Selection method of signal peptide while foreign protein is expressed by Chinese hamster ovary cells and application thereof
A technology of ovary cells and Chinese hamsters, applied in the biological field, can solve the problems of cleavage of target protein amino acids, cleavage site offset, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
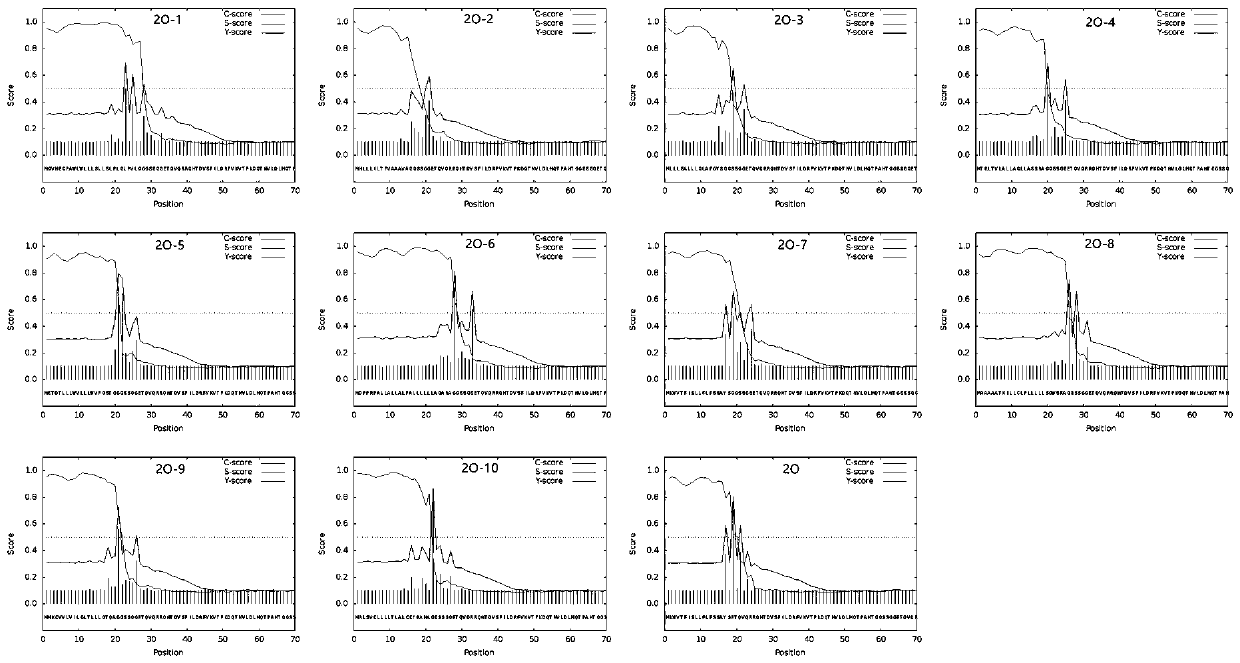
[0021] Informatics Analysis of Example 1 Signal Peptide Cleavage Site
[0022] Select 21-60AA, 141-160AA and 200-213AA of VP1 protein of type O foot-and-mouth disease (Gen Bank: QDJ95689.1) as dominant epitopes, and connect them rationally to construct a multi-epitope gene expression cassette. To prevent the formation of new epitopes after the ligation of two adjacent epitopes, a GGSSGG spacer sequence was introduced between the epitopes to ensure the correctness of the target antigenic site. The nucleotide sequences of signal peptides capable of effectively secreting and expressing foreign proteins were selected from the reported literature, and their nucleotide sequences were shown in Table 1, and they were connected with the multi-epitope gene expression cassette. The GGSSGG spacer and no GGSSGG spacer were introduced between the signal peptide and the multi-epitope gene expression cassette, and the amino acids of the recombinant protein were analyzed using analysis tools (...
Embodiment 2
[0023] Example 2 Construction of pCI-neo-O eukaryotic expression vector
[0024] 1. Synthesis of multi-epitope gene expression cassette
[0025] According to the gene sequence of O-type FMDV (Gen Bank: QDJ95689.1), 21-60AA, 141-160AA and 200-213AA on O-type FMDV VP1 were respectively selected as dominant epitopes, and each epitope was repeated twice in series, Linked through the linker sequence GGSSGG to form a new epitope cassette, the sequence of which is shown in SEQ ID: 01. And the gene sequence of the tandem epitope cassette was sent to Suzhou Jinweizhi Biotechnology Co., Ltd. for synthesis.
[0026] 2. The signal peptide is connected to the multi-epitope gene expression cassette
[0027] The selected 10 different secretory signal peptides were connected in series with the synthesized multi-epitope gene expression cassette by PCR method, among which Serum albumin preproprotein-F1 and Serum albumin preproprotein-F2 signal peptides were the same, but Serum albumin preprop...
Embodiment 3
[0030] Example 3 Recombinant protein accumulation analysis
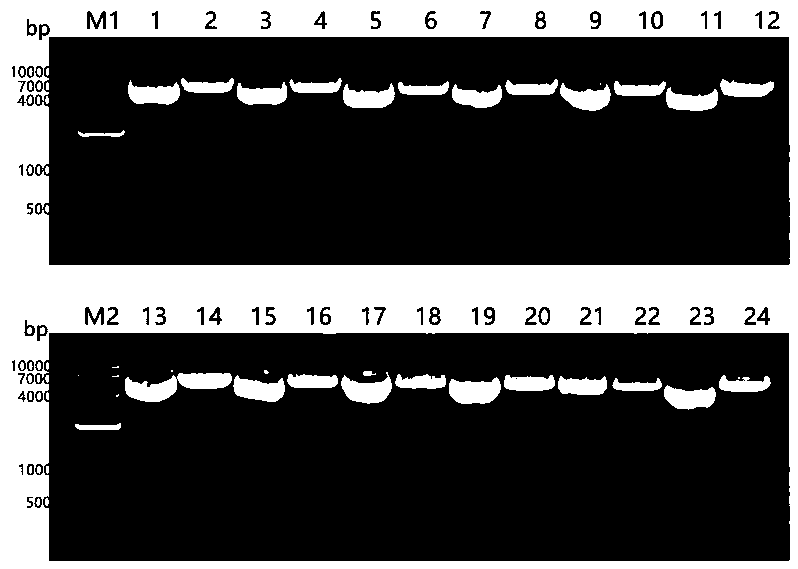
[0031] 1. Transfection of recombinant plasmids into CHO cells
[0032] The pCI-neo-O recombinant plasmid was transfected into CHO cells according to the instructions of the Lipofectamine 3000 kit. After 8 hours of transfection, the high-glucose DMEM medium containing 10% serum was replaced, and the culture was continued.
[0033] 2. Western blot identification of recombinant epitope protein accumulation in CHO cell culture medium
[0034] 48 hours after transfection, the CHO cell culture medium was collected, mixed with PMSF to a final concentration of 0.001mol / L, centrifuged at 800r / min for 5min to remove cell debris, and the supernatant was taken for BCA protein quantification. With a total protein amount of 60 μg, it was transferred to NC membrane by SDS-PAGE. Block with 5% skim milk for 2 hours at room temperature, add rabbit anti-FMD virus type O serum, incubate overnight at 4°C; wash 5 times with PBST for 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com