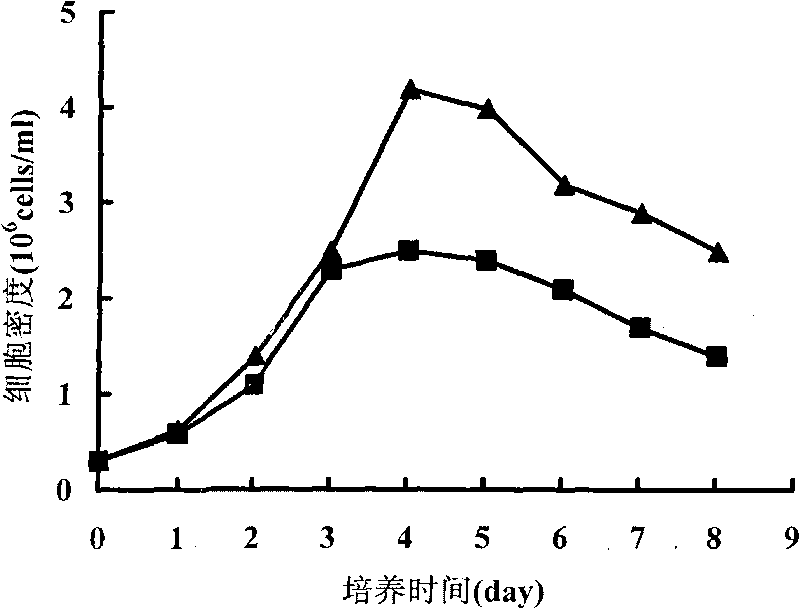
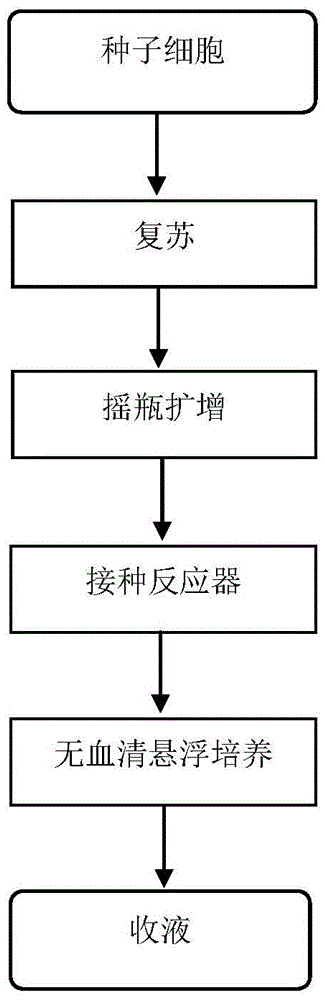
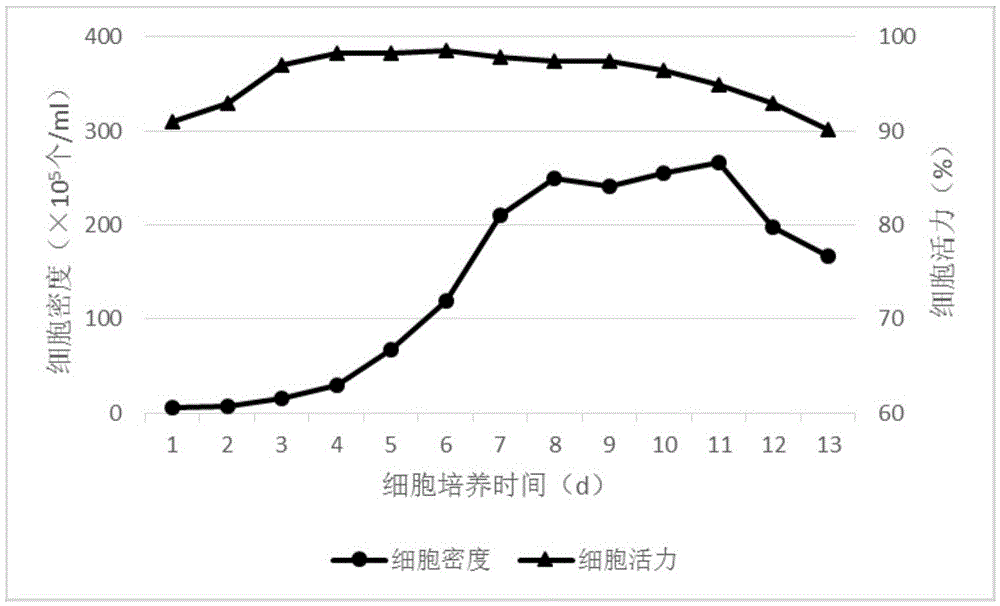
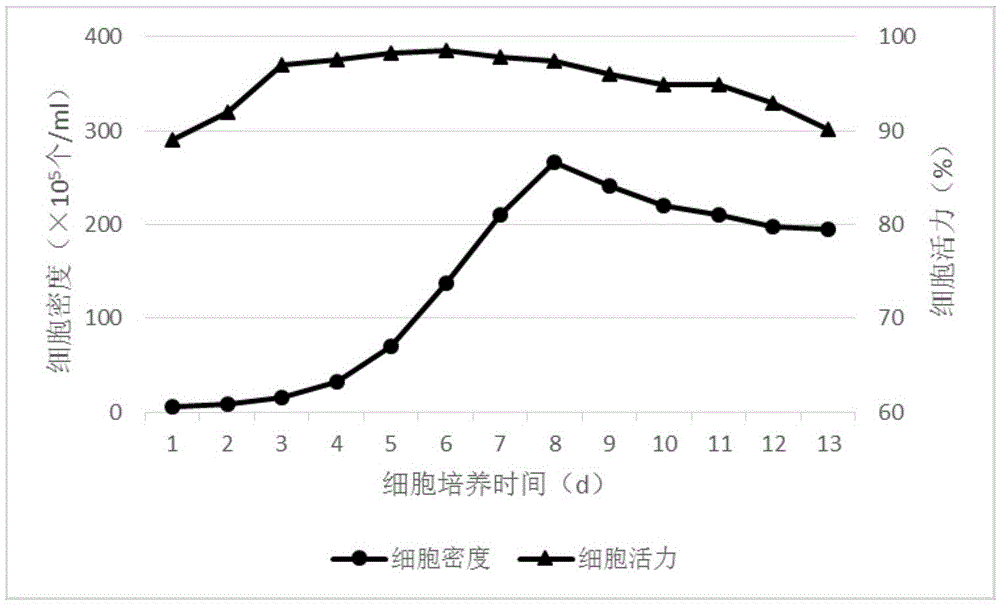
Patents
Literature
359 results about "Chinese hamster ovary cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chinese hamster ovary (CHO) cells are an epithelial cell line derived from the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of therapeutic proteins. They have found wide use in studies of genetics, toxicity screening, nutrition and gene expression, particularly to express recombinant proteins. CHO cells are the most commonly used mammalian hosts for industrial production of recombinant protein therapeutics.
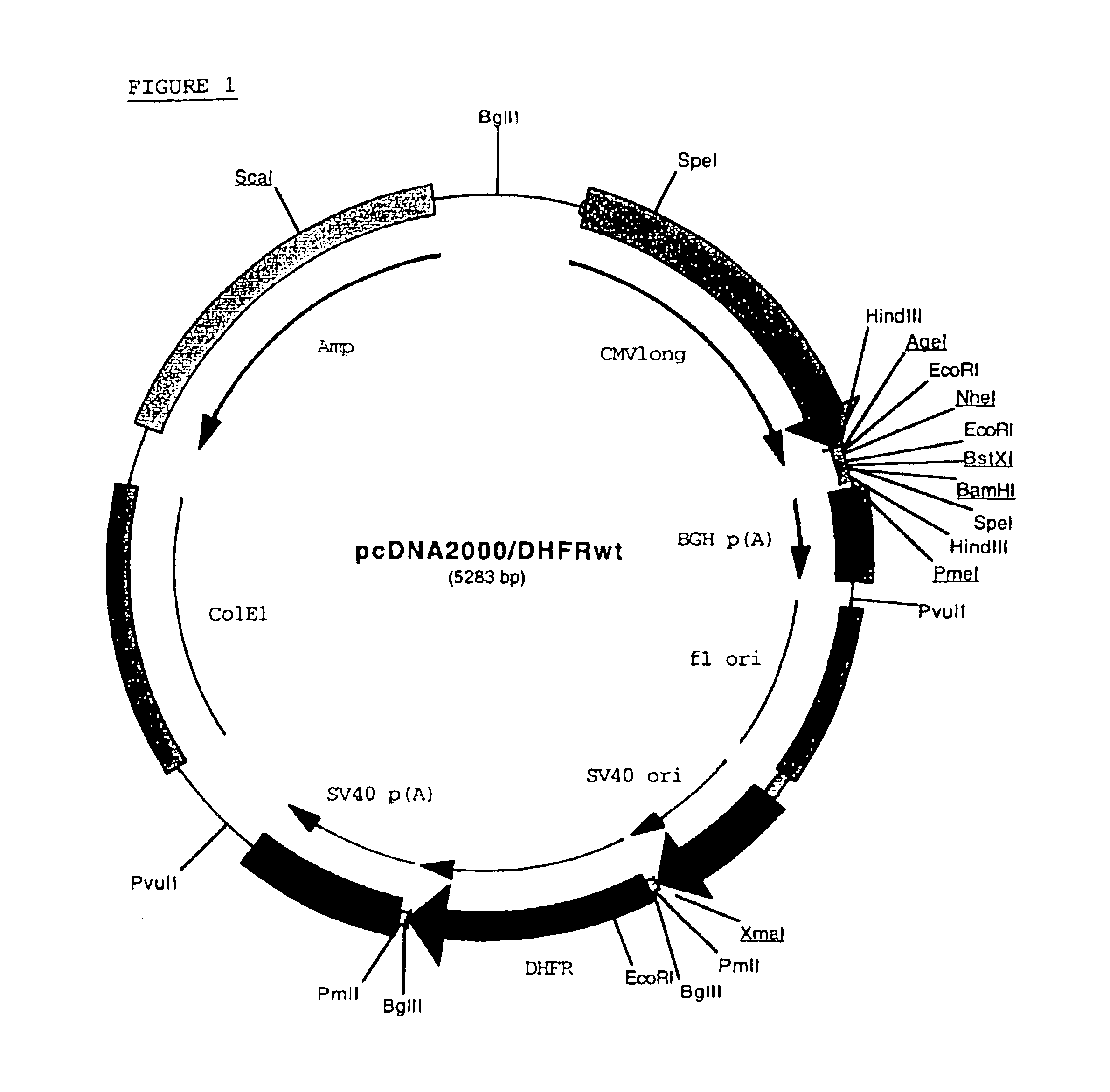
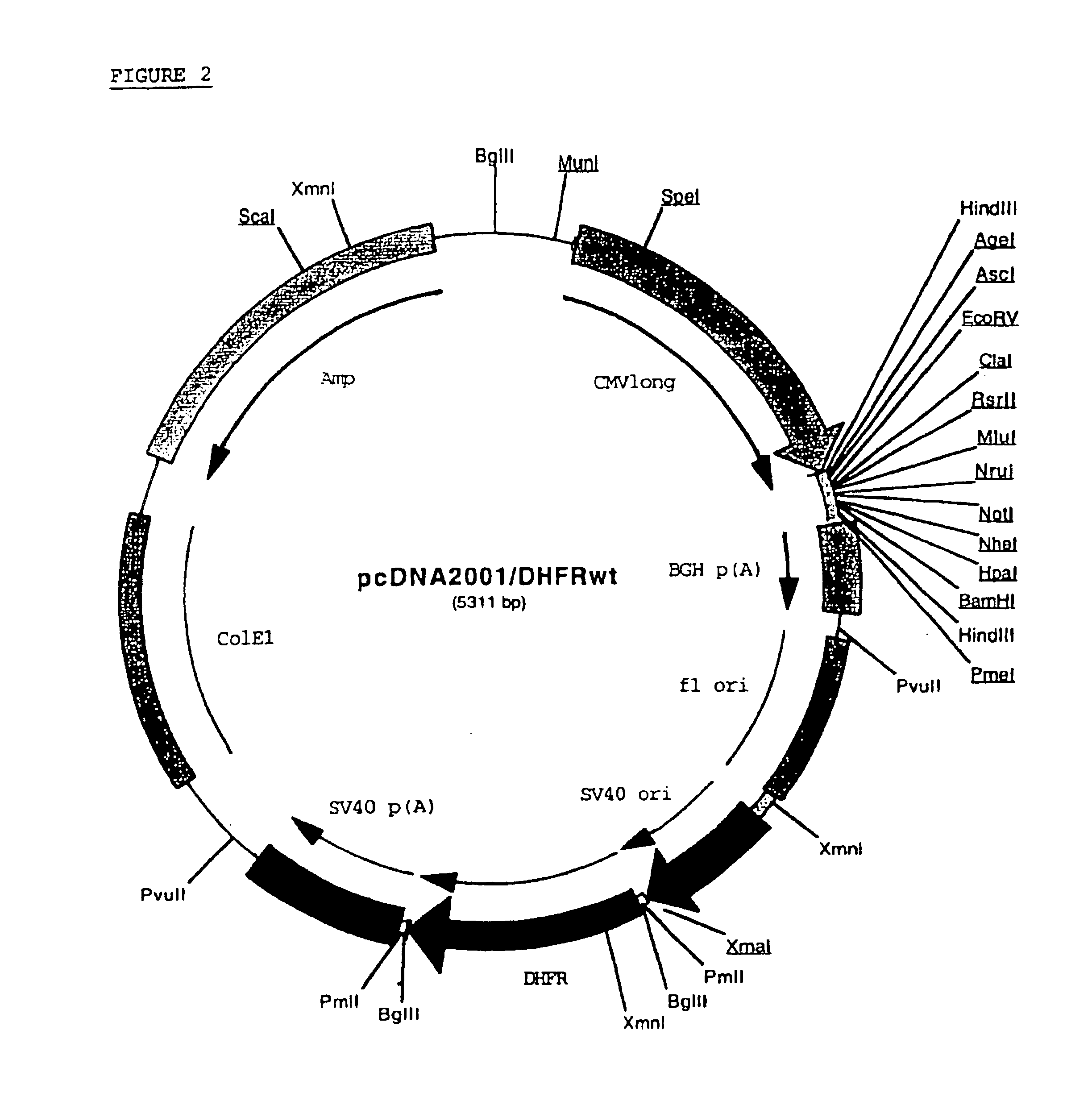
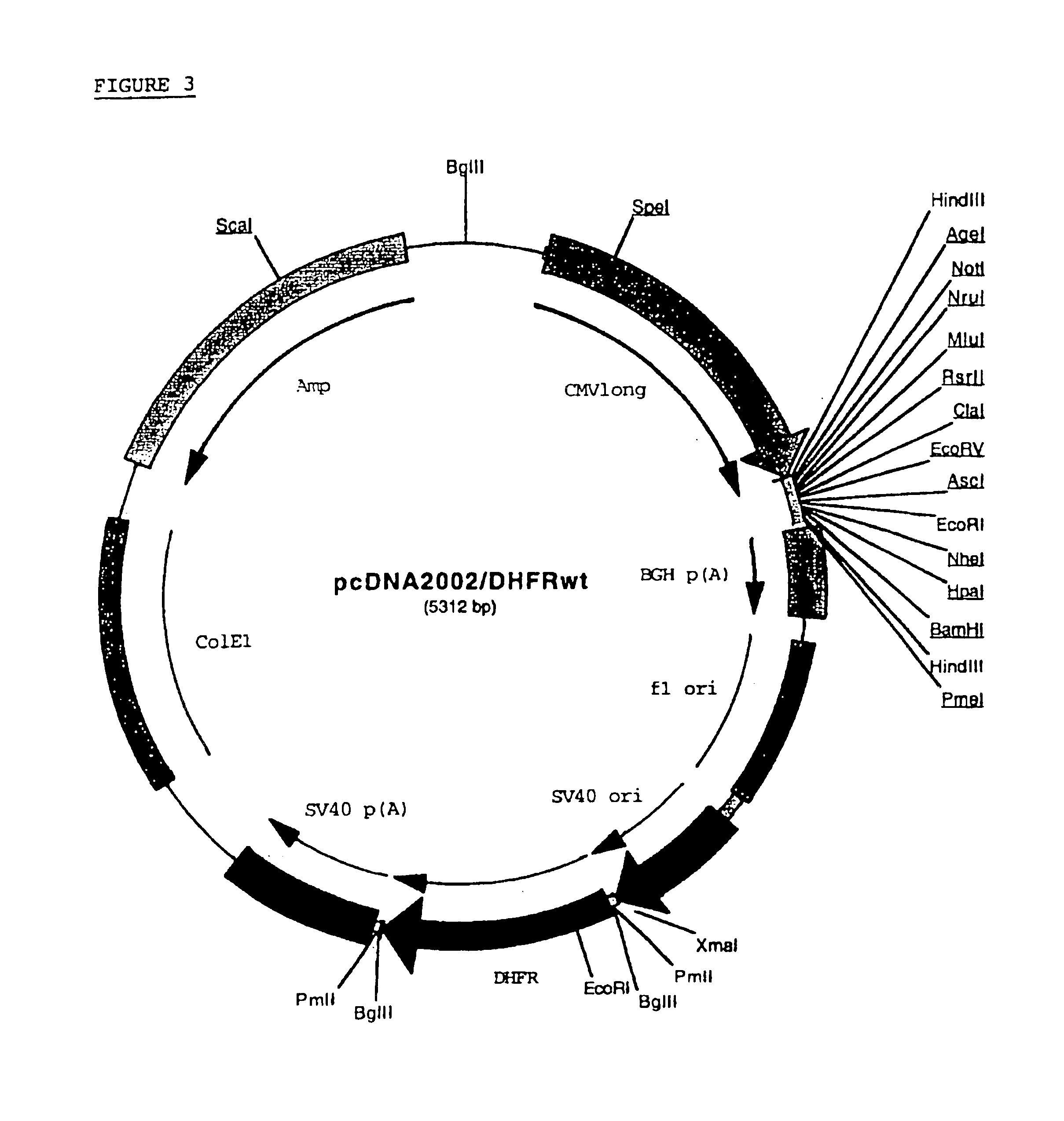
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
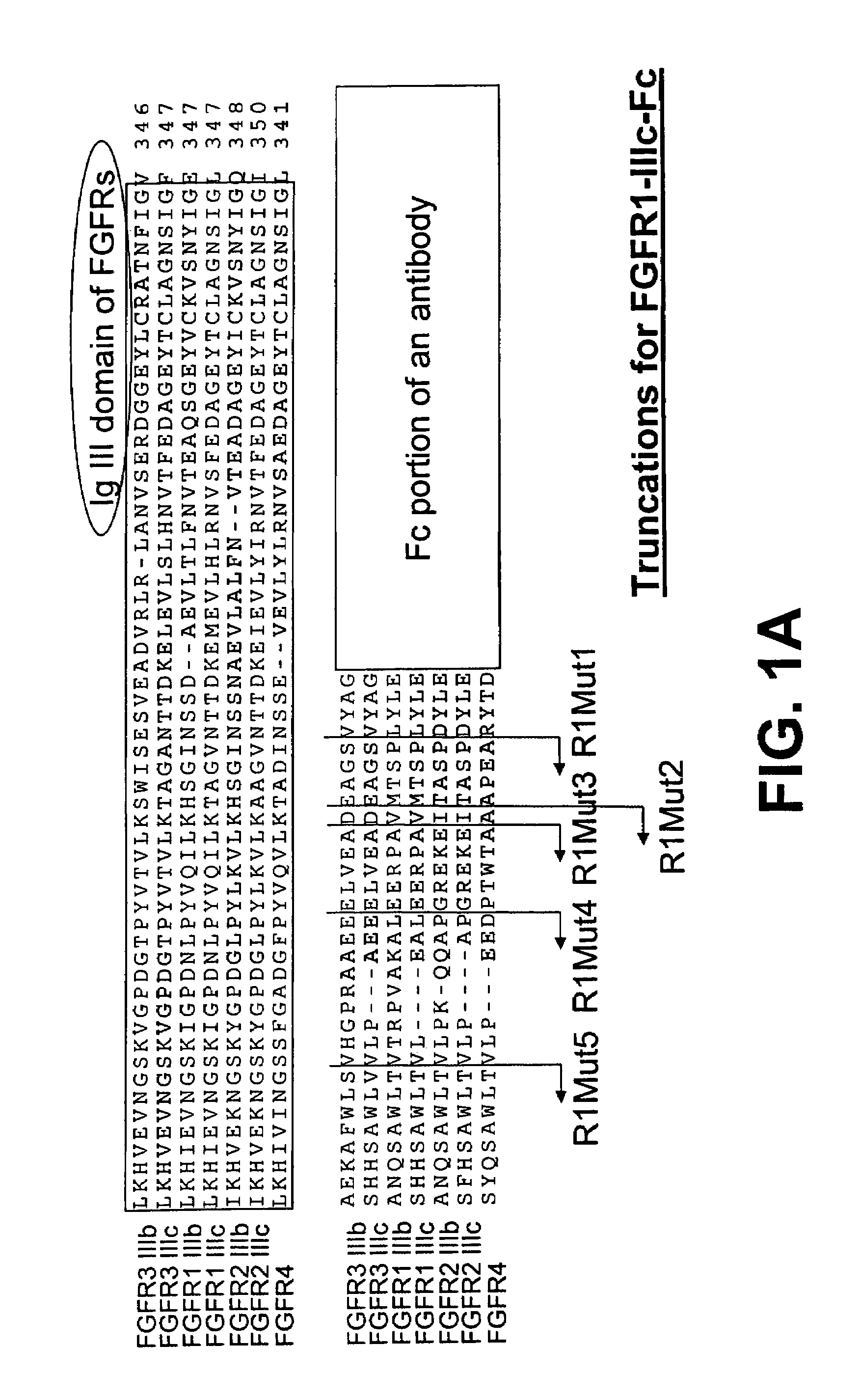
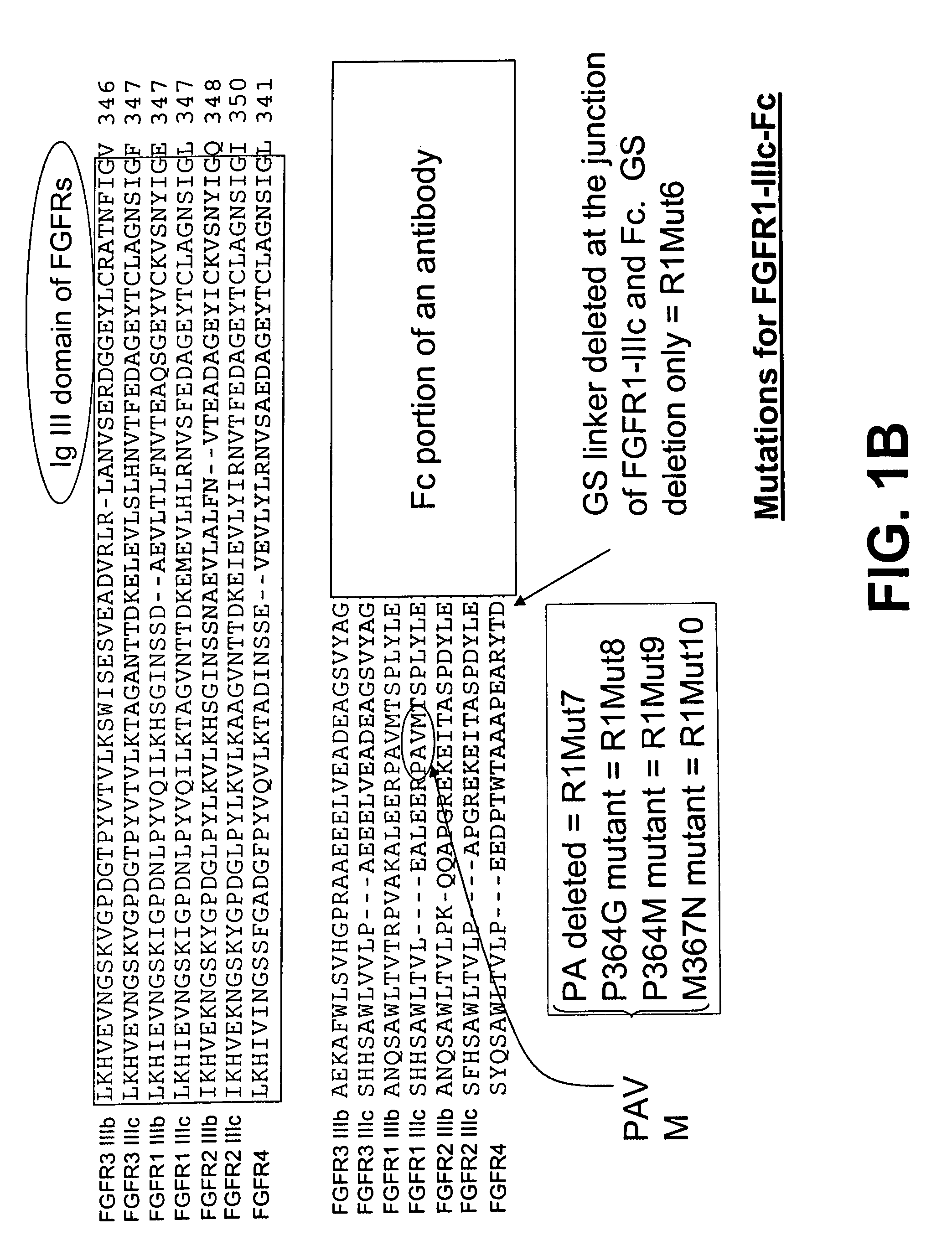
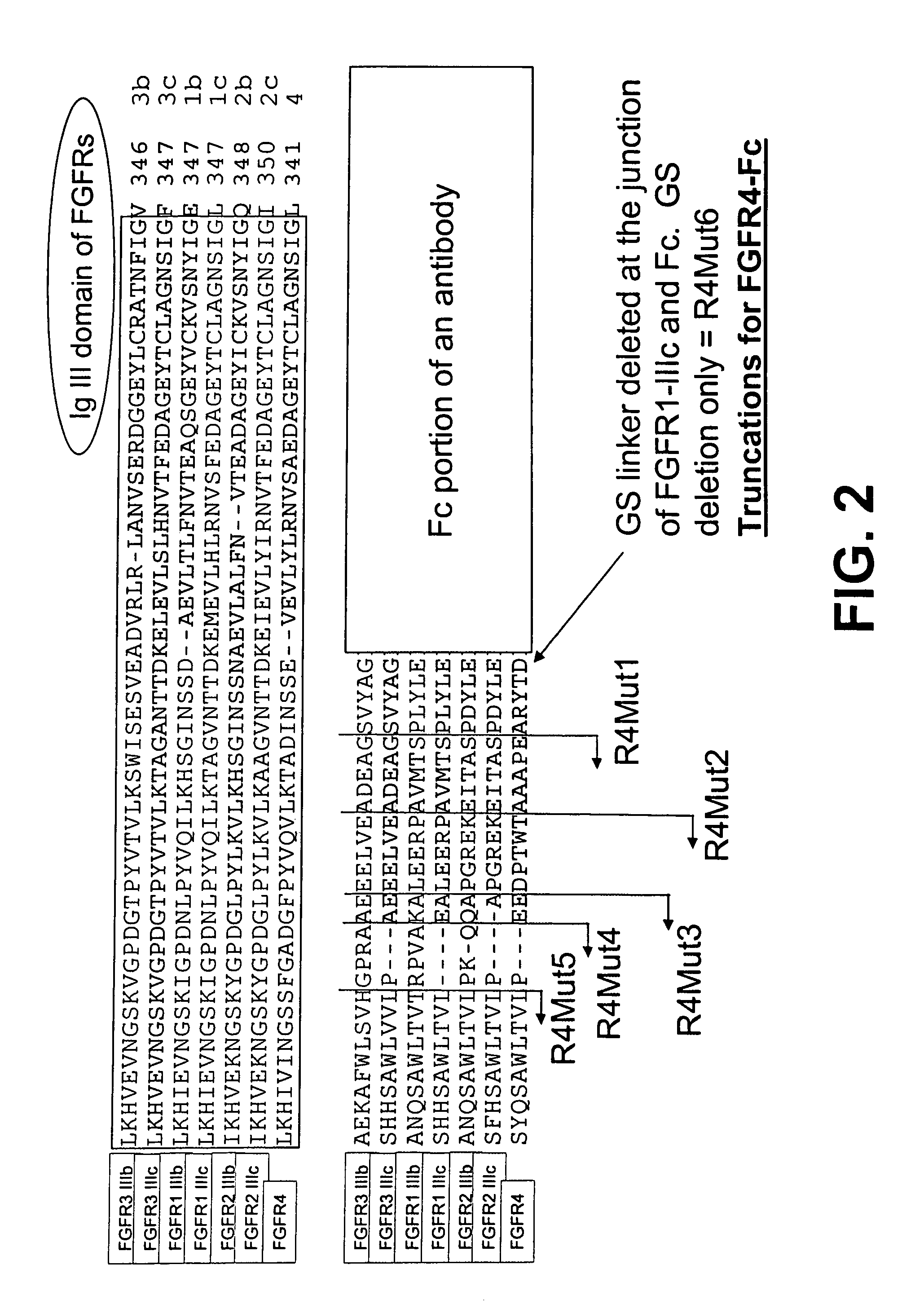
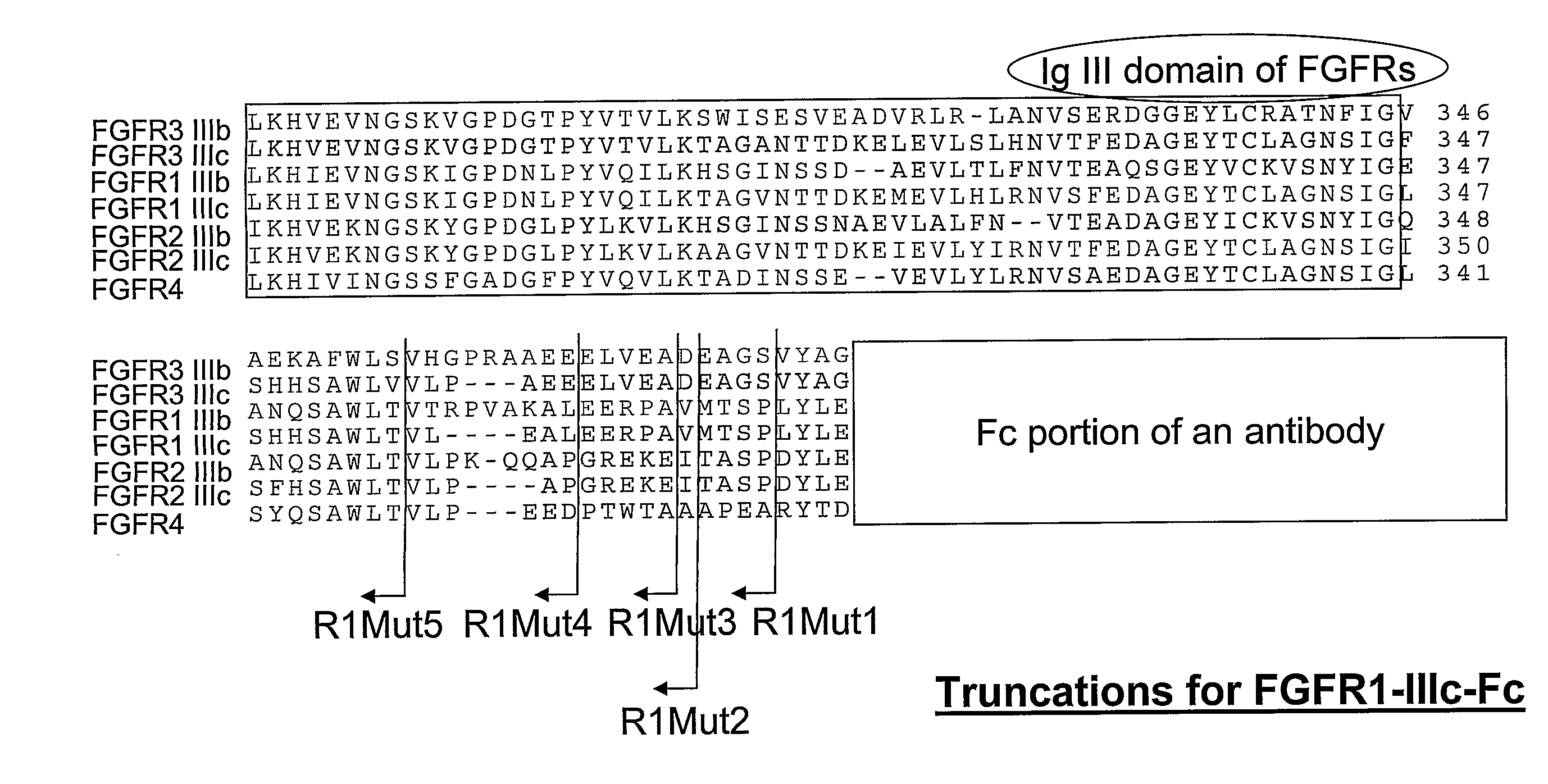
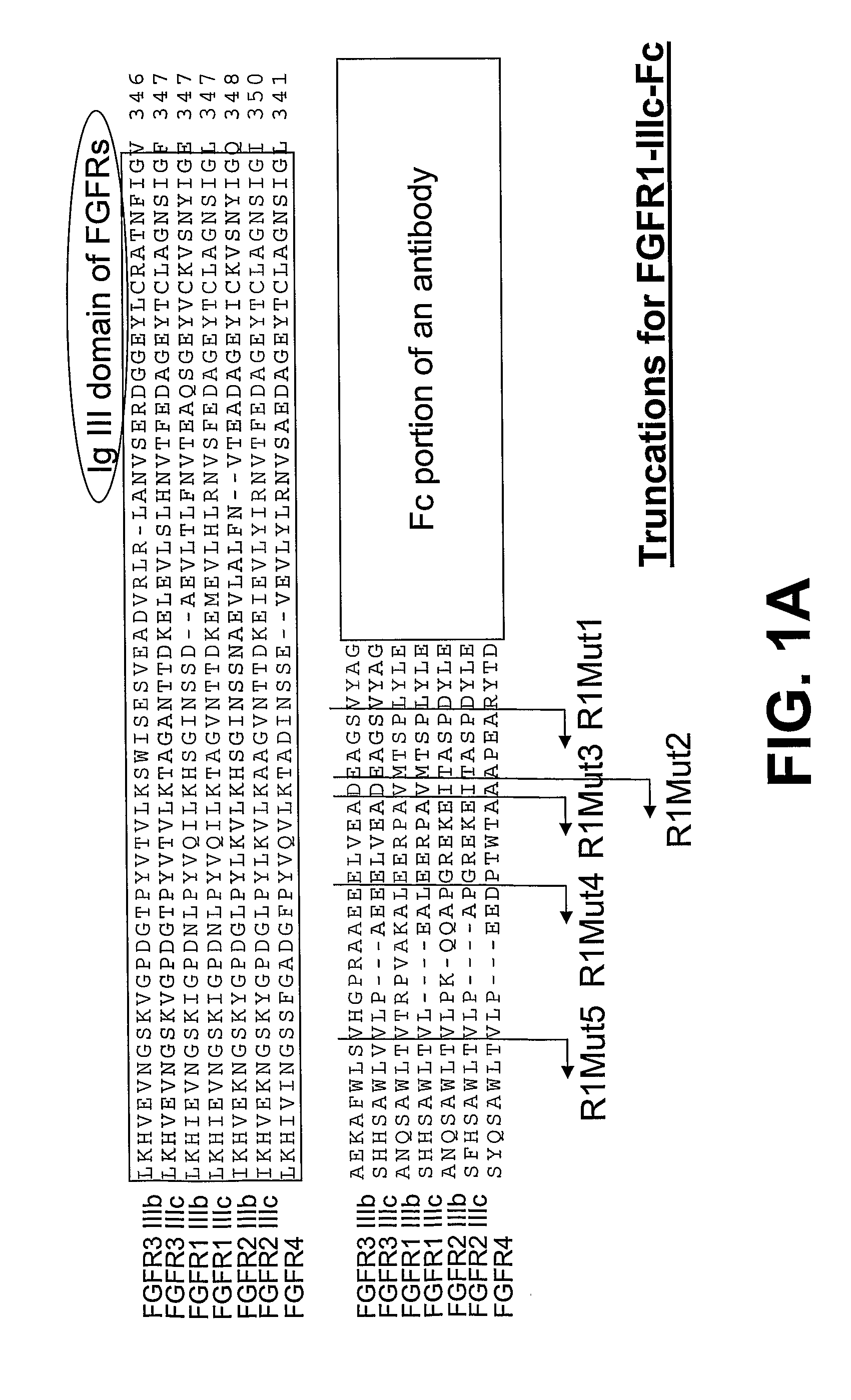
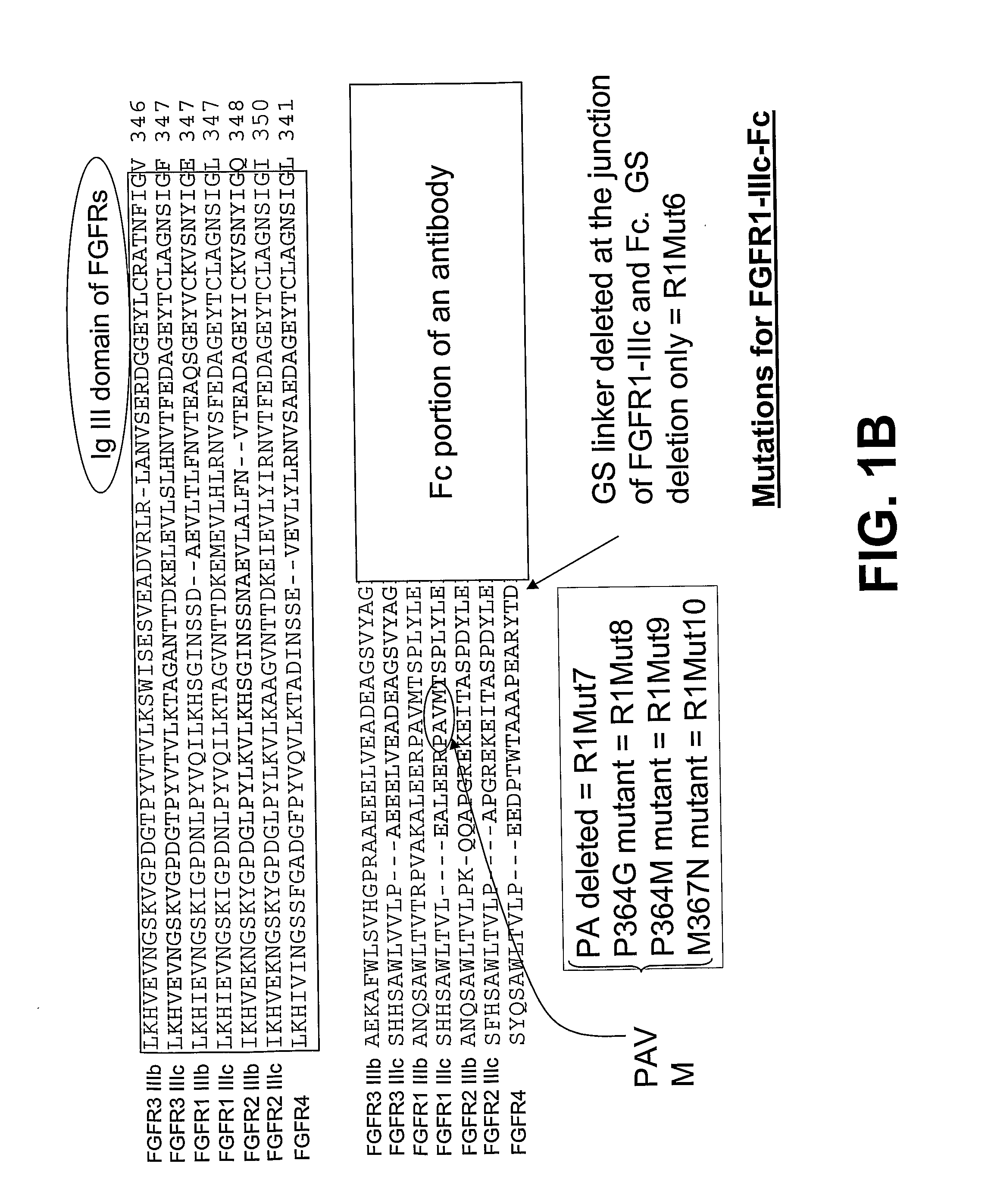
Compositions and methods of treating disease with FGFR fusion proteins
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
Compositions and Methods of Treating Disease with Fgfr Fusion Proteins
InactiveUS20080171689A1Less susceptible to cleavageSenses disorderPeptide/protein ingredientsCancer cellEphA Receptors
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
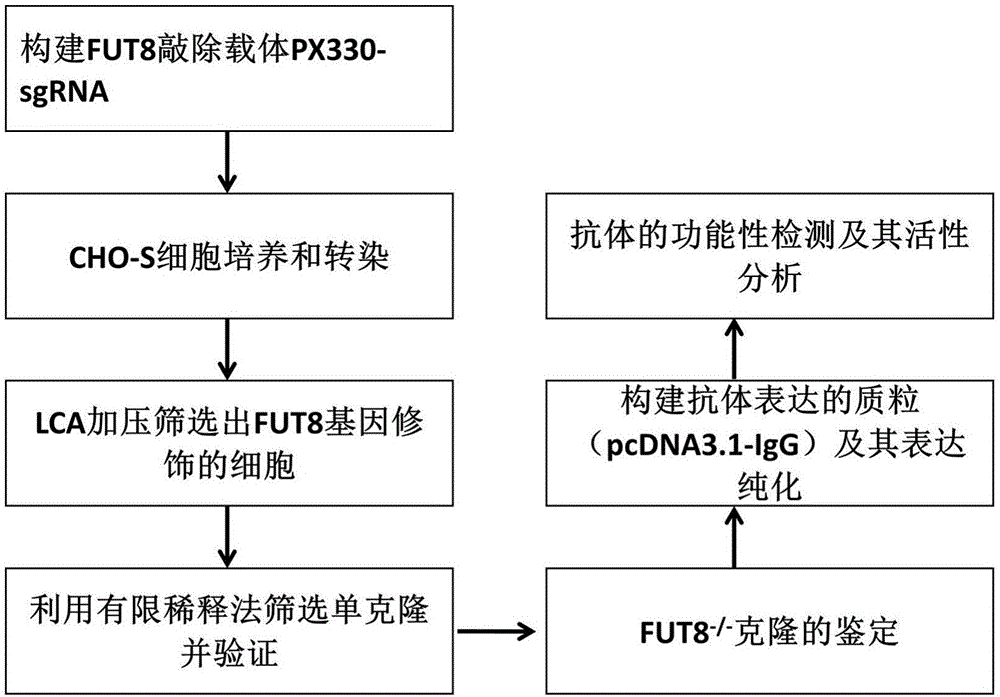
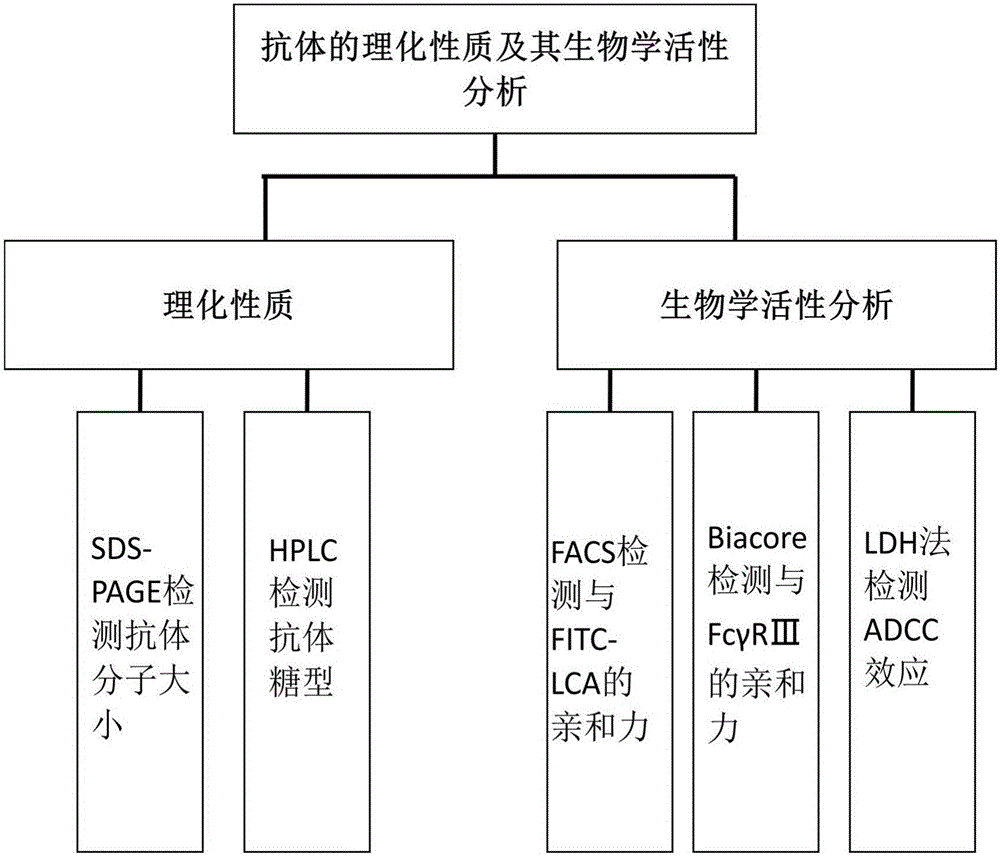
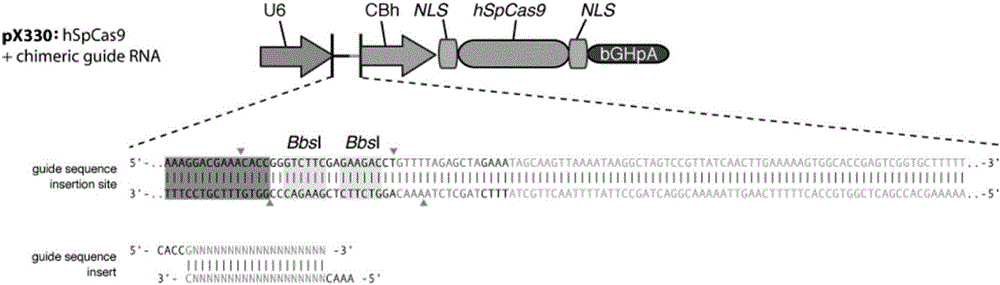
Establishment and application of CHO cell line for producing fucose-free monoclonal antibody
PendingCN106701823AEdit validSimple and fast operationNucleic acid vectorImmunoglobulinsAntigenWild type
The invention discloses establishment and application of a CHO cell line for producing a fucose-free monoclonal antibody. A CRISPR / Cas9 technology is utilized to encode an FUT8 gene of a CHO cell to obtain the FUT8 gene silenced CHO cell line for stabilizing FUT8 gene deletion (hereinafter referred to as FUT8- / -cell line). By adopting physicochemical property and bioactivity detection of an FUT8- / -cell expressed antibody, a glycoform detection result indicates that fucose of the FUT8- / -cell expressed monoclonal antibody is completely eliminated, a fucose-containing monoclonal antibody expressed by a wild CHO cell serves as a contrast, the affinity with a Fcgamma RIIIa antigen of the fucose-free monoclonal antibody is improved by 7 times, and the ADCC effect is improved by 25 times.
Owner:SHANGHAI JIAO TONG UNIV +2
Method for screening CHO cell line high-expression site
InactiveCN107557390AEasy to integrateHigh expression siteFermentationVector-based foreign material introductionForeign proteinFluorescence
The invention discloses a method for screening a CHO cell line high-expression site. The method comprises the following steps: integrating slow virus with a green fluorescence gene into a CHO cell genome, collecting monoclonal cells with high fluorescence expression quantity by a flow sorting method, performing expanding culture, and then screening out a corresponding high-expression integration site by using a chromosome walking technology. According to the method, a CRISPR / Cas9-mediated site-specific integration technology is combined, a to be expressed foreign gene can be quickly and accurately into a found site, and a foreign protein high-expression cell line can be quickly and efficiently obtained.
Owner:JIANGNAN UNIV
Truncated cd200
InactiveUS20040054145A1Increasing immune suppressionPrevent fetal lossOrganic active ingredientsPeptide/protein ingredientsDendritic cellWhite blood cell
A truncated form of the full-length, immunosuppression-inducing molecule, CD200, CD200tr, was expressed in CHO cells and the transduced cells used to produce mAbs to CD200tr. These mAbs detect simultaneous expression of full-length CD200 and CD200tr in LPS-stimulated dendritic cells (DCs). Moreover, CHO cells and DCs expressing CD200tr as well as soluble CD200tr are able to inhibit suppression of mixed leucocyte reactions in vitro which follows addition of the soluble form of CD200 (CD200:Fc) in culture, demonstrating that CD200tr is a physiological antagonist of CD200.
Owner:TRILLIUM THERAPEUTICS
Human antibody and expression thereof
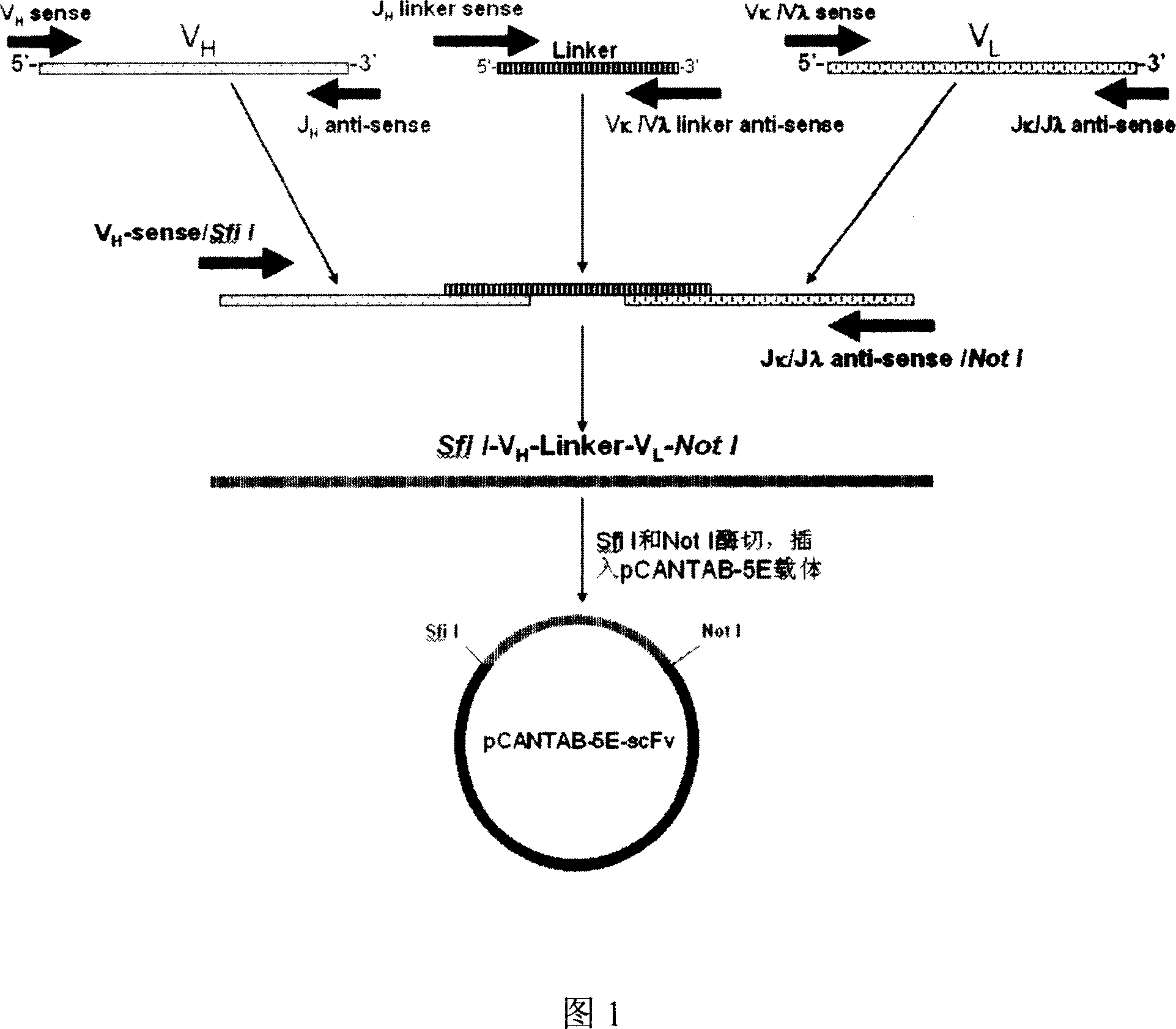
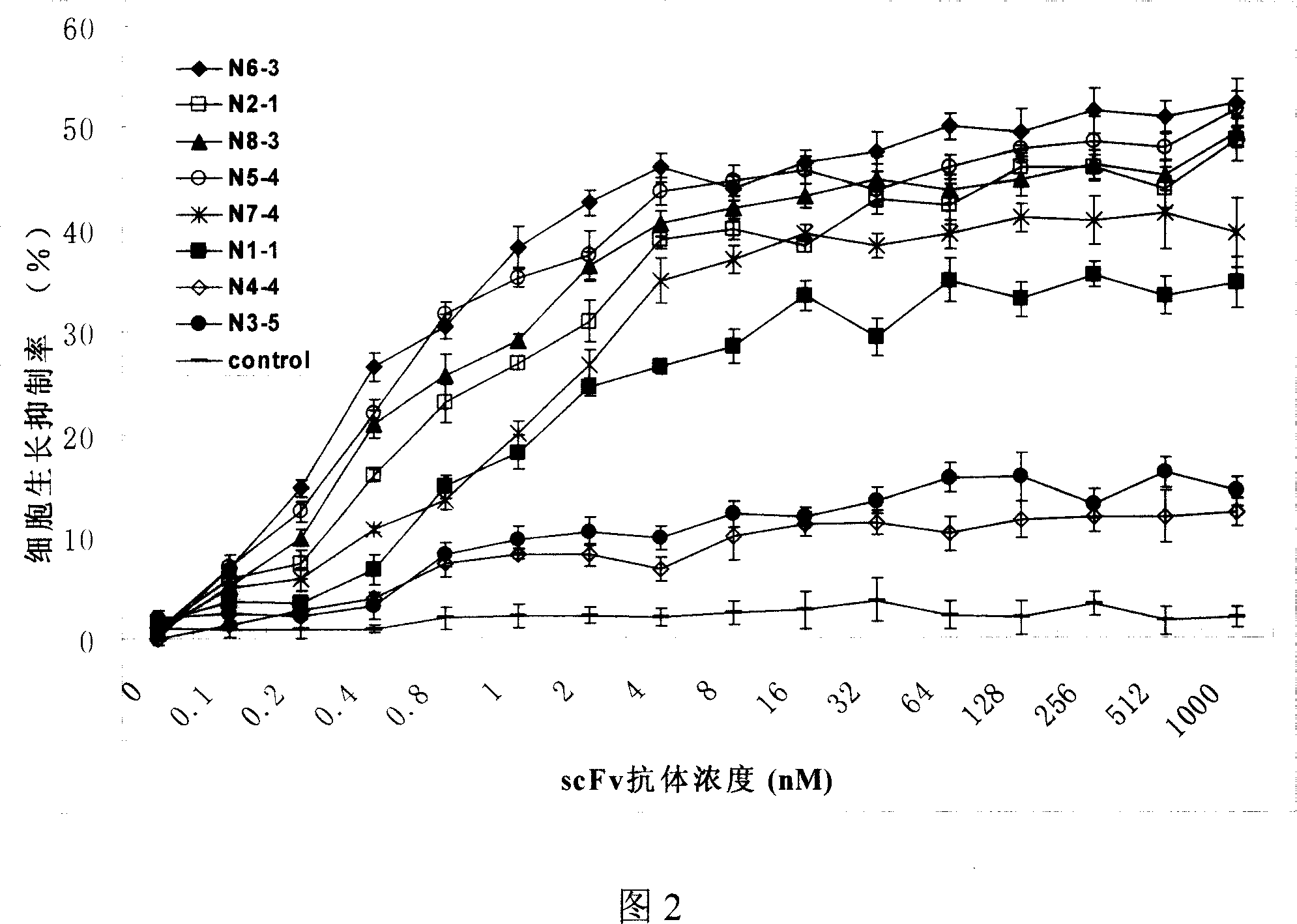
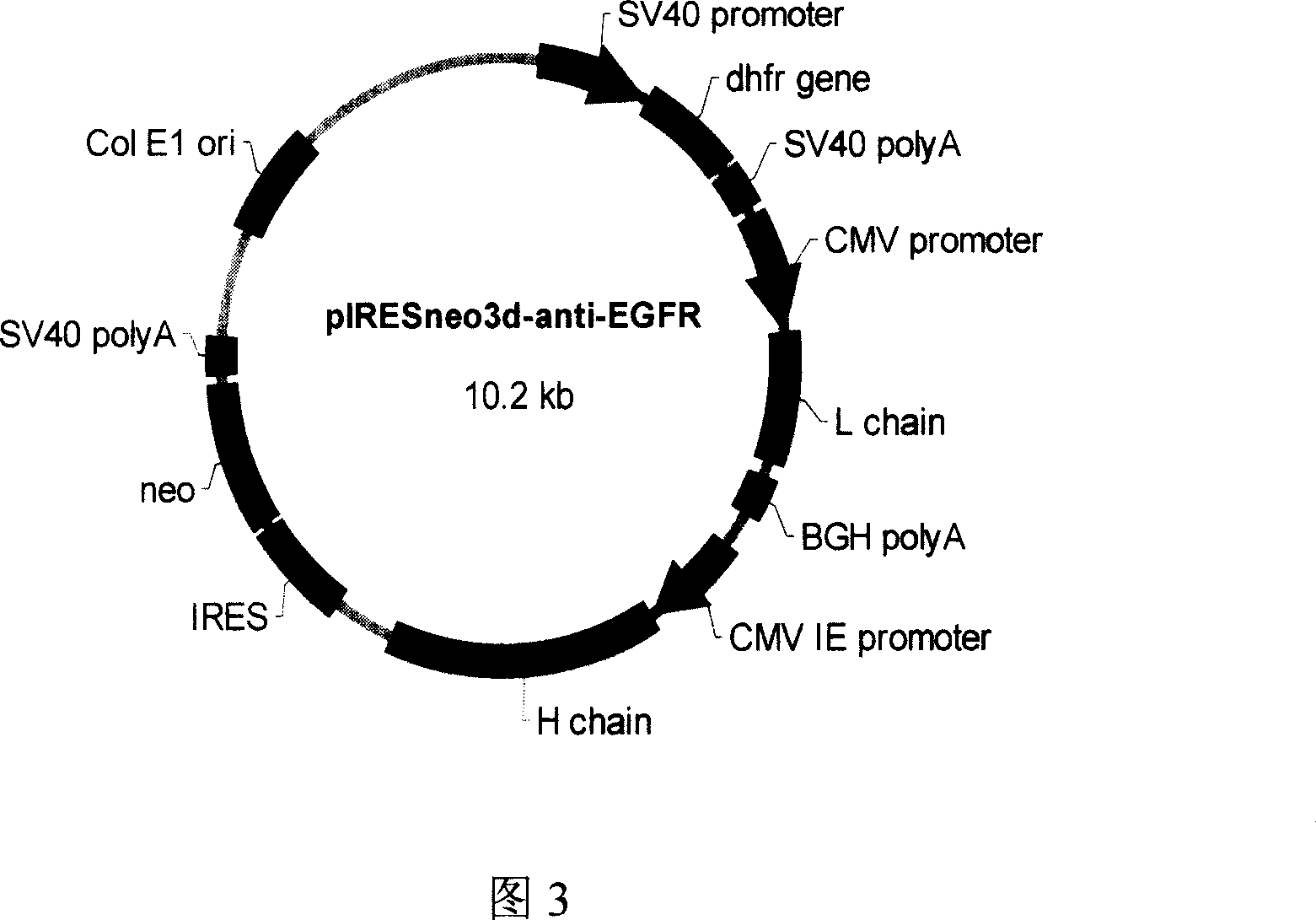
The invention discloses a full-human anti-EGFR scFv antibody segment and entire antibody, which contains heavy chain of antibody and nucleic acid and amino acid of variable area of light chain as well as high-effective expressing method of entire antibody in the CHO cell.
Owner:SINO CELL TECH INC
DNA Transmethylase defective CHO (Chinese hamster ovary) cell line and preparation method and application thereof
InactiveCN107828738AImprove expression levelIncrease productionTransferasesStable introduction of DNAInstabilityA-DNA
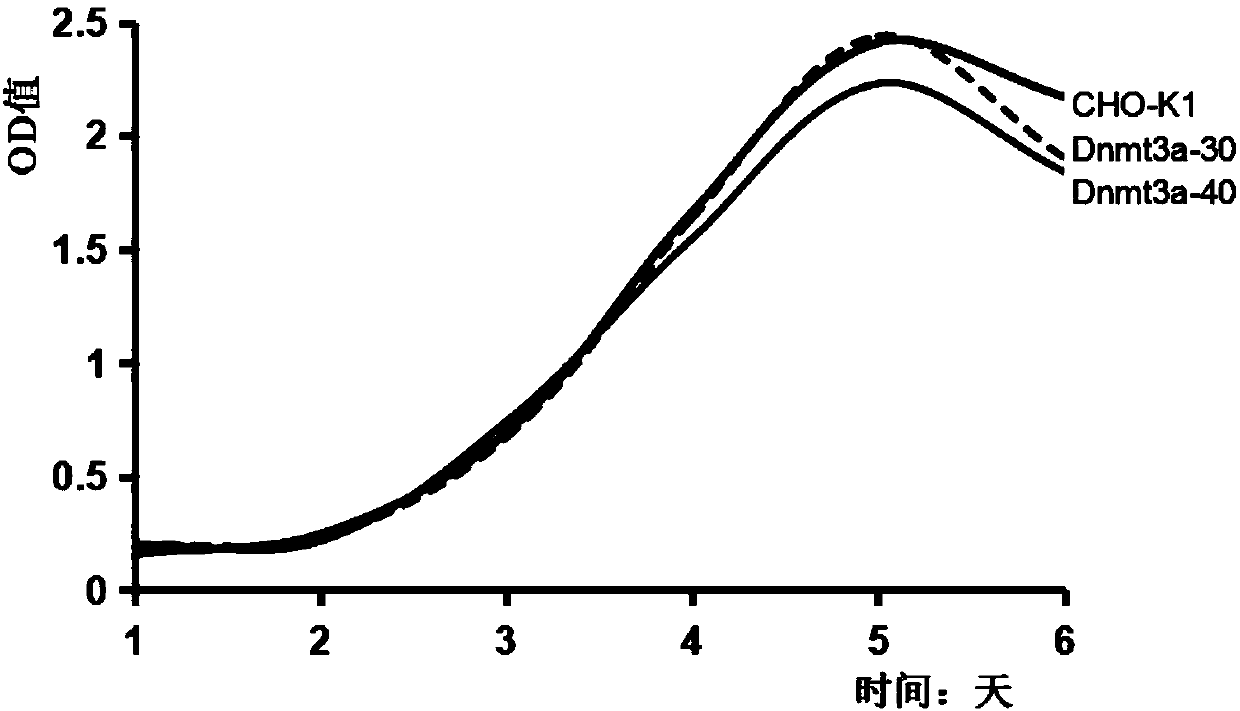
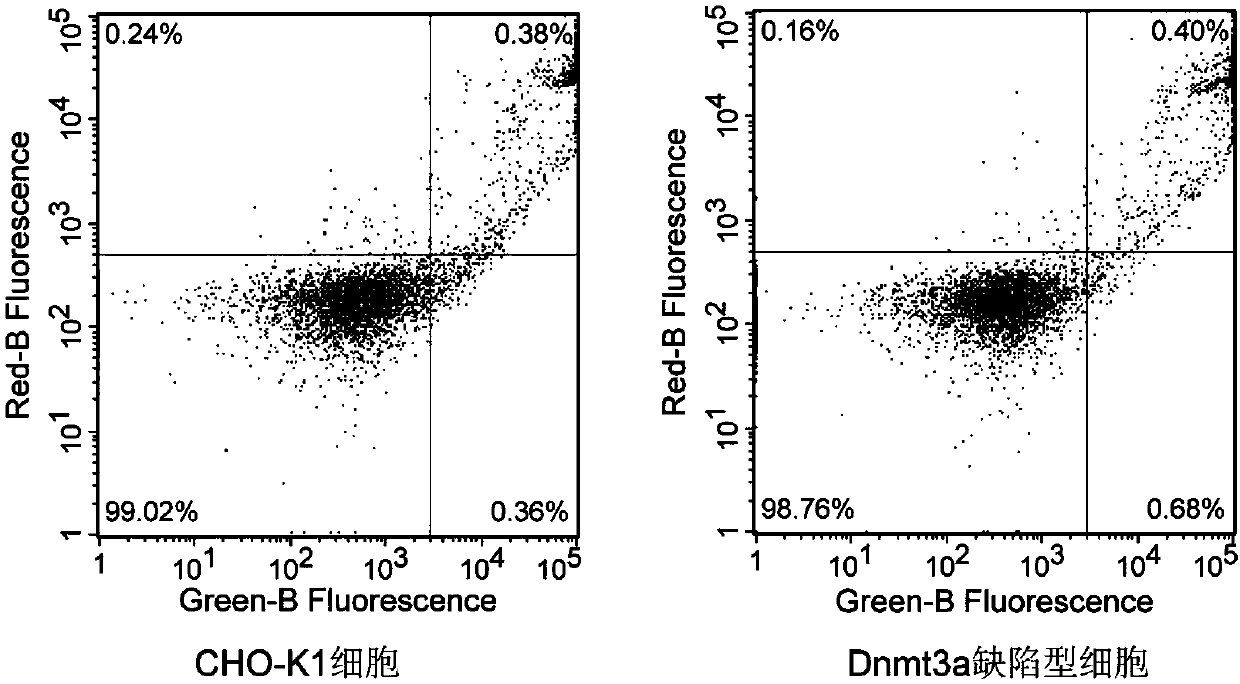
The invention relates to a DNA transmethylase defective CHO (Chinese hamster ovary) cell line and a preparation method and application thereof and belongs to the technical field of gene engineering. DNA Transmethylase Dnmt3a gene of CHO cells is knocked off by means of CRISPR / Cas9 gene editing technique, and screening and identifying are performed to obtain DNA transmethylase Dnmt3a defective CHOcells; the cells are transfected with a eukaryotic expression vector, stably expressed recombinant CHO cell strains are screened, and accordingly a novel CHO cell expression system based on DNA transmethylase deficiency is established. The recombinant gene CHO cell expression system is established via host CHO cell genetic modifications, expression level of recombinant proteins can be significantly increased, the problem of recombinant protein expression instability is solved, and recombinant protein expression stability is improved.
Owner:XINXIANG MEDICAL UNIV +1
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
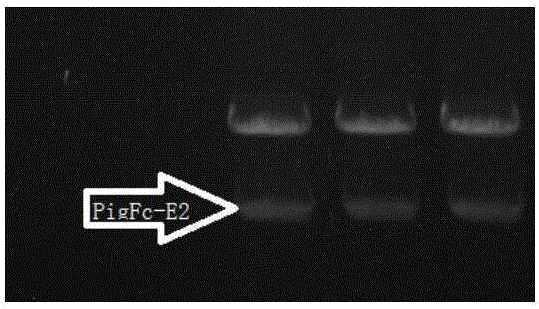
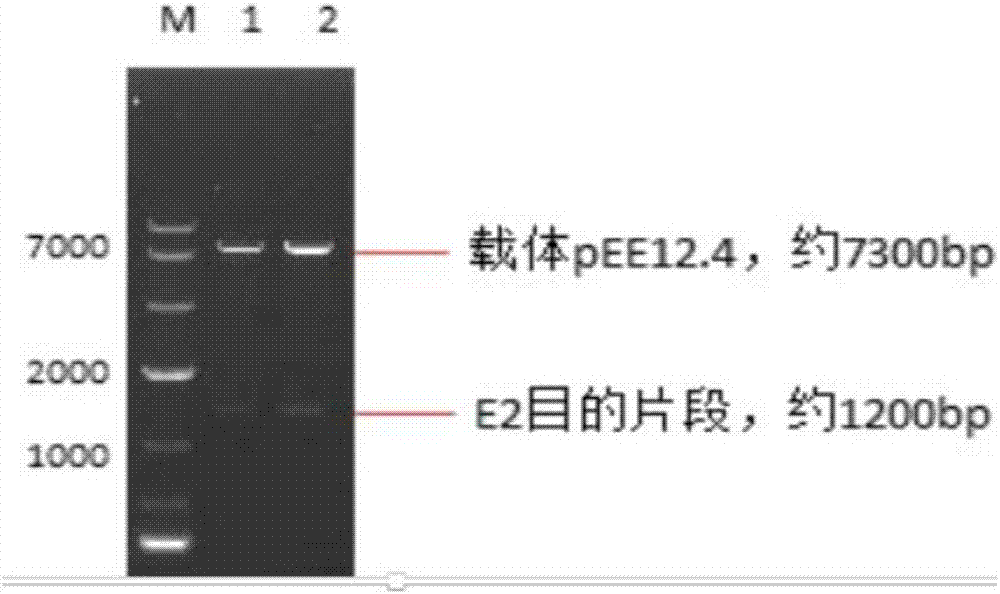
Establishing method of pig immunoglobulin Fc fragment-swine classical fever E2 fusion protein in CHO cell strain, as well as preparation method and application of fusion protein
ActiveCN106519041ASsRNA viruses positive-senseAntibody mimetics/scaffoldsFusion Protein ExpressionVaccine Production
The invention relates to a vaccine production technology in the technical field of biology, in particular to a CHO cell strain which is established by utilizing a gene engineering means and is used for expressing recombinant protein PigFC-pigSCFVE2, and a preparation method and application of the recombinant protein. The recombinant fusion protein PigFC-pigSCFVE2 provided by the invention is A1) or A2) shown as follows, wherein A1) is protein of which the amino acid sequence is as shown in SEQ ID No.2, and A2) is protein which is obtained by substituting, losing and / or adding one or several amino acid residues in the amino acid sequence of the protein of the A1) and has PigFC-pigSCFVE2 activity. A monoclonal cell strain which is obtained through the method and capable of carrying out secretory expression on PigFC-pigSCFVE2 is higher in fusion protein expression quantity, fusion protein obtained through affinity separation and purification of an antibody can be combined with a monoclonal antibody, animals can be immunized, the immunity of a generated neutralizing antibody is higher than that of a present market product, the fusion protein can be used for swine classical fever preventive vaccine, and the production cost and the immunity failure loss can be reduced.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
Blood serum-free culture medium for supporting suspension culture of CHO cells with large scale and high density
InactiveCN101724600AGrow fastThe added ingredients are clearGerm cellsVolumetric Mass DensityTransferrin
The invention discloses a blood serum-free culture medium suitable for the suspension culture of CHO cells with large scale and high density. The culture medium takes DMEM / F12 (v / v, 1:1) as a base culture medium, and adds the other substances such as insulin, putrescine, transferrin and microelement, etc. The blood serum-free culture medium has the advantages that: the culture medium can support the CHO cells to fast growth under the condition of suspension culture; the protein content is very low, and the chemical composition is basically clear; the cells are grown one by one by means of suspension, so that the living cell density and the cell living rate are higher than those of the serum culture and the similar commercialized serum-free culture; and the cost is lower.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Culture method for high-efficiency human follicle stimulating hormone expression CHO cells
ActiveCN105462909AStable batch-to-batch qualityThe cultivation process is stable and controllableMicroorganism based processesVertebrate cellsBottleHigh survival rate
The invention discloses a culture method for high-efficiency human follicle stimulating hormone expression CHO cells and belongs to the technical field of bio-pharmaceuticals. According to the culture method, seed cells are prepared into cell suspension, and subculture is performed after resuspension through a serum-free basal culture medium to obtain seed suspension; the obtained seed suspension is inoculated into a bioreactor to be cultured, the serum-free basal culture medium is fed at regular intervals in the culture process, growth situations of the cells are monitored, glucose solution is supplemented, and the cells are harvested after culture. According to the method, the serum-free basal culture medium is used throughout the process, culture in a fermentation tank is performed after reviving and amplification through bottle shaking, the process flow is simple, the cell culture density is high and can be as high as 4*107 / mL , the cell survival rate reaches more than 98%, maintaining time under high density and a high survival rate is long, the yield of end products can reach 40mg / L, and the method is applicable to industrial production.
Owner:哈药集团股份有限公司 +1
Method for recombinant protein production in mammalian cells
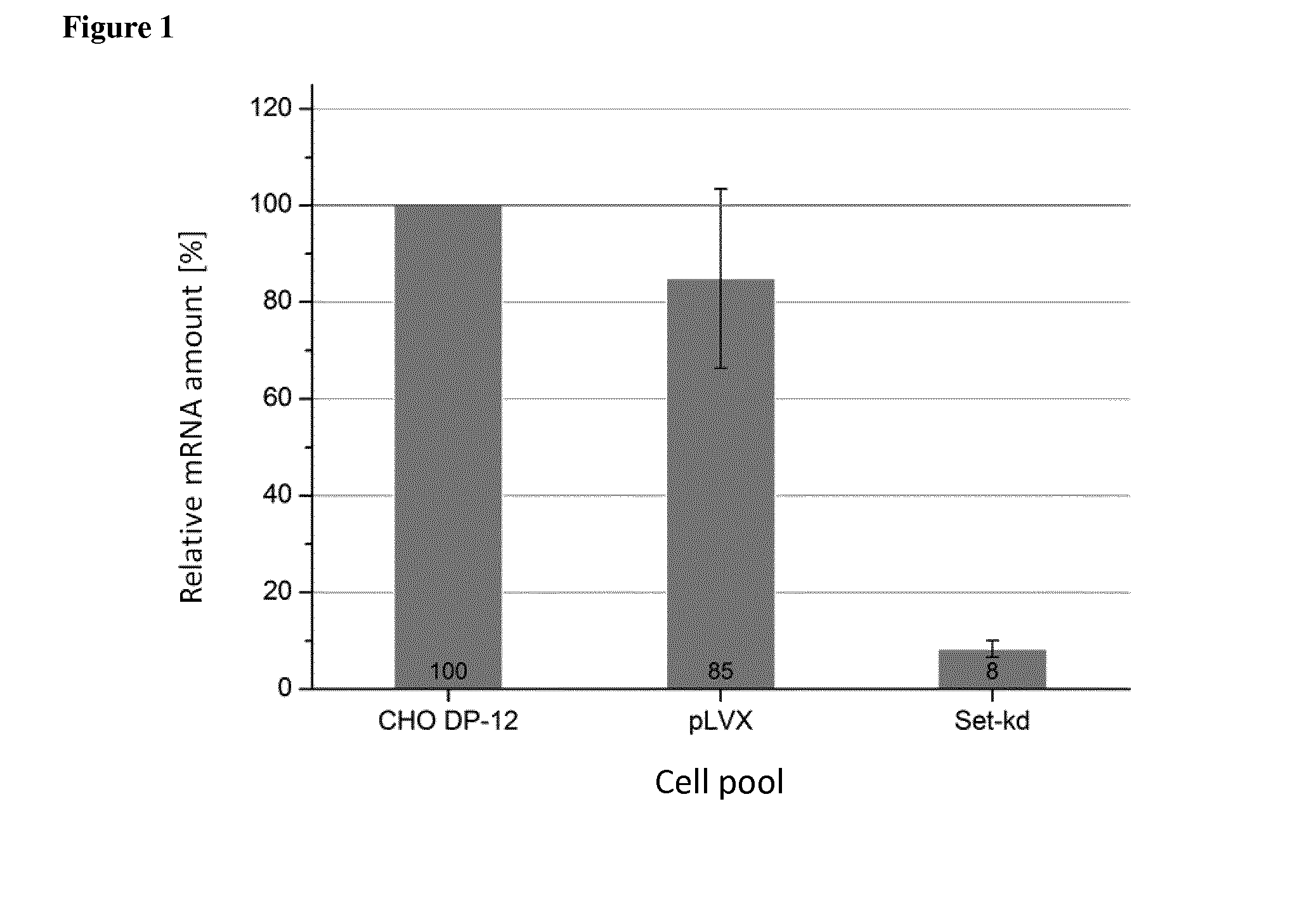
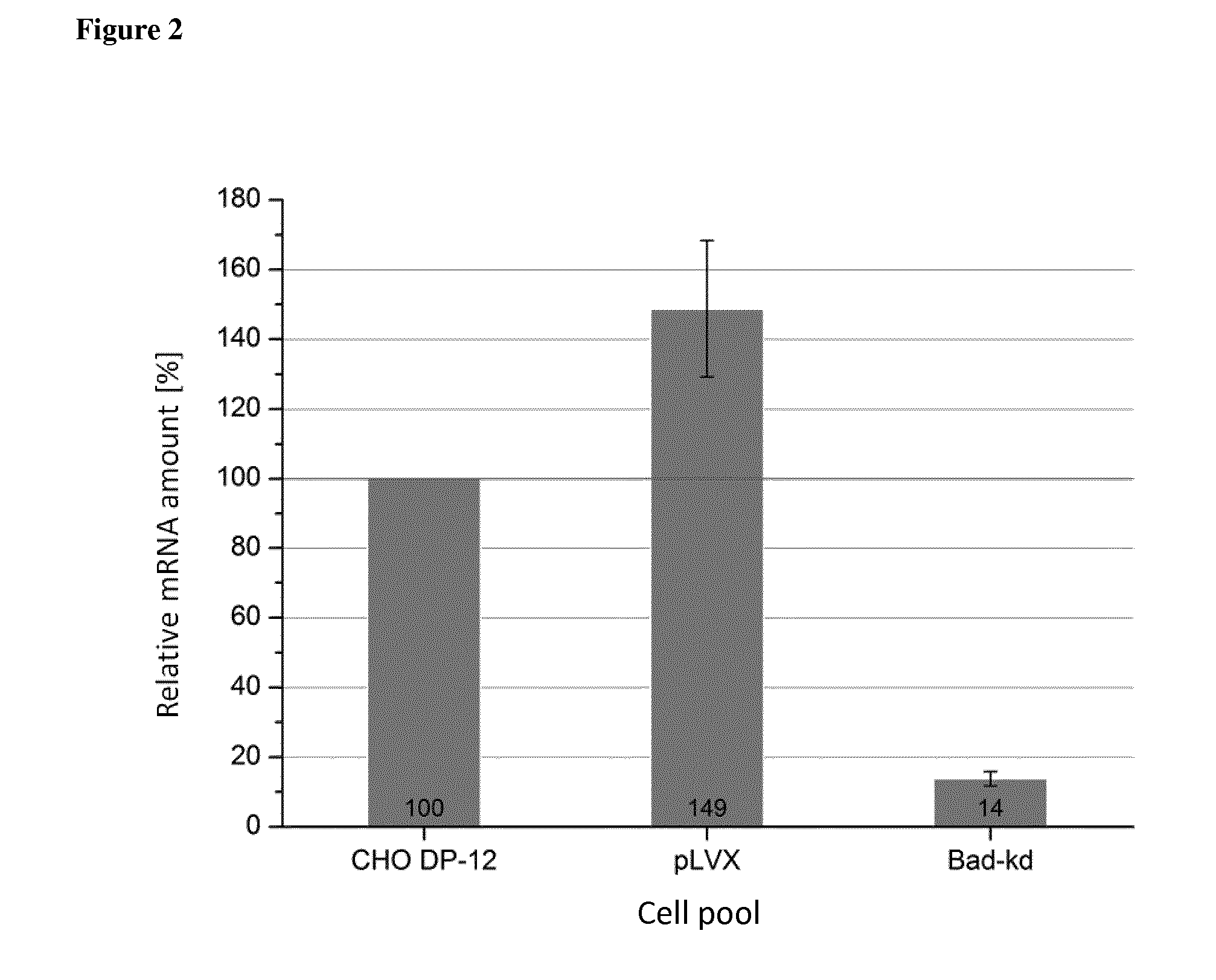
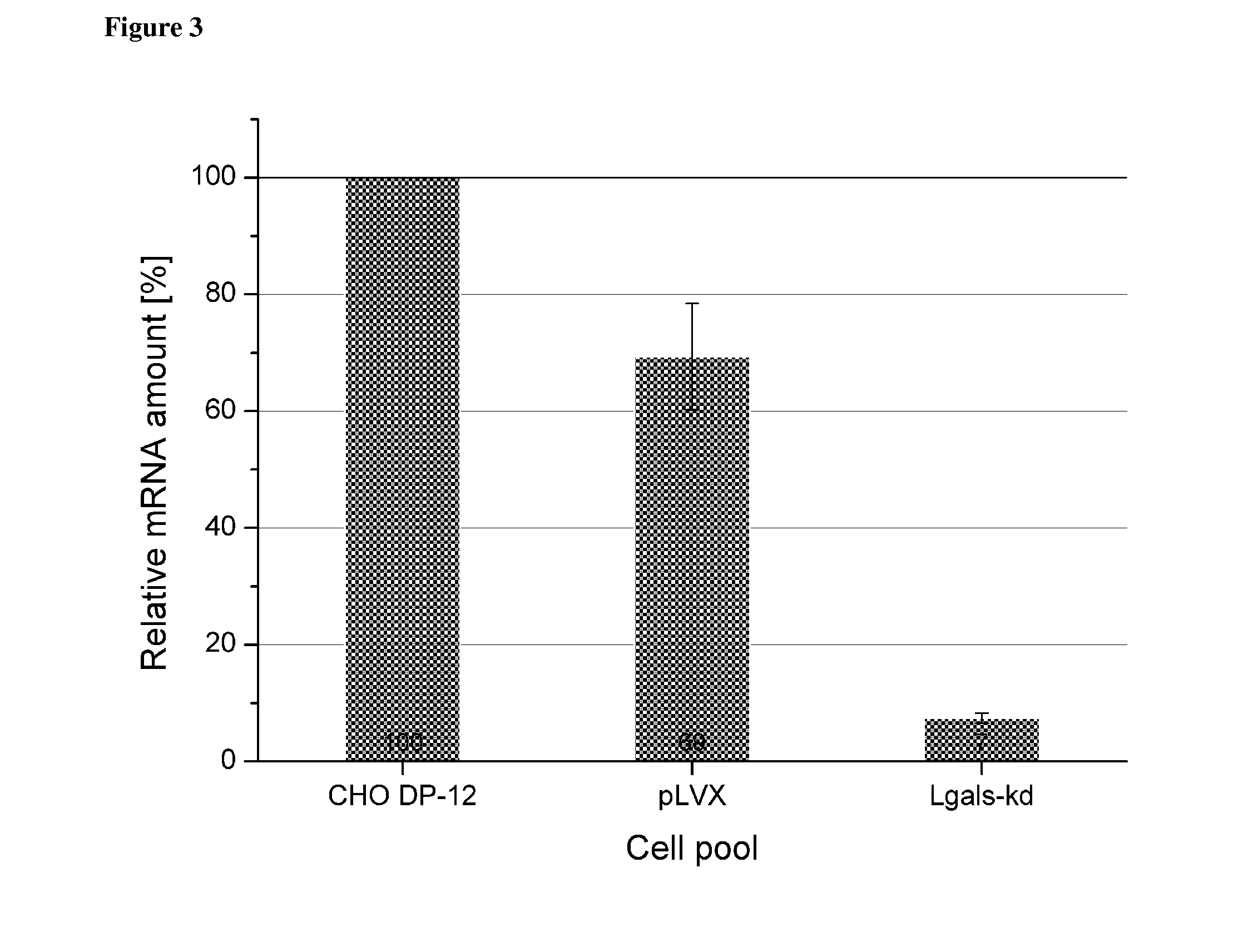
Some embodiments relate to methods for the recombinant expression of a protein of interest in a mammalian host cell, use of an sh RNA or an si RNA directed against the Galectin-1 gene for increasing the expression of a protein of interest in a mammalian host cell and kits comprising an shRNA or an si RNA and a CHO cell.
Owner:UNIVERSITY OF BIELEFELD
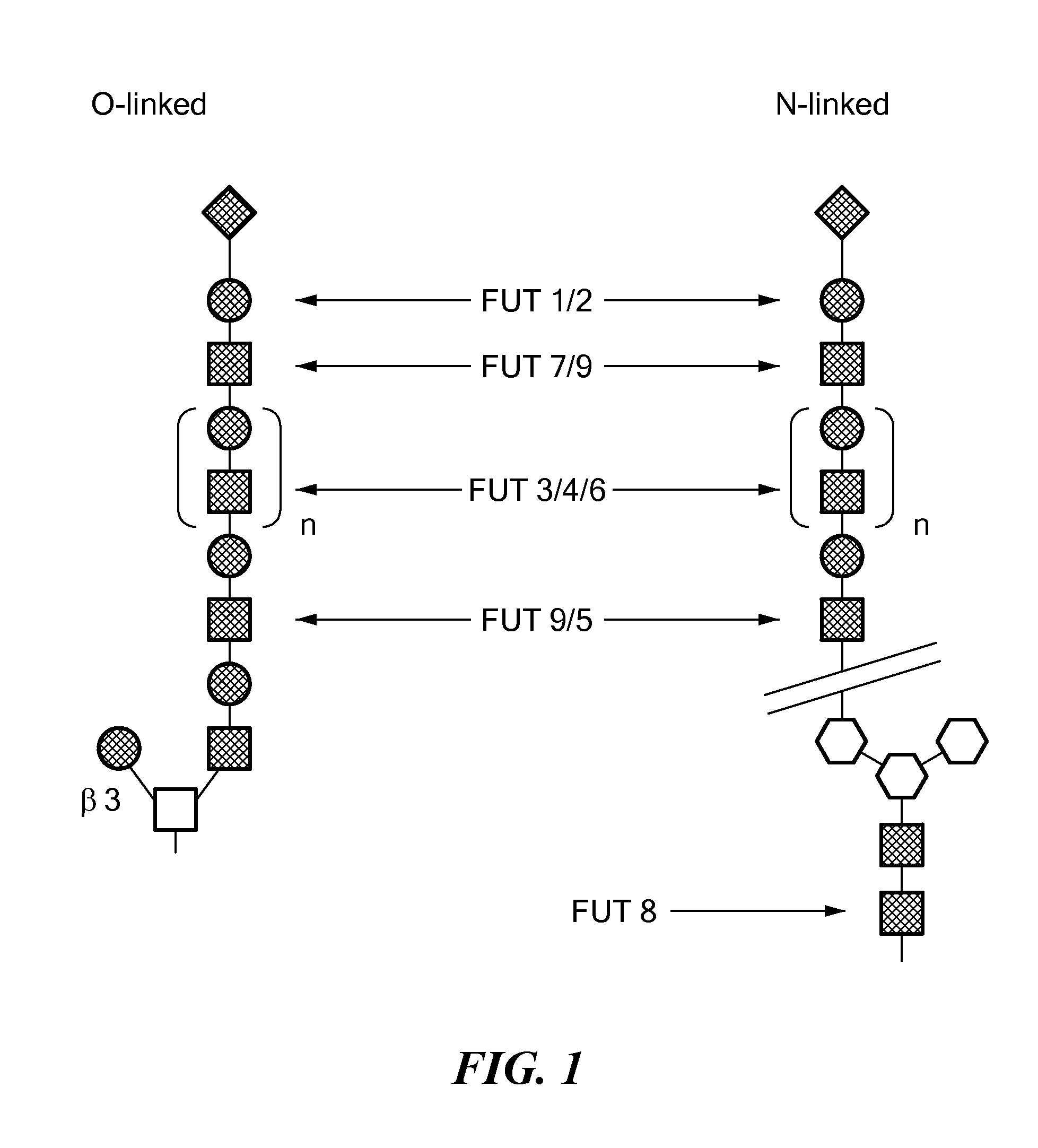
Antennary fucosylation in glycoproteins from cho cells
InactiveUS20110136682A1High and low levelHigh expressionOptical radiation measurementParticle separator tubesFucosylationRecombinant glycoprotein
Owner:MOMENTA PHARMA
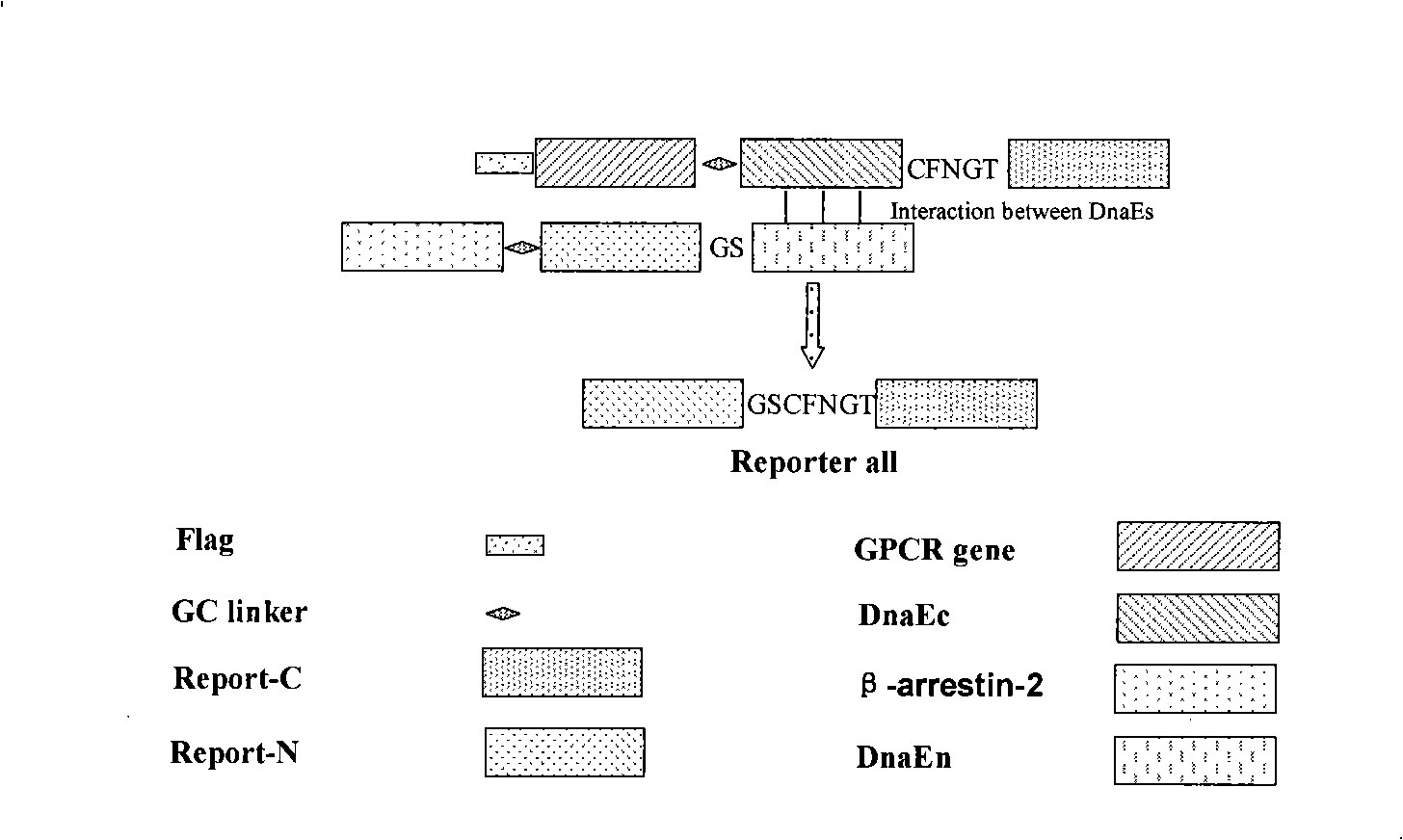
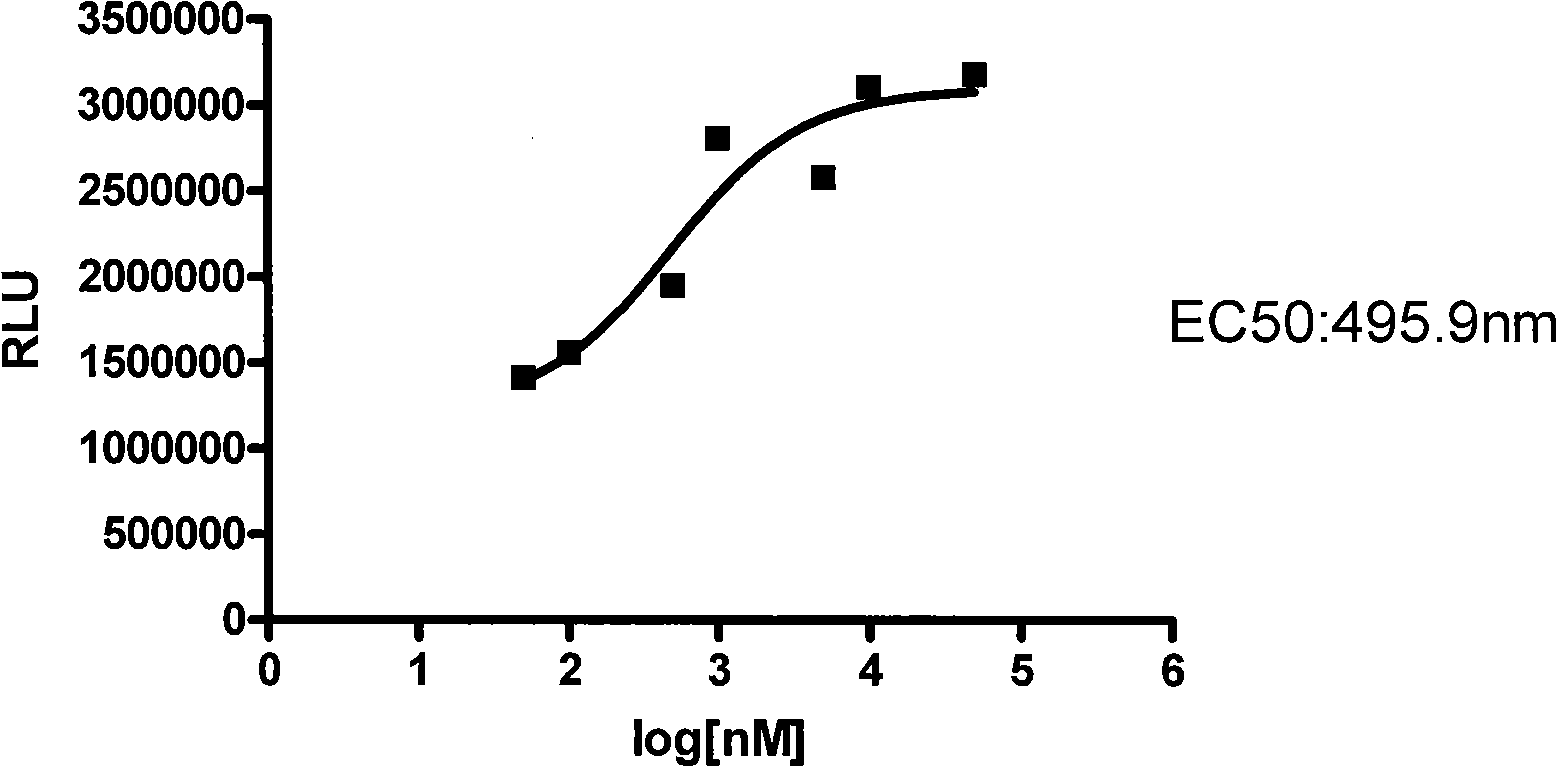
Novel horizontal screening system construct by recipient cell G protein coupling and applications
InactiveCN101333555AHigh sensitivityStrong specificityMicrobiological testing/measurementBiological testingDnaEC-terminus
The invention relates to a novel G protein-coupled receptor (GPCR) cell horizontal screening system as well as a construction method and an application. The screening system is constructed by HEK293 cells or CHO cells, and contains two fusion expression vectors constructed by gene sequences of different groups from the following sequences: the first group: (1) an assembly sequence of a C-terminal of a DnaE gene and a C-terminal of a report gene: DnaE-C-Report-C, (2) an assembly sequence of an N-terminal of the DnaE gene and an N-terminal of the report gene: Report-N-DnaE-N; and the second group: (1) GPCR gene, and (2)Beta-arrestin gene. The system and the method and the application of the invention mainly have the advantages of: high sensitivity and strong specificity when applying the screening system for G protein-coupled receptor (GPCR) cell horizontal screening, simple and fast operation and wide detection range, and can screen agonists and antagonists of any GPCR or GPCR signal transduction approach in any target cell type.
Owner:ZHEJIANG UNIV
Method for detecting DNA (deoxyribonucleic acid) content of CHO (cholesterol) cells by probe
ActiveCN102154503AHigh sensitivityStrong specificityMicrobiological testing/measurementBiologyChinese hamster ovary cell
The invention discloses a method for detecting DNA (deoxyribonucleic acid) content of CHO (cholesterol) cells by a probe, which comprises the following steps of: adopting a nucleotide sequence in a gene bank with accession number of EF 540878 so as to design a specific primer and a Taqman probe, carrying out the real-time fluorescence quantitative PCR (polymerase chain reaction) after extracting the DNA (deoxyribonucleic acid) from a sample under test, and obtaining the DNA (deoxyribonucleic acid) content of the CHO (cholesterol) cells in the sample under test by calculation according to an obtained standard curve. The invention further discloses a kit based on the method. The invention can accurately and quantificationally detect the residual DNA (deoxyribonucleic acid) content of CHO (cholesterol) cells in products derived from the CHO (cholesterol) cells such as therapeutic protein drugs, recombinant vaccines, monoclonal antibodies and the like. The method also provides an internalreference for analyzing copy numbers of specific genes in the CHO (cholesterol) cells.
Owner:SHANGHAI HENLIUS BIOTECH INC +1
Chinese hamster ovary genetic engineering cell line for performing high-level secretory expression on AcAPc2
InactiveCN102321588AAdaptableExtended half-lifeVector-based foreign material introductionForeign genetic material cellsHigh level expressionHamster
The invention discloses a Chinese hamster ovary (CHO) genetic engineering cell line for performing high-level secretory expression on AcAPc2 and relates to a CHO cell line for performing high-level secretory expression on the AcAPc2. The invention aims to solve the problems that the expression level of an exogenous gene product expressed by using CHO is low at present and the industrialized production cannot be realized. The CHO genetic engineering cell line for performing high-level secretory expression on the AcAPc2 is obtained by the following step of: screening dihydrofolate reductase-deficient CHO cells which are taken as host cells, and amplifying to obtain the cell line for performing stable and high-level expression on the AcAPc2. The CHO cell line can perform high-level expression on the AcAPc2; the expression level can be up to 10mg / L.72h; and the CHO cell line can be industrially produced. The cell line is used for resisting blood coagulation and treating tumor, septicaemiaand the like.
Owner:HEILONGJIANG UNIV
Method for high efficiency expressing destination protein
InactiveCN1869214AIncrease productionSimple methodRecombinant DNA-technologyFermentationFusion Protein ExpressionBiology
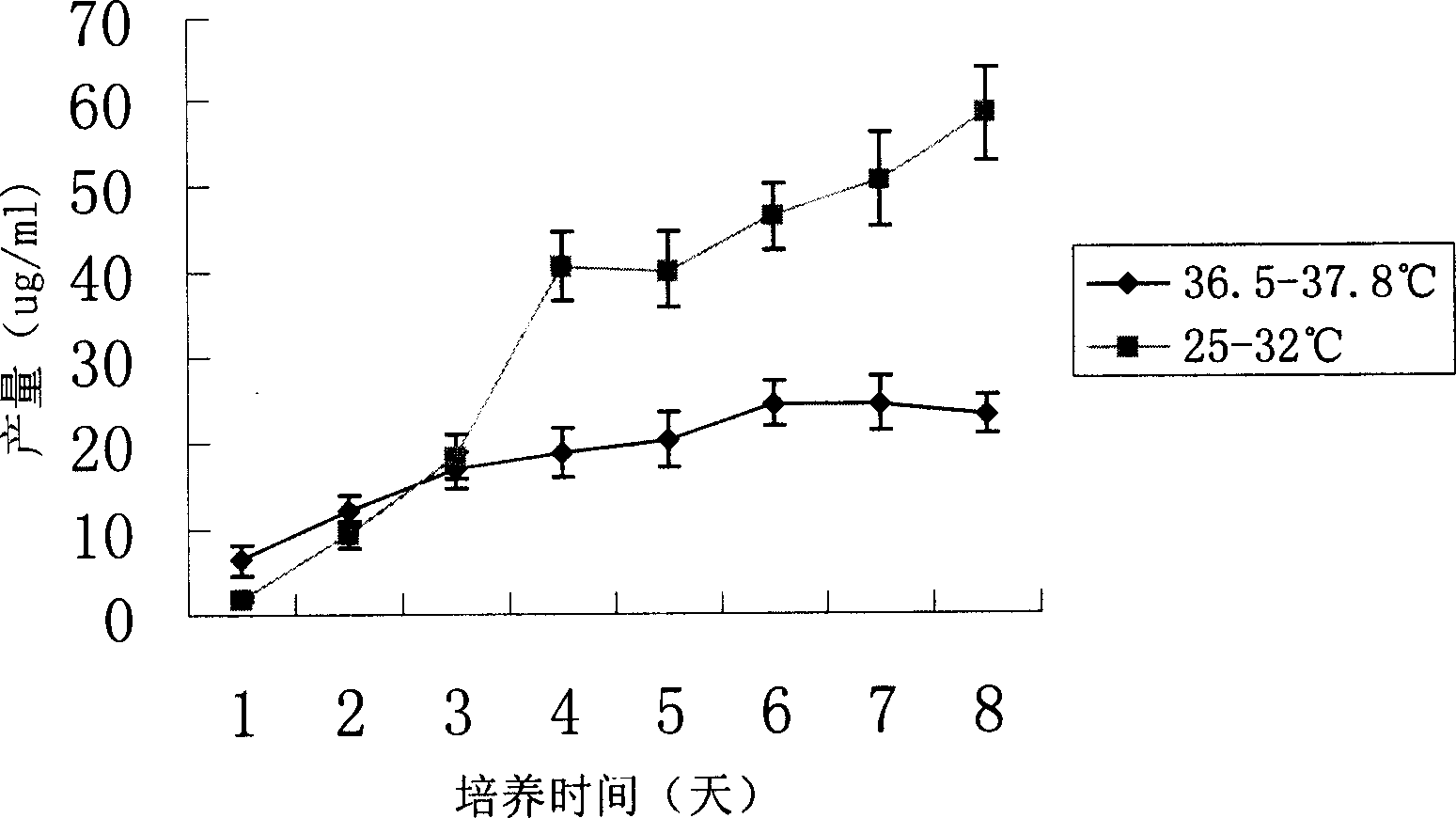
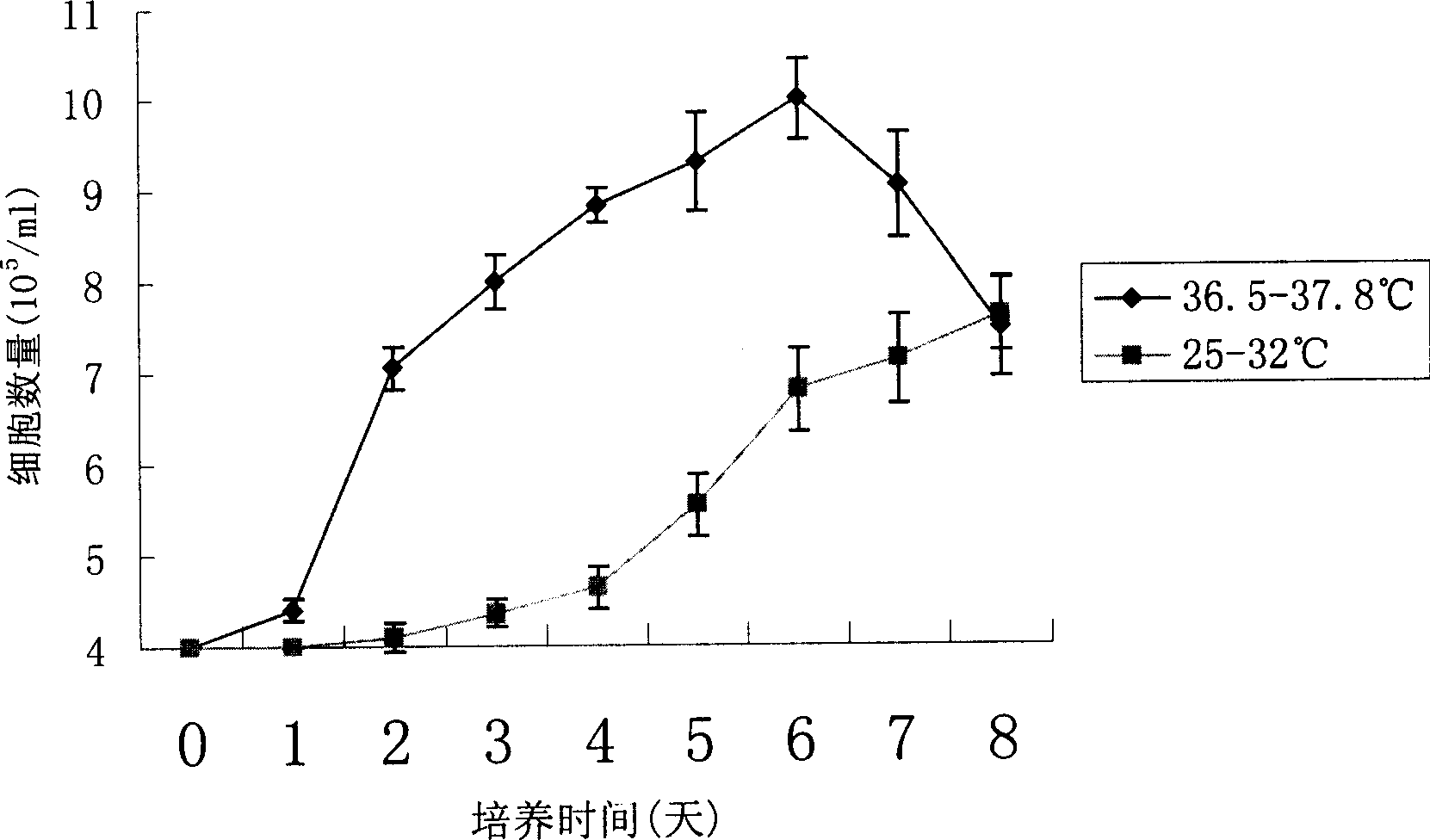
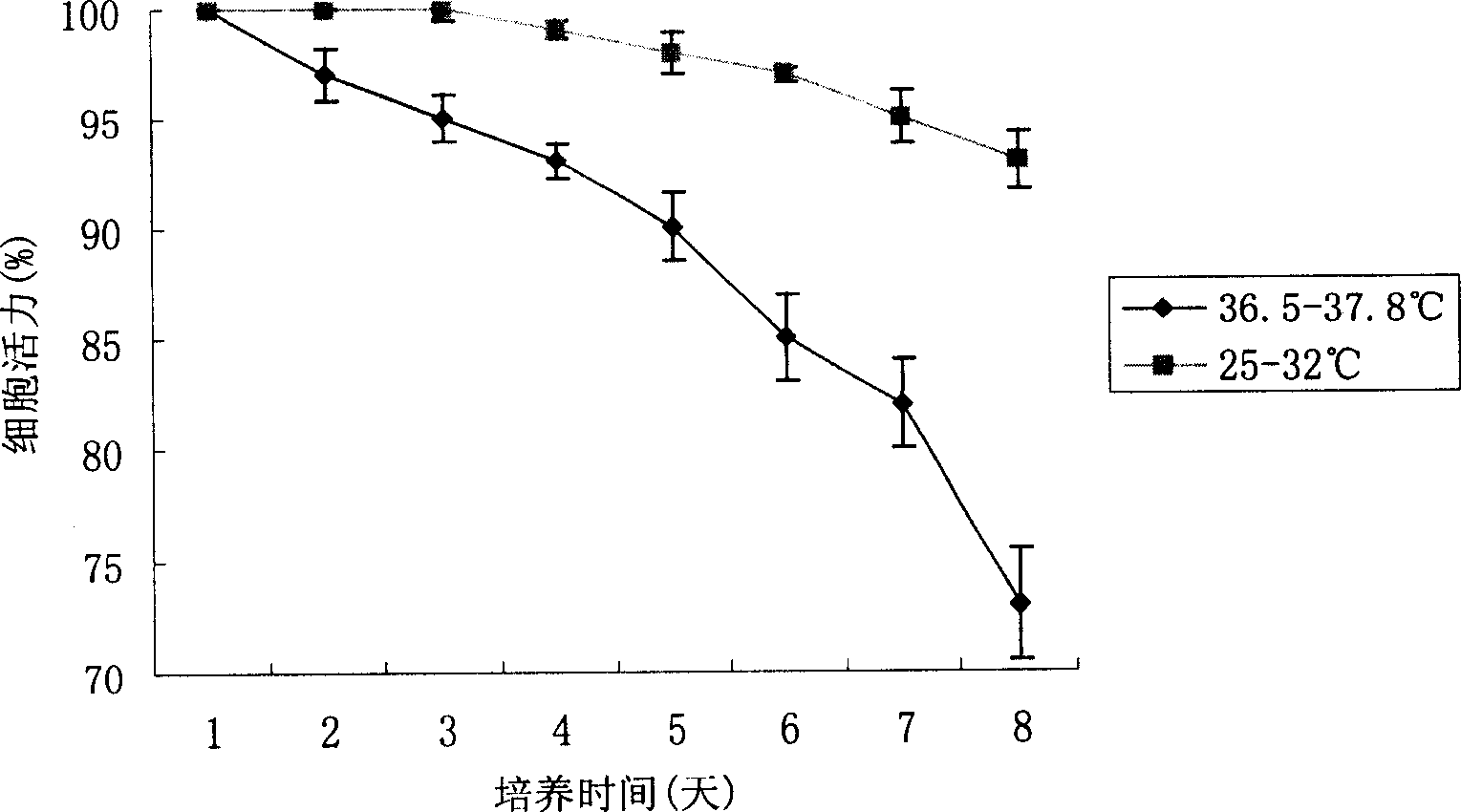
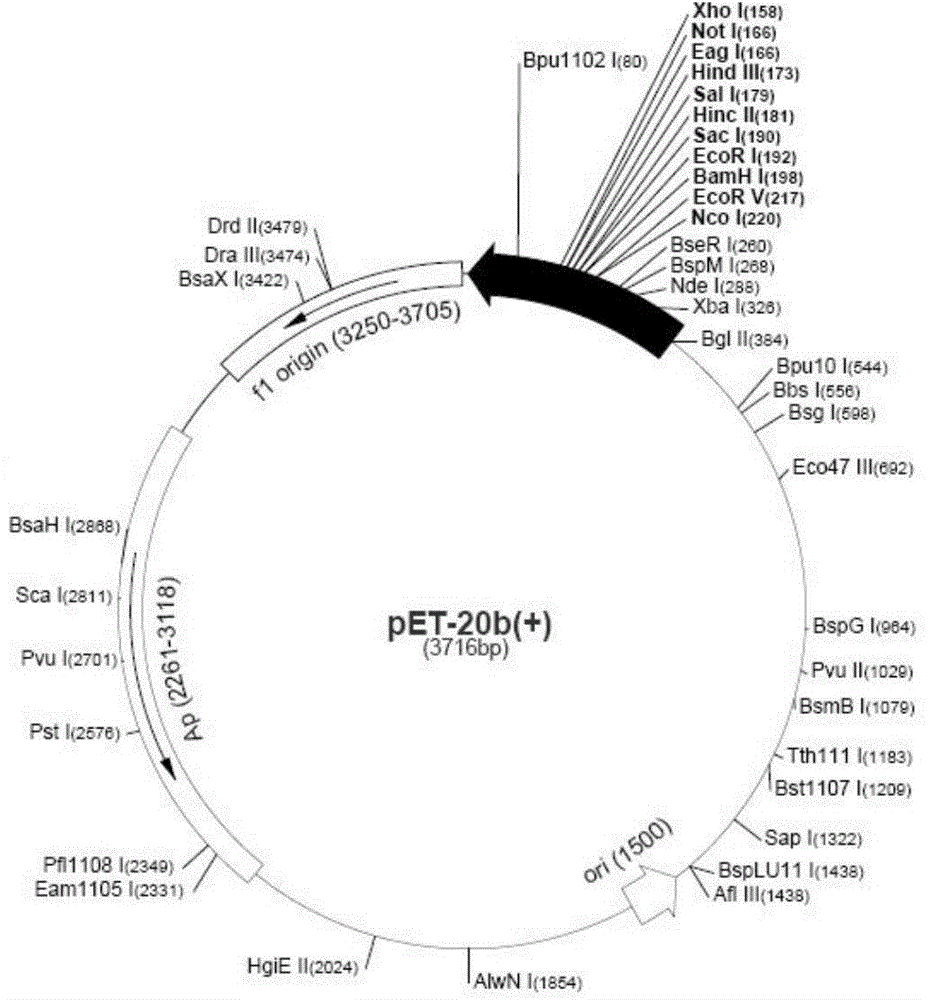
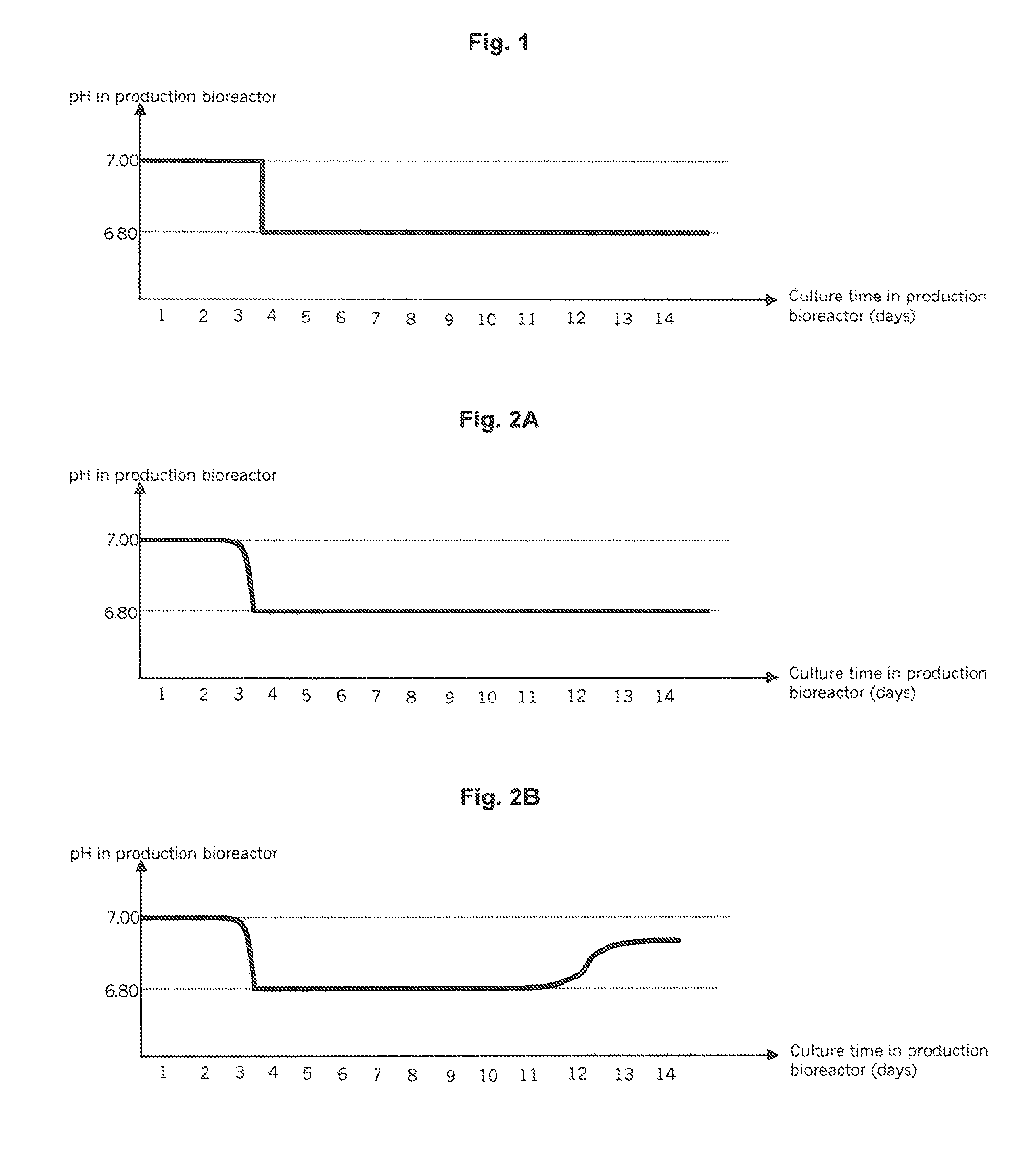
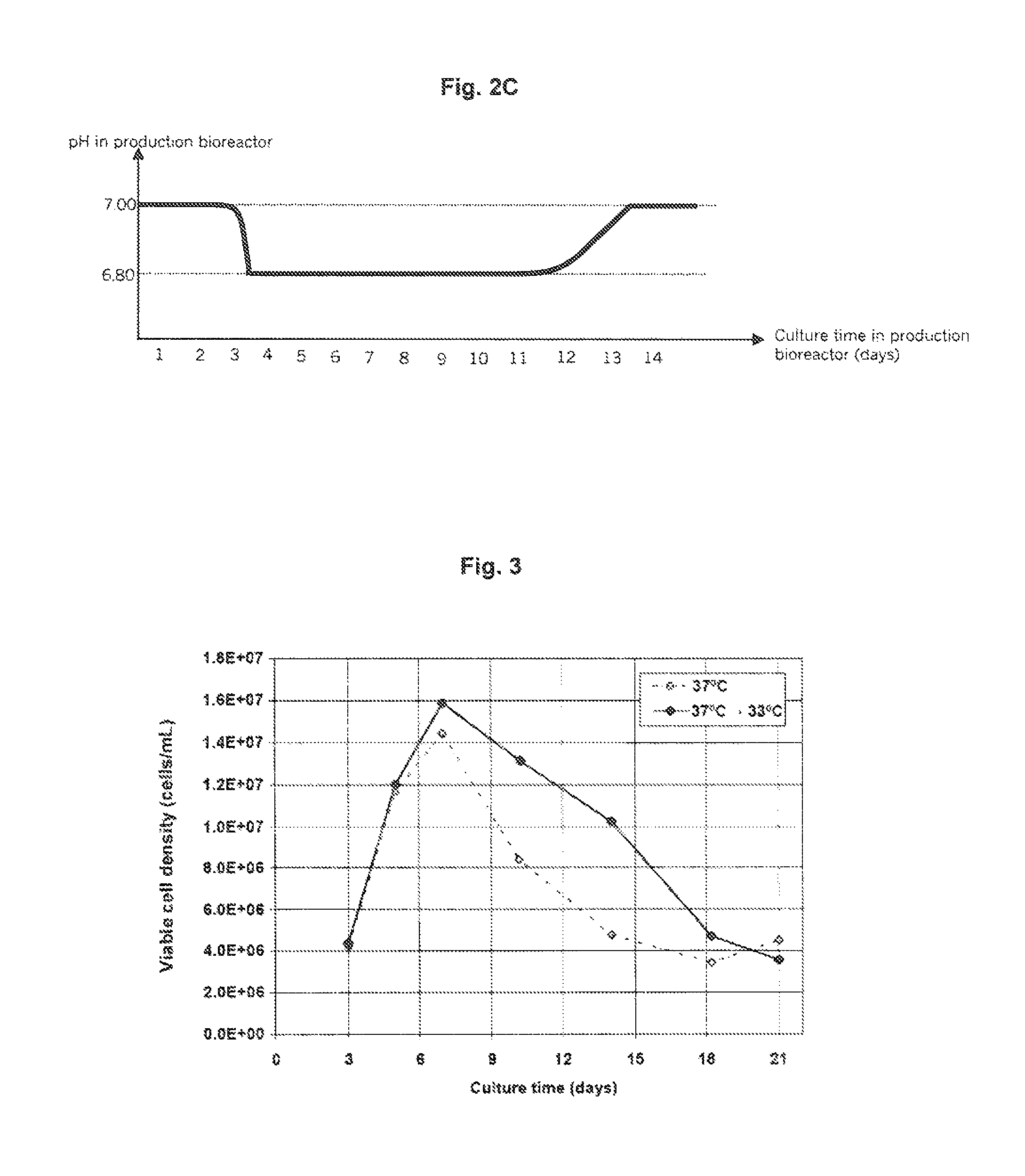
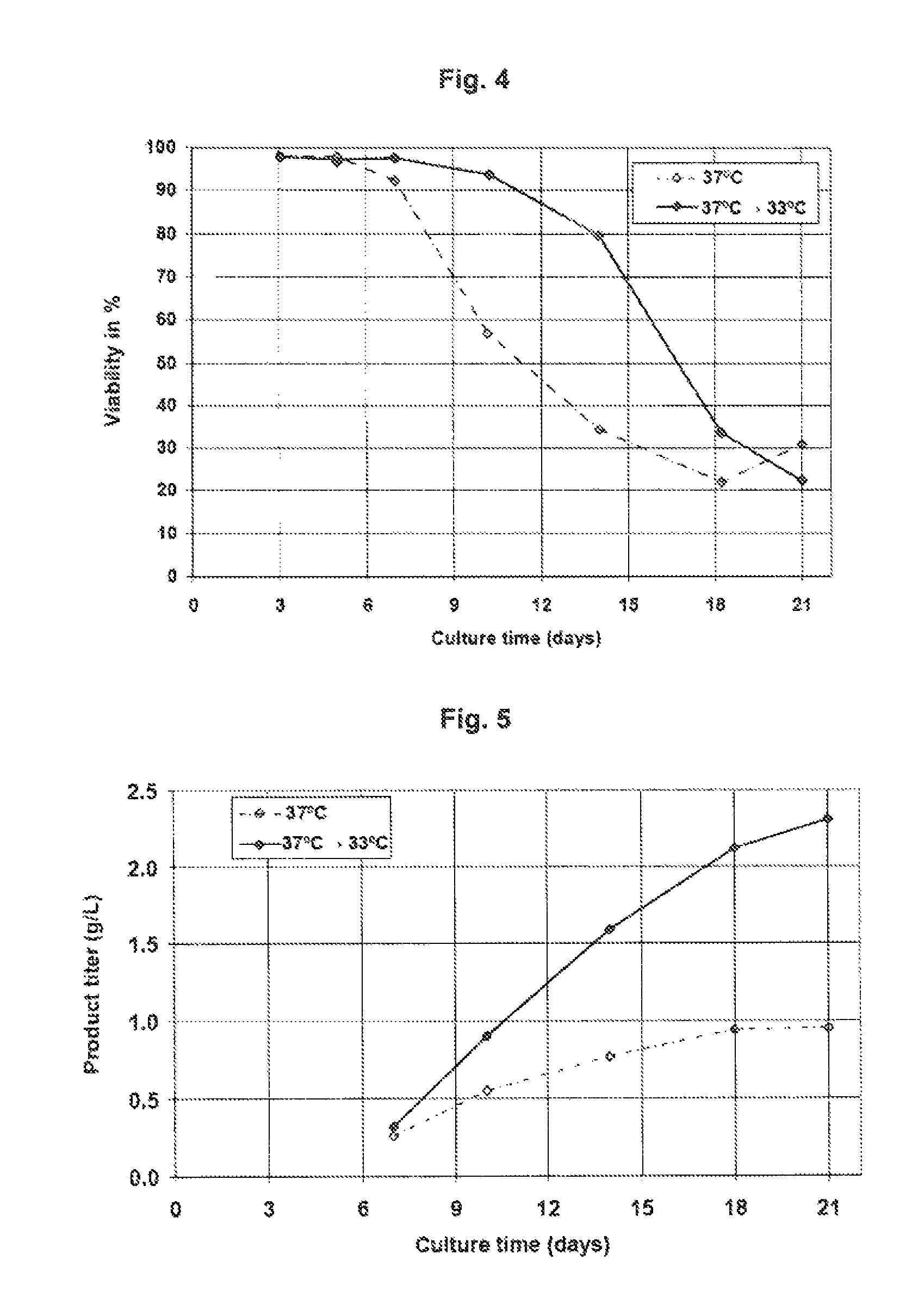
This invention discloses method of high efficiency expressing target albumen. Amalgamation albumen expression quantity can be greatly improved by using two steps culture strategy, and the culture temperature of the expression target albumen CHO cell is stabilized. The method in this invention is simple, and easy to realized, its efficiency is high, and cost is not improved. It has good application value not only to laboratory using or commercialization generating.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Cell culture method for reducing acidic peak content of antibody and improving glycoform of antibody
InactiveCN105779394AOptimizing Glycosylation LevelsGuaranteed efficacyCulture processImmunoglobulinsAntibody expressionEconomic benefits
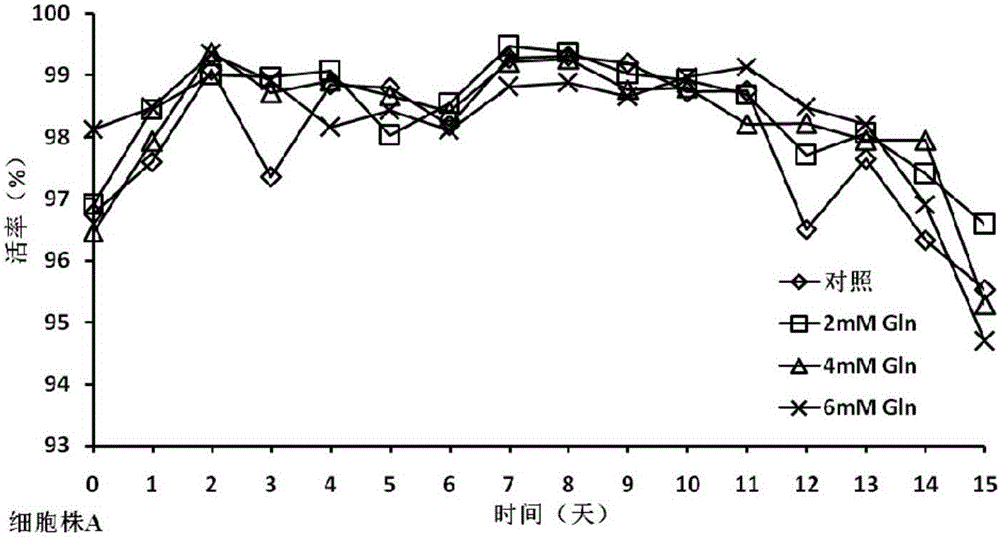
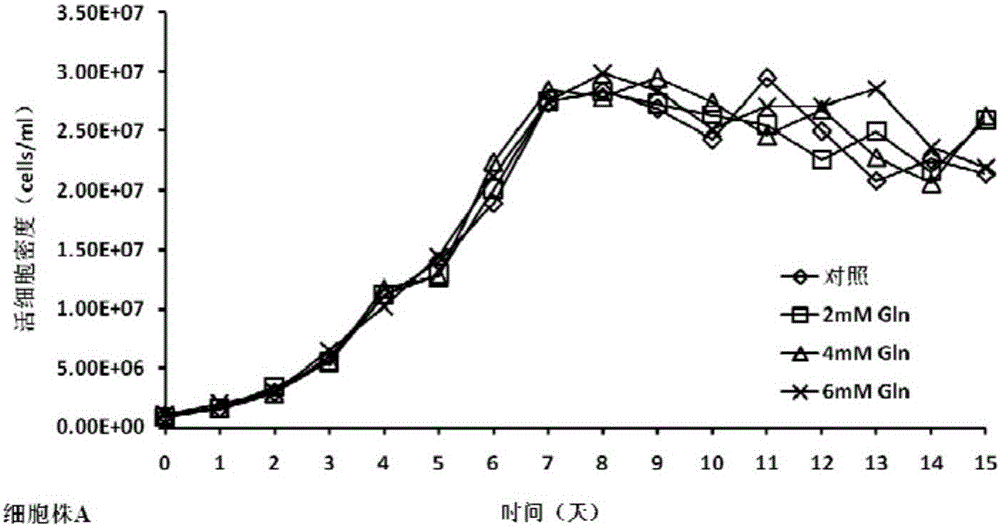
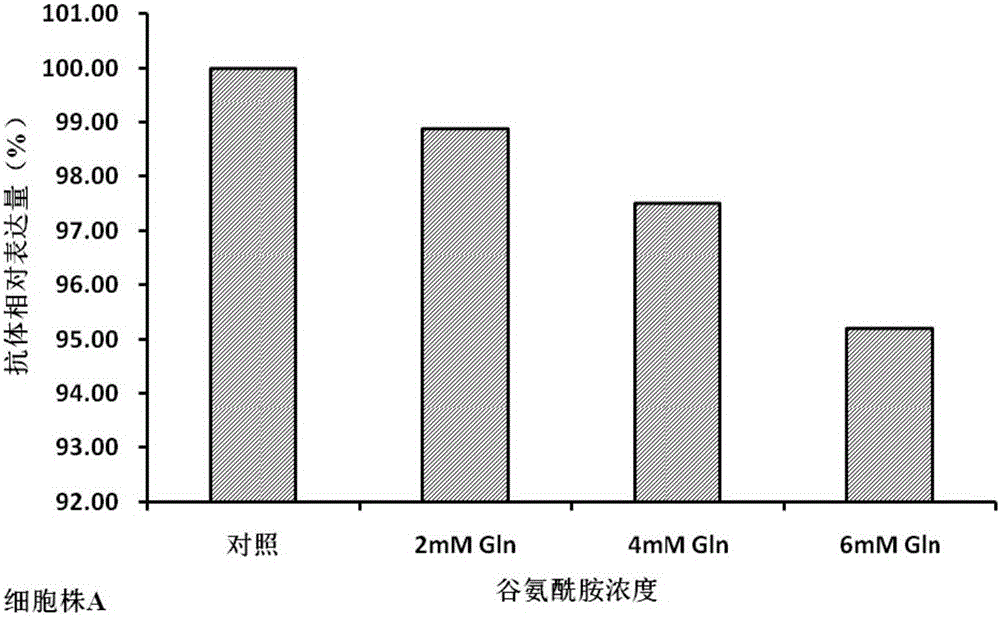
The invention discloses a cell culture method for reducing acidic peak content of an antibody and improving glycoform of the antibody. In the method, GS-CHO cells are used as an expression system, glutamine is added into a basic medium for antibody-expressed cell strain culture. After glutamine is added to the basic medium, it is possible to effectively reduce acidic peak content of the antibody and improve glycosylation level, thus modifying the activity and efficacy of antibody drugs and improving the quality of the antibody so that the quality is close to that of a standard product; the method is adapted to the development and study and later large-scale production of new antibody drugs and has a promising application prospect and good economic benefit.
Owner:SUNSHINE LAKE PHARM CO LTD
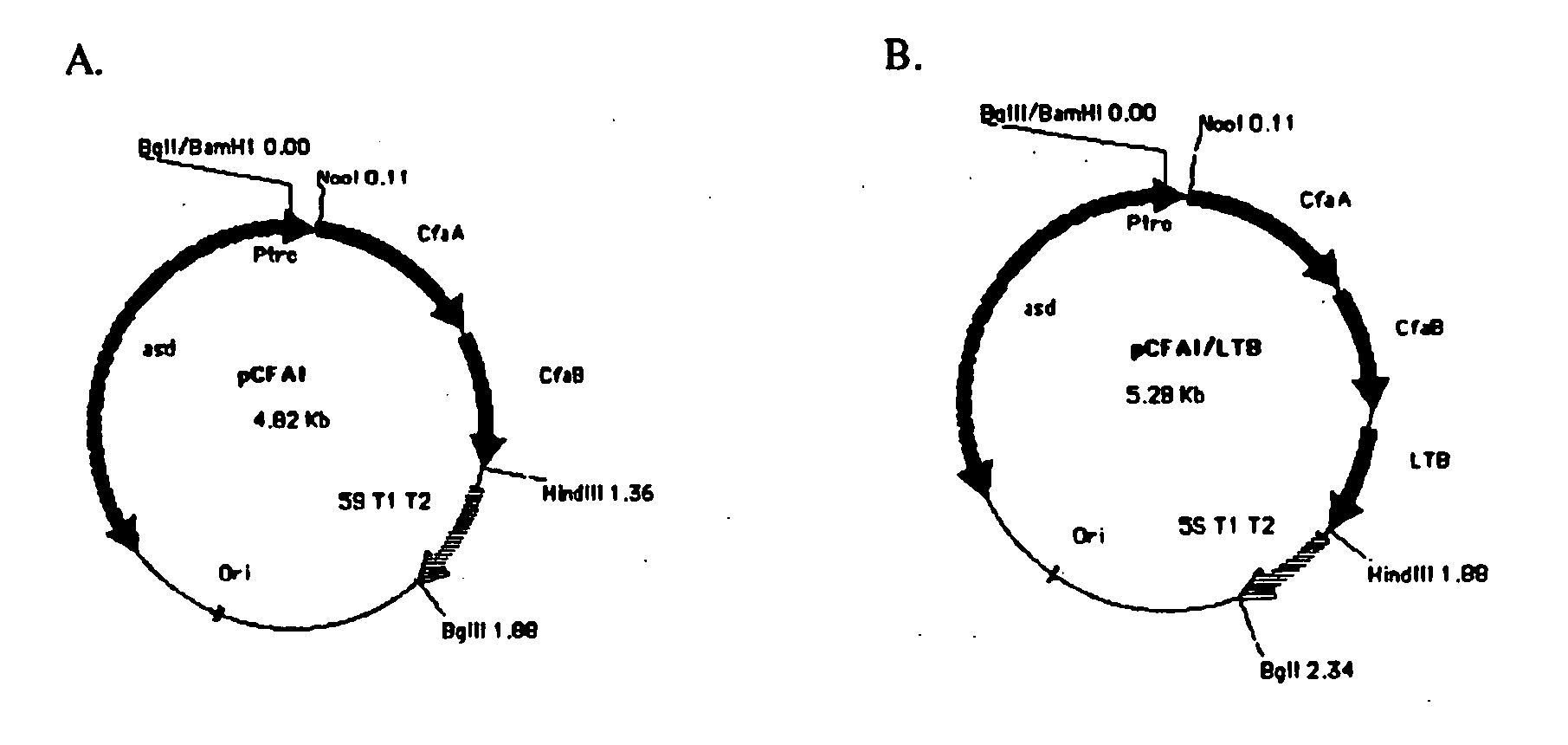
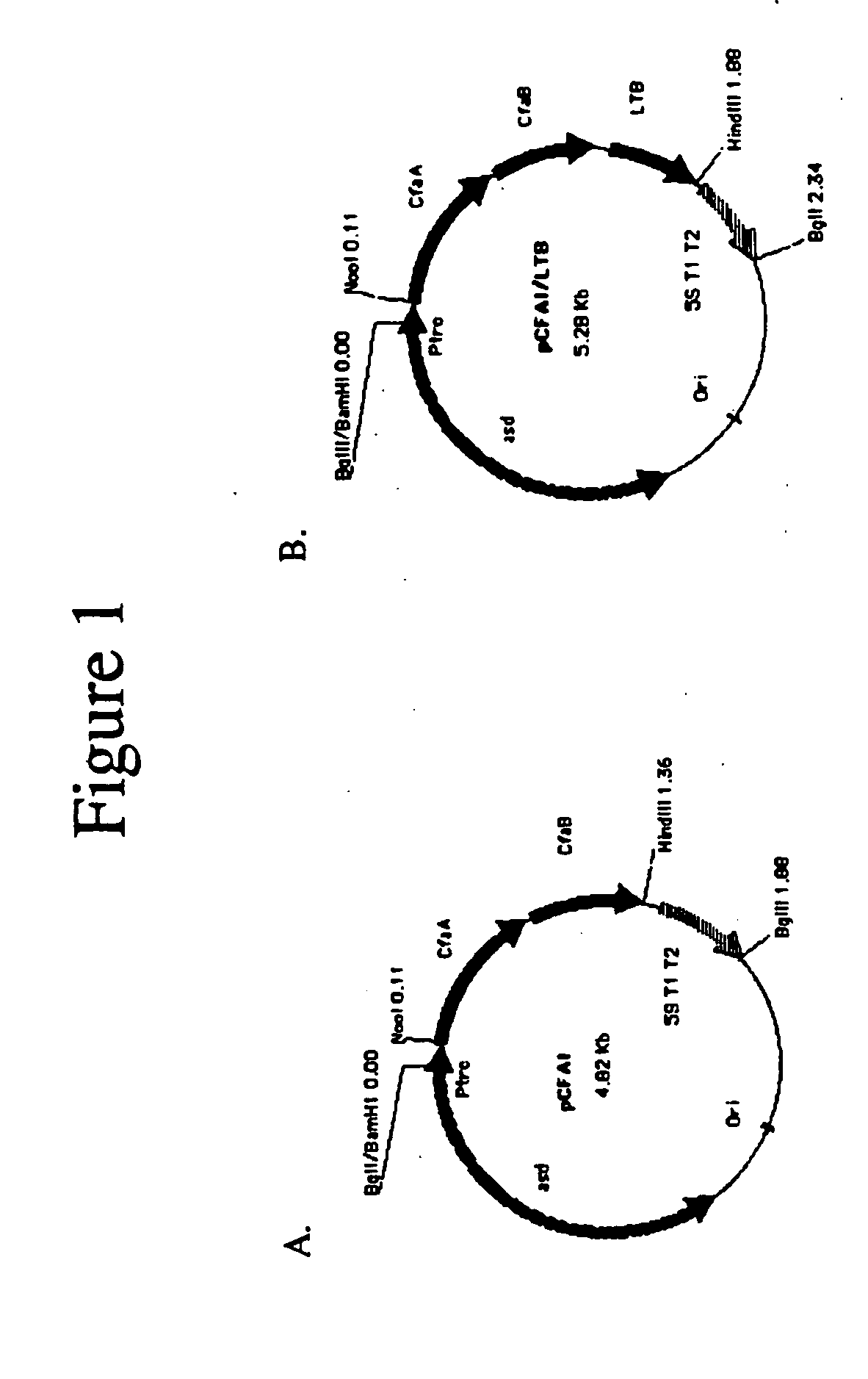
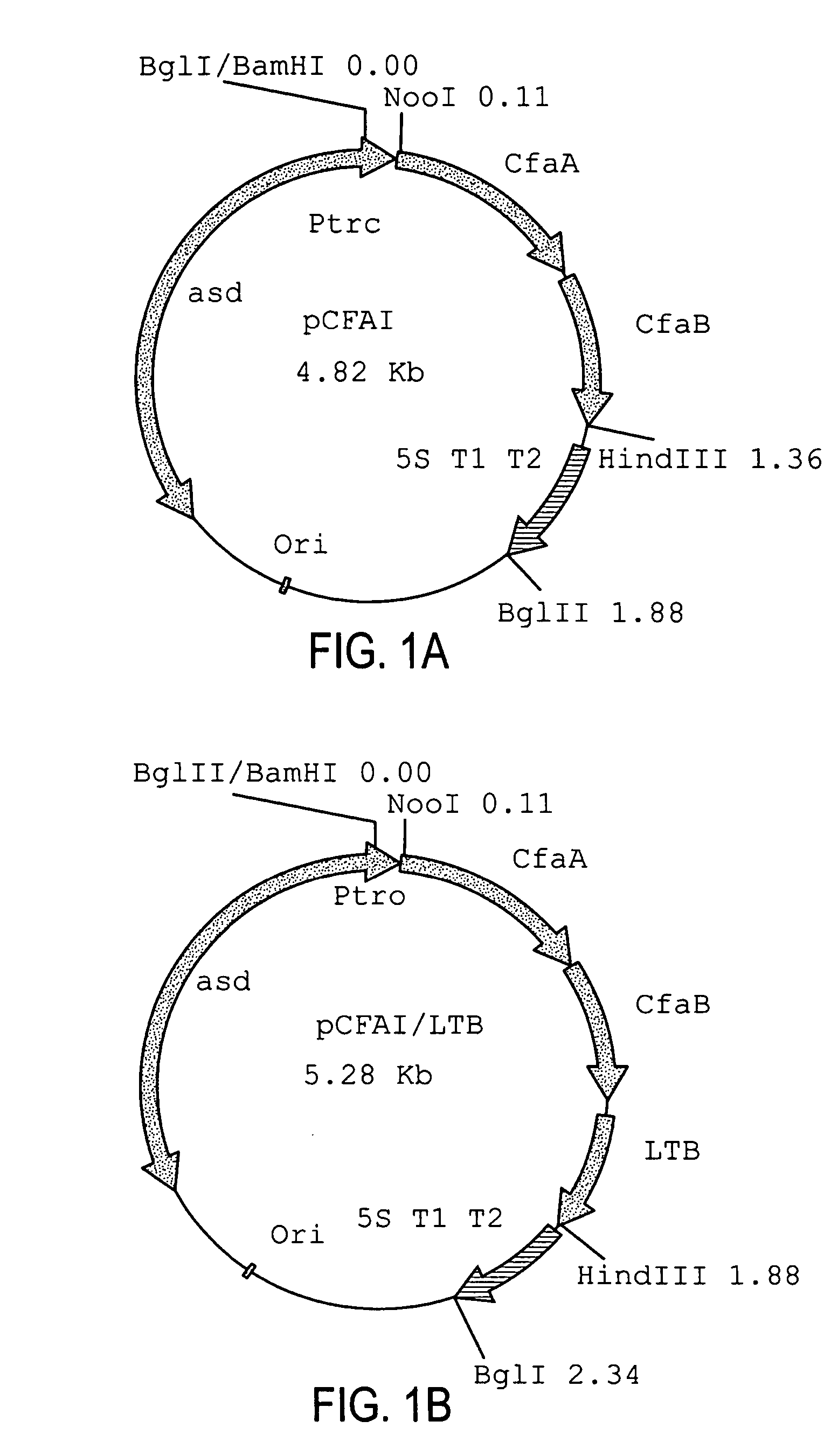
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (cfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E.coli
ActiveUS20070237791A1Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
With the goal of creating a combination vaccine against Shigella and other diarrheal pathogens we have constructed a prototype vaccine strain of Shigella flexneri 2a (SC608) that can serve as a vector for the expression and delivery of heterologous antigens to the mucosal immune system. SC608 is an asd derivative of SC602, a well-characterized vaccine strain, which has recently undergone several phase 1 and 2 trials for safety and immunogenicity. Using non-antibiotic asd-based plasmids, we have created novel constructs for the expression of antigens from enterotoxigenic E. coli (ETEC), including CFA / I (CfaB and CfaE) and the B-subunit from heat-labile enterotoxin (LTB) in Shigella vaccine strain SC608. Heterologous protein expression levels and cellular localization are critical to immune recognition and have been verified by immunoblot analysis. Following intranasal immunization (SC608(CFAI) and SC608(CFAI / LTB) of guinea pigs, serum IgG and IgA immune responses to both the Shigella LPS and ETEC antigens can be detected by ELISA. In addition, ELISPOT analysis for ASCs from cervical lymph nodes and spleen showed similar responses. All vaccine strains conferred high levels of protection against challenge with wild-type S. flexneri 2a using the Sereny test. Furthermore, serum from guinea pigs immunized with SC608 expressing CfaB and LTB contained antibodies capable of neutralizing the cytological affects of heat-labile toxin (HLT) on Chinese Hamster Ovary (CHO) cells. These initial experiments demonstrate the validity of a multivalent invasive Shigella strain that can serve as a vector for the delivery of pathogen-derived antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
PendingCN107674883AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetVaccine Production
The invention discloses preparation methods and application of recombinant swine fever E2 protein and a subunit vaccine of the recombinant swine fever E2 protein. The preparation method of the recombinant swine fever E2 protein comprises the following steps that (1) a swine fever E2 protein coding gene is cloned into an eukaryotic expression vector to obtain recombinant plasmid containing the swine fever E2 protein coding gene; (2) then, the recombinant plasmid containing the swine fever E2 protein coding gene is transfected into a CHO cell strain; (3) the CHO cell strain obtained in the step(2) is cultured, screened and domesticated; and (4) the cell strain in the step (3) is fermented and cultured; and the recombinant swine fever E2 protein is obtained after purification. The methods provided by the invention have the advantages that the target protein can be obtained from cell culture supernatant; the yield reaches up to 1g / L; the protein purification time is shortened; the vaccineproduction steps are simplified; and the vaccine production cost is also greatly reduced.
Owner:NOVO BIOTECH CORP
Eukaryotic expression vector for producing recombinant protein by using CHO cells, and system
ActiveCN103509823AAchieve integrationMicroorganism based processesFermentationGenetic engineeringGenome
The invention relates to the field of genetic engineering, and specifically discloses a gene expression system for producing recombinant protein by using CHO cells, and an eukaryotic expression vector, wherein the eukaryotic expression vector comprises a GS expression unit, and the GS expression unit comprises a TK promoter gene sequence aligned along a 5' to 3' direction, a glutamine synthetase gene sequence and a SV40polyA gene sequence. The expression system comprises the eukaryotic expression vector and CHO cells. With the expression system, integration and efficient expression of exogenous gene in CHO cell genome can be achieved, and broad application prospects are provided in the field of protein.
Owner:SHANGHAI HENLIUS BIOTECH INC +1
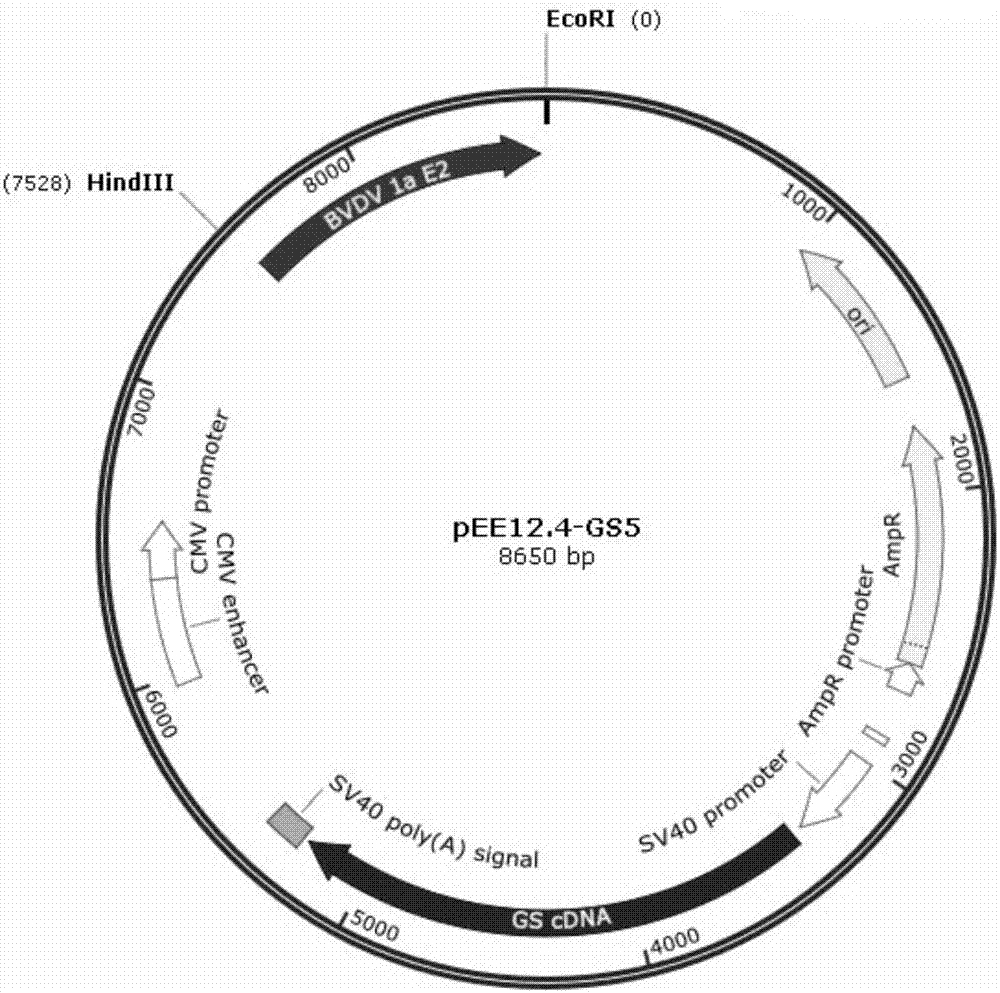
Preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and subunit vaccine and application
ActiveCN107973841AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetBovine Viral Diarrhea Viruses
The invention discloses preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and a subunit vaccine and applications and belongs to the technical fields of animal vaccines and veterinary biologicals. The object of the invention is to provide a preparation method capable of industrially producing the bovine viral diarrhea virus recombinant subunit vaccine ona large scale. The preparation method for the recombinant subunit vaccine, provided by the invention, comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein E2coding gene; 2) transfecting CHO cells, and performing selection, screening and acclimatizing to obtain suspending CHO cell strains, which stably and efficiently express the protein E2; 3) subjectingthe cell strains obtained in the step 2) to fermentation culture, and carrying out purification, so as to obtain recombinant protein E2; and 4) uniformly mixing the recombinant protein E2 and ISA 201VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches upto 500mg / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
Gonad cell amplification culture medium of Chinese hamster and uses thereof
InactiveCN101195816AImprove consistencyImprove stabilityVertebrate cellsArtificial cell constructsHamsterChinese hamster
The invention relates to a culture medium and the usage thereof, in particular to the Chinese hamster ovary cell enlarging culture medium and the usage thereof. The invention belongs to the biological product field. The Chinese hamster ovary cell enlarging non-protein culture medium of the invention, wherein comprises minimal medium, and also comprises microelement, Transferrin and Insulin subsistent. A serum-containing routine culture medium can be directly replaced with the serum free culture medium of the Chinese hamster ovary cells, and the Chinese hamster ovary cells are not required to adapt process.
Owner:天津百若克医药生物技术有限责任公司
Culture method for highly expressing erythropoietin in serum-free medium and cho cells
ActiveCN102268402AIncreased glycosylationIncrease the proportionMicroorganism based processesArtificial cell constructsCulture fluidChinese hamster
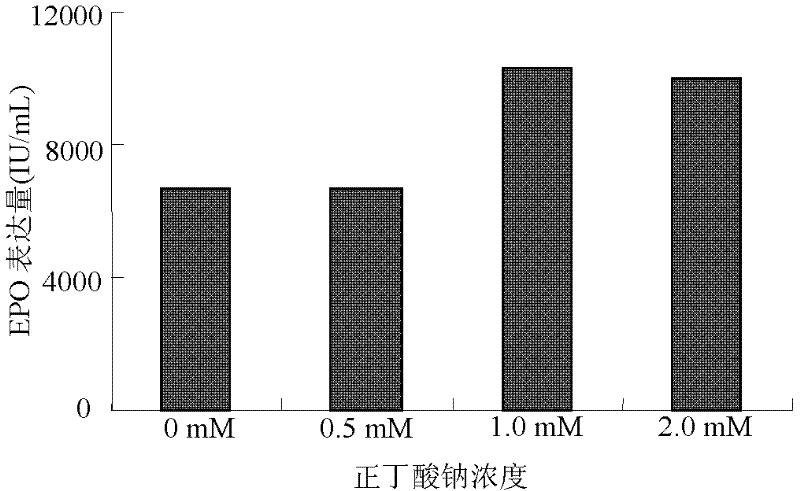
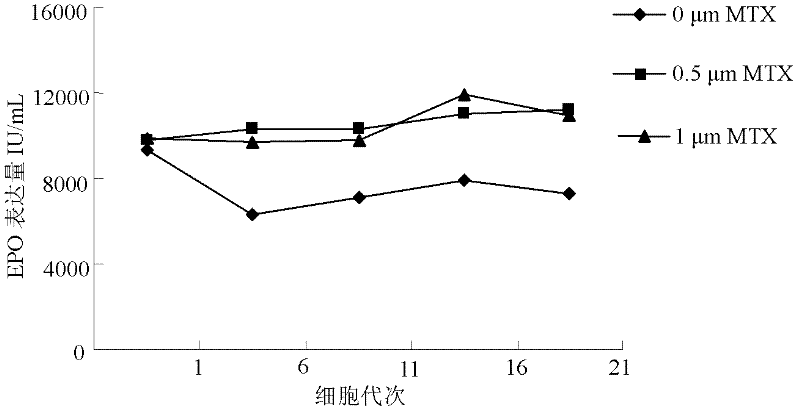
The invention discloses a serum free medium for expressing erythropoietin in CHO (Chinese hamster ovary) cells, and a culture method for high expression of erythropoietin in CHO cells. The serum free medium contains additives D-glucose and sodium butyrate, and also contains one or more of D-galactose, D-mannitose and N-acetylglucosamine. The culture method comprises the steps of: performing serumculture on activated seed cells by using a serum medium, and then performing serum free culture by the serum free medium. By adopting the medium and culture method disclosed by the invention, recombinant human erythropoietin protein of high and stable expression can be obtained, and the glycosylation degree of erythropoietin is improved, i.e. the specific gravity of EPO (erythropoietin) sialic acid is improved. As high as 1.0*107IU recombinant human erythropoietin can be obtained from one liter of culture fluid by the culture method provided by the invention, the expression is stable, and theculture method is simple and suitable for large-scale production.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Antibody fusion protein targeting VEGFR2 as well as preparation and use thereof
InactiveCN104628866AEnhanced ADCC effectPeptide/protein ingredientsPeptide preparation methodsAbnormal tissue growthNatural Killer Cell Inhibitory Receptors
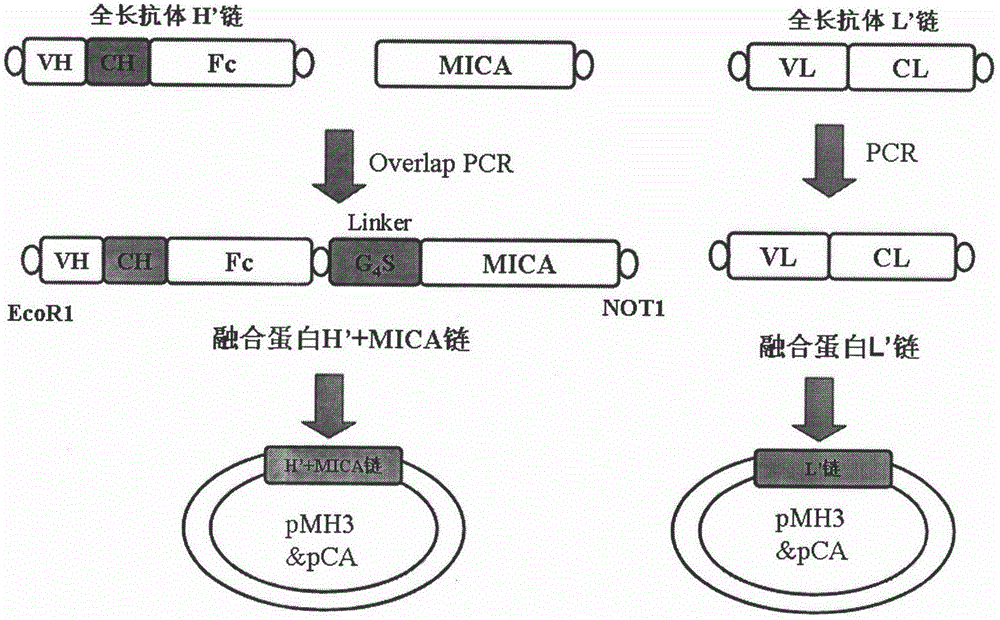
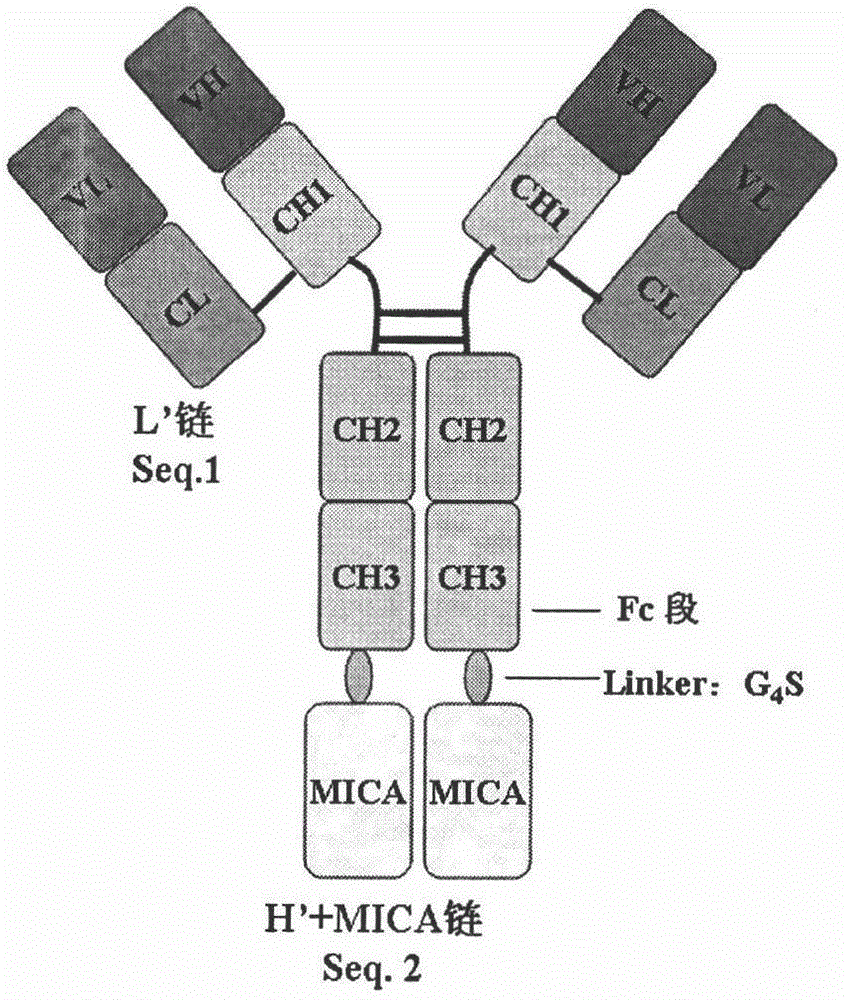
The invention belongs to the technical field of genetically engineered antibody, in particular relates to a fusion protein of tumor vascular endothelial growth factor receptor 2 (VEGFR2 / KDR3) antibody and MICA as well as a preparation method and use thereof. In the invention, a full-length antibody of VEGFR2 is linked with a ligand MHC I molecule associated protein A (MICA) of activated receptor of NK cell (NKG2D) through a flexible linker and expressed by CHO cells by utilizing the genetic engineering technology, and the formed fusion protein can act on VEGFR on the surface of a tumor cell to inhibit or kill tumor and inhibit or destroy tumor angiogenesis, and on the other hand, the formed fusion protein can increase the level of MICA on the surface of the tumor cell and stimulate the NK cells to kill tumor cells through identification of NKG2D on the surfaces of the NK cells, thereby reestablishing the immunological surveillance of an body activated by the NKG2D pathway, and enhancing effects of antibody-dependent cell-mediated cytotoxicity (ADCC) and the like mediated by the Fc region of the antibody to kill tumor cells.
Owner:CHINA PHARM UNIV
Recombinant protein production in a human cell
Owner:JANSSEN VACCINES & PREVENTION BV
CHO (Chinese Hamster Ovary) cell strain of efficiently-expressed recombinant human nerve growth factor and construction method thereof
ActiveCN102586190AImprove stabilityLow costMicroorganism based processesFermentationHuman bodyEmbryo
The intervention relates to a CHO (Chinese Hamster Ovary) cell strain of an efficiently-expressed recombinant human nerve growth factor and a construction method thereof and belongs to the field of biotechnology. The preservation number of the cell strain is CGMCC No. 4541, and the recombinant human nerve growth factor (rhNGF) with biological activity can be stably expressed. The invention also discloses a method for constructing the CHO cell strain; and through cell transfection, gene amplification and subcloning screening carried out on an amplified cell line, an efficiently-expressed monoclonal cell strain suitable for serum-free suspension culture is obtained. The invention simultaneously discloses a recombinant human nerve growth factor sequence secretly expressed by the CHO cell strain; and the secrete recombinant human nerve growth factor has good biological activity, so that PC12 multiplication can be kept outside a human body, PC12 cell differentiation is induced, and the chick embryo dorsal root ganglion nerve fiber growth is stimulated to lay a good foundation for the large-scale industrialization preparation of the recombinant human nerve growth factor.
Owner:北京福睿君安科技有限公司
Recombinant human-like collagen protein-human cell growth factor fusion protein and preparation method and application thereof
The invention discloses a recombinant human-like collagen protein-human original cell growth factor fusion protein and a preparation method and application thereof. The prepared fusion protein is provided with a special fibrous structure and good biological tissue compatibility, and can promote proliferation and differentiation of epidermis cells, endothelial cells and fibroblast cells. The preparation method comprises the following steps: obtaining encoded DNA sequence of the fusion protein, reconstructing and recombining fusion protein expressed by an expression vectors in escherichia coli, pichia pastoris or chinese hamster ovary cells. The fusion protein comprises a first zone and a second zone, wherein the first zone has at least 85% of sequence homology with human collagen protein and has the function of the human collagen protein, and the second zone has at least 85% of human original cell growth factor and has the function of the human original cell growth factor; connecting peptide is arranged between the first zone and the second zone; the general formula of the connecting peptide is (GGGS)n; preferably, the amino acid sequence of the fusion protein is SEQ ID NO. 15.
Owner:浙江肽源生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com