Recombinant varicella zoster virus vaccine
A technology for varicella zoster and virus vaccine, applied in the directions of viruses, viral peptides, antiviral agents, etc., can solve the problem of low purity of expression products, and achieve the effects of large molecular weight, maintaining spatial structure, and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the construction of plasmid expression vector:
[0066] 1. Gene sequence source
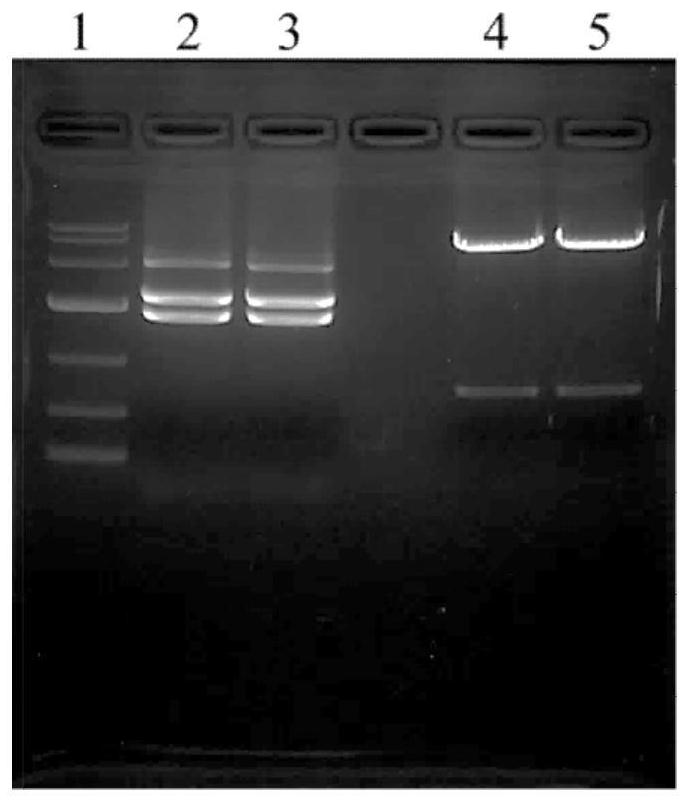
[0067] Varicella zoster virus E protein (VZV gE) of the present invention is the extracellular domain (ECD, 31-546aa) of gE protein, totally 516 amino acids; sequence 1). The varicella-zoster virus E protein gene and the human IgG1 Fc gene were concatenated (Appendix: DNA Sequence 2), and Nanjing GenScript Biotechnology Co., Ltd. was commissioned to synthesize the VZV gE-Fc fusion protein gene sequence and connect it to pUC57-1.8 On the K vector, the synthesized gene includes restriction site, Kozak sequence, signal peptide, target gene (2244bp) and stop codon, with a total length of 2355bp. Codon optimization was carried out when the recombinant gene was synthesized to facilitate expression in Chinese hamster ovary cell Cricetulus griseus (CHO cell).
[0068] 2. Construction of expression plasmid containing VZV gE-Fc gene
Embodiment 2
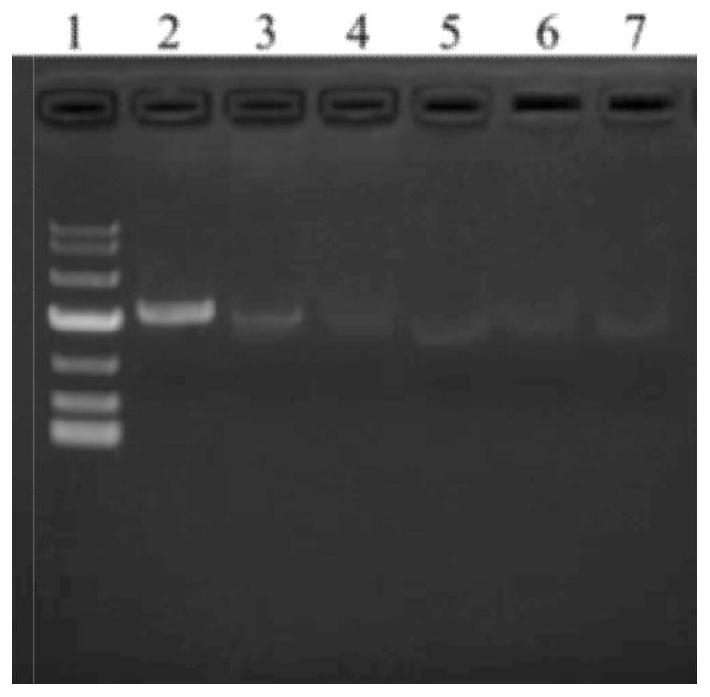
[0076] Embodiment 2, establishment and screening of stable clones
[0077]In the sterile laminar flow workbench, set the perforation voltage of the gene pulse generator Xcell (Bio-Rad) to 300V voltage, 900μF single pulse, and infinite resistance, and take out the disposable electric shock cup (Bio-Rad) with a gap of 4mm. Add 40μg linearized plasmid DNA (100μl) and 0.7ml CHO K1 cell suspension (1.5×10 7 cells / ml), the linearized plasmid VZV gE-Fc- was directly transfected into CHO K1 cells by electrotransfection, the cells in the electric shock cup were transferred to the Erlenmeyer flask, and 30ml CD CHO culture medium was added (GIBCO), at 36~37℃, 5%CO 2 Culture on a shaker at 135 rpm. After 24 hours of culture, collect the cells by low-speed centrifugation, replace with CD CHO medium (without glutamine) containing 50 μM MSX, and transfer the cells to a 96-well flat-bottom culture plate by limiting dilution Inside, place the culture plate at 37°C, 10% CO 2 Cultivate in an ...
Embodiment 3
[0078] Embodiment 3, expression and purification of target product
[0079] Inoculate the obtained 5B3 clone into a 2L Erlenmeyer flask containing 500ml of CD CHO culture medium, tighten the cap of the ventilated bottle, and store at 36-37°C, 5% CO 2 , 135r / min on a rotary shaker, after 4 days of culture, transfer 5B3 cells to a 5L automatic bioreactor containing 2.0L CD Opti-CHO culture medium, and set the culture parameters at 60r / min , temperature 36.5°C, pH7.0~pH7.4, dissolved oxygen DO 40%~60%, daily sampling to detect cell viability, cell density, glucose content, after 4 days of culture, the density of viable cells rose to 5×10 6 ~7×10 6 cells / ml, add 200ml~300ml CDEfficient Feed C, and then add it once every other day, measure the glucose content in the culture medium every day, if the glucose content is lower than 11.1mmole / L, add 40% glucose solution through a peristaltic pump Put it into a fully automatic bioreactor until the glucose content reaches 22mmole / L; cul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com