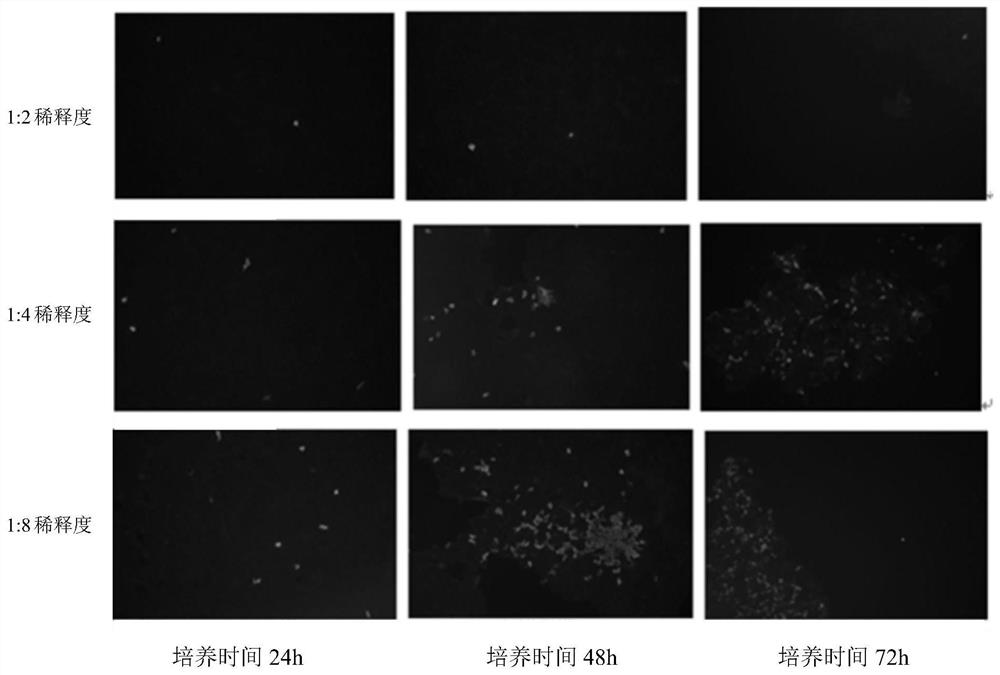
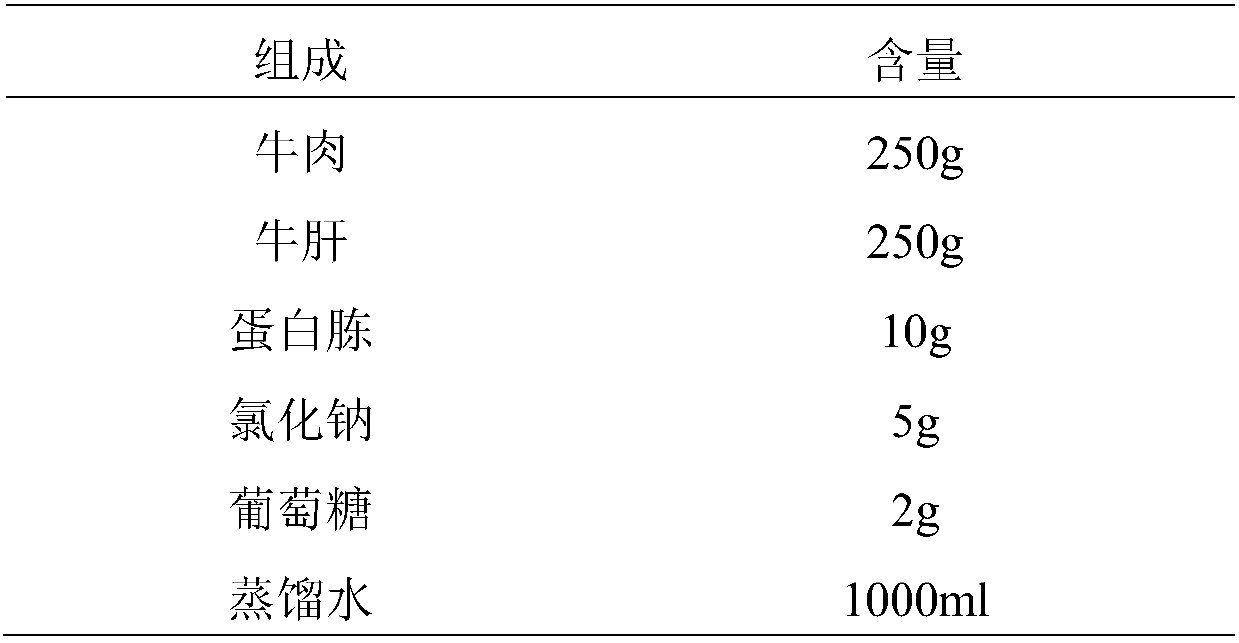
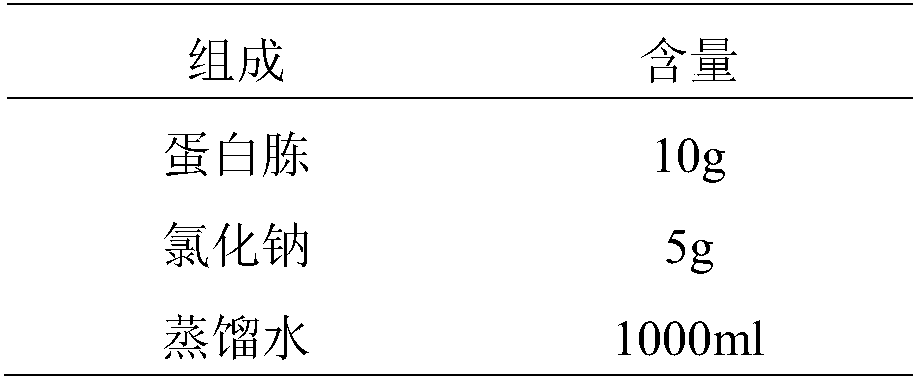
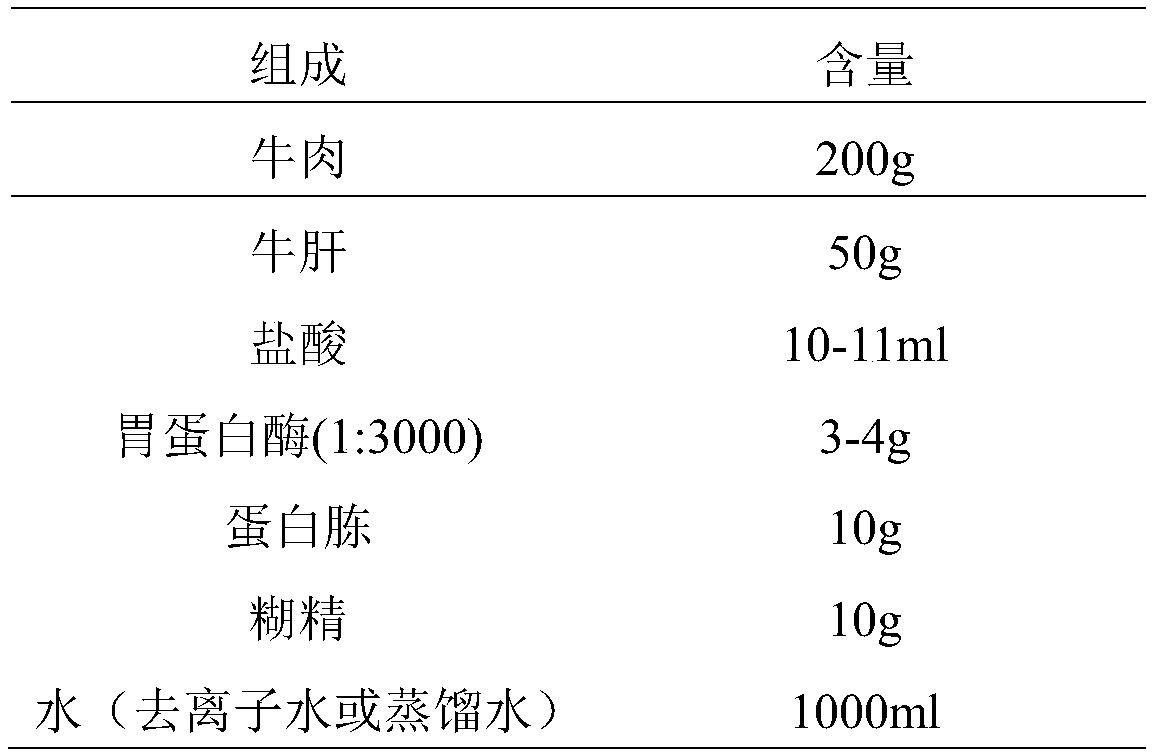
Patents
Literature
57 results about "Serum neutralization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
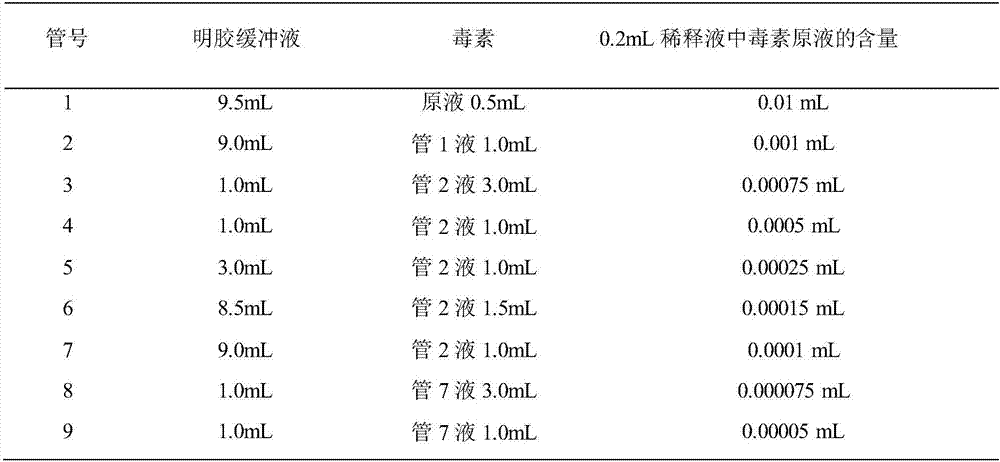
Serum neutralisation test. A test in which a patient’s serum, which may contain a neutralising antibody and a microorganism of interest (e.g., an adenovirus or enterovirus), is either placed in a cell culture or injected into a host organism so as to evaluate levels of protective antibodies present within the serum.
Immune-electrochemical sensor based on AuNPs@AgNCs nano composite material, construction and applications thereof
InactiveCN104267184AUniform sizeImprove stabilityDisease diagnosisMaterial electrochemical variablesAntigenBiotin-streptavidin complex
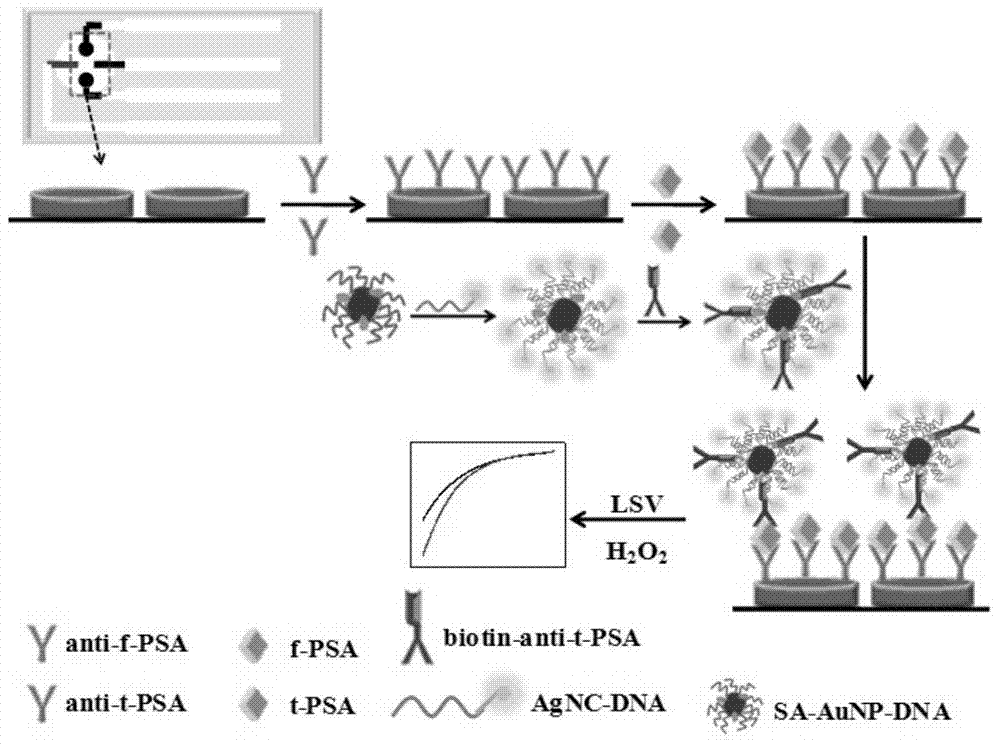
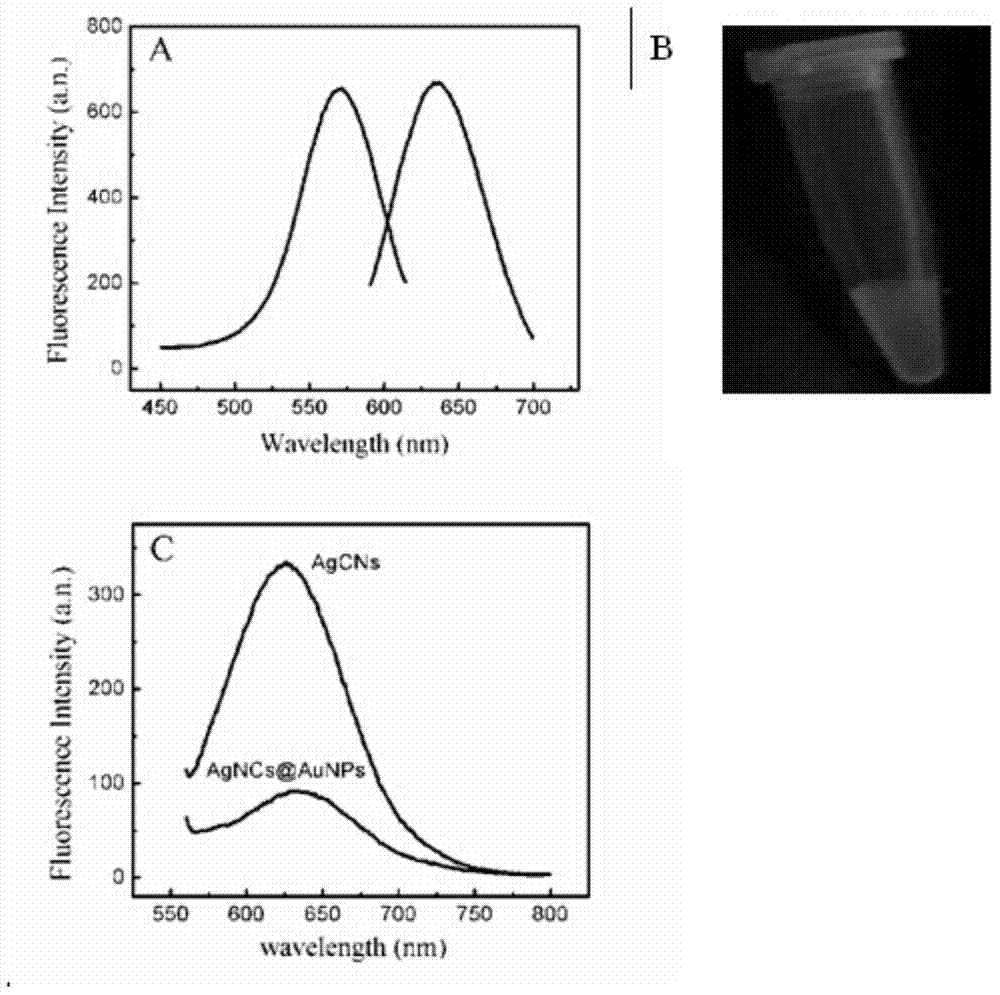
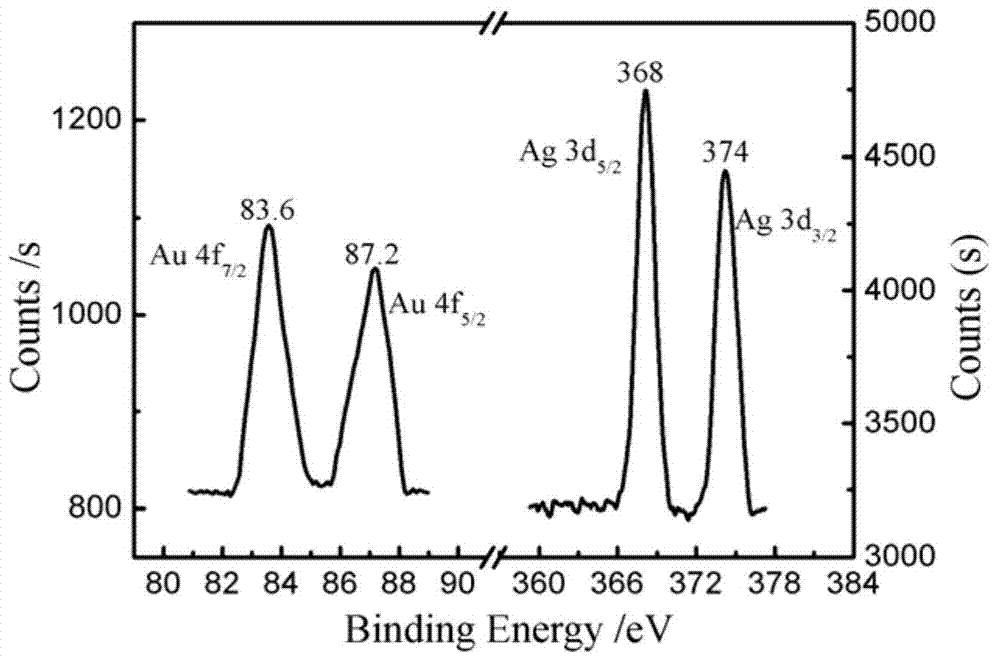
The invention discloses an immune-electrochemical sensor based on an AuNPs@AgNCs nano composite material, construction and applications thereof. Ab1 is fixed by graphene, PSA is taken as the target analyte, SA-AuNPs@AgNCs labeled by streptavidin is easily combined with monoclonal antibody Ab2(biotin-Ab2) which has been modified by biotin, and thus a PSA immunity sensor can be prepared by a sandwich interlaying method through the specific reactions between an antibody and an antigen. When the immune sensor is used, silver and H2O2 are precipitated on the sensor through electric catalytic reduction reactions, the detection is rapid and sensitive, has good specificity, and is capable of detecting the concentration of PSA with an ultralow content. The concentrations of f-PSA and t-PSA in serum of prostatic hyperplasia patients and prostatic cancer patients can also be detected by the sensor, so the sensor has an important meaning for diagnosis and identification on gray areas in prostatic cancer diagnosis.
Owner:CENT SOUTH UNIV
Vaccine for hand-foot-and-mouth disease viruses
ActiveCN101897963AEnsure safetyGood immune effectMicroorganism based processesAntiviralsAdjuvantInfectious Disorder
The invention relates to preparation of a vaccine for hand-foot-and-mouth disease viruses and an application method thereof, belonging to a novel vaccine for preventing infectious diseases. The vaccine of the invention mainly comprises the components of high-purified inactivated human enteropathogenic virus 71 (EV 71) and an aluminum adjuvant. The vaccine, which is prepared according to the method of the invention, has excellent immunogenicity, and after immunity, organisms can selectively generate a high titer serum neutralization antibody, thereby preventing infectious diseases caused by the human EV 71.
Owner:BEIJING LUZHU BIOTECH +1
Recombinant varicella zoster virus vaccine
ActiveCN112870344AHigh molecular weightImproving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsChickenpoxImmunogenicity
The invention discloses a recombinant varicella zoster virus vaccine, which comprises an amino acid sequence of a recombinant glycoprotein gE extracellular region of a live attenuated VZV strain (OKA strain) gene and a fusion protein formed by a human immunoglobulin Fc segment, and further comprises preparation and an application of the fusion protein, and a corresponding recombinant gene, an eukaryotic expression vector and the like. The fusion protein provided by the invention has good immunogenicity, and can induce generation of a high-level serum neutralizing antibody.
Owner:BEIJING LUZHU BIOTECH +1
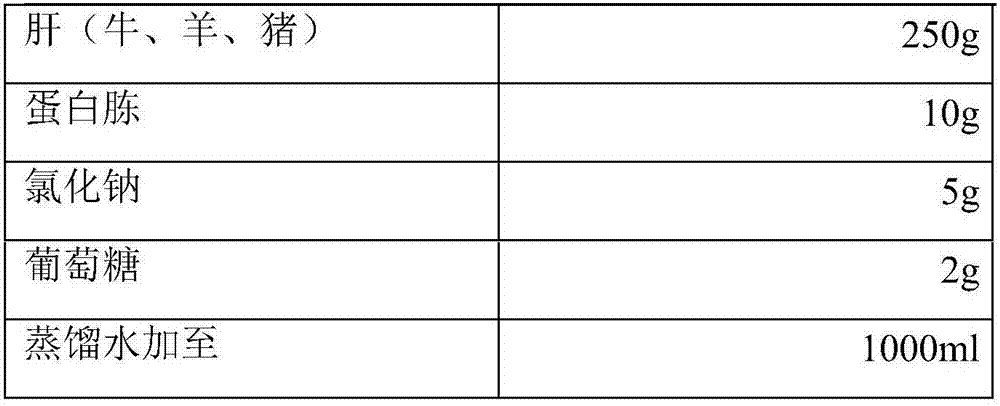
Veterinary D-type clostridium perfringen toxin, and preparation method and special culture medium of D-type clostridium perfringen toxin
ActiveCN107299070AStrong toxin production abilityToxic performance is stableAntibacterial agentsBacterial antigen ingredientsSerum neutralizationBiologic Products
The invention discloses a preparation method and a special culture medium of veterinary D-type clostridium perfringen toxin. The culture medium per 100ml comprises the following components: 1-1.5g soy peptone, 1-1.5g casein peptone, 0.5-0.75g yeast extract, 0.5-0.75g Na2HPO4.12H2O, 1-1.5g dextrin and the balance of water, wherein a pH (potential of hydrogen) value of the culture medium is 8.0-8.5. The preparation method of the D-type clostridium perfringen toxin comprises the steps of inoculating a D-type clostridium perfringen production strain into the culture medium, collecting a culture material, performing centrifugation, and then filtering a supernate. With the adoption of the method, the maximum toxicity can be improved to be 45 times a seedling standard in Regulations on National Veterinary Biological Products, and an output-input ratio can be increased to be 30-225 times that of the original traditional technology. In addition, corresponding serum neutralization titer of a toxoid vaccine prepared by the D-type clostridium perfringen toxin on a rabbit and a sheep is also improved to be 8.3 and 13.3 times a regulation standard respectively.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Detecting kit for hepatitis a virus IgM and IgG antibodies through colloidal gold method
InactiveCN102854316ARelieve painShorten the timeMaterial analysisAgainst vector-borne diseasesAntigenAssay
The invention discloses a detecting kit for hepatitis a virus IgM and IgG antibodies through a colloidal gold method. The detecting kit comprises a kit body, sample diluting liquid and detecting test paper. The detecting test paper is composed of a plastic supporting plate, a sample feeding pad, a colloidal gold pad of antigen VP1 marked with hepatitis a virus specifity, a nitrocellulose NC film, a detecting line T1, a detecting line T2, a quality control C line and a sample absorbing pad and arranged in the kit body. The kit detects that IgM and IgG in serum of the sample are positive simultaneously, the sample result is completely identical with an enzyme-linked immunosorbent assay (ELISA) reagent, no remarkable difference exists, and the kit is applicable to clinical detecting. The kit is low in cost and simple in preparation process and has practical value.
Owner:上海博沃生物科技有限公司
Preparation method of oral inactivated porcine epidemic diarrhea virus microcapsule
PendingCN108114277AAvoid infectionAvoid degradationSsRNA viruses positive-senseViral antigen ingredientsSerum igeVaccine antigen
The invention provides a preparation method of an oral inactivated porcine epidemic diarrhea virus microcapsule and relates to the field of preparation of microcapsules. The invention aims at overcoming the technical defects in an oral inactivated vaccine in domestic porcine epidemic diarrhea virus prevention and control at the present stage and provides a preparation method for the oral inactivated porcine epidemic diarrhea virus microcapsule based on inactive porcine epidemic diarrhea virus and oral microcapsule preparation technologies. Animal experimental results find that a serum neutralizing antibody titer detection result shows that the microcapsule vaccine can induce piglet mucosal immunization and systemic immune response, and serum and mucosa antibodies with relatively high levelare produced, so that the microcapsule vaccine is objectively explained to have a certain slow-release effect. After a microcapsule vaccine antigen substance is orally taken by piglets, expression ofspecific antibodies IgG and IgA of the piglets can be induced, resistance of the piglets on PEDV is improved, death rate of the piglets is reduced, and toxicity attack protection rate is 60-75% higher than that of a control group.
Owner:姜新鹏
Scutellaria barbata polysaccharide antitumor mechanism detection research method
InactiveCN105560266APromote activationPromote proliferationOrganic active ingredientsAntineoplastic agentsCell Surface ProteinsTumor therapy
The present invention discloses a scutellaria barbata polysaccharide antitumor mechanism detection research method which comprises the following detecting steps: an in-vivo tumor suppression experiment; ANAE method determination of the total number of T cells in peripheral blood of tumor-bearing mice; flow cytometry determination of T cell subsets and T cell surface protein-CD28 in the peripheral blood of the tumor-bearing mice; and determination of content of IL-2 and IFN-gamma in serum of the peripheral blood of the tumor-bearing mice; the scutellaria barbata polysaccharide in-vivo antitumor and cell immune function influence detection research method discloses a major role of scutellaria barbata polysaccharide in tumor treatment, and meanwhile the study on body cell immune mechanism of plant polysaccharides can be promoted.
Owner:HUAIAN COLLEGE OF INFORMATION TECH
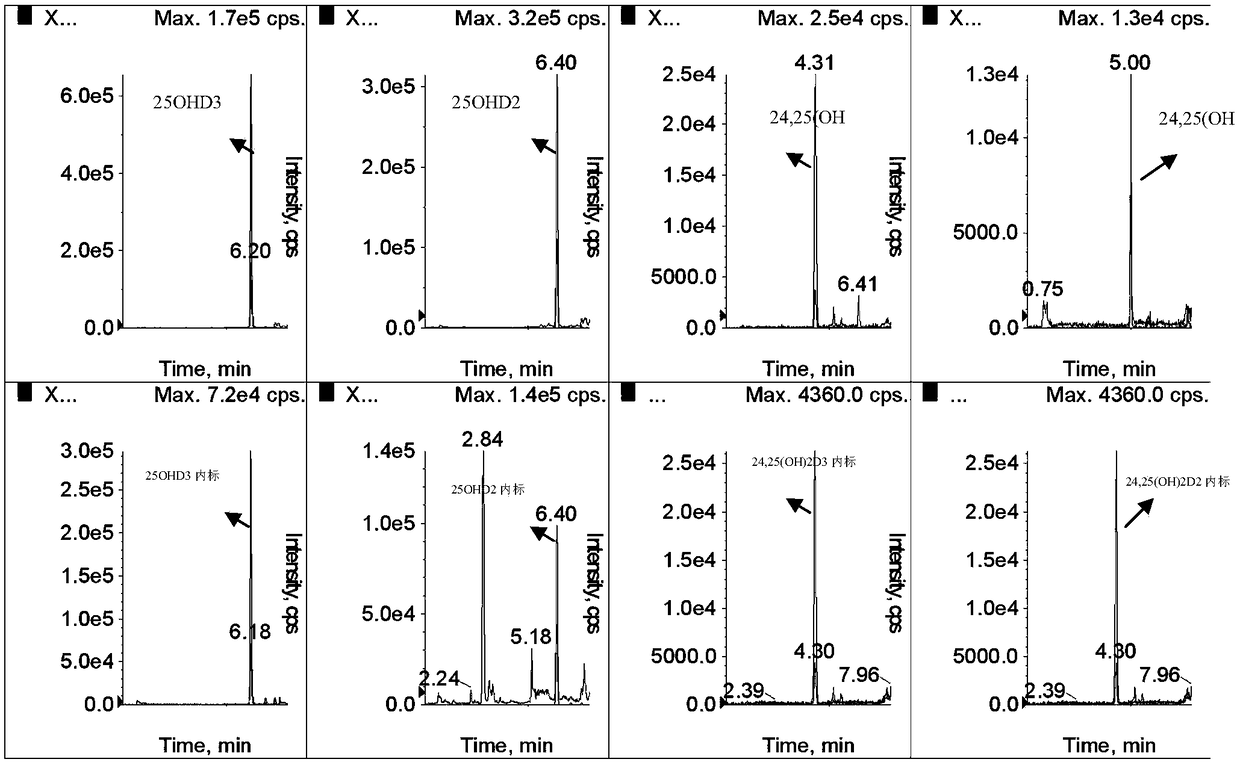
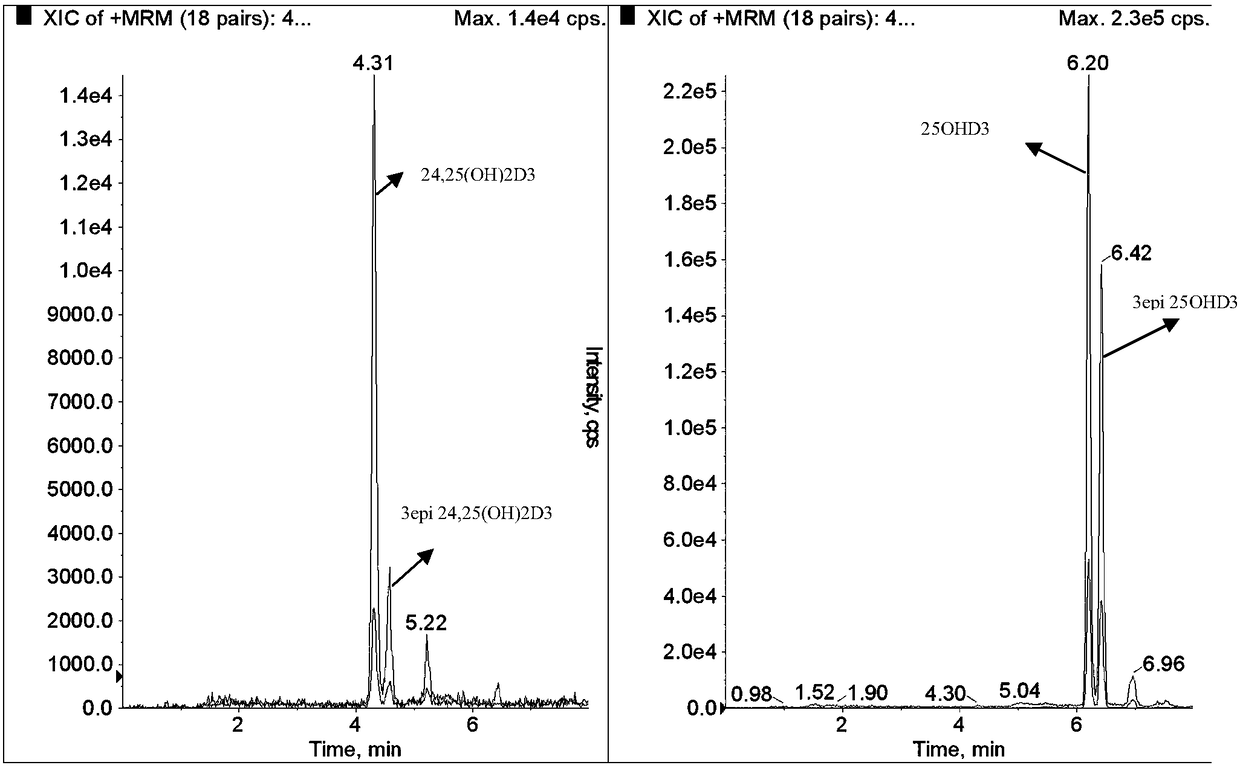
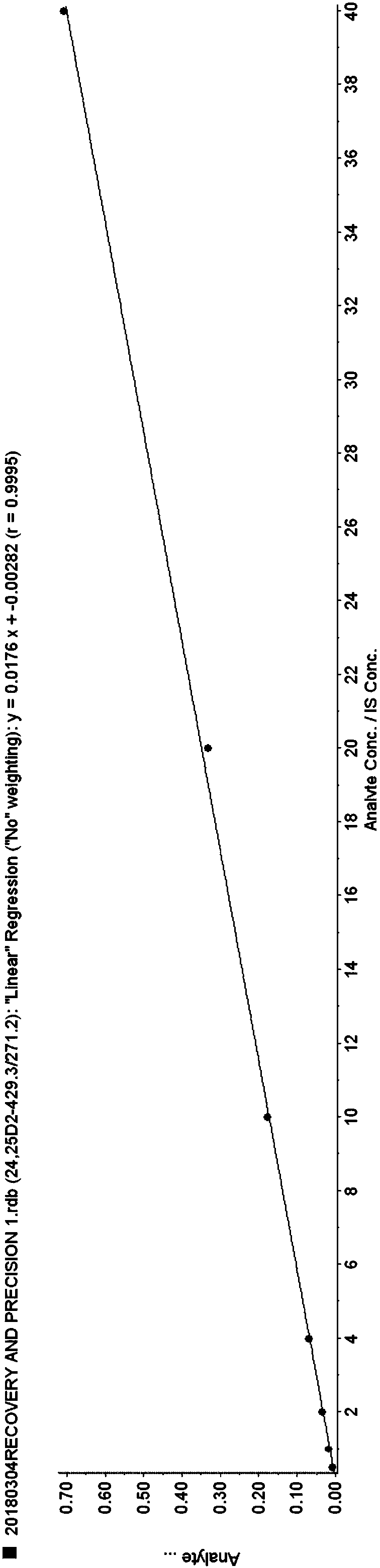
Method for simultaneously detecting serum 24,25(OH)2D and 25OHD
ActiveCN108593790AAccurate measurementEasy to handleComponent separationTandem mass spectrometryChemistry
The invention provides a method for simultaneously detecting serum 24,25(OH)2D and 25OHD by liquid chromatography tandem mass spectrometry. The method comprises the following steps: 1) preparing a serum sample; 2) separating the sample through liquid chromatogram, and removing an interference substance; 3) obtaining a detection signal through mass spectrometry; 4) drafting a standard curve; and 5)detecting the signal intensity of 24,25(OH)2D3, 24,25(OH)2D2, 25OHD2, and 25OHD3 in the to-be-measured sample, substituting the signal intensity in the above standard curve for calculation, and realizing the quantitative determination of 24,25(OH)2D and 25OHD in serum. The rapid detection method for simultaneously detecting 24,25(OH)2D and 25OHD by using less sample (200 [mu]l of serum) based onliquid chromatography tandem mass spectrometry, and can distinguish the interference of 3-epi 24,25(OH)2D3and 3-epi 25(OH)D3.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
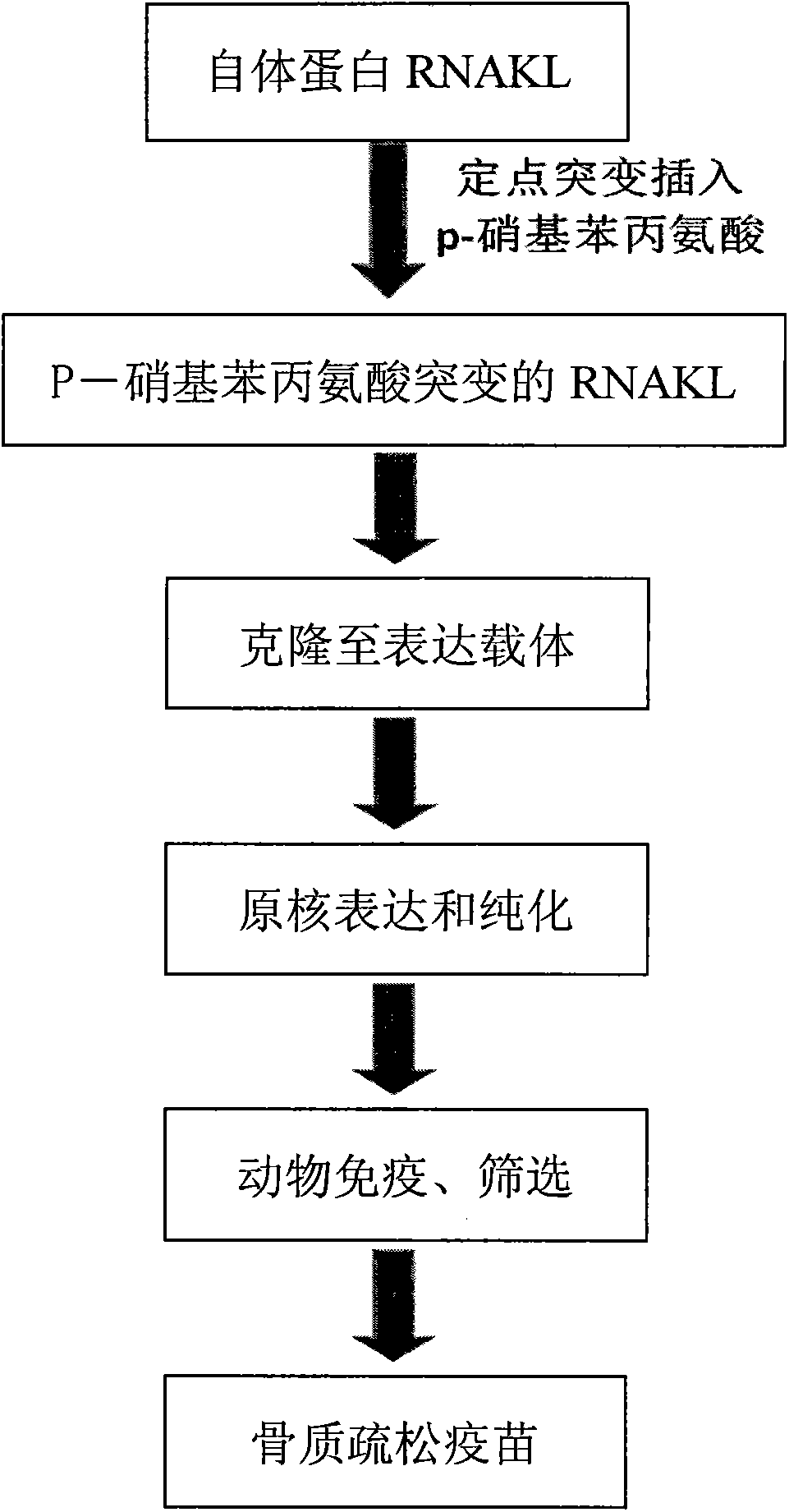
Osteoporosis vaccine built based on P-nitrobenzene alanine insertion method
InactiveCN103961694AAchieve healingOvercoming immune toleranceSkeletal disorderAntibody medical ingredientsHuman bodyImmune tolerance
The invention discloses an osteoporosis vaccine built based on a P-nitrobenzene alanine insertion method. The osteoporosis vaccine is characterized by being prepared through the following steps: 1), the construction of RANKL recombinant expression vector; 2), the fixed point insertion / replacement of the coded sequence of an unnatural amino acid, namely p-nitrobenzene alanine; 3), the recombination expression and purification of p-nitrobenzene alanine-RANKL albumen; 4), the animal immunization and screening; 5), the screening based on serum neutralization detection and osteoclast formation analysis; 6), the animal experiment, and the determination of required vaccine. According to the invention, the method can effectively break through immune tolerance of the human body to autologous albumen, and induces the human body to generate special neutralizing antibody for RANKL through an active immune means, so as to achieve the purpose of preventing osteoporosis well; besides, the problem of high cost for industrially producing antibodies is solved or avoided.
Owner:陶惠人 +1
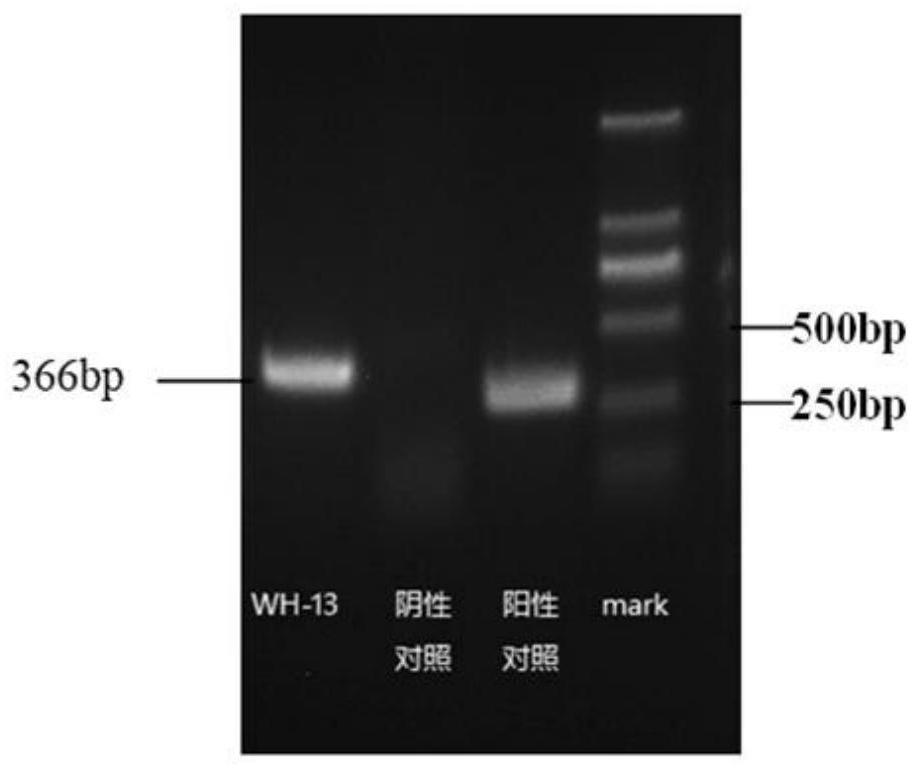
Porcine pseudorabies virus strain and application thereof
ActiveCN111748529AImprove purification effectImprove securityViral antigen ingredientsAntiviralsPig farmsRabies
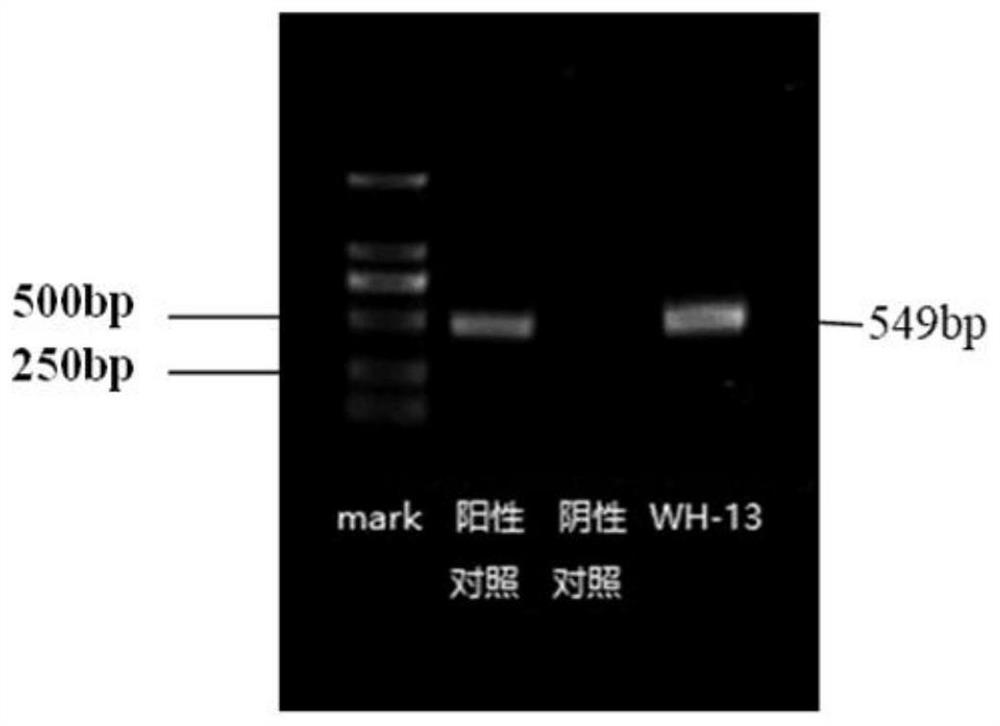
The invention discloses a porcine pseudorabies virus strain and an application thereof. The porcine pseudorabies virus WH-13 strain provided by the invention is separated in Wuhan pig farm in Hubei Province in 2013, and the identification shows that the strain is a prevalent strain with stronger virulence. The inactivated vaccine is prepared from the porcine pseudorabies virus WH-13 strain, piglets are immunized once (2ml), the serum neutralizing antibody level is not lower than 1: 128, and a protection period is as long as 6 months. The TK, gE and gI genes are deleted from the strain by adopting a genetic engineering method; the minimum immunization dose of the prepared porcine pseudorabies live vaccine piglet is 103.0 TCID50, the serum neutralizing antibody level of the piglet after immunization once is not lower than 1: 145, the immune protection rate of a classic strain and an epidemic strain is 100%, and the protection period is as long as 6 months. The preservation number of theporcine pseudorabies virus WH-13 strain is CCTCC NO: V202032.
Owner:WUHAN CHOPPER BIOLOGY
SARS-COV-2 Antigen Polypeptide, Recombinant Adeno-Associated Virus Expressing the Polypeptide, and Vaccine Containing the Virus
PendingUS20210346493A1Strong specificityImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseVaccine antigenCultured cell
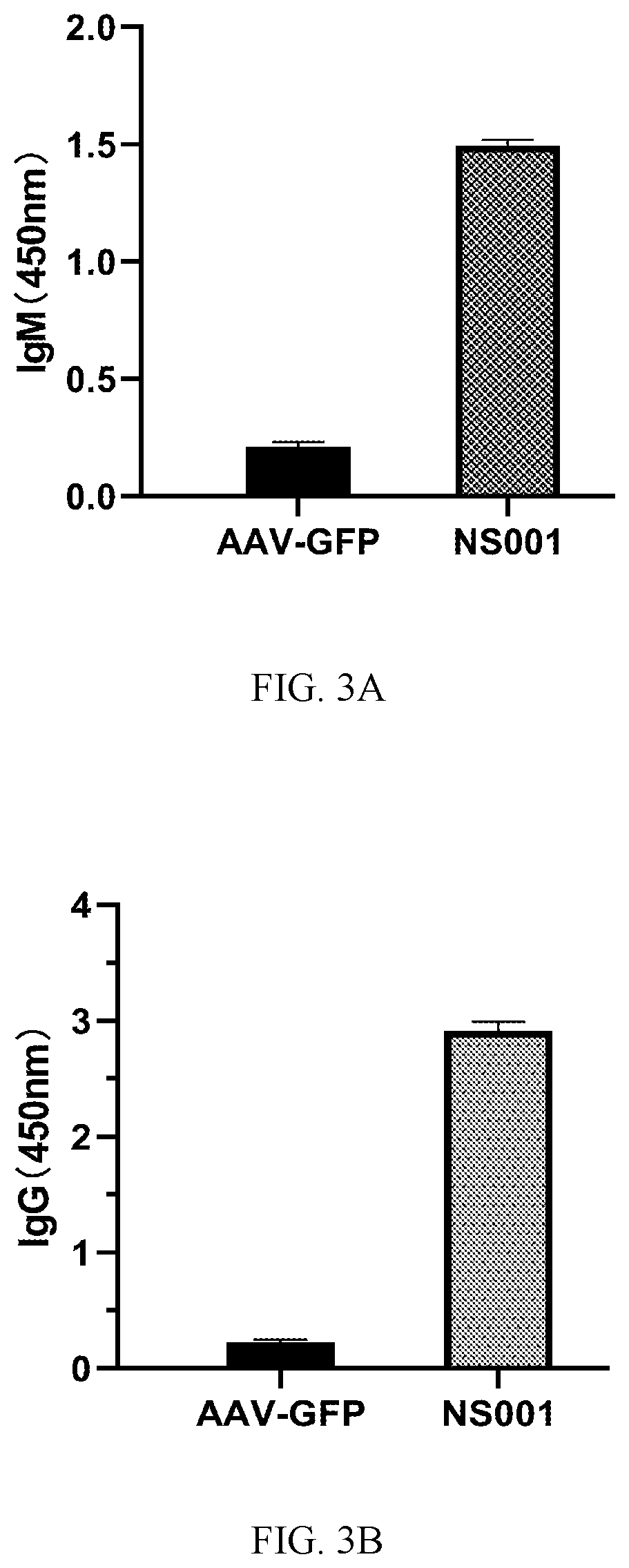
Disclosed are a SARS-COV-2 antigen polypeptide, a recombinant adeno-associated virus (rAAV) expressing the polypeptide, and a vaccine containing the virus. A sequence of the antigen polypeptide is shown in SEQ NO. 1. and SEQ ID NO. 2. A method for preparing the recombinant adeno-associated virus comprises co-incubating pHelper, pRep2Cap5, and an expression vector, transfecting a cell in the presence of polyethyleneimine as a transfection reagent; culturing the cell, then collecting the cell by centrifugation, performing lysis and purification to obtain a purified liquid comprising the recombinant adeno-associated virus. The rAAV is delivered and expressed in vivo to produce a fusion antigen polypeptide, induces the production of serum neutralizing antibodies, which have a neutralizing titer to the novel SARS-COV-2 coronavirus and are expressed continuously; the rAAV composition can be used to immunize humans against the novel coronavirus pneumonia COVID-19.
Owner:HENGDA BIOMEDICAL TECH CO LTD
Kit for detecting liver cancer, hepatitis and/or liver cirrhosis and application of kit in AKR1B10 and AFP combined quantitative determination
PendingCN111239410AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiseaseSerum neutralization
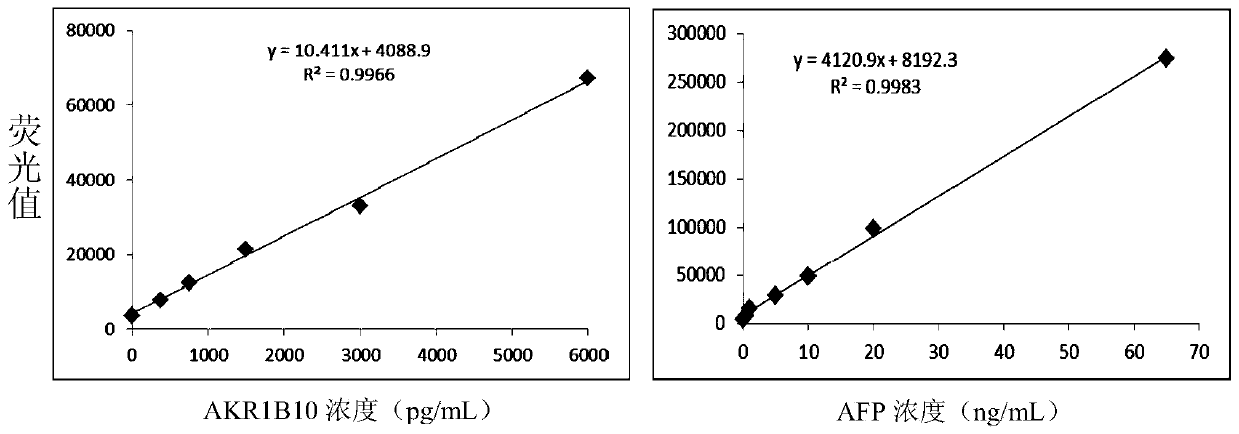
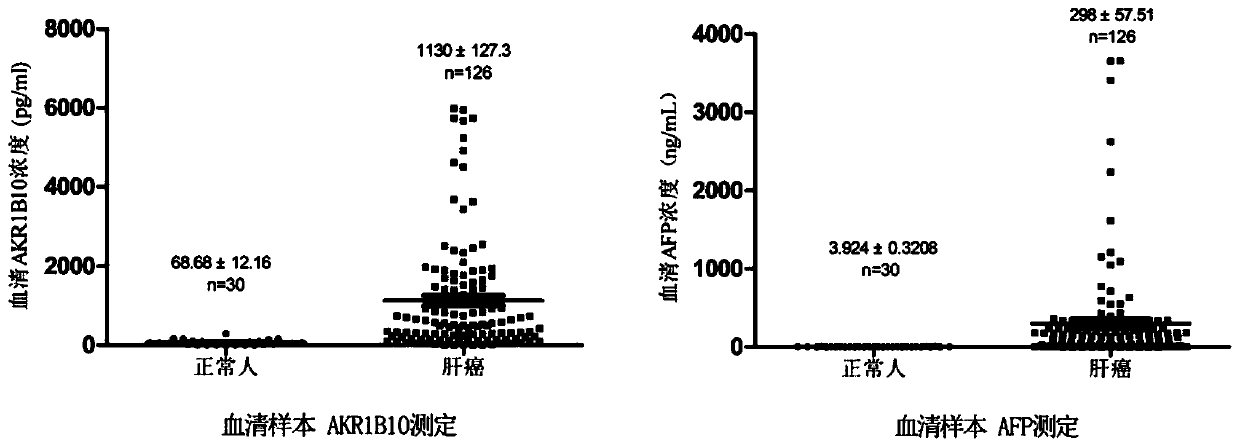
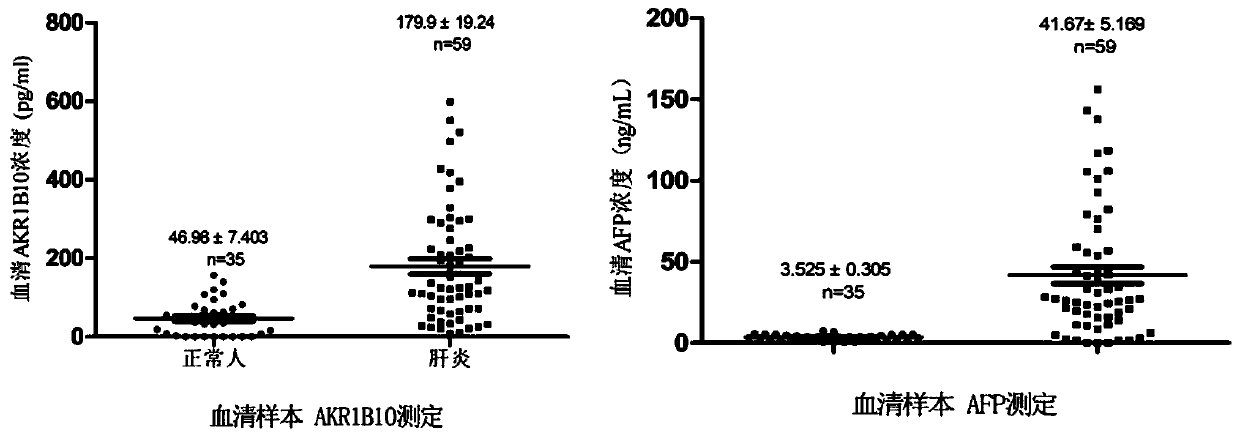
The invention belongs to the technical field of medical biology, and discloses a determination kit for simultaneously detecting contents of aldehyde ketoreductase 1B10 and alpha fetoprotein by using atime-resolved fluorescence immunoassay method, which can be used for screening, diagnosing, curative effect judging, prognosis evaluating or recurrence monitoring of diseases such as liver cancer, hepatitis, liver cirrhosis and the like. The kit comprises an elisa plate, a calibrator, a europium-labeled AKR1B10 detection antibody, a terbium-labeled AFP detection antibody and an enhancement solution. The AKR1B10 and the AFP in the serum to be detected are respectively combined with the AKR1B10 and the AFP coated antibody of the elisa plate, then two detection antibodies are added to form a double-antibody sandwich compound, an enhancement solution is added to dissociate europium ions and terbium ions on the compound, and high-intensity fluorescence is respectively emitted at wavelengths of340nm and 295nm. The fluorescence intensity is in direct proportion to the concentrations of AKR1B10 and AFP in the sample. By adopting the kit, the contents of the two markers can be detected at thesame time, and the detection sensitivity and specificity are greatly improved.
Owner:湖南莱拓福生物科技有限公司
Coxsackievirus A16-type strain and application thereof
ActiveCN113564132AGood intersectionImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsStructural proteinImmunogenicity
The invention relates to the technical field of biology, and particularly discloses a coxsackievirus A16-type strain and application thereof. The amino acid sequence of the P1 structural protein of the coxsackievirus A16-type strain is shown as SEQ ID NO.1. The strain is good in intra-type and inter-type cross property and stable in heredity, can serve as a strain for detecting the titer of a neutralizing antibody in coxsackievirus A16-type serum, and provides support for research and development of coxsackievirus A16-type related univalent / multivalent vaccines. The strain is good in immunogenicity, high in titer and high in toxicity, provides an attack strain for establishing a stable infected animal model, and is of great importance for establishing a CV-A16 mouse model.
Owner:BEIJING MINHAI BIOTECH
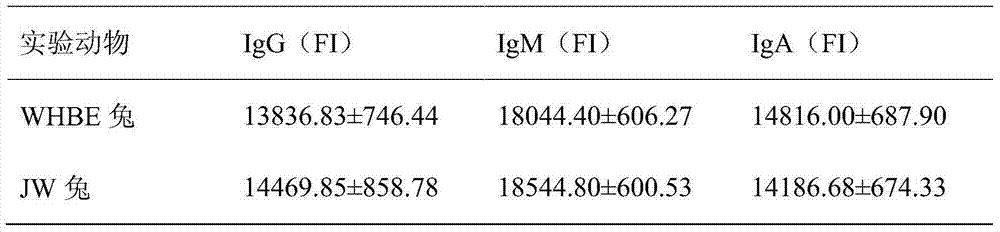
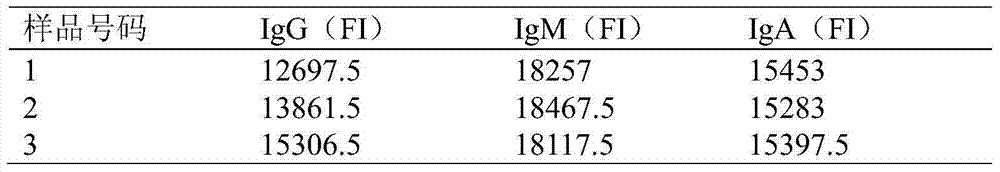
Rabbit serum immunoglobulin liquid-phase suspension chips and preparation and application thereof
The invention relates to the technical field of biology, and particularly relates to rabbit serum immunoglobulin IgM, IgG and IgA liquid-phase suspension chips and a preparation and application thereof. The rabbit serum immunoglobulin liquid-phase suspension chips comprise 42#, 48# and 57# microspheres which are respectively coupled to IgG, IgM and IgA capture antibodies. In addition, the rabbit IgM, IgG and IgA liquid-phase chips are prepared by the method, the IgM, IgG and IgA contents in WHBE (White Hair and Black Eyes) rabbit and JW rabbit are synchronous detected, and a method for detecting the rabbit serum immunoglobulin IgM, IgG and IgA liquid-phase suspension chips is built. The method for detecting the rabbit serum immunoglobulin IgM, IgG and IgA liquid-phase suspension chips can be applied to research and development of immunology and a biological diagnostic kit, and experimental materials and new resources are provided for development of the bioengineering and pharmaceutical industry, especially research and development and production of biological diagnostic reagents.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
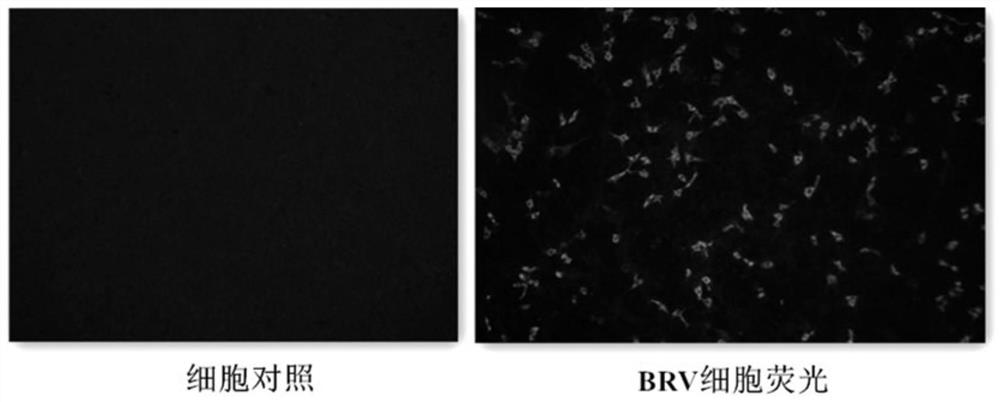
BRV serum neutralizing antibody titer level detection method based on IFA
PendingCN113552336AEasy to distinguishIncrease the proportionFluorescence/phosphorescenceSerum samplesVaccine Production
The invention discloses a BRV serum neutralizing antibody titer level detection method based on IFA, and belongs to the technical field of biology. According to the method provided by the invention, two DMEM maintenance solutions containing different concentrations of pancreatin are adopted to carry out two-stage two-fold dilution on a clinical serum sample on the basis of a method for detecting a neutralizing antibody by IFA to prepare a to-be-detected serum sample for co-incubation with a BRV neutralizing virus; and the influence on the growth of the BRV in the cell due to the neutralization of the pancreatin by the clinical serum sample can be effectively avoided so as to accurately detect the BRV serum neutralizing antibody titer level, and the method further has characteristics of rapidness, and can be effectively used for guiding the BRV vaccine production and the quality monitoring.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Clostridium perfringens type B exotoxin, preparation method thereof, toxigenic culture medium and application of clostridium perfringens type B exotoxin
ActiveCN109554420AToxic performance is stableStabilize Toxic EffectsAntibacterial agentsBacterial antigen ingredientsVaccine ProductionBacillus perfringens
The invention discloses a clostridium perfringens type B exotoxin, a preparation method thereof, a toxigenic culture medium and an application thereof. Each 1000 ml of the toxigenic culture medium isprepared from the following raw materials by weight: 15-25 g of peptone, 3-5 g of yeast extract, 3-4 g of NaCl, 5-10 g of Na2HPO4.12H2O, 5-10 g of dextrin or 5-10 g of coarse dextrin, and the balanceof water for injection. The virulence of the clostridium perfringens toxin liquid produced by the method according to the present invention is up to 1 mouse MLD of no more than 0.0006 mL, and the virulence is 3.3 times than the vaccine production standard. Moreover, a triple inactivated vaccine against bradsot, struck (or lamb dysentery) and enterotoxemia prepared by the clostridium perfringens type B exotoxin has serum neutralizing titer in rabbits and sheep equal to or higher than the relevant standard.
Owner:JINYUBAOLING BIO PHARM CO LTD
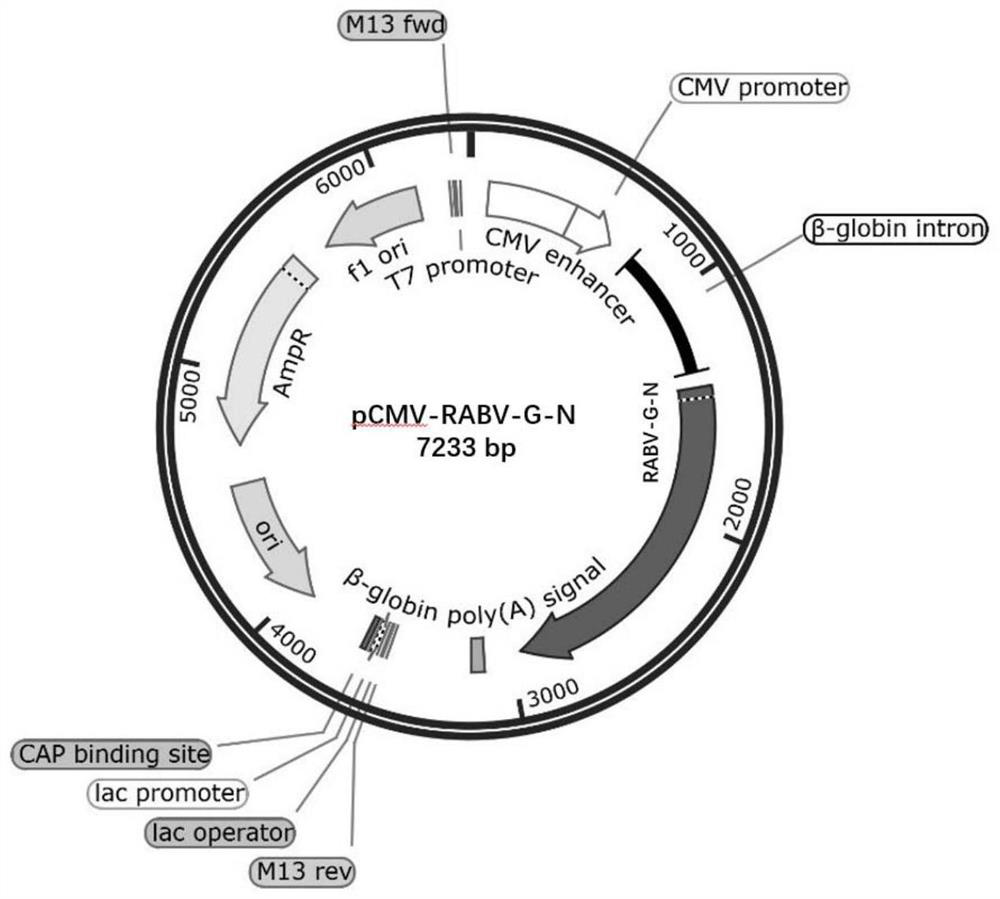
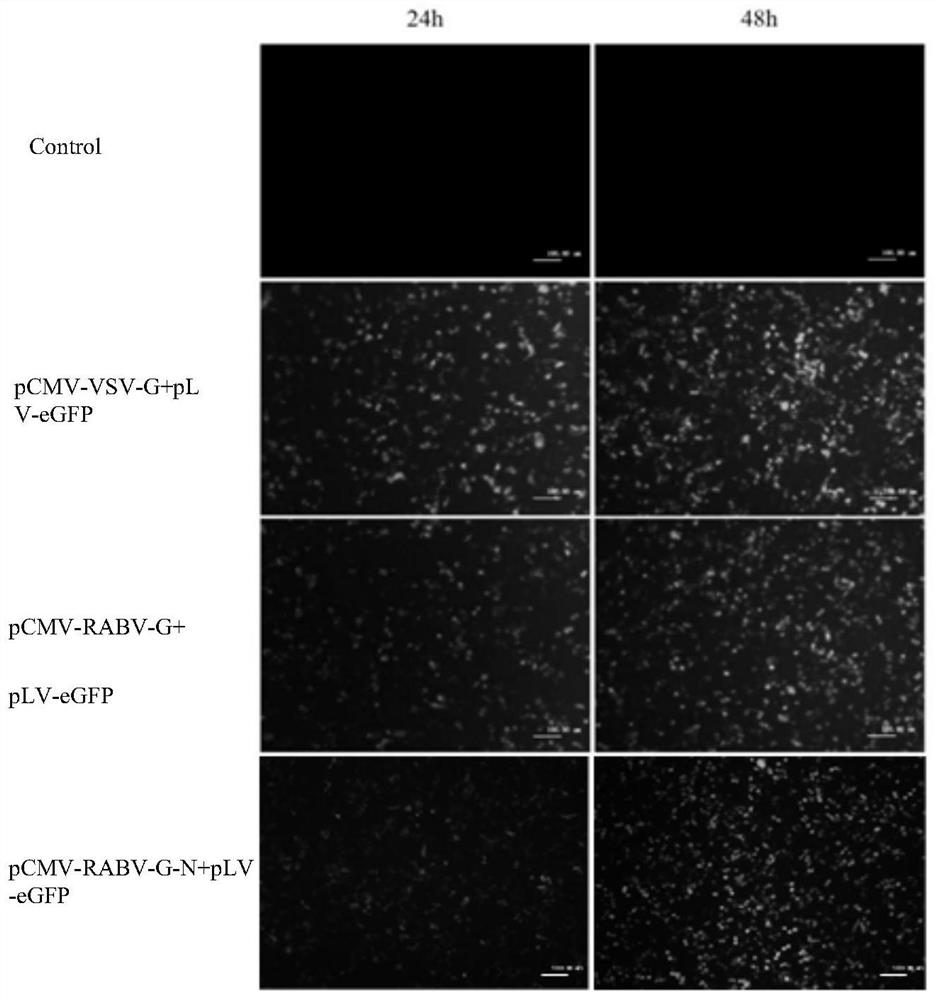
Recombinant gene of rabies virus, recombinant pseudovirus as well as construction method and application of recombinant pseudovirus
ActiveCN113174392AImproving immunogenicityHigh pack titersSsRNA viruses negative-senseViral antigen ingredientsNucleotideEmbryo
The invention relates to the technical field of gene engineering, in particular to a recombinant gene of a rabies virus, a recombinant pseudovirus and a construction method and application of the recombinant pseudovirus. The nucleotide sequence of the recombinant gene is as shown in SEQ ID NO: 1. The preparation method comprises the following steps: replacing VSV-G in a lentivirus envelope plasmid with a recombinant gene RABV-G-N to construct a recombinant envelope plasmid pCMV-RABV-G-N, and then co-transfecting a human embryonic kidney cell HEK-293T with the recombinant envelope plasmid, pLV-eGFP containing a green fluorescent protein reporter gene and psPAX2 to obtain the recombinant pseudovirus. The recombinant gene provided by the invention has biological characteristics of natural rabies virus, and can improve pseudovirus immunogenicity, and the prepared recombinant pseudovirus has high packaging titer, and can replace natural rabies virus to evaluate serum neutralizing antibody titer.
Owner:SOUTH CHINA INSTITUDE OF BIOMEDICINE
Vaccine strain rSN-R92G-E93K, construction method and applications thereof
The invention provides a vaccine strain rSN-R92G-E93K, and belongs to the technical field of influenza vaccine preparation. The construction method comprises: preparing an expression vector pHW-SN-HAfrom a HA gene fragment; carrying out point mutation on the expression vector; co-transforming cells by using the obtained expression plasmid SNHA-R92G-E93K and plasmids containing other genes; and carrying out recombination. According to the present invention, the vaccine strain rSN-R92G-E93K has the HI titer of 11.6+ / -0.5 log2 and the serum neutralizing titer of 296.50+ / -103.95 log2, such that the vaccine strain rSN-R92G-E93K is an ideal vaccine strain.
Owner:YANGZHOU UNIV +1
Bovine viral diarrhea virus low virulent strain and application thereof
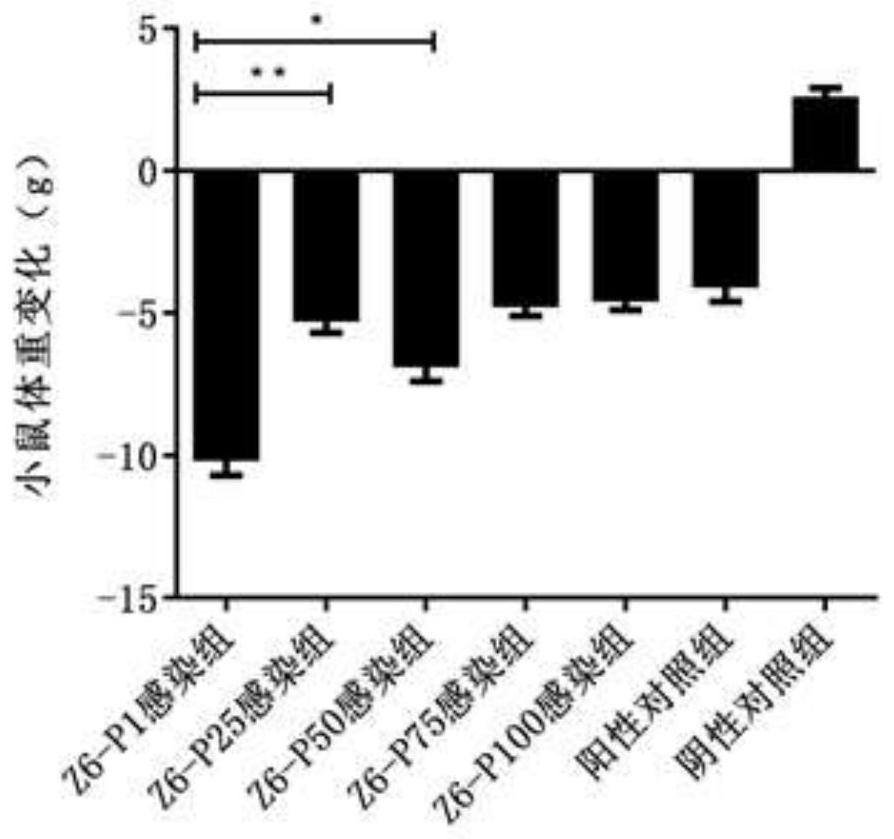
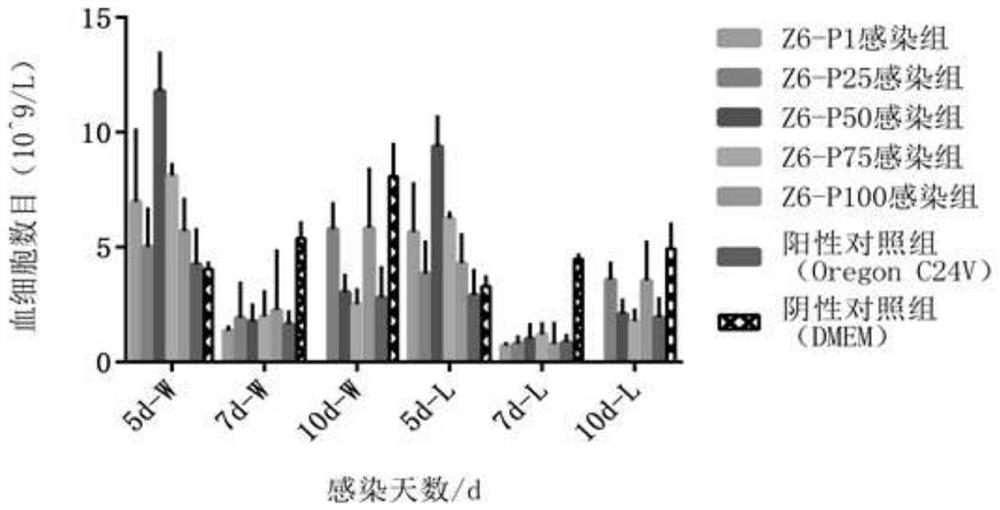
ActiveCN113174374AHigh proliferation rateHigh serum IgG levelsSsRNA viruses positive-senseViral antigen ingredientsBovine Viral Diarrhea VirusesNucleotide
The invention discloses a bovine viral diarrhea virus low virulent strain and application thereof.The low virulent strain is obtained after 100 generations of continuous passage culture of an SMU-Z6 / 1a / SC / 2016 strain, the nucleotide sequence of the low virulent strain is shown as SEQ ID NO.1, and the microbial preservation number of the low virulent strain is V202131. After BABL / c mice are immunized with the bovine viral diarrhea virus attenuated strain, high serum neutralizing antibody, serum IgG antibody, lung and intestinal sIgA antibody and splenic lymphocyte proliferation rate can be generated, and a good immune effect is achieved. The bovine viral diarrhea virus attenuated strain has a very good application prospect in prevention, treatment and diagnosis of the bovine viral diarrhea virus.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Adaptive culture method of rotavirus P(2) G3 strain and P(8) G1 strain on KMB17 cells and immunogenicity
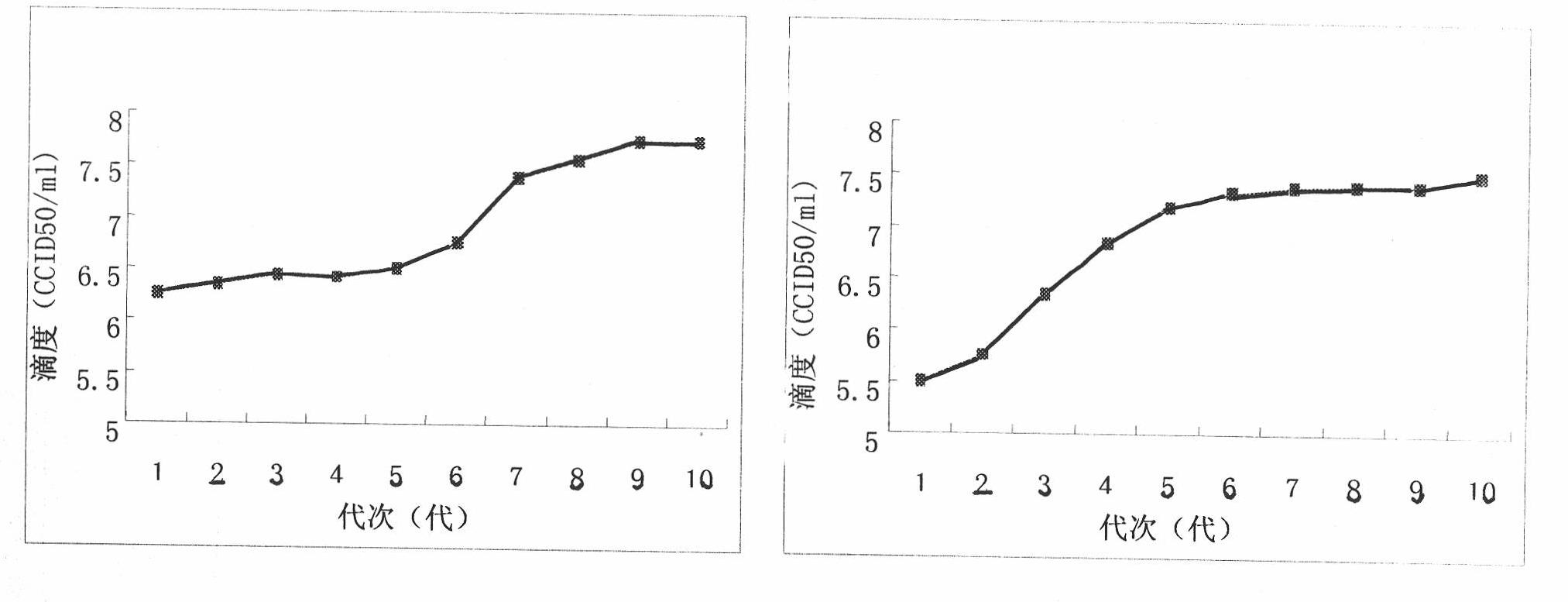
ActiveCN101899422ACopy helpsSuitable growth characteristicsMicrobiological testing/measurementInactivation/attenuationRotavirus RNABiological property
The invention relates to an adaptive culture method of a rotavirus P(2) G3 strain and a P(8) G1 strain on KMB17 cells and immunogenicity, belonging to the field of virus culture methods. 10 generations of adaptive culture of the two strains of rotavirus are continuously carried out on the KMB cells, thereby gradually increasing the cytopathy along with the virus generation, gradually increasing the infective titers and stabilizing the infective titers at the higher level. The virus titers respectively achieve 7.75CCID50 / ml and 7.33CCID50 / ml. The virus is applicable to the KMB17 cells and has a stable genome, and the neutralizing antibody titers in serum of mice immunized with the copied virus are 1:16384 and 1:8192 respectively. The rotavirus P(2) G3 strain and the P(8) G1 strain formed by the adaptive culture can be stabilized copied in the KMB17 cells, and the produced virus can keep the basic biological property of parent virus strains and have better immunogenicity.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Coxsackie virus a16 strain and its application
ActiveCN113564132BGood intersectionImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsStructural proteinImmunogenicity
The invention relates to the field of biotechnology, and specifically discloses the A16 strain of Coxsackie virus and its application. The amino acid sequence of the P1 structural protein of the Coxsackievirus A16 strain of the present invention is shown in SEQ ID NO.1. The strain has good intra-type and inter-type crossover and stable genetics. It can be used as a strain for testing the titer of serum neutralizing antibodies against Coxsackie virus A16 and provides support for the development of mono- / multivalent vaccines related to Coxsackie virus A16. . The strain has good immunogenicity, high titer, and strong virulence. It provides a challenge strain for the establishment of a stable infection animal model, which is very important for the construction of the CV‑A16 mouse model.
Owner:BEIJING MINHAI BIOTECH
Preparation method of rabbit anti-canine parvovirus 2a type polyclonal antibodies
InactiveCN102532308ASerum immunoglobulinsImmunoglobulins against virusesHemagglutininPolyethylene glycol
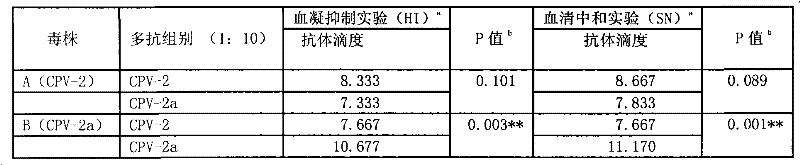
The invention discloses a preparation method of rabbit anti-canine parvovirus 2a type polyclonal antibodies. The method comprises the following steps of: A1) performing virus amplification; A2) concentrating antigens; A3) measuring the content of antigens; A4) adopting the immunization method; and A5) purifying serum; purifying the canine parvovirus (CPV)-2a virus strain, amplifying, concentrating the obtained virus liquid with polyethylene glycol (PEG)6000, emulsifying with Freud's adjuvant, and performing subcutaneous immunizations at ten different points, wherein basic immunization and booster immunization are totally performed four times. The prepared polyclonal antibodies have high titer, the hemagglutinin inhibition (HI) value is no less than 1:10240, and the serum neutralization (SN) value is no less than 1:51200; and compared with the CPV-2 polyclonal antibodies, the neutralizing CPV-2a antibodies have a stronger effect.
Owner:SICHUAN AGRI UNIV
Clostridium perfringens type d toxin for veterinary use and its preparation method and special medium
ActiveCN107299070BGood repeatabilityStrong toxin production abilityAntibacterial agentsBacterial antigen ingredientsClostridium perfringens toxoidBacillus perfringens
The invention discloses a preparation method and a special culture medium of veterinary D-type clostridium perfringen toxin. The culture medium per 100ml comprises the following components: 1-1.5g soy peptone, 1-1.5g casein peptone, 0.5-0.75g yeast extract, 0.5-0.75g Na2HPO4.12H2O, 1-1.5g dextrin and the balance of water, wherein a pH (potential of hydrogen) value of the culture medium is 8.0-8.5. The preparation method of the D-type clostridium perfringen toxin comprises the steps of inoculating a D-type clostridium perfringen production strain into the culture medium, collecting a culture material, performing centrifugation, and then filtering a supernate. With the adoption of the method, the maximum toxicity can be improved to be 45 times a seedling standard in Regulations on National Veterinary Biological Products, and an output-input ratio can be increased to be 30-225 times that of the original traditional technology. In addition, corresponding serum neutralization titer of a toxoid vaccine prepared by the D-type clostridium perfringen toxin on a rabbit and a sheep is also improved to be 8.3 and 13.3 times a regulation standard respectively.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Type b Clostridium perfringens exotoxin and its preparation method, toxin-producing medium and application
ActiveCN109554420BOptimizing Toxigenic Culture ConditionsStrong toxin production abilityAntibacterial agentsBacterial antigen ingredientsBiotechnologyClostridium perfringens toxoid
The invention discloses a type B Clostridium perfringens exotoxin, a preparation method thereof, a toxin-producing medium and an application thereof. Wherein every 1000ml described toxin-producing medium is made of the raw materials of following weight ratio: peptone 15-25g, yeast extract powder 3-5g, NaCl 3-4g, NaCl 2 HPO 4 12H 2 O 5‑10g, dextrin 5‑10g or crude dextrin 5‑10g, and the rest is water for injection. The virulence of the type B Clostridium perfringens toxin prepared by the method of the invention can reach up to 1 mouse MLD≤0.0006mL, which is 3.3 times higher than the seedling production standard. Moreover, the neutralization titer of the corresponding serum neutralization titers of rabbits and sheep in the triple inactivated vaccine of sheep rapid epidemic disease and sudden attack (or lamb dysentery) enterotoxemia also reaches or exceeds the standard of relevant regulations.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Preparation method of duck liver positive serum
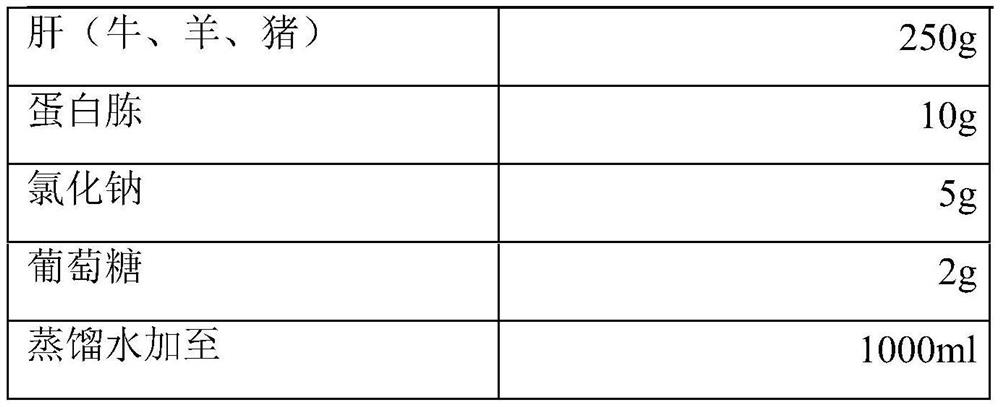
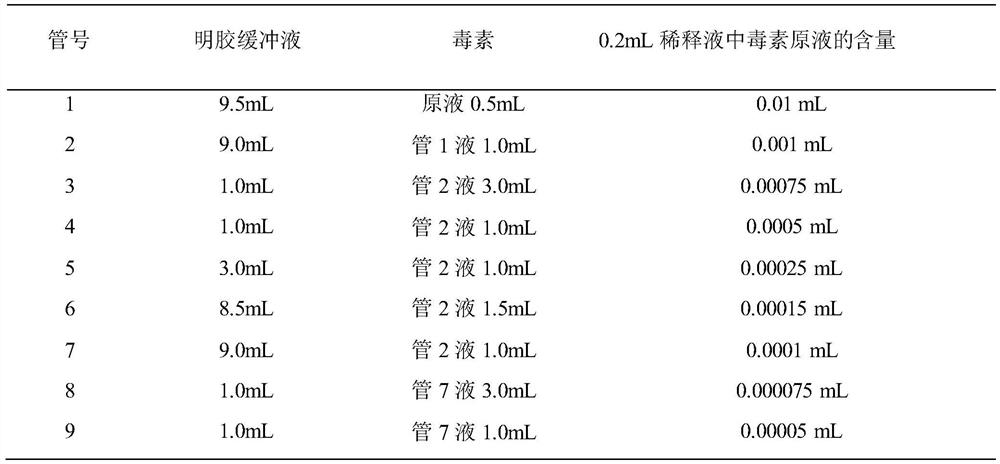
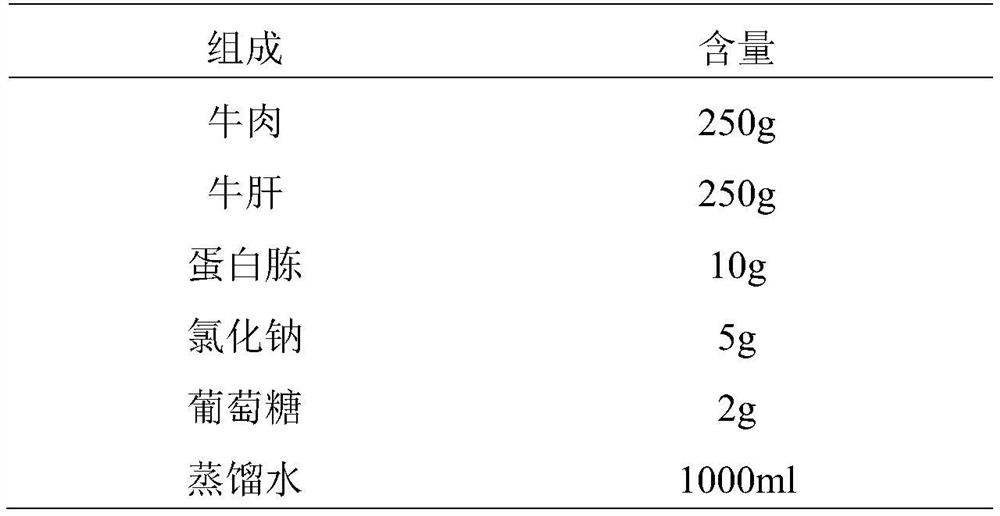
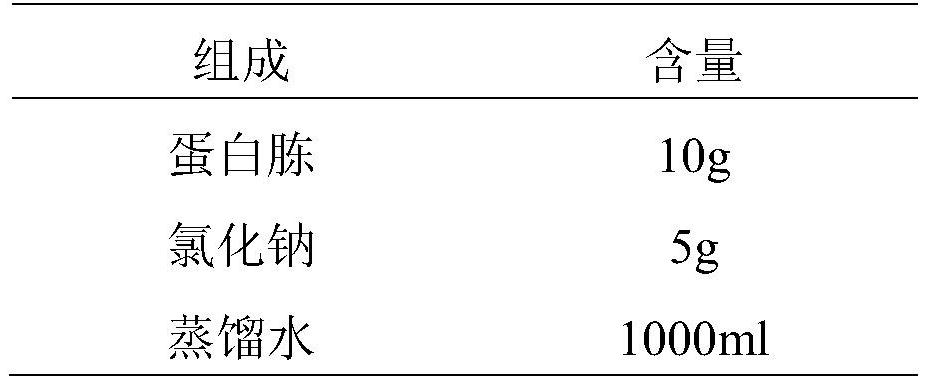
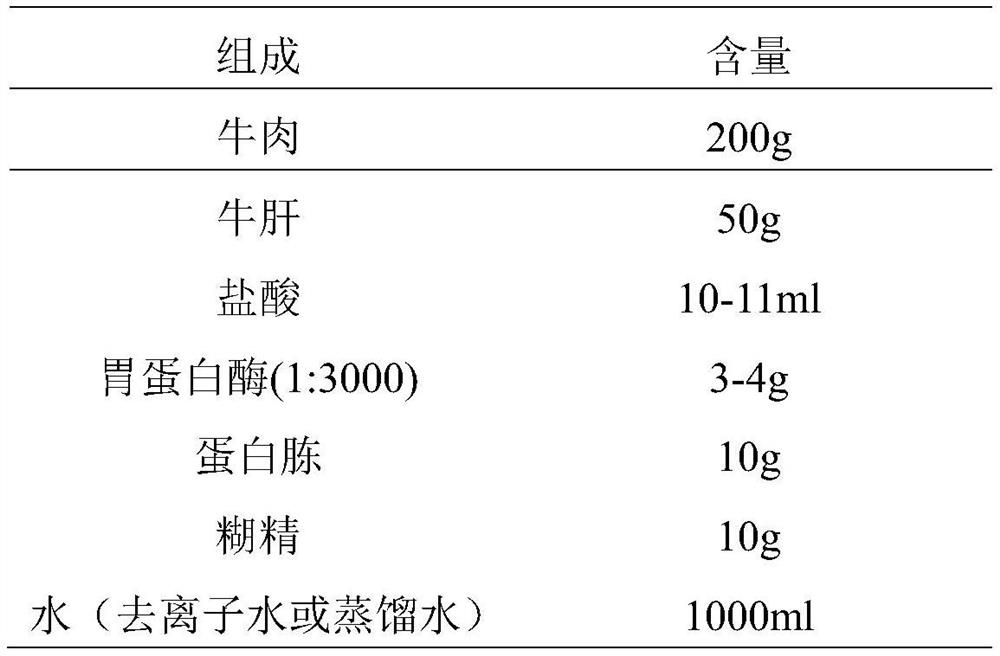
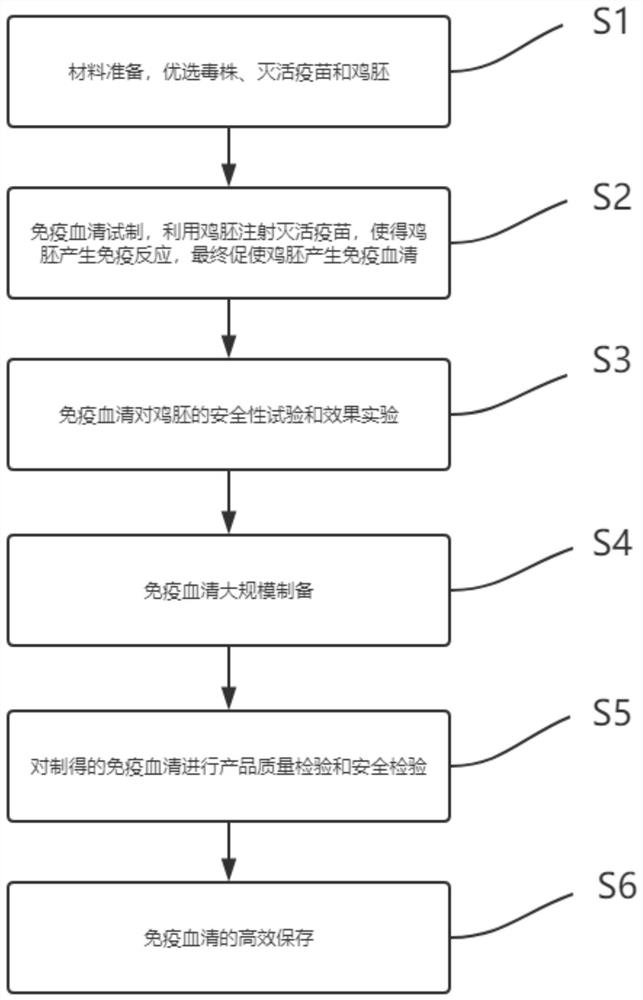
InactiveCN113698476ANo fatalitiesNo significant decrease in neutralizing titerSerum immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusEmbryo
The invention discloses a duck liver positive serum preparation method, and belongs to the technical field of virus detection. The duck liver positive serum preparation method comprises the steps that Step 1, materials are prepared, and a virus strain, an inactivated vaccine and a chick embryo are optimized; Step 2, immune serum is produced in a trail manner, the inactivated vaccine is injected into the chick embryo, so that the chick embryo generates immune reaction, and finally, the chick embryo is promoted to generate the immune serum; Step 3, a safety test and an effect test are performed on the chick embryo by using the immune serum; Step 4, the immune serum is prepared on a large scale; Step 5, product quality inspection and safety inspection are performed on the prepared immune serum; and Step 6, the immune serum is efficiently stored. According to the invention, the neutralizing reaction with the type 3 duck hepatitis virus is poor, the titer of the neutralizing antibody is 1:2896, the titer of the neutralizing antibody is stable after the repeated test, the antibody can be used for inspection, and during neutralization of the virus liquid with different contents by using the serum with different neutralizing antibody titers, and the virus liquid with the concentration of 104.0 ELD50 / 0.1 ml can be completely neutralized when the titer of the neutralizing antibody is 1:2048 according to the result.
Owner:山东和康源生物育种股份有限公司 +1
Kit for combined detection of 6 diabetic antibodies
ActiveCN102967704BEasy to operateHave confirmed valueMaterial analysisDiabetes mellitusAntiendomysial antibodies
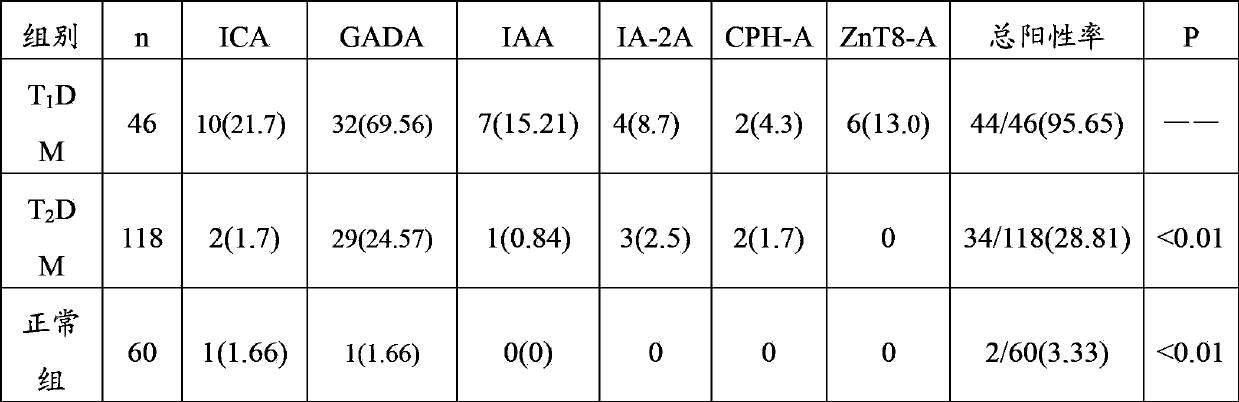
The invention relates to the technical field of biology, and in particular relates to a kit for combined detection of 6 diabetic antibodies. The kit is used for combined detection of 6 antibodies such as ICA, GADA, IAA, IA-2ACPH-A and ZnT8-A in T1DM, T2DM and serum of a normal person, has high sensitivity (98 percent) and specificity (99.6 percent) for I type diabetic diagnosis, and provides efficient and fast detection means for classificatory diagnosis of diabetes.
Owner:SHENZHEN BLOT BIOTECH
A joint screening test strip for early esophageal cancer
ActiveCN111505300BImprove the detection rateHigh detection sensitivityBiological material analysisBiological testingAutoantibodyOncology
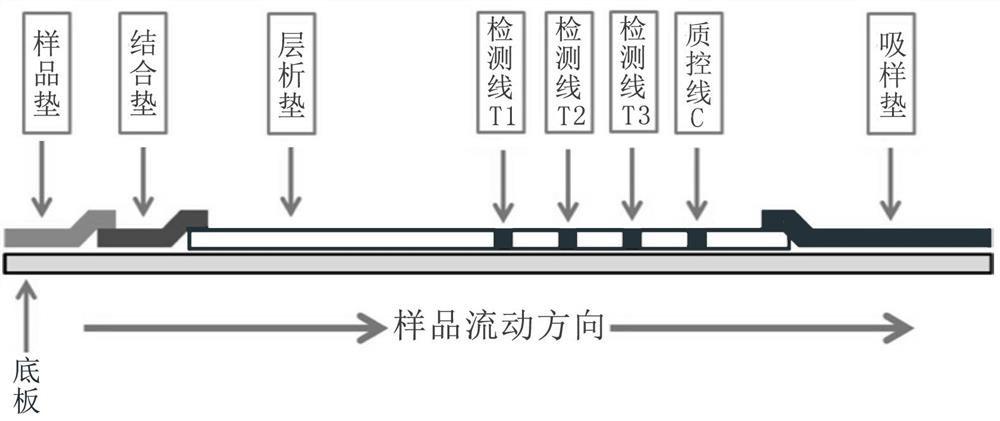
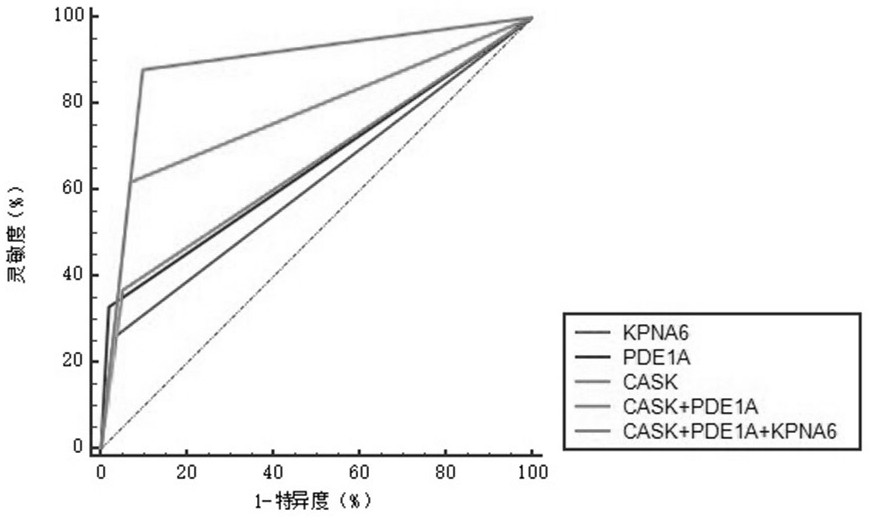
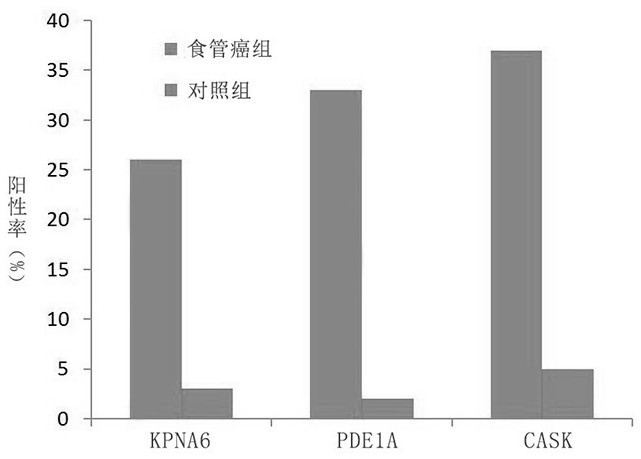
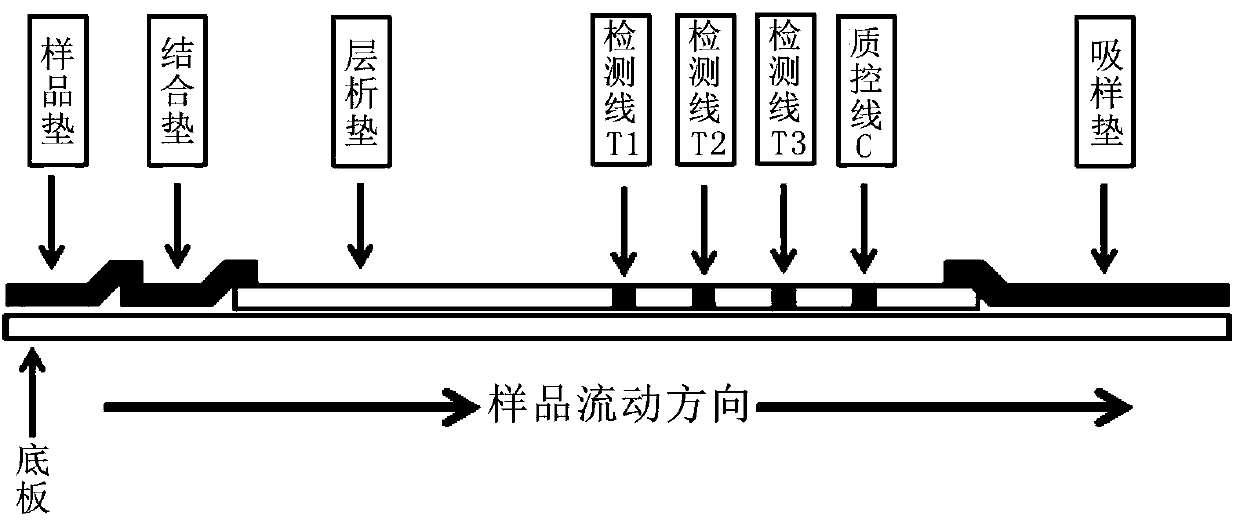
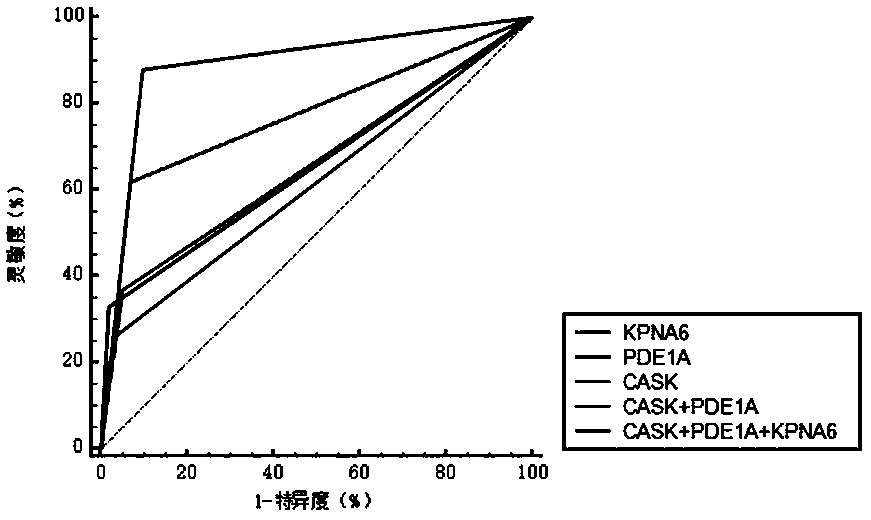
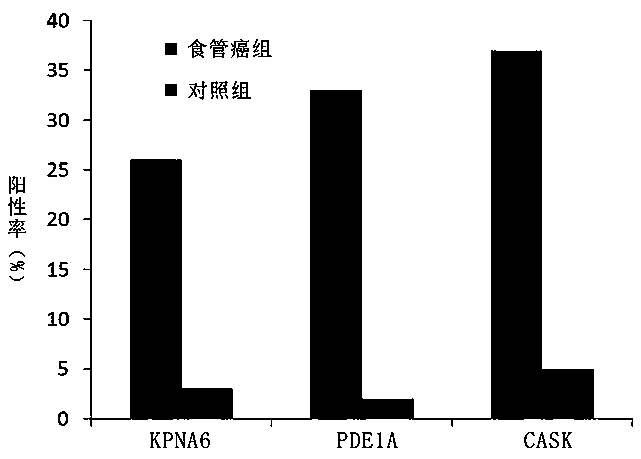
The invention belongs to the field of medical biotechnology and discloses a test strip for joint screening of early esophageal cancer. The test strip includes a binding pad and a chromatography pad, and the binding pad is coated with a mouse anti-human IgG monoclonal antibody and a rabbit IgG monoclonal antibody. Antibodies, mouse anti-human IgG monoclonal antibody and rabbit IgG monoclonal antibody all have detectable markers; there are three detection lines and one quality control line on the chromatography pad, and the detection antigens coated on the three detection lines are KPNA6 protein, PDE1A protein, CASK protein; the quality control belt is coated with goat anti-rabbit IgG. The test strip of the present invention combines KPNA6 protein, PDE1A protein, and CASK protein, three tumor-associated antigens, as markers for early esophageal cancer screening, and is used to detect the expression levels of autoantibodies of KPNA6, PDE1A, and CASK in serum, which can effectively Detection of esophageal cancer, especially early esophageal cancer, has greatly improved the detection rate of early esophageal cancer.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
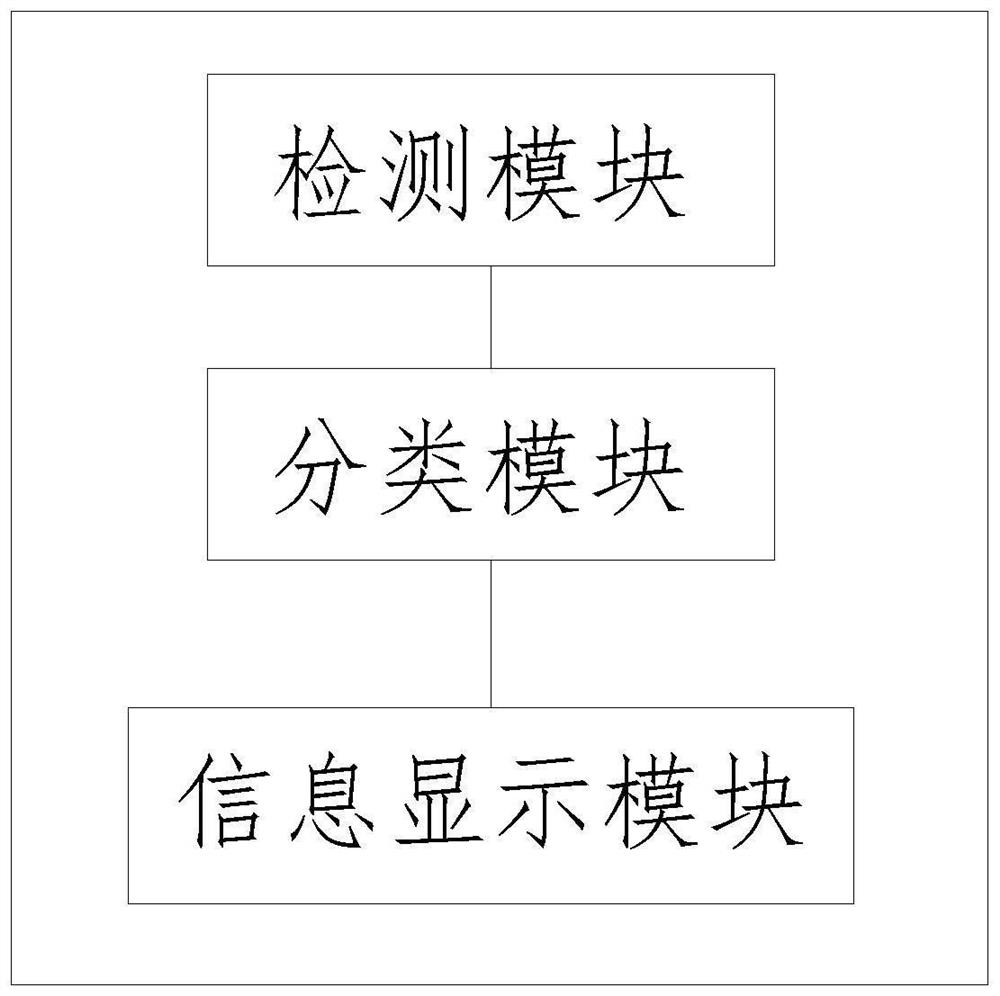
Device for classifying invasive glioma based on DNA methylation
PendingCN114540498AHigh sensitivityHigh precisionMicrobiological testing/measurementBiostatisticsDNA methylationA-DNA
The invention relates to the technical field of biomedicine, in particular to a DNA methylation-based invasive glioma classification device, which comprises a detection module, a classification module and an information display module, whether neuroglial cells have canceration or not and the canceration degree are predicted by measuring the methylation degree of a CpG island, neuroglioma is classified, and by adopting real-time quantitative methylation specific PCR, the sensitivity and the accuracy are high, the DNA methylation condition of formalin soaked specimen tissue can be detected, and the detection result is accurate. Therefore, the real-time quantitative methylation specific PCR has the potential of being used for glioma diagnosis, mi RNA can stably exist in serum and is easy to obtain and has the potential of becoming an early diagnosis molecular marker, and the content of mi R-25 and mi R-223 in the serum can be used as a diagnosis marker of a non-small cell lung cancer patient; a specific glioma mi RNA marker is screened out, DNA is extracted from blood of a patient, then the mi RNA expression level can be detected through PCR, and therefore early diagnosis can be conducted on glioma.
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
Recombinant Rsv Strains With Altered G Protein
The construction of recombinant respiratory syncytial virus (RSV) strains deleted of the region of G protein most likely to induce unwanted type 2 T cell responses in susceptible recipients is disclosed. Using reverse genetics, recombinant RSV strains were engineered with deletions of amino acids 151-221 and 178-219. Both RSV strains replicated in the respiratory tract of BALB / c mice and elicited serum neutralization and anti-F protein IgG titers that were equivalent to cp-RSV and contributed to a 3.9 log 10 reduction in RSV A2 four days after challenge. Importantly, pulmonary eosinophilia was significantly diminished in BALB / c mice primed with native G protein and challenged with either recombinant RSV strain. These findings are important for the development of immunogenic compositions against RSV.
Owner:WYETH HOLDINGS CORP
Early esophageal cancer combined screening test strip
ActiveCN111505300AImprove the detection rateHigh detection sensitivityBiological material analysisBiological testingAutoantibodySerum neutralization
The invention belongs to the technical field of medical biology, and discloses an early esophageal cancer combined screening test strip. The early esophageal cancer combined screening test strip comprises a combination pad and a chromatography pad, the combination pad is coated with a mouse anti-human IgG monoclonal antibody and a rabbit IgG monoclonal antibody, and the mouse anti-human IgG monoclonal antibody and the rabbit IgG monoclonal antibody are both provided with detectable markers. Three detection lines and a quality control line are arranged on the chromatography pad, the detection antigens coated by the three detection lines are respectively KPNA6 protein, PDE1A protein and CASK protein, and goat anti-rabbit IgG is coated on the quality control band. According to the test stripdisclosed by the invention, three tumor-associated antigens, namely the KPNA6 protein, PDE1A protein and CASK protein, are combined to serve as markers for screening early esophageal cancer, the teststrip is used for detecting the expression level of autoantibodies of KPNA6, PDE1A and CASK in serum, can effectively detect the esophageal cancer, especially early esophageal cancer, and greatly improves the detection rate of the early esophageal cancer.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com