Fusion protein of immune globulin binding structural domain and fluorescence protein and its uses
An immunoglobulin and fusion protein technology, which can be used in hybrid peptides, material testing products, instruments, etc., can solve the problems of long time consumption and high cost, and achieve the effect of simple production process, low cost, and easy mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
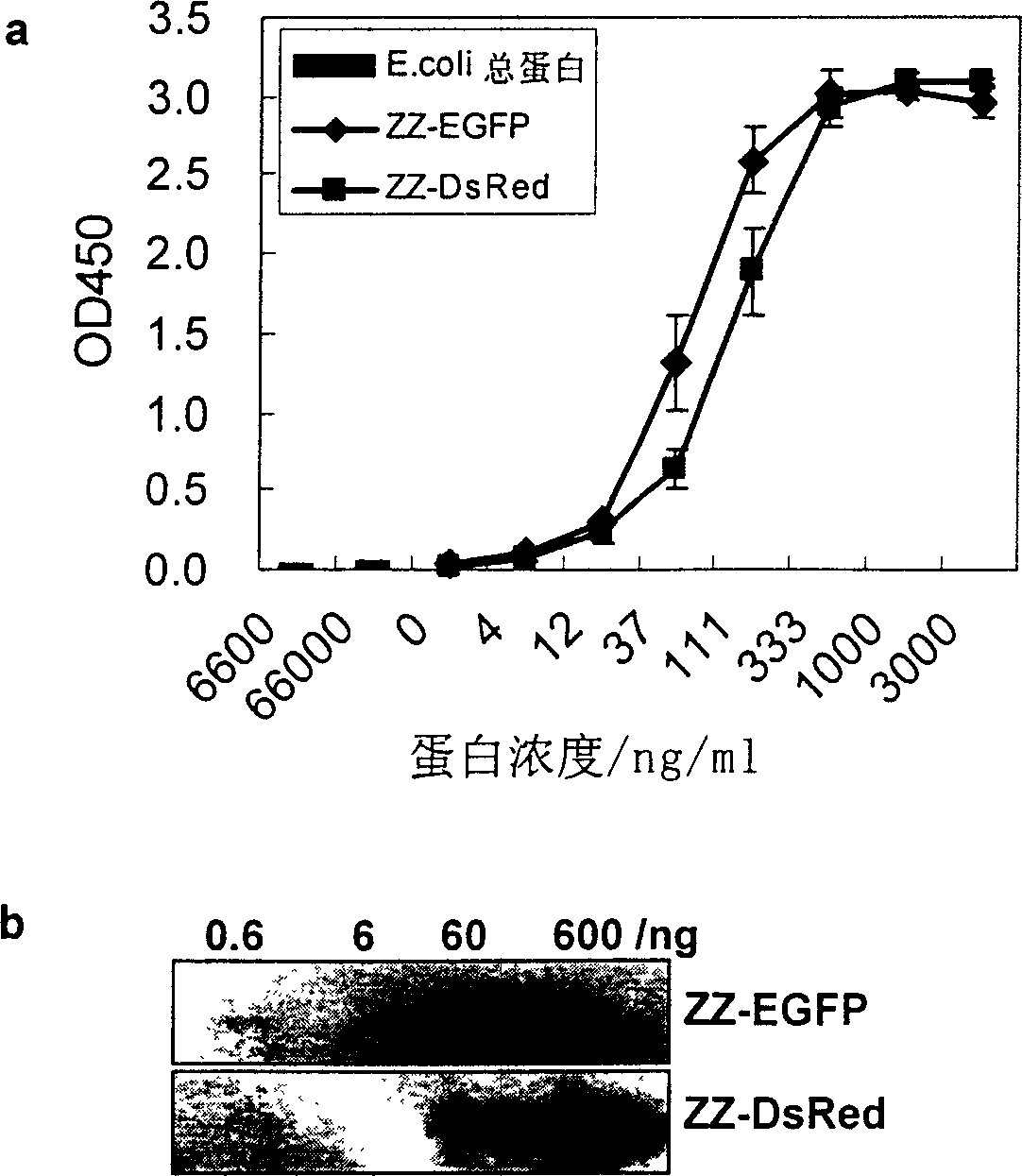
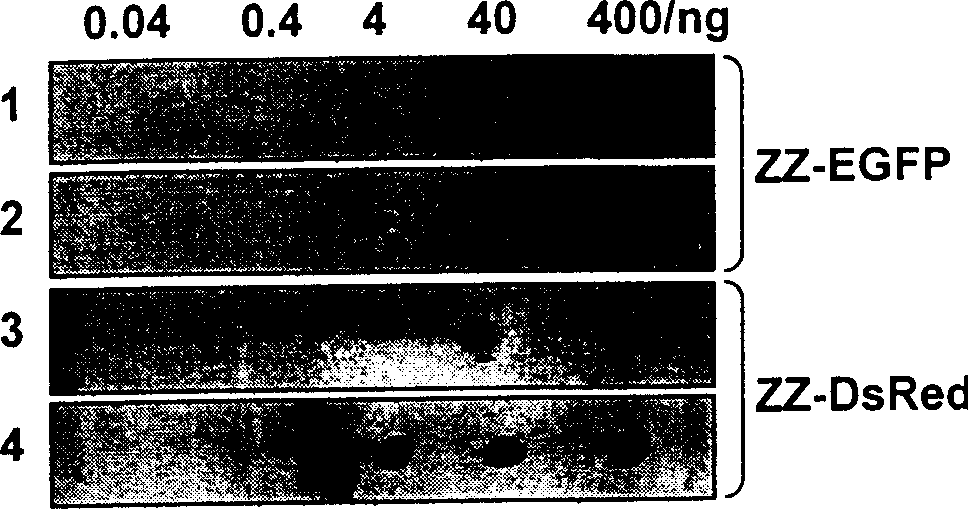
[0038] Example 1: Fusion of ZZ protein and green fluorescent protein (EGFP) and its application in immunoassay:
[0039] 1. Cloning of ZZ gene and construction of expression plasmid pET28a-ZZ:
[0040] According to the sequence of the ZZ gene, two primers ZZ-5' (5'CATGAATTCGCGCAACACGATGAAGCC 3') and ZZ-3' (5'CCCAAGCTTCTACCGAGCTCGAATTCGC 3') were chemically synthesized, and two primers ZZ-5' and ZZ-3' were obtained from pEZZ318 The coding sequence of the ZZ gene was obtained by PCR amplification from a plasmid (Amercia Company), and the amplified product was recovered by agarose gel electrophoresis and used for enzyme digestion and ligation. PCR conditions are: 94°C for 5 minutes; 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute, a total of 30 cycles; 72°C for 10 minutes; 4°C storage.
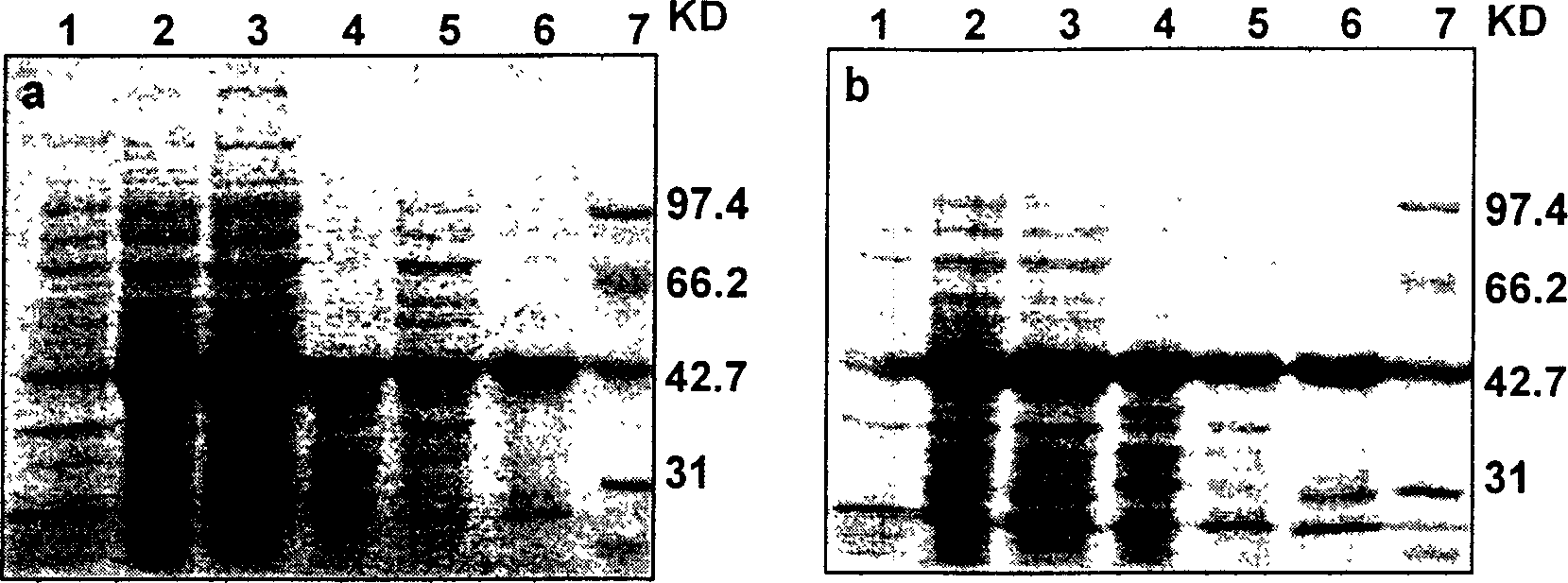
[0041] The recovered ZZ PCR product was digested with EcoR I and Hind III, and the plasmid pET28a (Novagen) was also digested with EcoR I and Hind III. The digested and purified ZZ frag...
Embodiment 2
[0056] Example 2: Fusion of ZZ protein and red fluorescent protein (DsRed-Express) and its application in immunoassay
[0057] 1. Cloning of ZZ-DsRed gene and construction of its expression vector:
[0058] The coding sequence of DsRed-Express was obtained from pDsRed-Express (Clontech) by Sal I and EcoR I double digestion, and then inserted into the pDsRed-Express (Clontech) that had been digested with XhoI, treated with alkaline phosphatase, and blunted with T4 DNA polymerase. and pET28a-ZZ filled in with T4 DNA polymerase. After the ligation product was transformed into Top10 competent cells, positive clones were screened by PCR with primers targeting the DsRed-Express coding sequence, and the direction of DsRed-Express gene insertion was checked by BamH I restriction enzyme digestion. DNA sequencing verified the correctness of the coding sequence and reading frame.
[0059] 2. Expression and purification of ZZ-DsRed in Escherichia coli:
[0060] The pZZ-DsRed expression...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com