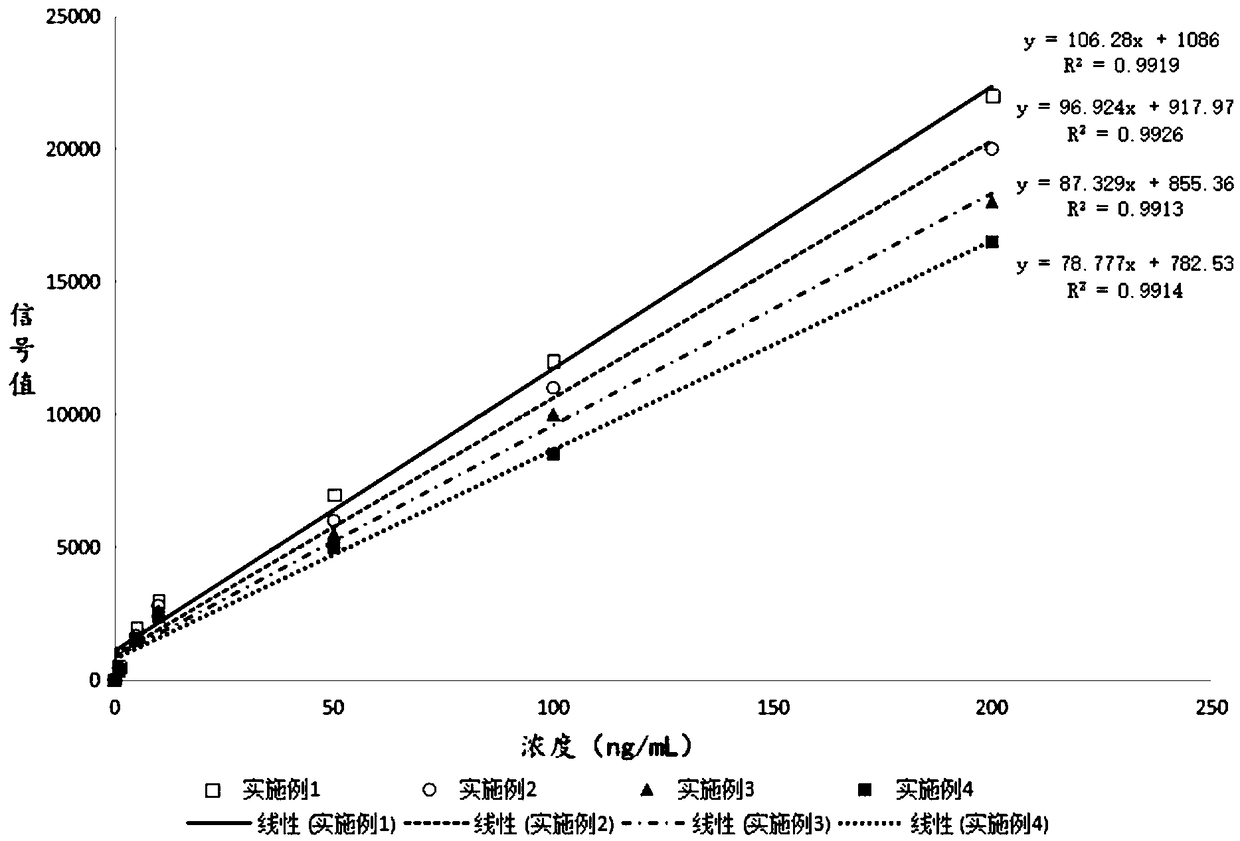
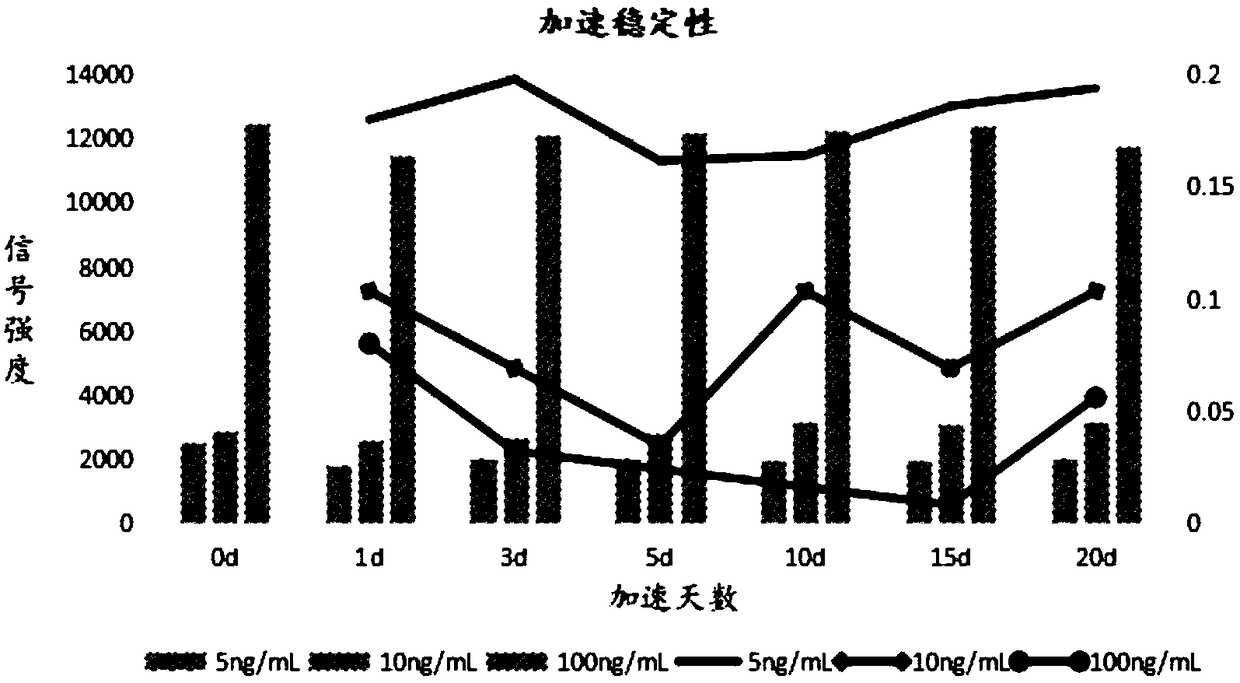
Patents
Literature
43 results about "Globin binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isolation of proteins
InactiveUS6919436B2Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
The present invention relates to a novel method for the isolation or purification of immunoglobulins (a special class of proteins) from a solution containing immunoglobulins, e.g. hybridoma cell culture supernatants, animal plasma or sera, or colostrum. The method includes the use of a minimum of salts, such as lyotropic salts, in the binding process and preferably also the use of small amounts of organic solvents in the elution process. The solid phase matrices, preferably epichlorohydrin activiated agarose matricees, are functionalised with mono- or bicyclic aromatic or heteroaromatic ligands (molecular weight: at the most 500 Dalton) which, preferably, comprises an acidic substituent, e.g. a carboxylic acid. The matrices utilised show excellent properties in a “Standard Immunoglobulin Binding Test” and in a “Monoclonal Antibody Array Binding Test” with respect to binding efficiency and purity, and are stable in 1M NaOH.
Owner:UPFRONT CHROMATOGRAPHY
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
Mutated immunoglobulin-binding protein
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
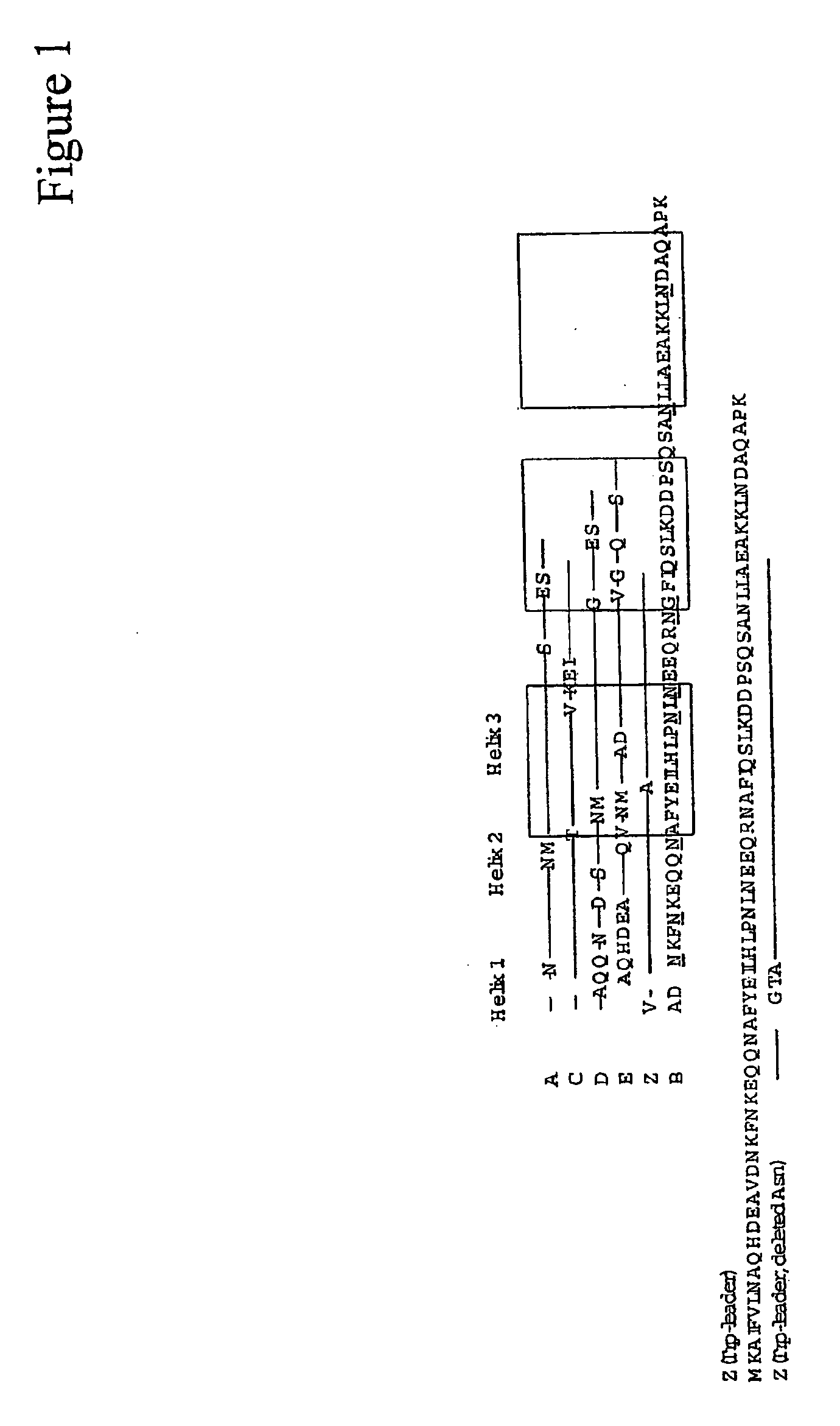
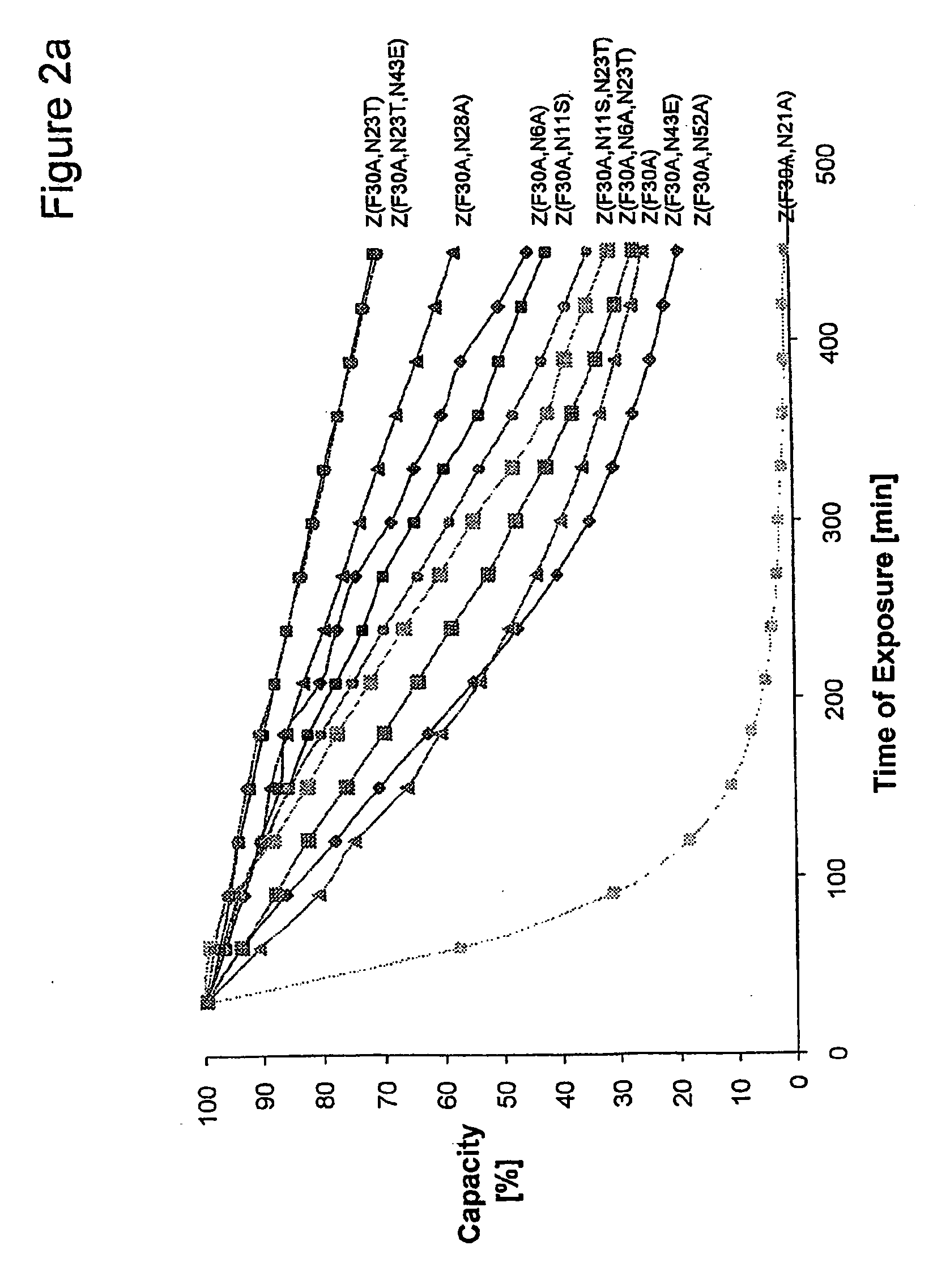
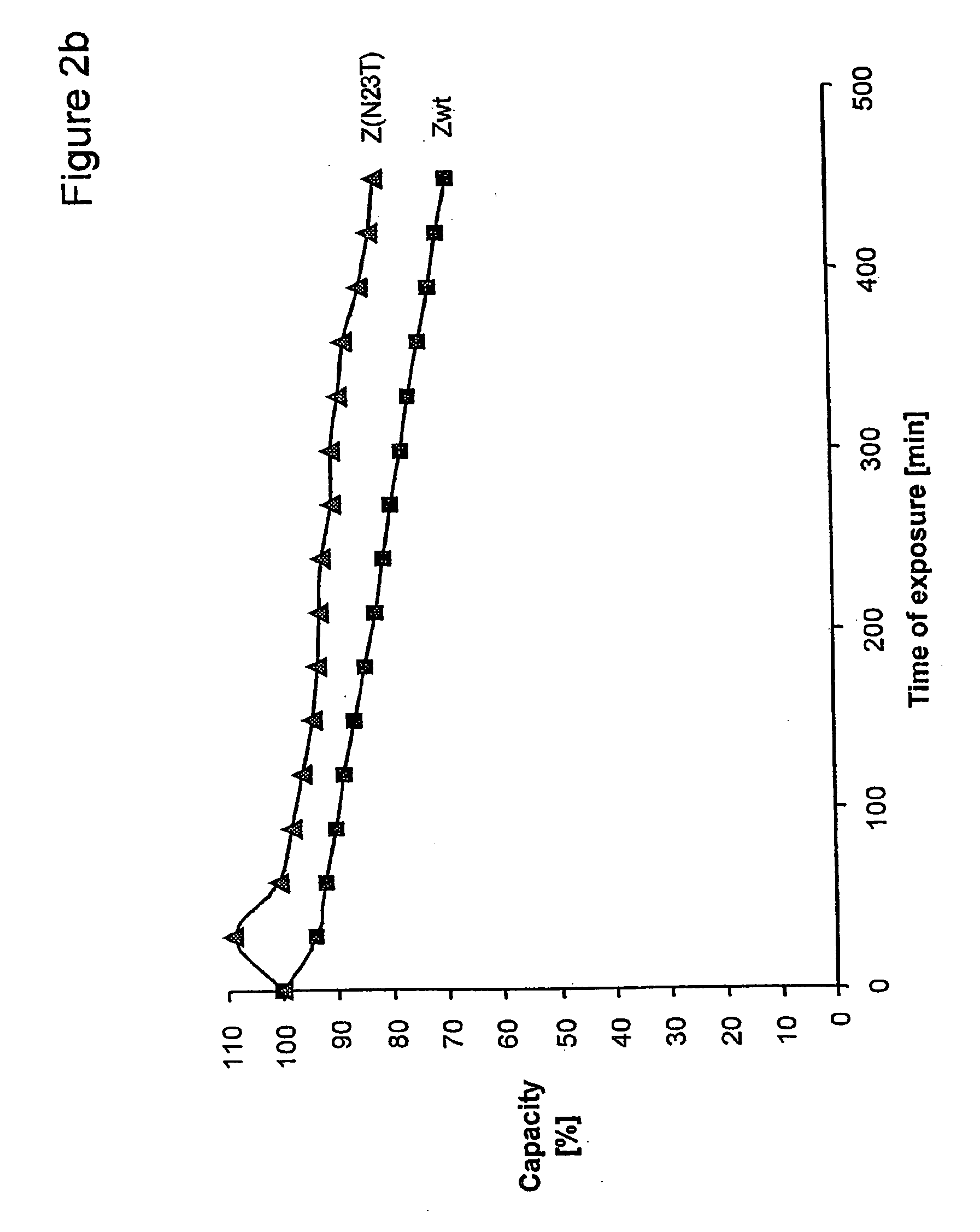
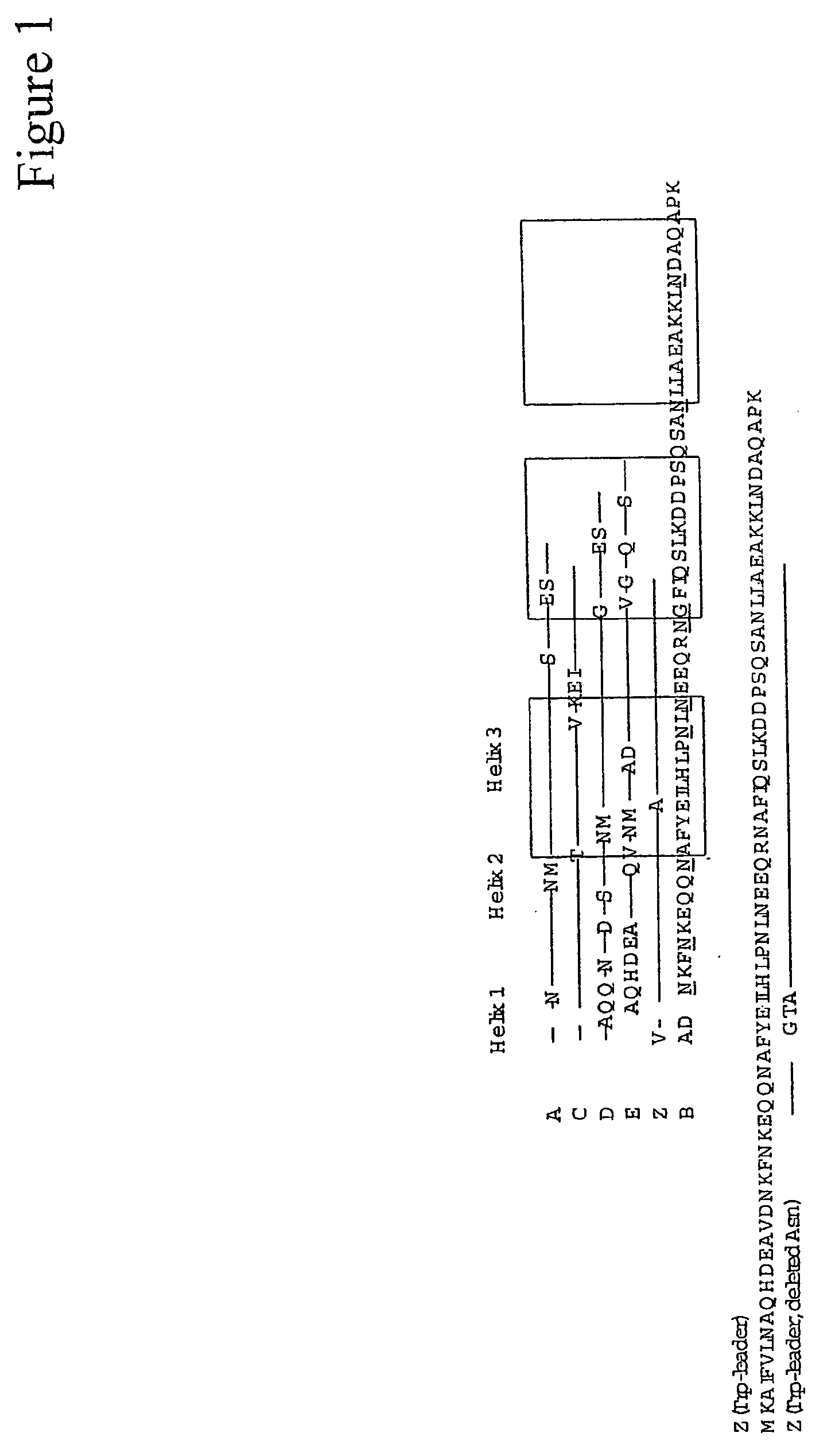
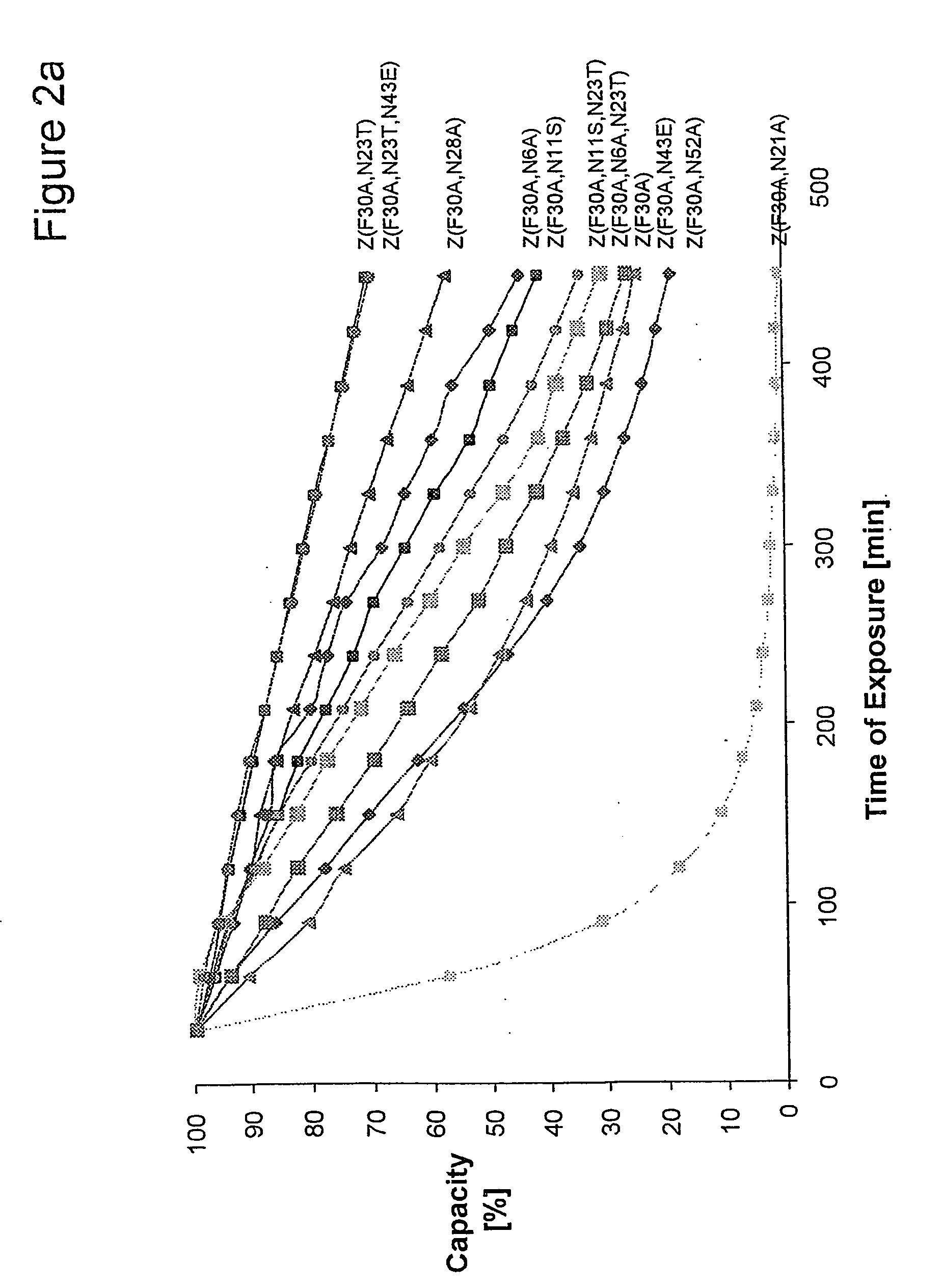
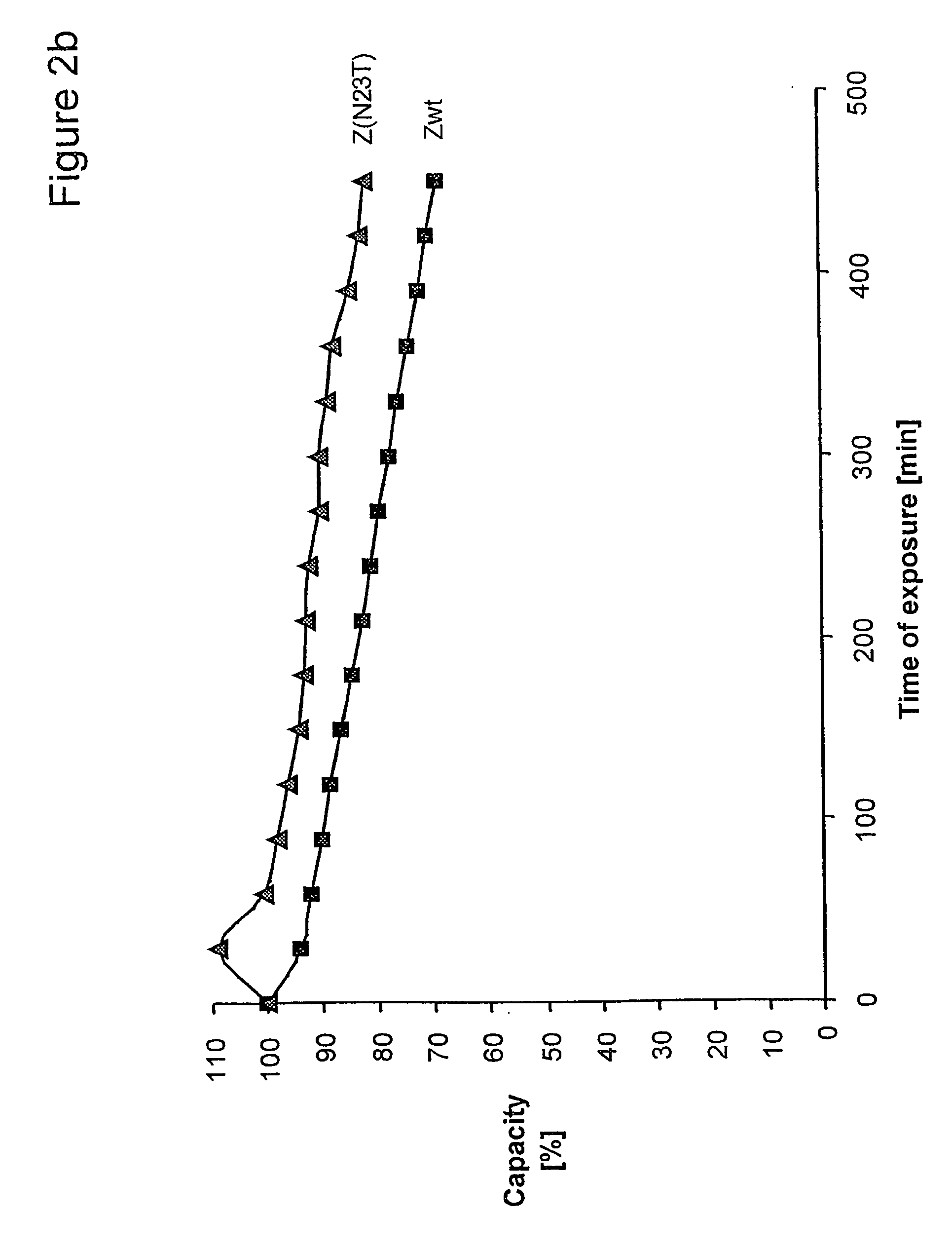
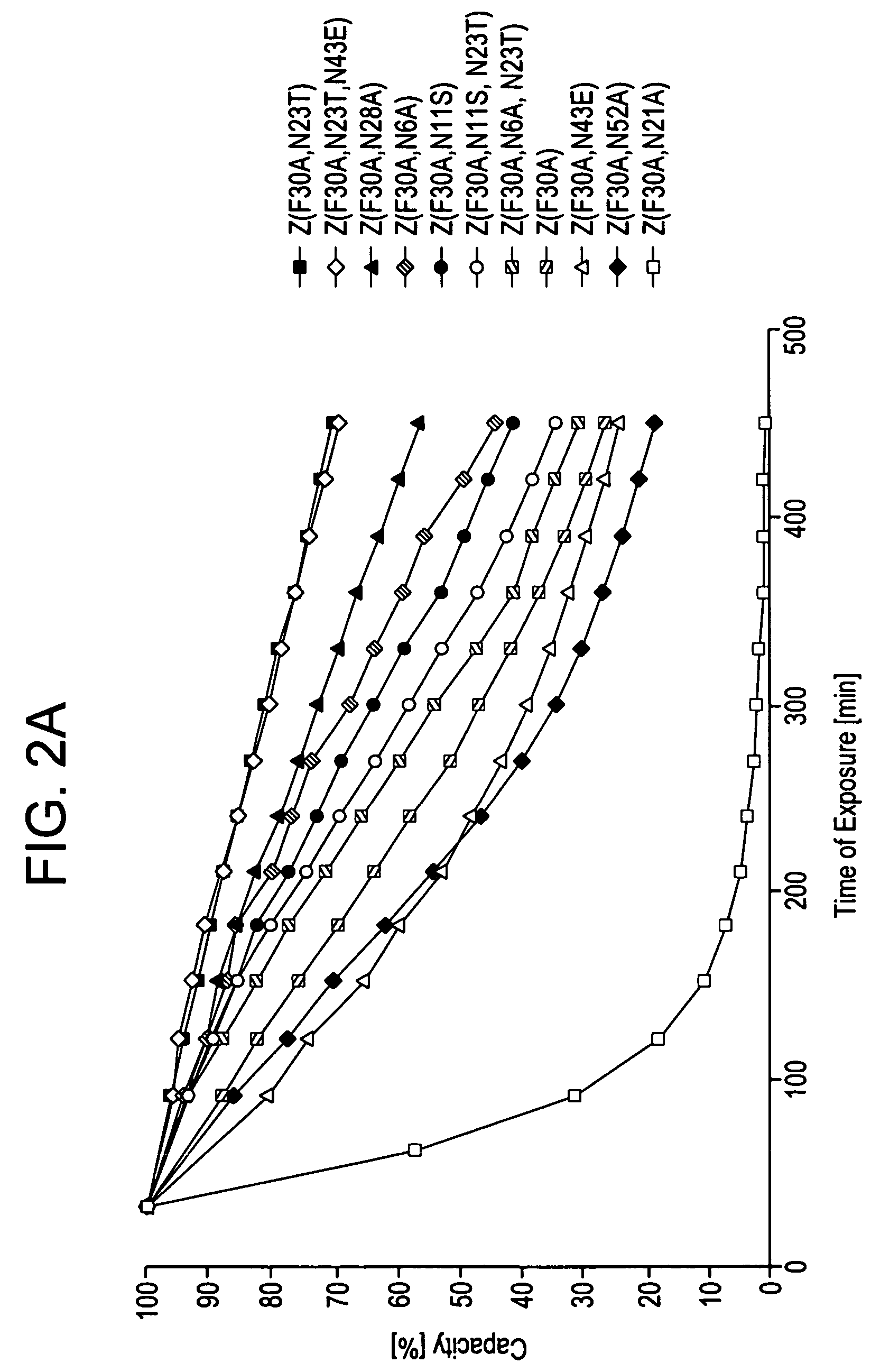
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Protein ligands
ActiveUS7709209B2Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionChemical stability
Owner:CYTIVA BIOPROCESS R&D AB
Native immunoglobulin binding reagents and methods for making and using same
InactiveUS20050142609A1Promote resultsPreferentially detect native immunoglobulinAnimal cellsPeptide librariesGlobin bindingReagent
Isolated native immunoglobulin binding reagents including antibodies are provided, along with articles of manufacture, compositions and kits that include the native immunoglobulin binding reagents. Labeled reagents and substrates that comprise samples or the reagents are provided. Methods of screening for, making and using the reagents are also provided.
Owner:EBIOSCIENCE (US)
Fusion protein of immune globulin binding structural domain and fluorescence protein and its uses
InactiveCN1807457AThe production process is simpleLow costRecombinant DNA-technologyBiological testingGenetic engineeringYellow fluorescent protein
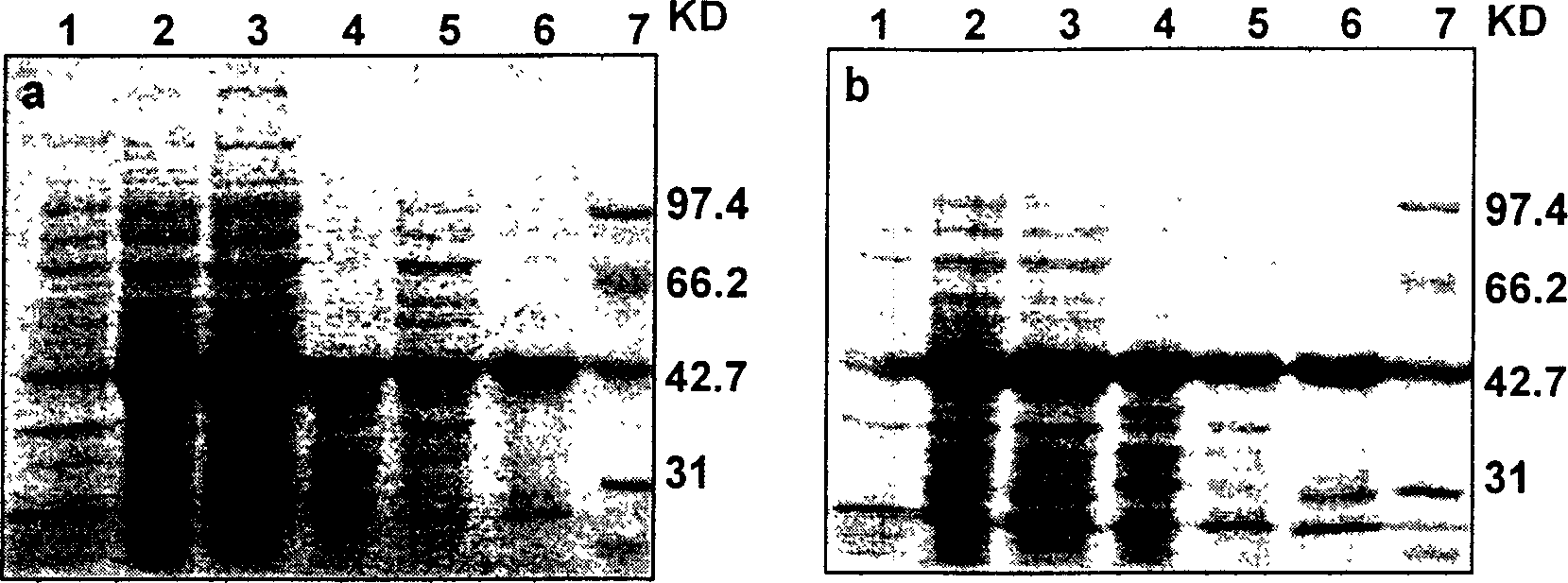
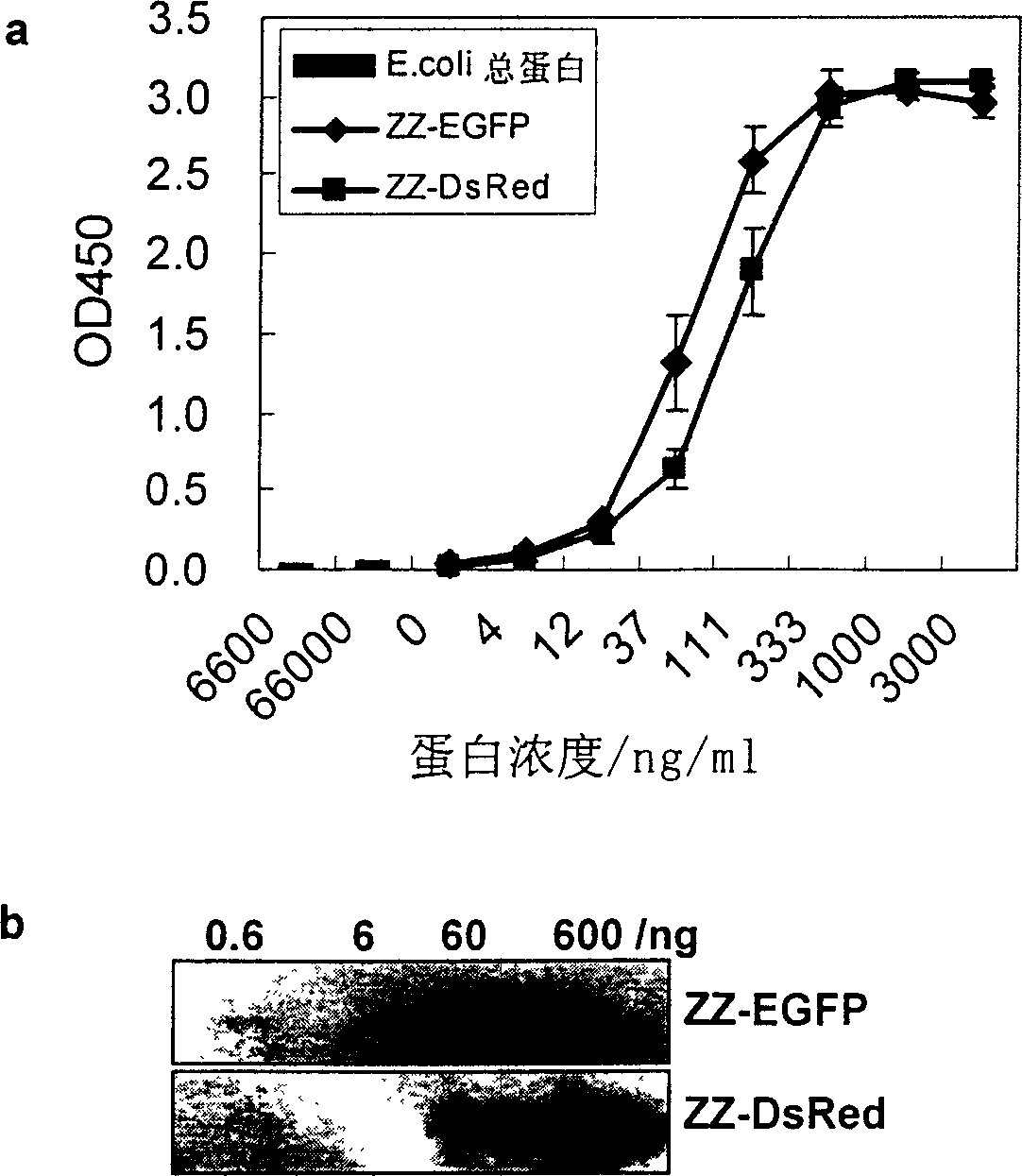
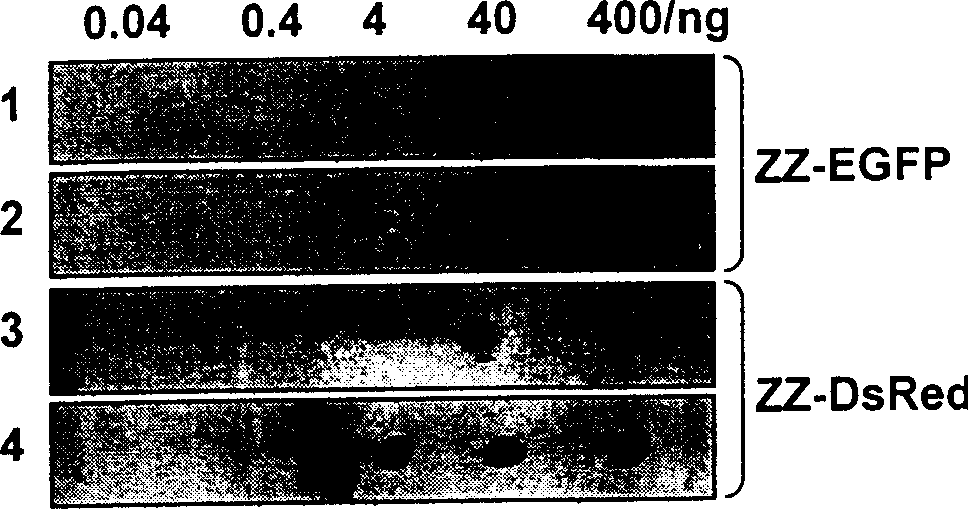
The invention belongs to bioengineering technical realm. This invention provides a merged protein which is made up of immunoglobulin combined structure field and fluorescent protein as well as the expression of genetic engineering and the method of separation and purification. Using the merged protein which is made up of immunoglobulin combined structure field and fluorescent protein of this invention expressed and purified in biology, medicine. ect realm can do various immunoassay and analysis, such as enzyme joined immunoadsorption analysis, Western Blotting analysis, spot hybridization detecting, immune combinatorial analysis, flowed cytoscopy and so on. This invention also provides two merged fluorescence protein's example of immunoglobulin combined structure field-green fluorescence protein (ZZ-EGF), immunoglobulin combined structure field-red fluorescence protein (ZZ-DsRed).
Owner:NANJING UNIV
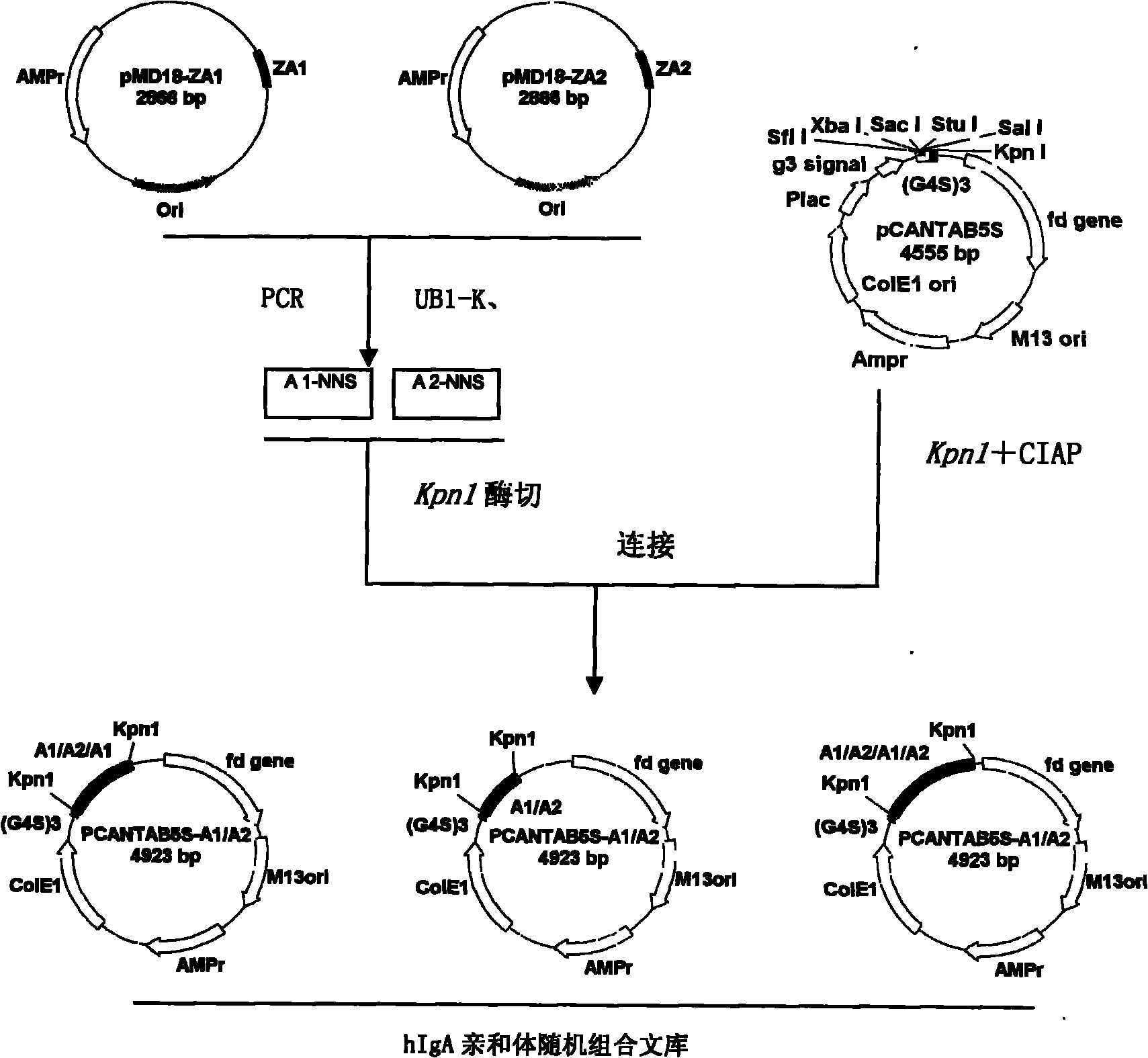
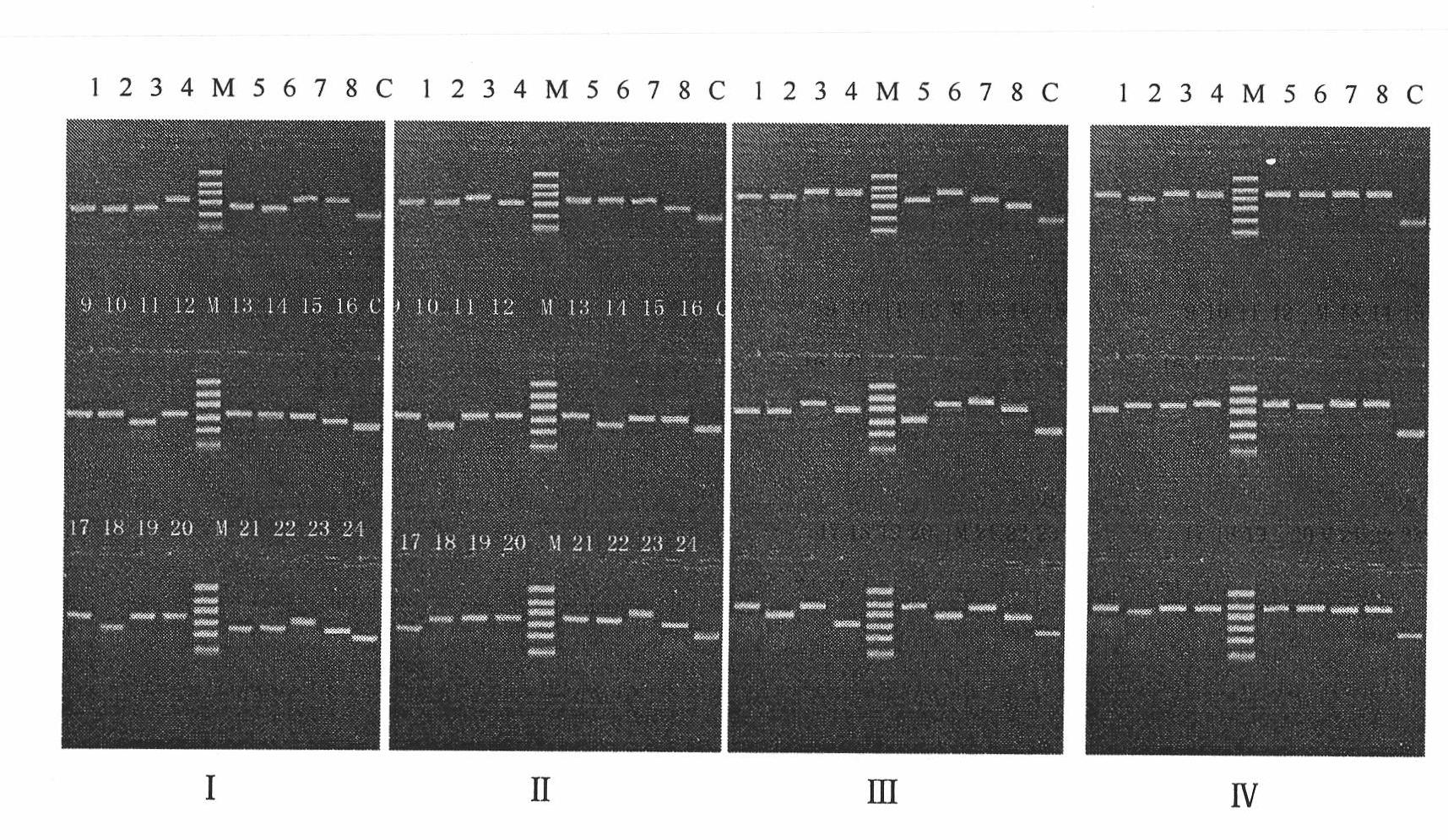
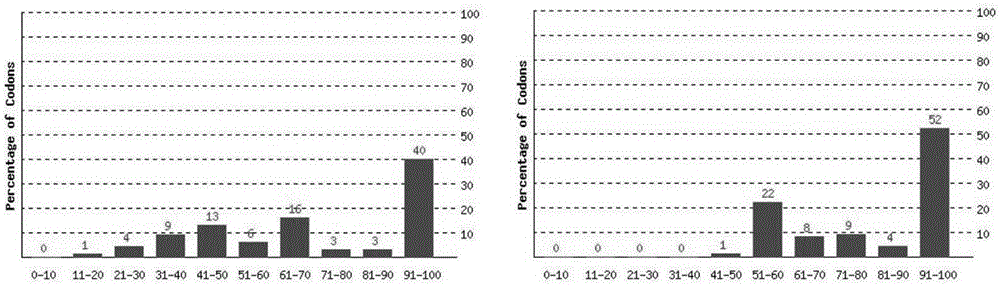
Human IgA immunoglobulin combination molecule having intramolecular affinity effect
InactiveCN102115497AHigh binding activityHigh activityBacteriaMicrobiological testing/measurementGlobin bindingBacteriophage
The invention discloses a human IgA immunoglobulin combination molecule having intramolecular affinity effect, a preparation method and an application thereof. The invention also discloses genes coding the human IgA immunoglobulin combination molecule, a preparation method and an application of the human IgA immunoglobulin combination molecule based on bacteriophage molecule evolution. The human IgA immunoglobulin combination molecule of the invention, especially repeated molecule of human IgA affibody, has intramolecular affinity effect when binding the human IgA, demonstrates very high human IgA binding activity, can be used for purification of high-specific IgA and research and development of detecting reagent, and purification for detection of human IgA antibody by ELISA adsorption method, immunity chromatography, immunohistochemical method and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Multi-species universal ELISA kit for differential diagnosis of foot and mouth disease virus infection
The invention discloses a multi-species universal AGL-ELISA kit for differential diagnosis of foot and mouth disease virus infection. The multi-species universal AGL-ELISA kit comprises an ELISA plate coated with 8BF protein and recombinant protein [AGL]n undergoing enzyme labeling. The recombinant protein [AGL]n is a multi-copy recombinant protein formed by performing gene optimization and series connection on three protein structural domains which are a B structural domain in a staphylococcus aureus A protein combination structural domain, a C2 structural domain in a streptococcus protein G protein immune globulin combination structural domain and a B3 structural domain in a peptostreptococcus magnus protein L protein immune globulin combination structural domain, wherein n is 1, 2 or 3. The recombinant protein [AGL]n undergoes enzyme labeling, an enzyme labeling product can be combined with multi-species mammal immune globulin through western-blot and ELISA detection, can serve as a universal antibody for serologic detection of various mammals, and can replace species specific antibodies to perform multi-species anthropozoonosis detection. According to an AGL-ELISA, various animal foot-and-mouth disease infections can be detected, vaccine immunity, naturally injected animals and persistently infected animals can be identified.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Evolved immunoglobulin binding molecule, and its preparation method and uses
InactiveCN1966526ACombined broad spectrumImprove bindingHybrid immunoglobulinsBacteriaNatural antibodyGenetic engineering
The invention relataes to novel evolutionary immunoglobulin binding molecules, as well as their preparation methods and applications. The invention discloses separated evolutionary immunoglobulin binding molecules, which are proteins with amino acid sequences as shown by SEQ ID NO : 1, or conservative variant proteins with immunoglobulin binding activity. The invention also discloses the gene encoding, genetic engineering preparation methods and applications of immunoglobulin binding molecules. The disclosed immunoglobulin molecule broad-spectrum combined with various immuneglobulin shows high immunoglobulin whole molecule binding activity, and can be used in large-scale purification of genetic engineering antibodies, purification of natural antibodies and monoclonal antibodies, enzyme-linked immunosorbent assay, and immuno-chromatography and immunohistochemical methods for immune antibody detection and diagnosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method and application of fusion protein with broad spectrum adsorption capacity to antibodies
ActiveCN102676562AImprove bindingAvoid non-specific adsorptionIon-exchange process apparatusBacteriaIntravenous gammaglobulinAnion-exchange chromatography
The invention discloses a preparation method and application of fusion protein with broad spectrum adsorption capacity to antibodies. The method includes the steps: obtaining a protein A and a protein G immune globulin binding zone gene by means of T vector connection and double digestion by the genetic engineering means and via PCR (polymerase chain reaction) amplification; then connecting onto an expression vector pET-23a to form a recombinant plasmid, converting E.coli BL21(DE3), and obtaining a great number of thalli containing the fusion protein AG after inducible expression; and finally, performing ultrasonication, high-temperature heating, precipitation of DNA (deoxyribonucleic acid) and purification of weak anion exchange chromatography and affinity chromatography so that the fusion protein AG is obtained. The fusion protein has dual advantages of the protein A and the protein B and is wider in spectrum combination and low in nonspecific adsorption of non-immune globulin substances of albumin in serum and the like. The fusion protein serving as a ligand is connected to a solid-phase vector matrix to be prepared into adsorbent so that the shortcoming of poor IgG3 binding force of protein A adsorbent is overcome, and can be applied to the fields of antibody purification, blood purification and the like.
Owner:DALIAN UNIV OF TECH
Multimeric immunoglobulin-binding domain
ActiveUS20160215027A1Prevent inhibiting immunoglobulin activityLow costImmunoglobulins against bacteriaPeptide preparation methodsGlobin bindingAntibody Affinity Chromatography
A multimeric immunoglobulin-binding protein having improved properties as an affinity ligand for affinity chromatography, and an insoluble support inmmobilizing such a multimer. The immunoglobulin-binding protein is represented by the formula: (R1)n-(R2)m, or (R2)m-(R1)n. R2 is an immunoglobulin-binding domain including an amino acid residue that covalently bonds to an insoluble support upon immobilization reaction with the insoluble support, and R1 is an immunoglobulin-binding domain without containing an amino acid residue the presence of which in the sequence compared to when it is absent in the sequence reduces the immunoglobulin-binding activity of the support yielded by the immobilization reaction. The immunoglobulin-binding protein satisfies: (1) n is an integer of 5 to 9; (2) m is an integer of 1 or 2; (3) the n (R1) domains may or may not have the same sequence; and (4) the total number of domains (n+m) is 6 to 10.
Owner:PROTENOVA
Magnetic Immunoglobulin-Binding Particles
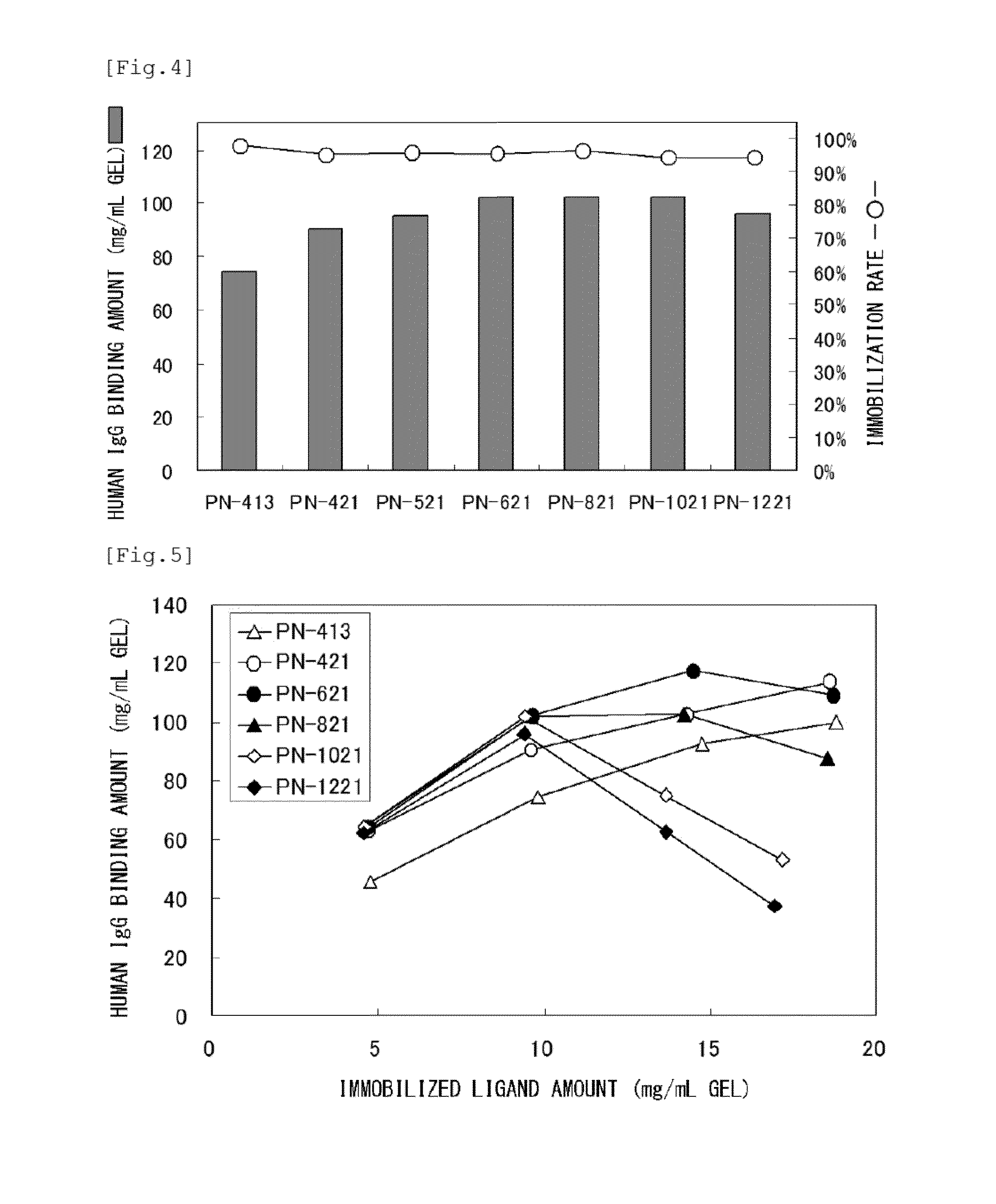
PendingUS20190339261A1Improve bindingHigh yieldBioreactor/fermenter combinationsBiological substance pretreatmentsGlobin bindingMagnetic bead
The invention discloses an immunoglobulin-binding magnetic bead, comprising a porous matrix and one or more magnetic particles embedded in said matrix, wherein said matrix comprises a porous polymer and at least 10 mg / ml Fc-binding proteinaceous ligands covalently coupled to said porous polymer.
Owner:CYTIVA SWEDEN AB
Immunoglobulin binding protein, and preparation method and application thereof
ActiveCN111057153AQuality improvementGuaranteed purityPeptide/protein ingredientsOther chemical processesProtein LProtein
An immunoglobulin binding protein provided by the invention comprises variants of the B structural domain of a protein A, the C2 structural domain of a protein G and the B3 structural domain of a protein L or a combination of any one to three of the variants. The protein A, the protein G and the protein L have different binding characteristics and certain complementarity, so the immunoglobulin binding protein is an alkali-resistant multifunctional IBP molecule with wide reactivity and high affinity. The protein is purified by a three-step chromatography method, so that the protein quality canbe stabilized, the protein purity is more than 97%, the endotoxin level is lower than 1 Eu / mg, and the requirement of clinical protein is met. The stability of an immunoadsorption filler synthesized from the purified immunoglobulin binding protein is improved, so the use frequency of the filler can be effectively increased, and the service life of the filler is prolonged.
Owner:GUANGZHOU KONCEN BIOSCI
Native immunoglobulin binding reagents and methods for making and using same
InactiveCN1867584AQuick checkEasy to parseAntibody ingredientsTissue cultureGlobin bindingScreening method
Isolated native immunoglobulin binding reagents including antibodies are provided, along with articles of manufacture, compositions and kits that include the native immunoglobulin binding reagents. Labeled reagents and subtrates that comprise samples or the reagents are provided. Method of screening for, making and using the reagents are also provided.
Owner:EBIOSCIENCE (US)
HIV protease substrate molecular with evolution immune globulin binding molecular, preparation method and application thereof
InactiveCN101045748AHigh positive rateImprove screening efficiencyBacteriaVirus peptidesBiologyAmino acid
This invention relates to system construction, preparation method and application of a new type of HIV proteolytic enzyme cutting model. This invention discloses a recomposed HIV proteolytic enzyme substrate molecule that carry with evolution immunoglobulin binding molecule, it is the protein which amino acid sequential showed as SEQ ID NO: 1 or has conservatism variation. This invention also discloses the preparation method and application of above protein, and the preparation method and application of its bacteriophage. This HIV proteolytic enzyme cutting model be able to used in judging HIV proteinase activity, judging sensibility of HIV proteolytic enzyme to proteolytic enzyme depressor drug, and screening HIV proteolytic enzyme depressor drug.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Immunomagnetic bead-based time-resolved fluorescence immunoassay kit of cardiac myosin binding protein C (cMyBP-C)
InactiveCN109298178AReduce dosageRealize instant detectionDisease diagnosisBiological testingCardiac myosinAntigen
The invention provides an immunomagnetic bead-based time-resolved fluorescence immunoassay kit of cardiac myosin binding protein C (cMyBP-C). The kit includes a calibrator, immunomagnetic beads, immunofluorescence microspheres, analysis buffering liquid and wash liquid. The magnetic beads are coupled with antibodies, and the time-resolved fluorescence microspheres are coupled with the antibodies;thus the two parts form immunomagnetic beads-cMyBP-C antigen-immunofluorescence microsphere complexes with cMyBP-C antigens in a sample in a reaction tube after shaking incubation; a time-resolved instrument is used to determine intensity of fluorescence emitted thereby under excitation of ultraviolet light; and comparison of a standard curve is carried out to determine the amount of the cMyBP-C antigens in the sample. The kit of the invention greatly shortens reaction time, and improves efficiency and sensitivity of detection.
Owner:江苏美克医学技术有限公司
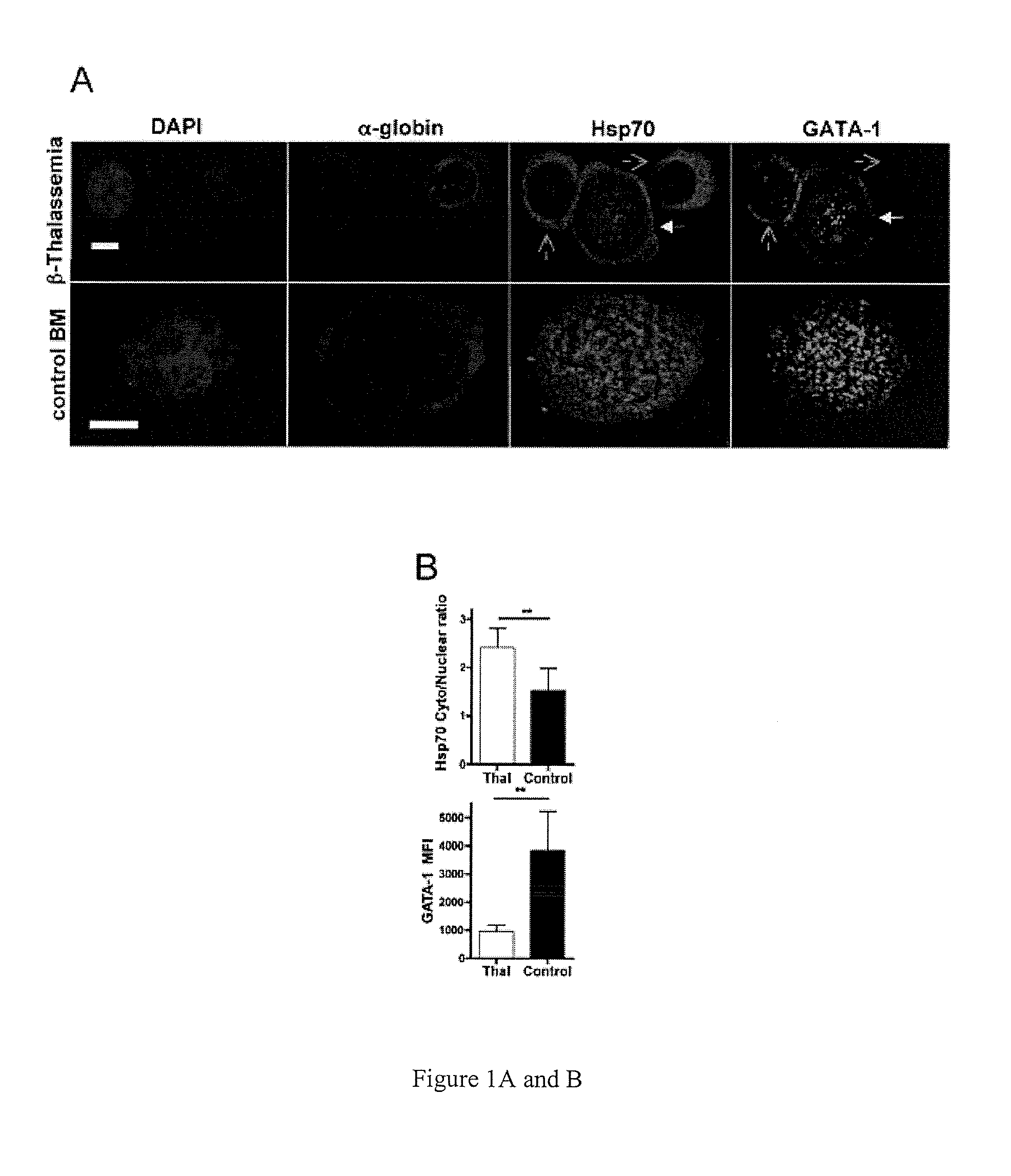
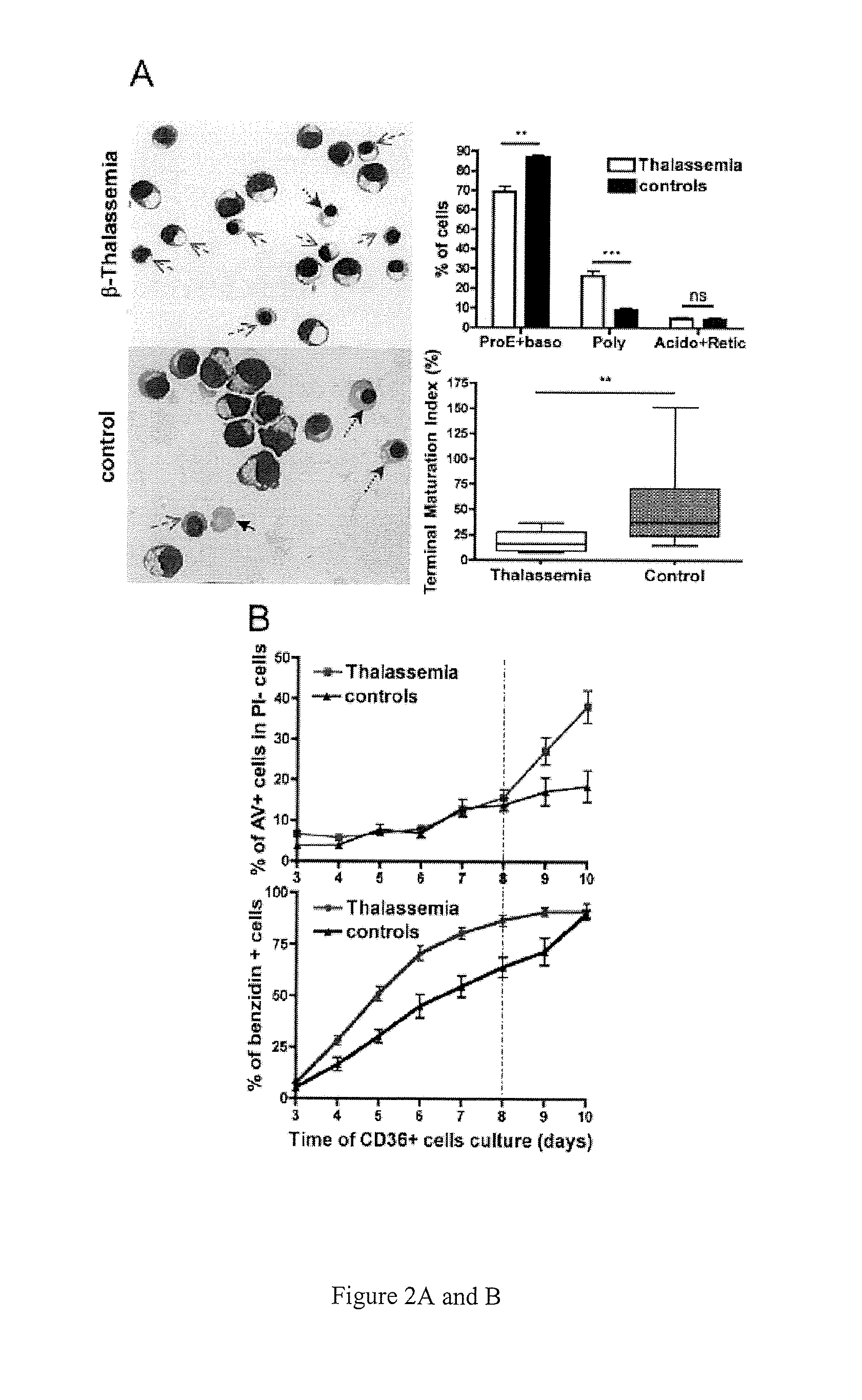
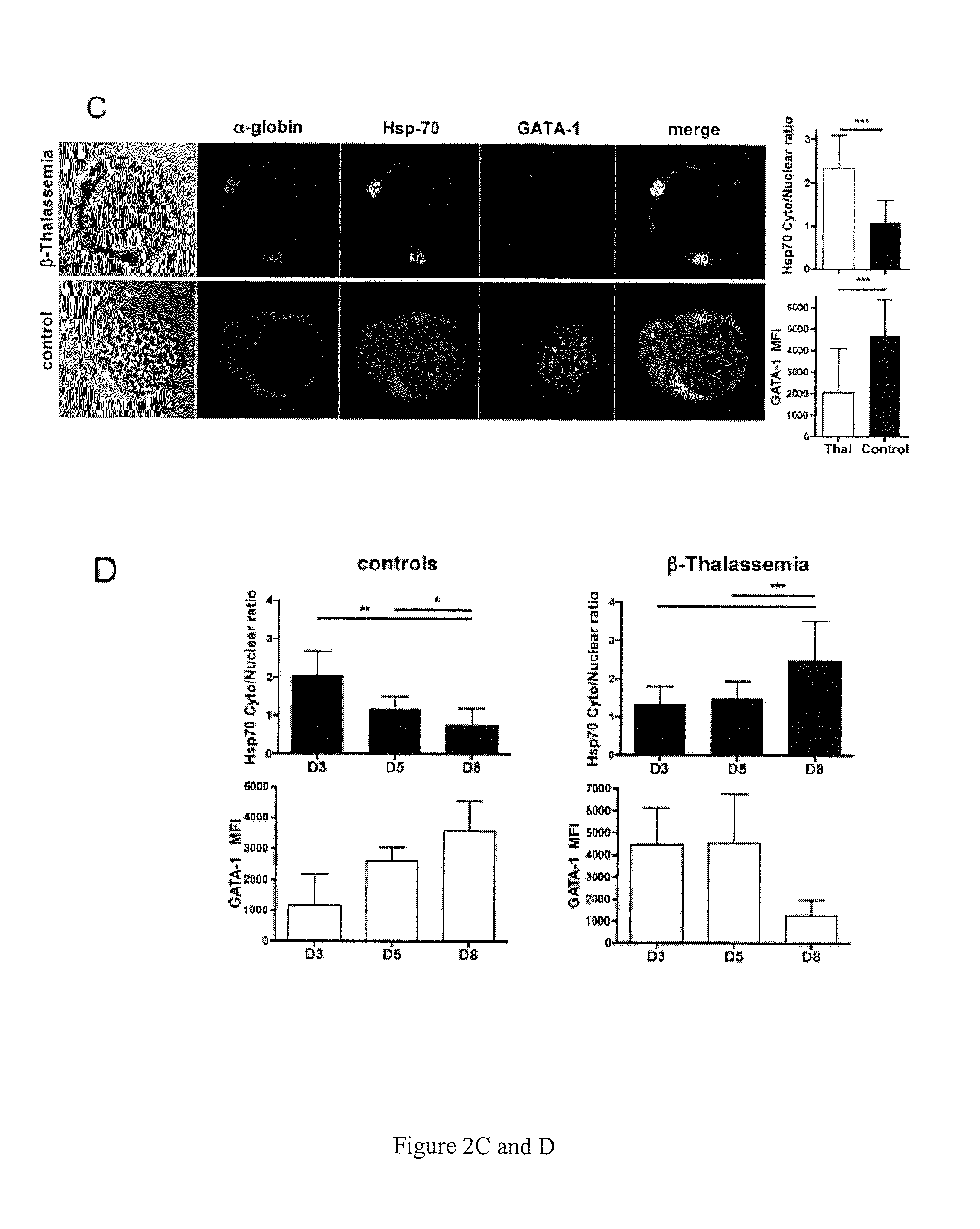
Methods and compositions for the treatment of beta-thalassemia
Methods and compositions for the treatment of .beta.-thalassemia are provided. Methods and compositions restore or increase erythrocyte maturation in individuals afflicted with .beta.-TM by preventing proteolysis of GATA-1 protein. Screening methods for identifying agents which bind heat shock protein 70 (HSP-70) and inhibit HSP-70 .alpha.-globin binding, but which allow GATA-1 protein-HSP-1 binding in a manner that prevents GATA-1 proteolysis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Mutated Immunoglobulin-Binding Protein Having Increased Alkaline Tolerance
ActiveUS20190202871A1Improved alkali toleranceImprove stabilitySolid sorbent liquid separationDepsipeptidesGlobin bindingA domain
The present invention relates to a mutated immunoglobulin-binding protein having increased alkaline tolerance and, more specifically, to an immunoglobulin-binding protein in which, with respect to the A-domain of Staphylococcal protein A, or a functional variant thereof, an amino acid at a specific site is mutated and thereby exhibits an increased chemical stability at an alkaline pH value in comparison to a parental molecule. The present invention can provide an antibody-purifying immunoglobulin-binding protein ligand and matrix which have enhanced alkaline tolerance and accordingly enhanced stability in multiple times of alkaline cleaning.
Owner:AMICOGEN INC
Evolved immunoglobulin binding molecule D-C-G3 and preparation method and application thereof
InactiveCN105153309AImprove bindingHigh sensitivityPeptide preparation methodsImmunoglobulinsGlobin bindingBond Force
The invention provides an evolved immunoglobulin binding molecule D-C-G3 characterized by having the amino acid sequence shown as SEQ ID NO:1. The invention also provides a cDNA sequence of the evolved immunoglobulin binding molecule D-C-G3 the code of which is shown as claim 1. The cDNA sequence is characterized in that the cDNA sequence is shown as SEQ ID NO:2. Moreover, the invention provides a preparation method of the evolved immunoglobulin binding molecule D-C-G3 as shown in claim 1. The evolved immunoglobulin binding molecule disclosed by the invention can bind total IgG of human and a plurality of animals and IgG of different subclasses in a broad-spectrum manner, and the bonding force of the evolved immunoglobulin binding molecule is higher than that of the immunoglobulin binding molecule compared with the prior art.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
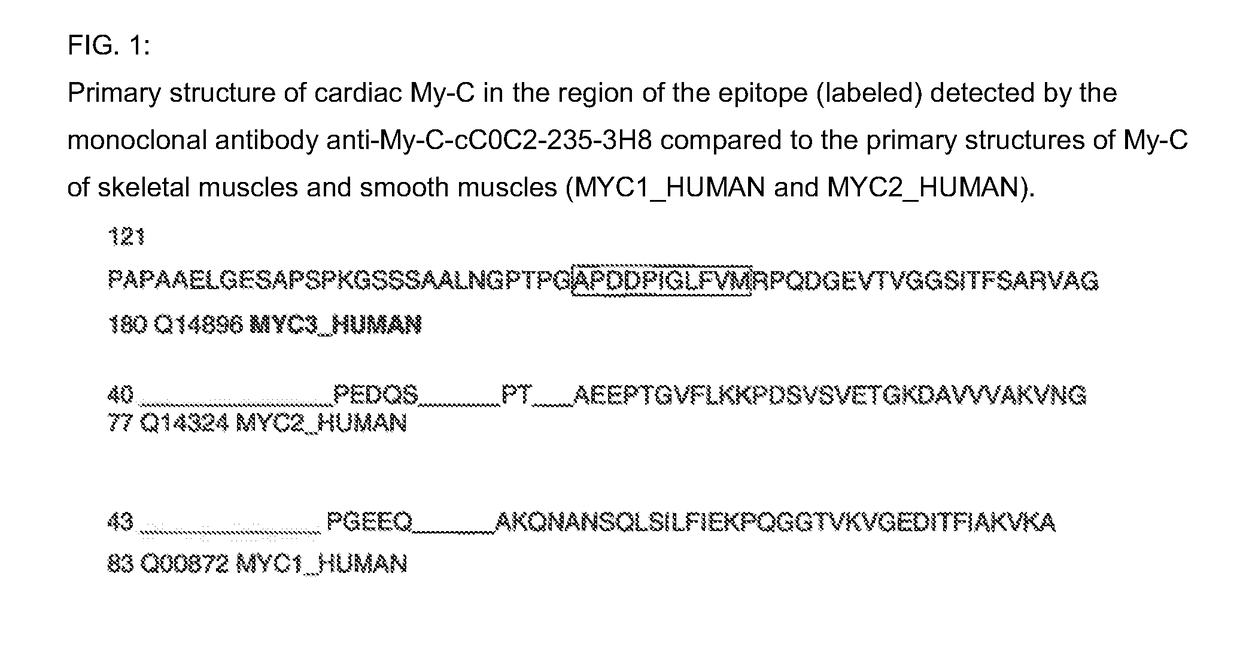
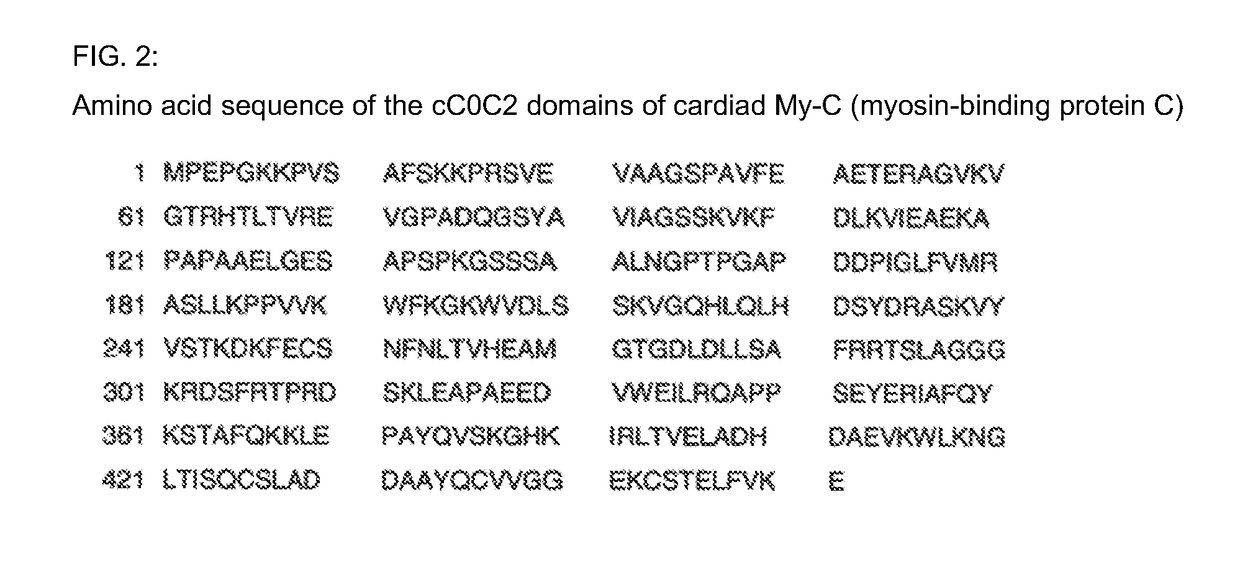
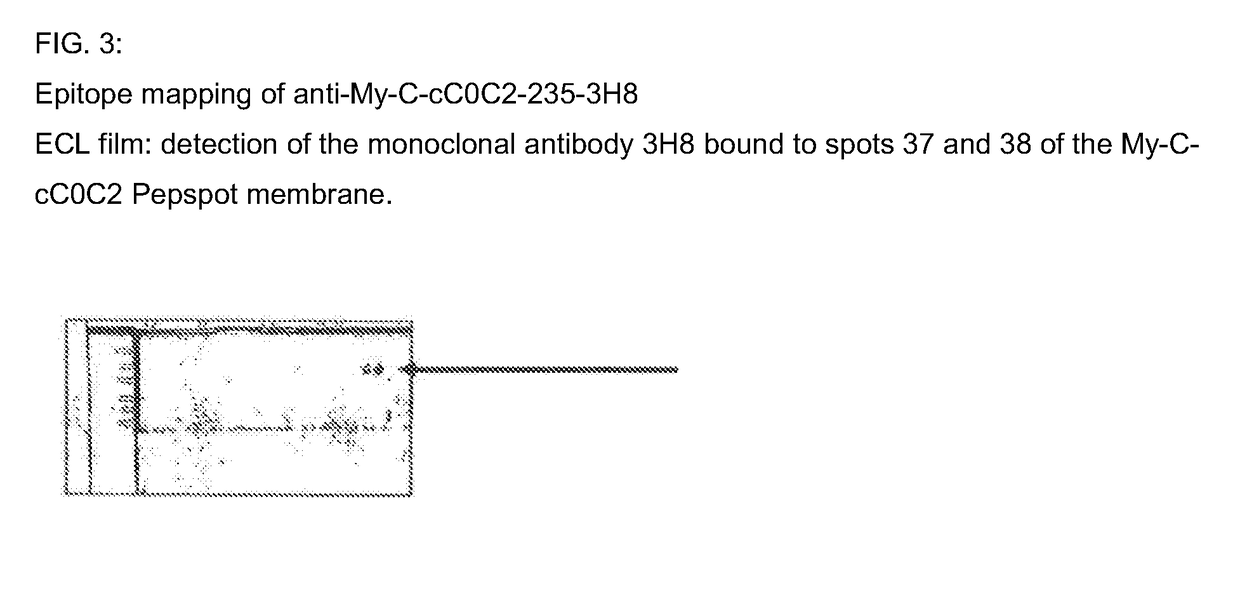
Hybridoma cell lines (my-c-cc0c2-235-3h8) and use thereof for producing a monoclonal antibody against the human cardiac myosin binding protein c (c-protein, mybpc3, cmybp-c or my-c)
ActiveUS20170088629A1Early diagnosis of cardiac infarctionsImmunoglobulins against animals/humansDisease diagnosisCardiac myosinBlood plasma
Monoclonal antibodies, which can be produced in vitro, against cardiac epitopes of the human My-C are produced by generating myeloma cell clones that produce such specific antibodies having epitope specificity. These monoclonal antibodies allow, among other things, the creation of an enzyme-linked immunosorbent assay (ELISA) for the specific, cross-reactivity-free quantitative determination of My-C in serum, plasma, whole blood or other body fluid. Specifically, a hybridoma cell clone producing a monoclonal antibody that detects and binds a cardiac epitope in the My-C is produced, which has no cross-reactivity with respect to the myosin-binding proteins of the skeletal muscles. The hybridoma cell line can be obtained by fusing myeloma cells with spleen cells of a test animal, in particular a mouse, immunized against recombinant My-C. The invention furthermore relates to epitope-specific antibodies produced by the hybridoma cell line, and to the use thereof.
Owner:KINGS COLLEGE LONDON
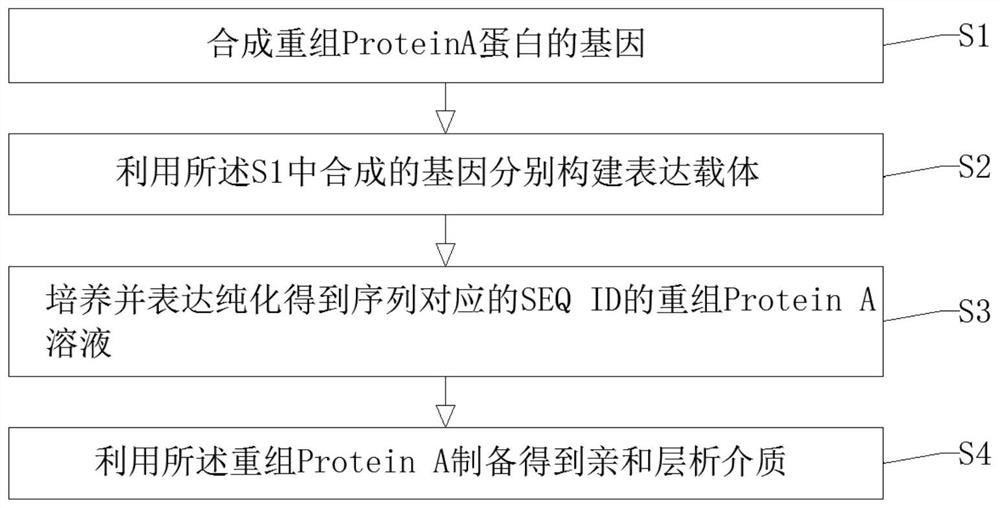
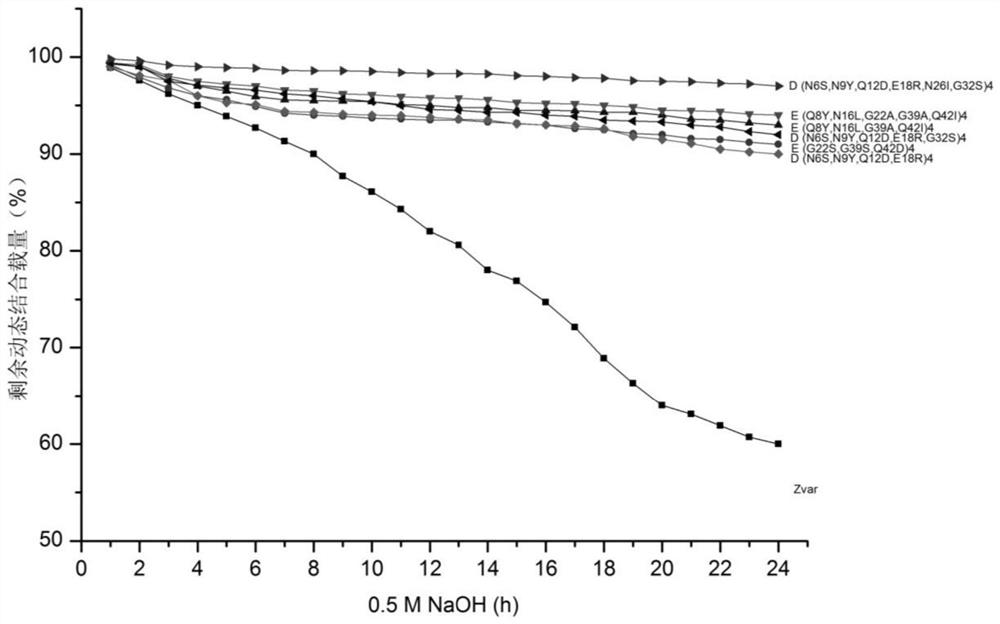
Recombinant Protein A protein and preparation method of affinity chromatography medium
ActiveCN113278052AGood chemical stabilityHigh binding capacityOther chemical processesPeptide preparation methodsMutantChemical stability
The invention discloses a recombinant Protein A protein and a preparation method of an affinity chromatography medium. The protein is a mutant of parent immune globulin-binding protein as defined by SEQ ID No 1 and SEQ ID No 2; and the preparation method of the affinity chromatography medium comprises the following steps: S1, synthesizing genes of the recombinant Protein A protein, S2, respectively constructing expression vectors by using the genes synthesized in the S1, S3, culturing, expressing and purifying to obtain a recombinant Protein A solution with a corresponding sequence of SEQ ID, and S4, preparing the affinity chromatography medium by using the recombinant Protein A. The preparation method has the advantages that the chemical stability of the Protein A in alkali liquor is greatly improved by redesigning an amino acid sequence, so that the Protein A with a specific binding effect on an antibody is provided, the Protein A has good chemical stability under an alkaline condition, the binding load of the recombinant Protein A and affinity chromatography medium is remarkably improved, in-place cleaning under 0.5-1.0 M NaOH can be tolerated, and the IgG binding load is 60-90 mg / ml.
Owner:PINGHU YOUPU BIOTECH CO LTD
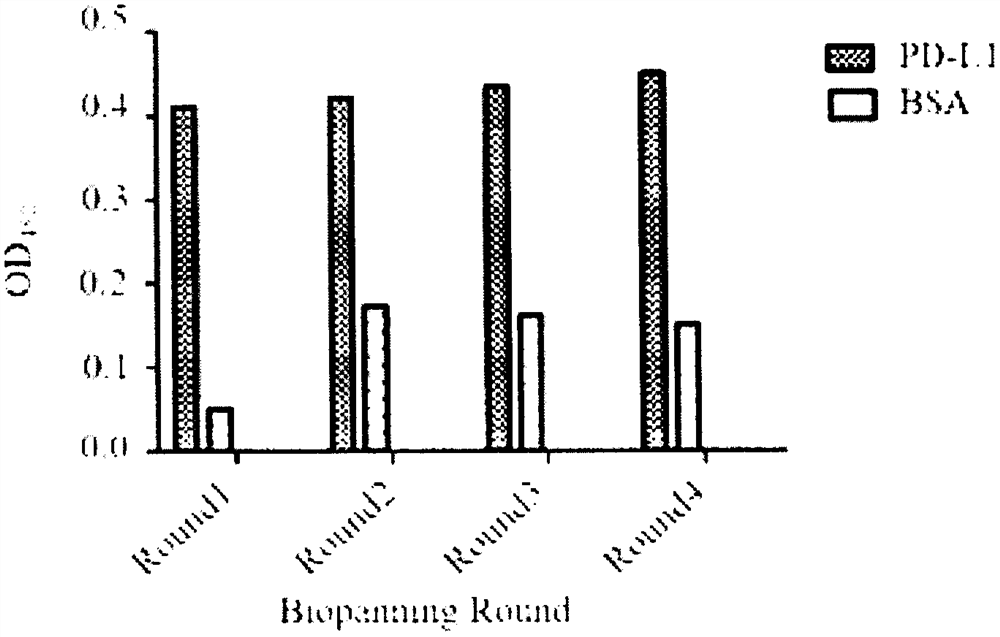
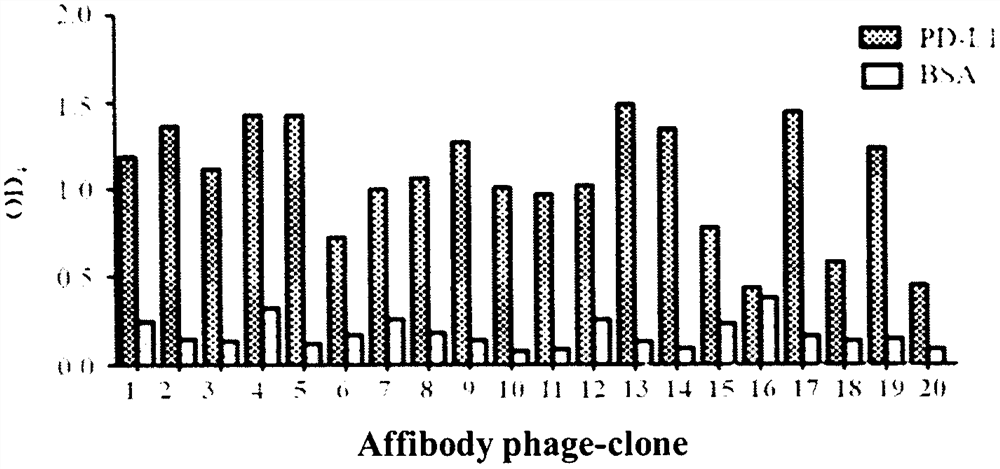
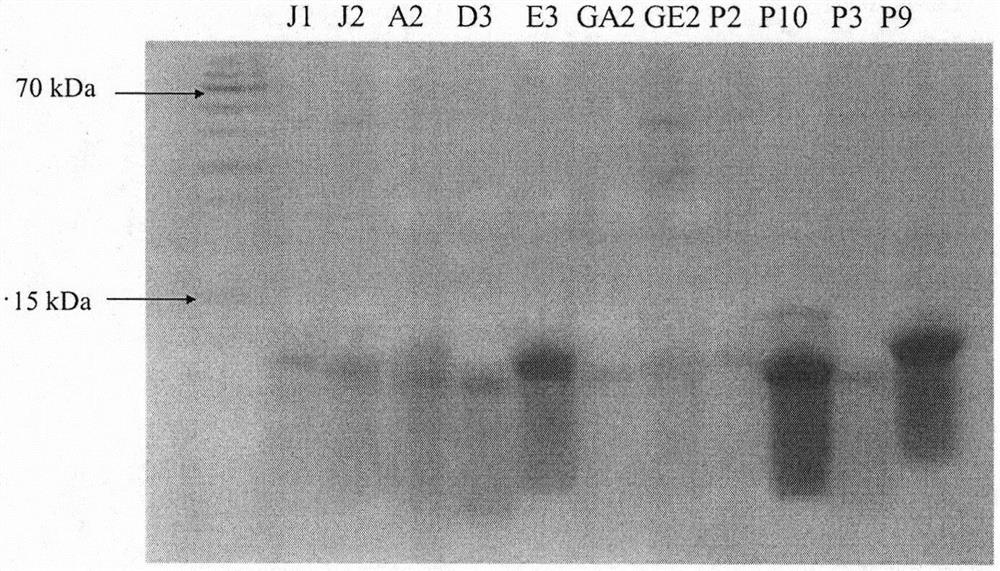
Programmed death receptor-ligand 1 (PD-L1) specific binding polypeptide and application thereof
ActiveCN113234152AGood specific affinityReduce manufacturing costBiological material analysisImmunoglobulins against bacteriaReceptorProgrammed death
The invention relates to a programmed death receptor-ligand 1 (PD-L1) specific binding polypeptide and application thereof, and belongs to the technical field of biology. The polypeptide is obtained by mutating 1-20 amino acid residues from a staphylococcus protein A (SPA) immunoglobulin binding region (the sequence of the polypeptide is shown in SEQ ID: 2-5), and the polypeptide can specifically bind to PD-L1 and block PD-1 / PD-L1 interaction. According to the invention, a PD-L1 specific binding polypeptide coding sequence is obtained by panning from a total synthesis affinity small body polypeptide molecular library through four rounds of enrichment, a prokaryotic expression system is utilized to prepare the obtained polypeptide in a large scale, and the polypeptide is specifically bound with PD-L1, has no obvious affinity with BSA protein, and can be used for PD-L1 detection. The polypeptide has the PD-1 / PD-L1 interaction blocking capability at the same time, and the PD-1 / PD-L1 interaction blocking capability of the polypeptide is enhanced along with the increase of the concentration.
Owner:TIANJIN UNIV OF SCI & TECH
Quantitative determination method of cardiac myosin binding protein C and detection kit
InactiveCN109444431AHigh detection sensitivityImprove linear rangeChemiluminescene/bioluminescenceBiological testingCardiac myosinElisa kit
The invention discloses a quantitative determination method of cardiac myosin binding protein C. According to the method, firstly, antibody coupling super paramagnetic micro particles and samples to be tested take specific combination reaction to obtain a first reactant, wherein the antibody coupling super paramagnetic micro particles are products obtained by coupling super paramagnetic micro particles and anti-cMyBP-C monoclonal antibodies A; then, the first reactant is taken to perform specific combination reaction with enzyme-labeled articles to obtain a second reactant, wherein the enzyme-labeled articles are products obtained by coupling enzymes and anti-cMyBP-C monoclonal antibodies B; finally, chemiluminescence substrates and the second reactant are taken to take enzymatic reaction;luminous signals are determined; the concentration value of cMyBP-C is obtained. The technology mode of the invention is a sandwich method. Compared with a cMyBP-C ELISA kit, the detection method andthe prepared kit have the advantages that the detection sensitivity and the linear range can be greatly improved. The technology evaluation on the prepared kit shows that the analysis sensitivity is0.05 ng / ml; the detection range is 0.1 to 50 ng / ml; high correlation and coincidence rate to clinic AMI patients are high.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Methods and compositions for the treatment of β-thalassemia
Methods and compositions for the treatment of β-thalassemia are provided. Methods and compositions restore or increase erythrocyte maturation in individuals afflicted with β-TM by preventing proteolysis of GATA-1 protein. Screening methods for identifying agents which bind heat shock protein 70 (HSP-70) and inhibit HSP-70 α-globin binding, but which allow GATA-1 protein-HSP-1 binding in a manner that prevents GATA-1 proteolysis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Affinity chromatography matrix
ActiveUS10189891B2Optimize purification stepsInhibition formationOther chemical processesSolid sorbent liquid separationThreonineHistidine
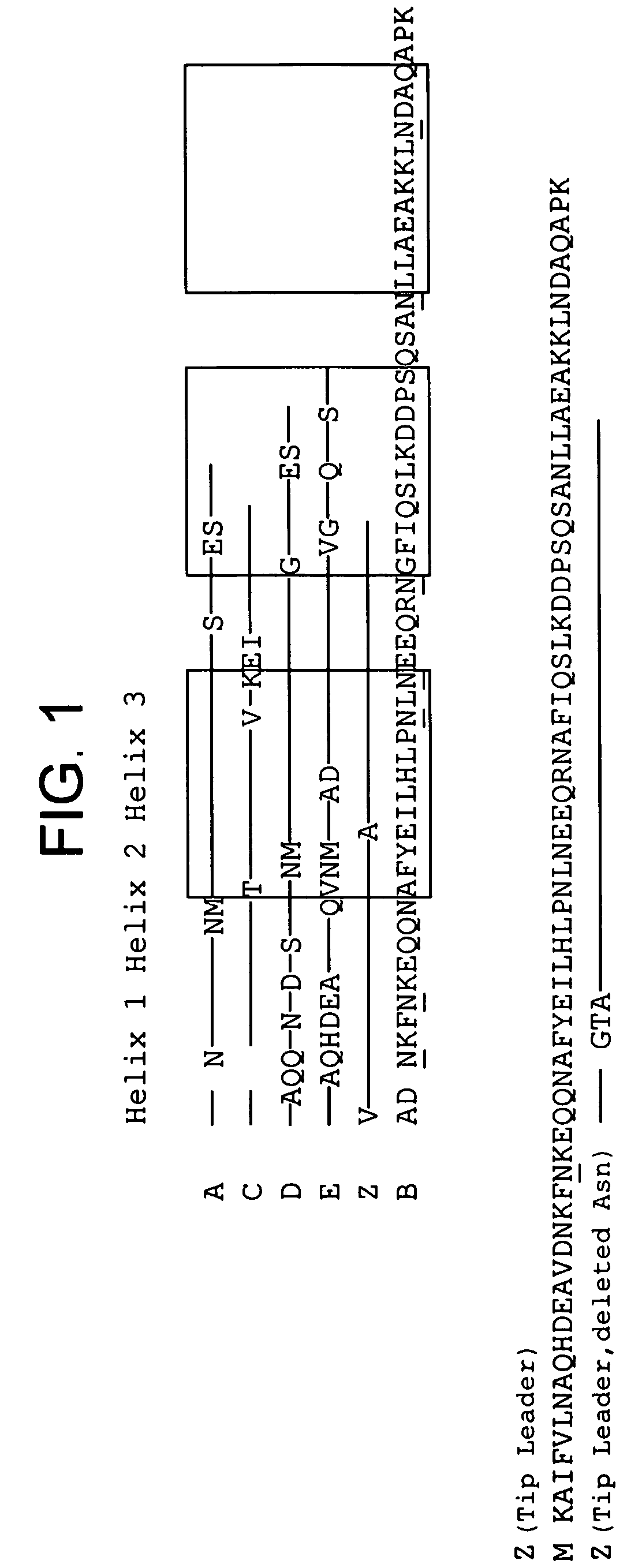
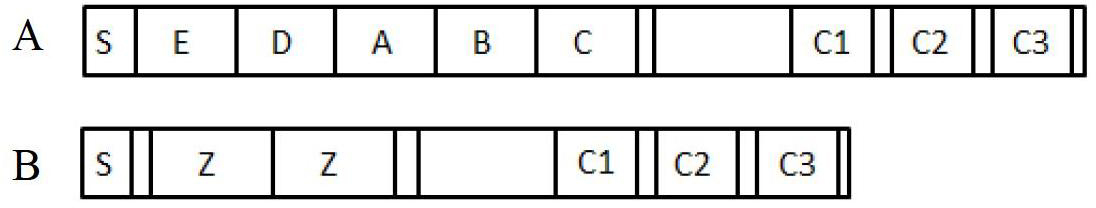
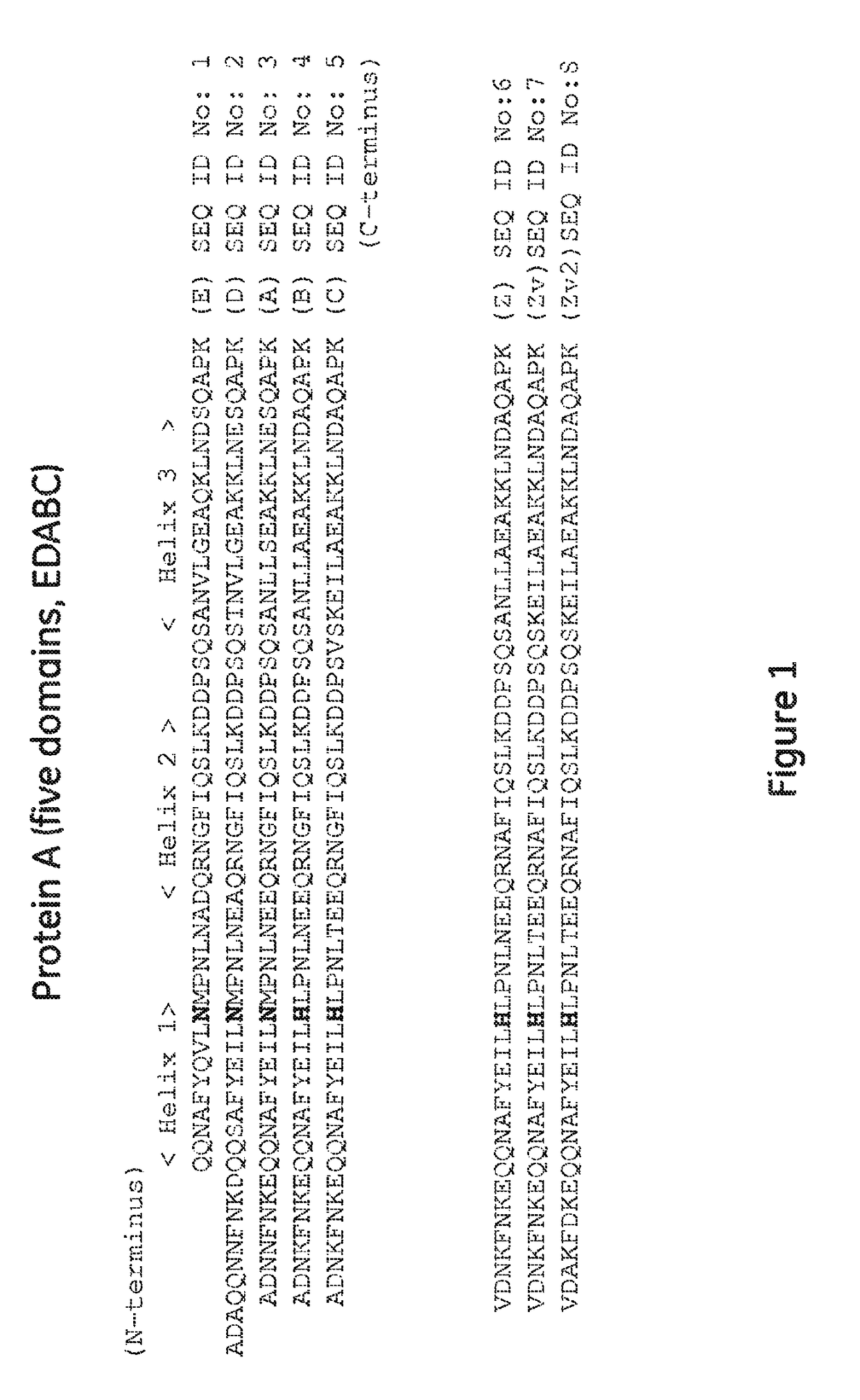
The invention discloses an immunoglobulin-binding protein comprising one or more mutated immunoglobulin-binding domains (monomers) of staphylococcal Protein A (E, D, A, B, C) or protein Z or a functional variant thereof, wherein in at least one of the one or more mutated monomers, the asparagine or histidine at the position corresponding to H18 of the B domain of Protein A or of Protein Z has been deleted or substituted with a first amino acid residue which is not proline or asparagine and wherein, if the amino acid residue at position 57 is proline and the amino acid residue at position 28 is asparagine, then the amino acid residue at the position corresponding to H18 of the B domain of protein A or of protein Z is not serine, threonine or lysine.
Owner:CYTIVA BIOPROCESS R&D AB
Multispecies universal detection protein with green fluorescence activity and application of universal detection protein
InactiveCN110615844AFluorescently activeEfficient captureMicroorganism based processesHybrid peptidesGlobin bindingFluorescence
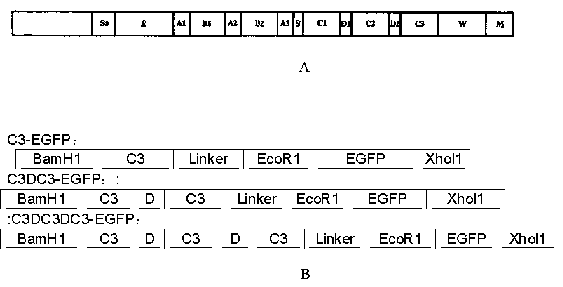
The invention provides a multispecies universal detection protein with green fluorescence activity and application of the universal detection protein. The universal detection protein is characterizedin that the detection protein comprises a streptococcus protein G (SPG) segment and a fluorescent protein; and an IgG binding segment of an SPG gene is reconstructed, only a C3 region where the protein G can be specifically bound with the Fc terminal of an antibody IgG is retained, the C3 region is divided into three groups of C3, C3-D-C3 and C3-D-C3-D-C3, all the groups are connected to EGFP, anda prepared recombinant protein has the fluorescence activity and dual activity of binding to antibodies of different species. Disclosed evolutionary immunoglobulin binding molecules can widely bind to total IgG and IgG of different subclasses in humans and many animals in a wide spectrum mode, and compared with immunoglobulin binding molecules in the prior art, the binding force of the evolutionary immunoglobulin binding molecules is also higher than that of the existing immunoglobulin binding molecules.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
High affinity immune globulin binding molecule and method for preparation
InactiveCN1319988CHigh affinityImmunoglobulins against plantsDepsipeptidesImmunglobulin eGlobin binding
This invention relates to high appetency immune globin combination molecule and its process method, wherein the method uses the structure units from the following fields: A, B, C, D, E fields of staphylococcus A protein; B1, B2, B3, B4, B5 structure fields of large digest streptococcus protein L; G1, G2 structure units of C and G streptococcus protein. The method connects the above structure units by random to recreate Ig combination molecule database expressed on the surface of the bacteriophage plaque. The immune globulin expresses the combination molecule to process appetency filtering for three to four times to get the aim combination molecule.
Owner:SHANGHAI NATURE STANDARD R&D & BIOTECH
A kind of immunoglobulin binding protein and its preparation method and application
ActiveCN111057153BQuality improvementGuaranteed purityPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein LProtein
An immunoglobulin binding protein provided by the present invention includes the B domain of protein A, the C2 domain of protein G, the variant of the B3 domain of protein L or any combination of 1-3 variants thereof, through protein A The different binding properties of protein G and protein L have certain complementarity, and they are alkali-resistant multifunctional IBP molecules with wider reactivity and higher affinity. In the present invention, the three-step chromatography is used to purify the protein, which can stabilize the protein quality, ensure the protein purity is greater than 97%, and the endotoxin level is lower than 1Eu / mg, meeting the requirements of clinical protein. The stability of the immunoadsorption filler synthesized by the purified immunoglobulin binding protein is increased, which can effectively increase the use times of the filler and prolong the service life of the filler.
Owner:GUANGZHOU KONCEN BIOSCI
Evolved immunoglobulin binding molecule, and its preparation method and uses
InactiveCN100486993CCombined broad spectrumImprove bindingBacteriaDepsipeptidesNatural antibodyGenetic engineering
The invention relataes to novel evolutionary immunoglobulin binding molecules, as well as their preparation methods and applications. The invention discloses separated evolutionary immunoglobulin binding molecules, which are proteins with amino acid sequences as shown by SEQ ID NO : 1, or conservative variant proteins with immunoglobulin binding activity. The invention also discloses the gene encoding, genetic engineering preparation methods and applications of immunoglobulin binding molecules. The disclosed immunoglobulin molecule broad-spectrum combined with various immuneglobulin shows high immunoglobulin whole molecule binding activity, and can be used in large-scale purification of genetic engineering antibodies, purification of natural antibodies and monoclonal antibodies, enzyme-linked immunosorbent assay, and immuno-chromatography and immunohistochemical methods for immune antibody detection and diagnosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Antibody directed against immunoglobulin-binding proteins of S. Aureus
InactiveCN108064241AImprove manufacturabilityImprove toleranceAntibacterial agentsSenses disorderBinding siteWild type
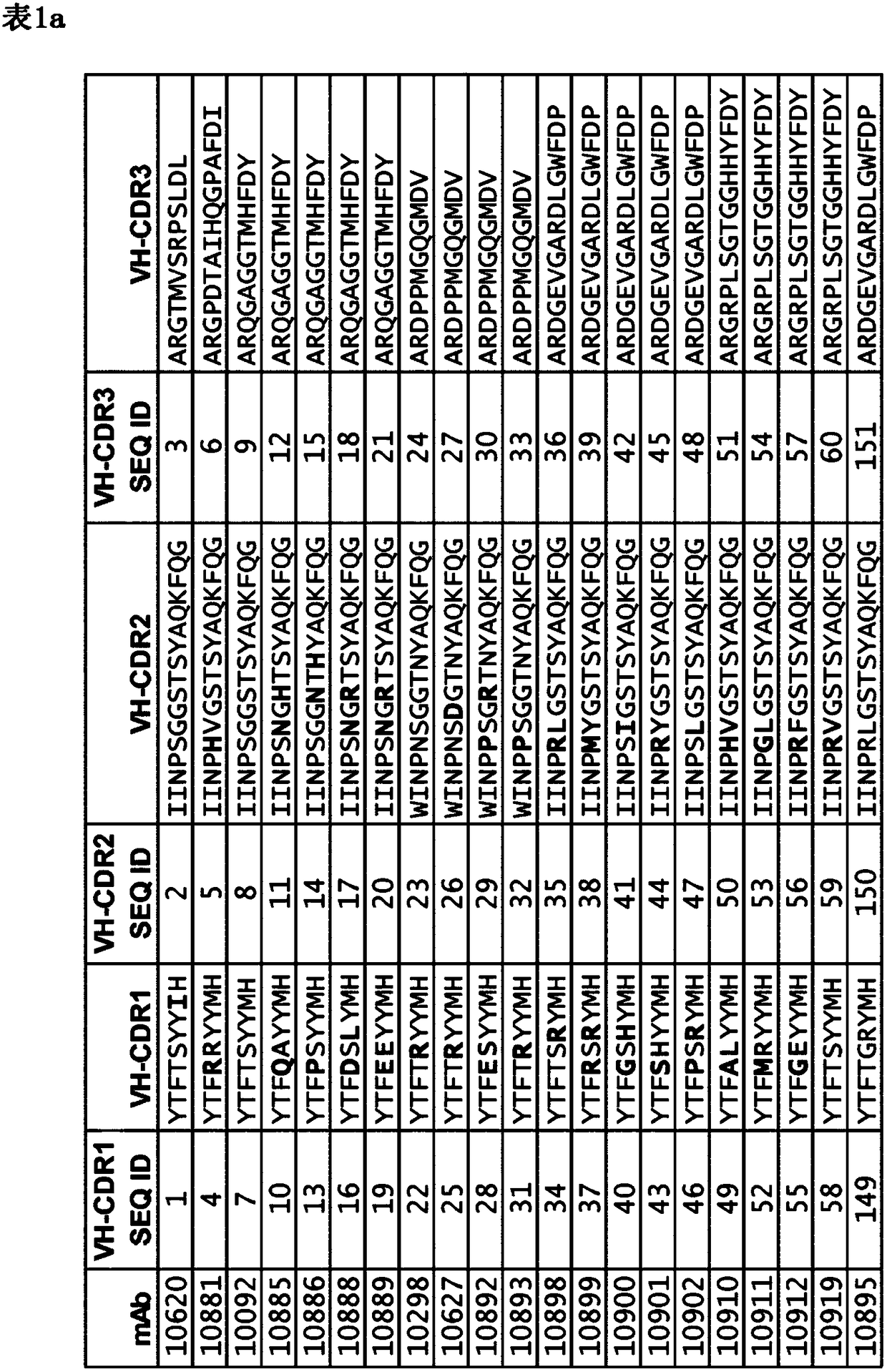
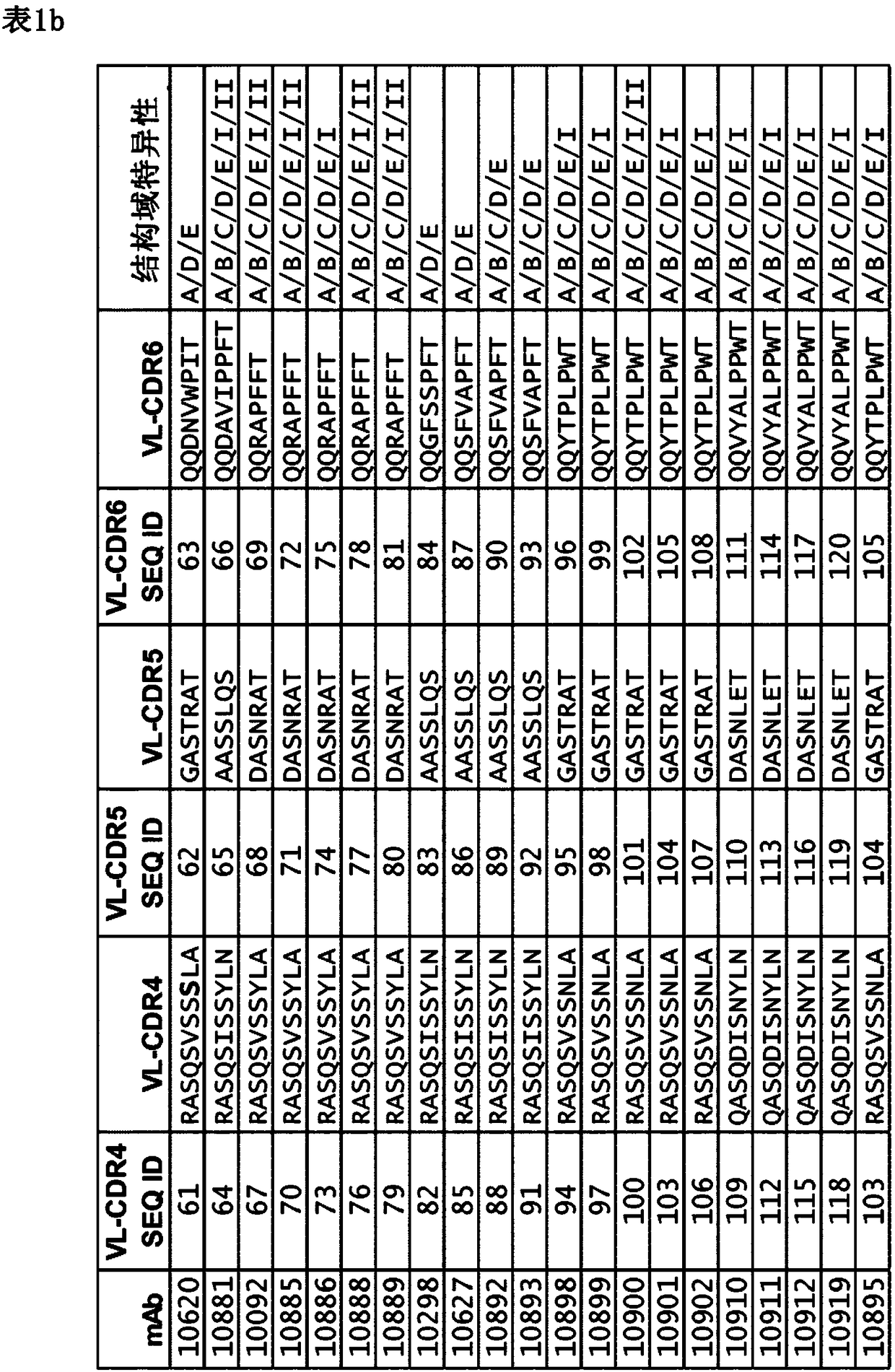
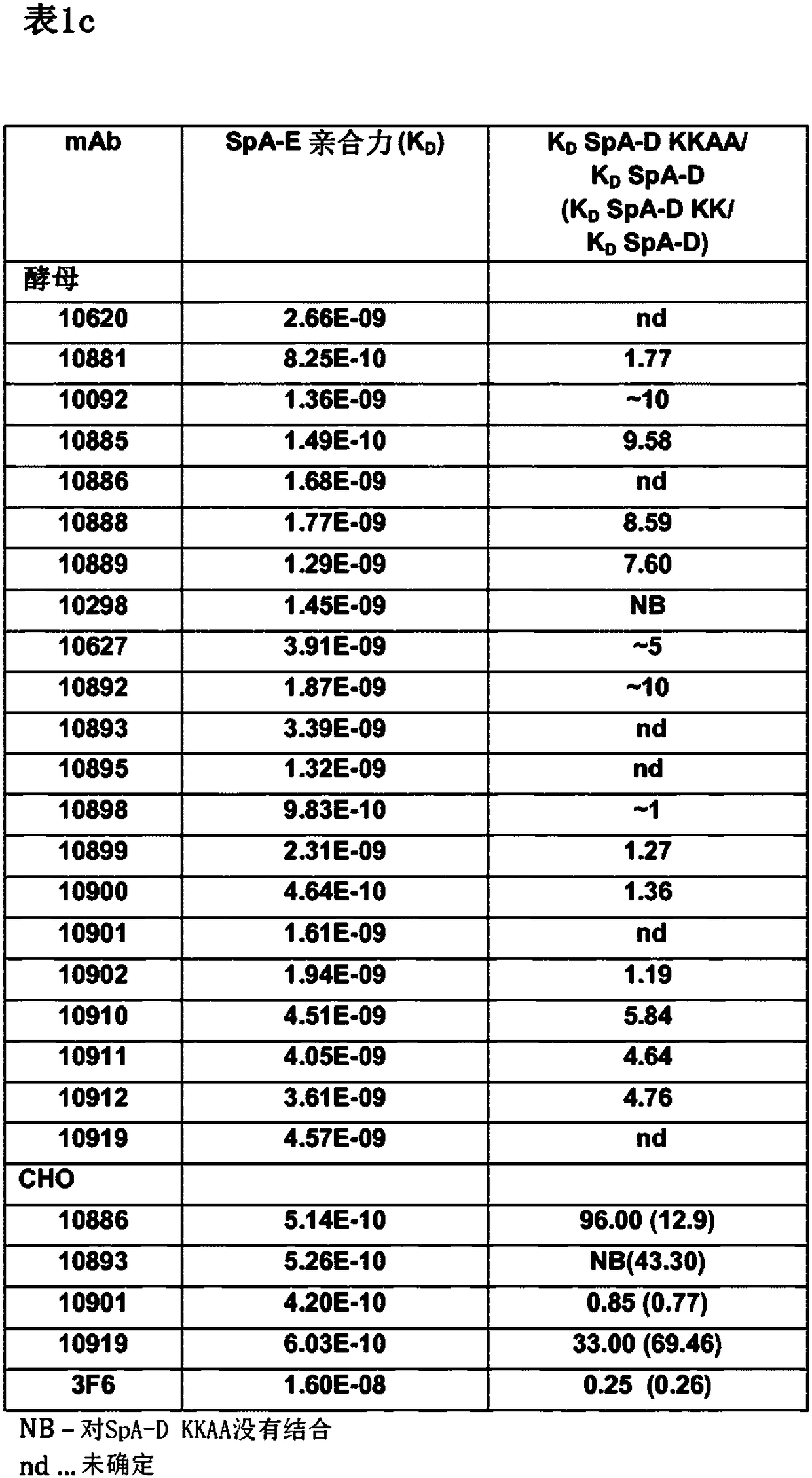
A monoclonal antibody that counteracts Staphylococcus aureus by specifically binding to wild-type immunoglobulin-binding proteins (IGBP) of S. aureus comprising a cross-specific CDR binding site recognizing at least three of the IGBP domains selected from the group consisting of Protein A (SpA) domains and immunoglobulin-binding protein (Sbi) domains SpA-A, SpA-B, SpA-C, SpA-D, SpA-E, Sbi-I, and Sbi-ll, wherein the antibody has an affinity to bind SpA-E with a KD of less than 5x10-9M as determined by a standard optical interferometry method for a F(ab)2 fragment, and preferably binds to wt SpAequally or better compared to mutant SpA-KKAA that lacks binding to IgG Fc or VH3.
Owner:ARSANIS BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com