Cell strain for producing biosimilar drug of Ustekinumab and production method thereof
A cell line and ovarian cell technology, applied in the field of biosimilar drugs for the production of ustekinumab, can solve the problems of physical and chemical properties, differences in biological activity, metabolic behavior in vivo, inability to pass similarity, low similarity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: vector construction
[0049] The heavy chain amino acid sequence (SEQ ID NO: 1) and light chain amino acid sequence (SEQ ID NO: 3) of ustekinumab were determined through public information query and peptide analysis. (Such as figure 1 )
[0050] The heavy chain amino acid sequence and light chain amino acid sequence of QX001S, which is a biosimilar drug of ustekinumab, are completely identical to ustekinumab.
[0051] Using the heavy and light chain amino acid sequence of ustekinumab as a template, the heavy and light chain gene sequence of QX001S was designed, and the expression plasmid was constructed.
[0052] The heavy chain gene sequence of the codon-optimized QX001S is SEQ ID NO: 2 (as shown in Figure 4).
[0053] The light chain gene sequence of the codon-optimized QX001S is SEQ ID NO: 4 (as shown in Figure 5).
[0054]In the expression plasmid system, the codon-optimized heavy chain gene and light chain gene of QX001S were respectively construc...
Embodiment 2
[0056] Embodiment 2: Screening of cell lines
[0057] Confirm that the constructed QX001S expression vector gene sequence and expressed protein are correct, and use CHO-S as the host cell to construct the stable expression cell line of QX001S. CHO-S host cells were purchased from ThermoFisher (formerly Invitrogen, Cat. No. A13696-01), recovered and cultured CHO-S TM cell. After the linearized expression vector is electroporated into the host cell, it is randomly integrated into the genome of the host cell through the homology arm on the vector.
[0058] Using glutamine synthetase as a screening marker, a CHO-S cell line with stable, high-yield expression and physicochemical properties consistent with ustekinumab was obtained by pressurized screening with methionine sulfone methylene (MSX).
[0059] 1. Through step-by-step amplification, ELISA detection and other operations, for the cell clones cultured in 40 plates and 96-well plates, a high-yielding cell pool (mixed cell cl...
Embodiment 3
[0065] Embodiment 3: Pharmaceutical similarity research
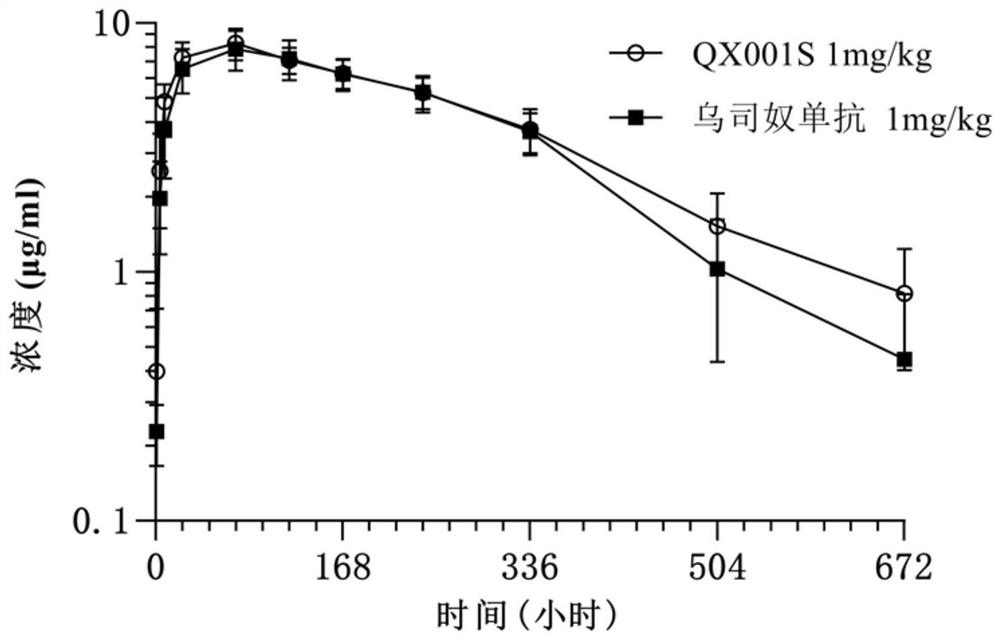
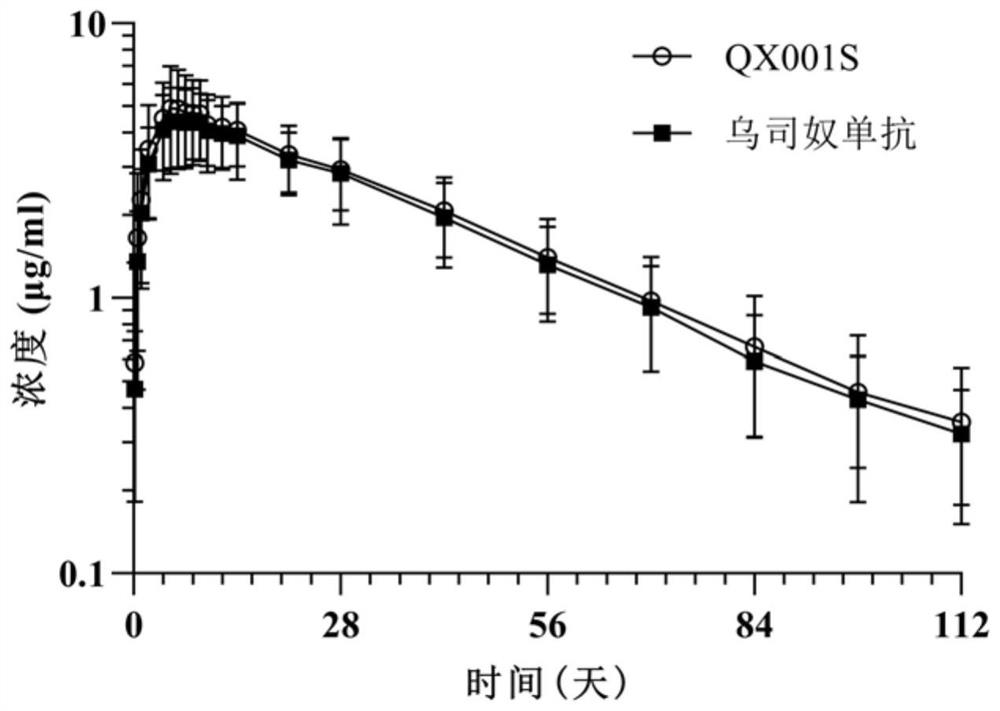
[0066] The pharmaceutical similarity between QX001S expressed by the CHO-S cell line of the above clone 4 and ustekinumab was studied. We comprehensively compared the primary structure, secondary and advanced structure, translation of QX001S and ustekinumab The similarities and differences in post-modification, charge heterogeneity, aggregates and degradation products, in vitro biological activity, and accelerated stability show that the two are highly similar in pharmaceutical research. The QX001S expressed by the CHO-S cell line is basically consistent with ustekinumab in 71 of the 78 indicators listed in Table 1 below.
[0067] The indicators that have differences between the two are mainly in post-translational modification (there are differences in 5 indicators), especially as determined, the proportion of sialylated components of ustekinumab is between 11.87% and 25.56%, while the CHO -S cell line expresses QX001...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com