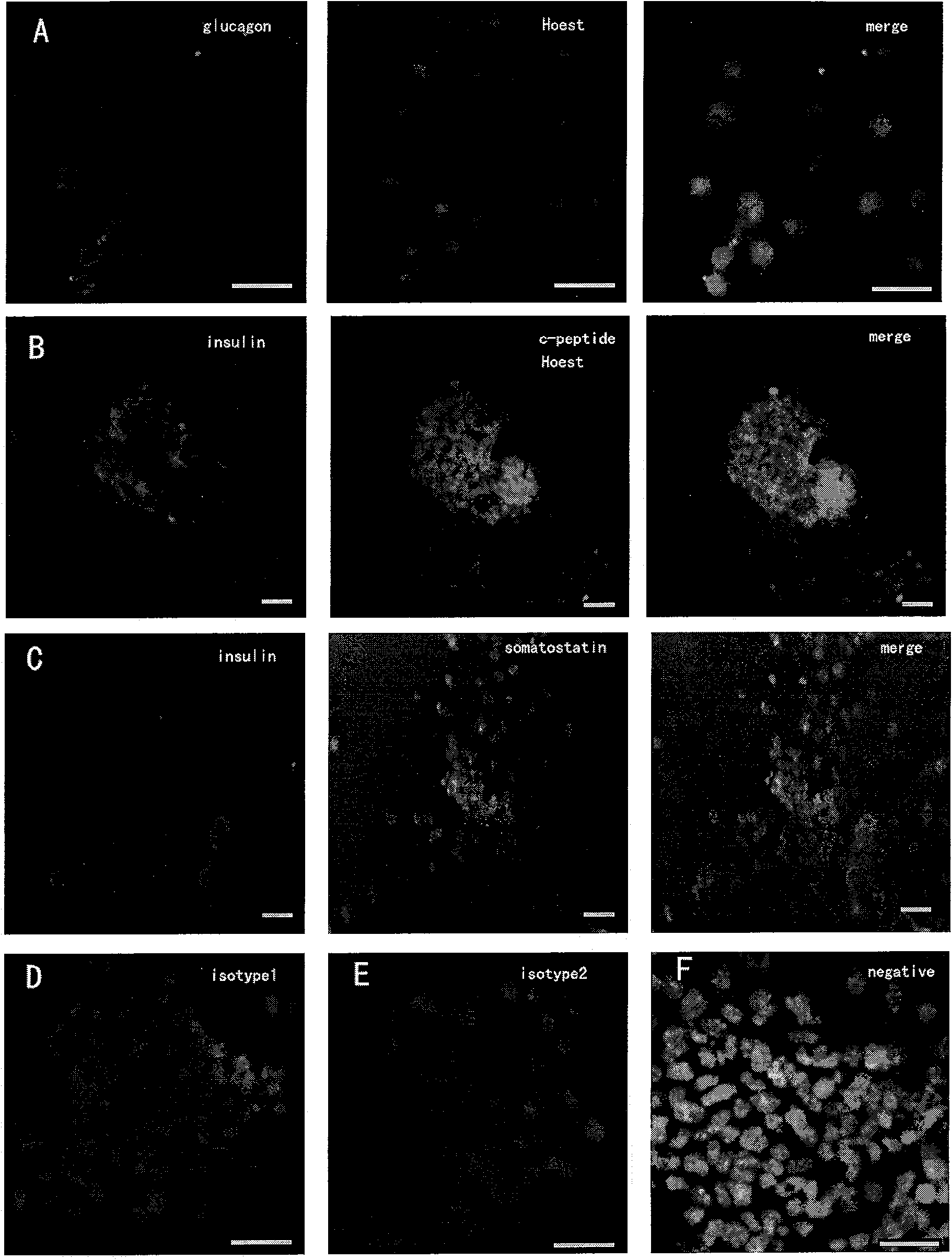
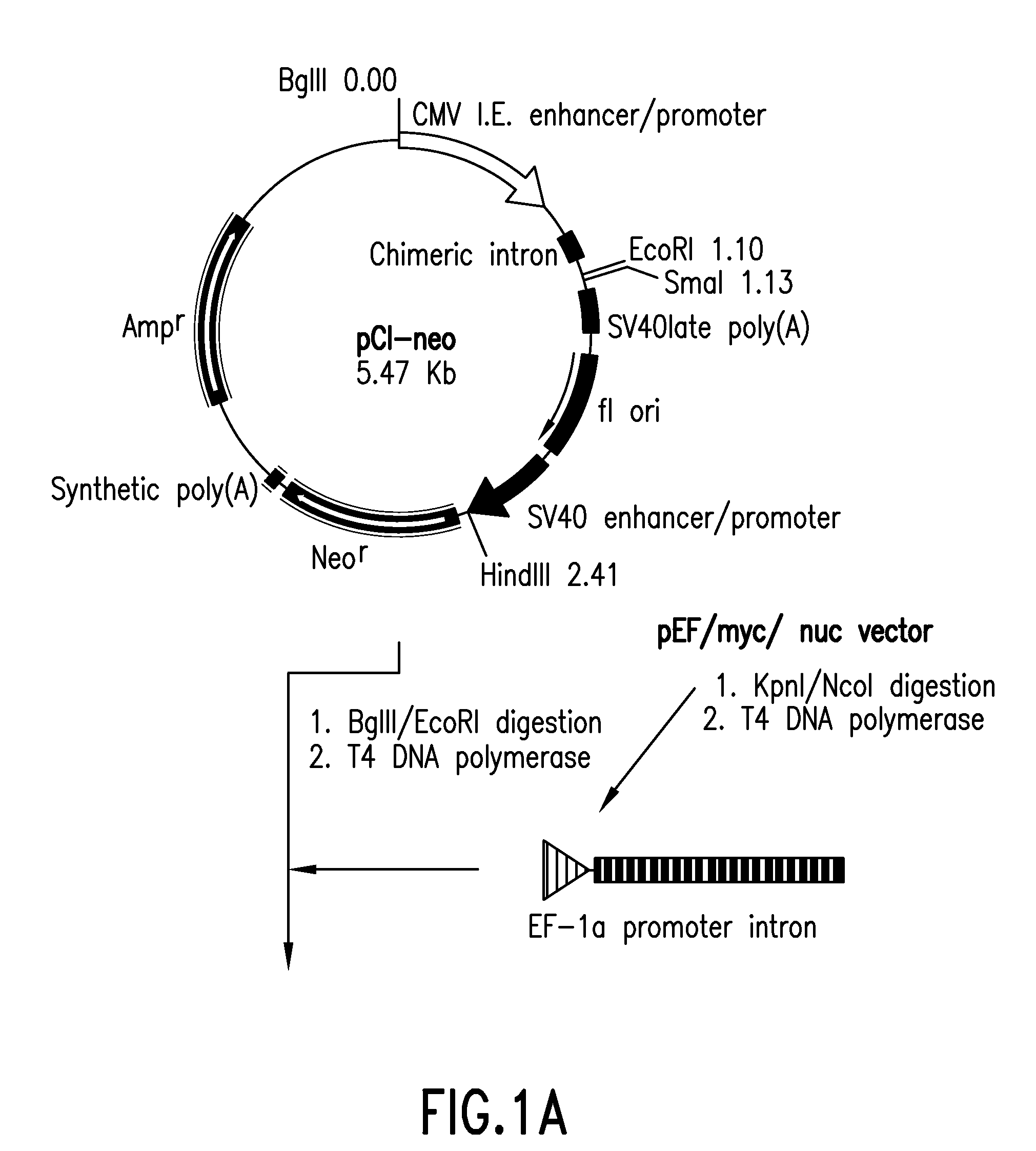
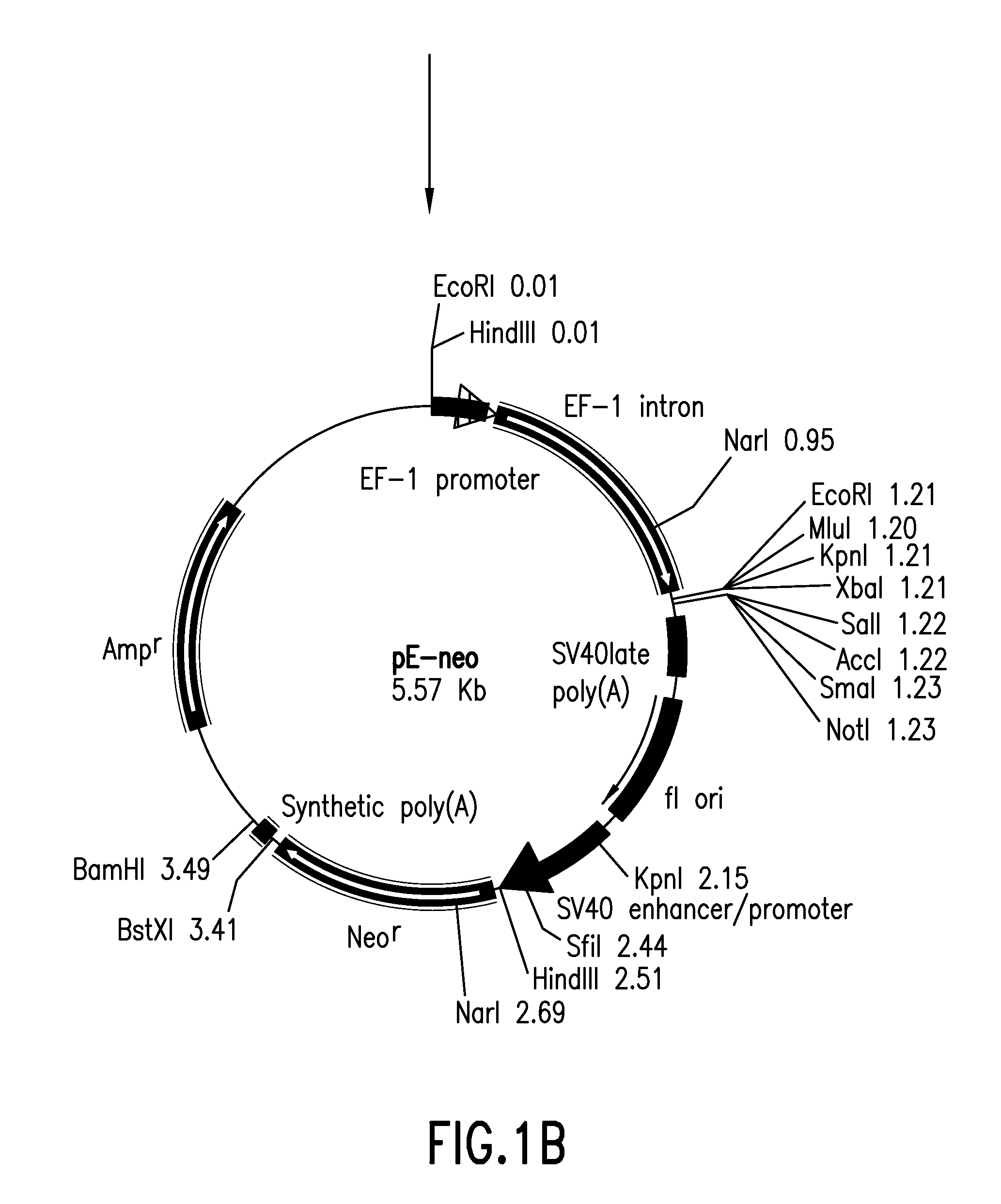
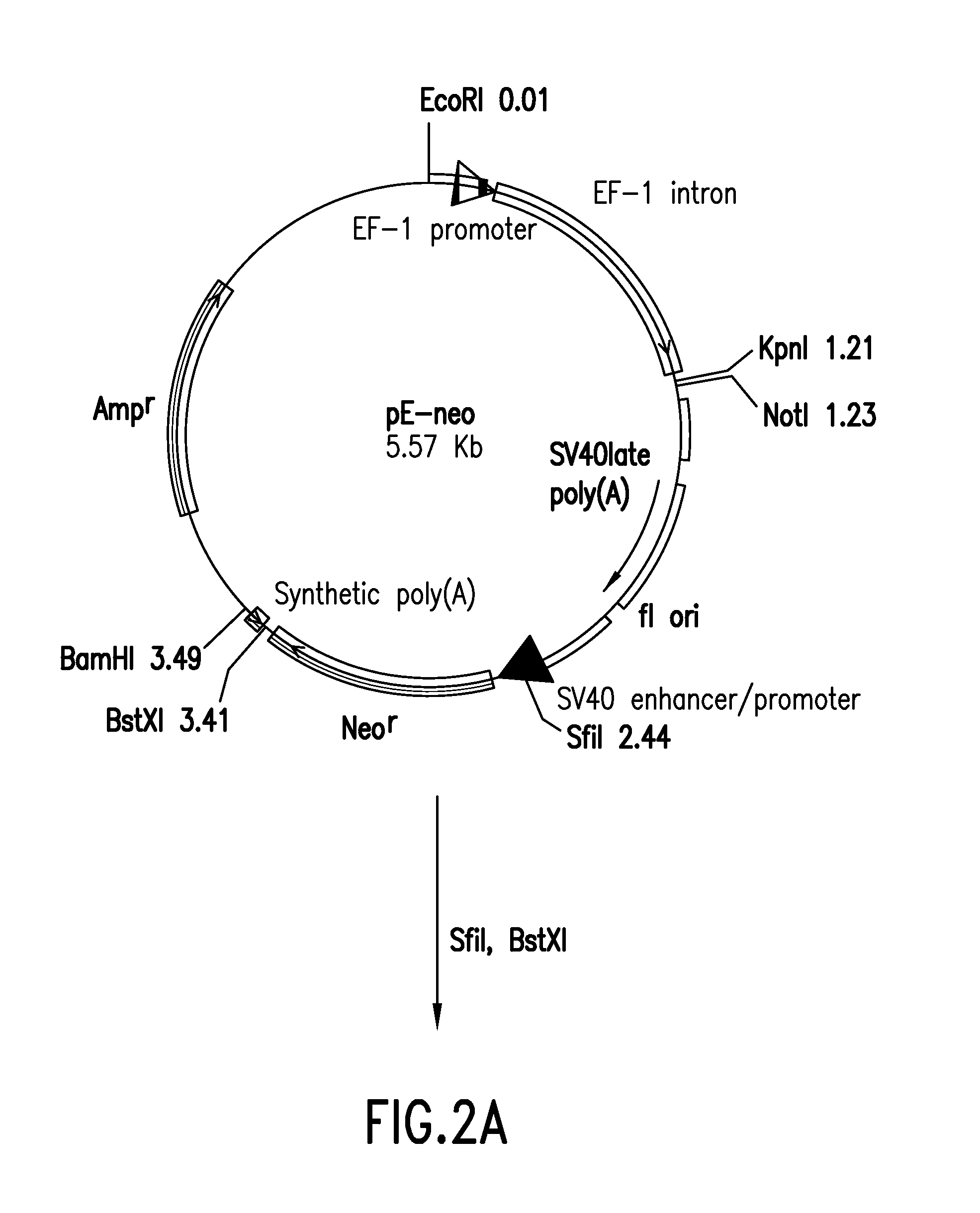
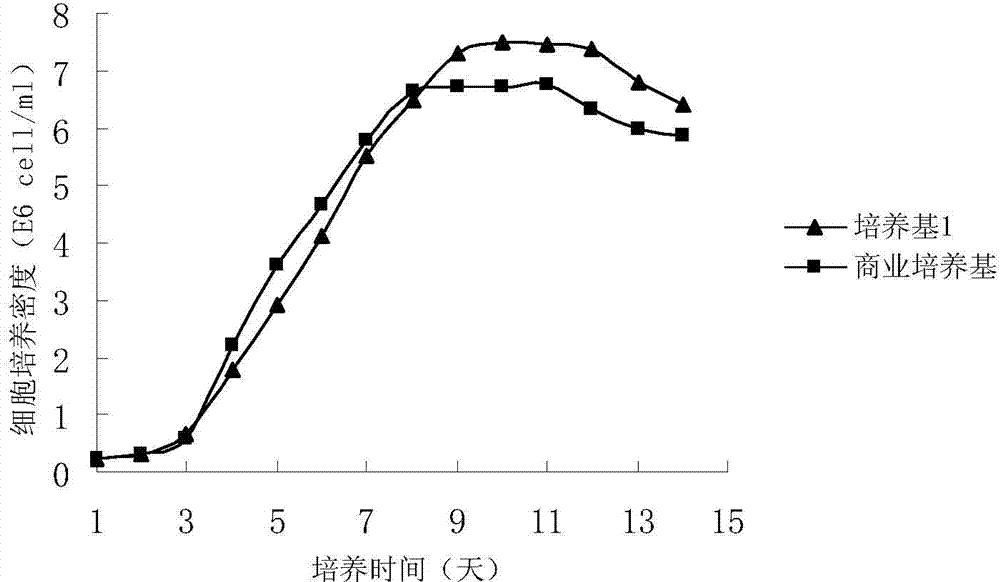
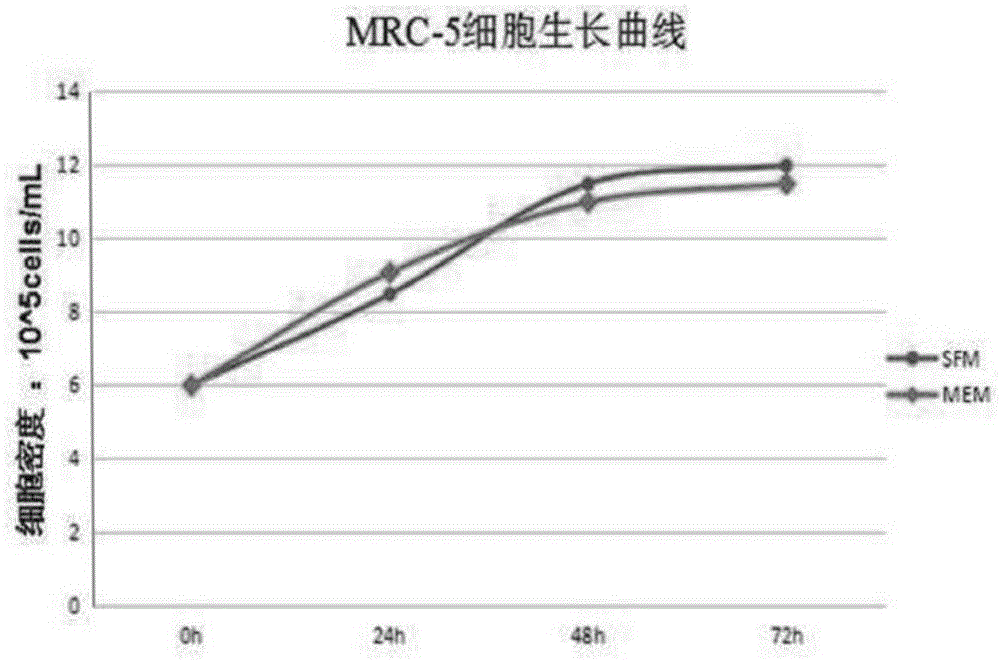
Patents
Literature
173 results about "Serum free medium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for producing polypeptide
InactiveUS7504256B1Efficient productionImprove metabolic efficiencyFused cellsImmunoglobulinsSerum igeSerum free media
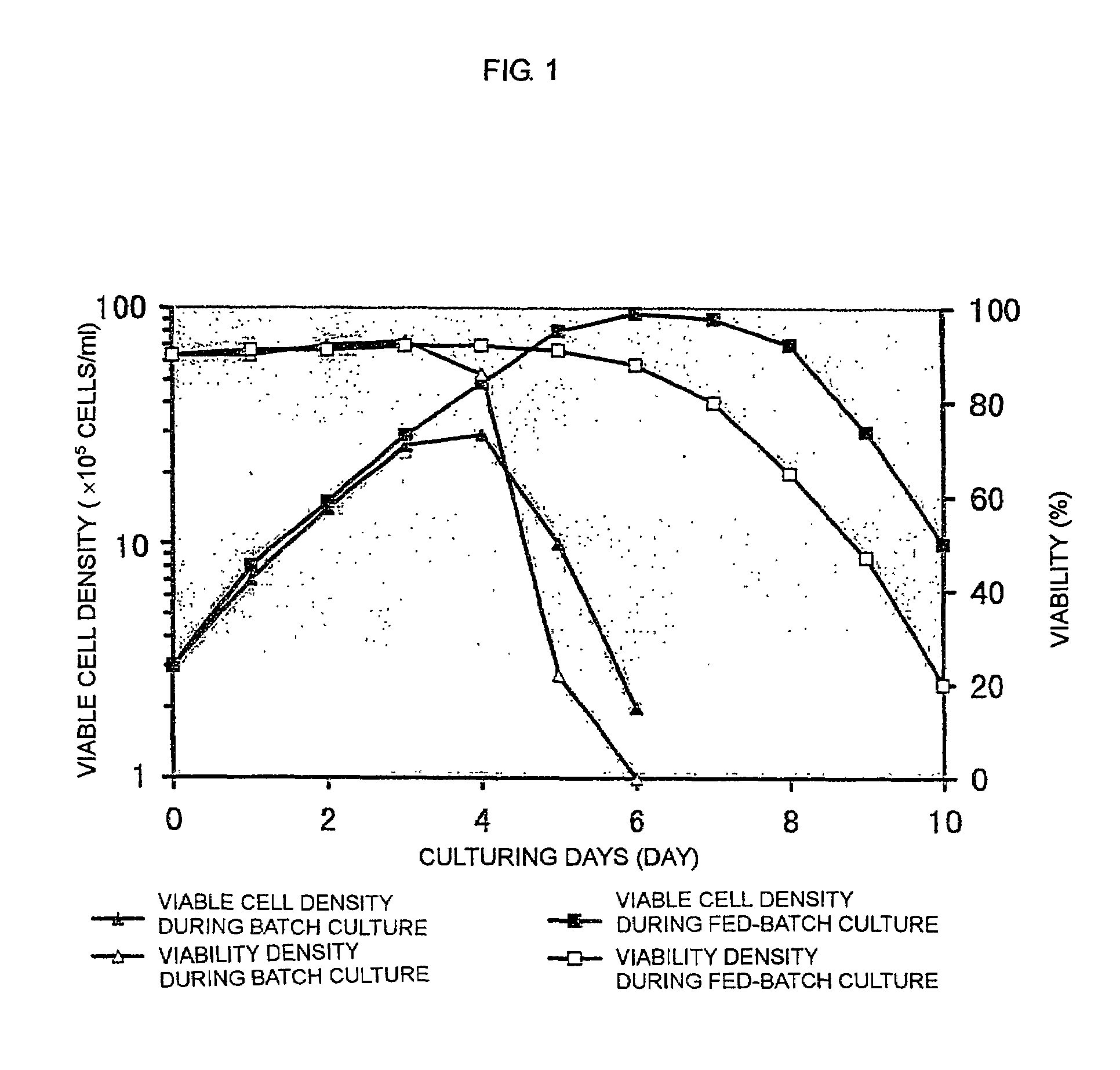
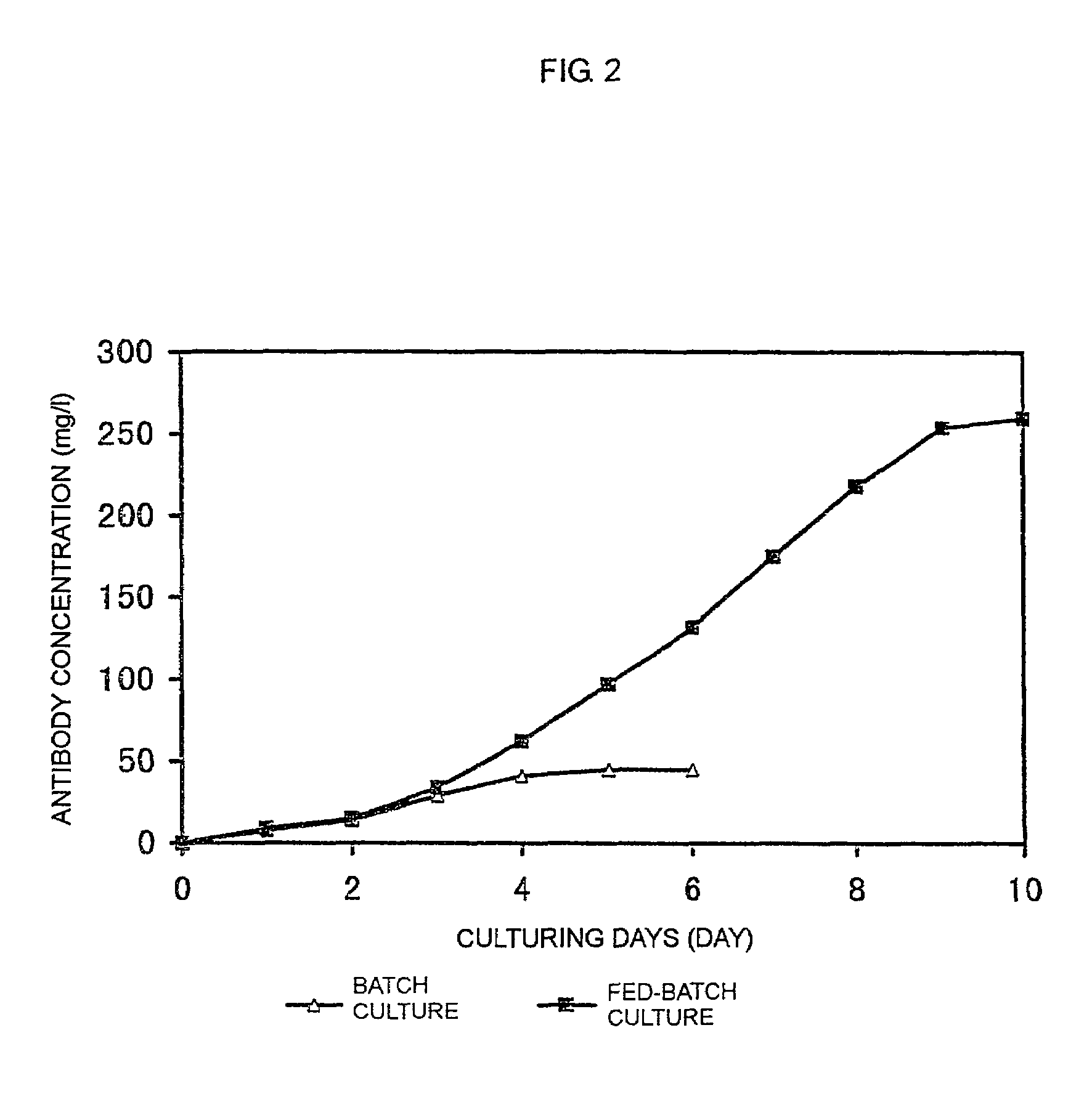
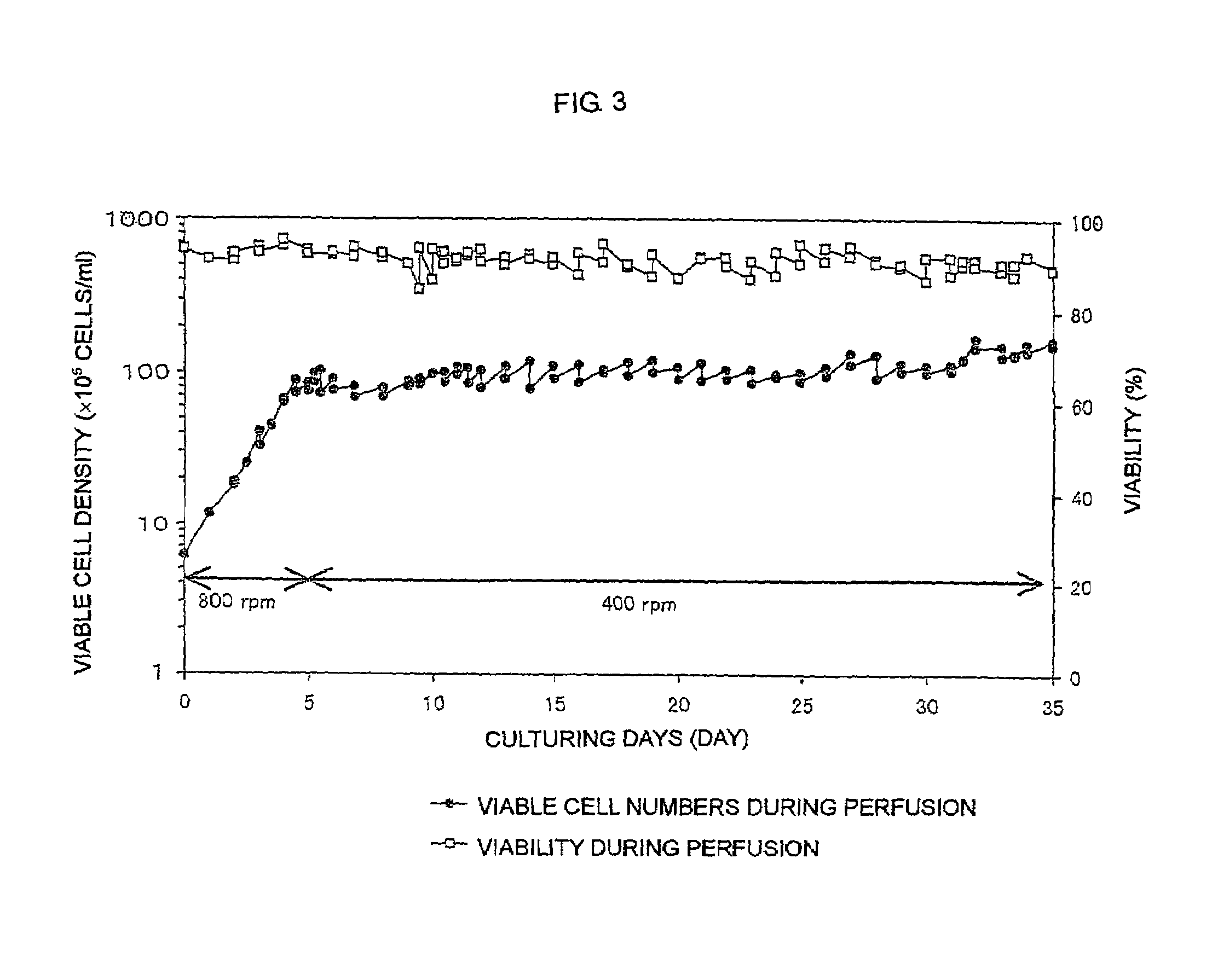
The present invention relates to a process for producing a desired polypeptide using rat cells. Specifically, the present invention relates to a process for producing the polypeptide which comprises culturing rat cells such as YB2 / 3HL.P2.G11.16Ag.20 (hereinafter referred to as YB2 / 0), preferably rat cells to which a recombinant DNA comprising DNA encoding a desired polypeptide such as an immunologically functional molecule is introduced, in a medium which does not contain serum (hereinafter referred to as a serum-free medium). Among the desired polypeptides obtained by the process of the present invention, an antibody obtained by using a transformant of YB2 / 0 has a high antibody-dependent cell-mediated cytotoxic activity (hereinafter sometimes referred to as ADCC activity) and is useful as a pharmaceutical agent.
Owner:KYOWA HAKKO KIRIN CO LTD
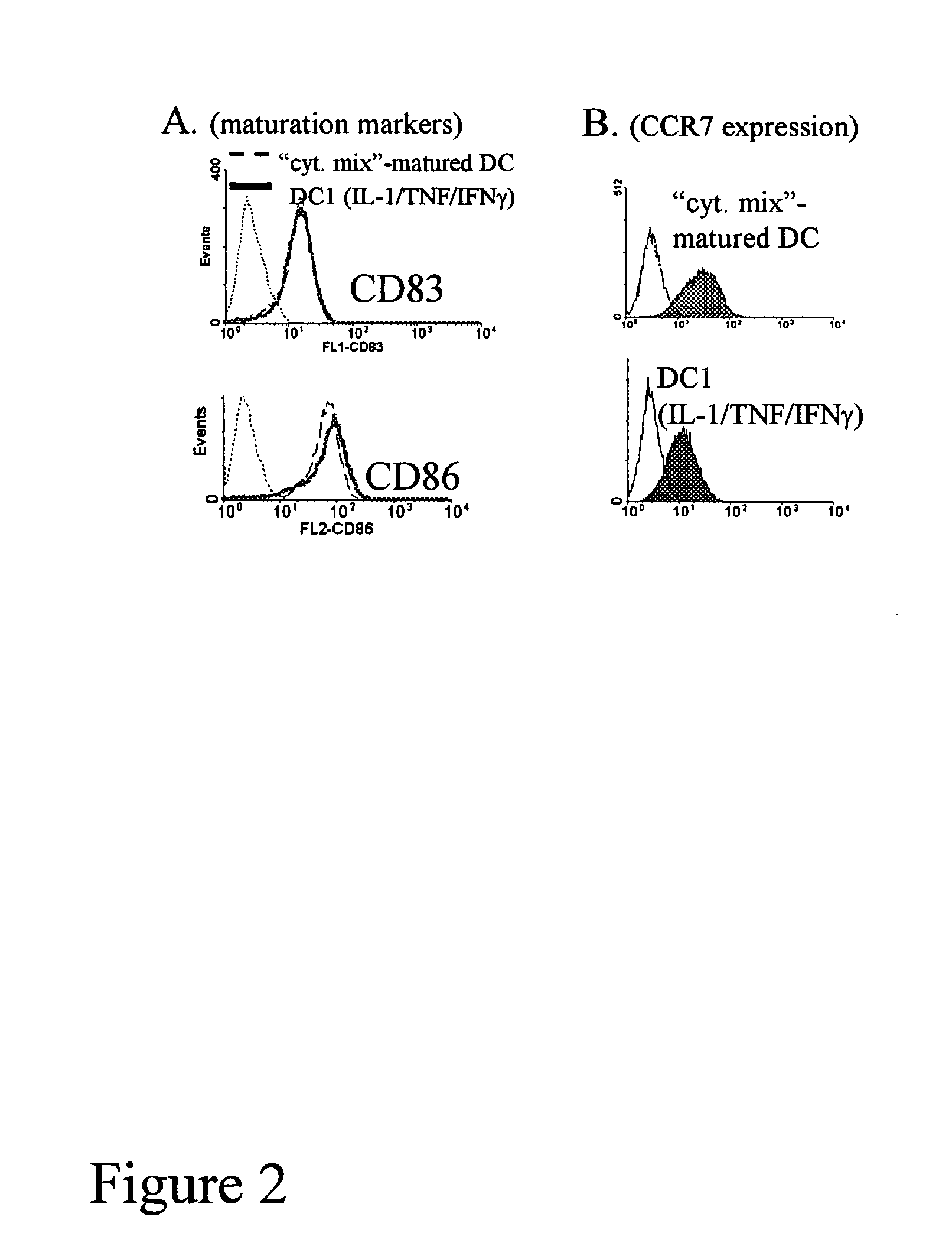
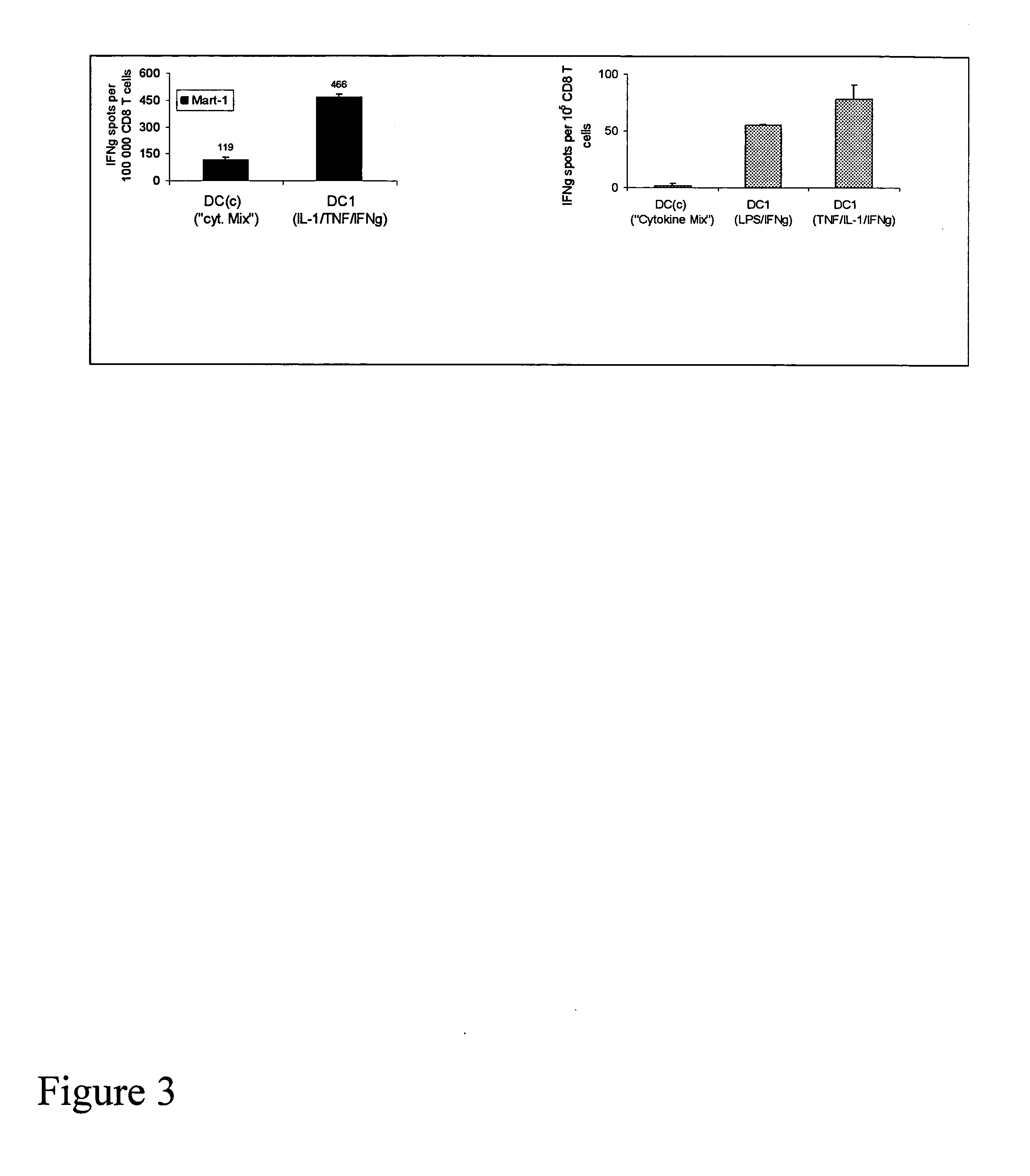
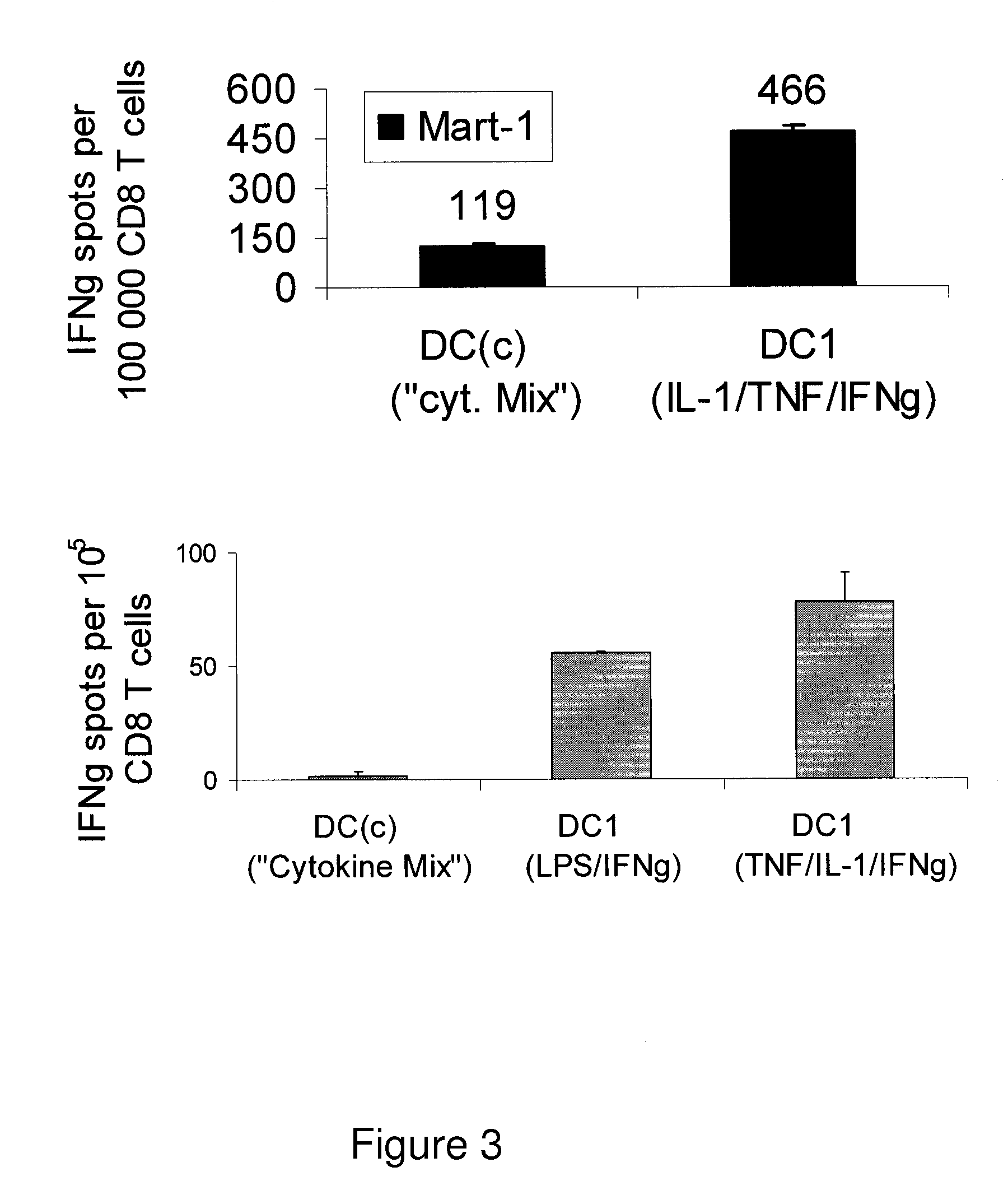
Mature type-1 polarized dendritic cells with enhanced IL-12 production and methods of serum-free production and use
InactiveUS20050003533A1Superior controlSuperiorArtificial cell constructsBlood/immune system cellsBlood serumSerum free
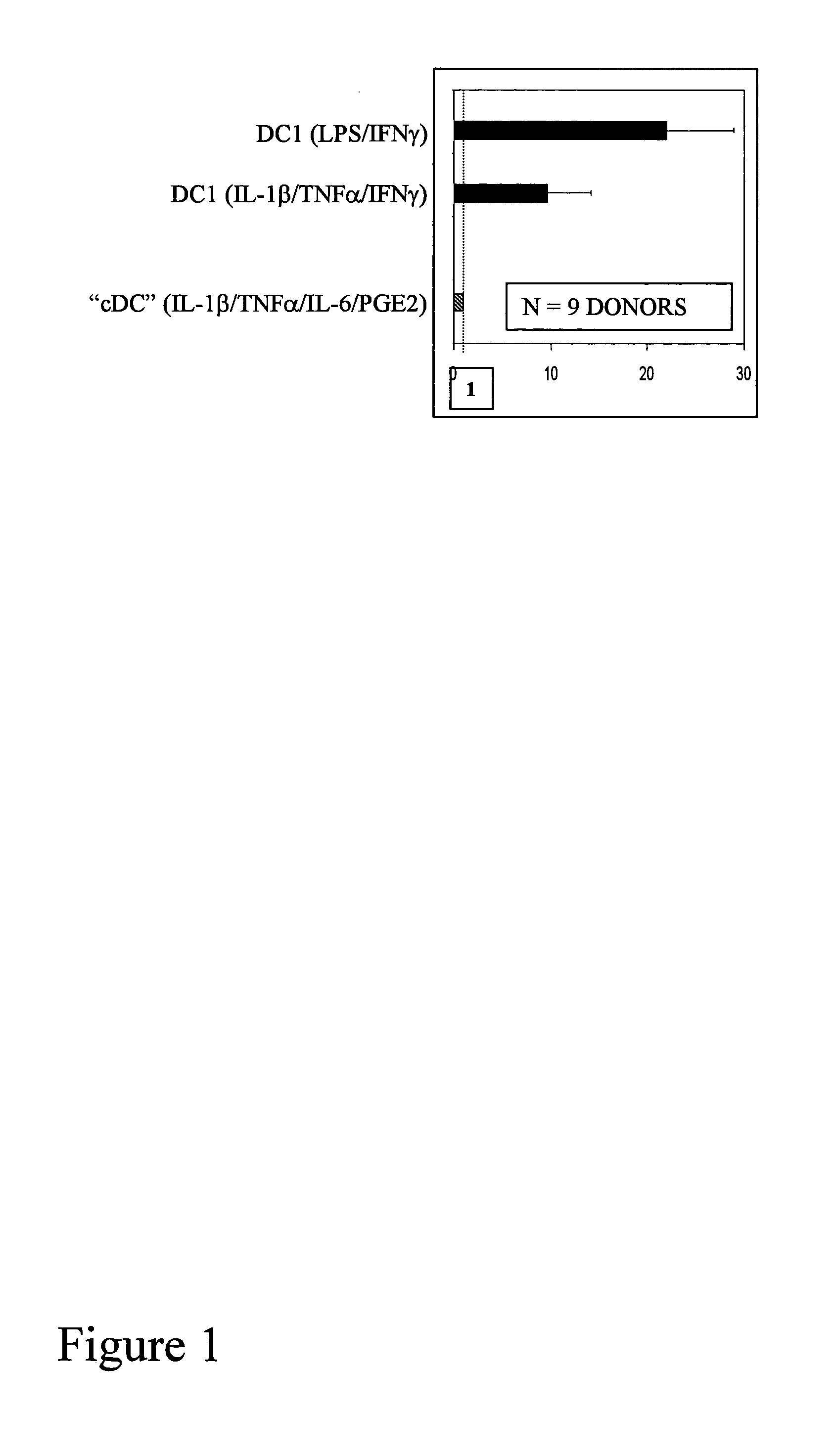
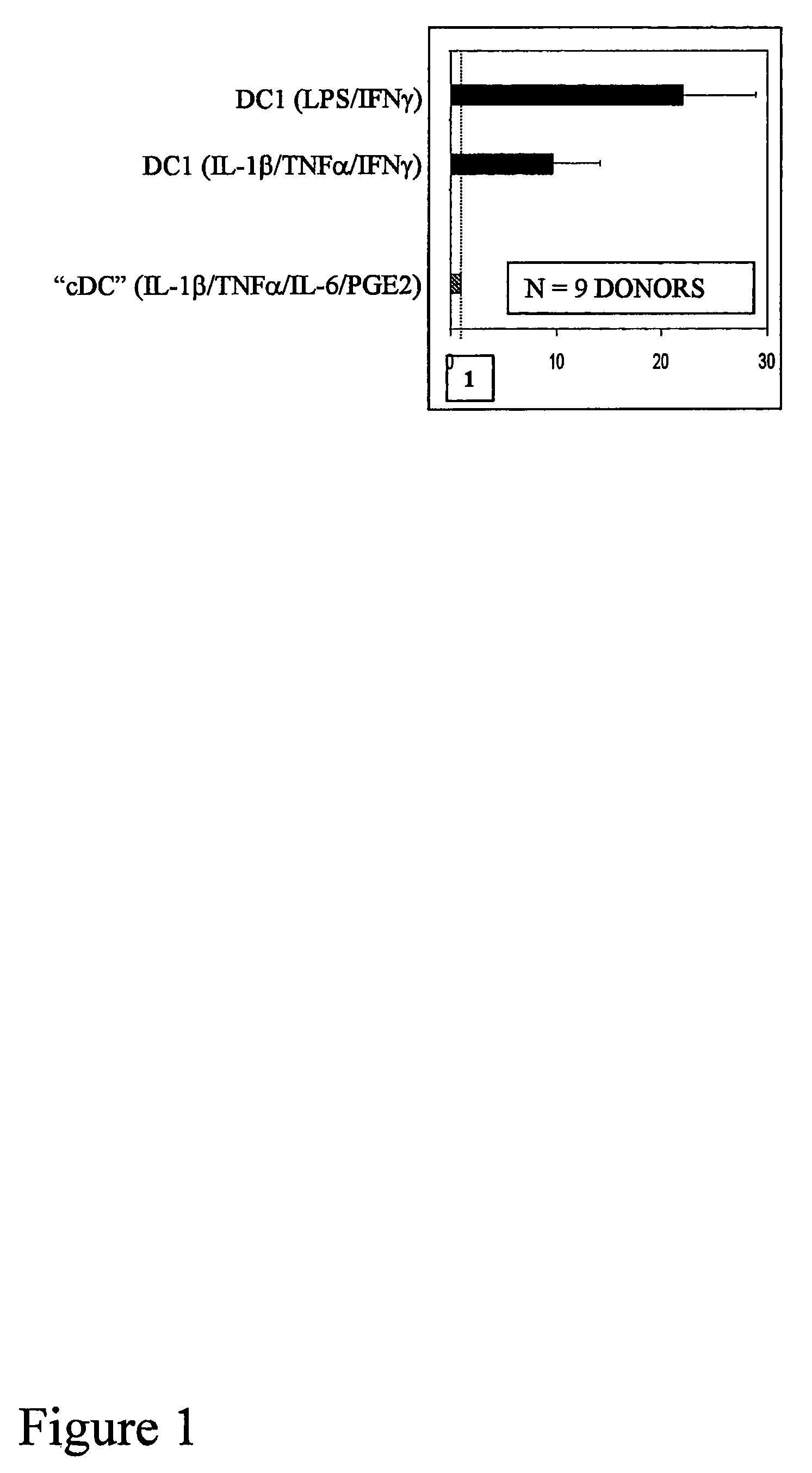
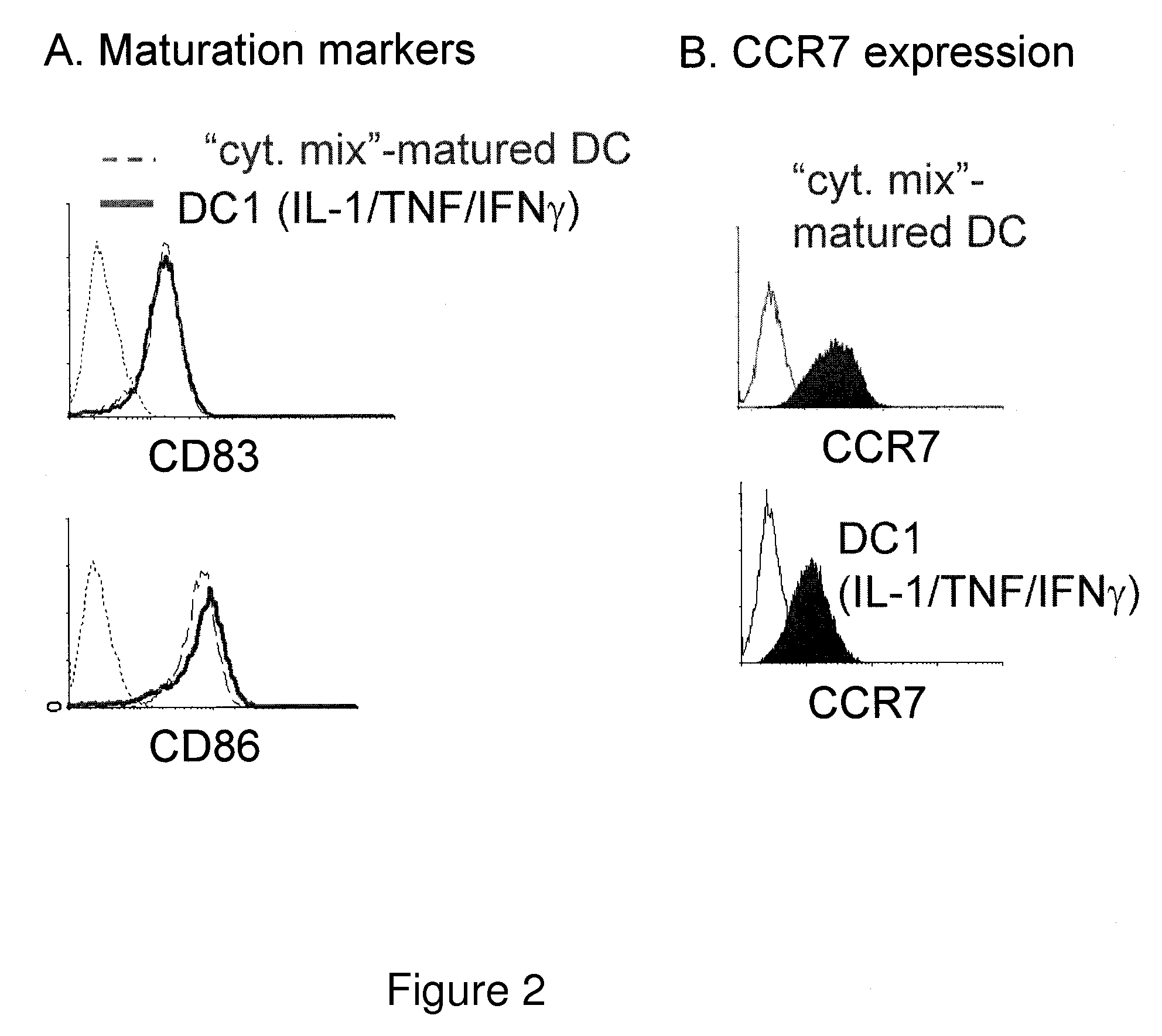
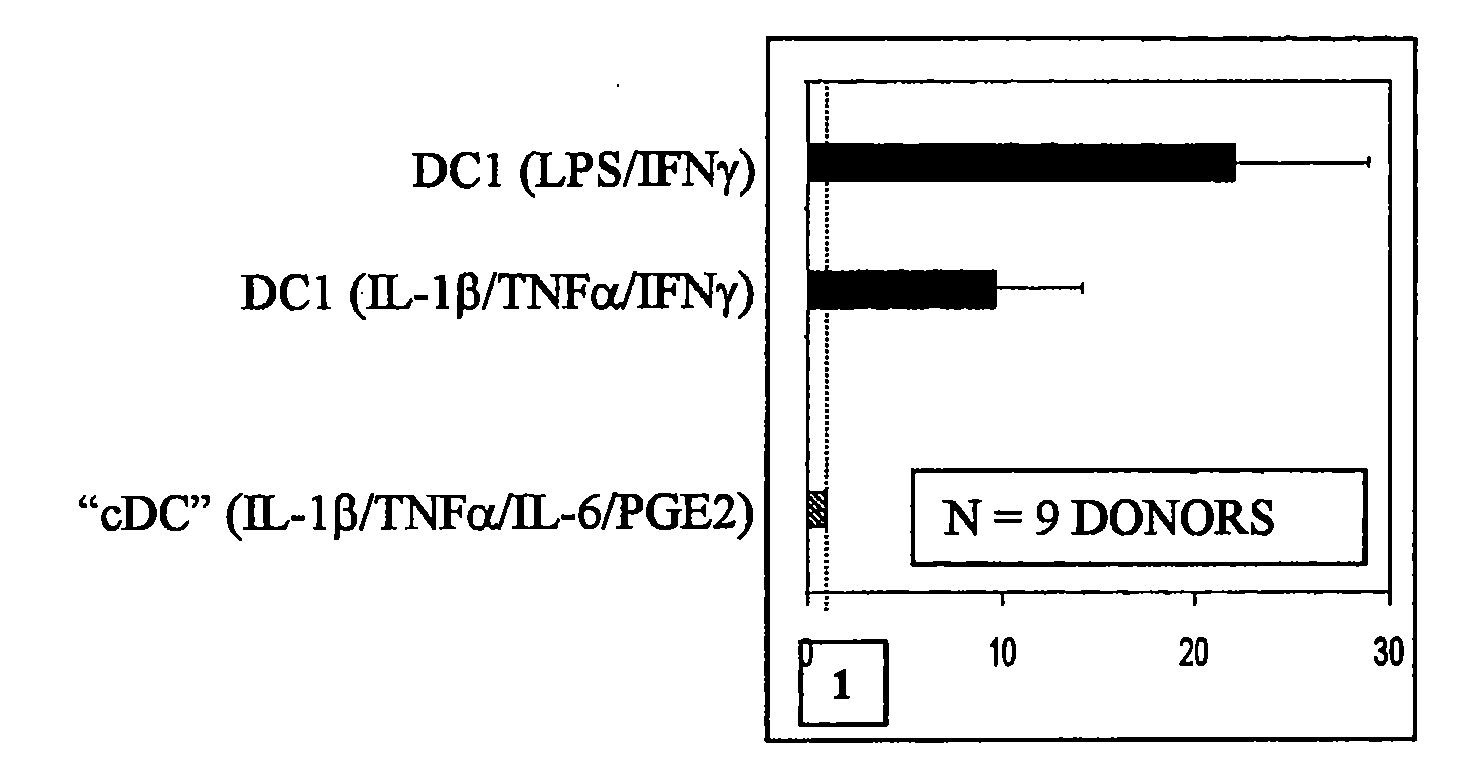
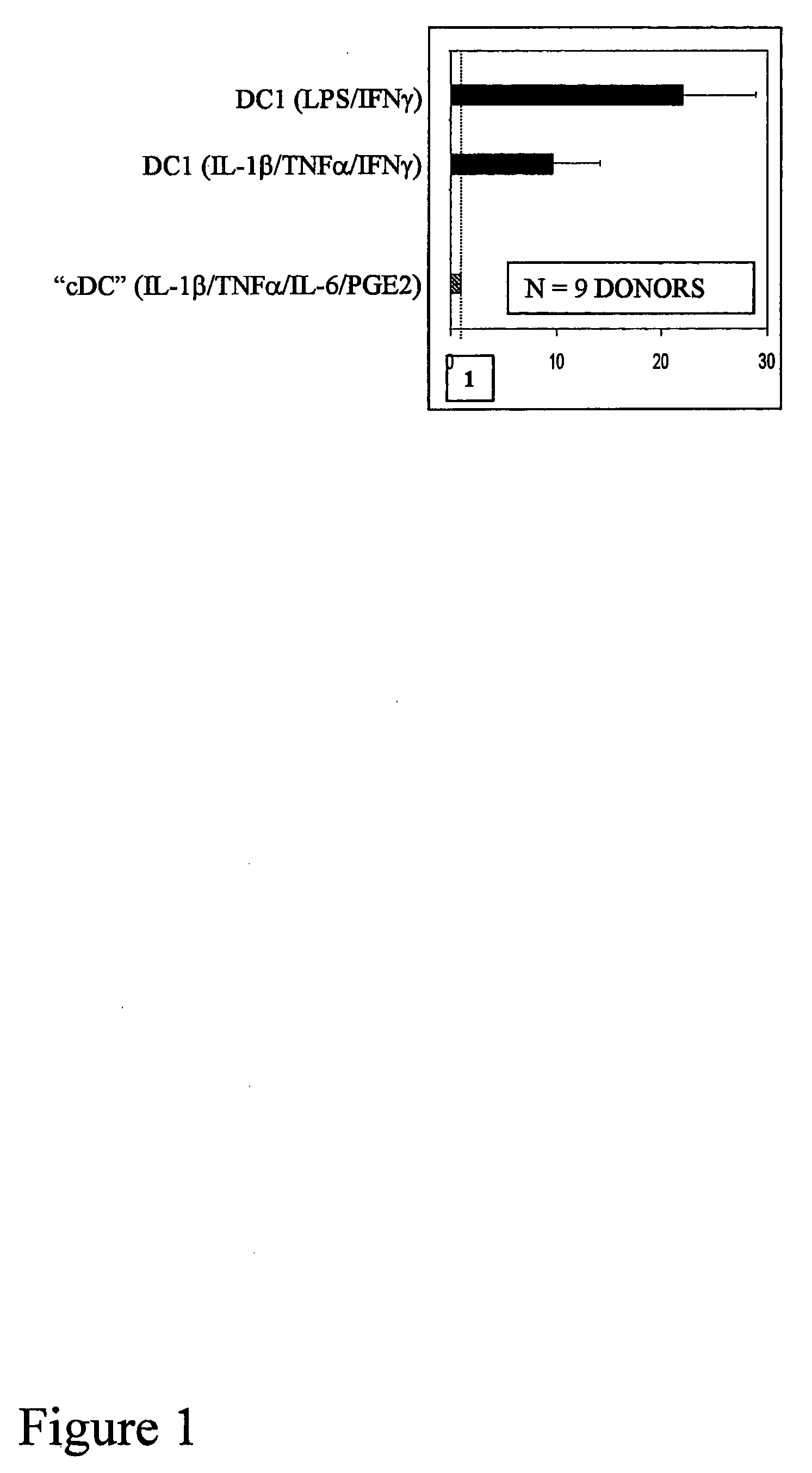
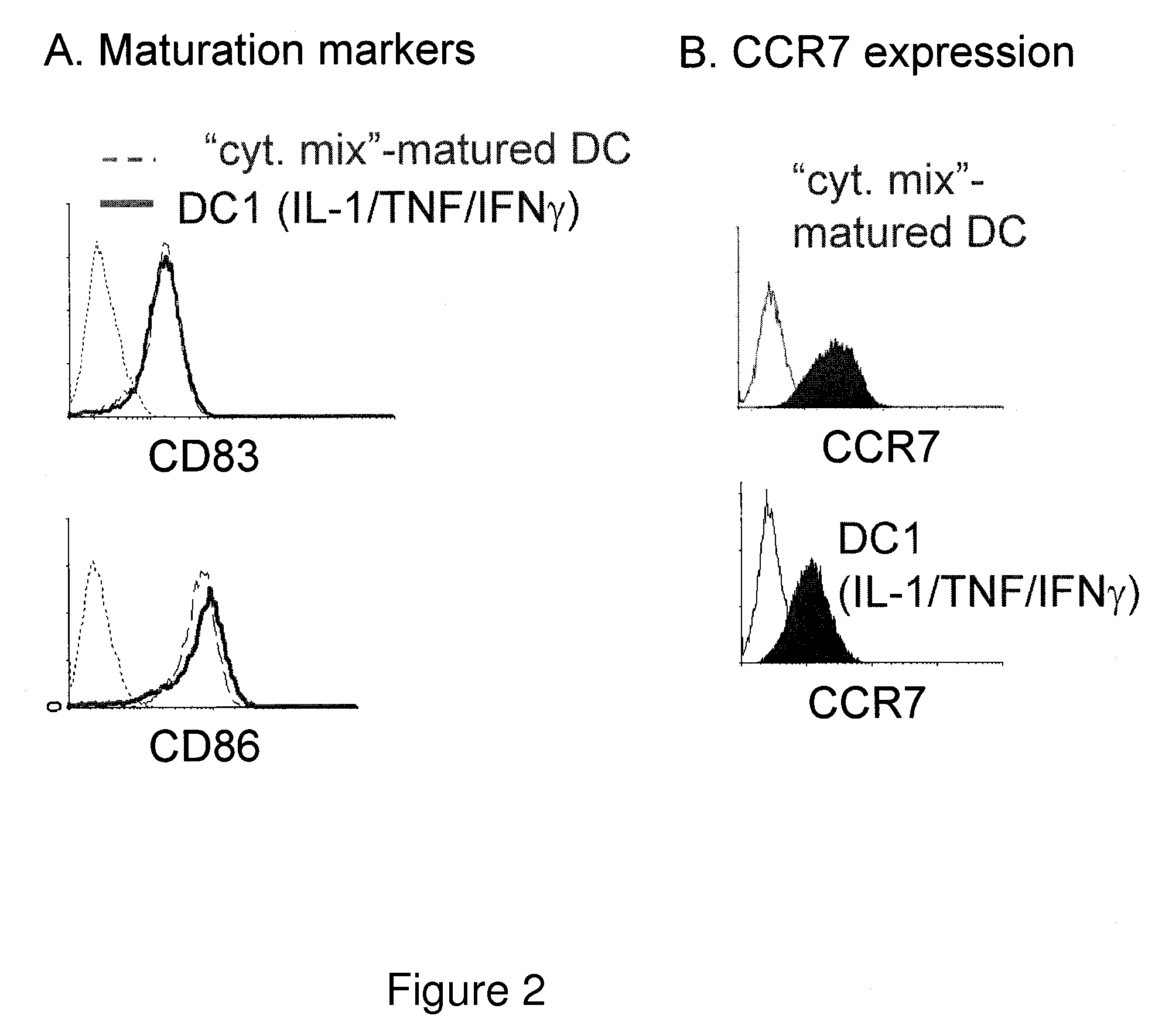
The present invention discloses novel dendritic cell maturation-inducing cytokine cocktails, and methods for inducting type-1 polarized dendritic cells in serum-free conditions which enhance the desirable properties of DC1s generated in serum-supplemented cultures. The invention further discloses methods and systems using IFNγ and other ligands of the IFNγ receptor, in combination with IFNα (or other type I interferons), poly I:C, and other IFNα (and IFNβ) inducers to enhance the IL-12-producing properties of dendritic cells. More specifically, the present invention discloses type-1 polarized dendritic cells that have a unique combination of a fully-mature status and an elevated, instead of “exhausted”, ability to produce IL-12p70. allows for the generation of fully-mature DC1s in serum-free AIM-V medium. The invention discloses systems that use the foregoing products and methods to facilitate the clinical application of DC1-based vaccines and the identification of novel factors involved in the induction of Th1 and CTL responses by DC1.
Owner:KALINSKI PAWEL
Serum-free medium for culturing placenta mesenchymal stem cells
ActiveCN103805562AIncrease growth rateMaintain stem cell propertiesSkeletal/connective tissue cellsFibroblast growth factor receptor 2Cell culture media
The invention discloses a serum-free medium for culturing placenta mesenchymal stem cells. The serum-free medium takes a DMEM (Dulbecco Modified Eagle Medium) culture solution as a basis and also contains a fibroblast growth factor receptor 2, growth hormone, insulin, transferrin, glutathione, BMP-4, L-glutamine, sodium pyruvate, non-essential amino acids and beta-mercaptoethanol. According to various serum-free media provided by the invention, growth and proliferation of the placenta mesenchymal stem cells in a serum-free medium system can be effectively promoted, the placenta mesenchymal stem cells have higher growth and proliferation rate in the serum-free medium system compared with a serum cell culture medium, the characteristics of the stem cells are preserved, the serum-free medium has multiple differential potentials, and the stem cells can be directionally induced into fat cells and osteoblasts.
Owner:章毅 +10
Gene transfer method with the use of serum-free medium
InactiveUS6287864B1Effective maintenanceImprove efficiencyBiocideMicroorganismsVirus-RetrovirusGene transfer
A method for transferring a gene into target cells by a retrovirus with the use of serum-free medium. This method comprises infecting target cells with a retrovirus in serum-free medium optionally containing low-density lipoprotein and / or cytokines in the presence of a functional substance such as fibronectin in an amount effective in elevating the gene transfer efficiency of the retrovirus into the target cells by co-localizing the retrovirus and the target cells.
Owner:TAKARA HOLDINGS
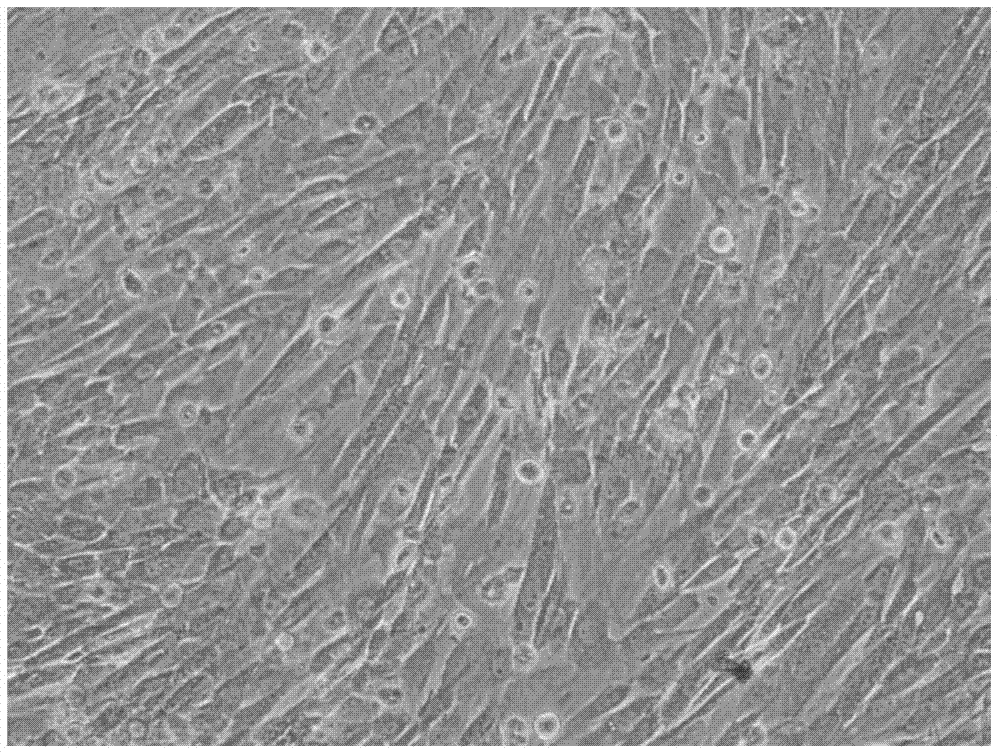
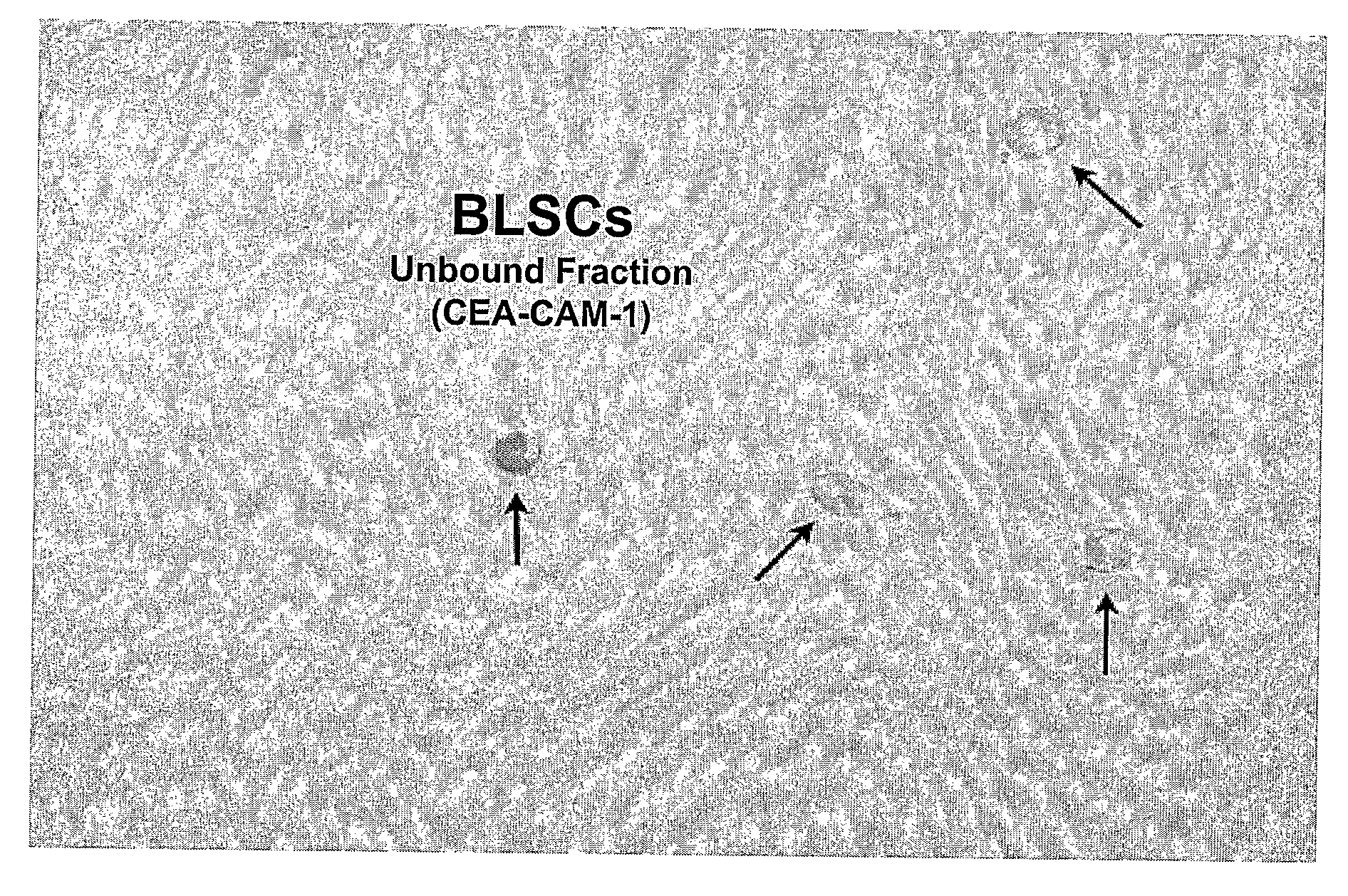
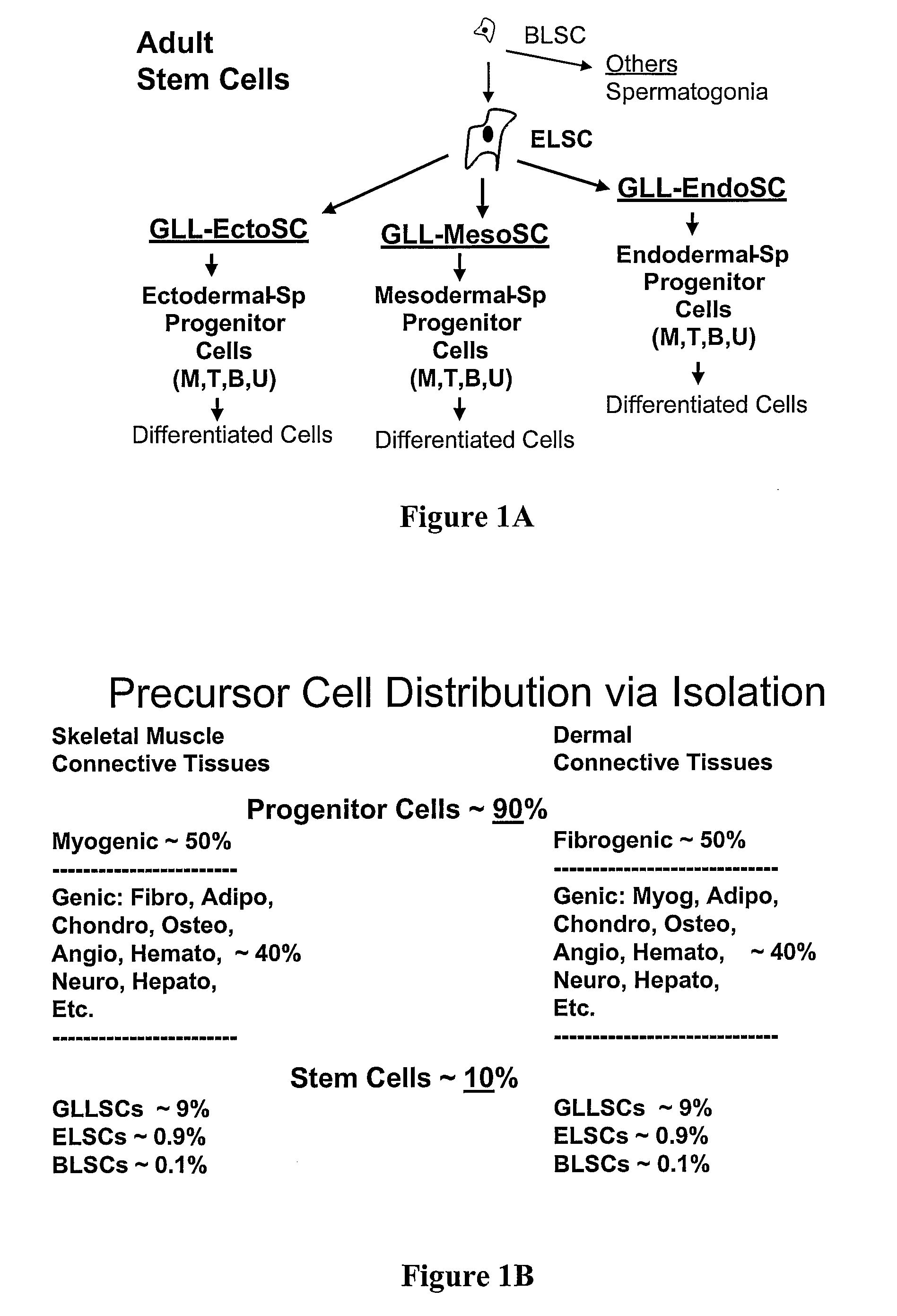
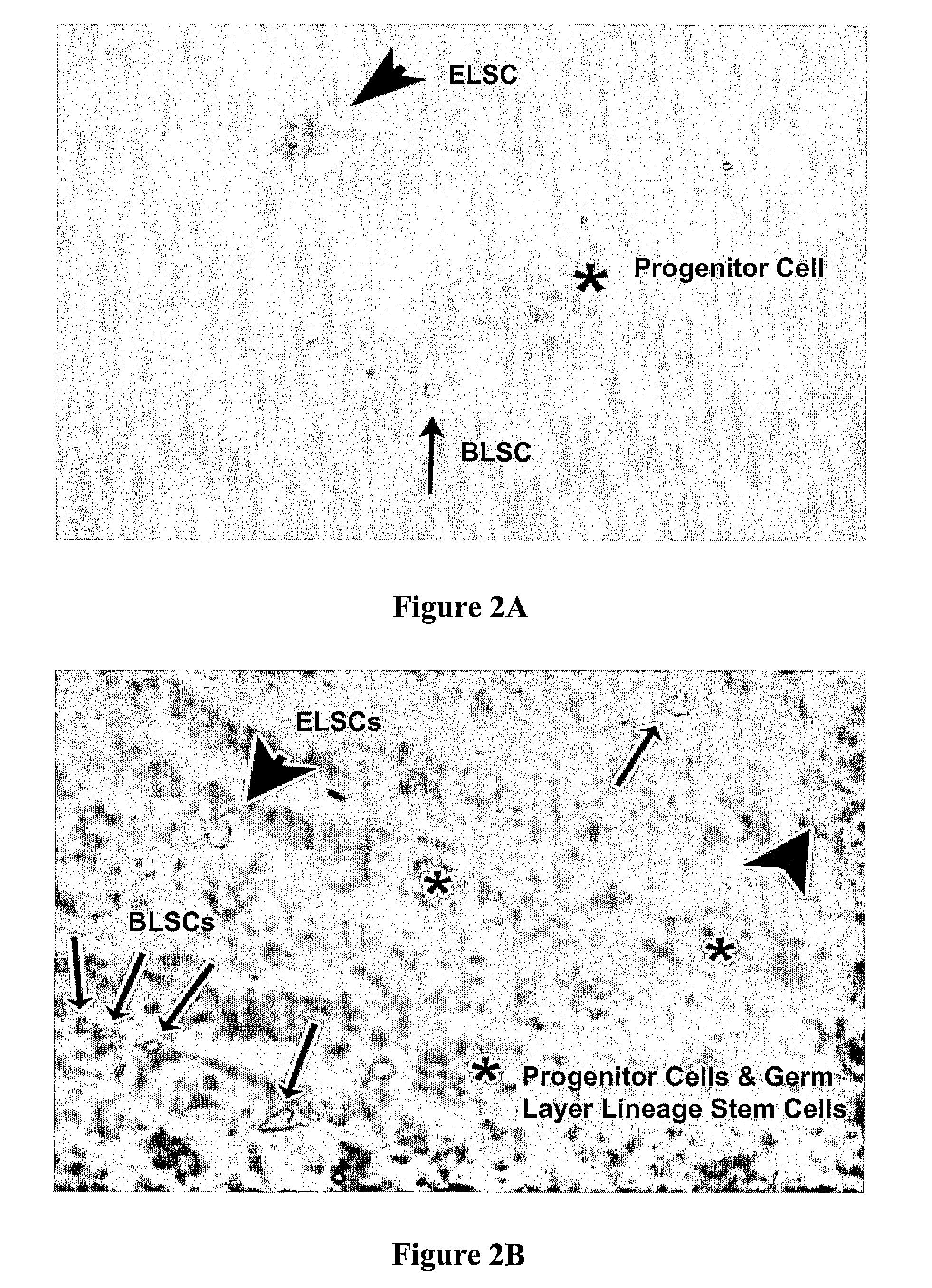
Non-Embryonic Totipotent Blastomere-Like Stem Cells And Methods Therefor
Non-embryonic blastomere-like totipotent stem cells are disclosed. Most preferably, such cells are obtained from various tissues of postnatal mammals (e.g., using tissue biopsied from the mammal), are smaller than 1 μm, have normal karyotype, and do not spontaneously differentiate in serum-free medium without differentiation inhibitors. These non-embryonic blastomere-like totipotent stem cells typically express CD66e, CEA-CAM-1 and telomerase, but do not typically express CD10, SSEA-1, SSEA-3, and SSEA-4. Such blastomere-like totipotent cells can be differentiated into ectodermal, mesodermal, or endodermal tissues, including placental tissues and germ cells. Moreover, when implanted into a mammal, such cells will not be teratogenic.
Owner:MORAGA BIOTECH CORP
Serum-free medium for full suspension cultivation of BHK (Baby Hamster Kidney)-21 cell
ActiveCN103555658AHigh densityClear ingredientsArtificial cell constructsVertebrate cellsBiotechnologyInorganic salts
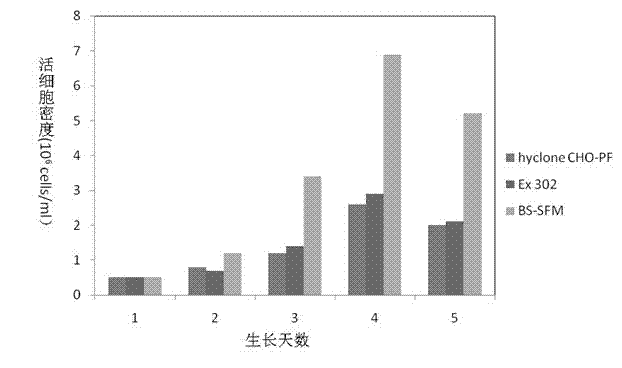
The invention discloses a serum-free medium for full suspension cultivation of a BHK (Baby Hamster Kidney)-21 cell. The serum-free medium for full suspension cultivation of the BHK-21 cell comprises an amino acid part, a vitamin part, an inorganic salt part and a part of other additives. The medium disclosed by the invention has the beneficial effects that the serum-free medium for full suspension cultivation of the BHK-21 cell is high in culture cell density, clear in component and free from animal serum, facilitates purification of downstream products, and improves the product quality. The medium is convenient to prepare and use, and is applicable to production of biological products on a large scale.
Owner:无锡市赛尔百灵生物技术有限公司
Culturing method of mesenchymal stem cells of menstrual blood
ActiveCN104560871APollution prevention methodsReduced Chances of ContaminationSkeletal/connective tissue cellsHydroxyethyl starchSurface marker
The invention provides a method for preparing mesenchymal stem cells of menstrual blood. The method comprises steps as follows: female menstrual blood is collected and taken as a raw material and is subjected to sterile processing; stem cells in the menstrual blood are separated; the mesenchymal stem cells of the menstrual blood are cultured; the mesenchymal stem cells of the menstrual blood are cryopreserved; and the mesenchymal stem cells of the menstrual blood are recovered. The method has the technical characteristics as follows: the female menstrual blood is collected and stored in a preservative fluid; by means of a bacterial pollution prevention method, the pollution probability is reduced from a collection source, a collecting cup is repeatedly washed by sterile water, and the pollution probability is effectively reduced; with the adoption of a differential centrifugation method, bacteria in the menstrual blood are removed as far as possible; a sample is repeatedly separated by HES (hydroxyethyl starch), and the maximum quantity of mesenchymal stem cells of the menstrual blood can be obtained; a serum-free medium is utilized for culturing, components of animal origin are reduced, the cell performance is stable, the in-vitro long-term culturing process of the mesenchymal stem cells of the menstrual blood can be kept, and cellular morphology, multiplication capacity, MSC (mesenchymal stem cell) surface marker expression, differentiation capacity and the like are maintained. The method is simple, practical and convenient to operate, the maximum quantity of required stem cells can be obtained, and the stem cells are successfully cultured.
Owner:SHENZHEN BEIKE BIOTECH
Serum-free medium suitable for large-scale production of influenza vaccines
ActiveCN103045533AEasy to separateEasy to purifyArtificial cell constructsVertebrate cellsBiotechnologyNutrition
The invention relates to the technical field of culture medium development and research of biotechnology and discloses a serum-free medium suitable for large-scale production of influenza vaccines. The serum-free medium comprises 23 basic metabolism nutrients, two nucleic acid compounds, 6 vitamins, 9 inorganic salt compounds, a shear force protective agent, two acidity and alkalinity buffer agents, an acidity and alkalinity indicator, 10 virus reproduction promoters and three additives. The conventional preparation method of the serum-free medium comprises the following steps of: dissolving the components in ultrapure water without a heat source so as to prepare the serum-free medium. The using method is a conventional method. The serum-free medium has the beneficial effects that the serum is not contained, the total protein content is lower than 10mg per liter, separation and purification of the product are promoted, and the serum-free medium is suitable for production of influenza vaccines, supports the normal growth and long-term continuous cell culture of animal cells and can be used without adaption; and moreover, because the components are clear, the serum-free medium is convenient to prepare, controllable in cost and suitable for large-scale production of the influenza vaccines.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing purified foot-and-mouth disease vaccine
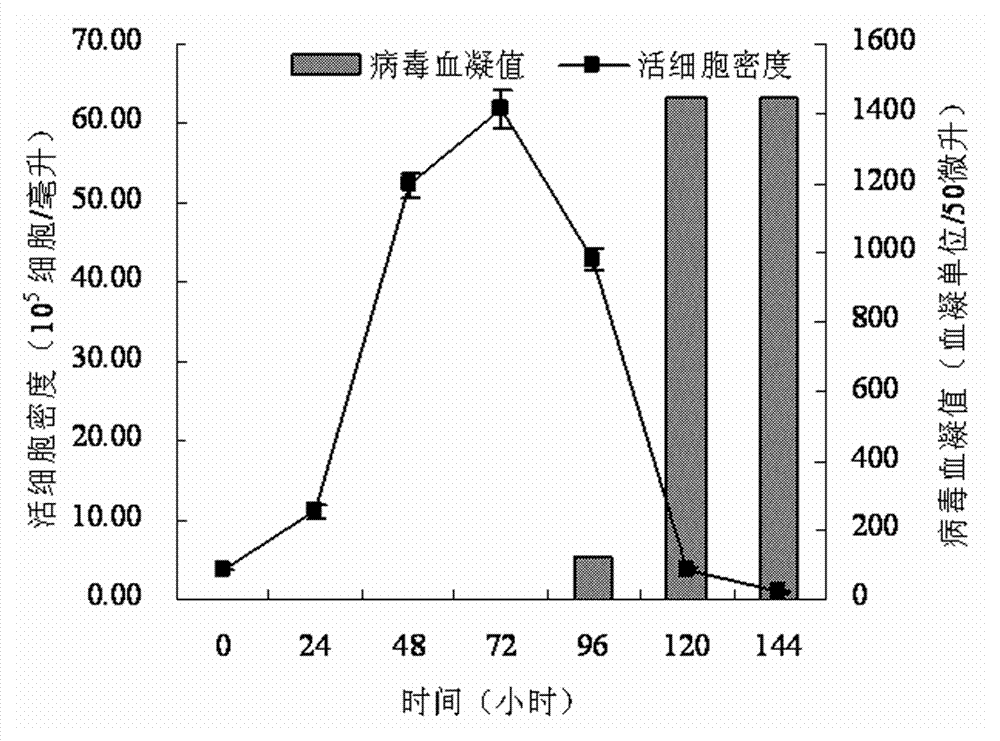
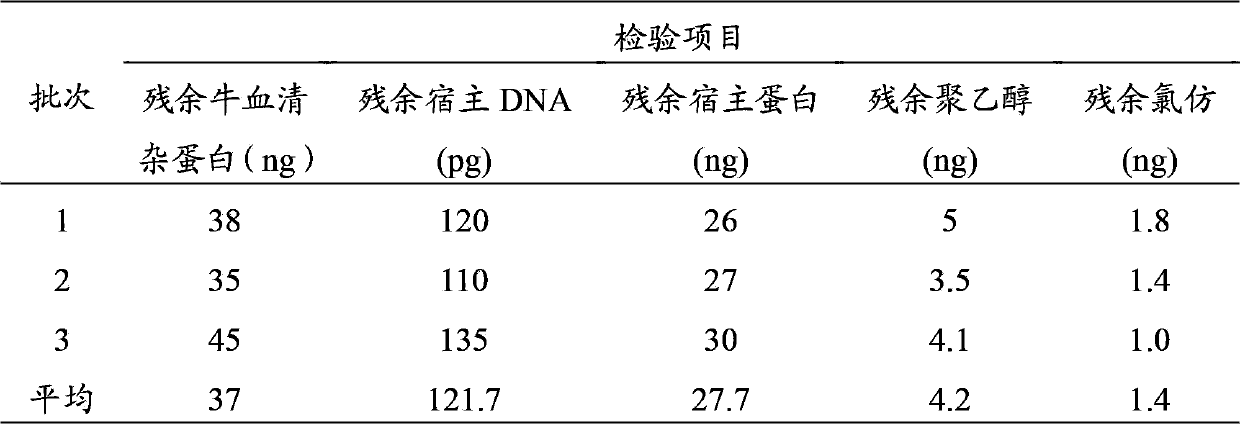
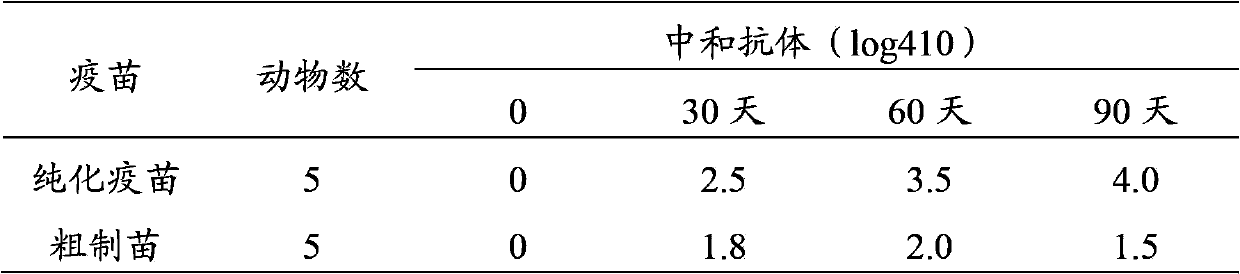
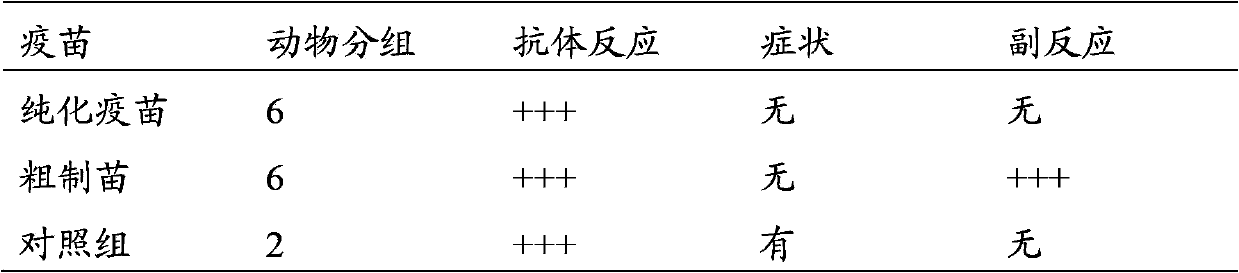
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
Separation and purification method of human umbilical cord mesenchymal stem cell exosome and application of human umbilical cord mesenchymal stem cell exosome
ActiveCN108103017AHigh protein purityGood repeatabilityMetabolism disorderSkeletal/connective tissue cellsSucrosePurification methods
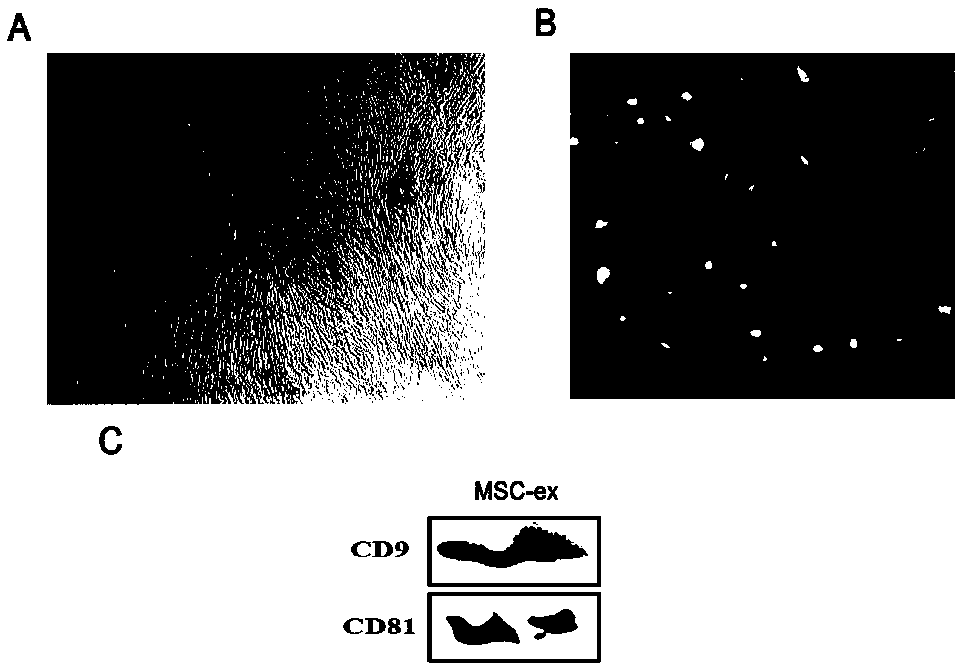
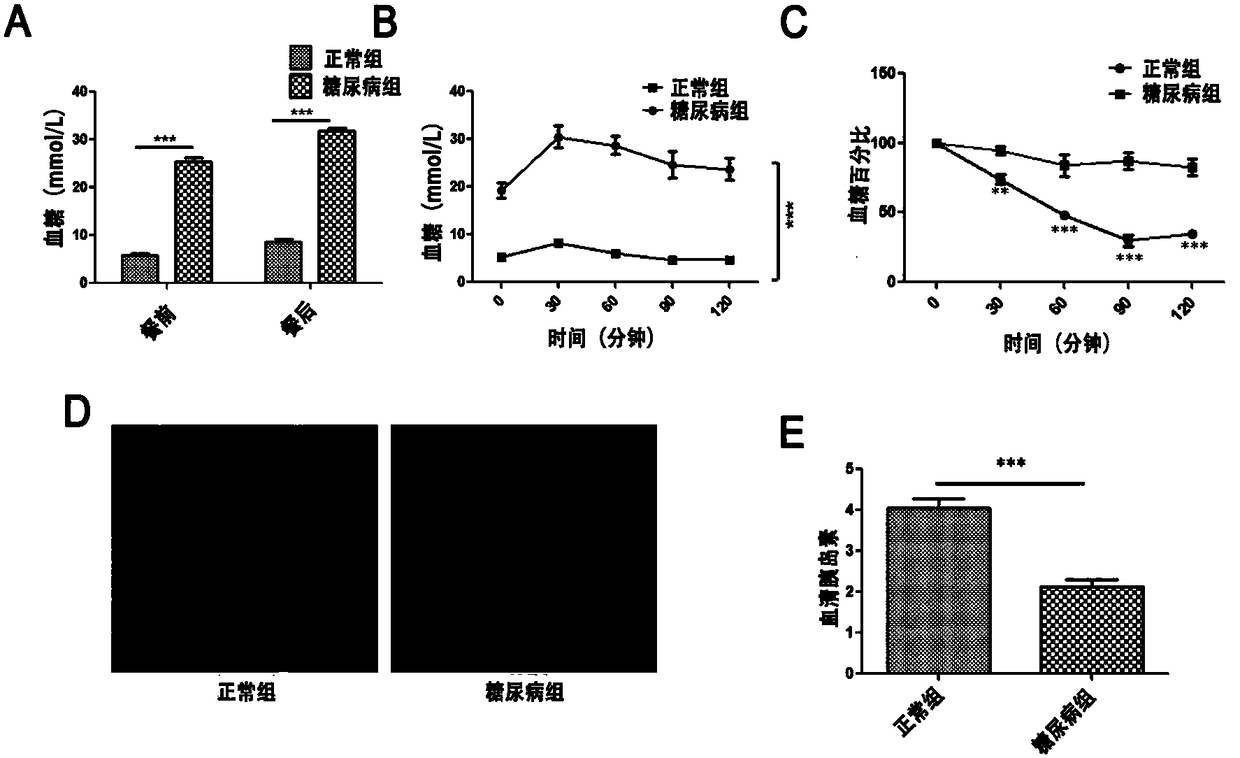
The invention provides a separation and purification method of a human umbilical cord mesenchymal stem cell exosome and an application of the human umbilical cord mesenchymal stem cell exosome, and belongs to the technical field of medicines. The human umbilical cord mesenchymal stem cell exosome provided by the invention is prepared by cultivating human umbilical cord mesenchymal stem cells via aserum-free medium, collecting supernatant liquid, implementing centrifuging as well as ultra-filtration and concentration, transferring a concentrated solution onto a 30% sucrose-heavy water densitypad and implementing further purification through sucrose density centrifuging, so that the human umbilical cord mesenchymal stem cell exosome is obtained. According to the separation and purificationmethod that the human umbilical cord mesenchymal stem cell exosome is obtained through separation and purification, immunoreactivity is effectively reduced, and a controllable dosage when the human umbilical cord mesenchymal stem cell exosome is used is guaranteed. The human umbilical cord mesenchymal stem cell exosome, by improving a degree of activating an insulin signaling pathway of a type IIdiabetes animal model, can inhibit composition and decomposition of hepatic glycogen, so that glucose metabolism homeostasis can be achieved; and meanwhile, the sensitivity of the type II diabetes animal model to insulin and an insulin secretion function of pancreatic [beta] cells can be improved and a blood glucose concentration can be reduced, so that the application of the human umbilical cordmesenchymal stem cell exosome to the preparation of medicines for treating type II diabetes can be achieved.
Owner:JIANGSU UNIV
Serum-free medium suitable for large-scale single-cell suspension culture of young hamster kidney cells
InactiveCN102268403AIncrease productivityEasy to separate and purifyArtificial cell constructsVertebrate cellsCulture mediumsAmino acid
The invention discloses a serum-free medium suitable for large-scale single-cell suspension culture of young hamster kidney cells (BHK cells), including 21 kinds of amino acids, 11 kinds of inorganic salts, 12 kinds of vitamins, 1 kind of protein hydrolyzate, 2 kinds of Lipids, 2 buffer components and 6 additives. The BHK cell serum-free medium of the present invention produces biological products by single-cell suspension culture, which can not only avoid various disadvantages of serum culture, but also eliminate the problem of difficult scale-up of roller bottle adherent culture, thereby improving the production efficiency of BHK cell culture And reduce production costs, while ensuring product quality, has good application value and huge market prospects.
Owner:上海米迪生物技术有限公司
Serum-free medium for human adipose-derived stem cells and preparation method thereof
ActiveCN105112364AFast adhesionStem cells are in good shapeSkeletal/connective tissue cellsVitamin CPancreatic hormone
The invention provides a serum-free medium for human adipose-derived stem cells, comprising a DMEM basic medium and further comprising L-glutamine, insulin-like growth factors, epidermal growth factors, transforming growth factors, platelet-derived growth factors, fibroblast growth factors, transferrin, vitamin C, human serum albumin and 1, 2, 3, 4, 6-O-pentagalloylglucose. The invention belongs to the technical field of stem cells; the serum-free medium for human adipose-derived stem cells provided by the invention is capable of promoting quick adherence and proliferation of the human adipose-derived stem cells and keeping good stem cell pedomorphism and stem cell characteristics.
Owner:广东美赛尔细胞生物科技有限公司
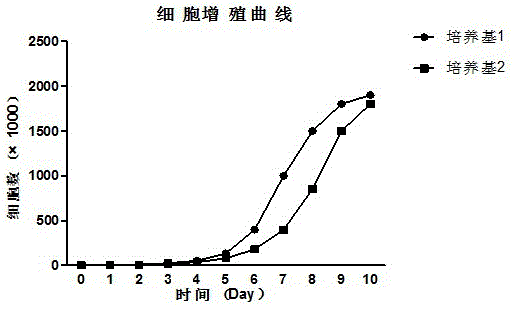
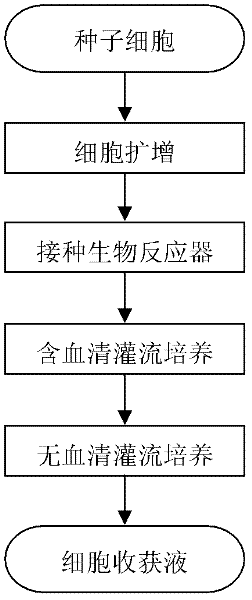
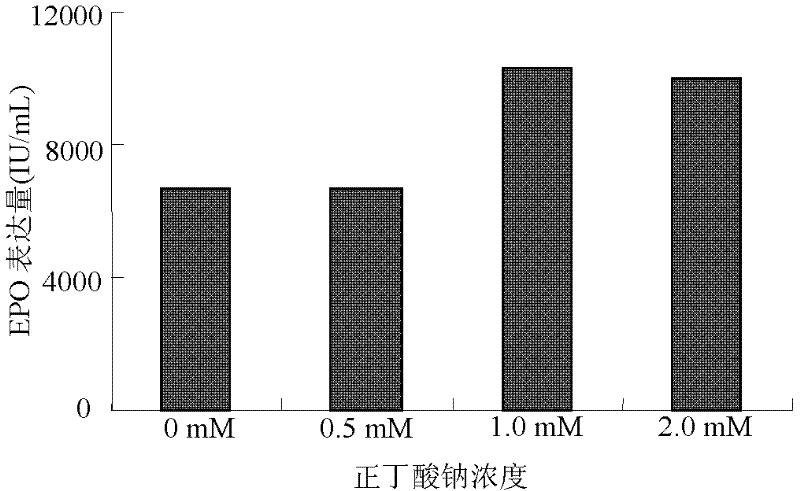
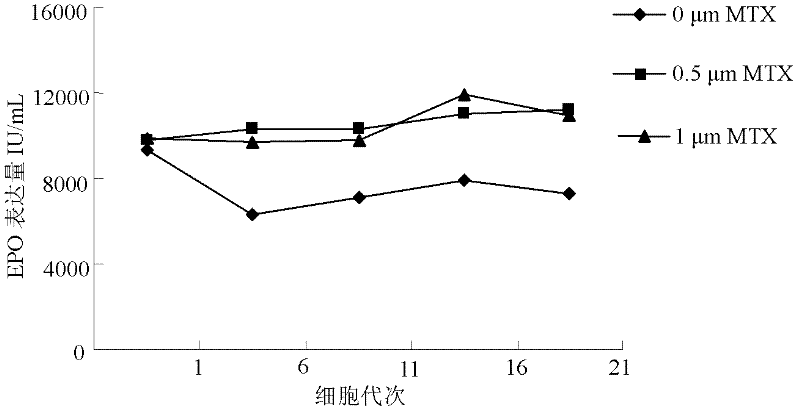
Culture method for highly expressing erythropoietin in serum-free medium and cho cells
ActiveCN102268402AIncreased glycosylationIncrease the proportionMicroorganism based processesArtificial cell constructsCulture fluidChinese hamster
The invention discloses a serum free medium for expressing erythropoietin in CHO (Chinese hamster ovary) cells, and a culture method for high expression of erythropoietin in CHO cells. The serum free medium contains additives D-glucose and sodium butyrate, and also contains one or more of D-galactose, D-mannitose and N-acetylglucosamine. The culture method comprises the steps of: performing serumculture on activated seed cells by using a serum medium, and then performing serum free culture by the serum free medium. By adopting the medium and culture method disclosed by the invention, recombinant human erythropoietin protein of high and stable expression can be obtained, and the glycosylation degree of erythropoietin is improved, i.e. the specific gravity of EPO (erythropoietin) sialic acid is improved. As high as 1.0*107IU recombinant human erythropoietin can be obtained from one liter of culture fluid by the culture method provided by the invention, the expression is stable, and theculture method is simple and suitable for large-scale production.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Method for separating primary adult hepatocytes, and special sterile apparatus box thereof
InactiveCN102220278AGuaranteed sterilityFor long-term storageBioreactor/fermenter combinationsBiological substance pretreatmentsSerum freeType IV collagen
The invention discloses a method for separating primary adult hepatocytes. The method comprises the following steps: (1) carrying out multi-point puncture on surface of isolated adult hepatic tissue through a needle syringe, and injecting a preperfusate, wherein connective tissues are removed from the isolated adult hepatic tissue; (2) carrying out the multi-point puncture on the surface of the isolated adult hepatic tissue through the needle syringe again, and injecting a IV collagenase solution; (3) separating the isolated adult hepatic tissue, followed by adding the IV collagenase solution and carrying out digesting through vibration at a temperature of 37 DEG C to obtain digest; (4) carrying out filtering for the digest, followed by centrifuging and collecting cell aggregate in the underlayer, then resuspending the adult hepatocyte aggregate through a hepatocyte wash buffer, followed by filtering and centrifuging, then abandoning supernatant and collecting the adult hepatocyte aggregate in the underlayer; (5) washing the adult hepatocyte aggregate in the underlayer from the step (4) through a serum-free DMEM medium to obtain the primary adult hepatocytes. The invention further discloses a disposable special sterile apparatus box for separating the primary adult hepatocytes. With the present invention, the disposable special sterile apparatus box is adopted, the primary adult hepatocytes are separated through the multi-point puncture on the surface of the tissue and the injection, such that operation is simplified, cost is reduced, and the method and the apparatus box are applicable for extracting the hepatocytes from small pieces of the irregular isolated adult hepatic tissues of recovery of liver resection.
Owner:SOUTHERN MEDICAL UNIVERSITY
Serum-free medium and preparation method thereof
InactiveCN104911147AComposition is stableGood functional consistencyBlood/immune system cellsAlbumin solutionCulture mediums
The invention relates to a serum-free medium and a preparation method thereof. The preparation method of the serum-free medium includes: loading fatty acid on FAF (fatty-acid-free) albumin, and then adding an obtained albumin solution into a basic culture medium. The serum-free medium obtained by using the preparation method of the serum-free medium is stable in component and good in functional consistency, and no difference can be caused due to difference of species origins or processing batches of the albumin.
Owner:英普乐孚生物技术(上海)有限公司
Obtaining method of stem cell conditioned medium, and application of stem cell conditioned medium in skin ageing resistance
Owner:河南中科干细胞基因工程有限公司
Mature type-1 polarized dendritic cells with enhanced IL-12 production and methods of serum-free production and use
InactiveUS7972847B2Improve propertiesEnhance IL-12-producing propertyArtificial cell constructsBlood/immune system cellsDendritic cellSerum free
Owner:PAWEL KALINSKI
Culture method of mesenchymal stem cells
ActiveCN112522191AImprove proliferative abilityComplete formCulture processSkeletal/connective tissue cellsMesenchymal stem cellSecretion
The invention relates to the technical field of cell culture, and particularly relates to a culture method of mesenchymal stem cells. In the culture method, a serum-free culture medium optimized by the invention is used for culturing the mesenchymal stem cells. In addition, the invention further provides a better culture method for 3D culture. The cells obtained by the culture method disclosed bythe invention are strong in proliferation capacity, intact in shape and strong in secretion capacity, and are in accord with the international quality control standards of the mesenchymal stem cells.Meanwhile, according to the culture method disclosed by the invention, a large number of mesenchymal stem cells (particularly human umbilical cord mesenchymal stem cells) can be obtained by amplifyinga small number of mesenchymal stem cells under the conditions of small space occupation, less culture medium consumption, simpler and more convenient operation and greatly reduced workload; and a solid technical scheme and a theoretical basis are provided for obtaining a large number of high-quality mesenchymal stem cells (especially human umbilical cord mesenchymal stem cells) and secretions thereof in the clinical field.
Owner:YUNNAN KEY LAB OF PRIMATE BIOMEDICINE RES
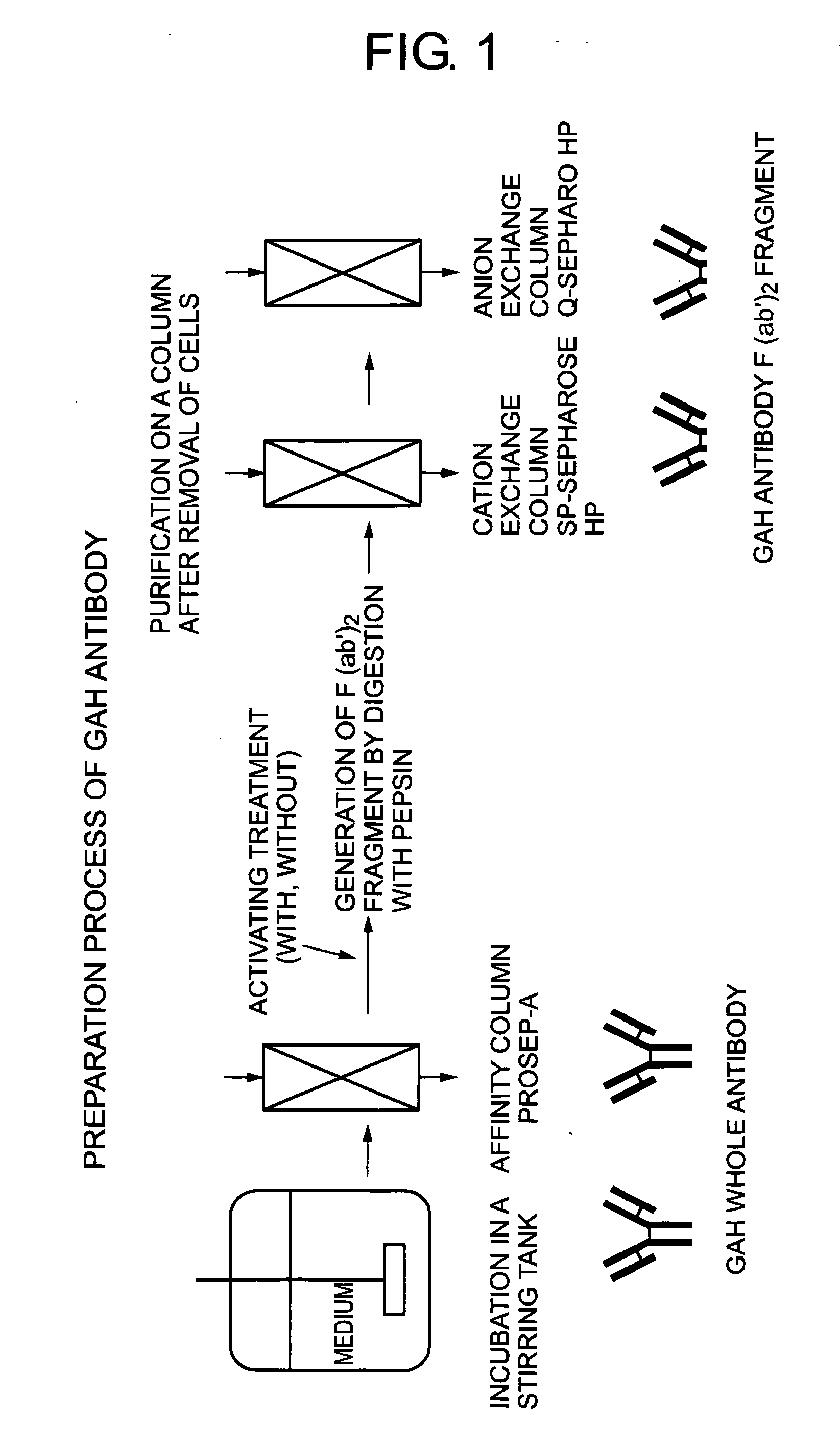
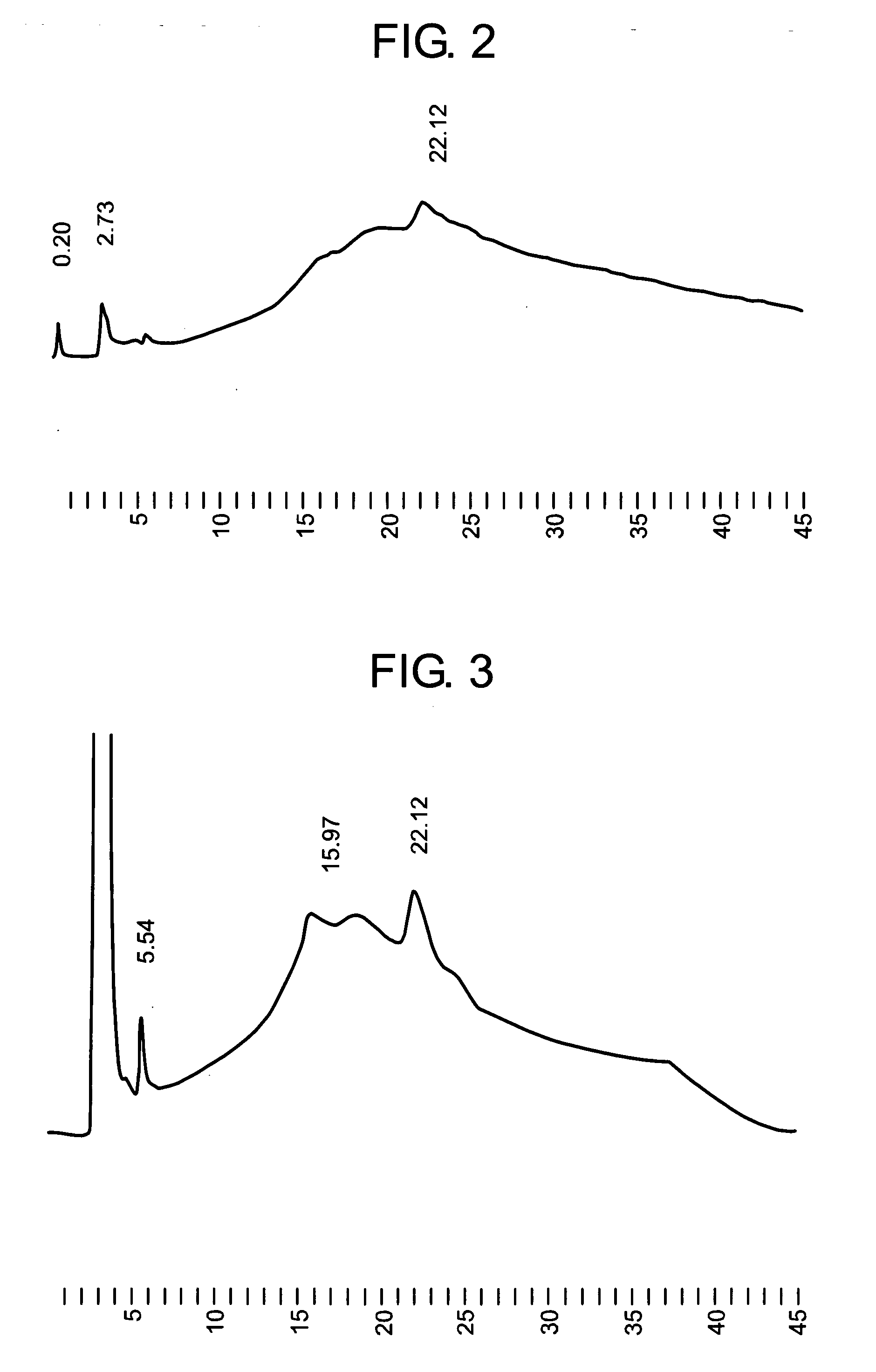
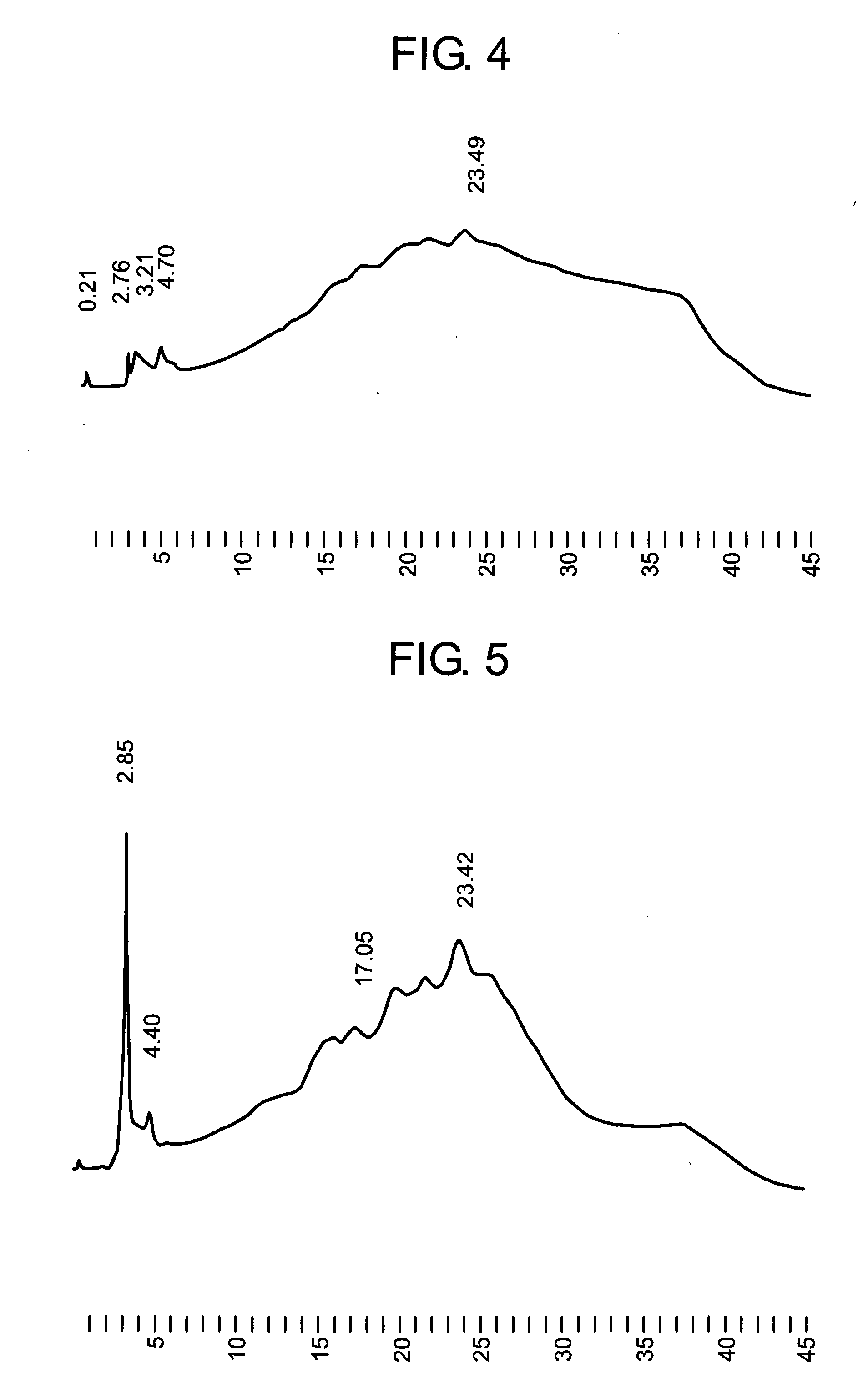
Method of activating protein
A method of producing a protein having free cysteine with the use of a serum-free medium, characterized in that the protein is produced in the activated state; a method of producing a protein by culturing in a serum-free medium in accordance with the above method; a method of activating a protein having free cysteine which has been produced in the inactivated state; and a protein obtained by any of the above methods. This protein shows physicochemical or biological properties comparable to a protein obtained by using a serum medium.
Owner:MITSUBISHI TANABE PHARMA CORP
Serum-free medium for placenta-derived mesenchymal stem cells and preparation method thereof
ActiveCN105112362AImprove securityImprove proliferative abilitySkeletal/connective tissue cellsINSULIN HUMANProliferative capacity
The invention belongs to the technical field of stem cells and relates to a serum-free medium for placenta-derived mesenchymal stem cells and a preparation method thereof. The serum-free medium comprises a DMEM basic medium and further comprises vitamin H, glutathione, recombinant human insulin, human serum albumin, transferrin, fibroblast growth factors, epidermal growth factors, stem cell growth factors, Human stem cell factors and magnolol. The serum-free medium provided by the invention has the advantages of high safety and capability for significant improvement of proliferative capability of placenta-derived mesenchymal stem cells, the serum-free medium is capable of well keeping cell forms and stem cell characteristics of the placenta-derived mesenchymal stem cells and is convenient to popularize and apply.
Owner:广东美赛尔细胞生物科技有限公司
Umbilical cord mesenchymal stem cells (MSCs) and culture method and application thereof
ActiveCN110157666AIncreased proliferationEnhanced clonogenicityOrganic active ingredientsPeptide/protein ingredientsComing outCell adhesion
The invention relates to umbilical cord mesenchymal stem cells (MSCs) and a culture method and application thereof, and belongs to the technical field of biological medicine. The culture method includes MSCs culture steps and phenotypic examination. The MSCs culture steps include primary culture and sub-culturing; during primary culture, Huatong gum tissue of the umbilical cord is obtained and cultured in a serum-free culture medium under hypoxic conditions; during sub-culturing, P1 primary cells are collected, a single cell suspension is prepared, cell precipitates are obtained after centrifuging, the serum-free culture medium is added into the cell precipitates, culturing is conducted under hypoxic conditions until next generations come out, continuous culturing is performed to obtain P2-P3 generations, ligustrazine hydrochloride and Shenmai injections are added in each sub-culturing stage, when the cells grow to a predetermined fusion degree, digestion is performed and the cells arecollected until the P6 generations come out. During phenotypic examination, the collected cells are subjected to phenotypic examination for use. An MSCs preparation obtained through culturing in themethod reduces the easy-to-aggregation characteristics of the stem cells, the occurrence of cell adhesion, erythrocyte rouleaux formation and cell mass embolism after intravenous infusion into the human body is thus avoided, and the stem cell (MSCs) technology can be better applied to clinical practice.
Owner:广东新南方干细胞再生医学科技有限公司
Method and combined medium for preparing insulin-like secreting cells
InactiveCN101570741ALower blood sugarIncrease weightTissue cultureBasic fibroblast growth factorFetal bovine serum
The invention discloses a combined medium for preparing insulin-like secreting cells, which consists of a medium I, a medium II and a medium III which are independent respectively, wherein, the medium I is a DMEM (Dulbecco's modified Eagle's medium) high-glucose medium prepared from fetal calf sera, basic fibroblast growth factors (bFGF), dimethyl sulfoxide (DMSO) and glucose; the medium II is a serum-free DMEM / F12 medium prepared from glucose, niacinamide, epidermal growth factors (EGF), bFGF, exendin-4, a B27 additive and an N2 additive; and the medium III is an RPMI (Roswell Park Memorial Institute) 1640 medium prepared from glucose, niacinamide, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, hepatocyte growth factors (HGF), activin A and exendin-4. The invention further discloses a method using the combined medium for preparing insulin-like secreting cells at the same time. The invention can facilitate the differentiation of hBM-MSC (human bone marrow mesenchymal stem cell) into insulin-like secreting cells having the effect of correcting diabetes hyperglycemia.
Owner:ZHEJIANG UNIV
Method for culturing skeletal muscle for tissue engineering
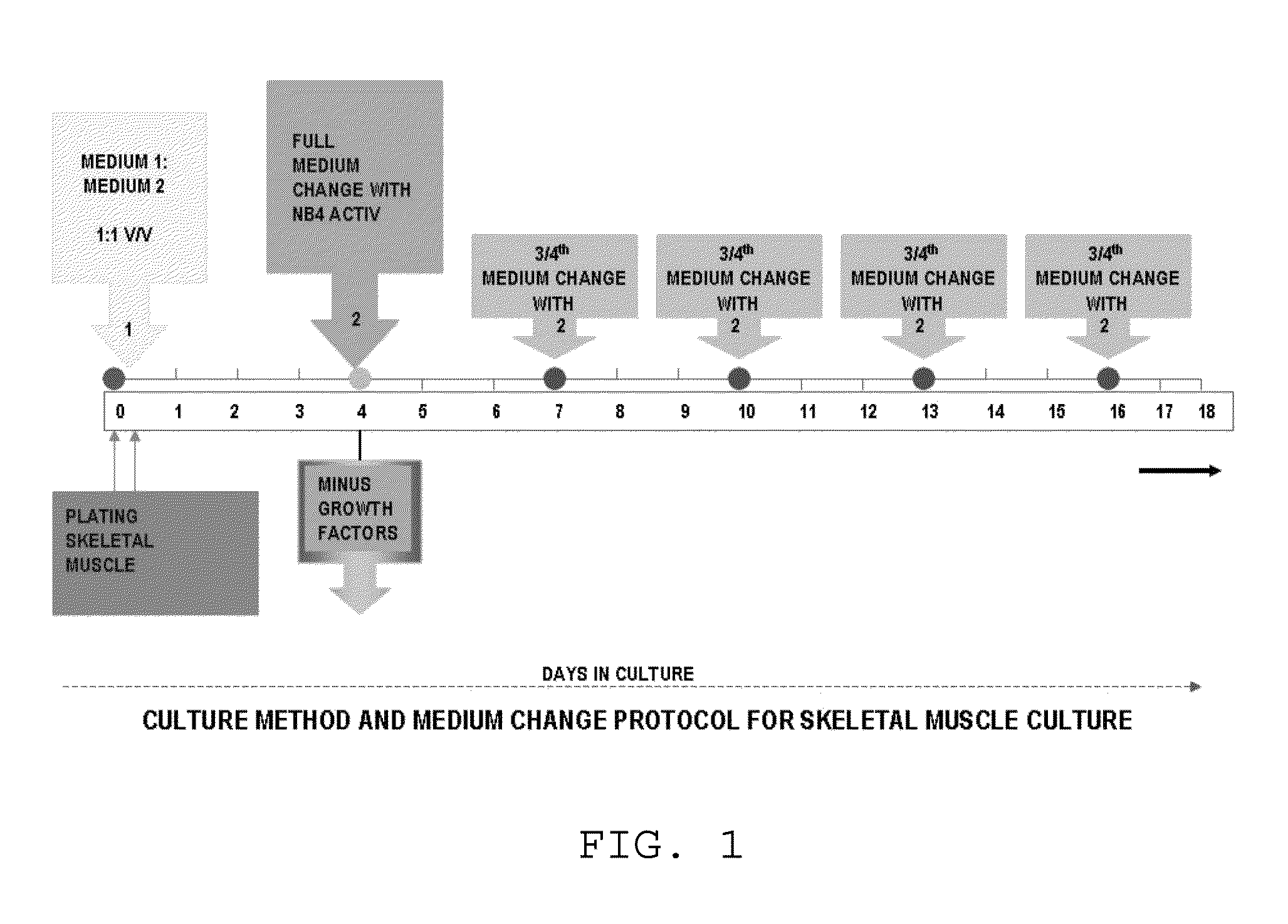
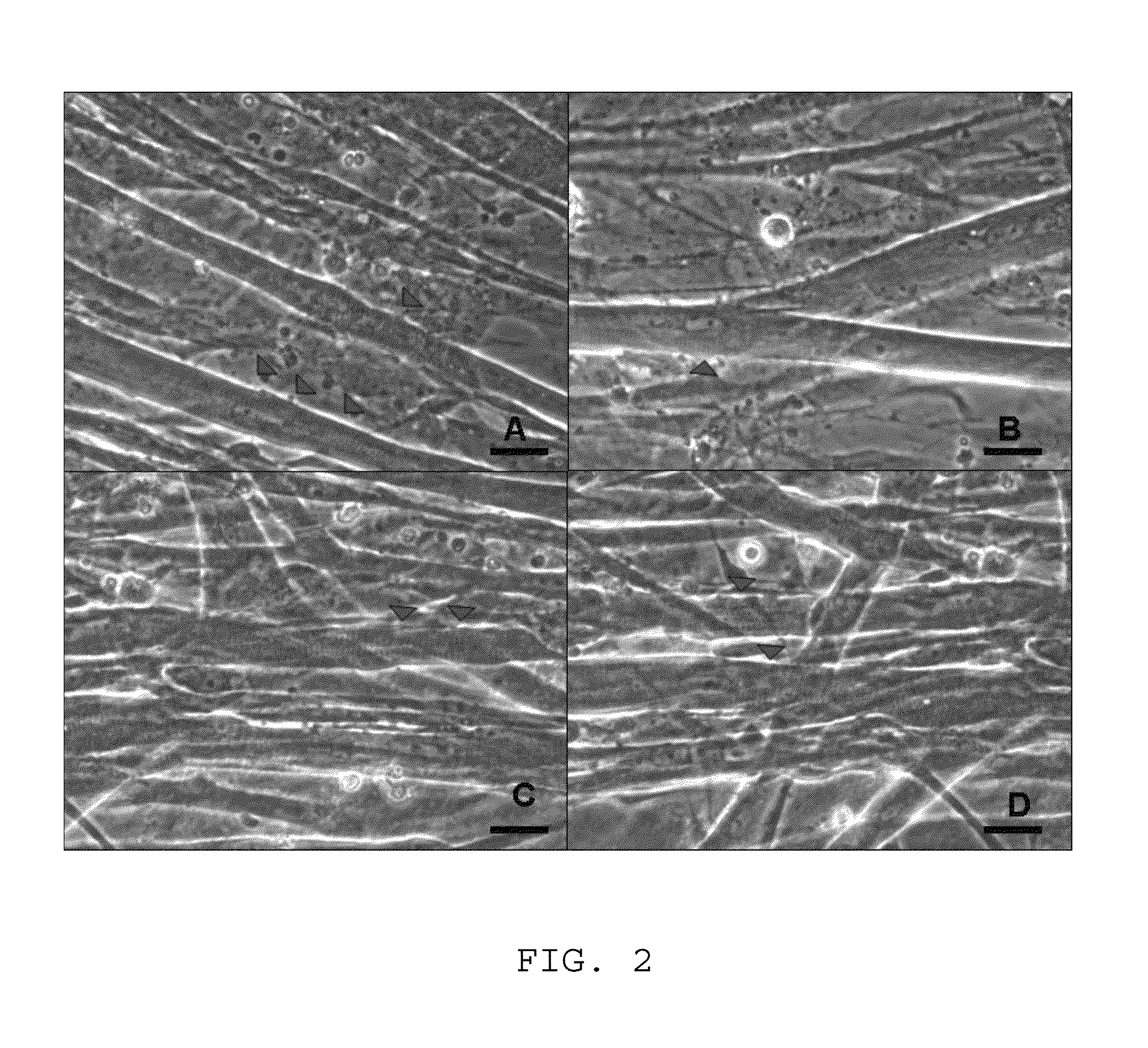
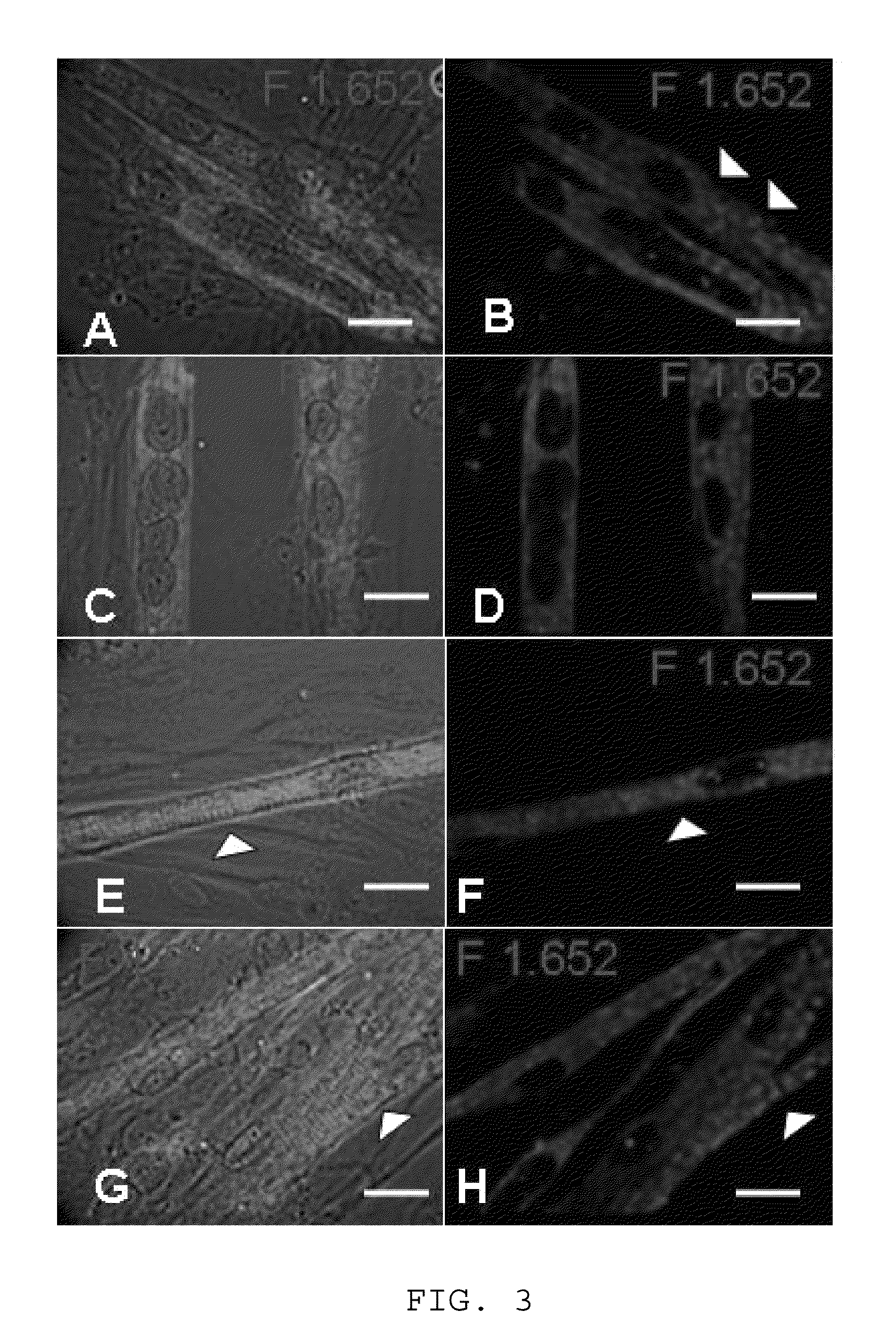
ActiveUS9163216B1Small sizeReduce complexityCulture processNervous system cellsInsulin-like growth factorSerum free media
The invention provides a nutrient medium composition and associated methods for lengthening the useful life of a culture of muscle cells. Disclosed is a method of culturing mammalian muscle cells, including preparing one or more carriers coated with a covalently bonded monolayer of trimethoxy-silylpropyl-diethylenetriamine (DETA); verifying DETA monolayer formation by one or more associated optical parameters; suspending isolated fetal rat skeletal muscle cells in serum-free medium according to medium composition 1; plating the suspended cells onto the prepared carriers at a predetermined density; leaving the carriers undisturbed for cells to adhere to the DETA monolayer; covering the carriers with a mixture of medium 1 and medium 2; and incubating. A cell nutrient medium composition includes Neurobasal, an antibiotic-antimycotic composition, cholesterol, human TNF-alpha, PDGF BB, vasoactive intestinal peptides, insulin-like growth factor 1, NAP, r-Apolipoprotein E2, purified mouse Laminin, beta amyloid, human tenascin-C protein, rr-Sonic hedgehog Shh N-terminal, and rr-Agrin C terminal.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Method for Production of Recombinant Human FSH
InactiveUS20090291473A1Eliminate riskImprove the environmentDepsipeptidesPeptide preparation methodsSerum free mediaMammal
Disclosed is a method for production of recombinant human FSH in high yield and a high purity. The method comprises the steps of: (a) culturing recombinant human FSH-producing mammalian cells in a serum-free medium, (b) collecting culture supernatant, (c) subjecting the culture supernatant to cation-exchange column chromatography, (d) dye affinity column chromatography, (e) hydrophobic column chromatography, and (f) gel filtration column chromatography to collect recombinant human FSH-active fractions, in the order.
Owner:JCR PHARMA
Method for producing anti CD20 antibody
ActiveCN104513805APromote growthHigh expressionMicroorganism based processesArtificial cell constructsAntibody expressionSide effect
The invention discloses a method for culturing animal cells for producing anti CD20 antibody, and specifically discloses inoculation quantity, medium formula and various culture process control parameters. The medium is a serum-free medium, so that no side effect is caused by serum in the process of cell culture, and antibody extraction and purification are further facilitated. By using the method for producing the anti CD20 antibody, differences between different batches are small, antibody expression quantity is high, production cost is low, and safety is good.
Owner:LUNAN PHARMA GROUP CORPORATION
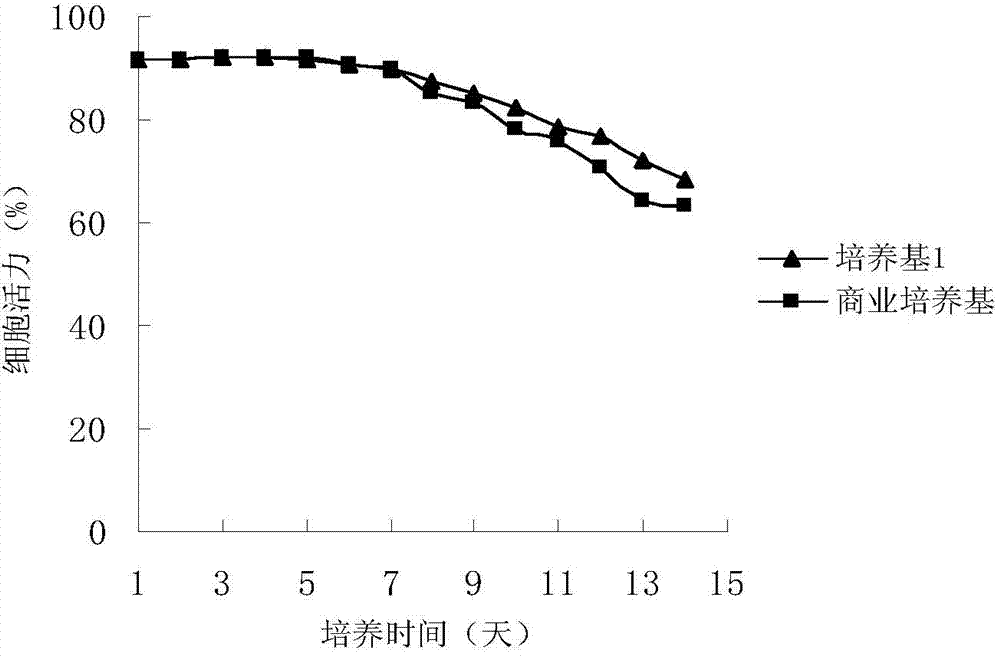
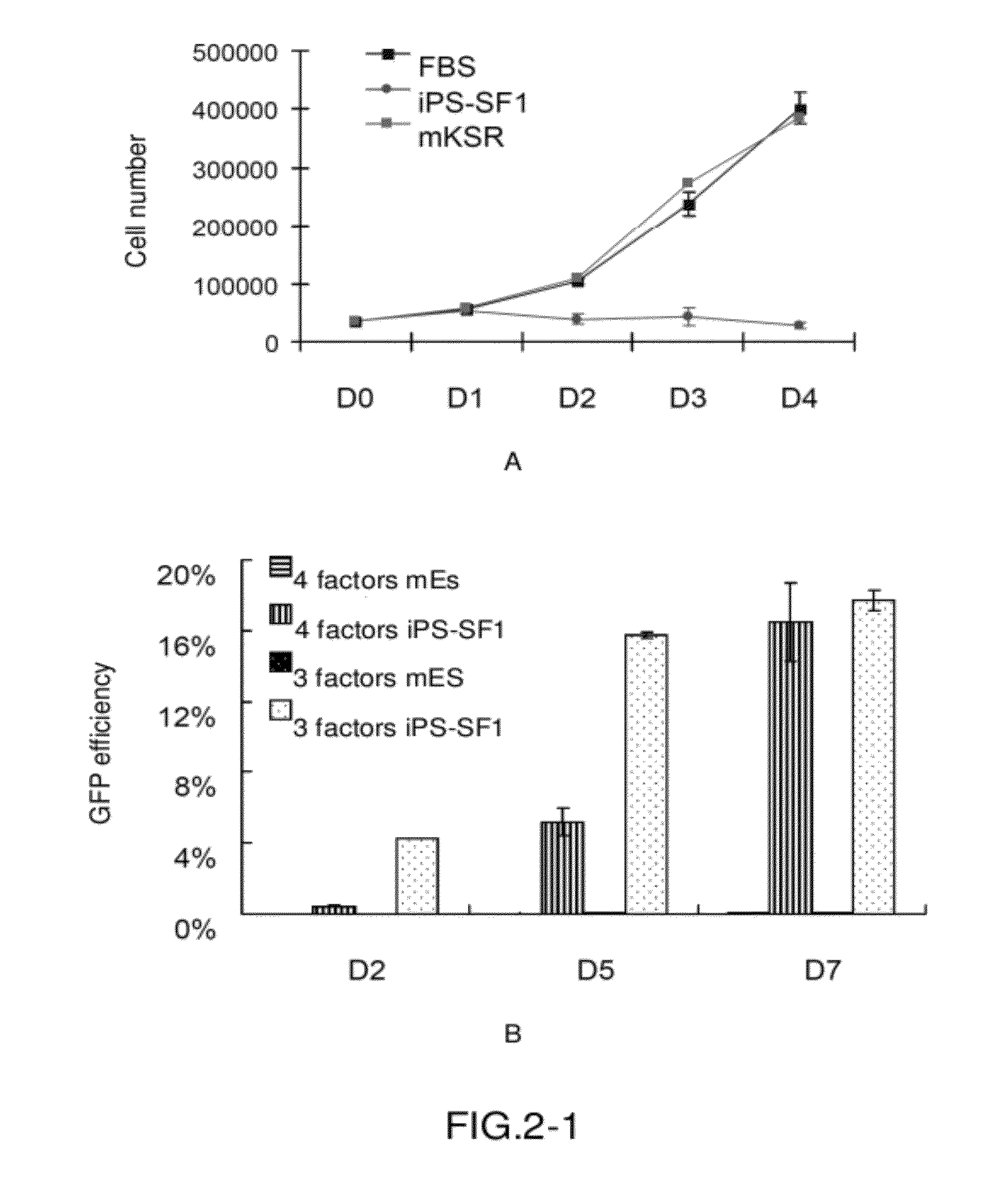
Serum-free medium for inducing pluripotent stem cells quickly with high efficiency and method using thereof
InactiveUS20120100568A1Improve efficiencyMicrobiological testing/measurementCulture processSerum free mediaHigh-Throughput Screening Methods
A serum-free medium for inducing and reprogramming somatic cells into induced pluripotent stem cells (iPS) quickly with high efficiency, and the method using thereof for inducing and reprogramming somatic cells without feeder are provided, wherein the rate and efficiency of whole process of inducing and reprogramming are greatly improved. The uses of the medium in inducing pluripotent stem cells, and the uses in the method for screening compounds, especially in the method for high throughput screening compounds are further provided.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Protein-free serum-free medium suitable for diploid cell culture and application
The invention provides a protein-free serum-free medium suitable for diploid cell culture and a preparing method thereof. The medium contains calcium chloride, magnesium sulfate, potassium chloride, sodium dihydrogen phosphate, L-arginine hydrochloride, L-asparaginate, L-cystine dihydrochloride, L-histidine hydrochloride, L-leucine, L-isoleucine and the like. The medium can meet the requirement of the growth or maintenance stage of diploid cells and prevents contact with exogenous viruses possibly existing in protein and serum to reduce potential safety hazards; as the components are simple and definite, standard products can be purchased in the market, the difference between different product batches is small, and the step of purifying down-stream products is simplified; a scientific basis is provided for large-scale standard production. In addition, the protein-free serum-free medium can be applied in the growth or maintenance stage of the diploid cells.
Owner:SICHUAN BAINUOJI TECH CO LTD
Culture method of bone marrow derived endothelial progenitor cells
ActiveCN106754650ASolve problems with low yield and high priceDoes not affect culture efficiencyCulture processArtificial cell constructsSerum free mediaBasic fibroblast growth factor
The invention discloses a culture method of bone marrow derived endothelial progenitor cells and belongs to the technical field of cell culture. The method comprises the steps of treating extracted bone marrow with a red blood cell lysis buffer, then, carrying out separating so as to obtain single karyocytes of the bone marrow, carrying out washing, then, inoculating a fibronectin precoated culture dish with the obtained single karyocytes, carrying out culture by using a complete culture medium, digesting the cells with trypsin after a period of time of culture, inoculating culture bottles with digested cells, and carrying out differential adherent culture and differential digestion culture. The used complete culture medium is prepared through adding fetal calf serum, a vascular endothelial growth factor, glucocorticoid, vitamin C, an epidermal growth factor, a basic fibroblast growth factor, a colony stimulating factor and a platelet-derived growth factor into a serum-free medium EGM2. The method has the characteristics that the operating steps are relatively simple, the spent time is short, the quantity of separation in one time is large, the number of transfer generations of the obtained cells is large, and the like.
Owner:哈尔滨中科赛恩斯生物技术有限公司
Mature type-1 polarized dendritic cells with enhanced il-12 production and methods of serum-free production and use
InactiveUS20090004157A1Improve propertiesEnhance IL-12-producing propertyBiocideArtificial cell constructsDendritic cellSerum free
The present invention discloses novel dendritic cell maturation-inducing cytokine cocktails, and methods for inducting type-1 polarized dendritic cells in serum-free conditions which enhance the desirable properties of DC1s generated in serum-supplemented cultures. The invention further discloses methods and systems using IFNγ and other ligands of the IFNγ receptor, in combination with IFNα (or other type I interferons), poly I:C, and other IFNα (and IFNβ) inducers to enhance the IL-12-producing properties of dendritic cells. More specifically, the present invention discloses type-1 polarized dendritic cells that have a unique combination of a fully-mature status and an elevated, instead of “exhausted”, ability to produce IL-12p70. allows for the generation of fully-mature DC1s in serum-free AIM-V medium. The invention discloses systems that use the foregoing products and methods to facilitate the clinical application of DC1-based vaccines and the identification of novel factors involved in the induction of Th1 and CTL responses by DC1.
Owner:PAWEL KALINSKI
Serum-free medium used for culture of elderly people cartilage cells
InactiveCN104120105AMaintain biological propertiesMeet the clinical applicationSkeletal/connective tissue cellsCartilage cellsVitamin C
The invention discloses a serum-free medium used for culture of elderly people cartilage cells, the-free medium comprises a basal medium and an additive comprising the components of the following concentration: 233muM-287 muM of vitamin C, 3.7mu M-8.9 muM of linoleic acid, 9muM-17 muM of cholesterol, 6nM-15 nM of dexamethasone, 43 muM-58mu M of acetyl cysteine, 18 mug / mL-32 mu g / mL of transferrin, 22nM-52 nM of sodium selenite, 13 muM-24 muM of sodium pantothenate, 28 muM-43 muM of biotin, 7 mug / mL-18 mu g / mL of insulin, 2 ng / mL-9 ng / mL of epidermal growth factor, 2 ng / mL-9 ng / mL of fiber growth factor, 2 ng / mL-9 ng / mL of platelet derived growth factor, and 0.5%-2.5% of human serum albumin. The cell culture time is short, and the cell biological performance is good.
Owner:HANGZHOU LONGHILL BIO MEDICATION TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com