Multispecies universal detection protein with green fluorescence activity and application of universal detection protein
A green fluorescence, protein technology, applied in the field of immunodetection, can solve the problems of biological macromolecular activity change, inactivation, green fluorescent protein fluorescence activity change, etc., and achieve the effect of simple preparation process and high-sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The construction of embodiment 1 recombinant protein
[0054] 1.1 Biomaterials
[0055] Escherichia coli BL21 was purchased from Nanjing Novizan Biotechnology Co., Ltd.; plasmid pET-28a(+) was preserved in the laboratory, SPG (Xu Rui, Zhao Dengyun, Hong Yang, Lu Ke, Li Hao, Lin Jiaojiao, Feng Jin Tao, Xu Yumei, Zhu Chuangang. Domain Remodeling, Expression and Identification of Streptococcal Protein G[J]. Chinese Journal of Zoonotic Diseases, 2015,23(05):46-52.) EGFP corresponds to the serial number on NCBI: U55762
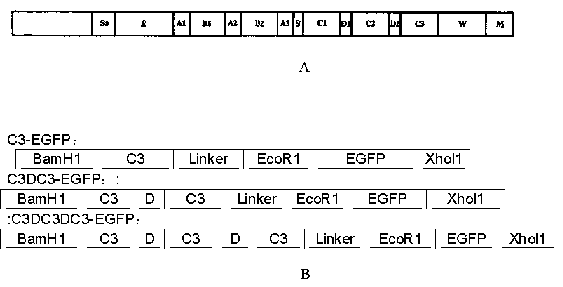
[0056] 1.2 Construction and synthesis of recombinant protein gene sequence (such as figure 1 B)
[0057] Detected from GenBank, the published gene fragments encoding EGFP and SPG region C, find out C1, C2, C3 region and D region, and obtain the gene fragments of C3 region. Analyze the signal peptide of the EGFP sequence to detect whether the gene sequence contains rare codons of Escherichia coli, that is, the frequency of use is less than 10%, and replac...
Embodiment 2
[0079] Expression and purification of embodiment 2 recombinant protein
[0080] 2.1 Expression of recombinant plasmids
[0081] phase
[0082] (1) Transfer the pET-28a(+)-C3-RFP recombinant plasmids with correct identification results into BL21(DE3), inoculate them in 5ml LB liquid medium containing Kan+, and place them in a shaking incubator at 37°C. Shake culture at 250rpm.
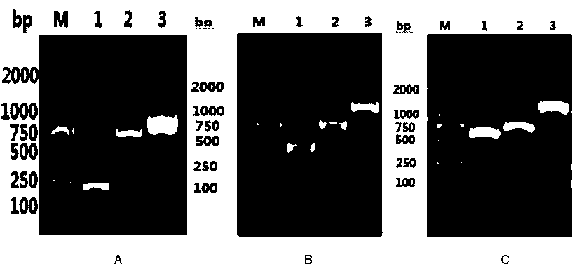
[0083] (2) When growing to the logarithmic phase (OD600 is about 0.6), add IPTG with a final concentration of 1 mmol / L to induce expression. Take 0.5ml bacterial liquid before induction and 1h, 2h, 4h, 6h, 8h after induction, and analyze the best induction time by SDS-PAGE electrophoresis. ( image 3 A,C,E)
[0084] Massive expression:
[0085] (1) Transform the pET-28a(+)-C3-RFP recombinant plasmids with correct identification results into BL21(DE3), inoculate them in 150ml LB liquid medium containing Kan+, and place them in a shaking incubator at 37°C. Shake culture at 250rpm.
[0086] (2) Whe...
Embodiment 3
[0098] Example 3 Activity identification of C3-RFP recombinant protein
[0099] 3.1 Fluorescence spectrum observation
[0100] The purified recombinant protein was prepared in different concentrations, and the excitation spectrum of the recombinant protein was scanned with a fluorescence spectrophotometer ( Figure 4 A), obtain the maximum excitation wavelength of the recombinant protein; use the maximum excitation wavelength to obtain the emission spectrum of the recombinant protein ( Figure 4 B). Observe the fluorescence intensity of the three recombinant proteins. It can be seen from the figure below that the fluorescence intensity of the three recombinant proteins is higher than that of the standard EGFP, among which the fluorescence intensity of C3-EGFP is the strongest, followed by C3DC3-EGFP, and C3DC3DC3-EGFP is the weakest, which may be related to the C3 region of the recombinant protein and the size of the EGFP fragment The C3 fragment in C3-EGFP is only one-thir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com