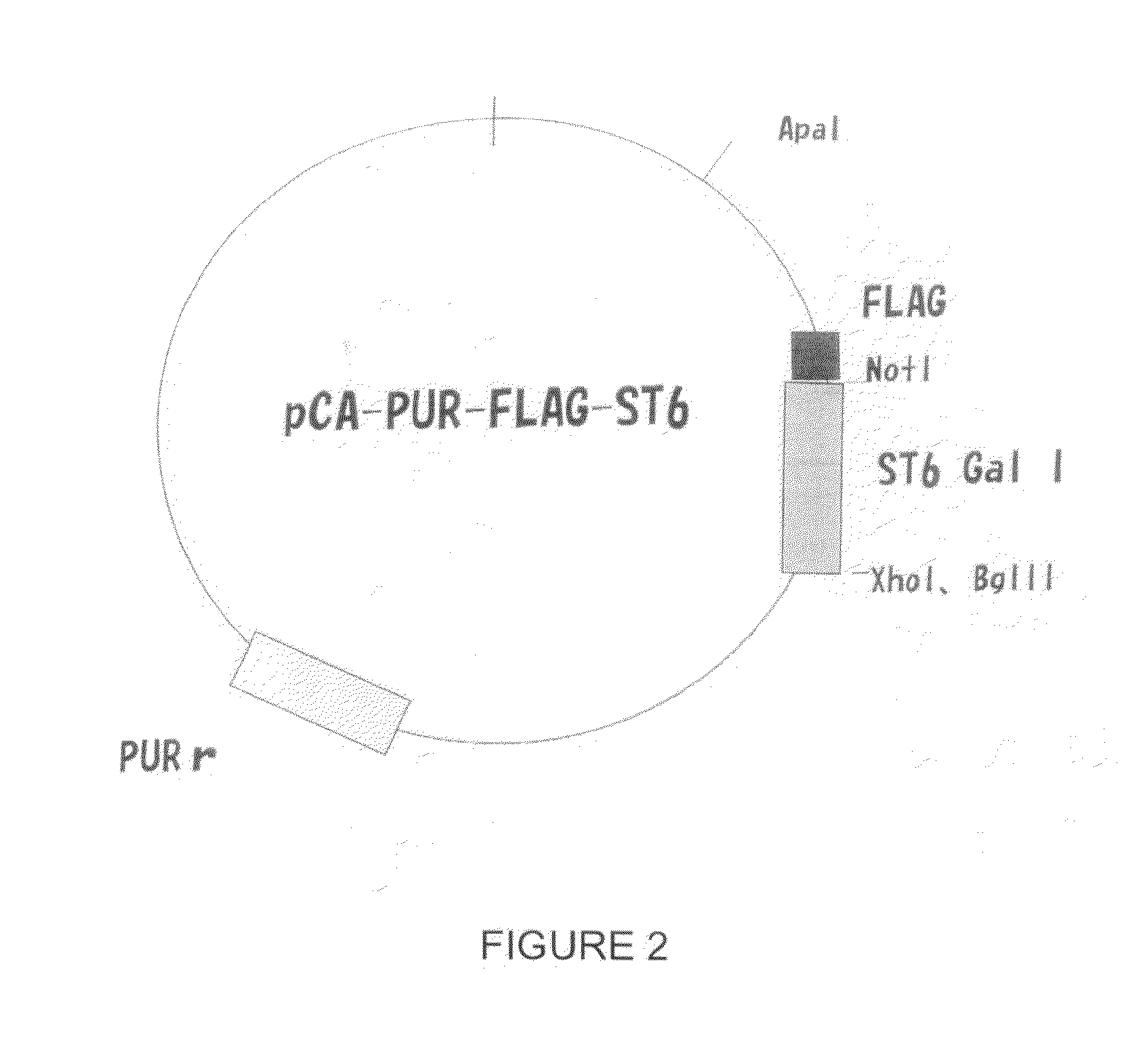
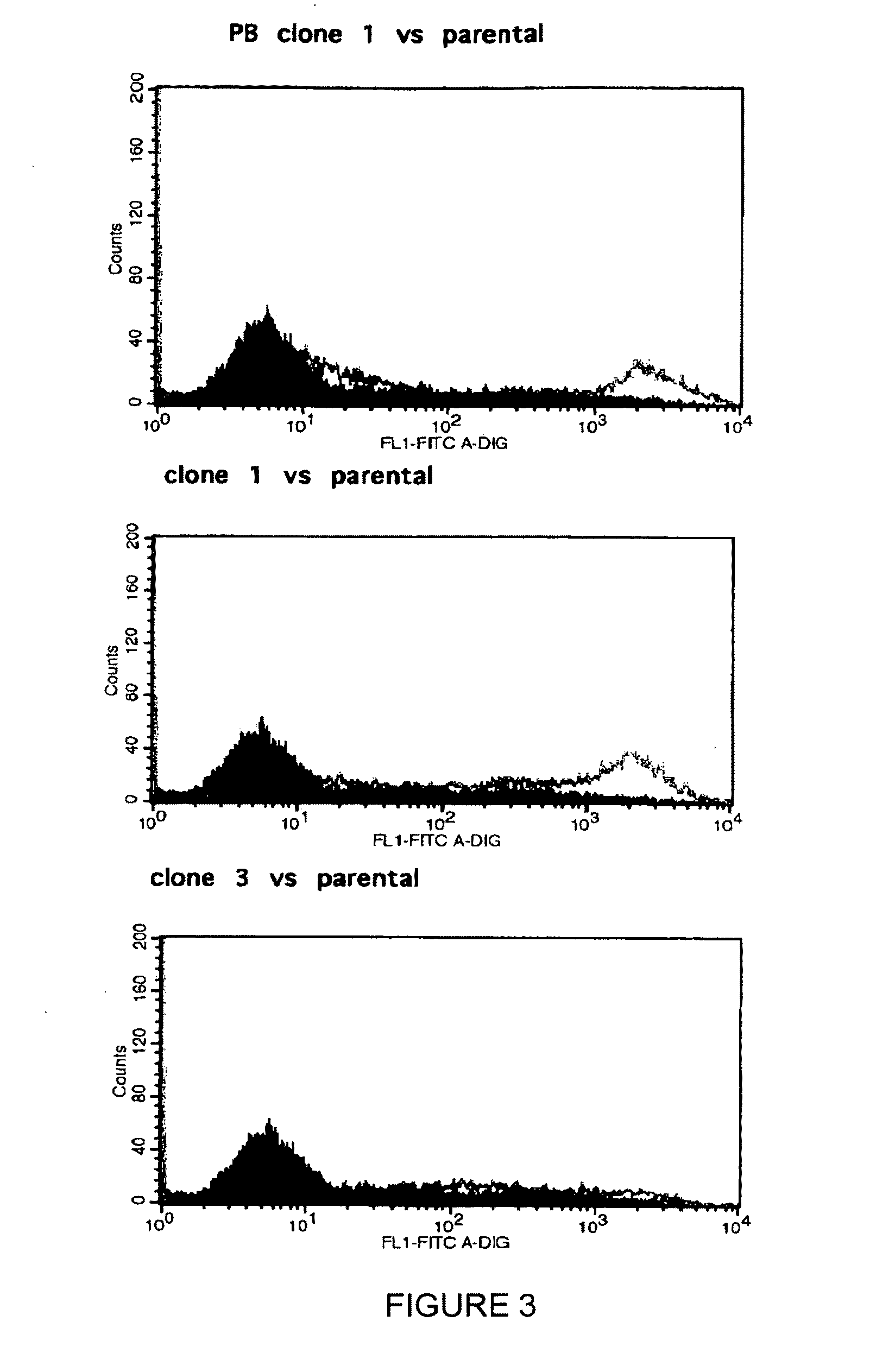
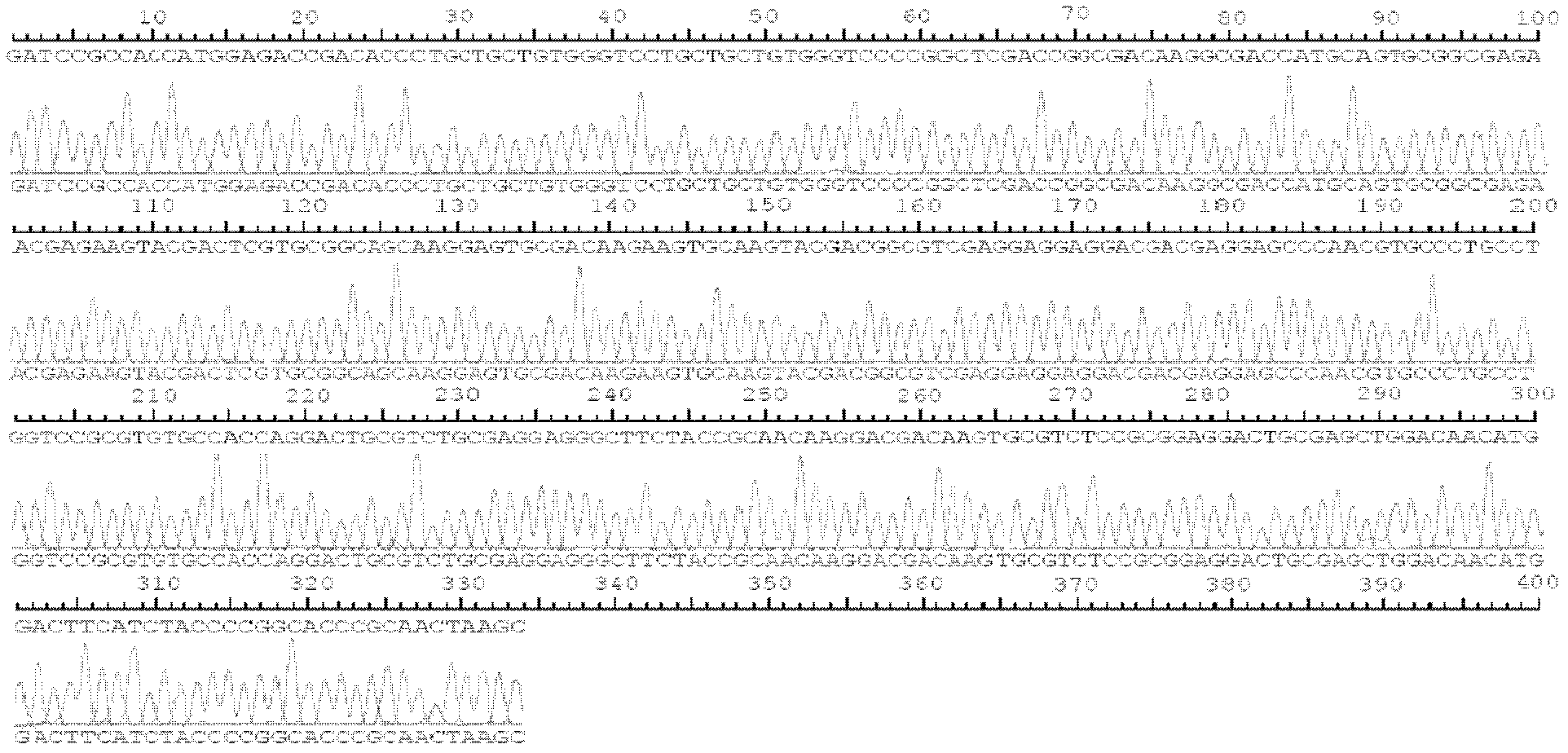
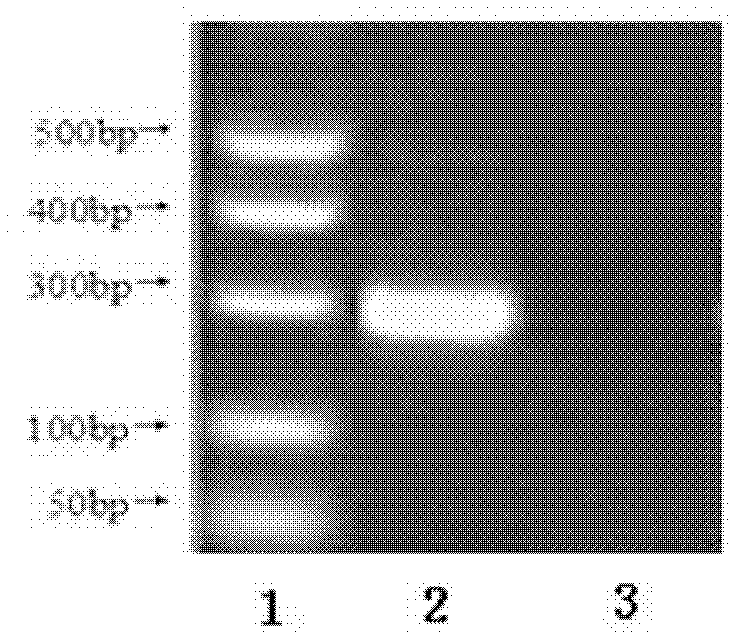
Patents
Literature
80 results about "Cricetulus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cricetulus is a genus of rodent in the family Cricetidae (voles and hamsters); it has seven member species that inhabit arid or semi-arid regions in Eurasia. They tend to be more ratlike in appearance than typical hamsters, hence the common name ratlike hamster. Many of the species are considered dwarf hamsters. However, members of the genera Allocricetulus, Tscherskia, and Cansumys are often called ratlike hamsters, and so are considered to be members of the genus Cricetulus by many authorities.
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Chinese hamster ovary genetic engineering cell line for performing high-level secretory expression on AcAPc2
InactiveCN102321588AAdaptableExtended half-lifeVector-based foreign material introductionForeign genetic material cellsHigh level expressionHamster
The invention discloses a Chinese hamster ovary (CHO) genetic engineering cell line for performing high-level secretory expression on AcAPc2 and relates to a CHO cell line for performing high-level secretory expression on the AcAPc2. The invention aims to solve the problems that the expression level of an exogenous gene product expressed by using CHO is low at present and the industrialized production cannot be realized. The CHO genetic engineering cell line for performing high-level secretory expression on the AcAPc2 is obtained by the following step of: screening dihydrofolate reductase-deficient CHO cells which are taken as host cells, and amplifying to obtain the cell line for performing stable and high-level expression on the AcAPc2. The CHO cell line can perform high-level expression on the AcAPc2; the expression level can be up to 10mg / L.72h; and the CHO cell line can be industrially produced. The cell line is used for resisting blood coagulation and treating tumor, septicaemiaand the like.
Owner:HEILONGJIANG UNIV
Method of producing factor viii proteins by recombinant methods
InactiveUS20120028900A1High expressionFacilitates secretionOrganic active ingredientsFungiElongation factorChinese hamster
Provided herein are methods and compositions for producing Factor VIII proteins. Such methods include introducing into a cell a nucleic acid molecule encoding a Factor VIII protein operably linked to a promoter, wherein the promoter is characterized by the ability to produce commercially viable Factor VIII protein; and incubating the cell under conditions for producing commercially viable Factor VIII protein. Also provided are nucleic acid molecules which encode a Factor VIII protein operably linked to a Chinese hamster elongation factor 1-α (CHEF1) promoter, which may be used in the methods provided herein.
Owner:CNJ HLDG +1
Reconstruction and application of targeted site-specific integrated CHO cell line
ActiveCN104818253AVector-based foreign material introductionForeign genetic material cellsLong armExogenous protein
The invention relates to an FRT-sCHO cell line with an integrated exogenous gene at a specific site and application of the FRT-sCHO cell line. The cell line is prepared by using a Chinese hamster ovary cell (CHO) as a host cell and inserting an identification tag into the terminal (1q12 and 1q13) regions of the long arm of chromosome No. 1 of the host cell. The FRT-sCHO cell line provided by the invention supports integration of exogenous genes at specific sites and maintains high-efficiency and stable expression of exogenous proteins for a long time.
Owner:成都金洛克锶生物技术有限公司
Chinese hamster ovary culture medium as well as preparation method and application thereof
InactiveCN102021139ACulture regulationCultivate delivery and controlTissue cultureAdditive ingredientCell culture media
The invention discloses a Chinese hamster ovary culture medium as well as a preparation method and application thereof. The culture medium comprises ascorbic acid, pyridoxine hydrochloride, vitamin B12, nicotinamide, riboflavin, thiamine chloride, choline chloride, folic acid, calcium pantothenate, sodium pyruvate, reduced glutathione, inositol, biotin, hypoxanthine, putrescine, lipoic acid, linoleic acid, thymine, glucose, HEPES (2-hydroxyethyl) buffer solution, dextran sulphate, Pluronic-F68, various amino acids and salts thereof and various inorganic salts. The culture medium has low cost, and the component does not contain animal origin ingredients, does not contain protein and has definite chemical ingredients. All ingredients are cooperated and blended, and the Chinese hamster ovary culture medium can satisfy the basic growth requirement of cells, and can effectively regulate, transfer and control the culture of cells so as to realize good high-density cell culture. Compared with the culture effect of the existing culture medium, the culture effect of culture medium disclosed in the invention is at least equivalent to even superior to the existing culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
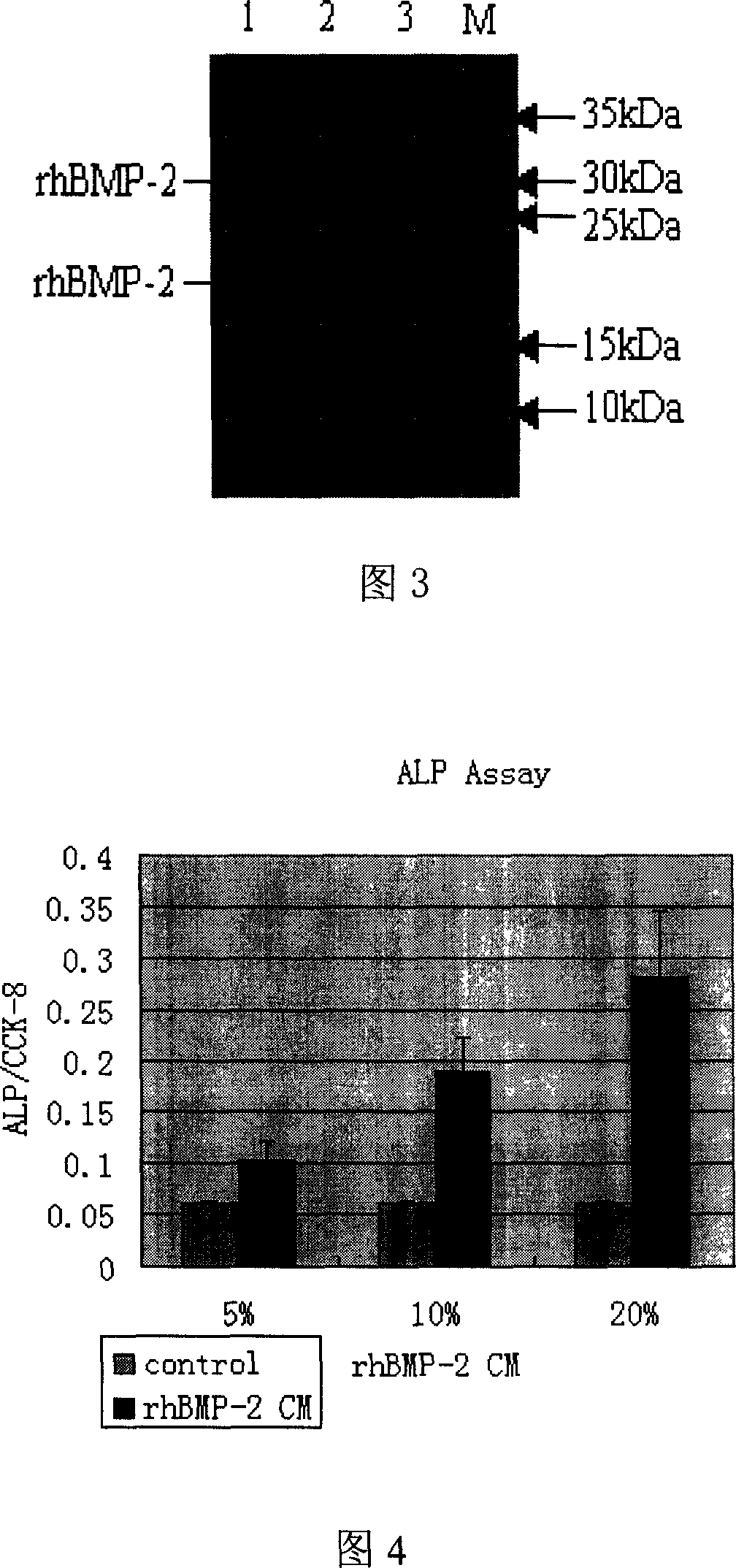
CHO cell strain for highly effective expressing rhBMP2 and establishing method thereof
InactiveCN101012453ASignificantly induces osteogenic activityPeptide/protein ingredientsGenetic material ingredientsDrugDisease
The invention discloses a CHO cellar strain of high-effective expressive exogenous rhBMP2, which is characterized by the following: adopting CHO-dhfr- in the dihydrofolic acid reductase flaw-typed Chinese hamster ovary cell as host cell; infecting exogenous hBMP2 plasmid; adopting secretory expressive product to induce myoblast system C2C12 to display the activity to stimulate sclerotomal cell; obtaining single-clone strain of CHO (rhBMP-2) with the content of BMP2 at 7.83ug / 24h*106; fitting for clinic treatment.
Owner:NANKAI UNIV
Process, vectors and engineered cell lines for enhanced large-scale transfection
ActiveUS8637315B2Enhanced transfectionHigh expressionAnimal cellsSugar derivativesValproic AcidHamster
Processes vectors and engineered cell lines for large-scale transfection and protein production in mammalian cells, especially Chinese Hamster Ovary (CHO) cells are described in which transfection efficiencies are realized through the use of a single vector system, the use of functional oriP sequences in all plasmids, the use of codon-optimized Epstein-Barr virus nuclear antigen-1 (EBNA1) constructs the use of a fusion protein between a truncated Epstein-Barr virus nuclear antigenen-1c (EBNA1c) protein and a herpes simplex virus protein VP16, the use of a 40 kDa fully deacetylated poly(ethylenimine) as a transfection reagent, the use of co-expression of a fibroblast growth factor (FGF) and / or the use of protein kinase B to potentiate heterologous gene expression enhancement by valproic acid (VPA).
Owner:NAT RES COUNCIL OF CANADA
Composition and method for site-specific recombination in hamster cells
ActiveCN103305504AForeign genetic material cellsDNA/RNA fragmentationSite-specific recombinationHamster
The invention provides a composition and a method for site-specific recombination in hamster cells, and particularly relates to a composition and a method for carrying out site-specific recombination on an exogenous sequence in a high-expression site of a target nucleotide sequence. The target nucleotide sequence can be a genome of hamster cells (such as Chinese hamster ovary cells, CHO cells). The invention also provides a host cell containing high-expression sites on chromosomes. The host cell contains the composition for site-specific recombination provided by the invention, or an exogenous sequence is subjected to site-specific recombination in a high-expression site of the composition.
Owner:ZHEJIANG ECHON BIOMEDICAL CO LTD
Gene sequence for expressing soluble recombinant human hyaluronidase PH20 in CHO (Chinese hamster ovary) cell
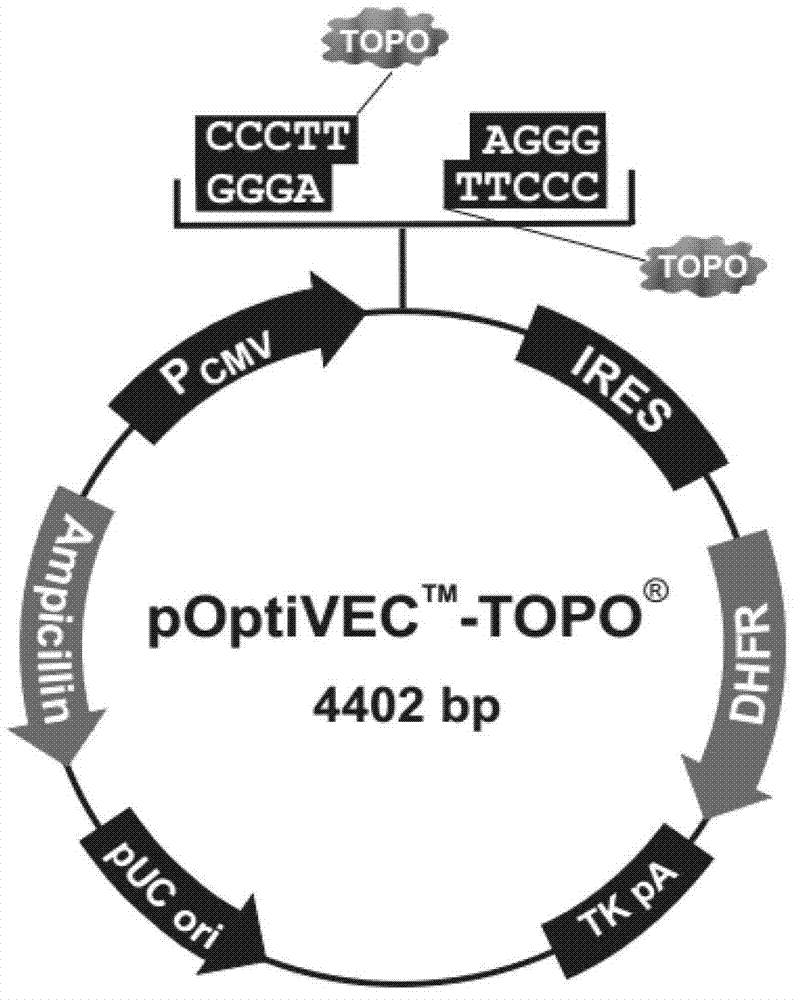
The invention discloses a gene sequence for expressing soluble recombinant human hyaluronidase PH20 by a CHO (Chinese hamster ovary) cell. The gene sequence has a nucleotide sequence shown in SEQ ID NO.2; after a kozak consensus sequence is added to 5' terminal of the optimized gene sequence, the optimized gene sequence is connected to pOptiVEC-TOPO carrier, and stably transferred to the Chinese hamster ovary cell improved by genetic engineering, so as to achieve high expression of the soluble recombinant human hyaluronidase PH20 in a mammalian cell.
Owner:广州白云山拜迪生物医药有限公司
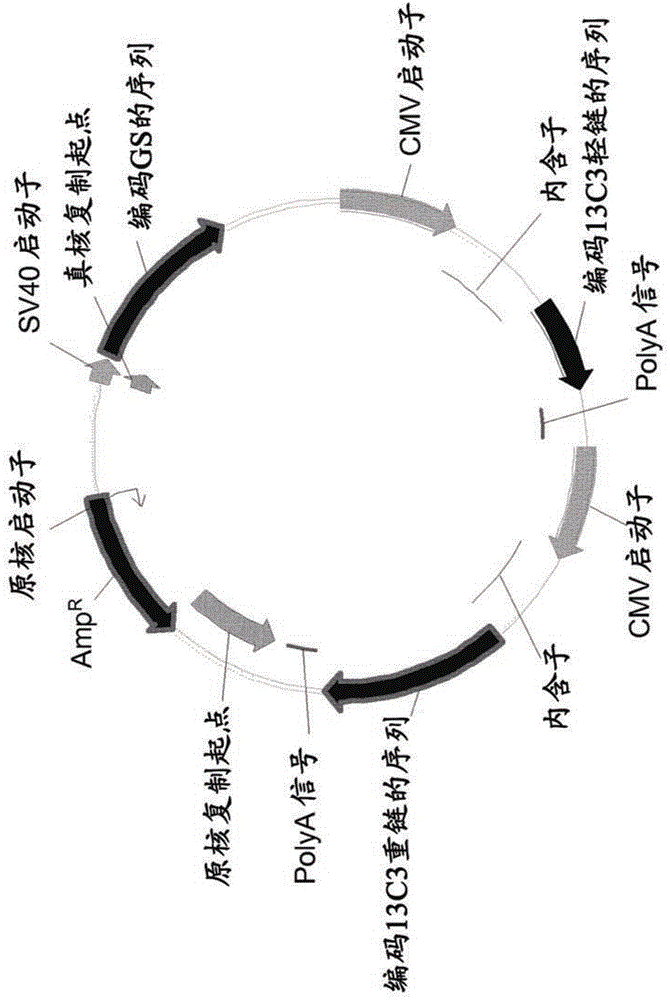
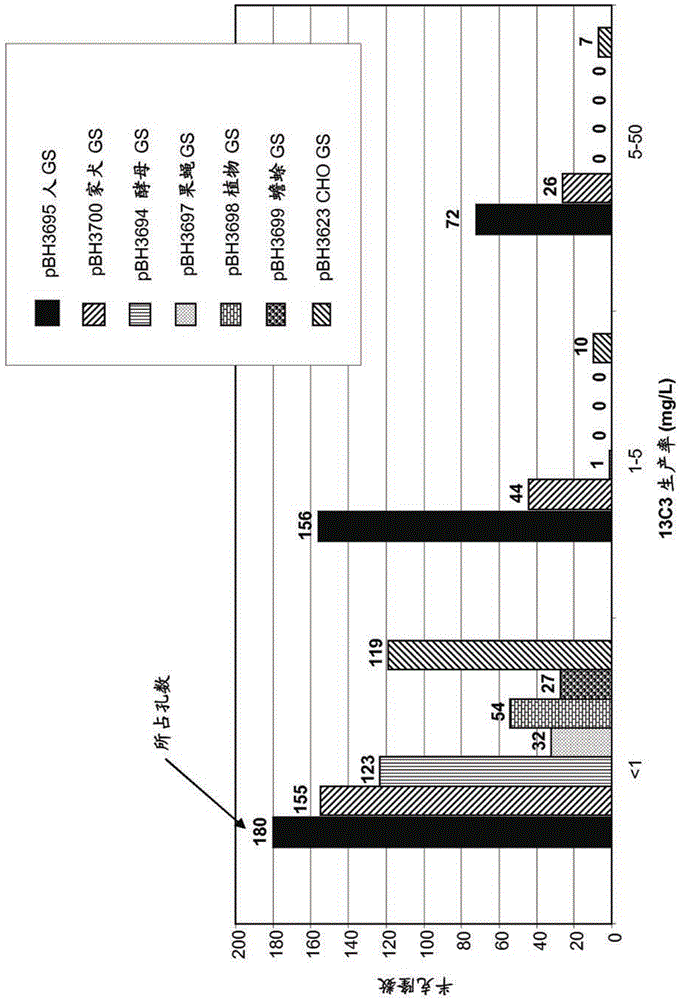
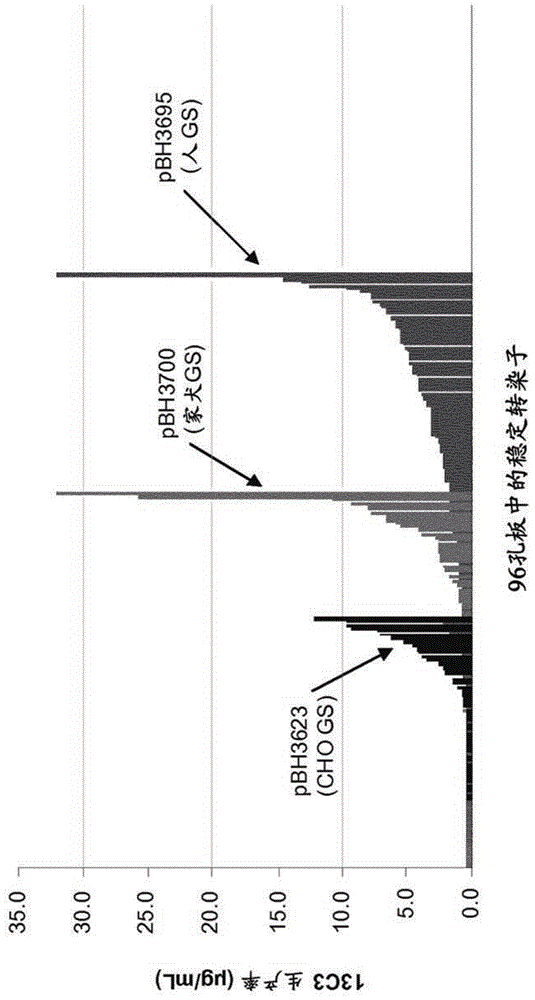
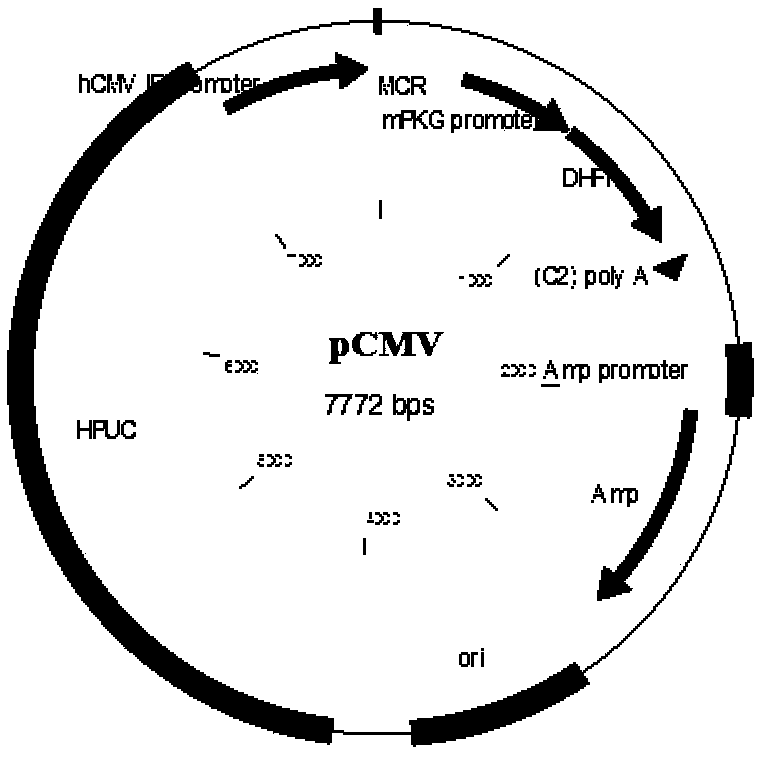
Cho expression system
The present invention is within the field of industrial protein production. The inventors have found a new expression system comprising an expression vector coding for a glutamine synthetase of human or dog origin, and a CHO cell line. More specifically, the invention pertains to a combination of (i) a DNA vector suitable for production of a recombinant protein, wherein said vector comprises a sequence coding for a glutamine synthetase, and (ii) a Chinese Hamster Ovary (CHO) cell line, wherein said GS comprises a sequence at least 94.5 % identical to the sequence of SEQ ID NO: 1 or to the sequence of SEQ ID NO: 2.
Owner:SANOFI SA
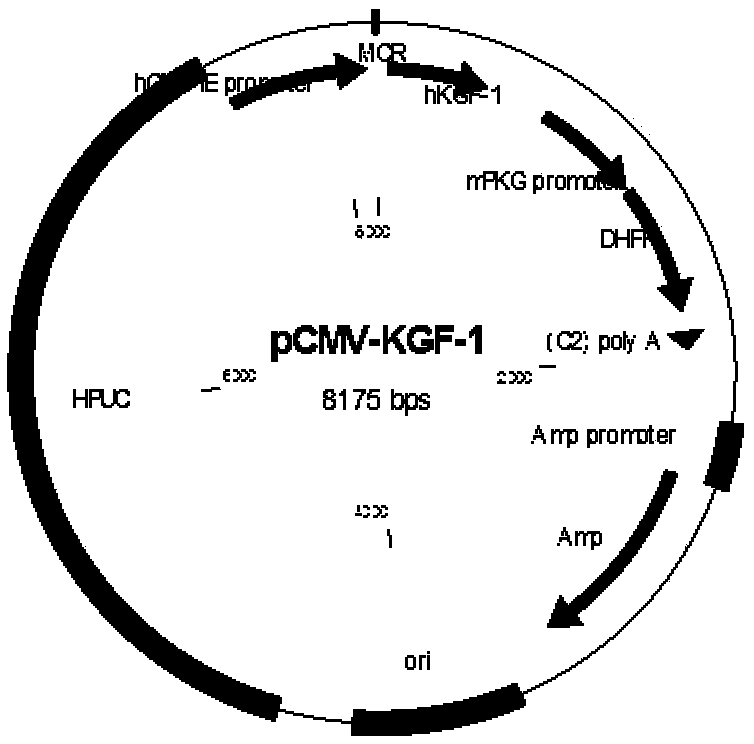
Recombinant human keratinocyte growth factor-1 and preparation and application methods thereof
InactiveCN109402130AImprove biological activityEasy to preparePeptide/protein ingredientsPeptide preparation methodsRecombinant Human Keratinocyte Growth FactorDisease
The invention relates to a production of a recombinant human keratinocyte growth factor-1 (KGF-1) and discloses a recombinant plasmid of the gene of the recombinant human keratinocyte growth factor-1,a method of expression of keratinocyte growth factors after the recombinant plasmid is electro-transfected into Chinese hamster ovary (CHO), and purification processes of the recombinant plasmid. Therecombinant human keratinocyte growth factor-1 can significantly promote proliferation of 4MBr-5 cells and accordingly is applicable to treating skin and mucosal tissue damage-related diseases, lungdamage-related diseases and bladder and urothelium damage-related diseases.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Methods for modeling chinese hamster ovary (CHO) cell metabolism
Embodiments of the present invention generally relate to the computational analysis and characterization biological networks at the cellular level in Chinese Hamster Ovary (CHO) cells. Based on computational methods utilizing a hamster reference genome, the invention provides methods for identifying a CHO cell line having a desired genetic trait, as well as for generating a desired CHO cell line having a genetic basis for a desired phenotype. Additionally, described herein are methods for constructing and analyzing in silico models of biological networks for CHO cells.
Owner:DANMARKS TEKNISKE UNIV
Selection method of signal peptide while foreign protein is expressed by Chinese hamster ovary cells and application thereof
ActiveCN110760541ADisadvantages of low levels of altered expressionImprove work efficiencyPolypeptide with localisation/targeting motifSsRNA viruses positive-senseWestern blotCricetulus
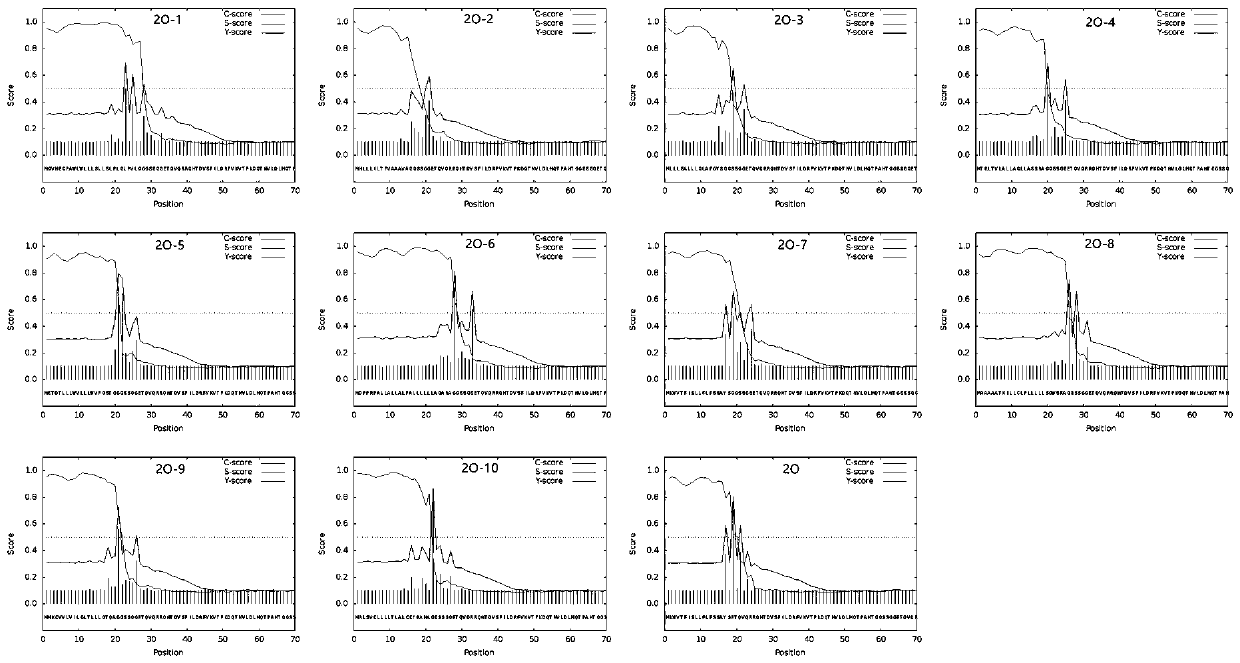
The invention relates to a selection method of a signal peptide while foreign protein is expressed by Chinese hamster ovary cells and an application thereof, which belong to the technical field of biology. An information analysis technology is adopted; 29 kinds of high-secretory expression signal peptides and foreign protein tandem sequences commonly used in CHO cells are analyzed; finally, 10 signal peptides with proper analysis results are selected, 10 different secretory signal peptides are connected with the 5'end of the tandem epitope gene through a PCR method, eukaryotic expression vectors are constructed respectively, mammalian cell CHO is transfected, Western blot is adopted to detect the cumulative difference of recombinant epitope proteins in a CHO culture medium, and the resultproves that the cumulative difference of the proteins conforms to the selection result of the method, so that the feasibility of the method is proved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
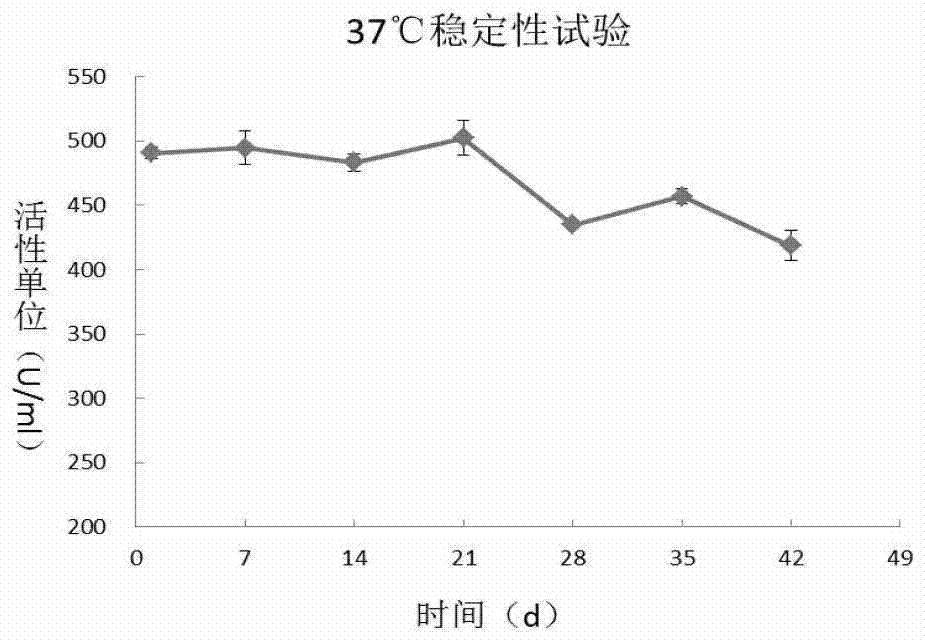
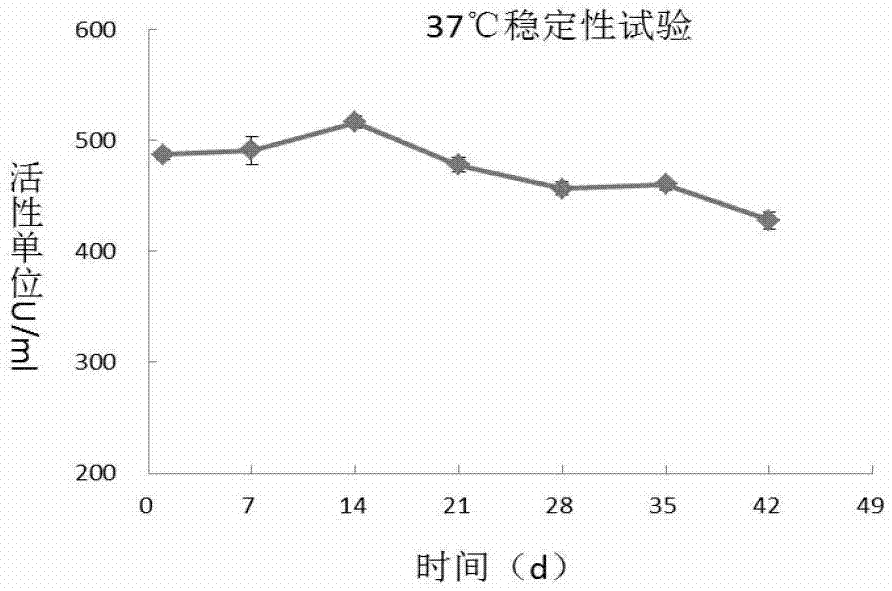
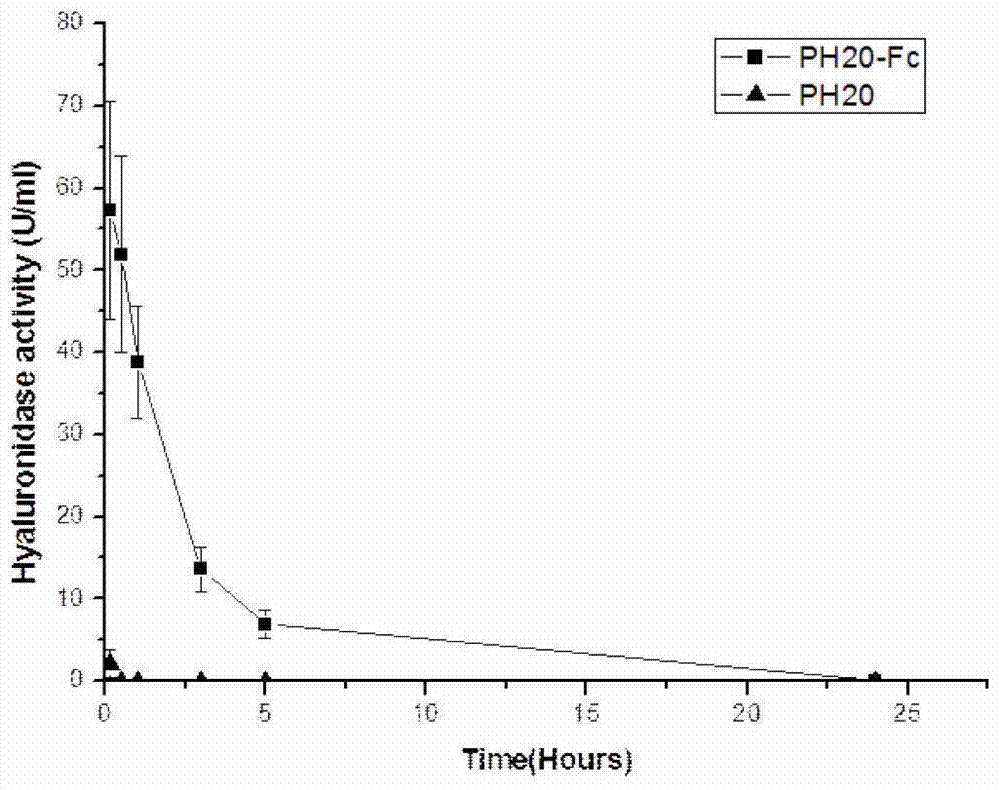
A recombinant long-acting human hyaluronidase, its coding gene, production method and application
The invention discloses a recombinant long-acting human hyaluronidase. The recombinant long-acting human hyaluronidase is a fusion protein PH20-HSA or PH20-IgFc of a human hyaluronidase PH20 extracellular part and a human protein (albumin HSA fragment or immunoglobulin IgG2Fc fragment), and the amino acid sequences of the PH20-HSA and the PH20-IgFc are represented by SEQ ID No. 4 and SEQ ID No. 5 respectively, and have the advantages of high in-vivo safety and long half-life. The invention also discloses an encoding gene, an expression vector, a host cell, a production method and a stable preparation of the recombinant long-acting human hyaluronidase. The achievement of industrial level and the obtaining of purified products with active energy are realized through expressing by using GC-rich high expression system and through producing Chinese hamster cells (CHO). The invention further discloses an application of the recombinant long-acting human hyaluronidase in cooperative dosage and the production of micro-molecular hyaluronic acid.
Owner:QINGDAO HUINUODE BIOLOGICAL TECH CO LTD
Gene engineering cell line receiving site-directed integration of exogenous genes
InactiveCN108441478ANucleic acid vectorVector-based foreign material introductionOvary cellGene engineering
The present invention relates to an engineering cell line for site-directed integration of exogenous genes, and uses thereof, wherein an identification tag is inserted into the Z4q3 region of Chinesehamster ovary cells (CHO) as host cells to obtain the cell line. According to the present invention, the engineering cell line supports the site-directed integration of exogenous genes, and can maintain the efficient and stable expression of exogenous genes for a long time.
Owner:成都金洛克锶生物技术有限公司
Protein-free culture medium for culturing microencapsulated recombinant Chinese hamster ovary (CHO) cells and preparation method thereof
InactiveCN104212768AAvoid Potential ContaminationReduce the difficulty and cost of separation and purificationOn/in organic carrierForeign genetic material cellsHamsterTransferrin
The invention discloses a protein-free culture medium for culturing microencapsulated recombinant Chinese hamster ovary (CHO) cells and a preparation method thereof. By using DMEM (dulbecco's modified eagle medium) / F12 as a basal culture medium, micromolecule substance iron salt with definite component, zinc salt, sodium selenite, sodium acetate, glutamine and glucose are added according to the characteristics of the microencapsulated cell culture. The culture medium does not influence the stability of the microcapsules, does not have insulin or transferrin in the traditional serum-free culture medium, and lowers the downstream technique and cost; and the product expression level is approximate to the culture level of the expensive specific culture medium, but the price is only one-tenth of the expensive specific culture medium. Thus, the protein-free culture medium can be used for microencapsulated recombinant CHO cells culture and protein expression, and greatly lowers the culture cost.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for constructing fusion protein TD-bFGF efficiently expressed in CHO cells and application of fusion protein TD-BFGF
The invention relates to a fusion cytokine production technology in the technical field of biology, and particularly relates to a method for constructing fusion protein TD-bFGF efficiently expressed in CHO (Chinese Hamster Ovary) cells by means of genetic engineering and application of the fusion protein TD-bFGF. The fusion protein TD-bFGF provided by the invention has an amino acid sequence as shown in SEQ ID No. 2, or has at least 75% of identity compared with the sequence as shown in SEQ ID No. 2. The invention further provides a biological material associated with the fusion protein TD-bFGF and a method for constructing a recombinant bFGF protein in the CHO cells. The TD-bFGF fusion protein obtained by the invention can penetrate the skin, has activity higher than that of the market product, can be used for cosmetic skin medicines, and has effects of reducing the production cost and improving the effect of bFGF; the method constructs CHO cell strains for stably and efficiently expressing a transdermal peptide fragment and cytokine bFGF fusion protein and provides a new application idea for the application of bFGF.
Owner:北京佳华戴维生物技术有限公司 +2
Integration sites in CHO cells
Owner:BOEHRINGER INGELHEIM INT GMBH
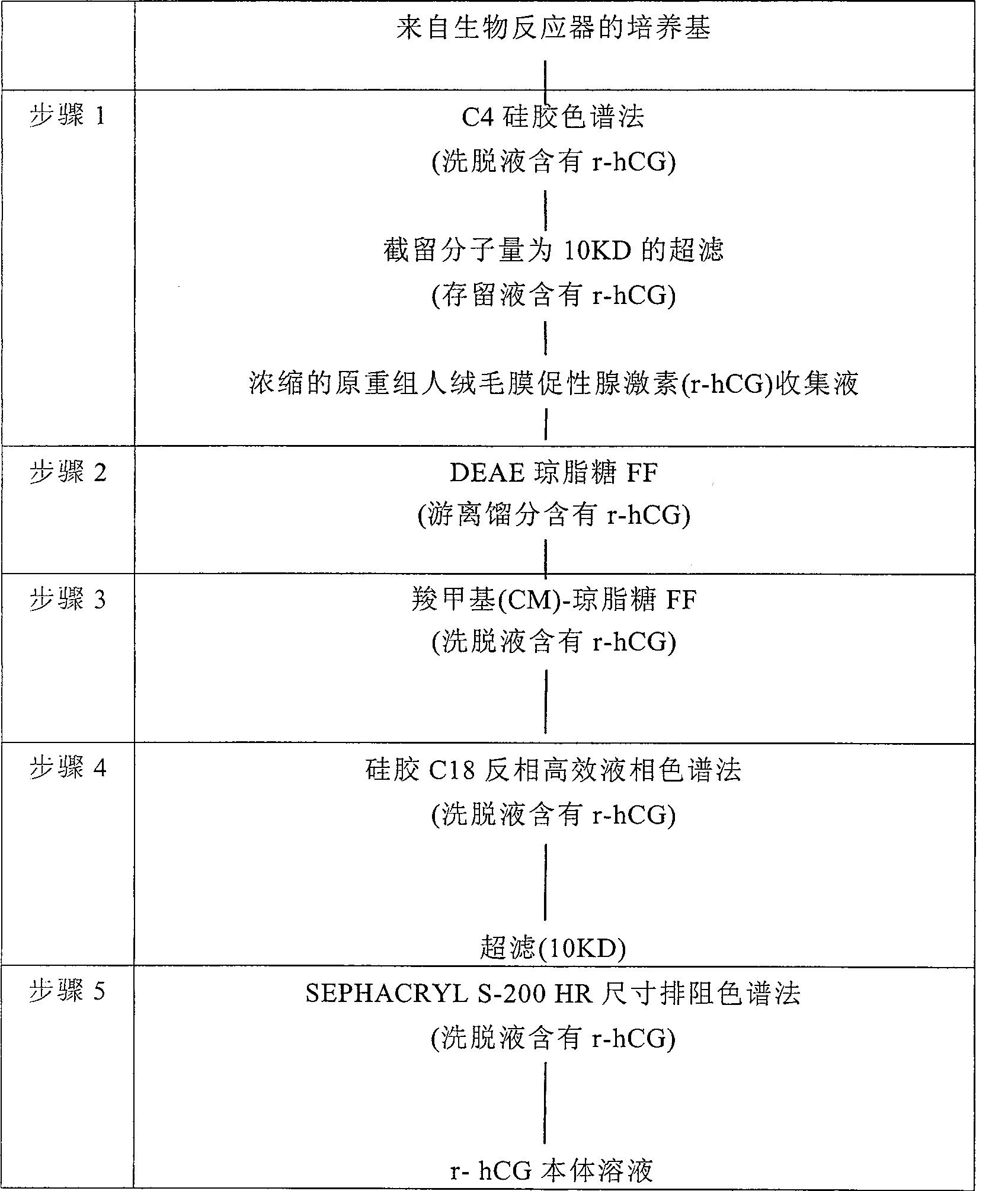
Process of purification of hCG and recombinant hCG purified by that method
InactiveCN100482679CPeptide/protein ingredientsOther chemical processesRecombinant Human Chorionic GonadotropinHCG - Human chorionic gonadotropin
A method for purifying recombinant human chorionic gonadotropin (hCG) from a raw recombinant human chorionic gonadotropin (hCG) sample of Chinese hamster ovary (CHO) cell supernatant, which includes the combined use of ion exchange chromatography and reversed-phase high-performance liquid Chromatography. Human chorionic gonadotropin is purified completely free of any contaminants by elution using ion exchange chromatography twice and finally by particle size exclusion chromatography. Highly pure human chorionic gonadotropin can be produced by this method, with a very high specific biological activity of approximately 25,000IU / mg.
Owner:MERCK SERONO SA
Feedstuff for cricetulus
InactiveCN1663427AFast growthHigh reproductive survival rateFood processingAnimal feeding stuffCricetulusHigh survival rate
The invention discloses a feedstuff for cricetulus comprising maize flour, rice flour, flour, wheat bran, bean powder, pig bone meal, calcium hydrogen orthophosphate, table salt, milk powder, white sugar, and multiple notorious minerals by a predetermined proportion. The invention realizes reasonable dispense prescription, low cost of preparation, fast speed of growth, and high survival rate in breeding.
Owner:INST OF SUBTROPICAL AGRI CHINESE ACAD OF SCI
Method for PCR primer design for sequence-unknown genes
InactiveCN102392015ASimple technologyAvoid Mass SequencingMicrobiological testing/measurementDNA preparationConserved sequenceNucleotide
The present invention belongs to the technical field of biological PCR detection, particularly a method for PCR primer design for sequence-unknown genes. By the principle that identical genes have conserved sequences across different species, the method performs nucleotide sequence alignment through BLAST software, so as to find the conserved sequence of a sequence-unknown gene and accordingly design a primer for PCR detection on the sequence-unknown gene. The present invention solves the problems of complex operation and high cost when PCR detection is performed on a sequence-unknown gene with the traditional method. In accordance with the cross-species PCR primer design method of the present invention, a sequence-unknown Chinese hamster gene is amplified successfully. The present invention has the advantages of simple feasible technical design, fewer operation limitations, wide application range, reliable results and low cost.
Owner:FUDAN UNIV
Culture medium for cloning formation of Chinese hamster ovary cells
PendingCN113122495AFast growthHigh rate of colony formationCulture processGerm cellsCricetulusBiochemistry
The invention provides a culture medium for cloning formation of Chinese hamster ovary cells. The culture medium provided by the invention does not contain serum or animal-derived components. The cloning culture medium provided by the invention can enable the CHO cells to be more compact in cloning agglomeration and less in diffusion, the growth speed of cloning is fast, and expression clones suitable for production and use purposes can be more efficiently selected.
Owner:SHANGHAI SINOMAB BIOTECHNOLOGY CO LTD +2
Method for recloning Chinese hamster ovary (CHO) cells
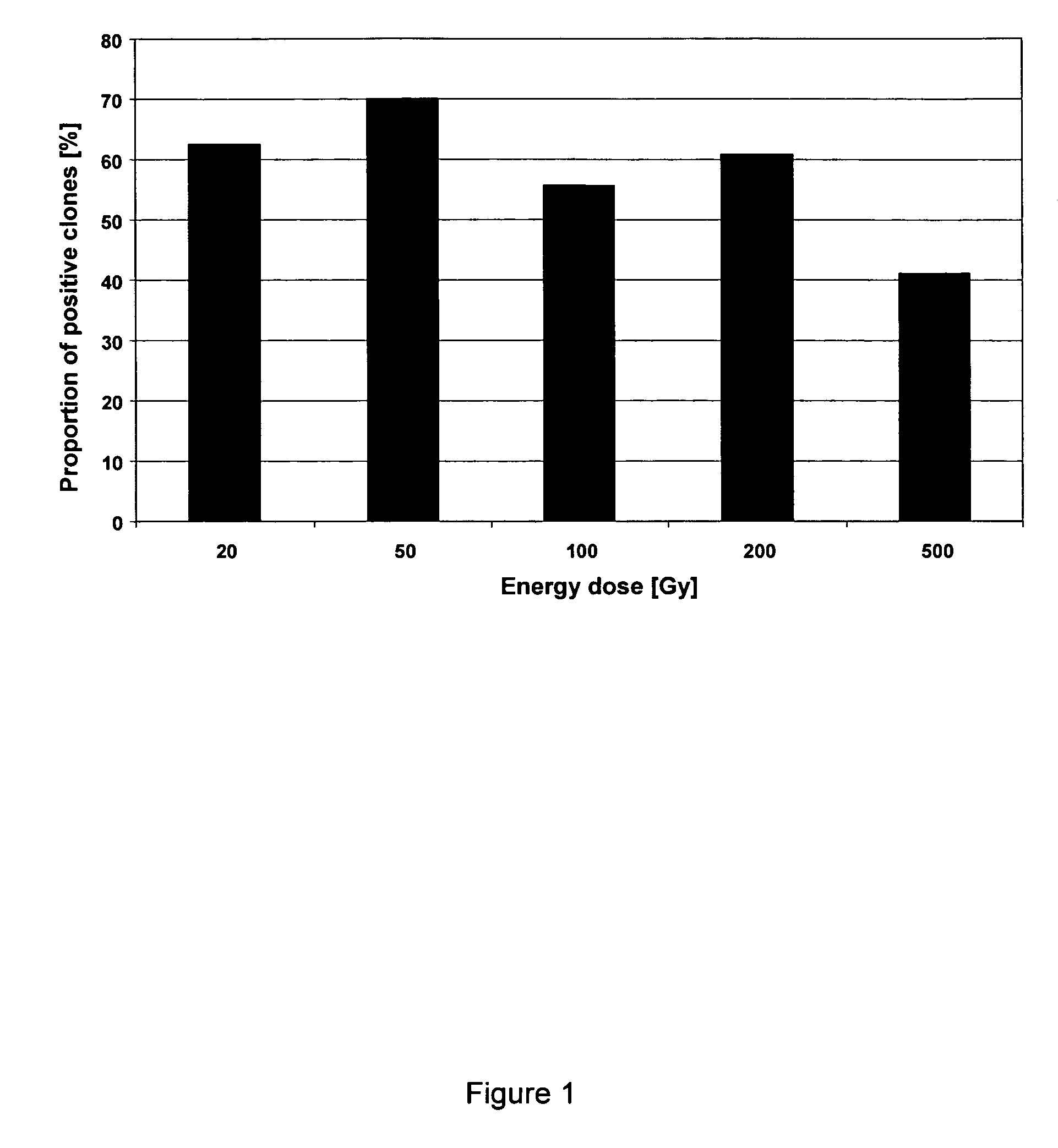
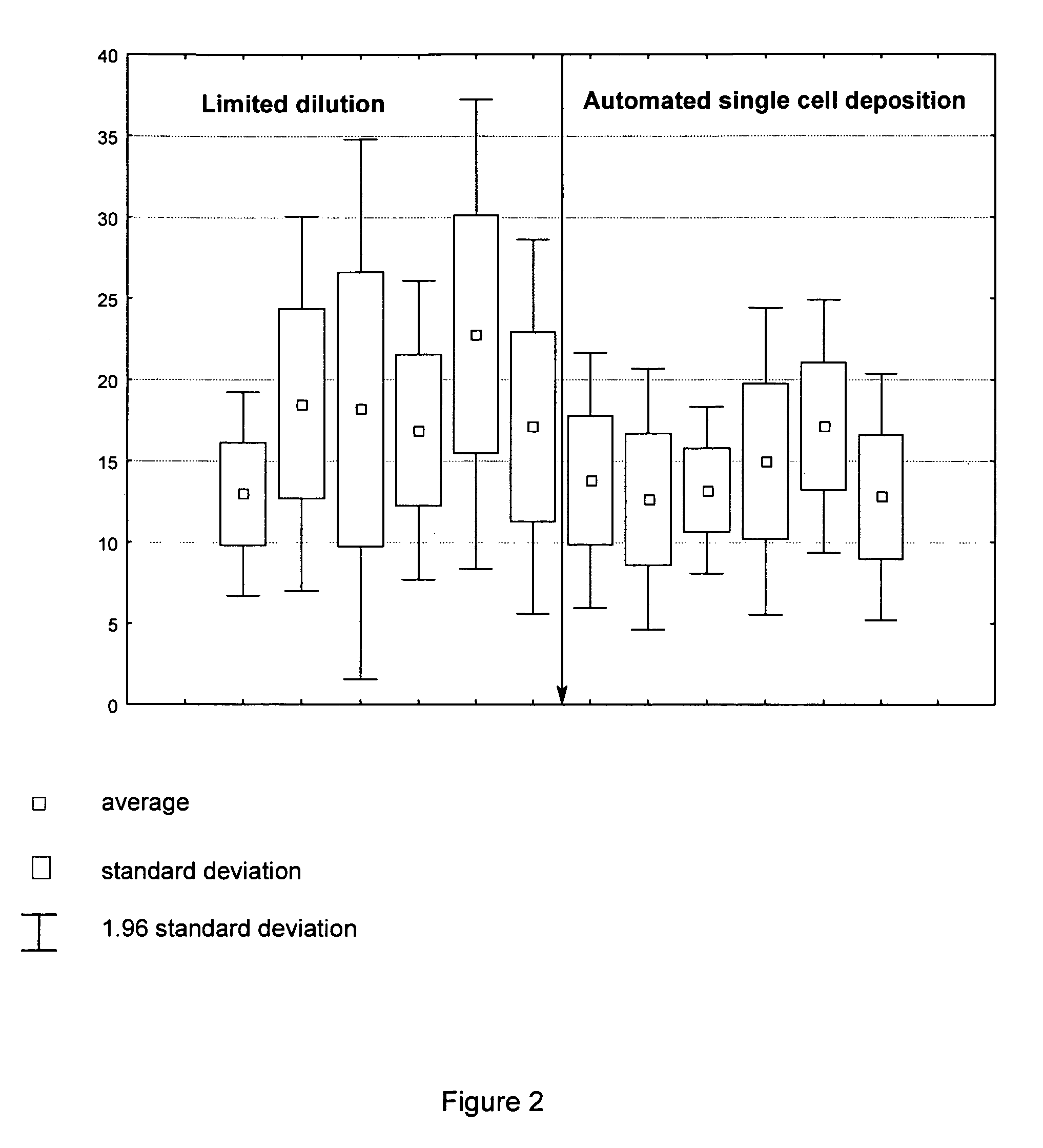
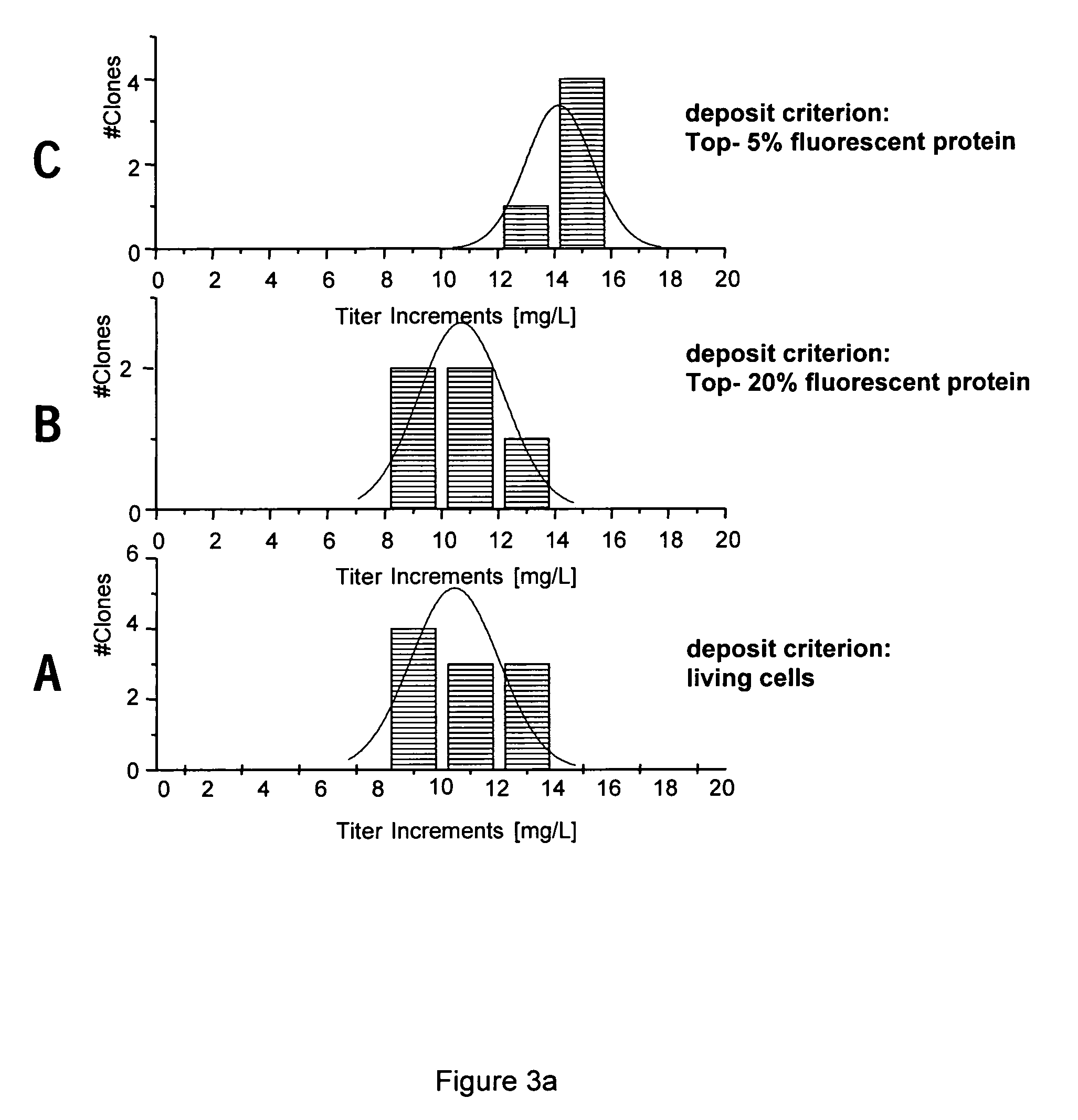
ActiveUS7833787B2Shorten development timeReduce riskGenetically modified cellsCulture processCricetulusBiopharmaceutical
A new method for selecting clones and recloning mammalian cells which are of importance for the production of biopharmaceuticals, preferably hamster or mouse myeloma cells, with a high degree of automation and throughput. The invention relates to methods of depositing and replicating single cell clones of the cells in question. The invention also relates to methods of preparing proteins using cells which have been obtained and replicated by single cell deposition as well as compositions which allow the replication of single cells.
Owner:BOEHRINGER INGELHEIM PHARM KG
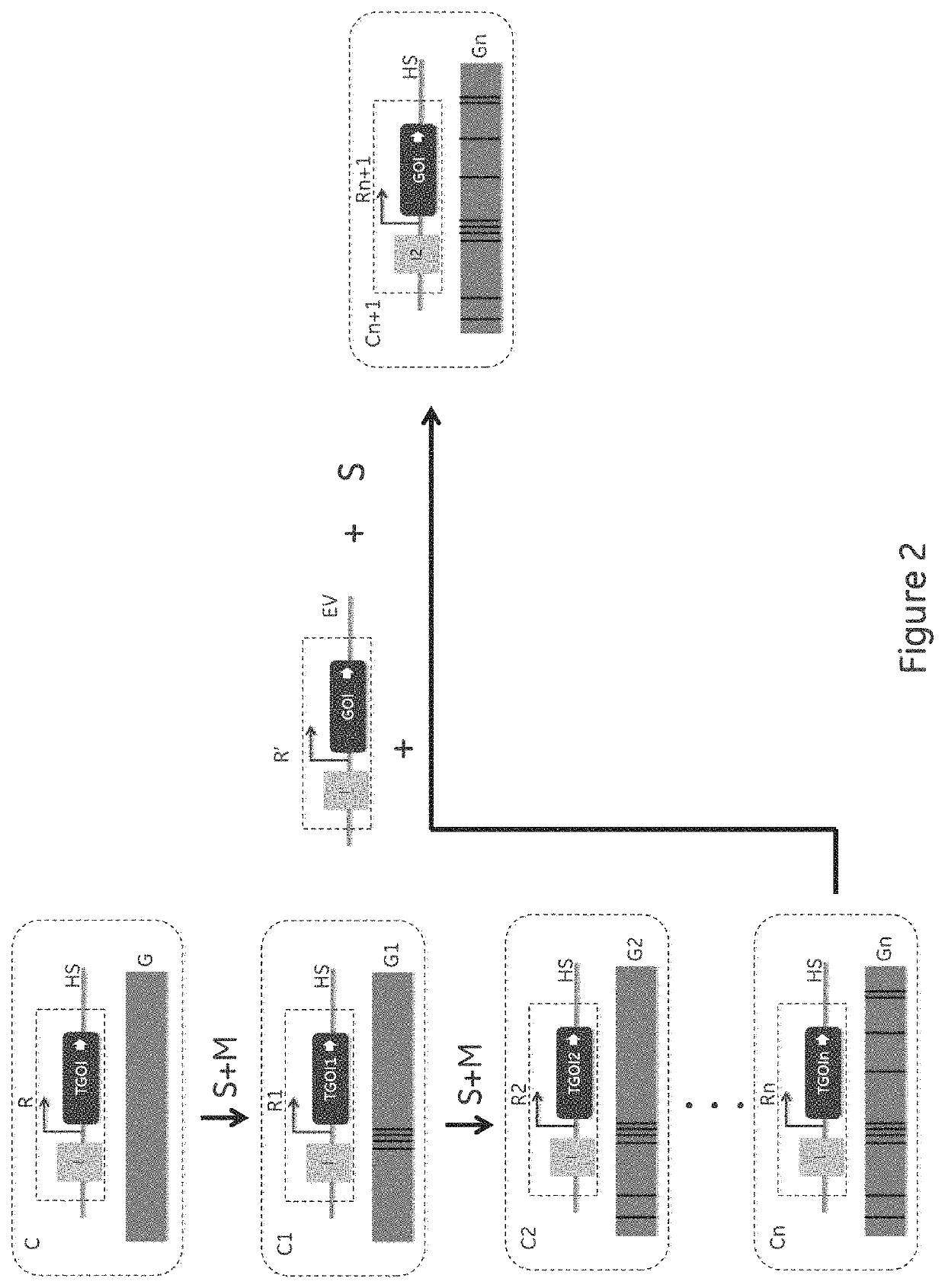
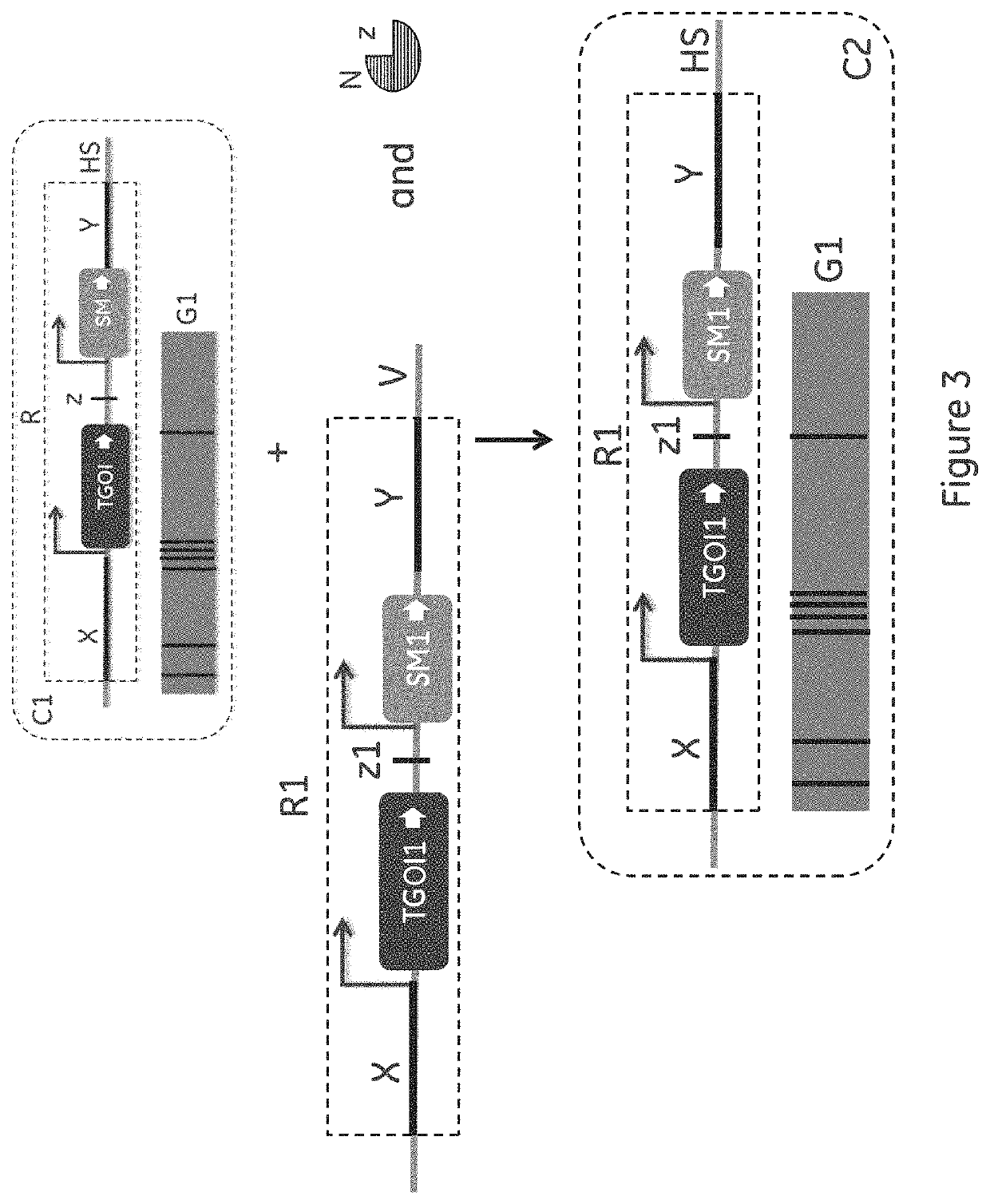
Method for cell line development
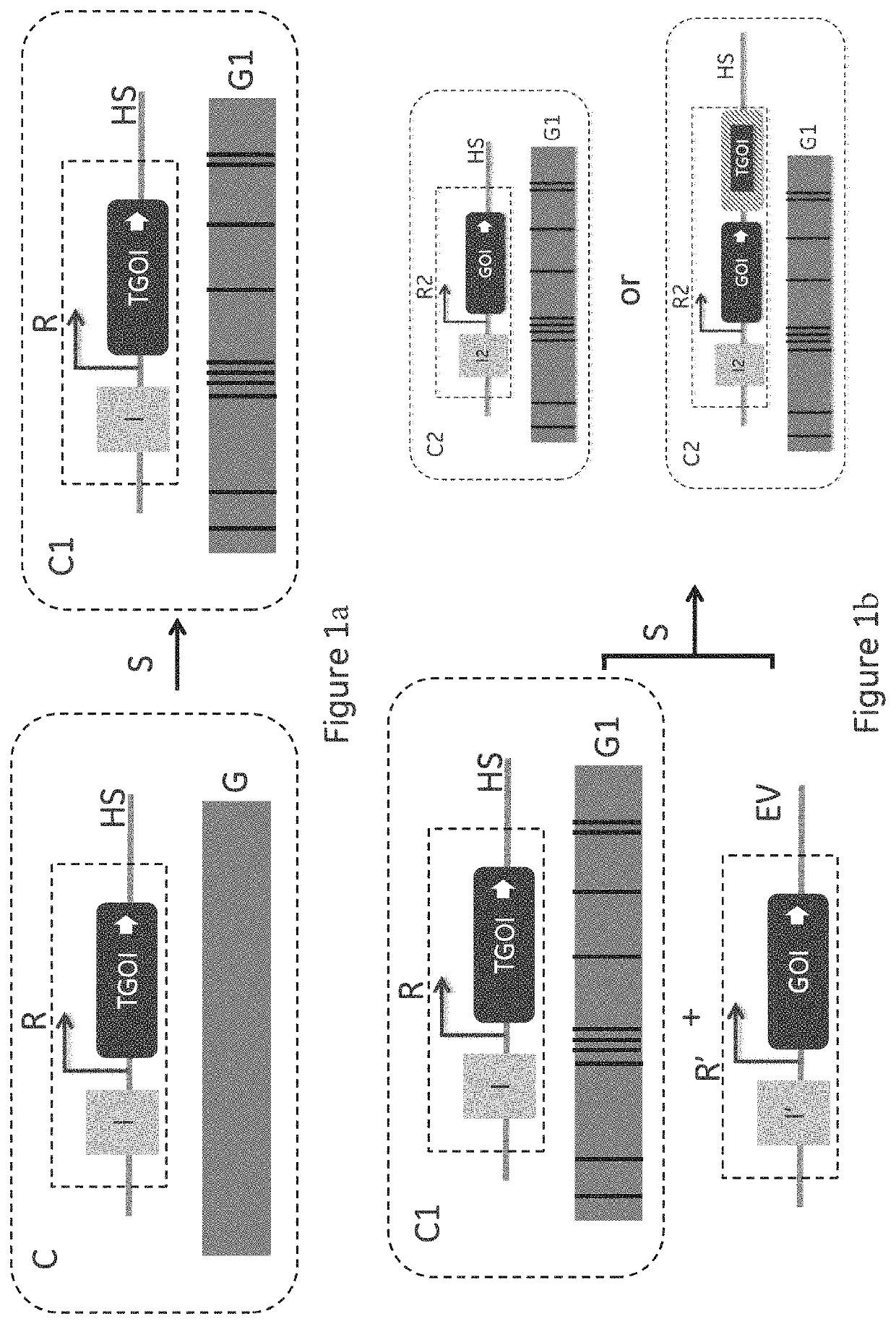
ActiveUS11136598B2Improving post-transcriptional processingInnovative designHydrolasesStable introduction of DNABiotechnologyCompetent cell
The present invention relates to an improved method for cell line development which is generally applicable to production of any therapeutic protein that can be produced using mammalian Cell lines and in particular Chinese Hamster Ovary (CHO) cells. The method combines site directed integration (SDI), expression construct components improving the post-transcriptional processing of the gene of interest (GOI), novel design of the GO genome target location and the introduction of a onetime pre-CLD host cell line selection workflow to generate a production competent cell line that can then be used in multiple CLD efforts from that point on.
Owner:CYTIVA SWEDEN AB
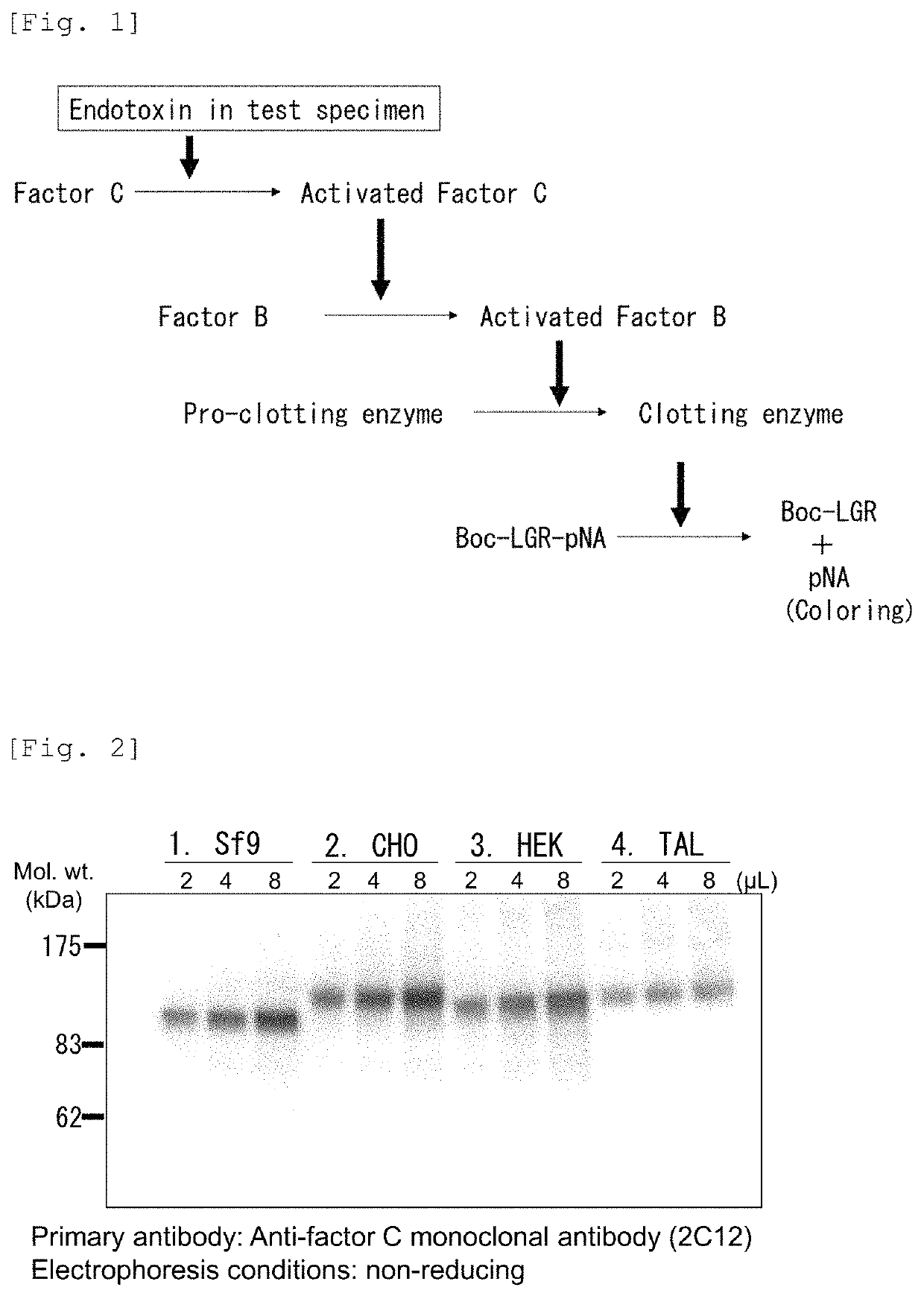
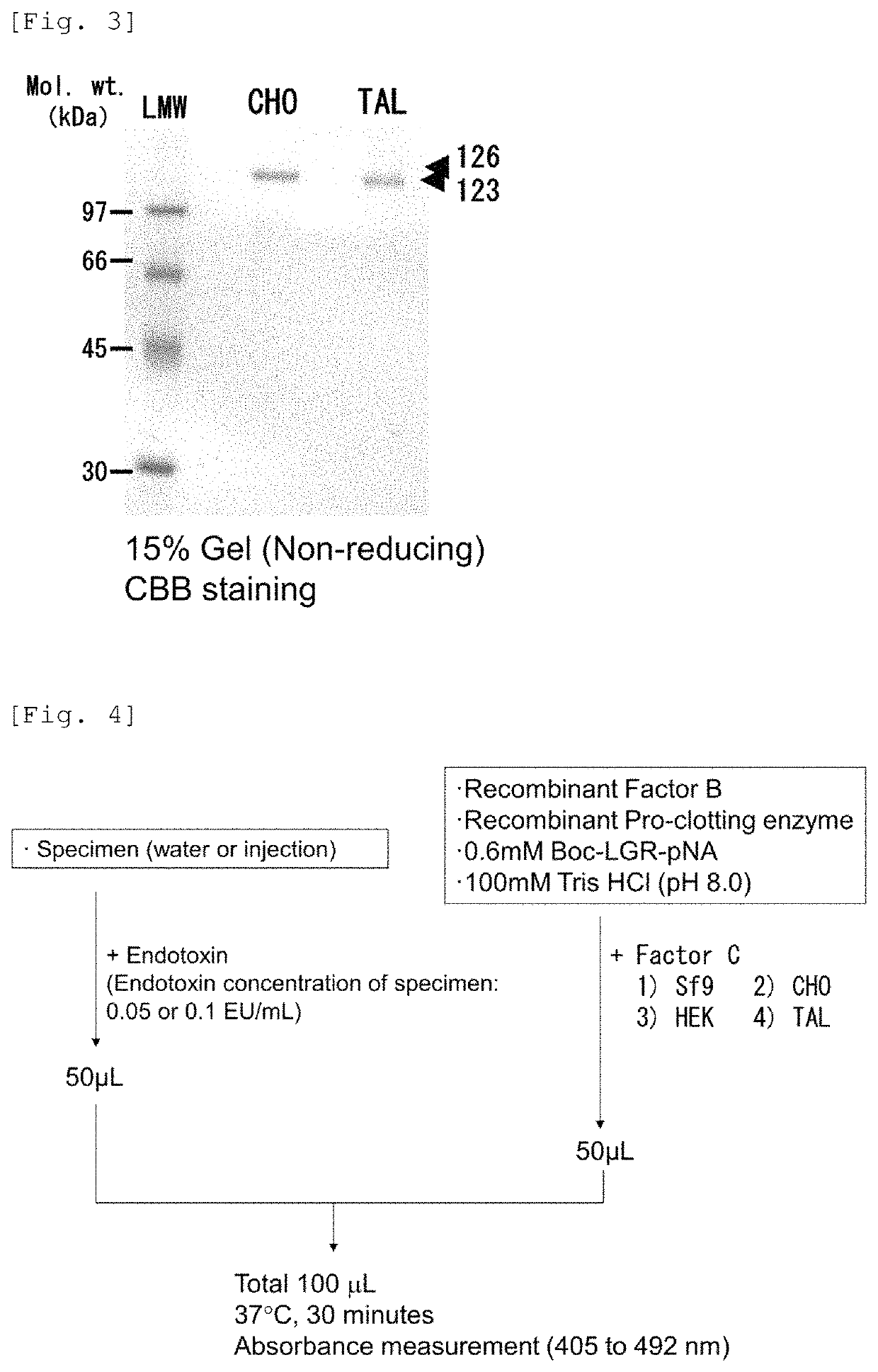
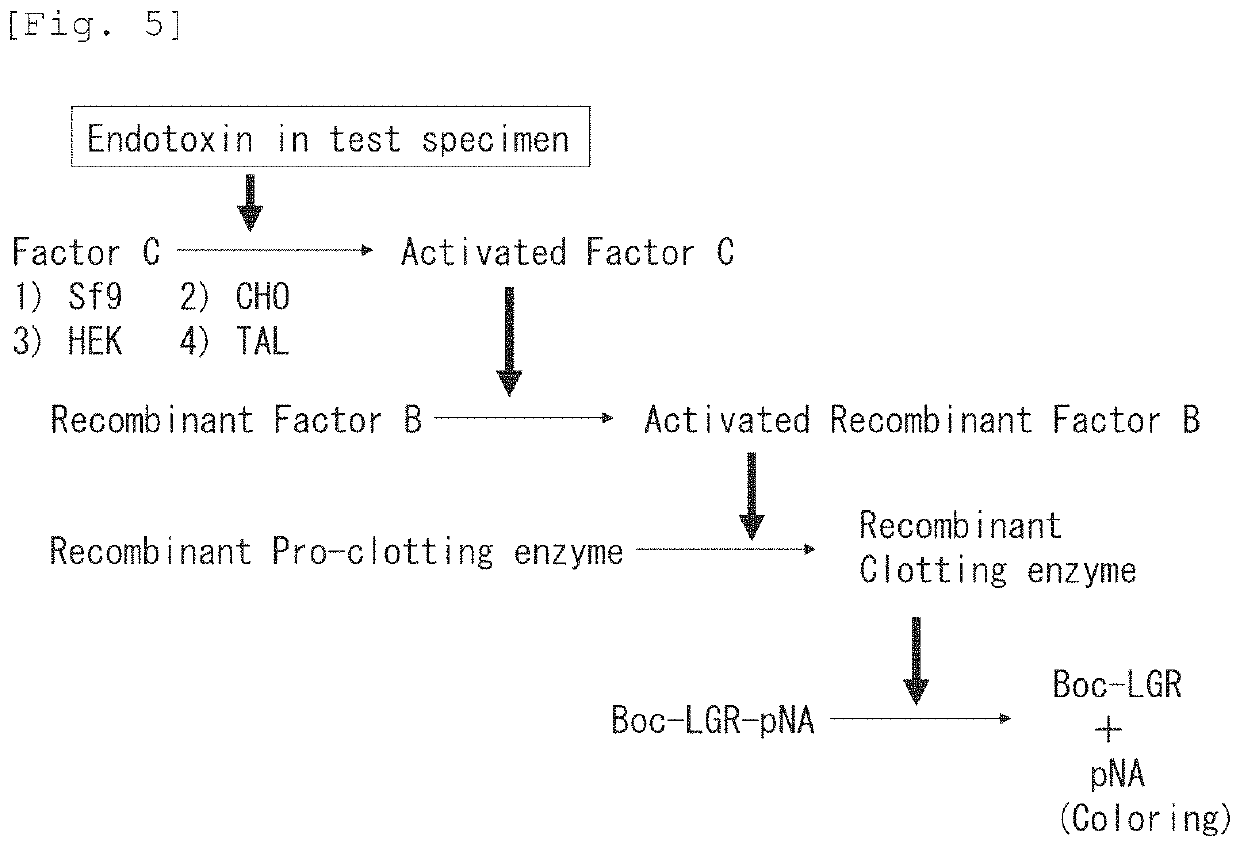
Recombinant Factor C and method for producing the same, and method for measuring endotoxin
ActiveUS11236318B2Microbiological testing/measurementBiological material analysisPolyphemusHorseshoe crab factor C
A horseshoe crab Factor C protein having activity of Factor C, wherein the horseshoe crab is selected from Tachypleus tridentatus, Limulus polyphemus, and Carcinoscorpius rotundicauda, and wherein the horseshoe crab Factor C protein is produced through being recombinantly expressed from a Chinese Hamster Ovary (CHO) DG44 cell or HEK cell.
Owner:SEIKAGAKU KOGYO CO LTD
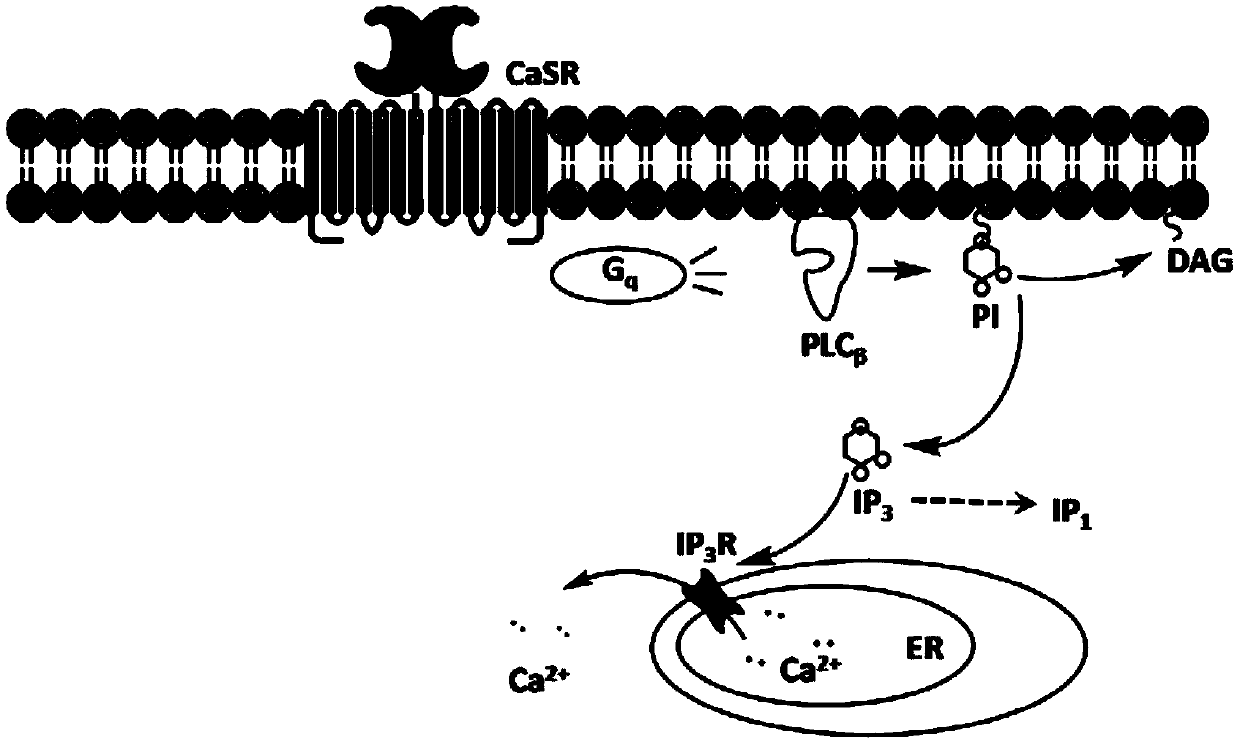
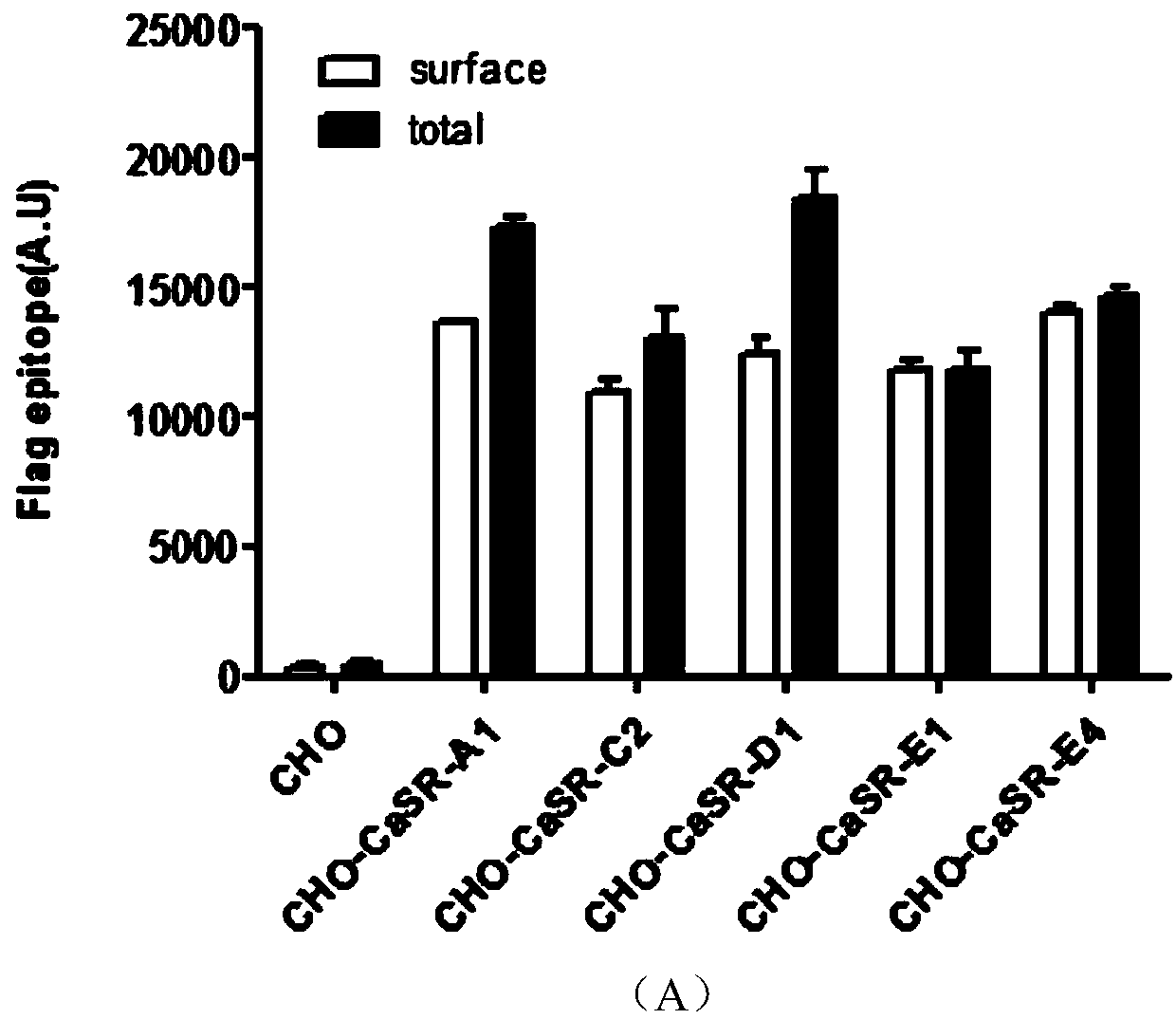
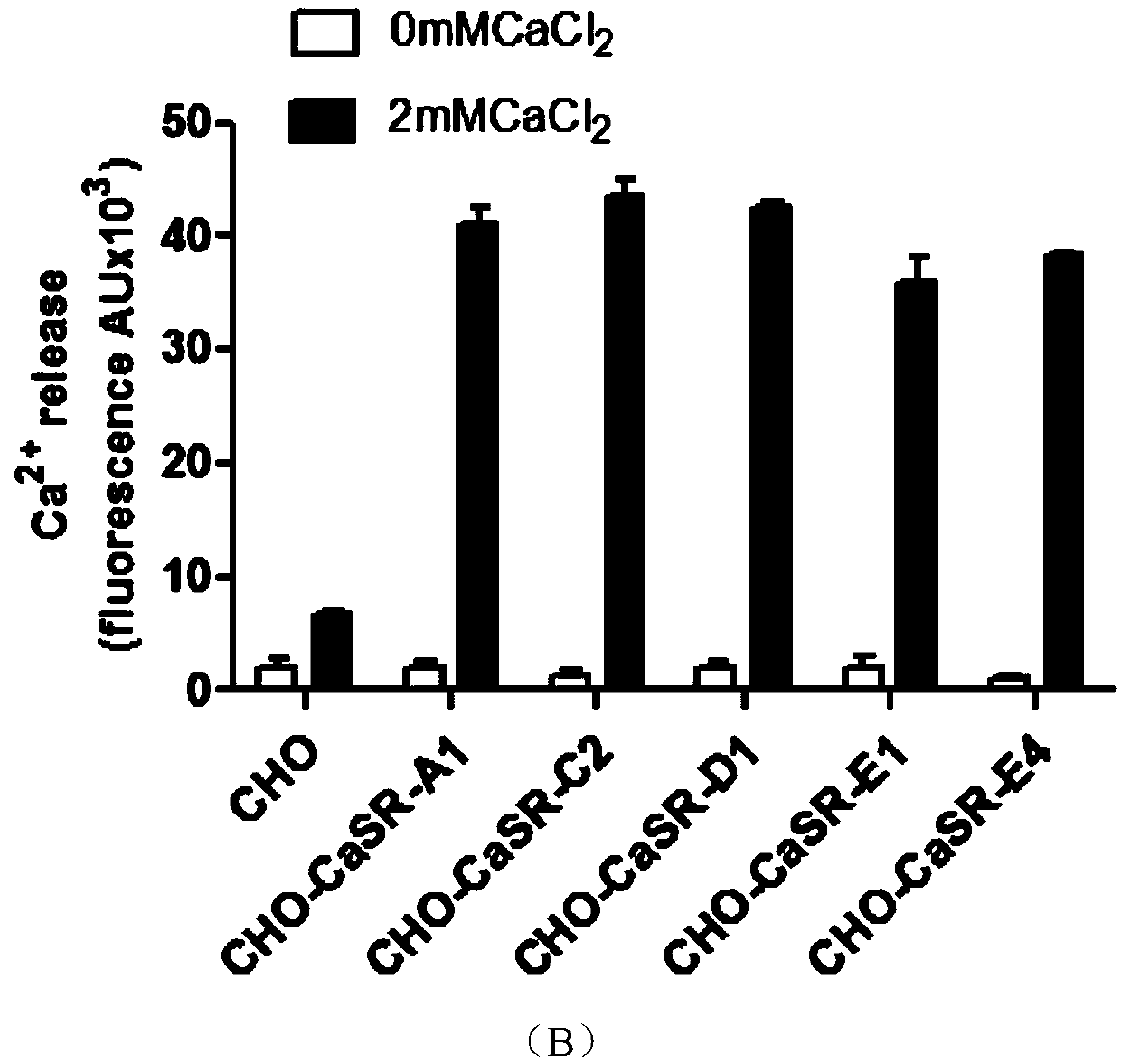
Cell model stably expressing human-derived CaSR gene and construction method of cell model
InactiveCN110656088ALow costImprove throughputGenetically modified cellsDiagnosticsSNAP-tagCricetulus
The invention provides a cell model stably expressing a human-derived CaSR gene. The cell model is preserved in China Center for Type Culture Collection and named as Chinese hamster ovary cell CHO-CaSR, with a preservation number being CCTCC NO: C201893. A construction method of the cell model is as follows: a SNAP tag and a CaSR gene are linked by a linking sequence cacgcgt to obtain the CaSR gene fused with the SNAP tag, the CaSR gene is linked with an expression vector to construct a recombinant plasmid containing the CaSR gene, the recombinant plasmid is transfected into mammalian cells, and a cell strain capable of stably expressing the CaSR gene is cultured by drug screening. The cell model can be used for screening CaSR-targeted active substances, and can also be used in conjunctionwith homogeneous time resolved fluorescence (HTRF) through SNAP-tag to accurately and quickly study interaction of CaSR with other proteins and oligomerization of the CaSR. The cell model can screenhigh-throughput and low-cost active substances targeting the CaSR, and the screening method is simple and controllable in steps, low in cost, high in throughput, and very good in stability and reliability.
Owner:HUAZHONG UNIV OF SCI & TECH
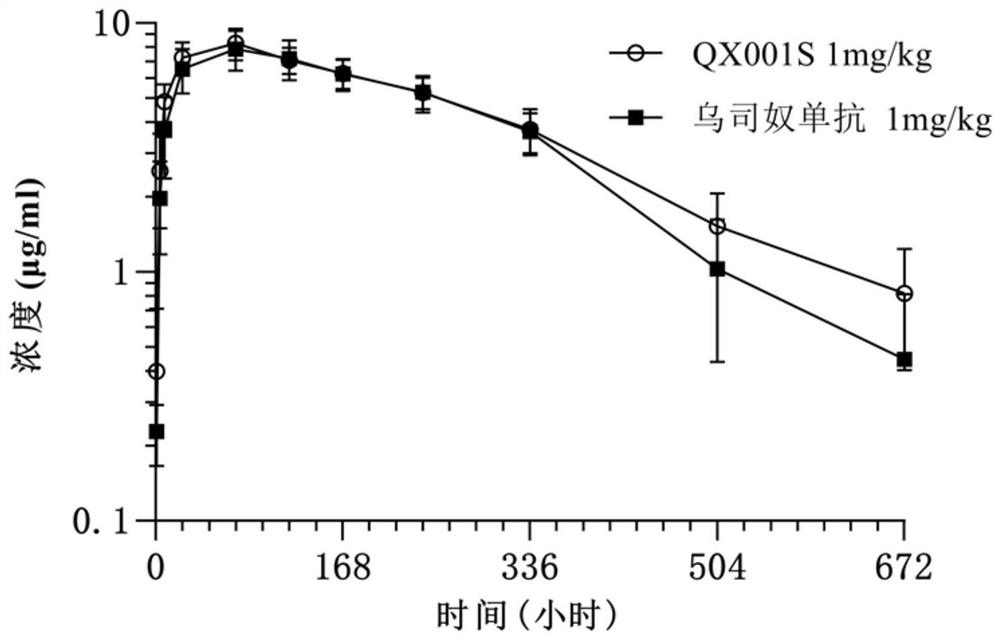
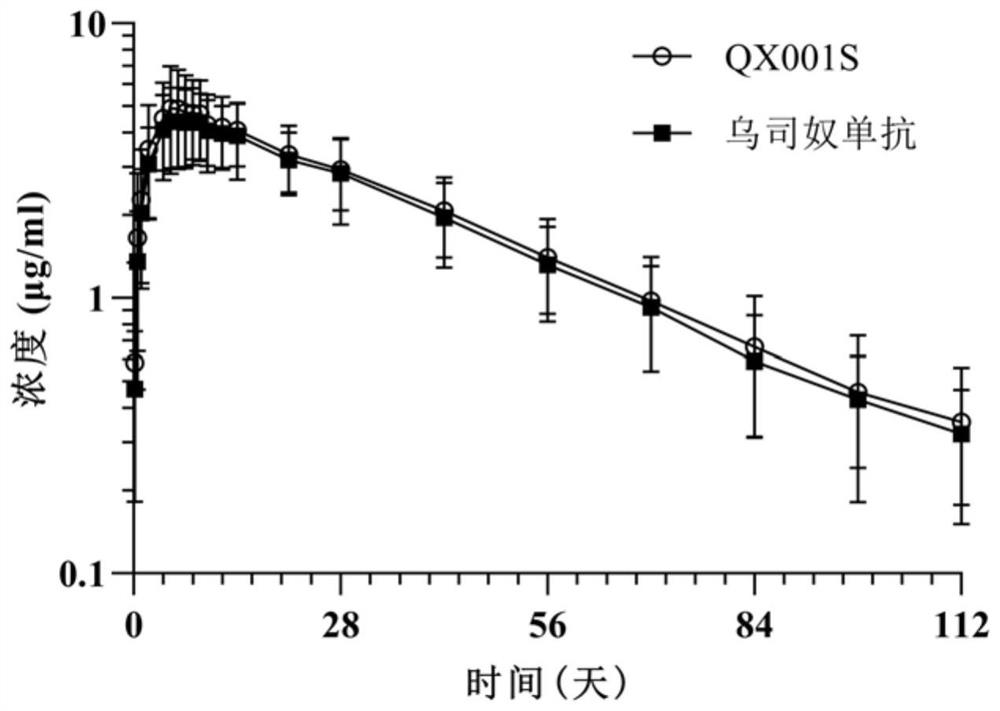
Cell strain for producing biosimilar drug of Ustekinumab and production method thereof
ActiveCN112852743AFacilitate rapid progressGenetically modified cellsImmunoglobulins against cytokines/lymphokines/interferonsUstekinumabAntiendomysial antibodies
The invention discloses a cell strain for producing a biosimilar drug of Ustekinumab and a production method thereof. The Chinese hamster ovary cell S cell strain is preserved in the China Center for Type Culture Collection (CCTCC), and the preservation number is CCTCC NO: C2020232. The cell strain expresses a fully human monoclonal antibody resisting a p40 sub-unit shared by human IL-12 and human IL-23. The fully humanized monoclonal antibody for resisting the p40 sub-unit shared by the human IL-12 and the human IL-23 is a biosimilar drug of Ustekinumab, not only shows high consistency with the Ustekinumab in pre-clinical research, but also passes pharmacokinetic bio-equivalence and safety similarity evaluation in clinical research, and the provided biosimilar drug of Ustekinumab is the first one that enter the clinical test period in China, is the only one that completes the I stage clinical test, and is one of the biosimilar drugs of Ustekinumab, which are the fastest in application in the international.
Owner:QYUNS THERAPEUTICS CO LTD
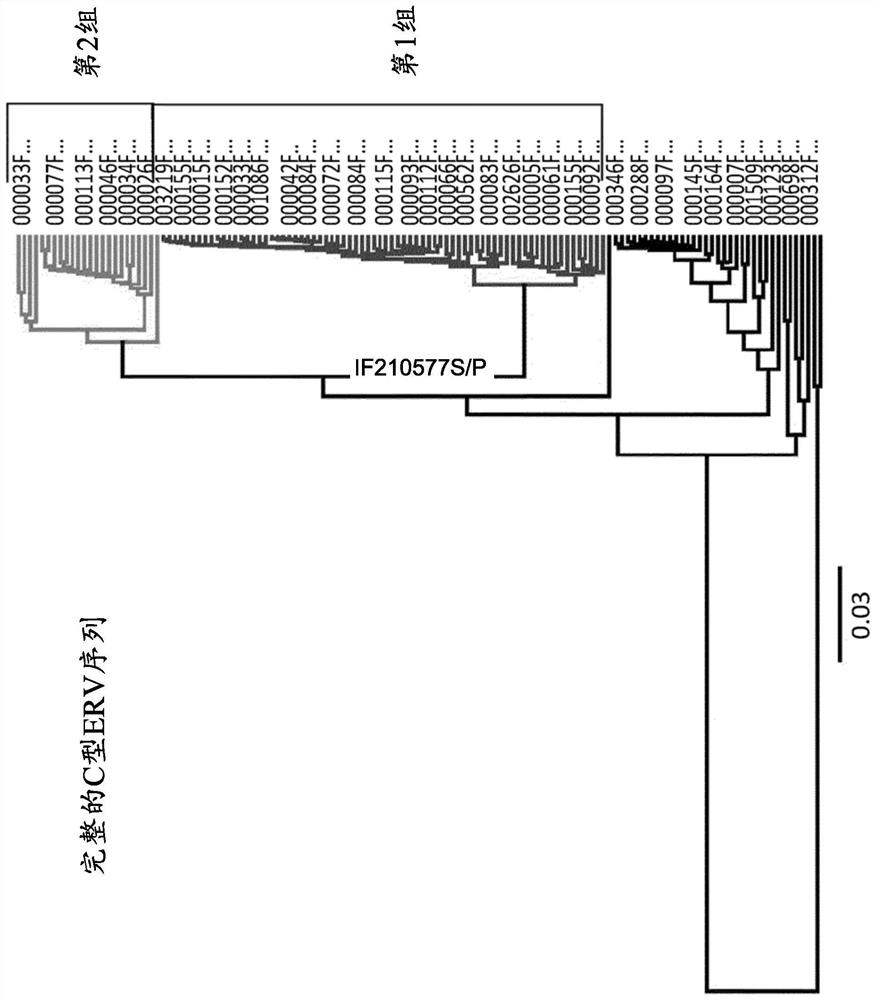
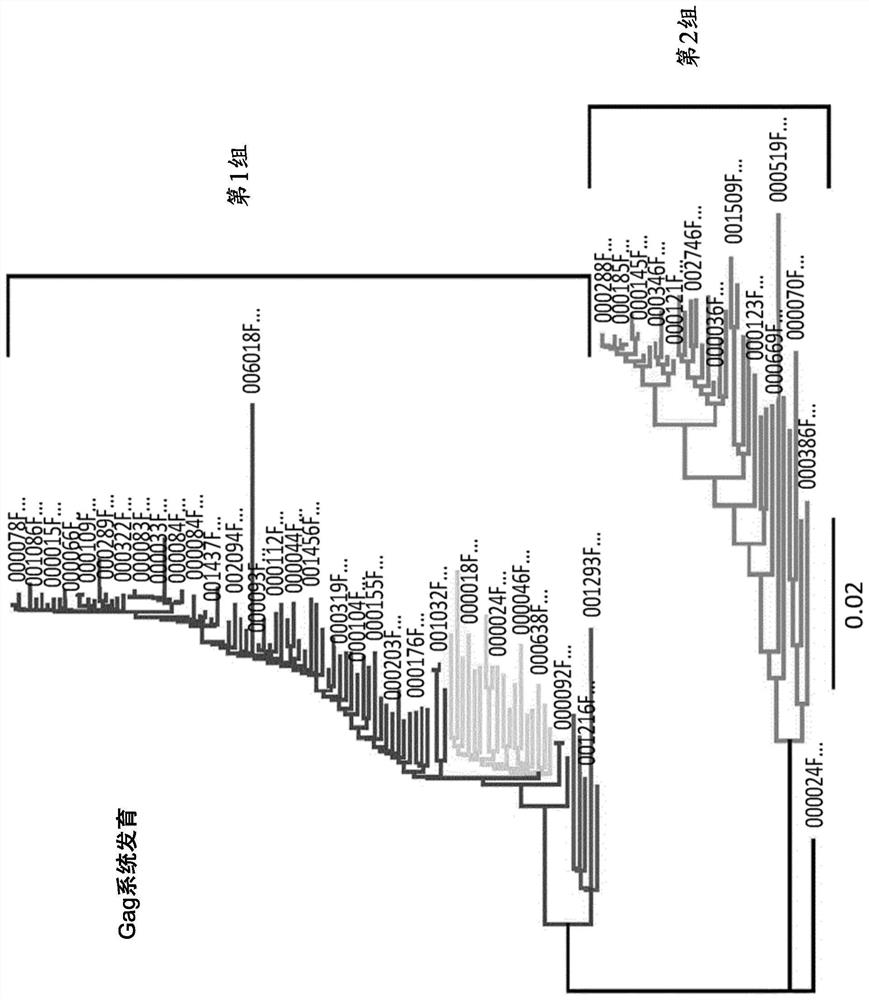
Characterization and inactivation of endogenous retroviruses in chinese hamster ovary cells
Type-C endogenous retroviruses (ERVs) embedded in Chinese hamster ovary (CHO) cells were altered to modify the release of retroviral and / or retroviral-like particles in the culture supernatant. Although evidence for the infectivity of these particles is missing, their presence has raised safety concerns. 173 type-C ERV sequences that clustered into functionally conserved groups were identified. Transcripts from one type-C ERV group were identified to be full-length with intact open reading frames, and to have corresponding viral RNA genomes that were loaded into retroviral-like particles. Also, sequence analysis of the genomic RNA from viral particles indicated that they may result from few expressed ERV sequences. Disclosed herein is the disruption / alteration of the gag gene of the expressed ERV group using CRISPR-Cas9 genome editing. Comparison of CRISPR-derived mutations at the DNA and mRNA level led to the identification of a single ERV locus responsible for the release of viral RNA-loaded particles from CHO cells. Clones bearing a Gag loss-of-function mutation in this particular ERV locus showed a reduction of viral RNA-containing particles in the cell supernatant by over 250-fold. Notably, ERV mutagenesis did not compromise cell growth, cell size or recombinant protein production. Provided herein is a new strategy and cells, in particular engineered CHO cells, to mitigate potential contaminations from CHO endogenous retroviruses during biopharmaceutical manufacturing.
Owner:SELEXIS SA
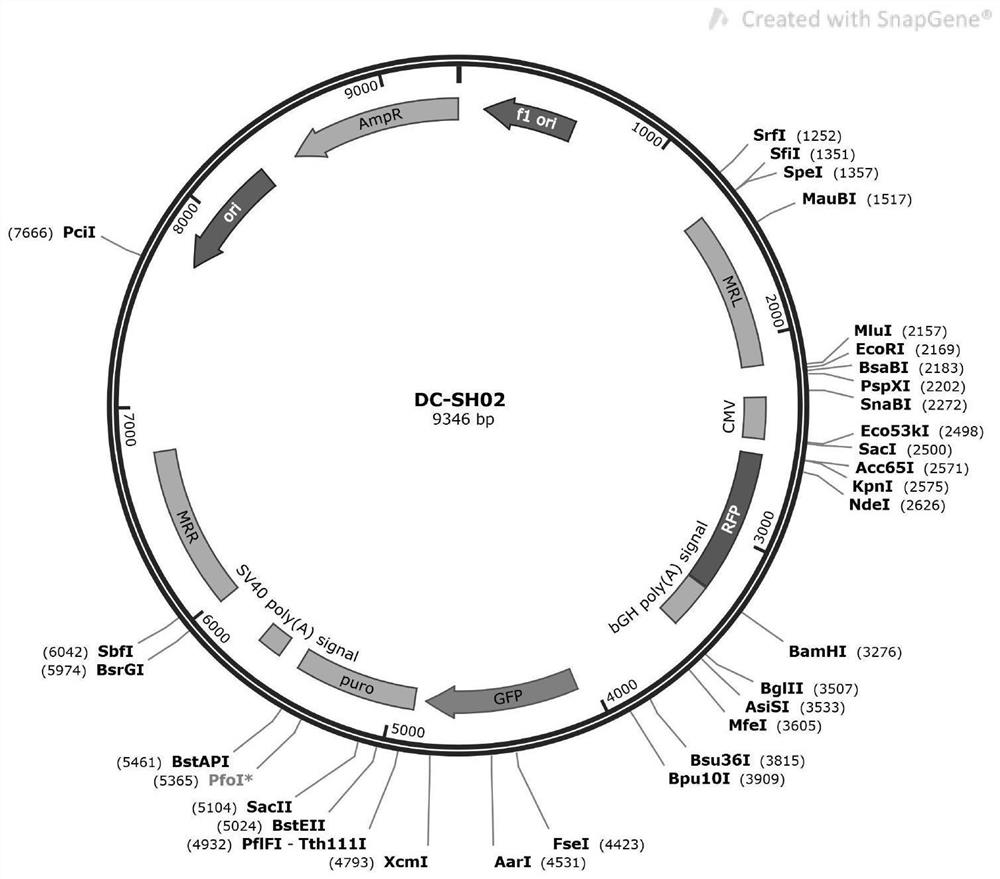
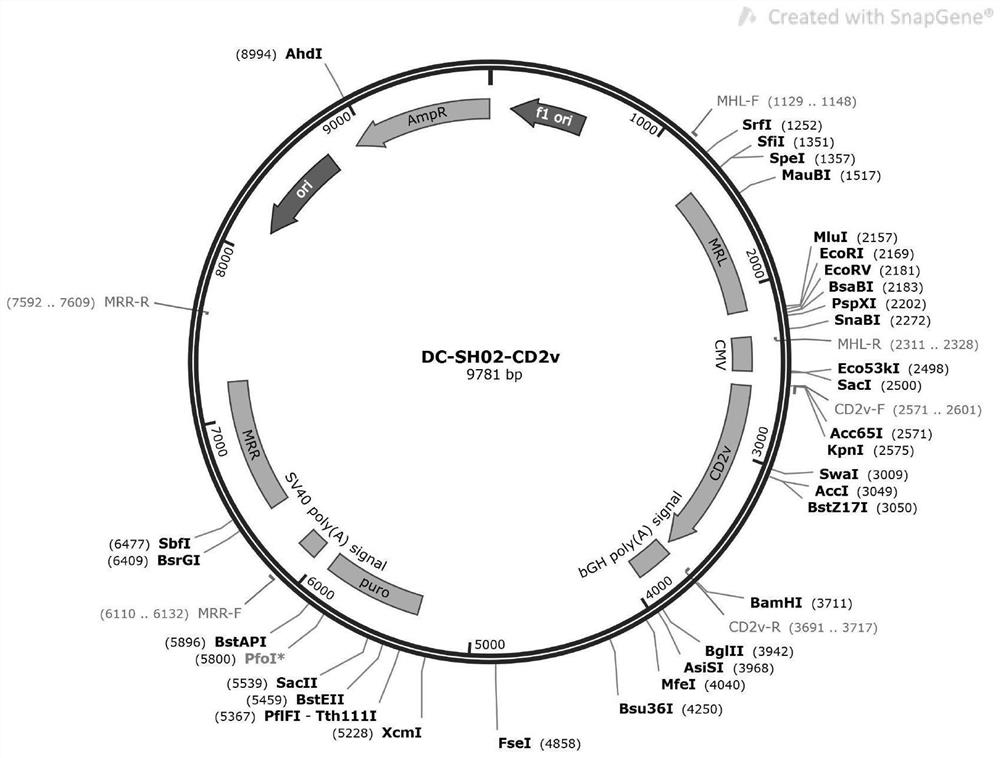
CHO cell line capable of stably expressing African swine fever CD2v protein as well as construction method and application of CHO cell line
PendingCN114107176ADoes not affect normal physiological functionSimplify the build processVirus peptidesDrug screeningAfrican swine feverVaccine Production
The invention belongs to the technical field of cell recombination and protein expression, particularly relates to a CHO cell line for stably expressing African swine fever CD2v protein as well as a construction method and application of the CHO cell line, and discloses the CHO cell line for stably expressing the African swine fever CD2v protein, which is a Chinese hamster ovary cell CHO-CD2v. The strain is sent to Guangdong Microbial Culture Collection Center for preservation on November 11, 2021, and the preservation number is GDMCC No: 62052. According to the cell line, an exogenous gene CD2v is integrated into a known safe site in a Chinese hamster genome, so that normal expression of a transferred gene is realized without influencing normal physiological functions of cells, and the cell line is simple in construction process and high in cloning accuracy. The cell line can be used in the aspects of high-yield CD2v protein, drug effect screening of African swine fever, vaccine production and the like, and has very important significance in diagnosis of African swine fever and vaccine production.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
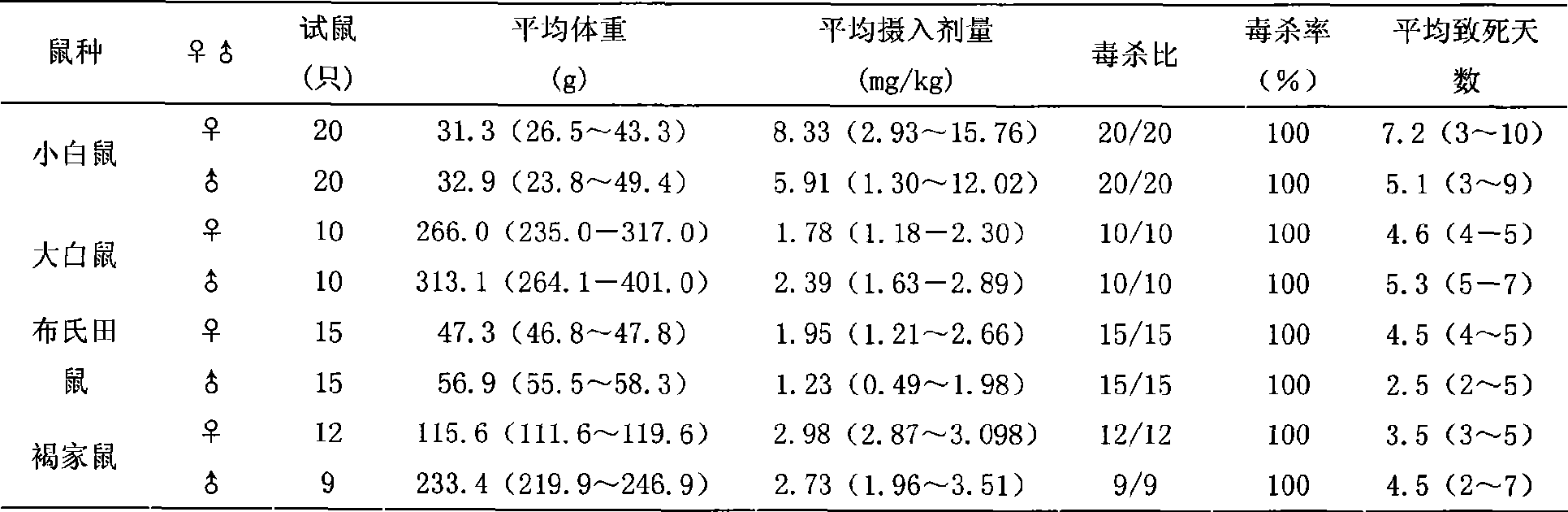
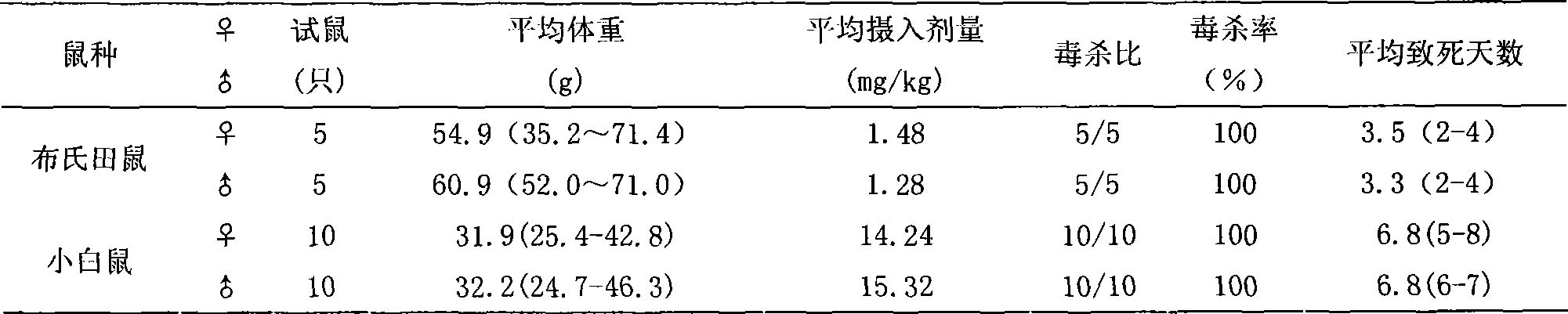
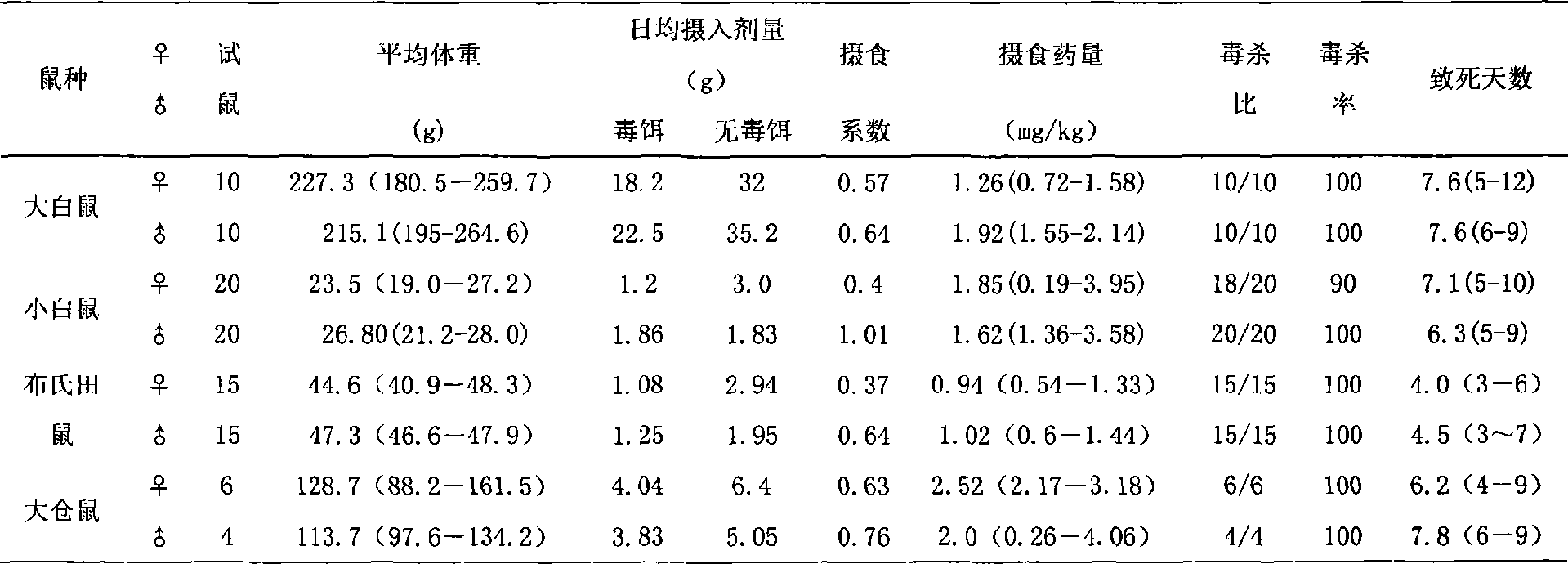
Rat eradication agent
The invention relates to development and application of anticoagulative compound synergistic rodenticide. The rodenticide mainly comprises bromadiolone and sodium dichlorophenolate, and is added with metronidazole, ethanol, glycerin and the like. The rodenticide can be prepared into liquid and powder. Bioassay shows that the rodenticide has good toxic effect for albino rats, mice, sewer rats, cricetulus tritons, microtus brandtis and apodemus agrariuses; the death ratio basically reaches 100 percent; and a large-are field test proves that prevention ratio for the sewer rats reaches more than 96 percent. When the bromadiolone content in the rodenticide is 0.002 percent, the control effect is better than or equal to the control effect of 0.005 percent of bromadiolone, so that cost is lowered greatly, environmental pollution is reduced, human and animal safety is ensured, and the national policy of energy saving and discharge reduction is firmly implemented. The rodenticide has the advantages of simple process and easy production, has special effect for antagonistic bandicoots, is fully suitable for control of various bandicoots, can be directly converted into productivity, and acquires economic benefit and social benefit as soon as possible.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com