Recombinant human-like collagen protein-human cell growth factor fusion protein and preparation method and application thereof
A kind of human-like collagen, human collagen technology, applied in the field of genetic engineering, can solve the problem of collagen easily carrying viruses, infecting health and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
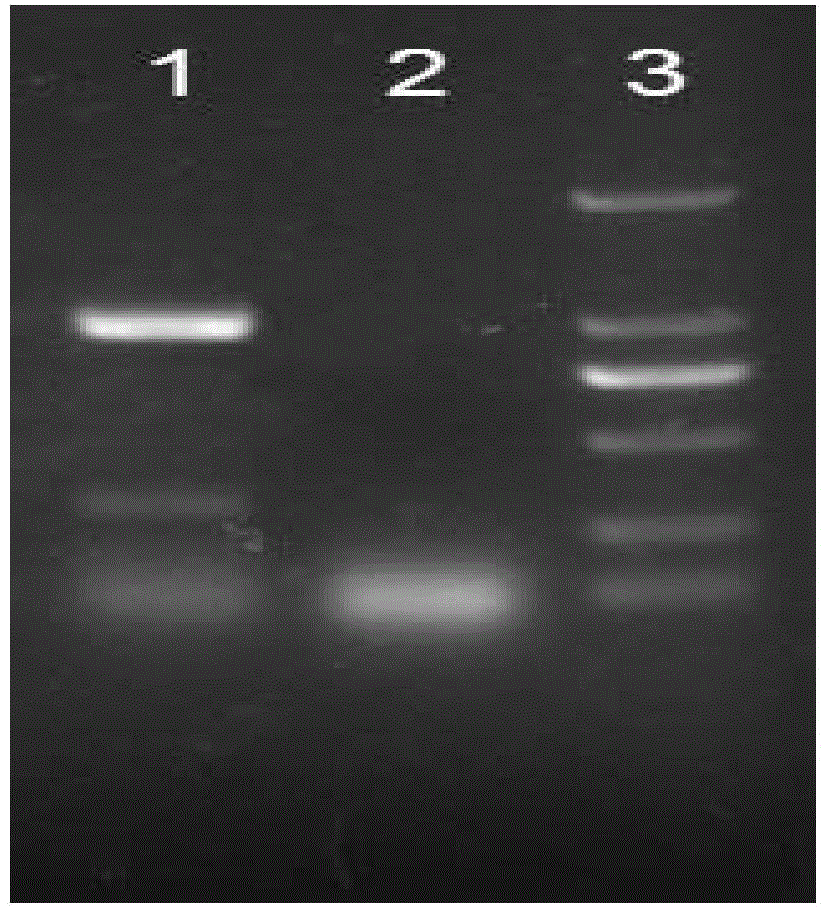
[0049] Expression of recombinant human-like collagen-human epidermal growth factor fusion protein in Escherichia coli
[0050] 1. Acquisition of gene encoding recombinant human-like collagen-human epidermal growth factor fusion protein
[0051] Send the DNA fragment (SEQ ID NO.2) encoding human-like collagen to Guangzhou Jetway Biotech Co. Ltd. (China) for whole gene synthesis, and use the DNA sequence and the whole gene The synthesized human epidermal growth factor (EGF) sequence (SEQ ID NO.3) was used as a template, and the two primers Ecoll F (SEQ ID NO.4) Ecoll R (SEQ ID NO.5) and the intermediate primer Ecoll-IN were added F (SEQ ID NO.6), Ecoll-IN R (SEQ ID NO.7) (primers synthesized by BGI) (primer concentration 10μM) each 1μL, then add dNTP (each 2.5μM) 10μL, 10x PCR pfu buffer Solution 10μL and pfu Taq DNA polymerase 1μL (5U / μL), add water to a total volume of 100μL, according to the touch-down PCR reaction conditions (94°C denaturation for 4min, enter the cycle, the...
Embodiment 2
[0057] Expression of Recombinant Human Collagen-Epidermal Growth Factor Fusion Protein in CHO Cells
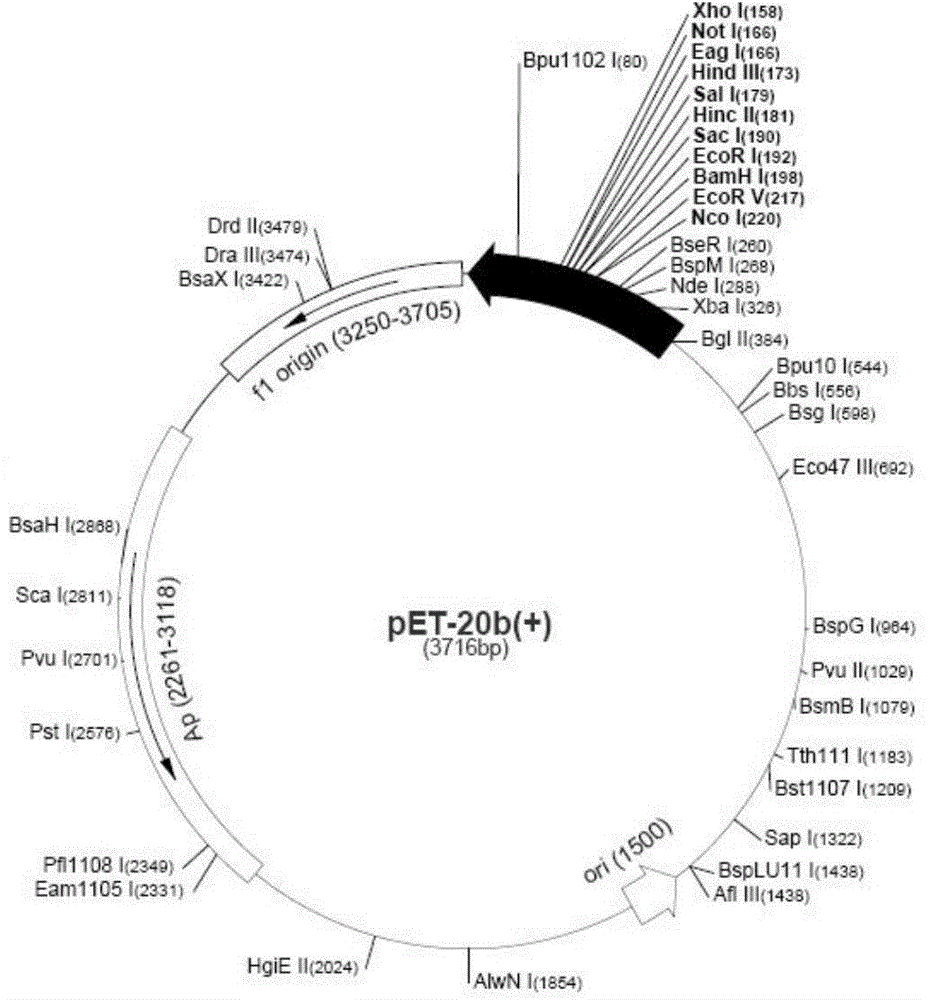
[0058] First construct transfection CHO-dhfr according to the method described in Example 1 - Cell expression vector pSV2-dhfr-Ecoll. Using the Ecoll described in Example 1 as a template to utilize the upstream primer Ecoll F2 (SEQ ID NO.8) and the downstream primer Ecoll R2 (SEQ ID NO.9) to amplify the coding fusion protein sequence Ecoll, with TOPO TA Cloning Kit (purchased from Invitrogen Enzyme amplification in ), ligated to T vector (pCR TM 2.1-TOPO vector), verified by sequencing, obtained the pT-Ecoll recombinant plasmid of the Ecoll gene fragment containing the HindIII (aagctt) restriction site at both ends. For the construction of recombinant fusion proteins in CHO-dhfr - The expression vector pSV2-dhfr in the cell was single cut by HindIII, the vector was dephosphorylated with CIAP alkaline phosphatase (TAKARA), and then ligated with pT-Ecoll, the small fragment r...
Embodiment 3
[0062] Expression of recombinant human-like collagen-growth factor fusion protein in yeast cells
[0063] First, the recombinant expression vector pPICZαA-Ecoll for transforming yeast cell GS115 was constructed according to the method described in Example 1. Using the Ecoll described in Example 1 as a template, use the upstream primer Ecoll F3 (SEQ ID NO.10) and the downstream primer Ecoll R3 (SEQ ID NO.11) to amplify the Ecoll containing the α-factor secretion signal peptide recognition site sequence at the N-terminus The gene was digested with Xho I and Xba I, connected with the large fragment pPICZαA of the vector recovered after the same double digestion, transformed into Top10 competent cells, and the transformants were screened on bleomycin-resistant YPD solid medium and identified by sequencing After the plasmid is correct, extract the plasmid, linearize the recombinant plasmid pPICZαA-Ecoll with Sac I, purify the plasmid by ethanol precipitation, transform GS115 compet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com