Immunoglobulin binding protein, and preparation method and application thereof
An immunoglobulin and binding protein technology, applied in the field of immunoglobulin binding protein and its preparation, can solve the problems of unsatisfactory treatment effect of autoimmune diseases, large toxic and side effects, cross infection, etc., and achieves high affinity and stable protein quality. , the effect of prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
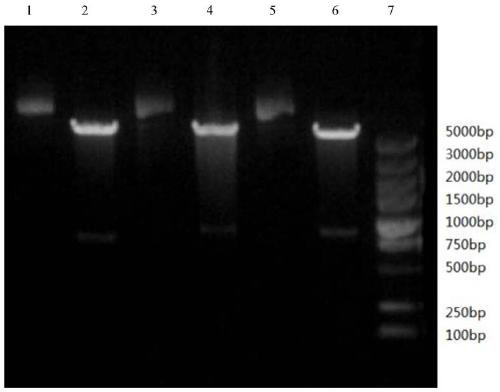
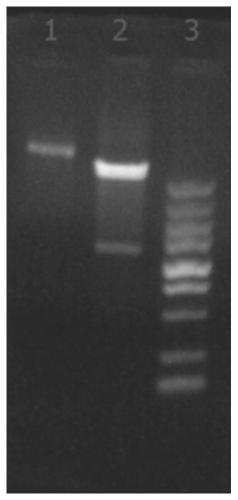
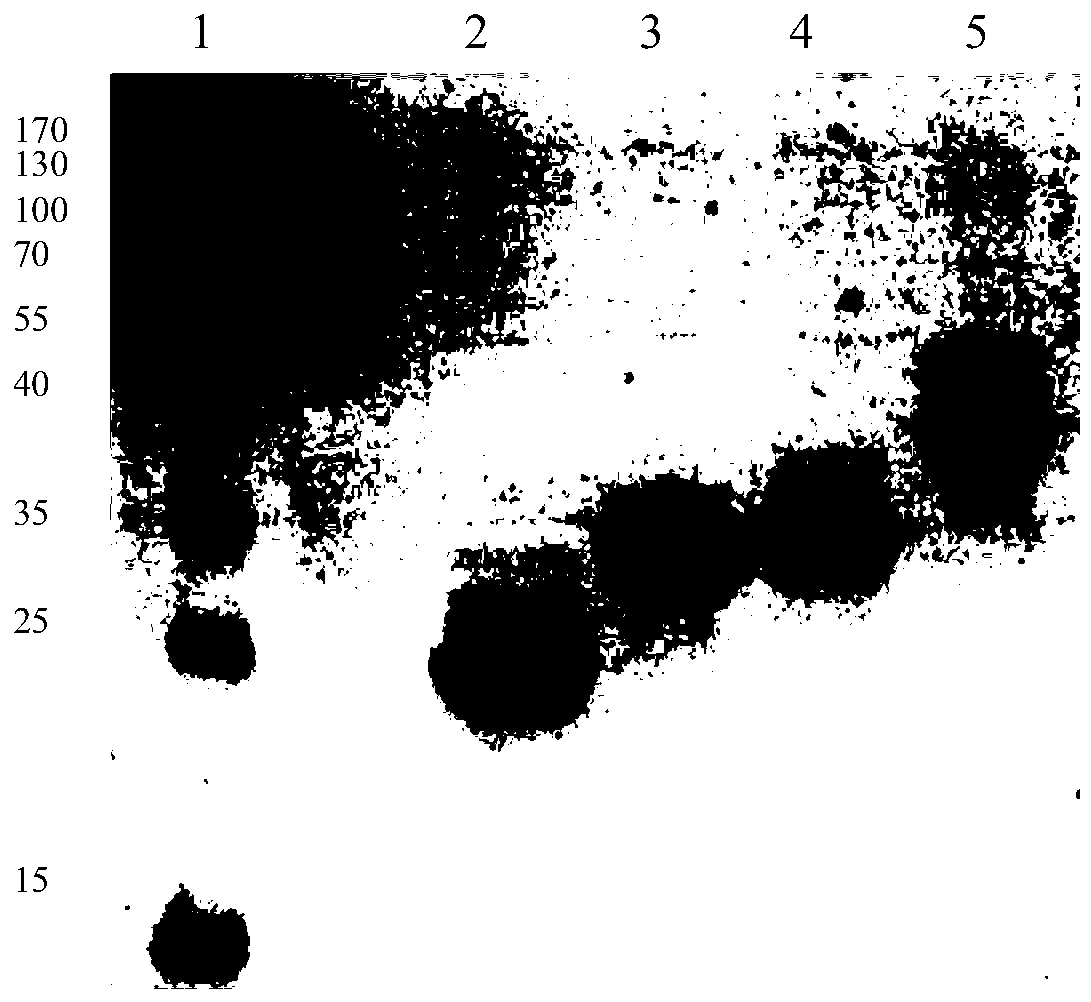
Image
Examples
Embodiment 1
[0043] Example 1 Vector Construction and Expression of Immunoglobulin Binding Proteins
[0044] Artificially synthesized amino acid sequences such as SEQ ID No: 1-13, the recombinant plasmid PET30a (Novagen) that can encode the gene sequence of the amino acid sequence of interest was ligated with restriction sites NdeI and XhoI, and then transformed into competent Escherichia coli strain BL21 (DE3) (merck millipore). Put 50mL of LB medium in a 250mL Erlenmeyer flask with a final concentration of kanamycin of 50 μg / mL, culture at 37°C and 180 rpm for 4 hours, then add IPTG with a final concentration of 50 μg / mL to induce expression, and culture for another 5 hours. Collect bacteria.
Embodiment 2
[0045] The crushing treatment of embodiment 2 thalline
[0046] Get 1 kg of thalline containing immunoglobulin binding protein, add the thalline to the PBS buffer solution of pH=7.2 according to the ratio of mass:volume=1kg:10L, break it with a high-pressure homogenizer after stirring evenly, and break it with a pressure of 800bar 2 Once, centrifuge at 4°C, 8000rpm for 15min to collect about 9.5L of supernatant. Add hydrochloric acid to the obtained solution to adjust the pH to 3.0, centrifuge at 4°C, 8000rpm for 15min, collect the supernatant and adjust the pH to 7.0 with sodium hydroxide at 8000rpm, centrifuge for 15min to obtain the supernatant of the broken cells.
Embodiment 3
[0047] Example 3 Purification of Immunoglobulin Binding Proteins
[0048] (1) Affinity chromatography: equilibrate the chromatographic column packed with TALON superflow metal ion chelating filler with 10mMbis-tris[bis(2-hydroxyethyl)triimino(hydroxymethyl)methane], pH 7.2 5 times the column volume, then load the broken supernatant obtained in Example 2, equilibrate 5 times the column volume with a solution containing 10mM bis-tris, pH=7.2 after loading the sample, wash 1 times the column volume with 0.01M NaOH, The column volume was then equilibrated for 5 times with a solution containing 10 mM bis-tris, pH=7.2, and finally the collected protein was eluted with a solution containing 180 mM imidazole and 10 mM bis-tris, pH 7.2. The main purpose of affinity chromatography is to capture proteins. Using metal ion chelating fillers and the alkali-resistant properties of proteins to be purified, proteins with weak binding forces are washed away with low-concentration sodium hydroxi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com