Method for producing anti CD20 antibody
A CCTCCNO.C201158, vitamin technology, applied in the field of biopharmaceuticals, can solve the problems of large differences in different batches, high cost, microbial contamination, etc., and achieve the effect of increasing antibody expression and promoting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
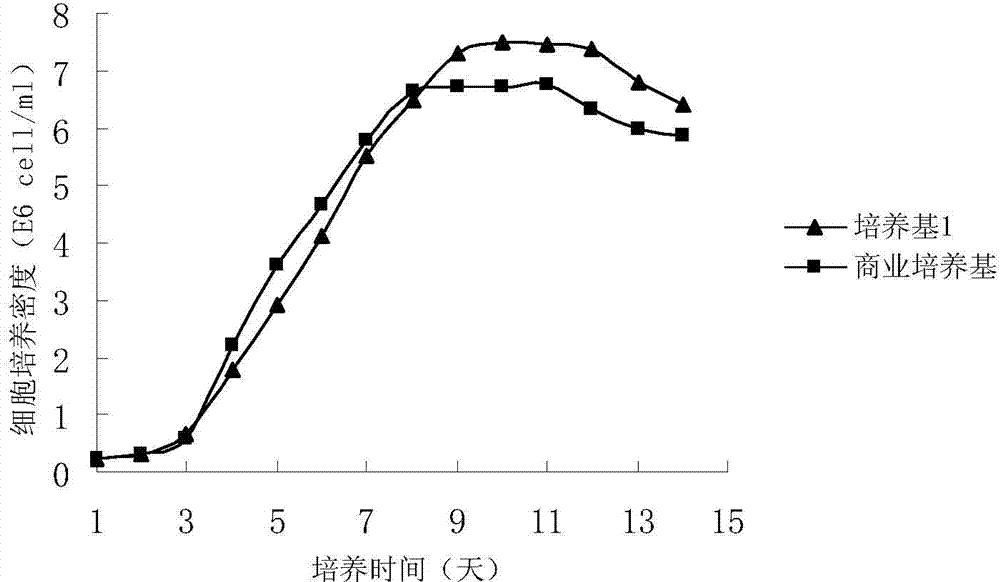
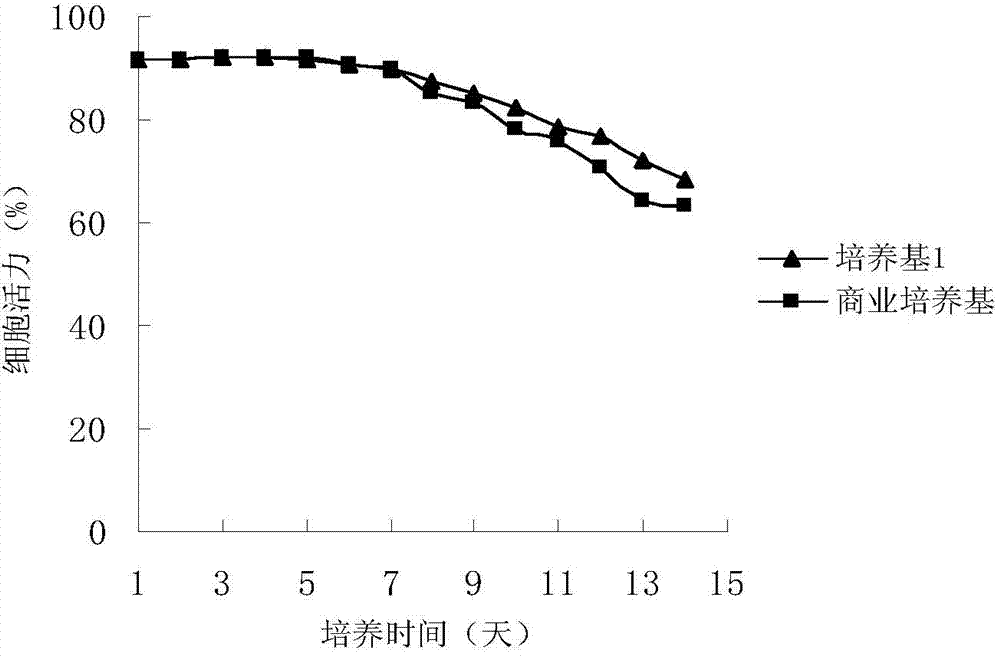
[0027] The medium was prepared according to the components and contents described in Table 2, and the medium was sterilized by filtration with a 0.22 μm bacterial filter membrane before being used for production. A cryopreserved CHO cell was taken out from the liquid nitrogen of the production cell bank, revived and inoculated into a 100 mL spinner bottle for culture. In the spinner bottle culture stage, the shaker speed was 85 rpm and the culture temperature was 37°C.
[0028] Amplify the spinner bottle step by step, after 100mL→250mL→500mL passage expansion, when the total number of cells reaches 1.0×10 8 One hour, inoculated into a 5.0L reactor for cultivation, the inoculum size was 0.3×10 6 cells / ml culture medium. During the fermentation process, the glucose concentration was maintained at 0.5-3g / L, and the dissolved oxygen was 30%. From the initial stage of culture to the logarithmic growth phase, control the culture temperature to 37°C, pH to 7.2, and stirring speed t...
Embodiment 2
[0033] The medium was prepared according to the components and contents described in Table 3, and the medium was sterilized by filtration with a 0.22 μm bacterial filter membrane before being used for production. A cryopreserved CHO cell was taken from the liquid nitrogen of the production cell bank, which is a cell with high expression of anti-CD20 antibody. After recovery, it was inoculated into a 100mL spinner bottle for culture. During the spinner bottle culture, the shaker speed was 75rpm and the culture temperature was 36°C.
[0034] Amplify the spinner bottle step by step, after 100mL→250mL→500mL passage expansion, when the total number of cells reaches 1.0×10 8 One hour, inoculated into a 5.0L reactor for cultivation, the inoculum size was 0.5×10 6 cells / ml culture medium. During the fermentation process, the glucose concentration was maintained at 0.5-3g / L, and the dissolved oxygen was 50%. From the initial stage of culture to the logarithmic growth phase, control t...
Embodiment 3
[0040] The medium was prepared according to the components and contents described in Table 4, and the medium was sterilized by a 0.22 μm filter membrane before being used for production. Take out a cryopreserved CHO cell from the liquid nitrogen of the production cell bank, which is a high-expression anti-CD20 antibody cell. After recovery and expansion, it is inoculated into a 100ml spinner bottle for culture. During the spinner bottle culture, the shaker speed is 75rpm and the culture temperature is 37. ℃.
[0041] Amplify the spinner bottle step by step, after 100mL→250mL→500mL passage expansion, when the total number of cells reaches 1.0×10 8 One hour, inoculated into a 5.0L reactor for cultivation, the inoculum size was 0.5×10 6 cells / ml culture medium. During the fermentation process, the glucose concentration was maintained at 0.5-3g / L, and the dissolved oxygen was 60%. From the initial stage of culture to the logarithmic growth phase, control the culture temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com