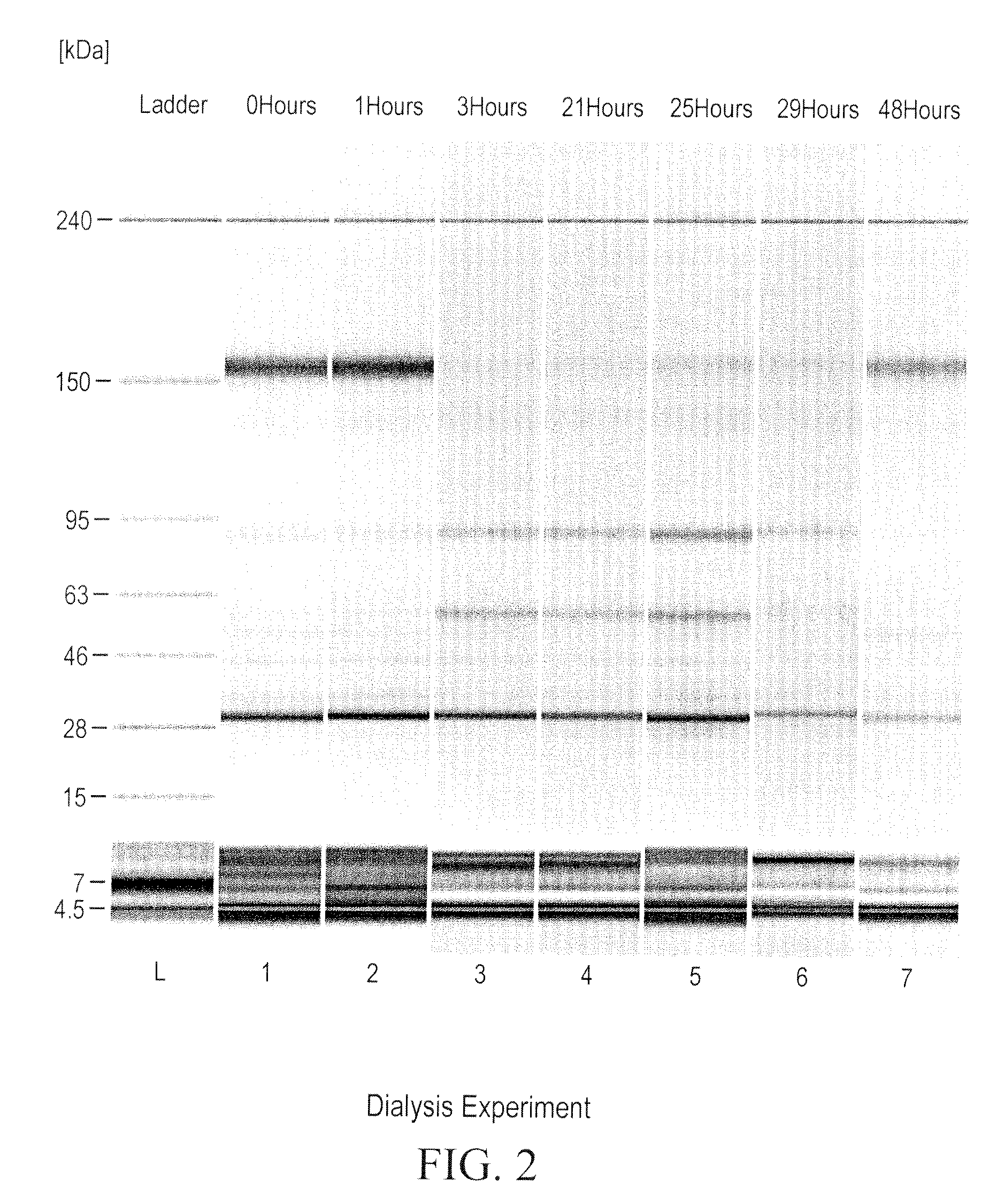
Patents
Literature
199 results about "Recombinant protein production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant protein production is the expression of proteins that have been produced by recombinant DNA techniques. This process enables these substances to be made in large quantities. Such mass production is done both for laboratory study and for industrial production.
Protein expression systems
InactiveUS20050186666A1High protein yieldEfficient productionBacteriaHydrolasesBiotechnologyRecombinant protein production
The present invention provides an improved expression system for the production of recombinant polypeptides utilizing auxotrophic selectable markers. In addition, the present invention provides improved recombinant protein production in host cells through the improved regulation of expression.
Owner:PFENEX
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
Prevention of disulfide bond reduction during recombinant production of polypeptides
The invention concerns methods and means for preventing the reduction of disulfide bonds during the recombinant production of disulfide-containing polypeptides. In particular, the invention concerns the prevention of disulfide bond reduction during harvesting of disulfide-containing polypeptides, including antibodies, from recombinant host cell cultures.
Owner:GENENTECH INC
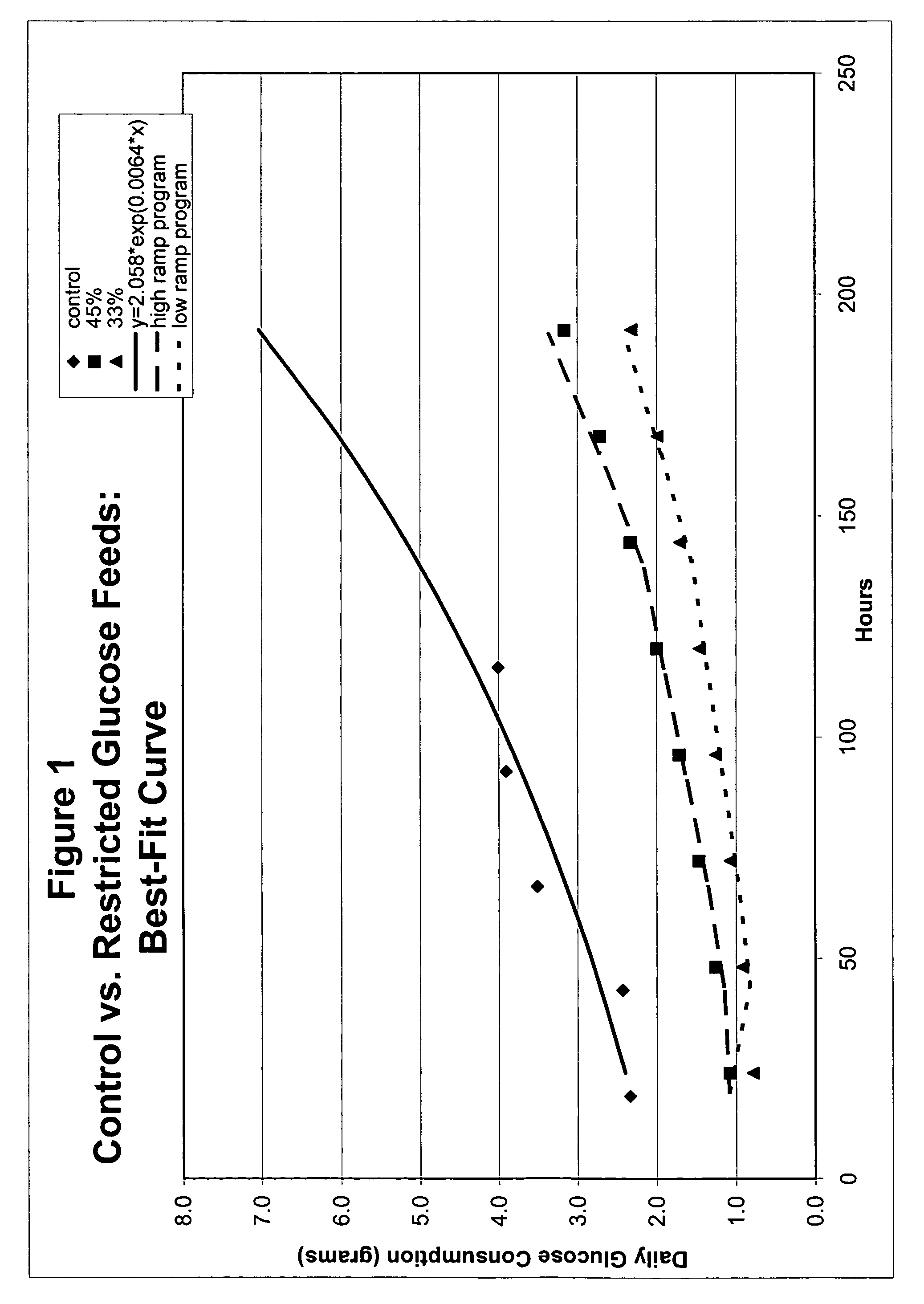
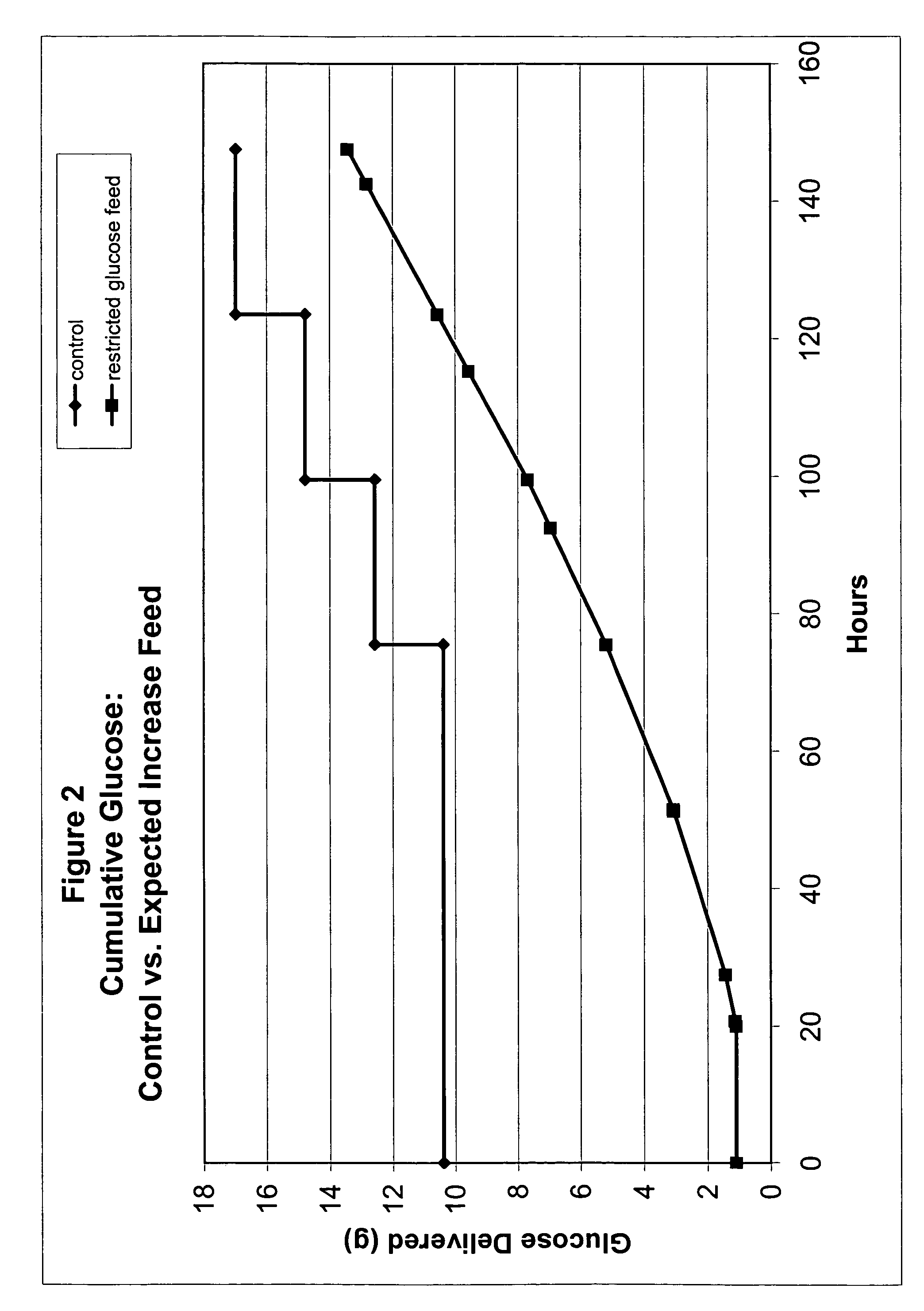
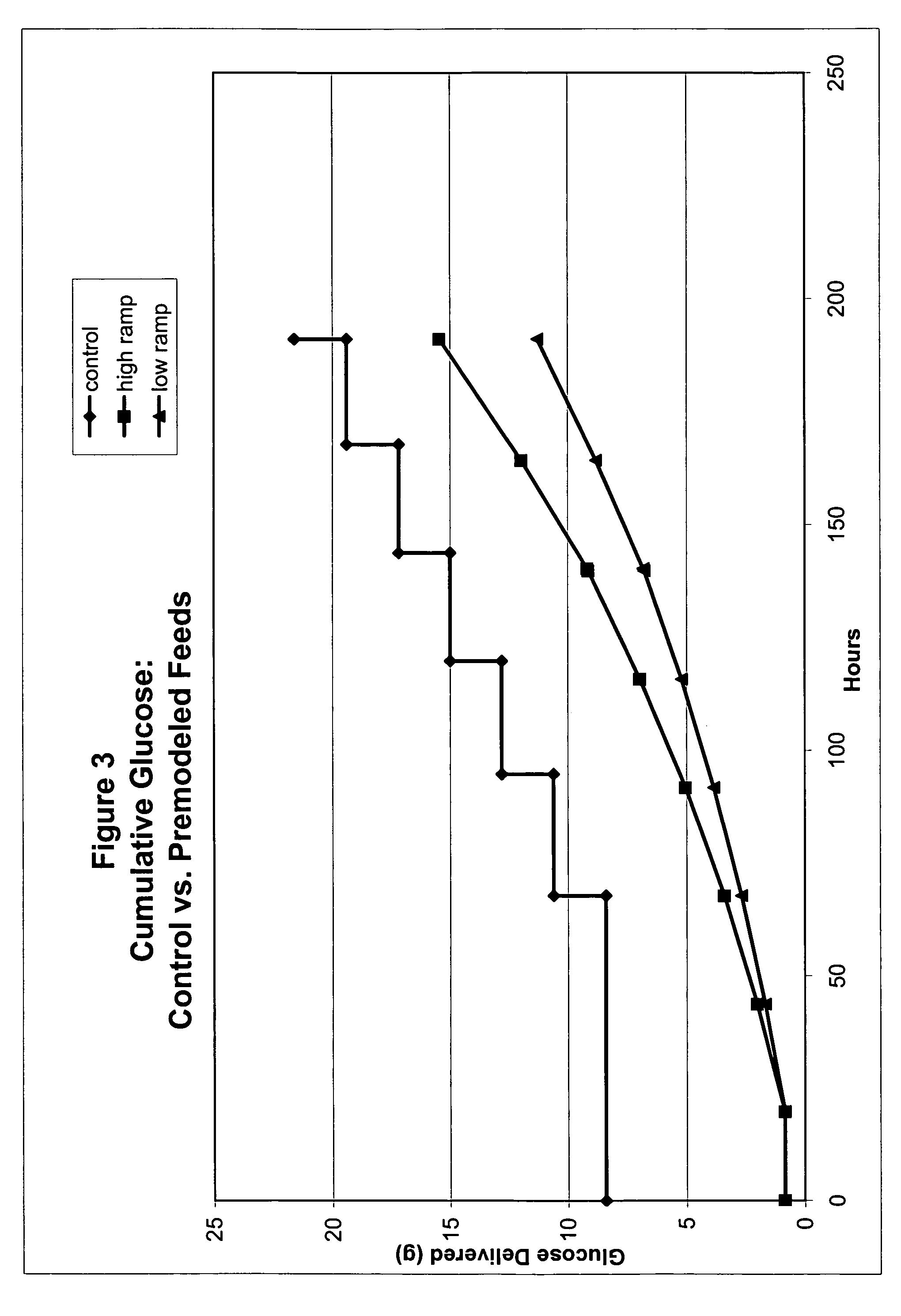
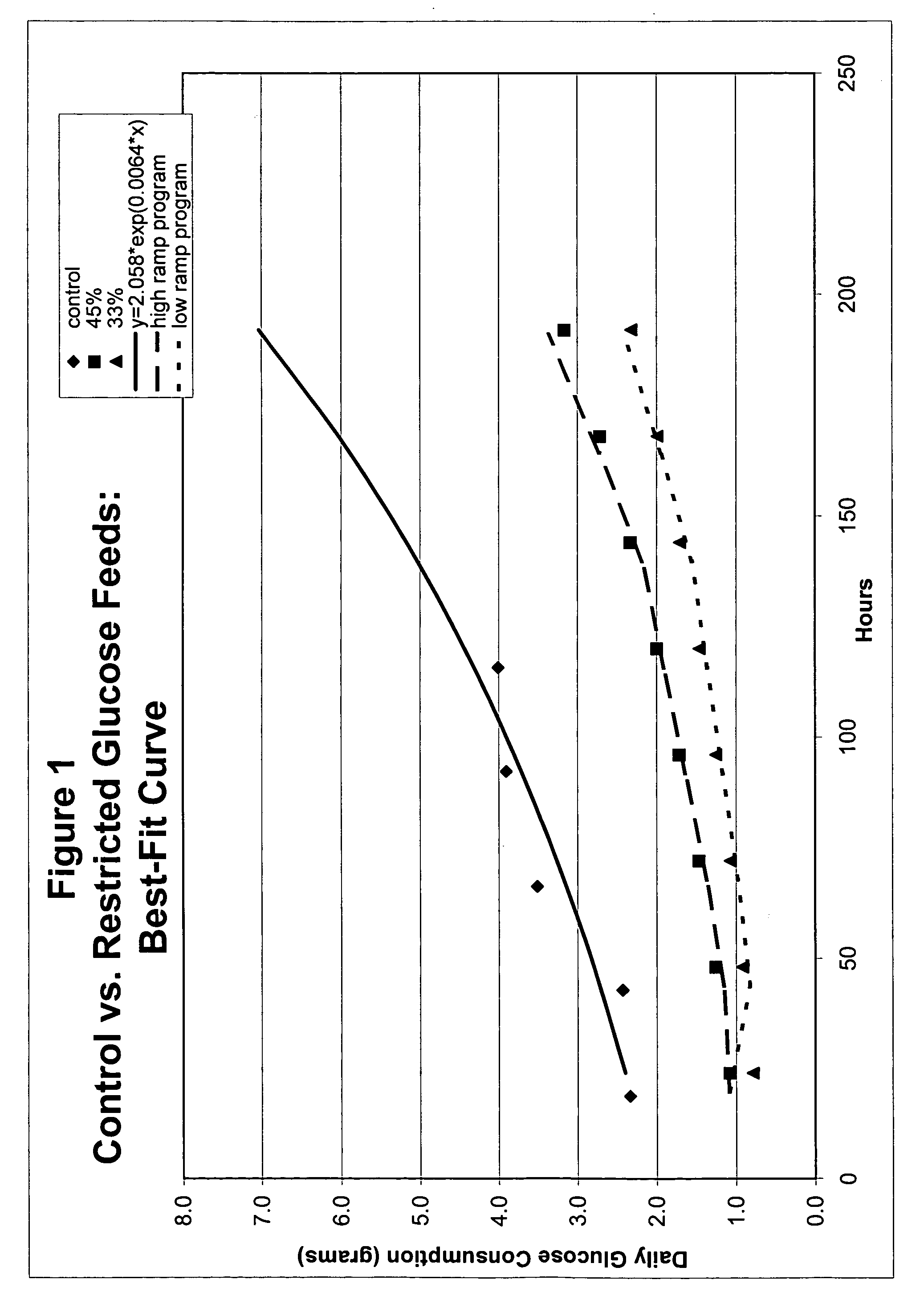
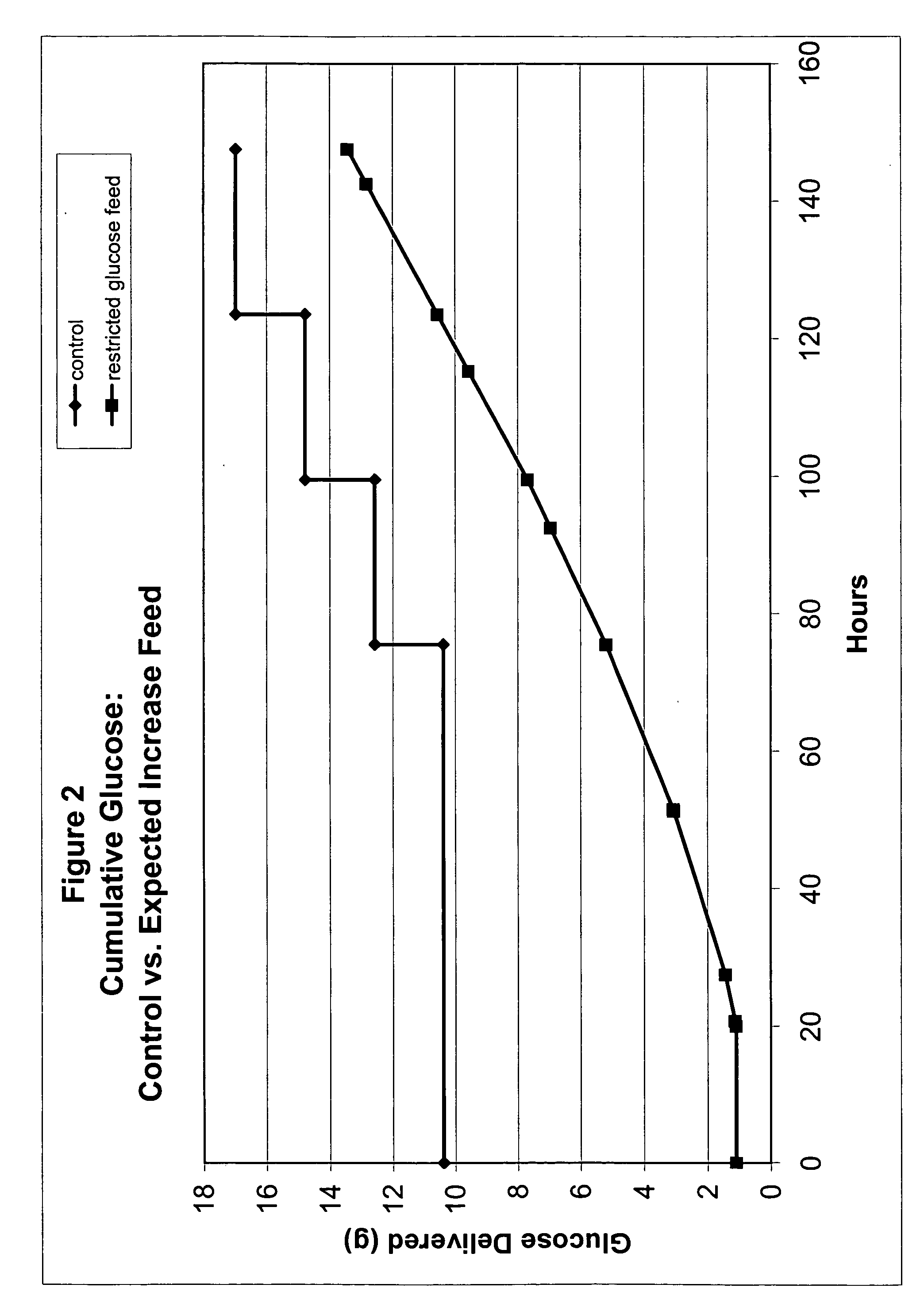
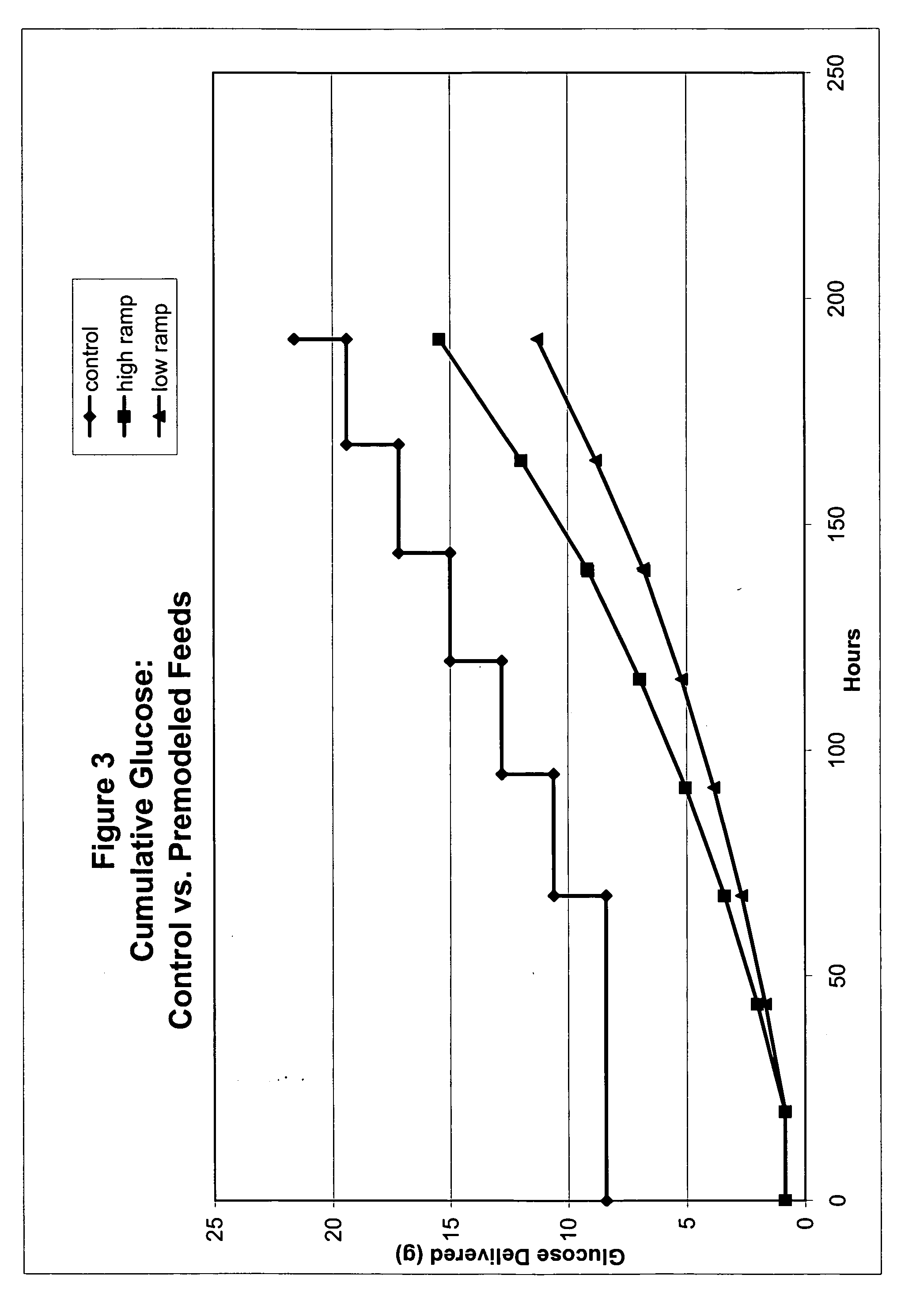
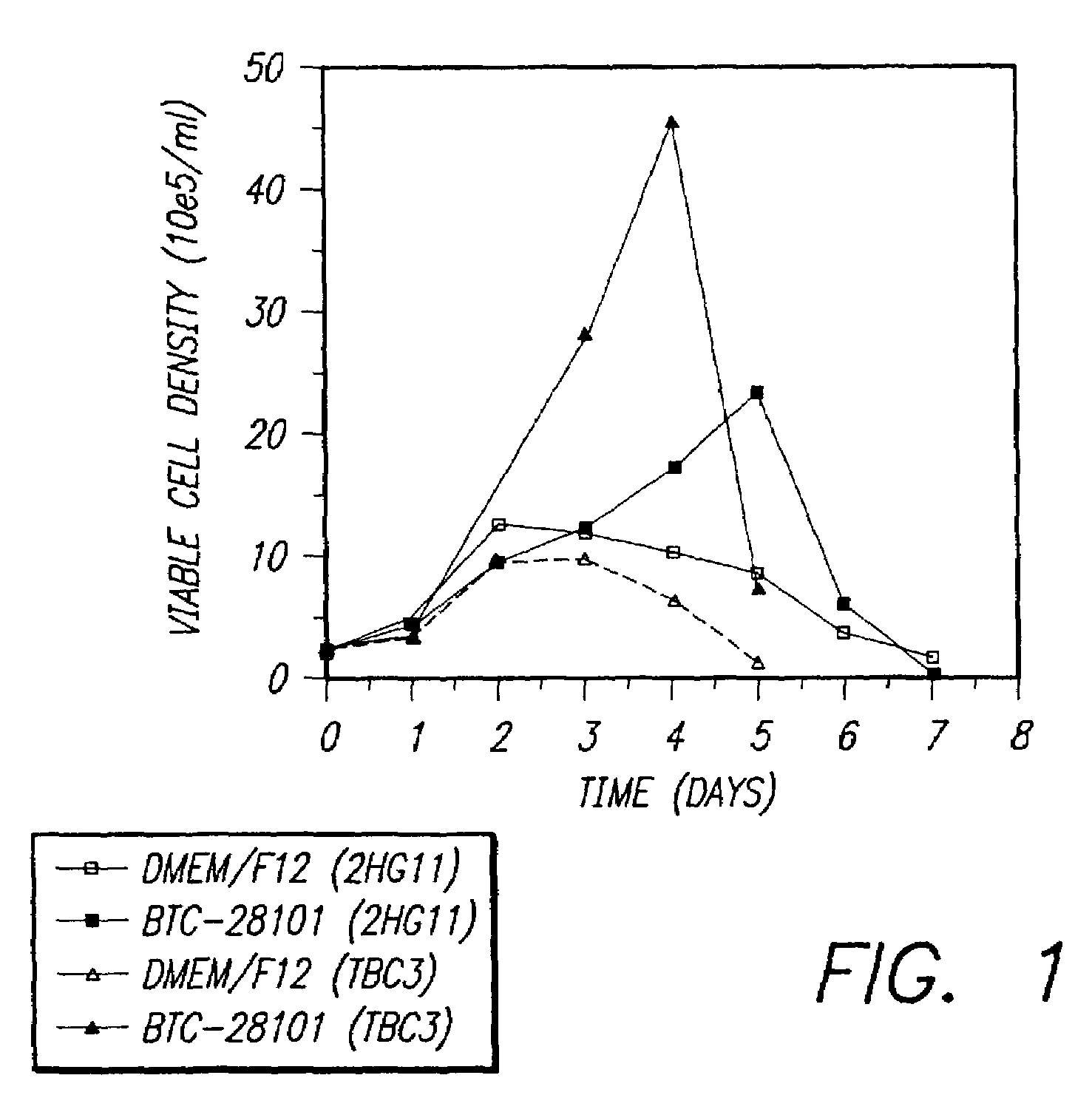
Restricted glucose feed for animal cell culture
ActiveUS7429491B2Promote productionFlexible controlCulture processCell culture mediaBiotechnologyBiochemistry
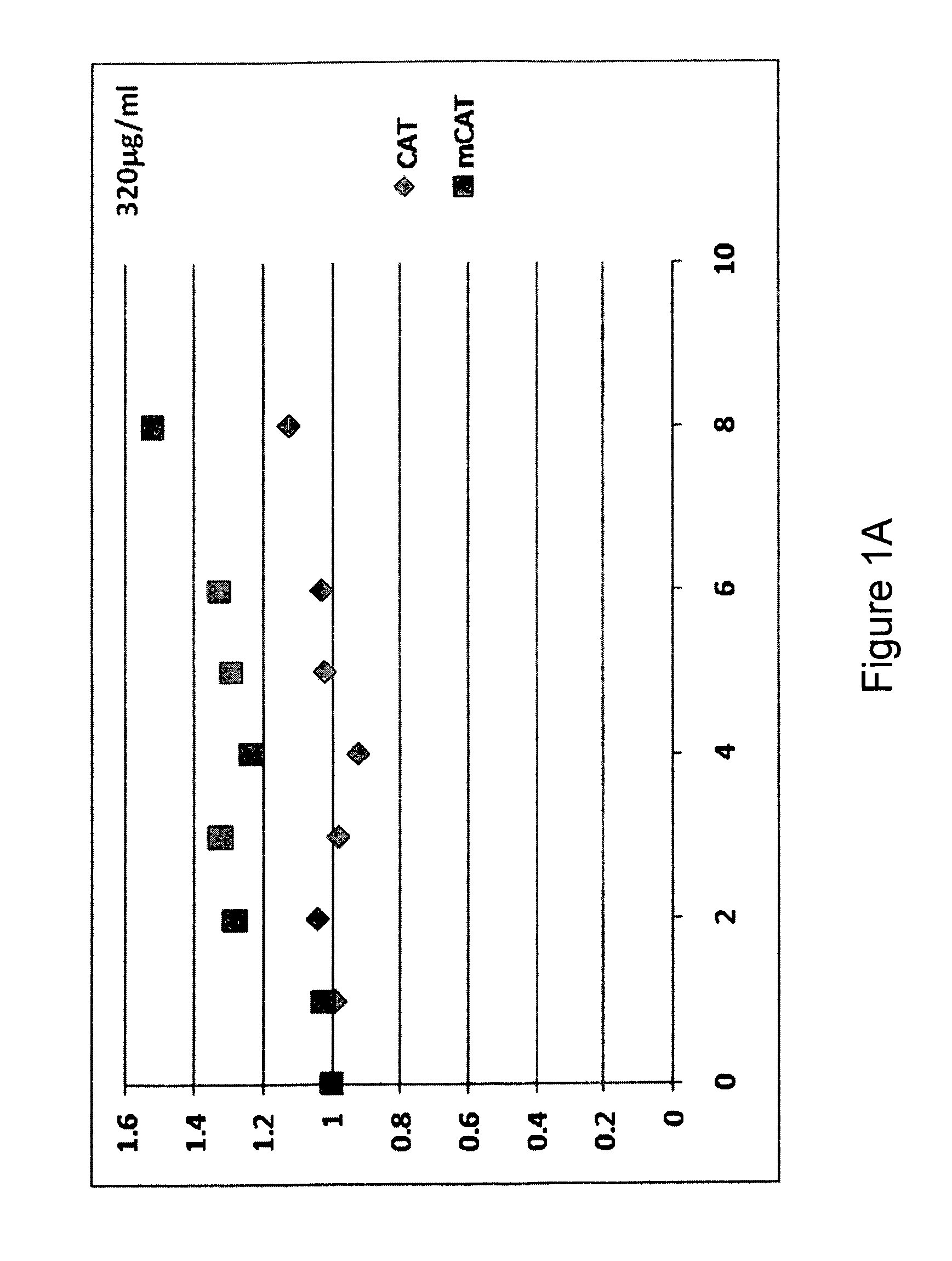
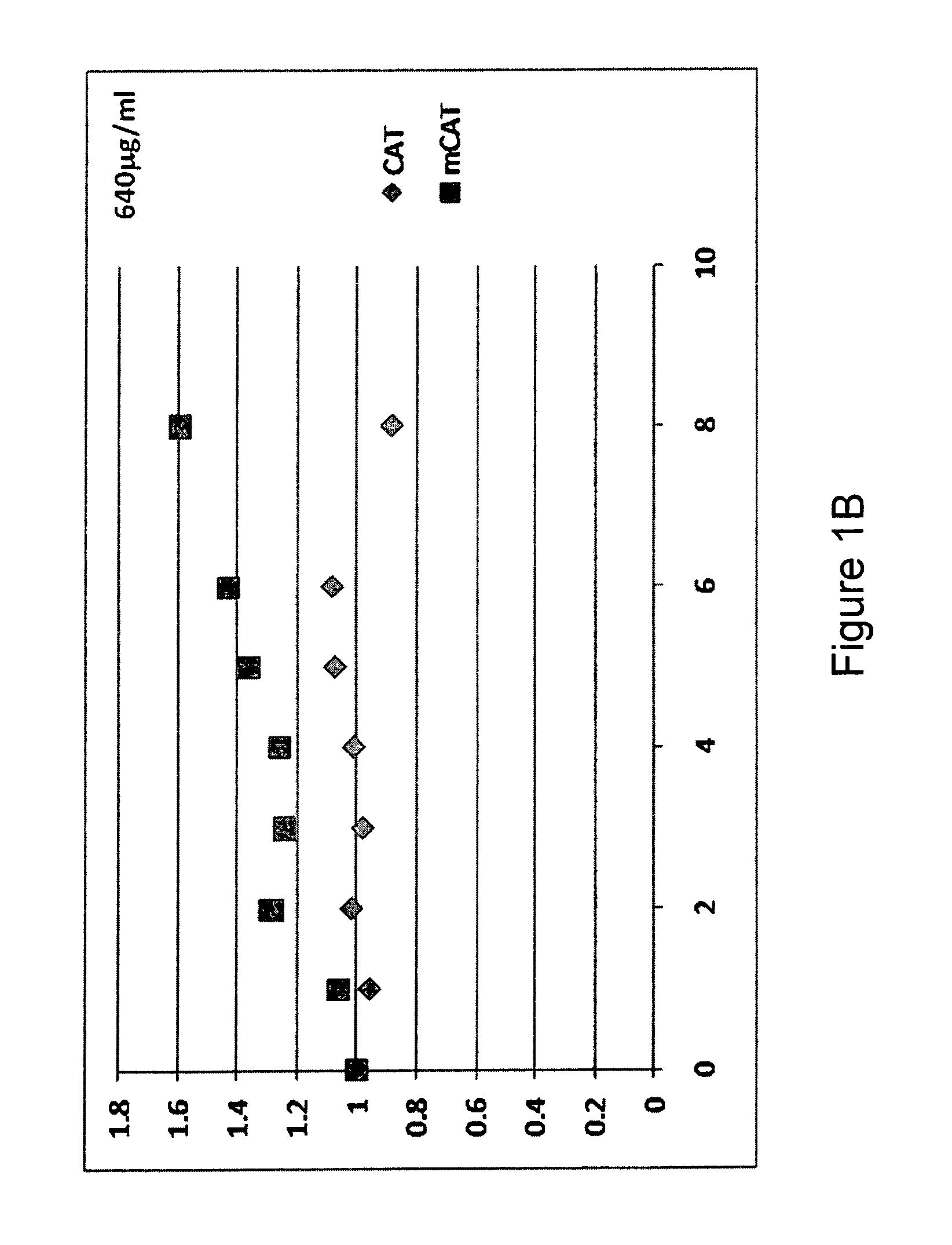
Methods of improving protein production in animal cell cultures are provided. Cell culture methods are presented wherein glucose is fed in a restricted manner to cell culture; this restricted feeding of glucose to the cell culture results in lactate production being controlled to a low level. The restricted feeding of glucose in a fed-batch process is not accomplished through a constant-rate feeding of glucose, and the restricted feeding need not depend on sampling. Instead, restricted feeding of glucose to the culture is accomplished through feeding of glucose to the culture at a rate that is a function of an expected or a premodeled rate of glucose consumption by the animal cells when exposed to medium containing a high level of glucose. Because lactate production is controlled to low levels, recombinant protein production is increased.
Owner:WYETH LLC
Translation enhancer element of the human amyloid precursor protein gene
The present invention is directed to a DNA element that enhances the translation of the human amyloid precursor protein (APP) gene. The enhancer may be incorporated into expression vectors to enhance recombinant protein production. In addition, the invention is directed to an assay that utilizes vectors containing the translation enhancer element for the purpose of identifying agents that modulate the expression of the human amyloid precursor protein. These agents will ultimately be used to suppress APP expression in patients with Alzheimer's disease.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
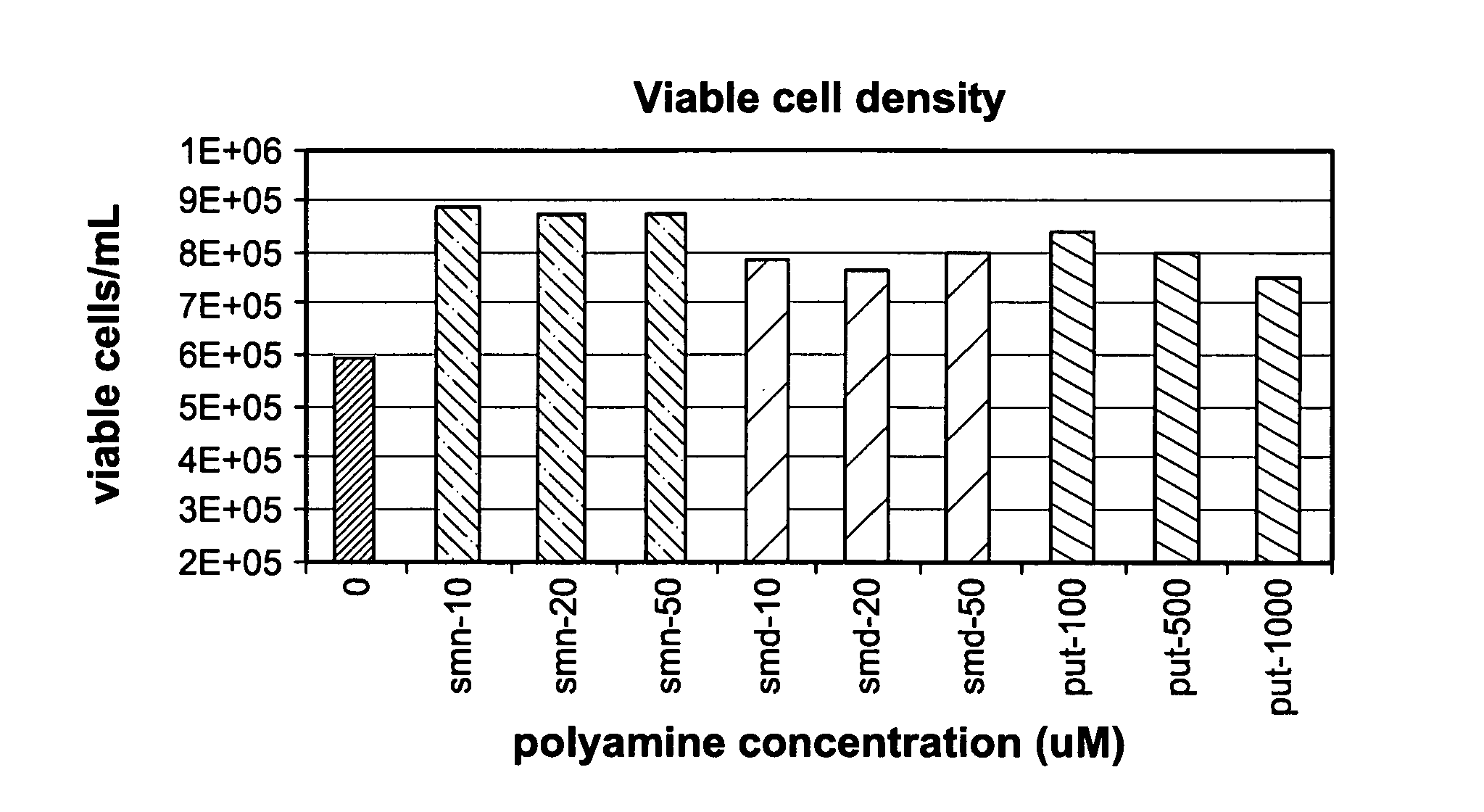
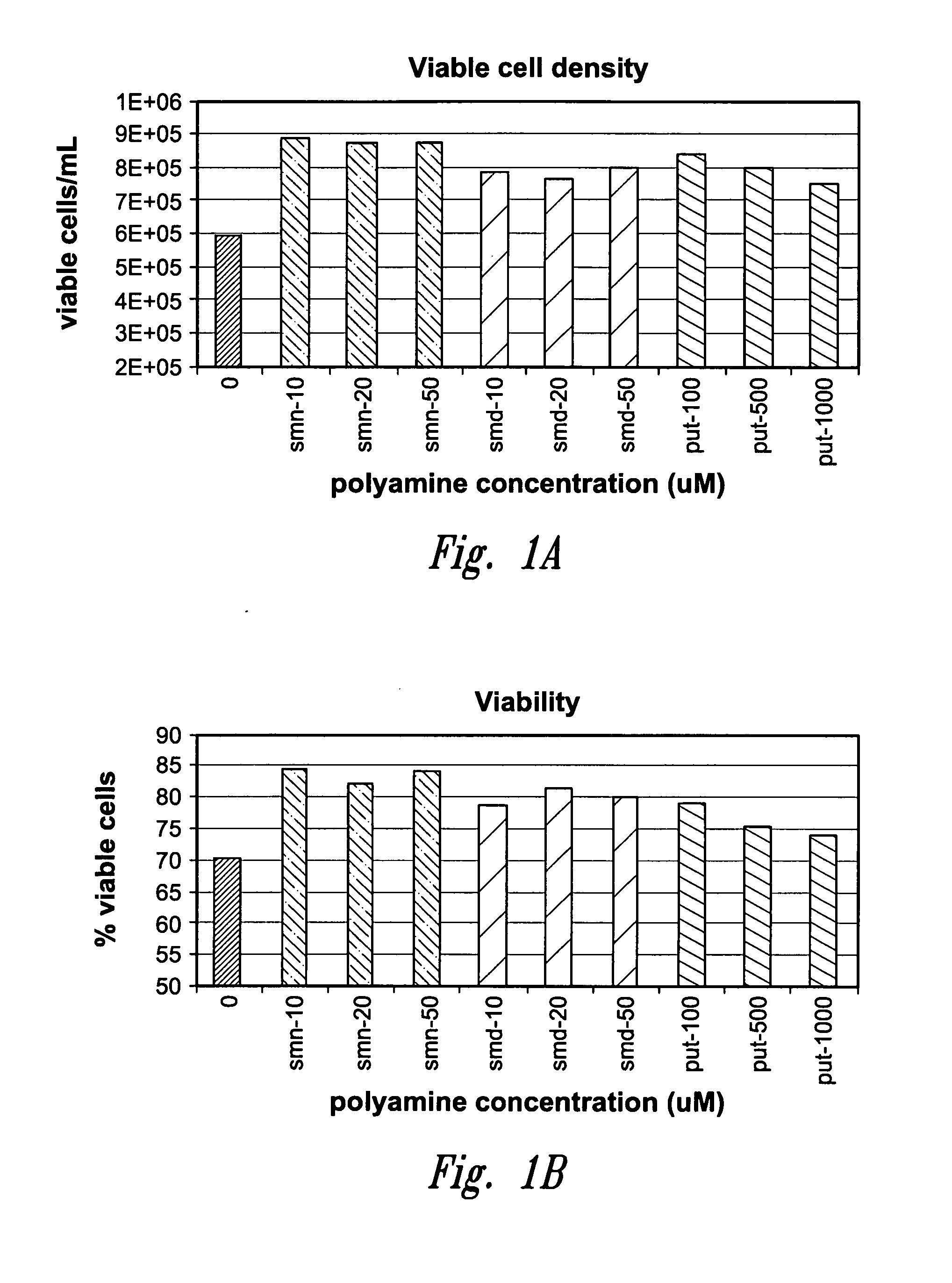
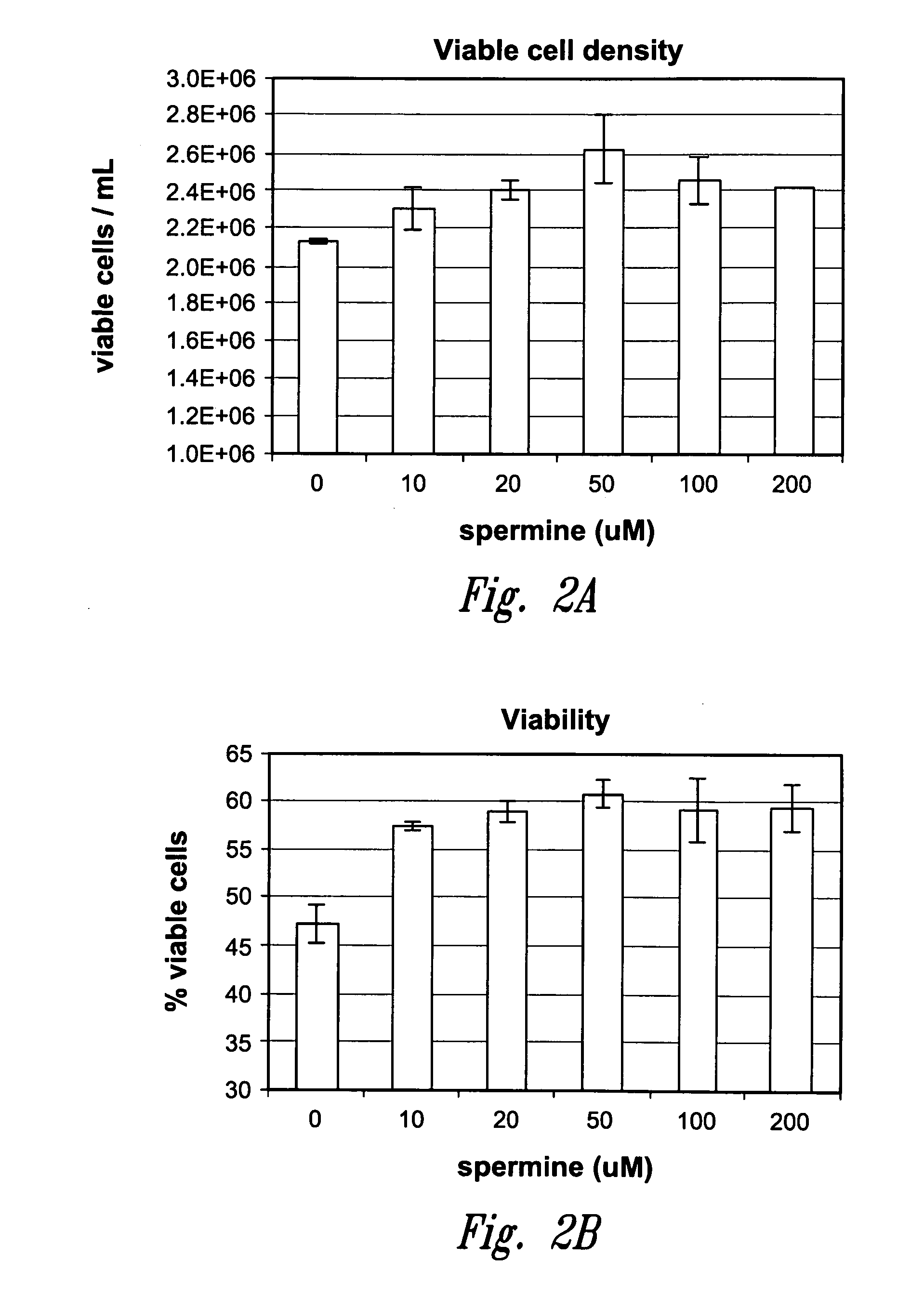
Method for culturing mammalian cells to improve recombinant protein production
InactiveUS20100221823A1Enhance cell viabilityExpression of protein improvedGenetically modified cellsCulture processMicrobiologyMammalian cell
The present invention relates to methods for mammalian cell culture, wherein the methods make use of media containing polyamines, such as putrescine, spermidine and spermine.
Owner:AMGEN INC
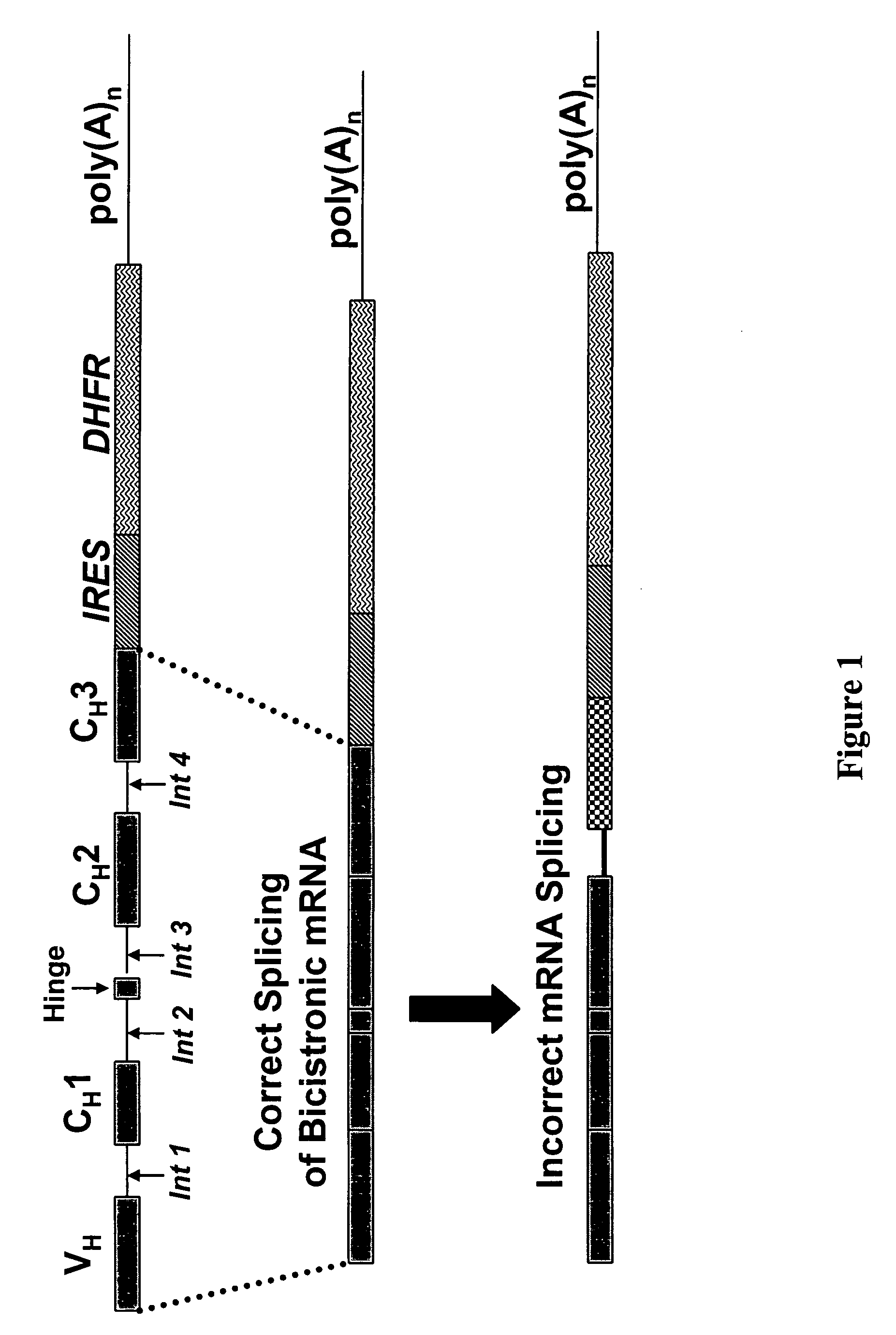
Methods and compositions for improving recombinant protein production
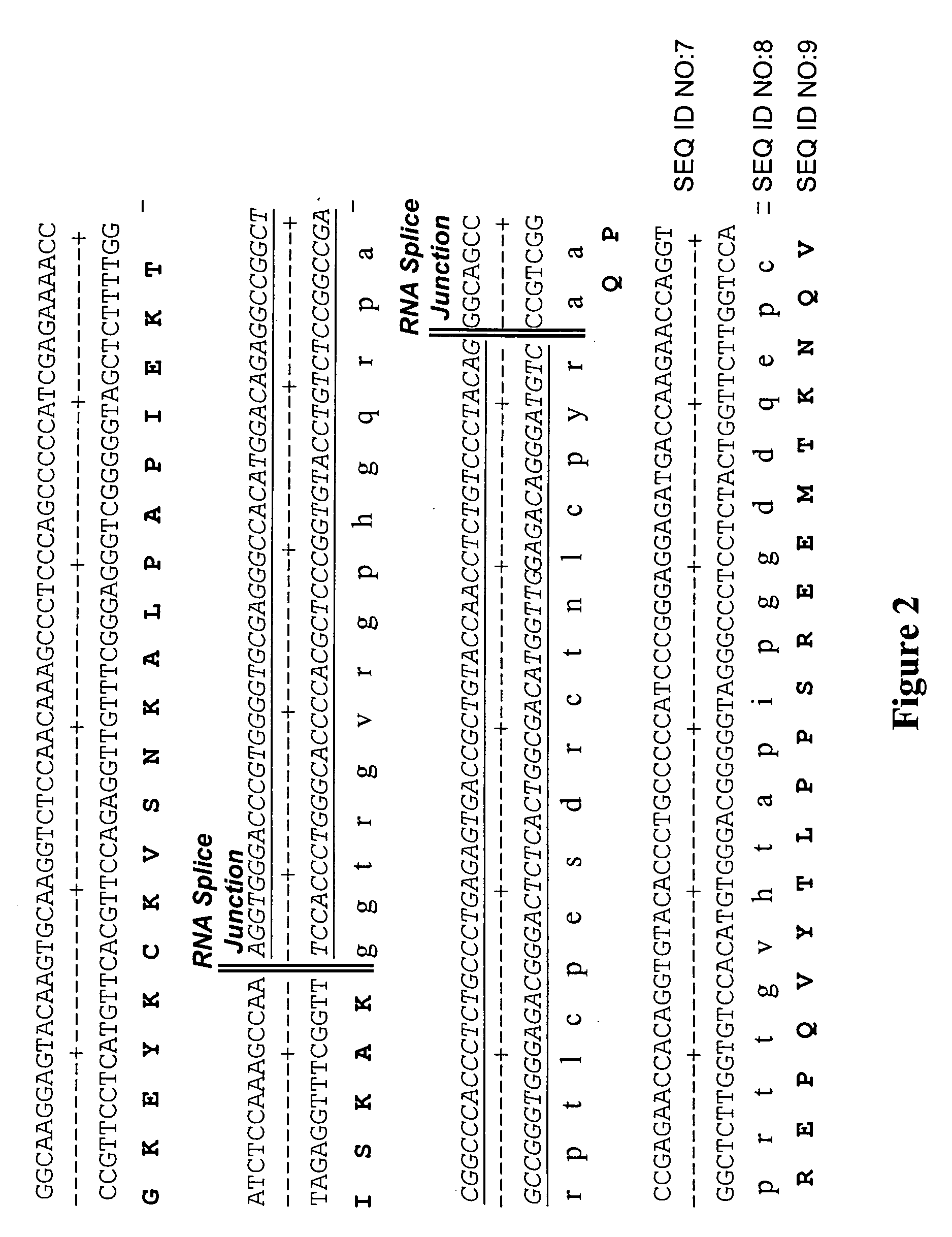
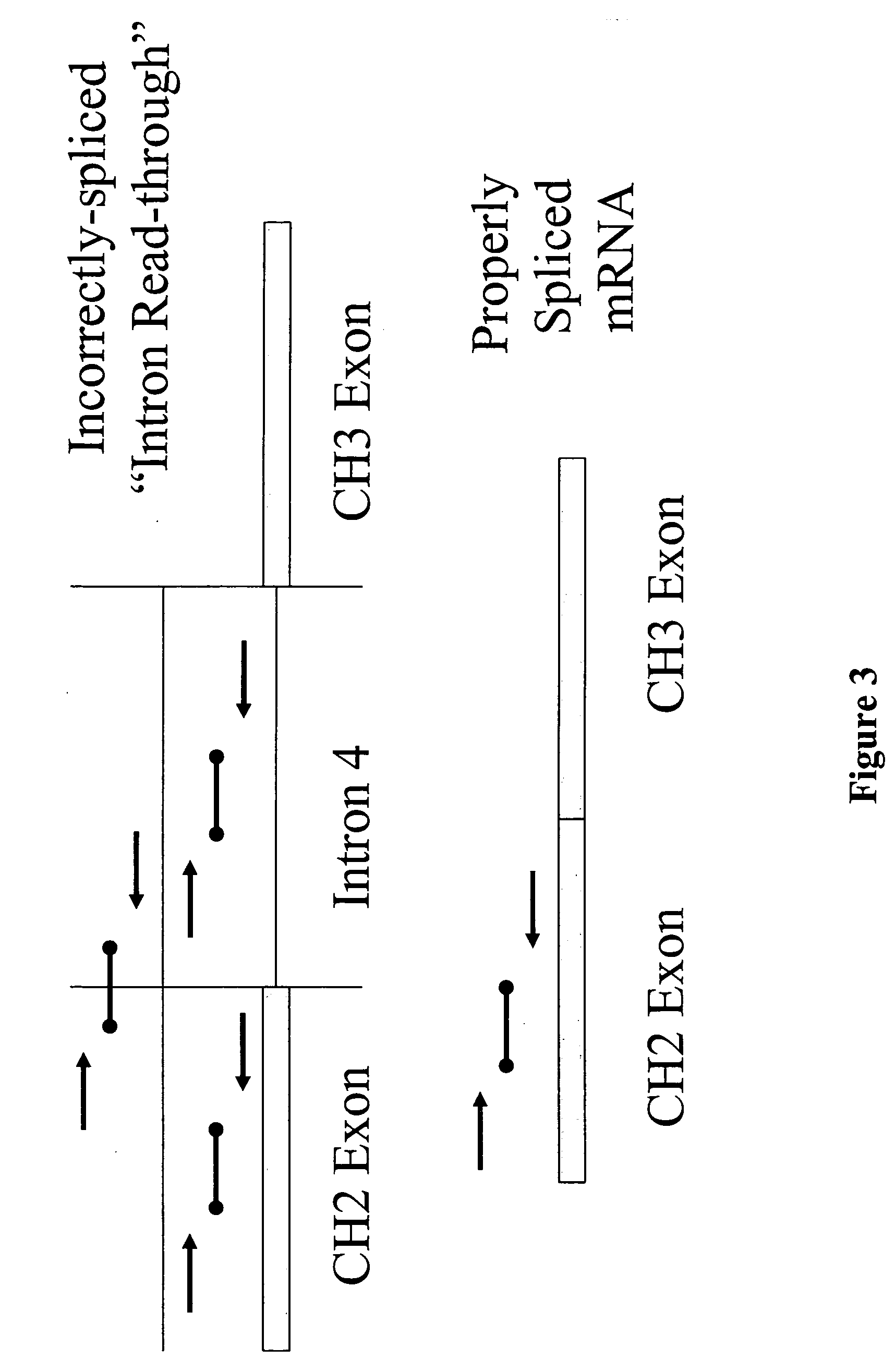
InactiveUS20060099206A1Enhance protein expressionReduction and elimination of mis-splicedAnimal cellsNervous disorderAntibody expressionRead through
Nucleic acid molecules modified to enhance recombinant protein, e.g., antibody, expression and / or reduce or eliminate mis-spliced and / or intron read-through (IRT) by-products are disclosed. The invention also provides methods for producing proteins devoid of mis-spliced and / or intron read-through by-products by the use of such vectors in host cells under cell culture conditions suitable for recombinant protein expression.
Owner:WYETH
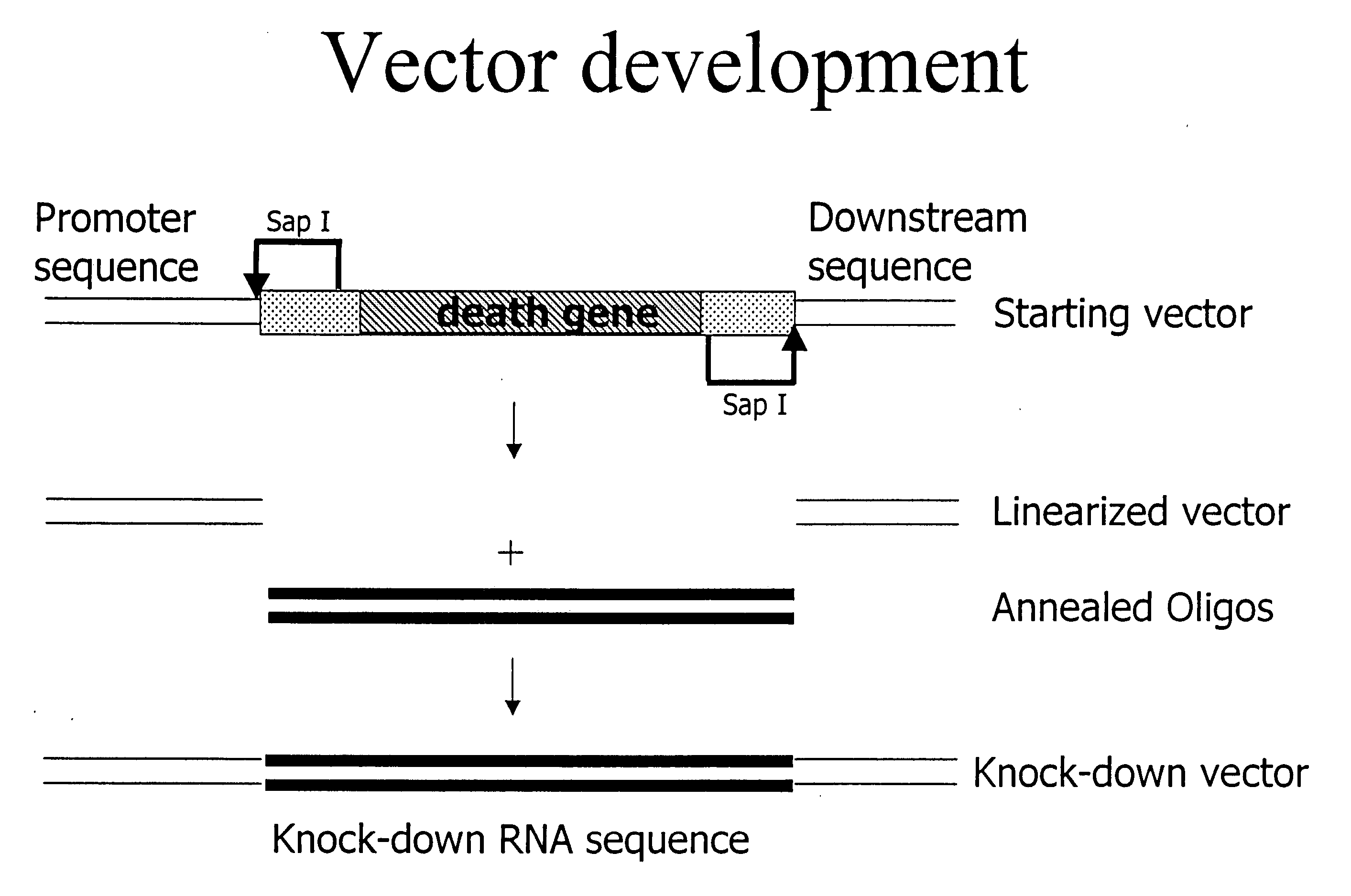
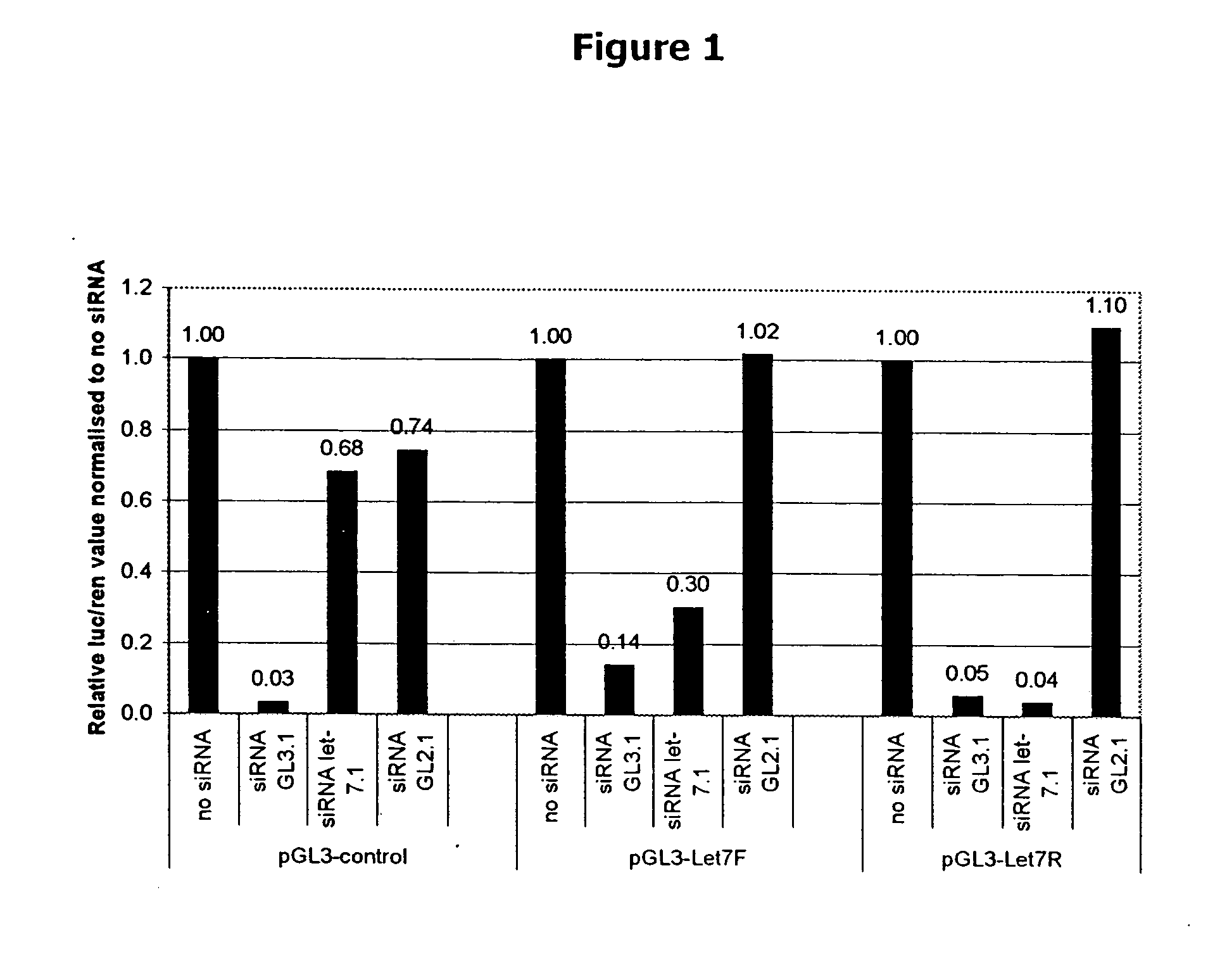
siRNA knockout assay method and constructs
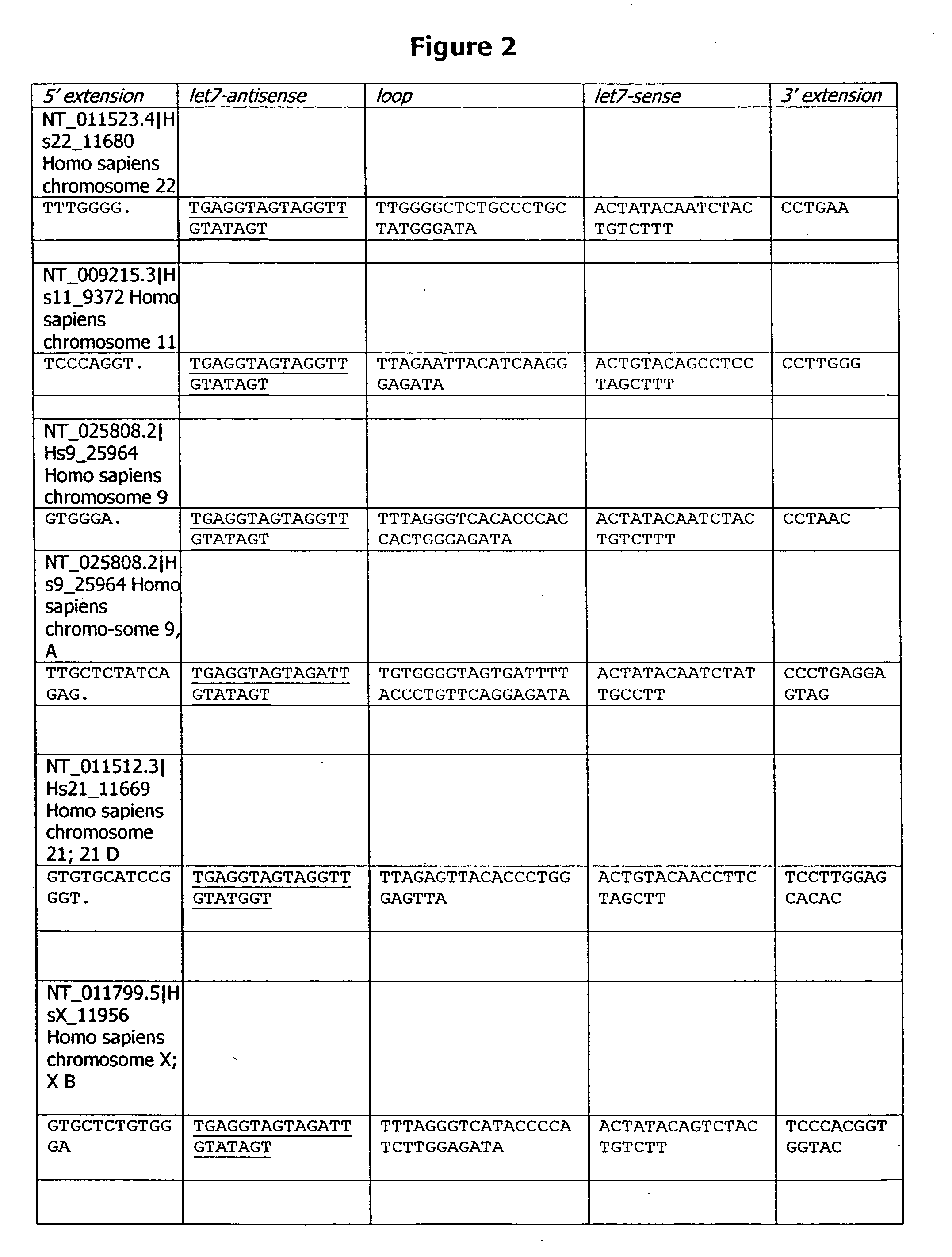
Isolated polynucleotides, and vectors including the same, are disclosed as useful for down-regulation of specific RNA in cells, including a first sequence of about 17 to about 23 nucleotides, complementary to said RNA, and linked to a second sequence capable of forming a loop when said second sequence is RNA. The polynucleotides include self-complementing single-stranded polynucleotides, including a third sequence linked by said second sequence where all nucleotides in said first and said third sequences are complementary. Functional genomic, diagnostic and therapeutic methods are disclosed that involve reducing the amount of a unique RNA sequence in cells using a vector encoding the self-complementing polynucleotide including a first sequence complementary to said RNA sequence. Methods are also disclosed for preparing the polynucleotides, vectors, libraries of vectors, and the temporary knock-down of proteins, such as lethal proteins, during virus or recombinant protein production.
Owner:GALAPAGOS NV
Reengineering mRNA primary structure for enhanced protein production
ActiveUS8853179B2Improve efficiencyImprove protein stabilitySugar derivativesActivity regulationStart codonTranslational efficiency
Described herein are rules to modify natural mRNAs or to engineer synthetic mRNAs to increase their translation efficiencies. These rules describe modifications to mRNA coding and 3′ UTR sequences intended to enhance protein synthesis by: 1) decreasing ribosomal diversion via AUG or non-canonical initiation codons in coding sequences, and / or 2) by evading miRNA-mediated down-regulation by eliminating one or more miRNA binding sites in coding sequences.
Owner:THE SCRIPPS RES INST
Method for increasing the efficiency of recombinant AAV production
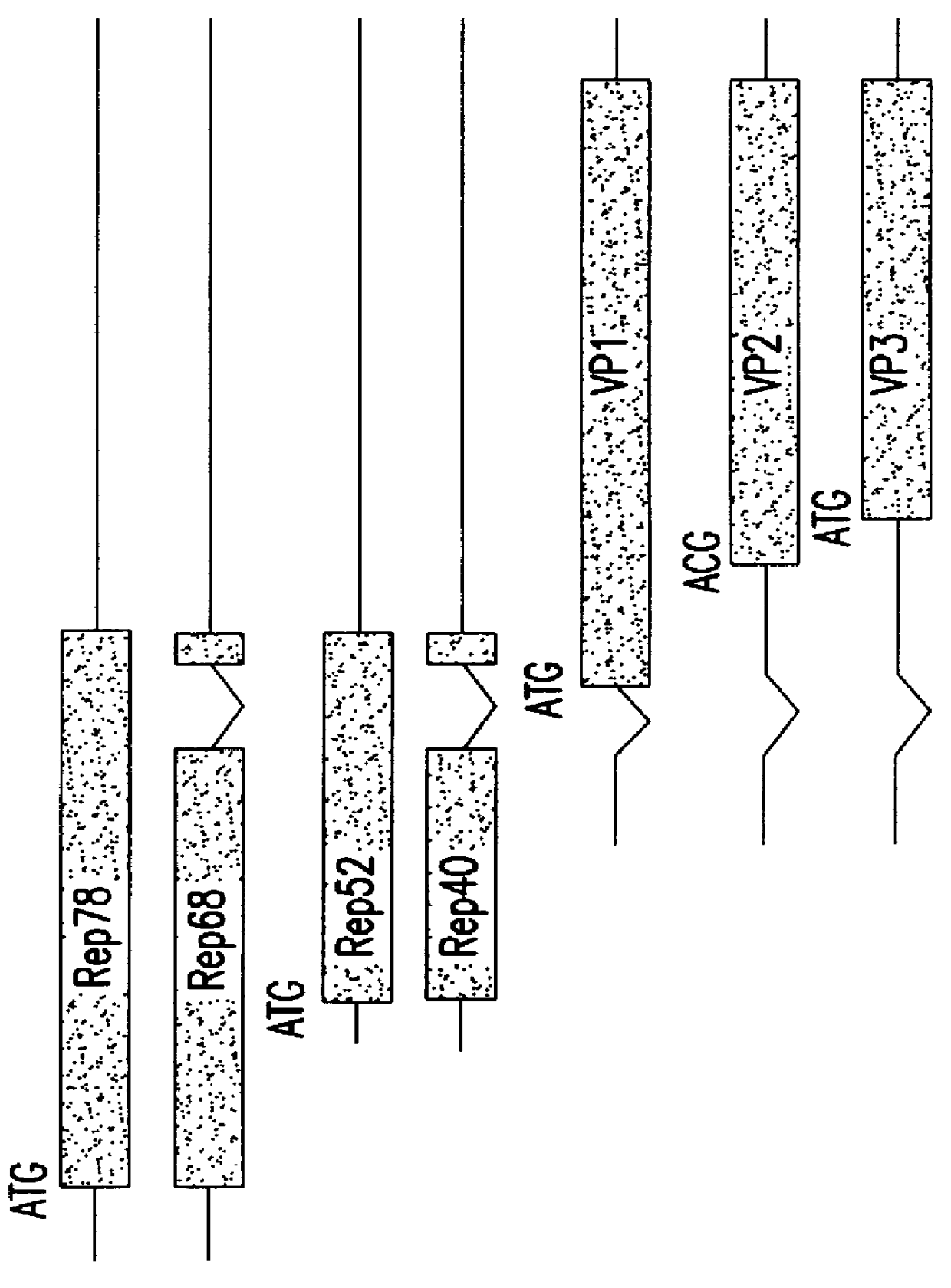
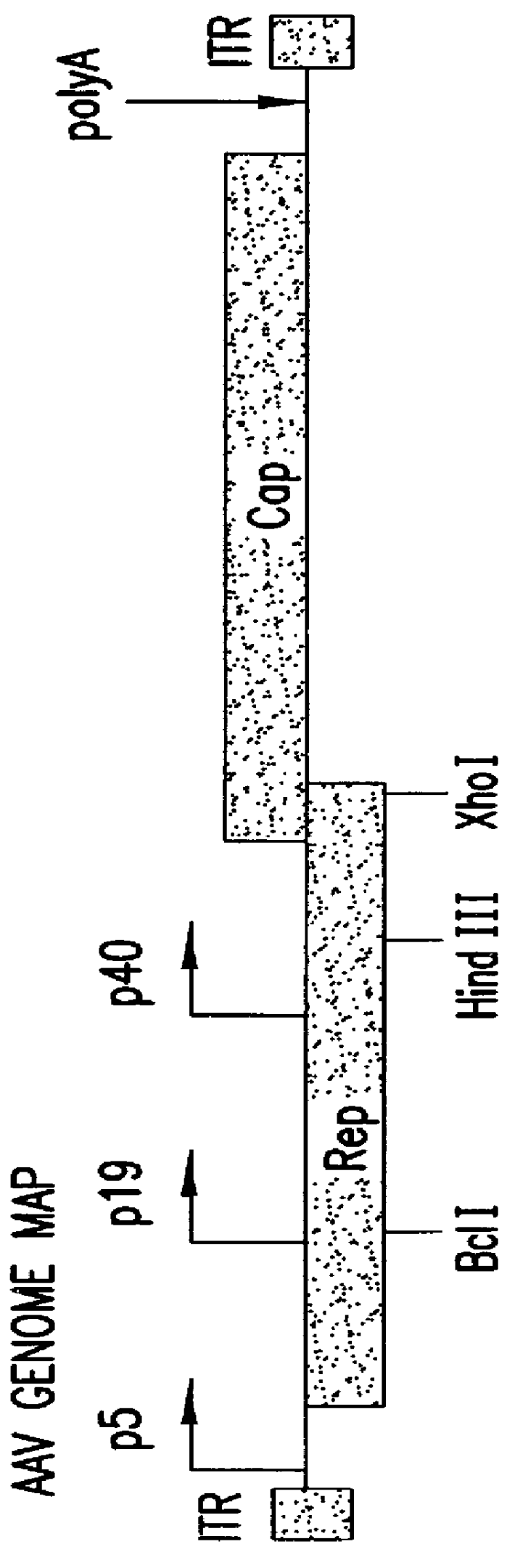
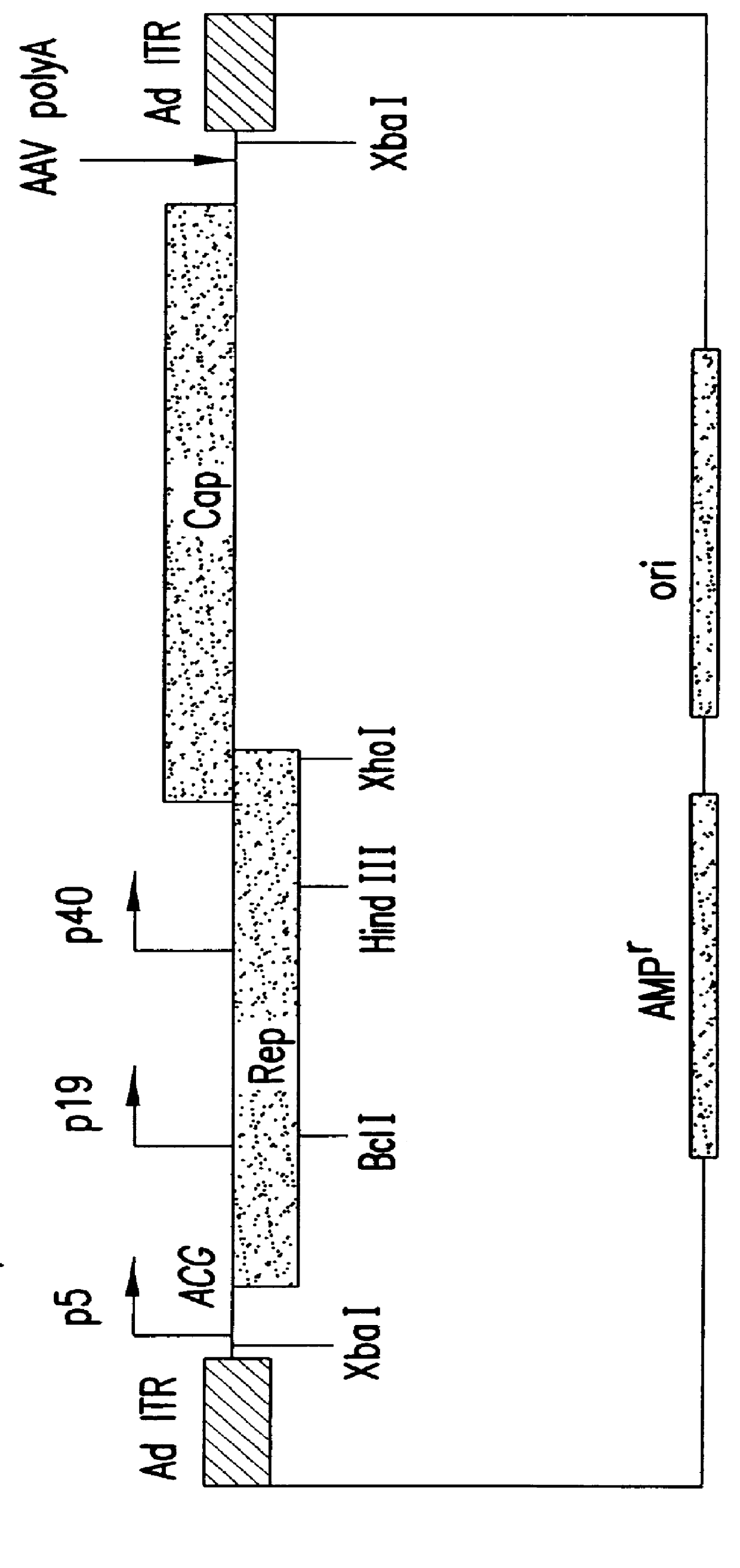
InactiveUS6037177AReduce expressionIncreased of replicationArtificial cell constructsVertebrate cellsPost translationalTiter
The present invention relates to methods and compositions for increasing the production of high titre stocks of recombinant AAV (rAAV) through regulation of expression of the AAV REP and CAP proteins. The methods and compositions of the invention are based on the observation that the low level expression of the AAV REP 78 / 68 protein increases the production of AAV viral capsid protein and efficiency of packaging resulting in production of higher titre recombinant viral stocks. The invention encompasses recombinant AAV vectors that direct the expression of AAV REP and CAP proteins and the use of such vectors for the production of novel stable cell lines capable of generating high titre rAAV vectors. The invention provides methods for regulating the expression of the AAV REP 78 / 68, REP 40 / 52 and CAP gene at the transcriptional and post-translational level. The methods and compositions of the invention can be used to produce high titre stocks of rAAV which can be used in gene therapy for the purpose of transferring genetic information into appropriate host cells for the management and correction of human diseases including inherited and acquired disorders.
Owner:CELLS GENESYS INC
Protein expression systems
ActiveUS20090325230A1High protein yieldEfficient productionBacteriaSugar derivativesBiotechnologyRecombinant protein production
The present invention provides an improved expression system for the production of recombinant polypeptides utilizing auxotrophic selectable markers. In addition, the present invention provides improved recombinant protein production in host cells through the improved regulation of expression.
Owner:PELICAN TECH HLDG INC
Method for culturing mammalian cells to improve recombinant protein production
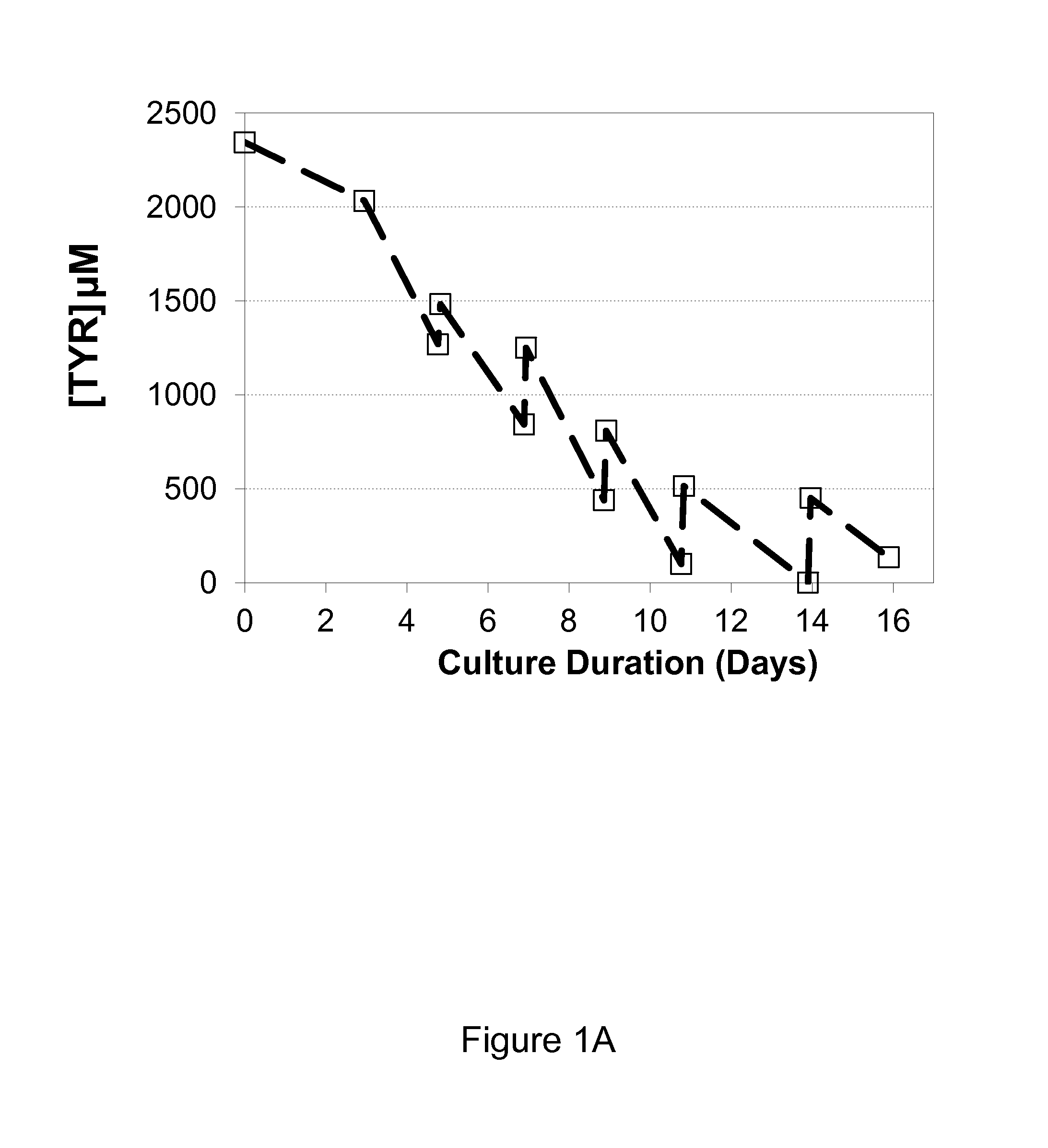
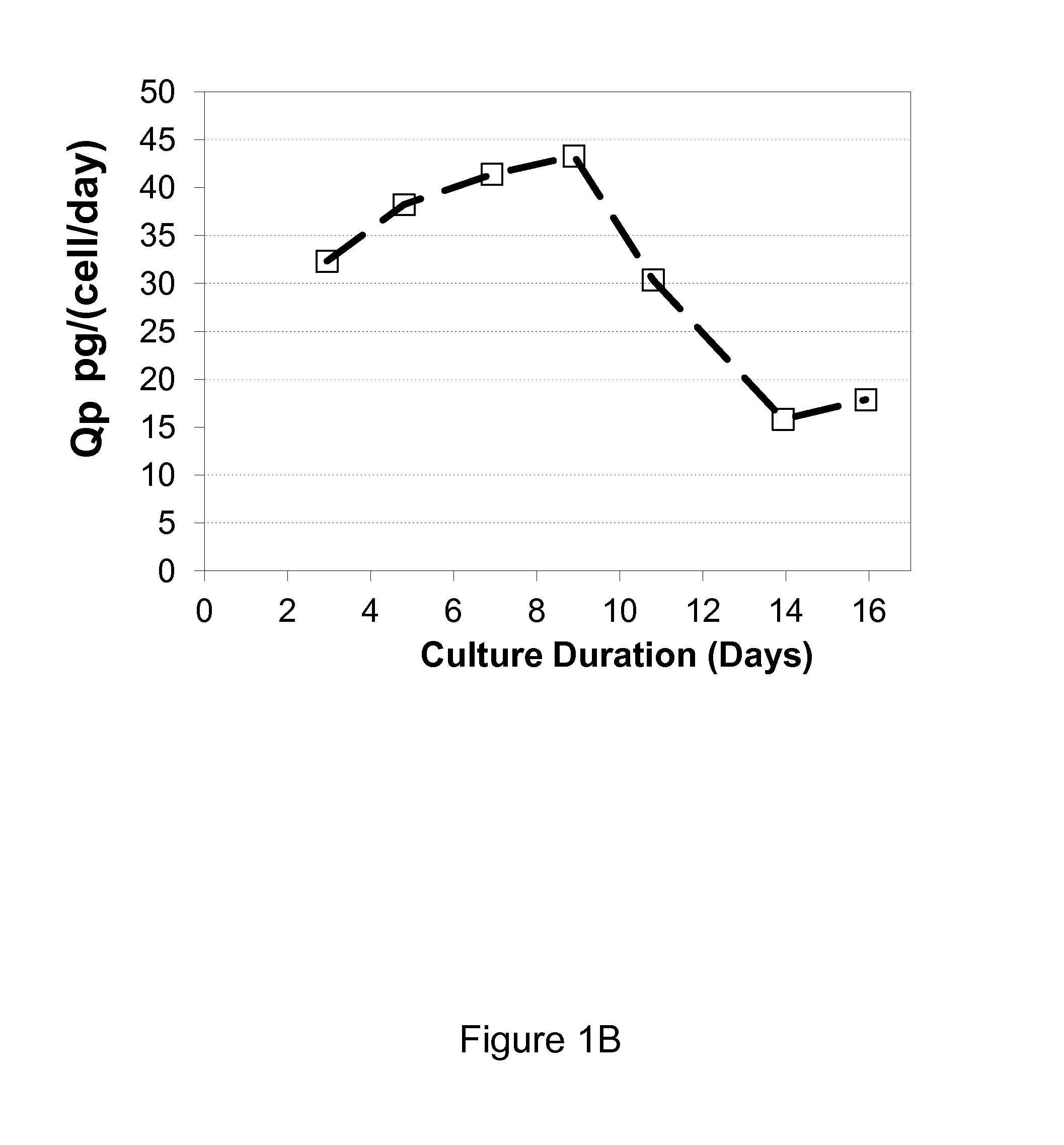
ActiveUS20140178984A1Increase productivityImprove viabilityAnimal cellsGenetically modified cellsBiotechnologyTyrosine
The present invention relates to methods for mammalian cell culture. The methods make use of independent tyrosine and cystine feed streams.
Owner:AMGEN INC
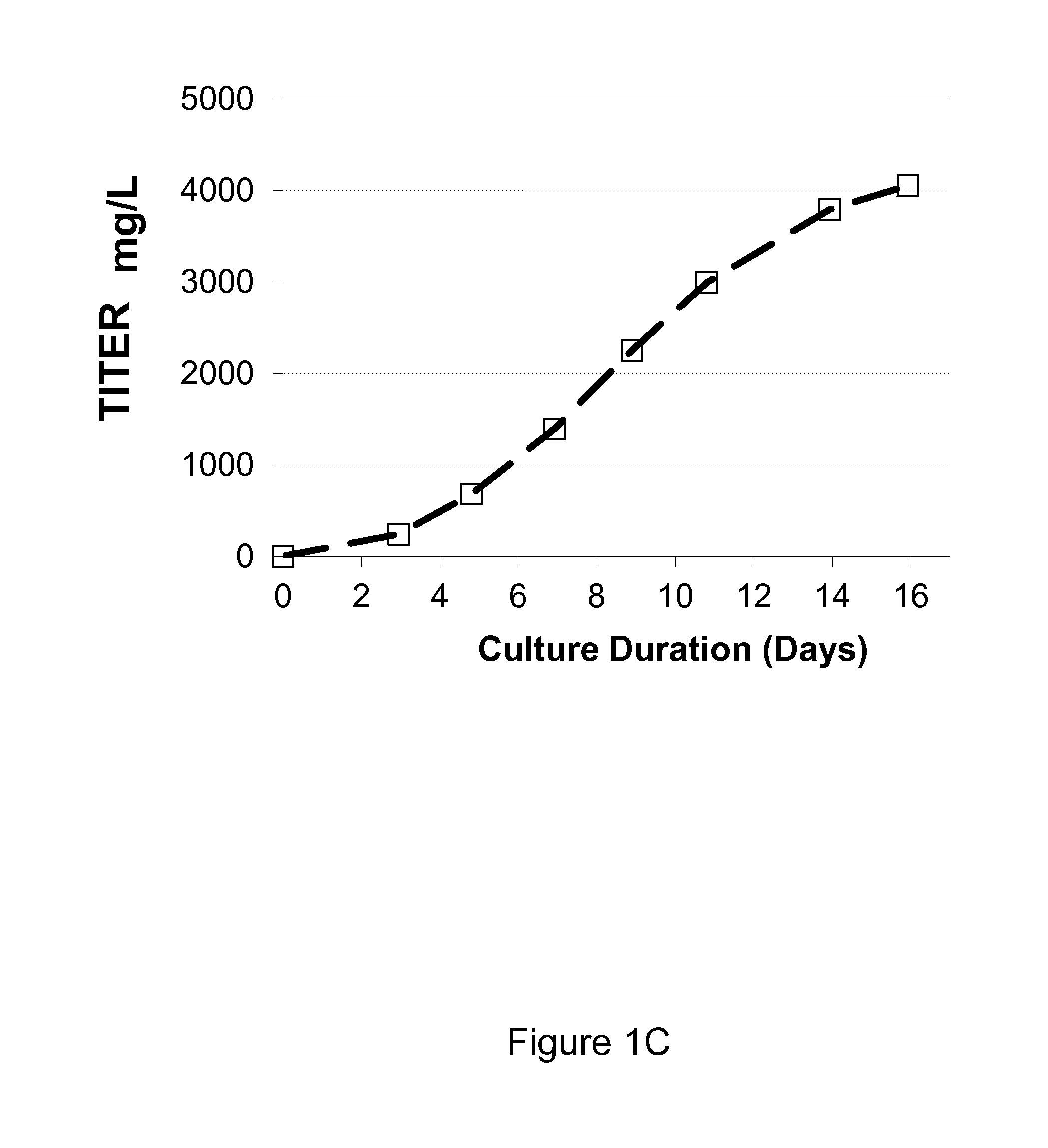
Process of expressing and isolating recombinant proteins and recombinant protein products from plants, plant derived tissues or cultured plant cells
A process of expressing a recombinant protein in a plant and of isolating the recombinant protein from the plant, the process is effected by (a) providing a plant, a plant derived tissue or cultured plant cells expressing a fusion protein including the recombinant protein and a cellulose binding peptide being fused thereto, the fusion protein being compartmentalized within cells of the plant, plant derived tissue or cultured plant cells, so as to be sequestered from cell walls of the cells of the plant, plant derived tissue or cultured plant cells; (b) homogenizing the plant, plant derived tissue or cultured plant cells, so as to bring into contact the fusion protein with a cellulosic matter of the plant, plant derived tissue or cultured plant cells, to thereby effect affinity binding of the fusion protein via the cellulose binding peptide to the cellulosic matter, thereby obtaining a fusion protein cellulosic matter complex; and (c) isolating the fusion protein cellulosic matter complex.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Restricted glucose feed for animal cell culture
ActiveUS20050070013A1Promote production of recombinant proteinFlexible controlCulture processCell culture mediaAnimal scienceGlucose polymers
Methods of improving protein production in animal cell cultures are provided. Cell culture methods are presented wherein glucose is fed in a restricted manner to cell culture; this restricted feeding of glucose to the cell culture results in lactate production being controlled to a low level. The restricted feeding of glucose in a fed-batch process is not accomplished through a constant-rate feeding of glucose, and the restricted feeding need not depend on sampling. Instead, restricted feeding of glucose to the culture is accomplished through feeding of glucose to the culture at a rate that is a function of an expected or a premodeled rate of glucose consumption by the animal cells when exposed to medium containing a high level of glucose. Because lactate production is controlled to low levels, recombinant protein production is increased.
Owner:WYETH
Process for improved protein expression by strain engineering
ActiveUS20140162279A1Increasing recombinant protein productionReduce energy consumptionMicrobiological testing/measurementFermentationBiotechnologyFusion Protein Expression
This invention is a process for improving the production levels of recombinant proteins or peptides or improving the level of active recombinant proteins or peptides expressed in host cells. The invention is a process of comparing two genetic profiles of a cell that expresses a recombinant protein and modifying the cell to change the expression of a gene product that is upregulated in response to the recombinant protein expression. The process can improve protein production or can improve protein quality, for example, by increasing solubility of a recombinant protein.
Owner:PFENEX
Method for recombinant protein production in mammalian cells
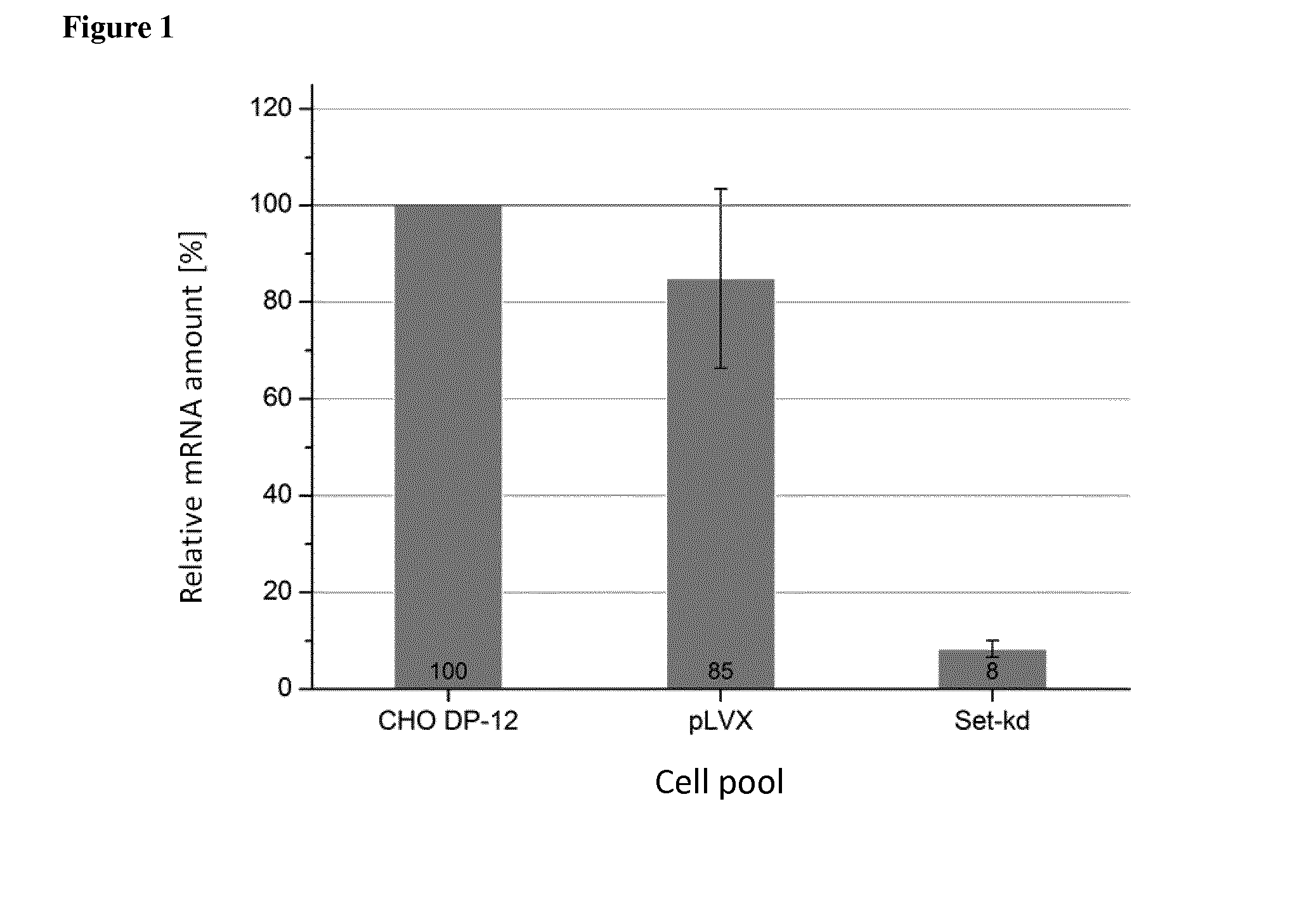
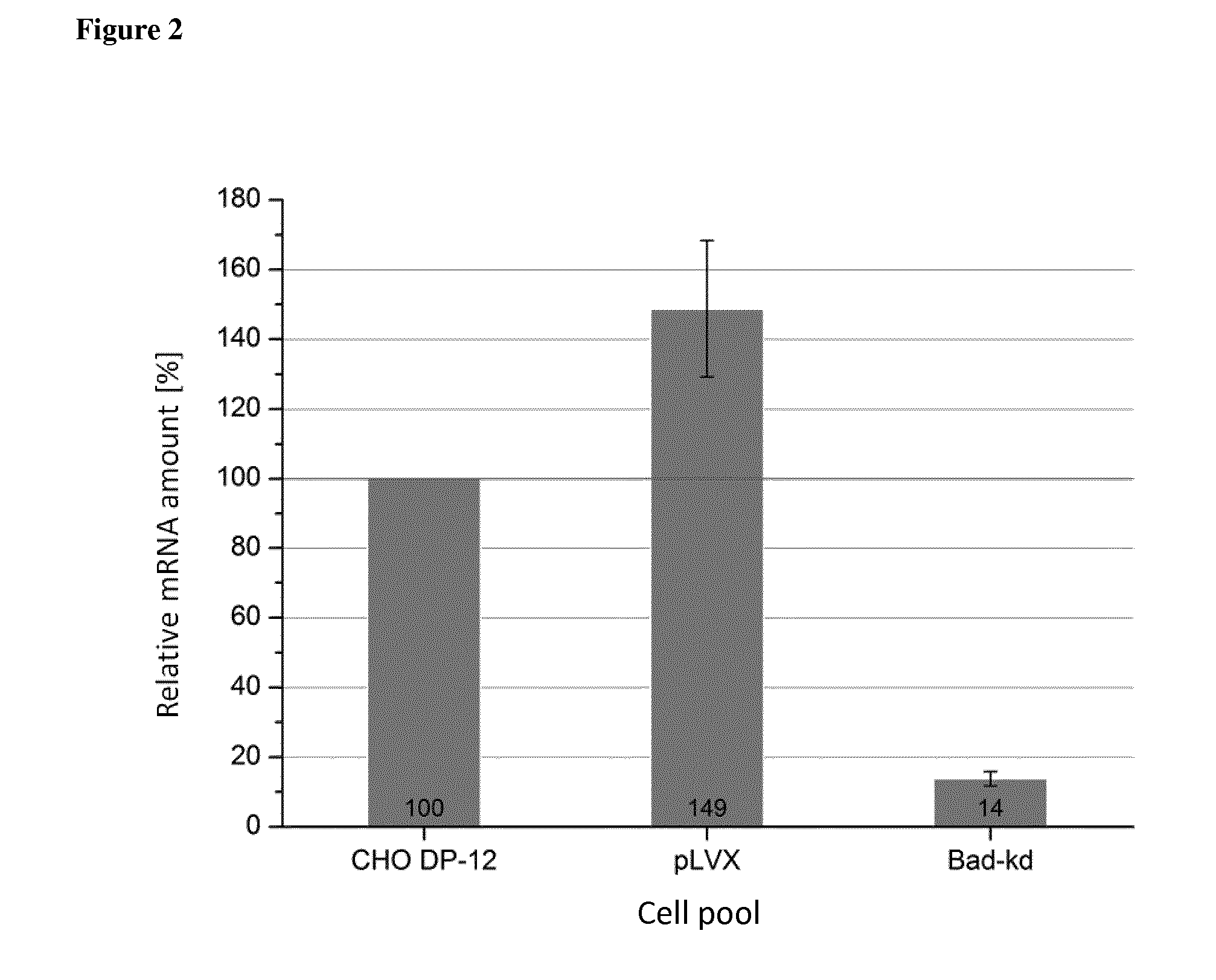
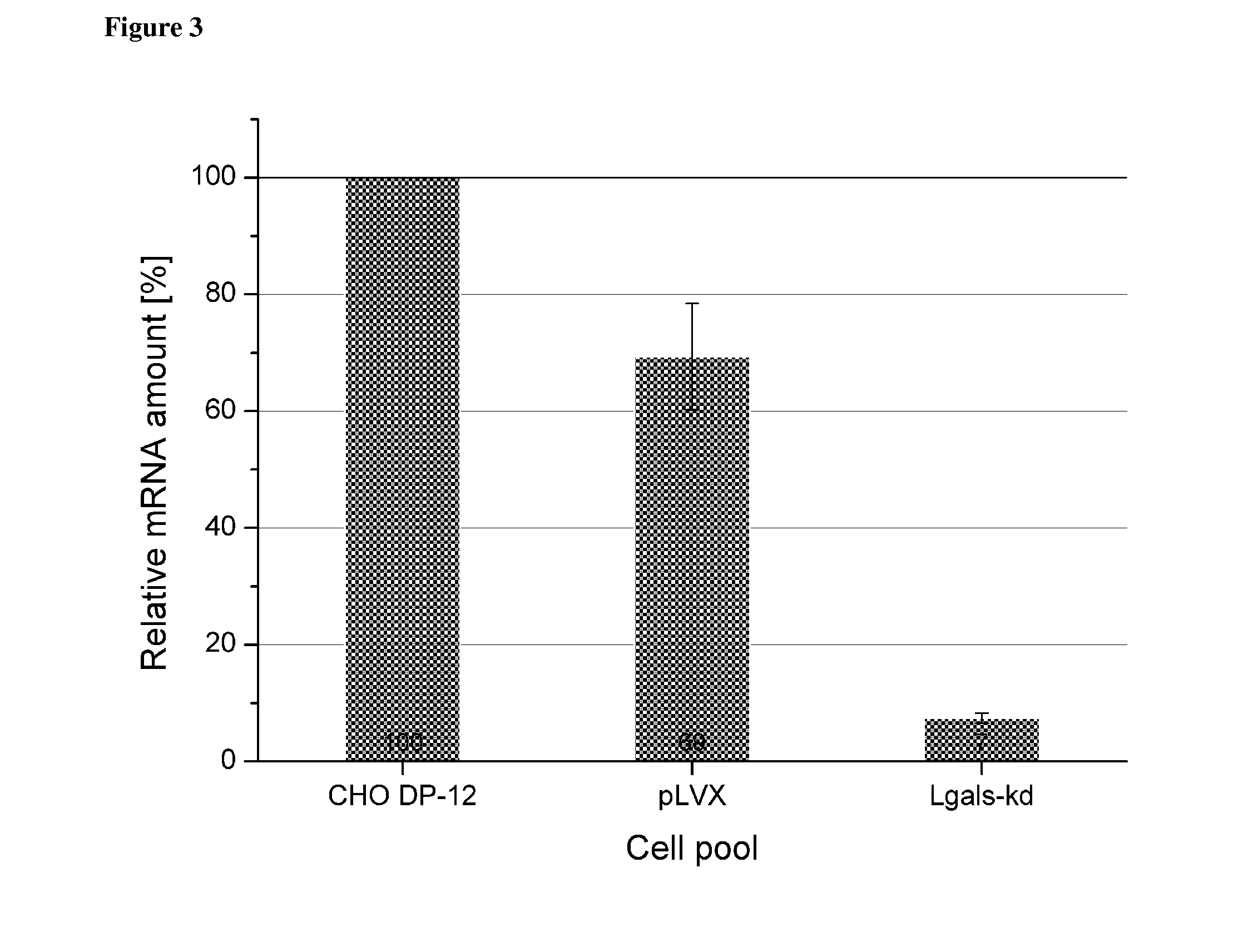
Some embodiments relate to methods for the recombinant expression of a protein of interest in a mammalian host cell, use of an sh RNA or an si RNA directed against the Galectin-1 gene for increasing the expression of a protein of interest in a mammalian host cell and kits comprising an shRNA or an si RNA and a CHO cell.
Owner:UNIVERSITY OF BIELEFELD
Cell culture media for enhanced protein production
InactiveUS7601535B1High protein yieldInhibit cell growthCulture processCell culture mediaBiotechnology3D cell culture
Culture media for in vitro cell culture which contain substantially saturated amounts of selected amino acids improve protein production, constrain cell growth and extend cell longevity, methods for the production and use of such media, and systems for the production of protein utilizing such media and methods.
Owner:WENG STEVE OH KAH +1
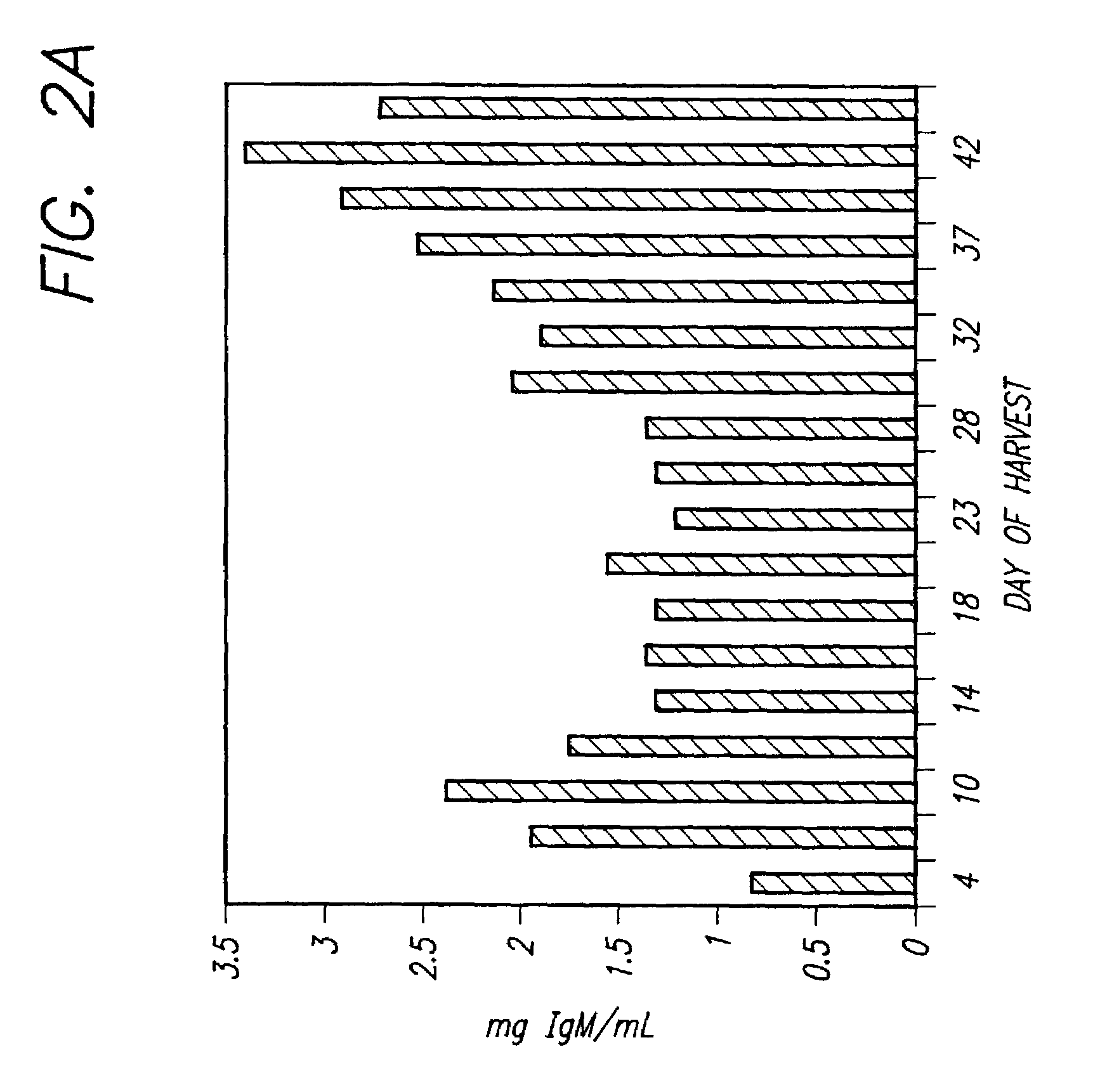
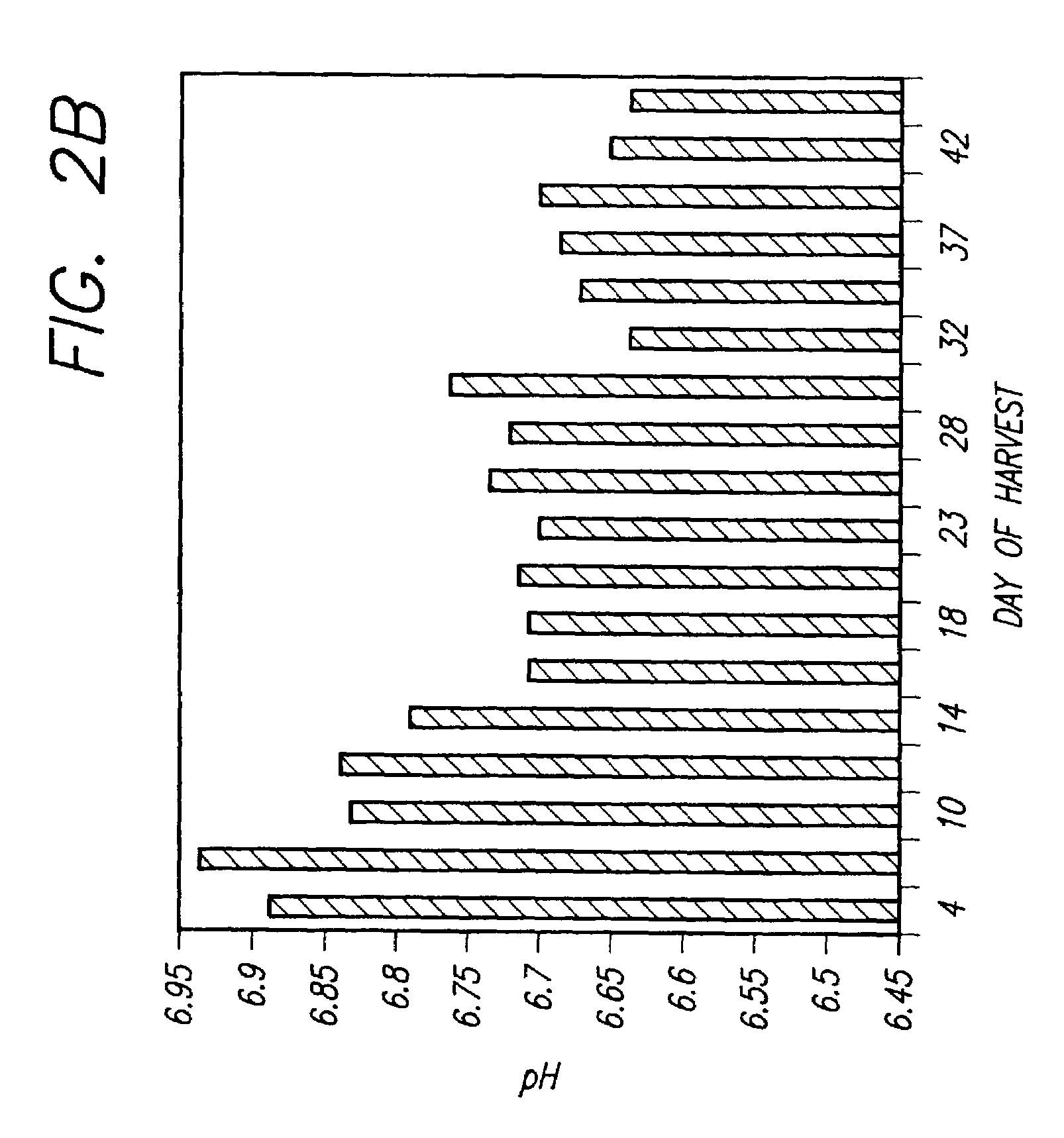
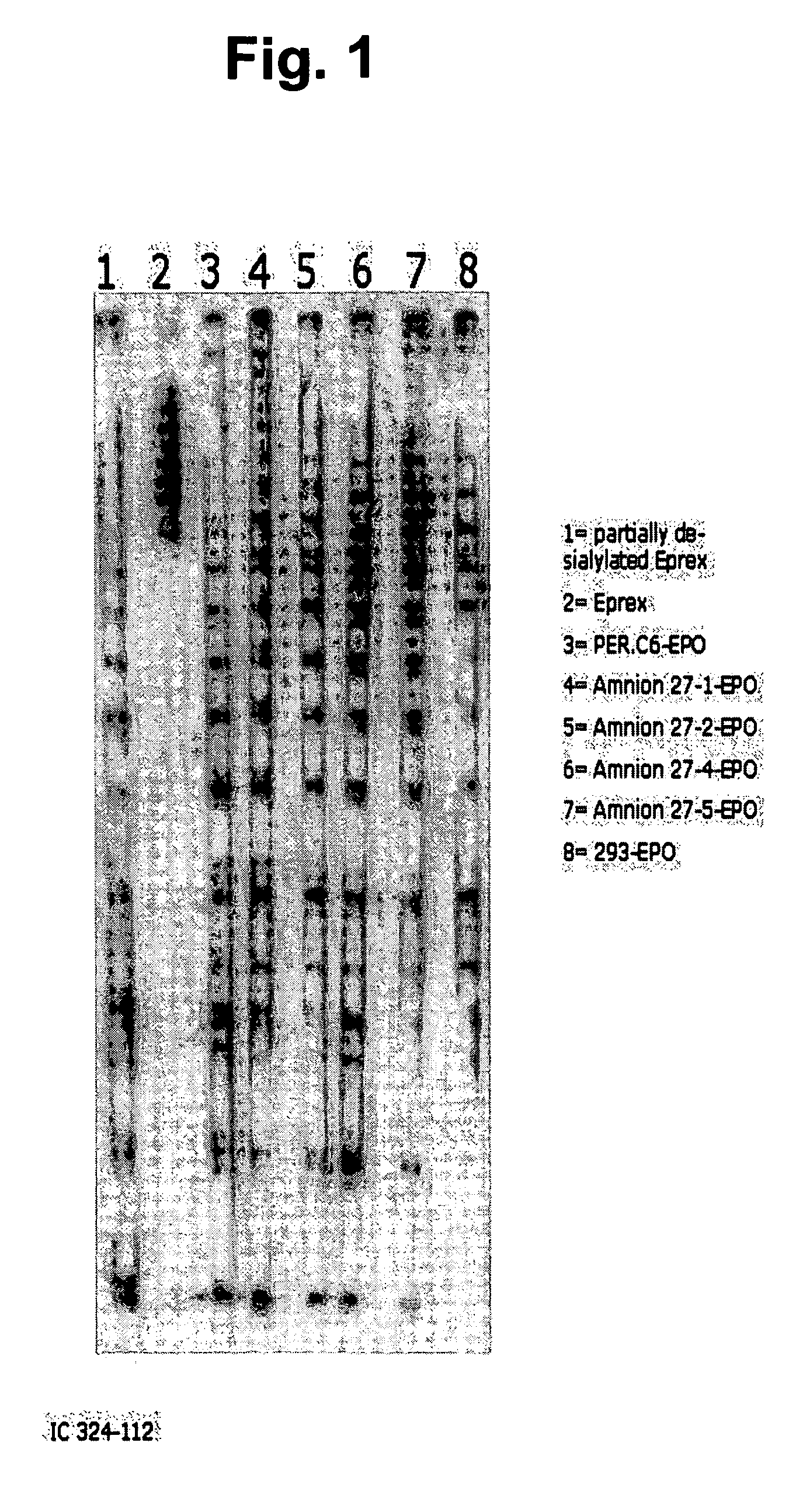
Recombinant protein production in permanent amniocytic cells that comprise nucleic acid encoding adenovirus E1A and E1B proteins
InactiveUS20050170463A1Good of suspension growthGood of growth factor independenceSsRNA viruses negative-sensePeptide/protein ingredientsHeterologousNucleic acid sequencing
The invention provides a method for producing at least one polypeptide of interest in a eukaryotic cell, the method comprising: providing a eukaryotic cell comprising nucleic acid encoding the polypeptide of interest; culturing the cell in a suitable medium; and harvesting the at least one polypeptide of interest from the eukaryotic cell, from the suitable medium, or from both the eukaryotic cell and the suitable medium, wherein the eukaryotic cell is a permanent amniocytic cell comprising a nucleic acid sequence encoding adenoviral E1A and E1B proteins. The invention also provides a eukaryotic cell comprising nucleic acid encoding a polypeptide of interest under control of a heterologous promoter, the eukaryotic cell not comprising a structural adenoviral protein, wherein the eukaryotic cell is a permanent amniotic cell comprising a nucleic acid sequence encoding adenoviral E1A and E1B proteins.
Owner:BOUT ABRAHAM +1
Nucleic acid structure and expression carrier for enhancing recombinant protein production and mass-production of recombinant protein
InactiveCN1712534AImprove propertiesProtection from decompositionHaemoglobins/myoglobinsAntibody mimetics/scaffoldsNucleic acid structureGene product
Nucleic acid construction and expression carrier for improving recombinant protein production and production of recombinant protein are disclosed. The process is carried out by cloning first nucleic acid sequence of coded thioredoxin and second nucleic acid sequence of coded ferrohemoglobin into recombinant host cell, reinforcing the recombinant host cell production and helping to release intracellular stress.
Owner:FENG CHIA UNIVERSITY
Recombinant protein production in a human cell
Owner:JANSSEN VACCINES & PREVENTION BV
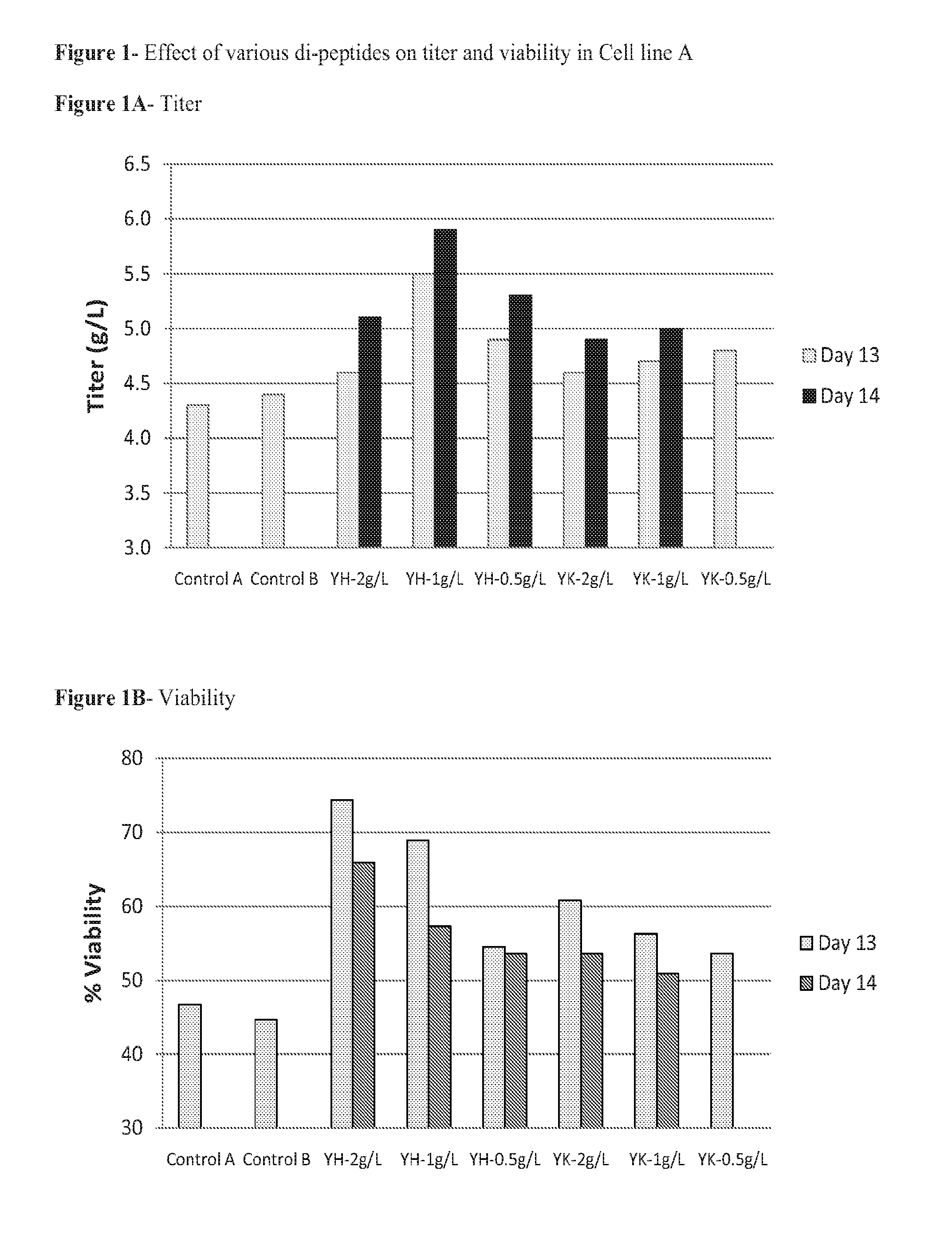
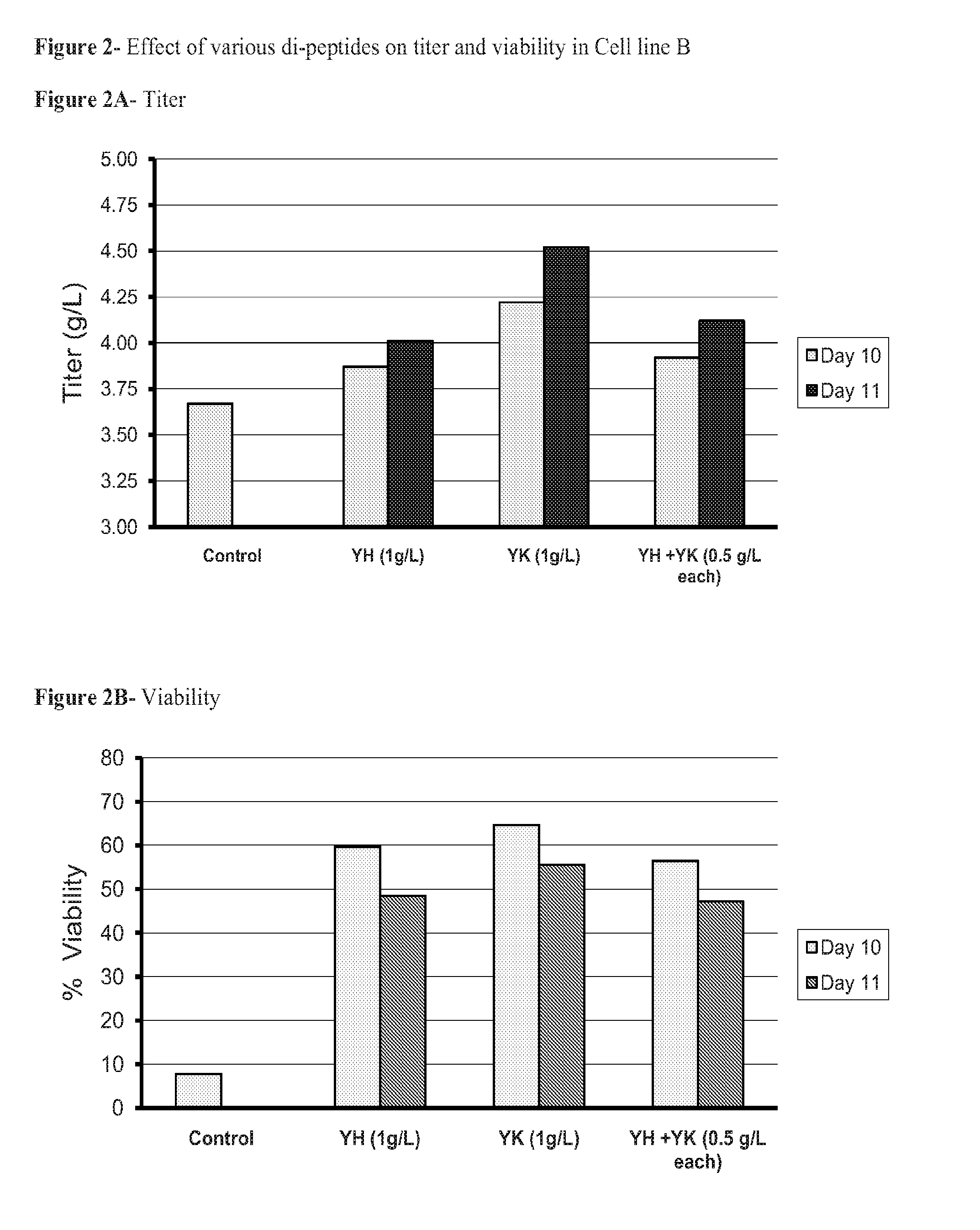
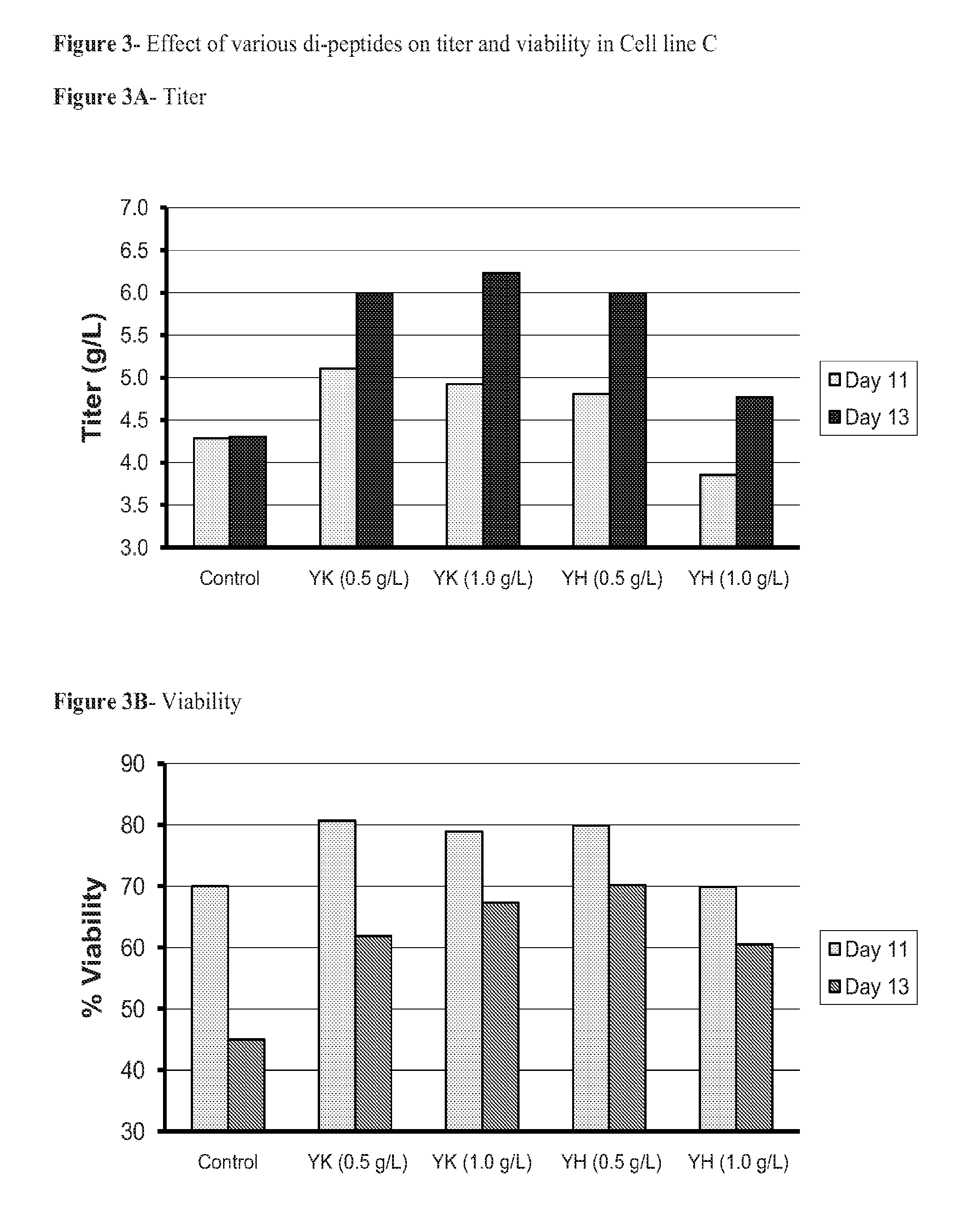
Dipeptides to enhance yield and viability from cell cultures
ActiveUS20130189737A1Improve viabilityEnhanced viable cell densityAnimal cellsGenetically modified cellsDipeptide3D cell culture
The present invention relates to the culture of animal cells in serum-free culture medium. The present invention provides particular dipeptides that can improve recombinant protein production and cell viability in such cultures, especially in the absence of peptones.
Owner:AMGEN INC
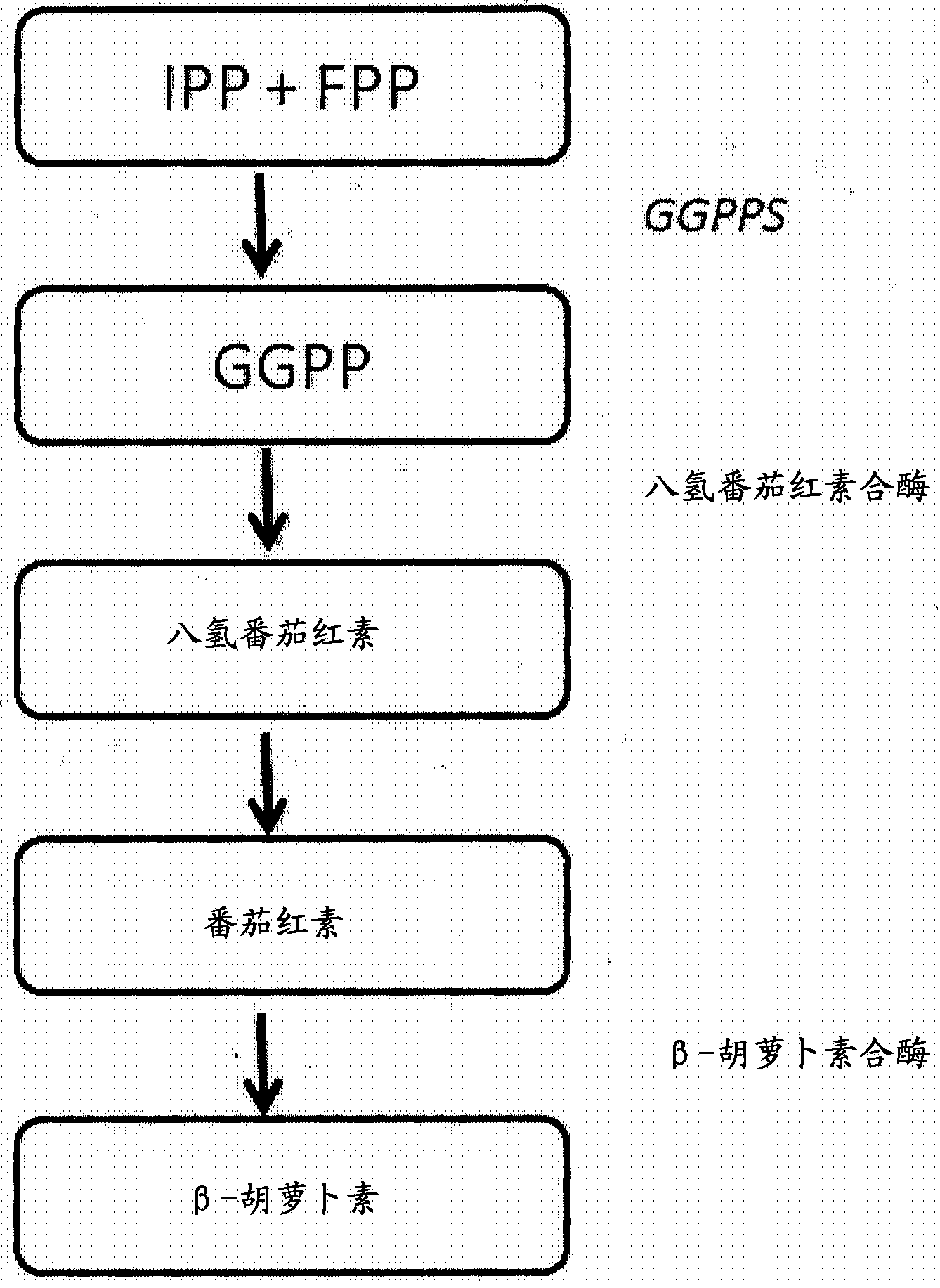
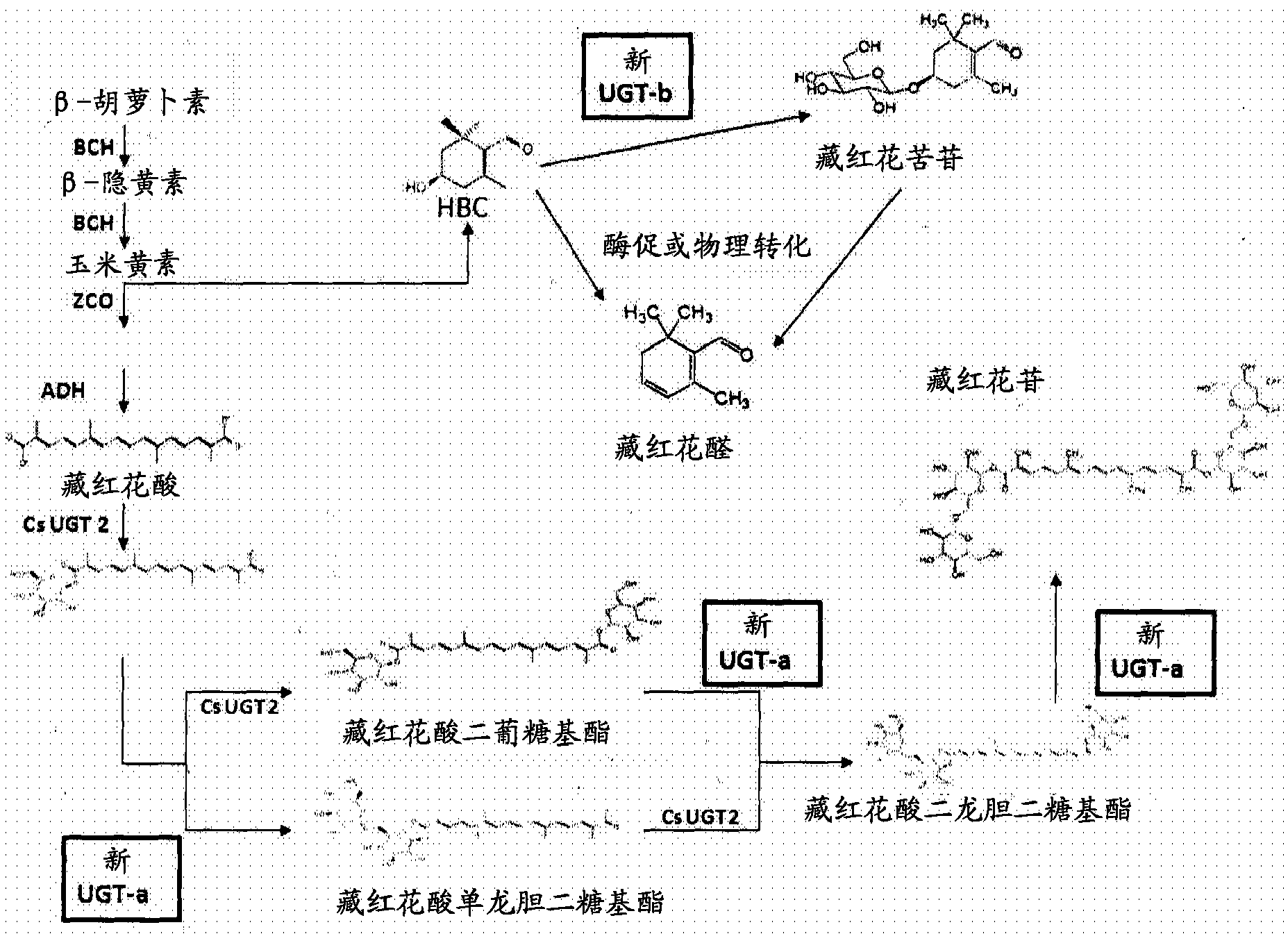
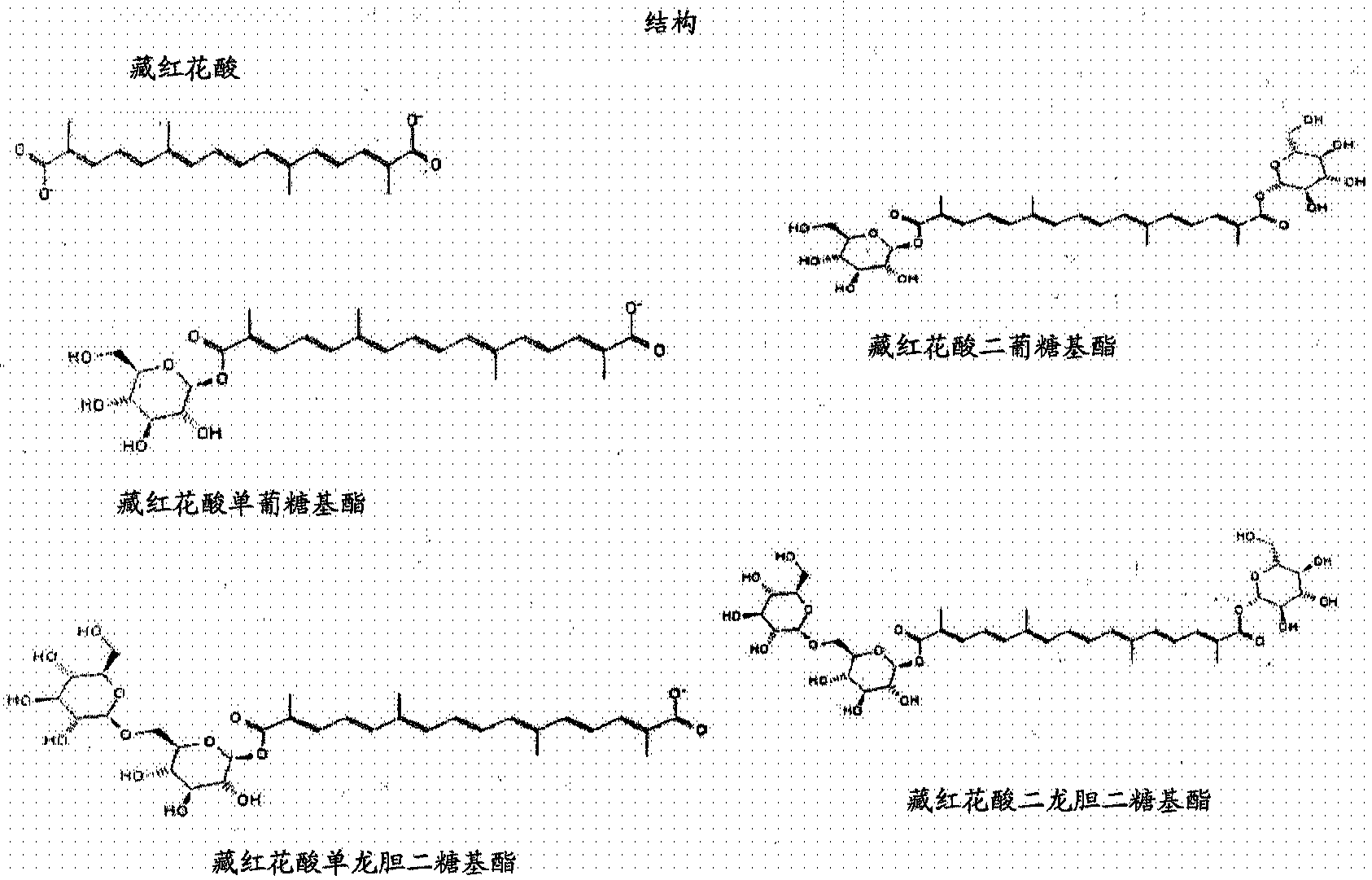
Methods and materials for recombinant production of saffron compounds
Recombinant microorganisms, plants, and plant cells are disclosed that have been engineered to express a zeaxanthin cleavage dioxygenase alone or in combination with recombinant genes encoding UDP-glycosyltransferases (UGTs). Such microorganisms, plants, or plant cells can produce compounds from saffron such as crocetin, crocetin dialdehyde, crocin, or picrocrocin.
Owner:EVOLVA SA
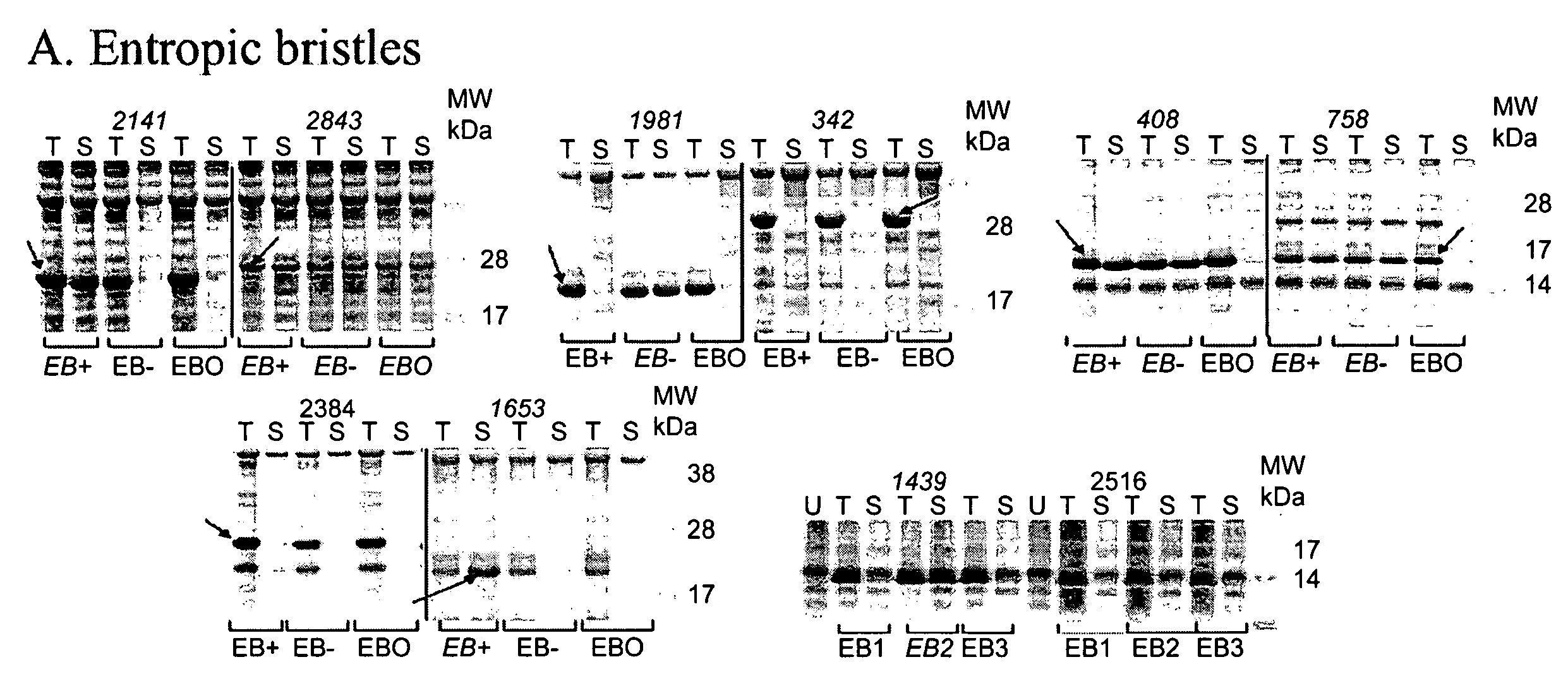
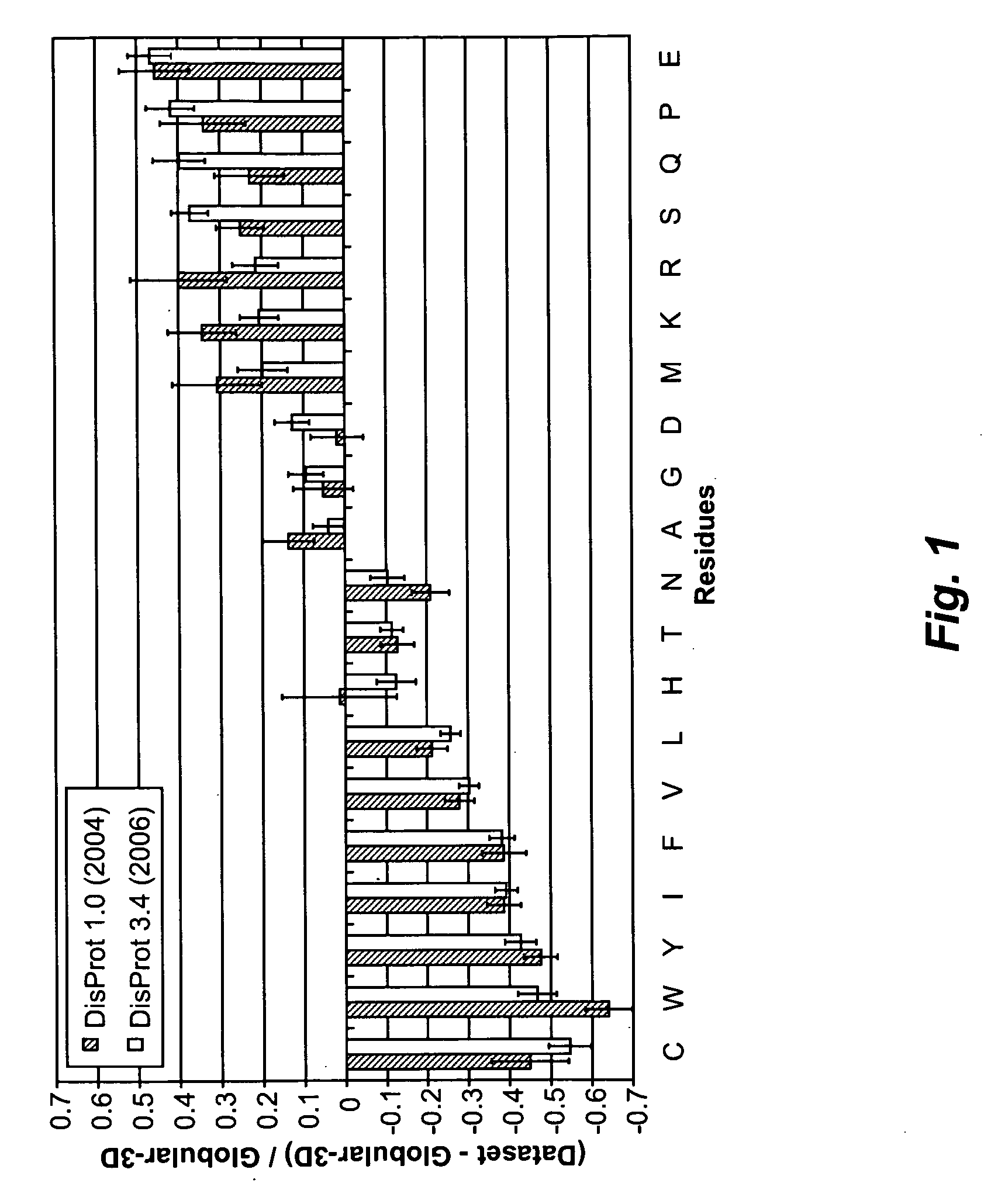
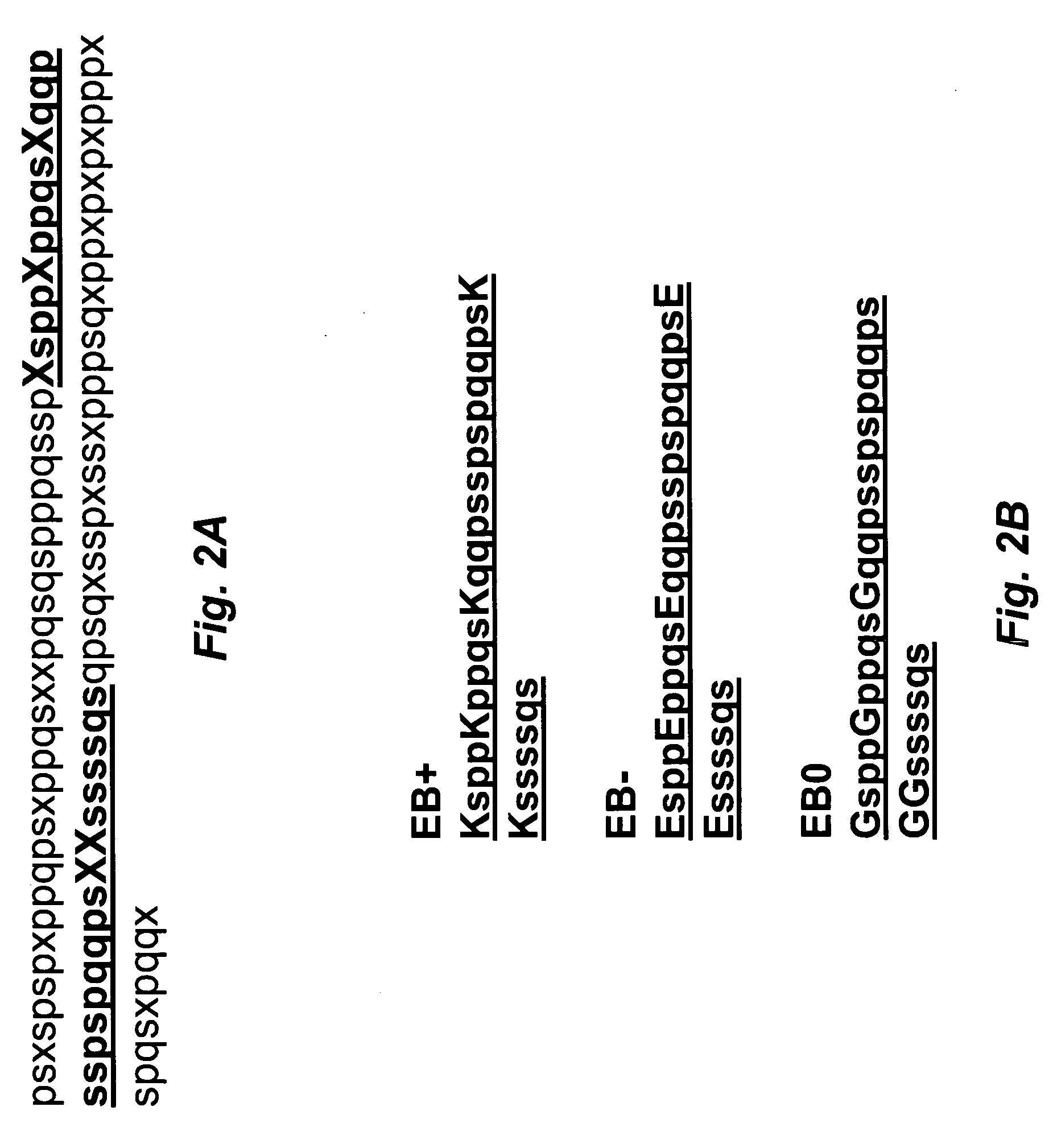
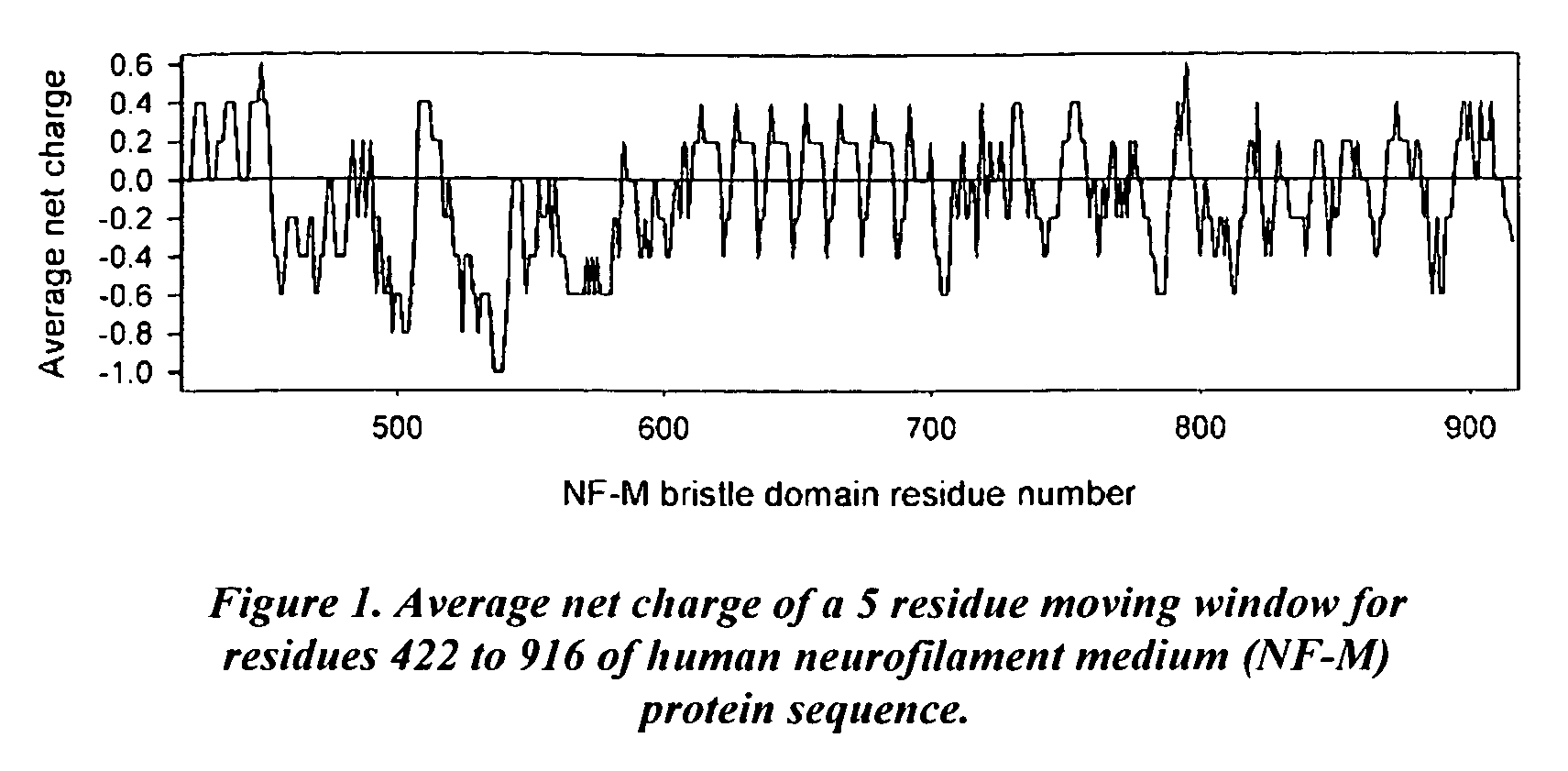
Artificial entropic bristle domain sequences and their use in recombinant protein production
InactiveUS20090137004A1Improve solubilityReduce aggregationSugar derivativesBacteriaBristlePolynucleotide
Compositions and methods for recombinant protein production and, more particularly, fusion polypeptides, polynucleotides encoding fusion polypeptides, expression vectors, kits, and related methods for recombinant protein production.
Owner:MOLECULAR KINETICS
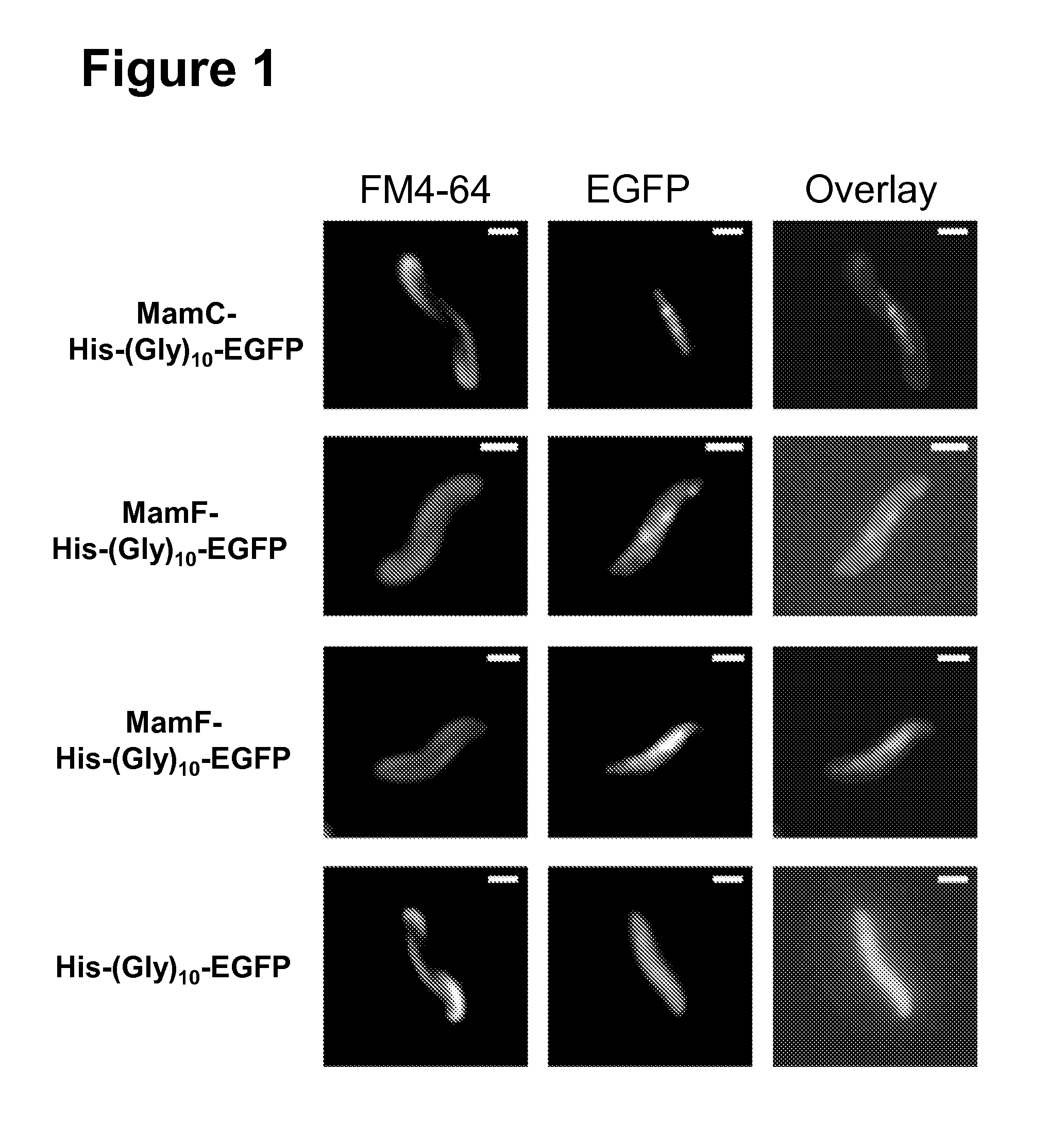
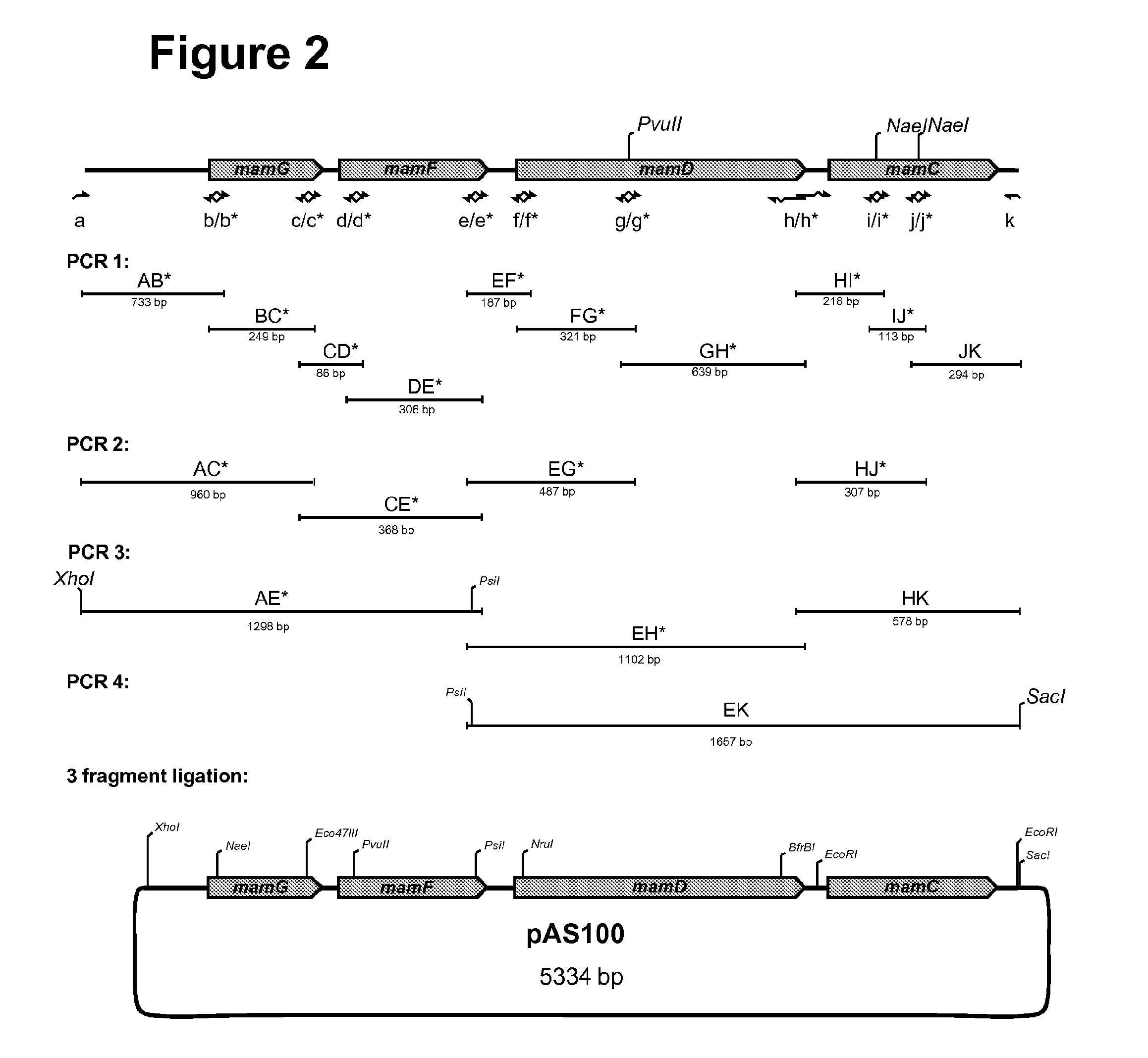
Method for the recombinant production of magnetic nanoparticles
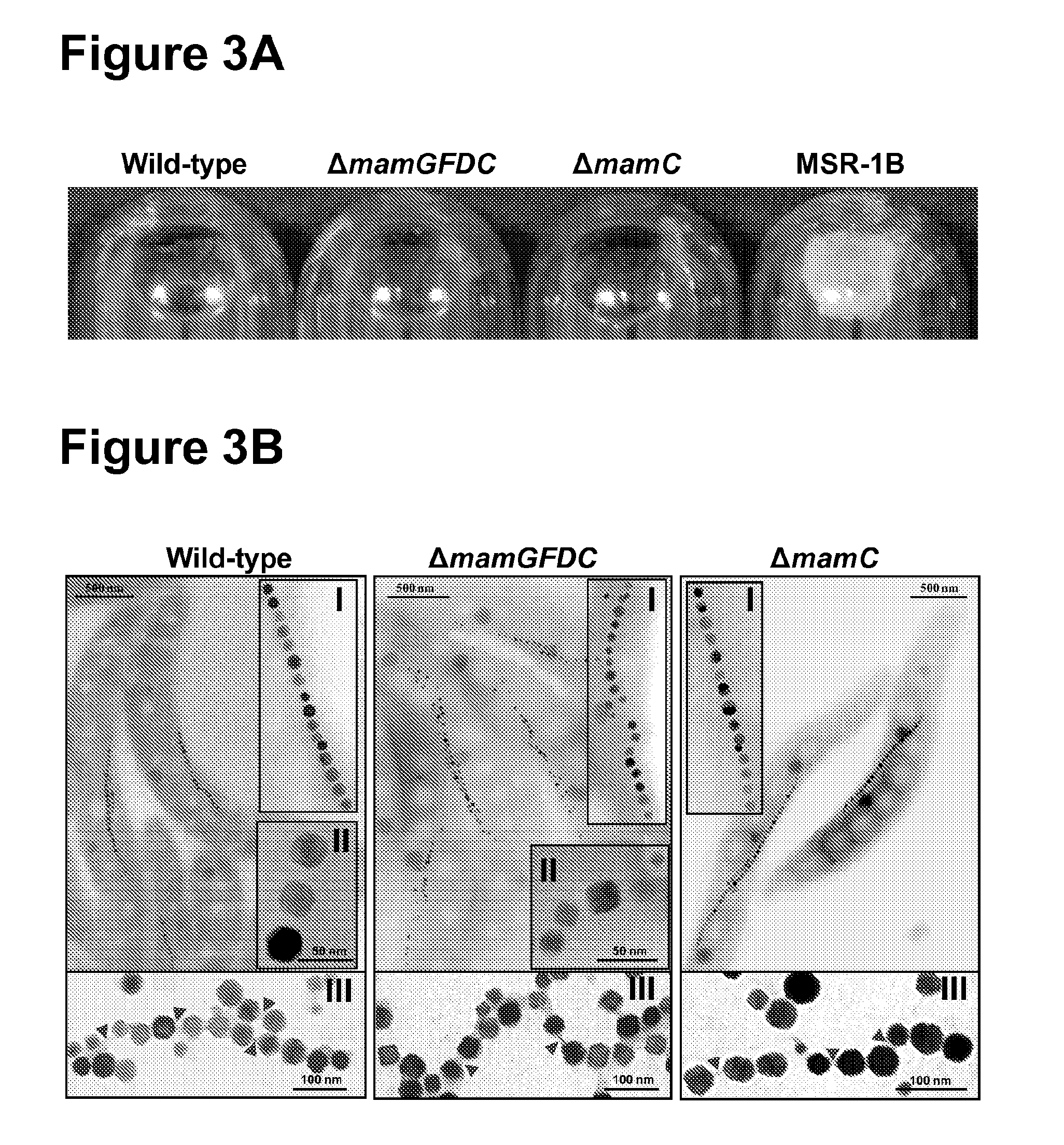
The present invention relates to methods and compositions for the size-adjusted recombinant production of magnetic nanoparticles. More particular, the invention relates to a method, comprising: providing one or more cells being capable of producing magnetic nanoparticles; modifying in the one or more cells the expression of one or more genes involved in the formation of the magnetic nanoparticles; cultivating the modified cells obtained in step (b); and isolating the magnetic nanoparticles from the cultivated cells, wherein the magnetic nanoparticles have a defined size. In preferred embodiments, the method comprises modifying the expression of one or more genes of the mamGFDC operon in magnetotactic bacterial cells. The invention is further directed to host cells bearing said modifications, the recombinant magnetic particles isolated from such cells as well as to the use of such particles for the detection and / or separation of biomolecules or as a contrast agent in magnetic resonance imaging.
Owner:LUDWIG MAXIMILIANS UNIV MUNCHEN +1
Pichia pastoris fermentation medium for large scale production of recombinant human collagen
ActiveCN106256911AHigh expressionShort induction periodMicroorganismsMicroorganism based processesPichia pastorisCollagen VI
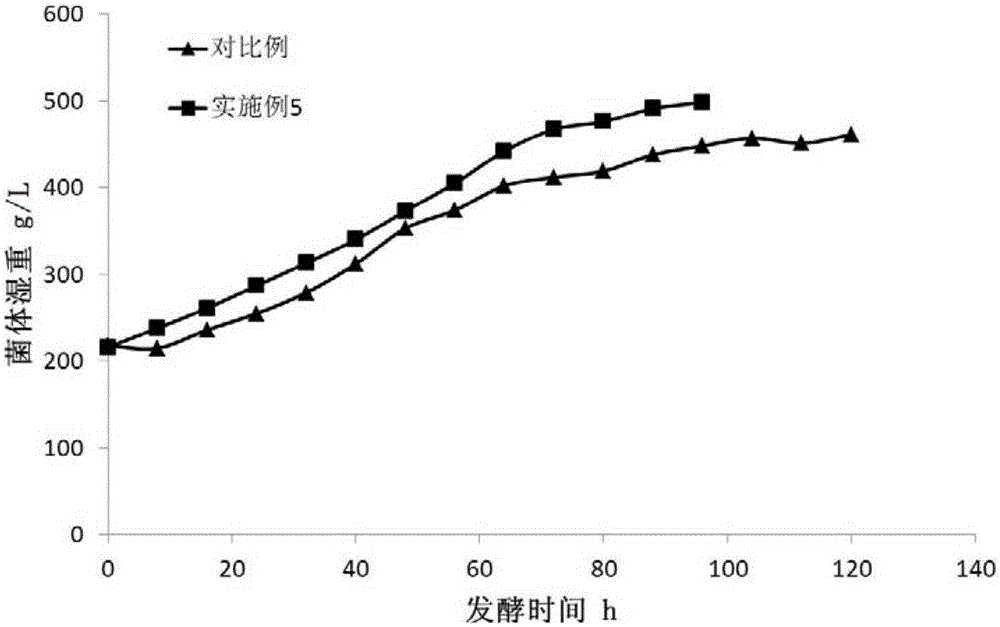
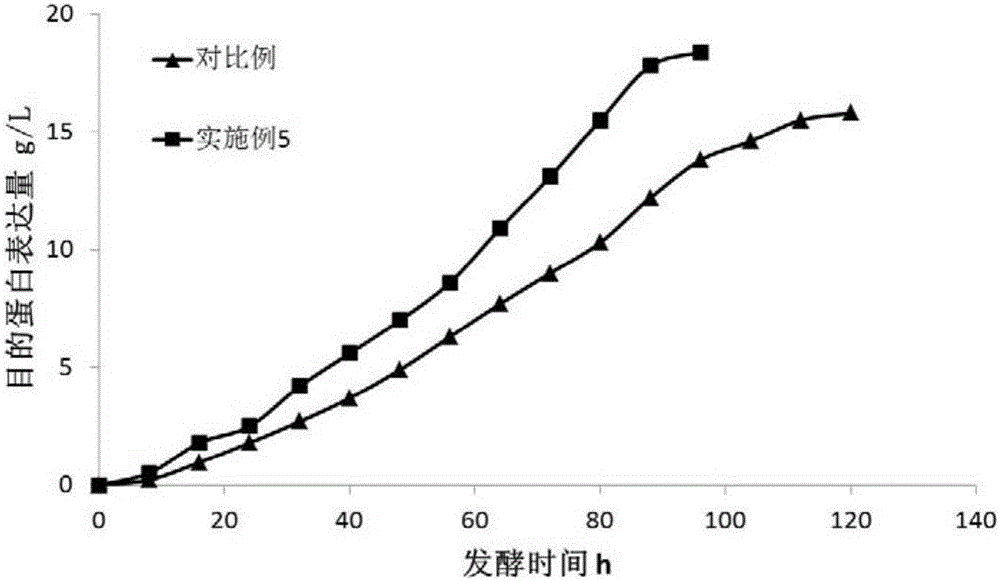
The invention discloses a pichia pastoris fermentation medium for large scale production of recombinant human collagen. 0.01-10g / L of a metabolism conditioning agent is used in the medium so that pichia pastoris metabolism is adjusted, recombinant protein expression is accelerated, a fermentation period is shortened, energy consumption is reduced, an error risk is reduced and stable industrial production is guaranteed. The medium realizes large scale production of recombinant human collagen through 1000L of a fermentation cylinder, shortens a methanol induction period by 20% and improves a recombinant human collagen expression level by 16.5%. The pichia pastoris fermentation medium is especially suitable for a pichia pastoris methanol induction stage of recombinant human collagen industrial high density fermentation expression, effectively reduces a production cost, produces substantial economic benefits and has a very important meaning for industrial production of the recombinant human collagen.
Owner:浙江诸暨聚源生物技术有限公司
Method for enhancing yield of recombinant protein production from plants
InactiveUS20050251885A1Inhibit expressionPromote recoveryHydrolasesOther foreign material introduction processesBiotechnologyProteinase activity
The present invention relates to a method for enhancing the yield of recombinant protein produced in genetically transformed plants. The invention most particularly relates to a method for preventing the undesirable proteolysis of recombinant proteins after harvest of the plant, during processing of the products from the plants. Especially, this invention focuses on introducing protease inhibitors in plants to prevent undesirable proteolysis of recombinant proteins at the time of cell disruption during the extraction process.
Owner:UNIV LAVAL
Recombinant production of peptides
ActiveUS20120088268A1Peptide/protein ingredientsAntibody mimetics/scaffoldsNucleic acid sequencingSelf assembling
The present invention relates to repetitive self-assembling precursor proteins, nucleic acid sequences and expression constructs encoding the same, and to methods for recombinant production of peptides using such precursor proteins.
Owner:BASF AG
Methods and compositions for preventing norleucine misincorporation into proteins
Owner:GENENTECH INC
Entropic bristle domain sequences and their use in recombinant protein production
InactiveUS7494788B2Efficient cleaningImproved self-foldingSugar derivativesPeptide preparation methodsBristlePolynucleotide
Owner:MOLECULAR KINETICS
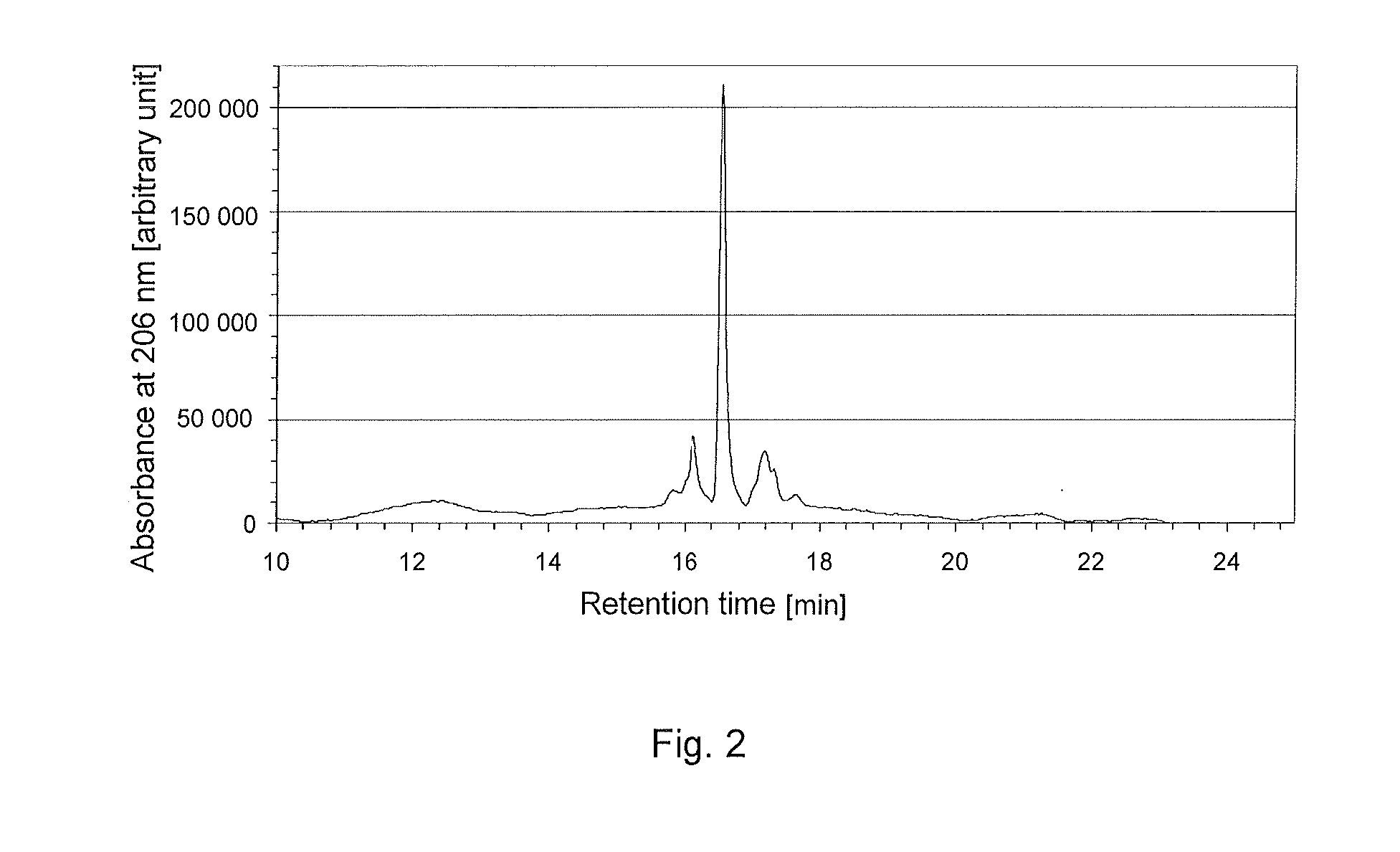
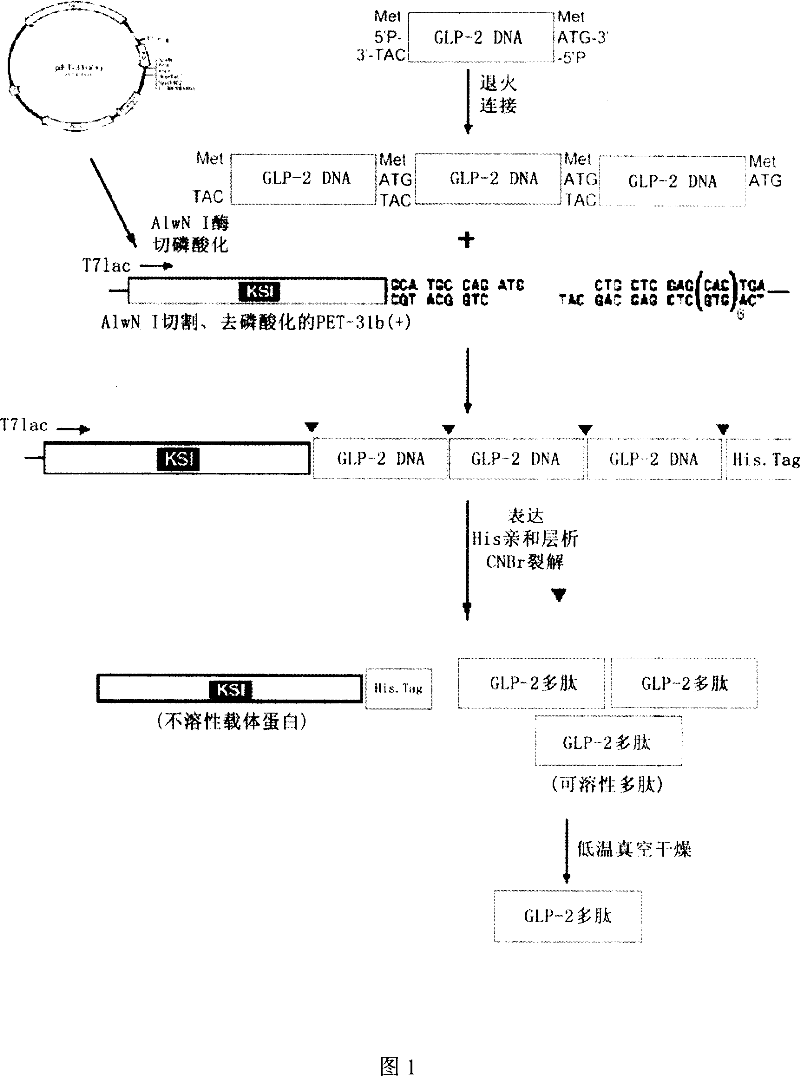
Method for recombinant production of pancreatic glucagons sample peptide-2
InactiveCN101041818AIncrease contentBacteriaPeptide/protein ingredientsPancreatic glucagonDrug biological activity
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com