Method of conferring a protective immune response to norovirus
a technology of immune response and norovirus, which is applied in the field of vaccines, can solve the problems of lack of reproducibility, no studies have reported being able to achieve protective immunity against i>norovirus/i>, etc., and achieves the effects of enhancing immune response, enhancing antigen uptake, and increasing antigen retention tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GLP Toxicity Study of Norovirus Vaccine Formulations in Rabbits
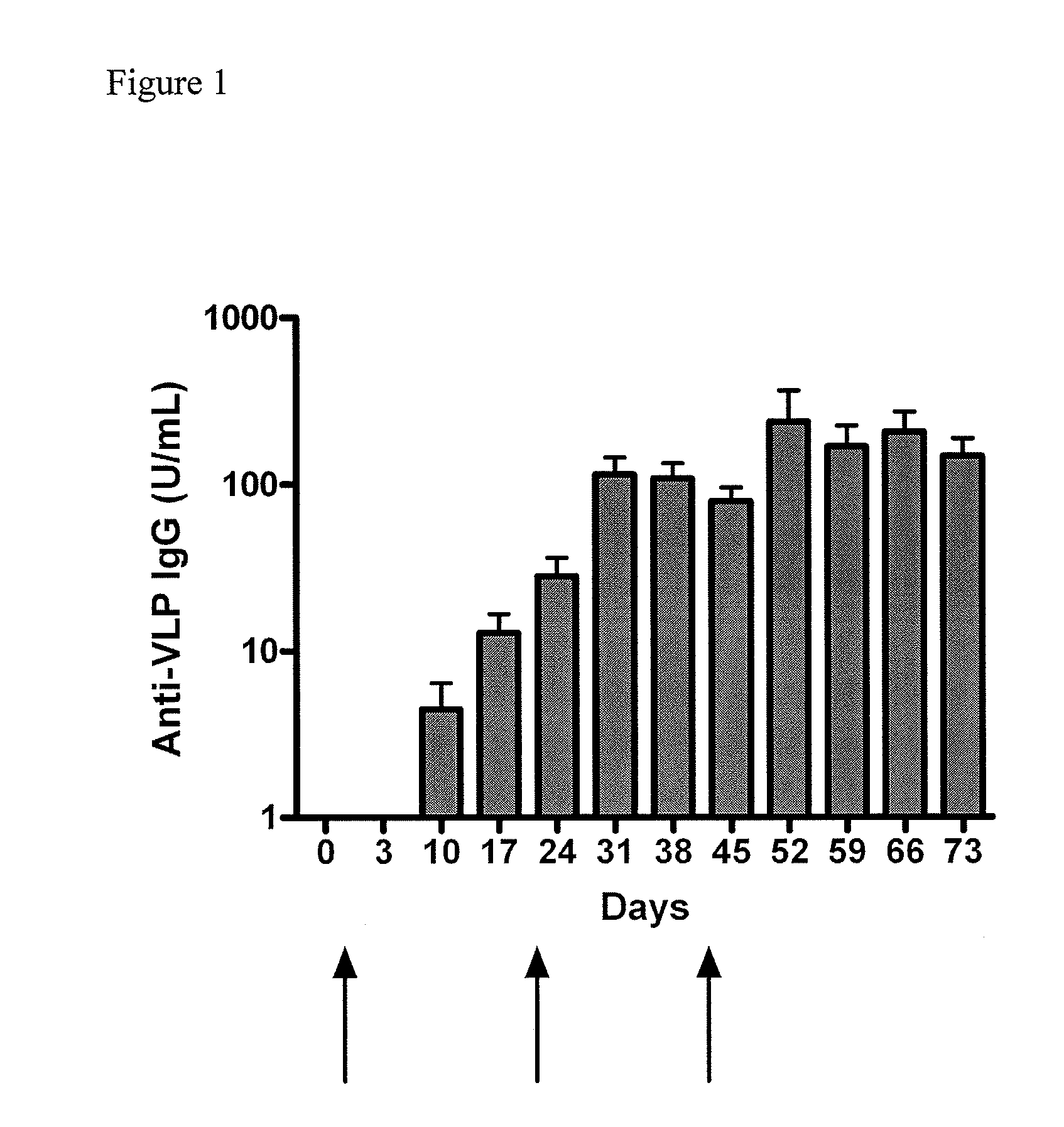
[0070]The purpose of this study was to evaluate the potential toxicity of a Norwalk virus-virus-like particle (NV-VLP) vaccine following three intranasal doses in rabbits. The NV-VLP vaccine contained (per 10 mg dry powder) 25 μg of a Genogroup I VLP, 25 μg MPL, 7 mg chitosan glutamate, 1.475 mg mannitol, and 1.475 mg sucrose. The study was conducted over an eight week period. The persistence, reversibility, or delayed onset of any effects were assessed after a four-week, no-treatment recovery interval. Sixty New Zealand White rabbits (30 / sex) were randomly assigned to three groups (10 rabbits / sex / group). Group 1 animals were not dosed (i.e. naïve). Group 2 animals were administered 10 mg / nostril (20 mg total) of placebo (i.e. adjuvant / excipient: MPL, chitosan, sucrose, and mannitol). Group 3 animals were administered 10 mg / nostril (20 mg total) of NV-VLP vaccine, which represented 25 μg of antigen per nostril (50 μg tot...
example 2
Dose Escalation Safety Study of Norwalk Vaccine Formulation in Humans (LV01-101 Study)
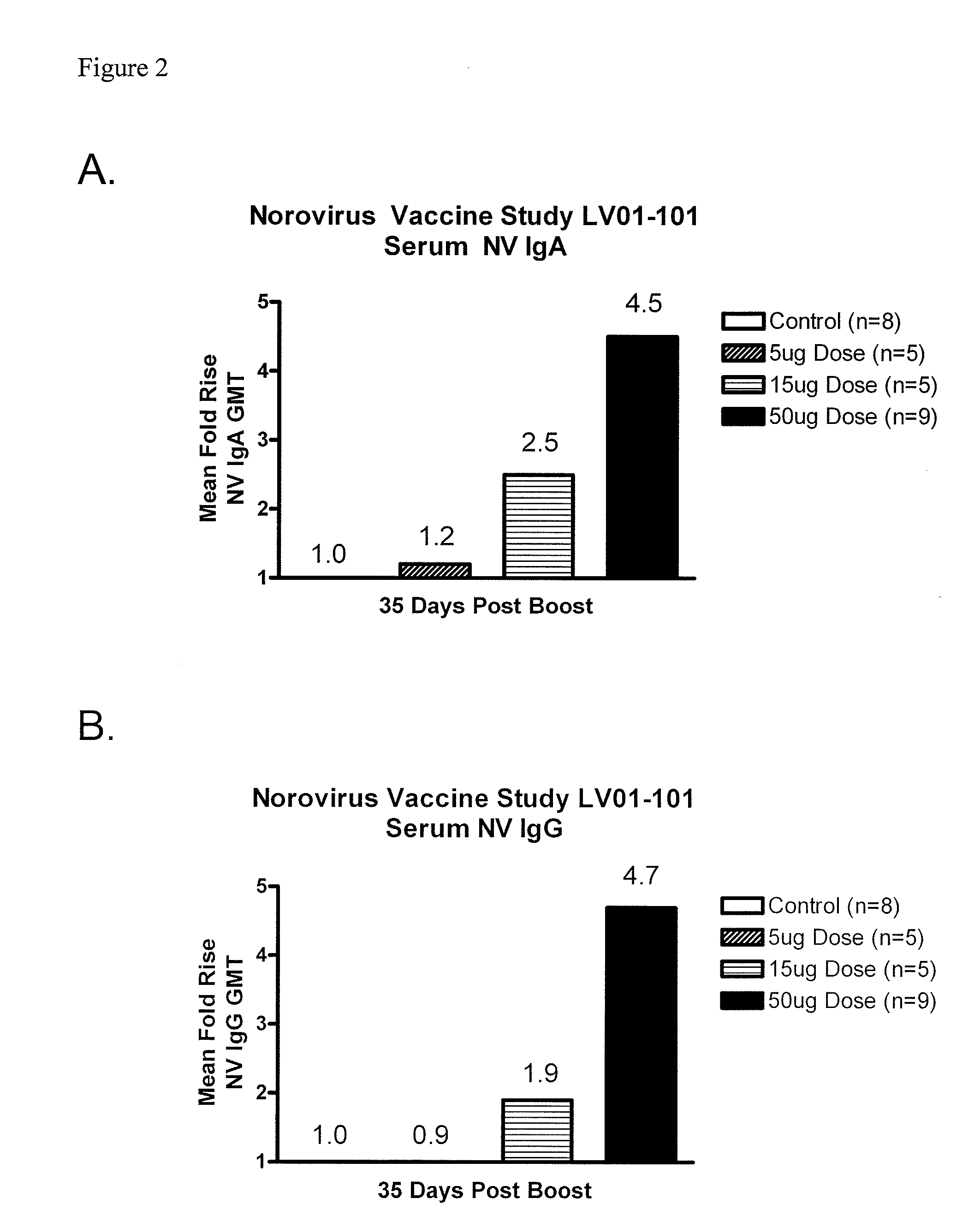
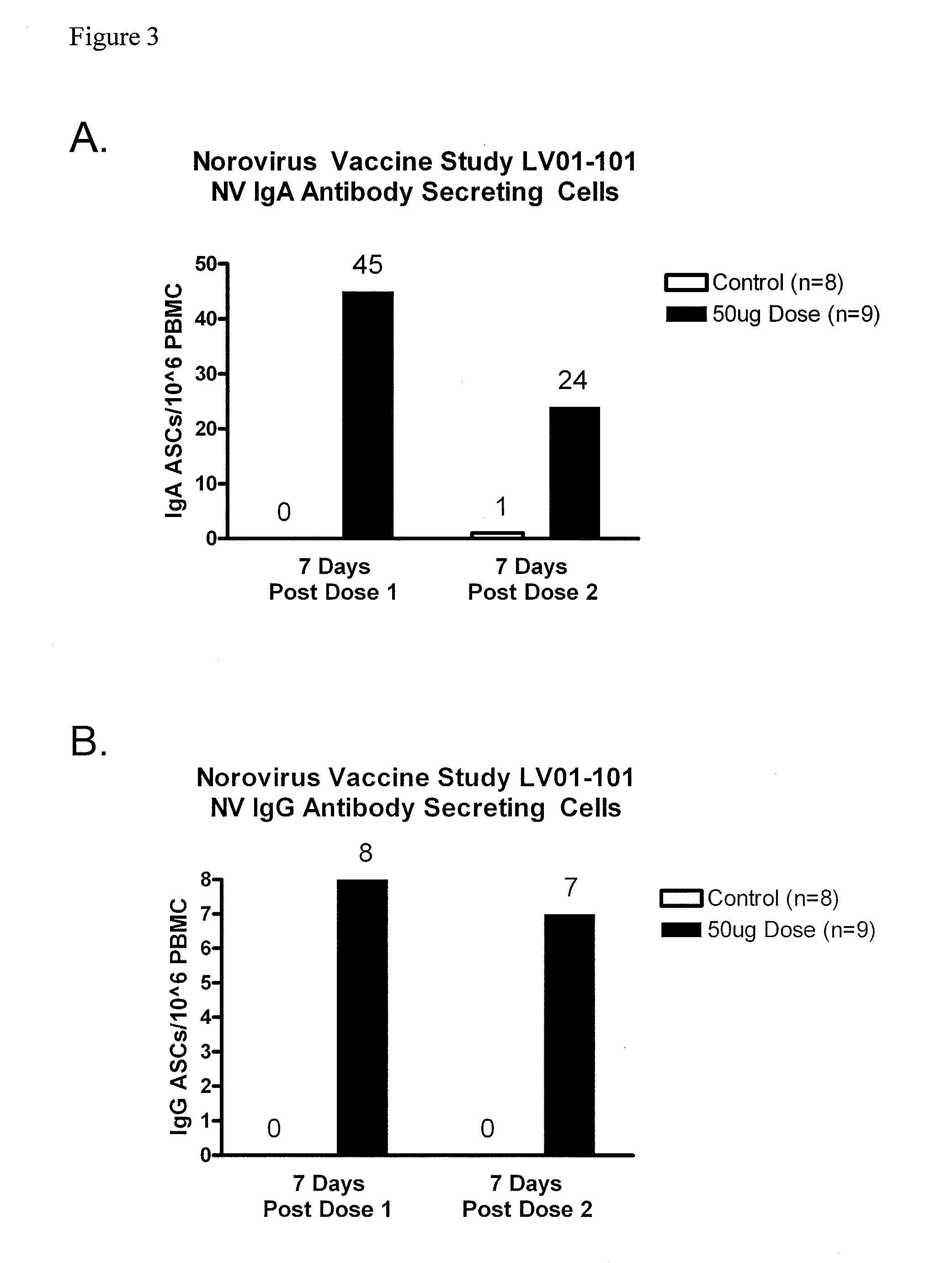
[0074]A double-blind, controlled, dose-escalation phase 1 study of the safety and immunogenicity of a Norovirus genogroup 1 vaccine was conducted. The vaccine consisted of lyophilized Norwalk virus-like particles (VLPs) in a dry powder matrix designed for intranasal administration. Vaccines included healthy adult volunteers who were H type 1 antigen secretors. The rationale for enrollment of H type 1 antigen secretors is that H type 1 antigen secretors are susceptible to Norwalk viral infections while non-secretors are resistant. Saliva was collected from volunteers to determine H type 1 antigen secretor status. As a control, 2 additional volunteers at each dosage level received matrix alone. The dry powder matrix included 25 μg MPL® adjuvant, 7 mg chitosan, 1.5 mg mannitol, and 1.5 mg sucrose. Volunteers were dosed on days 0 and 21 and were required to keep a 7-day diary of symptoms after each dos...
example 3
Safety and Immunogenicity Study of Two Dosages of Intranasal Norwalk VLP Vaccine in Humans (LV01-102 Study)
[0087]A randomized, double blind, multi-center study in healthy adults was conducted to compare the safety and immunogenicity of two dosage levels (50 μg and 100 μg) of a Norwalk virus-like particle (VLP) vaccine with adjuvant / excipients and placebo controls (empty device). The vaccine consisted of Norwalk virus-like particles (VLPs) in a dry powder matrix designed for intranasal administration as described in Example 2. Vaccines included healthy adult volunteers ages 18-49 who were H type 1 antigen secretors. Saliva was collected from volunteers to determine H type 1 antigen secretor status. Further, only subjects whose blood type was A or O (not type B or AB) were included in the study as those with B blood type are reported to be less susceptible to Norwalk infection (Glass et al. (2009) N. Engl. J. Med., Vol. 361: 1776-1785). The human volunteers were randomly assigned to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com