A kind of polypeptide compound with high affinity to glp-1 receptor and its preparation method and application
A technology for GLP-1 and peptide compounds, which is applied in the fields of pharmaceutical preparations and biomedicine, can solve the problems of limited clinical application, cannot meet the needs of long-term storage and transportation of medicines, etc., and achieves a simple and easy preparation method, easy storage, and obvious curative effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Preparation of maleimide Evans blue derivatives:
[0048] a. Preparation of intermediate maleyl xylidine Mal-tolidine
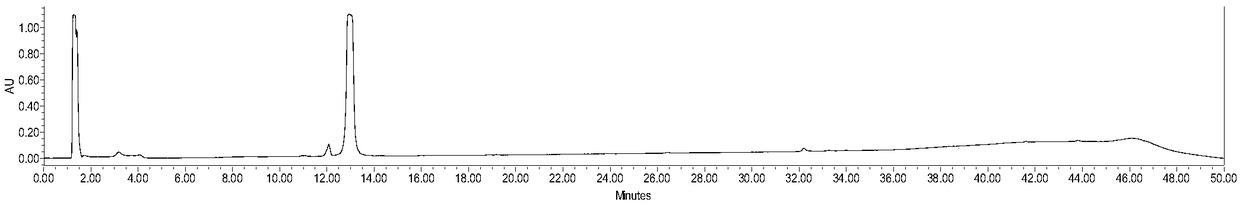
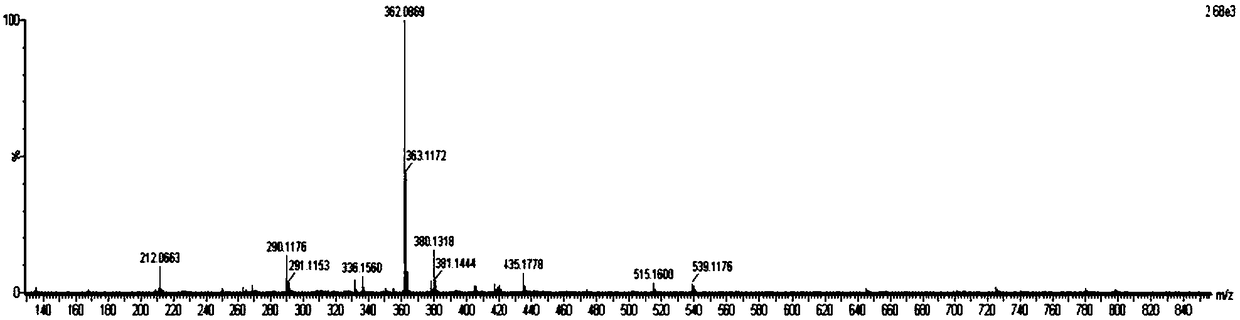
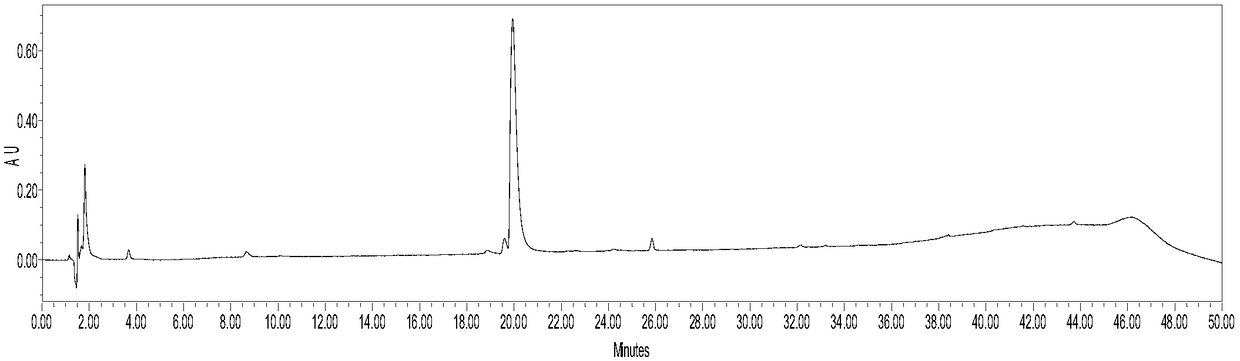
[0049] 3-Maleimidopropionic acid (10.0mmol), HATU (20mmol) and DIPEA (50.0mmol) were successively cast into acetonitrile (500mL) solution of 3,3'-dimethylbenzidine tolidine (50.0mmol) . After stirring at room temperature for 48 h, the reaction mixture was filtered, and the filtrate was spin-dried to remove the solvent to obtain a yellow crude product. The residue was purified by a silica gel column to obtain a pale yellow intermediate Mal-C3-Tolidine, whose characterization is shown in figure 1 , 2 , yield 60%. The reaction process is as follows:
[0050]
[0051] b. Preparation of Evans blue (MEB) with maleimide groups
[0052] Dissolve Mal-tolidine (5.0 mmol) prepared in step a into a mixed solvent of acetonitrile and water to obtain a yellow solution. Under the condition of ice bath, HCl was slowly added dropwise to the above Mal-tolidi...
Embodiment 2
[0069] Add 5mL [Leu] to the sodium acetate solution (50mM, pH 5.5) of Evans blue (1mmol) with aldehyde group 14 Exendin-4 (1mmol) was dissolved in 50mM sodium acetate solution (50mM, pH 5.5), and then 20mM NaCNBH was added 3 reducing agent. Under the condition of avoiding light, react at room temperature for 2 hours. Terminate the reaction with 0.1% aqueous trifluoroacetic acid (TFA), react the mixture, lyophilize the reaction mixture to obtain a lyophilized mixture, then dissolve the lyophilized mixture in PBS buffer with a pH of 7.0, and remove unreacted by dialysis substance, and finally Evan's blue-modified [Leu] 14 Exendin-4.
Embodiment 3
[0071] Add 5mL of [Leu] 14 Exendin-4 (1mmol) was dissolved in 50mM sodium acetate solution (50mM, pH 5.5), and then 20mM NaCNBH was added 3 reducing agent. Under the condition of avoiding light, react at room temperature for 2 hours. Terminate the reaction with 0.1% aqueous trifluoroacetic acid (TFA), react the mixture, freeze-dry the reaction mixture to obtain a freeze-dried mixture, then dissolve the freeze-dried mixture in PBS buffer with a pH of 8.5, and remove the unreacted mixture by dialysis substance, and eventually Evan's blue modified [Leu] 14 Exendin-4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com