Method for high efficiency expressing destination protein
A high-efficiency expression and recombinant protein technology, applied in chemical instruments and methods, cells modified by introducing foreign genetic material, peptides, etc. Relative quantity and other issues, to achieve the effect of improving the yield of target protein, good application value and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
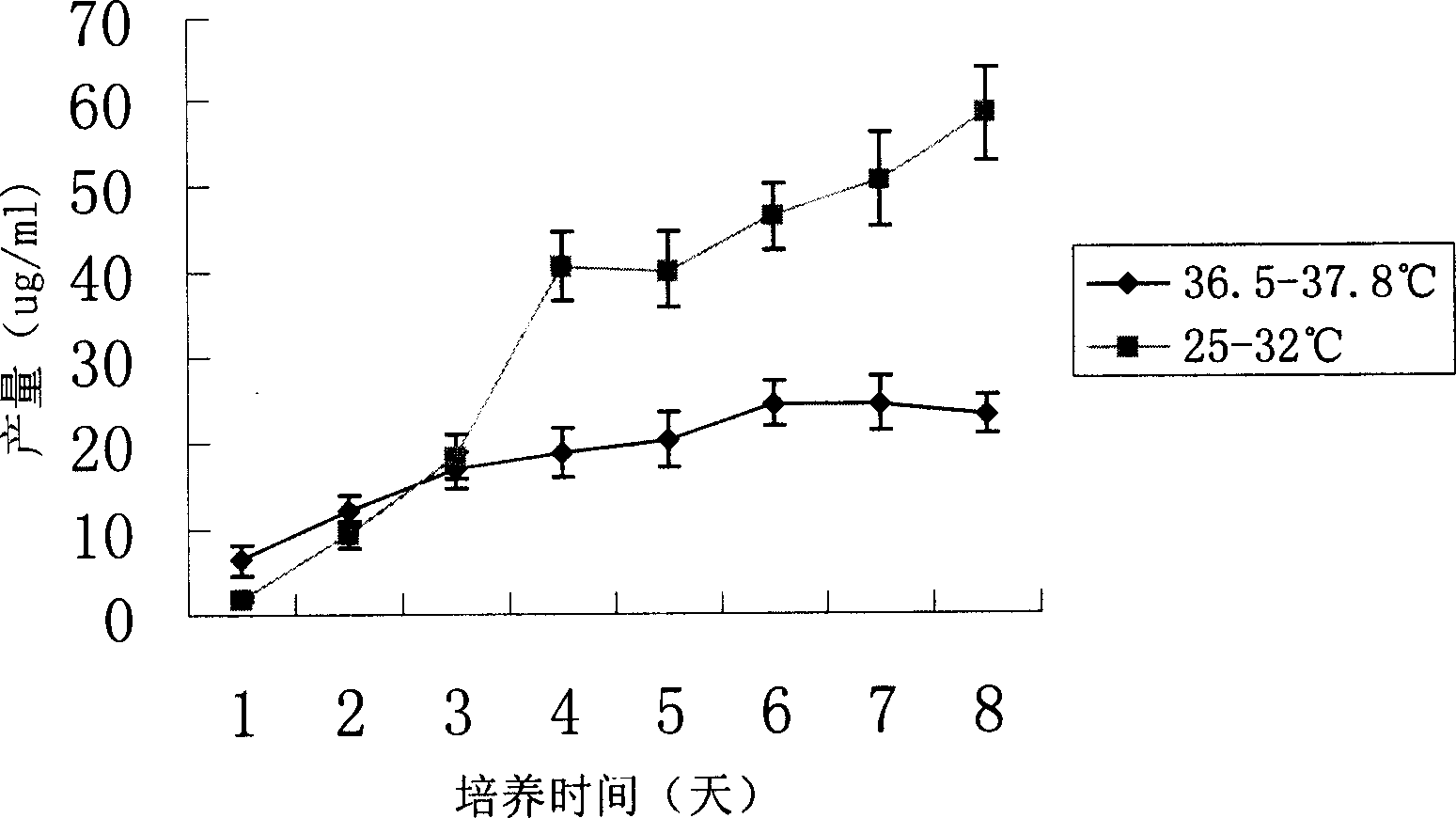
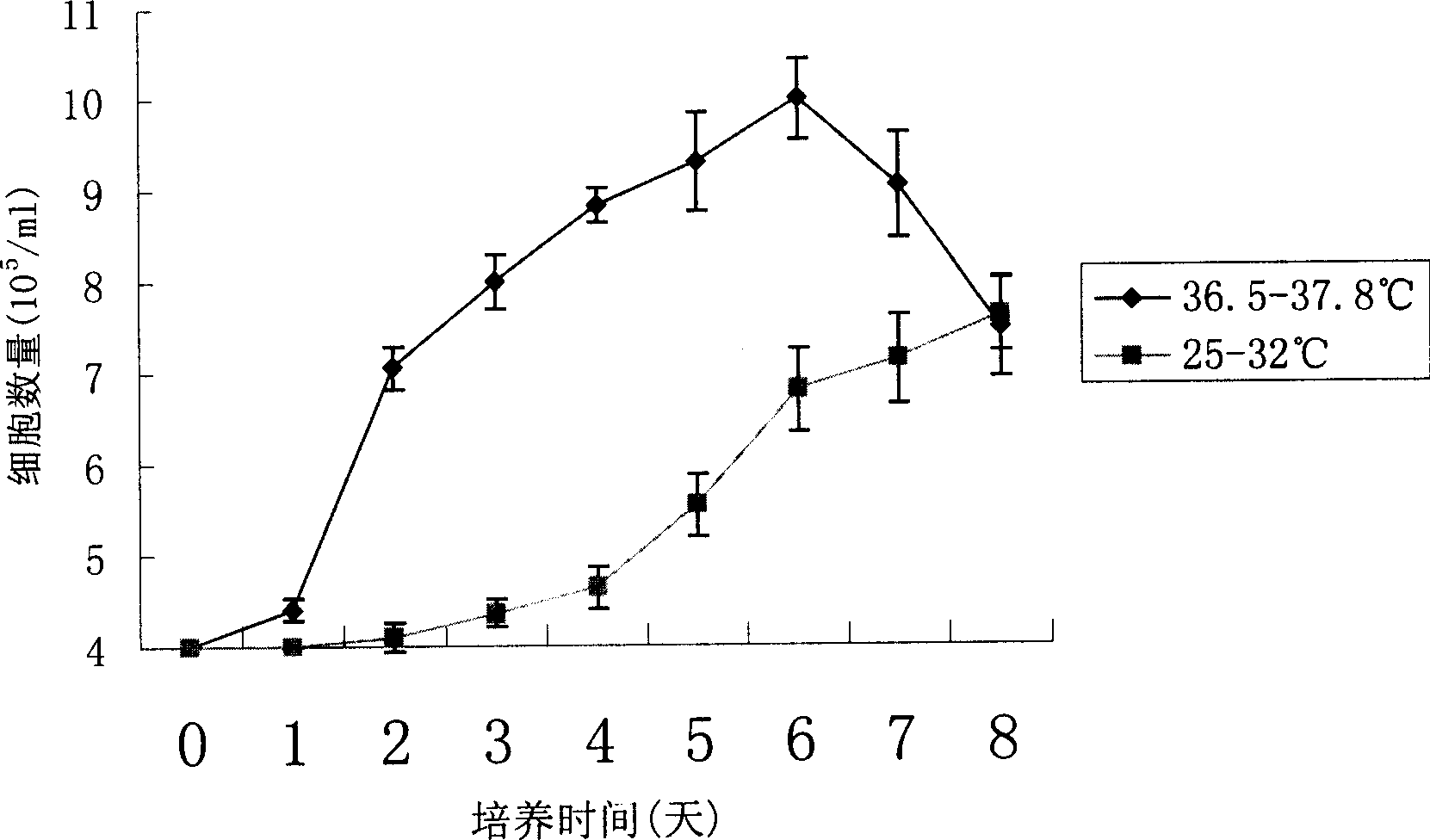
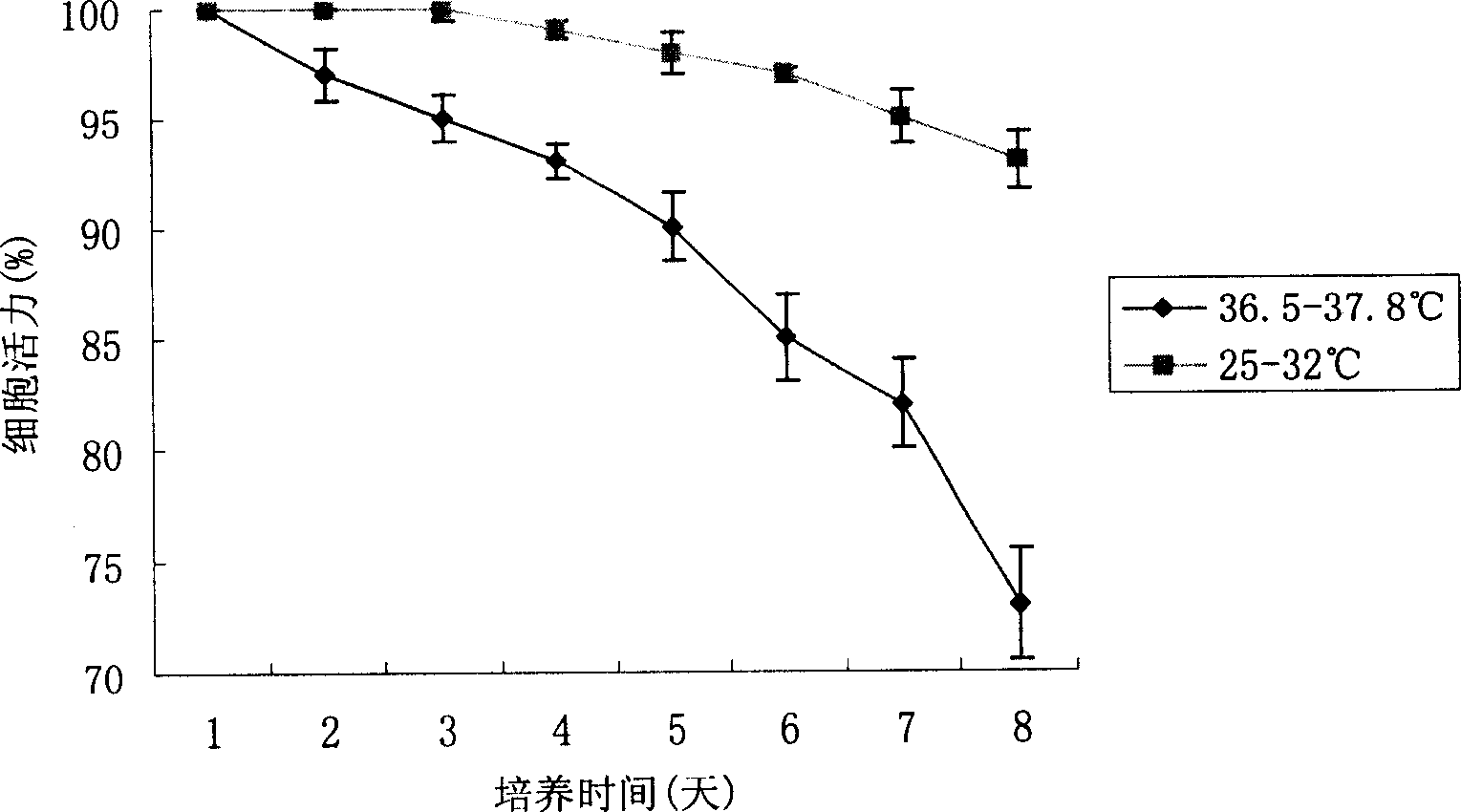
[0018] Use a two-stage culture method to improve the fusion protein (HFI) in recombinant CHO cells
[0019] Expression
[0020] 1. Material:
[0021] A CHO cell line stably expressing the fusion protein HFI (Shi, M., Xie, Z., Feng, J., Sun, Y., Yu, M., Shen, B., Guo, N., 2003. A recombinant anti -ErbB2, scFv-Fc-IL-2 fusion protein retains antigenspecificity and cytokine function. Biotechnol. Lett. 25, 815-819); DMEM medium and protein-free medium HyQSFM4CHO are produced by HyClone; goat anti-human IgG, horseradish Peroxidase-labeled goat anti-human IgG was purchased from Beijing Zhongshan Biotechnology Company.
[0022] 2. Methods and results
[0023] The recombinant CHO cell line stably expressing the fusion protein anti-erbB2-ScFv-Fc-IL-2 was divided into 2×10 6 / Number of bottles, inoculated on the bottom area of 25cm 2 There are a total of 16 flasks in the culture flasks. The applied medium is DMEM+10% FBS. After the cells are cultured at 36.5°C-37.8°C t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com