Monoclonal antibody for identifying EB virus gH glycoprotein, and application of monoclonal antibody
A monoclonal antibody and antigen technology, applied in the direction of antiviral immunoglobulin, antibody, antiviral agent, etc., can solve the problems of monoclonal antibody marketing and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation of embodiment 1 anti-Epstein-Barr virus gLgH protein monoclonal antibody (monoclonal antibody)
[0073] (1) Preparation of protein antigen: refer to the complete gene sequence of Epstein-Barr virus M81 strain (KF373730.1) and connect the C-terminus of the gL protein ectoregion sequence (corresponding to virus BKRF2 gene aa24-aa137) to the gH protein ectoregion through a flexible amino acid sequence Sequence (corresponding to the virus BXLF2 gene aa19-aa678), the N-terminal of the gL protein is connected to the signal peptide coding sequence, and the C-terminal of the gH protein is connected to the polyhistidine polypeptide (6×His) that is convenient for affinity chromatography purification. The above sequence is constructed into a suitable eukaryotic expression vector, and the successfully constructed recombinant plasmid is transfected into 293F cells for expression and purified to finally obtain gLgH protein (the amino acid sequence of gLgH protein is sh...
Embodiment 2
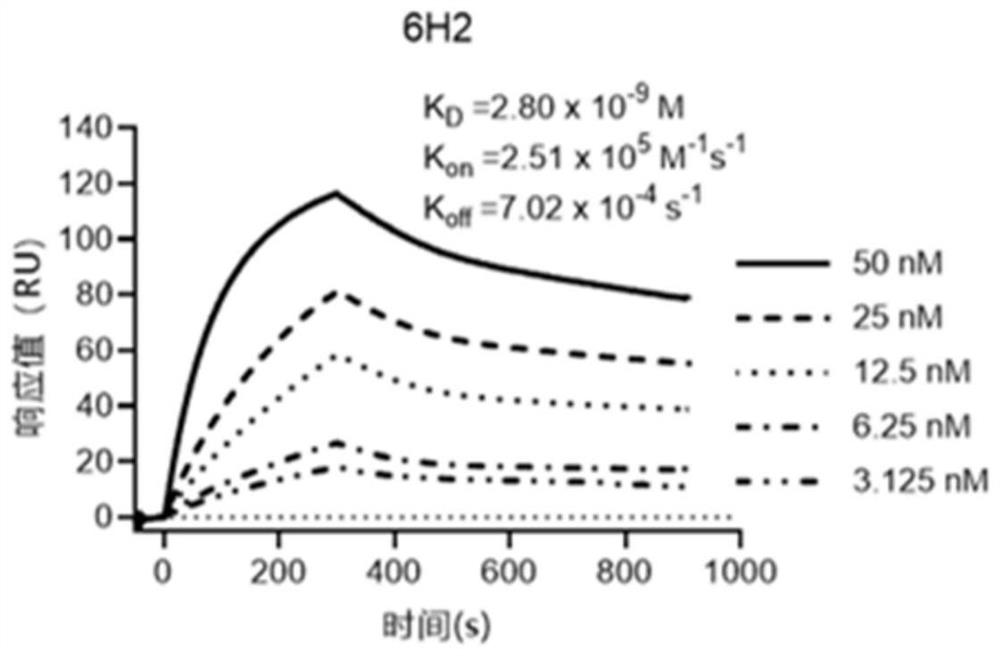
[0080] Example 2 Isolation and sequence analysis of the monoclonal antibody 6H2 light chain gene and heavy chain gene against Epstein-Barr virus gLgH protein
[0081] Semi-adherent culture about 10 7Blow up the adherent cells to make them suspend, transfer the suspension to a new 4mL centrifuge tube, centrifuge at 1500rpm for 3min, collect the cell pellet, resuspend in 100μL sterile PBS, and transfer to a new 1.5mL centrifuge tube Add 800uL Trizol (Roche, Germany), gently invert and mix, let stand for 10min; add 200μL chloroform, shake vigorously for 15s, let stand for 10min, centrifuge at 12000rpm at 4°C for 15min, transfer the upper layer to a new 1.5mL centrifuge tube , add an equal volume of isopropanol, mix well, let stand for 10 minutes; centrifuge at 12,000 rpm at 4°C for 10 minutes, discard the supernatant, add 600 μL of 75% ethanol to wash, centrifuge at 12,000 rpm at 4°C for 5 minutes, discard the supernatant, and dry the precipitate at 60°C for 5 minutes ; The tran...
Embodiment 3
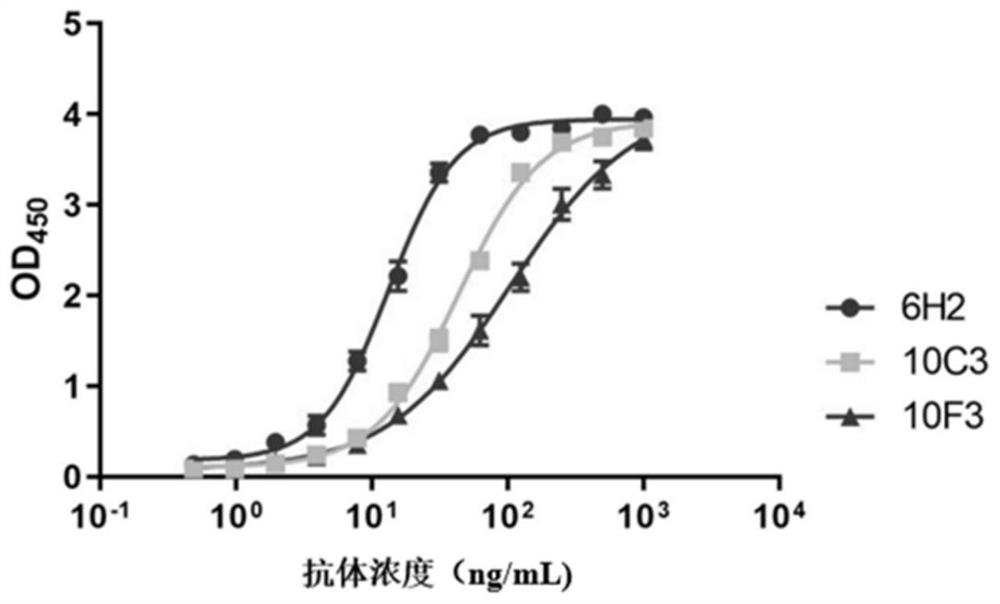
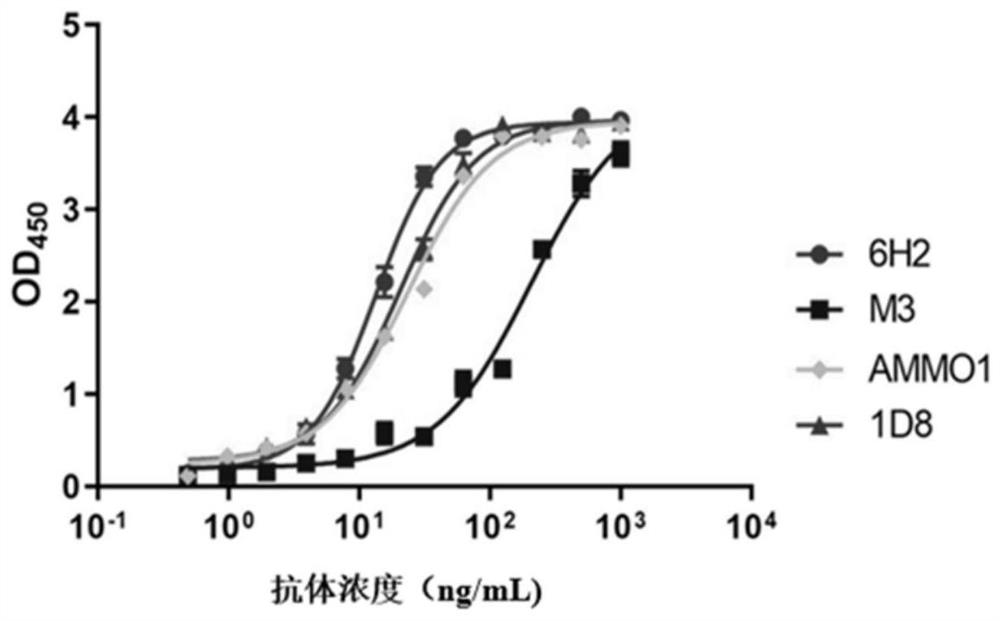
[0083] Example 3 Comparison of EC50 of neutralizing antibody 6H2 and other antibodies AMMO1, M3, 1D8
[0084] The gLgH protein was washed with CB buffer (NaHCO 3 / Na 2 CO 3 buffer, the final concentration is 50mM, the pH value is 9.6) and the final concentration is 2 μg / mL; add 100 μL of coating solution to each well of a 96-well microplate plate, and coat at 37 ° C for 2 hours; wash with PBST ( 20mM PBS7.4, 150mM NaCl, 0.1% Tween20) to wash once; then add 200μL blocking solution (20mM NaCl with pH value of 7.4 containing 20% calf serum and 1% casein) 2 HPO 4 / NaH 2 PO 4 buffer solution), placed at 37°C for 2 hours; discard the blocking solution; dry and store in an aluminum foil bag at 2-8°C for later use. Monoclonal antibodies 6H2, AMMO1 (references for the preparation method of AMMO1 Snijder et al., 2018, Immunity 48,799–811; PDB of AMMO1 light chain: 6C5V_L; PDB of AMMO1 light chain: 6C5V_H), M3 (already published in patent document CN111548411A Disclosed in), 1D8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com