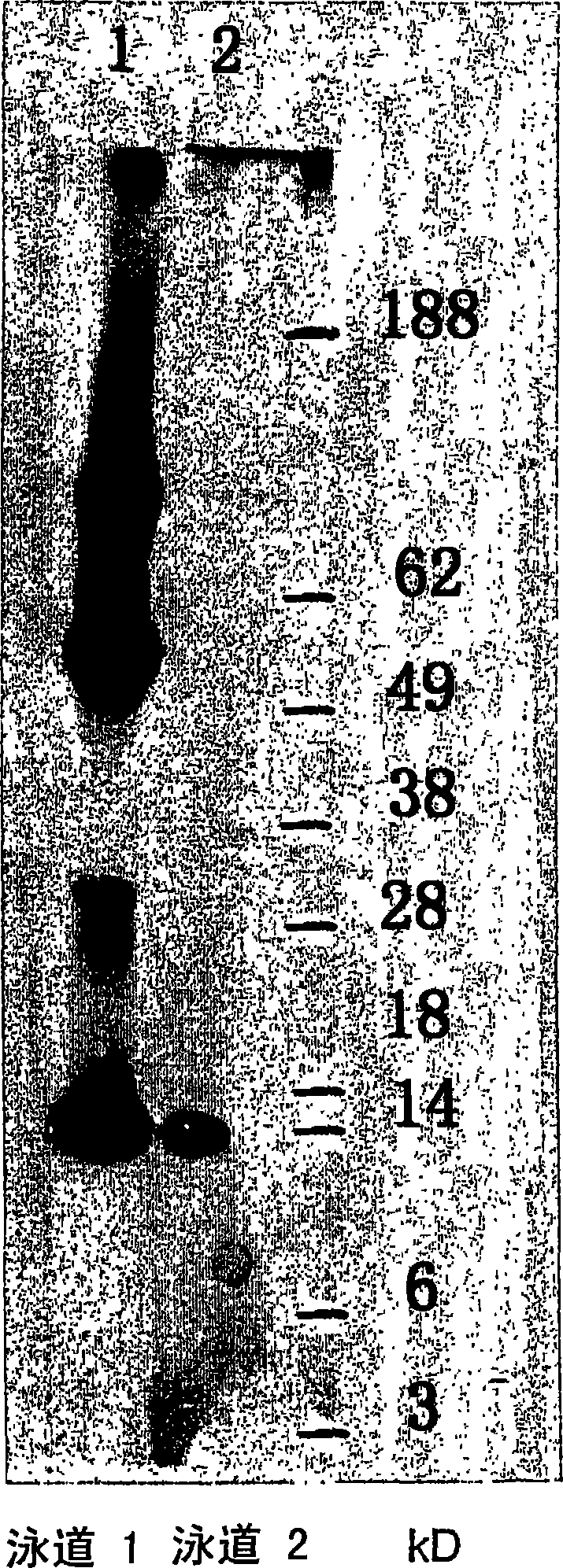
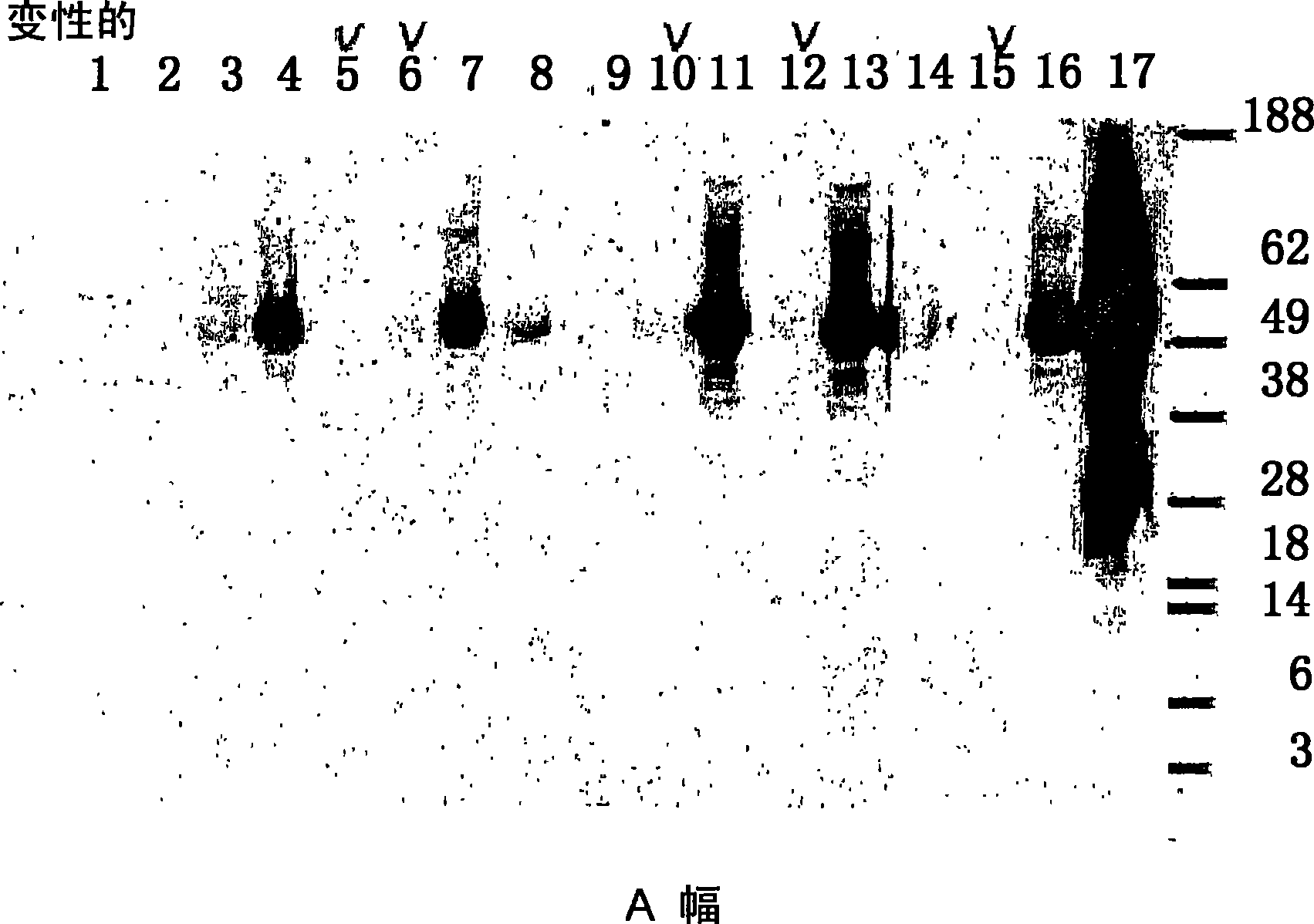
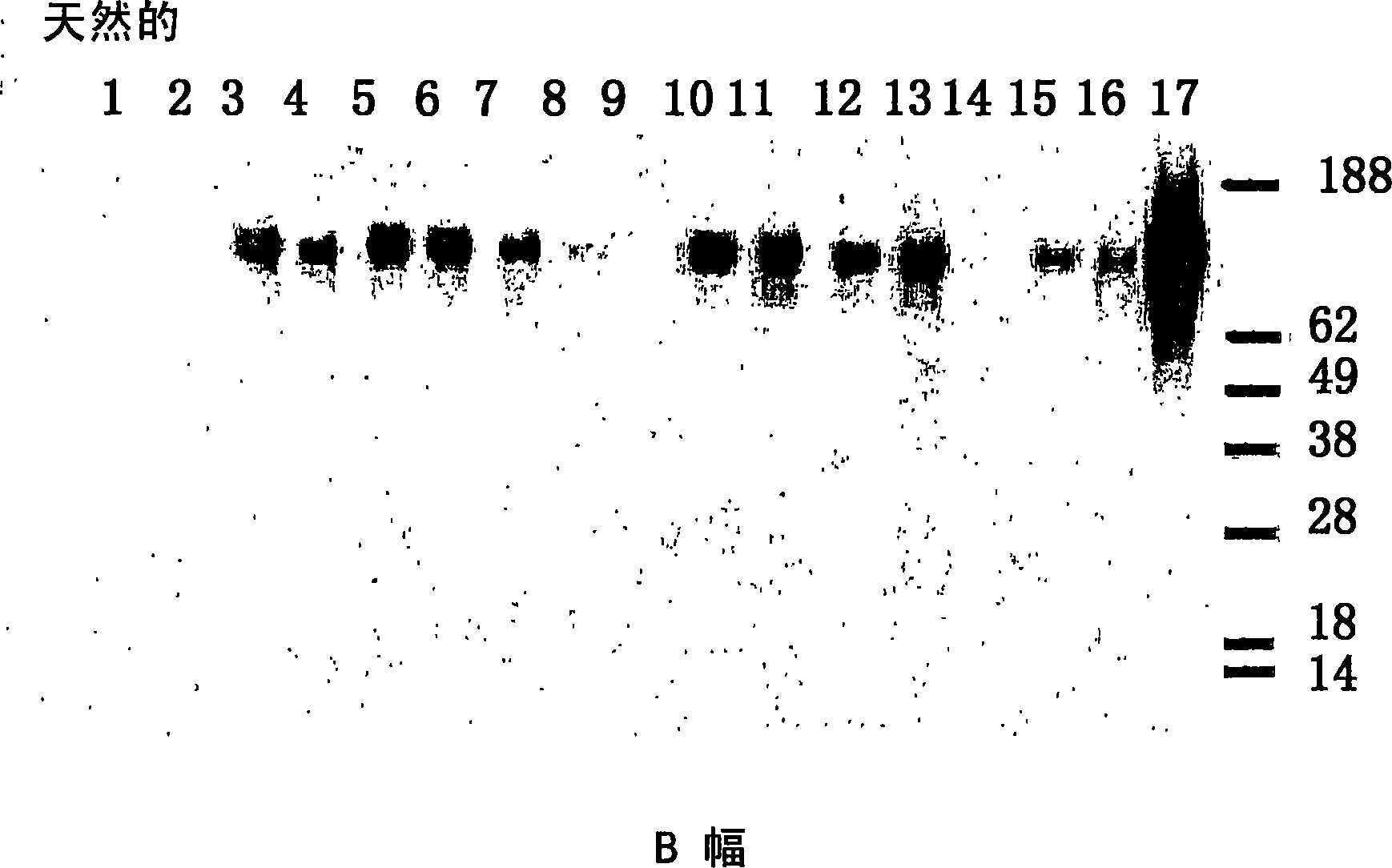
Native immunoglobulin binding reagents and methods for making and using same
A technology of immunoglobulin and natural immunity, which is applied in the field of binding reagents, can solve the problems of increased time for immunoprecipitation, no preferential identification of natural immunoglobulin reagents available, and increased complexity of other steps to achieve the effect of unique ability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Antibody production
[0133] Immunization and Fusion Methods
[0134] Lou / M rats were immunized with mouse serum immunoglobulin. Balb / c mice were immunized with rabbit serum immunoglobulin. Serum titers were tested after three immunizations. Spleens from animals with high serum titers were fused to the mouse myeloma fusion partner SP2 / O.
[0135] fusion method
[0136] A. Culture medium preparation
[0137] 1) IMDM basal medium:
[0138] 2) 20% FBS complete medium:
[0139] 500ml IMDM basal medium+100ml FBS+6.5ml P / S (stock solution concentration: 10,000 units / ml penicillin; 100,000 units / ml streptomycin)+6.5ml glutamine (stock solution concentration: 200μM).
[0140] 3) Supplements:
[0141] 100×HAT
[0142] 50 x HES (Hybridoma Enhanced Supplement)
[0143] 4) fusion medium:
[0144] 500ml complete medium
[0145] 5ml 100×HAT
[0146] 10ml 50×HES
[0147] 5) 50% PEG (Sigma, MW 1500)
[0148] B. Preparation of Myeloma Cells
[0149] 1) Myeloma cell l...
Embodiment 2
[0248] Preparation of Polyclonal Anti-NIgSAB Subtraction Immunization
[0249] method:
[0250] Day 1: 6 mice were injected (i.p.) with a tolerogen (denatured immunoglobulin) (25-50 mg) not required for final antibody production in complete adjuvant. Ten minutes later, cyclophosphamide (SJGMA) in sterile phosphate-buffered saline was injected at 100 mg / kg body weight. A 2 mg / ml solution of cyclophosphamide was prepared for this purpose.
[0251] Day 2: Another injection of cyclophosphamide (100 mg / kg body weight).
[0252] Day 3: Repeat injection of cyclophosphamide.
[0253] Day 7: Mice were bled and tested for antibody titers by ELISA.
[0254] Day 14: Tolerogen (25-50 mg) not required for final antibody production in incomplete adjuvant was used to inject 6 mice (i.p.). Ten minutes later, cyclophosphamide in sterile phosphate-buffered saline was injected at 100 mg / kg body weight.
[0255] Day 15: Another injection of cyclophosphamide (100 mg / kg body weight).
[025...
Embodiment 3
[0263] Cloning and Expression of Anti-NIGSAB in Mammalian Cells
[0264] A typical mammalian expression vector contains at least one promoter element that mediates the initiation of transcription of the mRNA, the antibody coding sequence and signals required for transcription termination and polyadenylation of the transcript. Other elements include enhancers, Kozak sequences, and intervening sequences flanked by donor and acceptor sites for RNA splicing. Efficient transcription can be achieved with the early and late promoters of SV40, the long terminal repeat (LTRS) of retroviruses (eg, RSV, HTLVI, HIVI), and the early promoter of cytomegalovirus (CMV). However, cellular elements (eg, the human actin promoter) can also be used. Suitable expression vectors for practicing the invention include, for example, vectors such as pIRESlneo, pRetro-Off, pRetro-On, PLXSN or pLNCX (Clonetech Labs, Palo Alto, California), pcDNA3.1(+ / -), pcDNA / Zeo (+ / -) or pcDNA3.1 / Hygro (+ / -) (Invitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com