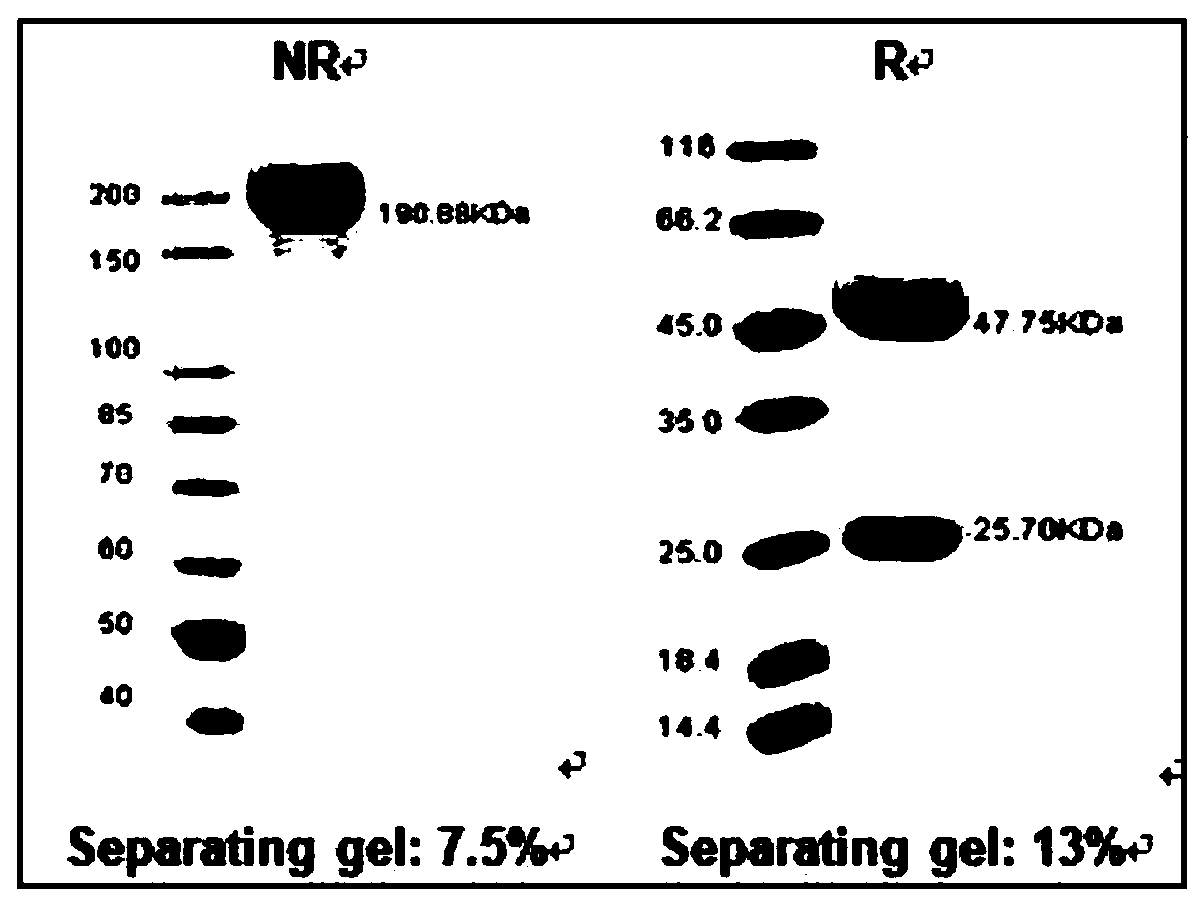
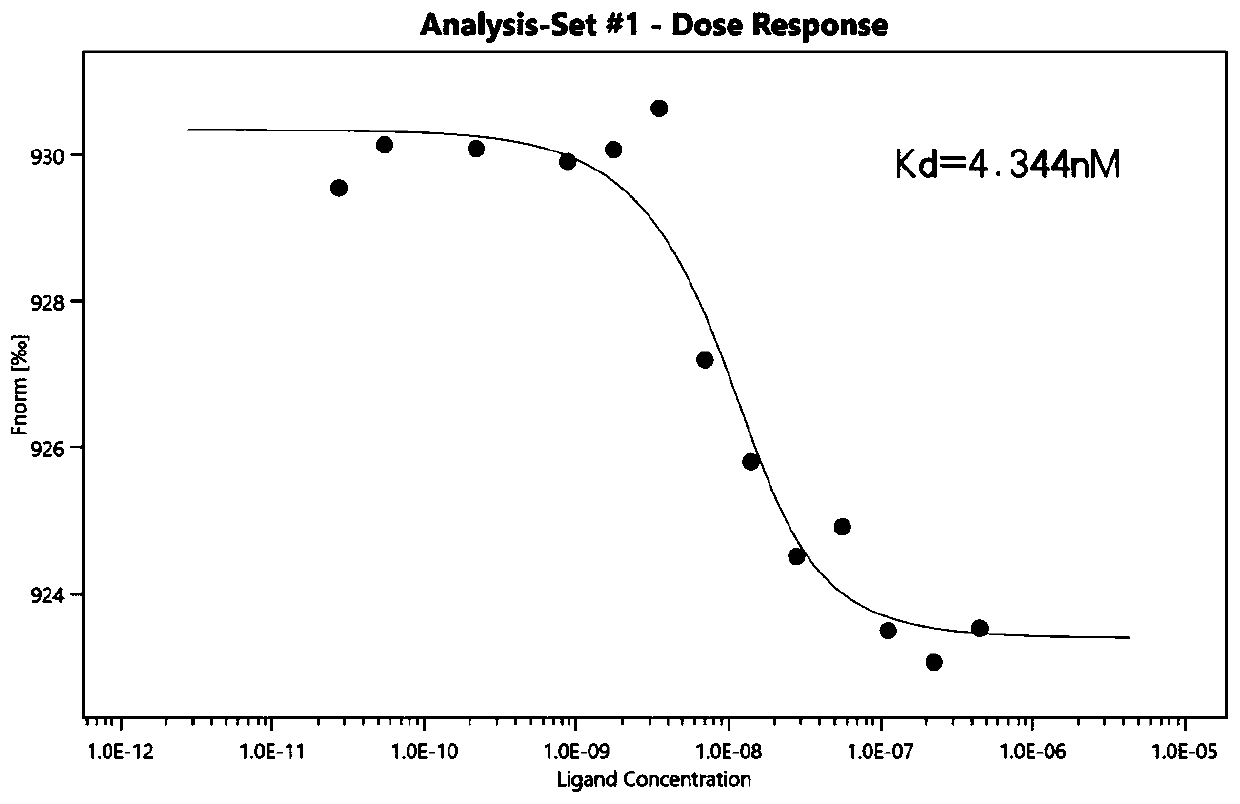
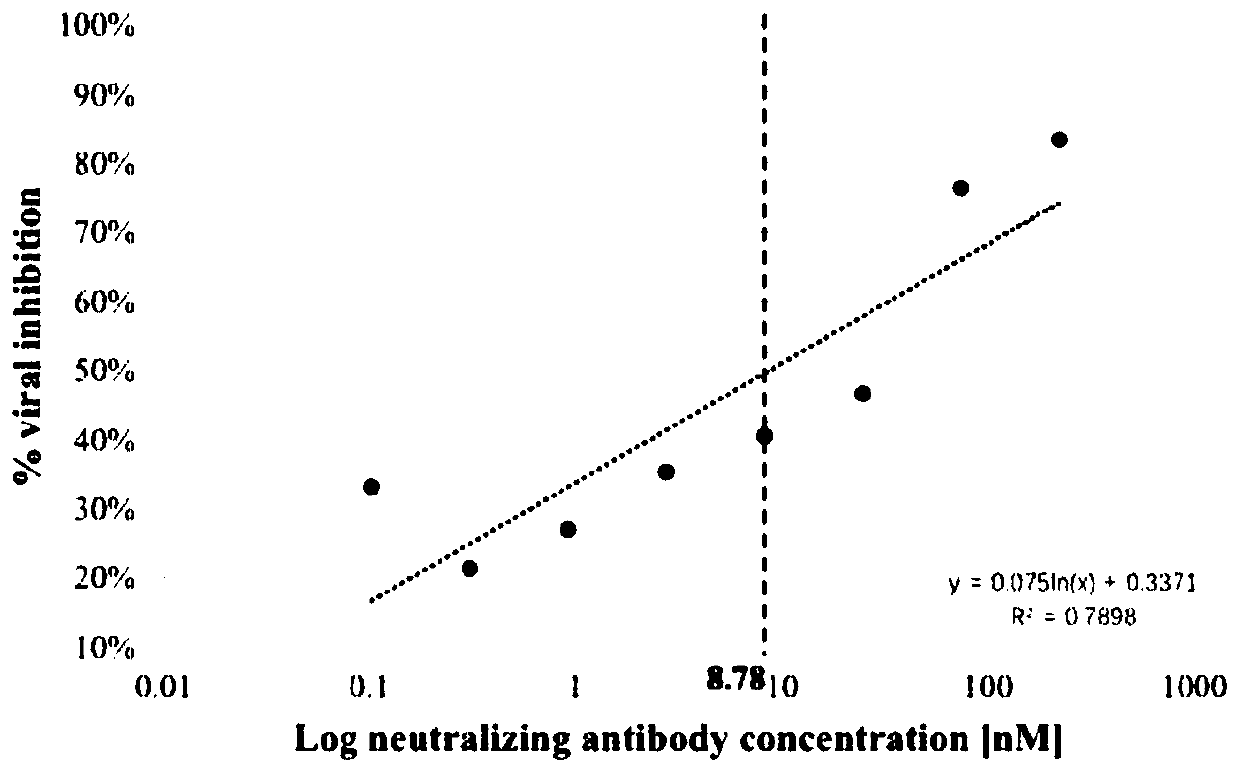
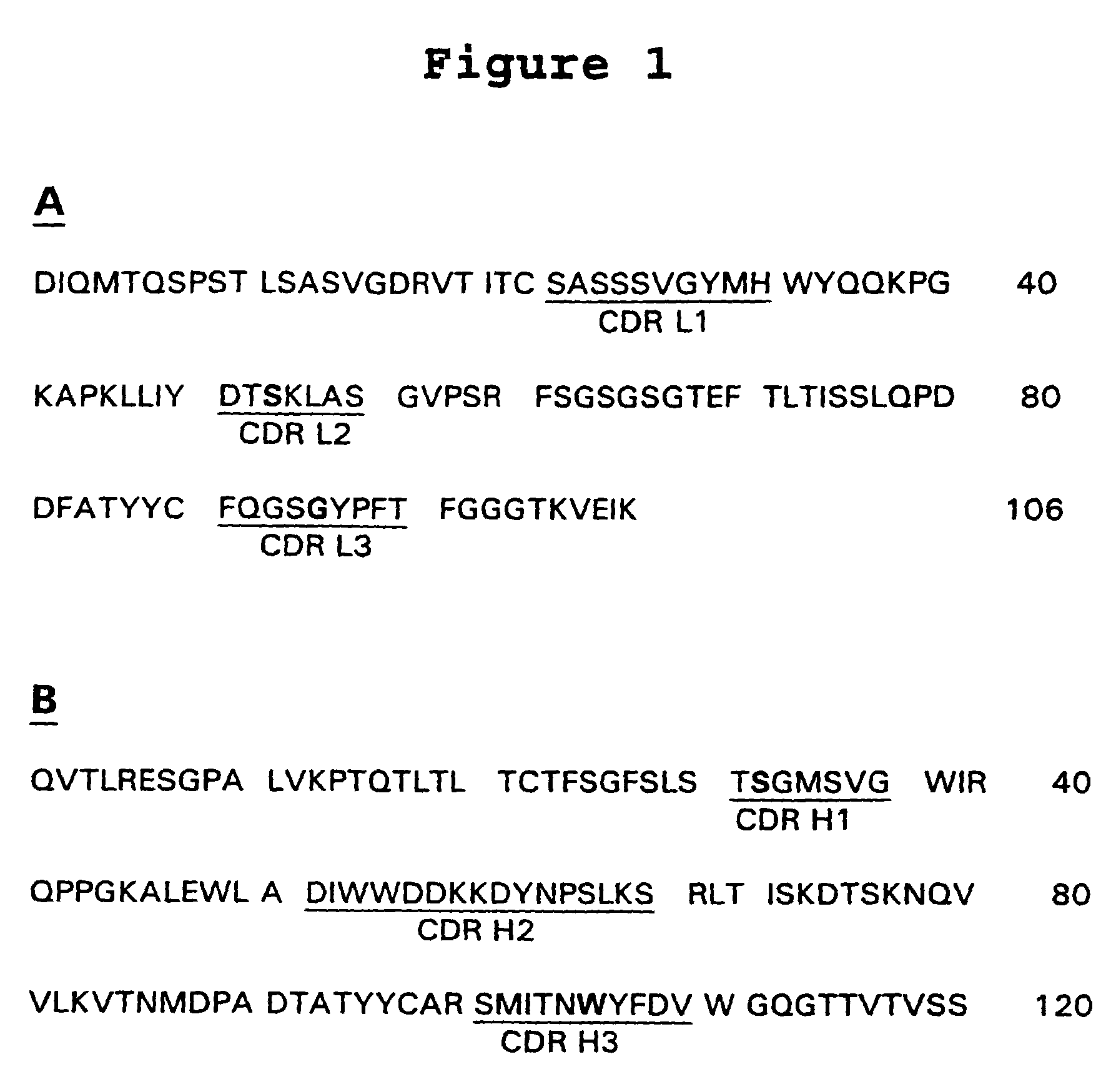
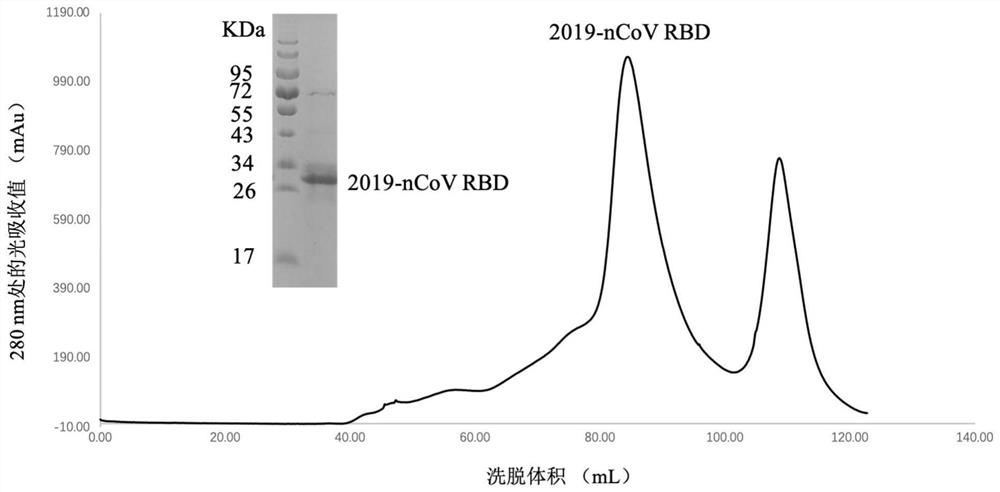
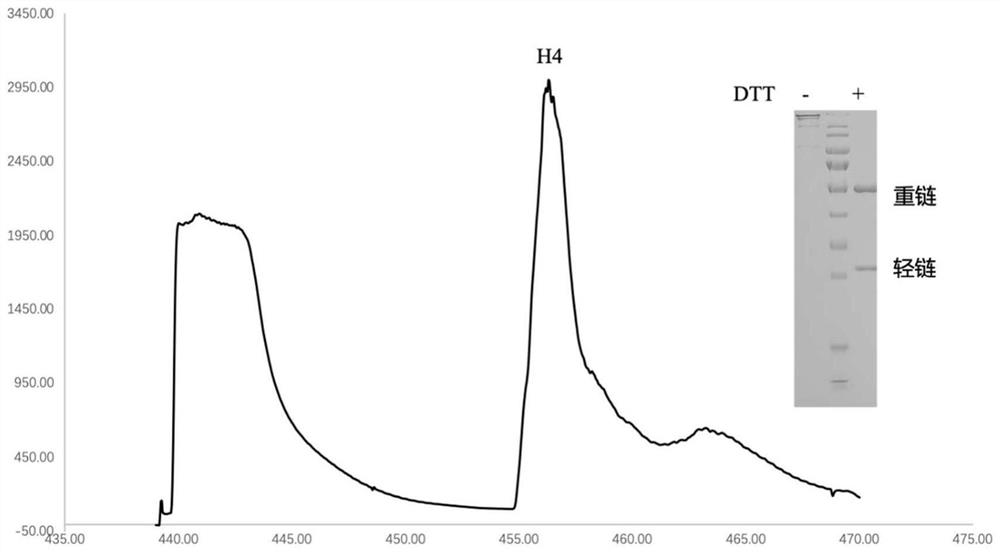
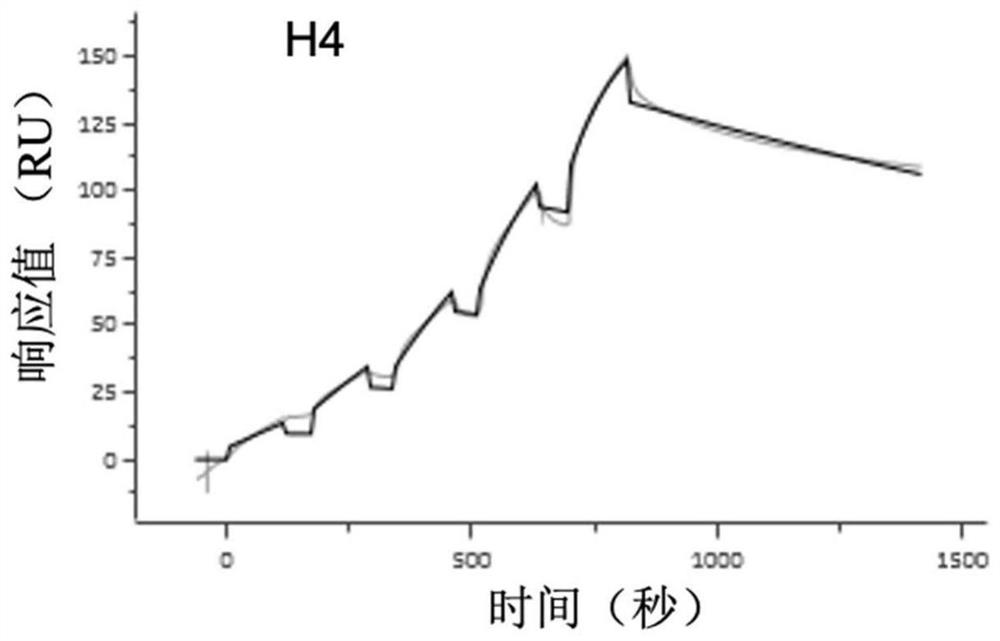
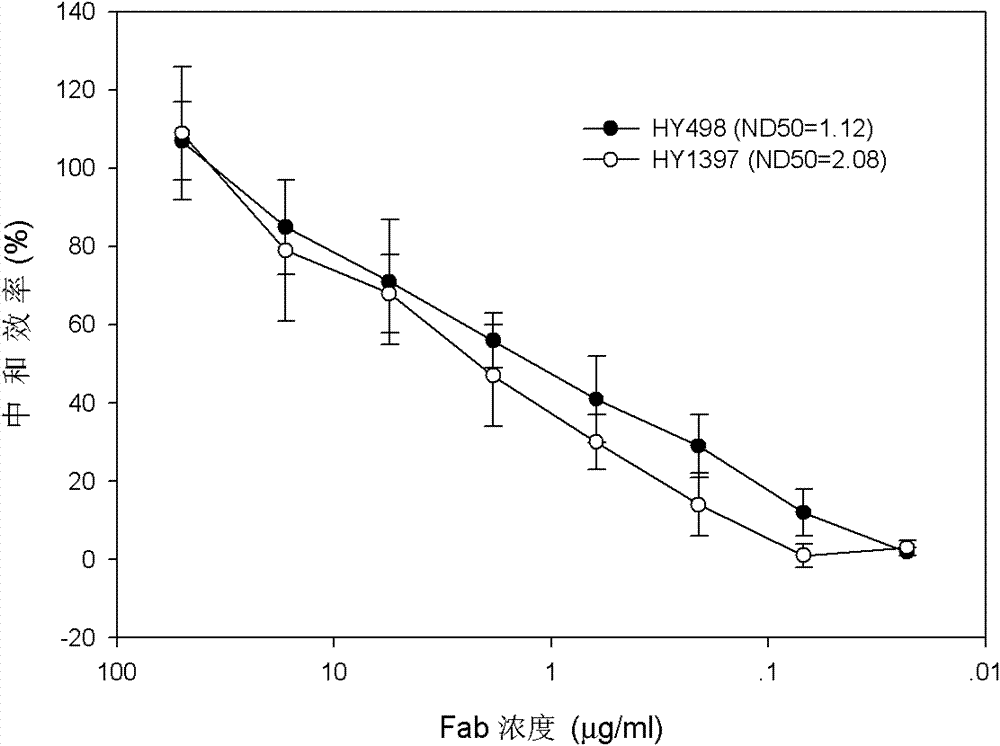
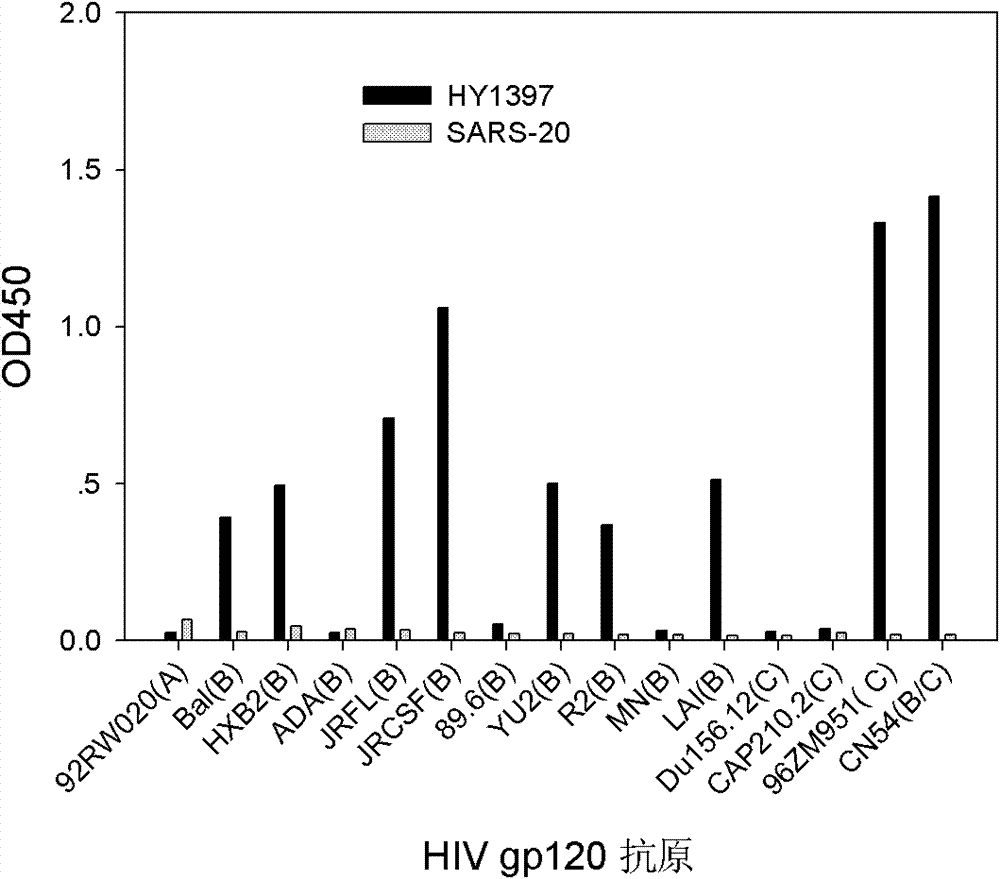
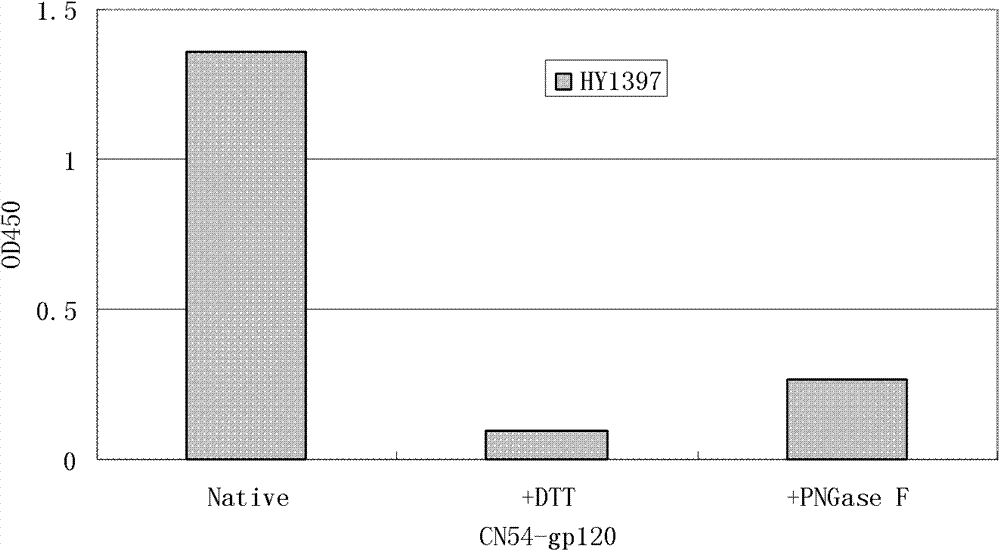
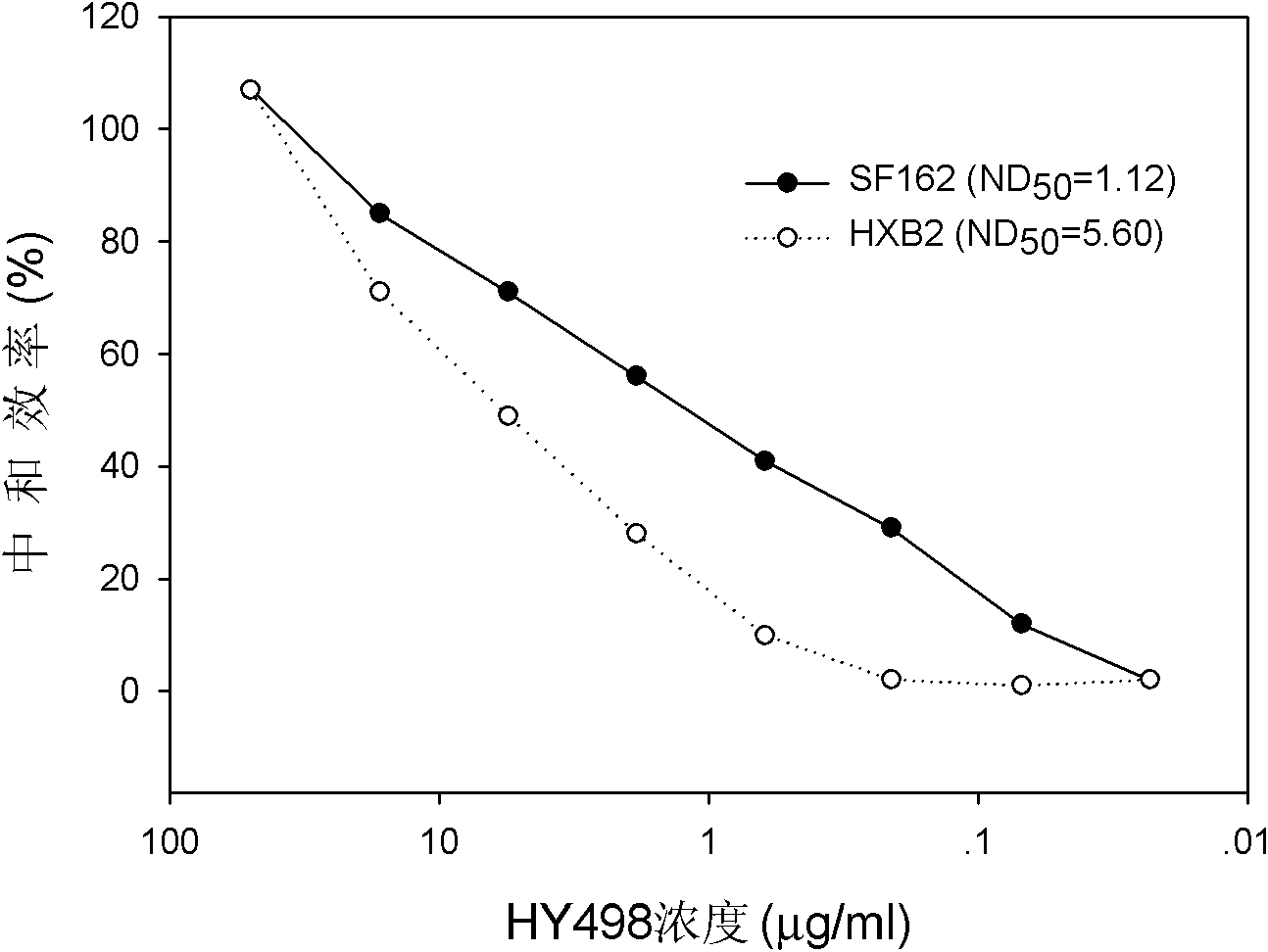
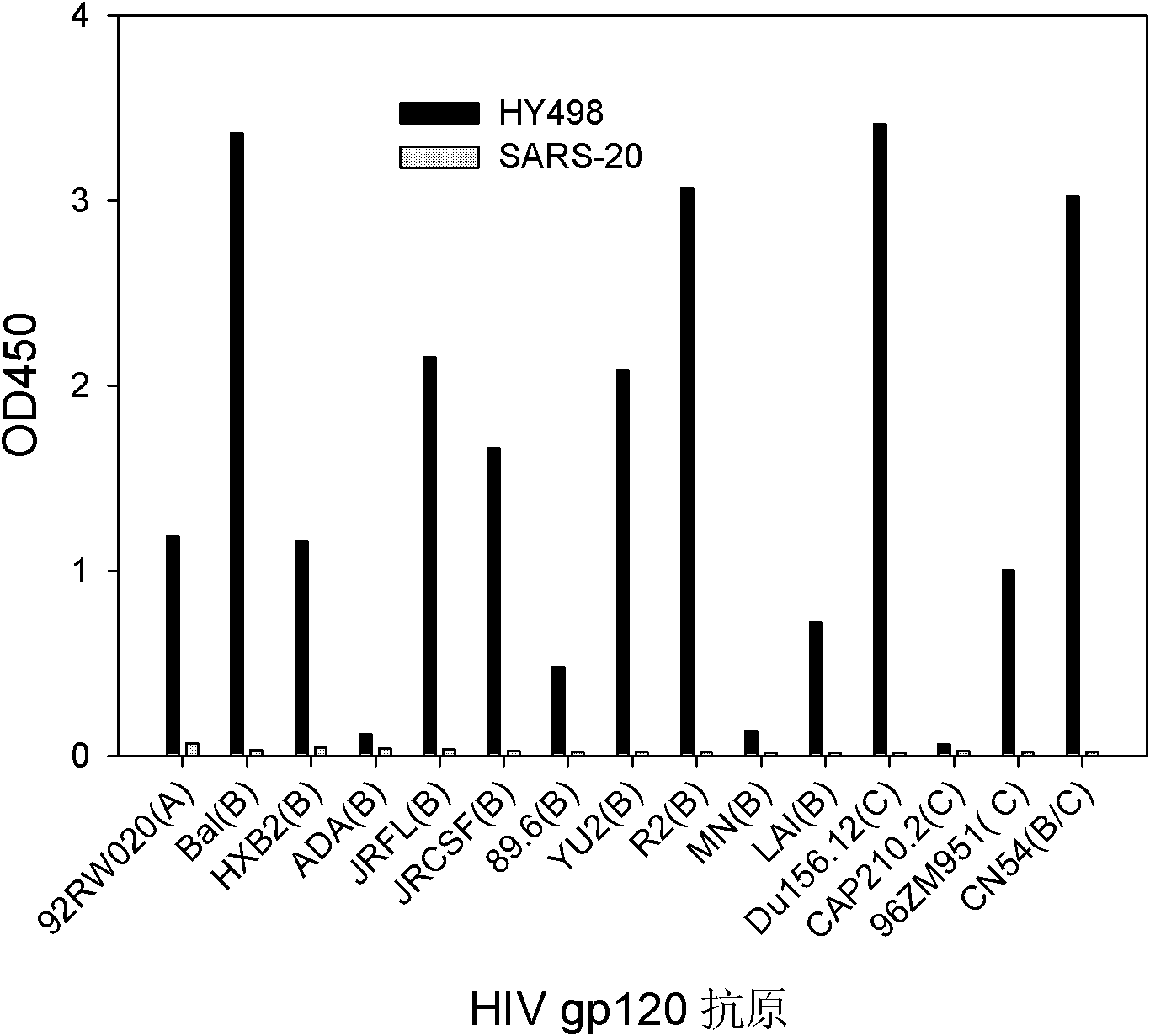
Patents
Literature
41results about How to "Strong neutralizing activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
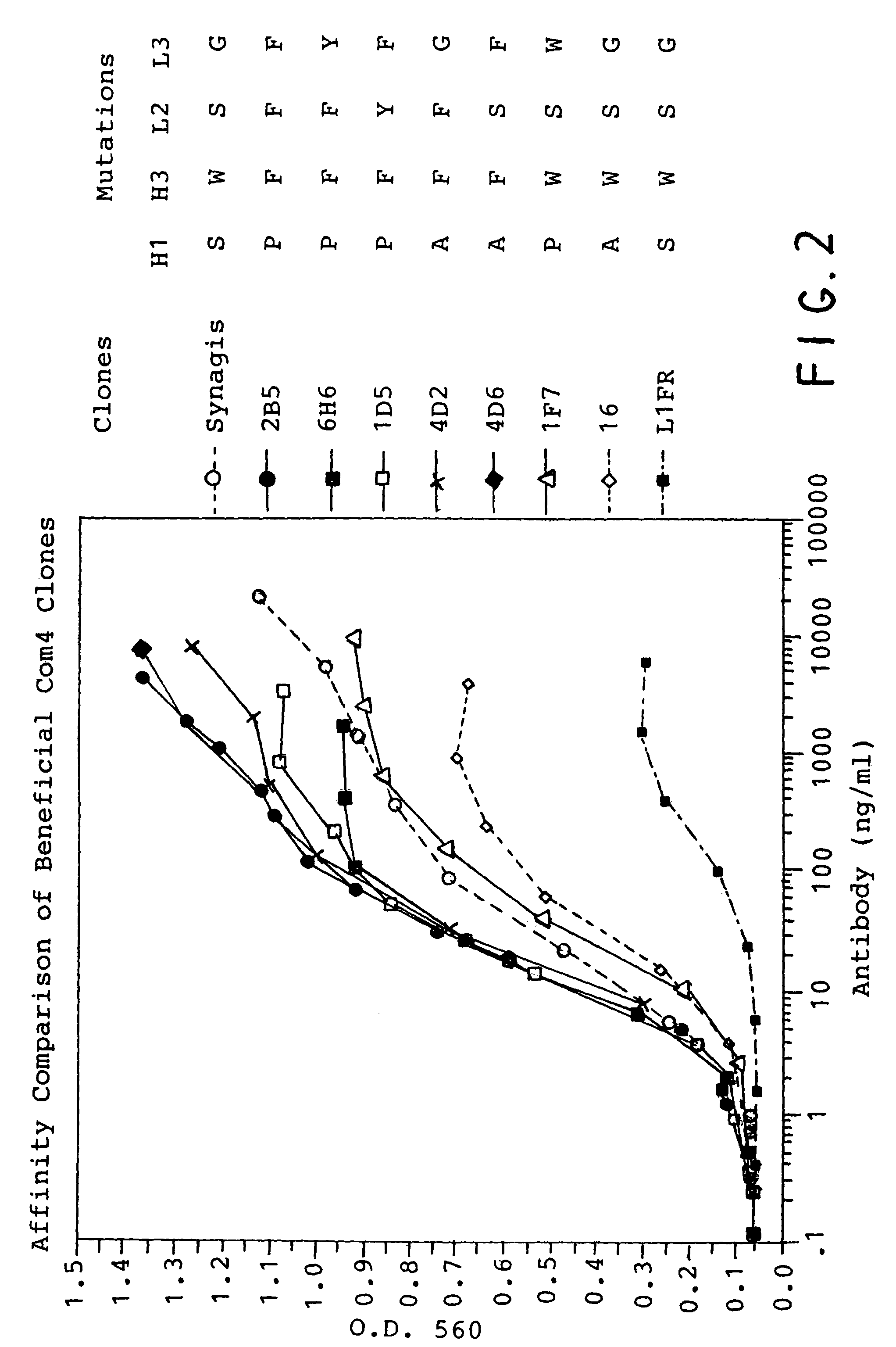
Anti-novel coronavirus monoclonal antibody and application thereof
ActiveCN111592594AStrong neutralizing activityImmunoglobulins against virusesAntiviralsDiseaseInfection induced
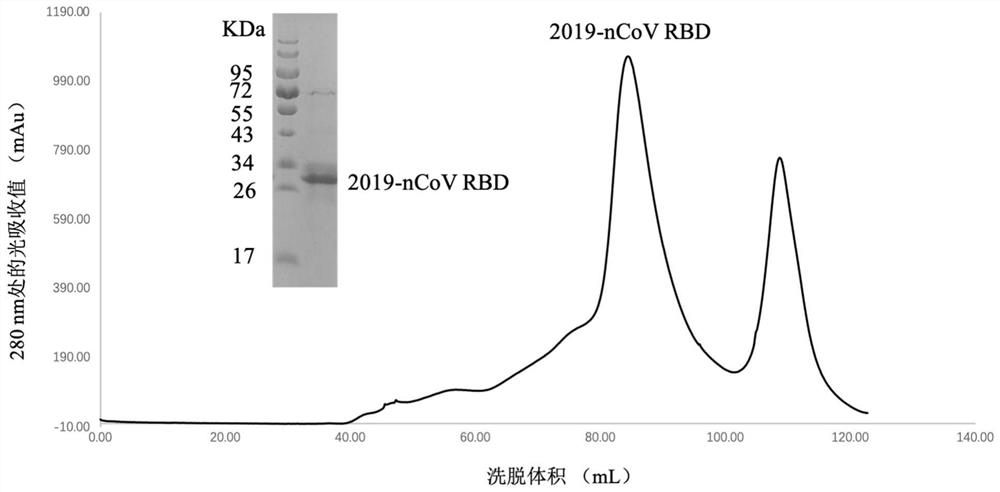
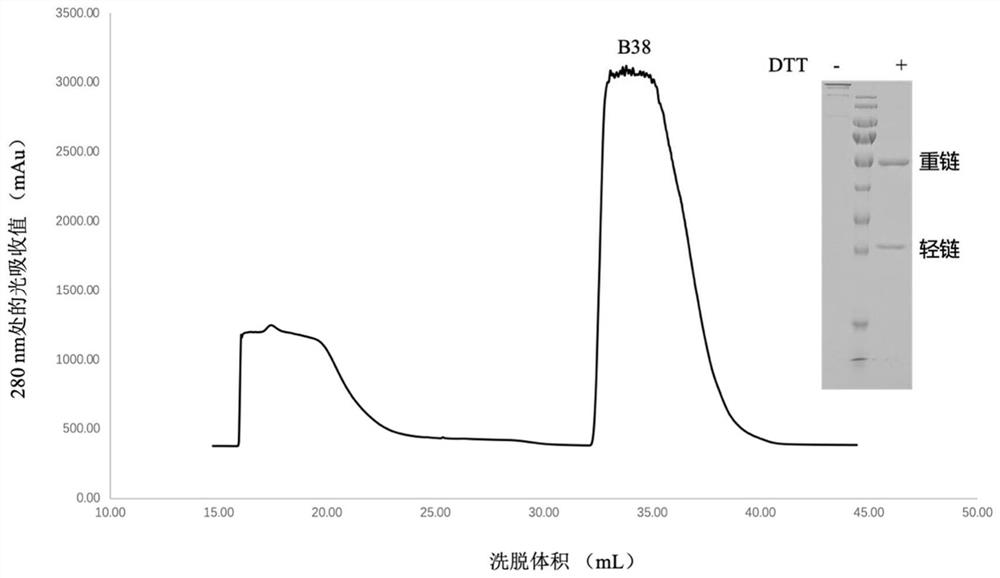
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of novel coronaviruses. Specifically, the present inventionrelates to an anti-novel coronavirus monoclonal antibody, and a composition (e.g., a diagnostic agent and a therapeutic agent) comprising the antibody. Furthermore, the invention also relates to an application of the antibody. The antibody provided by the invention can be applied to diagnosis, prevention and / or treatment of infection of the novel coronavirus and / or diseases (e.g., novel coronavirus pneumonia) caused by the infection.
Owner:PEKING UNIV
Ultra high affinity neutralizing antibodies
InactiveUS7740851B2Low costReduce efficacyHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseComplementarity determining region
Ultra high affinity antibodies with binding affinities in the range of 1010 M−1, and even 1011 M−1 are disclosed. Such antibodies include antibodies having novel high affinity complementarity determining regions (CDRs), especially those with framework and constant regions derived from either humans or mice. Methods of preparing and screening such antibodies, as well as methods of using them to prevent and / or treat disease, especially virus-induced diseases, are also disclosed.
Owner:MEDIMMUNE LLC
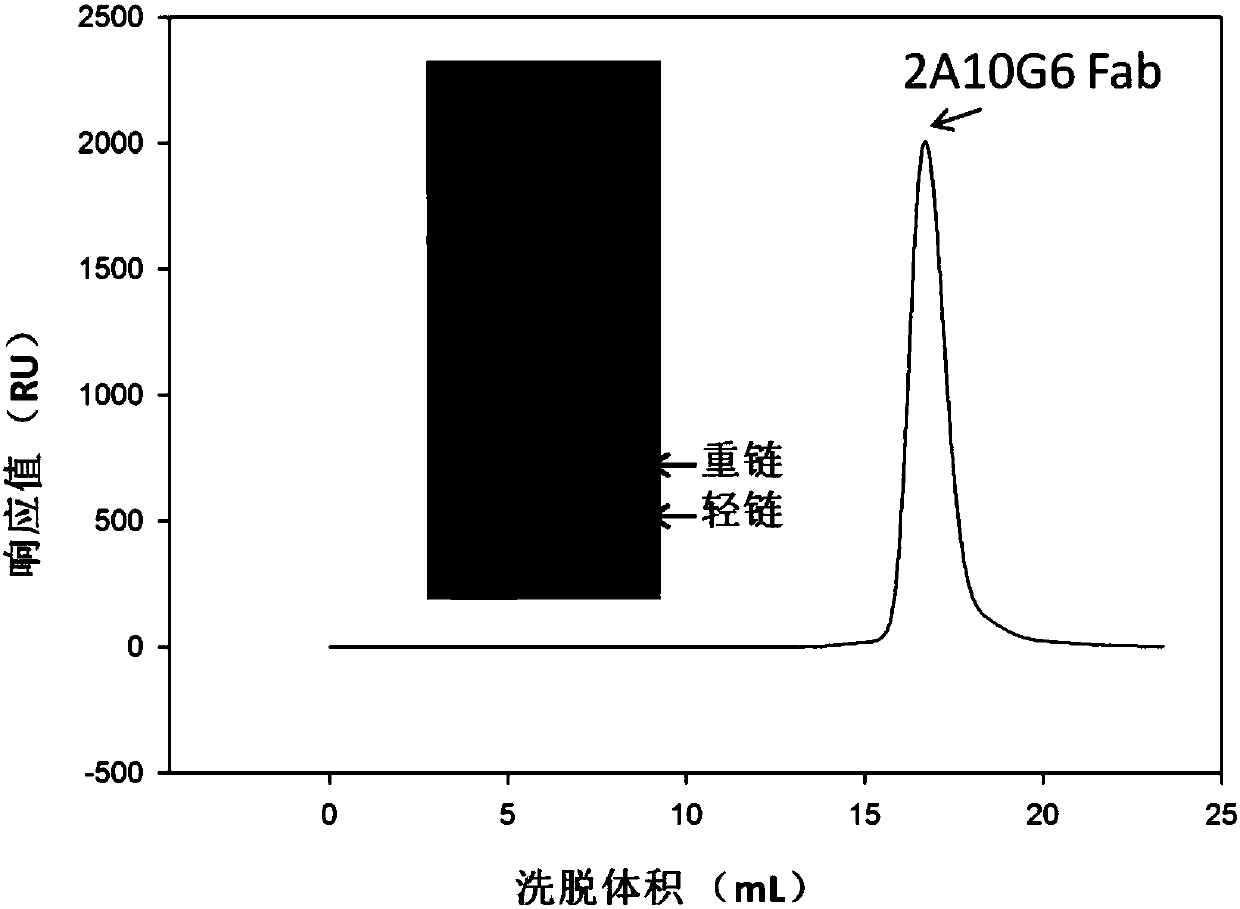
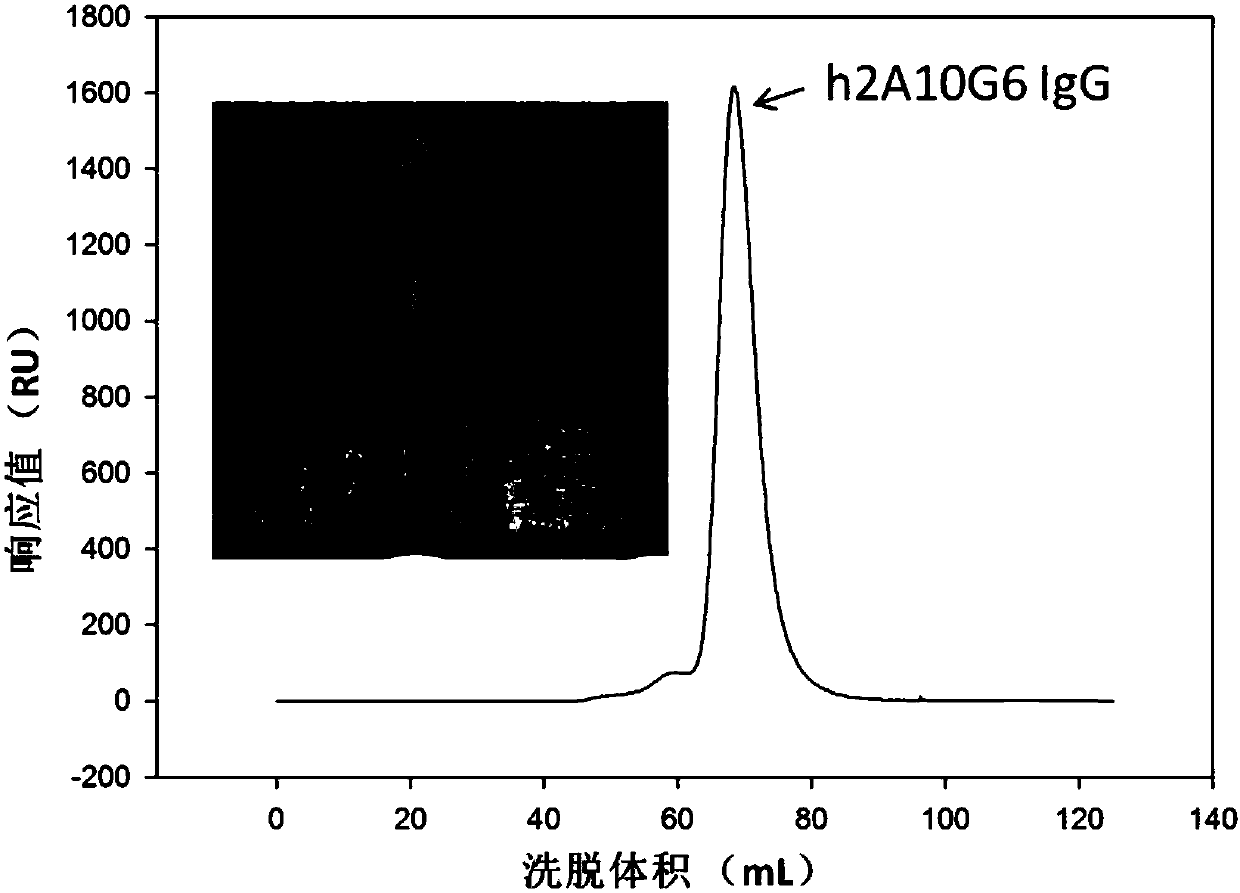
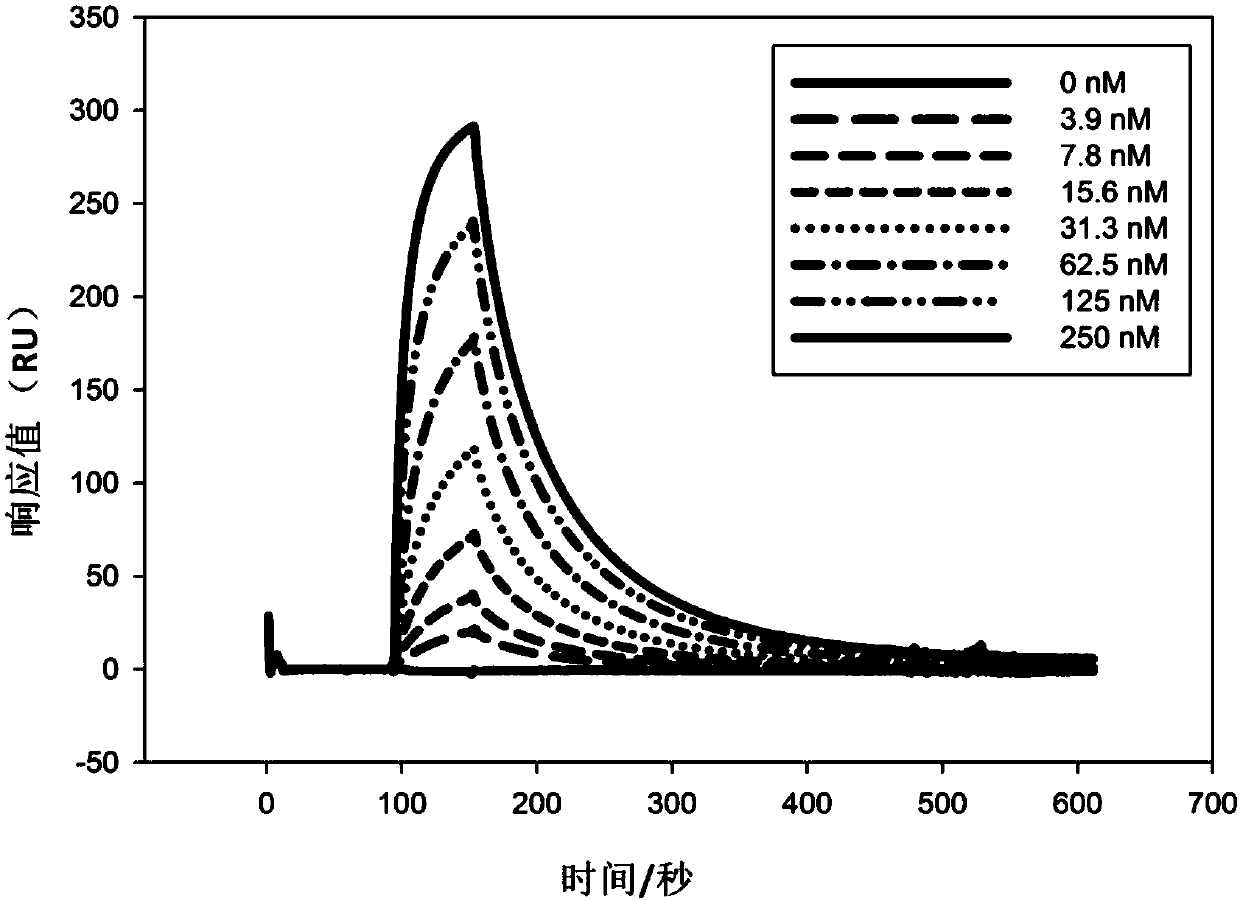
Humanized monoclonal antibody and application thereof
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
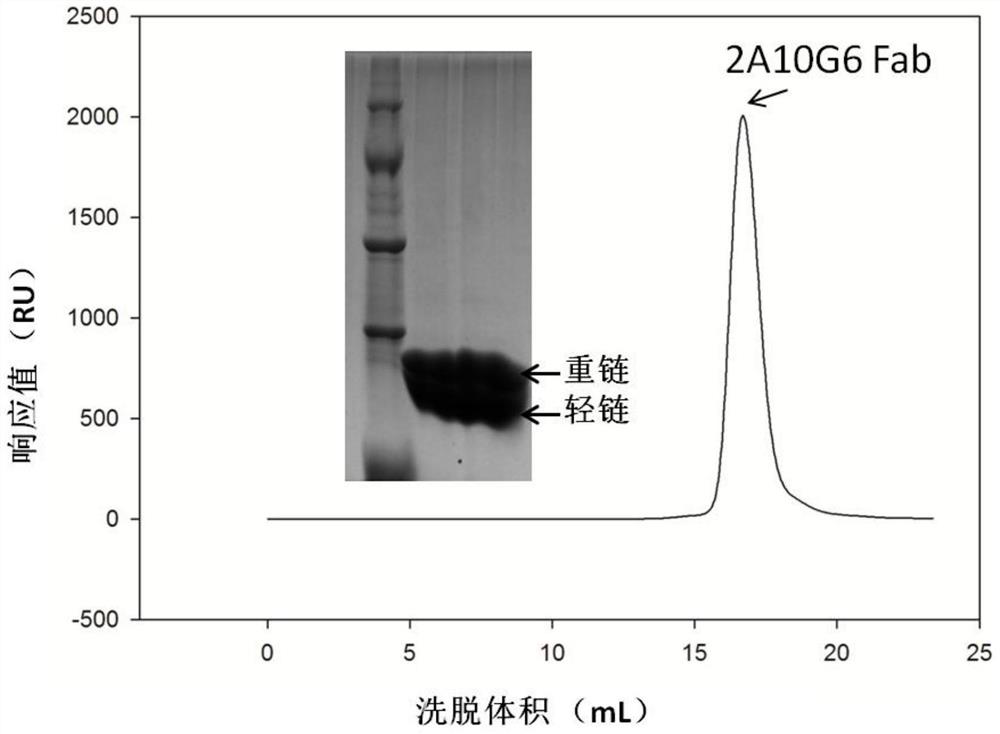
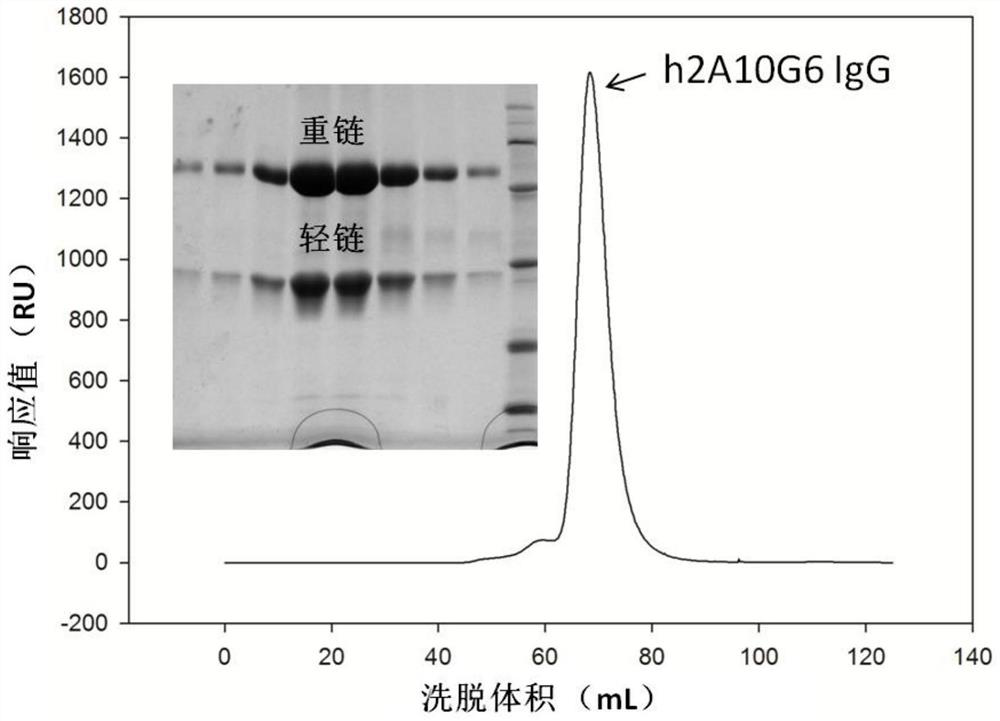
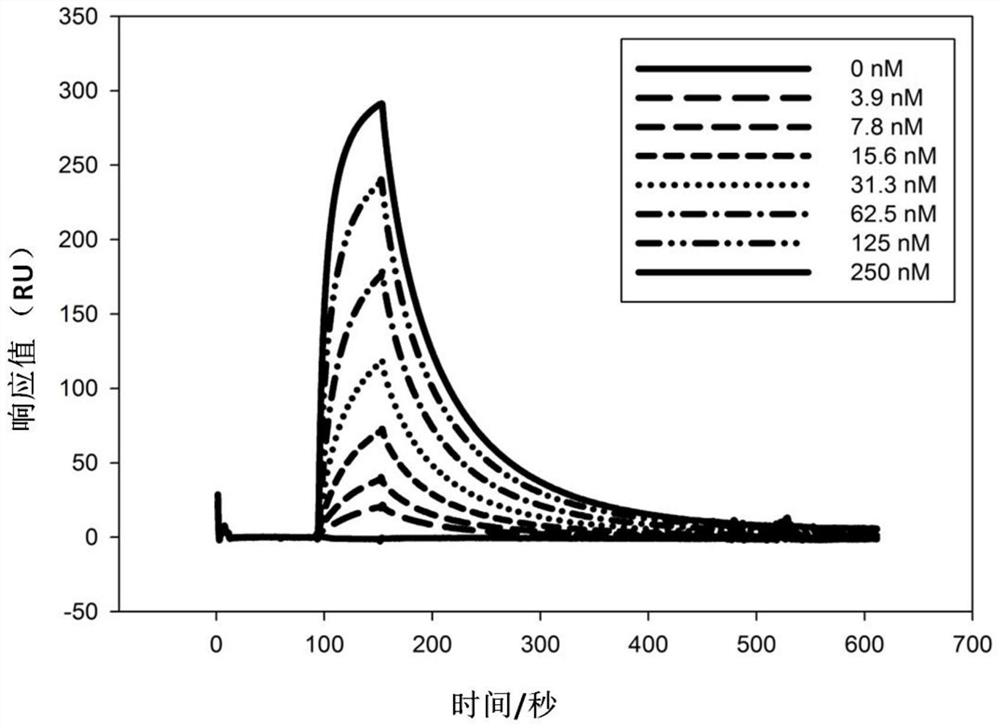
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Anti fgf23 antibody and a pharmaceutical composition comprising the same
ActiveUS20090148461A1Suppress actionPrevent and treat diseaseMuscular disorderSkeletal disorderCancer researchAntibody
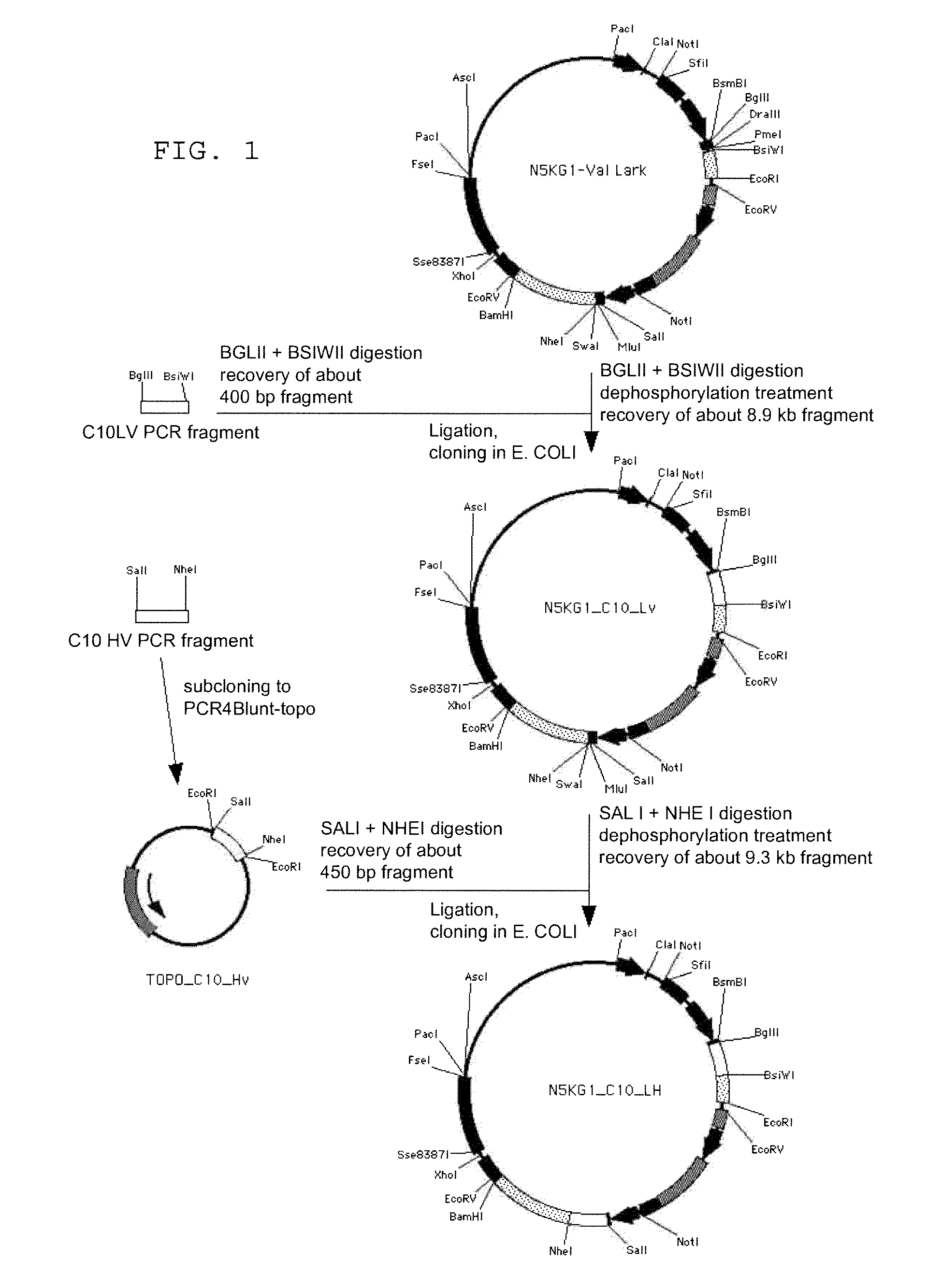
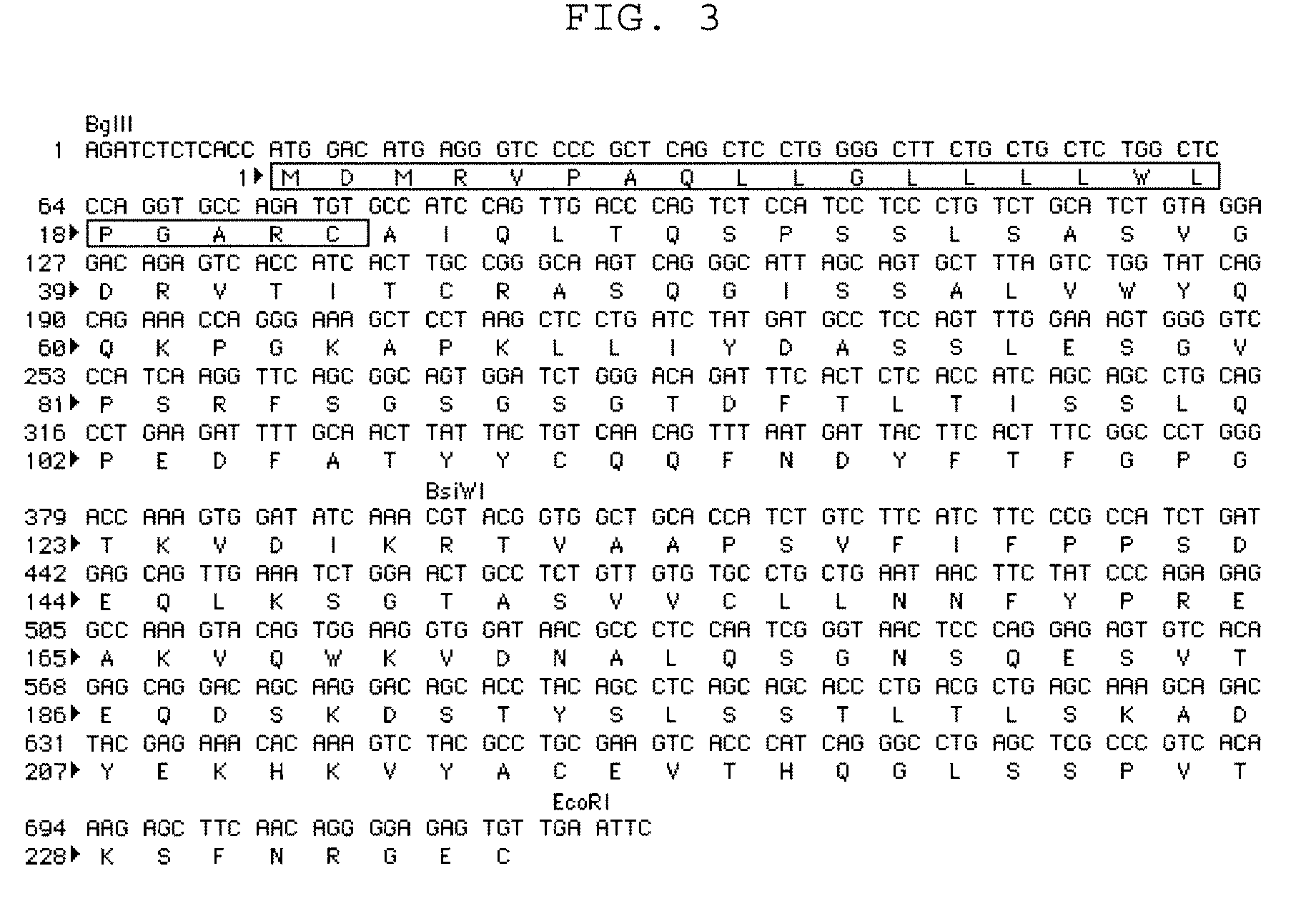
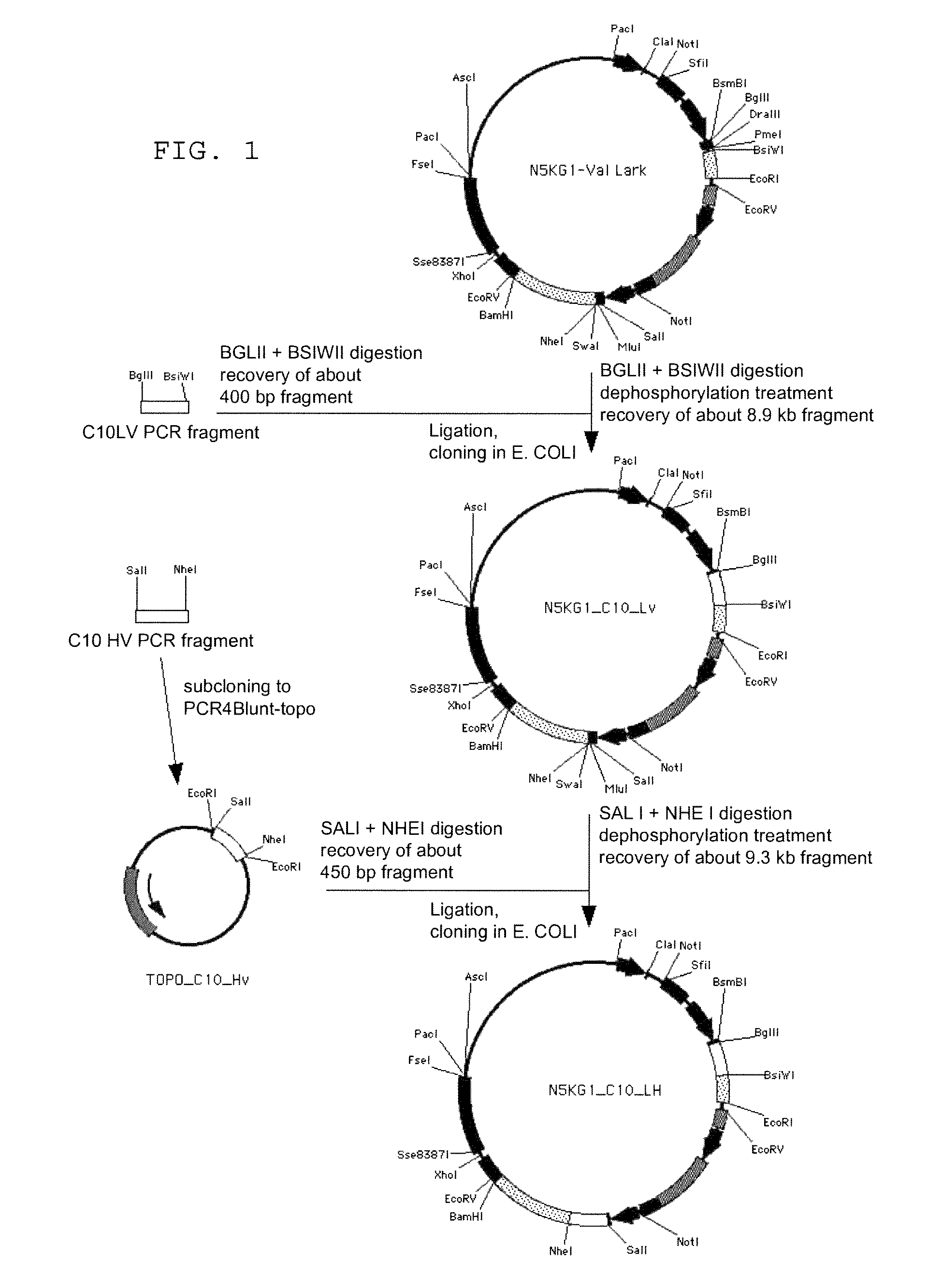
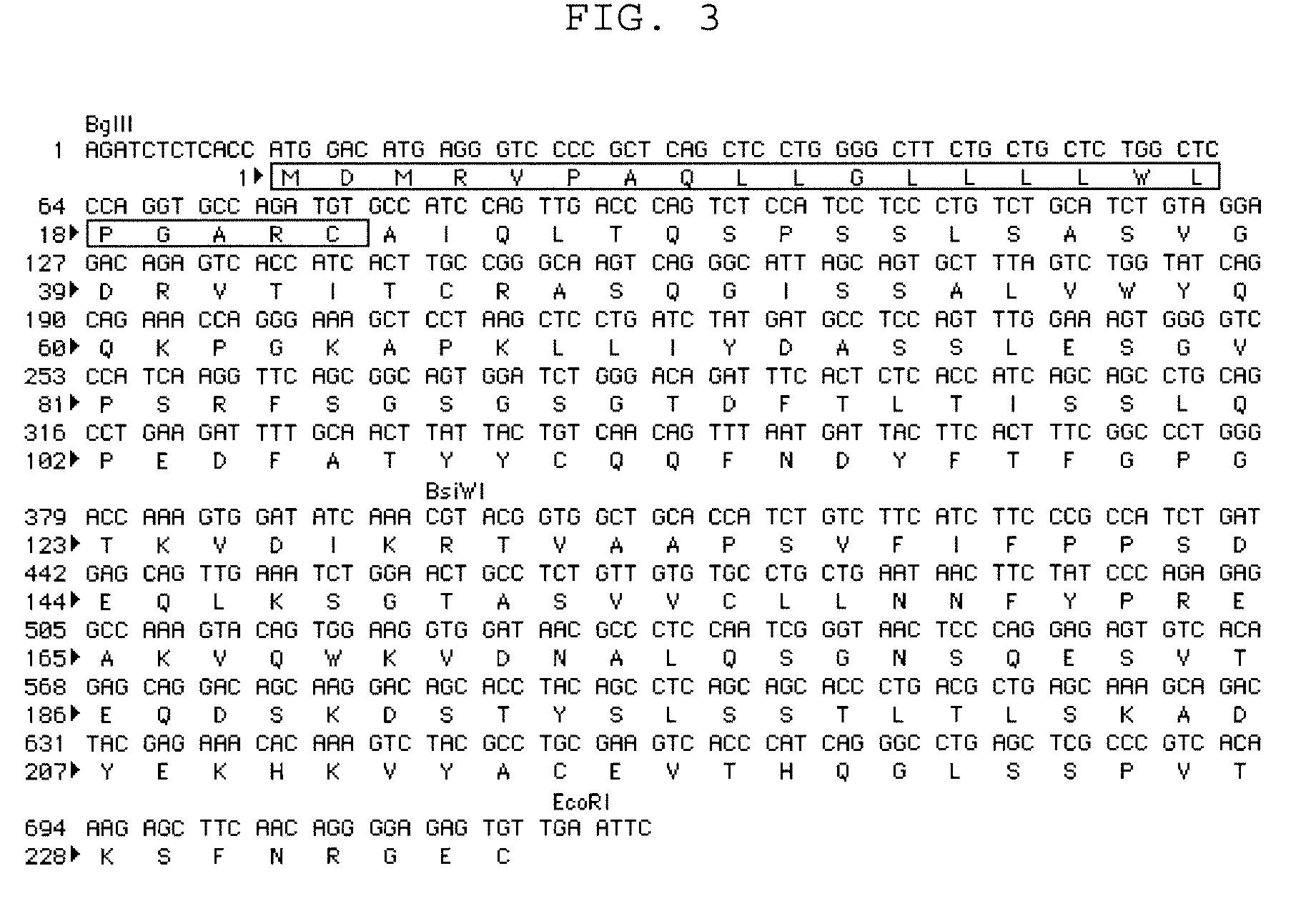
To provide an antibody against FGF23 and a pharmaceutical composition such as a preventive or therapeutic agent which can prevent or treat by suppressing an action of FGF23 by using the antibody. An antibody or its functional fragment against human FGF23 produced by hybridoma C10 (Accession No. FERM BP-10772).
Owner:KYOWA HAKKO KIRIN CO LTD
Anti FGF23 antibody and a pharmaceutical composition comprising the same
ActiveUS7883705B2Suppress actionLess side effectsMuscular disorderSkeletal disorderAntiendomysial antibodiesPharmaceutical drug
To provide an antibody against FGF23 and a pharmaceutical composition such as a preventive or therapeutic agent which can prevent or treat by suppressing an action of FGF23 by using the antibody. An antibody or its functional fragment against human FGF23 produced by hybridoma C10 (Accession No. FERM BP-10772).
Owner:KYOWA HAKKO KIRIN CO LTD
Monoclonal antibody of novel coronavirus and mutant thereof and application of monoclonal antibody
ActiveCN113943368AHigh affinityHigh neutralizing activityImmunoglobulins against virusesAntiviralsAntigenComplementarity determining region
The invention relates to the technical field of immunology and molecular virology, and particularly discloses a monoclonal antibody of novel coronavirus and a mutant thereof and application of the monoclonal antibody. Complementary determining regions CDR1, CDR2 and CDR3 of a heavy chain variable region of the monoclonal antibody or an antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3; and complementary determining regions CDR1, CDR2 and CDR3 of a light chain variable region of the monoclonal antibody or the antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 4, SEQ ID NO: 5 and SEQ ID NO: 6. The monoclonal antibody disclosed by the invention can be combined with the S protein RBD of the novel coronavirus and various mutant strains thereof with high affinity, is strong in neutralizing activity, and has ideal clinical application value for preventing and treating infection of the novel coronavirus and various mutant strains thereof.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Monoclonal antibody, vaccine composition thereof and application thereof
ActiveCN106188280AStrong neutralizing activityBroad-spectrumImmunoglobulins against virusesAntiviralsMonoclonal antibodyVariant strain
The invention provides a Pseudorabies virus resisting monoclonal antibody, a vaccine composition containing the monoclonal antibody and an application of the vaccine composition. The monoclonal antibody has very high neutralizing potency and can be used for effectively neutralizing swine classical and variant strains of Pseudorabies virus; and the vaccine composition containing the monoclonal antibody has the characteristic of broad spectrum, application objects are free from limitations to animal species, and the preventing and treating efficacy (effect) of display of swine and canine animal experiments is excellent.
Owner:LUOYANG PULIKE WANTAI BIOTECH +1
Rabies virus resistant specific humanized antibody and application thereof
ActiveCN104193823AStrong neutralizing activityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
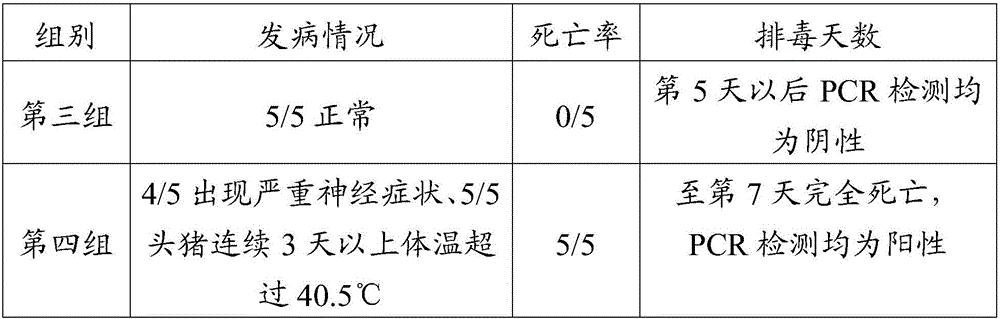
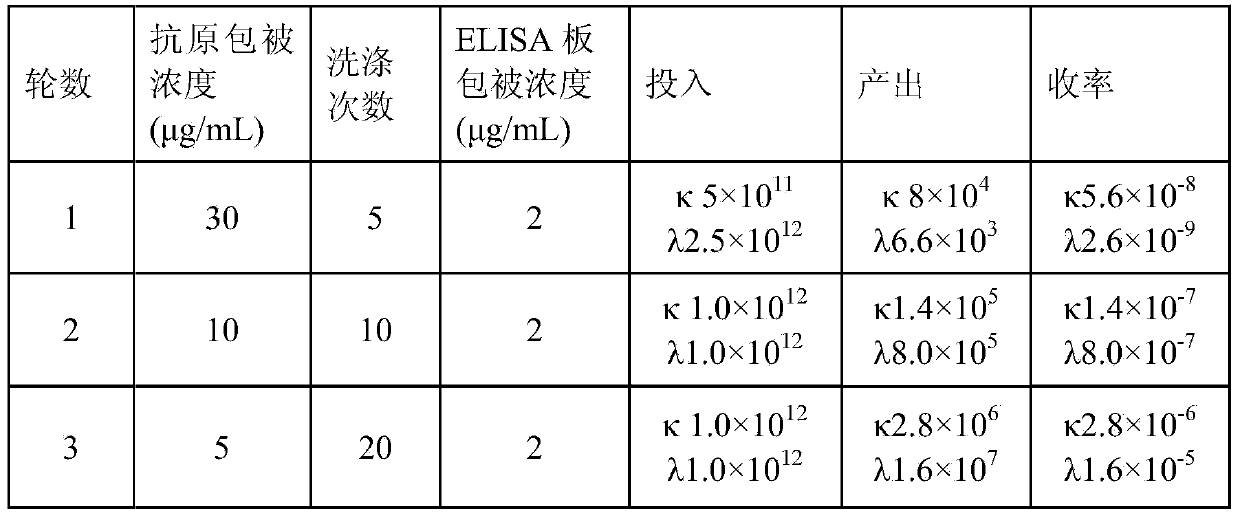
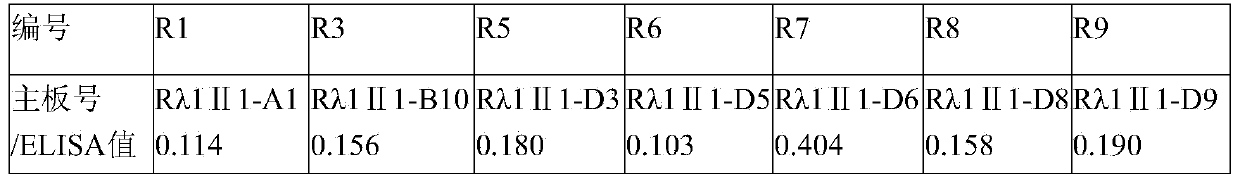
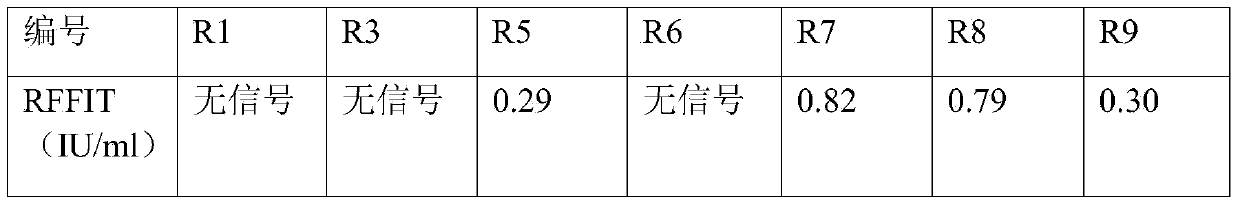
The invention aims at providing a rabies virus resistant neutralizing antibody, and particularly provides a humanized or completely humanized monoclonal antibody to meet the requirement for clinically diagnosing and / or treating rabies. A phage antibody library is prepared by adopting a phage antibody library technology and taking 32 parts of high-potency healthy human peripheral blood inoculated with rabies vaccines as a raw material; 7 ELISA positive antibodies are obtained through three rounds of screening from the phage antibody library; and furthermore, the neutralizing activity of the 7 ELISA positive antibodies is measured through an RFFIT method, wherein four ELISA positive antibodies, namely R5, R7, R8 and R9, have higher neutralizing activity in all. The rabies virus resistant neutralizing antibody with high affinity, which is provided by the invention, can be used for substituting ERIG and HRIG to carry out active and / or passive immune therapy on rabies virus seriously-exposed persons.
Owner:LANZHOU INST OF BIOLOGICAL PROD
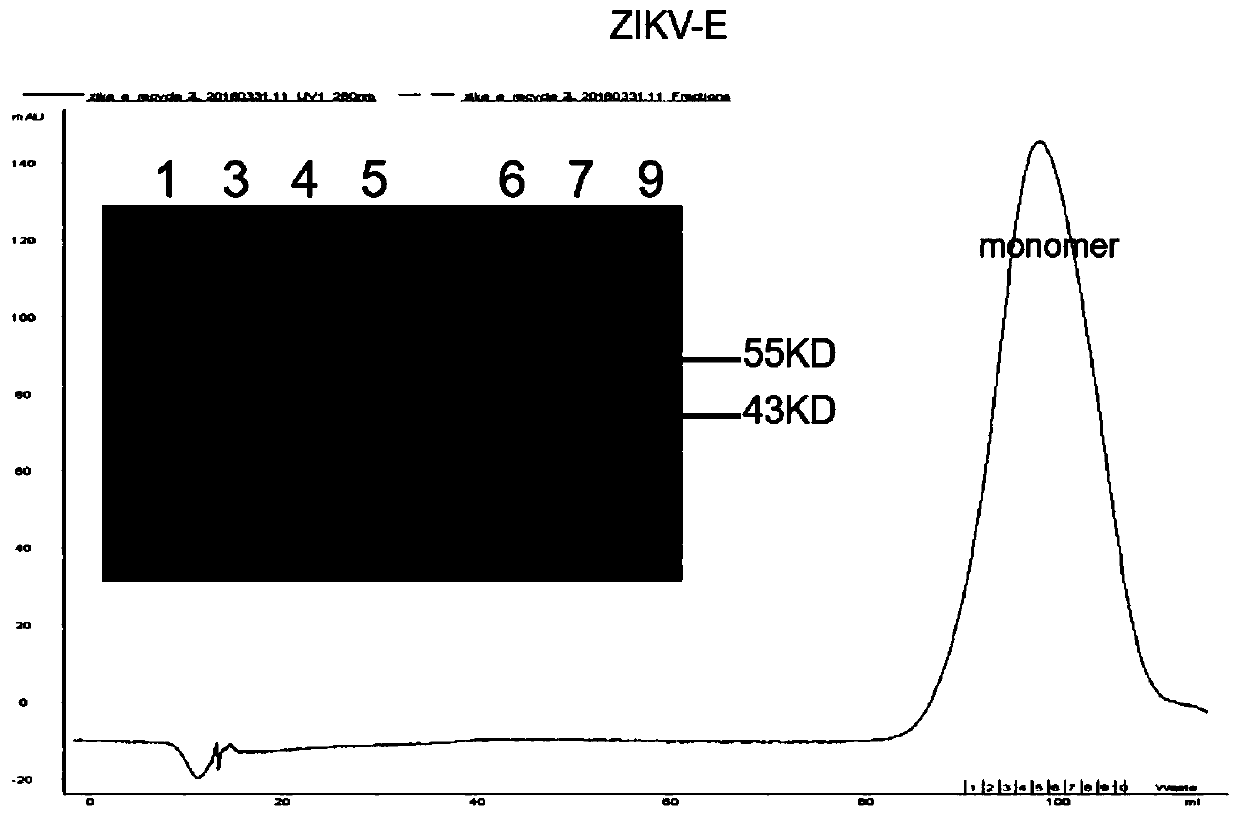
Human monocolonal antibody with high neutralization activity for Zika virus and application thereof
ActiveCN110172095AFree from attackStrong neutralizing activityImmunoglobulins against virusesAntiviralsAntigenEscherichia coli
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Monoclonal antibody 20D8 of anti-SARS-CoV-2 epidemic mutant strain
ActiveCN113354733AStrong neutralizing activityBiological material analysisImmunoglobulins against virusesAntiendomysial antibodiesWild type
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Specific Zika virus neutralizing antibodies and application thereof
ActiveCN110066333AFree from attackStrong neutralizing activityImmunoglobulins against virusesAntiviralsEscherichia coliAntigen
The invention discloses specific Zika virus neutralizing antibodies and application thereof, and belongs to the technical field of medicine. Zika E protein expressed by colibacillus serves as an antigen, through fluorescence-activated cell sorting, memory B cells capable of being specifically bound with the Zika E protein are screened in PBMCs of a Zika patient in the rehabilitation stage, the screened single B cells are subjected to a RT-PCR, the sequence and segments of the variable region of the antibody are obtained, and the variable region and the constant region are connected in an expression vector. After mammalian cell expression and purification, a series of function detection is conducted, such as the binding force of the ZIKE-E protein, the in-vitro neutralizing effect and the in-vivo protection capacity, and the three human monoclonal antibodies capable of completely protecting Zika virus infection are obtained.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Fab segment of human HIV antibody, and coding gene and application thereof
The invention discloses a Fab segment of a human HIV antibody, and a coding gene and application thereof. The Fab segment of the antibody consists of a heavy chain variable region VH and a constant region subunit CH1 of the antibody, and a light chain of the antibody, wherein the light chain consists of a variable region VL and a constant region CL; the VH and VL respectively consist of a complementary-determining region (CDRs) and a framework region (FRs); the complementary-determining region consists of CDR1, CDR2 and CDR3; the amino acid sequences of the CDR1, CDR2 and CDR3 of the VL are shown as the 27th-32nd position, 50th-52nd position and 89th-98th position in a sequence 2; and the amino acid sequences of the CDR1, CDR2 and CDR3 of the VH are shown as the 26th-33rd position, 51st-57th position and 96-109th position in a sequence 3. The Fab segment and the coding gene thereof prepare gene engineering antibodies in different forms so as to prepare medicines, vaccines and diagnostic reagents for treating, preventing and diagnosing HIV infection and human immunodeficiency virus.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
E2 recombinant protein and application thereof
PendingCN110746495AImprove purification effectStrong neutralizing activitySsRNA viruses positive-senseVirus peptidesBovine Viral Diarrhea VirusesBlot
The invention provides an E2 recombinant protein. The E2 recombinant protein has an amino acid sequence of a sequence table SEQ.ID.No.1, and the E2 recombinant protein has a base sequence of a sequence table SEQ.ID.No.2. The invention also provides application to an indirect ELISA kit for detecting bovine viral diarrhea virus antibodies. The E2 recombinant protein provided by the invention has high accuracy, not only is the neutrality of the protein ensured, but also the trouble of high mutation rate is avoided, and the recombinant protein is more beneficial to the development of a BVDV antibody detection technology. The E2 recombinant protein uses a traditional protein prokaryotic expression technology, the cost is low, the expression quantity is large, and after the successfully expressed E2 recombinant protein is subjected to urea gradient renaturation, the West blot detection reactionogenicity is strong. The E2 recombinant protein is applied to the indirect ELISA kit for detectingbovine viral diarrhea virus antibodies, and an established BVDV antibody ELISA detection method is high in sensitivity and good in specificity, is more beneficial to popularization and application ingrass-roots farmers, and is more beneficial to purification of BVDV in cattle herds in China.
Owner:SHIHEZI UNIVERSITY
Neutralizing antibody against novel coronavirus receptor binding regions and application thereof
ActiveCN113150135AHas a neutralizing effectGood neutralizing activityImmunoglobulins against virusesAntiviralsHeavy chainReceptor
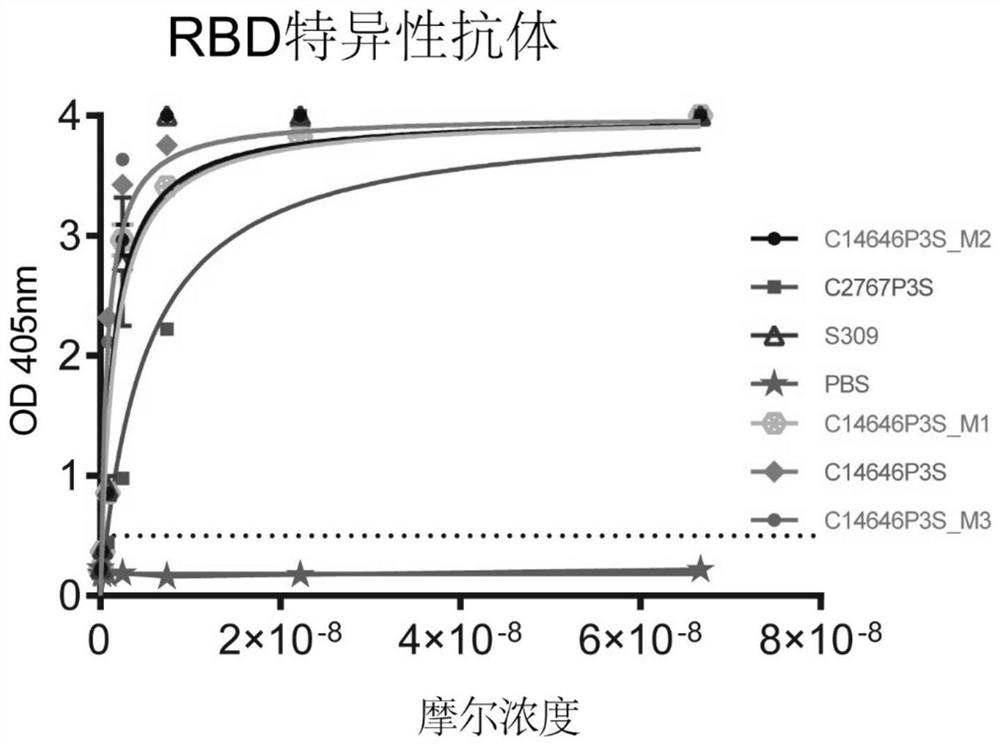
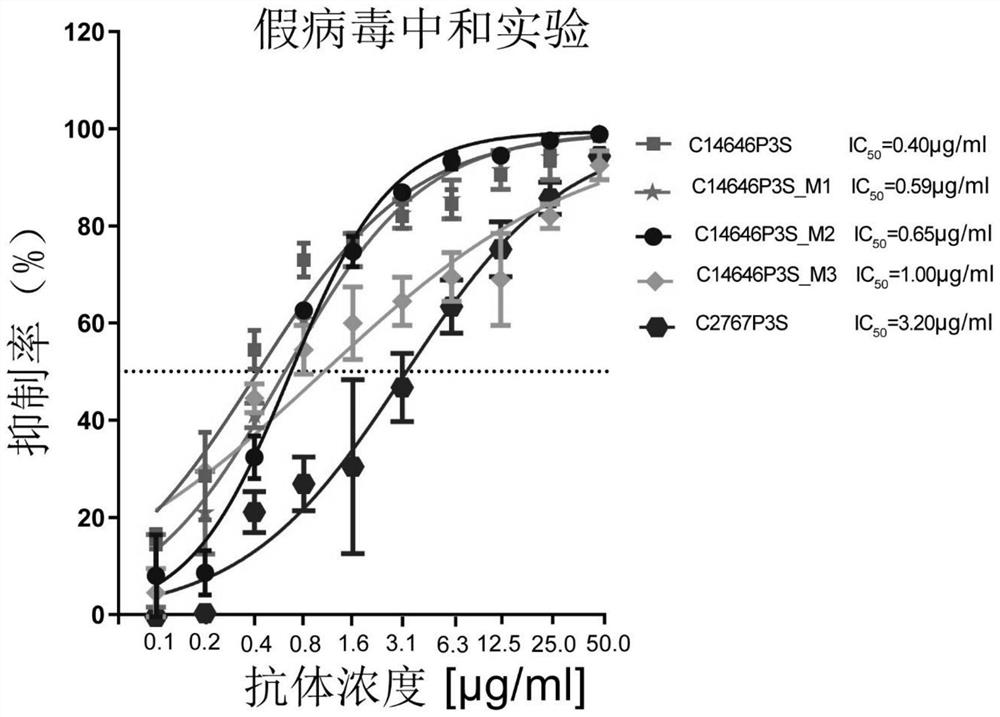
The invention provides a neutralizing antibody against novel coronavirus receptor binding regions and application thereof, and belongs to the technical field of biological products. The neutralizing antibody comprises a heavy chain variable region and a light chain variable region; and the heavy chain variable region comprises a heavy chain CDR3 as shown in SEQ ID NO: 3 or SEQ ID NO: 13, and the light chain variable region comprises a light chain CDR3 as shown in SEQ ID NO: 6 or SEQ ID NO: 16. According to the neutralizing antibody against the novel coronavirus receptor binding regions and the application thereof, the neutralizing antibodies C14646P3S and C2767P3S of the SARS-CoV-2 receptor binding region are obtained by utilizing LIBRA-seq high-throughput screening; and the neutralizing antibodies C14646P3S and C2767P3S have relatively strong neutralizing capability on SARS-CoV-2 pseudoviruses and live viruses in vitro, can well block the binding of a novel coronavirus receptor binding region and a receptor thereof, and have important application value in the aspects of prevention, treatment and diagnosis of SARS-CoV-2 infection.
Owner:SUN YAT SEN UNIV
IgA CD4i ANTIBODIES AND METHODS OF TREATMENT USING SAME
InactiveUS20150175678A1Improve bindingReduce the valueAnimal cellsSugar derivativesEpitopeVariable domain
The present invention relates to isolated IgA antibodies, or fragments thereof, which have variable domains derived from an antibody that specifically binds to a CD4-induced (CD4i) epitope. In particular, the isolated IgA antibodies display enhanced neutralization activity relative to their IgG, non-chimeric counterparts. The invention also provides methods for therapy with the isolated IgA antibodies for the treatment of a subject having a viral infection or having an increased risk of a viral infection.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Monoclonal antibody against novel coronaviruses and application of monoclonal antibody
ActiveCN113354729AStrong neutralizing activityClinical application value of prevention and treatmentImmunoglobulins against virusesAntiviralsInfection inducedMolecular virology
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of novel coronaviruses. In particular, the invention relates to a monoclonal antibody against novel coronaviruses, and a composition (e.g., diagnostic and therapeutic agents) comprising the antibody. In addition, the invention also relates to an application of the antibody. The antibody can be used for diagnosing, preventing and / or treating infection of the novel coronaviruses and / or diseases caused by the infection (e.g., novel coronavirus pneumonia).
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN +1
Hydroxy-aluminum montmorillonite chewable tablet as well as preparation method and application thereof
ActiveCN105055355AReduced activityPromote healingDigestive systemPill deliveryCurative effectMontmorillonite
The invention discloses a hydroxy-aluminum montmorillonite chewable tablet as well as a preparation method and application thereof. The hydroxy-aluminum montmorillonite chewable tablet disclosed by the invention is characterized in that hydroxy-aluminum montmorillonite is taken as an effective component of a medicament; the mass percentage of the hydroxy-aluminum montmorillonite in the chewable tablet is 50 to 80 percent; proper pharmaceutic adjuvants are added into the hydroxy-aluminum montmorillonite to prepare a chewable tablet medicament. By administrating the chewable tablet through a gastrointestinal tract, the effective component of the hydroxy-aluminum montmorillonite chewable tablet can be used for neutralizing a pH value of gastric juice of a patient and further reducing the activity of pepsase, so that the actions of alleviating gastralgia and promoting gastric ulcer healing can be realized; then, the hydroxy-aluminum montmorillonite is discharged outside through a digestive tract, so that the aim of resisting gastric acid is achieved; the hydroxy-aluminum montmorillonite chewable tablet disclosed by the invention is higher in dissolution rate and good in clinical curative effect.
Owner:SHANDONG SBOND PHARMA
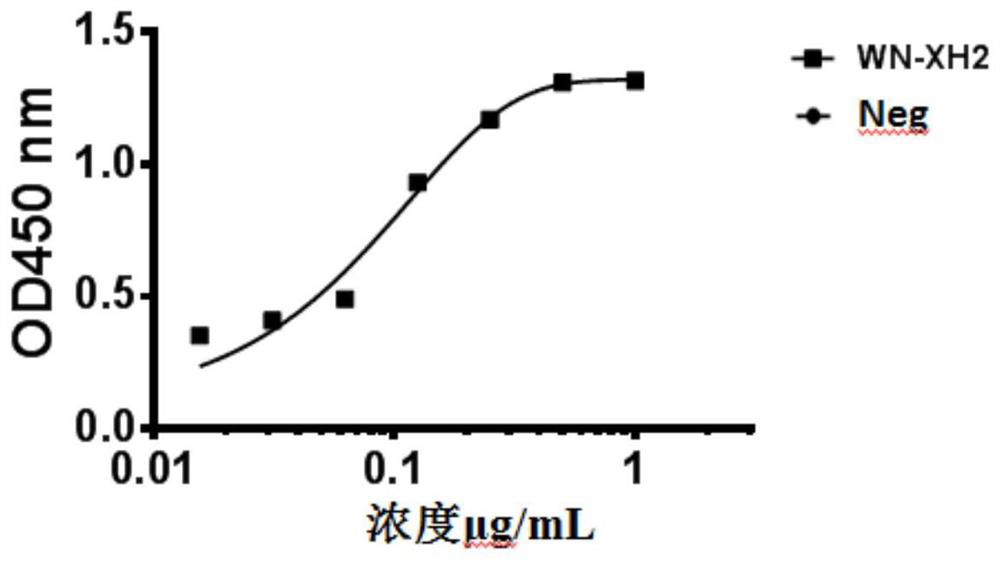
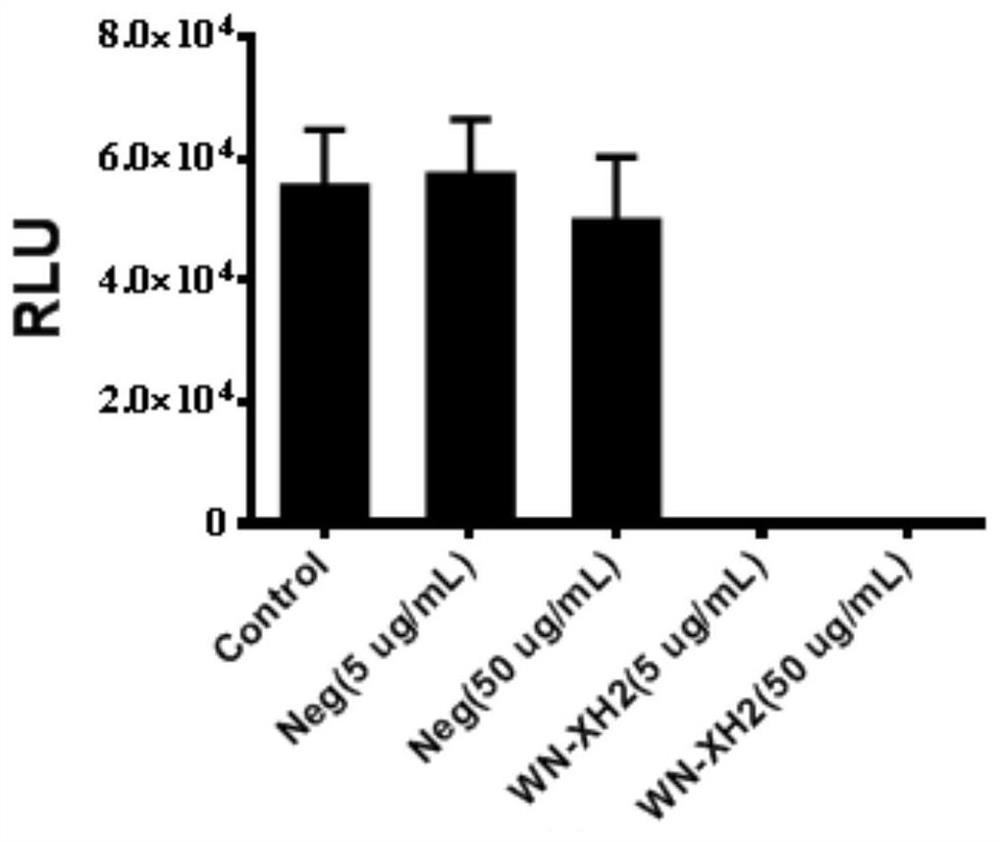
Binding molecule for resisting WNV infection
ActiveCN112159470AHigh potencyStrong specificityImmunoglobulins against virusesAntiviralsPharmaceutical drugAnti infectives
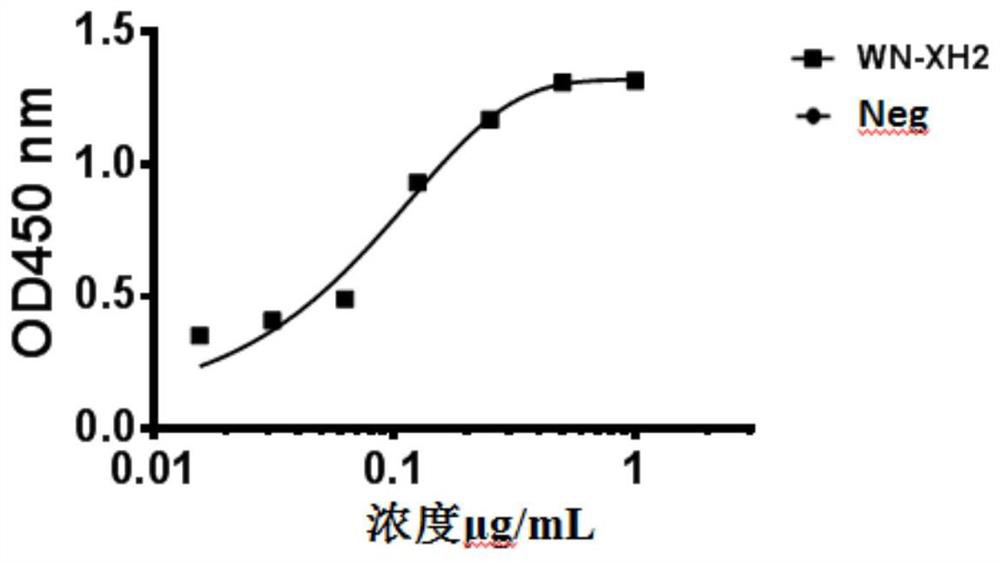
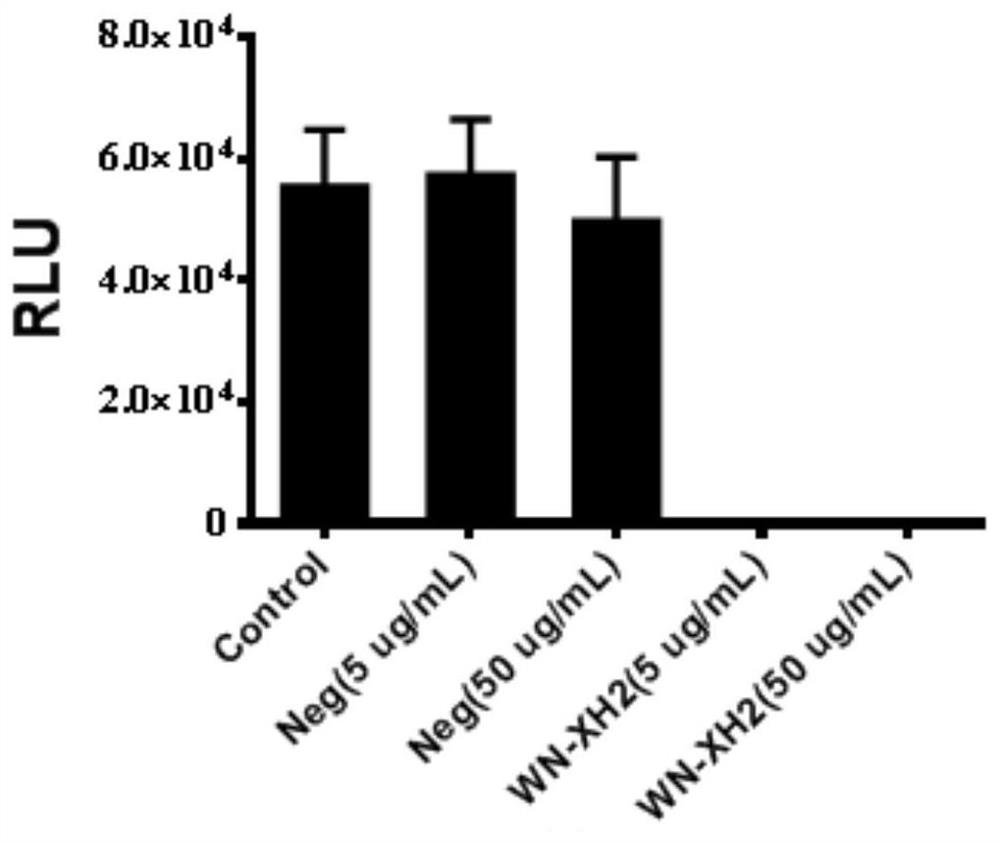
The invention discloses a binding molecule for resisting WNV infection. The binding molecule is a monoclonal antibody aiming at WNV E protein. In-vitro experiments prove that the monoclonal antibody can be specifically combined with WNV E protein, has relatively high affinity activity and can neutralize WNV viruses. On one hand, the research result provides a new method for clinical diagnosis, andon the other hand, a candidate drug is provided for clinical WNV infection resistance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Monoclonal antibody against novel coronaviruses and application of monoclonal antibody
ActiveCN113354730AStrong neutralizing activityClinical application value of prevention and treatmentImmunoglobulins against virusesAntiviralsInfection inducedMolecular virology
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of novel coronaviruses. In particular, the invention relates to a monoclonal antibody against novel coronaviruses, and a composition (e.g., diagnostic and therapeutic agents) comprising the antibody. In addition, the invention also relates to an application of the antibody. The antibody can be used for diagnosing, preventing and / or treating infection of the novel coronaviruses and / or diseases caused by the infection (e.g., novel coronavirus pneumonia).
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN +1
Monoclonal antibody for identifying EB virus gH glycoprotein, and application of monoclonal antibody
ActiveCN113372440AHigh binding activityHigh affinityImmunoglobulins against virusesAntiviralsViral glycoproteinAntigen Binding Fragment
The invention belongs to the technical field of antibodies, and discloses a monoclonal antibody for recognizing EB (Epstein-Barr) virus gH glycoprotein, and application of the monoclonal antibody. The monoclonal antibody or the antigen binding fragment thereof is subjected to specific binding with the 573th, 625th, 627th and 655th amino acids of the gH protein, wherein the amino acid sequence of the gH protein is as shown in SEQ ID NO. 11. The binding activity of the monoclonal antibody or the antigen binding fragment thereof is superior to the binding activity of other monoclonal antibodies (AMMO1, M3 and 1D8); affinity to the gLgH protein is high; strong neutralizing activity is realized in all epithelial cell infection models; an obvious inhibition effect for cell membrane fusion is achieved; and a way that the monoclonal antibody or the antigen binding fragment thereof is subjected to the specific binding with the 573th, 625th, 627th and 655th amino acids of the gH protein is different from other reported gLgH neutralizing antibody recognition and binding epitopes.
Owner:SUN YAT SEN UNIV CANCER CENT +1
A kind of monoclonal antibody against novel coronavirus and application thereof
ActiveCN113354729BStrong neutralizing activityClinical application value of prevention and treatmentImmunoglobulins against virusesAntiviralsInfection inducedMolecular virology
The present invention relates to the field of immunology and molecular virology, especially the field of diagnosis, prevention and treatment of novel coronavirus. In particular, the present invention relates to monoclonal antibodies against novel coronaviruses, and compositions (eg, diagnostic and therapeutic agents) comprising said antibodies. Furthermore, the present invention also relates to the use of said antibody. The antibody of the present invention can be used for diagnosing, preventing and / or treating novel coronavirus infection and / or diseases caused by said infection (for example, novel coronavirus pneumonia).
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN +1
Fab segment of human HIV antibody, and coding gene and application thereof
The invention discloses a Fab segment of a human HIV antibody, and a coding gene and application thereof. The Fab segment of the antibody consists of a heavy chain variable region VH and a constant region subunit CH1 of the antibody, and a light chain of the antibody, wherein the light chain consists of a variable region VL and a constant region CL; the VH and VL respectively consist of a complementary-determining region (CDRs) and a framework region (FRs); the complementary-determining region consists of CDR1, CDR2 and CDR3; the amino acid sequences of the CDR1, CDR2 and CDR3 of the VL are shown as the 27th-32nd position, 50th-52nd position and 89th-98th position in a sequence 2; and the amino acid sequences of the CDR1, CDR2 and CDR3 of the VH are shown as the 26th-33rd position, 51st-57th position and 96-109th position in a sequence 3. The Fab segment and the coding gene thereof prepare gene engineering antibodies in different forms so as to prepare medicines, vaccines and diagnostic reagents for treating, preventing and diagnosing HIV infection and human immunodeficiency virus.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Fab segment of human-source human immunodeficiency virus (HIV) antibody and coded gene and application thereof
InactiveCN102212133AStrong neutralizing activityBacteriaGenetic material ingredientsHiv diseaseGenetic engineering
The invention discloses an Fab segment of a human-source human immunodeficiency virus (HIV) antibody, a coded gene thereof and application thereof. The Fab segment of the antibody consists of a variable region (VH) and a constant region subunit CH1 of a heavy chain of the antibody and a light chain of the antibody, wherein the light chain consists of a light chain variable region (VL) and a lightchain constant region (CL); both of the VH and the VL consist of complementary determining regions (CDRs) and framework regions (Framework Region, FRs); the complementary determining region consists of a CDR1, a CDR2 and a CDR3; the amino acid sequences of the CDR1, the CDR2 and the CDR3 of the VL are respectively shown as the 27th to the 32nd positions of the sequence 2, the 50th to the 52nd positions of the sequence 2 and the 89th to 98th positions of the sequence 2; and the amino acid sequences of the CDR1, the CDR2 and the CDR3 of the VH are respectively shown as the 26th to the 33rd positions of the sequence 3, the 51st to the 58th positions of the sequence 3 and the 97th to 116th positions of the sequence 3. The Fab segment and the coded gene thereof are used for preparing genetic engineering antibodies in different forms and medicaments, vaccines and diagnostic reagents for treating, preventing and diagnosing HIV infection and acquired immune deficiency syndrome.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Hydroxy aluminum montmorillonite chewable tablet and its preparation method and application
ActiveCN105055355BDoes not cause alkalemiaStrong neutralizing activityDigestive systemPill deliveryUlcer healingGastric pepsin
The invention discloses a hydroxy-aluminum montmorillonite chewable tablet as well as a preparation method and application thereof. The hydroxy-aluminum montmorillonite chewable tablet disclosed by the invention is characterized in that hydroxy-aluminum montmorillonite is taken as an effective component of a medicament; the mass percentage of the hydroxy-aluminum montmorillonite in the chewable tablet is 50 to 80 percent; proper pharmaceutic adjuvants are added into the hydroxy-aluminum montmorillonite to prepare a chewable tablet medicament. By administrating the chewable tablet through a gastrointestinal tract, the effective component of the hydroxy-aluminum montmorillonite chewable tablet can be used for neutralizing a pH value of gastric juice of a patient and further reducing the activity of pepsase, so that the actions of alleviating gastralgia and promoting gastric ulcer healing can be realized; then, the hydroxy-aluminum montmorillonite is discharged outside through a digestive tract, so that the aim of resisting gastric acid is achieved; the hydroxy-aluminum montmorillonite chewable tablet disclosed by the invention is higher in dissolution rate and good in clinical curative effect.
Owner:SHANDONG SBOND PHARMA
A kind of humanized monoclonal antibody and its application
ActiveCN107586335BStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
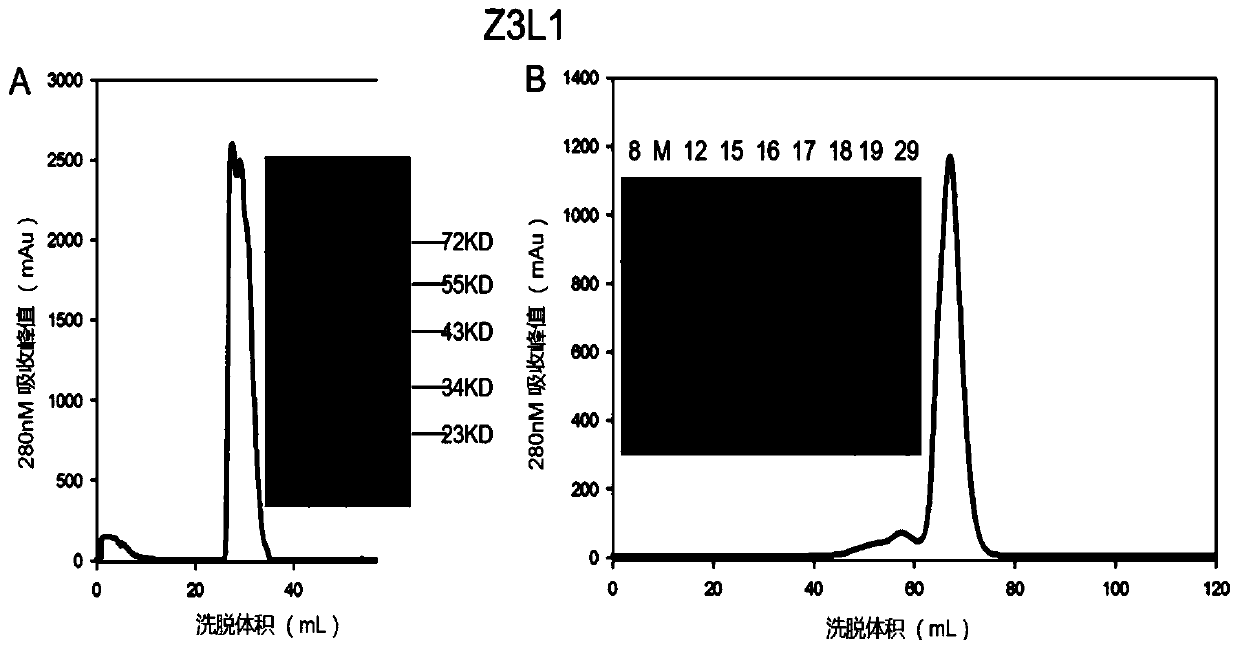
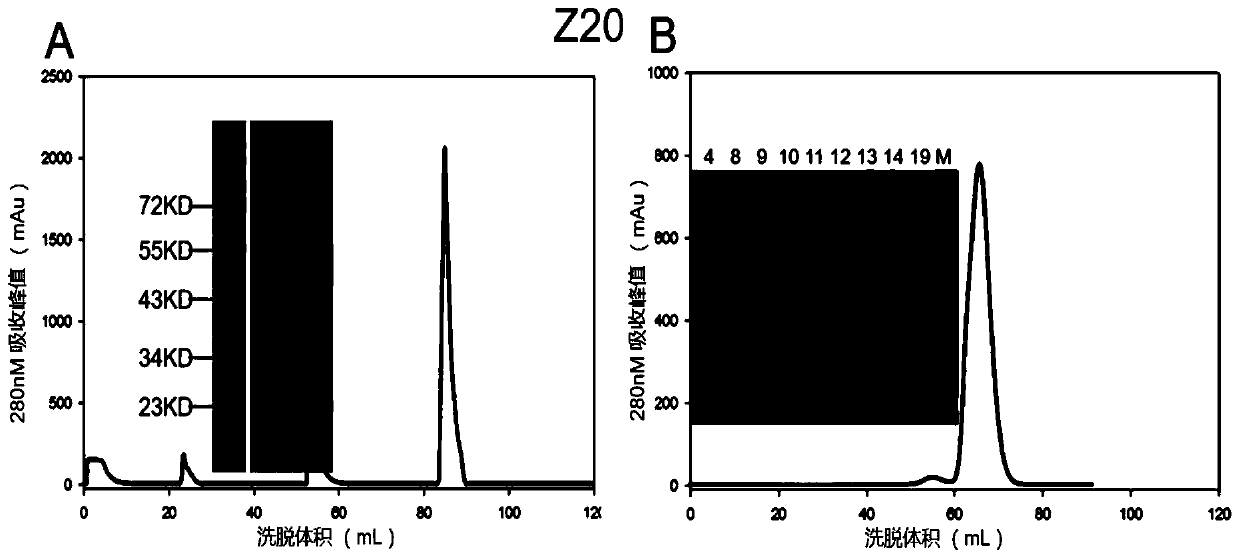
Murine monoclonal antibody against Zika virus envelope protein
ActiveCN111100201BStrong specificityStrong neutralizing activityImmunoglobulins against virusesAntiviralsZika virusGene Modification
The invention discloses a mouse-derived monoclonal antibody directed at Zika virus envelope protein. After screening and genetic modification, the obtained antibody has good specificity, strong neutralizing activity, and increased anti-aggregation property, and is suitable for Zika virus prevention and treatment.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
A binding molecule against wnv infection
ActiveCN112159470BHigh potencyStrong specificityImmunoglobulins against virusesAntiviralsPharmaceutical drugAnti infectives
Owner:ACADEMY OF MILITARY MEDICAL SCI
A kind of neutralizing antibody against tetanus toxin and its application
ActiveCN108623681BStrong neutralizing activityHigh affinity activityAntibacterial agentsImmunoglobulins against bacteriaDiseaseClostridium tetani
Owner:CHANGCHUN BCHT BIOTECH
A kind of monoclonal antibody against novel coronavirus and application thereof
ActiveCN113354730BStrong neutralizing activityClinical application value of prevention and treatmentImmunoglobulins against virusesAntiviralsInfection inducedMolecular virology
The present invention relates to the field of immunology and molecular virology, especially the field of diagnosis, prevention and treatment of novel coronavirus. In particular, the present invention relates to monoclonal antibodies against novel coronaviruses, and compositions (eg, diagnostic and therapeutic agents) comprising said antibodies. Furthermore, the present invention also relates to the use of said antibody. The antibody of the present invention can be used for diagnosing, preventing and / or treating novel coronavirus infection and / or diseases caused by said infection (for example, novel coronavirus pneumonia).
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN +1
Multifunctional hybrid peptide with antibacterial, anti-inflammatory, endotoxin neutralizing and immunomodulatory activities as well as preparation method and application thereof
ActiveCN111944058AEasy to adjustFunctionalAntibacterial agentsPeptide/protein ingredientsNutritionCytotoxicity
The invention relates to the technical field of protein engineering, in particular to a multifunctional hybrid peptide with antibacterial, anti-inflammatory, endotoxin neutralizing and immunomodulatory activities as well as a preparation method and application thereof. The hybrid peptide provided by the invention comprises a peptide I and a peptide II fused with the peptide I, wherein an amino acid sequence of the peptide I is as shown in SEQ ID NO.1, and an amino acid sequence of the peptide II is as shown in 19th-29th sites of SEQ ID NO.2. The hybrid peptide has antibacterial, endotoxin neutralizing, immunoregulation and anti-inflammatory functions, has the advantages of low cytotoxicity, high safety, convenience in preparation and low cost, can be used as an ideal antibacterial agent, endotoxin neutralizing agent, immunoregulator and anti-inflammatory agent, is widely applied to the fields of medicines, foods, health care, feeds, nutrition and the like of human beings and animals, and has good application potential and value.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com