Truncated Evans blue modified fibroblast activating protein inhibitor as well as preparation method and application thereof
A technology of fibroblasts and Evans blue, applied in the field of nuclear medicine and molecular imaging, can solve the problems of limited FAPI tumor uptake dose and retention time, insufficient combination of drugs and targets, and inability to meet therapeutic purposes, etc., to achieve prolonged blood Effects of Circulating Half-Life, Enhanced Tumor Uptake Enrichment, and Retention Time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Preparation of tEB-FAPI Linker (Compound 20)
[0094] Synthesis of compound 2:
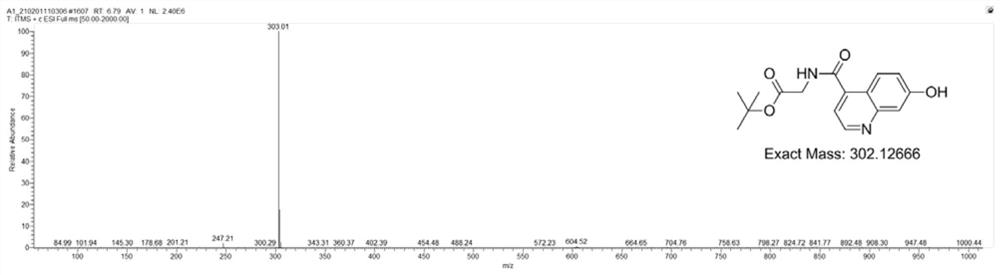
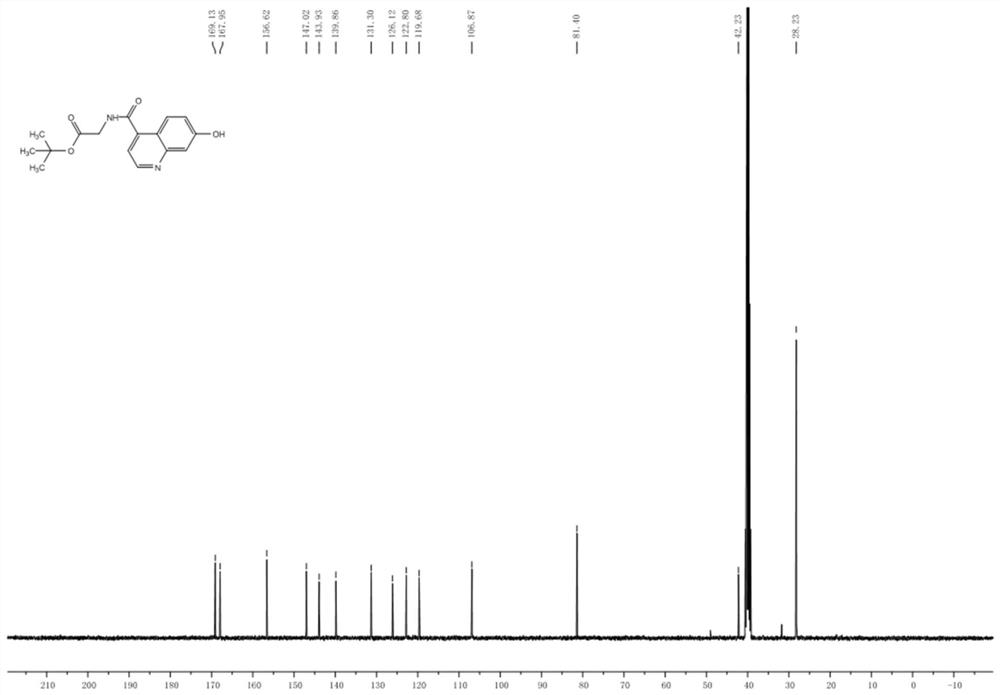
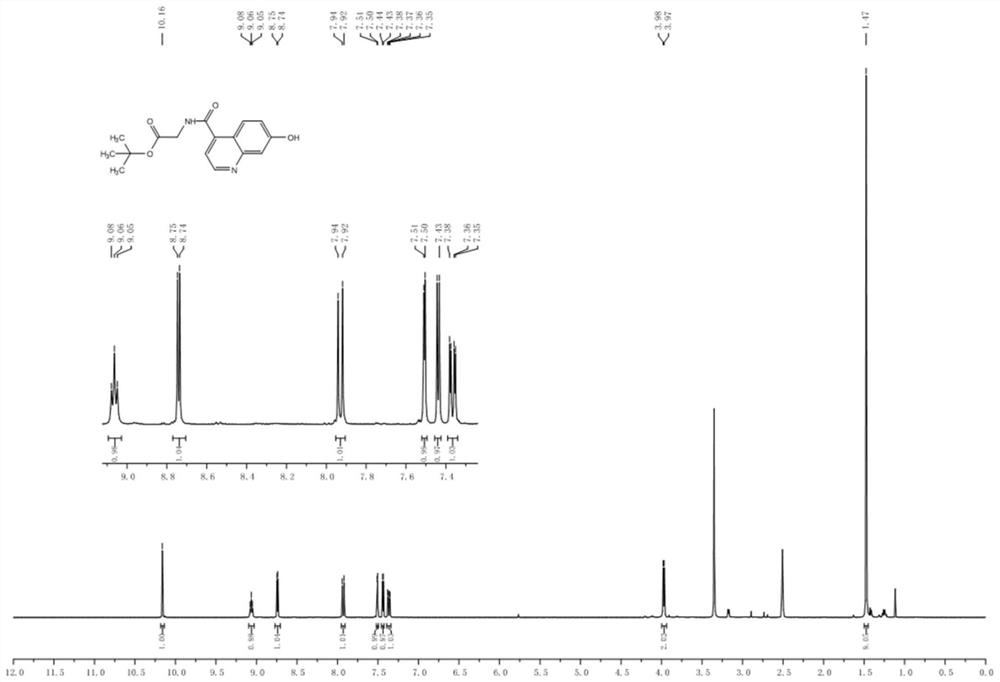
[0095] Drop into compound 1 (7-hydroxyl-4-quinoline carboxylic acid, 1.89g, 10.0mmol), glycine tert-butyl ester (1.89g, 10.0mmol), HATU (3.8g, 10.0mmol) and N respectively in a 100mL flask. N-Diisopropylethylamine (2.6 g, 20.0 mmol) was successively added to 30 mL of N,N-dimethylformamide. The reaction mixture was stirred overnight, and the solvent was distilled off under reduced pressure to obtain a crude product. Purified by silica gel column (dichloromethane / methanol=30:1) to obtain compound 2 as a white solid with a yield of 87%, figure 1 is the mass spectrum of compound 2, figure 2 The H NMR spectrum of compound 2 is shown, image 3 The C NMR spectrum of compound 2 is shown.
[0096] Synthesis of compound 3:
[0097] In a 100mL flask, compound 2 (1.51g, 5.0mmol)), 1-bromo-3-chloropropane (1.55g, 10.0mmol), and potassium carbonate (1.38g, 10.0mmol) were successively dr...
Embodiment 2-8
[0135] The compound structures of Examples 2-8 are shown in formula (II-2) to formula (II-8), and their preparation methods can refer to Example 1, wherein 5,8,11,14 reacted with compound 8 -Tetraoxa-2-azaheptadecadioic acid-1-tert-butyl ester was replaced by 5,8,11-trioxa-2-azatridecanedioic acid-1-tert-butyl ester, 9- Amino-4,7-dioxanonanoic acid tert-butyl ester, glycine tert-butyl ester or other suitable compounds, or replace (S)-pyrrolidine-2-carbonitrile hydrochloride reacted with compound 6 with 3, 3-Difluoropyrrolidine hydrochloride, or both, give the corresponding structures as follows:
[0136]
[0137]
Embodiment 9-13
[0139] The compound structure of embodiment 9-13 is shown in formula (III-1) to formula (III-4), wherein It is partly prepared according to the preparation method of compound 3BP-4089 disclosed in the patent document WO2021005131A1, and the rest of the preparation steps can refer to Example 1 and Examples 2-8.
[0140]
[0141]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com