Human monocolonal antibody with high neutralization activity for Zika virus and application thereof
An antibody and sequence technology, applied in the field of medicine, can solve the problems of lack of vaccines and treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
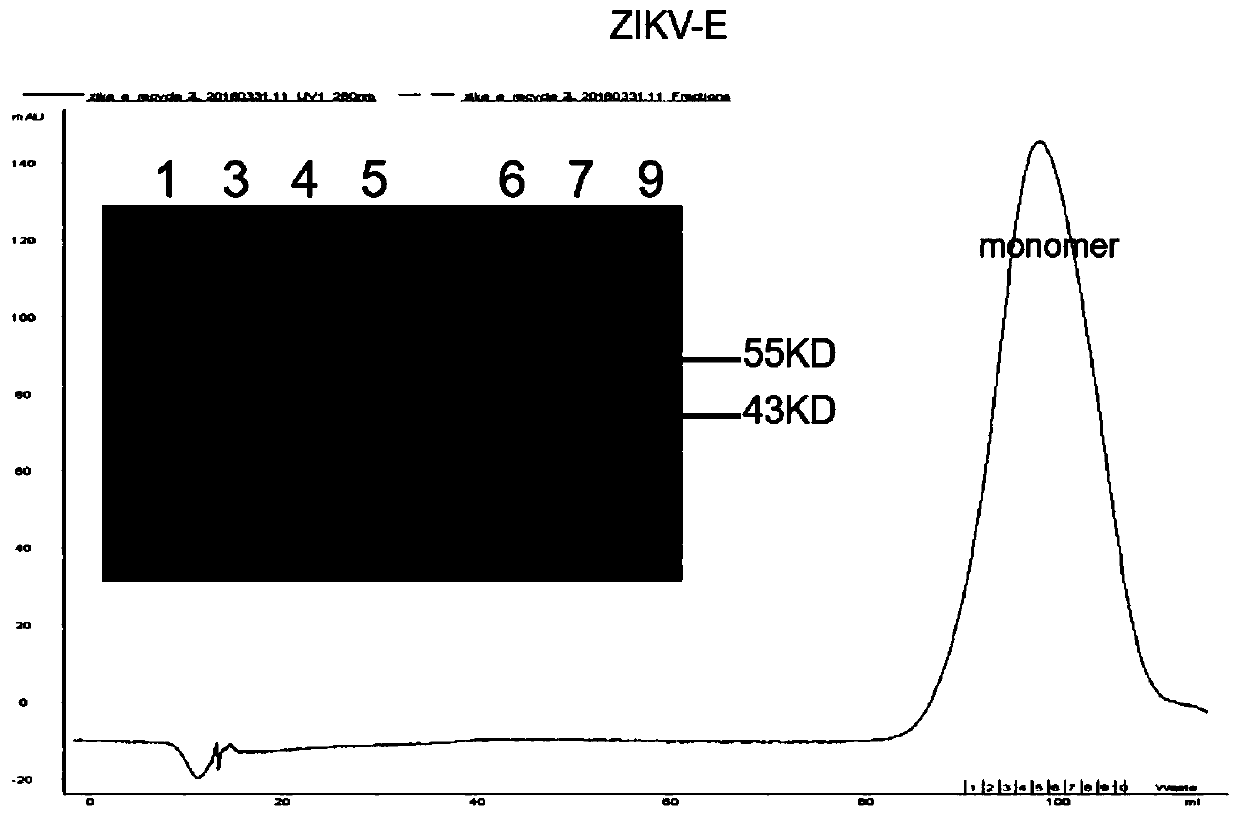
[0036] Example 1: Expression and purification of Zika E protein
[0037]The DNA fragment of the extracellular region of ZIKV E (the amino acid sequence is shown in SEQ ID NO: 21, and the nucleotide sequence is shown in SEQ ID NO: 22) was digested with NdeI and XhoI, and then connected to the pET21a vector. The 3' end of the ZIKV E protein coding region is connected with the coding sequence of 6 histidine tags (hexa-His-tag) and the translation stop codon. Then the ligation product was transformed into BL21 Escherichia coli competent cells. Single clones were inoculated into 40mL LB medium and cultured for 6-8 hours. Inoculate into 4L of LB medium, culture at 37°C until OD600=0.4-0.6, add IPTG to a final concentration of 1 mM, and continue culturing at 37°C for 4-6 hours. Inclusion bodies were harvested and refolded by dilution. The refolding solution was concentrated and replaced with 20mM Tris, 150mM NaCl, pH8.0 buffer. The concentrated protein solution was further purifi...
Embodiment 2
[0038] Example 2: Isolation of ZIKV-E protein-specific memory B cells
[0039] With the patient's informed consent, 15 mL of blood was collected and PBMCs were isolated. Separated PBMCs in 10 7 / mL density and final concentration of 100nM ZIKV-E protein was incubated on ice for half an hour, then washed twice with PBS, and then incubated with the following antibodies: anti-human CD3 / PE-Cy5, anti-human CD16 / PE- Cy5, anti-humanCD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-humanCD38 / APC, anti-humanIgG / FITC, and anti-His / PE. After the antibody was incubated on ice for half an hour, it was washed twice with PBS.
[0040] Collect PE-Cy5 by FACSAria III sorting - APCs - APC-Cy7 + Pacific Blue + FITC + PE + The cells were directly collected into a 96-well plate, 1 cell / well.
Embodiment 3
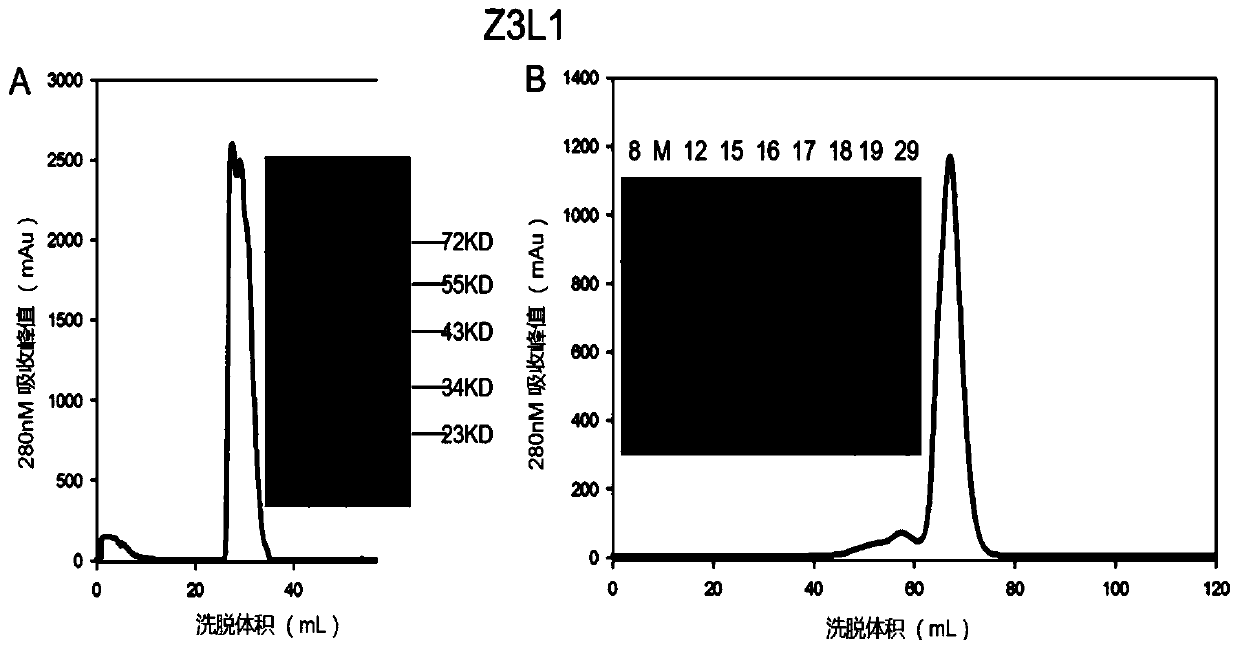
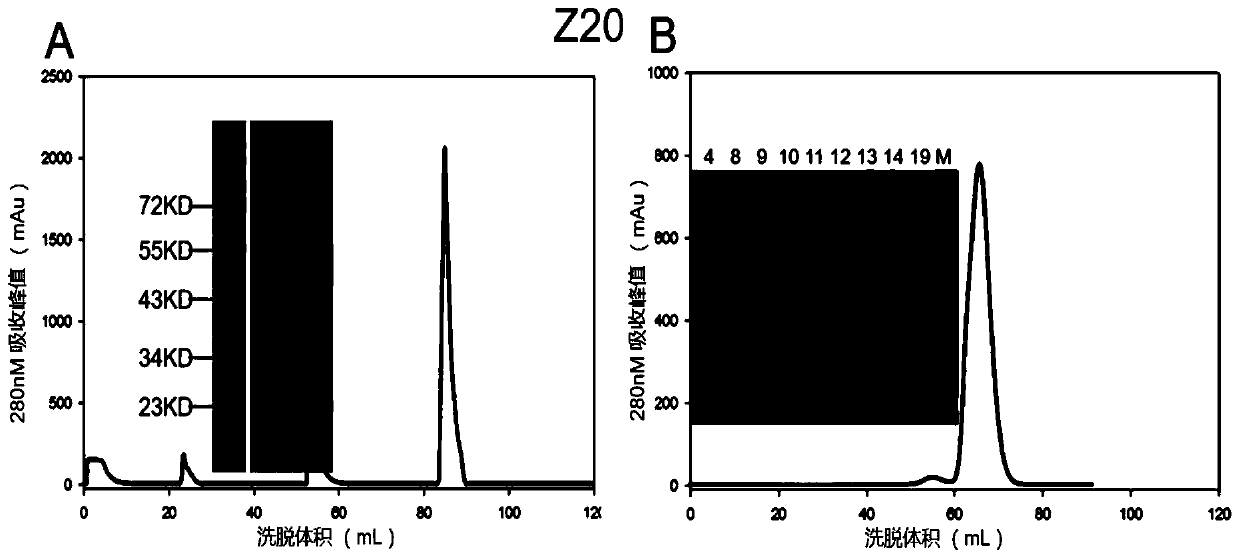
[0041] Example 3: Single B cell PCR, sequence analysis and human antibody design
[0042] The B cells obtained in Example 2 were reverse-transcribed by Superscript III reverse transcriptase (Invitrogen). The reverse transcription primers are shown in Table 1 (sequences are shown in SED ID NO.23 to SED ID NO.30), and reacted at 55° C. for 60 min.
[0043] Table 1. Primers for reverse transcription reactions
[0044]
[0045] Using this reverse transcription product as a template, PCR was performed with HotStar Tap Plus enzyme (QIAgen) to amplify the antibody variable region sequence (PCRa). The corresponding primers were designed, and the reaction conditions were as follows: 95°C, 5min; 95°C for 30s, 55°C (heavy chain / κ chain) / 50°C (λ chain) for 30s, 72°C for 90s, 35 cycles; 72°C, 7min. Use this as a template for another round of PCR (PCRb), the conditions are as follows: 95°C, 5min; 95°C for 30s, 58°C (heavy chain) / 60°C (κ chain) / 64°C (λ chain) for 30s, 72°C for 90s , 35 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com