Nanovesicle drug formed by self-assembly and its preparation method and application
A technology of nanovesicles and drugs, applied in the direction of nano drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Self-assembly of EB-CPT alone.
[0079] The self-assembly process of EB-CPT is quite simple. We tested two strategies for the self-assembly of EB-CPT into micelles. The first method was previously described as spontaneous self-assembly by directly suspending EB-CPT powder in deionized water (ACS Nano, 2017, 11, 8838-8848). To complete and compare the co-assembly process of EB-CPT and PTX, we also tested the self-assembly process of EB-CPT by solvent evaporation. In a typical procedure, 100 μL of 5 mg / mL EB-CPT was dissolved in methanol and added dropwise to a solution of 2 mL deionized water. The solution was left open to evaporate methanol under a fume hood, and the residual organic solvent was further removed with a rotary evaporator at low pressure. A clear solution was obtained without obvious precipitation. Both methods yielded uniform EB-CPT micelles, while the diameter of the micelles of the evaporation method was slightly larger than that of the m...
Embodiment 2
[0080] Example 2: Co-assembly of EB-CPT to PTX in a weight ratio (w / w) of 1:0.5.
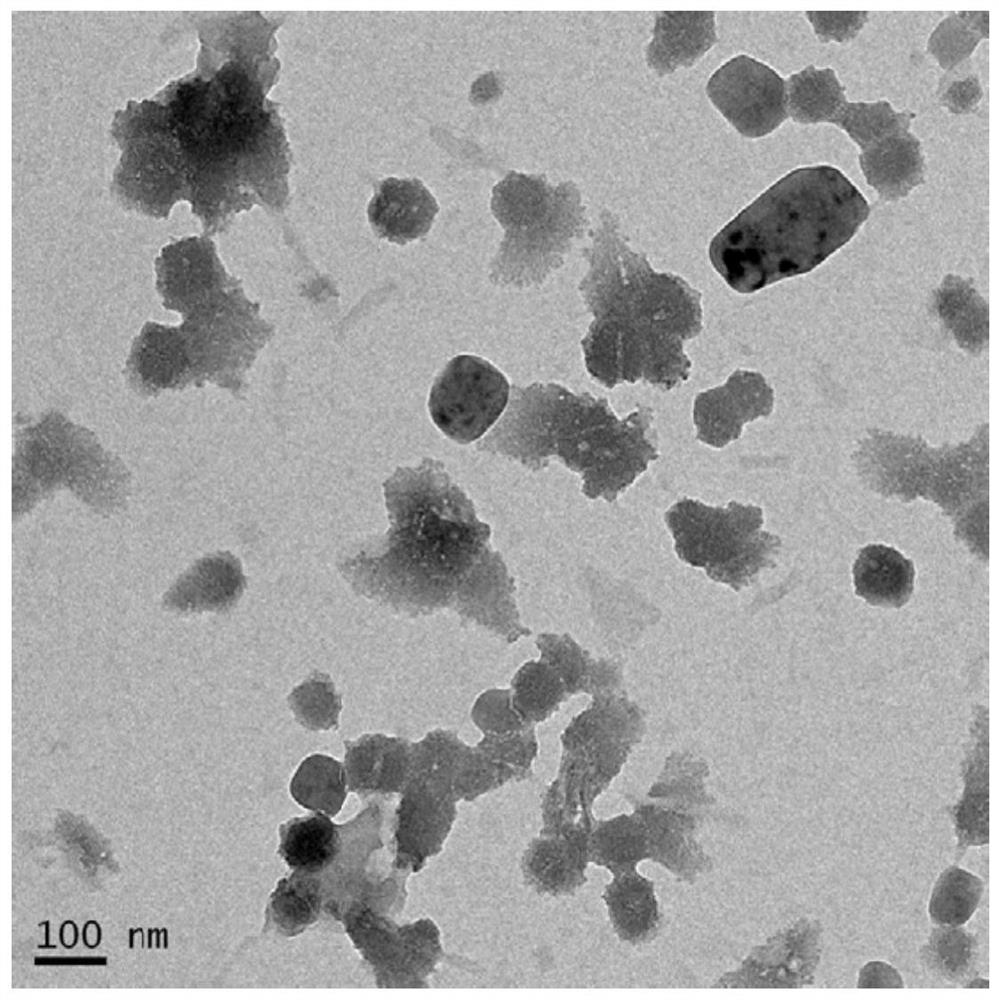
[0081] 40 μL of EB-CPT (5.0 mg / mL) in methanol and 20 μL of PTX (5.0 mg / mL) in methanol were mixed and added dropwise to 2 mL of deionized water. The organic solvent methanol was mainly evaporated under a fume hood, and the remaining organic solvent methanol was further removed by a rotary evaporator under low pressure. The obtained solution was clear without obvious precipitation, indicating that the co-assembly of EB-CPT and PTX was successful. The nanoparticles co-assembled in the solution of this example are marked as "ECX (1:0.5) NVS", and the transmission electron microscope image is as follows image 3 As shown, it can be seen that the nanoparticles basically form vesicles, and although there are certain differences in the size of the vesicles, the diameter of any cross-section of each vesicle is basically below 100 nm.
Embodiment 3
[0082] Example 3: Co-assembly of EB-CPT to PTX in a weight ratio (w / w) of 1:1.
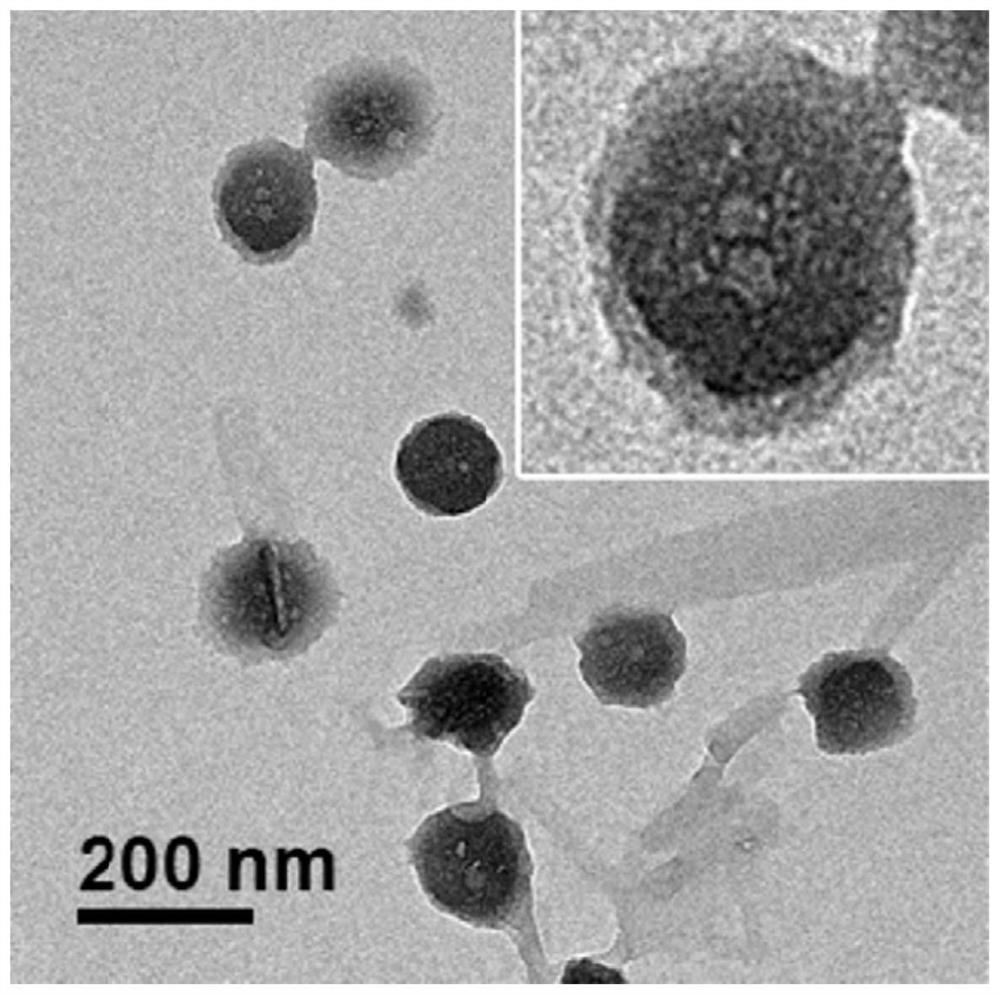
[0083] 40 μL of EB-CPT (5.0 mg / mL) in methanol and 40 μL of PTX (5.0 mg / mL) in methanol were mixed and added dropwise to 2 mL of deionized water. The organic solvent methanol was mainly evaporated under a fume hood, and the remaining organic solvent methanol was further removed by a rotary evaporator under low pressure. The obtained solution was clear without obvious precipitation, indicating that the co-assembly of EB-CPT and PTX was successful. The ECX transmission electron microscope images of nanoparticles co-assembled in solution are as follows: Figure 4 As shown, it can be seen that the nanoparticles are almost all formed into vesicles, and the size of the vesicles is relatively uniform, the diameter of each vesicle in any section is basically below 80 nm, and the diameter ratio of each dimension section does not exceed 2: 1. It is spherical as a whole.
[0084] In this example, EB-CPT a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com