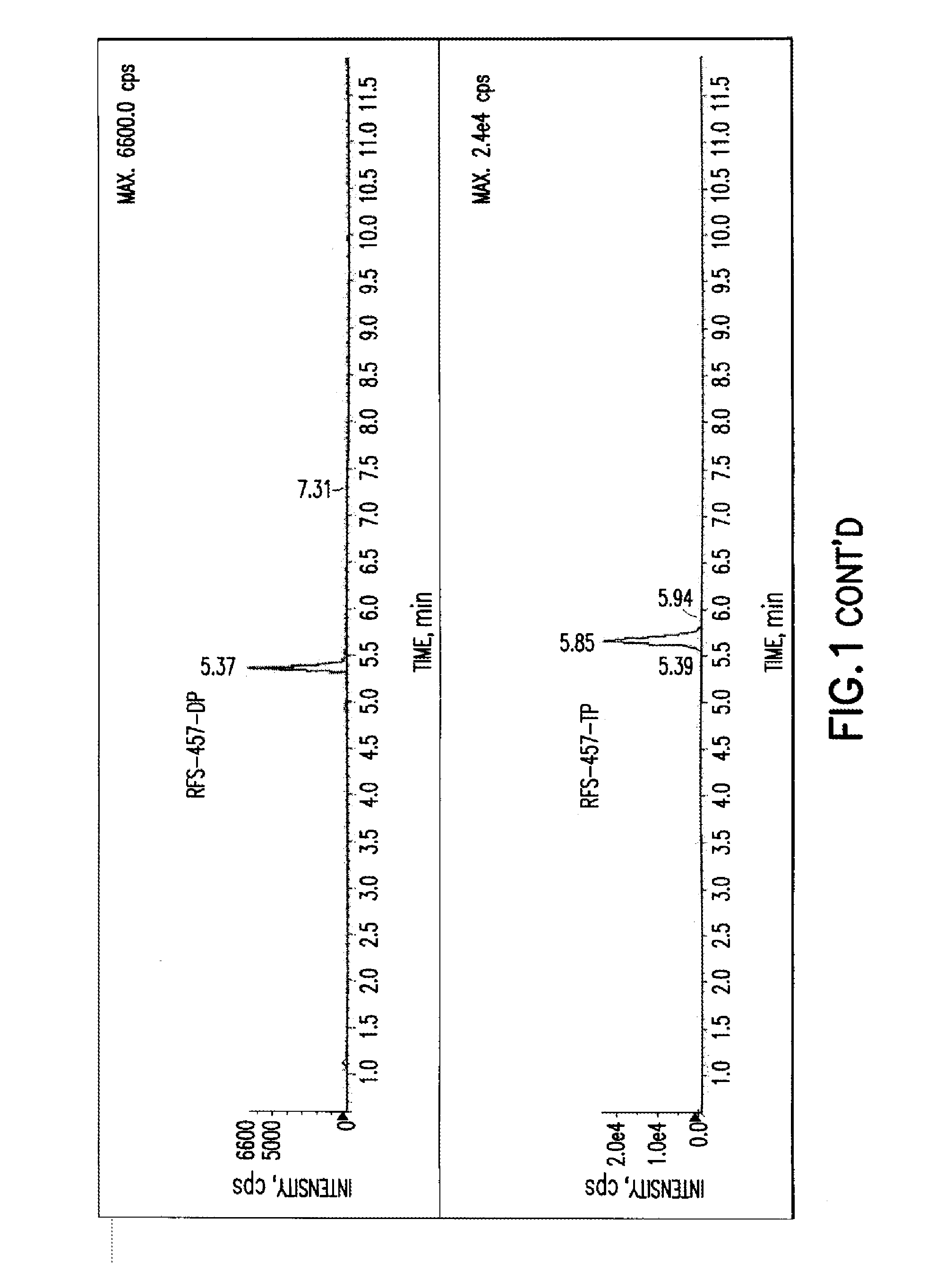
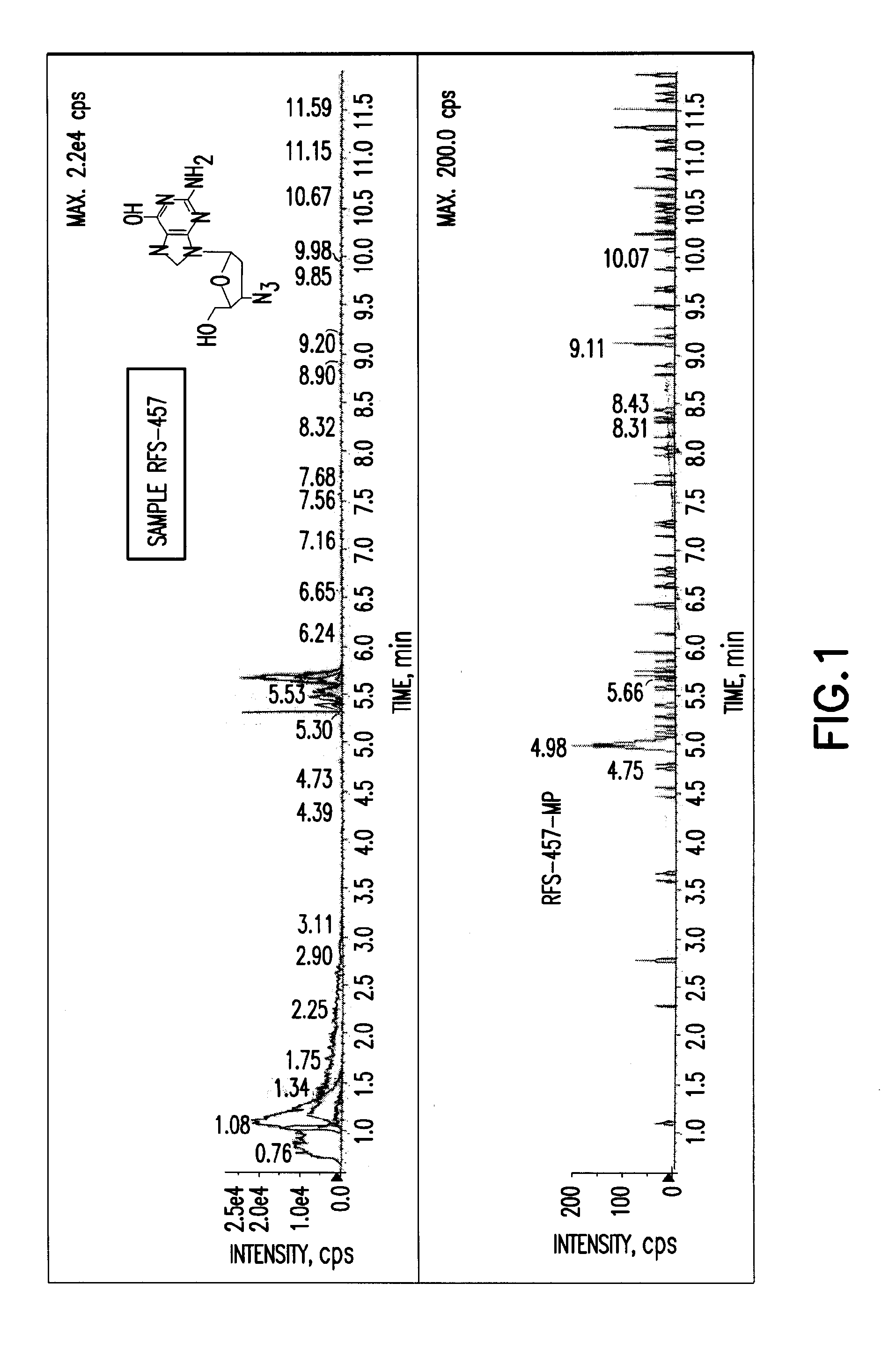
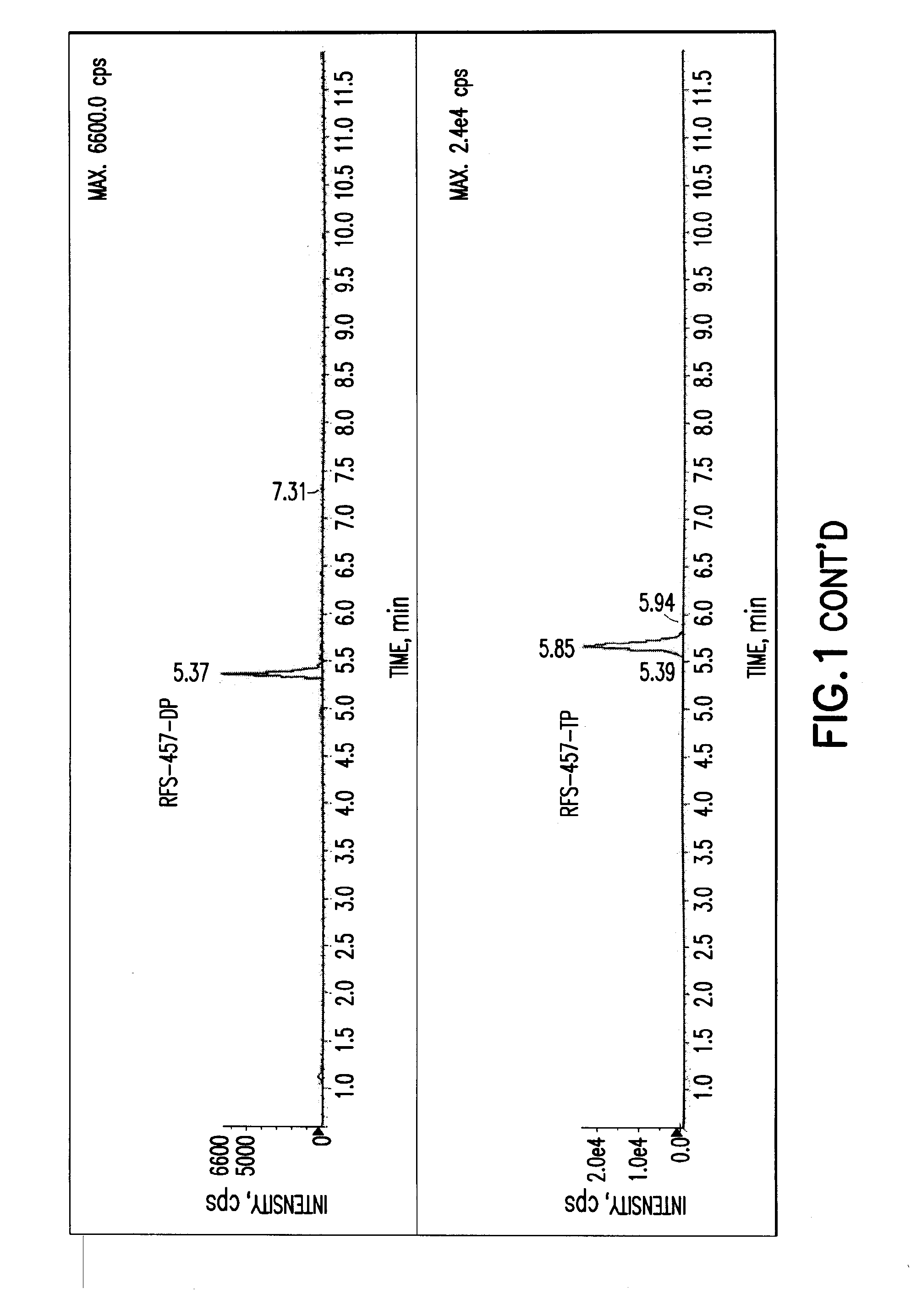
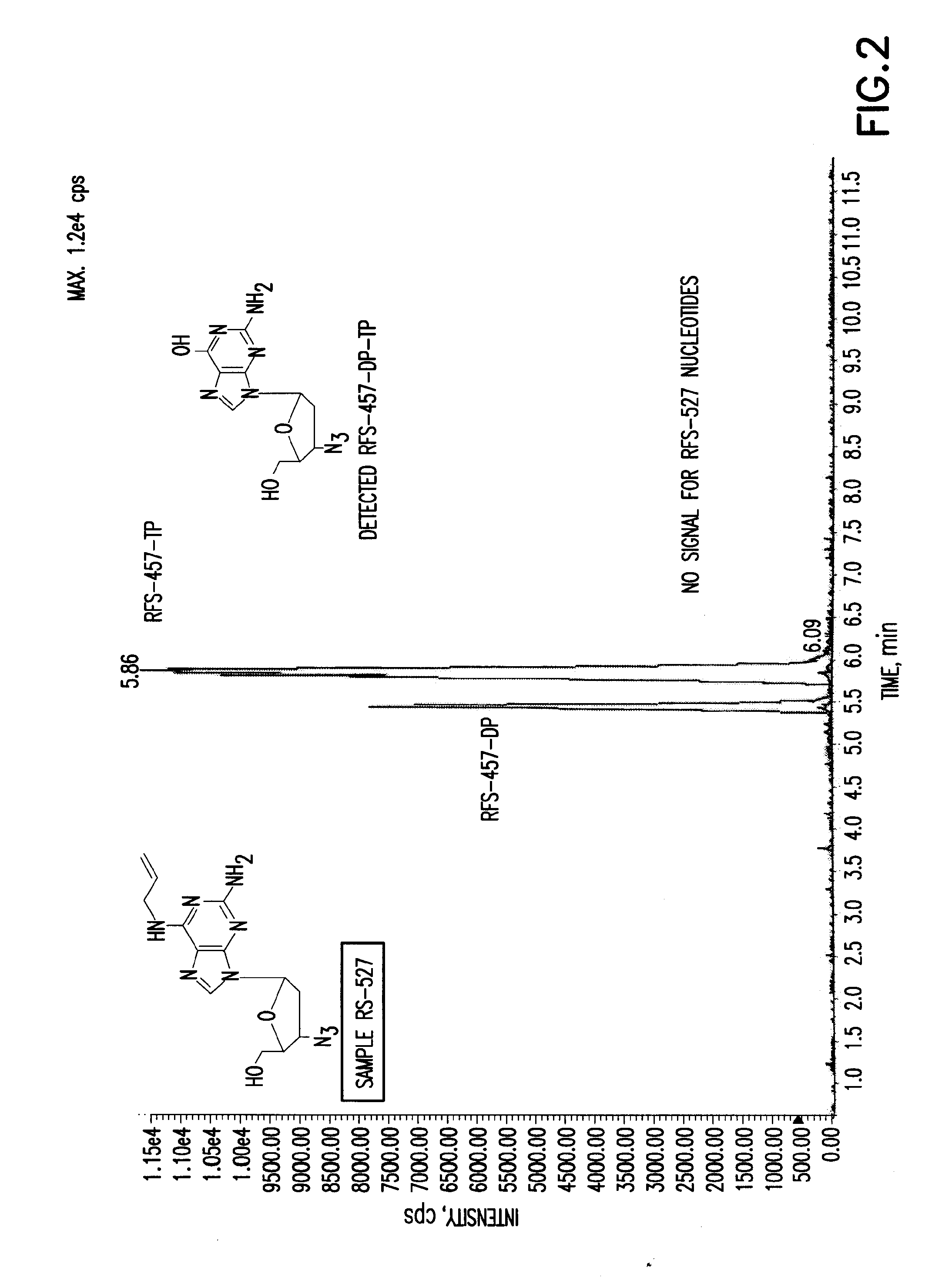
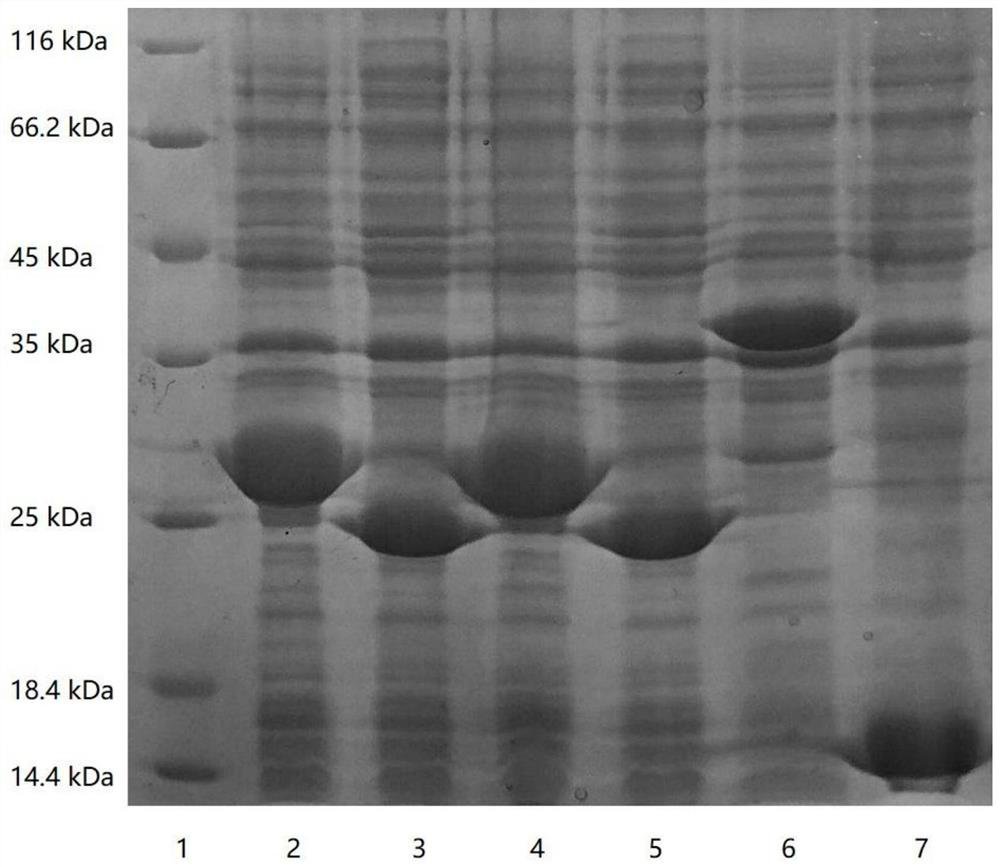
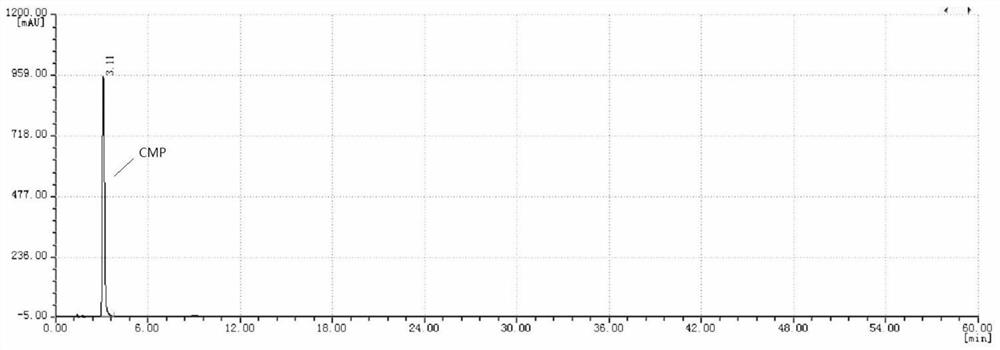
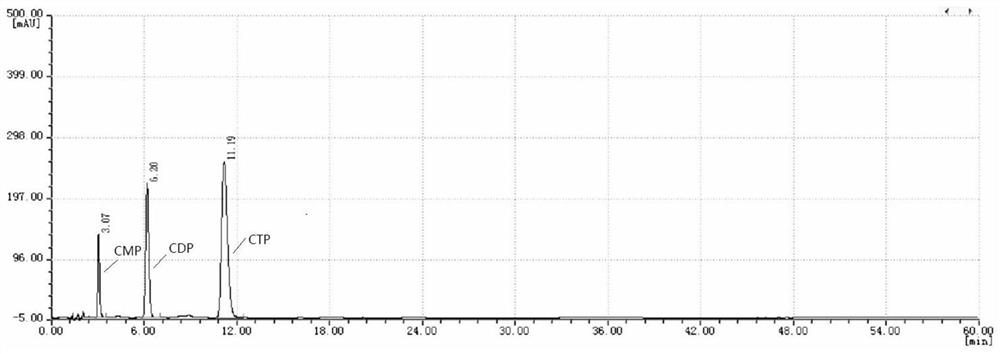
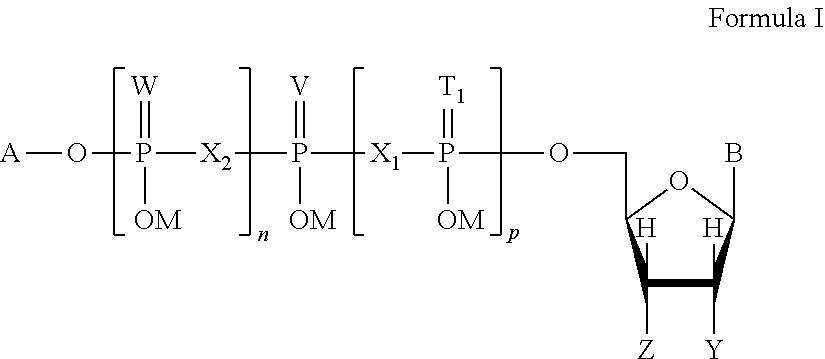Patents
Literature
43 results about "Nucleoside monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Structural elements of three nucleotides—where one-, two- or three-phosphates are attached to the nucleoside (in yellow, blue, green) at center: 1st, the nucleotide termed as a nucleoside monophosphate is formed by adding a phosphate group (in red); 2nd, adding a second phosphate group forms a nucleoside diphosphate; 3rd, adding a third phosphate ...
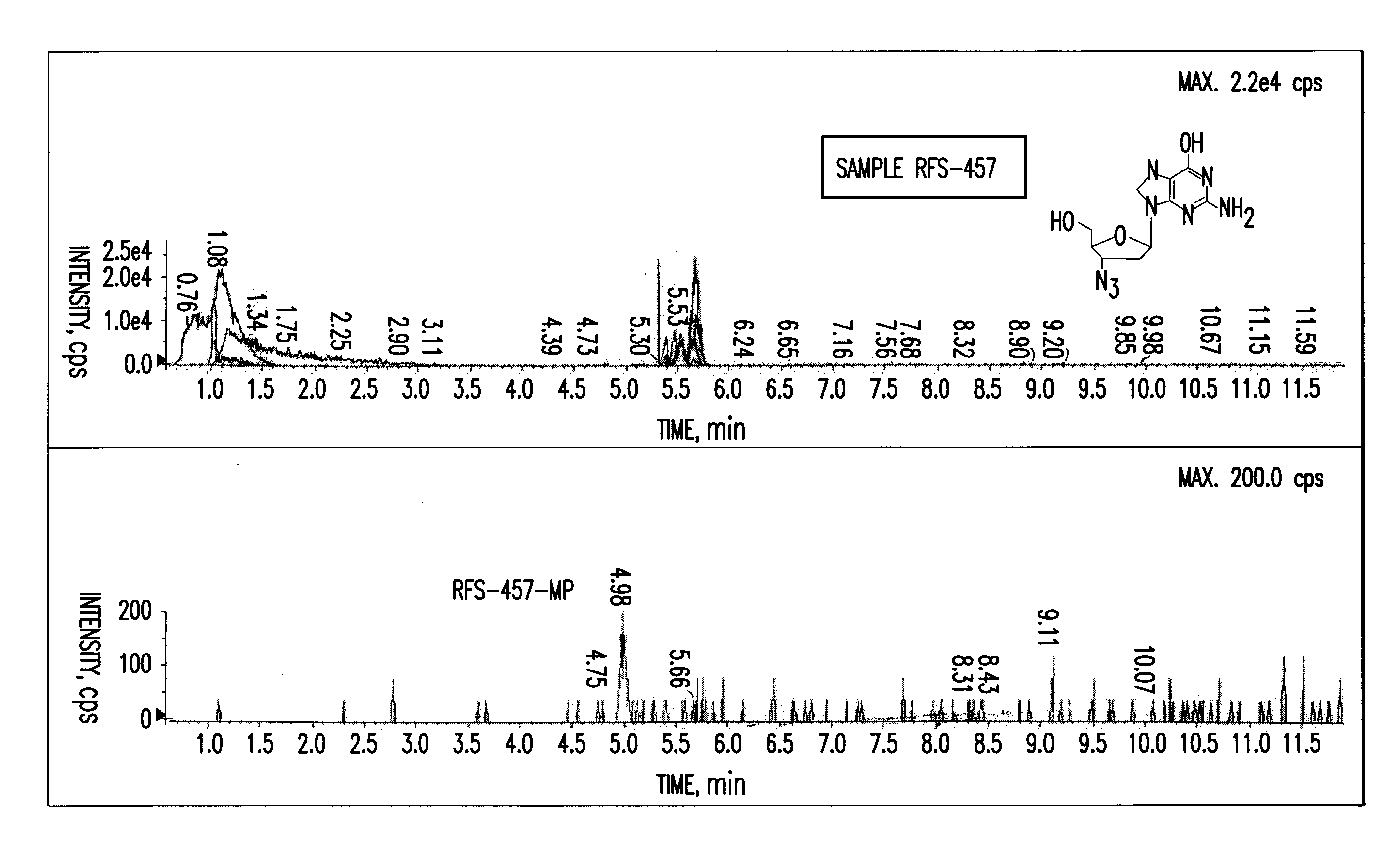
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2′-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:COCRYSTAL PHARMA INC
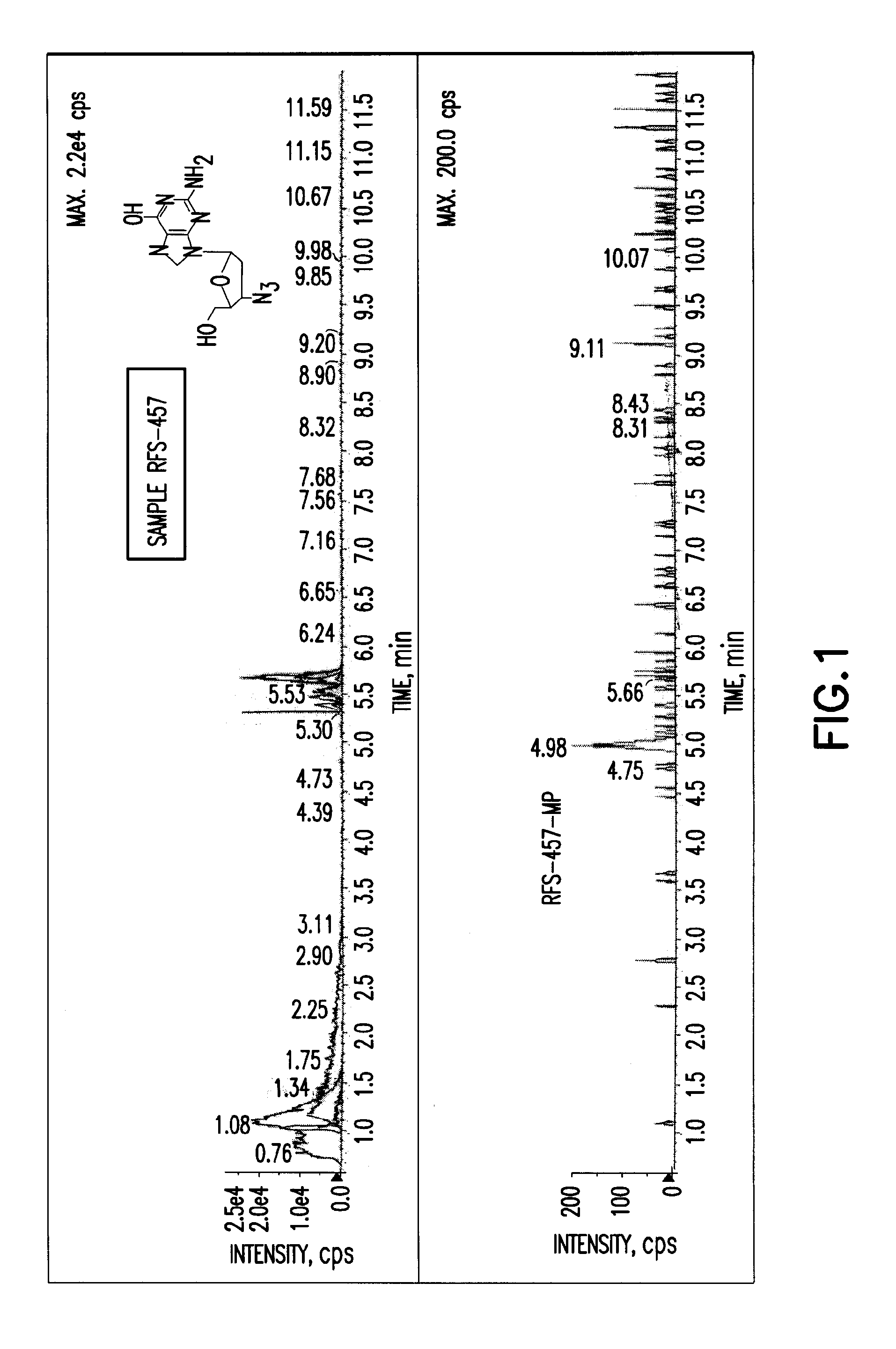
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
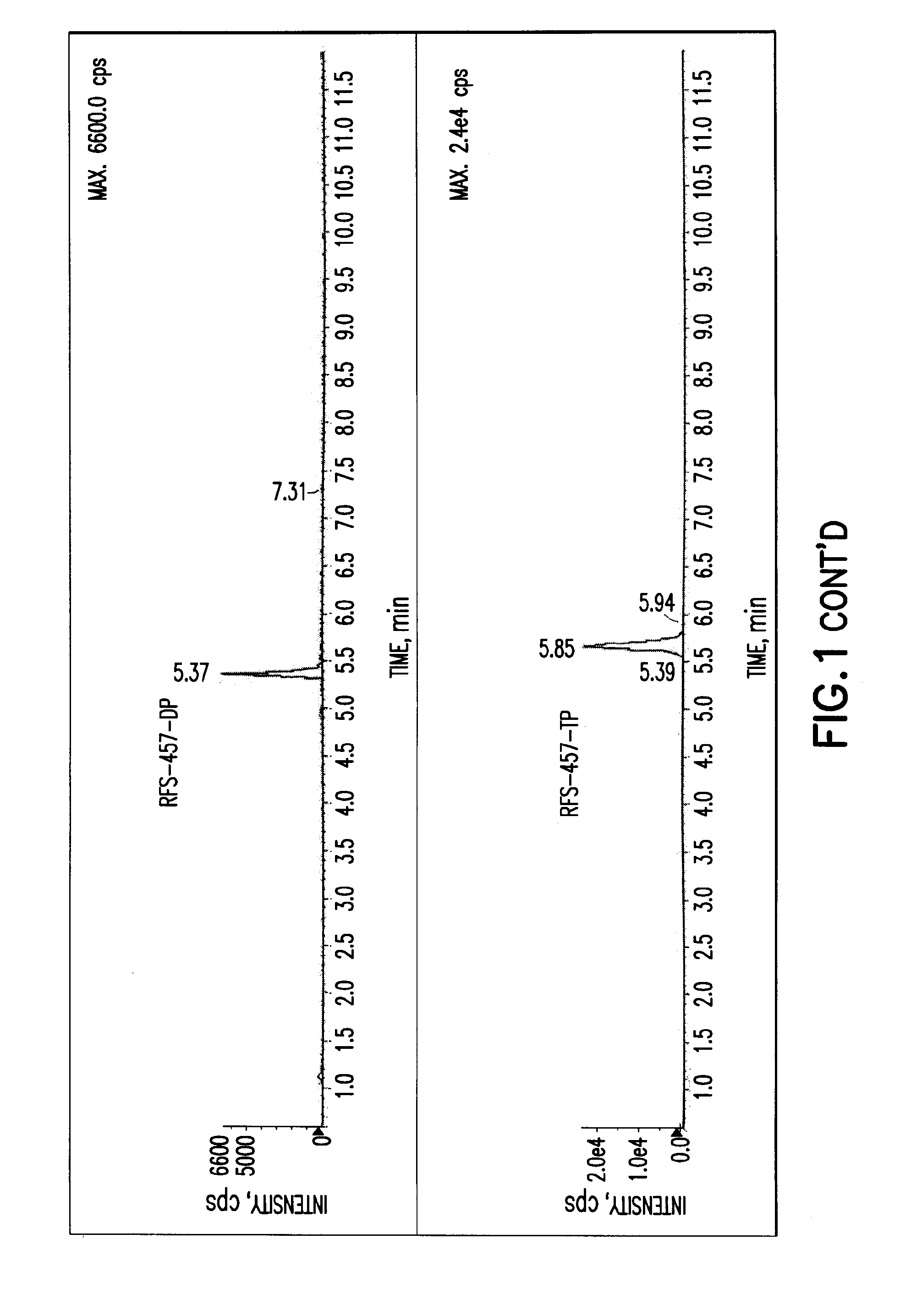
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
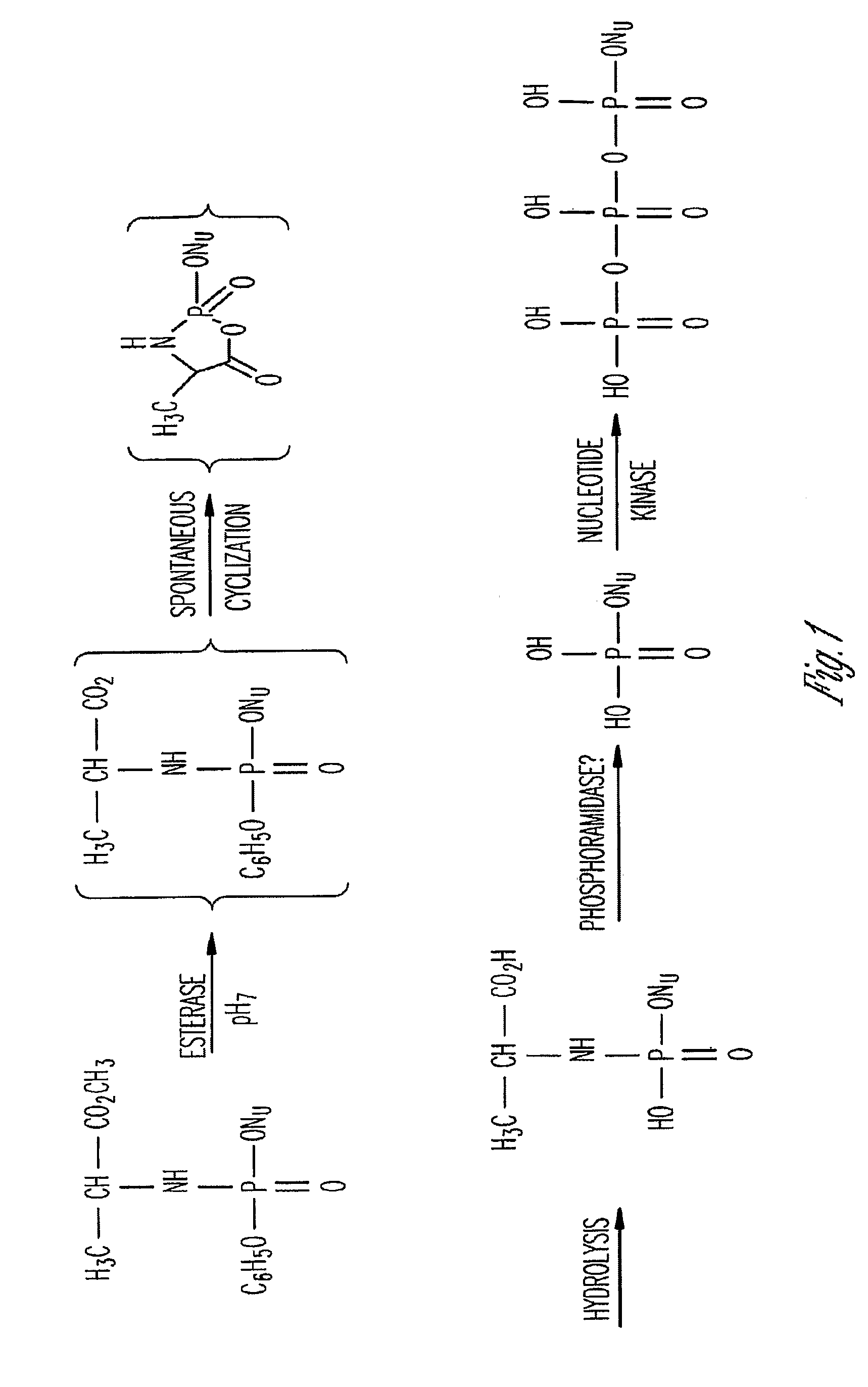
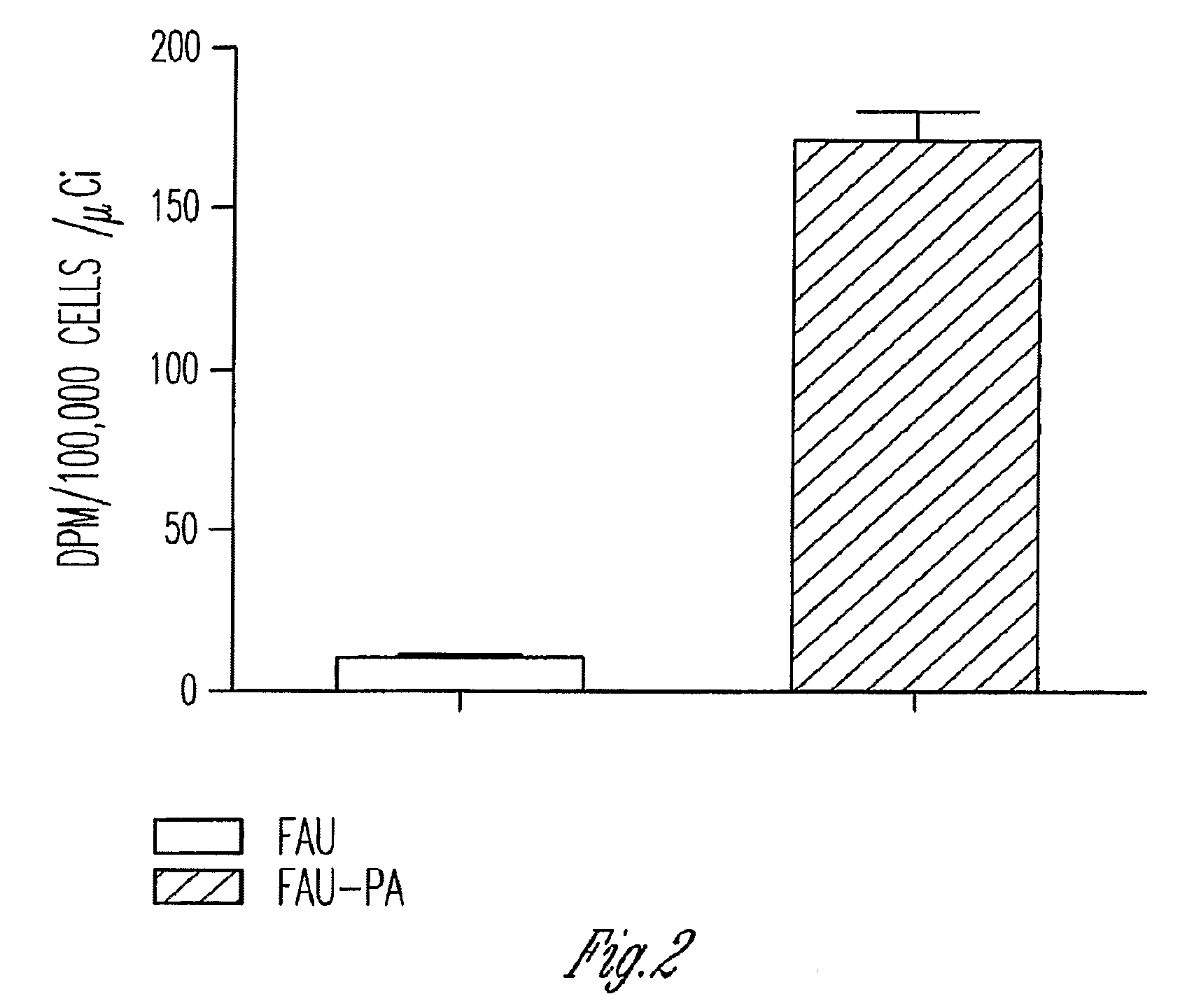
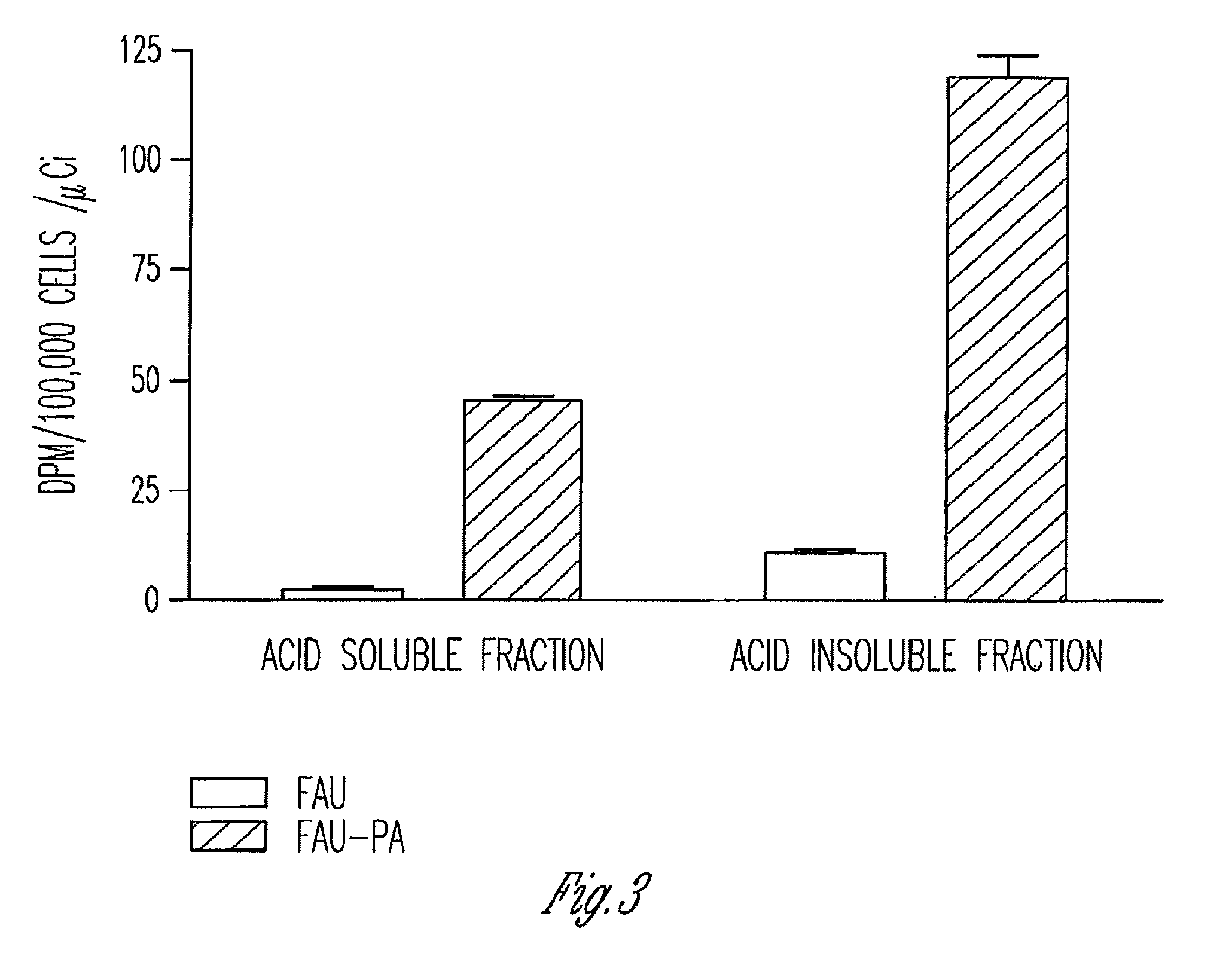
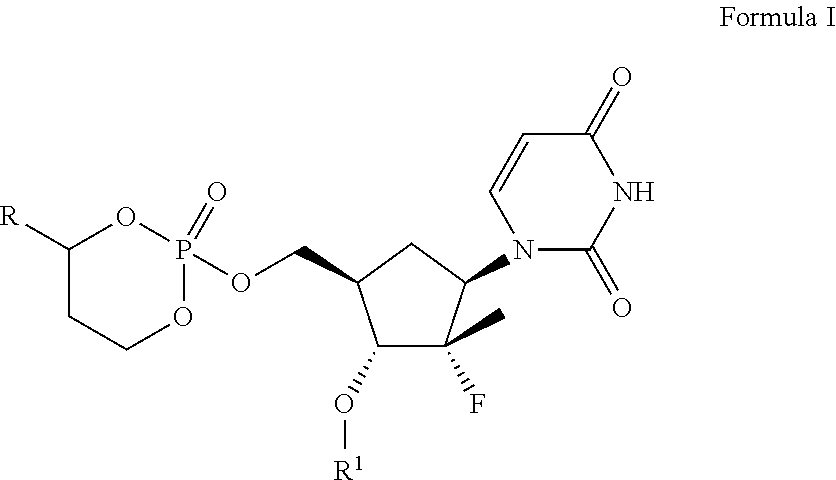
Phosphoramidate derivatives of FAU
InactiveUS7888330B2Efficient deliveryIncreased formationBiocideIn-vivo radioactive preparationsNucleoside monophosphatePhosphorylation
The present invention provides phosphoramidate derivatives of a furanosyluracil analog, FAU, that can effectively deliver FAU monophosphate, or a derivative thereof, intracellularly. FAU-Phosphoramidate diesters can bypass the first step of phosphorylation and be activated intracellularly so as to be converted to nucleoside monophosphates. This results in improved formation of nucleoside triphosphates, and higher incorporation into DNA. The compounds of the invention can be used to treat cancer.
Owner:WAYNE STATE UNIV
P2Y6 receptor agonists for treating lung diseases
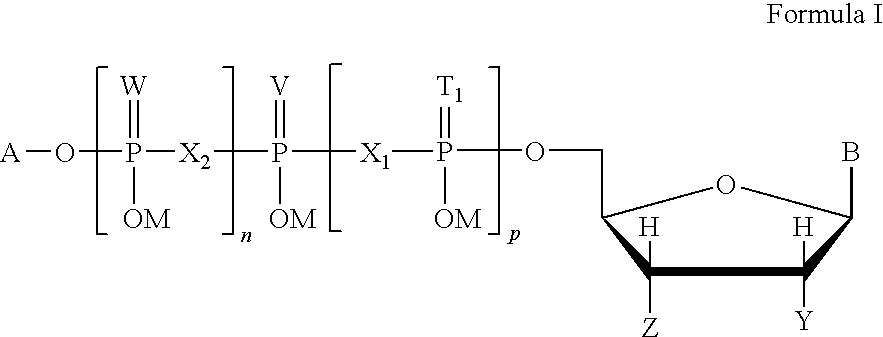
This invention is directed to a method of enhancing or facilitating the clearance of the lung mucus secretions in a subject. This invention is also directed to a method of facilitating the hydration of the lung mucus secretions in a subject. This invention is further directed to a method of preventing or treating diseases or conditions associated with impaired lung or airway function in a human or other mammal. The method comprises administering to a subject a pharmaceutical composition comprising a therapeutic effective amount of P2Y6 receptor agonist compound, wherein said amount is effective to activate the P2Y6 receptors on the luminal surface of lung epithelia. The P2Y6 receptor agonist compounds useful for this invention include mononucleoside 5′-diphosphates, dinucleoside monophosphate, dinucleoside diphosphates, or dinucleoside triphosphates of general Formula I, or salts, solvates, hydrates thereof.
Owner:MERCK SHARP & DOHME LLC
Crystallization process of 5'-nucleoside-sodium phosphate
InactiveCN1861624AQuality improvementImprove crystallization yieldSugar derivativesSugar derivatives preparationNucleoside monophosphateAlcohol
A crystallizing process for 5'-nucleoside-sodium phosphate includes such steps as proportionally adding the inorganic Na salt and solution educing agent, stirring while crystallizing at 15-40 deg.C, filtering, alcohol washing and vacuum drying. It has high output and controllable size of crystal.
Owner:NANJING UNIV OF TECH
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2'-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:RFS PHARMA +1
Radiologic Agents for Monitoring Alzheimer's Disease Progression and Evaluating a Response to Therapy and Processes for the Preparation of Such Agents
ActiveUS20090117041A1Effective imagingSugar derivativesRadioactive preparation carriersNucleoside monophosphatePositron emitters
Disclosed are certain cycloSalingenyl pyrimidine nucleoside monophosphates comprising positron emitters or gamma-emitting radiohalides, uses thereof for monitoring Alzheimer's disease progression and evaluating response to therapy and process for their preparation.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Crystallization process of 5'-nucleoside-sodium phosphate
InactiveCN100395256CQuality improvementImprove crystallization yieldSugar derivativesSugar derivatives preparationNucleoside monophosphateSodium phosphates
Owner:NANJING TECH UNIV
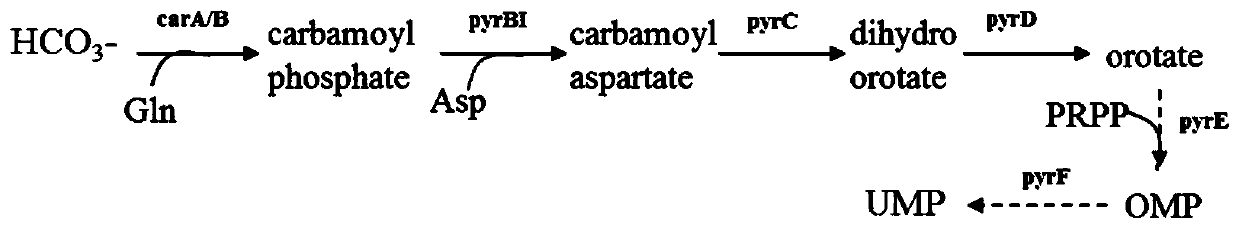
Recombinant microorganisms for producing orotic acid and method thereof
ActiveCN110564660AEnhanced metabolic fluxIncrease productionBacteriaHydrolasesOrotic acid metabolismOrotic aciduria
The invention provides a plurality of recombinant microorganisms for producing orotic acid and a method thereof. The recombinant microbial strains have one or more or all of the following characteristics: (1) the synthesis of phosphoribosyltransferase or orotic acid nucleoside monophosphate decarboxylase in an orotic acid metabolic pathway is weakened or completely lost; (2) a corresponding enzymeamount is increased through overexpression of five genes of enzymes participating in the orotic acid synthesis route; and (3) the corresponding transport protein is increased by overexpression of a transport protein coding gene for intracellular transport of orotic acid.
Owner:SUZHOU BIOSYNTHETICA CO LTD
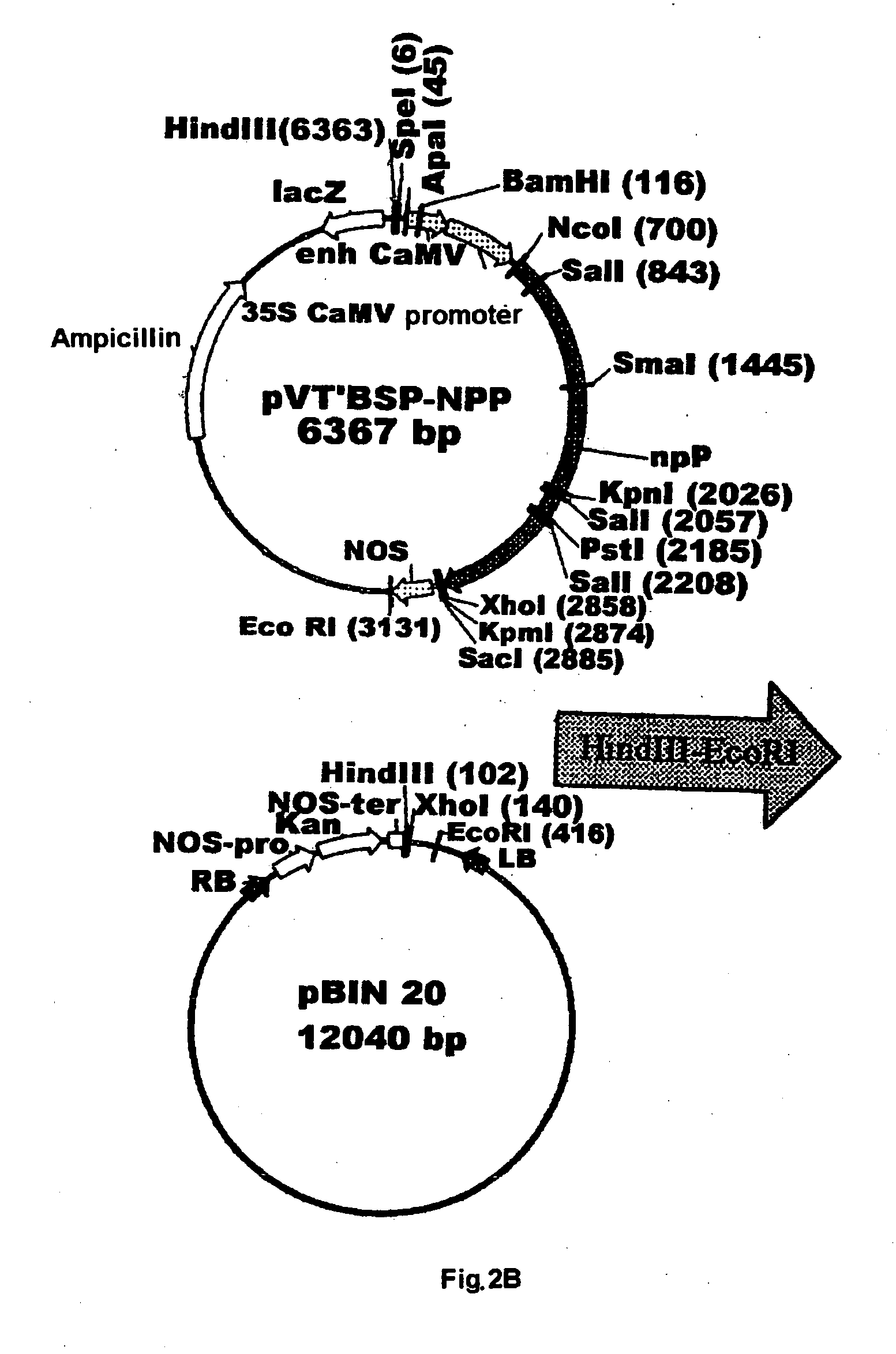
Plant nucleotide-sugar pyrophosphatase/phosphodiesterase (nppase), method of obtaining same and use of same in the production of assay devices and in the production of transgenic plants
InactiveUS20060242739A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHigh resistancePhosphate
Plant nucleotide pyrophosphatase / phosphodiesterase (NPPase), method of production, use in the manufacture of testing devices and in the production of transgenic plants. NPPase is an enzyme that catalyses the hydrolysis of a wide range of small molecules with phosphodiester and phosphosulphate bonds, in particular ADPG (adenosine diphosphate glucose) and APS (adenosine 5′-phosphosulphate). The enzyme obtained from plant extracts is used in assay devices for determining levels of nucleoside diphosphate sugars, based either on the sugar-1-phosphate released, or on the nucleoside monophosphate, both of which are products formed by the reaction catalysed by NPPase, as well as the detection of sulphonucleotides such as 3′-phosphoadenosine 5′-phosphosulphate (PAPS) and APS. The amino acid sequence of the enzyme is also described, as well as the nucleotide sequence of a complete cDNA and another incomplete cDNA. Finally, it describes the production of transgenic plants that overexpress NPPase and that have a high content of sugars, low content of starch and cell-wall polysaccharides and high resistance to high concentrations of salts and high temperature.
Owner:UNIV PUBLICA DE NAVARRA PAMPLONA +1
Bitterness screening agent as well as preparation method and application thereof
The invention provides a bitterness screening agent for eliminating the bitterness of a leaven enzyme extract and a seafood enzyme extract as well as a preparation method of the bitterness screening agent. The bitterness screening agent comprises nucleoside or nucleoside monophosphate, ethyl maltol, a licorice extract and amino acetic acid. According to the invention, substances with different debitterizing effect mechanisms are compounded to enable the agent to achieve an effect of comprehensively screening and shielding the bitter peptides in yeast extracts or seafood hydrolysate, so that the acceptance of substrate is higher, and the application range of the substrate is enlarged.
Owner:楚大波
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464294AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdenosine diphosphateAbsorbance
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, adenosine diphosphate, an oxaloacetic acid, a ketoglutaric acid, pyruvate carboxylase, isocitrate dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
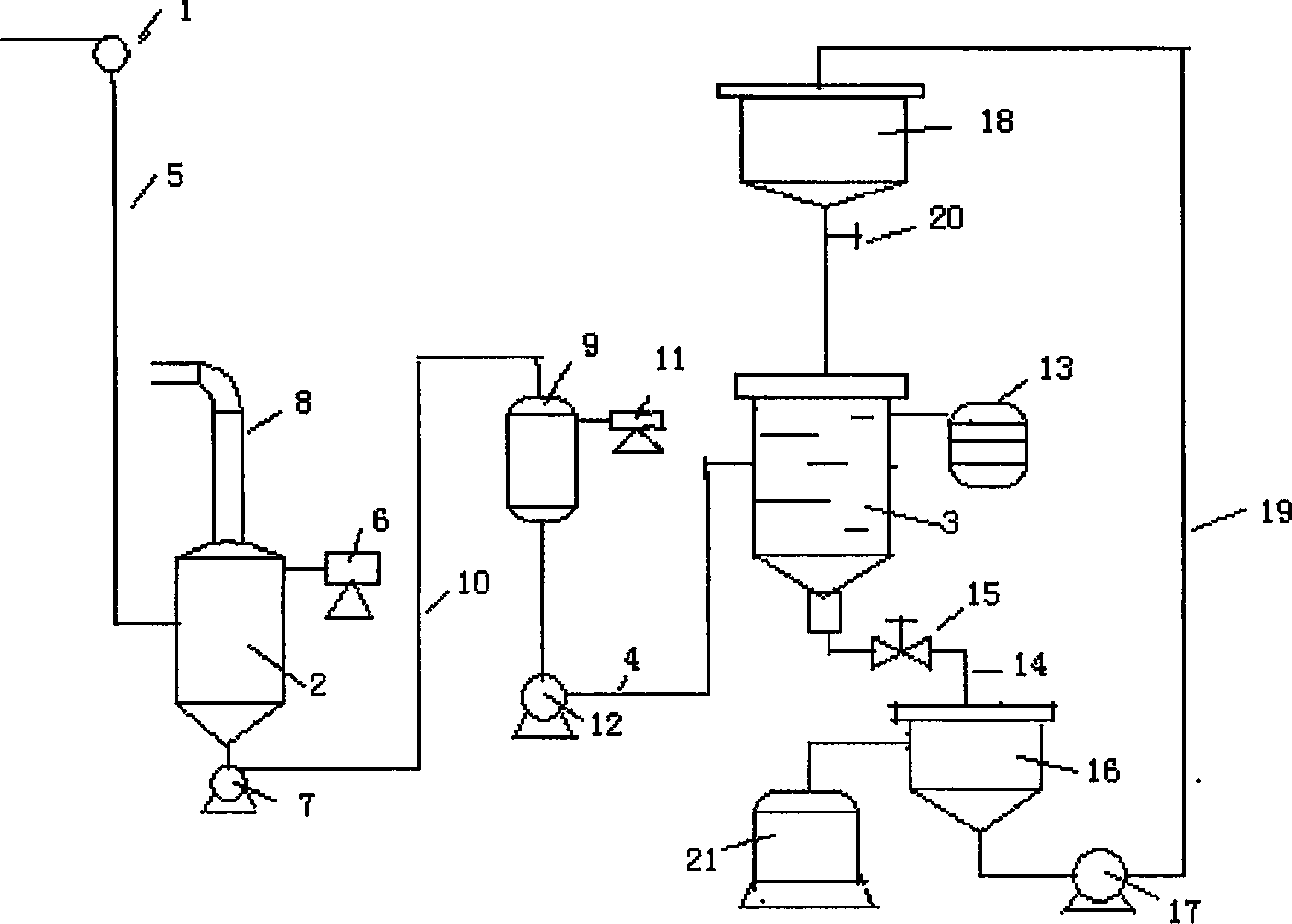
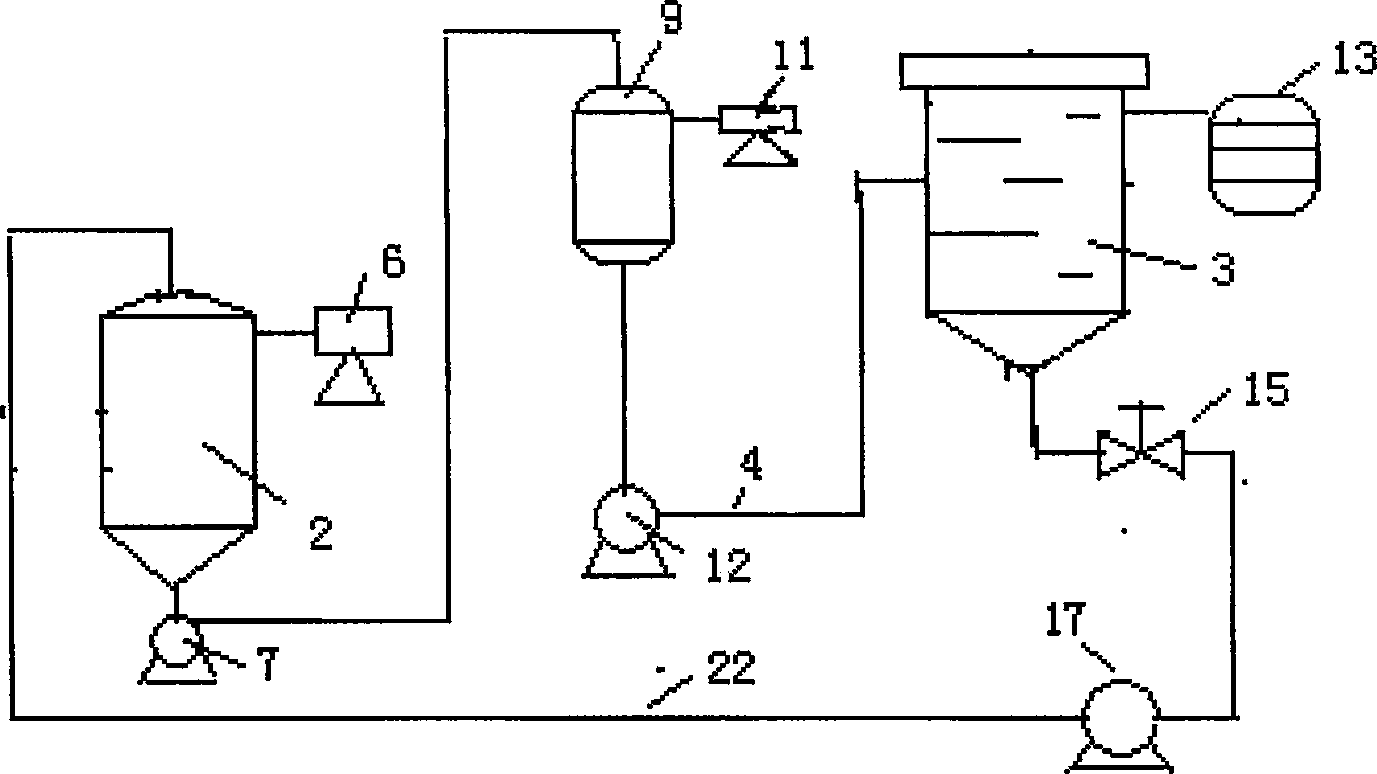
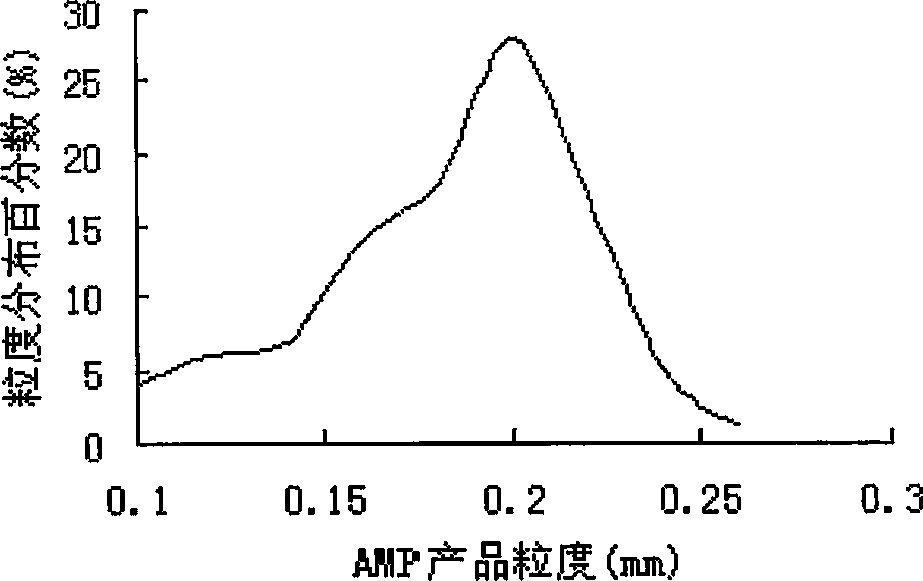
Technique and equipment for crystallizing nucleotide
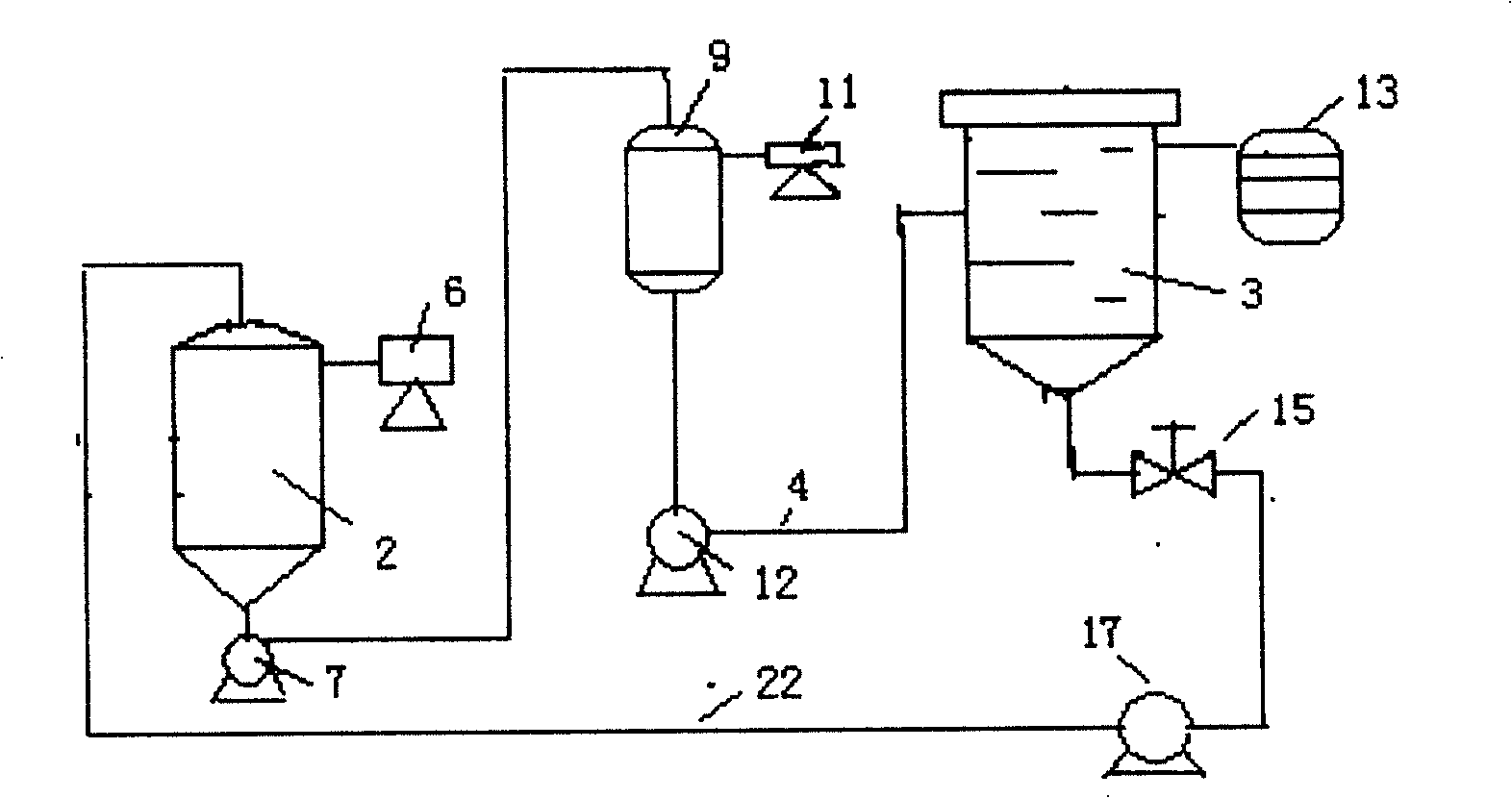
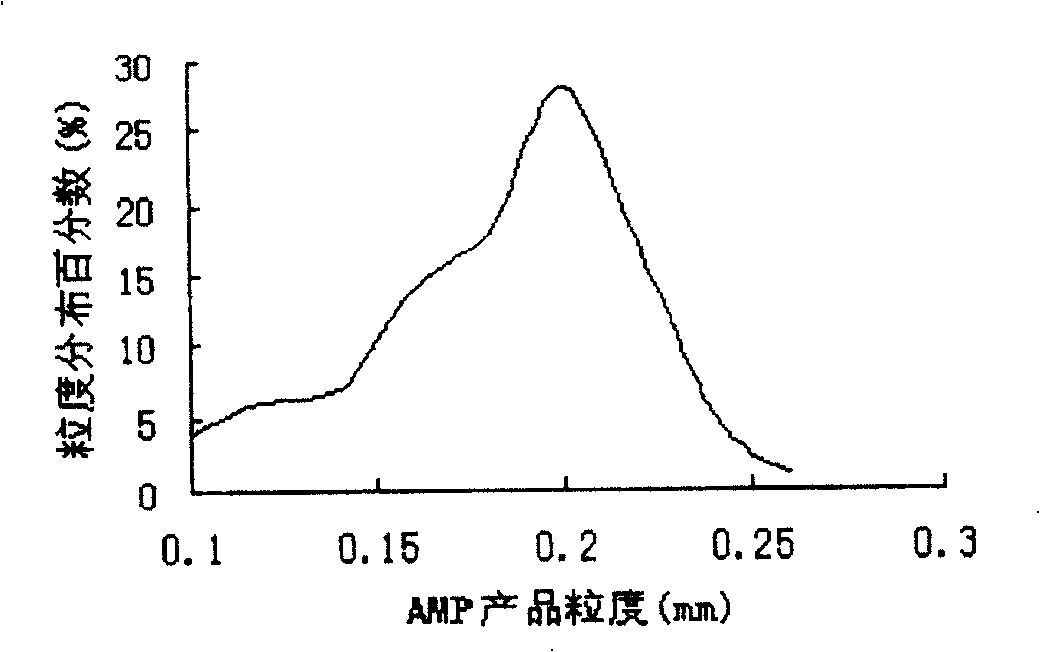
ActiveCN1873004ALarge and uniform particlesHigh purityDNA preparationNucleoside monophosphateCrystal structure
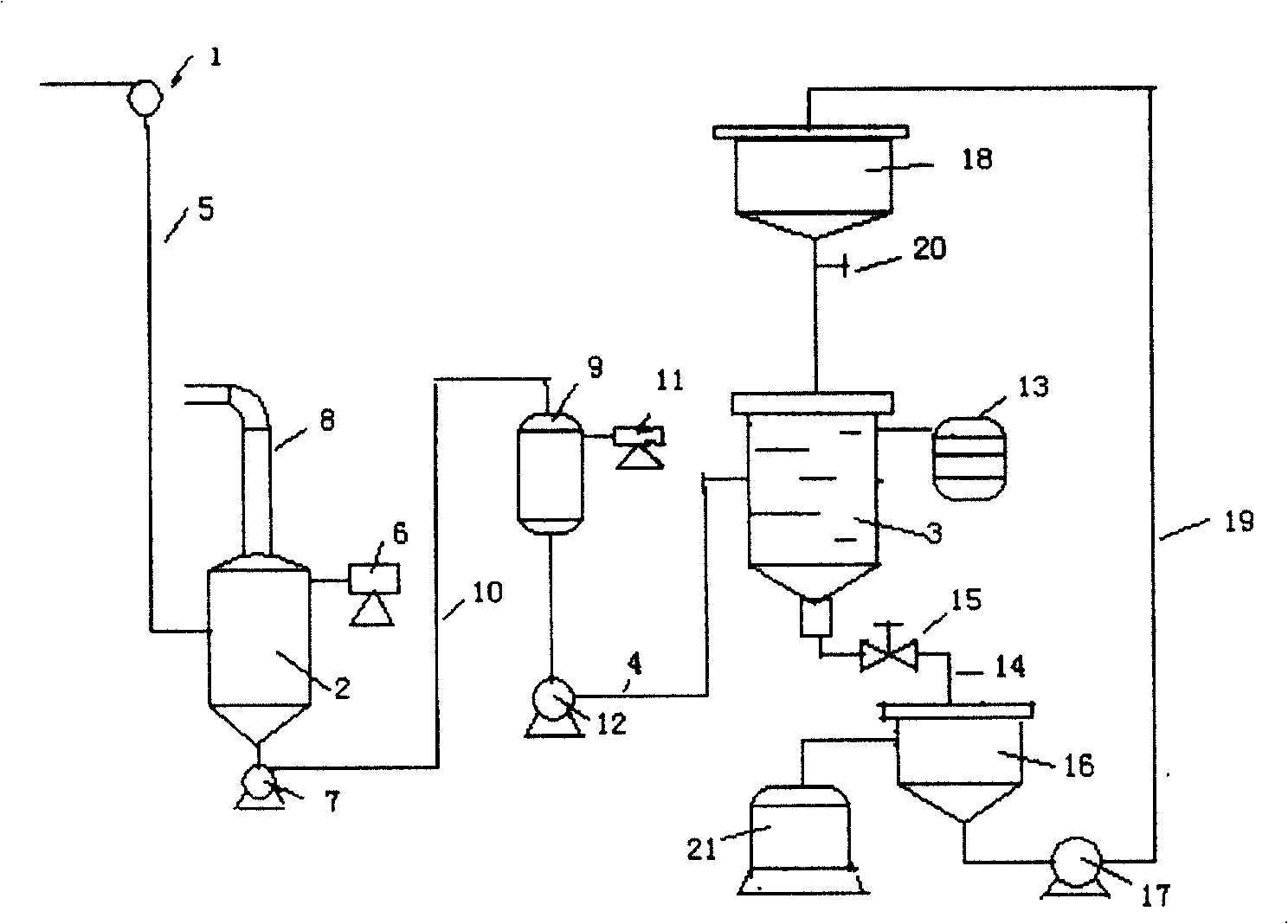
This invention provides method and device for directly crystallizing nucleoside monophosphate (NMP) from its solution. The process comprises: (1) vaporizing and concentrating crude NMP solution to obtain an oversaturate solution; (2) introducing the oversaturated solution to crystallizer by a heat-insulating pipe, and crystallizing at constant temperature and stirring speed to obtain NMP crystals. The process has such advantages as low cost, high crystal quality, good crystal structure, high crystal purity and high yield.
Owner:NANJING UNIV OF TECH
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464286AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsNucleotidaseAdenosine diphosphate
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, coenzyme, nucleoside monophosphate, glutamine, adenosine diphosphate, glutamine synthetase, glutamate dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the increase in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Polyphosphate:AMP phosphotransferase
ActiveUS7329522B2High specific activityEfficient ATP generation/regeneration systemSugar derivativesBacteriaKilodaltonPhosphoric acid
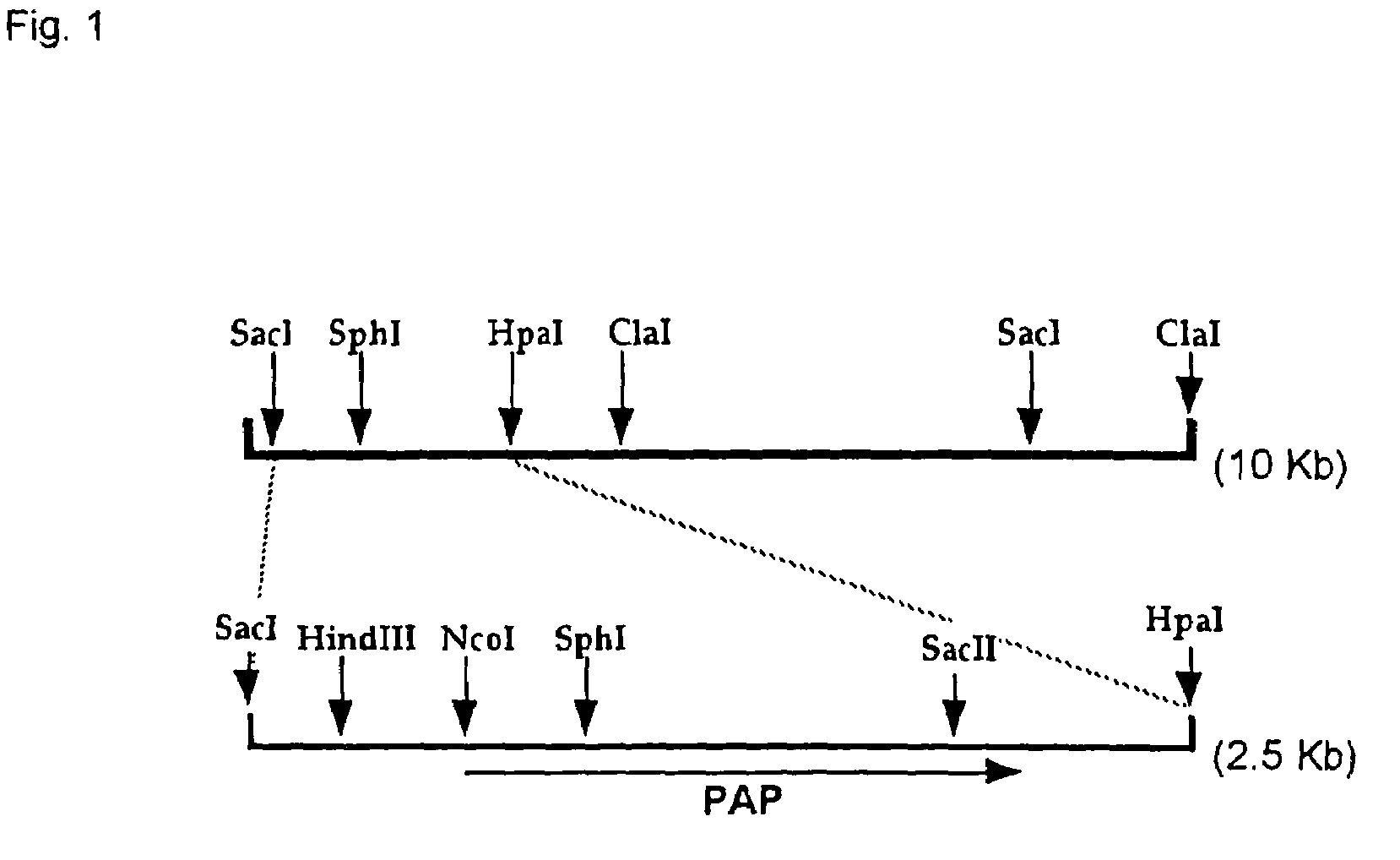
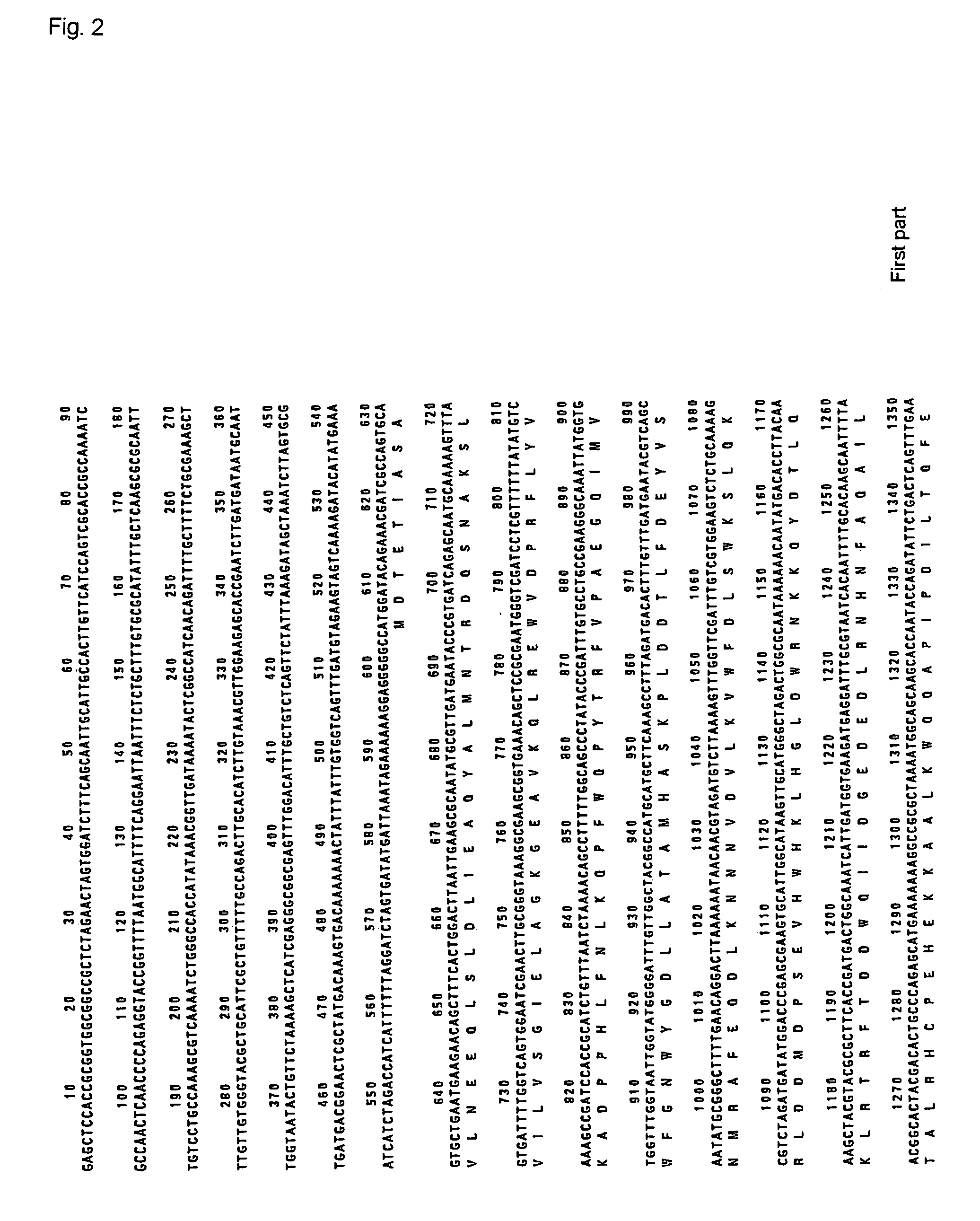
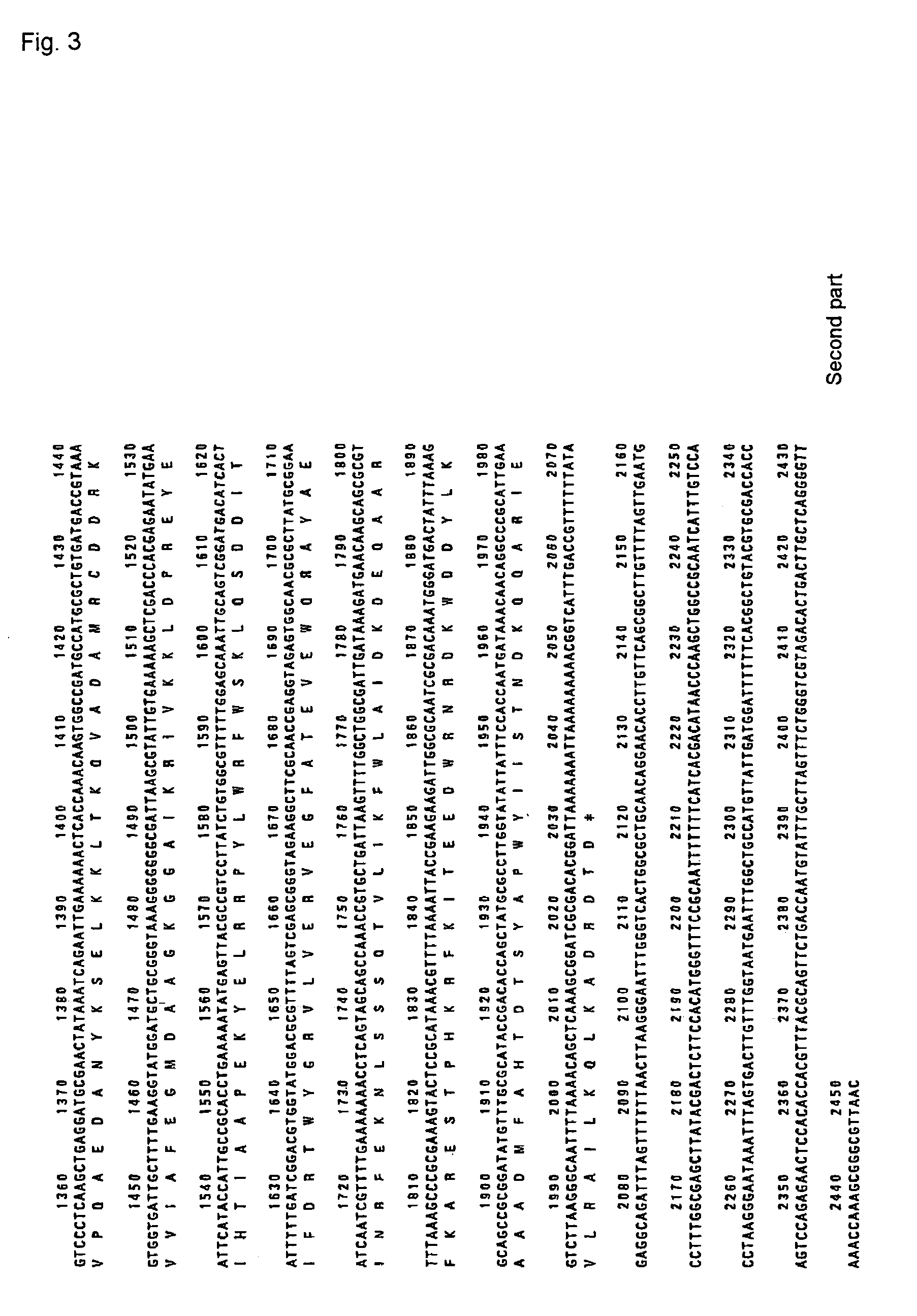
This invention relates to a novel polyphosphate: AMP phosphotransferase (PAP), a gene coding this PAP, and their use. The PAP has the following properties:(A) action: catalyzing of the following two reactions:NMP+PolyP(n)→NDP+PolyP(n-1) dNMP+POlyP(n)→dNDP+PolyP(n-1) (wherein NMP represents nucleoside monophosphate, NDP represents nucleoside diphosphate, dNMP represents deoxynucleoside monophosphate, dNDP represents deoxynucleoside diphosphate, n represents degree of polymerization of the polyphosphate which is an integer of up to 100);(B) substrate specificity: specific to AMP, GMP, IMP, dAMP, and dGMP, also acting with CMP, UMP, dCMP, and TMP;(C) molecular weight: about 55 to 56 Kd (kilodalton); and(D) specific activity: at least 70 units per 1 mg of enzyme protein.
Owner:YAMASA SHOYU CO LTD
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464260AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsActivity concentrationNucleoside monophosphate
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, coenzyme, nucleoside monophosphate, sucrose, sucrose phosphorylase, fructose dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the increase in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Technique and equipment for crystallizing nucleotide
ActiveCN100422324CLarge and uniform particlesHigh purityDNA preparationNucleoside monophosphateCrystal structure
This invention provides method and device for directly crystallizing nucleoside monophosphate (NMP) from its solution. The process comprises: (1) vaporizing and concentrating crude NMP solution to obtain an oversaturate solution; (2) introducing the oversaturated solution to crystallizer by a heat-insulating pipe, and crystallizing at constant temperature and stirring speed to obtain NMP crystals. The process has such advantages as low cost, high crystal quality, good crystal structure, high crystal purity and high yield.
Owner:NANJING TECH UNIV
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464291AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsPhosphoenolpyruvate carboxylaseAbsorbance
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, an oxaloacetic acid, acetylcoenzyme A, phosphoenolpyruvate carboxykinase, pyruvate dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
5'nucleotidase diagnostic kit and method for measuring 5'nucleotidase activity concentration
InactiveCN101609008AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsWavelengthAdenosine diphosphate
The invention relates to a 5'nucleotidase diagnostic kit utilizing technologies of an enzymatic-colorimetric method and an enzyme-link method, also relates to a method and a principle of measuring the activity concentration of 5'nucleotidase and compositions and components of reagents, and belongs to the technical field of testing and measuring of medical science. The kit mainly comprises the following compositions: buffer solution, reduced coenzyme, nucleoside monophosphate, adenosine diphosphate, oxaloacetic acid, pyruvate carboxylase, formaldehyde dehydrogenase and stabilizer; samples are mixed with the reagents in certain volume ratio to perform a series of enzymatic reactions; then reactants are placed under a UV / visible analyzer; and the descending speed of absorbance is tested at the position where dominant wave length is 340nm so as to measure and calculate the activity concentration of the 5'nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
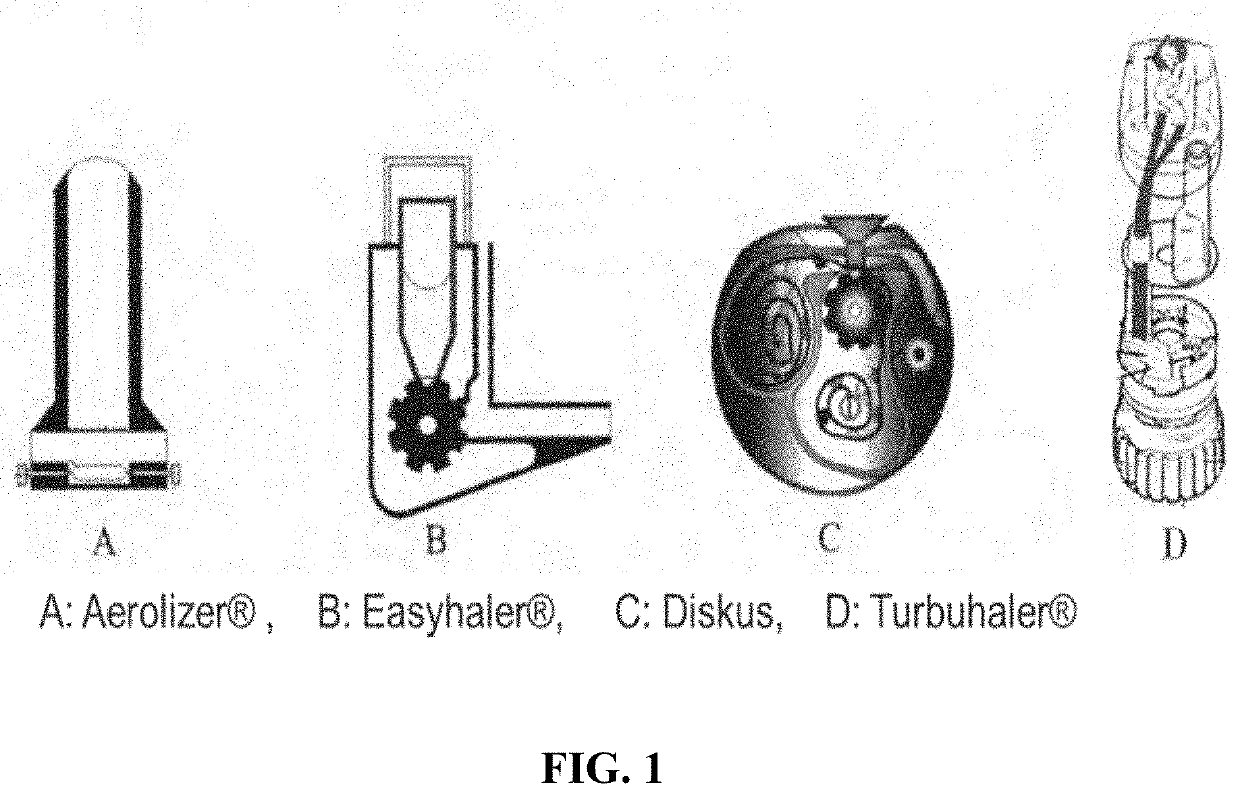
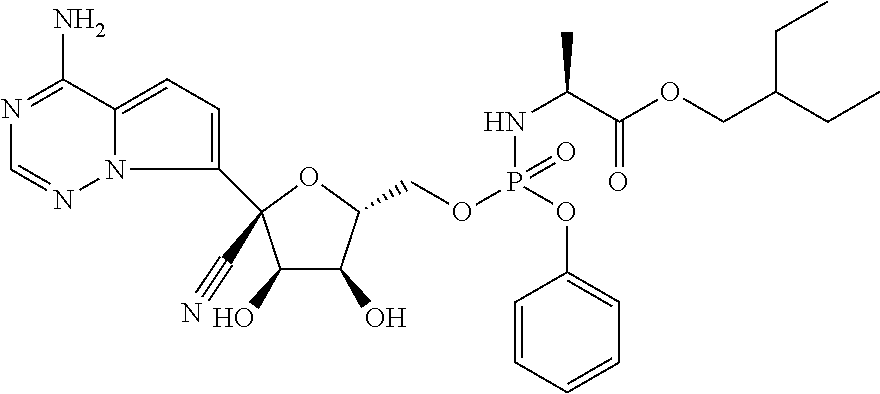
Pharmaceutical formulation containing remdesivir and its active metabolites for dry powder inhalation
InactiveUS20210353650A1Decrease and remove viral loadEffective and easy to administerPowder deliveryOrganic active ingredientsDiseaseRespiratory disease
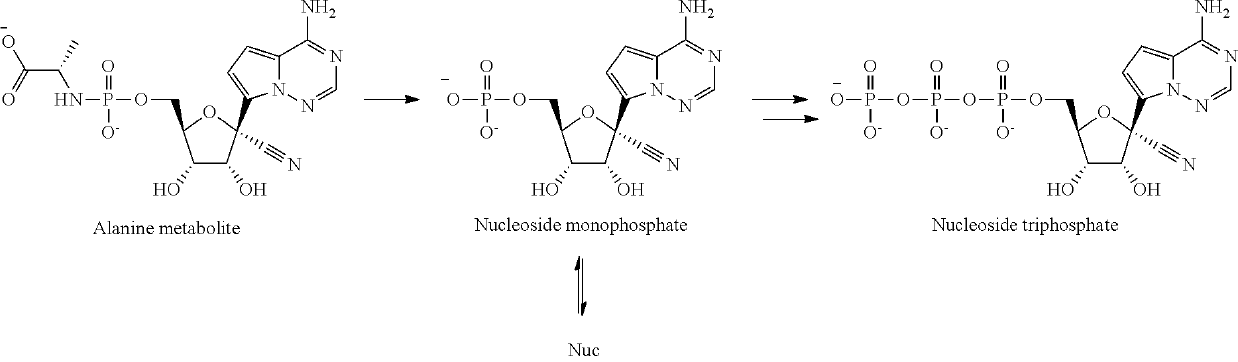
The invention provides a pharmaceutical composition for dry powder inhalation and a preparation method thereof, wherein the composition comprises, a carrier material, preferably lactose and micronized remdesivir and / or its active metabolites (such as Alanine metabolite (Ala-met), Nucleoside monophosphate, and Nucleoside Triphosphate (NTP)) and / or its analog GS-441524 and pharmaceutically acceptable salts thereof. The active pharmaceutical ingredient may be an anti-viral, taken as remdesivir and / or its active metabolites (such as Alanine metabolite (Ala-met), Nucleoside monophosphate. and Nucleoside Triphosphate (NTP)) and / or its analog GS-441524. The dry powder inhalation containing Remdesivir and / or its active metabolites and / or its analog GS-441524 as active ingredients, further consisting of a breath-powered, dry powder inhaler, and a cartridge for delivering a dry powder formulation deep into the lungs for the treatment of respiratory disorders. The inhaler and cartridge can be provided with a drug delivery formulation comprising, for example, an active ingredient, including, small organic molecules, including, remdesivir and / or its active metabolites (such as Alanine metabolite (Ala-met), Nucleoside monophosphate, and Nucleoside Triphosphate (NTP)) and / or its analog GS-441524 and pharmaceutically acceptable salts thereof for the treatment of disease and disorders, for example, COVID-19 and other viral respiratory infections.
Owner:ANOVENT PHARM U S LLC
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464257AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsPhosphoenolpyruvate carboxylaseNucleotidase
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, adenosine diphosphate, an oxaloacetic acid, phosphoenolpyruvate carboxykinase, formaldehyde deoxygenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Derivatives of uridine 5'-cyclophosphate useful to treat hepatitis c viral infections
Some embodiments of the present invention include nucleoside 5′-monophosphate derivative compounds, their preparation and their uses. In some embodiments, such compounds are useful to treat hepatitis C viral infections.
Owner:LIGAND PHARMA INC
5'nucleotidase diagnostic kit and method for measuring activity concentration of 5'nucleotidase
InactiveCN101609007AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdenosine diphosphateWavelength
The invention relates to a 5'nucleotidase diagnostic kit utilizing technologies of an enzymatic-colorimetric method and an enzyme-link method, also relates to a method and a principle of measuring activity concentration of 5'nucleotidase and compositions and components of reagents, and belongs to the technical field of testing and measuring of medical science. The kit mainly comprises the following compositions: buffer solution, coenzyme, nucleoside monophosphate, glutamine, adenosine diphosphate, glutamine synthetases, glutamate synthetase and stabilizer; samples are mixed with the reagents in certain volume ratio to perform a series of enzymatic reactions; then reactants are placed under a UV / visible analyzer; and the descending speed of absorbance is tested at the position where dominant wave length is 340nm so as to measure and calculate the activity concentration of the 5'nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464287AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdenosine diphosphateAbsorbance
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, adenosine diphosphate, an oxaloacetic acid, ammonium chloride, pyruvate carboxylase, alanine dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method for preparing nucleoside triphosphate and deoxynucleoside triphosphate from polyphosphate
PendingCN113122593AThe synthesis process is simplePromote safe productionFermentationOrganosolvPhosphoric acid
The invention discloses a method for preparing nucleoside triphosphate and deoxynucleoside triphosphate from polyphosphate. The method comprises the following steps: (1) preparing nucleoside monophosphate kinase and PPK enzyme required by reaction; (2) reacting to generate NTP and dNTP: adding polyphosphate into a reaction system taking NMP and dNMP as substrates, and reacting the NMP, dNMP and polyphosphate under the combined action of nucleotide monophosphate kinase and PPK enzyme to generate dNTP and NTP; and (3) separating a target product. The high-efficiency and specificity of the biological enzyme of the invention enables the whole synthesis process to be simple, stable and rapid, and the whole process is free of addition of pollutants such as organic solvents, the whole process is green and environment-friendly, the production is safe, the period is short and the cost is low. ATP does not need to be added as an energy source in the reaction, and the raw material cost is saved by 50% or above; there are few by-products generated in the reaction, and it is easy for later purification without the interference of ATP, AMP and ADP; the enzyme dosage is small, and the reaction is rapid.
Owner:ANHUI GSH BIO TECH CO LTD
5'-nucleotidase diagnosis reagent kit and 5'-nucleotidase active concentration determination method
InactiveCN101464292AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdenosine diphosphateAlanine dehydrogenase
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, glutamine, adenosine diphosphate, a pyruvic acid, glutamine synthetase, alanine dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
5'-NT diagnosing kit and method for measuring 5'-NT activity concentration
InactiveCN101464293AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdenosine diphosphateAbsorbance
The invention relates to a kit for diagnosing 5'-nucleotidase by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the active concentration of 5'-nucleotidase, and belongs to the technical field of medical inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, nucleoside monophosphate, glutamine, adenosine diphosphate, a ketoglutaric acid, glutamine synthetase, glutamate dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of 5'-nucleotidase.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
P2y6 receptor agonists for treating lung diseases
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com