Quantitative determination method of cardiac myosin binding protein C and detection kit
A quantitative detection method, myosin technology, applied in the quantitative detection kit of cardiac myosin binding protein C, the field of quantitative detection of cardiac myosin binding protein C, can solve the problem of narrow linear range, long reaction time and sensitivity Low-level problems, achieve high correlation and coincidence rate, improve detection sensitivity and linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of a kit for quantitative detection of cardiac myosin binding protein C (enzyme conjugate using horseradish peroxidase)
[0025] 1. Preparation of antibody-coupled superparamagnetic particles
[0026] Take the superparamagnetic microparticles (1um~3um in diameter) whose surface active groups are carboxyl groups, wash them with coating buffer (50mmol / L, pH 7.6 phosphate buffer) for 5 times, add 10% glutaraldehyde Activation; after washing, coat with cMyBP-C antibody diluted to 100-500 μg / mL, shake and react for 2 hours; wash, then block with pH4.4, 0.02mol / L buffer containing 50mmol / L acetic acid for 1h ; After removing the blocking solution, add pH 7.4, 0.02 mol / L phosphate buffer containing 0.5% Tween-20, 1% BSA, 0.1% Procline300 for storage, and store at 2-8°C for later use.
[0027] 2. Preparation of antibody-conjugated horseradish peroxidase (HRP) conjugate
[0028] Weigh 5mg HRP and dissolve it in 1ml distilled water, add 0.06mol / L NaIO 4 A...
Embodiment 2
[0034] Embodiment 2 The detection method of kit of the present invention
[0035] A full-automatic chemiluminescence immunoassay analyzer was used, and the kit prepared in Example 1 was used as a detection tool: 25 μL of sample, 20 μL of cMyBP-C antibody-coated magnetic particles and 100 μL of horseradish peroxidase-labeled cMyBP-C antibody, after reacting for 15 minutes, carry out magnetic separation, the instrument sends the reaction mixture into the dark room, and sequentially add the luminescent substrate A solution (containing 0.1M luminol) and B solution (containing 0.5% H2O2) to carry out the luminescent reaction , and finally record the luminous intensity, and calculate the cMyBP-C content of the tested sample from the standard curve.
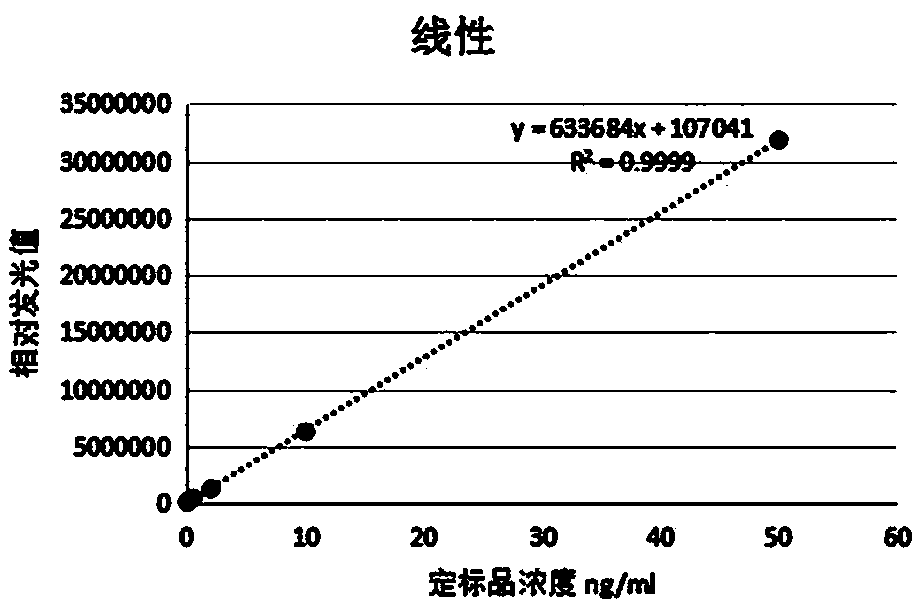
[0036] The cMyBP-C calibrator is detected by the above method, and the standard curve drawn is as follows figure 1 shown.
Embodiment 3
[0037] Embodiment 3 The performance test of kit of the present invention
[0038] 1. Sensitivity detection
[0039]The sensitivity of the cMyBP-C chemiluminescence immunoassay kit was calculated by referring to the recommended experimental scheme in the CLSI EP17-A document, and the obtained sensitivity was 0.05ng / mL.
[0040] 2. Linear detection
[0041] Perform linear analysis on standard substances with concentrations of 0ng / mL, 0.05ng / mL, 0.5ng / mL, 2ng / mL, 10ng / mL and 50ng / mL, and calculate the linear correlation coefficient, r 2 =0.99, the linear range of the kit for detection of cMyBP-C samples is 0.1-50ng / ml.
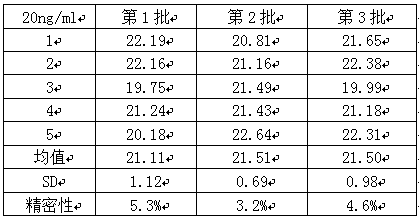
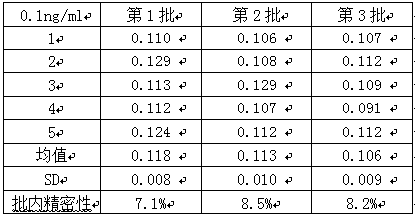
[0042] 3. Precision testing
[0043] Two cMyBP-C samples with a concentration of 0.1ng / mL and 20ng / mL were taken, and five parallel samples were made for each concentration of each sample. Three batches of kits were used for detection, and the intra-assay and inter-assay differences of the kits were calculated. The results See Table 1, Table 2, and Table 3 be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com