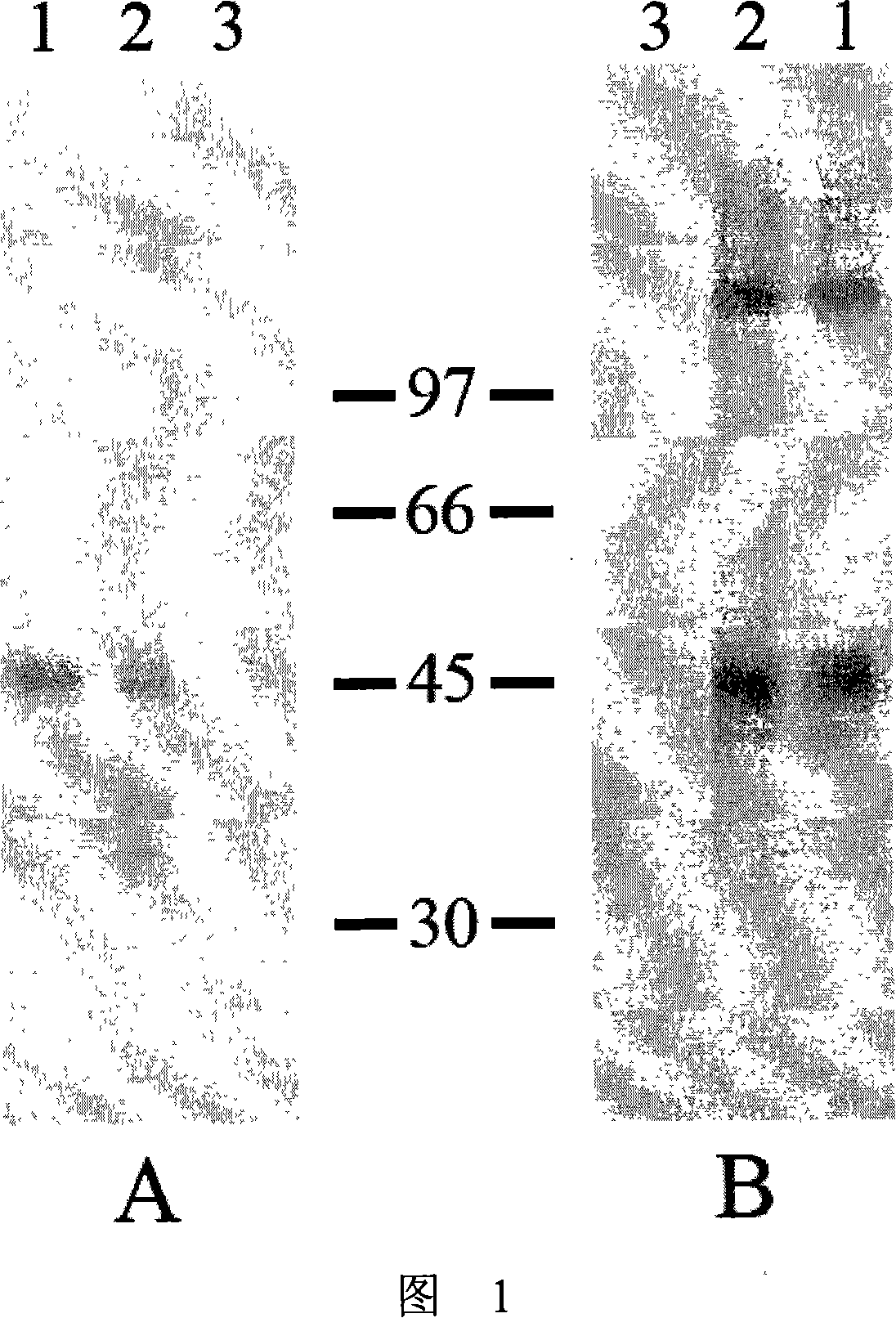
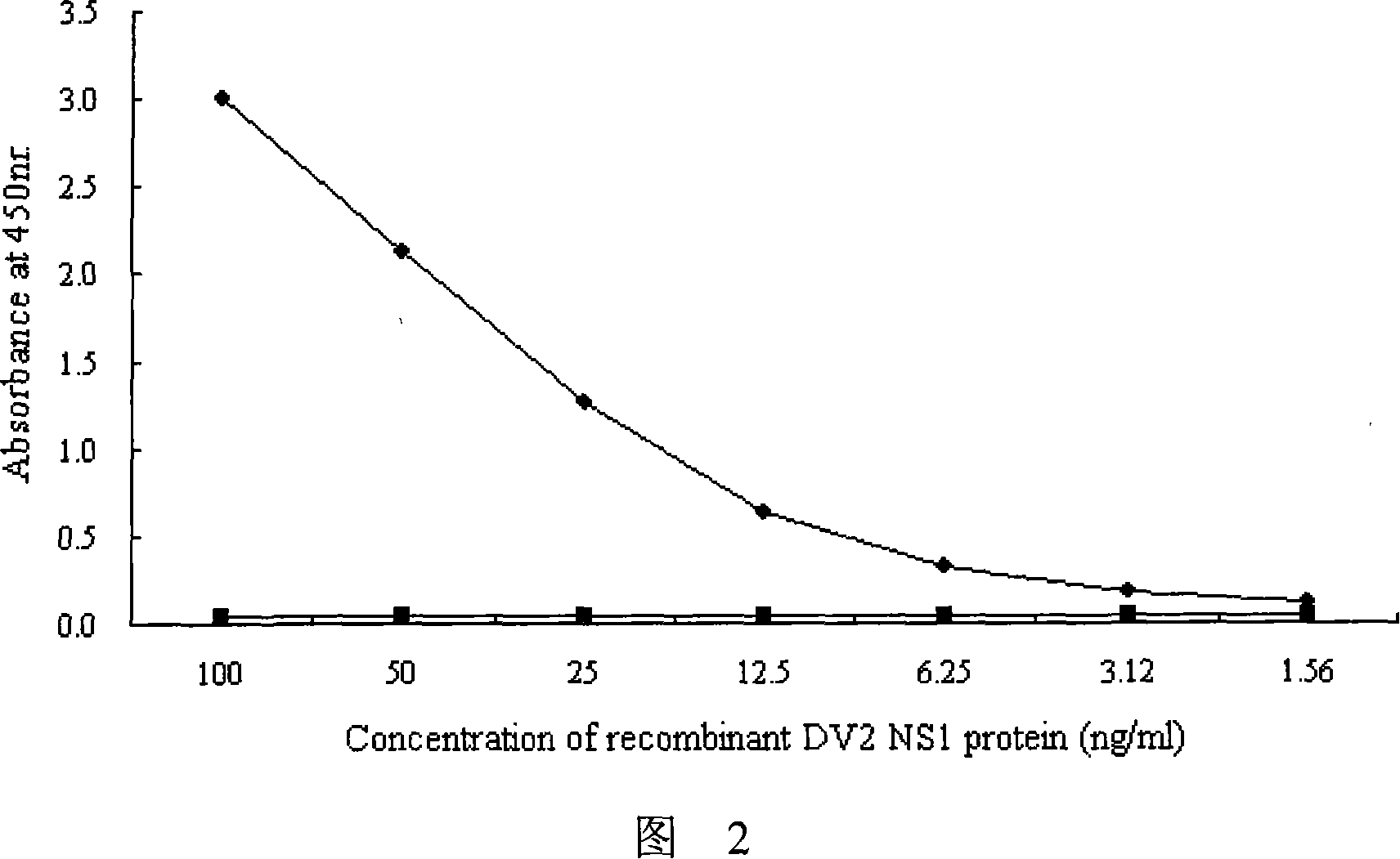
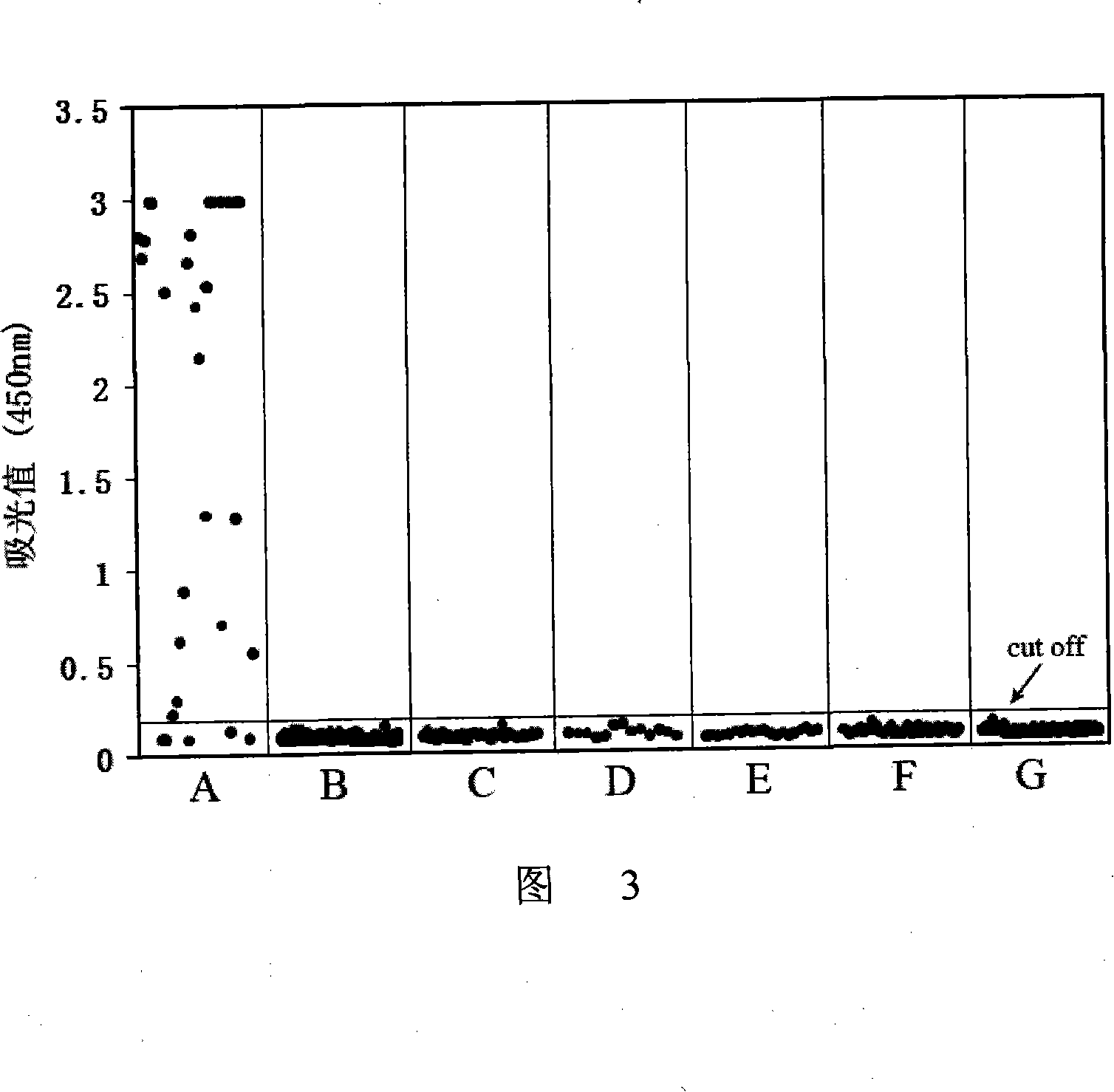
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
A dengue virus and immunodiagnosis technology, applied in the field of dengue virus detection kits, can solve the problem of dengue virus that cannot distinguish four serotypes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] 1. The kit of the present invention consists of the following reagents:
[0018] (1) Microwell reaction plate coated with monoclonal antibody DV2-M6;
[0019] (2) Sample treatment solution: composed of sample treatment solution A and sample treatment solution B, wherein A is a 1.5M glycine solution (PH2.8), and B is a 1.5M Tris-Hcl solution (PH9.7);
[0020] (3) Biotin-labeled monoclonal antibody DV2-M14;
[0021] (4) horseradish peroxidase-labeled avidin, purchased from Zymed Company;
[0022] (5) Concentrated lotion: 20×PBS containing 2% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02gNa 2 HPO 4 .12H 2 O, 175.3gNaCl, 15 lbs, 20min autoclave, add 20ml Tween-20, stir well, dilute 20 times when used;
[0023] (6) Positive control: 1:1000 dilution of DV2NS1 antigen;
[0024](7) Negative control: 10mM PH7.4PBS containing 0.1% Tween-20, that is, 4.56gNaH in 1L solution 2 PO 4 , 58.02g Na 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds 20min autoclave, add 0...
example 2
[0091] 1. The kit of the present invention consists of the following reagents:
[0092] (1) Microwell reaction plate coated with monoclonal antibody DV2-M6;
[0093] (2) Composed of sample treatment solution A and sample treatment solution B, wherein A is a 1.5M glycine solution (PH2.8), and B is a 1.5M Tris-Hcl solution (PH9.7);
[0094] (3) Mab DV2-M14 labeled with horseradish peroxidase;
[0095] (4) Concentrated lotion: 20×PBS containing 2% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02gNa 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds of 20min after autoclaving, add 20ml Tween-20 and stir well, dilute 20 times when used;
[0096] (5) Positive control: 1:1000 dilution of DV2NS1 antigen;
[0097] (6) Negative control: 10mM PH7.4PBS containing 0.1% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02g Na 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds 20min autoclave, add 0.1% Tween-20 after 20-fold dilution;
[0098] (7) Chromogenic solution: It is compose...
example 3
[0109] 1. The kit of the present invention consists of the following reagents:
[0110] (1) Microwell reaction plate coated with monoclonal antibody DV2-M6;
[0111] (2) Sample treatment solution: composed of sample treatment solution A and sample treatment solution B, wherein A is a 1.5M glycine solution (PH2.8), and B is a 1.5M Tris-Hcl solution (PH9.7);
[0112] (3) Alkaline phosphatase-labeled monoclonal antibody DV2-M14;
[0113] (4) Concentrated lotion: 20×PBS containing 2% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02gNa 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds of 20min after autoclaving, add 20ml Tween-20 and stir well, dilute 20 times when used;
[0114] (5) Positive control: 1:1000 dilution of DV2NS1 antigen;
[0115] (6) Negative control: 10mM PH7.4PBS containing 0.1% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02g Na 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds 20min autoclave, add 0.1% Tween-20 after 20-fold dilution;
[0116] (7)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com