Preparation and application of mouse monoclonal antibody against noroviruses GII.4
A technology of antibodies and vectors, applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problems of norovirus lack of cell culture models, no small animal models, vaccines and antiviral drug research obstacles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0128] Preparation of monoclonal antibodies
[0129] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. For monoclonal antibodies, hybridoma technology can be used to prepare (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981) or can be prepared by recombinant DNA methods (US Patent No. 4,816,567).
[0130] Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-11 mouse tumors (...
Embodiment 1
[0181] Example 1 Screening of hybridoma cells secreting GII.4 specific antibody
[0182] Spleen cells from mice immunized with GII.4 virus-like particles were used to prepare hybridoma cells. The hybridoma cell supernatant was screened by Elisa test, so as to obtain a hybridoma cell line capable of secreting virus-binding ability. Finally, five monoclonal antibodies with excellent binding ability were screened out, all of them could bind GII.4 VLP. Subtype identification showed that G9, D11, 7D8 and 8E1 belonged to IgG1, and 2D8 belonged to IgG3.
[0183] Table 1. Identification of hybridoma cell lines secreting monoclonal antibody
[0184] hybridoma cell line
light chain
Binding ability to GII.4VLP*
G9
IgG1
kappa
+++
D11
IgG1
kappa
+++
2D8
IgG3
kappa
+++
7D8
IgG1
kappa
+++
8E1
IgG1
kappa
+++
[0185] All samples used for analysis were 50ul hybridoma ...
Embodiment 2
[0187] Example 2 Specific Analysis of Anti-GII.4 Monoclonal Antibody
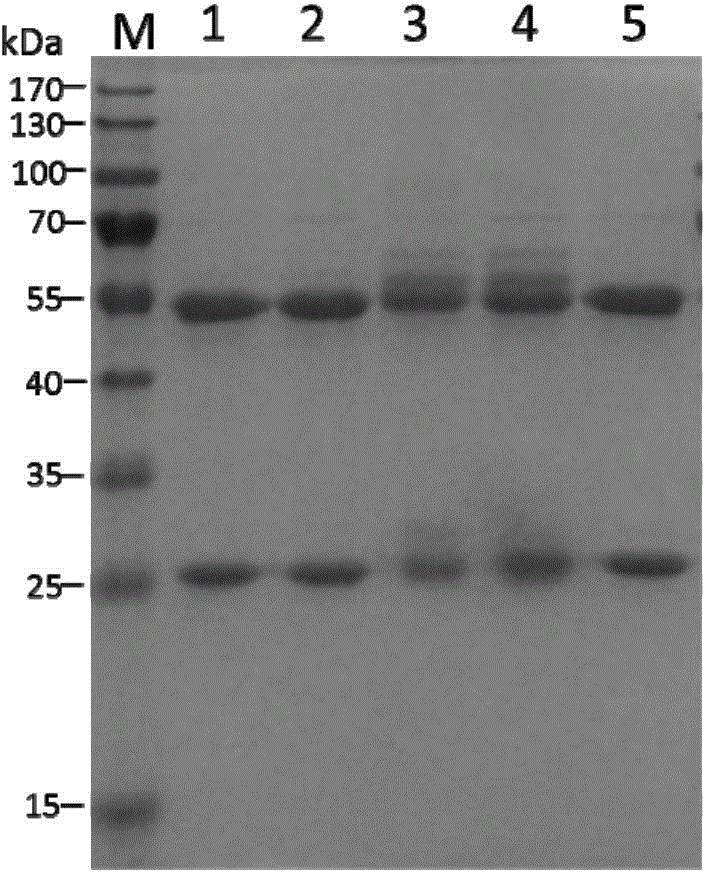
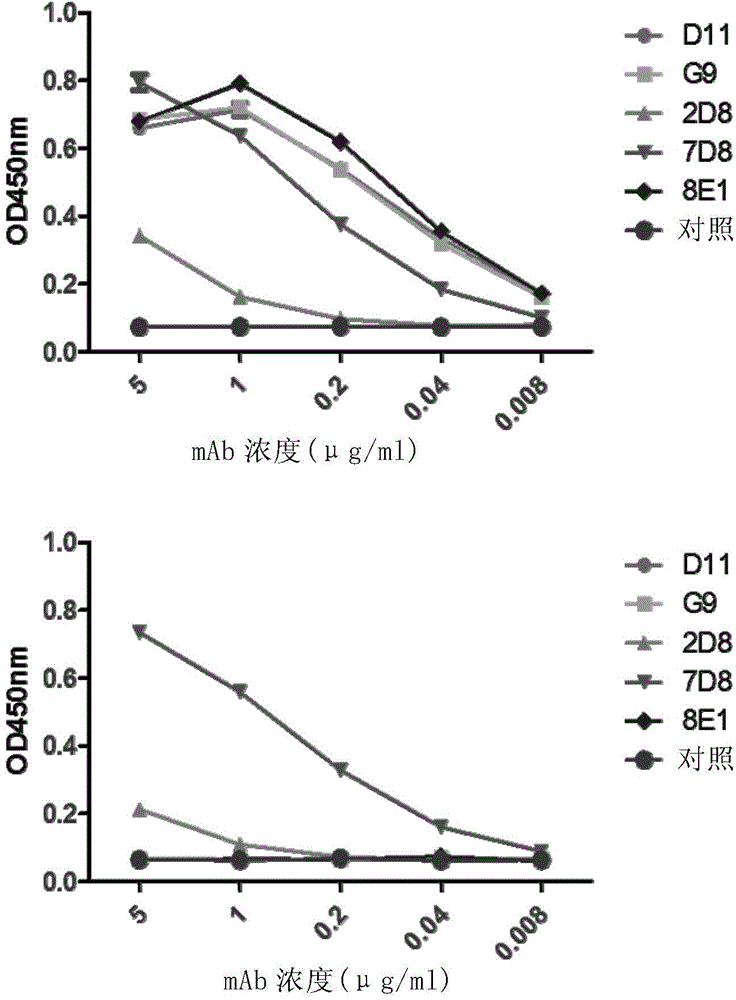
[0188] The purity and integrity of GII.4 mAb purified from ascitic fluid were first identified by SDS-PAGE. figure 1 It shows that the heavy chain and light chain of the five monoclonal antibodies are about 50KD and 25KD respectively. Next, the reactivity of the monoclonal antibody with different antigens, including GI.1 virus-like particles and GII.4 virus-like particles, was detected by Elisa method. figure 2 It shows that G9, D11, 2D8 and 8E1 can specifically recognize GII.4 virus-like particles, and there is no cross-reaction with GI.1 virus-like particles, while some monoclonal antibodies (such as 7D8) can also bind to GI.1 virus-like particles Combined with GII.4 virus-like particles, it cannot specifically recognize GII.4 virus.
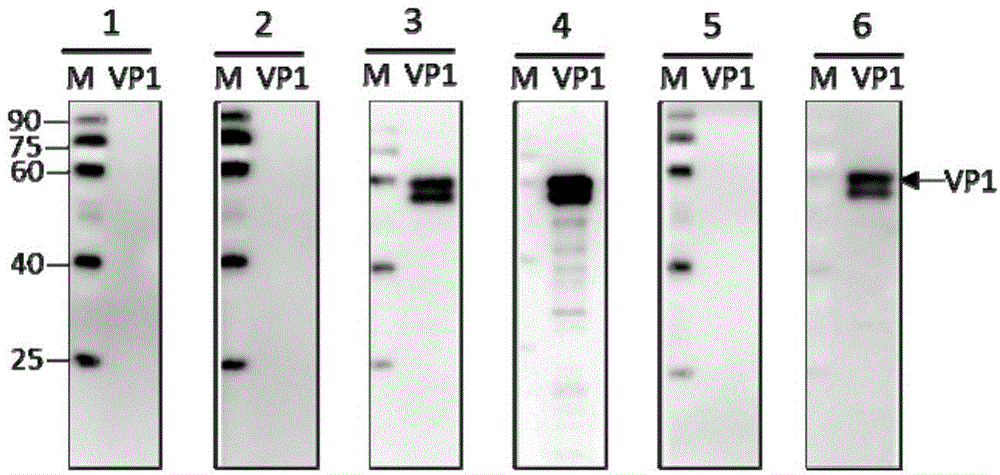
[0189] Finally, the binding of monoclonal antibody to GII.4 was analyzed by Western blot, image 3 It shows that among the five antibodies: D11, G9 and 8E1 can recogn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com