Methods and materials for recombinant production of saffron compounds
A technology of saffron and saffron acid, applied in the field of recombinant production of compounds from saffron, a saffron plant, can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
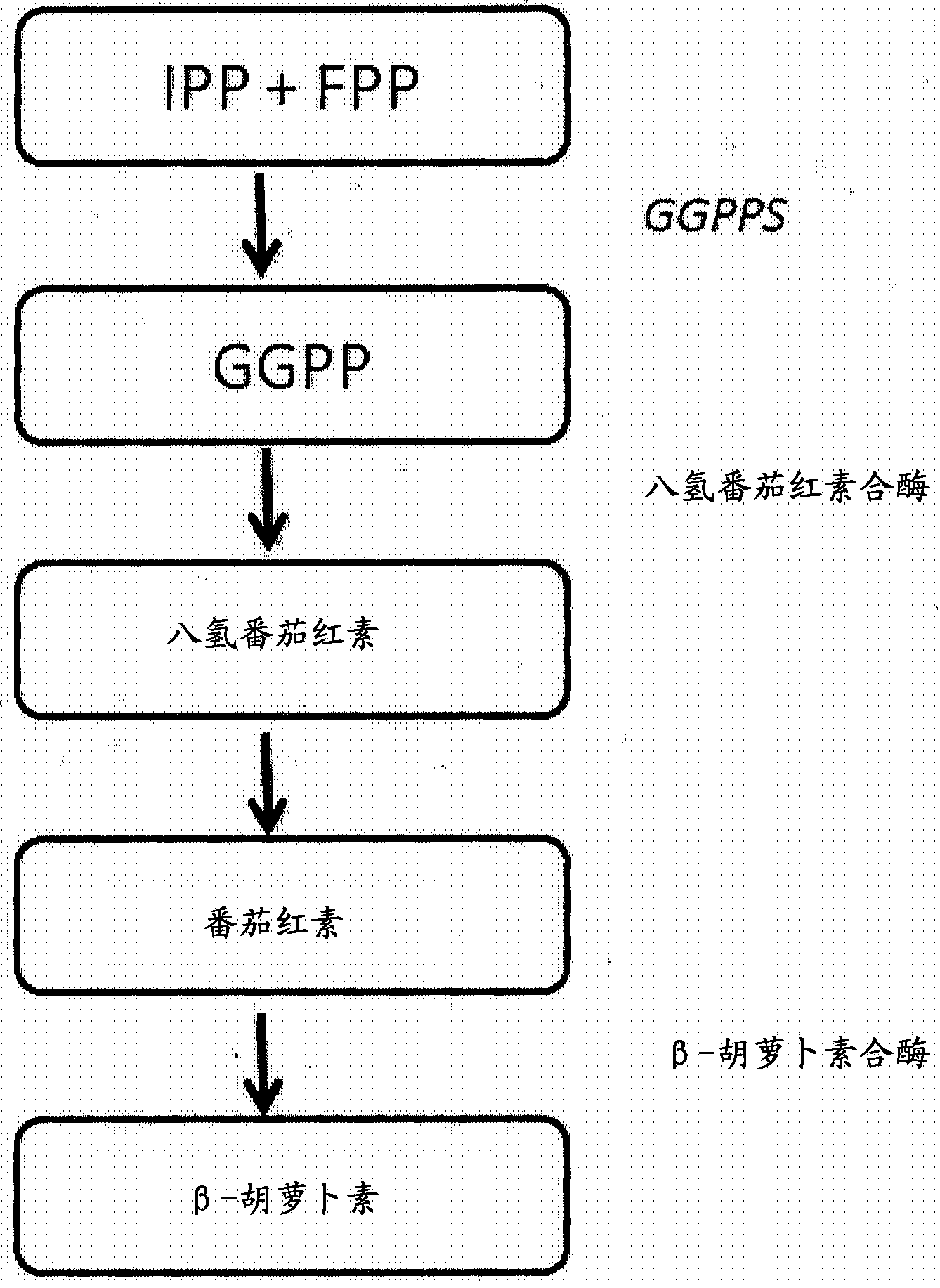
[0122] Example 1: Production of beta-carotene in yeast
[0123] Construction of β-carotene-producing yeast reporter strains for use in eYAC experiments designed to discover optimal combinations of saffron biosynthetic genes. Neurospora crassa phytoene desaturase (also known as phytoene desaturase) (accession number XP_964713) and Phaffia GGDP synthase (also known as geranylgeranyl Pyrophosphate synthase or CrtE) (accession number DQ012943) and Phaffia phytoene-β-carotene synthase CrtYB (accession number AY177204) genes were inserted into expression cassettes, and these expression cassettes were integrated into the experimental In the genome of the chamber yeast strain Saccharomyces cerevisiae CEN.PK113-11. Phytoene desaturase and CrtYB were overexpressed under the control of the strong constitutive GPD1 promoter, and CrtE was overexpressed using the strong constitutive TPI1 promoter. Chromosomal integration of the Phaffia CrtE and Neurospora crassa phytoene desaturase expres...
Embodiment 2
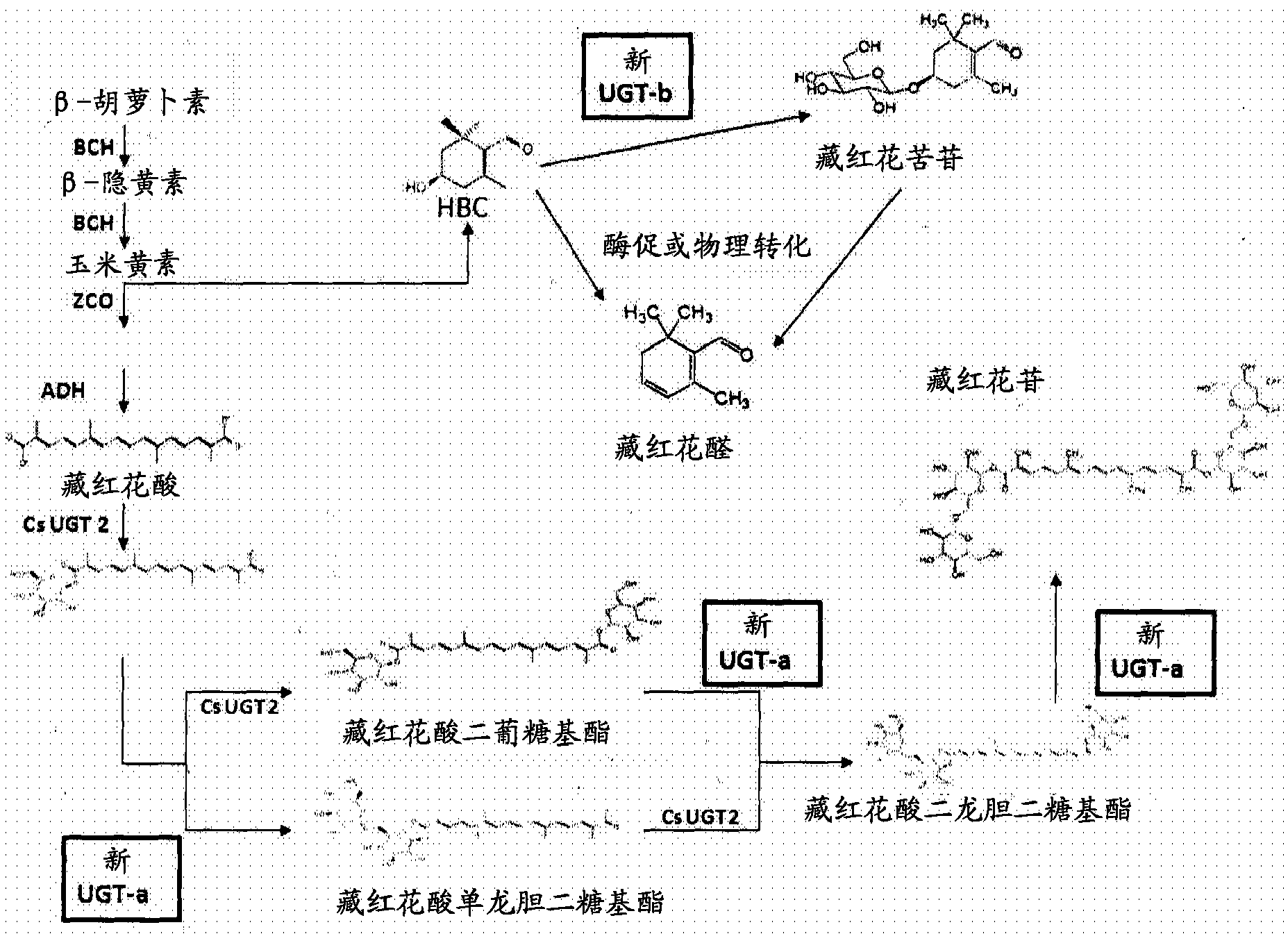
[0125] Example 2: Yeast production of optimized HBC and crocetin dialdehyde
[0126] Crocetin is known to be formed from crocetin dialdehyde, and crocetin dialdehyde and hydroxy-β-cyclic citral (HBC) are produced after cleavage of zeaxanthin with the enzyme zeaxanthin-cleaving dioxygenase (ZCD) . Using the eYAC described in Example 1 and the β-carotene-producing yeast strain, a batch of genes was assembled into the eYAC to establish the optimal pathway for the biosynthesis of crocindialdehyde and HBC.
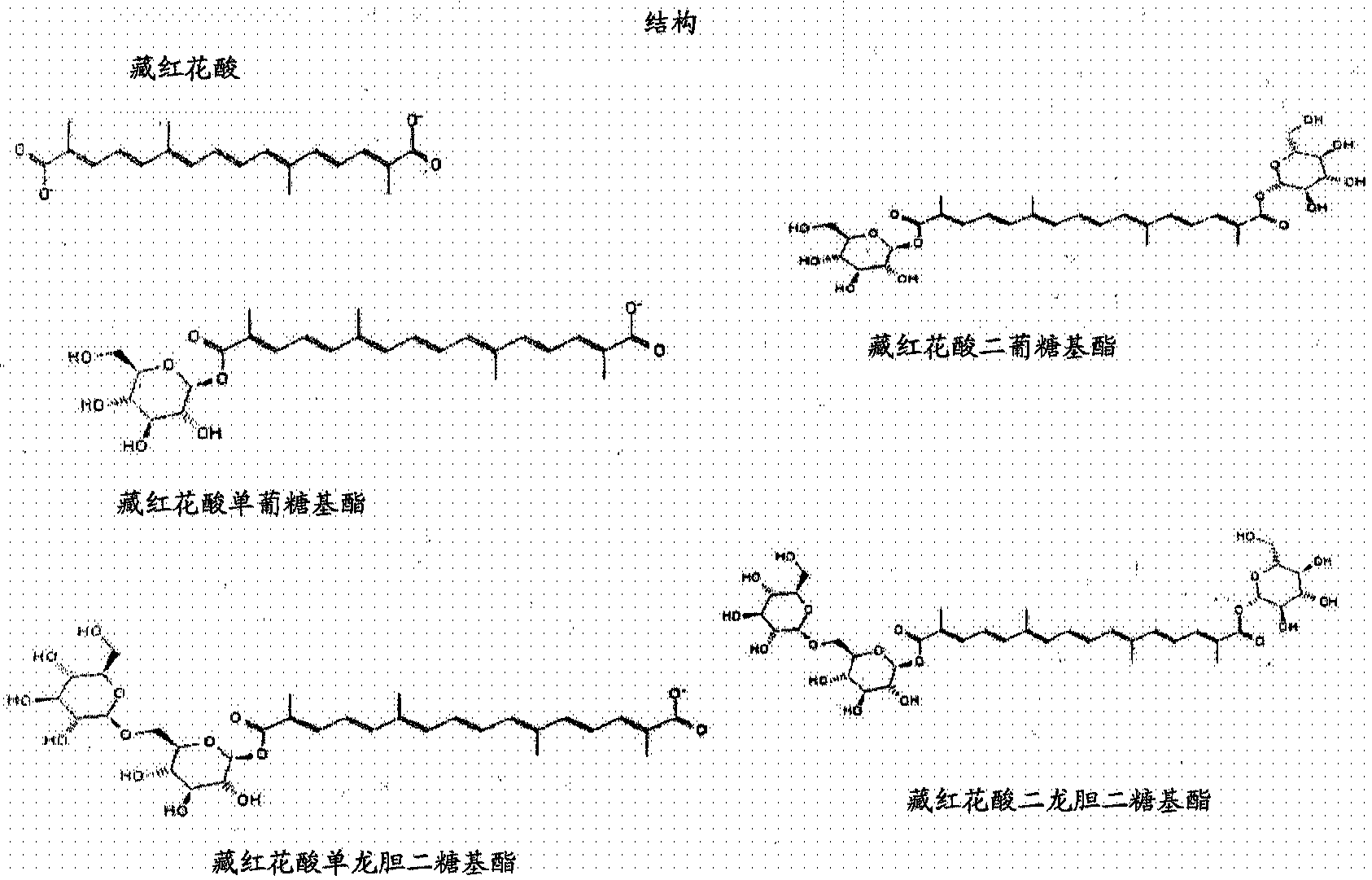
[0127] A panel of genetic analogs of the enzyme for the conversion of β-carotene to crocindialdehyde was generated by yeast codon-optimized synthesis (DNA2.0) and inserted under the promoters of various methionine-repressible genes eYAC enters the vector (Entry Vector). Naesby et al., Microb Cell Fact. 8:45 (2009) describes the use of eYAC technology. The expression cassettes for the 37 saffron biosynthetic genes shown in Table 1 were ligated (with or without UGT genes) ...
Embodiment 3
[0134] Example 3: Discovery of UGTs that form crocinpicrin
[0135] Glucosyltransferases are required to form saffron from hydroxy-β-cyclocitral (HBC). This reaction is aglycone glucosylation rather than glucose-glucose bond formation, and there are families of glycosyltransferases that utilize UDP-glucose to screen for this type of activity.
[0136] Source of HBC substrate
[0137] HBC was synthesized and the desired compound was purified by chiral column chromatography (GVK, Hyderabad).
[0138] Screening for UGT enzymes
[0139] Crocinopicroside formation was assayed for the following UGTs: Stevia 88B1, 76G1, 74G1, 91D2e, 85C2, 73EV12; Vinca UGT2; and Arabidopsis UGT75B1 and Arabidopsis hybridases UGT353 and UGT354 ( image 3 sequence provided).
[0140] Genes encoding these UGTs were cloned into plasmids utilizing the T7 promoter and transformed into E. coli BL21 cells for expression studies. Strains with these UGTs were induced with 0.1 mM IPTG and the induced cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com