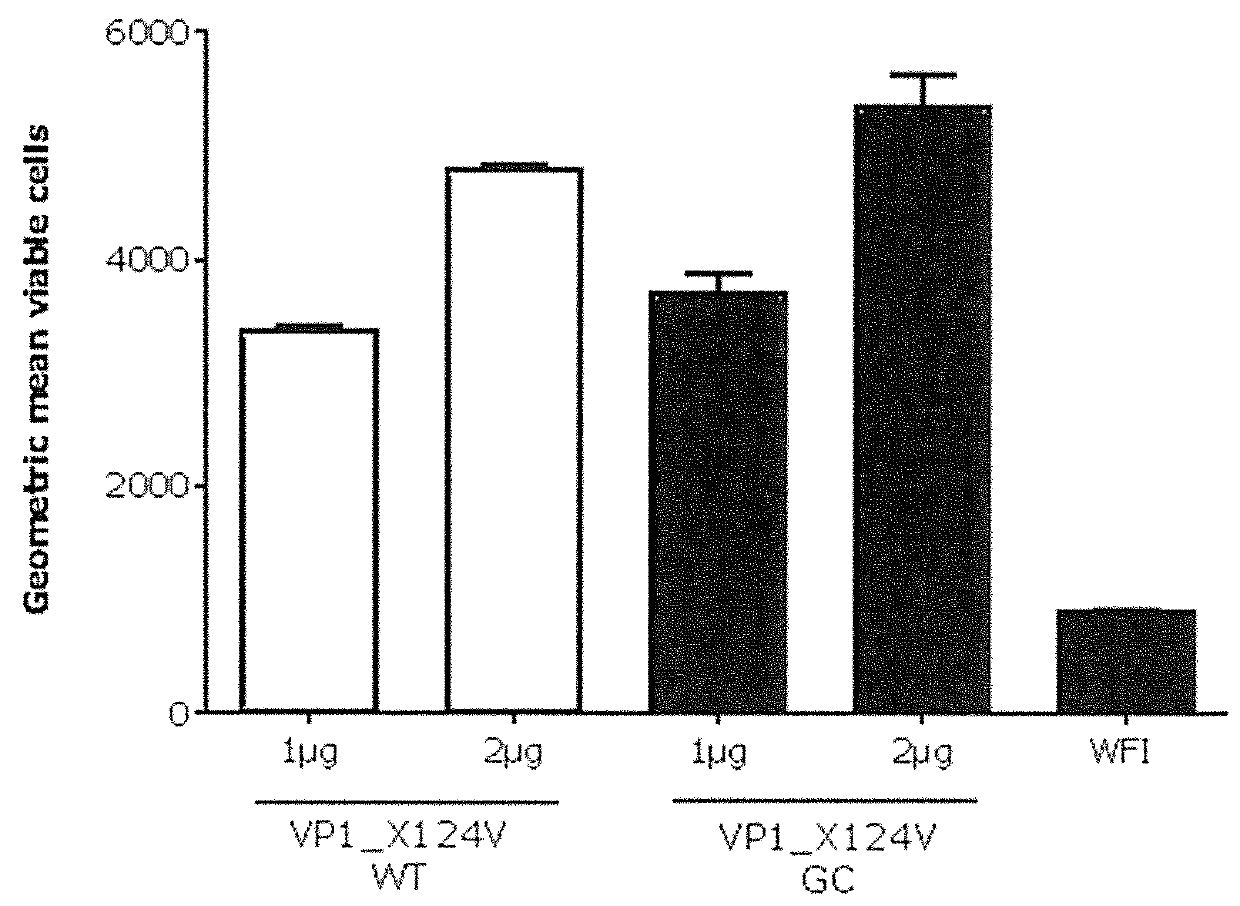
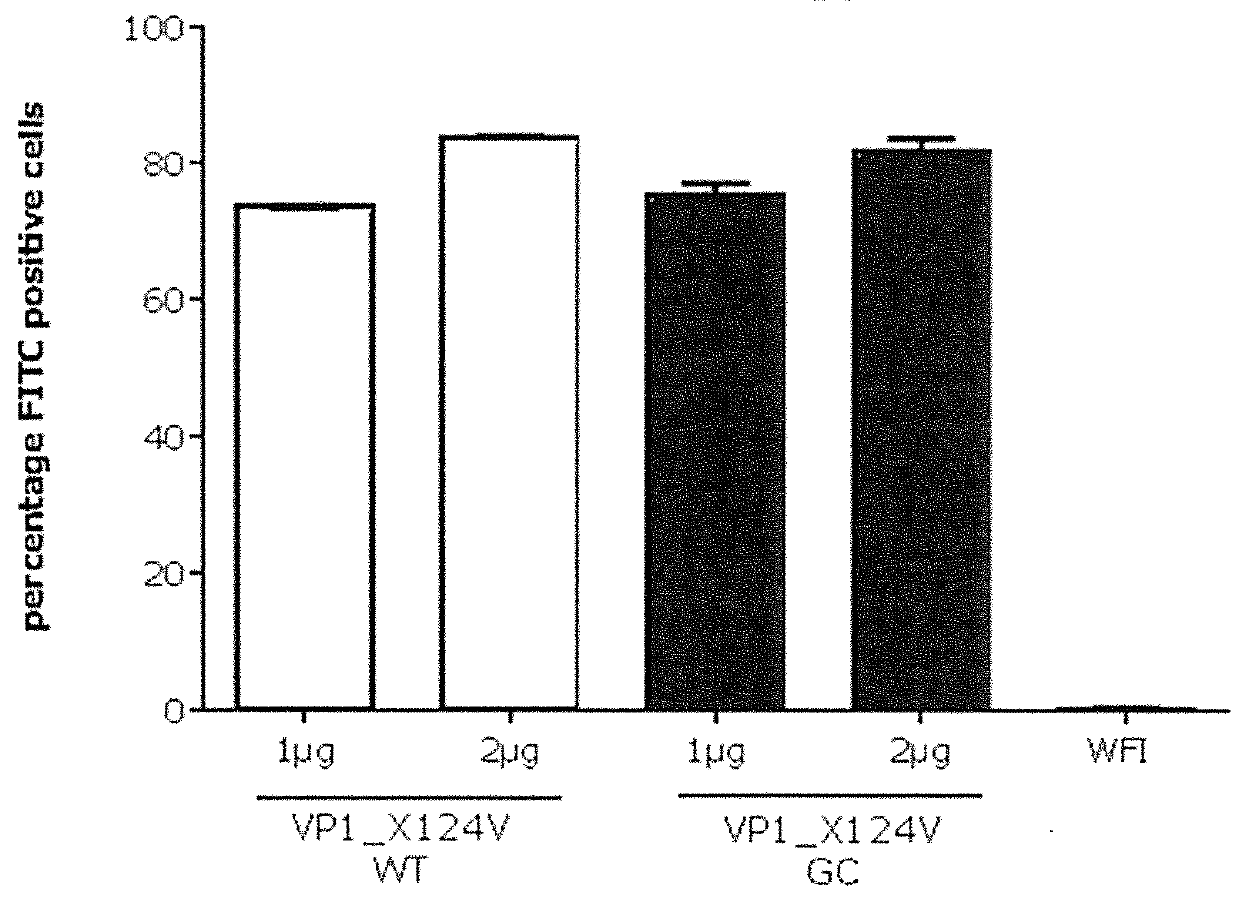
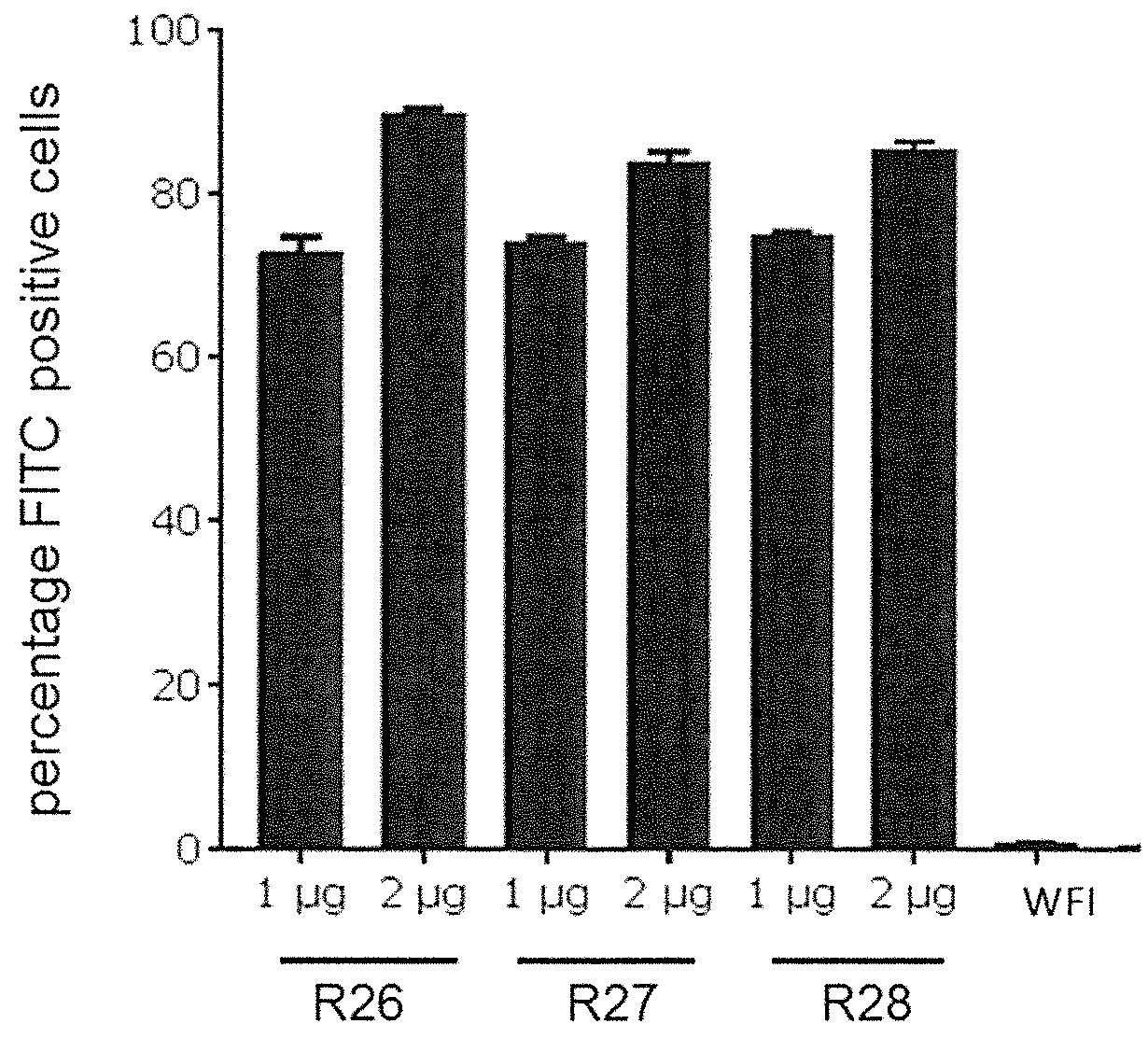
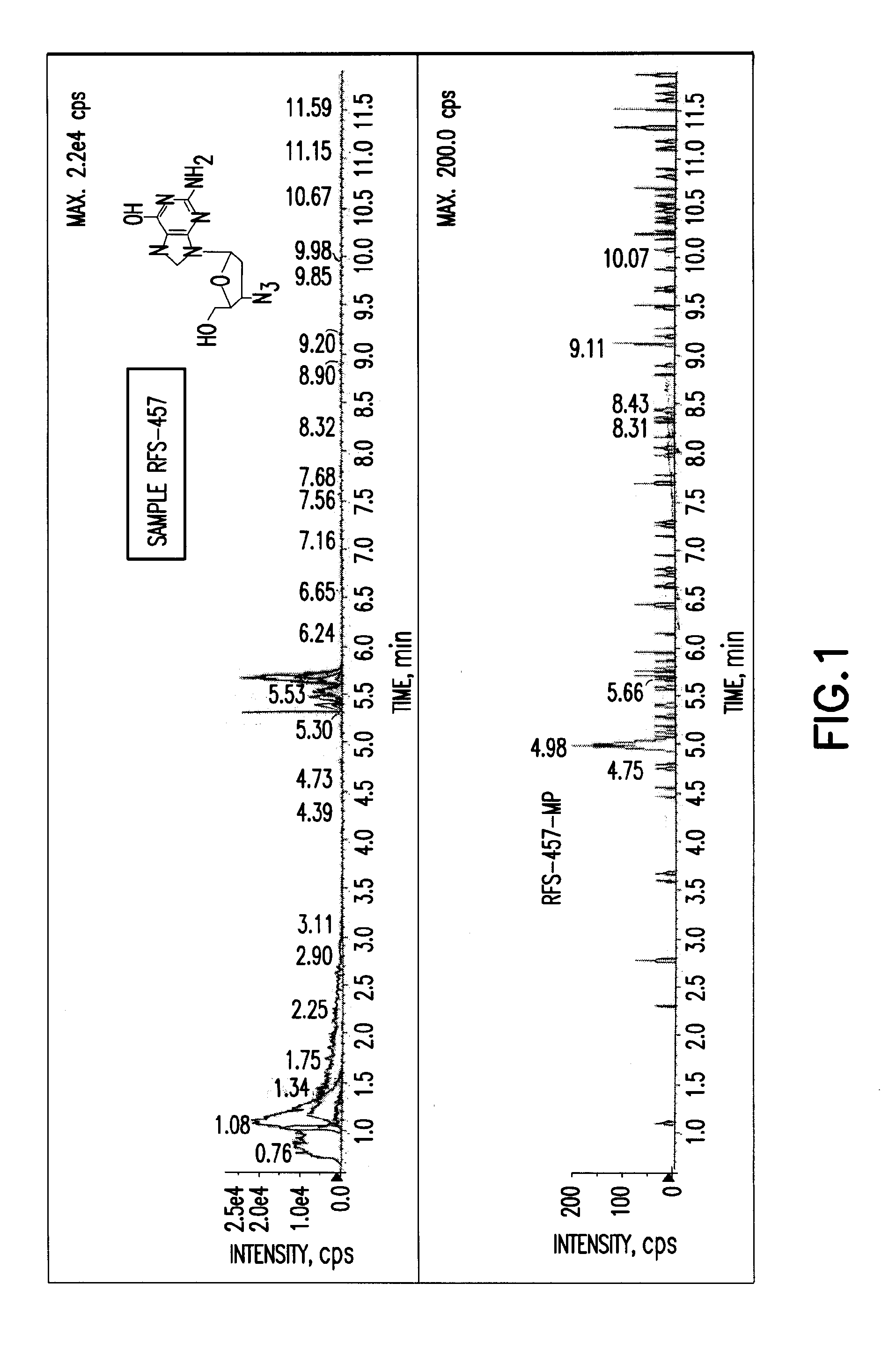
Patents
Literature
252 results about "Norovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Norovirus, sometimes referred to as the winter vomiting bug, is the most common cause of gastroenteritis. Infection is characterized by diarrhea, vomiting, and stomach pain. Blood is not usually present. Fever or headaches may also occur. This usually develops 12 to 48 hours after being exposed. Recovery typically occurs within 1 to 3 days. Complications may include dehydration.
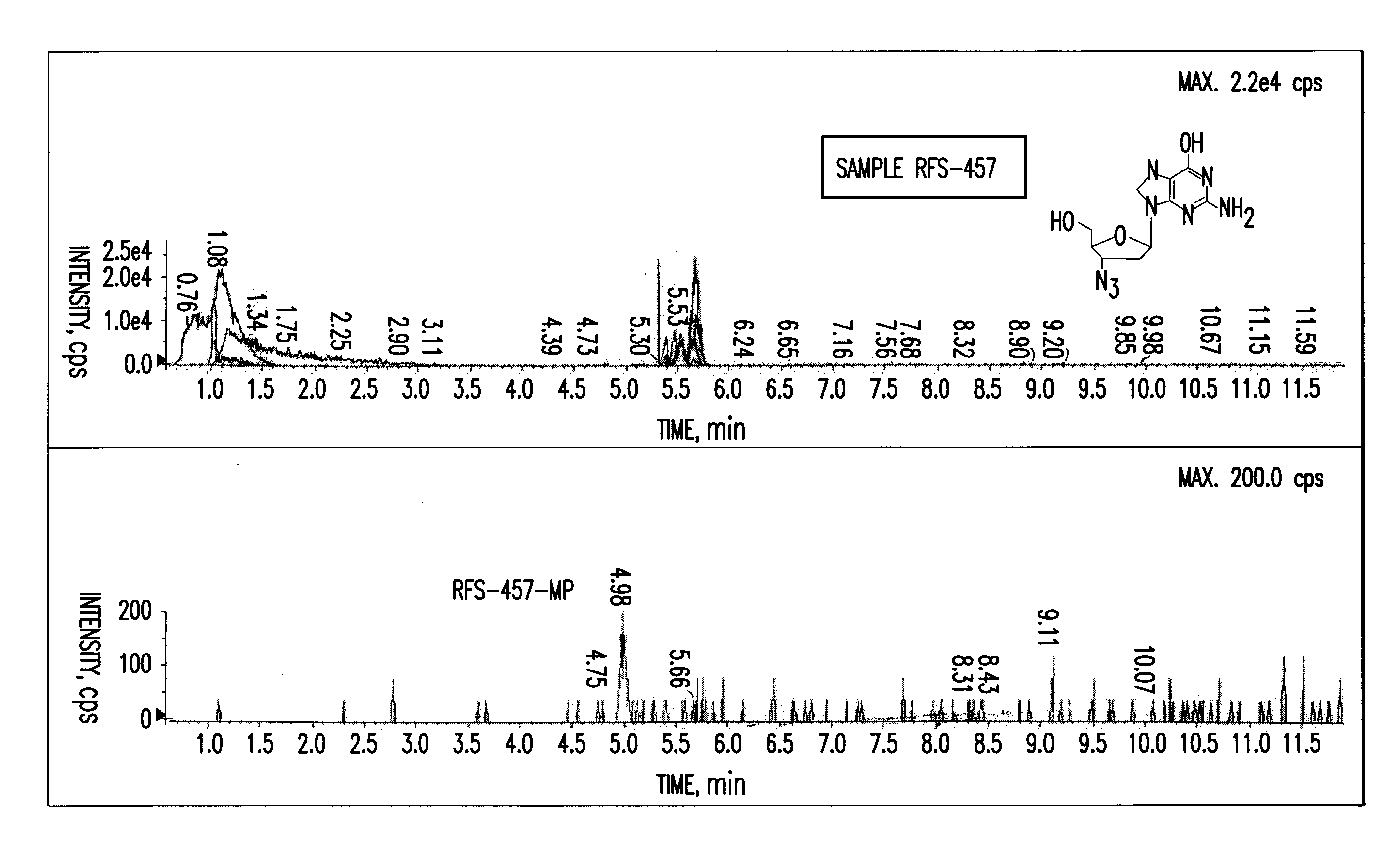
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
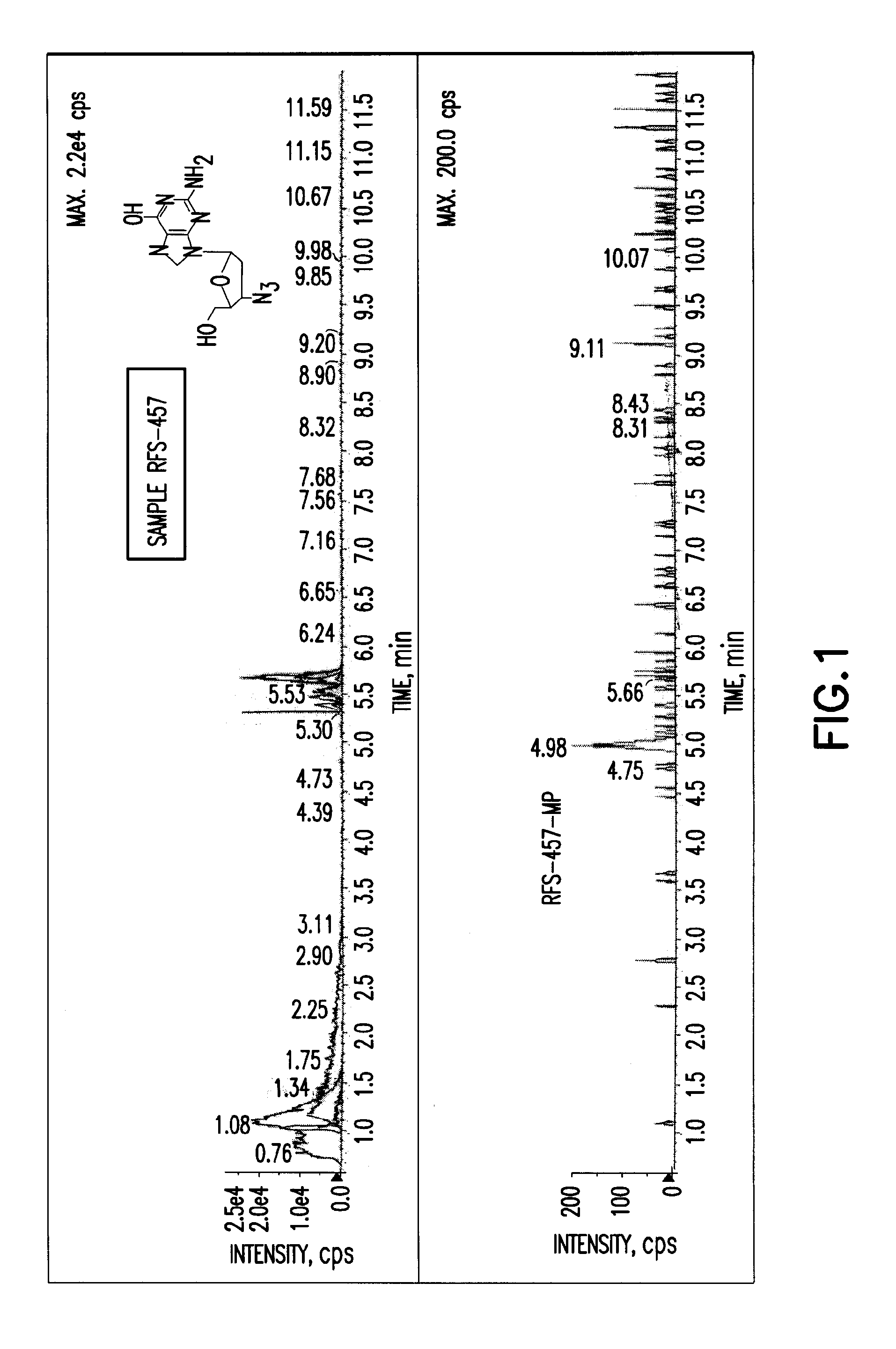
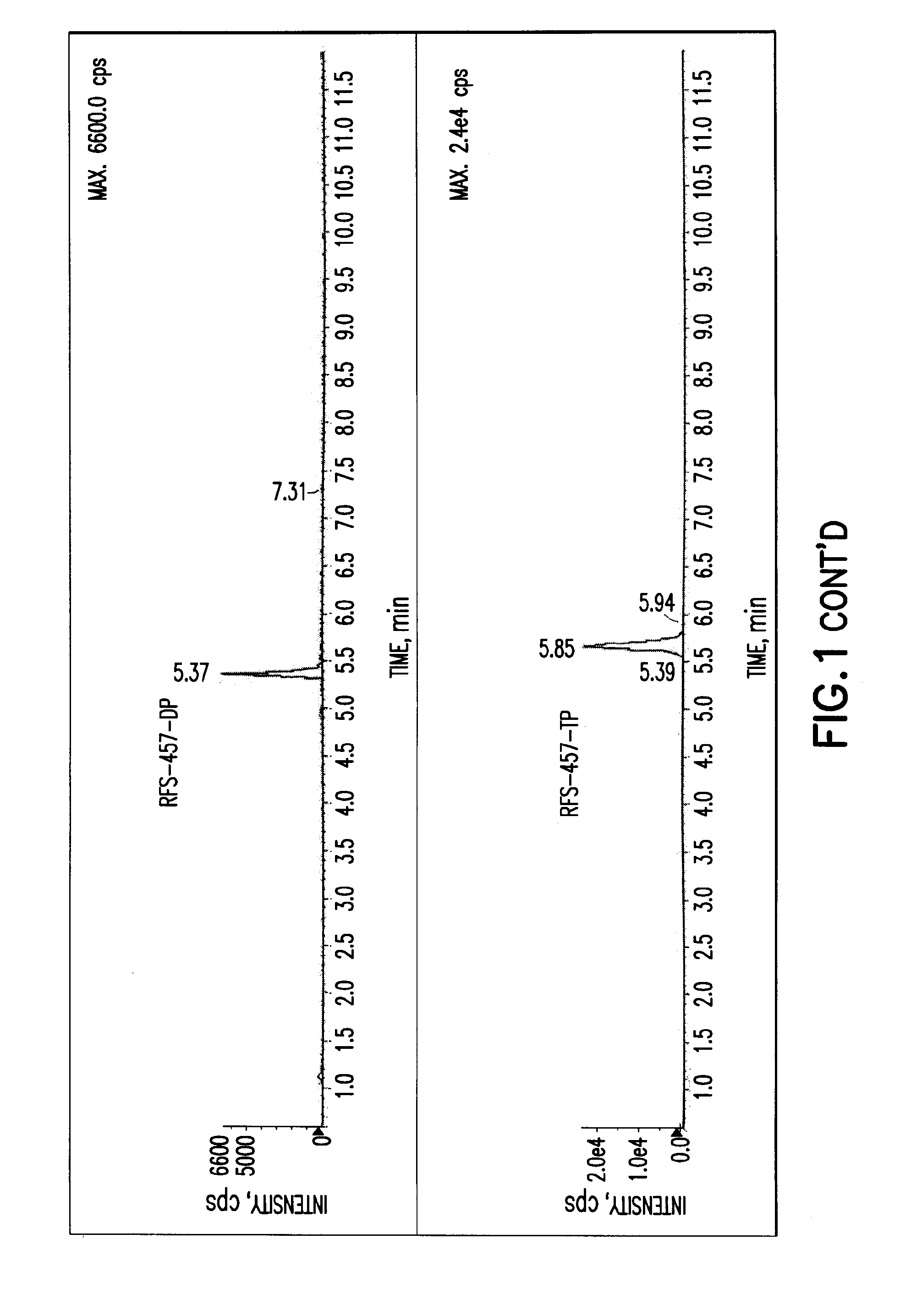
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2′-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:COCRYSTAL PHARMA INC
Pyrimidine nucleosides and their monophosphate prodrugs for treatment of viral infections and cancer
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, or HBV in human patients or other animal hosts. The compounds are certain N4-hydroxycytidine nucleosides derivatives, modified monophosphate and phosphonates prodrugs analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Nucleic acid molecules and uses thereof
ActiveUS20190125857A1Efficient inductionEfficient combinationSsRNA viruses positive-senseVirus peptidesDiseaseTGE VACCINE
The present invention is directed to an artificial nucleic acid and to polypeptides suitable for use in treatment or prophylaxis of an infection with Norovirus or a disorder related to such an infection. In particular, the present invention concerns a Norovirus vaccine. The present invention is directed to an artificial nucleic acid, polypeptides, compositions and vaccines comprising the artificial nucleic acid or the polypeptides. The invention further concerns a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial nucleic acid, polypeptides, compositions and vaccines. Further, the invention is directed to a kit, particularly to a kit of parts, comprising the artificial nucleic acid, polypeptides, compositions and vaccines.
Owner:CUREVAC SE
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Broad-spectrum antivirals against 3c or 3c-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
ActiveUS20140243341A1Preventing and inhibiting replicationBiocideSsRNA viruses positive-senseEnterovirusDisease
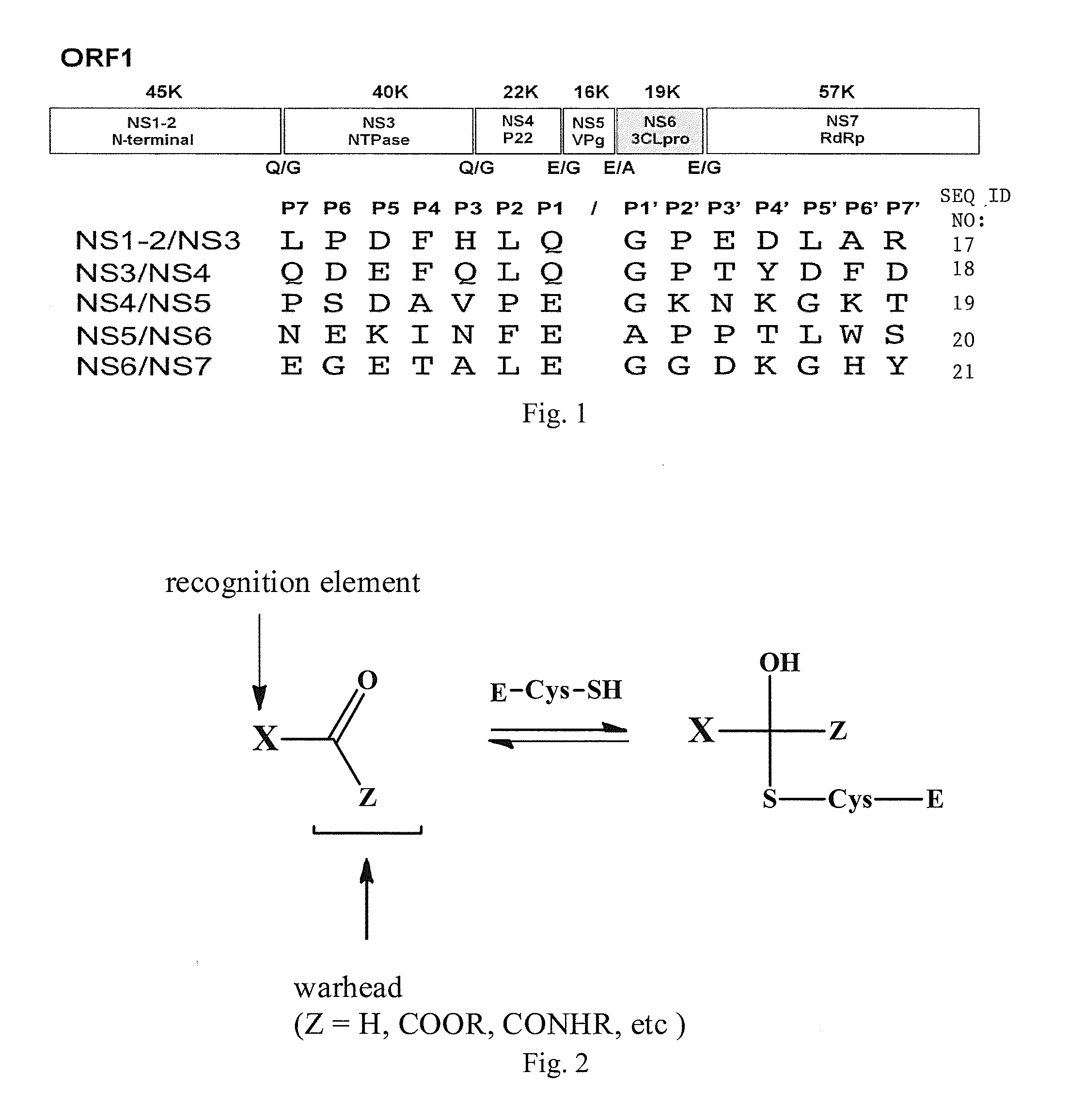
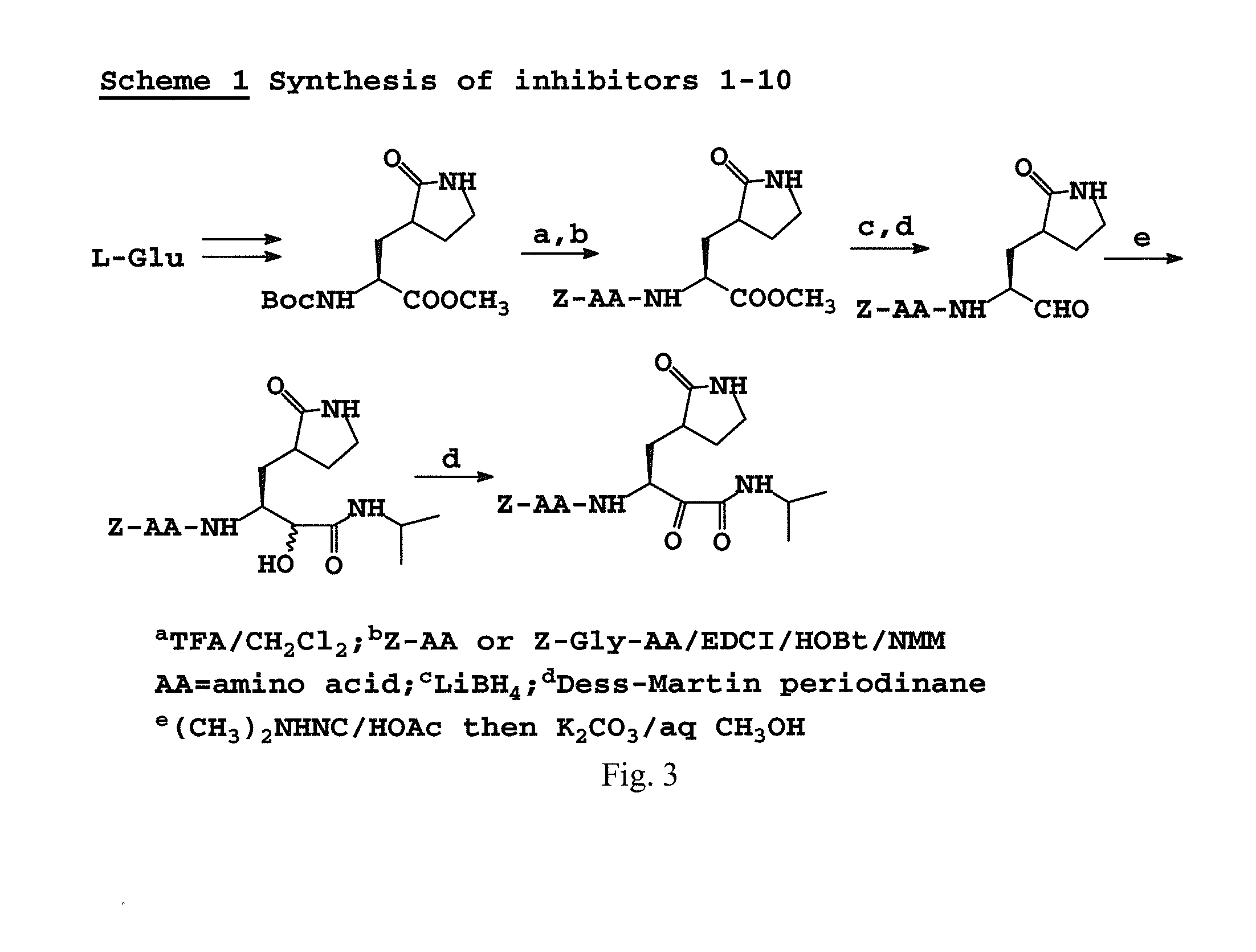
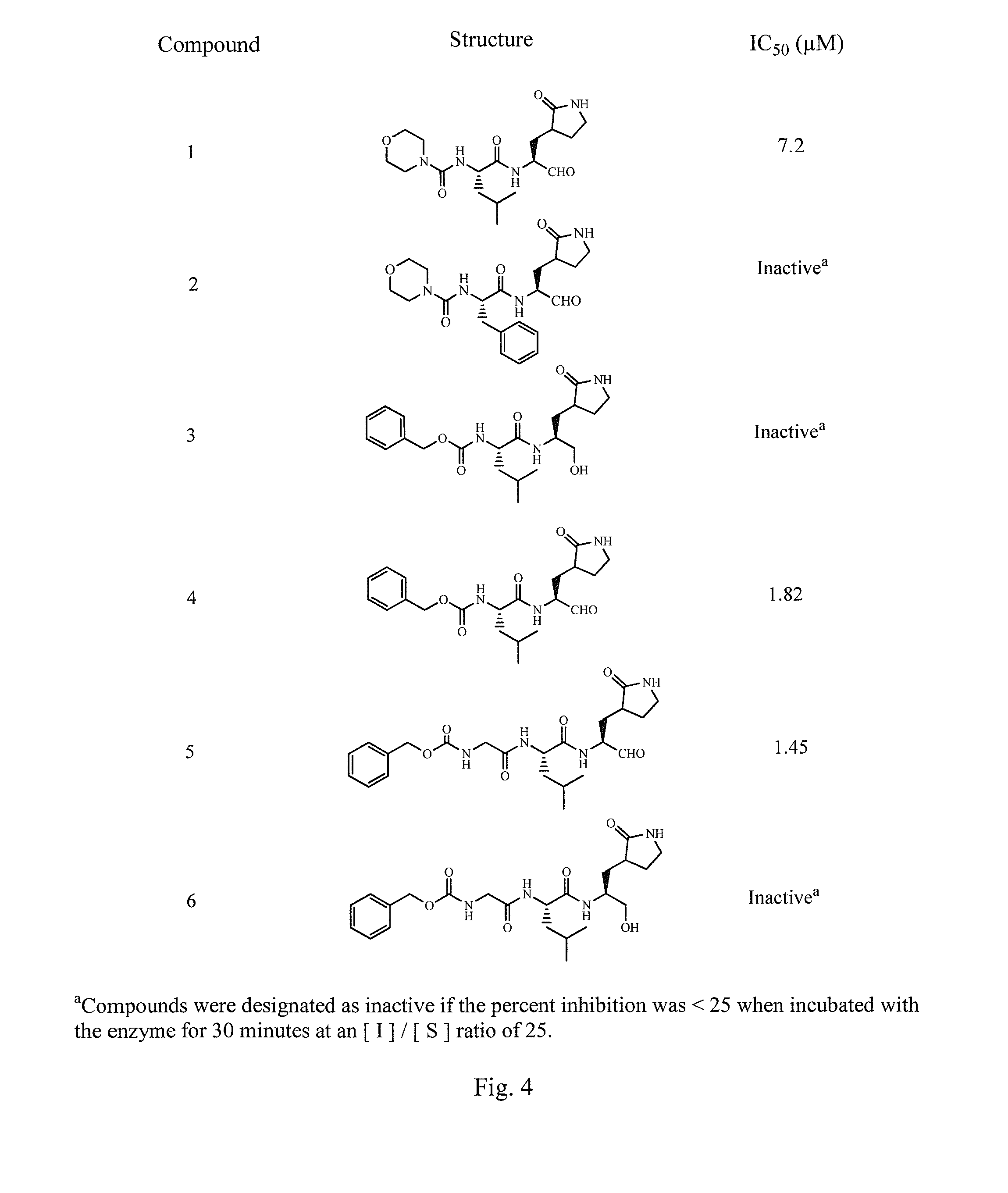
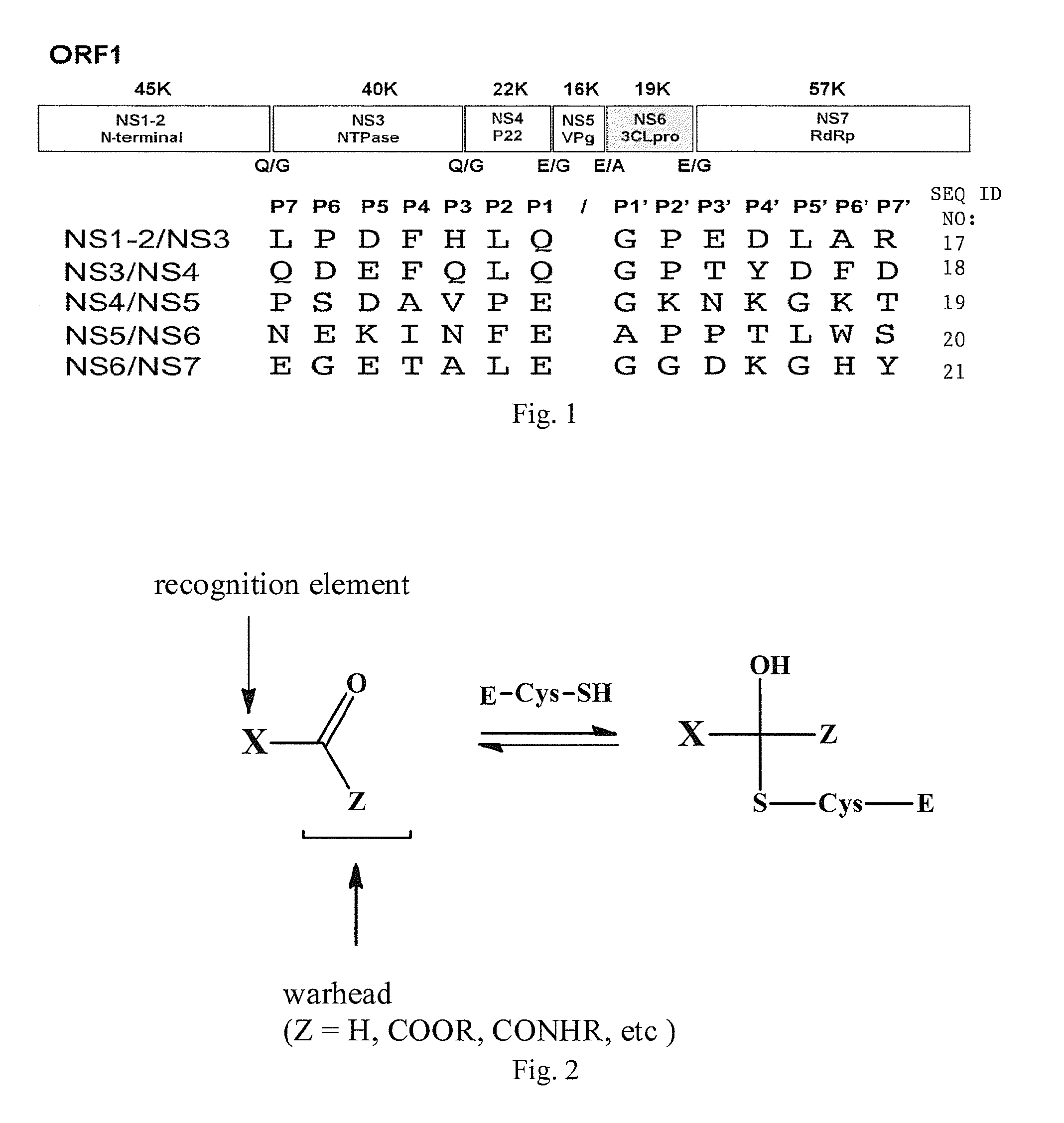
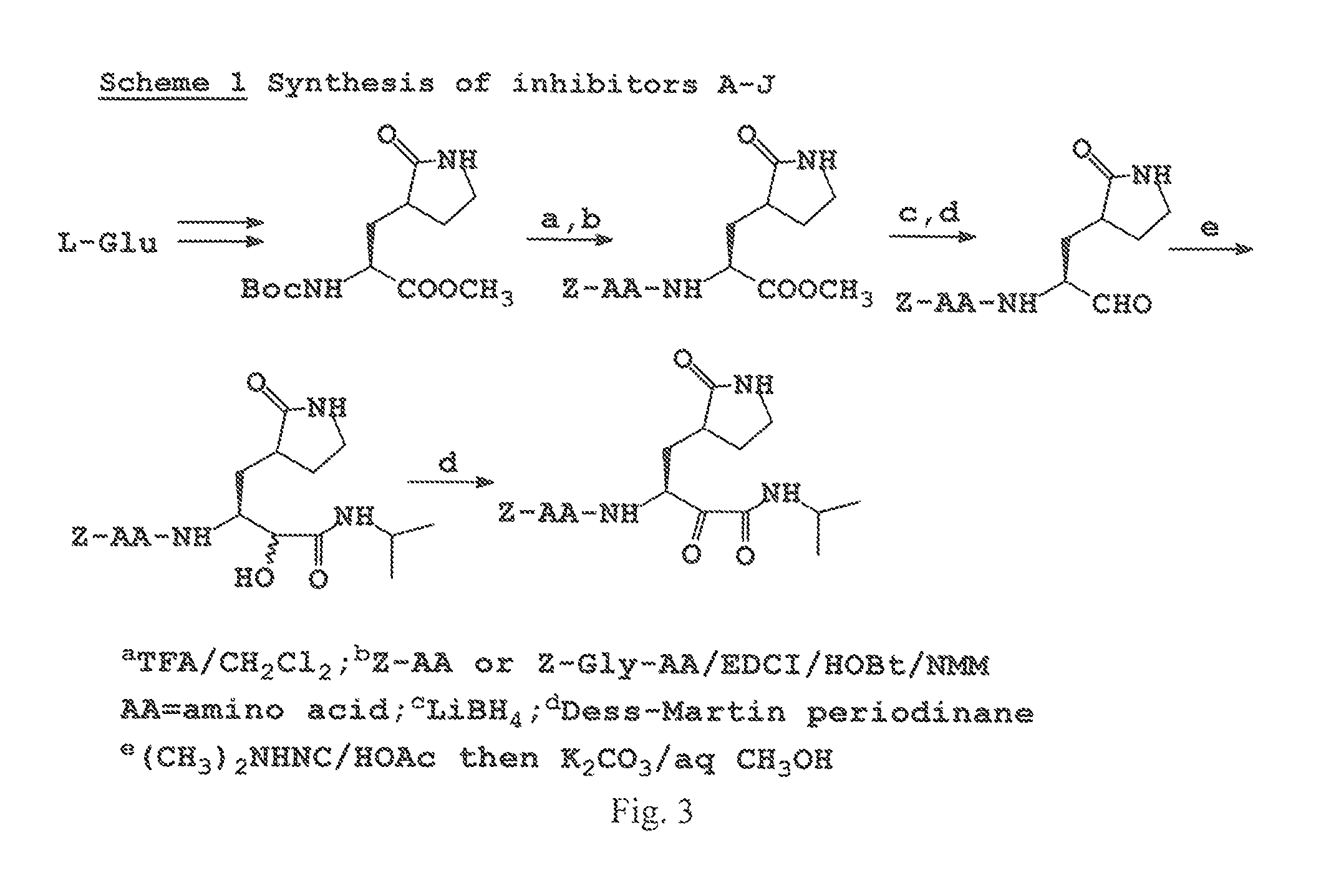
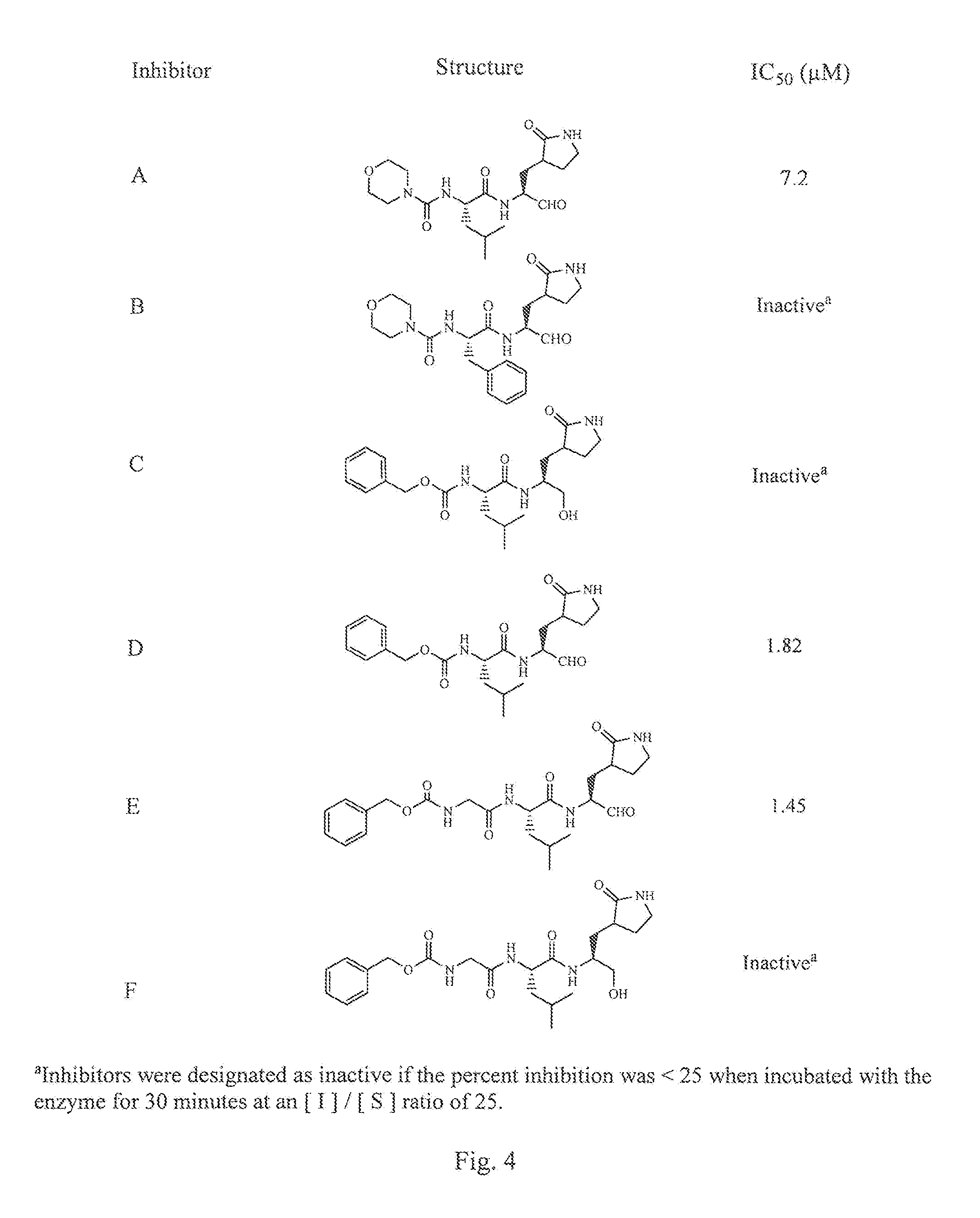
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Kit for detecting GII type norovirus and applications thereof
InactiveCN103397105AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationQuantitative determinationAquatic product
The invention relates to a kit for detecting GII type norovirus and applications thereof, concretely a primer pair and a probe for detecting the GII type norovirus, and a kit comprising the primer pair and the probe. The GII type norovirus in a sample cab be successfully detected by a recombinase polymerase amplification reaction, and can be quantified by fluorescence intensity. The kit has advantages of high sensitivity, strong singularity, short detection time, and constant and low reaction temperature, can be used for qualitative screening and quantitative determination for GII type norovirus in the sample, such as a clinic sample, foodstuff, an aquatic product, a water body, etc. accordingly, the kit can be prepared to be a diagnostic kit for GII type norovirus.
Owner:SHANGHAI OCEAN UNIV
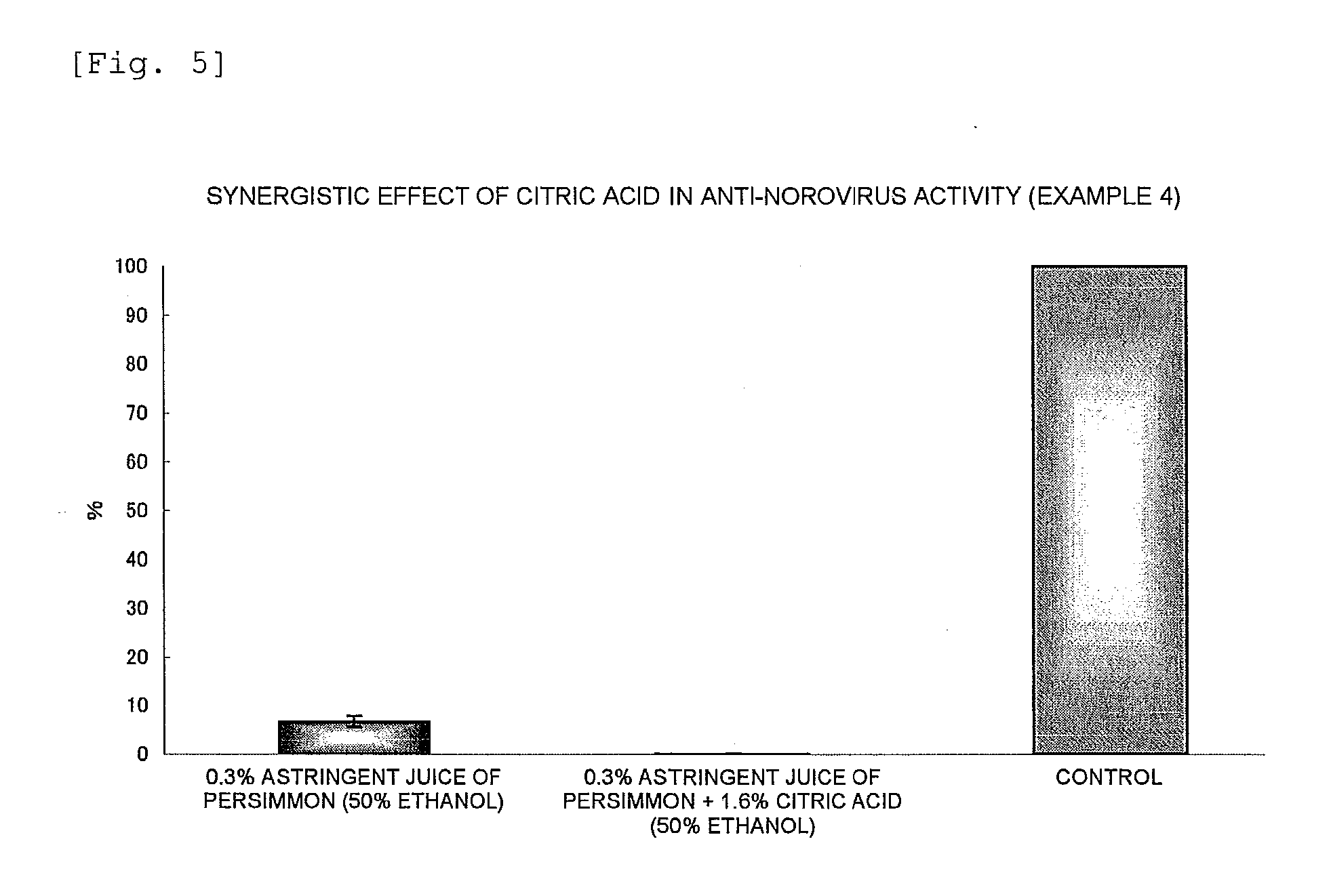
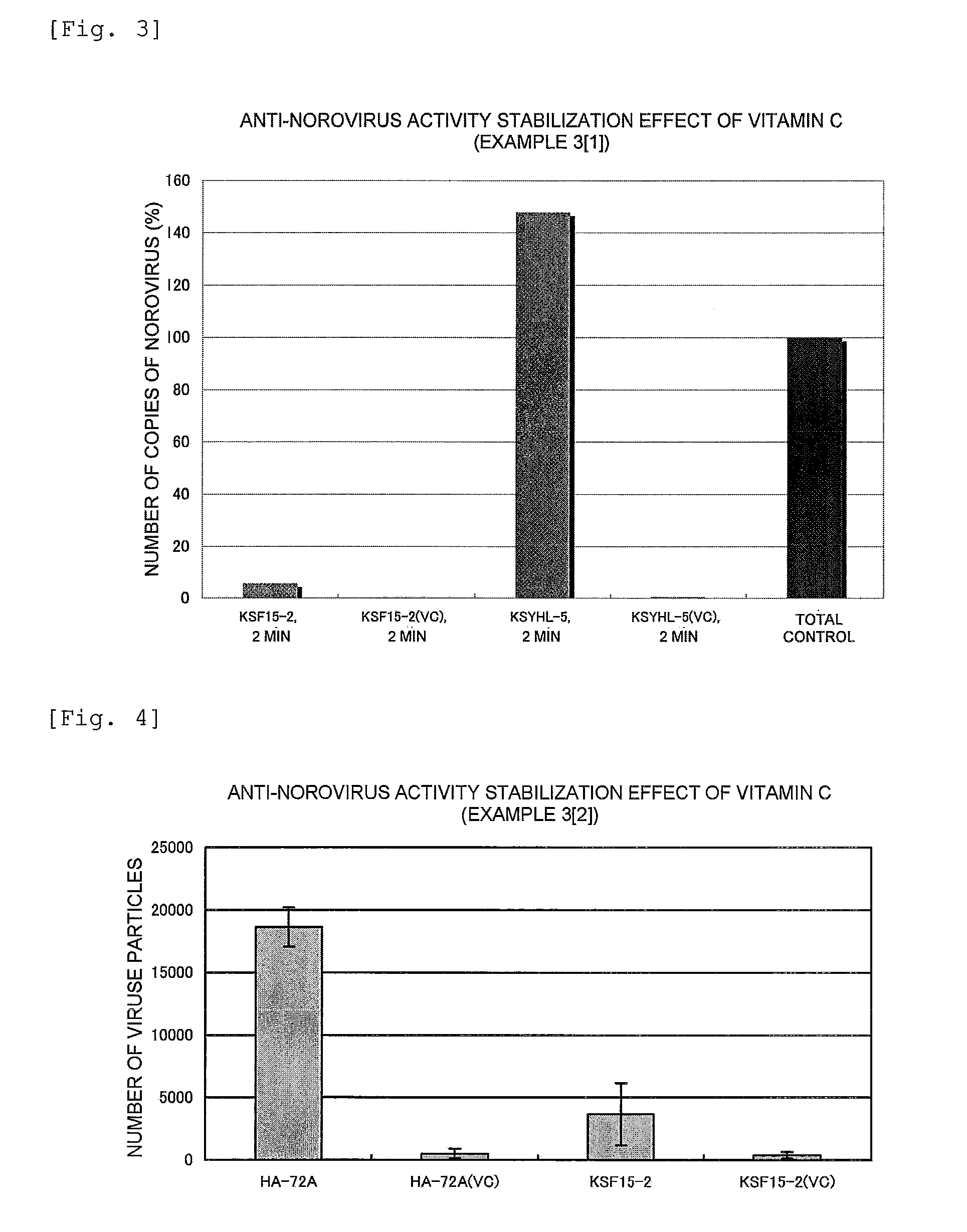
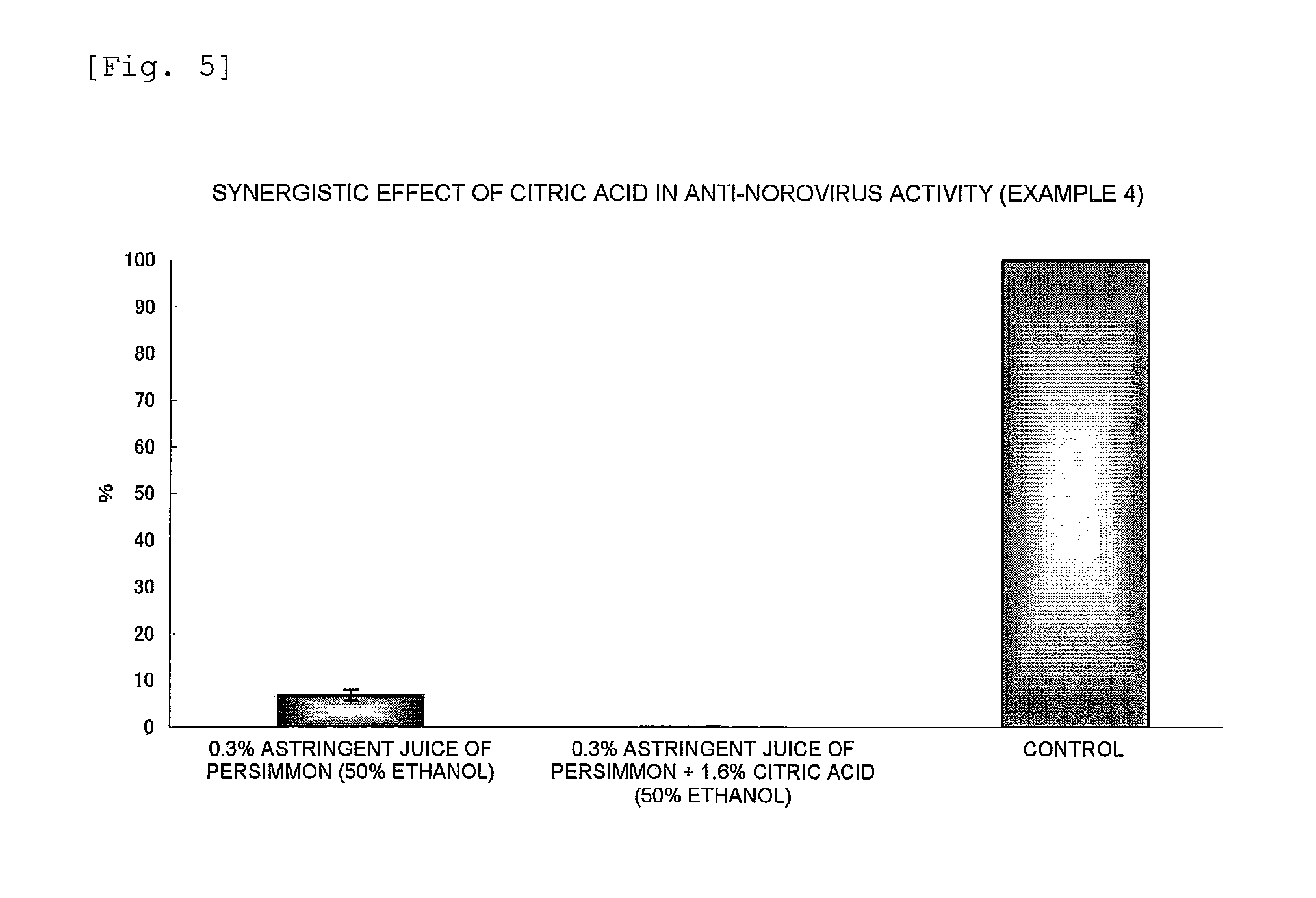
Anti-Norovirus Agent and Composition Containing the Same
ActiveUS20100240600A1Excellent anti-norovirus characteristicEffective disinfectionAntibacterial agentsBiocideFruit juiceVitamin C
Provided is an anti-norovirus agent that has high norovirus-inactivating activity and is safe for the human body, and an anti-norovirus composition that contains the anti-norovirus agent and is useful for disinfection and infection control against the norovirus. The anti-norovirus agent includes, as an active ingredient, an extract from a plant of the genus Diospyros containing tannin (hereinafter referred to as a “persimmon extract”), preferably a persimmon extract produced by heating squeezed juice or an extract from the fruit of a plant of the genus Diospyros or treating the squeezed juice or the extract with an alcohol. The anti-norovirus composition contains the anti-norovirus agent and at least one selected from the group consisting of alcohols, surfactants, antimicrobial agents, humectants, and cosmetic fats and oils, and preferably further containing an organic acid, such as citric acid, and / or a salt thereof or vitamin C.
Owner:HIROSHIMA UNIVERSITY +1
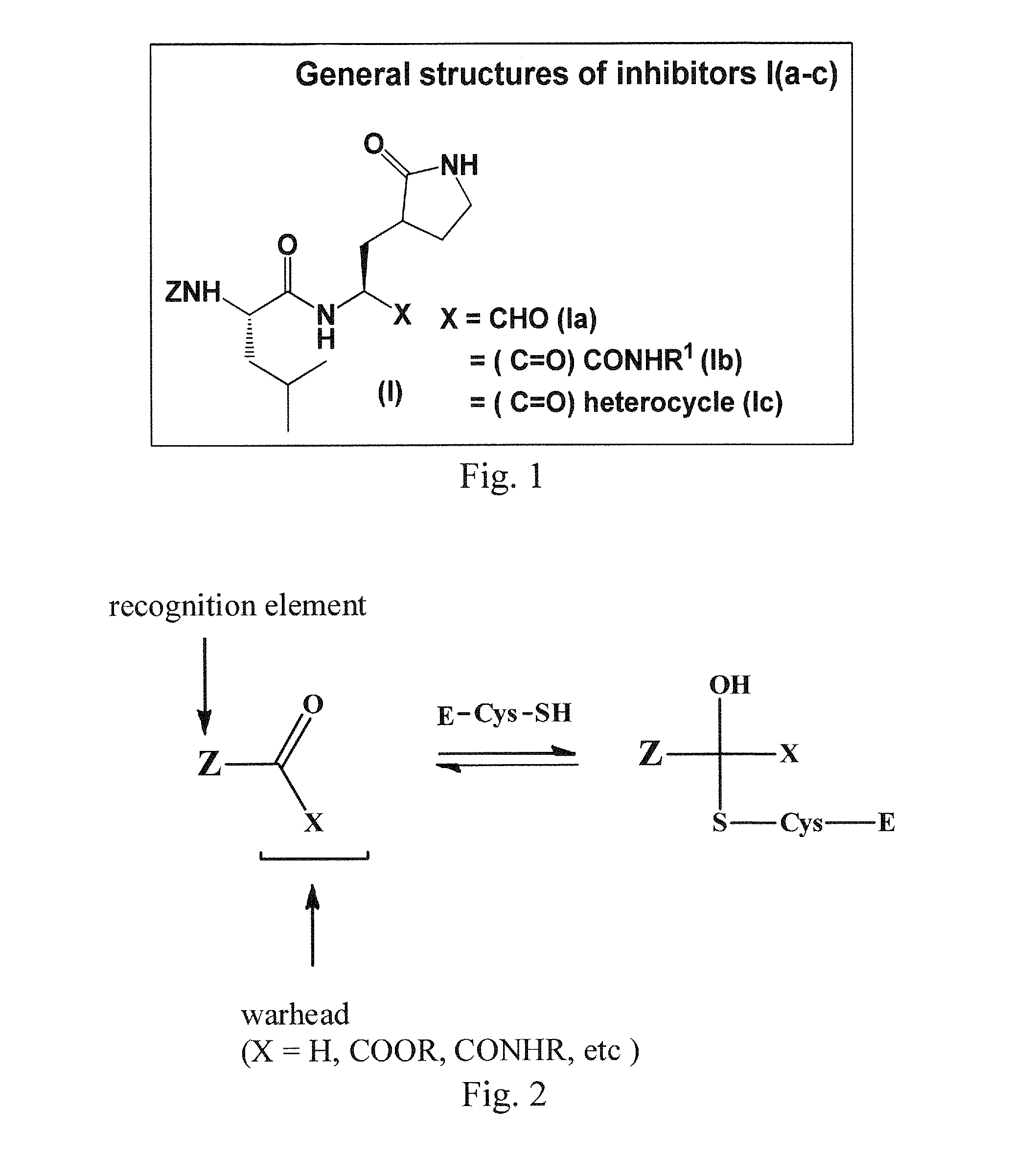
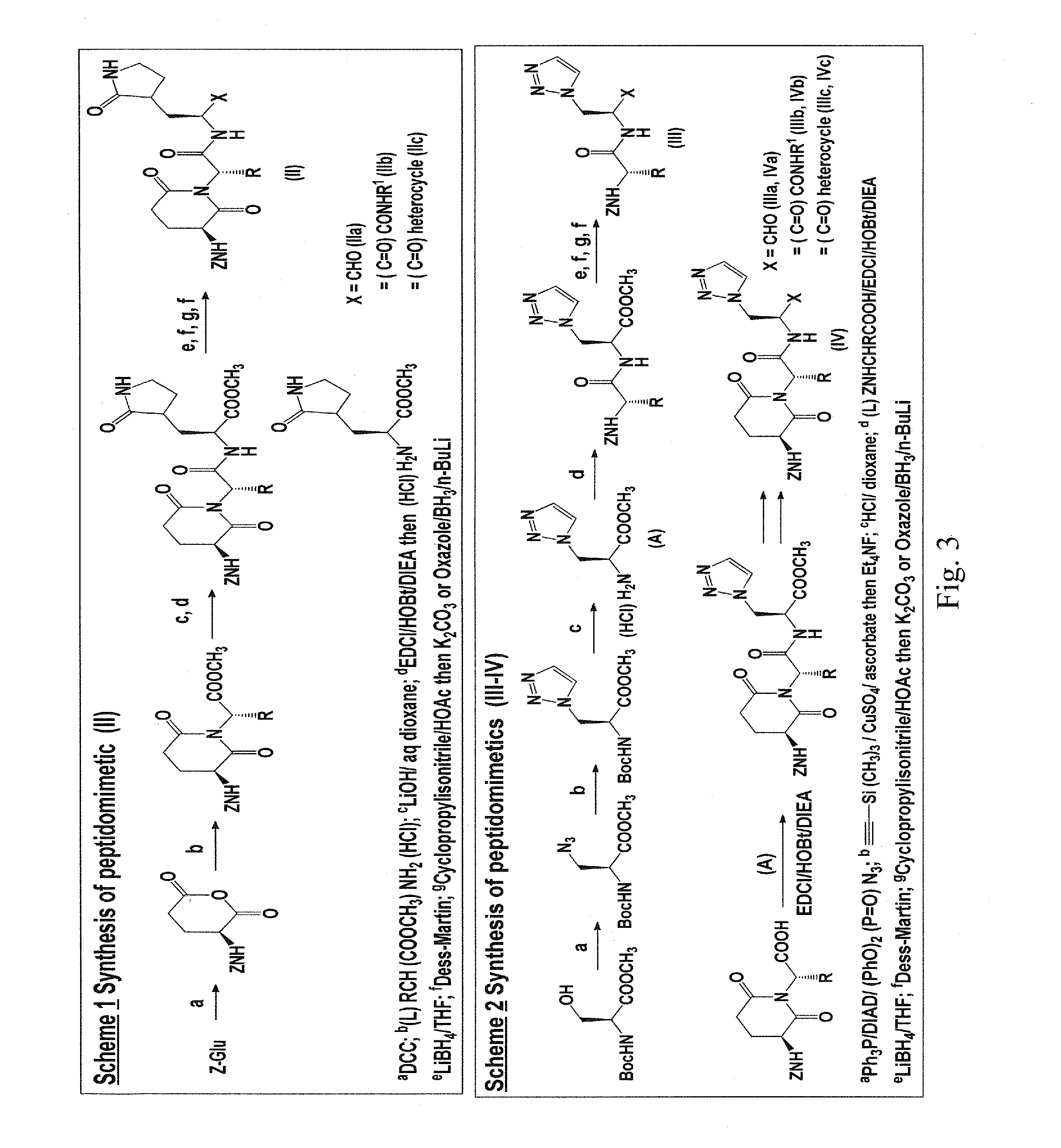
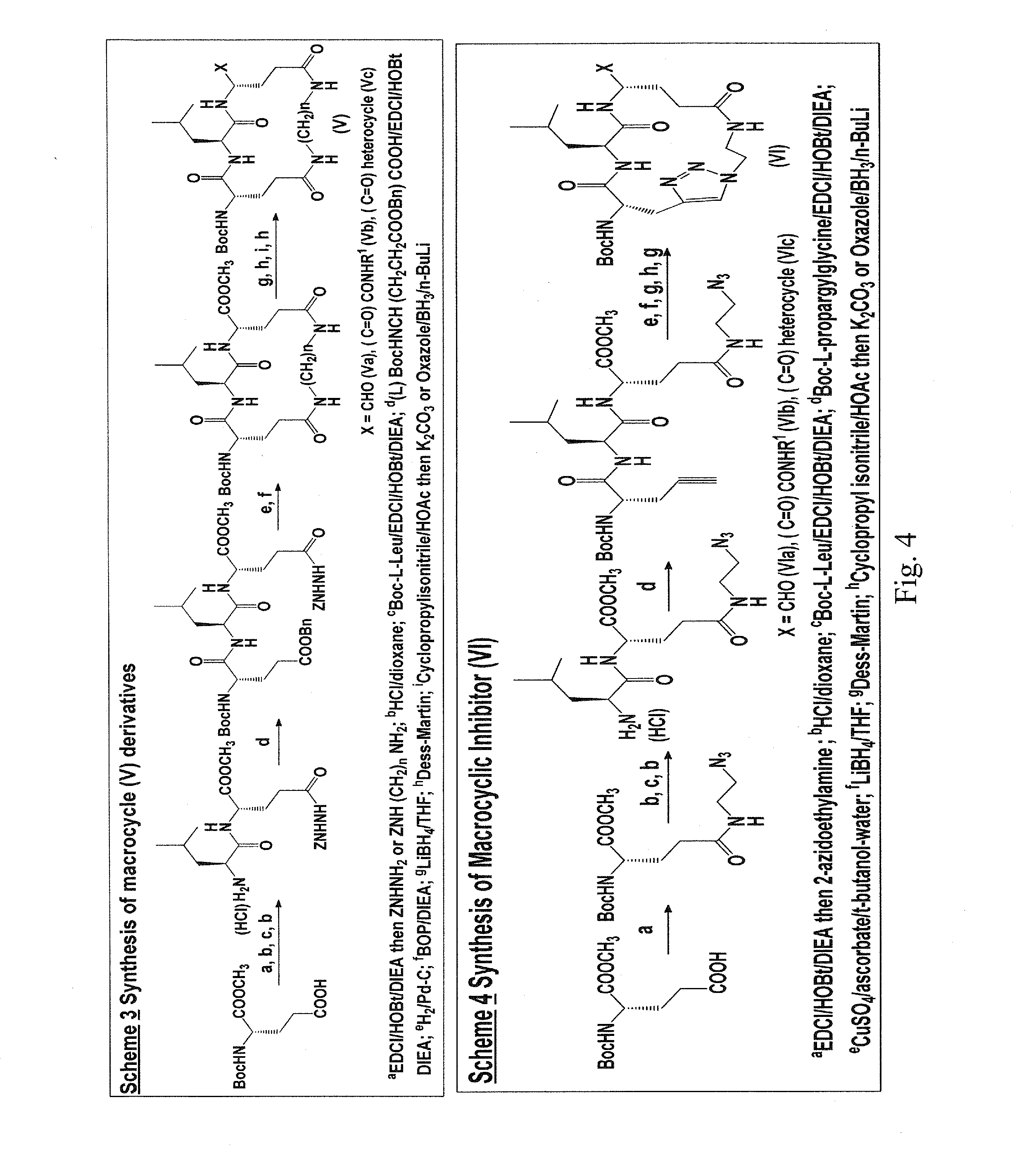
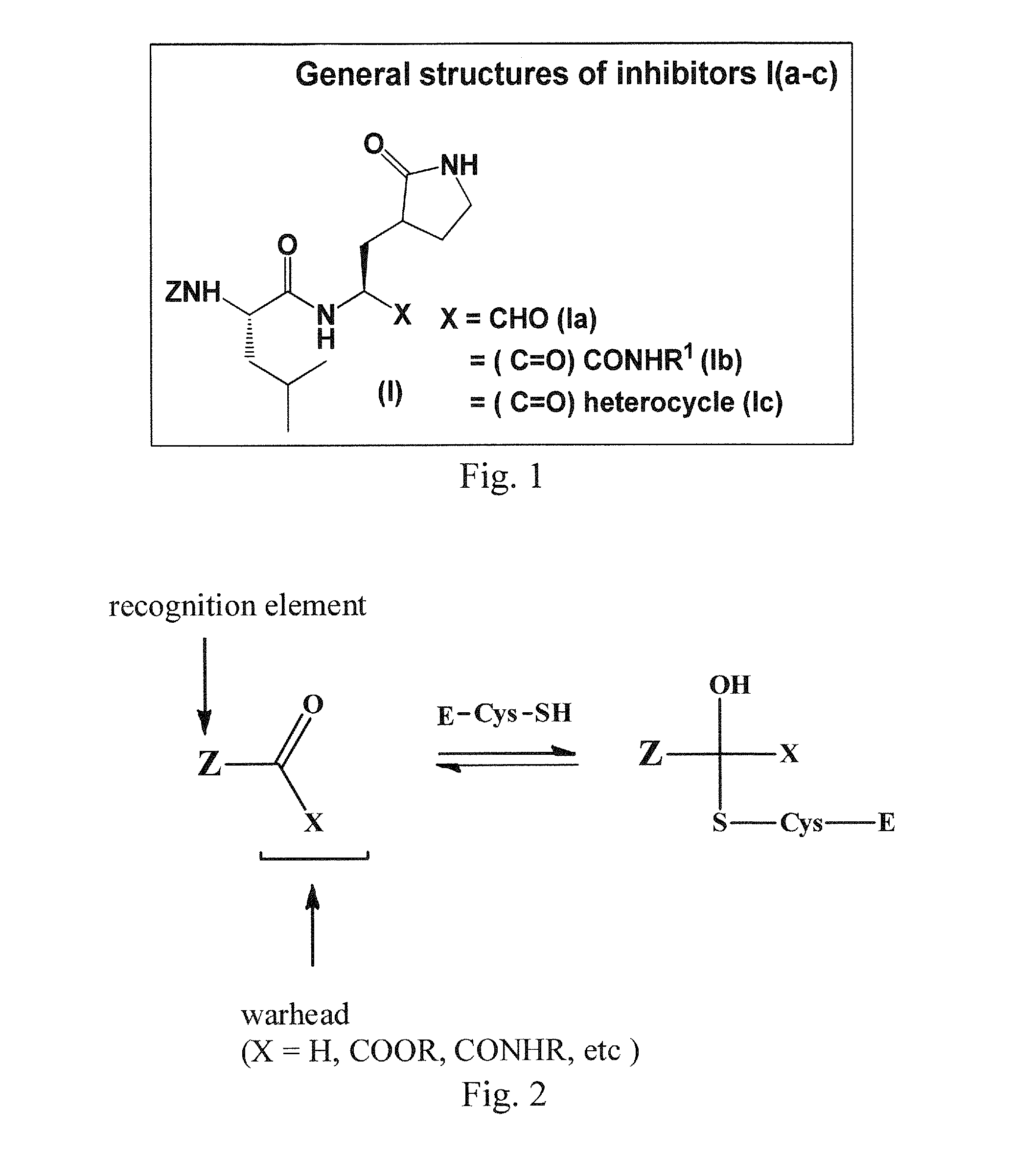
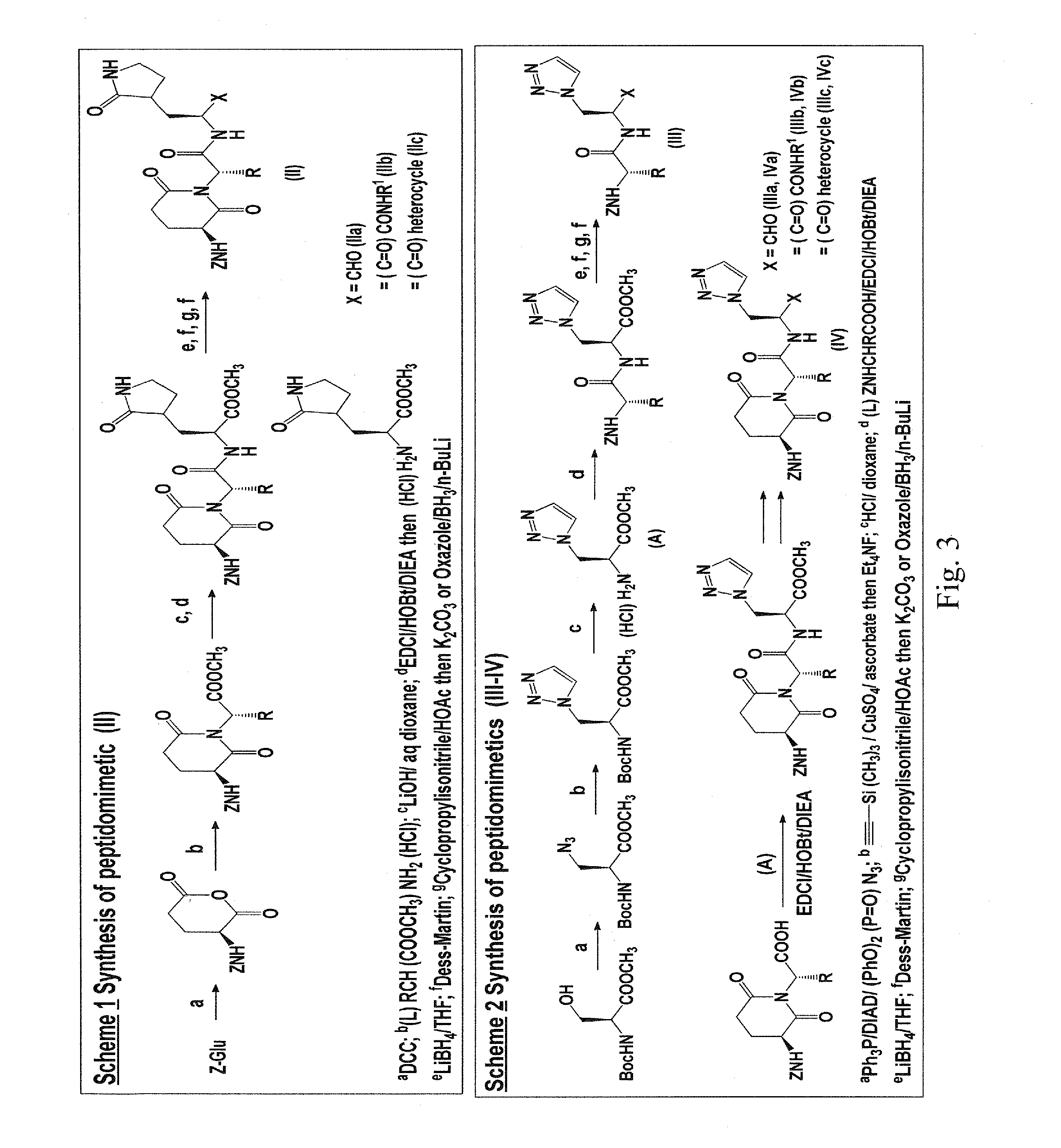
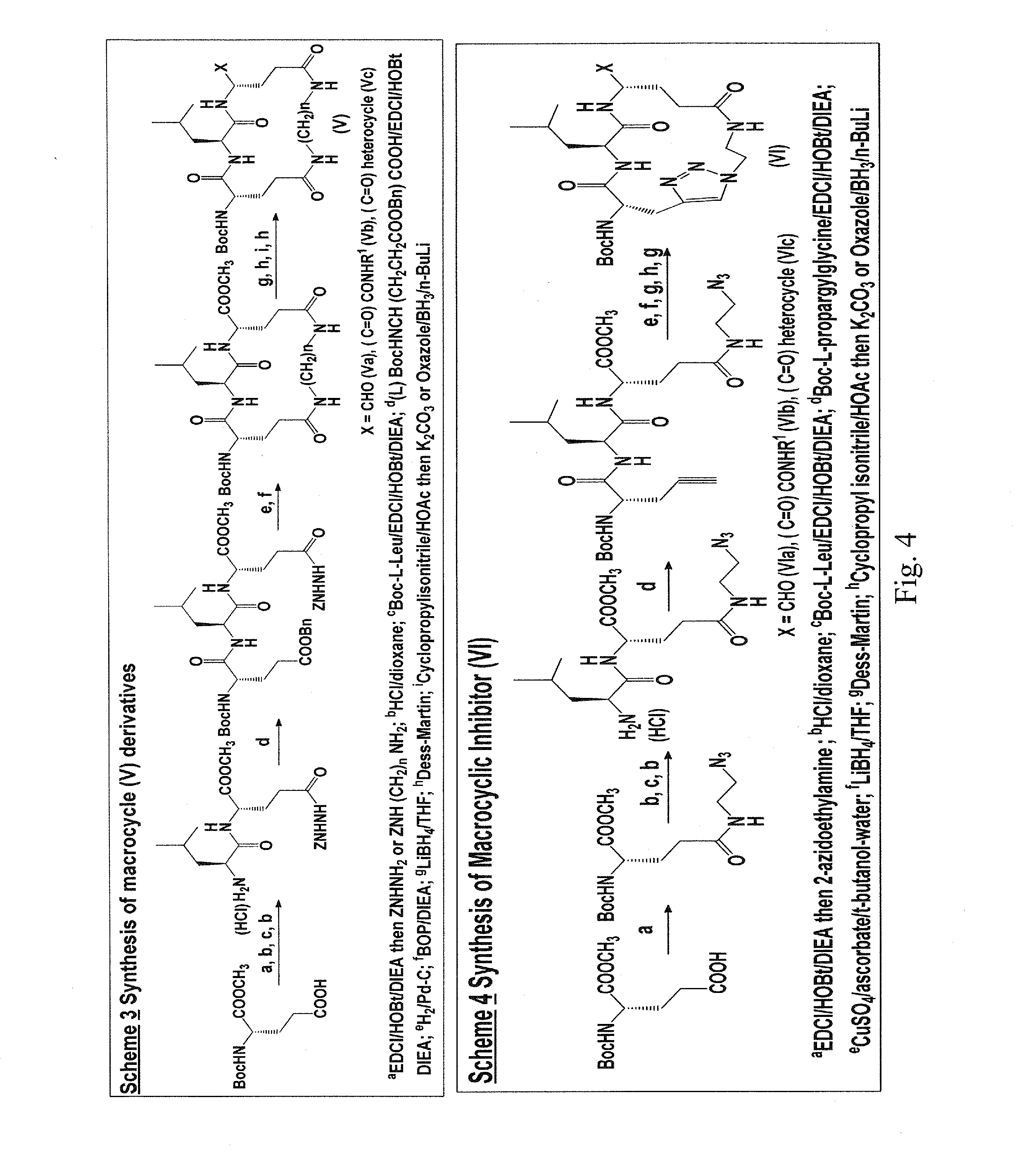
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfate salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
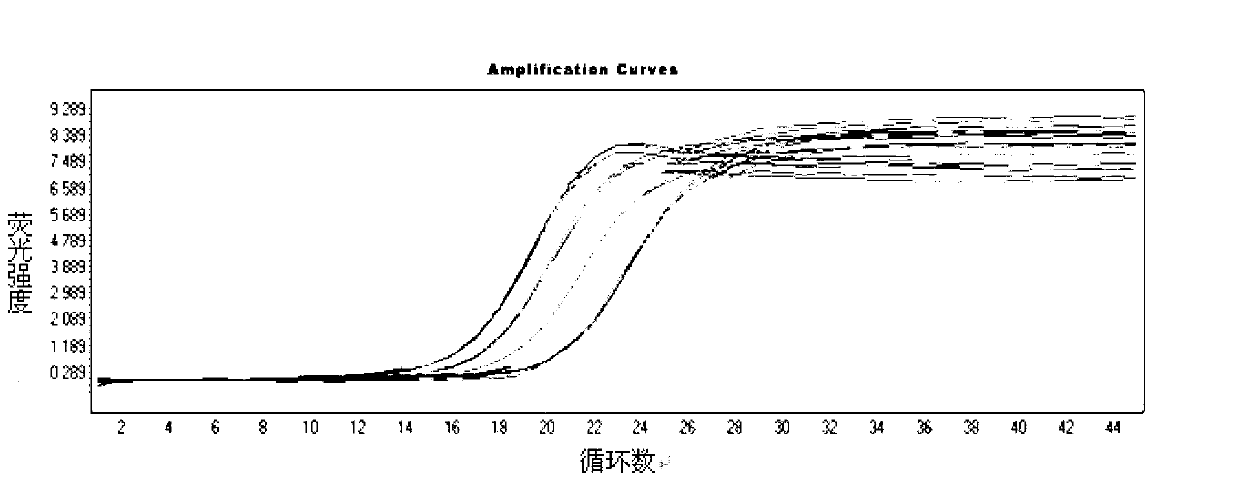
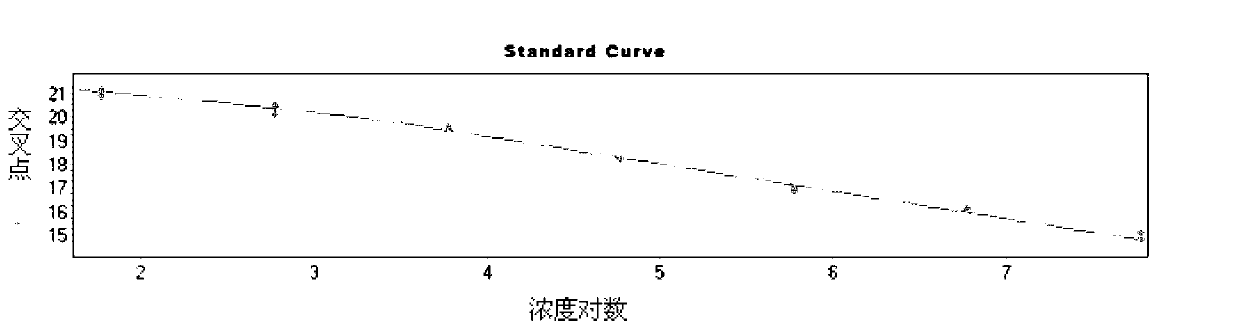
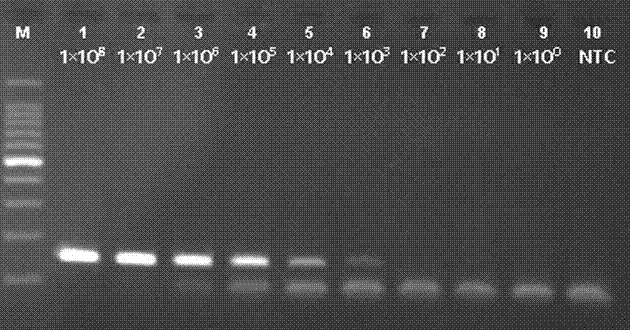
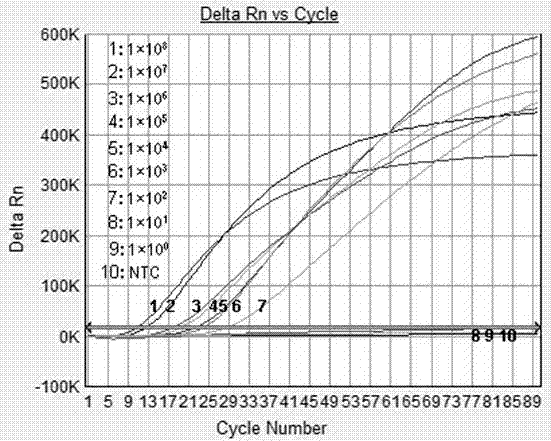
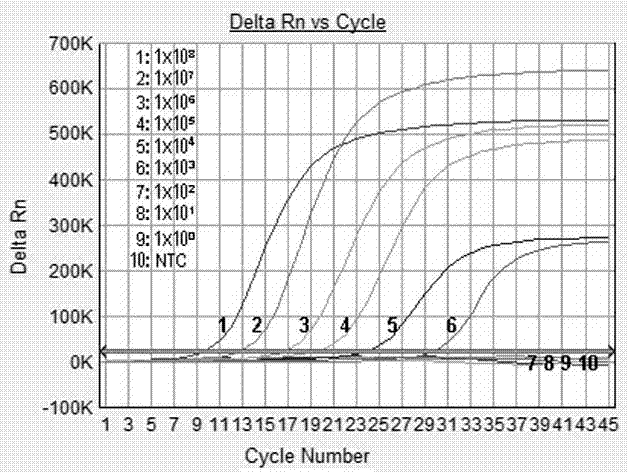
Norovirus real-time fluorescent RT-PCR detection kit and application thereof
InactiveCN103131798AEasy to judgeAvoid cross reactionMicrobiological testing/measurementMicroorganism based processesOligonucleotide primersTrue positive rate
The invention relates to a norovirus real-time fluorescent RT-PCR detection kit and an application thereof. The invention belongs to the field of gene detection. The kit provided by the invention comprises a pair of oligonucleotide primers and an oligonucleotide probe aiming at type I norovirus obtained by screening, and / or a pair of oligonucleotide primers and an oligonucleotide probe aiming at type II norovirus obtained by screening. With a one-step real-time fluorescent RT-PCR, detectable minimal concentration of type I and / or type II norovirus is 1.0*10<2>copies / mL. Therefore, sensitivity and specificity of the kit provided by the invention are both high. With the invention, rapid early-stage detection and quantitative analysis of norovirus in samples such as stools and rectal swabs are realized. With the kit provided by the invention, detection period is short, detection efficiency is high, virus detection specificity is high, accuracy is high, and virus qualitative analysis and quantitative analysis can be carried out simultaneously. The sensitivity of the kit is higher than common PCR and immunological detection methods. The operation is simple, and the kit is easy to popularize. Experiment result repeatability is good.
Owner:湖北朗德医疗科技有限公司
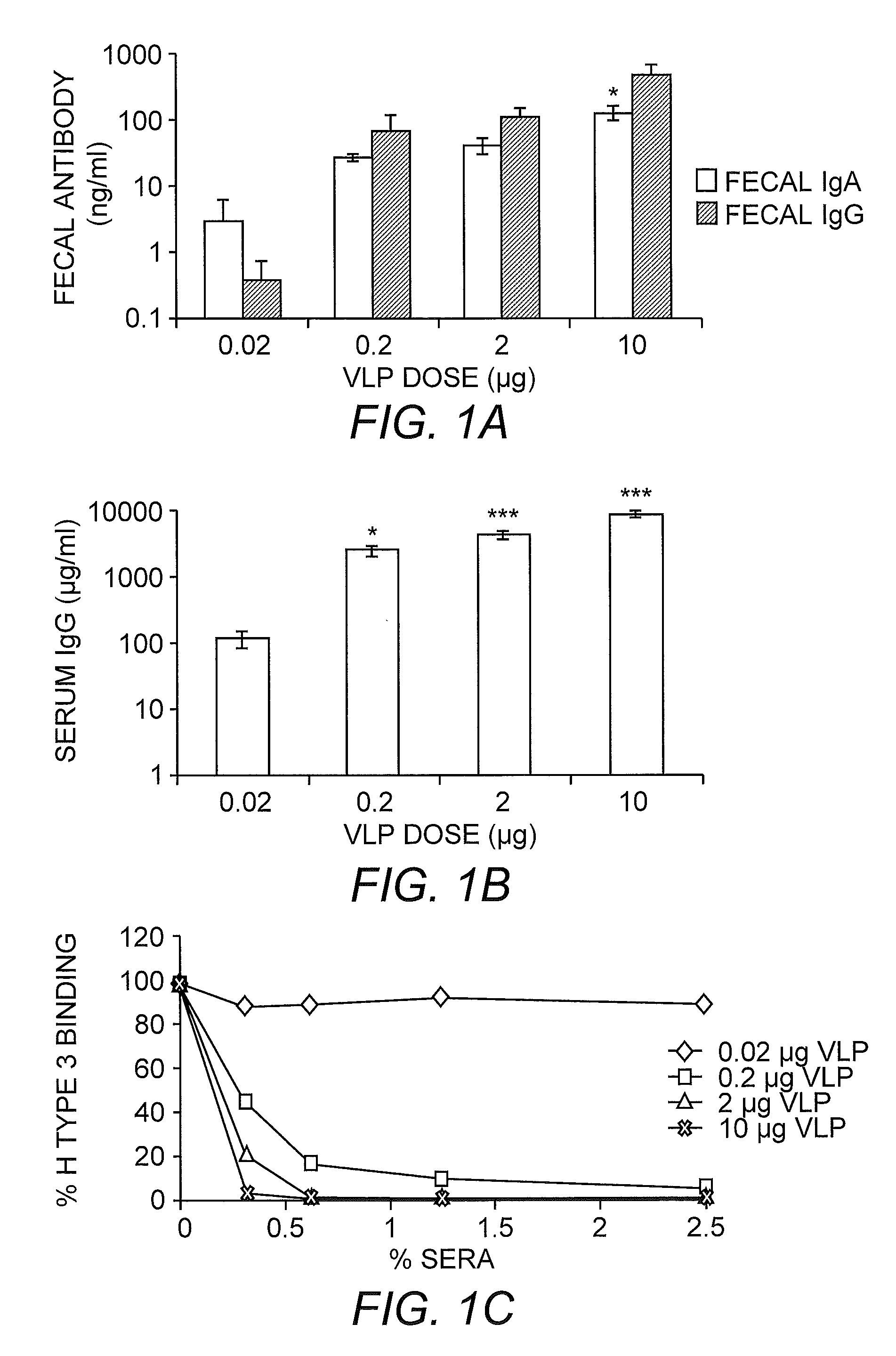
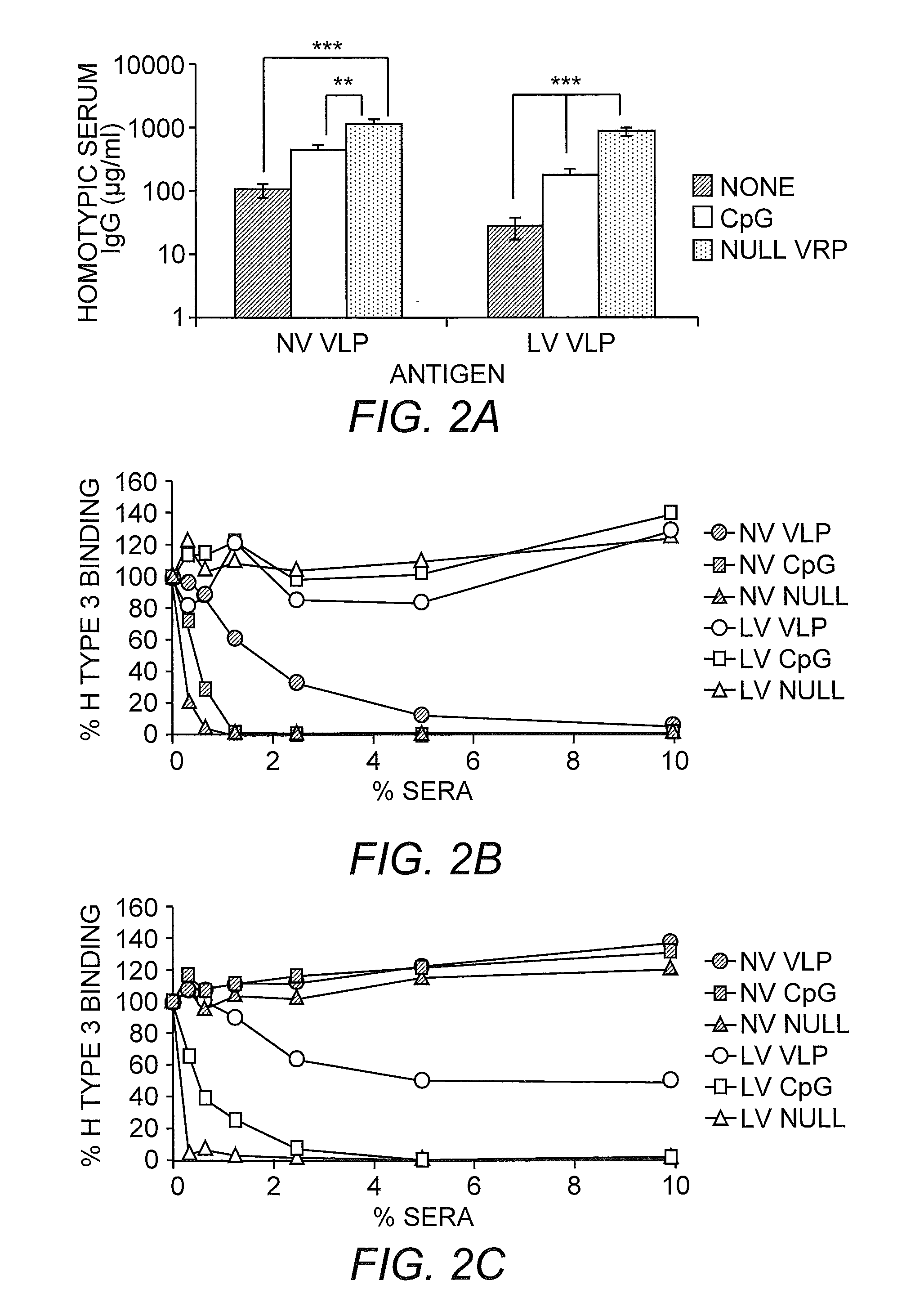
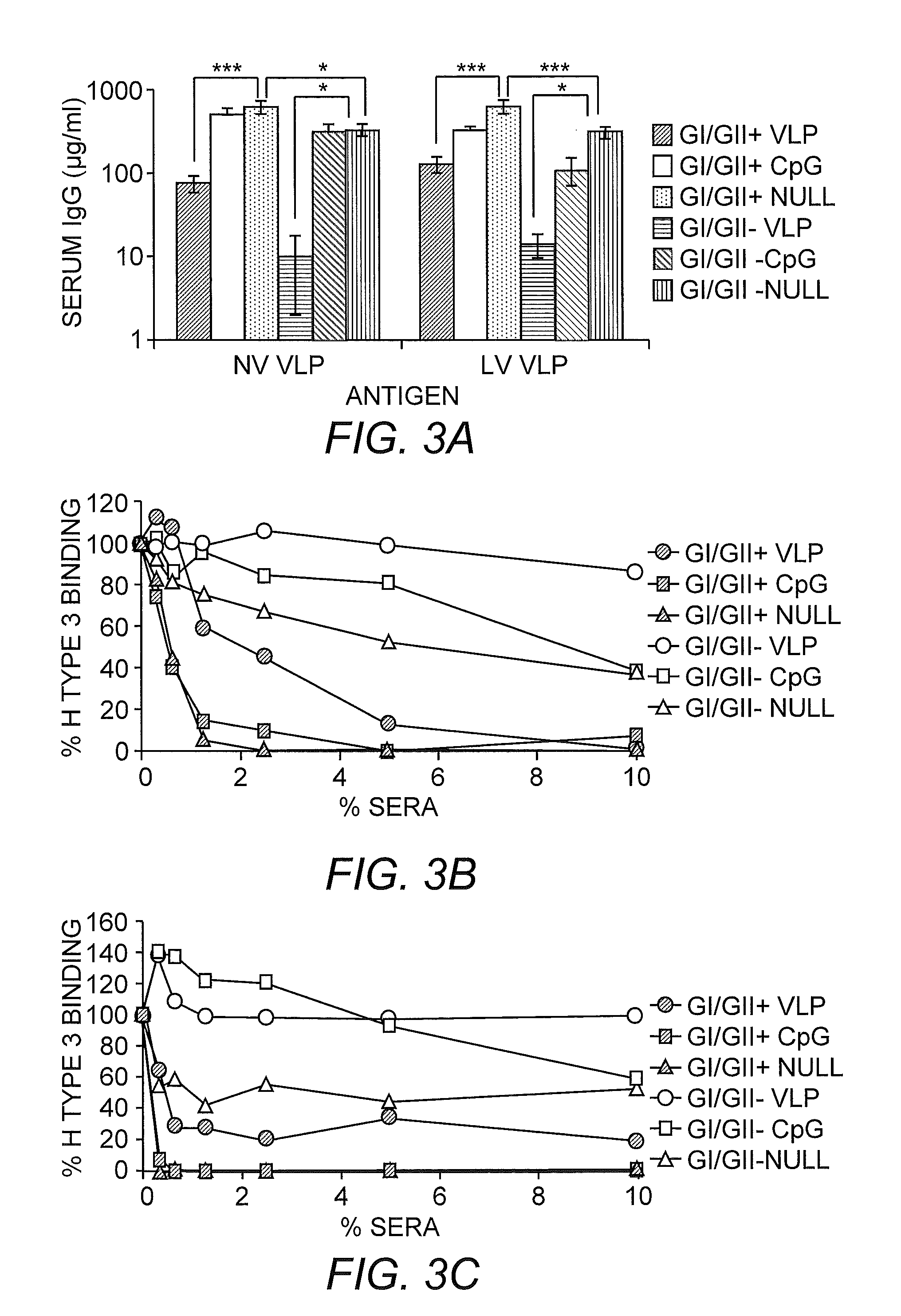
Multivalent Immunogenic Compositions Against Noroviruses and Methods of Use
InactiveUS20110070260A1Effective amountSsRNA viruses positive-senseViral antigen ingredientsAdjuvantVirus-like particle
The invention provides immunogenic formulations comprising virus-like particles (VLPs) from two or more genoclusters and / or strains of norovirus in a pharmaceutically acceptable carrier. In representative embodiments, the formulation also comprises an adjuvant, for example, a viral adjuvant or CpG. The invention also provides methods of inducing an immune response to one or more noroviruses.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Primer, probe and kit for detecting rotavirus and Norovirus liquid phase chips
InactiveCN102154528AAccurate detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationRotavirus RNAFluorescence
The invention provides a primer, a probe and a kit for detecting rotavirus and Norovirus liquid phase chips. The primer comprises three pairs of primers of a specific amplification A type rotavirus sequence, a GI type Norovirus sequence and a GII type Norovirus sequence. The probe comprises three specificity detection probes of A type rotavirus, GI type Norovirus and GII type Norovirus. The kit comprises the three pair of primers and a specificity detection microsphere mixture prepared by coupling the three specificity detection probes and a fluorescence coding microsphere. The experiment proves that the primer and the probe disclosed by the invention are characterized in that a method of combining multiple PCR (polymerase chain reaction) with liquid phase chip detection can simultaneously and accurately detect the A type rotavirus, the GI type Norovirus and the GII type Norovirus. The primer, the probe and the kit have the advantages of strong specificity and high sensitivity.
Owner:何雅青 +2
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:THE WICHITA STATE UNIV +1
Acid/anionic antimicrobial and virucidal compositions and uses thereof
ActiveUS20190090483A1Dilutable and non-flammable and no-rinse efficacyDilutable, non-flammable, no-rinseBiocideAnimal repellantsPersonal protective equipmentFood contact
Antimicrobial compositions including at least one acid and at least one anionic surfactant are provided. In particular, food contact antimicrobial compositions including at least one acid and at least one anionic surfactant provide a no-rinse compositions efficacious against Norovirus, having acceptable use solution pH that do not require use of personal protective equipment (PPE), are surface compatible and do not leave residues on treated surfaces are provided. Methods of cleaning a surface with the compositions are also provided.
Owner:ECOLAB USA INC
Method for detecting norovirus
ActiveCN113155924AImprove conductivityEffective combinationMaterial electrochemical variablesBlack phosphorusEngineering
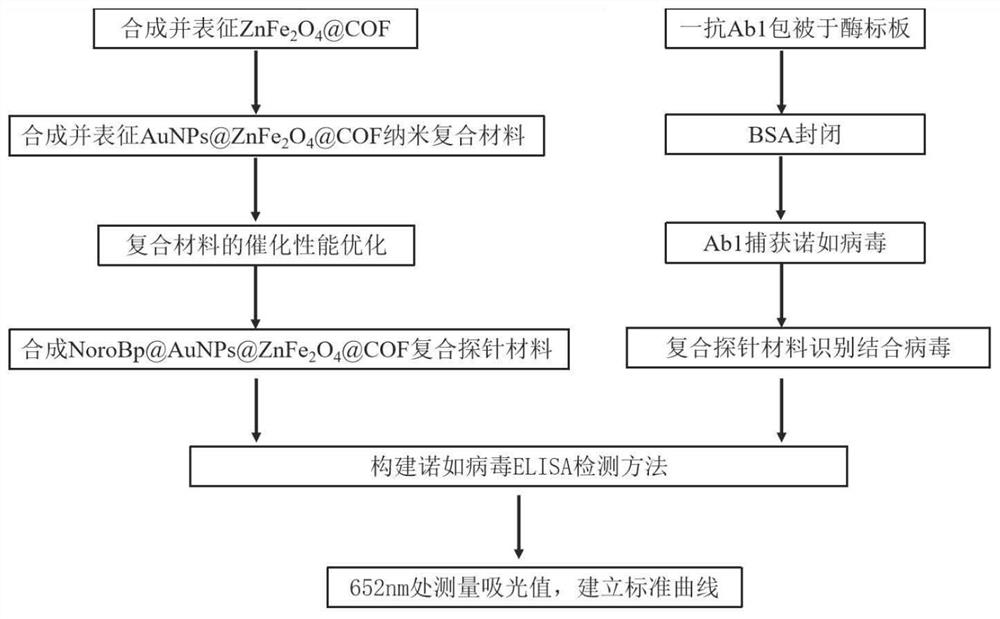
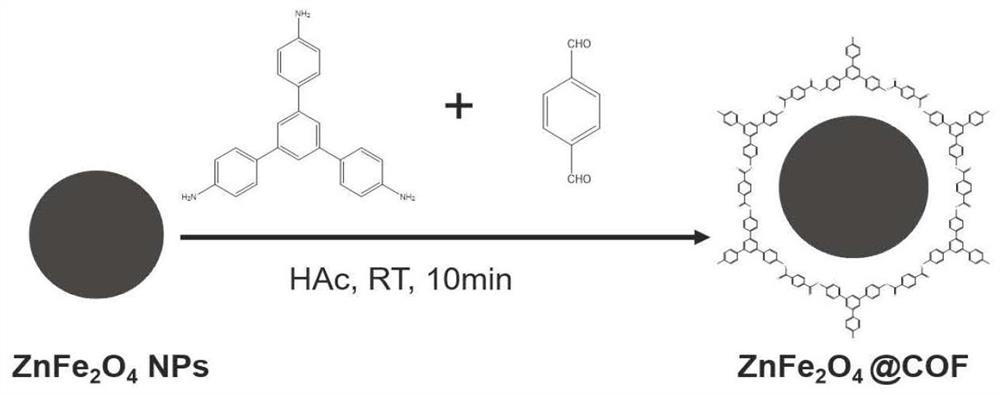
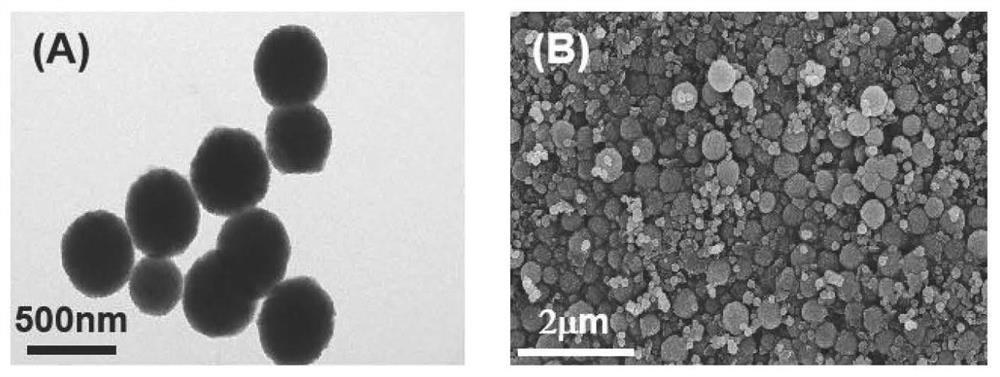
The invention relates to a method for detecting norovirus. According to the method, three substances, namely AuNPs@ZnFe2O4@COF, Apt@AuNPs@ZnFe2O4@COF and AuNPs@BP@Ti3C2MXene, are utilized. The method comprises the following steps: 1) synthesizing ZnFe2O4@COF; synthesizing AuNPs@ZnFe2O4@COF; and synthesizing apt@AuNPs@ZnFe2O4@COF; 2) stripping black phosphorus BP; synthesizing BP@Ti < 3 > C < 2 > MXene; synthesizing AuNPs@BP@Ti < 3 > C < 2 > MXene; immobilizing the polypeptide (NoroBP); and 3) constructing the norovirus electrochemical sensor. The invention constructs an electrochemical sensor capable of rapidly and effectively detecting the concentration of norovirus in a sample.
Owner:YUNNAN UNIV
Primer and method for detecting GI type and GII type norovirus by utilizing primer
InactiveCN102304514AEasy to operateShorten detection timeMicrobiological testing/measurementMicroorganism based processesReverse transcription polymerase chain reactionBiology
The invention relates to a primer and a method for detecting GI type and GII type norovirus by utilizing the primer, belonging to the technical field of biological detection. The sequence of the primer is as follows: Nov-F: 5-GTGAATGAWGATGGCGTCKA-3, Nov-R: 5-GTHAGWATCCAGGGGTCAAT-3. The invention focuses on the design of the sequence of the primer, the composition of an RT-PCR (reverse transcription-polymerase chain reaction) system, the selection of reaction conditions and the judgment of reaction results can be performed according to the conventional methods in the field. By adopting the primer and the method provided by the invention, a pair of primers can simultaneously detect and differentiate the GI type and the GII type norovirus in a same reaction tube, thereby not only simplifying the operation steps, but also shortening the detection time.
Owner:湖州市疾病预防控制中心
Magnetic nano-enzyme material with peroxidase catalytic activity and kit for detecting norovirus and application thereof
ActiveCN113333024AEasy to operateGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsBiological material analysisPeroxidaseMedicine
The invention provides a magnetic nano-enzyme material with peroxidase catalytic activity and a kit for detecting norovirus and an application thereof, and belongs to the technical field of virus detection. The magnetic nano-enzyme material with peroxidase catalytic activity is prepared from ZnFe2O4@ COF and gold nanoparticle raw materials. The invention also provides a magnetic nano-composite enzyme for specifically detecting the norovirus. The magnetic nano-composite enzyme is formed by combining a specific norovirus recognition biological material on gold nanoparticles of the magnetic nano-enzyme material, can specifically recognize and combine the norovirus, and has efficient horseradish peroxidase catalytic activity; and a horseradish peroxidase-labeled secondary antibody in traditional ELISA detection can be completely replaced, and detection of norovirus under non-physiological conditions is realized. The detection kit provided by the invention has the advantages of convenience in detection operation, low cost, high detection throughput and higher use value.
Owner:YUNNAN UNIV
Parenteral norovirus vaccine formulations
ActiveUS20160000899A1SsRNA viruses positive-senseViral antigen ingredientsVirus-like particleProtection sex
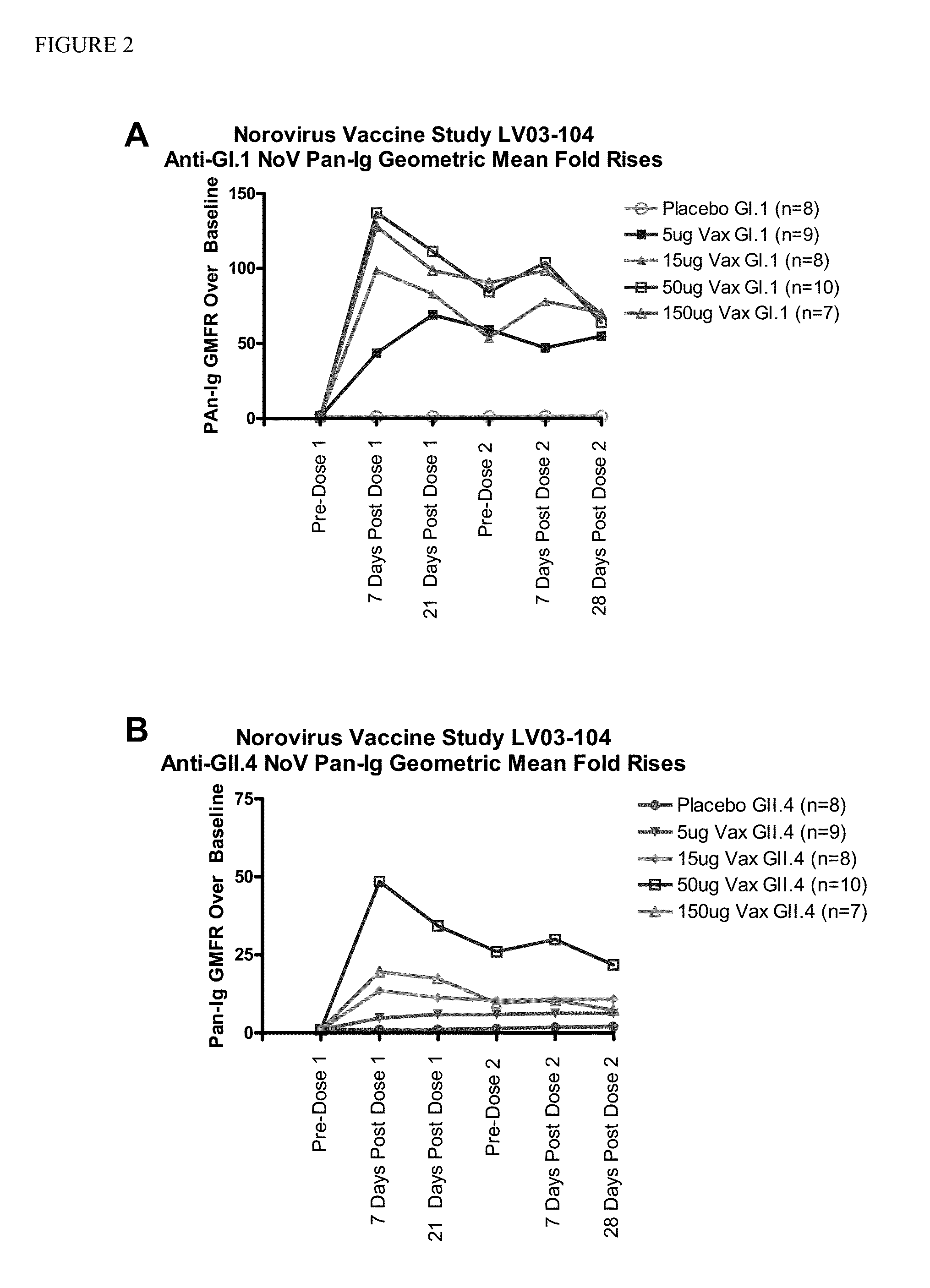
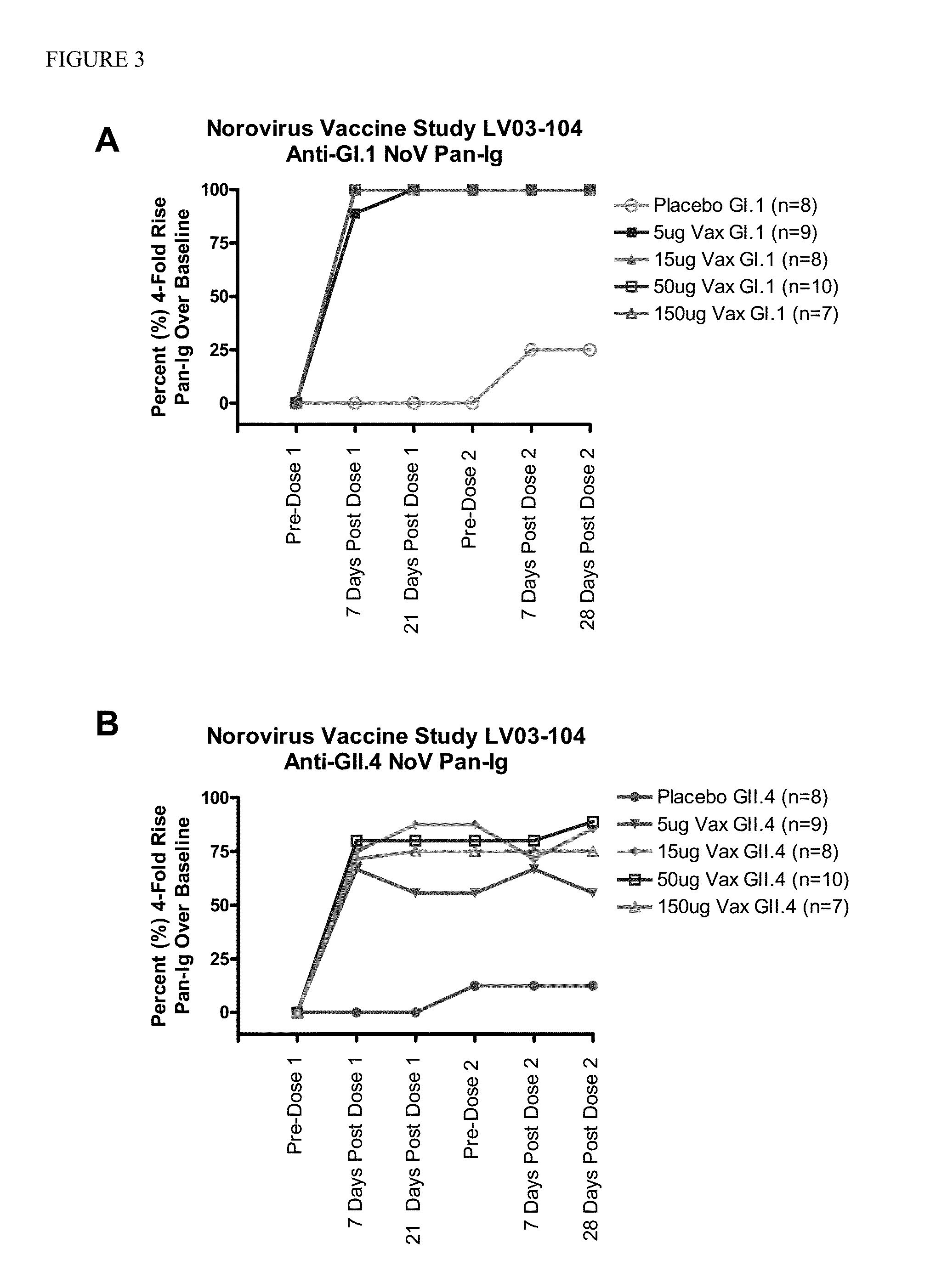
The present invention relates to single dose parenteral vaccine compositions comprising mixtures of monovalent Norovirus virus-like particles. Methods of conferring protective immunity against Norovirus infections in a human subject by administering such compositions are also disclosed.
Owner:TAKEDA VACCINES INC
Preparation and application of mouse monoclonal antibody against noroviruses GII.4
ActiveCN106188281AImmunoglobulins against virusesAntiviralsVirus-like particleMouse monoclonal antibody
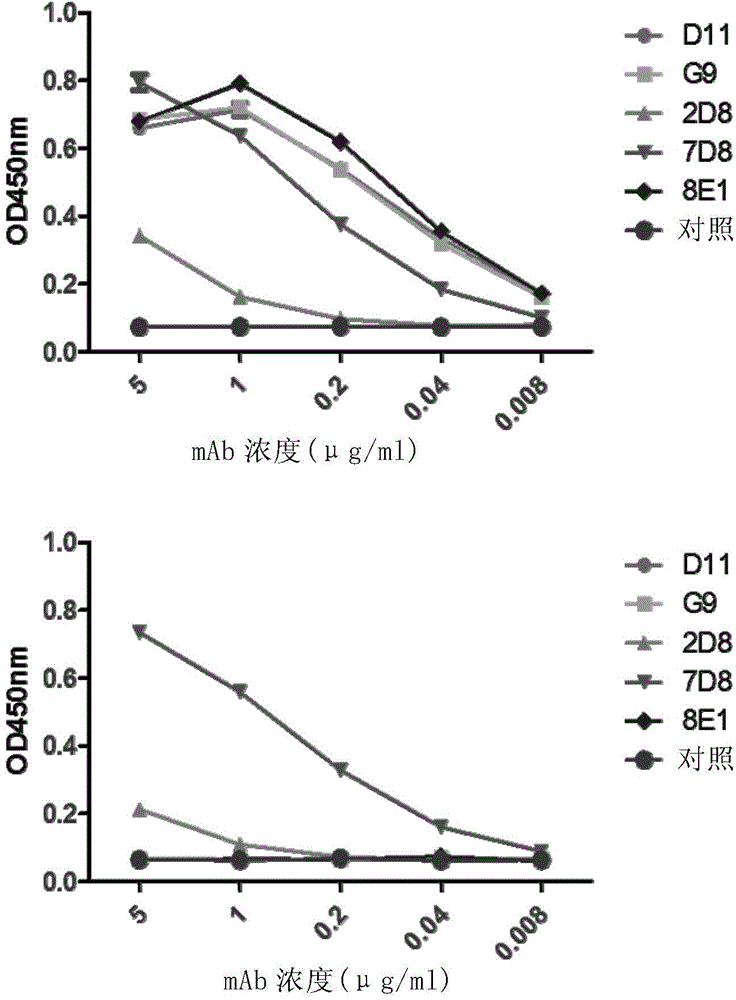
The invention discloses preparation and application of a mouse monoclonal antibody against noroviruses GII.4. Experimental results indicate that the monoclonal antibody has high neutralizing activity for the noroviruses GII.4, does not generate a cross reaction with GI.1 virus-like particles, and can specifically recognize the GII.4 virus-like particles.
Owner:中国科学院上海免疫与感染研究所
Materials and methods for detection of enterovirus and norovirus
The invention provides polynucleotides and methods for detecting and quantifying RNA viruses, such as enteroviruses and noroviruses. In one aspect, the invention provides amplification primers and labeled molecular beacons for amplification of viral nucleic acid sequences. In another aspect, the invention provides a synthetic RNA internal control. In another aspect, the invention provides a kit for detecting the presence of enterovirus and / or norovirus in a sample.
Owner:UNIV OF SOUTH FLORIDA
Purine monophosphate prodrugs for treatment of viral infections
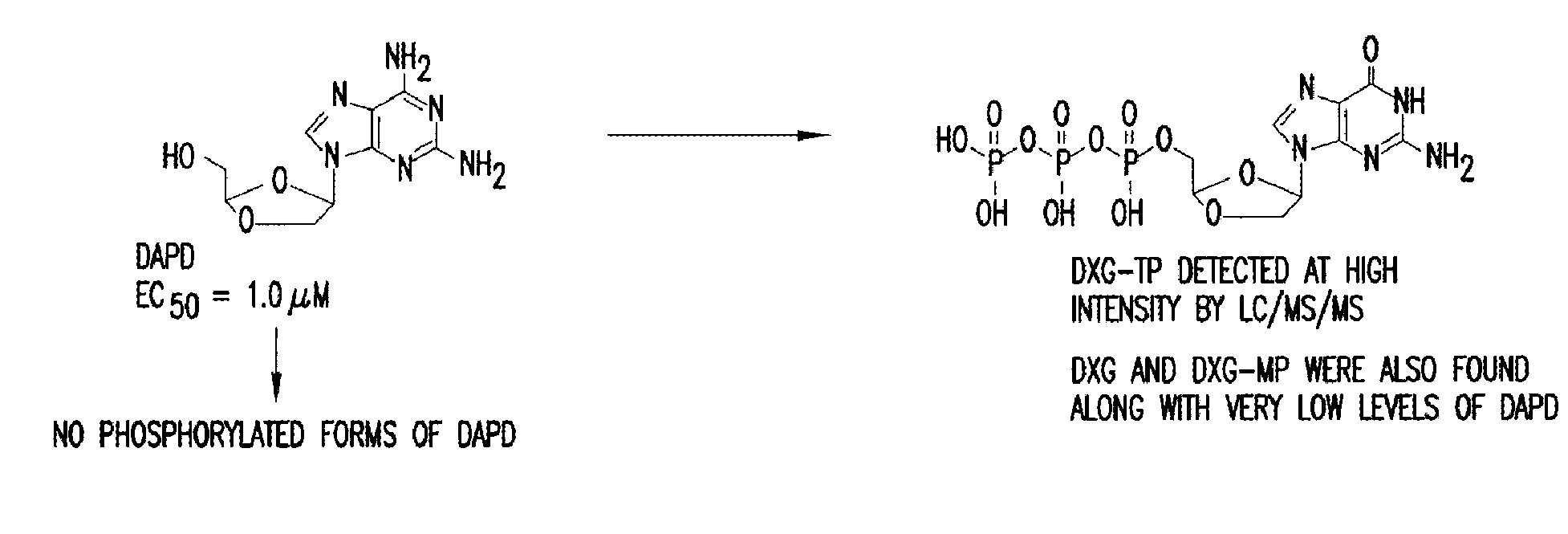
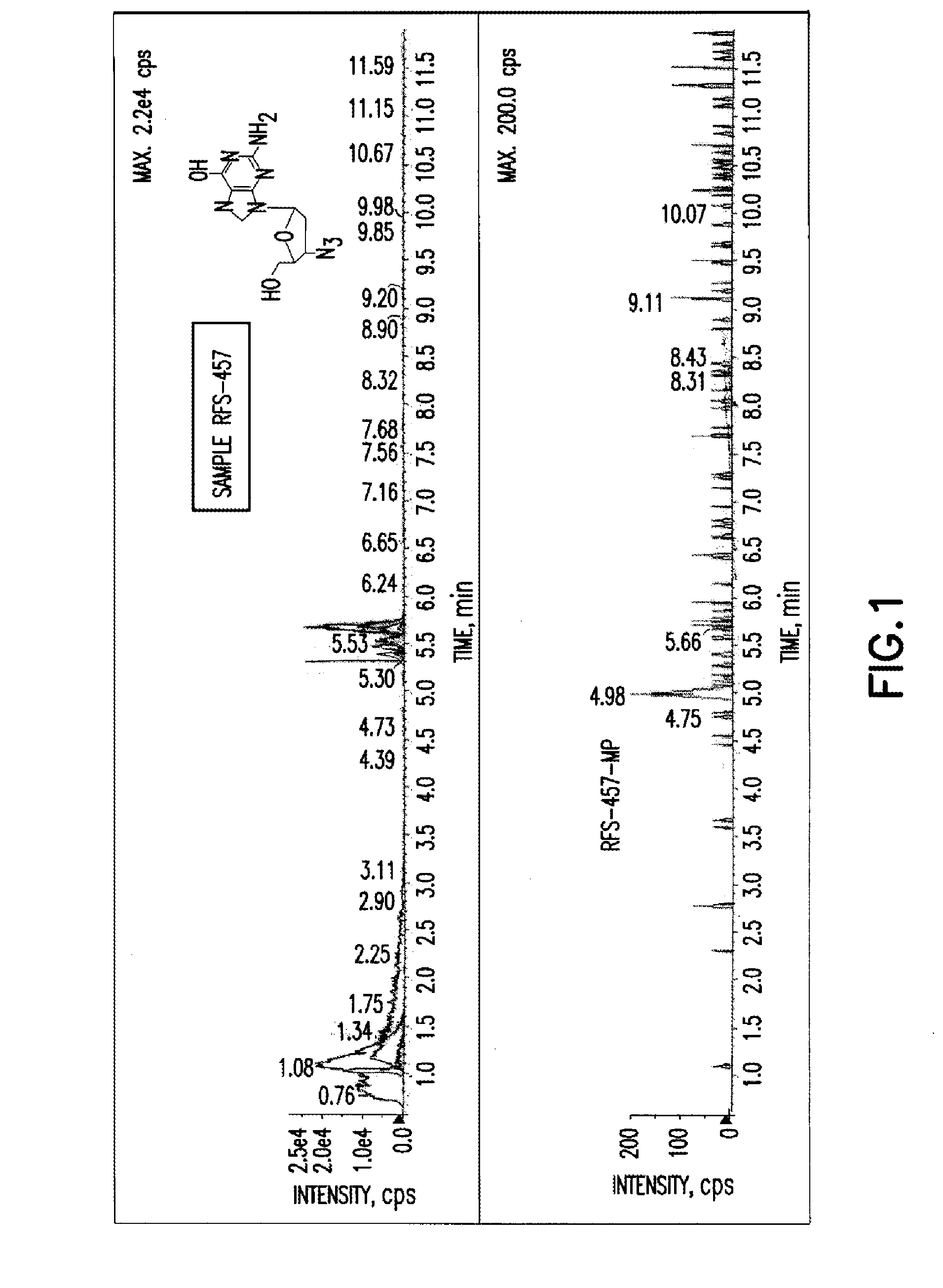
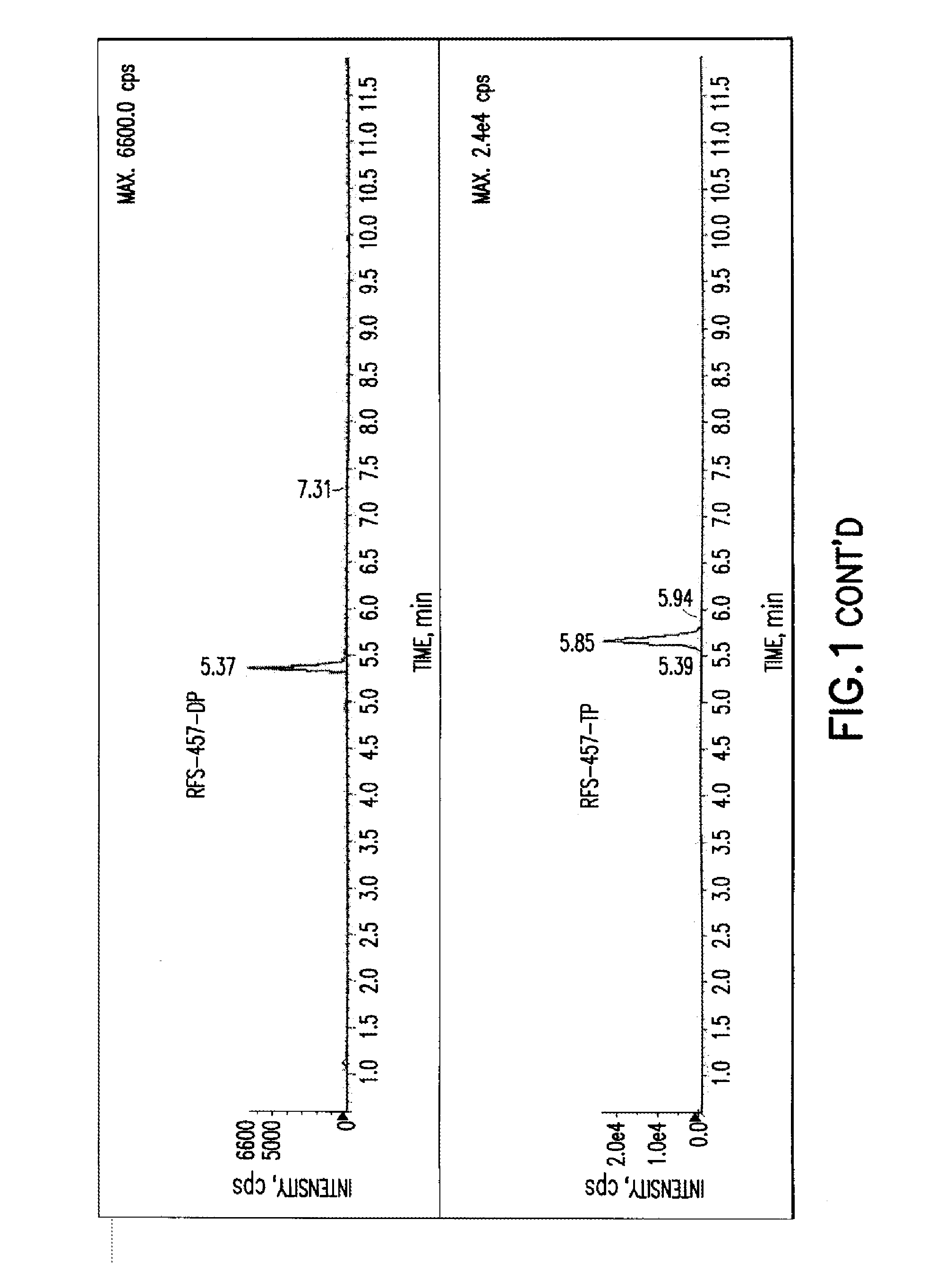
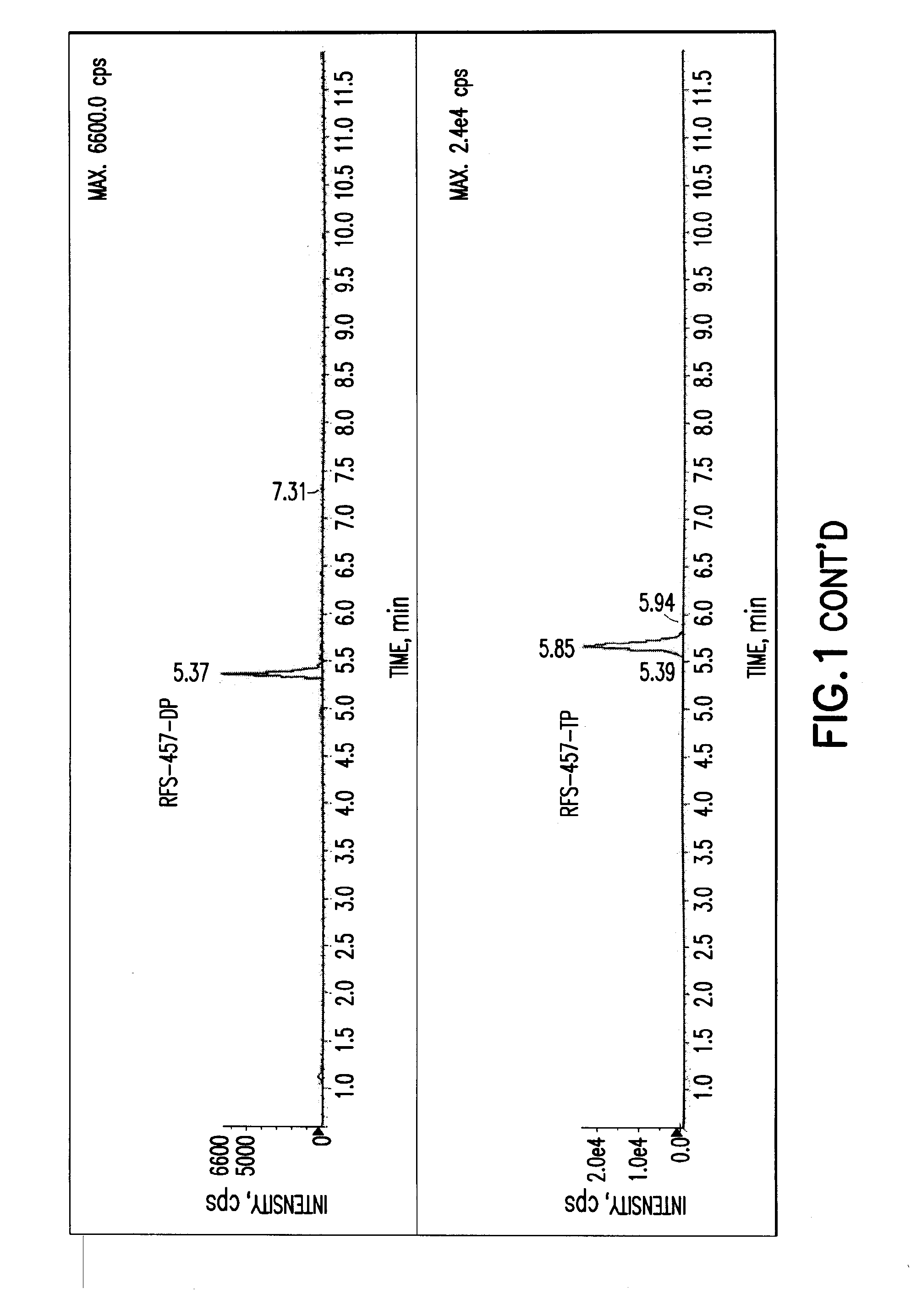
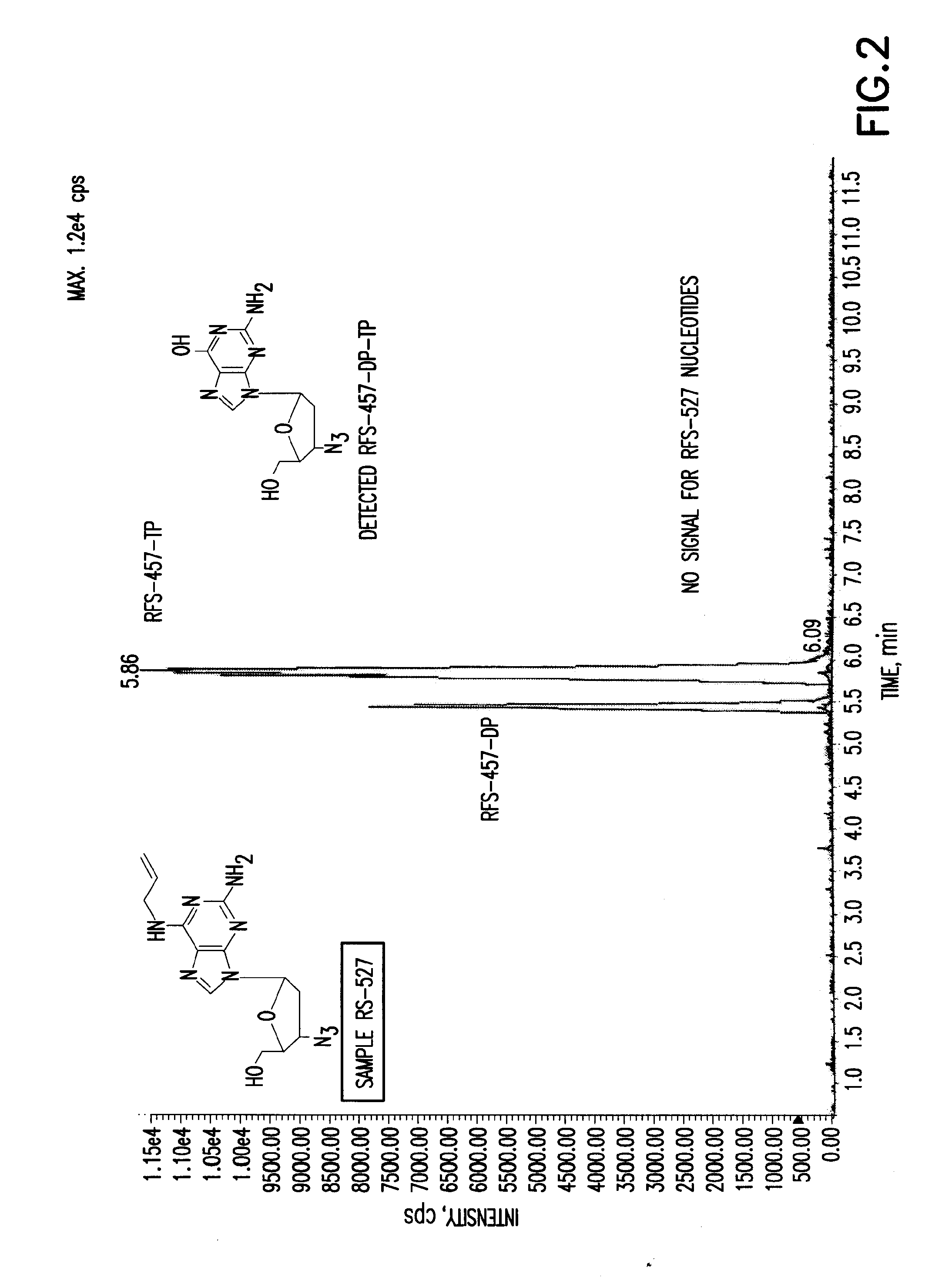
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2'-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:RFS PHARMA +1
Antiviral agent and cleansing agent
InactiveUS20120046362A1Good effectExcellent in inactivation abilityCosmetic preparationsBiocideAllergic dermatitisMedicine
It is an object to provide an antiviral agent that can be used for persons having sensitive skin or on the face, inactivates viruses such as a norovirus and an influenza virus, and is excellent in germicidal properties. Further provided is a cleansing agent that does not lead to environmental pollution since the cleansing agent is easily decomposed in the natural environment, scarcely causes eczema and allergic dermatitis since no germicidal agent is added, and has an antiviral performance. The antiviral agent containing a surface-active agent having a C18 unsaturated alkyl group as an active component. It is not always necessary to lather or rinse off with water like cleansing agents such as medicated soaps since the antiviral agent of the present invention at a very low concentration can inactivate the virus.
Owner:SHABONDAMA SOAP +1
Method of Disinfection or Infection Control Against Norovirus
InactiveUS20130023582A1Excellent anti-norovirus characteristicReduce morbidityAntibacterial agentsBiocideVitamin CFruit juice
An anti-norovirus agent that has high norovirus-inactivating activity and is safe for the human body, and an anti-norovirus composition that contains the anti-norovirus agent and is useful for disinfection and infection control against the norovirus. The anti-norovirus agent includes, as an active ingredient, an extract from a plant of the genus Diospyros containing tannin (hereinafter referred to as a “persimmon extract”), preferably a persimmon extract produced by heating squeezed juice or an extract from the fruit of a plant of the genus Diospyros or treating the squeezed juice or the extract with an alcohol. The anti-norovirus composition contains the anti-norovirus agent and at least one selected from the group consisting of alcohols, surfactants, antimicrobial agents, humectants, and cosmetic fats and oils, and preferably further containing an organic acid, such as citric acid, and / or a salt thereof or vitamin C.
Owner:HIROSHIMA UNIVERSITY +1
Norovirus real-time isothermal amplification detection kit, its primers and probe
InactiveCN102965452AAvoid inconvenienceStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseaseMicrobiology
The invention relates to an enteropathogen rapid detection technology based on real-time nucleic acid sequence-based amplification (NASBA). Specifically, the invention provides a Norovirus real-time isothermal amplification detection kit, and a pair of primers and a molecular beacon probe thereof. The kit includes: a 2*real-time NASBA reaction solution containing the primers and the probe, a 5*enzyme mixed solution and a positive control template, a negative control and a blank control. The sequences of the primers and the probe are the sequences numbered as SEQIDNO:1-3, and the primers and the probe can specifically amplify and detect a Norovirus ORFs1 gene fragment. The kit provided in the invention has the characteristics of fastness, high efficiency, sensitivity and specificity, and real-time detection analysis, etc., and can be used in the fields of conventional detection and disease control and prevention in clinical practice and ports.
Owner:珠海国际旅行卫生保健中心
Aptamers with binding affinity to norovirus
ActiveUS20170114420A1Effective treatmentPrevent and reduce infectionMaterial nanotechnologyMicrobiological testing/measurementAptamerVirus Binding
The instant disclosure provides norovirus-binding aptamers, compositions comprising such aptamers, and methods of using and producing such aptamers. The aptamers are useful, for example, for detecting the presence of norovirus in test samples, for capturing and / or concentrating norovirus from test samples, for evaluating the efficacy of therapeutic agents in patients diagnosed with a norovirus infection, and for evaluating the efficacy of norovirus vaccines.
Owner:NORTH CAROLINA STATE UNIV
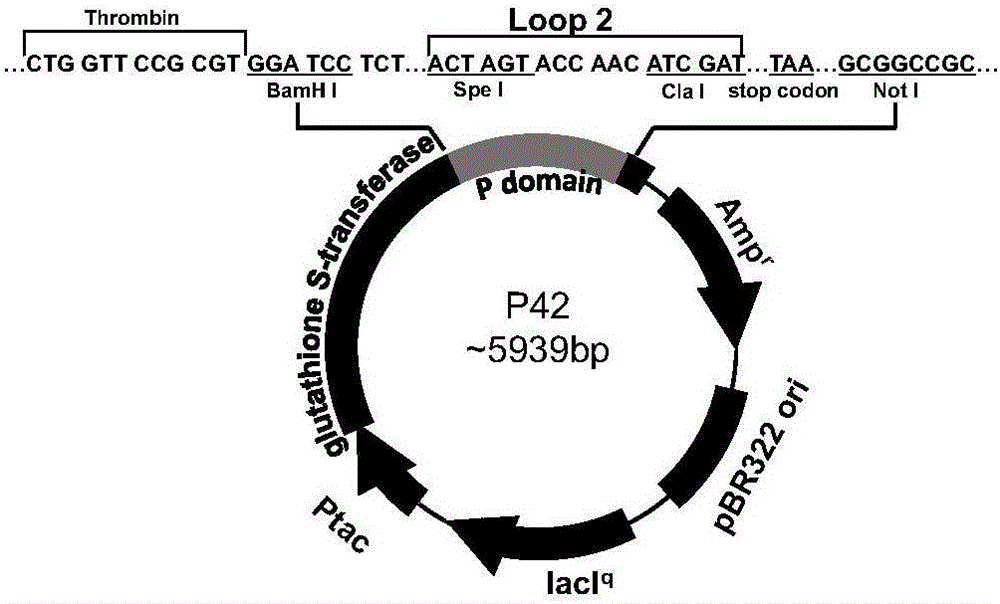
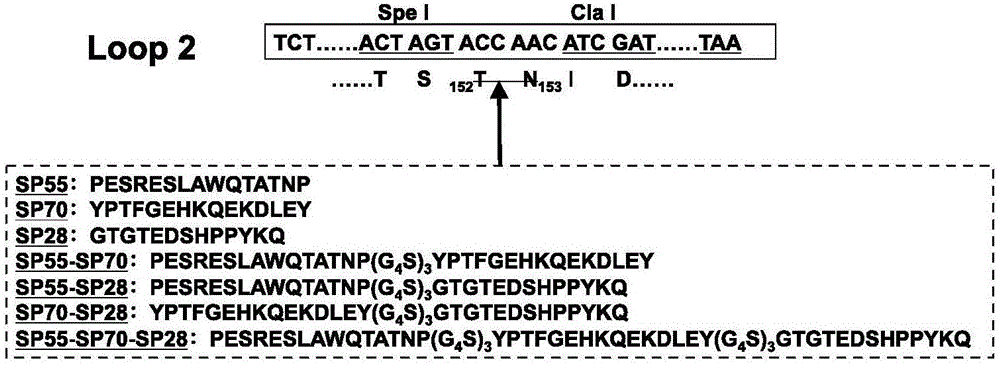
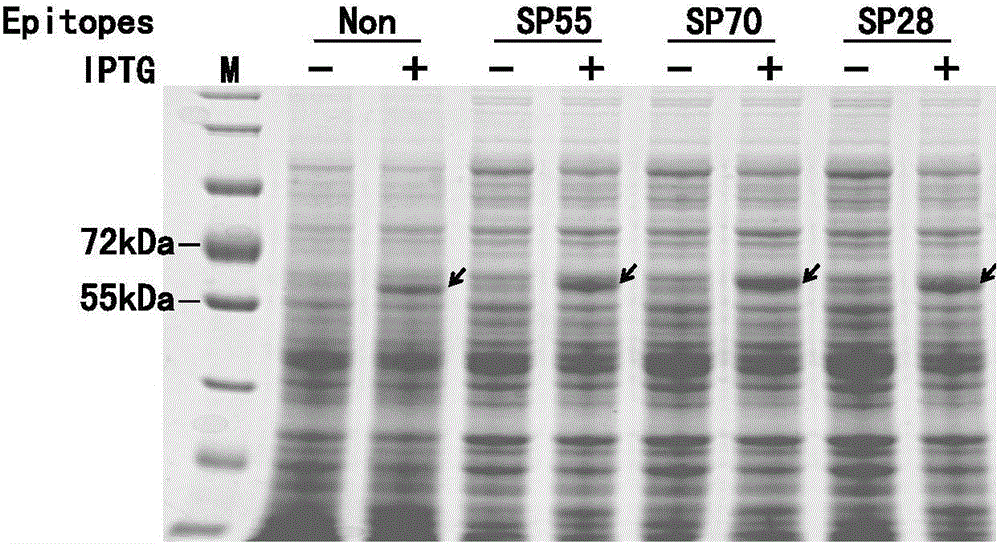
Antigen-norovirus P-domain monomers and dimers, antigen-norovirus P-particle molecules, and methods for their making and use
ActiveUS8486421B2Easy to produceImproving immunogenicitySsRNA viruses positive-sensePeptide/protein ingredientsDiseaseAntigen
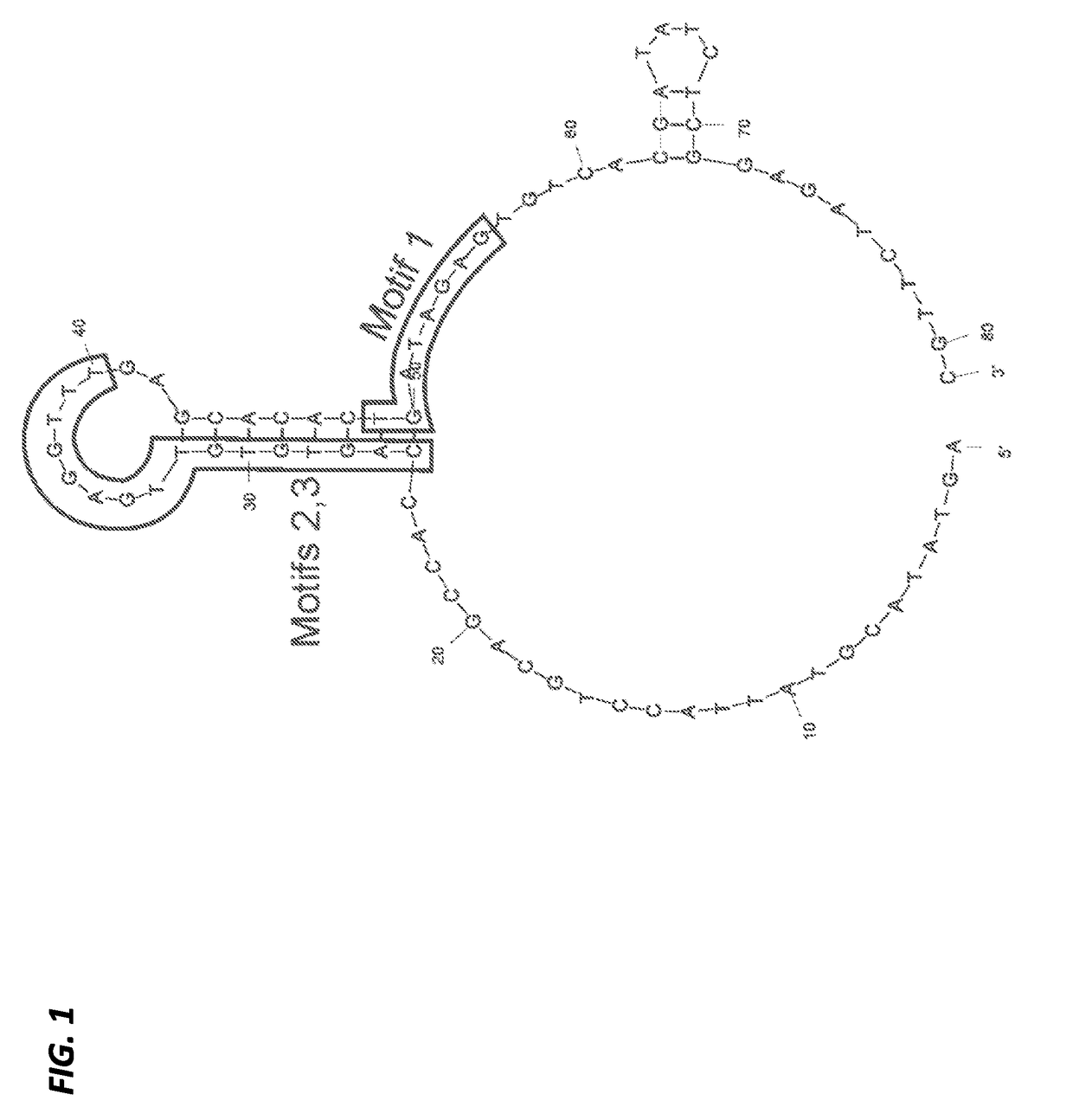
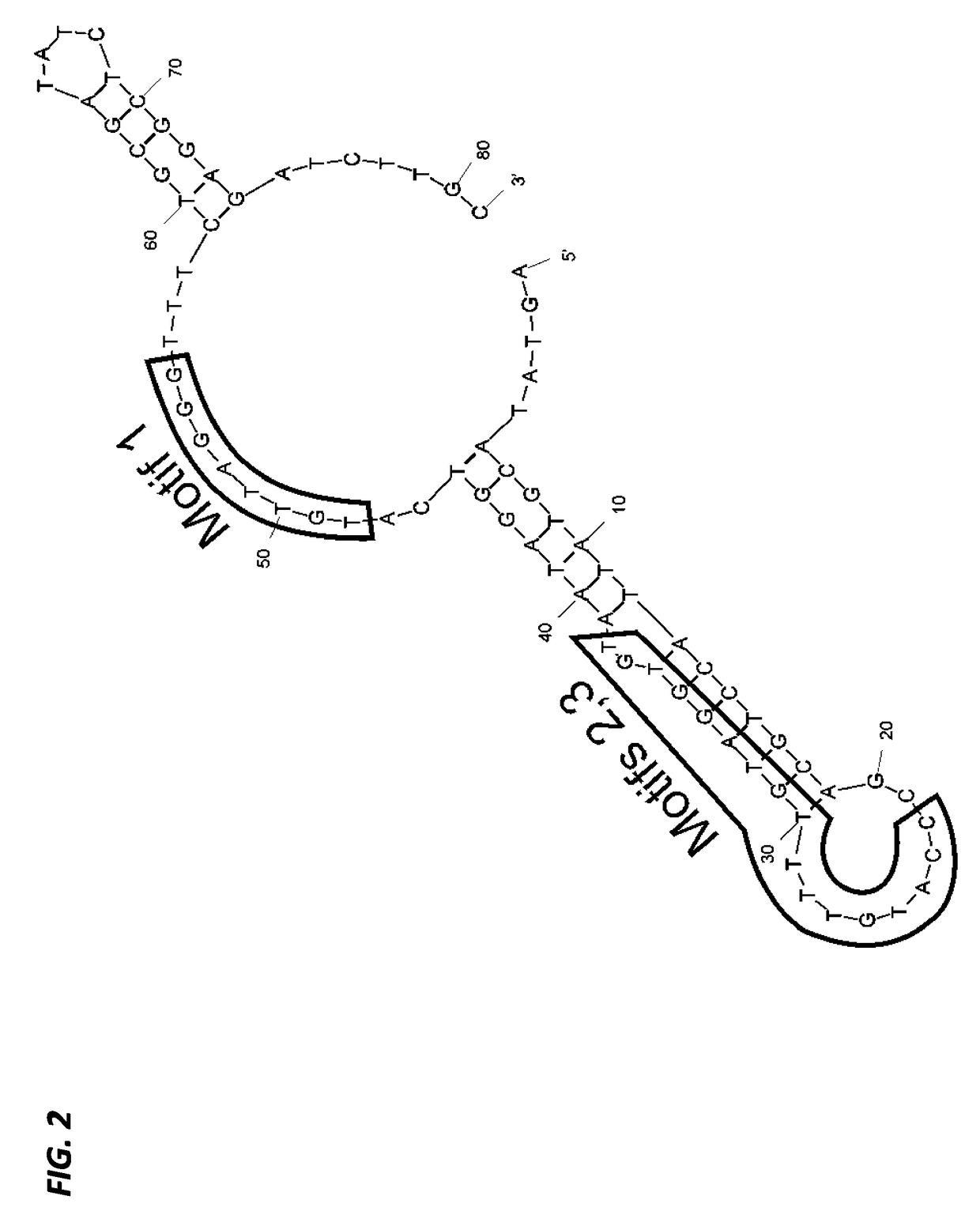
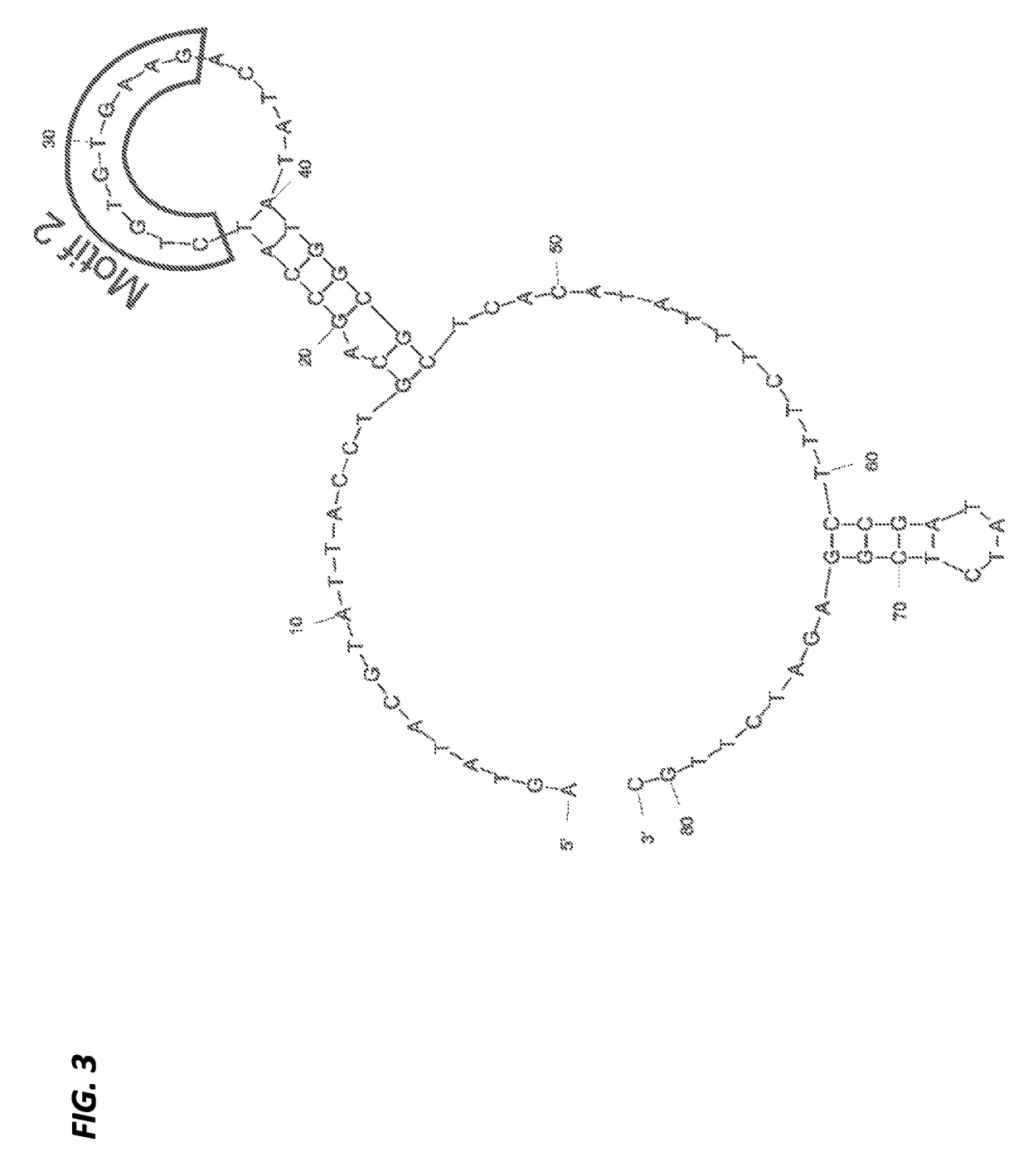
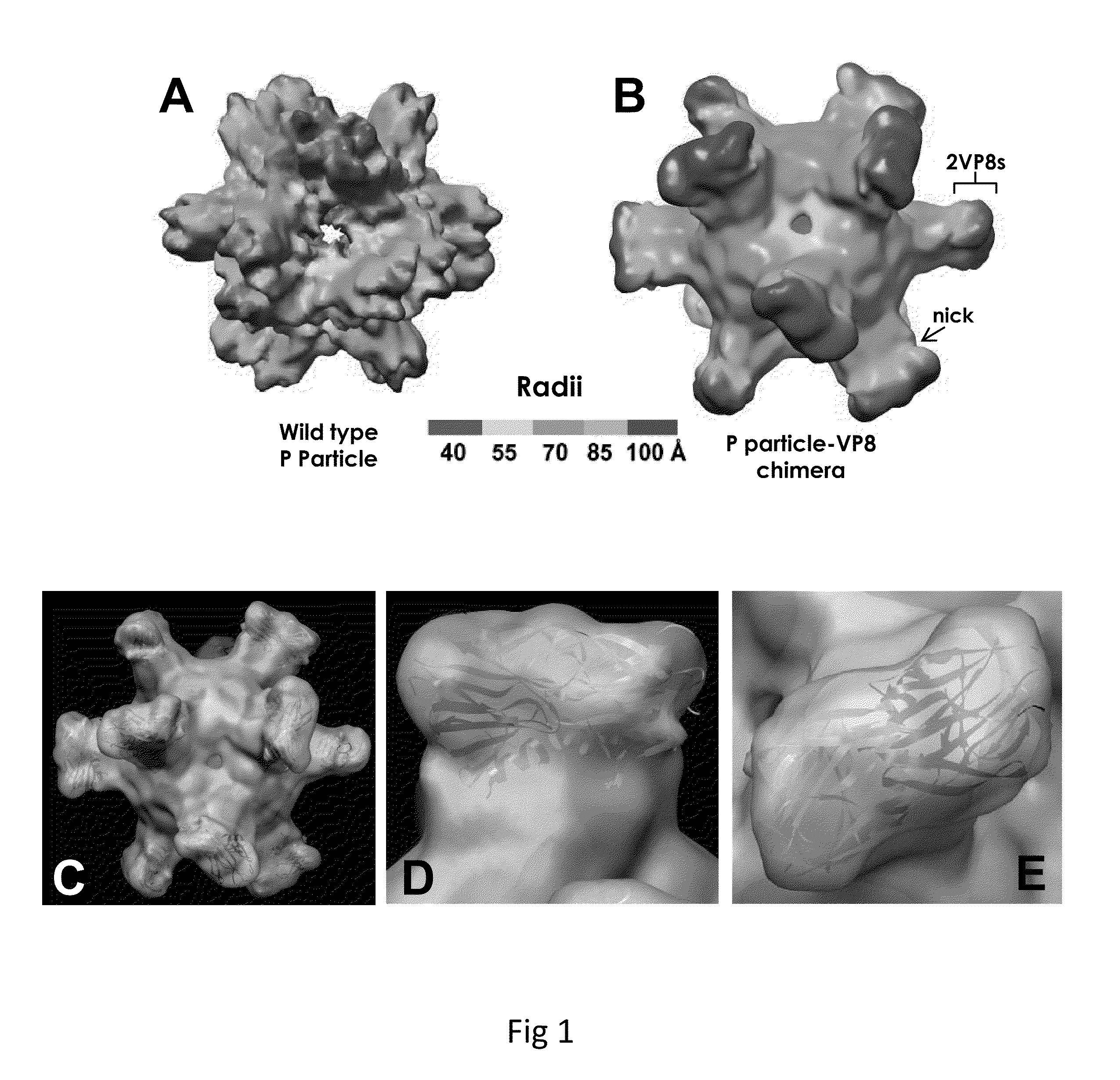
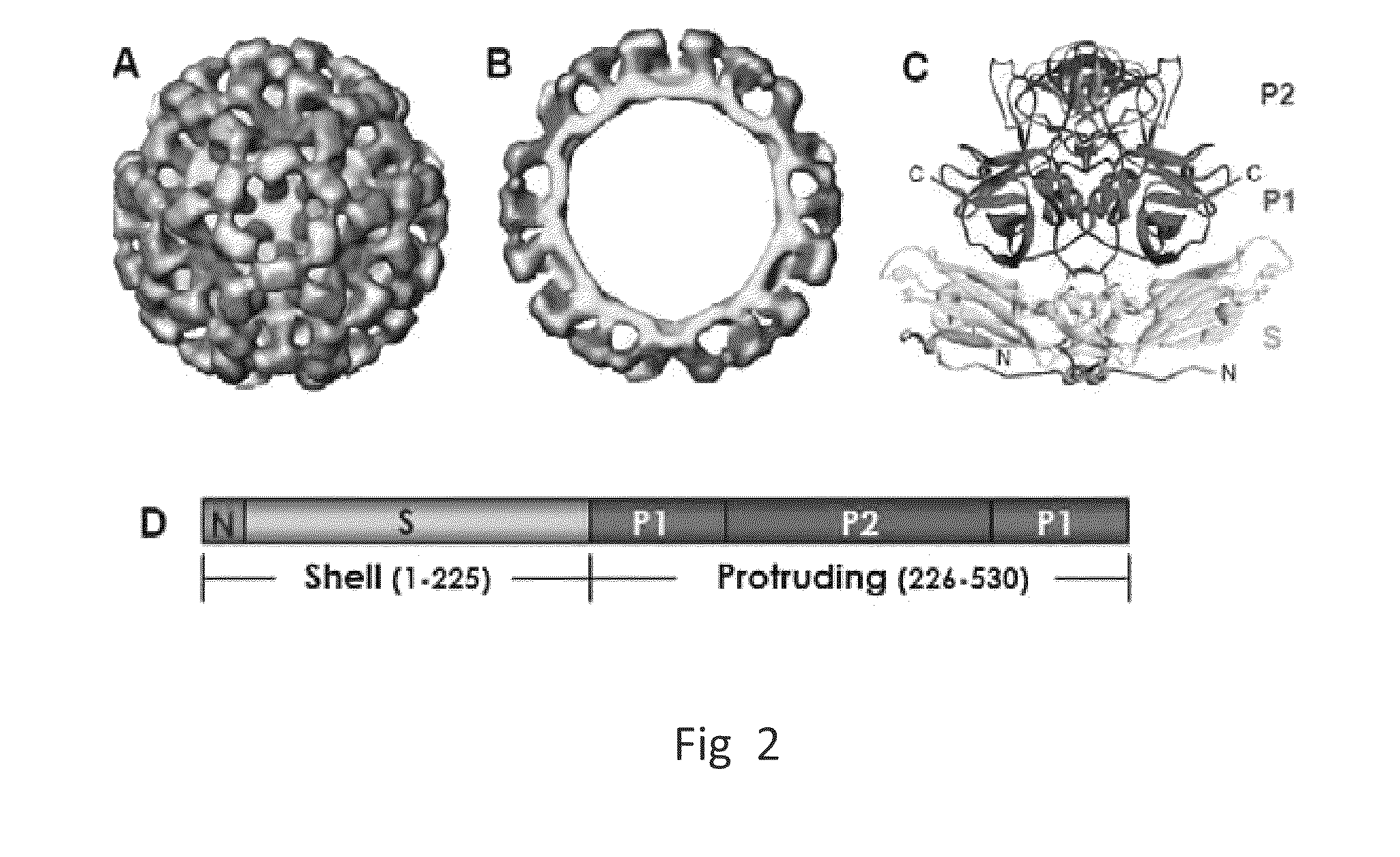
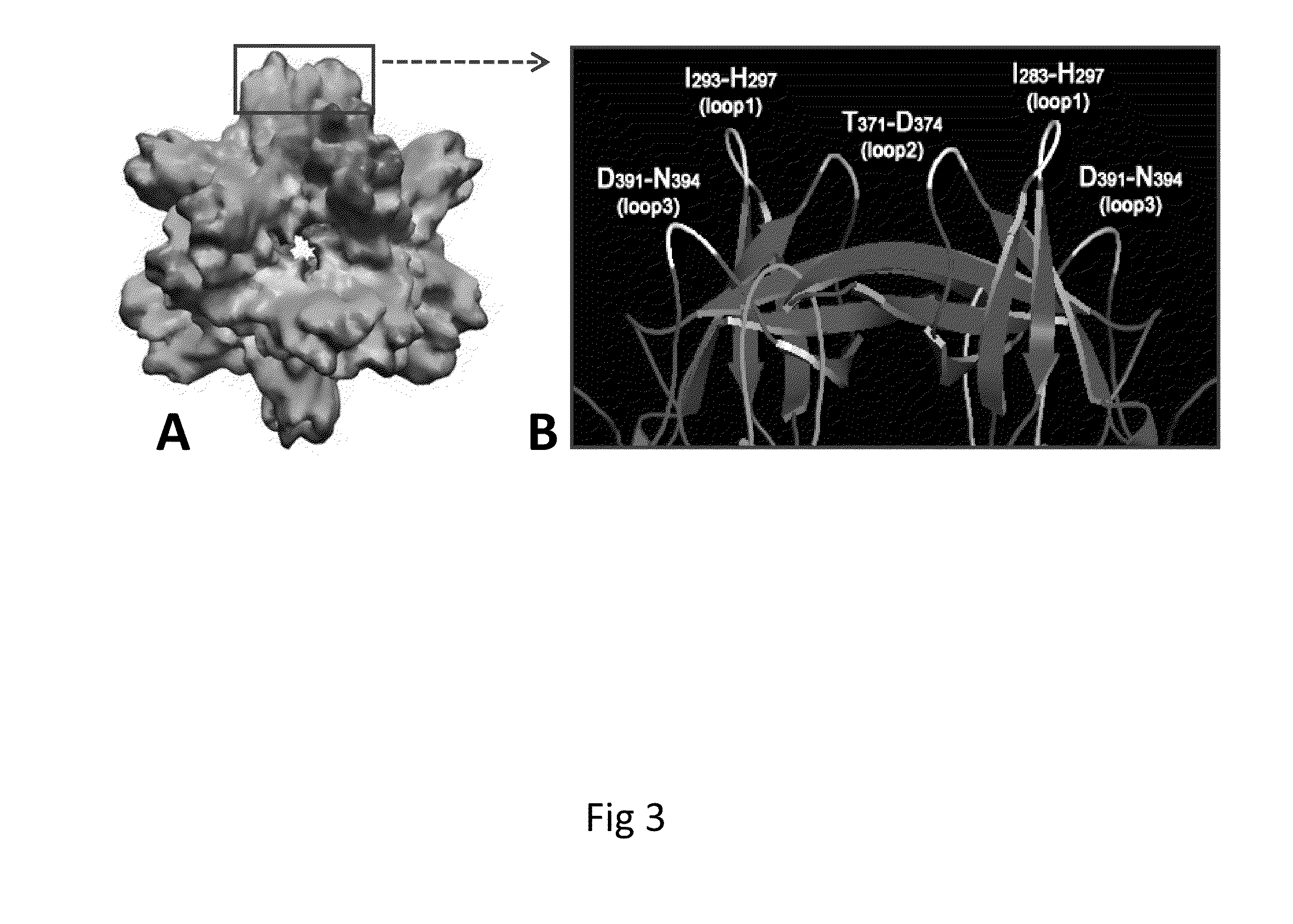
A substituted Norovirus capsid protein monomer, having only the P-domain and called an antigen-Norovirus P-domain monomer, includes a foreign antigen inserted into one or more of three surface loops present on each P-domain monomer by molecular cloning. The antigen-P-domain monomer can assemble spontaneously into an octahedral form, called an antigen-Norovirus P-particle, that is composed of 24 copies of the antigen-P-domain monomer. Each substituted P-domain monomer will contain one to three copies of the foreign antigen, for a total of 24-72 antigen copies on each antigen-P-particle. The antigen-P-particle is useful in methods for diagnosing, immunizing and treating individuals infected with a foreign virus, for example Rotavirus, and can serve as a carrier for presentation of foreign antigens for development of novel vaccines against many infectious and non-infectious diseases. The substituted Norovirus P-particles can be readily produced in E. coli and yeast, are highly stable and tolerate a wide range of physio-chemical conditions. A modified Norovirus P-domain monomer includes one or more restriction recognition sites inserted within one or more of the three loops of the P-domain monomers, to provide user-friendly cloning cassettes for conveniently inserting candidate foreign antigens into the surface loops. The P-particle-VP8 chimeras may also serve as a dual vaccine against both rotavirus and norovirus.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
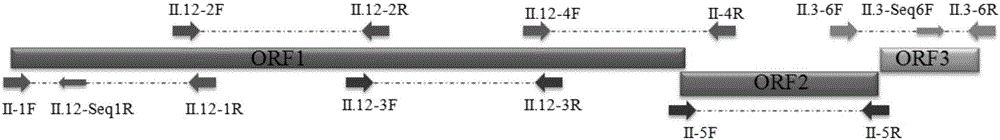
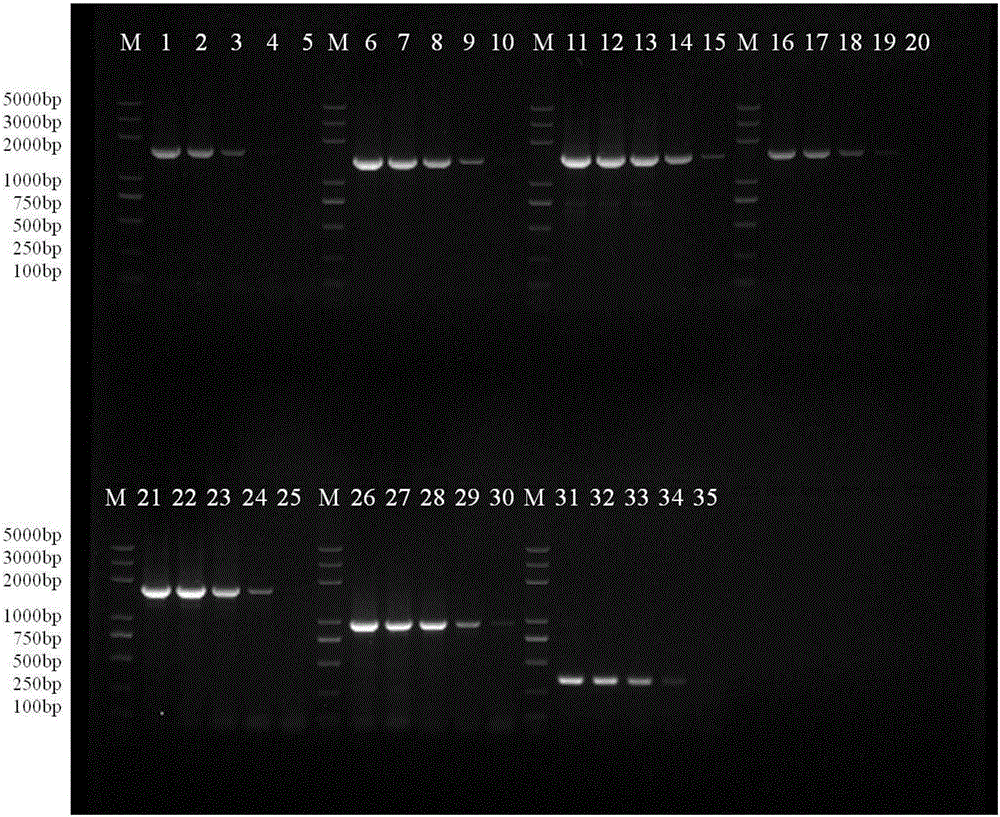
GII.P12/GII.3 recombinant norovirus genome amplification primer and amplification method
The invention discloses a GII.P12 / GII.3 recombinant norovirus genome amplification primer and an amplification method. The amplification method comprises the following steps: by taking six pairs of amplification primers as forward and reverse primers of amplification primers respectively, and by taking RNA of GII.P12 / GII.3 recombinant norovirus as a template, performing RT-PCR amplification so as to obtain amplification products respectively, performing nucleotide sequencing on the amplification products by using the six amplification primers and two sequencing primers II.12-Seq1R and II.3-Seq6F, and then carrying out jointing and comparison, thereby obtaining full-length sequences of a GII.P12 / GII.3 recombinant norovirus genome. For the GII.P12 / GII.3 recombinant norovirus which occurs frequently in China, a sectional amplification strategy of '4+1+1'is designed according to three opening reading frames included in the genome, corresponding amplification primers are designed according to conserved regions, the amplification primers are applied to practical detection samples, and GII.P12 / GII.3 recombinant norovirus genome sequences can be acquired. The GII.P12 / GII.3 recombinant norovirus genome amplification primer and the amplification method can be widely applied to fields such as medical treatment and public health and inspection and quarantine with norovirus detection requirements, and related scientific fields.
Owner:GUANGDONG INST OF MICROORGANISM +1
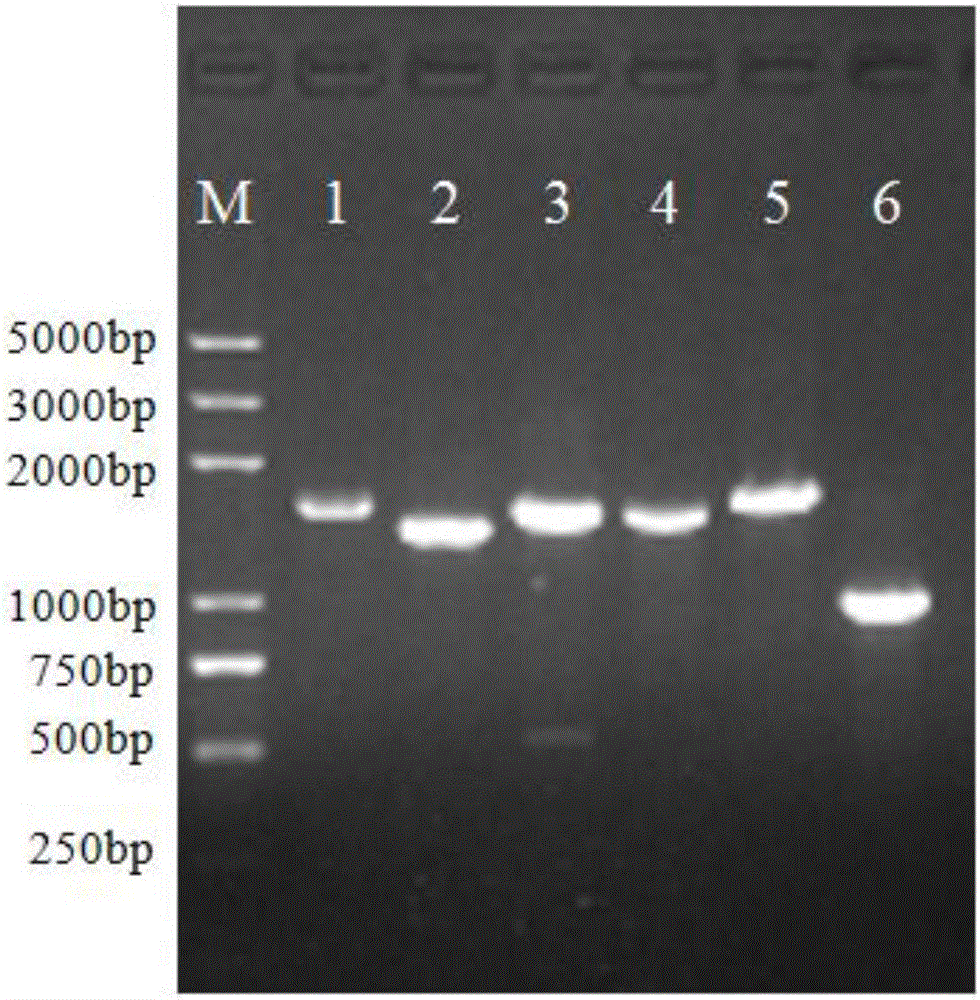
Establishing and expressing method for chimeric vector of EV71 neutralization epitope and norovirus P structure domain
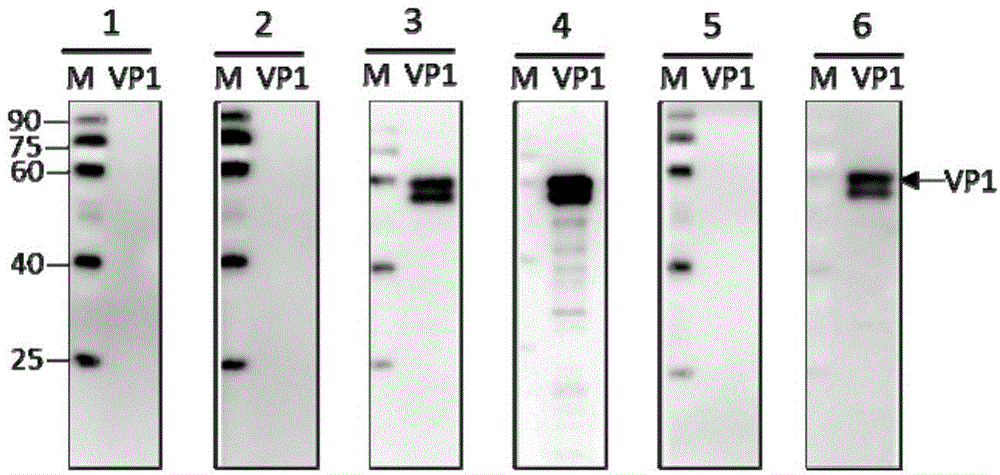
PendingCN106220738AHigh purityEasy to makePeptide preparation methodsHybrid peptidesEnterovirusAntigen
The invention aims at providing chimeric protein of enterovirus EV71 neutralization epitope and a human norovirus P structure domain and an establishing method of a chimeric vector. By means of immunoblotting, it is verified that the chimeric protein of the enterovirus EV71 neutralization epitope and the norovirus P structure domain can be used for research and development of detection kits of EV71 and norovirus and novel bivalent subunit vaccines.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com