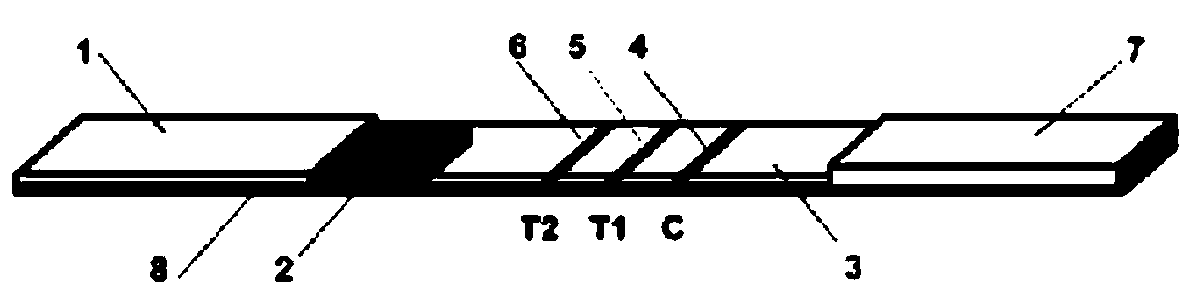
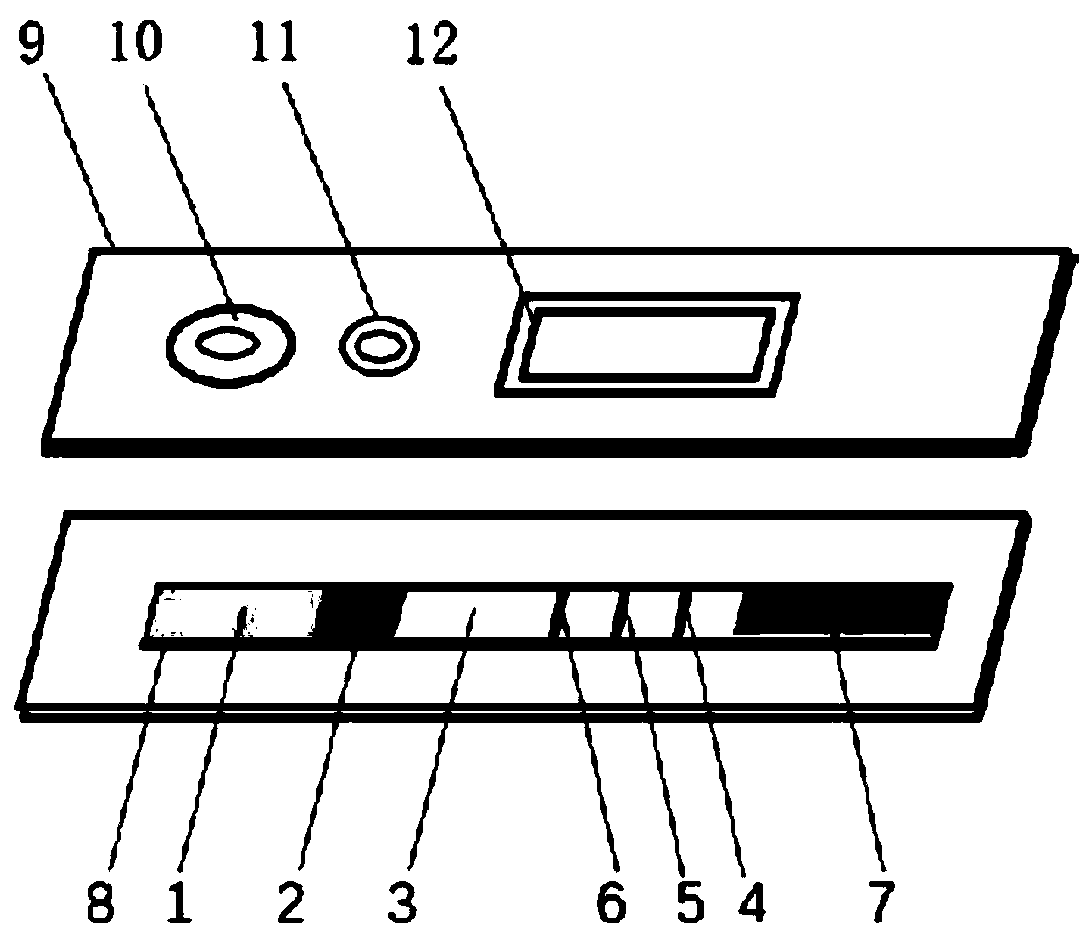
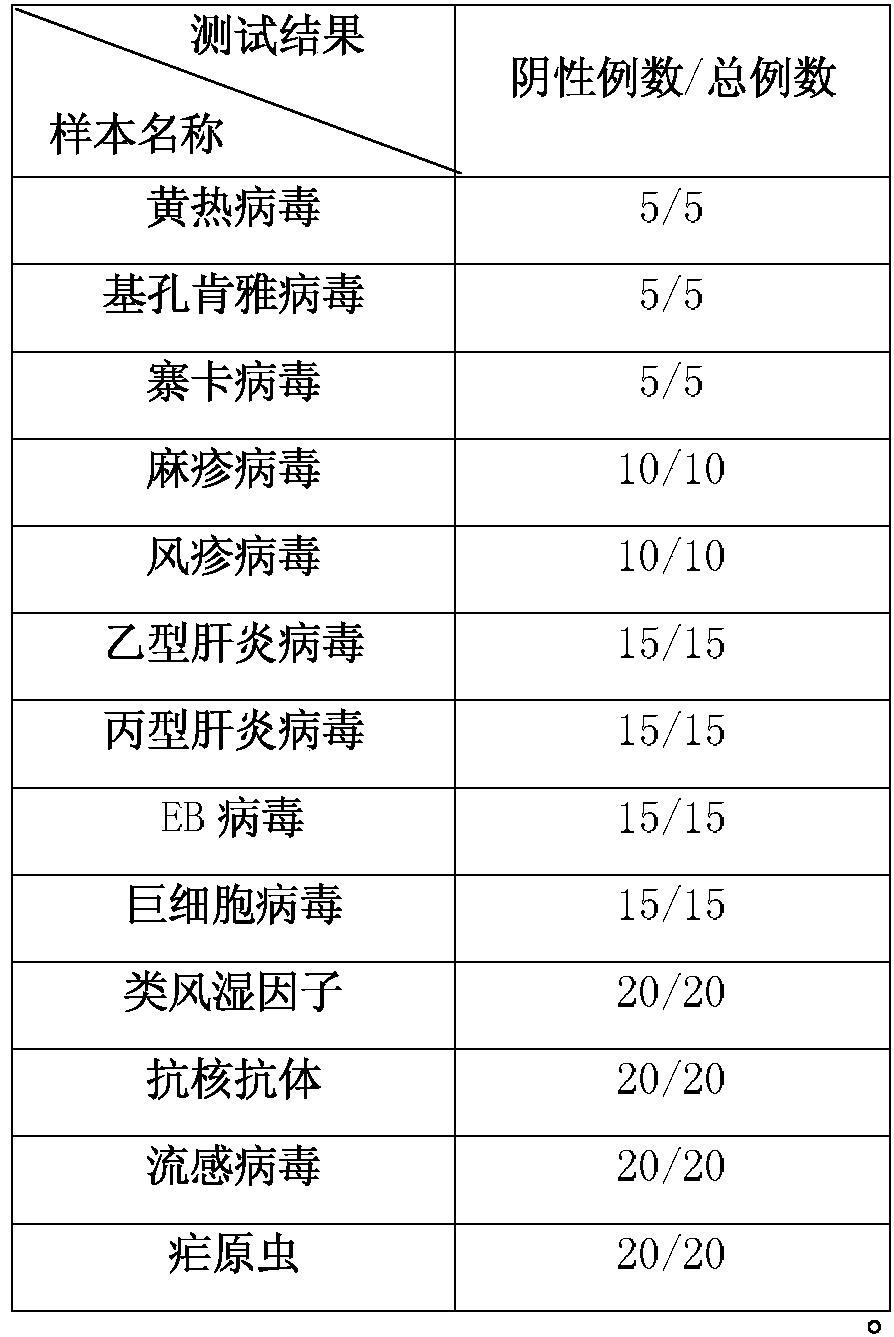
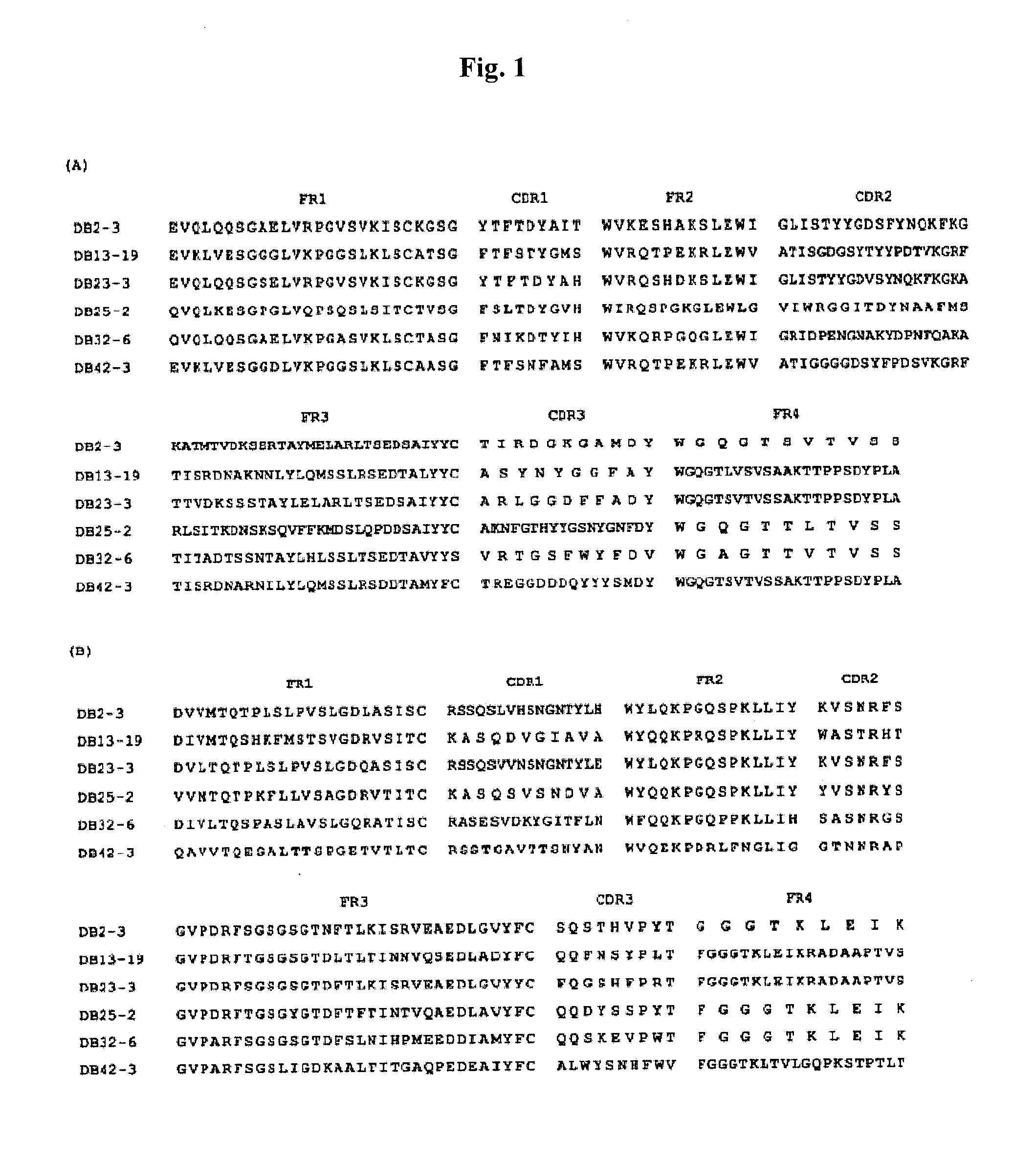
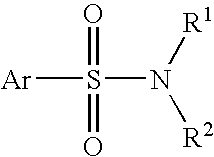
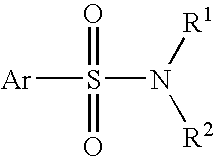
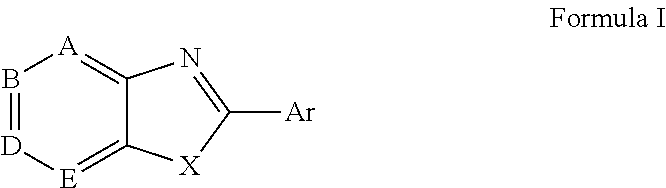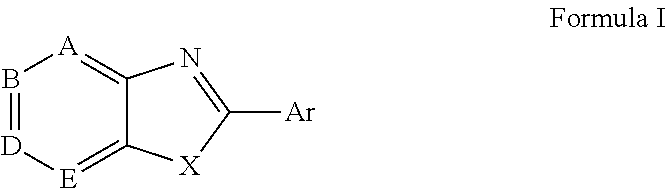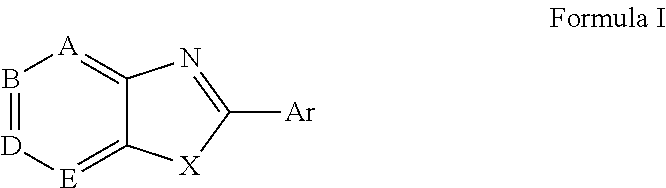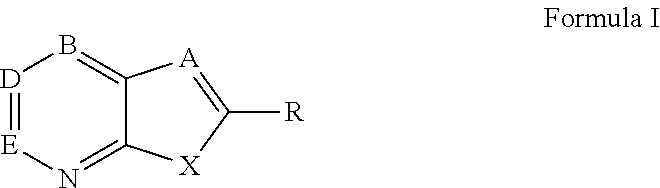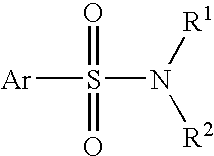Patents
Literature
598 results about "Dengue virus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
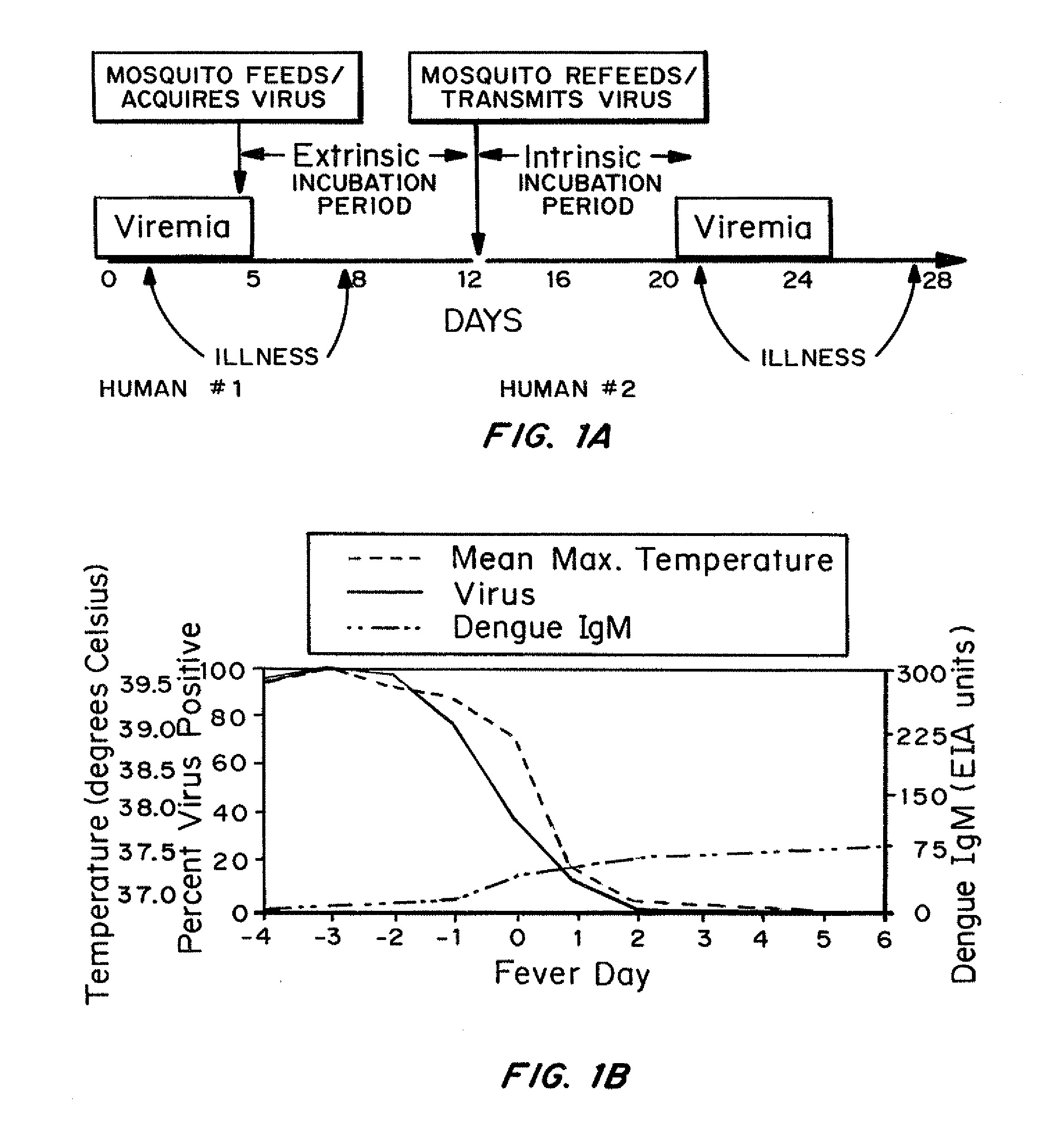
Jump to navigation Jump to search. Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Five serotypes of the virus have been found, all of which can cause the full spectrum of disease.
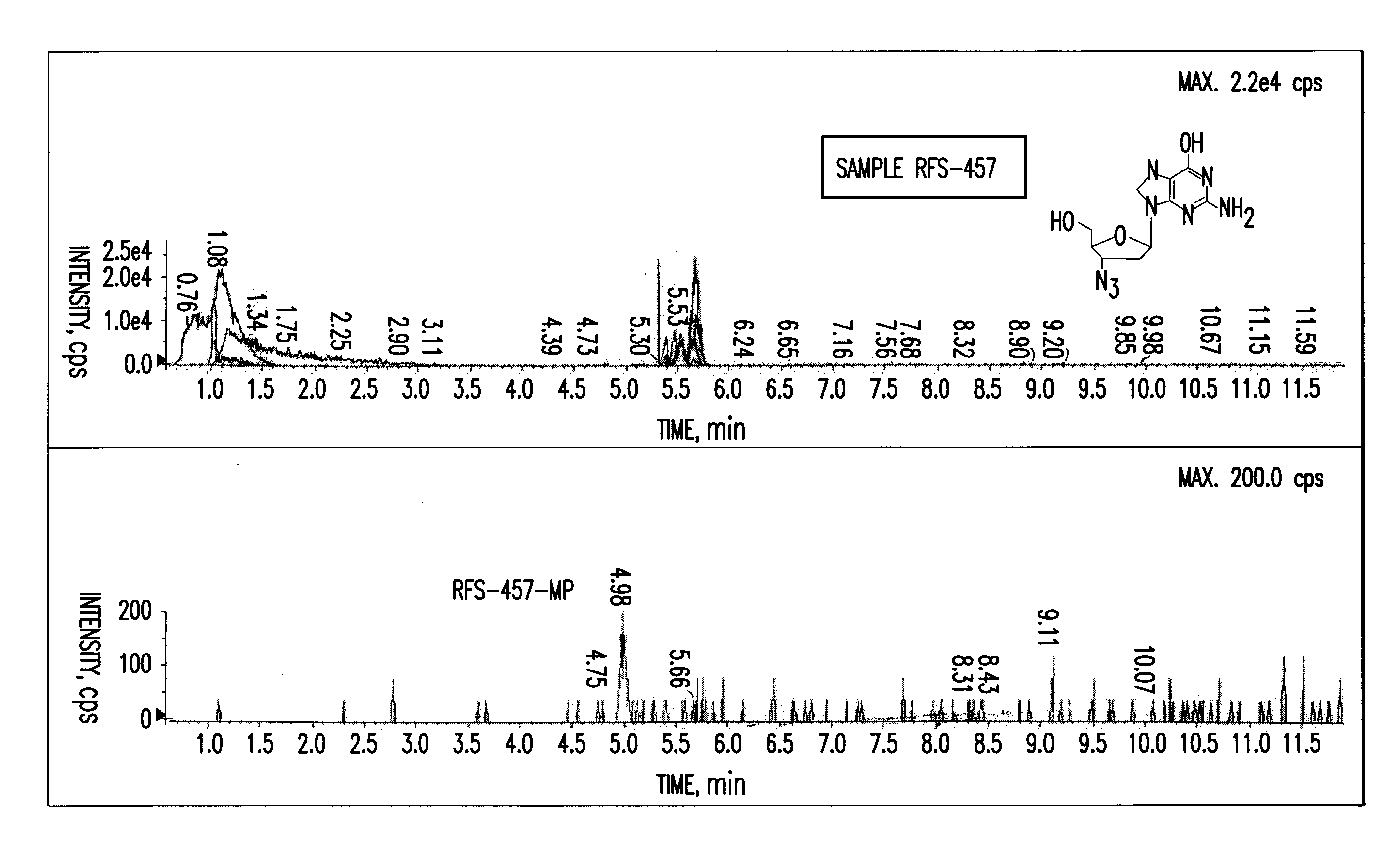
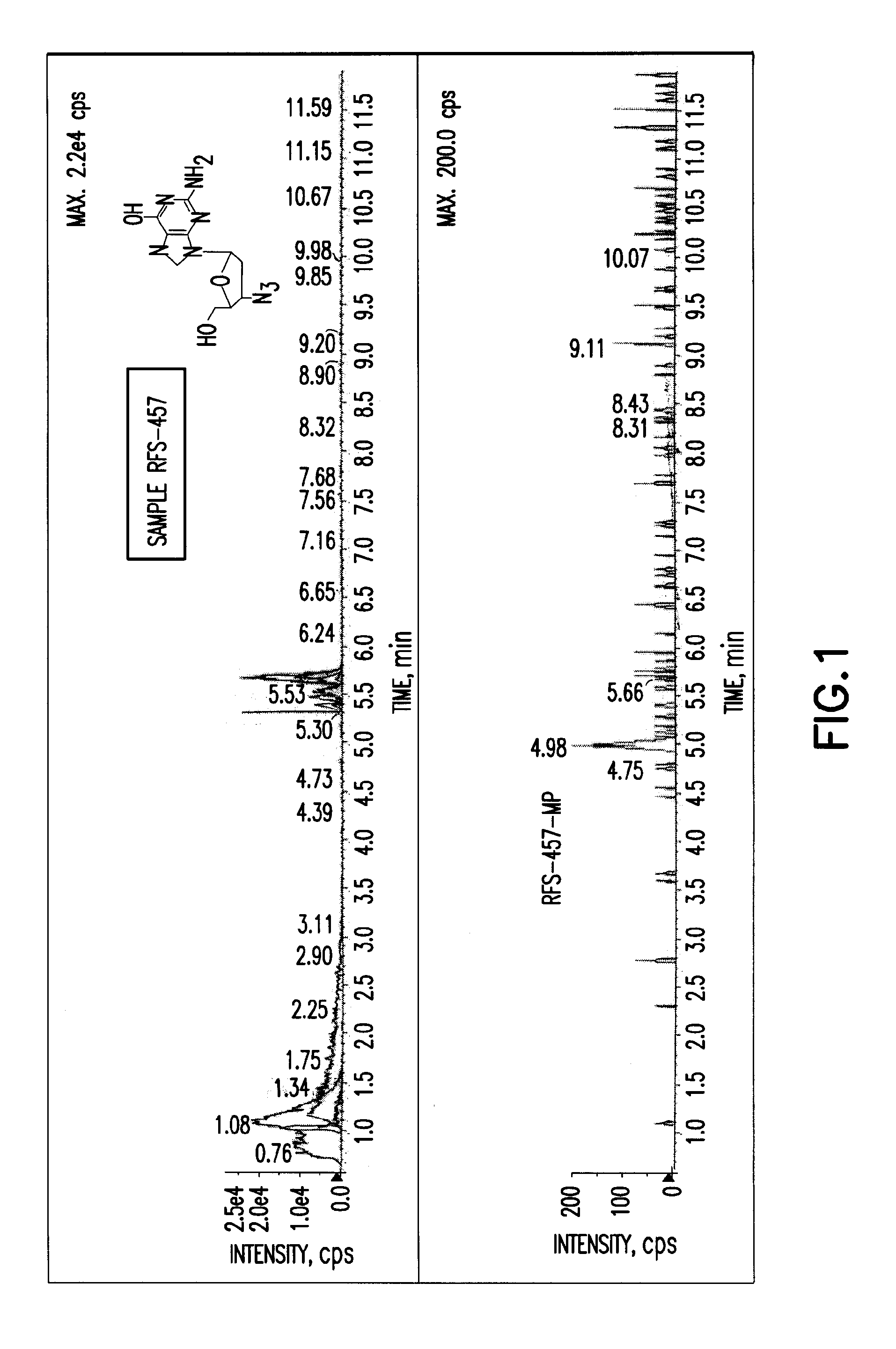
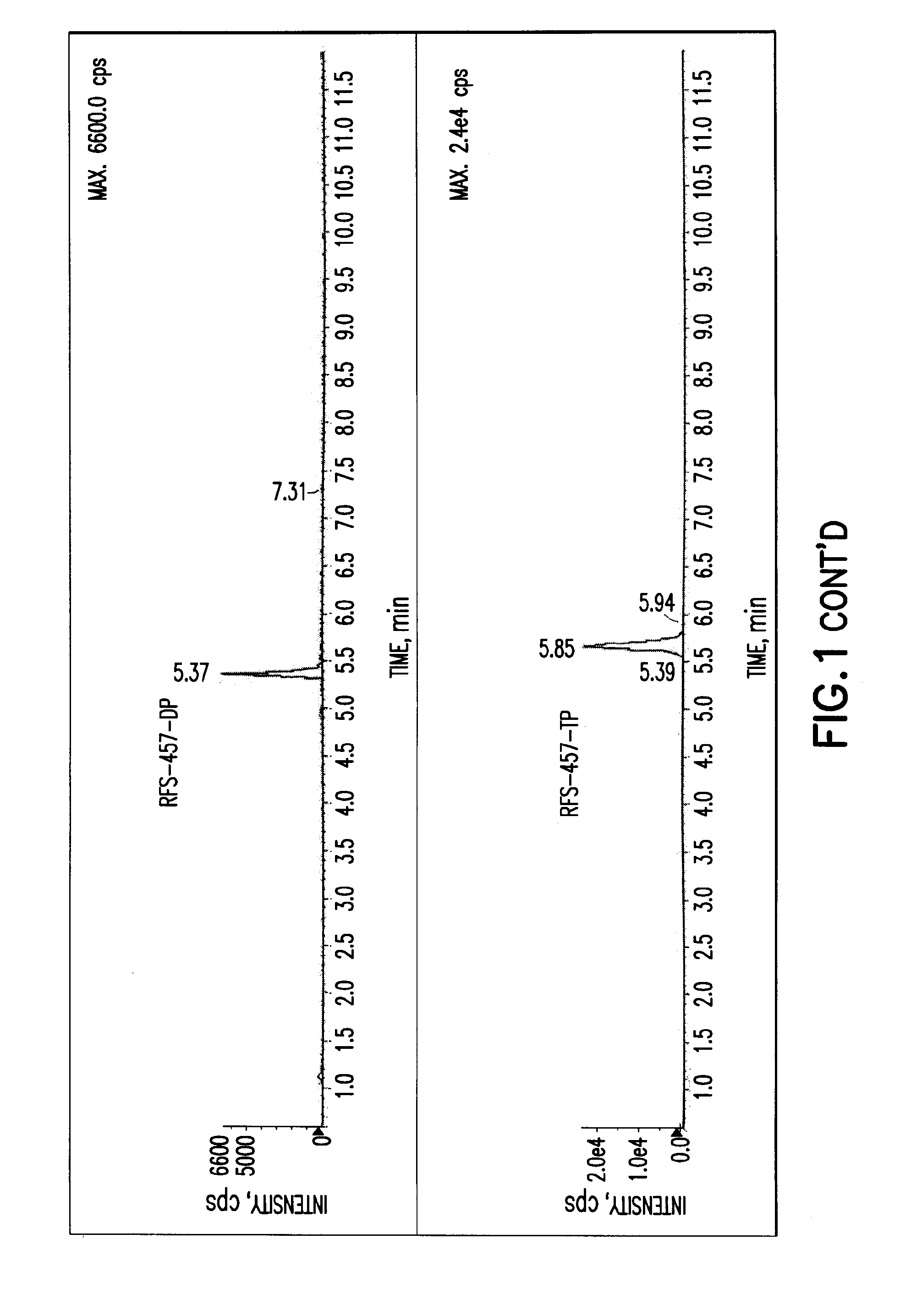
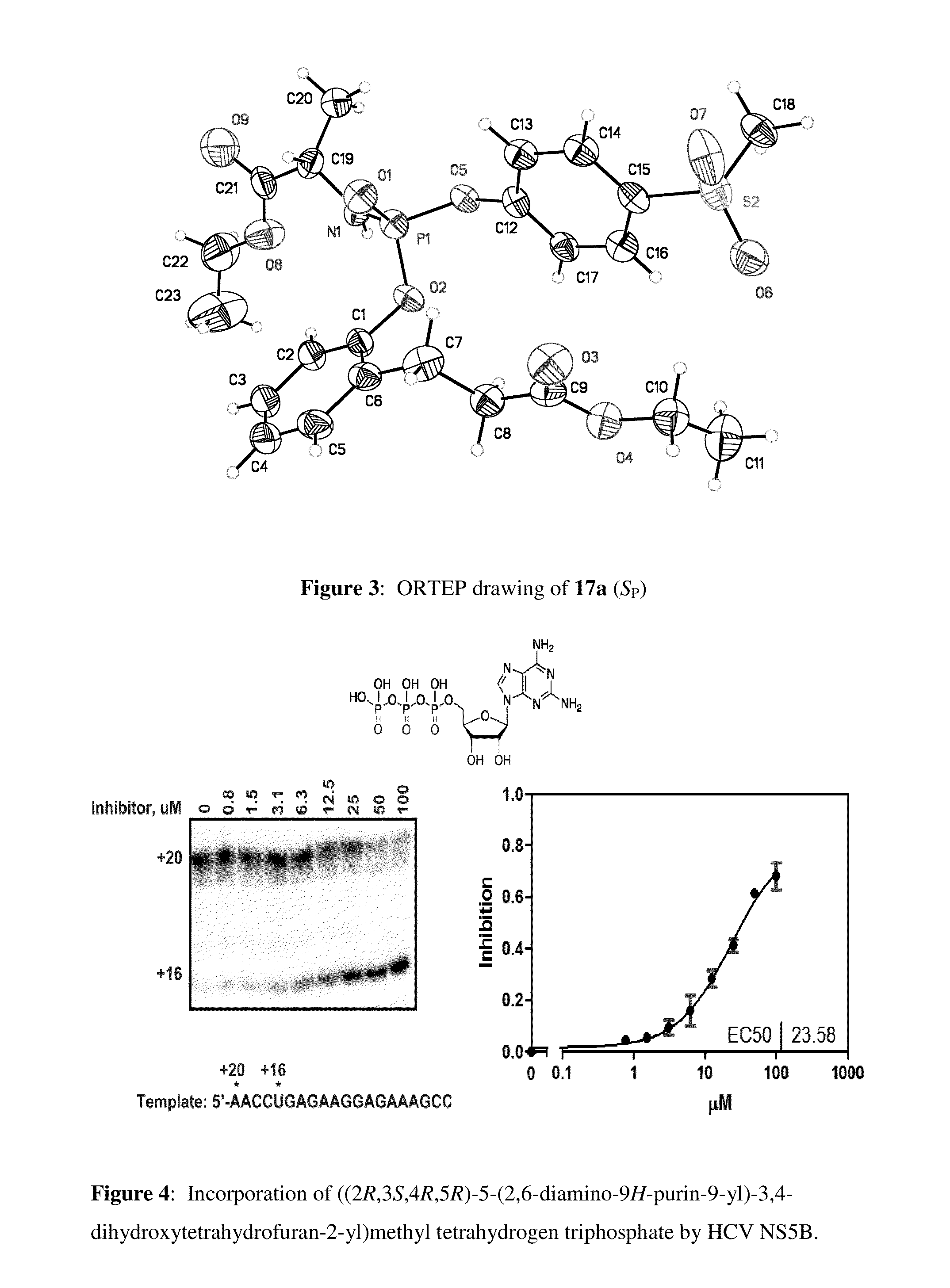
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
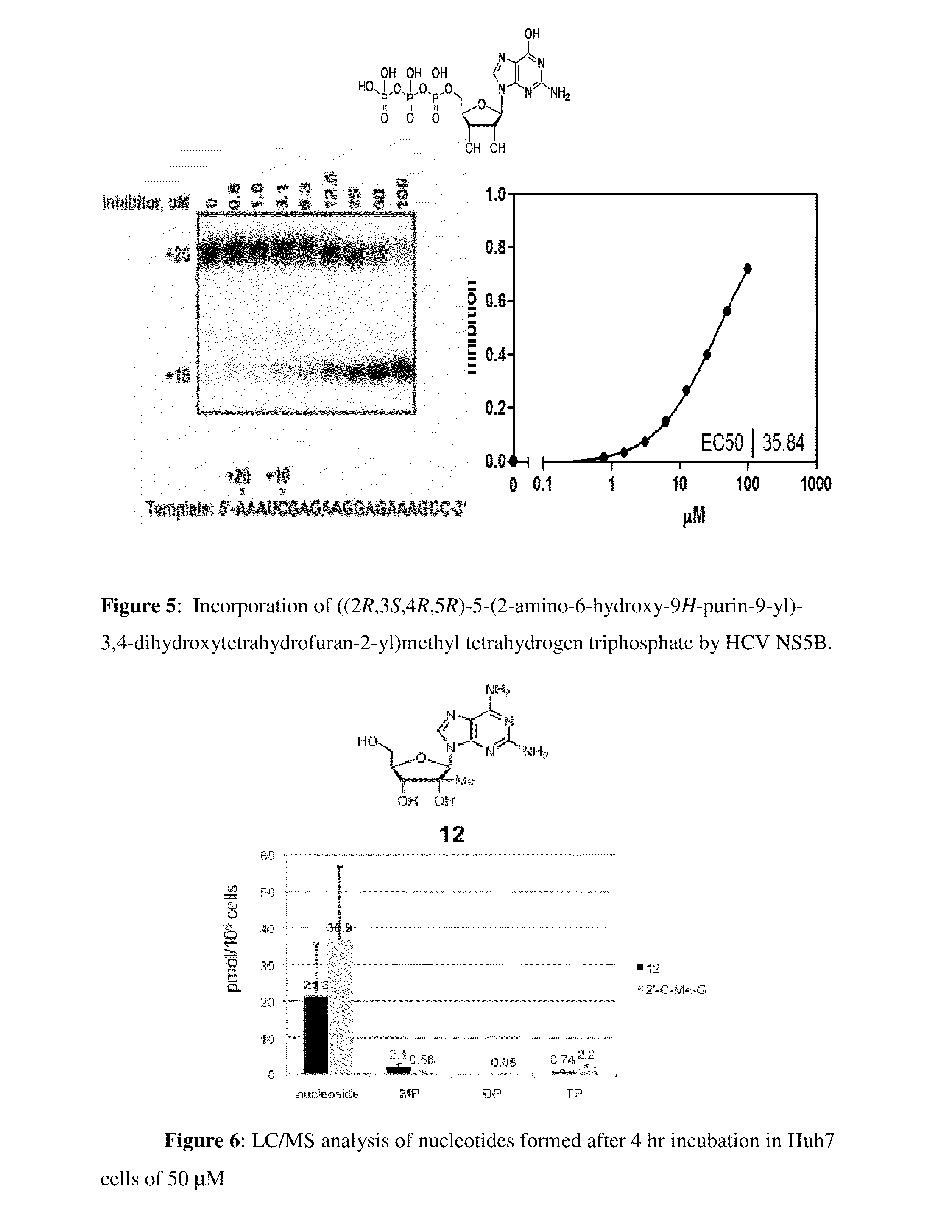
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2′-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:COCRYSTAL PHARMA INC
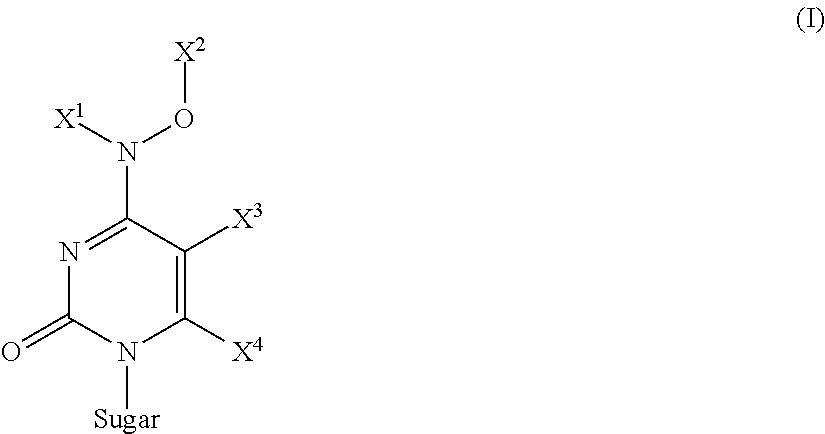
Pyrimidine nucleosides and their monophosphate prodrugs for treatment of viral infections and cancer
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, or HBV in human patients or other animal hosts. The compounds are certain N4-hydroxycytidine nucleosides derivatives, modified monophosphate and phosphonates prodrugs analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
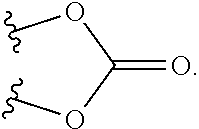
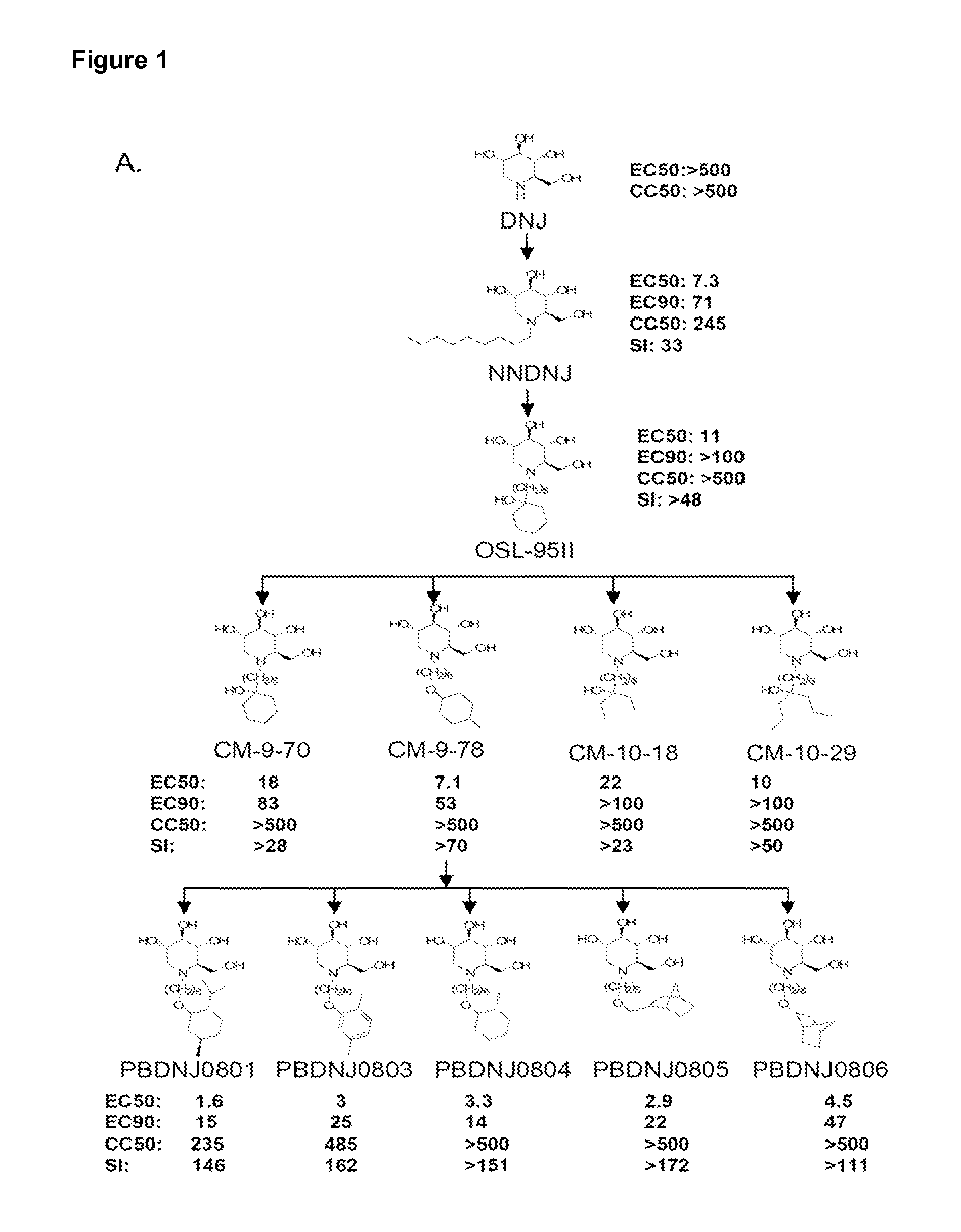
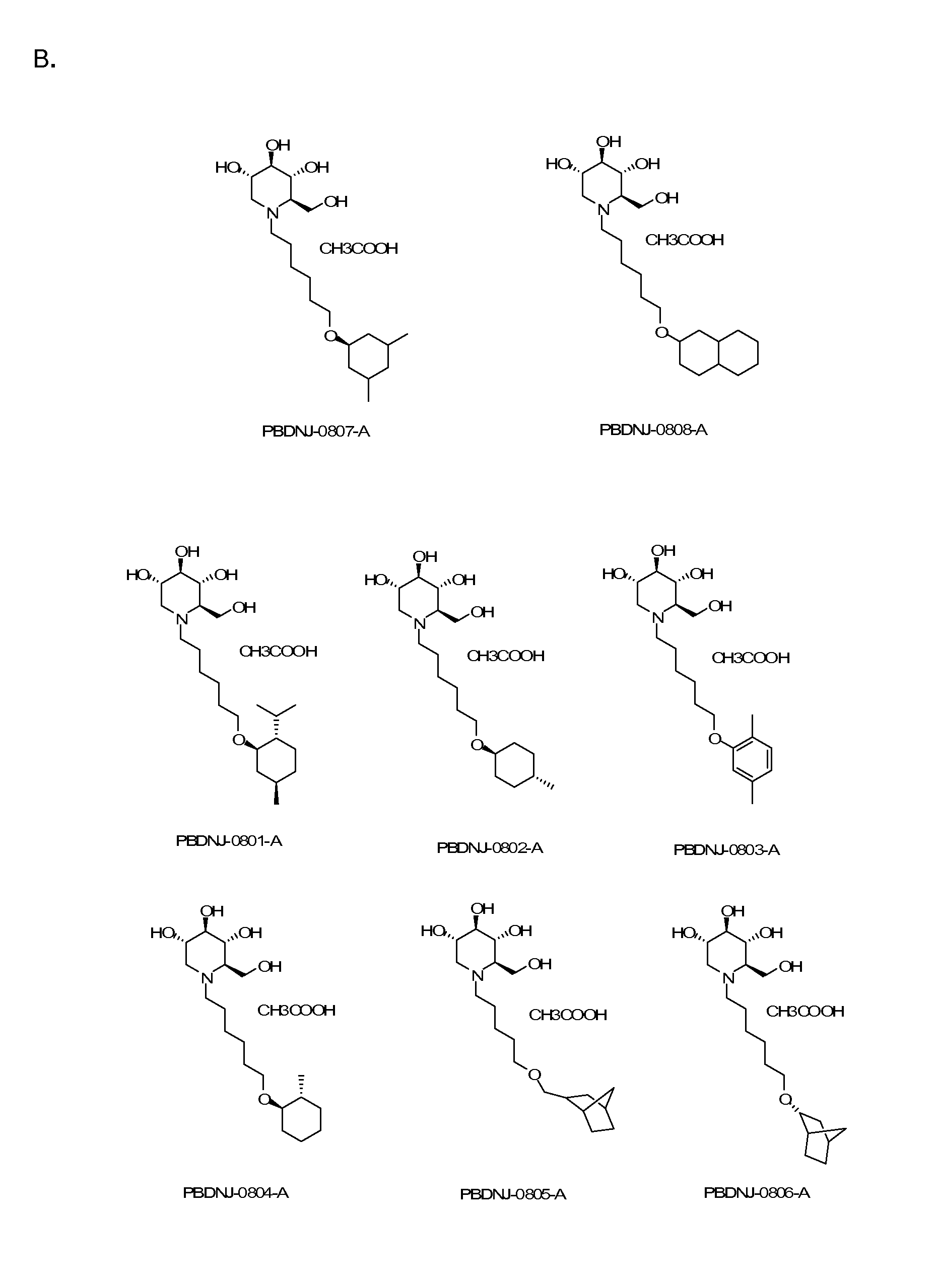
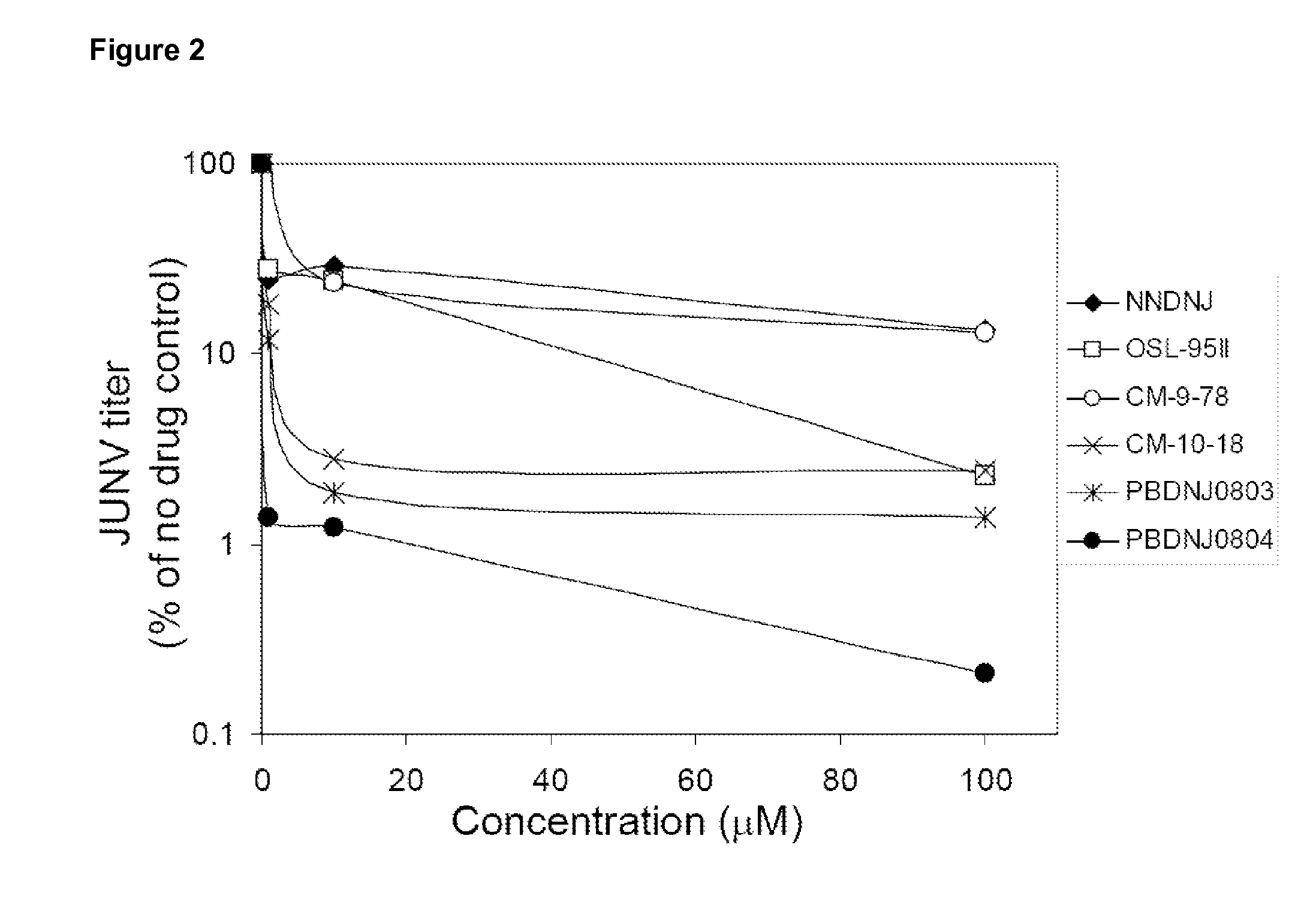
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Treatment and prevention of dengue virus infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain 2-aryl-benzothiazole or 2-heteroaryl-benzothiazole derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
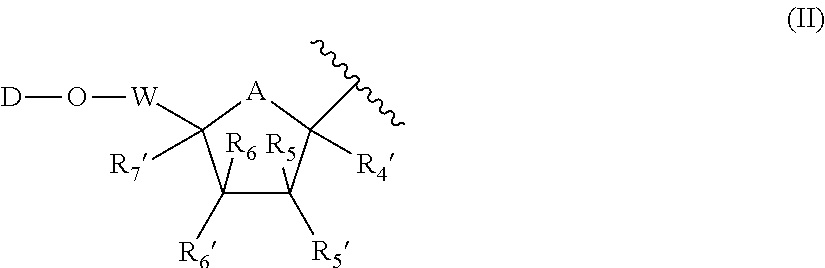
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
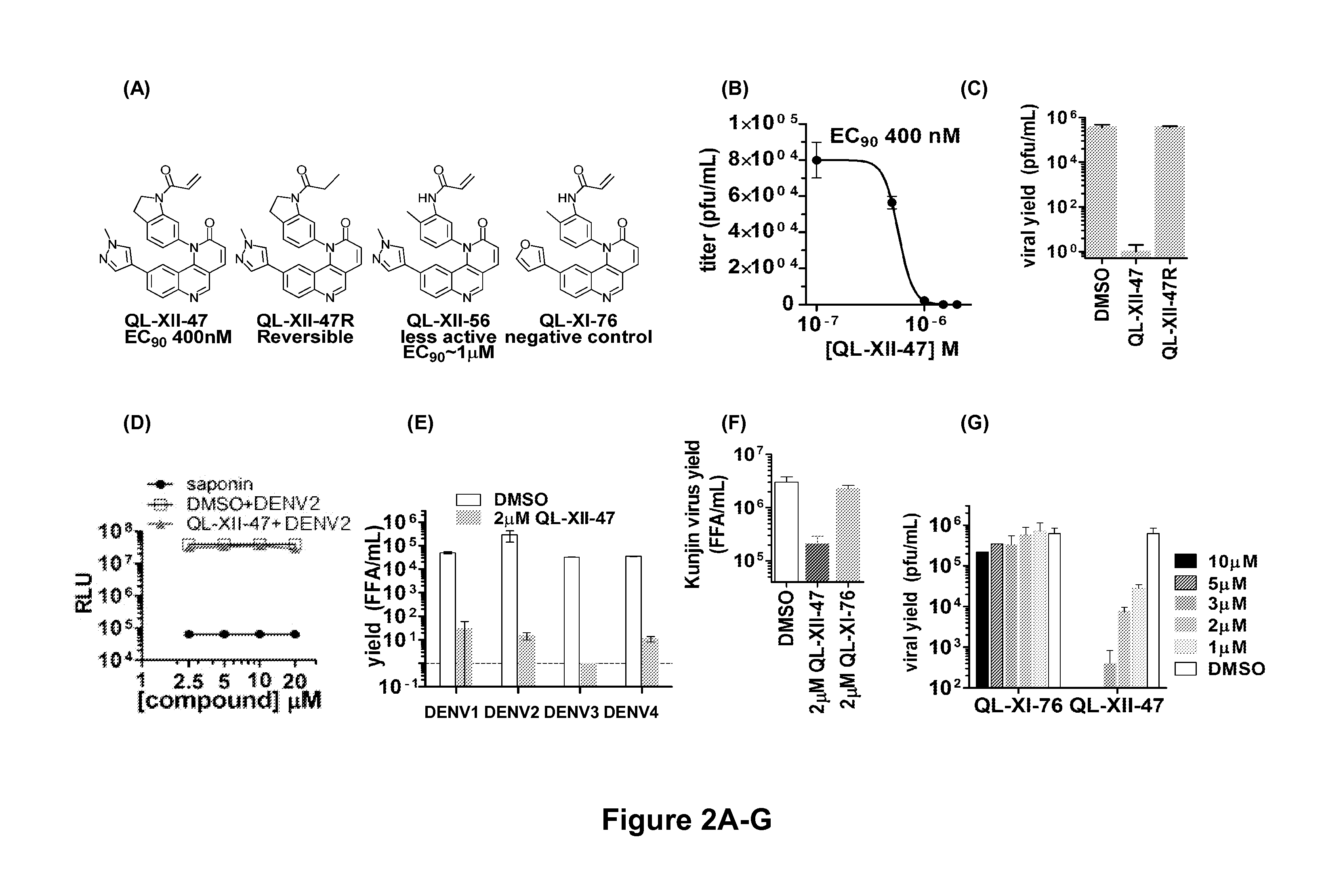
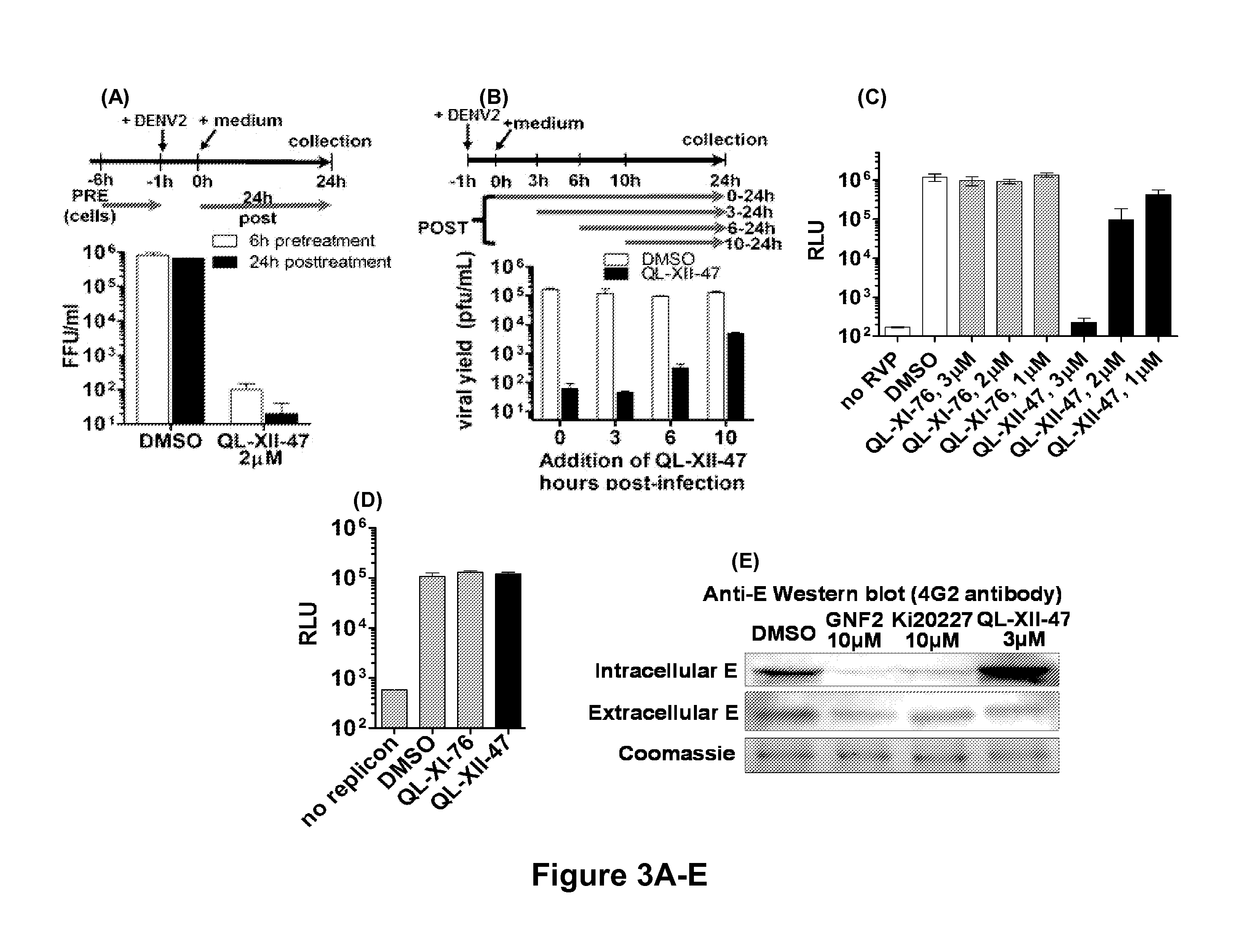
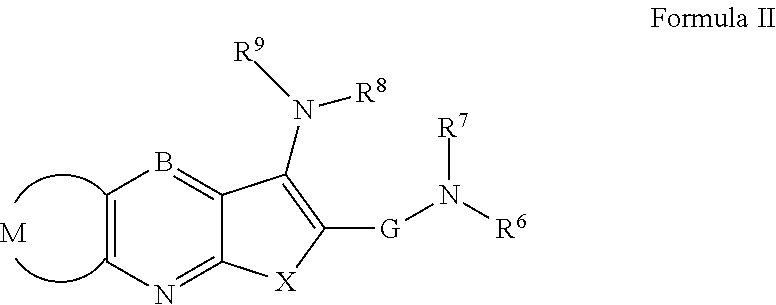
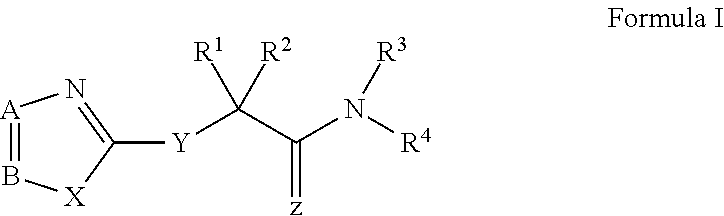
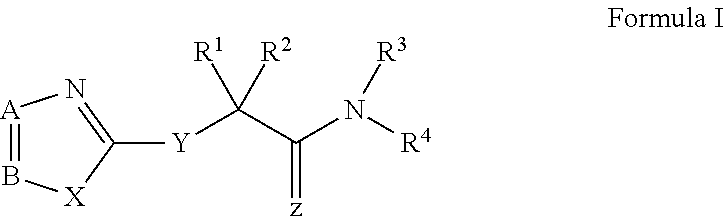
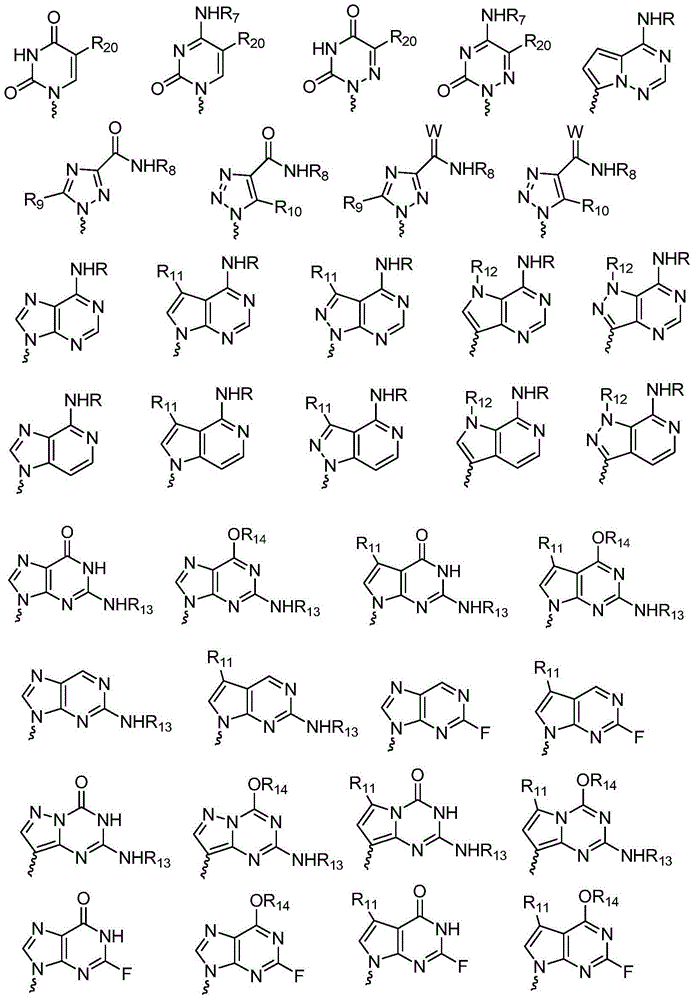
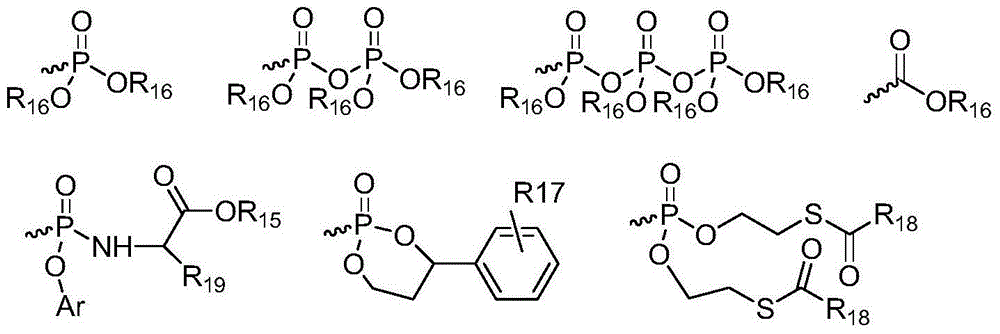
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Integrated microchip sensor system for detection of infectious agents
InactiveUS20110136262A1Rapid direct early detectionHigh sensitivityMaterial analysis using sonic/ultrasonic/infrasonic wavesComponent separationSingle crystalMicrofluidic channel
An integrated multiplexed acoustic wave biosensor chip system with enhanced sensitivity has been developed. The biosensor system incorporates one or more microfluidic channels, coated with target-specific binding films enabling rapid and early detection of viral, bacterial or parasitic targets such as Dengue virus and sexually transmitted diseases in specimens from potentially infected patients. The biosensors are used in portable analytical systems that are suitable for real-time point of care (POC) clinical diagnosis in cost sensitive and / or resource limited settings. The highly sensitive biosensors utilize thinned single crystal piezoelectric substrates that propagate layer guided shear horizontal acoustic plate mode (LG-SH-APM) waves in sensing regions bearing immobilized binders that provide simultaneous and direct detection of mass changes due to multiple bound target pathogens or molecules.
Owner:AVIANA MOLECULAR TECH
Purine nucleoside monophosphate prodrugs for treatment of cancer and viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV in human patients or other animal hosts. The compounds are certain 6-substituted purine monophosphates, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, HSV-1, HSV-2, Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
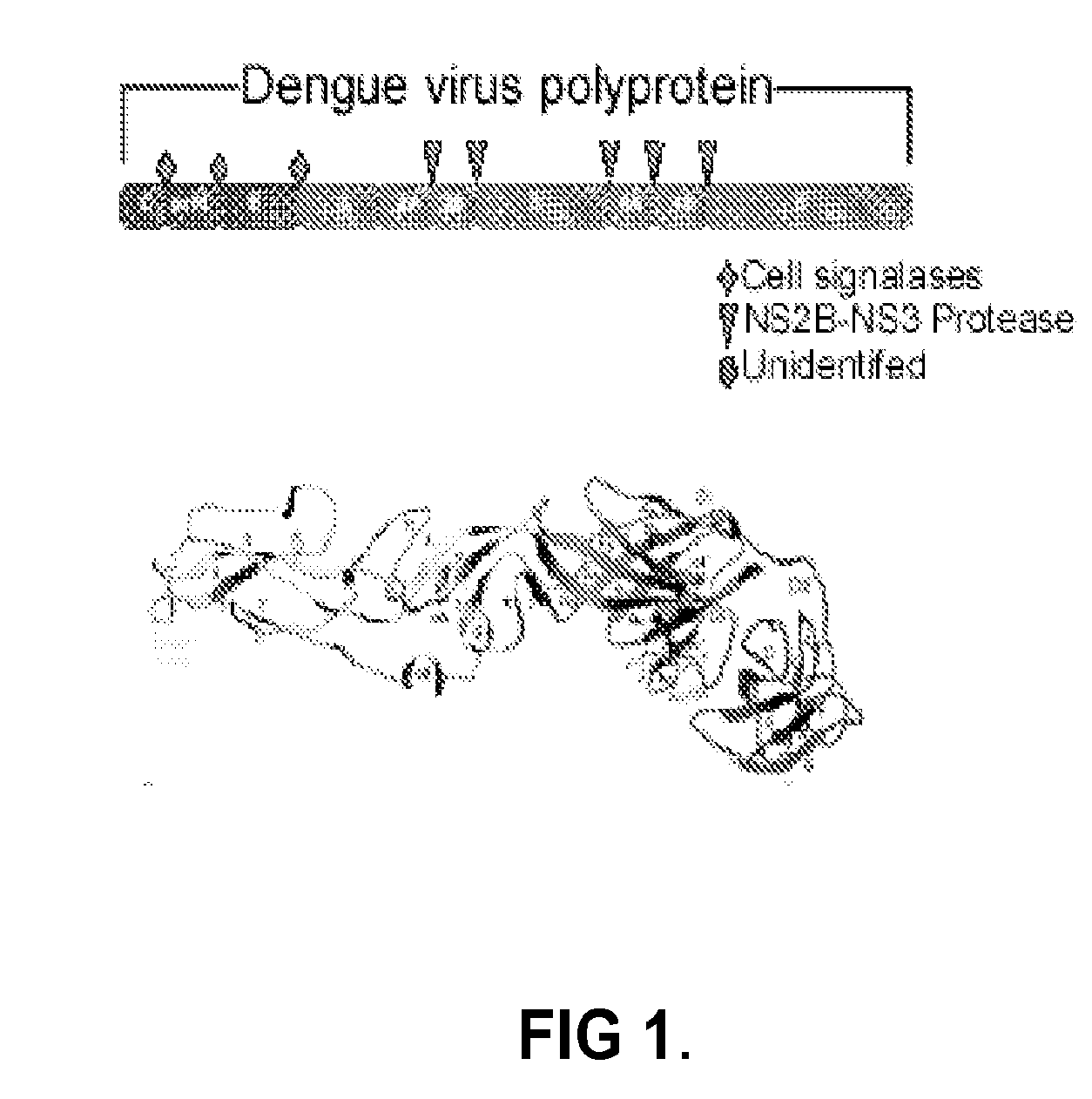
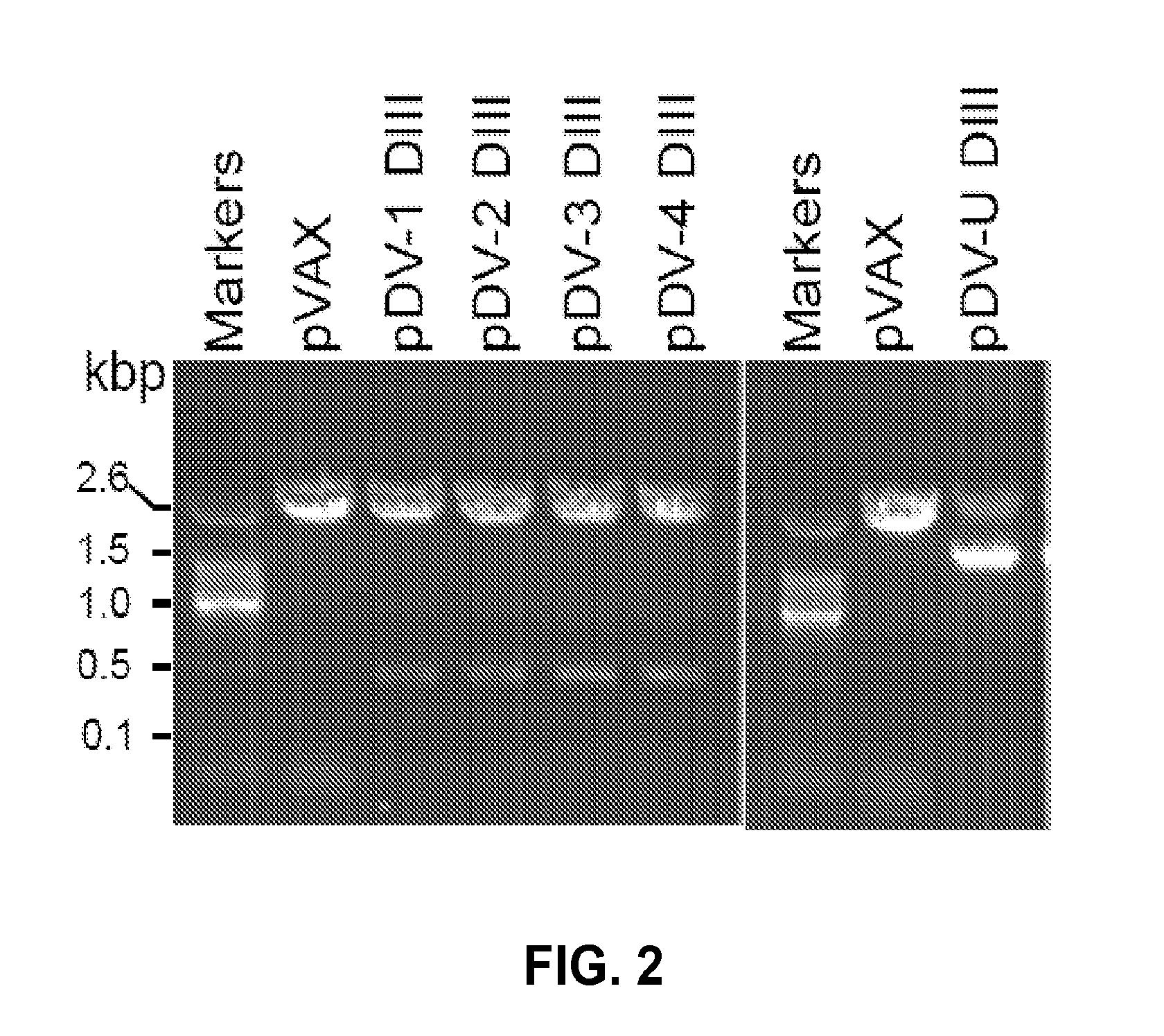
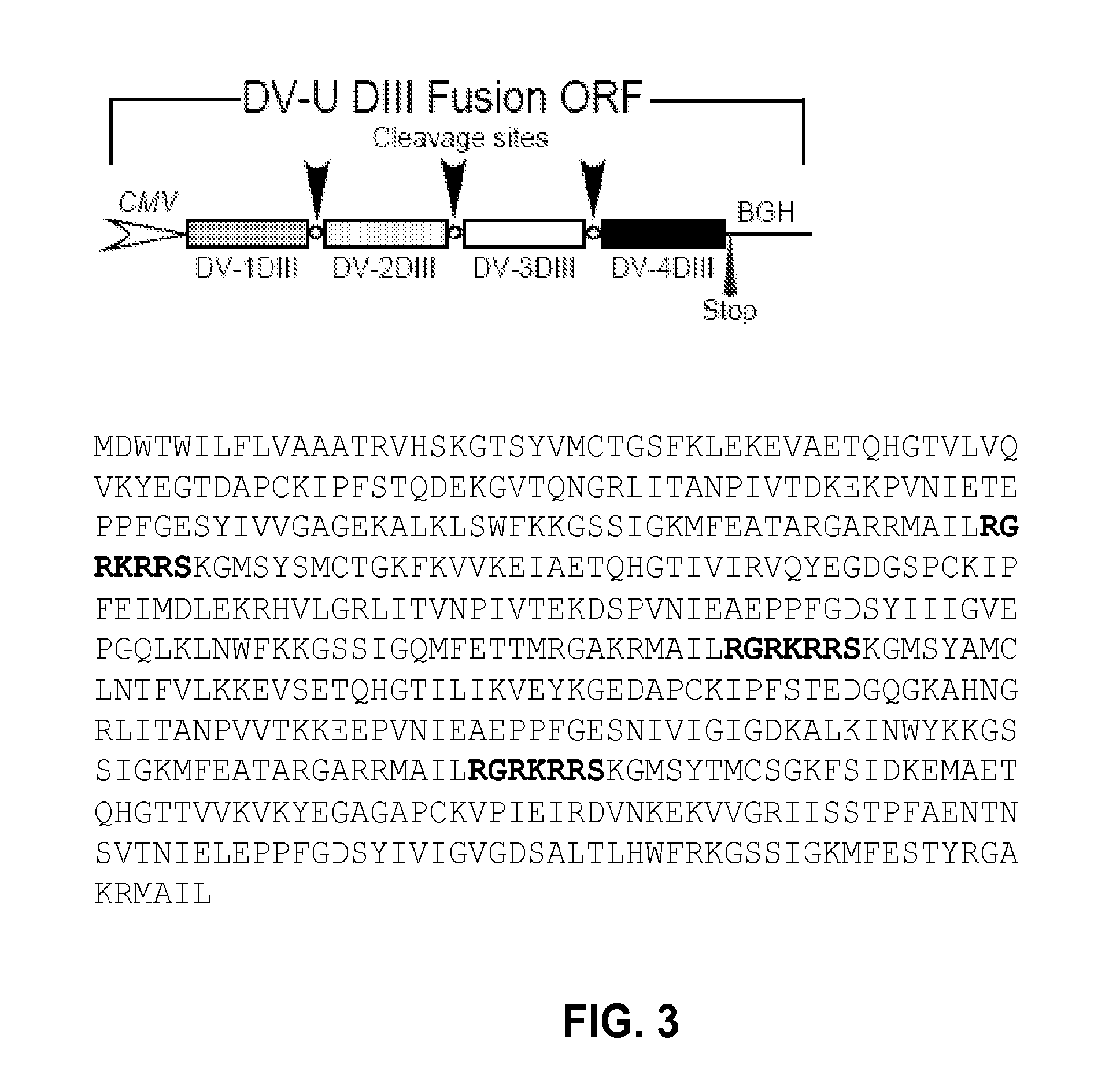
Novel vaccines against multiple subtypes of dengue virus
ActiveUS20100291144A1Elicit immune responseSsRNA viruses positive-senseViral antigen ingredientsAntigenMammal
An aspect of the present invention is related to nucleic acid constructs capable of expressing a polypeptide that elicits an immune response in a mammal against more than one subtype of dengue virus, and methods of use thereof. Additionally, there are DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of dengue virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient, and methods of use thereof. The DNA plasmid is capable of expressing a consensus dengue antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal.
Owner:VGX PHARMA
Anti-dengue virus antibodies
ActiveUS8637035B2Viral antigen ingredientsMicrobiological testing/measurementDengue virus antibodyAntigen Binding Fragment
Provided herein are monoclonal antibodies specific to dengue virus as well as their antigen-binding fragments, and functional variants. Also disclosed are uses thereof for treating or diagnosing dengue virus infection.
Owner:ACAD SINIC
Thienopyridine Derivatives for the Treatment and Prevention of Dengue Virus Infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain thienopyridine derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Methods and materials for the detection of dengue virus infection
ActiveUS20130164734A1Easy to useQuick controlMaterial analysis by observing effect on chemical indicatorImmunoglobulins against virusesStructural glycoproteinPolyclonal antibodies
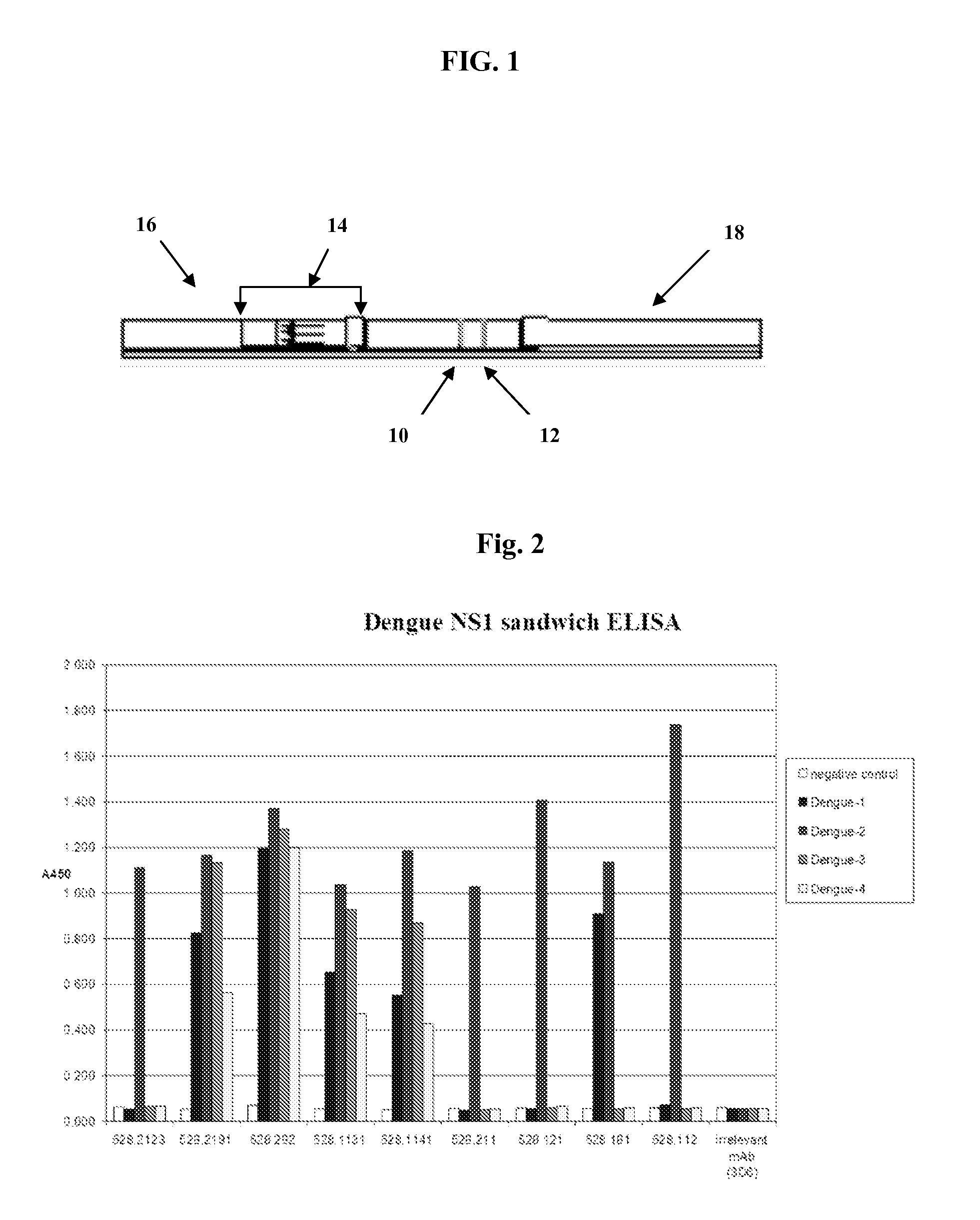
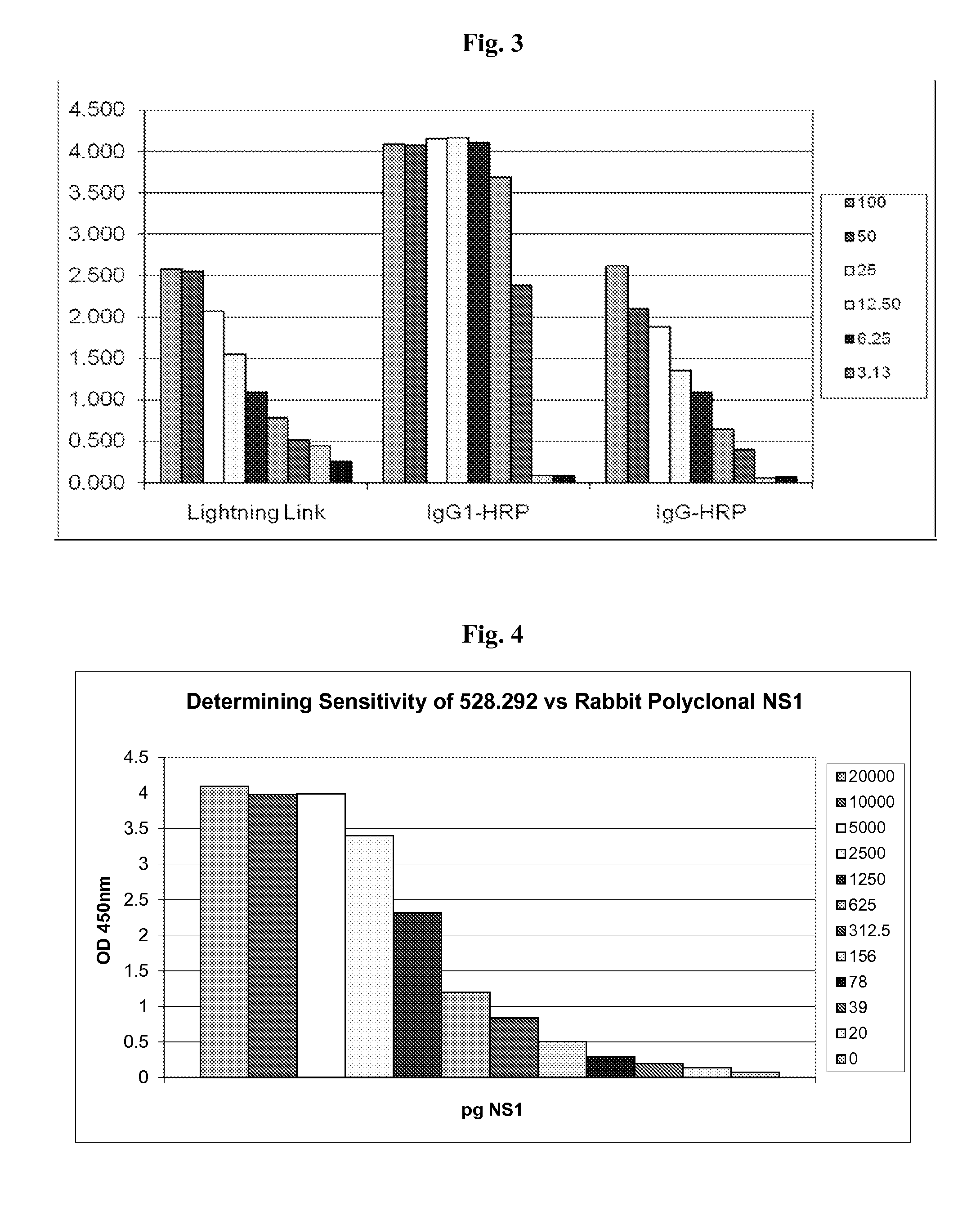
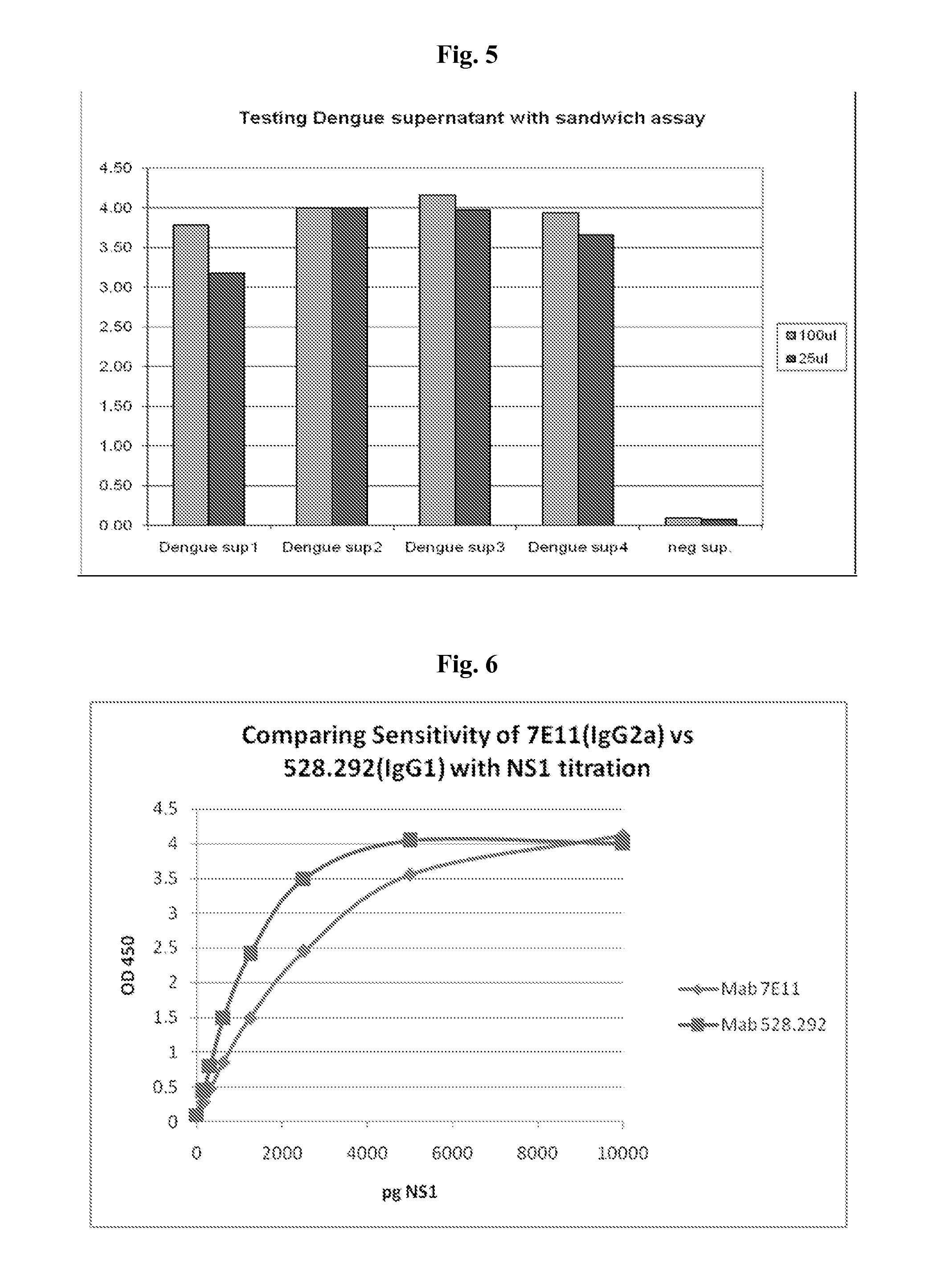
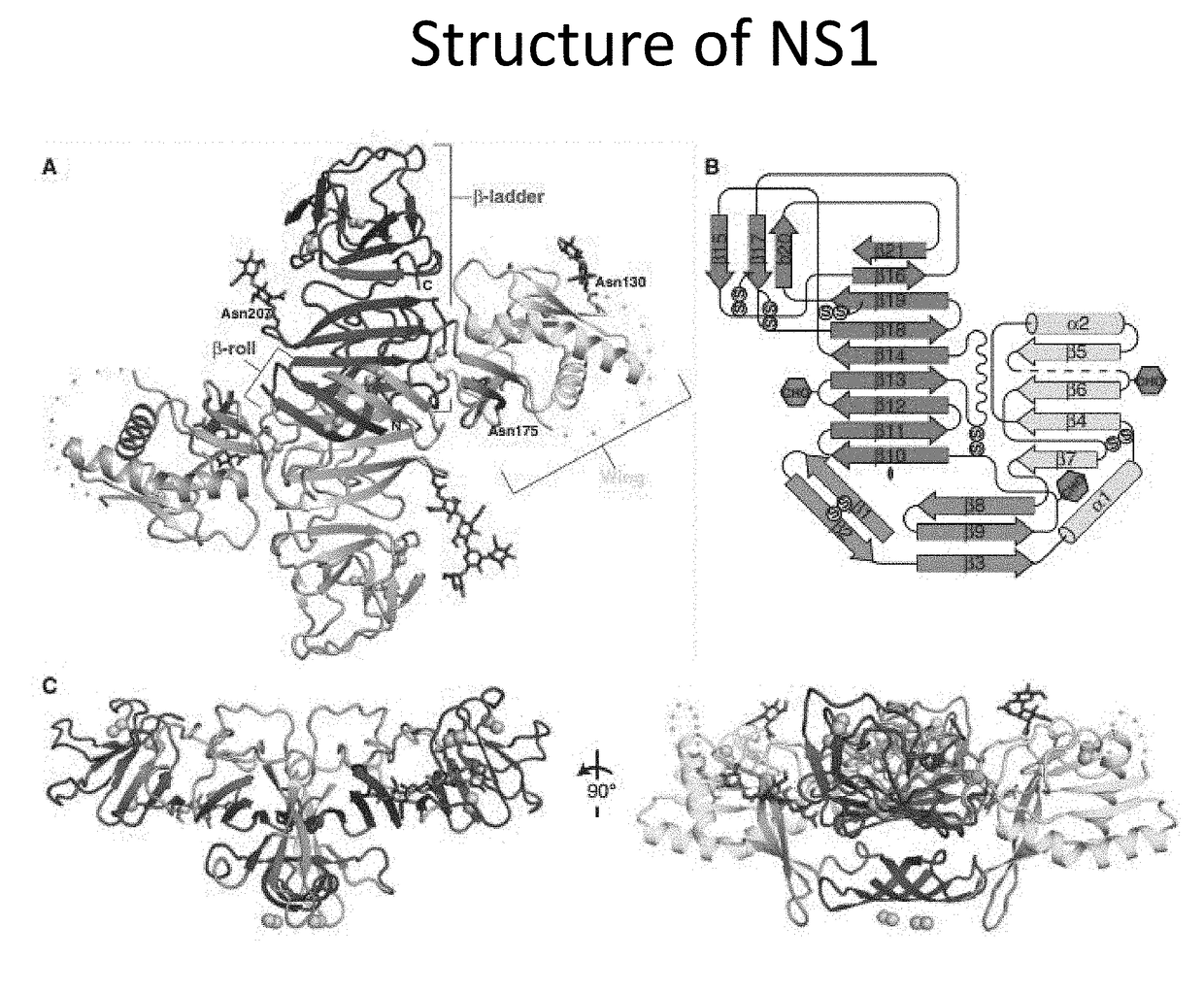
The present invention provides monoclonal antibodies that are specific for the Dengue non-structural glycoprotein NS1 in monomeric and / or oligomeric (primarily dimeric) form, together with methods, including ELISA and lateral flow assays, that employ the disclosed antibodies for the early detection of Dengue virus infection. Diagnostic kits for the detection of Dengue infection are also provided, such kits including the disclosed monoclonal and / or polyclonal antibodies.
Owner:INBIOS INT
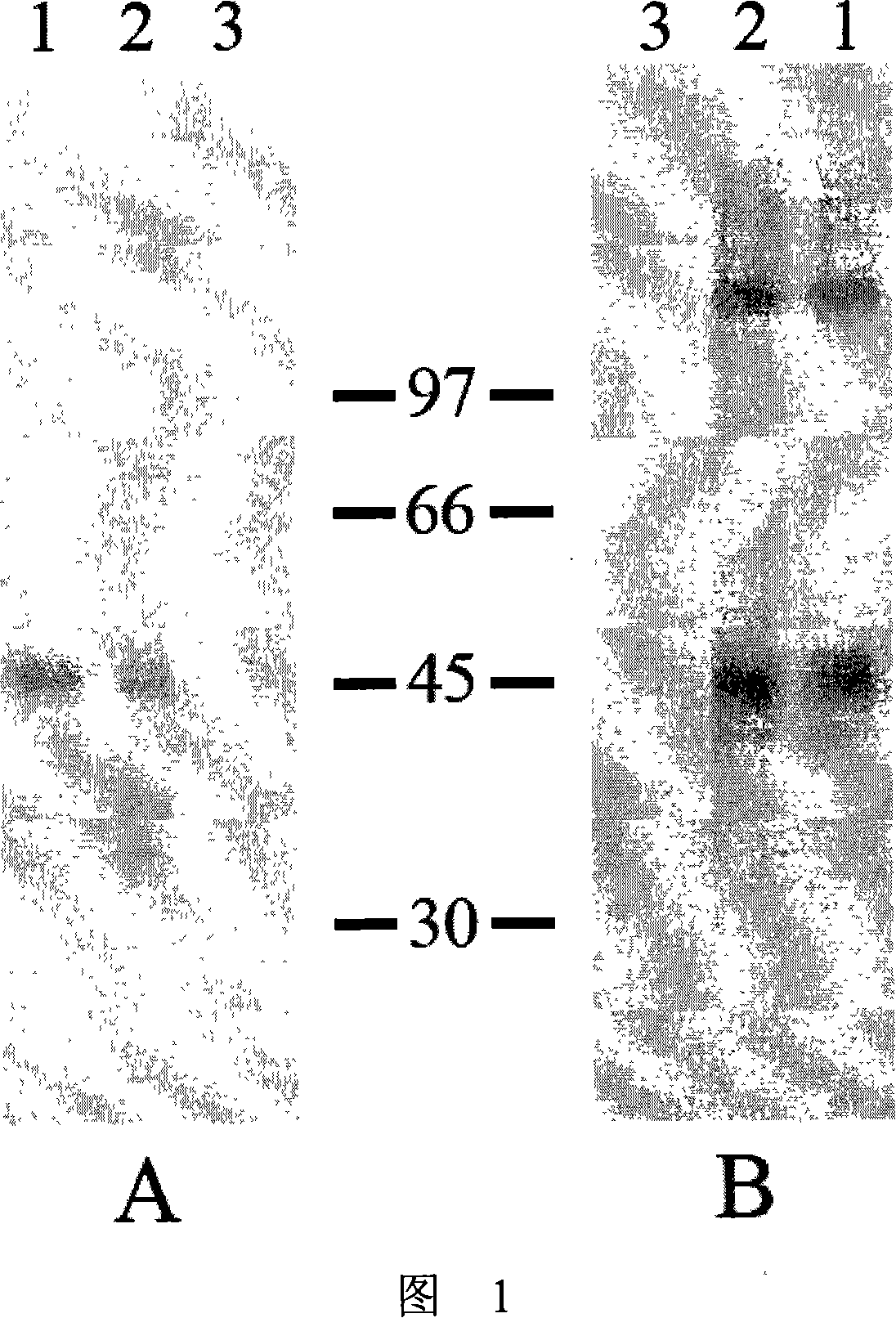
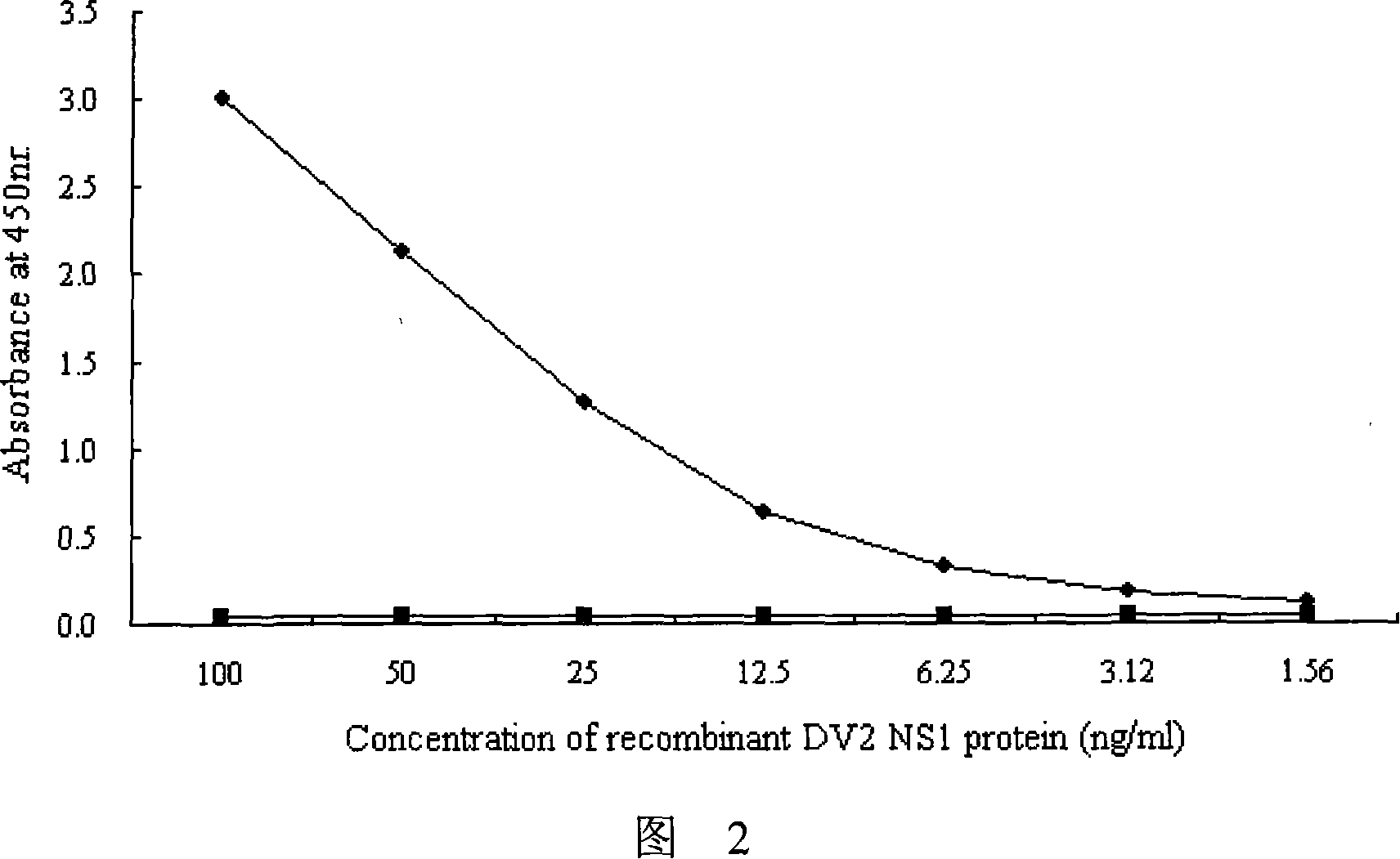
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
Humanized monoclonal antibody and application thereof
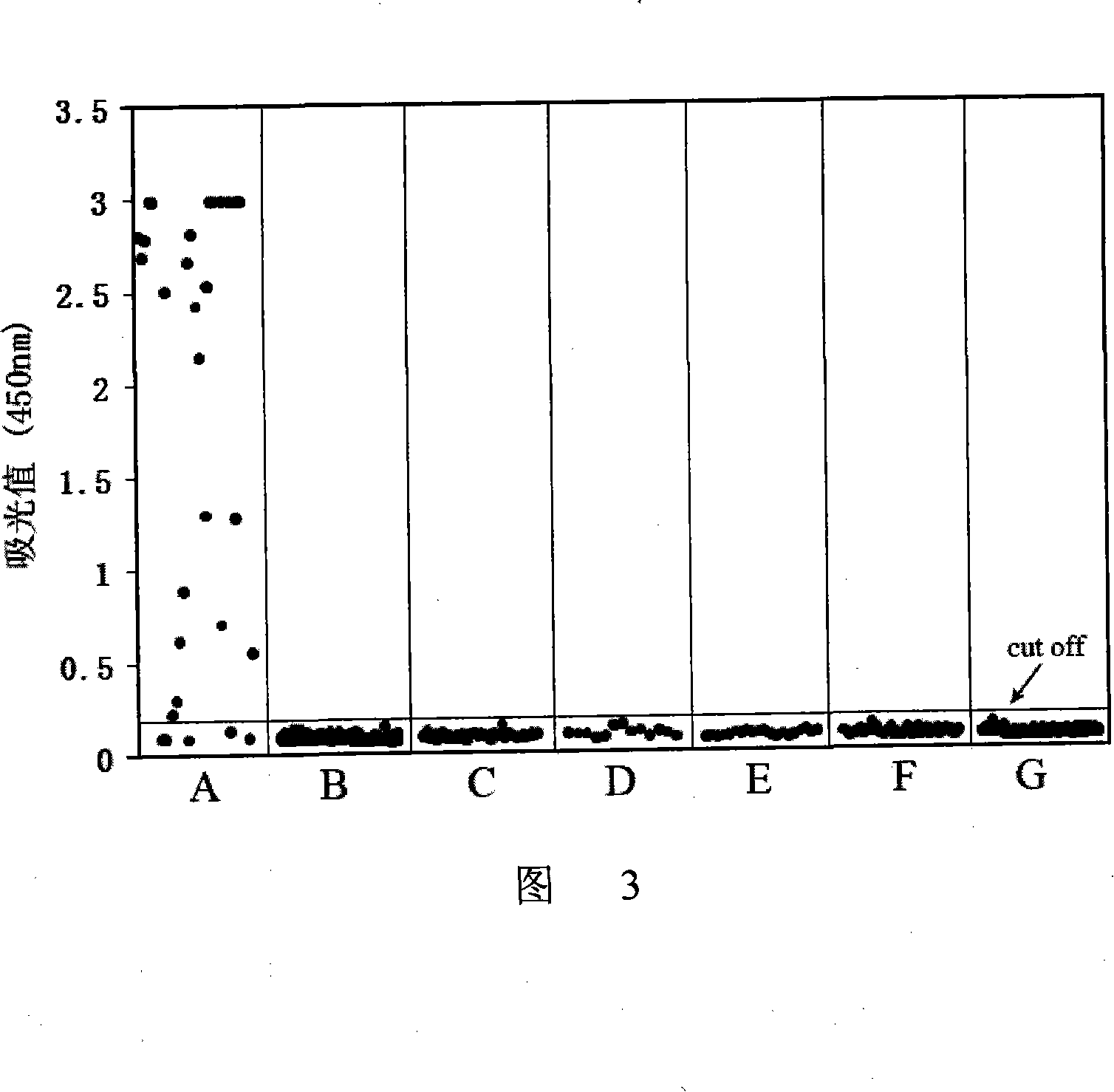
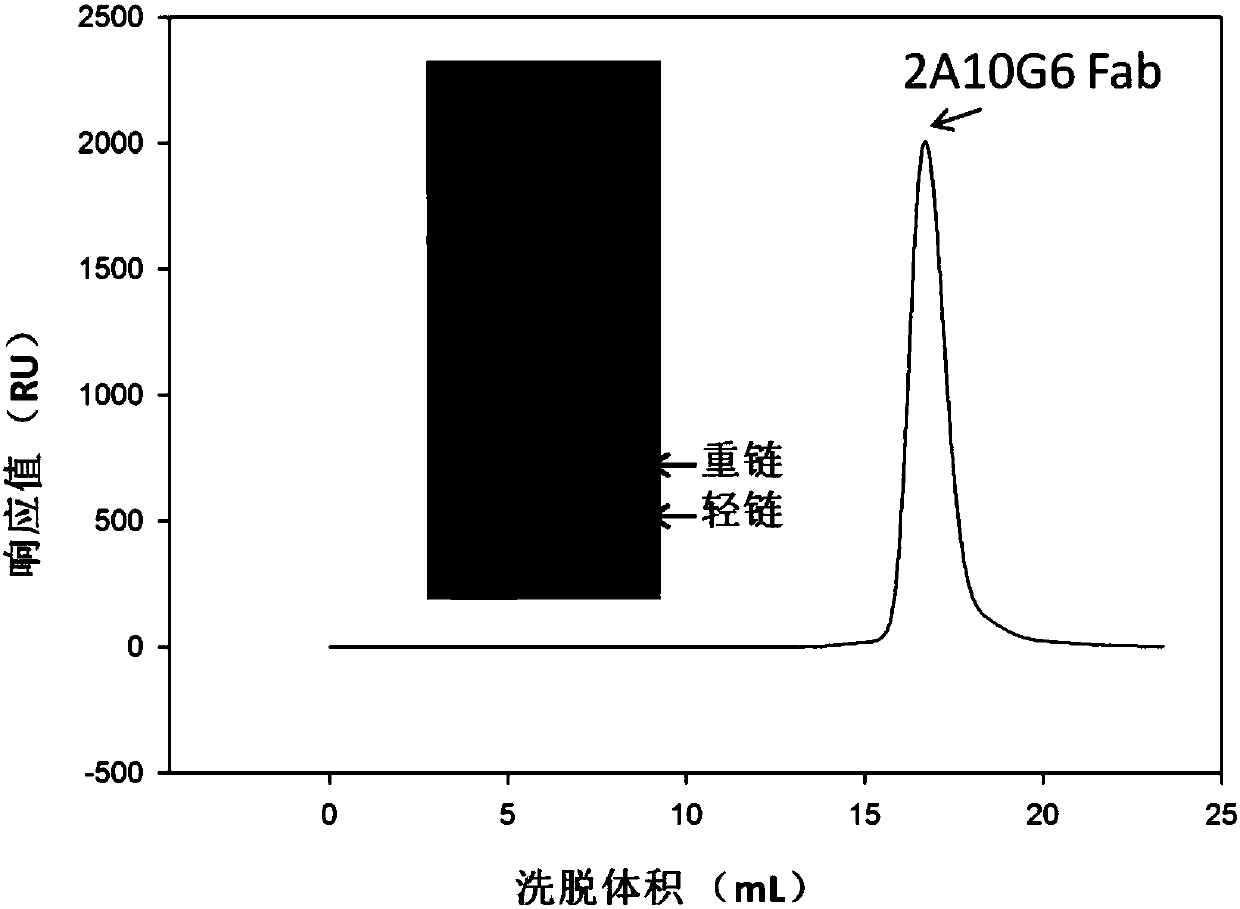
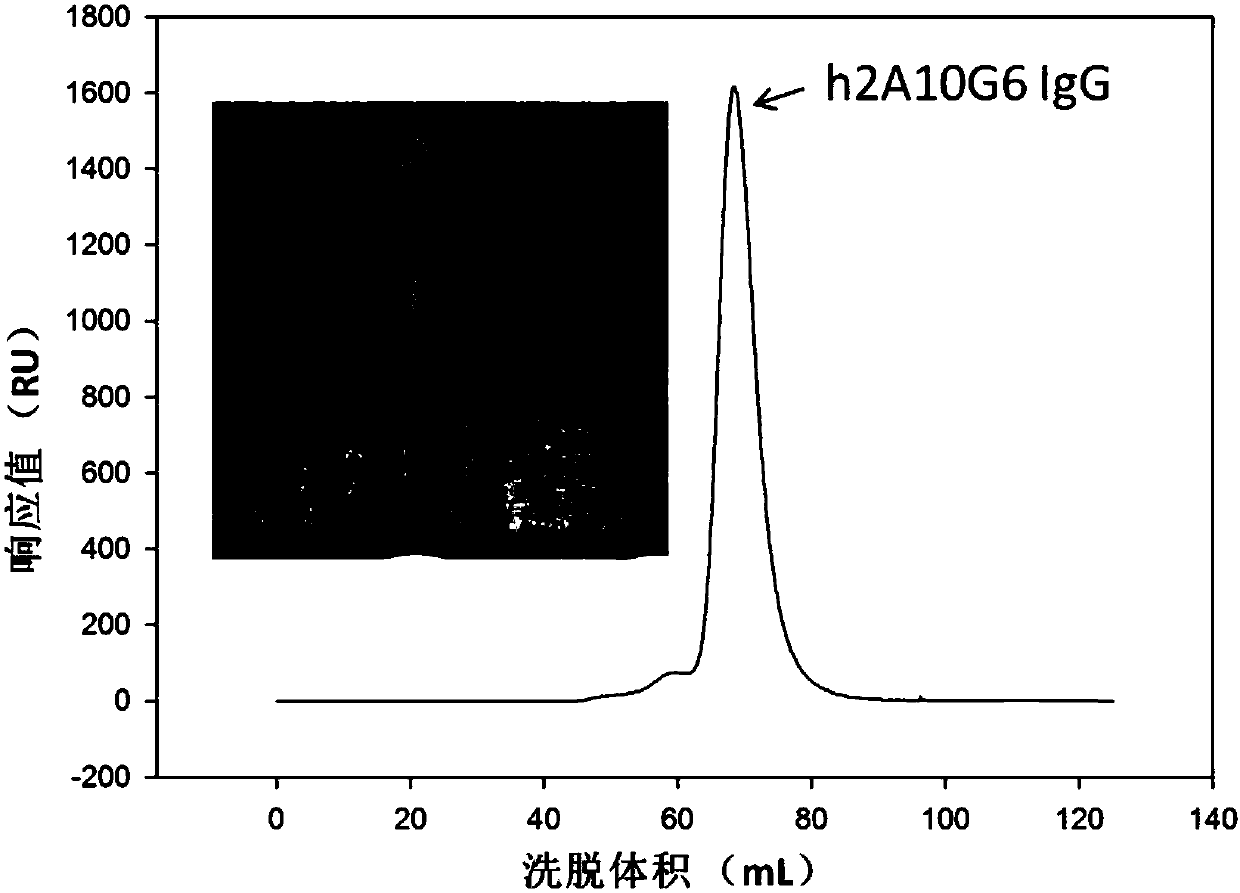
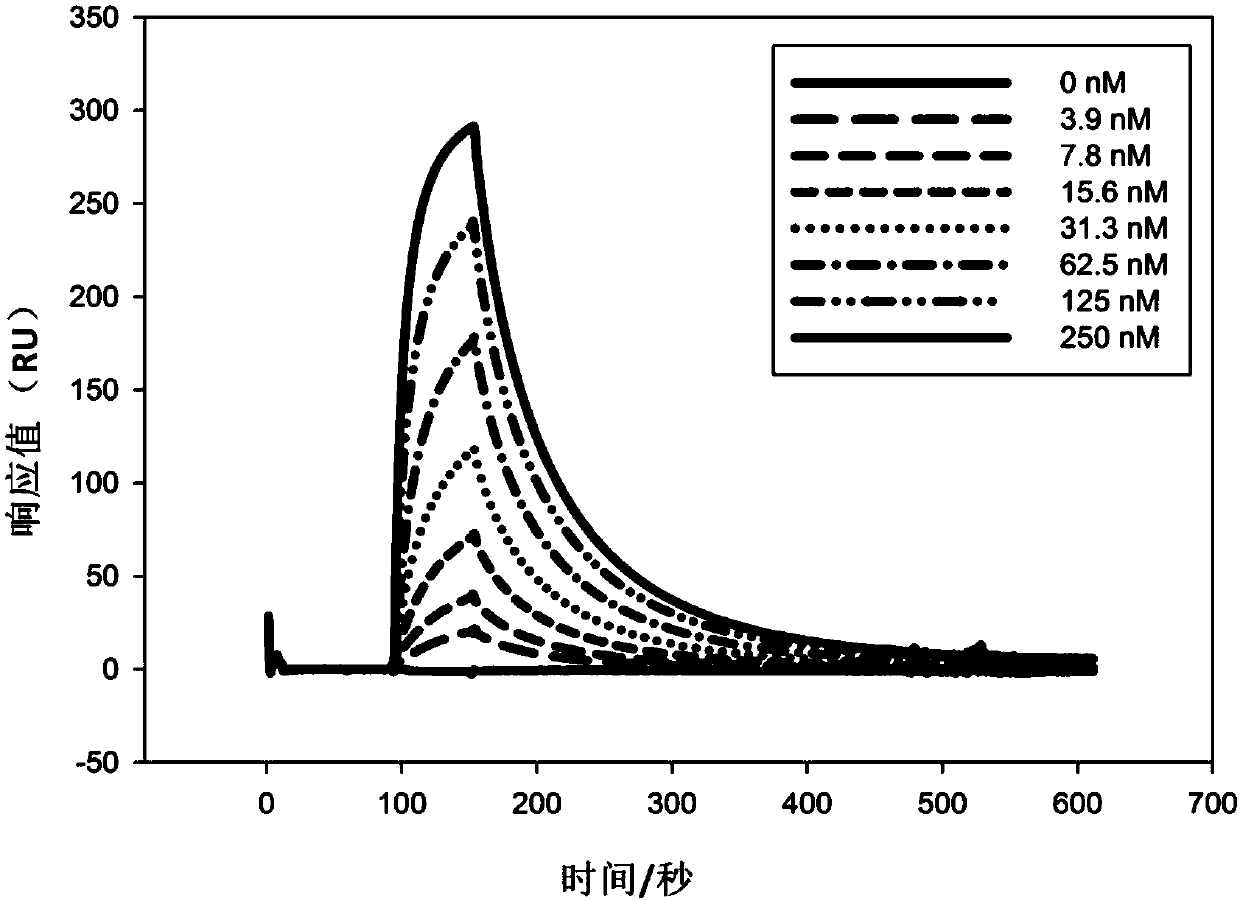
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Dengue virus IgG/IgM antibody detection test strip, kit and preparation method thereof
InactiveCN110007096ASmall sample sizeHigh detection sensitivityBiological testingAgainst vector-borne diseasesIgm antibodyDisease course
The invention discloses a dengue virus IgG / IgM antibody detection test strip, kit and preparation method thereof, and relates to the technical field of dengue virus detection. The dengue virus IgG / IgMantibody detection test strip of the invention comprises a sample pad, an immune combination pad, a nitrocellulose membrane and absorbent paper, wherein the sample pad is sequentially stuck to the bottom of polyvinyl chloride; the immune combination pad is coated with dengue virus recombinant antigen marked by colloidal gold and chicken IgY polyclonal antibody; the nitrocellulose membrane is coated with dengue virus anti-human IgM antibody, a detection line of IgG antibody and a quality control line of anti-chicken IgY polyclonal antibody. The test strip can detect whether dengue virus IgG / IgM antibody exists in a sample to be detected through a method for detecting a marker. The test strip and the detection card comprising the test strip can be used as supplement for antigen detection onthe early acute infection of the dengue virus, covering the middle and later stages of the disease course and reducing the risk of missed detection.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Anti-Dengue Virus Antibodies
ActiveUS20120014945A1Microbiological testing/measurementBiological material analysisDengue virus antibodyAntigen Binding Fragment
Provided herein are monoclonal antibodies specific to dengue virus as well as their antigen-binding fragments, and functional variants. Also disclosed are uses thereof for treating or diagnosing dengue virus infection.
Owner:ACAD SINIC
Recombinant MVA virus expressing dengue virus antigens, and the use thereof in vaccines
InactiveUS6869793B2Efficient and exceptionally safeSsRNA viruses positive-senseSugar derivativesVaccinationDengue virus Antigen
Recombinant MVA viruses containing and / or capable of expressing dengue virus antigens and the use of such recombinant MVA for vaccination.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT +2
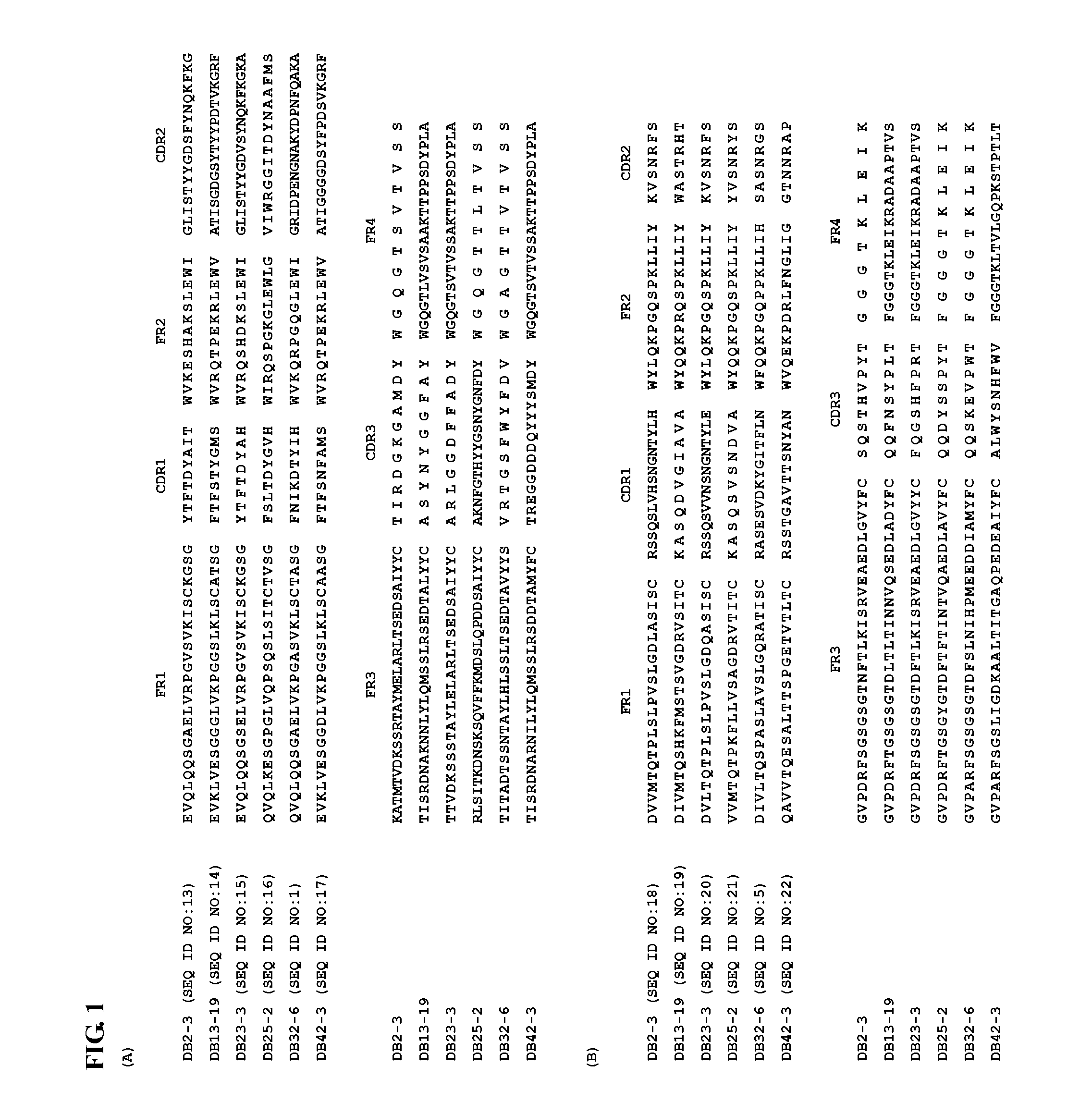
Monoclonal antibodies that bind or neutralize dengue virus
The present invention relates to monoclonal antibodies that bind or neutralize dengue type 1, 2, 3, and / or 4 virus. The invention provides such antibodies, fragments of such antibodies retaining dengue virus-binding ability, fully human or humanized antibodies retaining dengue virus-binding ability, and pharmaceutical compositions including such antibodies. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. Additionally, the invention provides for prophylactic, therapeutic, and diagnostic methods employing the antibodies and nucleic acids of the invention.
Owner:HEALTH & HUMAN SERVICES THE GOVERMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF
Antiviral Drugs for Treatment or Prevention of Dengue Infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Anti-Dengue Virus NS1 Protein Monoclonal Antibodies
ActiveUS20170233460A1Improve developmentUseful in therapyImmunoglobulins against virusesAntibody ingredientsProtein.monoclonalSpecific detection
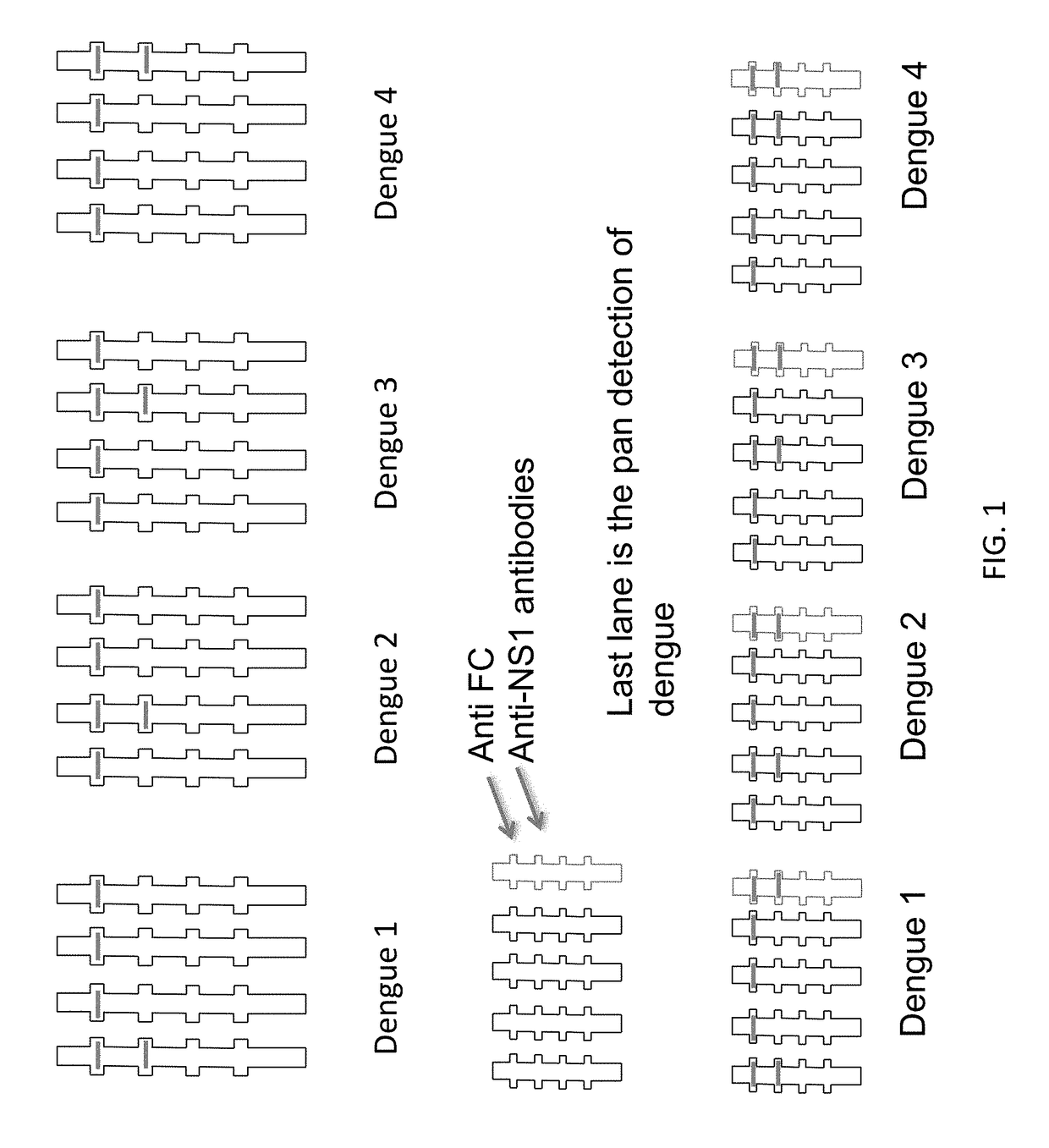
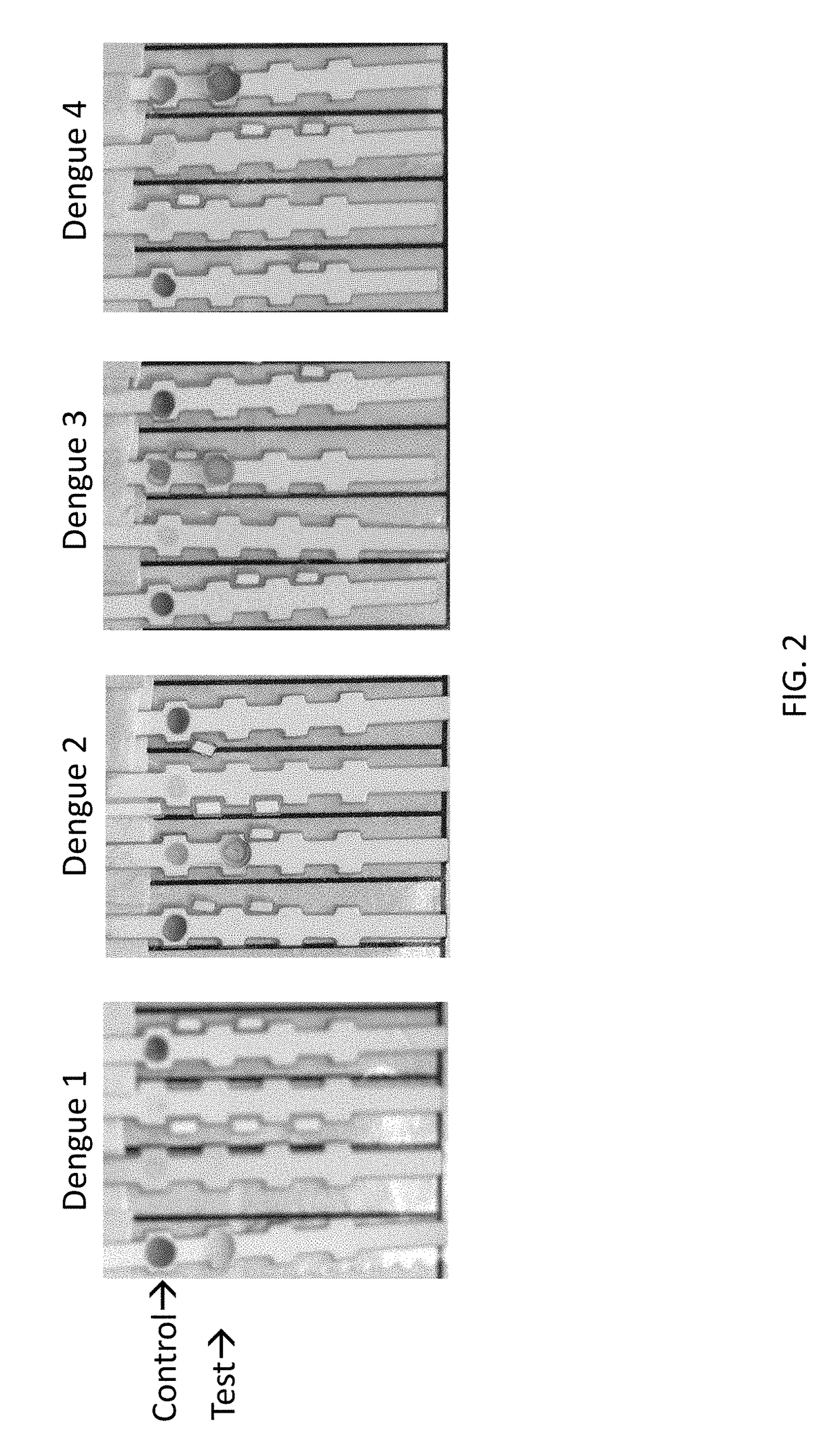
The present invention provides matched antibody pairs for the specific detection of one or more of the four dengue virus serotypes in a biological sample that may contain one or more of such dengue virus serotypes. Each matched antibody pair is capable of detecting not more than one serotype of dengue virus NS1 protein that may be present in the sample and will not cross react with other serotypes that may be present in the sample. Multiple matched pairs may be used to detect one or more dengue virus serotypes that may be present in a sample. Such matched pair antibodies, facilitate the development of confirmatory in vitro diagnostic tests such as sandwich immunoassays, that detect and distinguish the presence of one or more dengue virus serotypes in a biological sample, preferably a sample derived from human subject. The invention also provides kits comprising the matched antibody pairs of the invention and methods for using the kits for immunoassays for the specific detection of one or more serotypes of dengue virus in a patient population. The present invention also provides monoclonal antibodies specific for the NS1 protein of dengue virus and therapeutic compositions and methods for treating dengue virus infection.
Owner:THE FOOD & DRUG ADMINISTATION +1
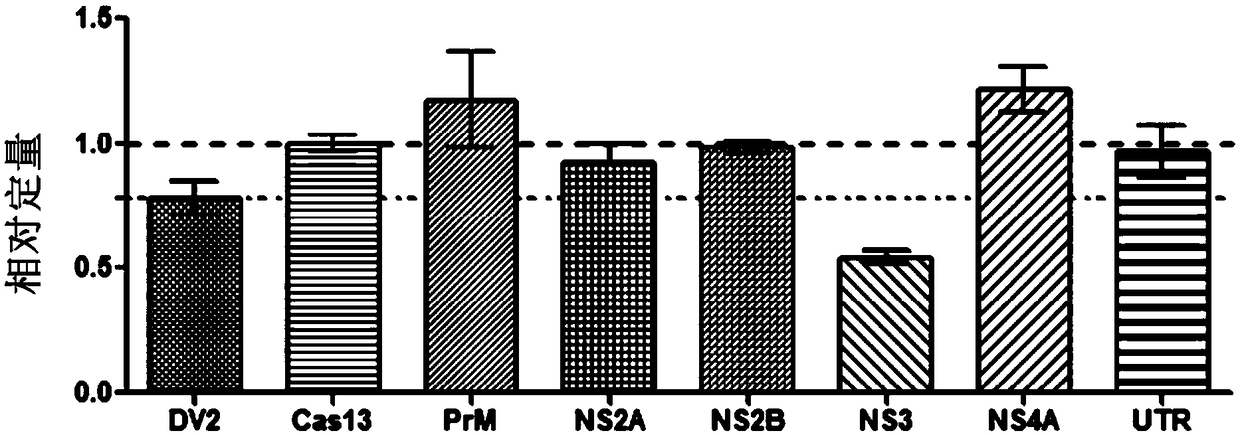
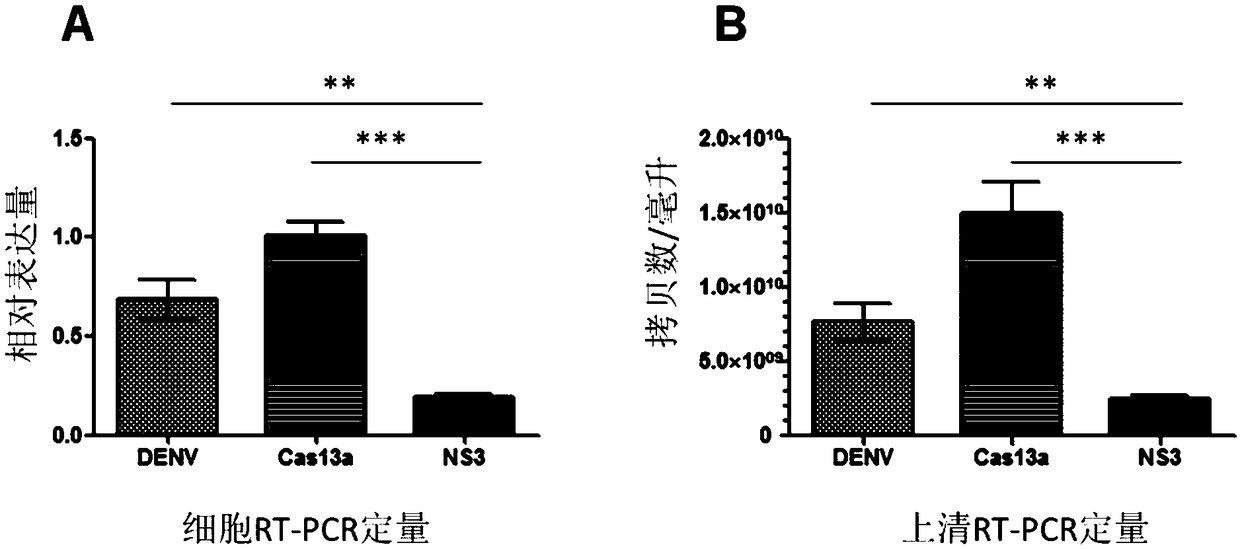
Effective Cas13a-based anti-dengue virus nucleic acid target and application thereof
ActiveCN108715849ALower resistanceImprove efficiencyOrganic active ingredientsSsRNA viruses positive-senseAnti virusGene targets
The invention discloses an effective Cas13a-based anti-dengue virus nucleic acid target and application thereof. The invention provides a CRISPR-Cas13a system for inhibiting dengue virus, wherein theCRISPR-Cas13a system comprises a Cas13a protein and a crRNA corresponding to an NS3 gene target of the dengue virus, or a complex formed by the Cas13a protein and crRNA; and NS3 gene targets of the dengue virus are 4657th-4685th nucleotide sequences of the NS3 gene. In the invention, a novel anti-dengue virus method is found, is different from a traditional anti-virus method, directly targets a viral nucleic acid, specifically degrades a target gene, and makes the virus lose the replication ability; therefore, the effective Cas13a-based anti-dengue virus nucleic acid target has characteristicsof high efficiency, high specificity and programmability, is not easy to produce drug resistance generated by traditional antiviral drugs, and may become a novel antiviral drug in the future.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Samll molecule inhibitors for the treatment or prevention of dengue virus infection
Methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Vectors for regulating gene expression
InactiveUS20060239971A1Reduction in expression of target geneBiocideViral antigen ingredientsSuppressorRegulator gene
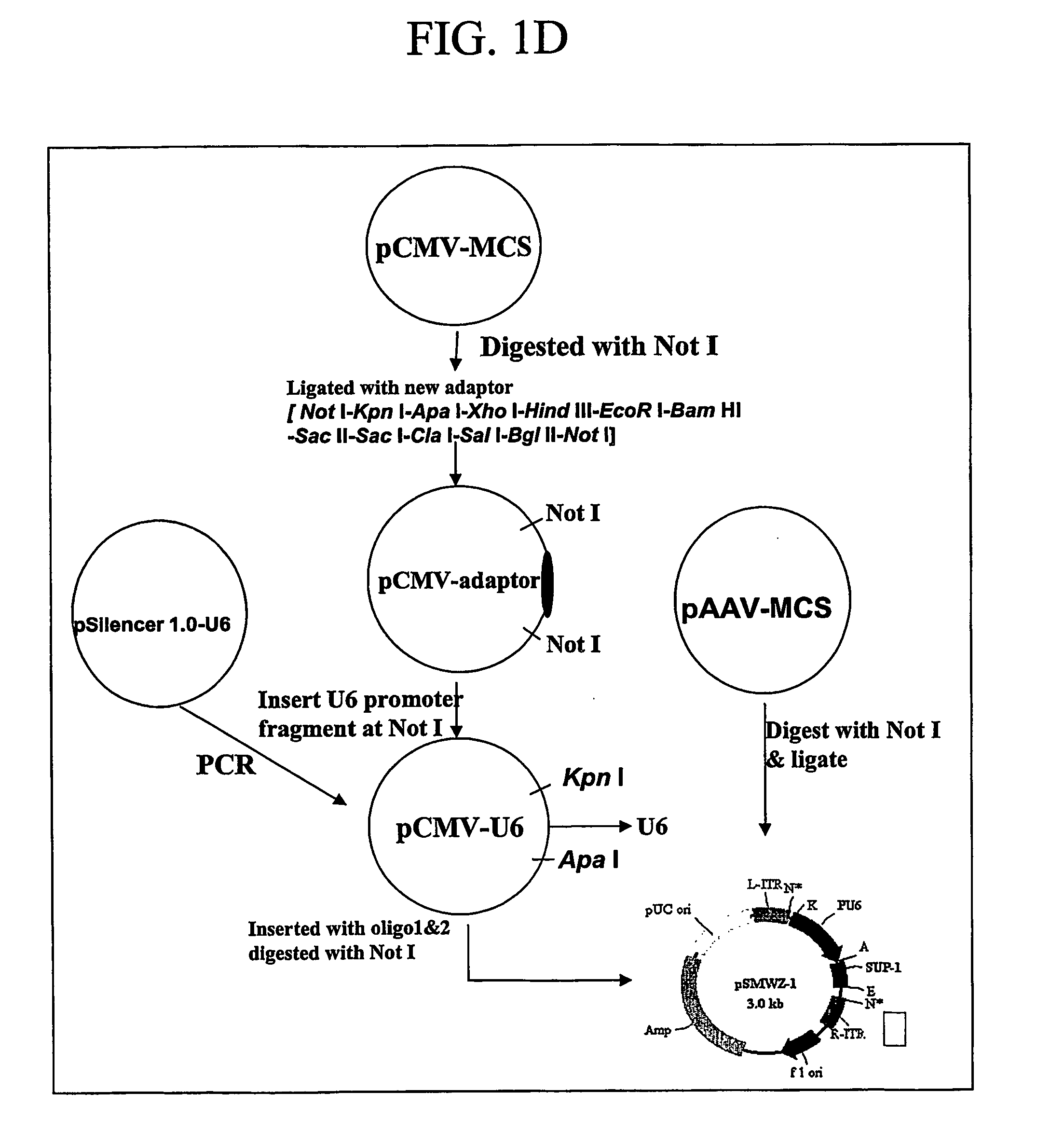
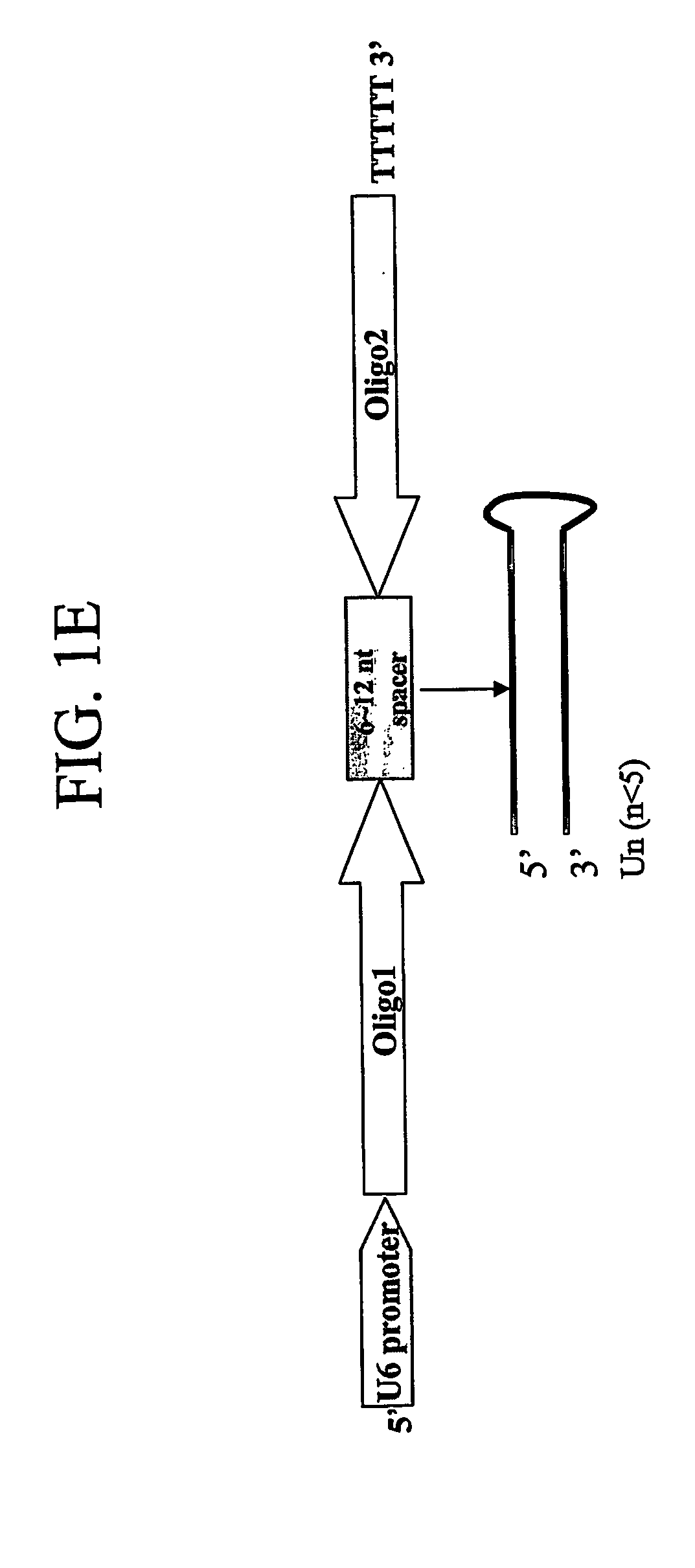
The present invention pertains to vectors for regulating gene expression having at least one gene expressing cassette and at least one gene suppressing cassette, wherein the gene expression cassette encodes a polypeptide of interest, and wherein the gene suppressing cassette encodes a short interfering RNA (siRNA) molecule that reduces expression of a target gene by RNA interference. The present invention further includes vectors that contain suppressor cassettes in conjunction with cassettes upregulating gene expression regulated by either a constitutive promoter, such as a general CMV promoter, or a tissue specific promoter. The present invention further includes vectors that contain Dengue virus gene suppression cassettes. The present invention further includes pharmaceutical compositions containing such vectors, methods of modulating the expression of genes in a host using such vectors, and method of producing such vectors.
Owner:UNIV OF SOUTH FLORIDA
L-nucleoside compounds and application thereof
InactiveCN105646629AInhibitory activityOrganic active ingredientsSugar derivativesEbola virusRNA Virus Infections
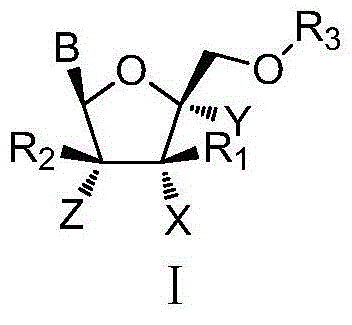
The invention discloses L-nucleoside compounds having the structure characteristic represented by the formula (I) or pharmaceutically acceptable salts thereof, and belongs to the technical field of pharmaceutical chemistry. The compounds can inhibit the activity of RNA viral polymerase, so the compounds can be used as potential drugs for prevention and treatment of infection of RNA viruses such as HCV, influenza virus, HRV (rhinovirus), RSV, Ebola virus, dengue virus, intestinal virus and the like.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Multivalent dengue virus vaccine
InactiveUS20070087015A1Enhance immune responseSufficient amountSsRNA viruses positive-senseViral antigen ingredientsSerial passageSerotype
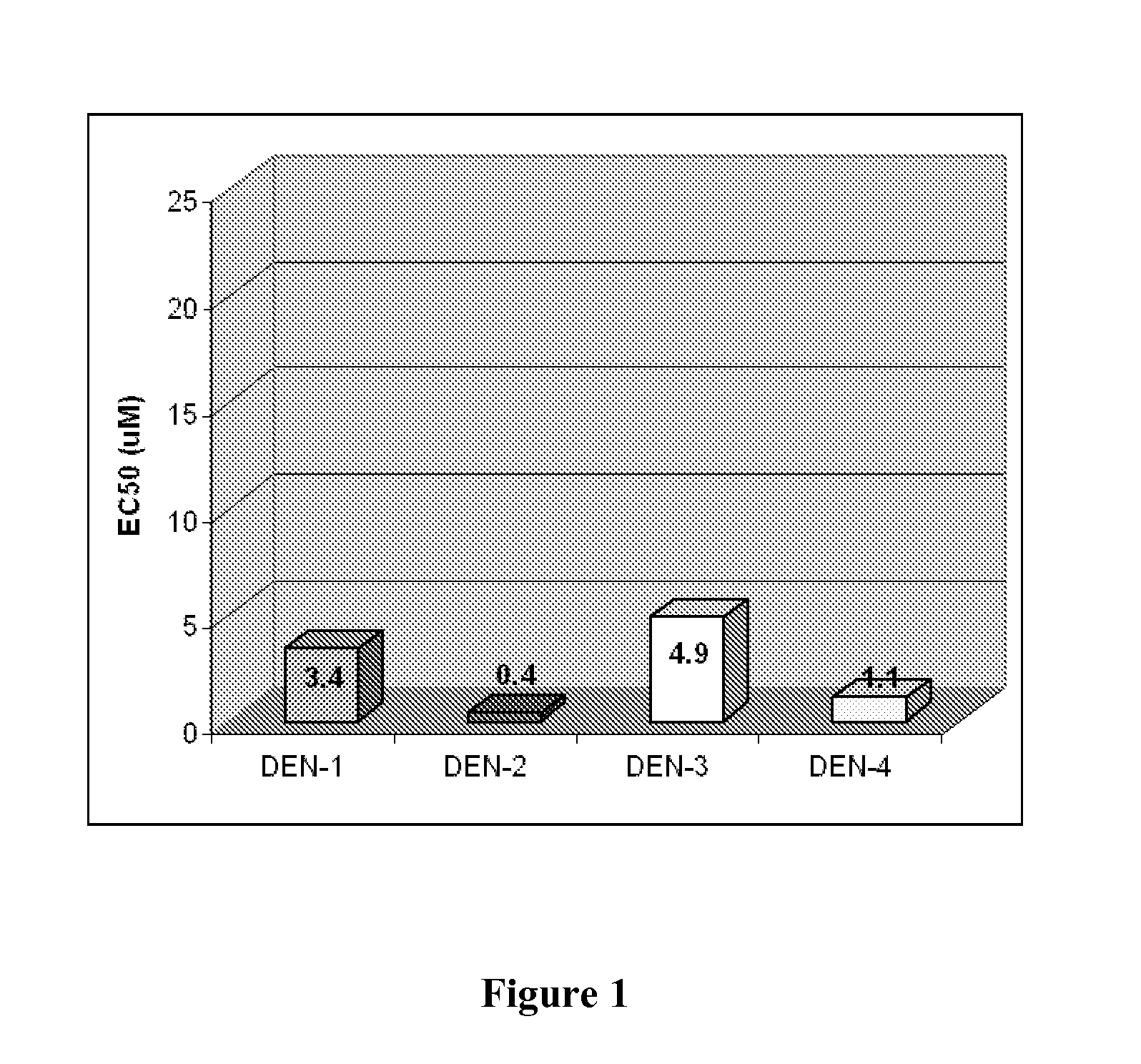
The present invention provides vaccine compositions of attenuated dengue virus. More specifically, the attenuated virus is produced by serial passage in PDK cells. The invention also provides methods for stimulating the immune system of an individual to induce protection against all four dengue virus serotypes by administration of attenuated dengue-1, dengue-2, dengue-3, and dengue-4 virus.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com