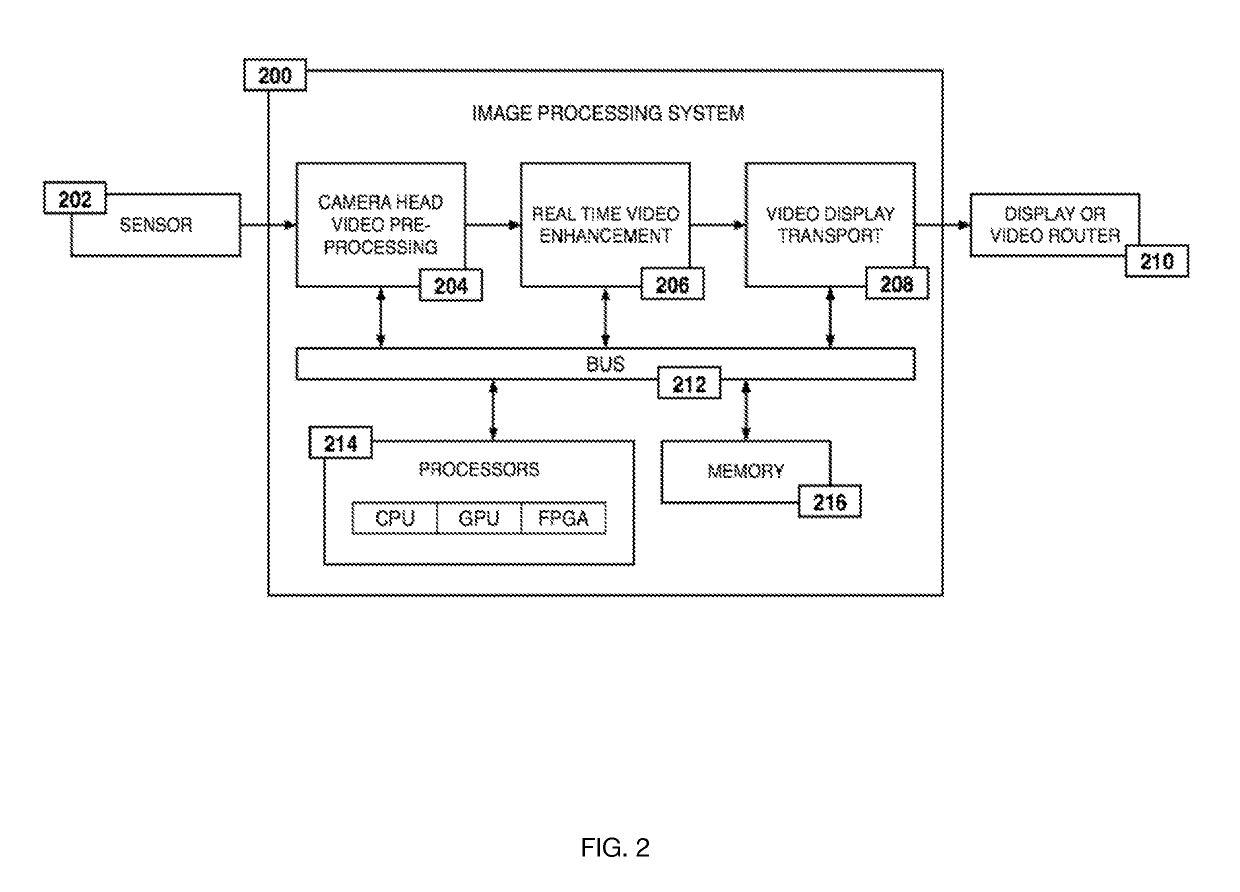
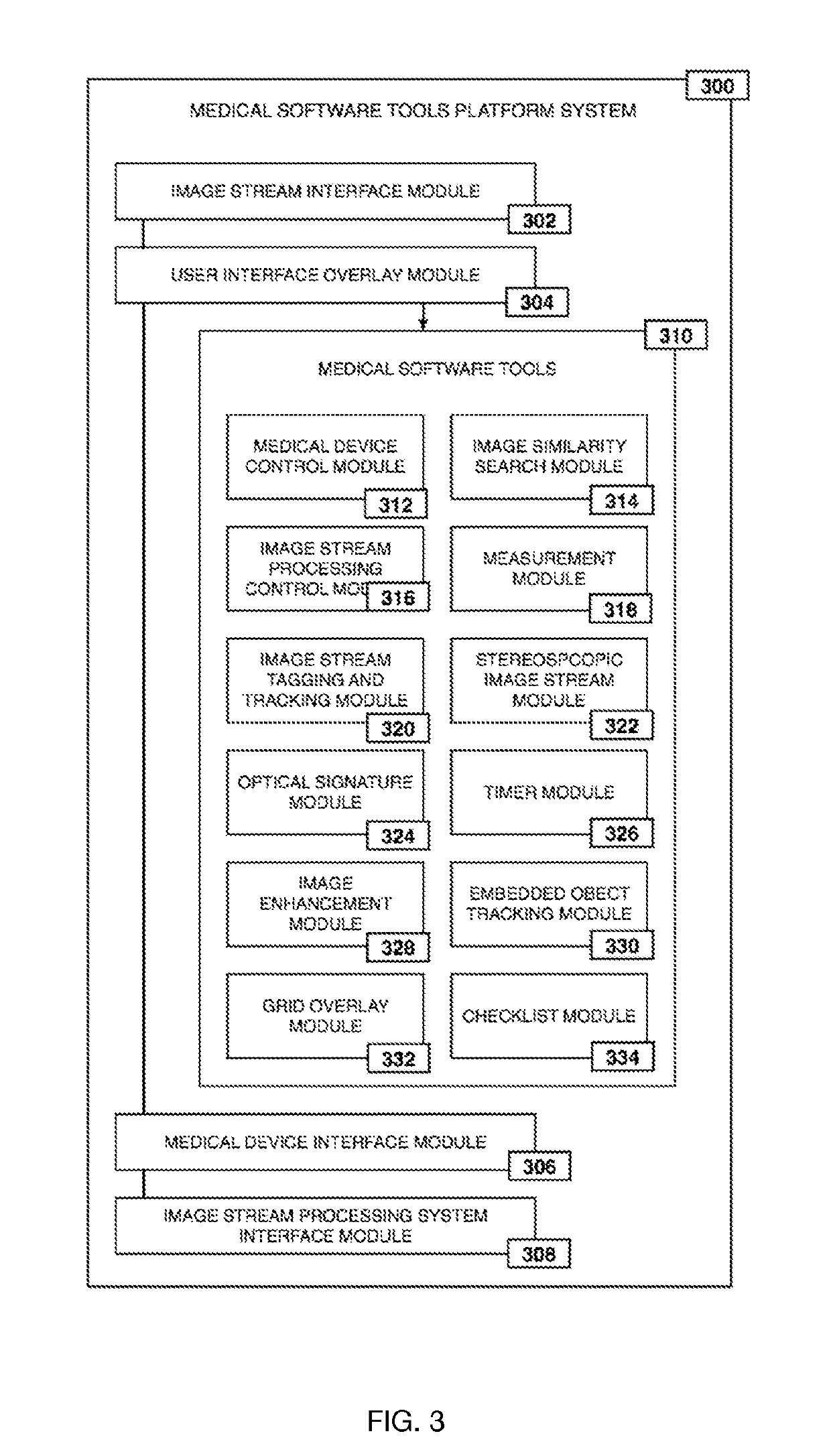
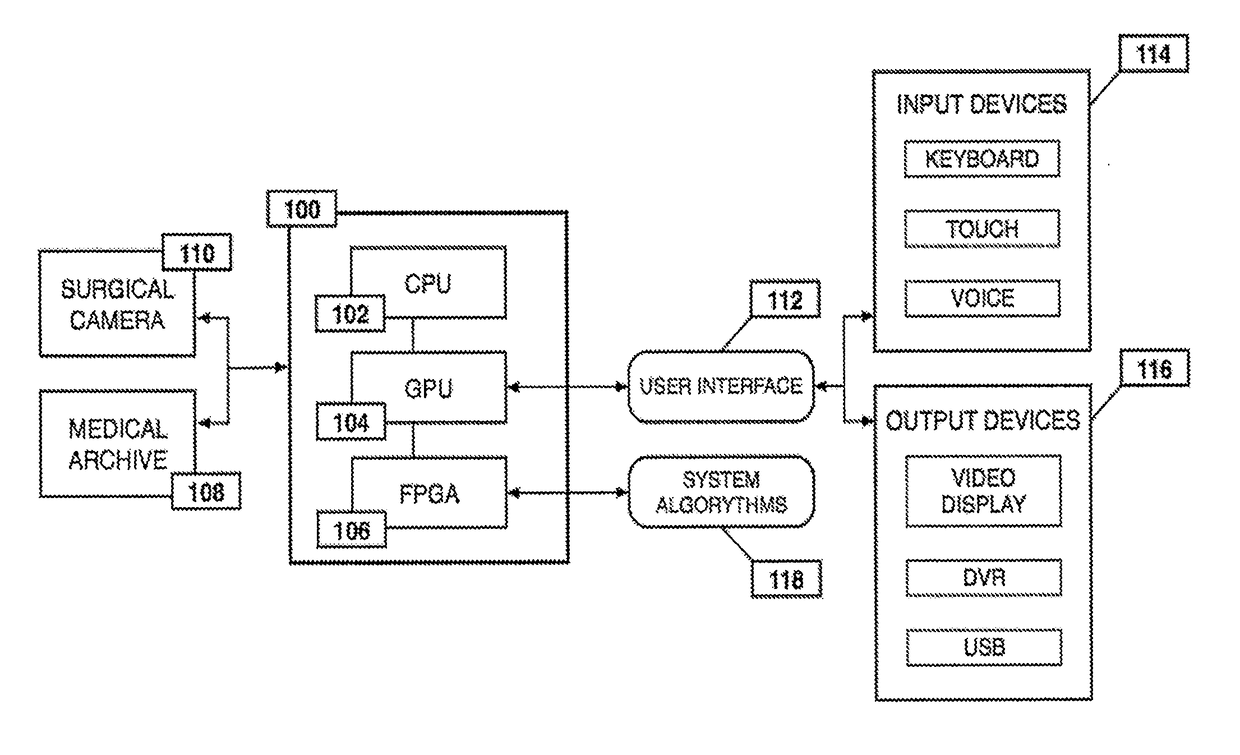
ActiveUS20190133711A1Easy to integrateConvenient computer operationImage enhancementImage analysisDiseaseAbnormal tissue growth
Owner:WADE JACK
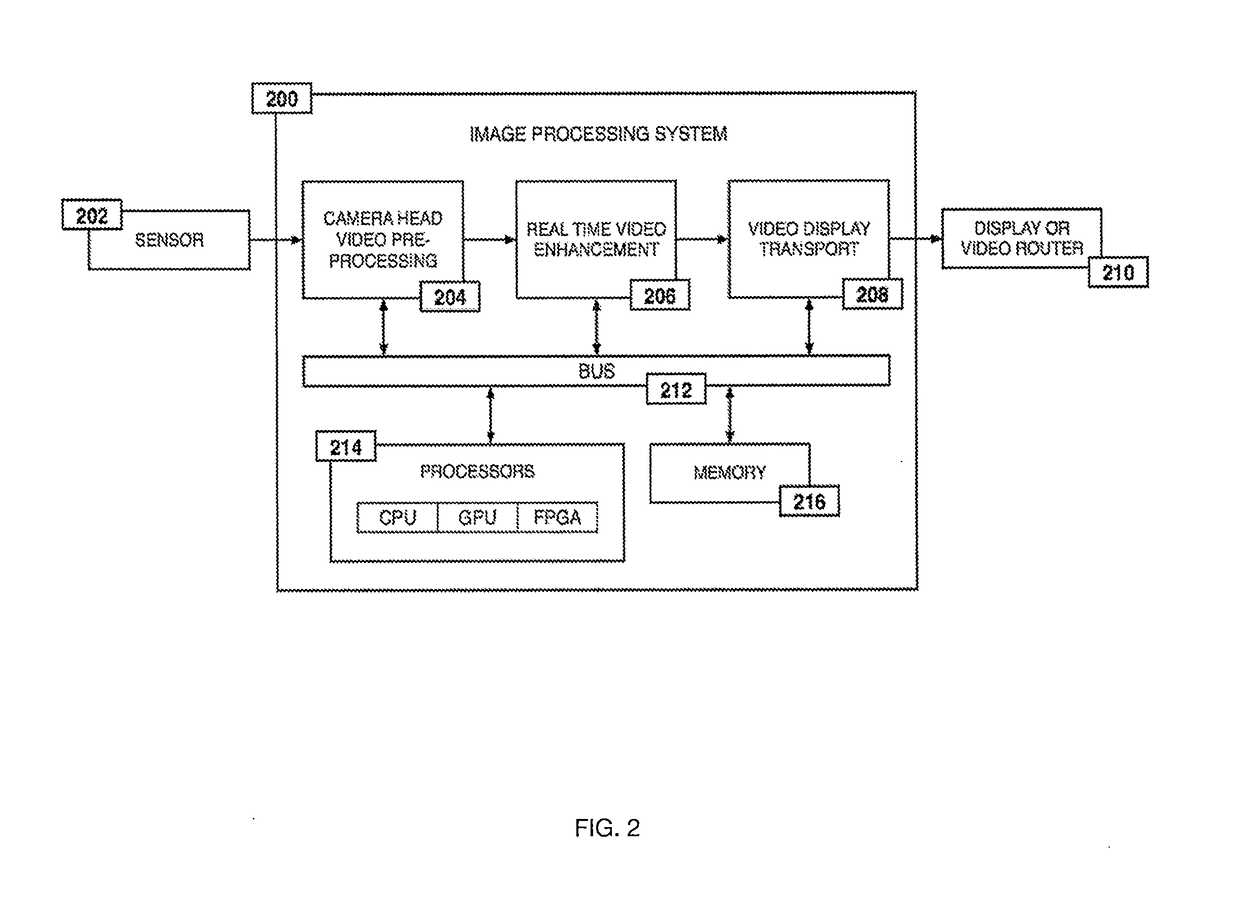
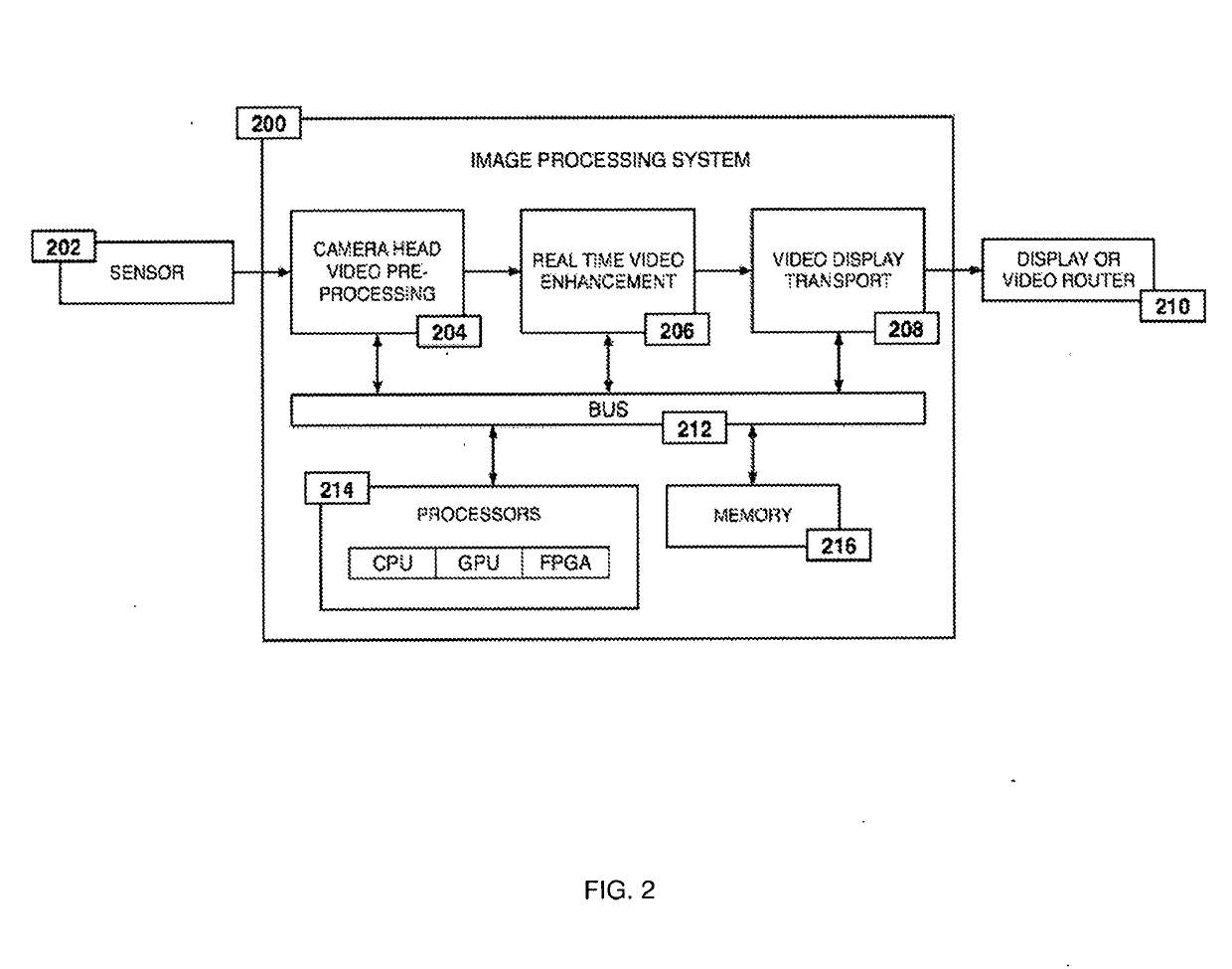
System and method for enhanced data analysis with specialized video enabled software tools for medical environments
InactiveUS20180289443A1Easy to integrateIncrease awarenessImage enhancementImage analysisDisplay deviceEndoscopic camera
Medical software tools platforms utilize a surgical display to provide access to specific medical software tools, such as medically-oriented applications or widgets, that can assist surgeons or surgical team in performing various procedures. In particular, an endoscopic camera may register the momentary rise in the optical signature reflected from a tissue surface and in turn transmit it to a medical image processing system which can also receive patient heart rate data and display relevant anomalies. Changes in various spectral components and the speed at which they change in relation to a source of stimulus (heartbeat, breathing, light source modulation, etc.) may indicate the arrival of blood, contrast agents or oxygen absorption. Combinations of these may indicate various states of differing disease or margins of tumors, and so forth. Also, changes in temperatures, physical dimensions, pressures, photoacoustic pressures and the rate of change may indicate tissue anomalies in comparison to historic values.
Owner:WADE JACK
System and method for enhanced data analysis with specialized video enabled software tools for medical environments
ActiveUS20190090976A1Easy to integrateIncrease awarenessImage enhancementImage analysisDiseaseDisplay device
Medical software tools platforms utilize a surgical display to provide access to specific medical software tools, such as medically-oriented applications or widgets, that can assist surgeons or surgical team in performing various procedures. In particular, an endoscopic camera may register the momentary rise in the optical signature reflected from a tissue surface and in turn transmit it to a medical image processing system which can also receive patient heart rate data and display relevant anomalies. Changes in various spectral components and the speed at which they change in relation to a source of stimulus (heartbeat, breathing, light source modulation, etc.) may indicate the arrival of blood, contrast agents or oxygen absorption. Combinations of these may indicate various states of differing disease or margins of tumors, and so forth. Also, changes in temperatures, physical dimensions, pressures, photoacoustic pressures and the rate of change may indicate tissue anomalies in comparison to historic values.
Owner:WADE JACK
Brain tumor surgery incision locating and approach planning method
ActiveCN105147362AShort access pathRequirements for achieving precise positioning of incisionsDiagnosticsExcision instrumentsSurgical approachShortest distance
The invention belongs to the field of computer-assisted surgery, and in particular relates to a brain tumor surgery incision locating and approach planning method which is used for incision localization and surgical approach planning before a brain tumor resection surgery on the basis of a medical image. The method comprises the following steps: acquiring a tumor central point; locating a point, which keeps the shortest distance from the tumor central point, on the scalp; locating a point, which keeps the shortest distance from the tumor central point, within a region; acquiring tumor marginal points; determining a surgical incision location; and designing a surgical approach. Compared with other methods of locating brain tumor on scalp, the method disclosed by the invention, under the circumstance of effectively avoiding intracranial important tissues, guarantees the shortest route of the designed surgical approach and meets the requirement of precise localization of incision with the minimum additional wound.
Owner:HARBIN ENG UNIV
System for enhanced data analysis with specialized video enabled software tools for medical environments
ActiveUS20180344424A1Easy to integrateConvenient computer operationImage enhancementMechanical/radiation/invasive therapiesDiseaseDisplay device
Medical software tools platforms utilize a surgical display to provide access to specific medical software tools, such as medically-oriented applications or widgets, that can assist surgeons or surgical team in performing various procedures. In particular, an endoscopic camera may register the momentary rise in the optical signature reflected from a tissue surface and in turn transmit it to a medical image processing system which can also receive patient heart rate data and display relevant anomalies. Changes in various spectral components and the speed at which they change in relation to a source of stimulus (heartbeat, breathing, light source modulation, etc.) may indicate the arrival of blood, contrast agents or oxygen absorption. Combinations of these may indicate various states of differing disease or margins of tumors, and so forth. Also, changes in temperatures, physical dimensions, pressures, photoacoustic pressures and the rate of change may indicate tissue anomalies in comparison to historic values.
Owner:WADE JACK
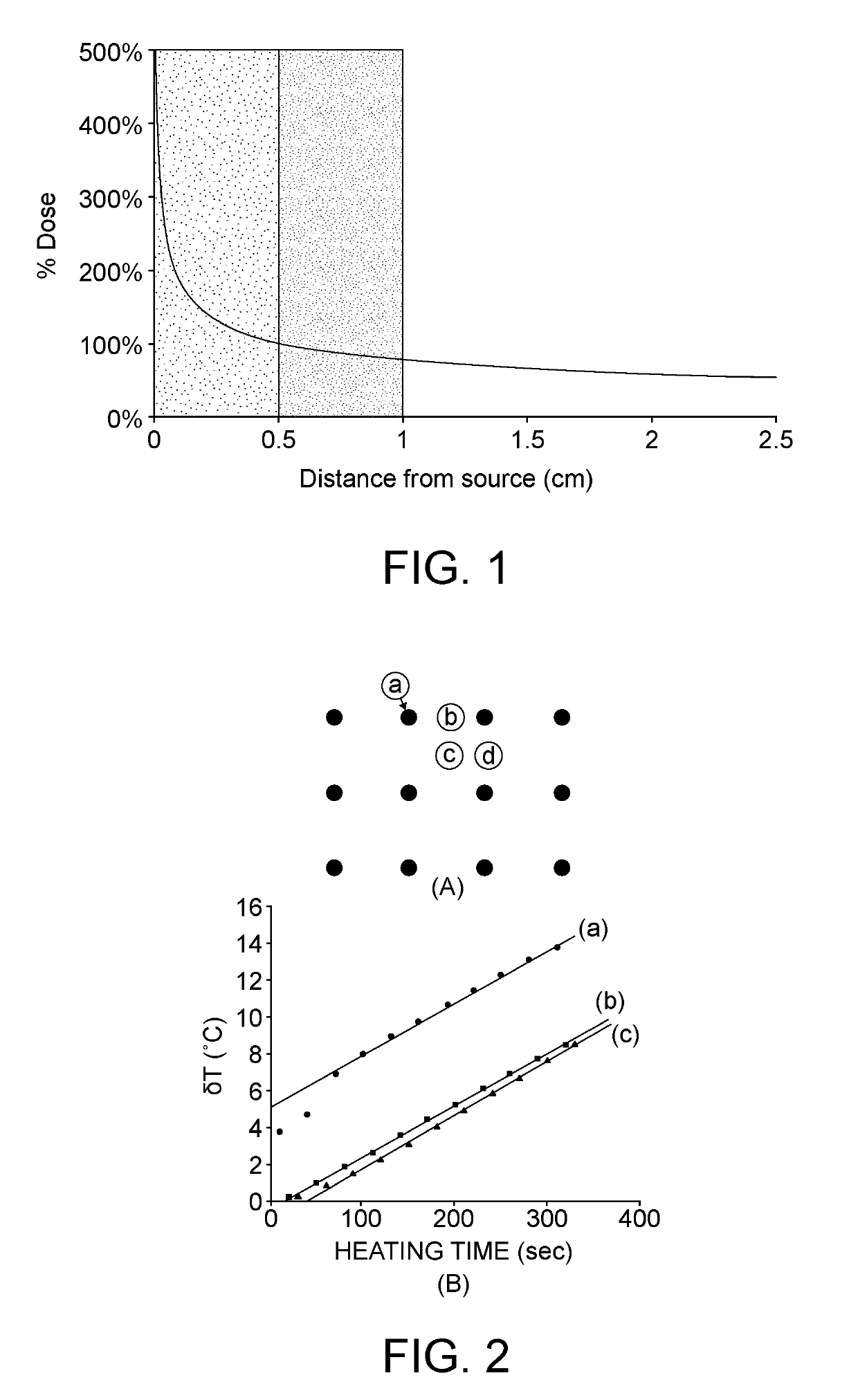
Reverse temperature control combined probe type tumor thermal-therapeutic apparatus
InactiveCN101803948AComplete heat therapyHeat therapy without omissionSurgical instruments for heatingProbe typeEngineering
The invention provides combined probe type tumor thermal-therapeutic apparatus capable of accurately measuring and controlling temperature. The combined probe type tumor thermal-therapeutic apparatus mainly adopts a reverse temperature control inactivation tumor therapeutic method which controls and determines the heating action in the tumor according to the induced temperature on the tumor margins. The apparatus has a function of performing selective thermal damage to biological tissues, so that by using the apparatus, any length of heating time for clinical treatment of tumors of any irregular shape can be obtained, and the probability that 100 percent of tumor tissues are completely inactivated for one time, but normal tissues around the tumors are not harmed, which is an important medical aim in clinical biomedicine, is realized.
Owner:韩俊峰
Human colon cancer nude mouse skin subcutaneous transplantation tumor model establishing method
InactiveCN103976803APersonalizeHigh tumor survival rateSurgical veterinaryNecrosis tumourColorectal cancer specimen
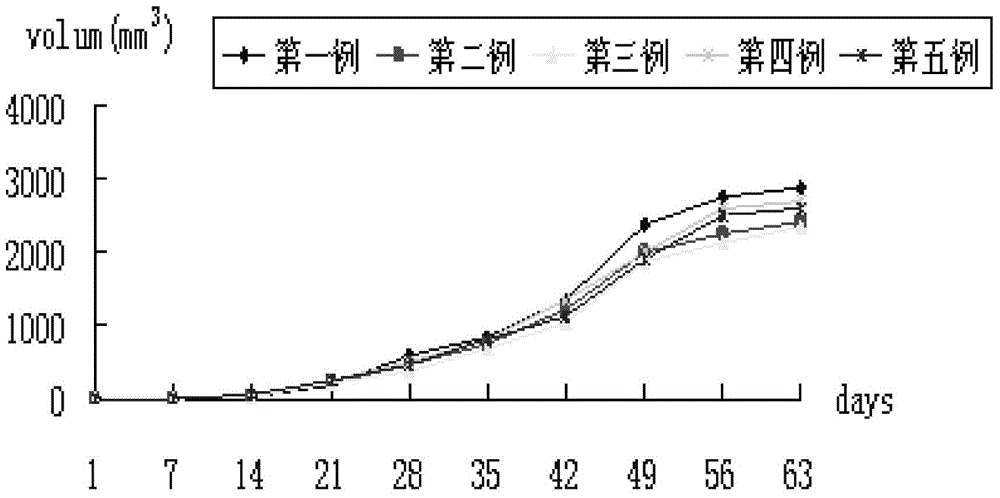
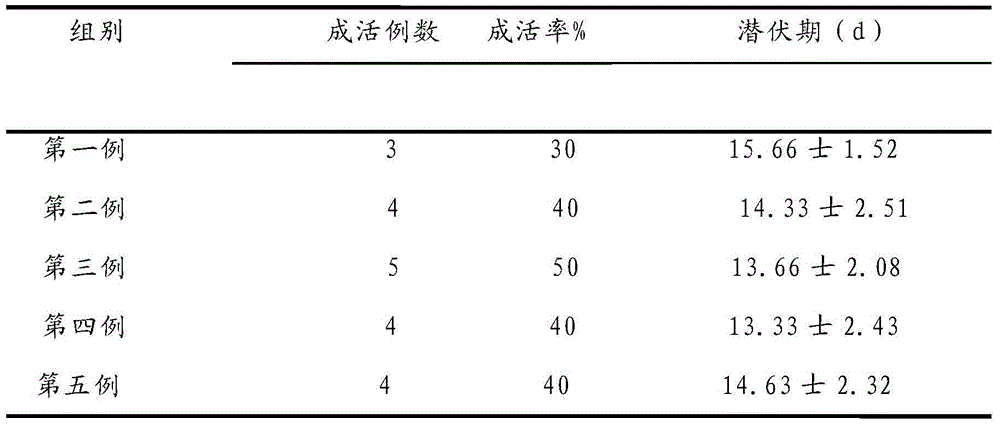
The invention provides a human colon cancer nude mouse skin subcutaneous transplantation tumor model establishing method. The method comprises the steps off cutting a tumor edge fish flesh shaped non-necrosis tumor tissue from a colon cancer specimen; shearing the tumor tissue into 0.8-1.3mm3 small blocks, performing primary transplantation and passage transplantation and finally studying individual specificity through a tumor model subjected to passage transplantation, such as the sensibility of a certain individual to chemotherapeutic drugs or radiotherapy.
Owner:贵阳医学院附属医院
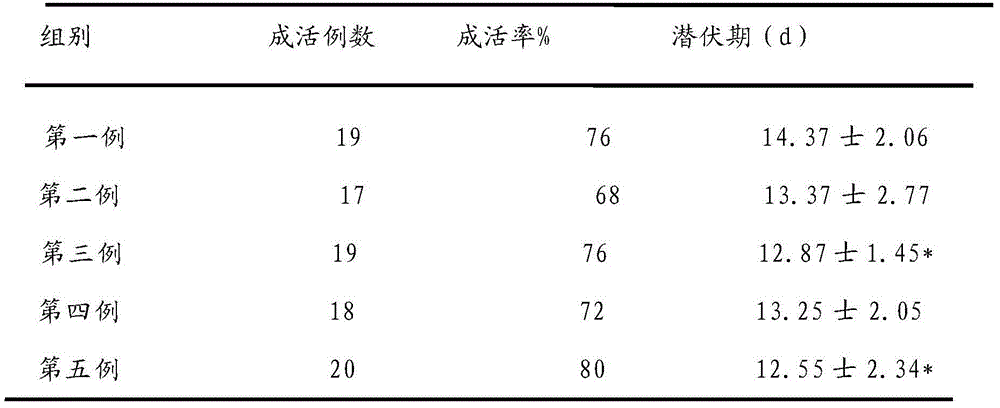
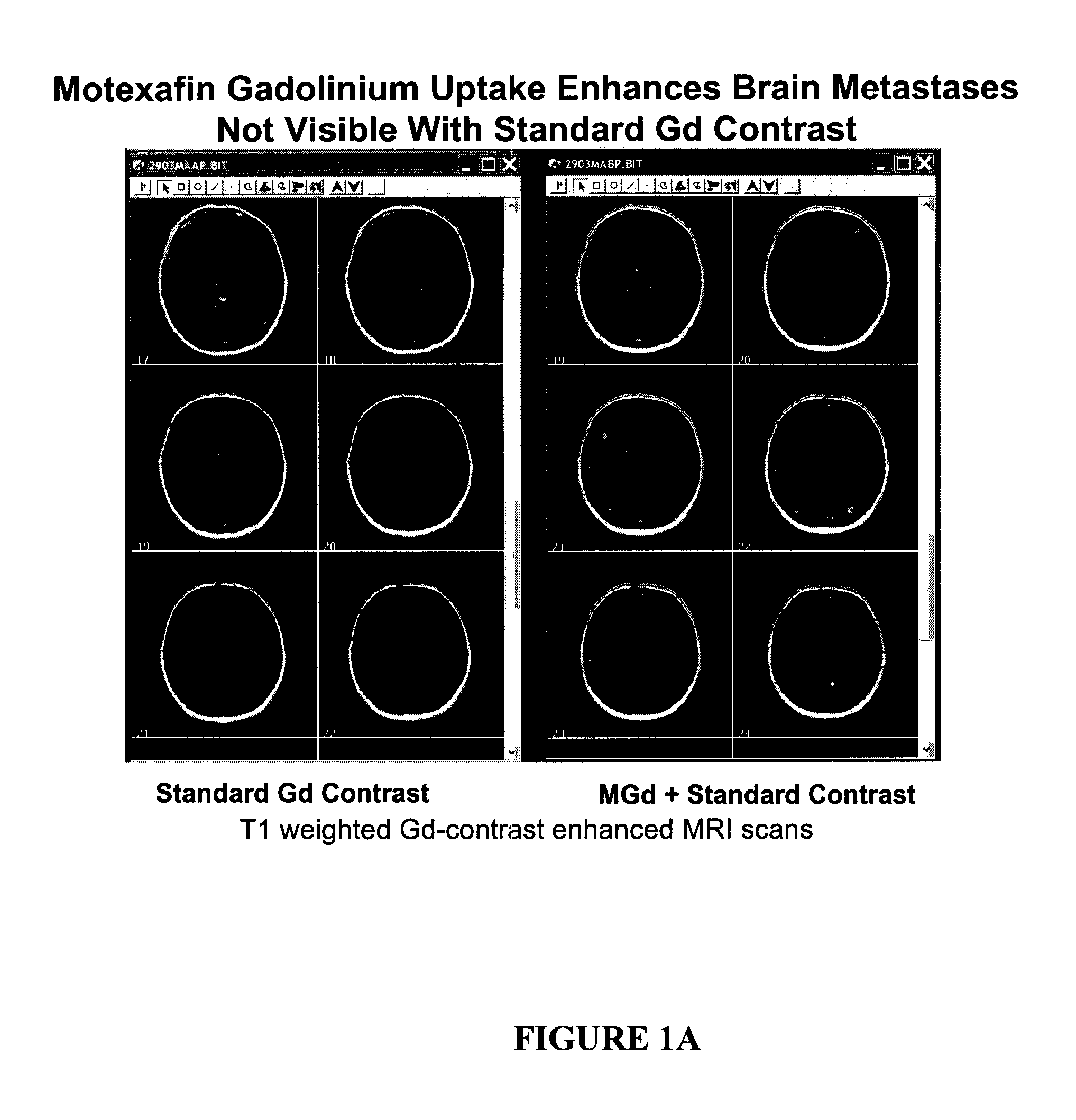
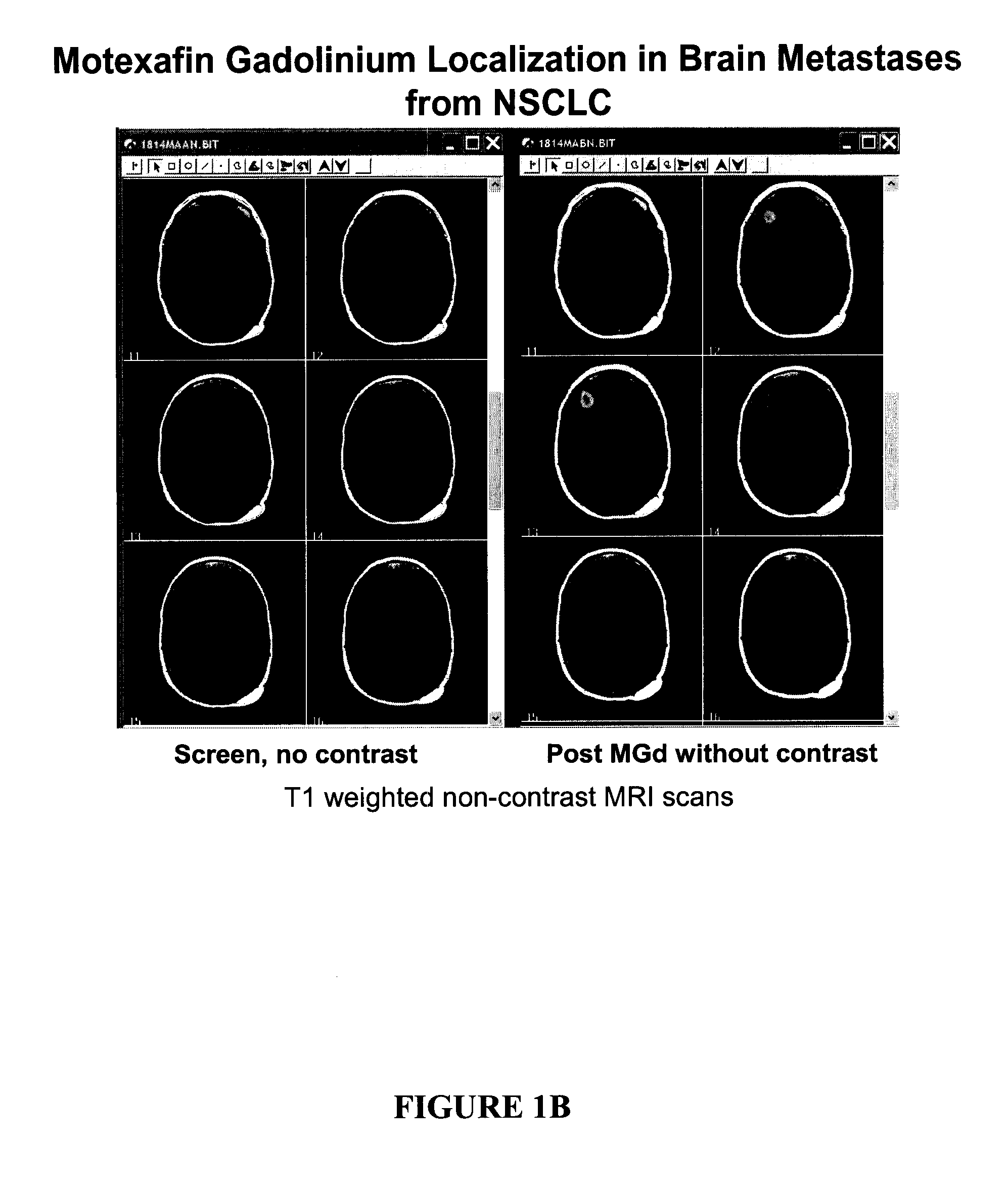
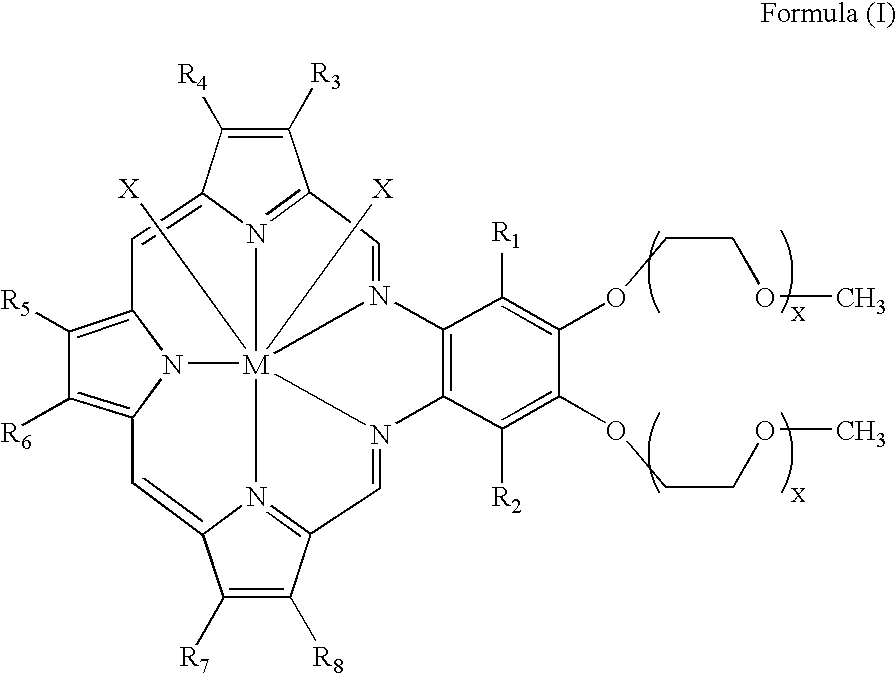
Methods of treating cancer using hypofractionated radiation and texaphyrins
InactiveUS20070219174A1Increase image brightnessHigh imaging sensitivityOrganic active ingredientsBiocideMedical physicsAbnormal tissue growth
Described herein are methods of treating cancer by administering a therapeutically effective amount of at least one texaphyrin metal complex or a pharmaceutically acceptable derivative, and performing stereotactic radiosurgery to the patient. In some embodiments, at least one texaphyrin metal complex or a pharmaceutically acceptable derivative is administered while the patient is undergoing a radiation therapy. Also described herein are methods for detecting various cancerous tumors, lesions, or metastases in a patient by administering an effective amount of at least one texaphyrin metal complex or a pharmaceutically acceptable derivative before a patient undergoes stereotactic radiosurgery. Also described herein are methods for visualizing tumors not otherwise visualized; methods for detecting the margins of a tumor; and methods for improving the targeting of the SRS radiation.
Owner:PHARMACYCLICS
Tumor bed implant for multimodality treatment of at risk tissue surrounding a resection cavity
PendingUS20190099618A1Low toxicityIncrease doseElectrotherapyBalloon catheterLocal HyperthermiaThick wall
A device having a biocompatible polymer as a body and regularly spaced radiation seeds and regularly spaced magnetic materials disposed of within the body of the biocompatible polymer, or uniformly distributed liquid radiation and magnetic fluid materials within a polymer slab, hollow thick wall polymer shell, or thin wall polymer balloon, as suitable for surgical placement within a resection cavity for treatment of at-risk tissue in the tumor margin with local hyperthermia in combination with radiation and potentially also with chemotherapy and / or immunotherapy that is slowly released from the biocompatible polymer.
Owner:THOMAS JEFFERSON UNIV +1
Compositions And Methods For Treatment Of Cancer
InactiveUS20070142287A1Inhibit proliferation and metastasisPeptide/protein ingredientsDepsipeptidesLymphatic SpreadCancer cell
The present invention provides a pharmaceutical composition that includes a) one or both of i) a peptide ligand that binds a zeta receptor and ii) at least one cancer chemotherapeutic agent; and b) a sustained release formulation. The invention also provides a combination including at least a portion of a tissue comprising one or more cells harboring a zeta receptor, such as a cancer cell or a precancerous cell, in contact with a pharmaceutical composition of the invention. Additionally the invention provides a method of treating a pathology that responds to a peptide ligand that binds a zeta receptor that includes a step of contacting a tissue characteristic of the pathology and containing cells harboring a zeta receptor, with a pharmaceutical composition of the invention. The invention further provides methods of inhibiting proliferation or metastasis of a cancer in a subject, including a) surgically excising a tumor characterizing the cancer from the subject; b) ensuring maximal removal of a tumor margin from the tumor site; and c) disposing a pharmaceutical composition of the invention upon a tissue remaining at a tumor margin wherein the tissue contains one or more tumor cells (such as a cancer cell or a precancerous cell) harboring a zeta receptor. The peptide ligand of a zeta receptor may be an enkephalin, an endorphin, a dynorphin, or a derivative thereof. Additionally the pharmaceutical composition may include one or more cancer chemotherapeutic agents.
Owner:BIOMED SOLUTIONS
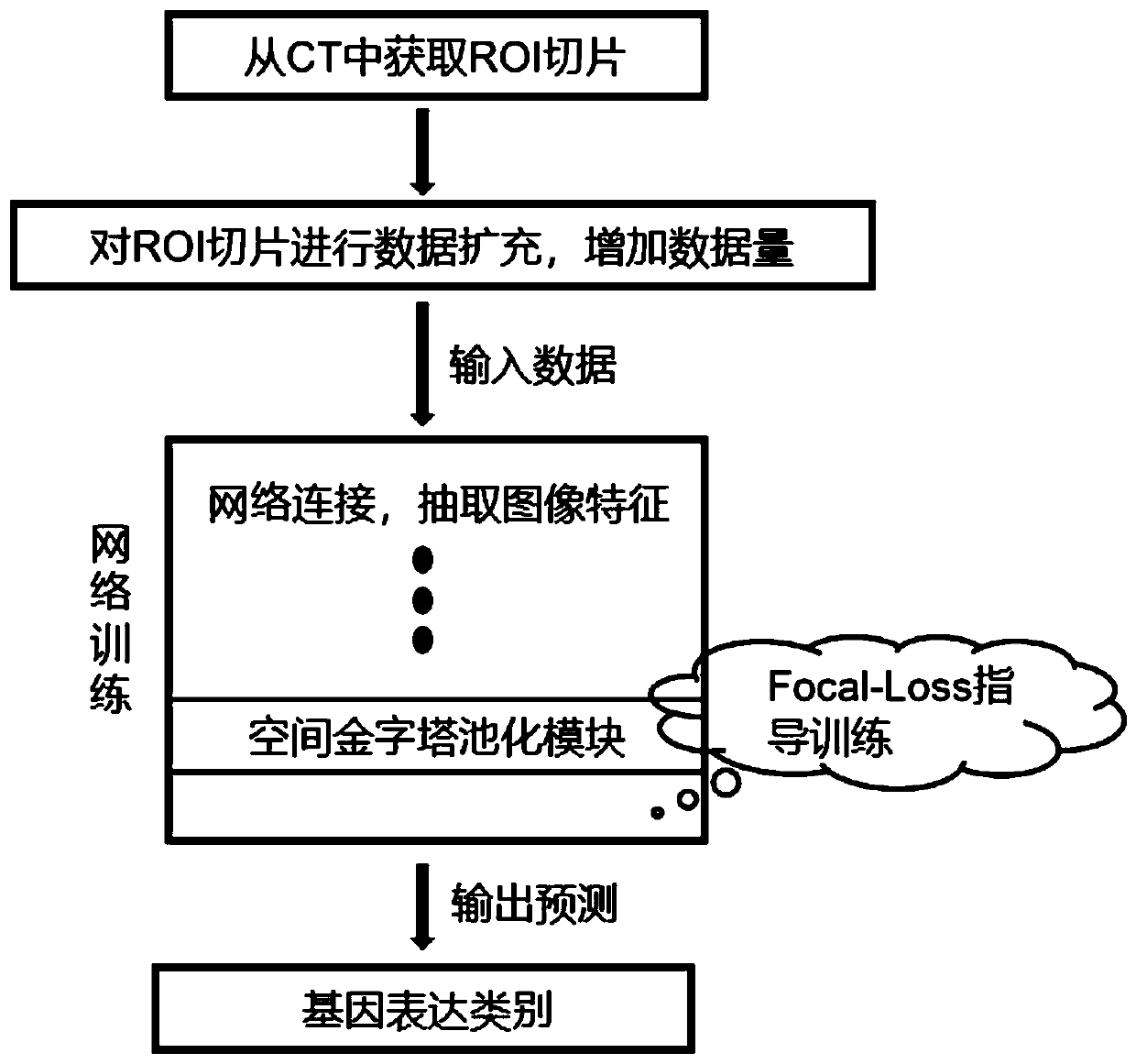
Method for automatically predicting gene expression categories based on cancer CT images
InactiveCN111583271AEase of useNon-invasiveImage enhancementImage analysisGenes mutationData expansion
The invention discloses a method for automatically predicting a gene expression category based on a cancer CT image. The method comprises the following steps: a) acquiring ROI slices and expanding thenumber by 48 times; b) constructing a neural network based on DenseNet-12 and a spatial pyramid module; c) performing training by using a focusing loss function; and d) comprehensively judging the model prediction to obtain a final prediction result. The data expansion technology adopted by the invention can greatly expand the data volume without changing the property of the CT image. A spatial pyramid pooling module with four dimensions extracts multi-level image features, including global semantics and detail features. Focal-Loss is used for guiding a network to pay more attention to slicesof which effective features are difficult to mine at the tumor edge, namely the head end and the tail end, and a training strategy of which the precision is gradually improved is used, so that accurate and efficient CT image gene mutation prediction is finally realized.
Owner:EAST CHINA NORMAL UNIV
Multi-blade collimator of ray accelerator treatment head and tumor radiotherapy equipment
PendingCN110755762AReduce Radiation LeakageEffectively closeX-ray/gamma-ray/particle-irradiation therapyTumor marginRadiation leakage
The invention discloses a multi-blade collimator of a ray accelerator treatment head. The multi-blade collimator comprises a tank body which is arranged in a multi-layer manner, wherein openings whichpenetrate through the tank body are formed in the upper surface and the lower surface of each layer of the tank body; all the openings are coaxially formed and are used for enabling rays to penetrate; a plurality of vertical blades are arranged in each layer of the tank body; the blades are divided into two groups, and are respectively arranged on two ends of the tank body; the front ends of every two groups of the blades in the horizontal direction are opposite and are located at the corresponding opening; a driver is independently connected to the rear end in the horizontal direction of each blade; the blades in each group are sequentially arrayed, and the side surfaces of the blades are attached to each other; and a right angle beveling is arranged at the front end edge of the side surface of each blade. Through the adoption of the multi-blade collimator disclosed by the invention, multi-blade collimator can be more effectively attached to the edge of the tumor, so that better radiation field shape adapting effect is realized, radiation field shape adapting resolution is increased, ray radiation leakage is reduced, and radiation field transformation efficiency is improved. Theinvention also discloses tumor radiotherapy equipment comprising the multi-blade collimator of the ray accelerator treatment head.
Owner:SHINVA MEDICAL INSTR CO LTD
A method for adaptively extracting an MR image of a cervical tumor
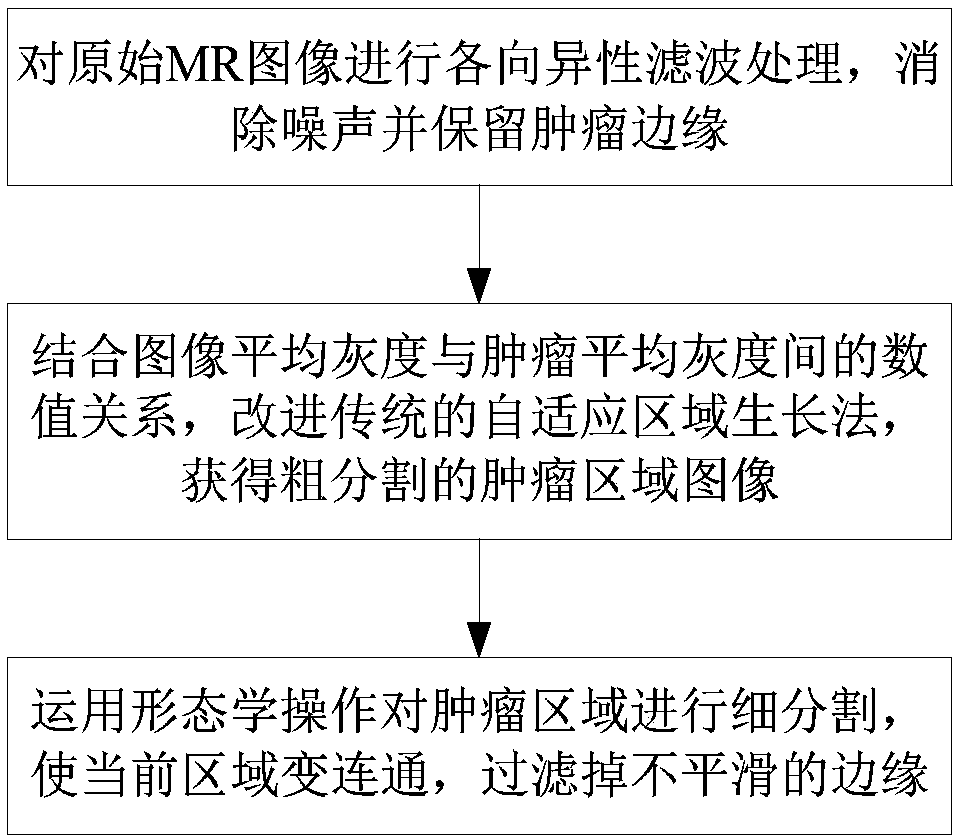
InactiveCN109636827AReduce workloadDecrease productivityImage enhancementImage analysisTumor marginImaging processing
The invention relates to the technical field of medical image processing, and provides a method for adaptively extracting an MR image of a cervical tumor, and the method comprises the steps: 1, carrying out the anisotropic filtering of an original MR image, eliminating noise, and keeping the edge of a tumor; Step 2, for the filtered MR image, setting a proportionality coefficient in combination with the numerical relationship between the average gray scale of each image and the average gray scale of the tumor, and improving a traditional adaptive region growth method to obtain a roughly segmented tumor region image; And step 3, performing fine segmentation on the tumor region by using morphological operation to enable the current region to be communicated and smooth the edge. According tothe method, an improved adaptive region growth method is utilized, the coincidence rate of an obtained segmentation result and a tumor region gold standard manually drawn by a doctor is high, the complexity of manually setting a threshold value is avoided, the artificial participation degree is reduced, artificial subjective deviation is reduced, universality is achieved, and a reasonable reference is provided for clinical diagnosis.
Owner:NORTHEASTERN UNIV
Gemcitabine-containing anti-cancer medicine sustained-release injection
The invention relates to cancer therapy drug sustained-release injection containing gemcitabine. The injection comprises sustained-release microspheres and dissolvent, wherein, the sustained-release microspheres comprise active ingredients for cancer therapy and sustained-release auxiliary materials, and the dissolvent is special dissolvent containing suspending agent. The active ingredients for cancer therapy comprise antimetabolite of gemcitabine or antimetabolite selected from zalcitabine, emtritabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite synergist selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogs and / or DNA repair enzyme inhibitor; the sustained-release auxiliary materials comprise polifeprosan, bi-fatty acid, decanedioic copolymer, polylactic copolymer and EVAc; the viscocity of the suspending agent is 100cp to 3000cp (at 20 to 30 DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The sustained-release microspheres can also be produced into sustained-release implant, and by injecting or positioning the sustained release agent in tumor or tumor margin, the efficacy of non-operative treatment such as radiation treatment and chemical treatment can be enhanced.
Owner:SHANDONG LANJIN PHARMA +1
Prior boundary-fused brain tumor segmentation method
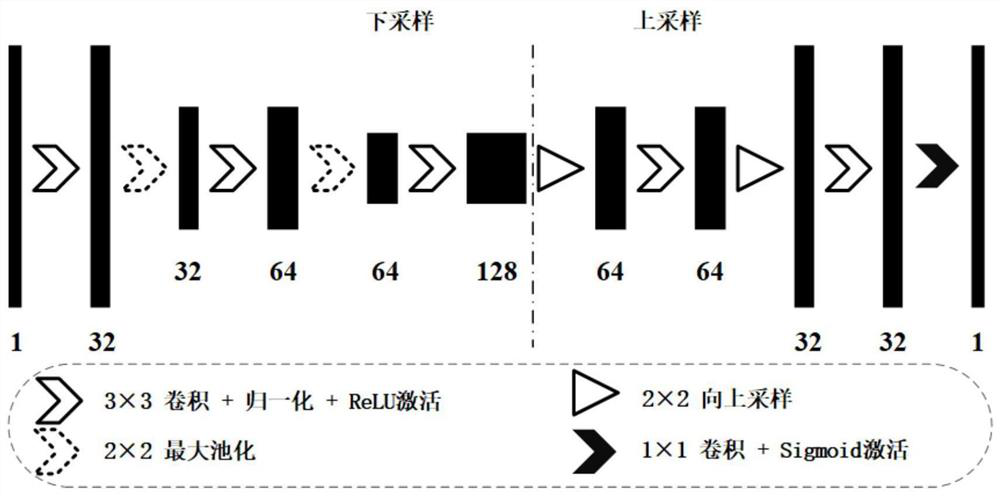
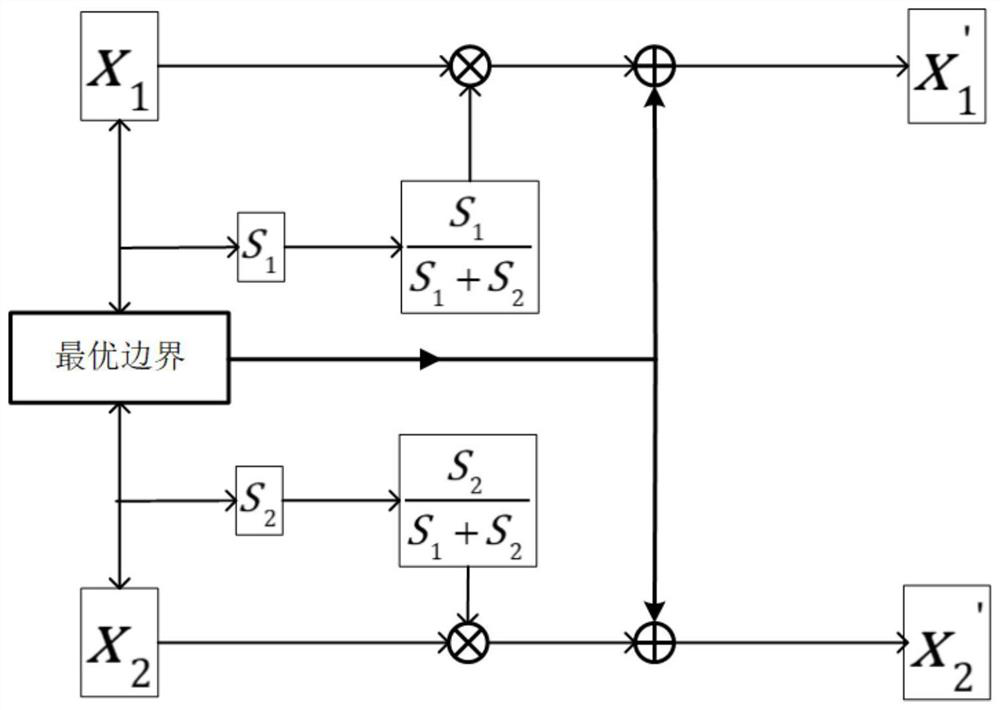
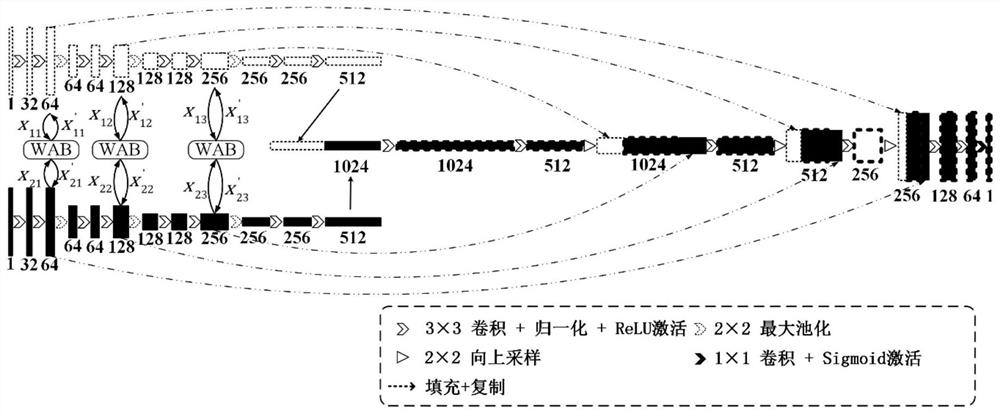
PendingCN113160142AImprove comprehensivenessImprove accuracyImage enhancementImage analysisTumor marginBrain tumor
The invention discloses a prior boundary-fused brain tumor segmentation method, and aims to overcome the defects that the generated brain tumor segmentation boundary is rough and the tumor is easy to reconstruct due to the fact that an existing convolutional network cannot fully utilize global image information. An optimal boundary of a tumor truth value is obtained from tumor prior knowledge, an optimal boundary generation network is constructed, an optimal boundary is added to a 3D U-net network of multiple down-sampling channels to carry out weight distribution and boundary enhancement on each layer of the network, and the similarity between a generated tumor edge and a tumor truth value edge is used as a loss item to be added to an original loss function to improve the accuracy of edge segmentation. According to the method, the prior knowledge is fused into the network and the loss function by using the tumor information of different modal nuclear magnetic images through multiple down-sampling channels, so that the comprehensiveness of tumor information utilization and the accuracy of tumor edge segmentation are improved.
Owner:ZHEJIANG UNIV OF TECH
Engraftable Neural Progenitor and Stem Cells for Brain Tumor Therapy
One of the impediments to the treatment of some human brain tumors (e.g. gliomas) has been the degree to which they expand, migrate widely, and infiltrate normal tissue. We demonstrate that a clone of multipotent neural progenitor stem cells, when implanted into an experimental glioma, will migrate along with and distribute themselves throughout the tumor in juxtaposition to widely expanding and aggressively advancing tumor cells, while continuing to express a foreign reporter gene. Furthermore, drawn somewhat by the degenerative environment created just beyond the infiltrating tumor edge, the neural progenitor cells migrate slightly beyond and surround the invading tumor border. When implanted at a distant sight from the tumor bed (e.g., into normal tissue, into the contralateral hemisphere, into the lateral ventricles) the donor neural progenitor / stem cells will migrate through normal tissue and specifically target the tumor cells. These results suggest the adjunctive use of neural progenitor / stem cells as a novel, effective delivery vehicle for helping to target therapeutic genes and vectors to invasive brain tumors that have been refractory to treatment.
Owner:CHILDRENS MEDICAL CENT CORP
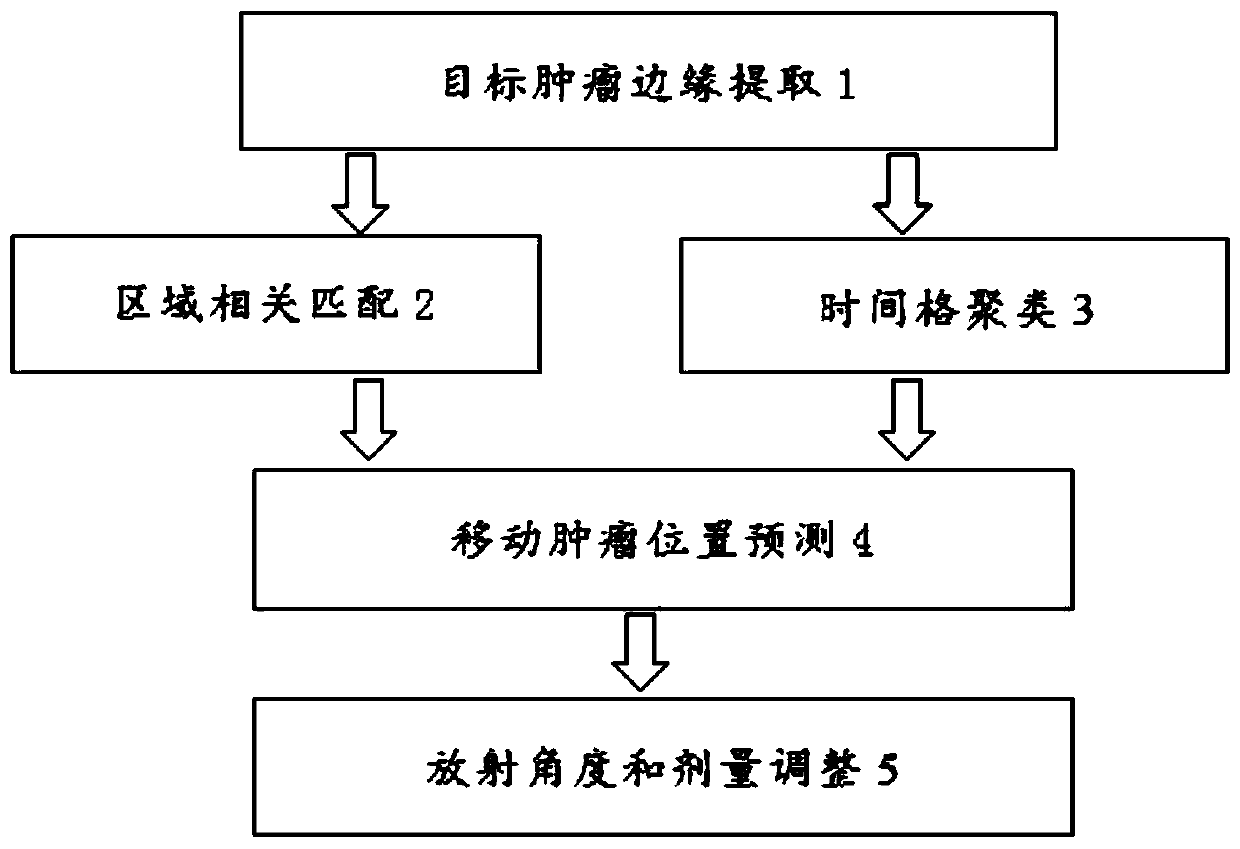
Method for dynamically adjusting radioactive angle and dosage according to movement of tumor
ActiveCN111388882AImprove accuracyHigh precisionX-ray/gamma-ray/particle-irradiation therapyTumor marginTreatment targets
The invention provides a method for dynamically adjusting the radioactive angle and dosage according to the movement of a tumor. According to the method, the moving position and spreading area of a dynamic tumor are precisely predicted and tracked, and the deflection of a radioactive angle is adjusted in time, and the radioactive dosage of a treatment target can be accurately controlled. The method comprises the following steps: (1) extracting the edge of a target tumor; (2) performing area related matching; (3) performing time grid clustering; (4) predicting the moving tumor position; and (5)adjusting the radioactive angle and dosage.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT +2
Motion-adaptive interactive imaging method
ActiveUS10993622B2Easy to detectImprove image qualityTelevision system detailsRadiation diagnostic clinical applicationsHigh frame rateTumor margin
Methods and systems are provided for the detecting and presenting of faint signals from a real-time interactive imager. A real-time view of a subject is controlled by a user by entering commands that move the subject or a camera used to record the real-time view. The rate of this movement is monitored, and when the rate descends below a preset value, a trigger is sent to the camera. In response to the trigger, the camera switches to a lower frame frequency for capturing the real-time view of the subject, thereby increasing the amount of light collected in each frame, and increasing the sensitivity of the camera to faint signals. The methods and systems are particularly relevant for surgical or biopsy imaging, in which both a higher frame rate preview image of a subject for 3D visualization, and a higher sensitivity fluorescence image of the subject for tumor margin assessment, are desired.
Owner:LI-COR BIOTECH LLC
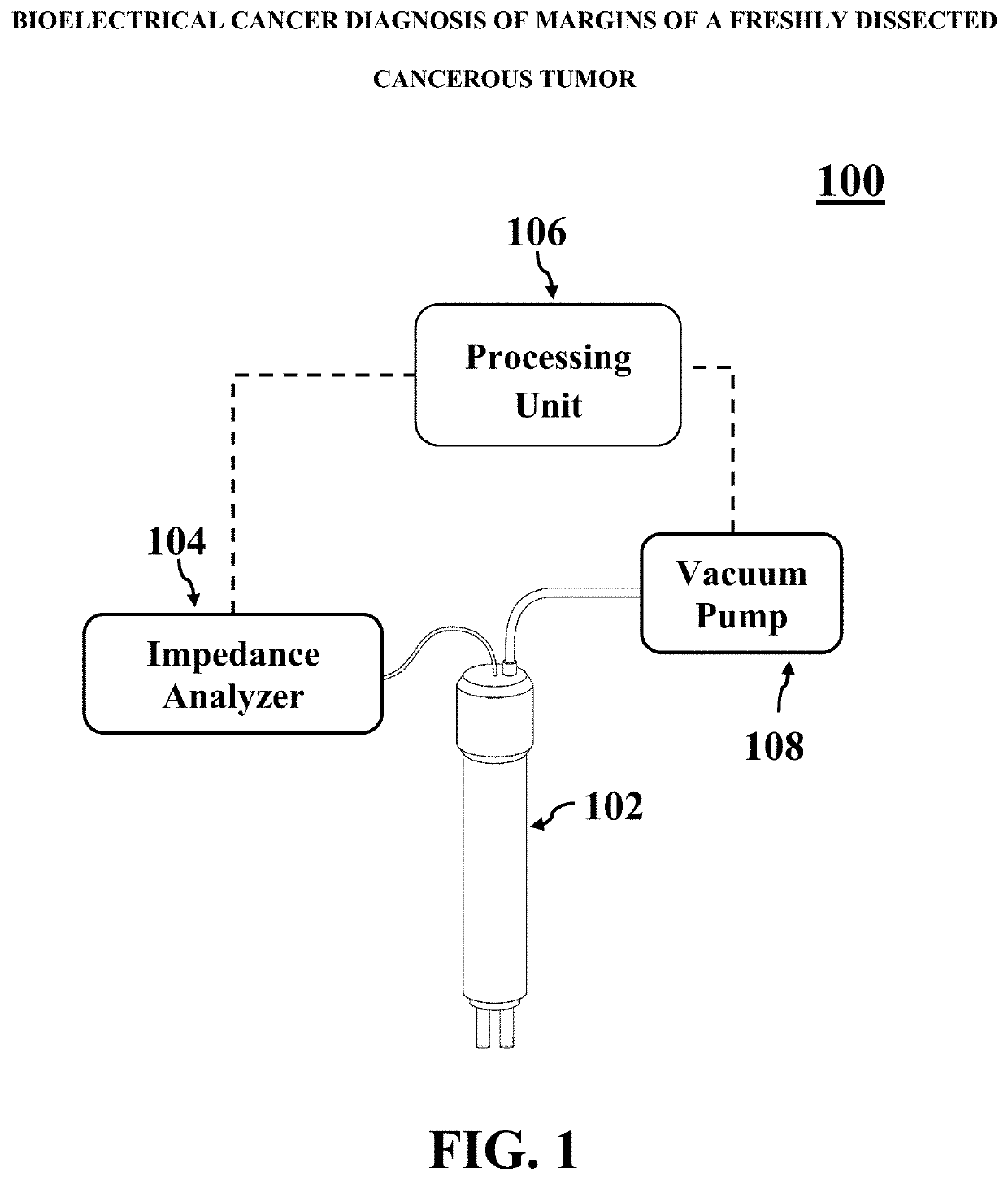
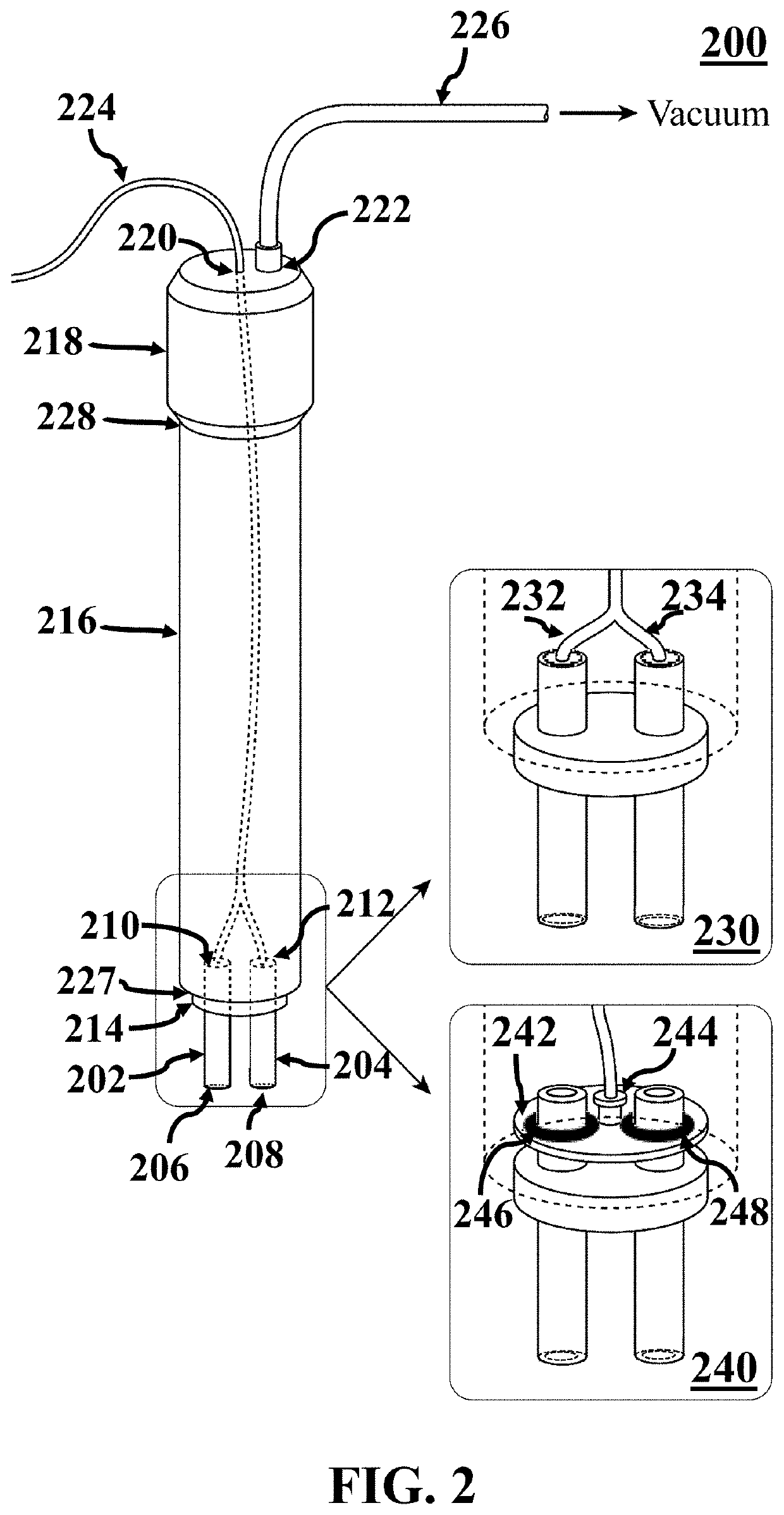
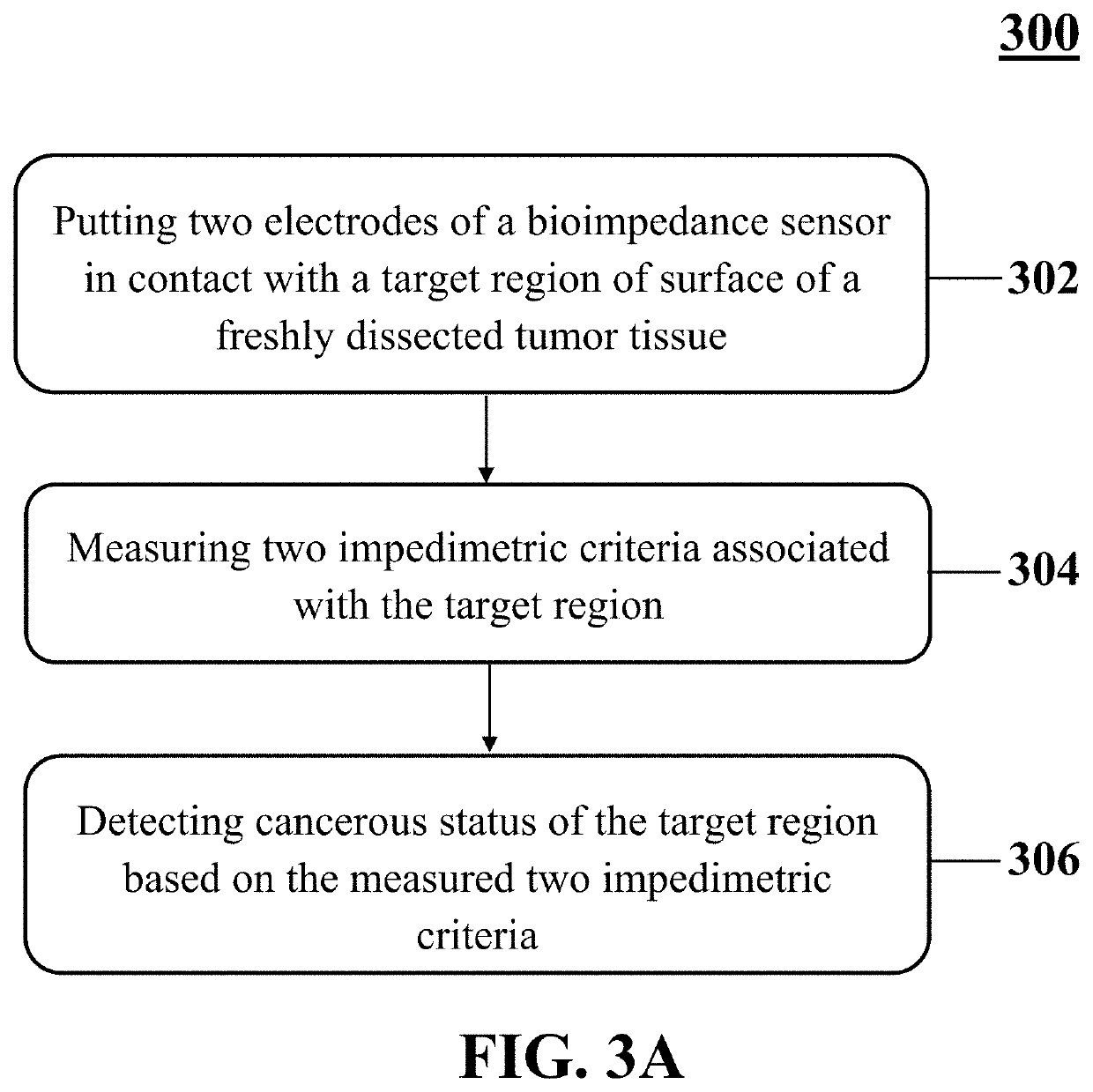
Bioelectrical cancer diagnosis of margins of a freshly dissected cancerous tumor
A method for identifying cancerous status of margins of a tumor. The method includes putting at least two electrodes of a bioimpedance sensor in contact with a target region of surface of a freshly dissected tumor tissue, measuring two impedimetric criteria associated with the target region, and detecting cancerous status of the target region based on the two measured impedimetric criteria. The two measured impedimetric criteria includes an electrical impedance magnitude of the target region at a frequency of 1 kHz (Z1 kHz) and an impedance phase slope (IPS) of the target region in a frequency range of 100 kHz to 500 kHz.
Owner:NANO HESGARSAZAN SALAMAT ARYA
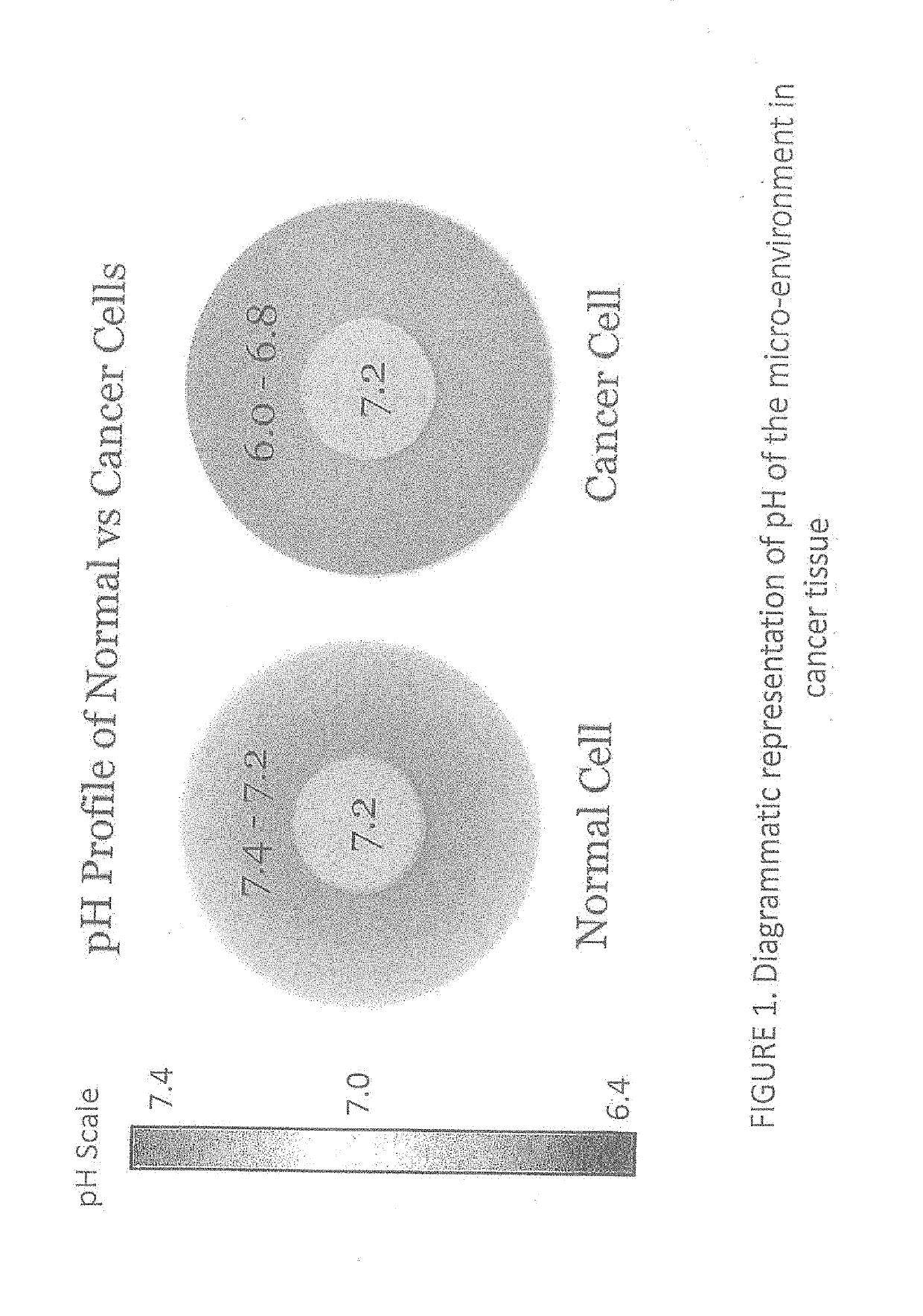
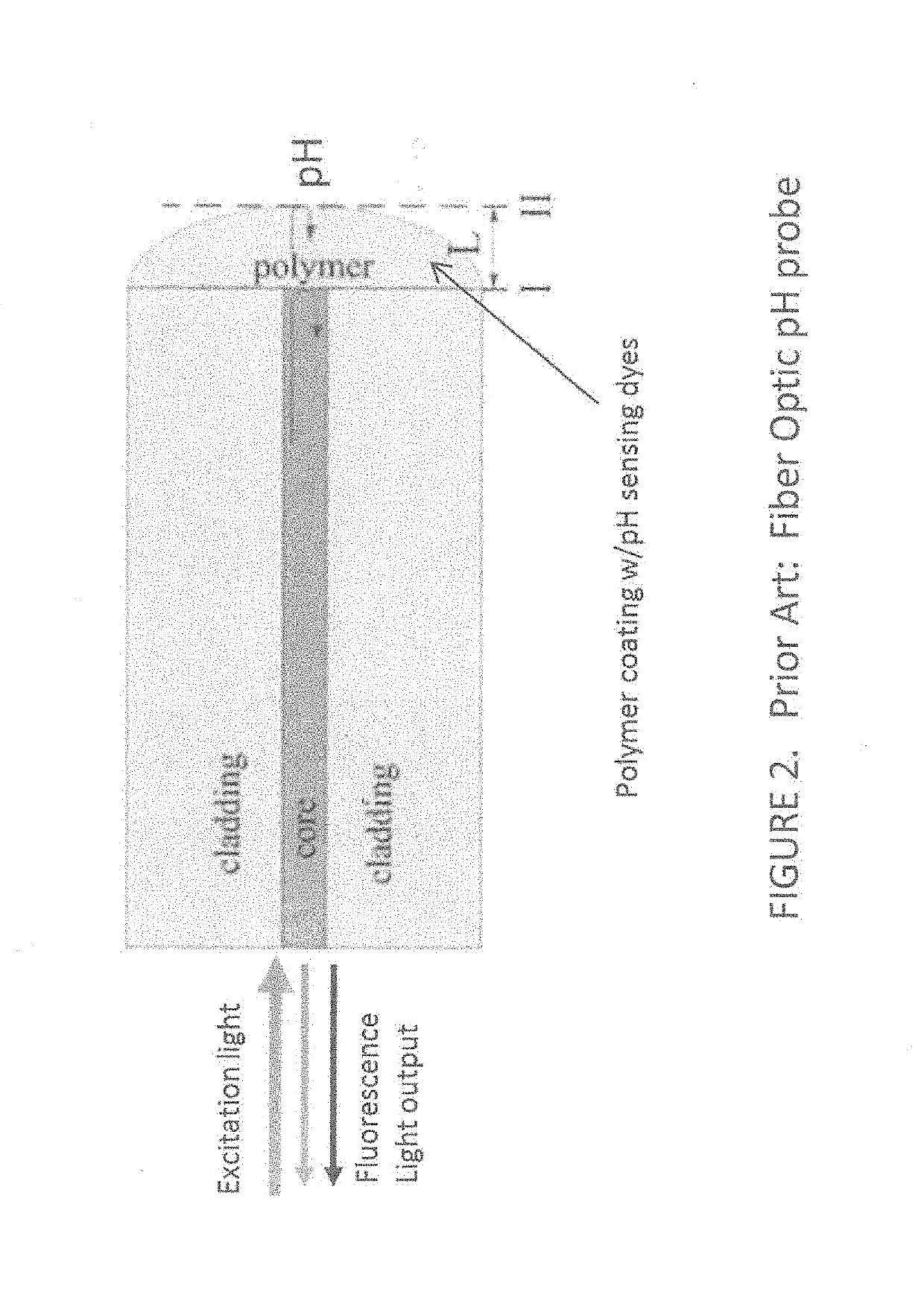
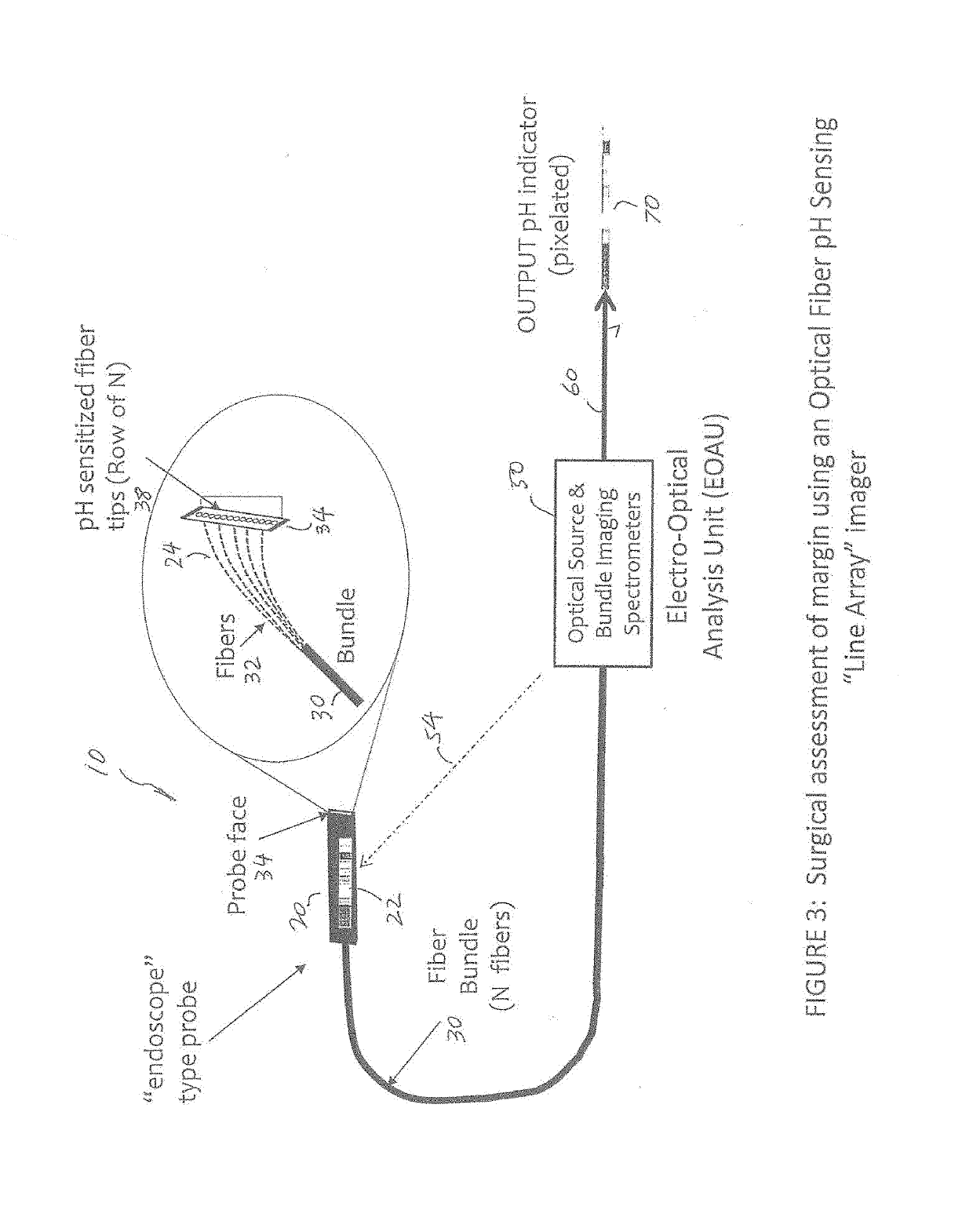
Apparatus and method for assessment of cancer margin
An apparatus for inspecting a biological tissue uses a pH-sensitive coating material to determine whether the tissue is normal or cancerous. The coating material is placed in contact with the tissue to be excited by an excitation light. The coating material is arranged to provide a response signal indicative of the pH value of the tissue. Using a fiber bundle having a plurality of optical fibers forming a linear array or a two-dimensional array adjacent the coating material, the imaging of localized surface pH in the biological tissue can be achieved using the response signal through each of the optical fibers. The fiber bundle can be arranged as a probe to examine the tissue for providing direct mapping of the tumor margin via a display, so that a surgeon can inspect the tissue in real-time.
Owner:KERSEY ALAN D
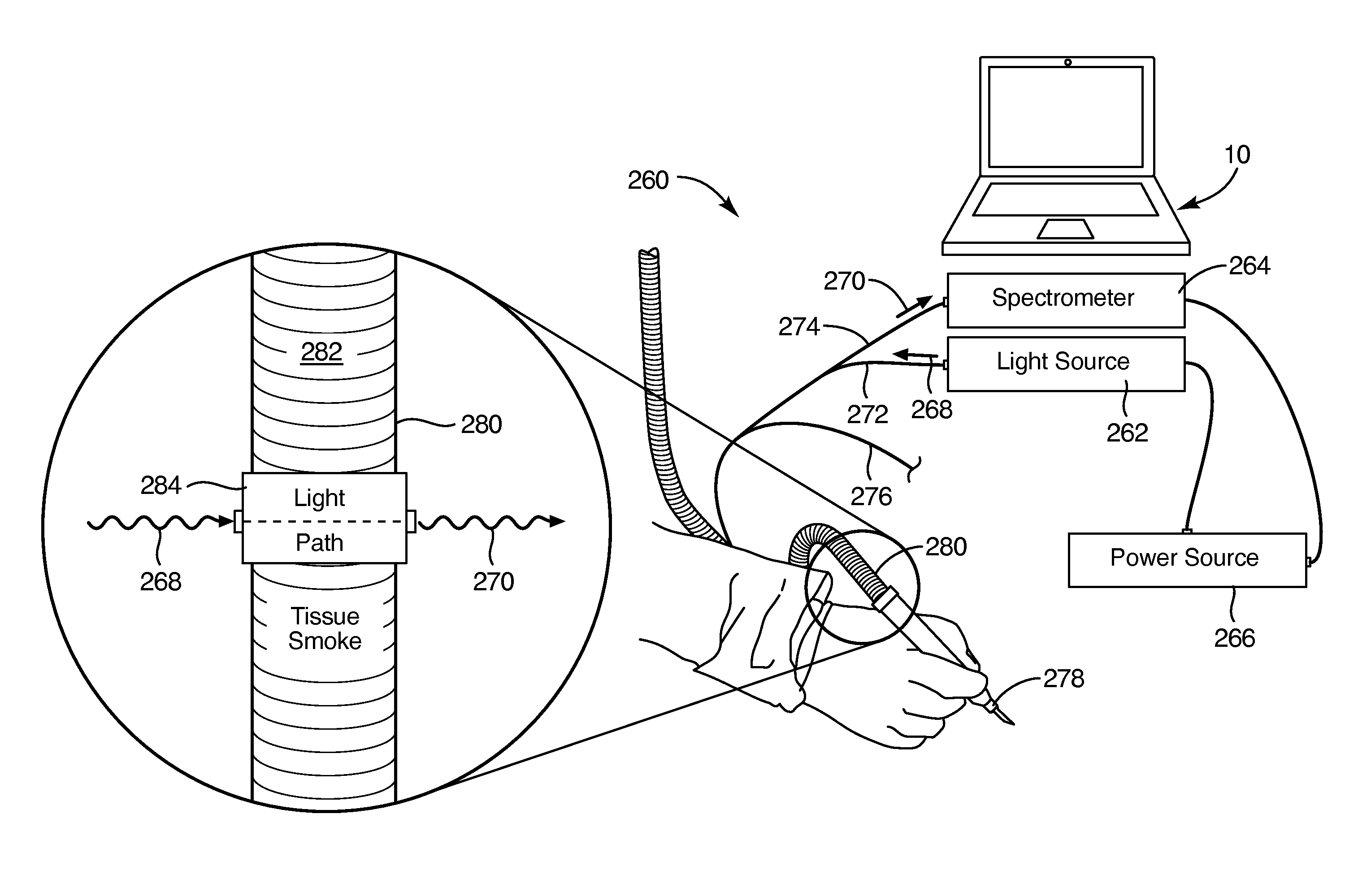
Method and device for in situ cancer margin detection
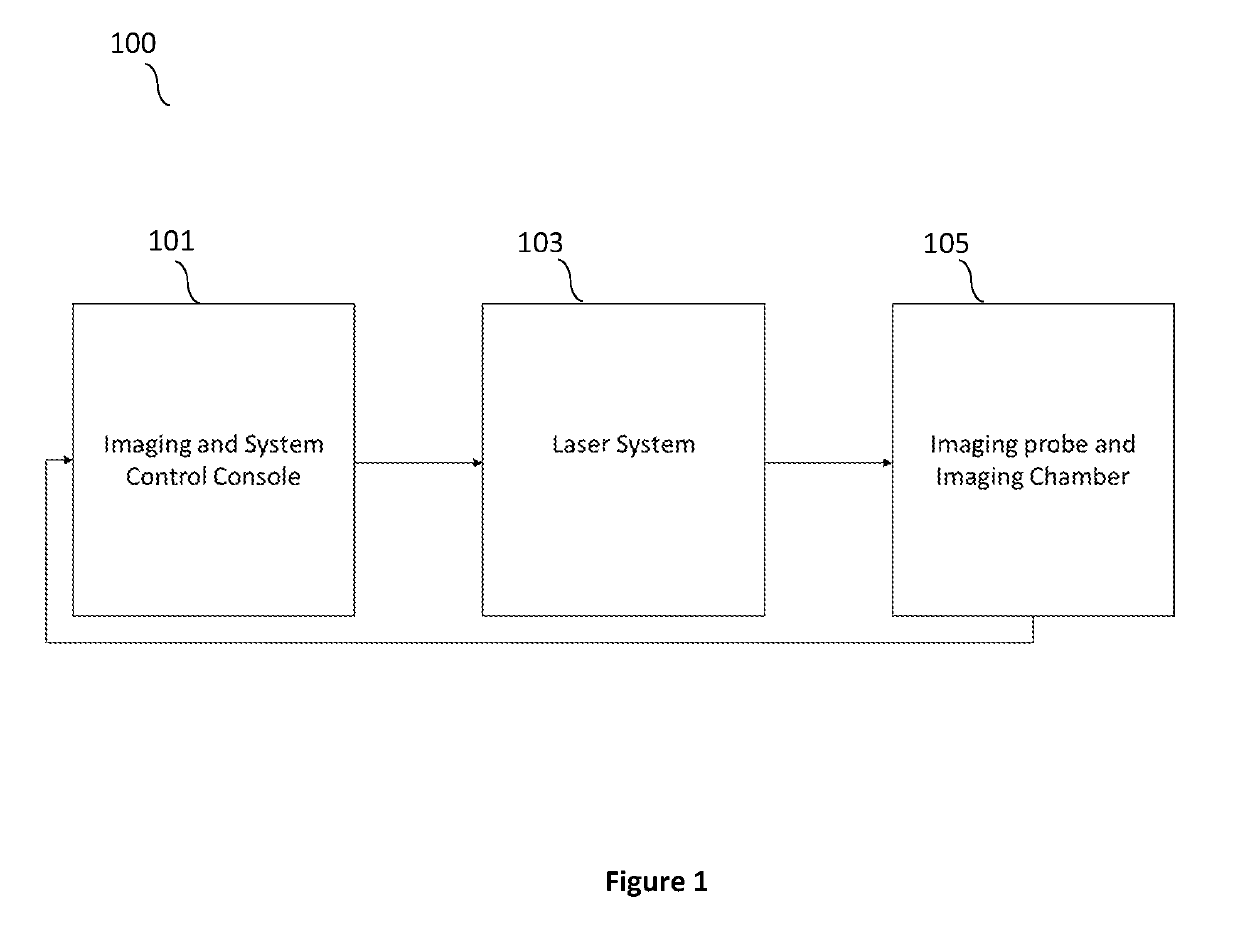
A multimodal ultrasound / photoacoustic tumor margin detection system. The system can be comprised of an imaging and system control console, which can include an ultrasound and data acquisition subsystem including an ultrasound transmitter and receiver configured to transmit and receive ultrasonic energy. A host-control computer configured to provide a function generator function and a delay generation function can also be a part of the imaging and system control console.
Owner:PURDUE RES FOUND INC
A brain tumor surgical incision and approach planning method
ActiveCN105147362BShort access pathRequirements for achieving precise positioning of incisionsExcision instrumentsComputer-aided planning/modellingSurgical approachShortest distance
The invention belongs to the field of computer-assisted surgery, and in particular relates to a brain tumor surgery incision locating and approach planning method which is used for incision localization and surgical approach planning before a brain tumor resection surgery on the basis of a medical image. The method comprises the following steps: acquiring a tumor central point; locating a point, which keeps the shortest distance from the tumor central point, on the scalp; locating a point, which keeps the shortest distance from the tumor central point, within a region; acquiring tumor marginal points; determining a surgical incision location; and designing a surgical approach. Compared with other methods of locating brain tumor on scalp, the method disclosed by the invention, under the circumstance of effectively avoiding intracranial important tissues, guarantees the shortest route of the designed surgical approach and meets the requirement of precise localization of incision with the minimum additional wound.
Owner:HARBIN ENG UNIV
Automated tumour-stroma interface zone detection for Anti-tumour response assessment by immunogradient indicators
PendingUS20220138955A1Extraction of informationImprove statistics performanceImage enhancementImage analysisDiseaseAnalysis data
We present a new method to automatically sample the tumour / stroma interface zone (IZ) from microscopy image analysis data. It first delineates the tumour edge using a set of explicit rules in grid-subsampled tissue areas; then the IZ of controlled width is sampled and ranked by the distance from the edge to compute TIL density profiles across the IZ. From this data, a set of novel Immunogradient indicators are computed to reflect TIL “gravitation” towards the tumour. We applied the method on CD8 immunohistochemistry images of surgically excised breast and colorectal cancers to predict overall patient survival. In both patient cohorts, we found strong and independent prognostic value of the Immunogradient indicators, outperforming methods currently available. We conclude that data-driven, automated, human operator-independent IZ sampling enables precise spatial immune response measurement in the tumour / host interaction frontline for prediction of disease and therapy outcomes.
Owner:VILNIUS UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com