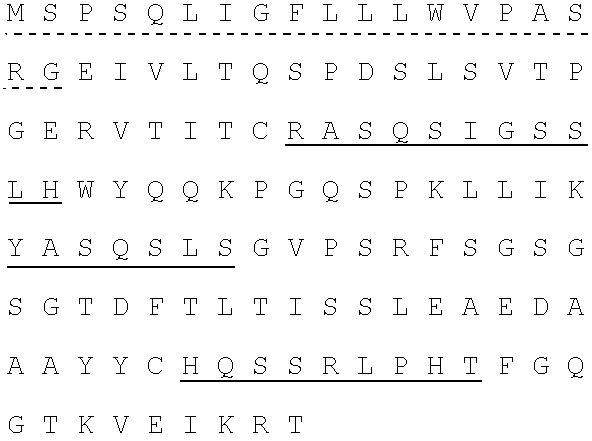
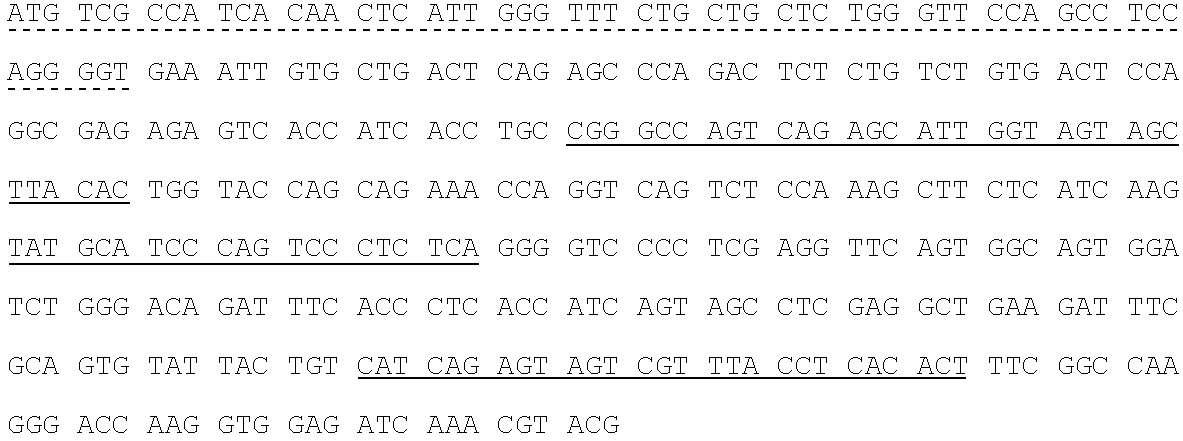
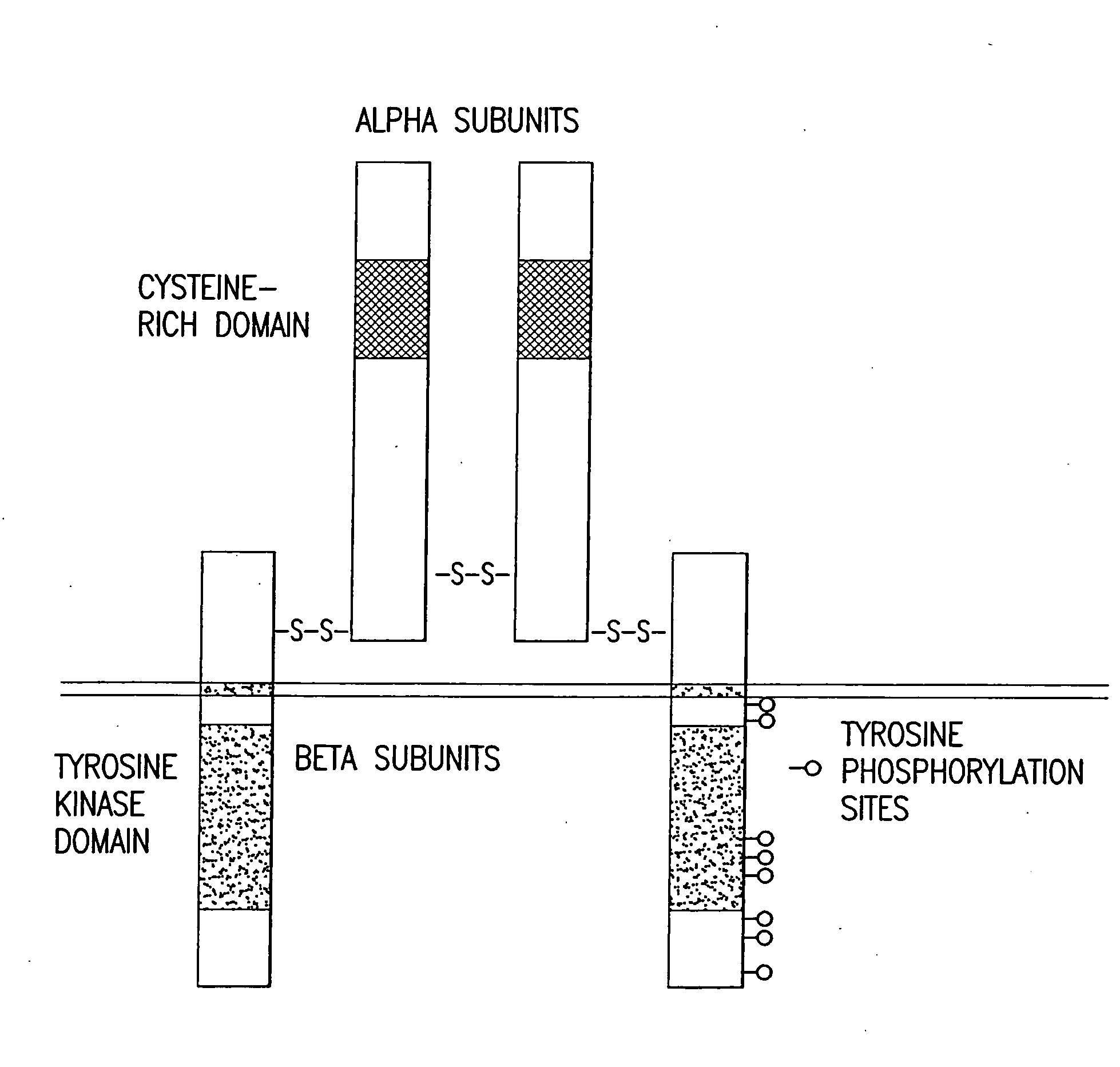
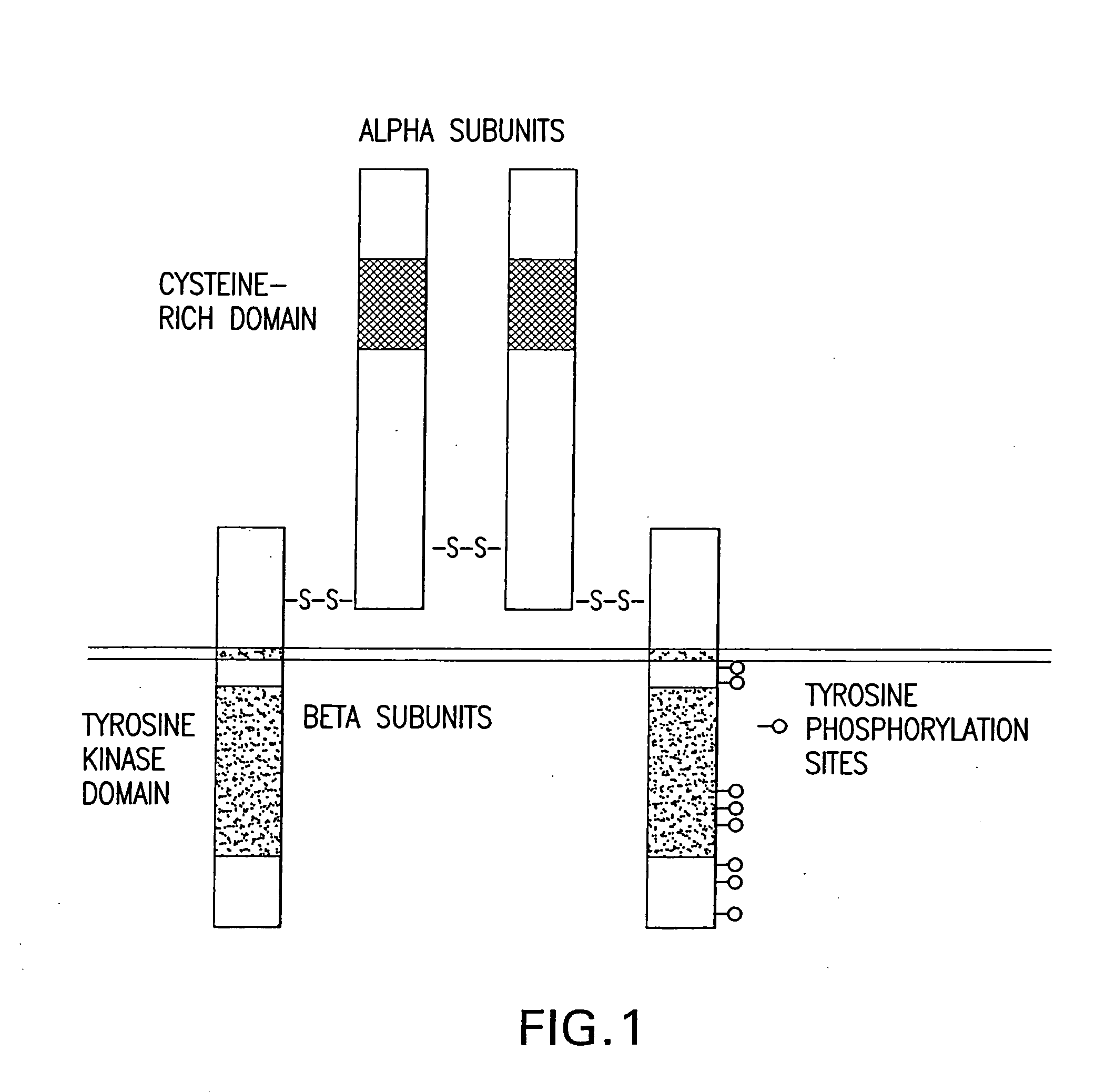
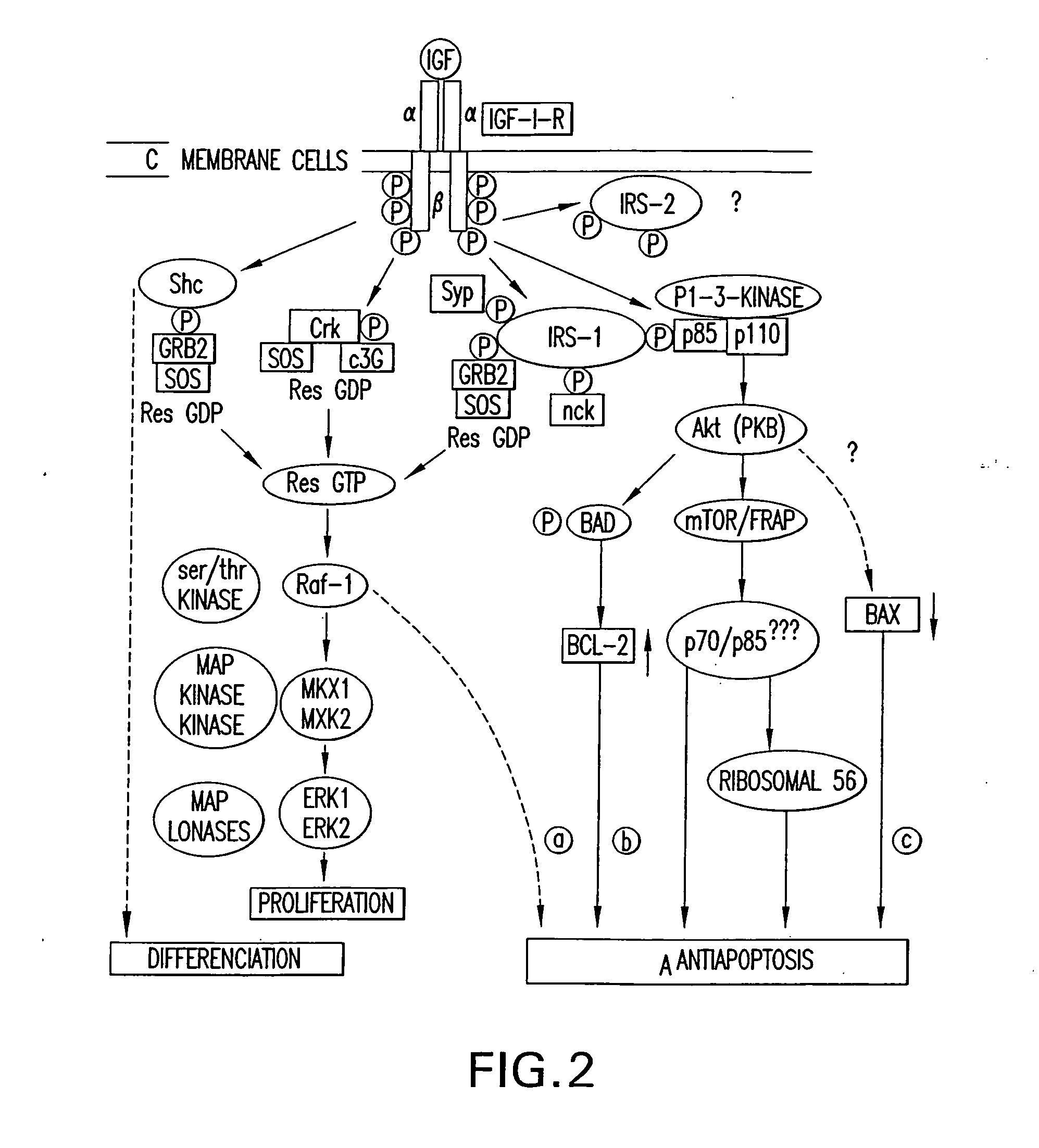
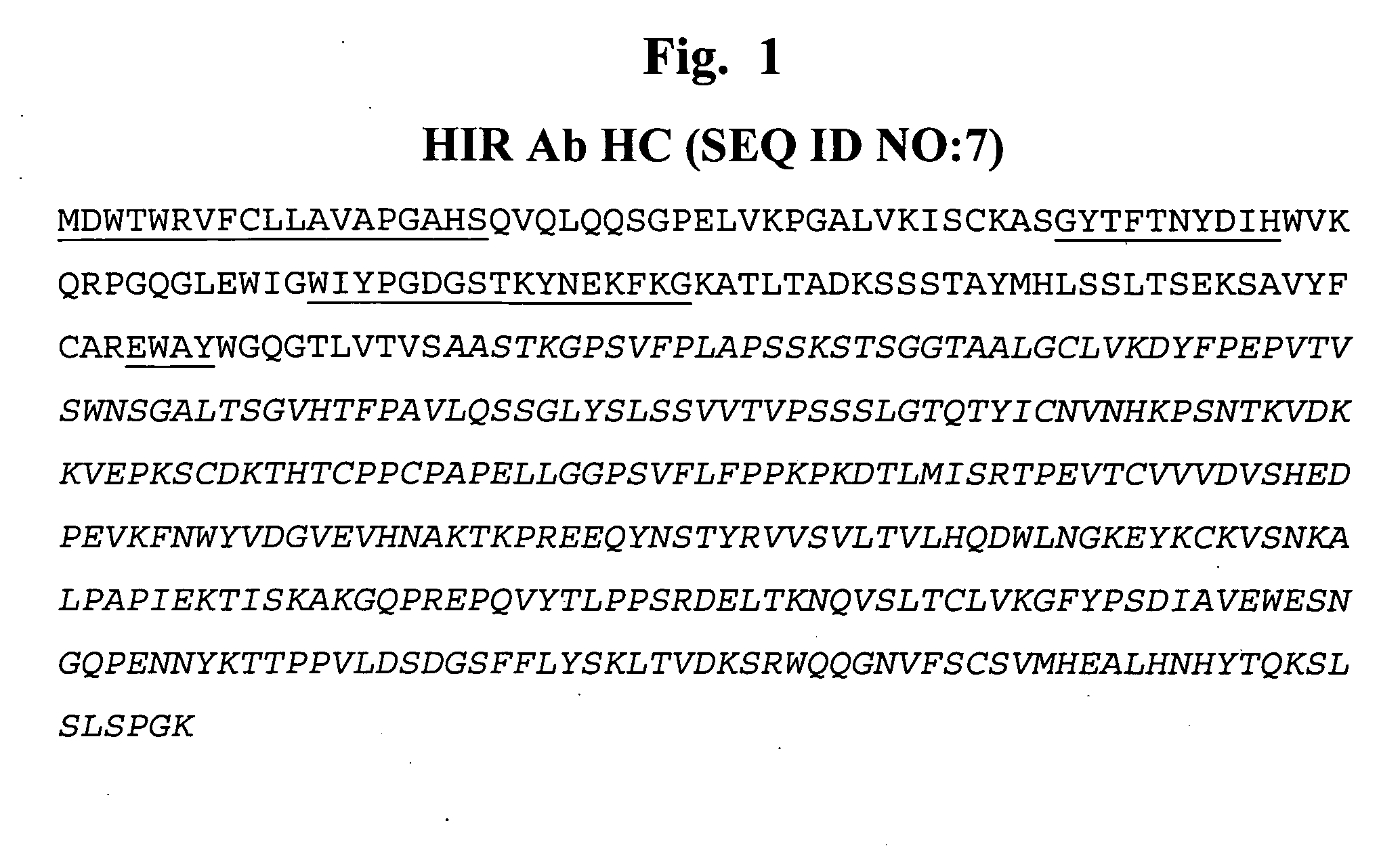
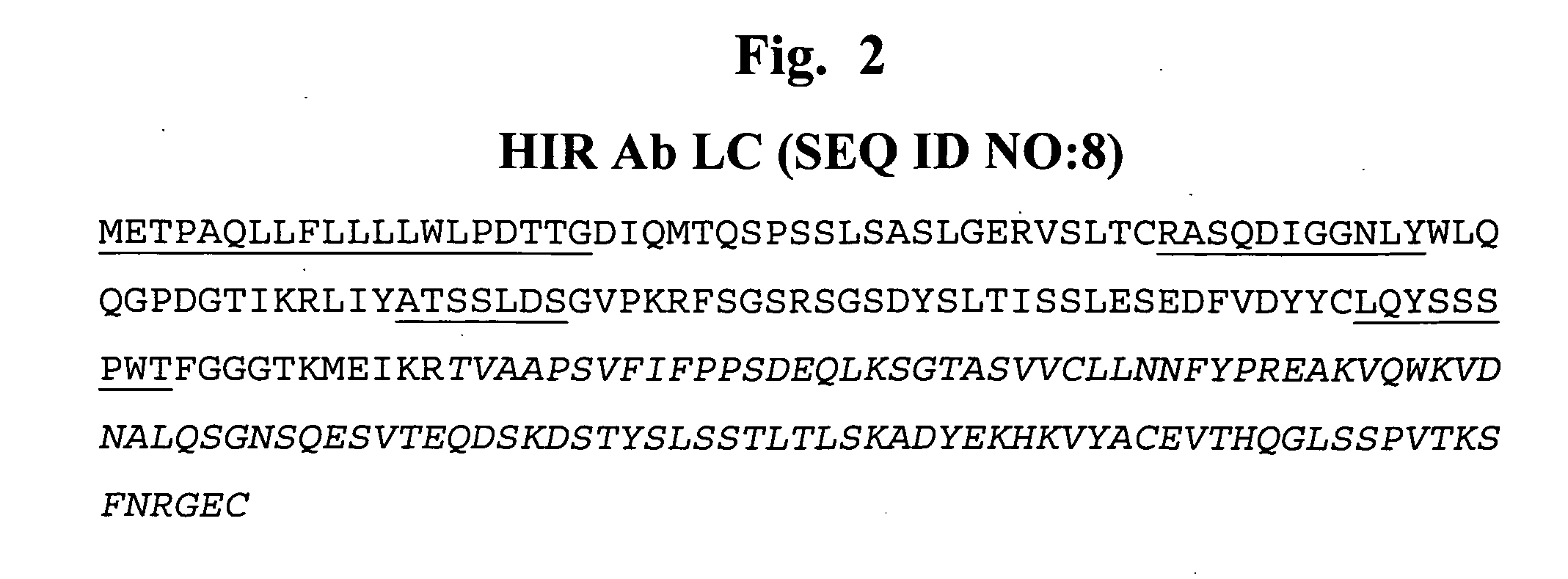
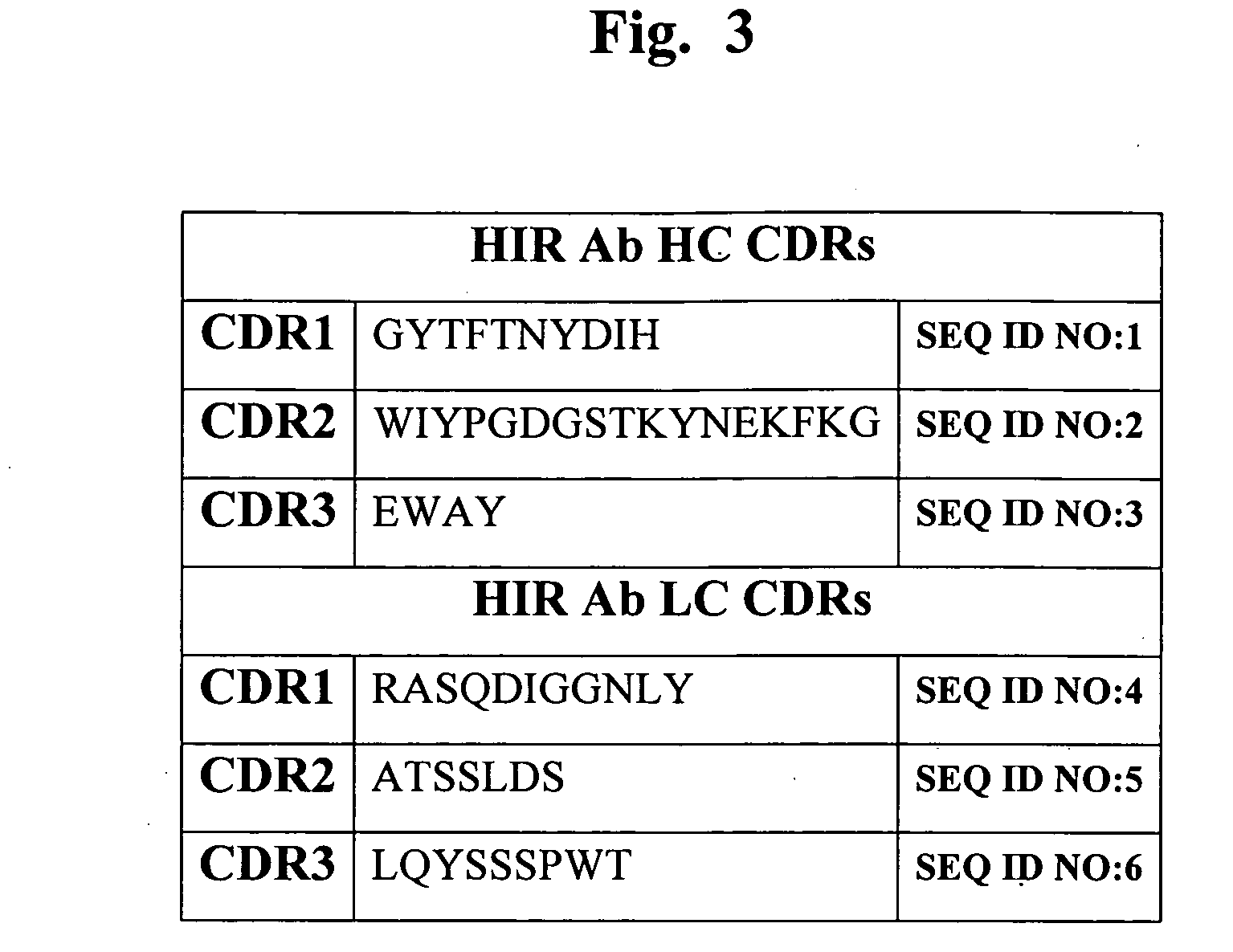
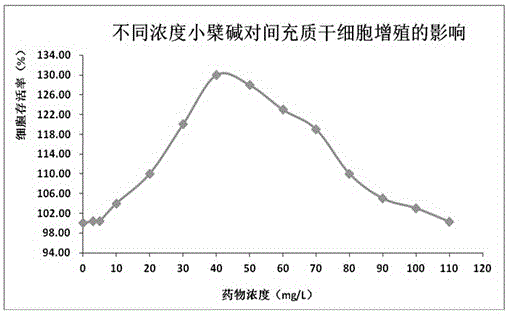
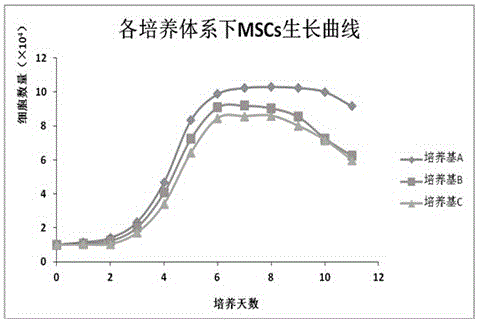
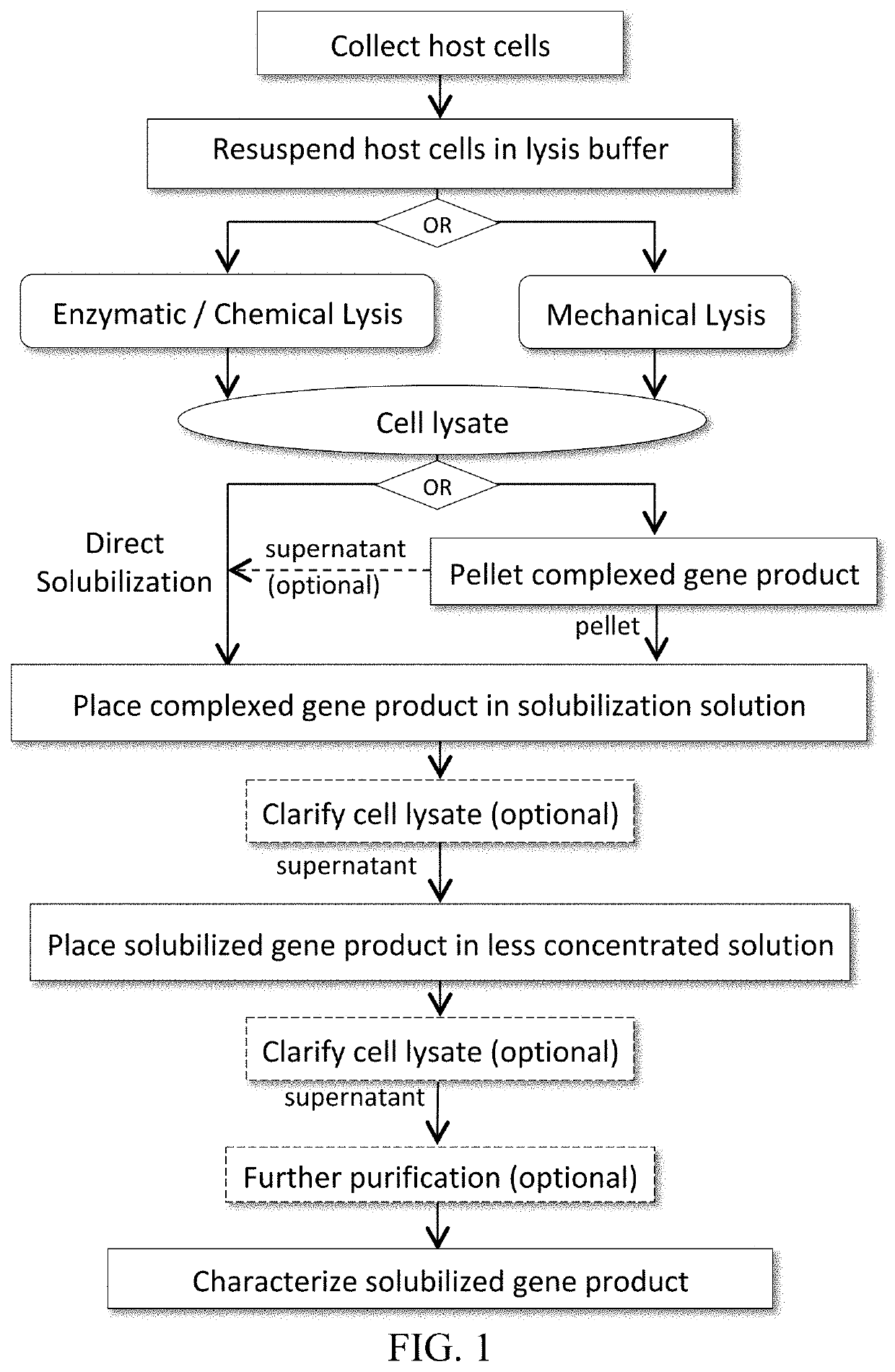
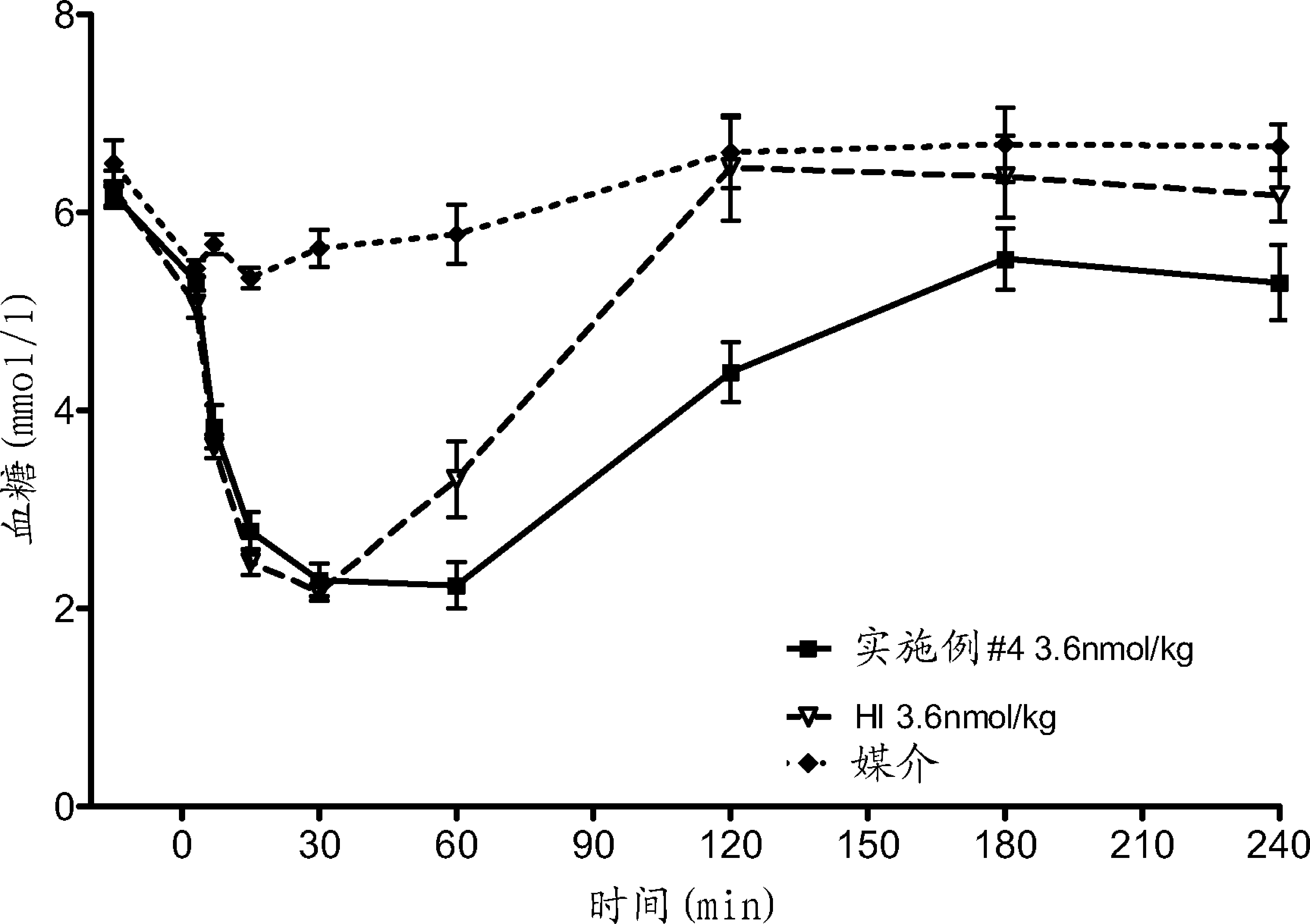
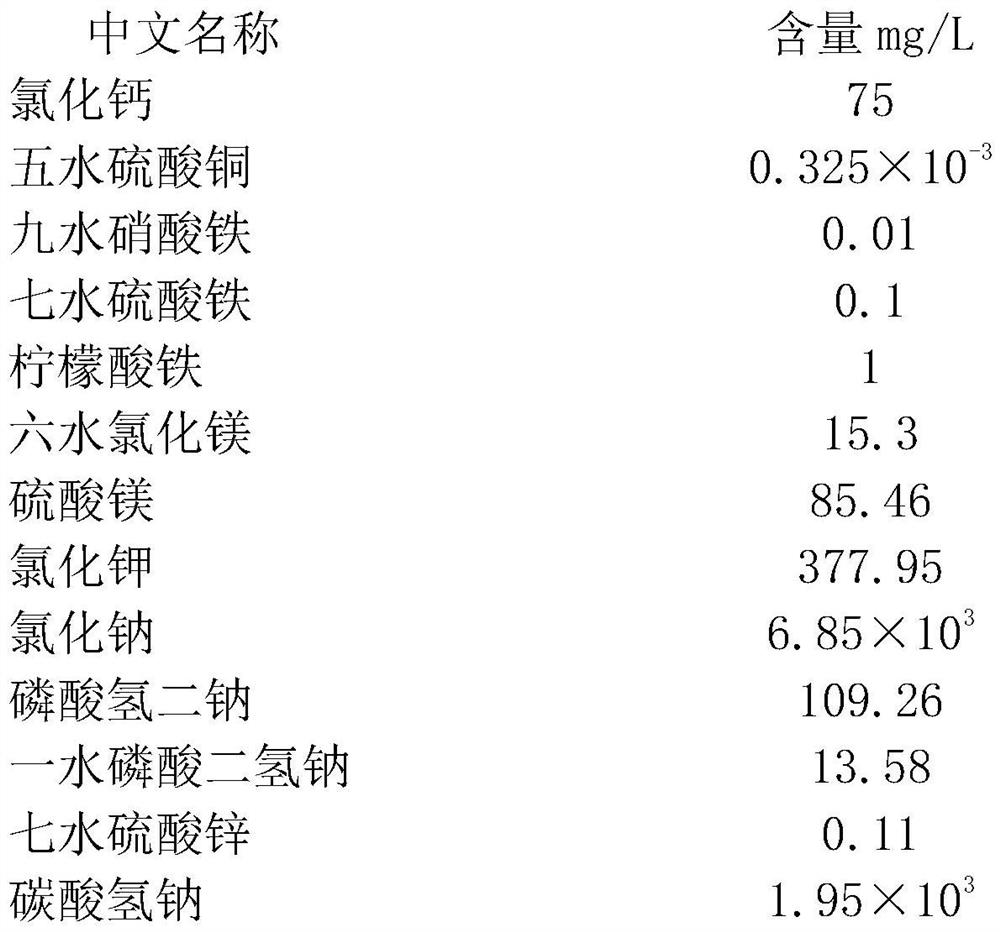
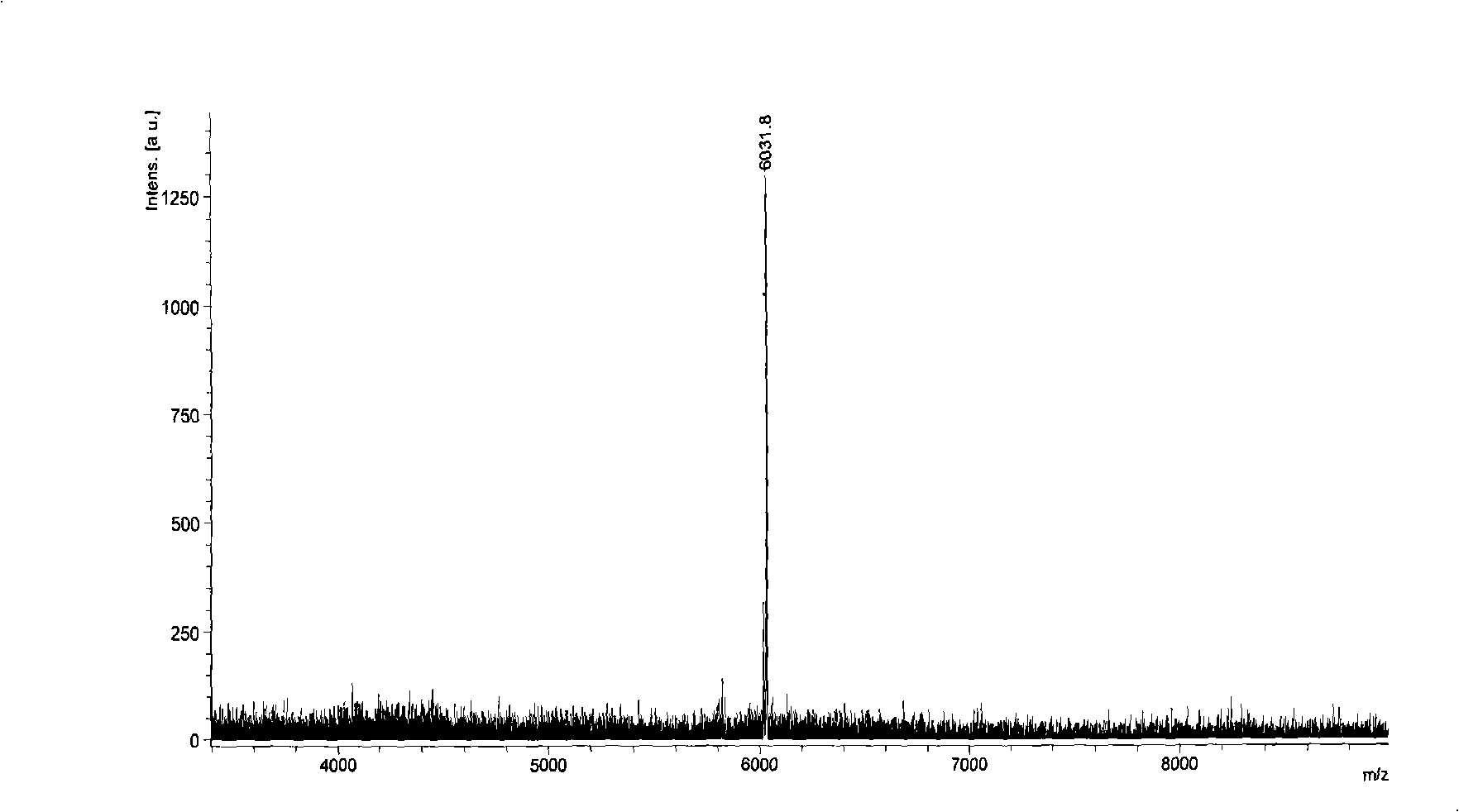
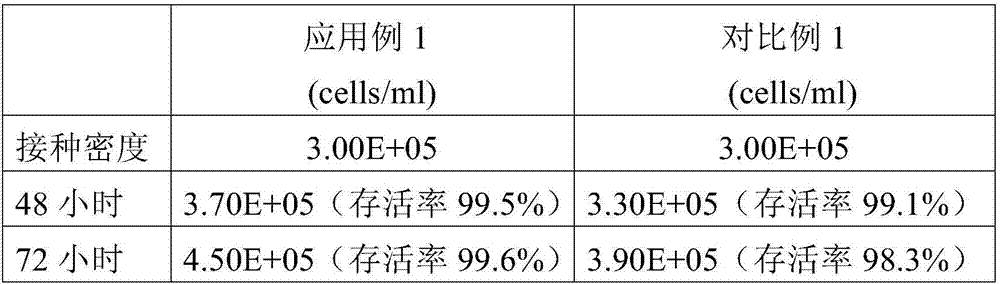
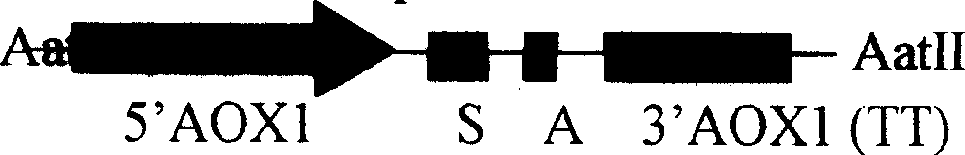Patents
Literature
66 results about "INSULIN HUMAN" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
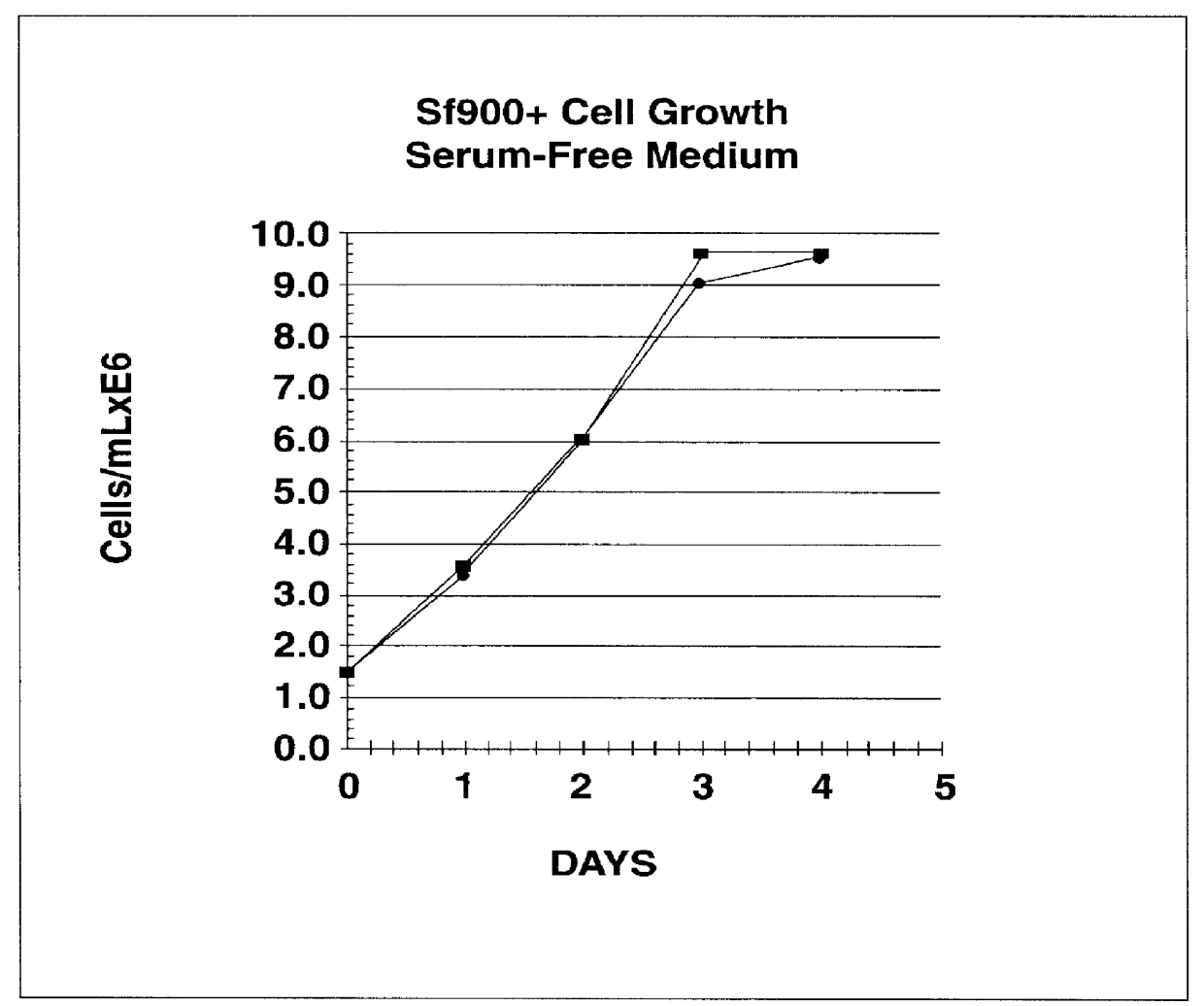
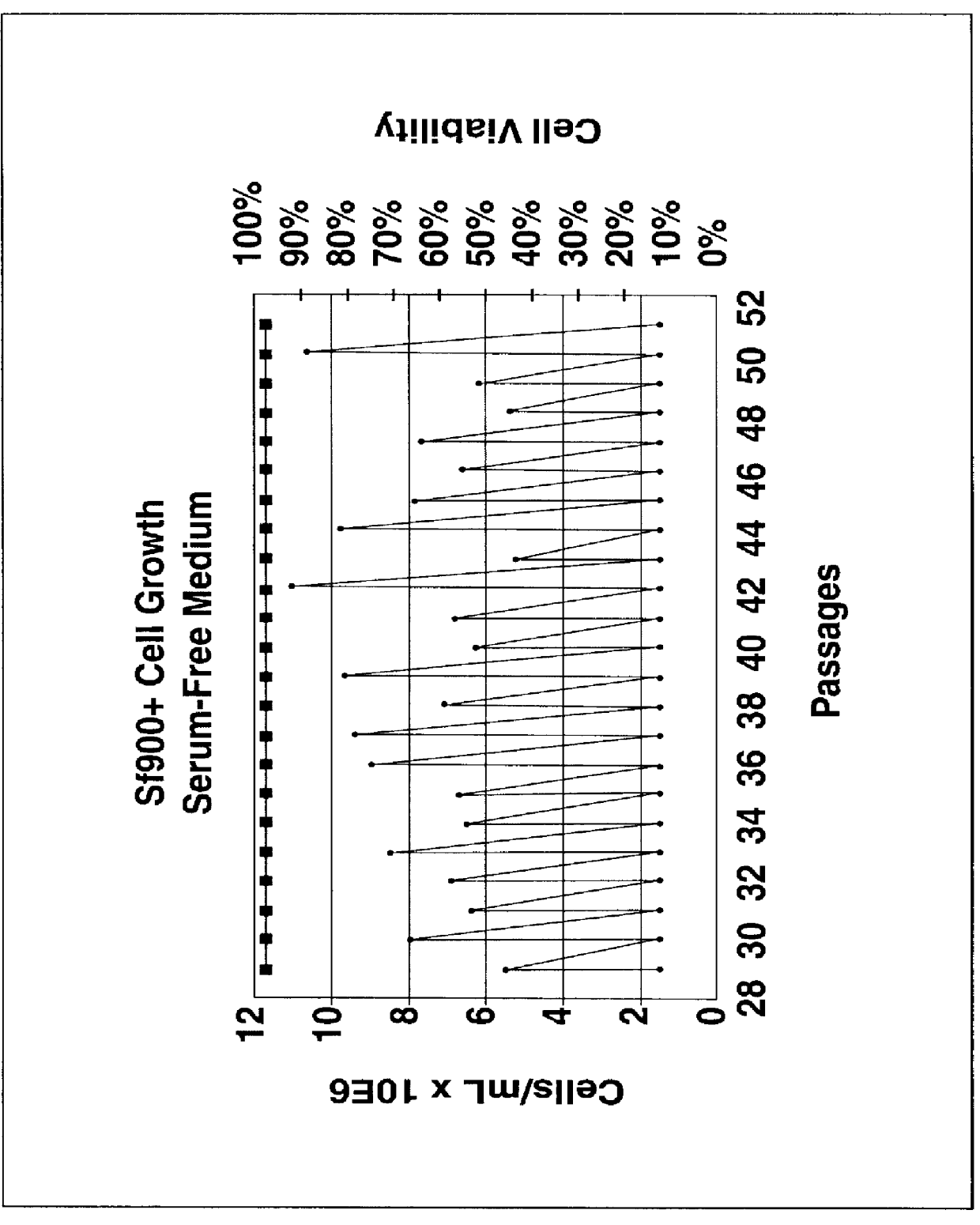
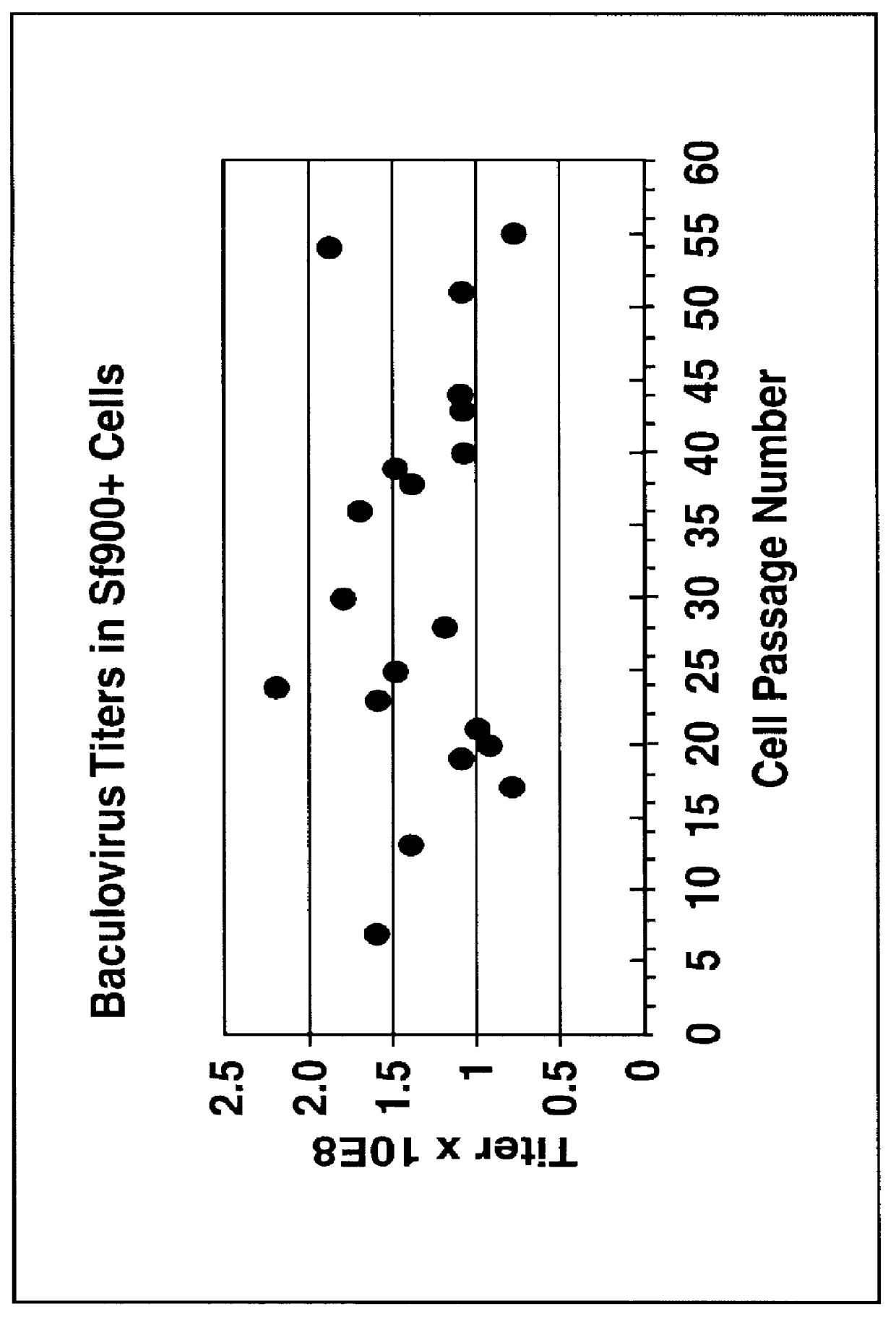
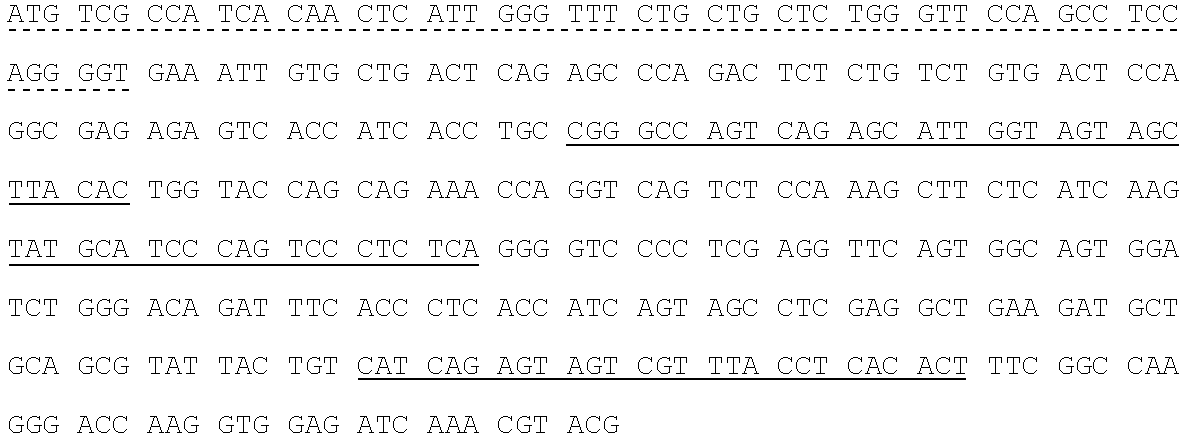
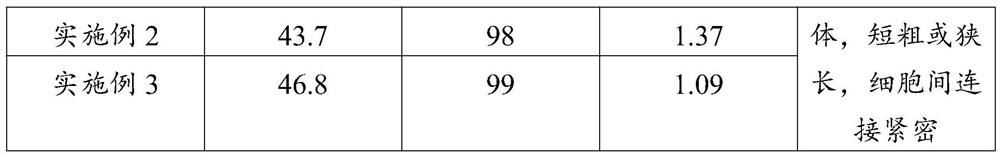
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Neutralizing human anti-IGFR antibody
The present invention includes fully human, neutralizing, monoclonal antibodies against human Insulin-like Growth Factor Receptor-I (IGFR1). The antibodies are useful for treating or preventing cancer in a subject. Also included are methods of using and producing the antibodies of the invention.
Owner:MERCK SHARP & DOHME CORP
Novel anti-IGF-IR antibodies and uses thereof
InactiveUS20080193445A1Stimulate interestPromote secretionMicrobiological testing/measurementImmunoglobulins against growth factorsDiseaseAnticarcinogen
The present invention relates to novel antibodies capable of binding specifically to the human insulin-like growth factor I receptor IGF-IR and / or capable of specifically inhibiting the tyrosine kinase activity of said IGF-IR receptor, especially monoclonal antibodies of murine, chimeric and humanized origin, as well as the amino acid and nucleic acid sequences coding for these antibodies. The invention likewise comprises the use of these antibodies as a medicament for the prophylactic and / or therapeutic treatment of cancers overexpressing IGF-IR or any pathology connected with the overexpression of said receptor as well as in processes or kits for diagnosis of illnesses connected with the overexpression of the IGF-IR receptor. The invention finally comprises products and / or compositions comprising such antibodies in combination with anti-EGFR antibodies and / or compounds and / or anti-cancer agents or agents conjugated with toxins and their use for the prevention and / or the treatment of certain cancers.
Owner:INST DE RECH PIERRE FABRE +1
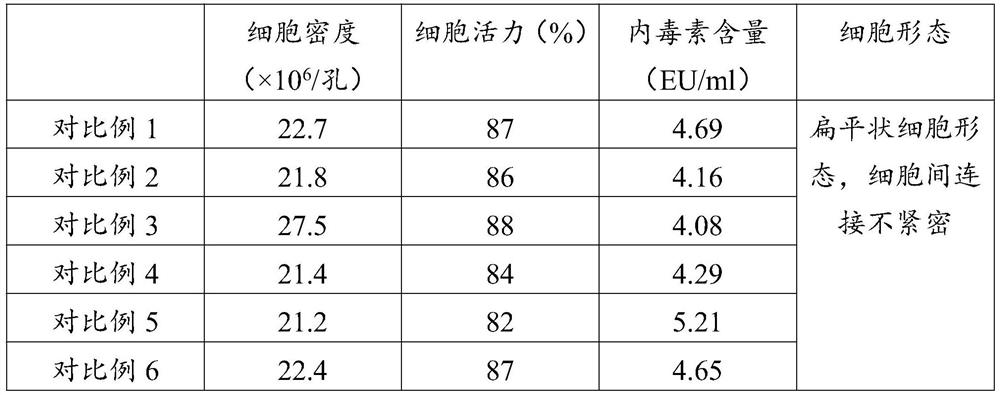
Serum-free culture medium for mesenchymal stem cells
ActiveCN102433302AGood growthFast growthSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
The invention discloses a serum-free culture medium for mesenchymal stem cells. The serum-free culture medium for the mesenchymal stem cells comprises the following ingredients: fibronectin at the final concentration of 25mu g / ml, basic fibroblast growth factors at the final concentration of 10ng / ml, human epidermal growth factors at the final concentration of 15ng / ml, recombinant human insulin at the final concentration of 1mg / ml, human transferrin at the final concentration of 0.55mg / ml, human blood albumin in a volume ratio of 5 percent, sodium selenite at the final concentration of 0.67mug / ml, L-carnitine at the final concentration of 5mM and resveratrol at the final concentration of 30mu M. When the mesenchymal stem cells are cultured by the serum-free culture medium for the mesenchymal stem cells, animal-derived serum is not contained, so infection risks can be controlled; the L-carnitine and the resveratrol which are added into the serum-free culture medium for the mesenchymalstem cells can effectively improve the growth state of the mesenchymal stem cells; and the growth speed of the mesenchymal stem cells is remarkably improved, and the biological characteristics of themesenchymal stem cells are kept unchanged.
Owner:CHENGDU QINGKE BIOTECH
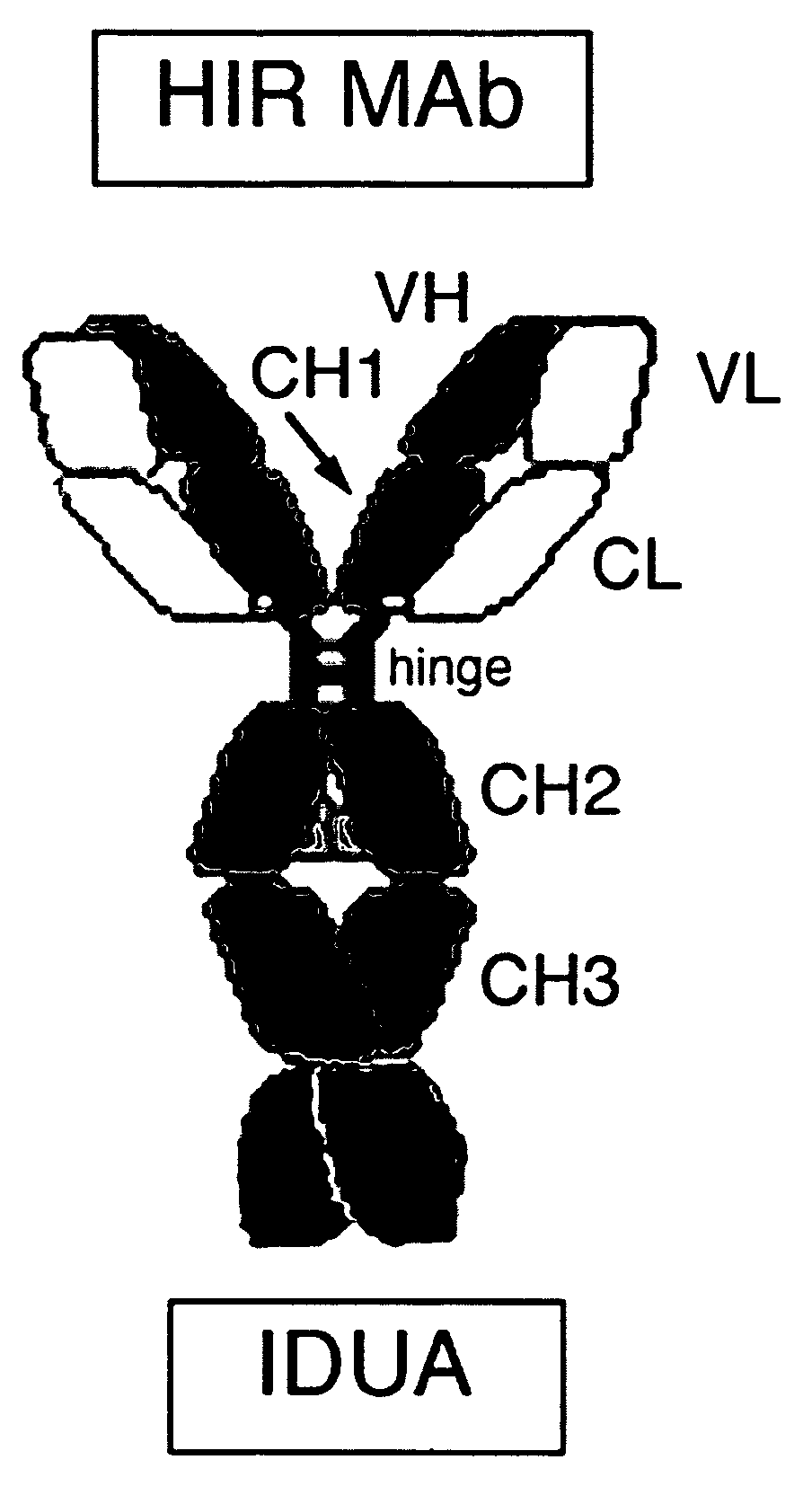
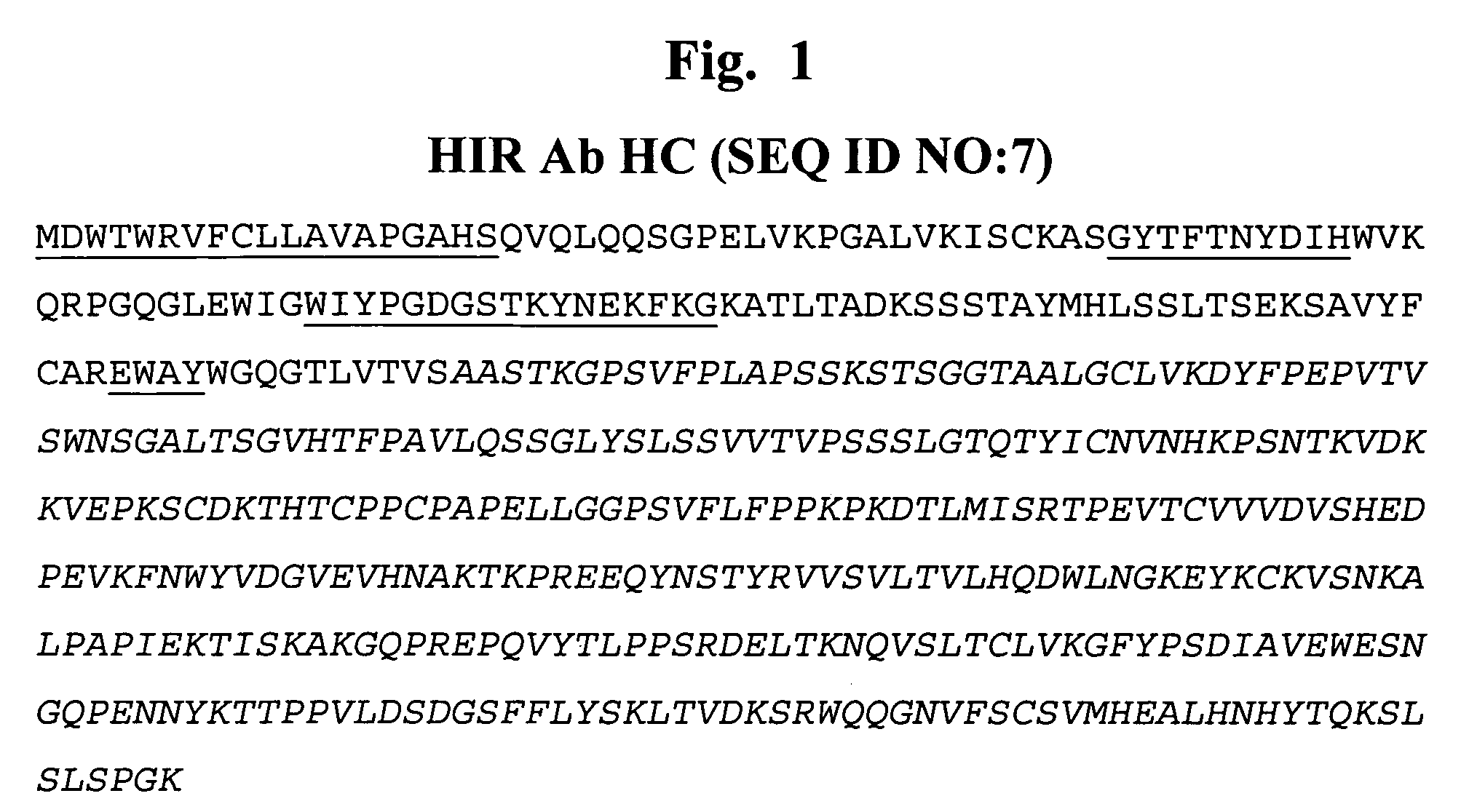
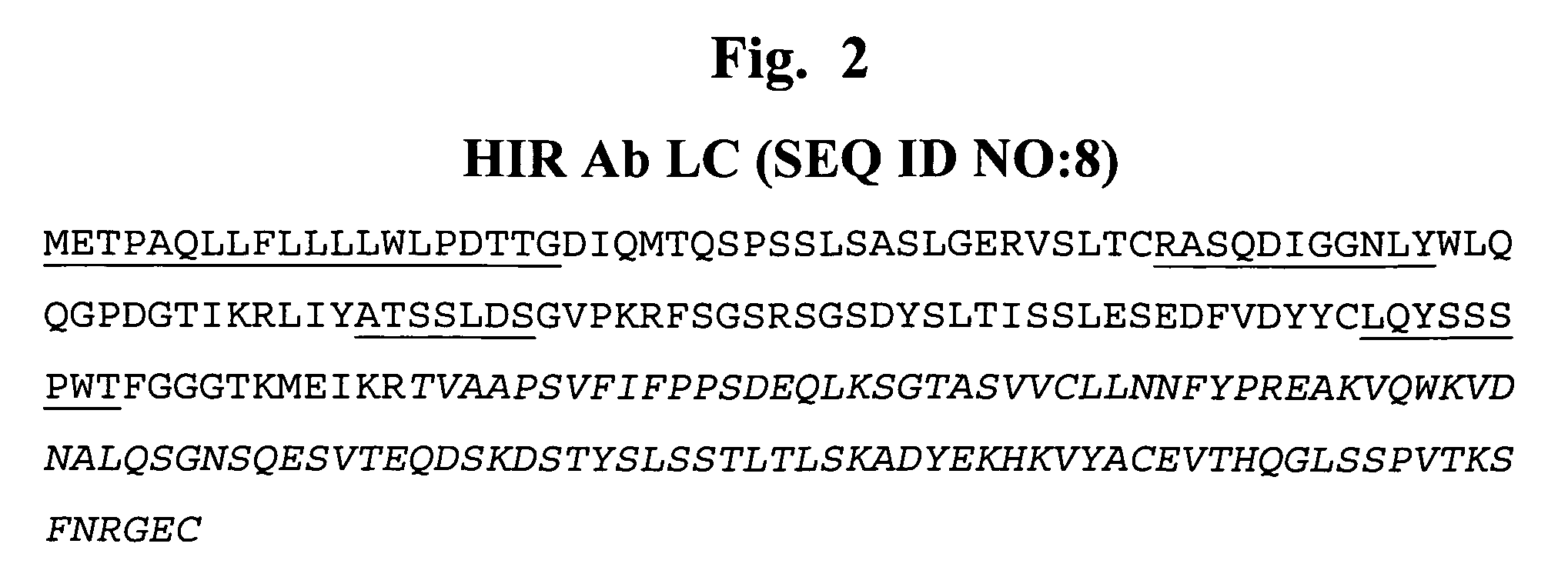
Methods and compositions for increasing alpha-l-iduronidase activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in α-L-Iduronidase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an α-L-Iduronidase. A therapeutically effective systemic dose is based on the specific CNS uptake characteristics of human insulin receptor antibody-α-L-Iduronidase fusion antibodies as described herein.
Owner:ARMAGEN INC +1
Neural stem cells medium and method for performing human neural stem cells in-vitro long-term culture and amplification by using neural stem cells medium
ActiveCN105062972AGenetic stabilitySolve the easy differentiation of in vitro cultureNervous system cellsInsulin activityCuticle
The invention relates to a neural stem cells medium and a method for performing human neural stem cells in-vitro long-term culture and amplification by using the neural stem cells medium. The neural stem cells medium comprises the following ingredients by weight proportion: 100-1000 micrograms of heparin sodium, 10-100 micrograms of vitamin E, 5-50 milligrams of insulin human recombinant, 0.5-5 milligrams of putrescine, 2-10 micrograms of sodium selenite, 2-10 milligrams of human transferrin, 2-10 micrograms of progestin, 300 milligrams of L-glutamine, 5.9 grams of 2-[4-(2-Hydroxyethyl)-1-piperazine]ethanesulfonic acid, 10-100 micrograms of recombinant human epidermal growth factors, 10-100 micrograms of recombinant human basic fibroblast growth factors, 20-200 milligrams of vitamin C glucoside and 40,000-400,000 IU (international unit) of gentamicin. By the neural stem cells medium, the technical problems that human neural stem cells are easy to differentiate when cultured in vitro and long-term culture and amplification are difficult to implement are solved.
Owner:ZHEJIANG ORIGIN BIOTECH
Berberine-containing serum-free medium for mesenchymal stem cells
ActiveCN105112366ALong growth cycleGuaranteed Biological PropertiesSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
Owner:广东美赛尔细胞生物科技有限公司
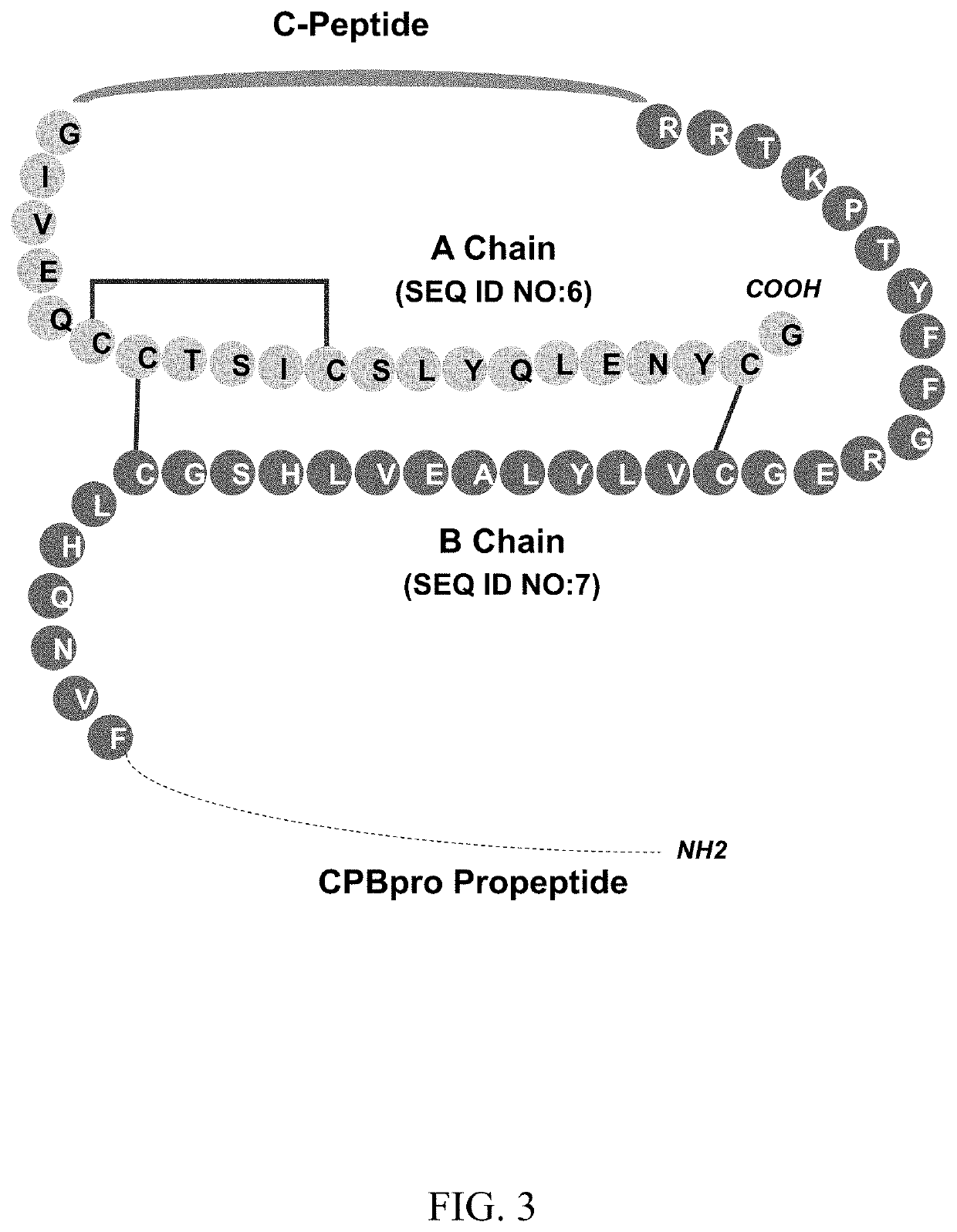
C-peptides and proinsulin polypeptides comprising the same
A connecting polypeptide has SEQ ID NO:73. A proinsulin polypeptide includes a mature insulin A-chain, a mature insulin B-chain, and a connecting peptide comprising SEQ ID NO: 73 linking the mature A-chain and the mature B-chain, wherein the connecting peptide is not a native human proinsulin C-peptide. The proinsulin polypeptides according to the invention can be made in high titers and in high purity.
Owner:ABISCI LLC
Serum-free medium for placenta-derived mesenchymal stem cells and preparation method thereof
ActiveCN105112362AImprove securityImprove proliferative abilitySkeletal/connective tissue cellsINSULIN HUMANProliferative capacity
The invention belongs to the technical field of stem cells and relates to a serum-free medium for placenta-derived mesenchymal stem cells and a preparation method thereof. The serum-free medium comprises a DMEM basic medium and further comprises vitamin H, glutathione, recombinant human insulin, human serum albumin, transferrin, fibroblast growth factors, epidermal growth factors, stem cell growth factors, Human stem cell factors and magnolol. The serum-free medium provided by the invention has the advantages of high safety and capability for significant improvement of proliferative capability of placenta-derived mesenchymal stem cells, the serum-free medium is capable of well keeping cell forms and stem cell characteristics of the placenta-derived mesenchymal stem cells and is convenient to popularize and apply.
Owner:广东美赛尔细胞生物科技有限公司
Immunological adjuvant with immunity vegulating agent for treating and preventing diabetic from insulin-dependent
InactiveCN1831012AReduce immune damageDoes not induce atherosclerosisPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesInsulin dependent
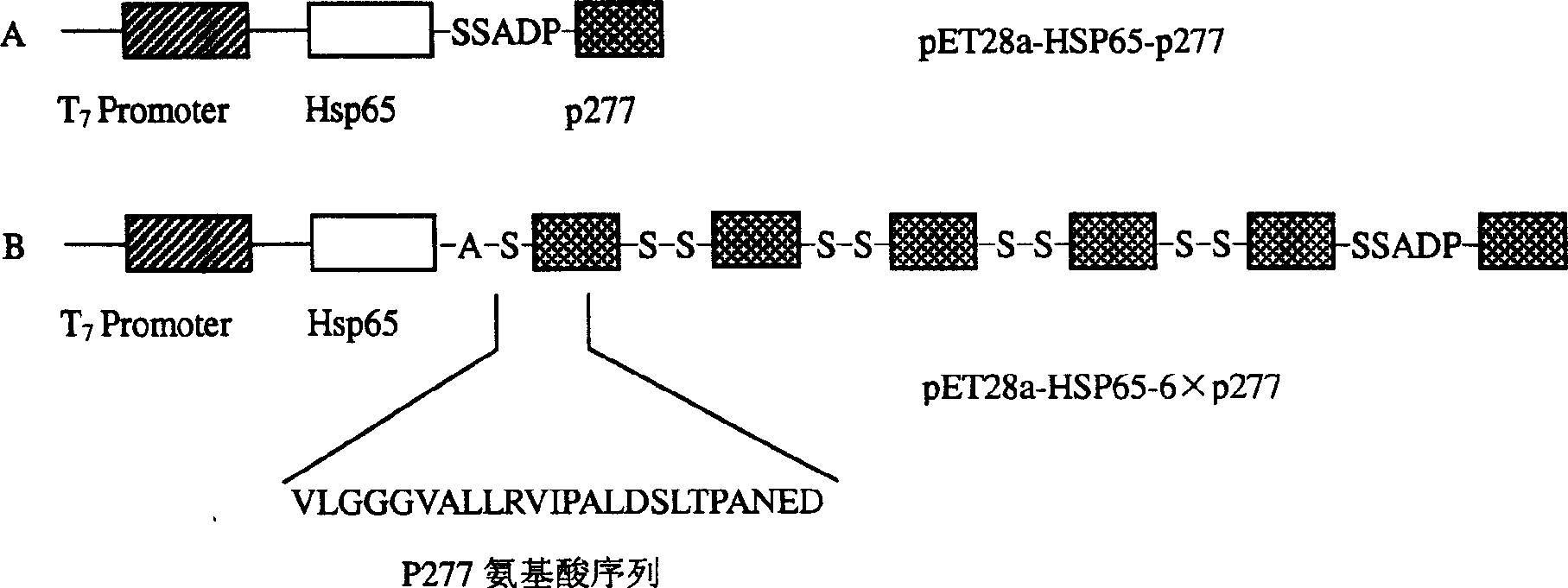
The invention provides the immunomodulator with the free adjuvant having the function of preventing and curing the insulin-dependent diabetes mellitus. Aiming at the weak immunogenicity, the antigen epitope polypeptide gene (a length of antigen epitope p277 rooted from human HSP60) is associated repeatedly six times and inserted repeatedly into the backward position of the heat shock protein HSP65 gene rooted from the mycobacterium bovis, the fusing expression between the repeat series connecting antigen polyeptide and the HSP65 is realized. The produced fusing albumen did not need the adjuvant; the antigen polyeptide don't need be coupled with the carrier chemistry to the immunity. The fusing albumen can inspire the organism to produce the high titer aiming at p277 by means of the mucous membrane immunity, so the nosogeny of NOD chmice diabetic are depressed highly. The invention can prevent the 1 type and 1.5 type diabete characterized in depending on the insulin.
Owner:CHINA PHARM UNIV
Culture medium, application thereof and culture method
ActiveCN105154402AIncrease multiplierExtend the number of culture daysBlood/immune system cellsSurface markerVitamin C
The invention relates to the technical field of cell culture, in particular to a culture medium, application thereof and a culture method. The culture medium comprises transferrin, recombinant human insulin, progesterone, fetal calf serum, stem cell growth factors, vitamin C, interleukin 2, interleukin 4, zinc sulfate and a DMEM / F12 culture medium. When the culture medium is used for culturing CD19-CAR-T cells, the cell proliferation multiple can be improved, the number of cell culture days is increased, and the CD19CAR and other surface markers can be kept expressed.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Methods and compositions for increasing α-L-iduronidase activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in α-L-Iduronidase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an α-L-Iduronidase. A therapeutically effective systemic dose is based on the specific CNS uptake characteristics of human insulin receptor antibody-α-L-Iduronidase fusion antibodies as described herein.
Owner:ARMAGEN INC +1
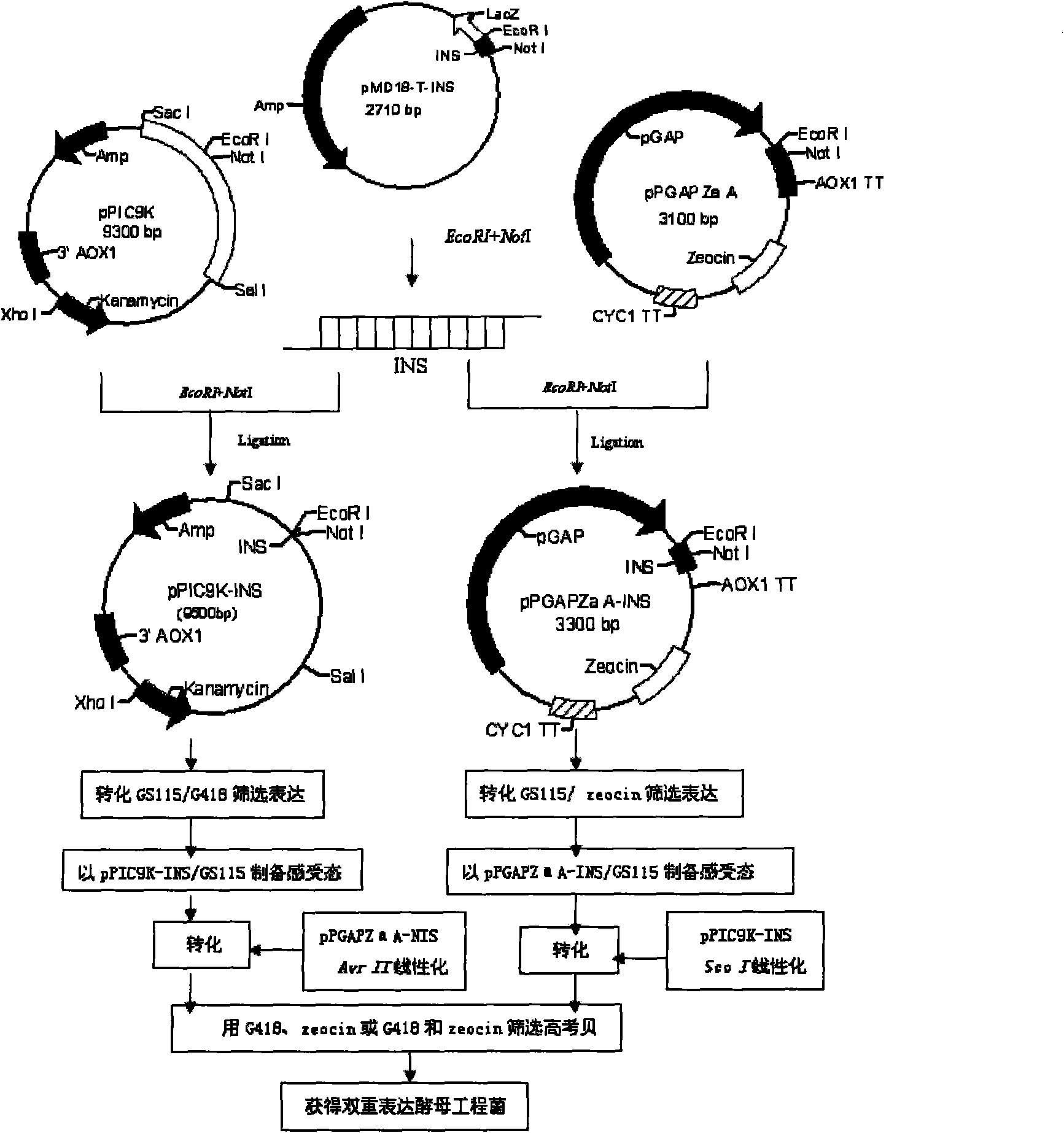
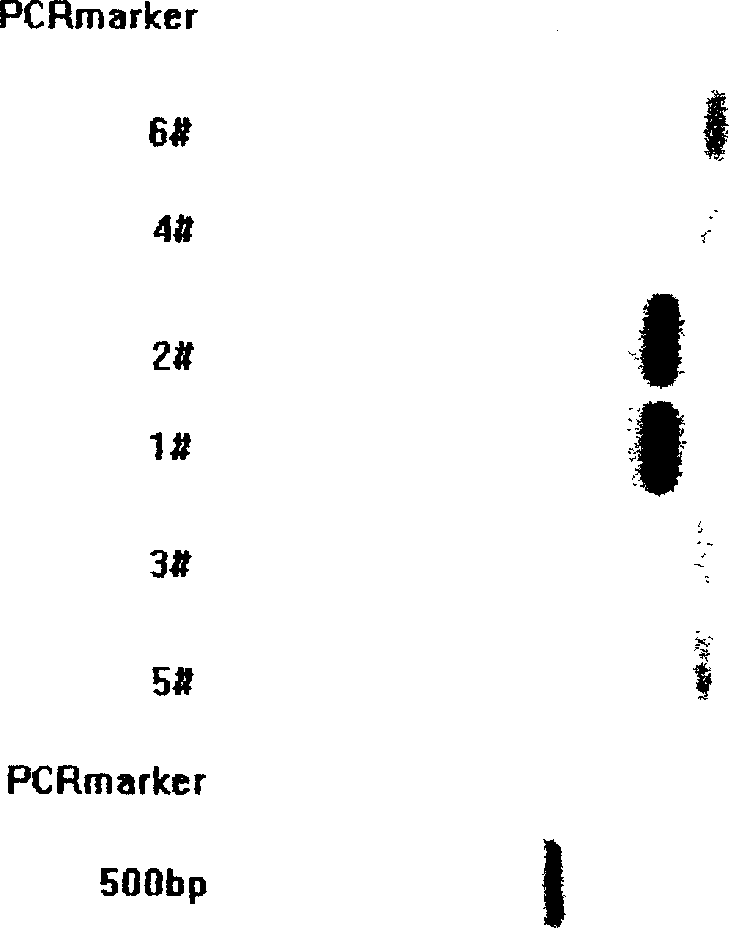
Expression of human insulin and analogues thereof in dual-promoter of methanol yeast and preparation of human insulin and analogues thereof
ActiveCN102051372AEfficient preparationReduce manufacturing costMicroorganism based processesFermentationINSULIN HUMANDual promoter
The invention discloses expression of human insulin and analogues thereof in a methanol yeast containing dual promoters and a high-efficient preparation method of the human insulin and the analogues thereof, and the method is characterized in that recombinant human insulin and the analogues thereof are prepared by constructing an expression vector containing the dual promoters and engineering cells in host cells of the methanol yeast, and performing the optimal fermentation technology and the high-efficient purification technology, so that the production cost of the recombinant human insulin and the analogues thereof can be effectively reduced.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Culture medium used for culturing adipose mesenchymal stem cells, and applications thereof
InactiveCN107267453AHigh purityGood stem cell propertiesCulture processSkeletal/connective tissue cellsHuman insulinTransferrin
The invention discloses a culture medium used for culturing adipose mesenchymal stem cells, and applications thereof. The culture medium comprises insulin human, human serum albumin, transferring, fibronectin, ascorbic acid, biotin, PDGF, bFGF, and TGF-beta. It is confirmed by experiments that culturing with the culture medium is capable of obtaining a large amount of high quality mesenchymal stem cells, and the mesenchymal stem cells possess excellent stem cell characteristics, higher immunosuppression activity, and low immunogenicity, and are more suitable to be used for allogeneic cell therapy. The potential risk of clinical applications of exogenous animal serum is avoided, cell pollution rate is reduced, proliferation of human adipose mesenchymal stem cells is promoted, aging speed of human adipose mesenchymal stem cells in in-vitro culture is reduced. Requirements on large scale industrialized production of adipose mesenchymal stem cells needed by clinical therapy are satisfied, and problems such as unstable properties of different batches and high cost are solved at the same time.
Owner:北京康爱瑞浩细胞技术有限公司
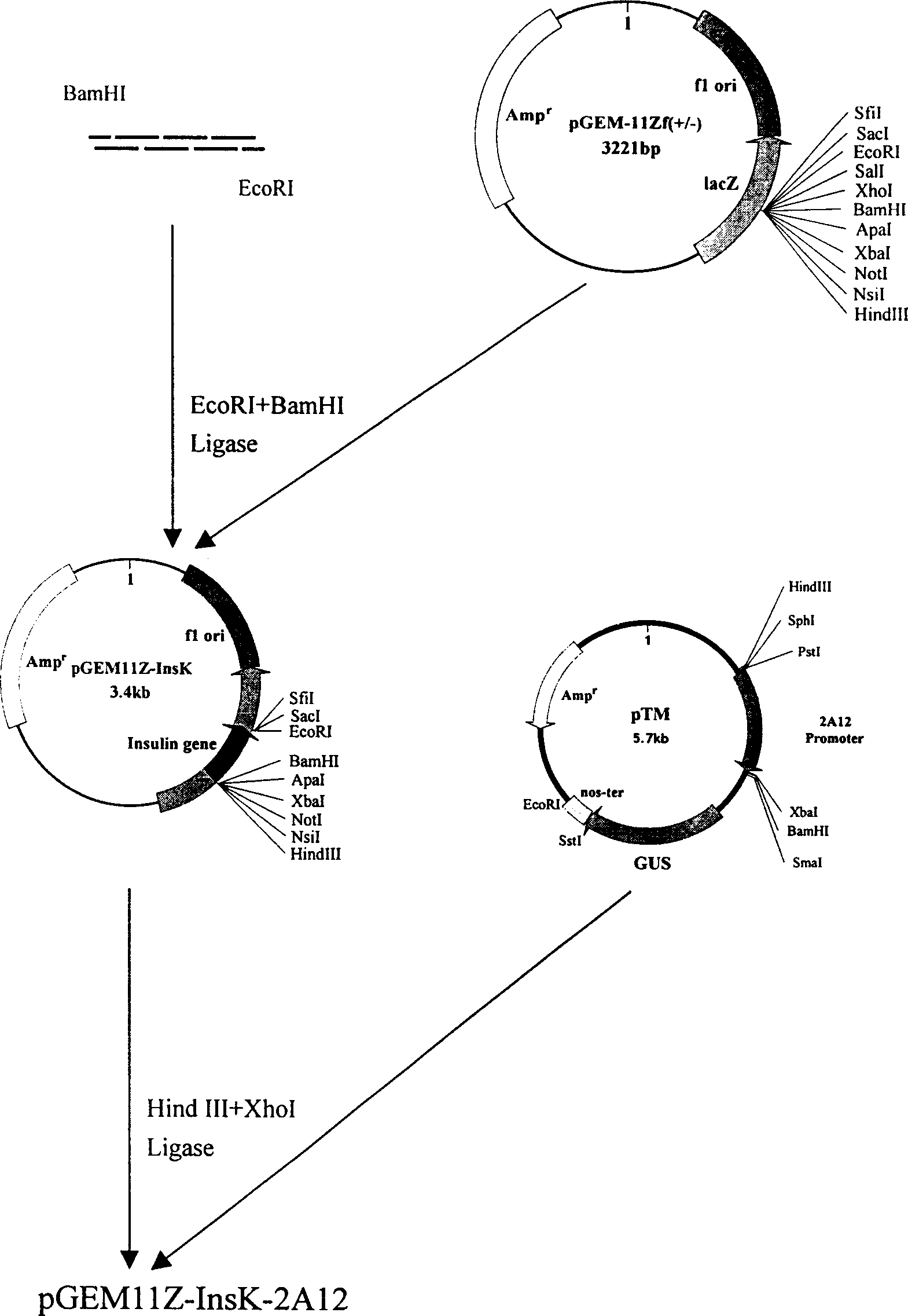
Process for producing human insulin using transgenic tomato
InactiveCN1458161AAvoid degradationLow costSugar derivativesMetabolism disorderReticulum cellPlant cell
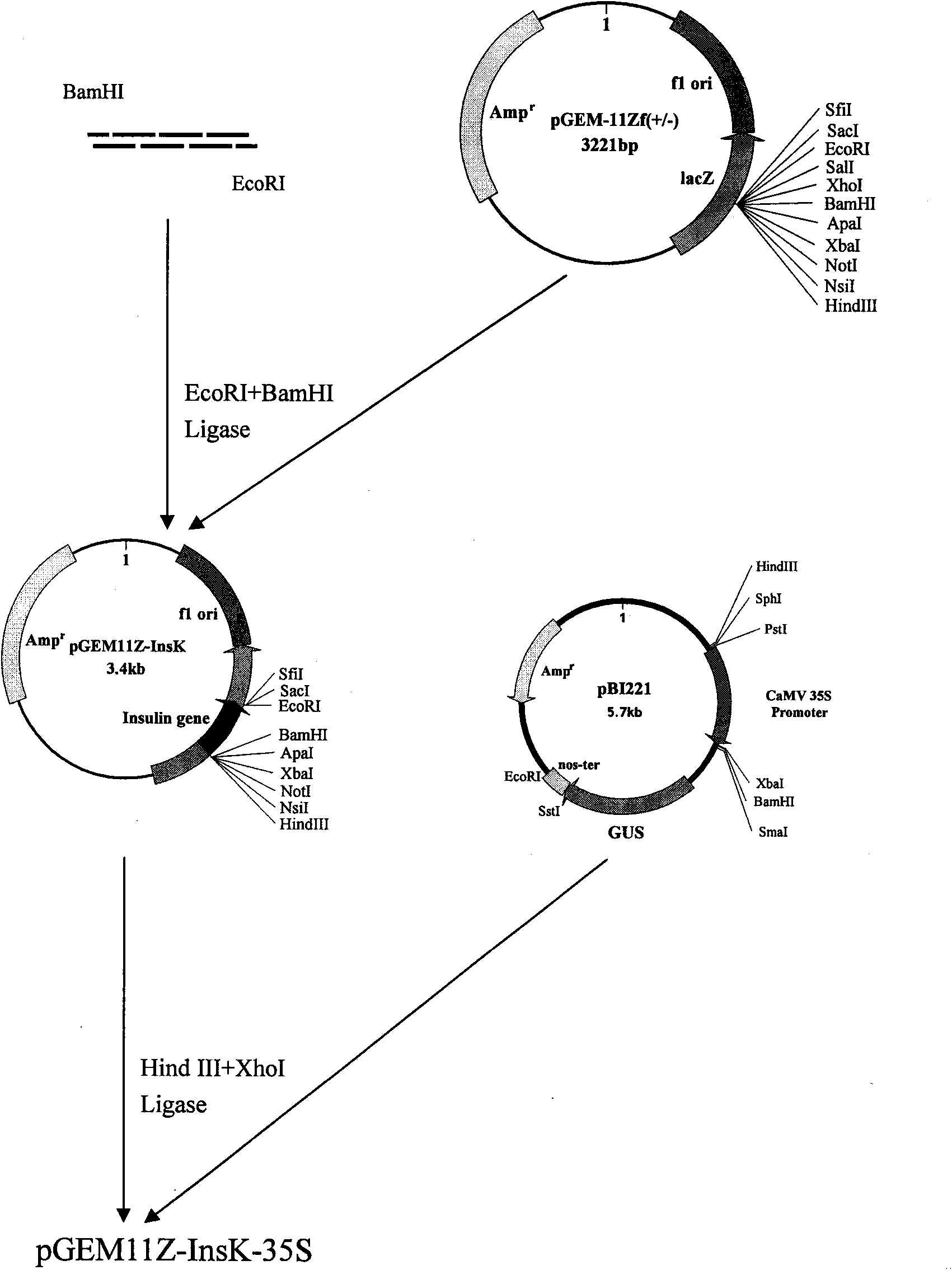
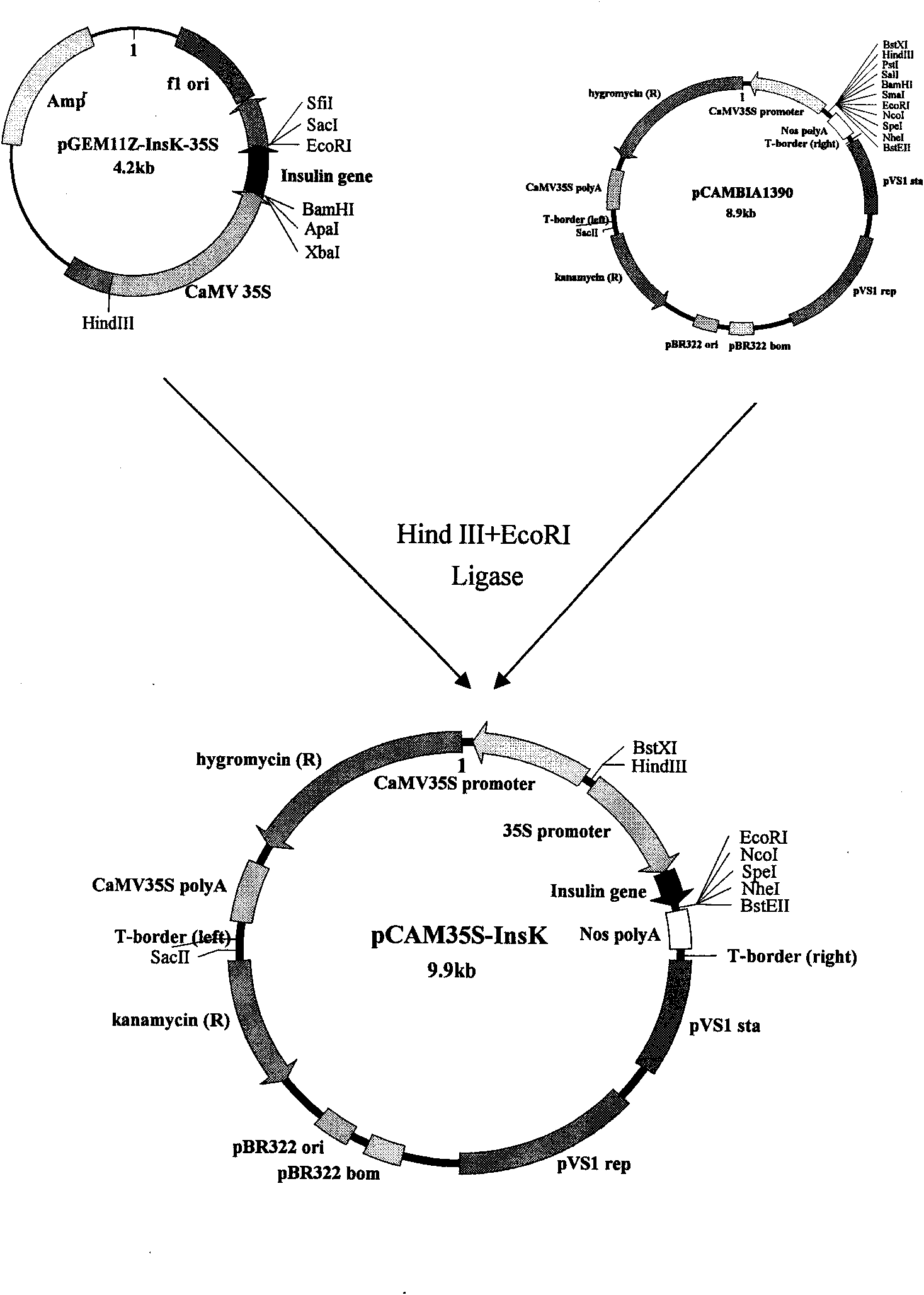
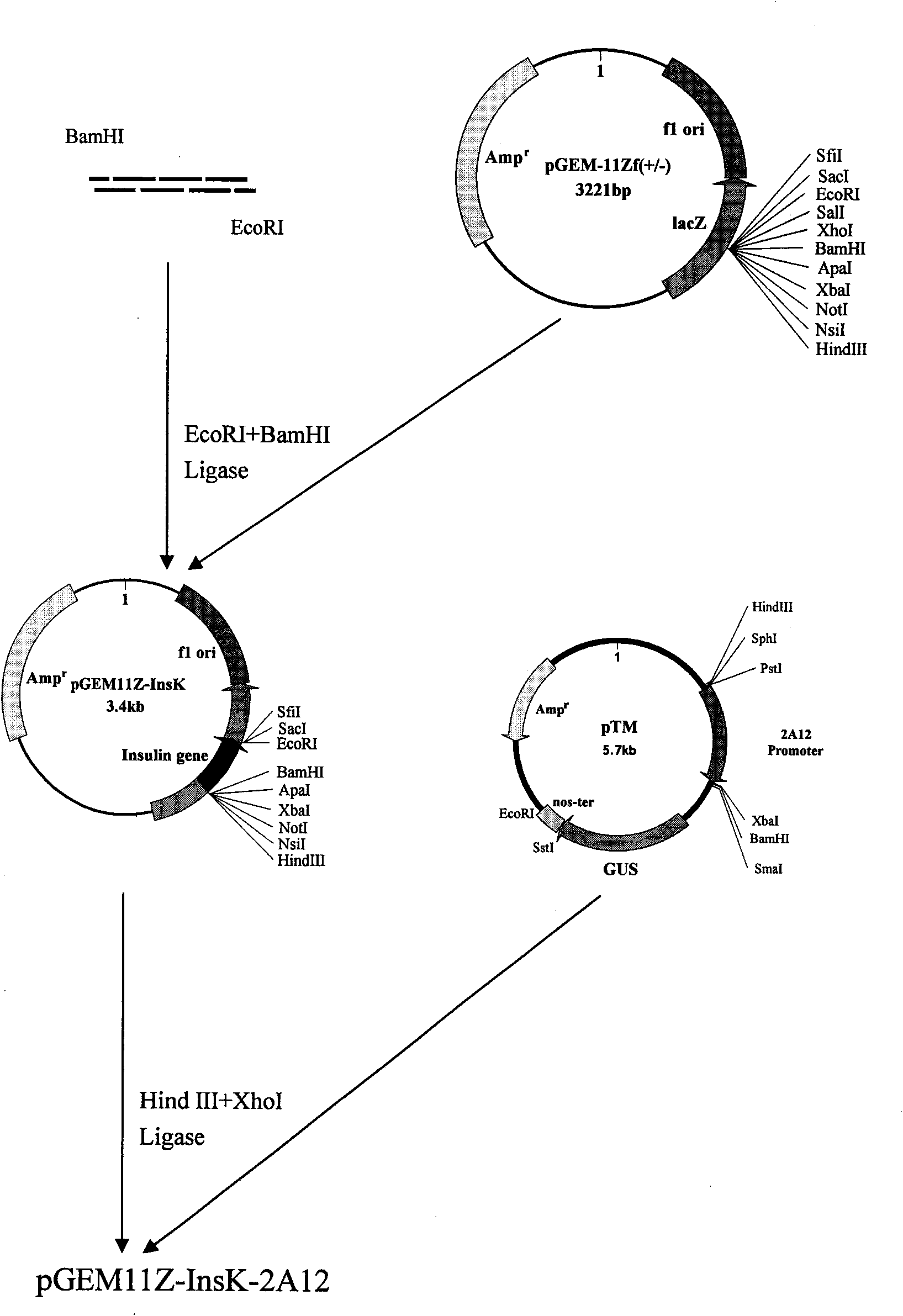
The present invention utilizes transgenic tomato as bioreactor to produce human insulin. The human insulin is produced through designing plant preference codon, artificially synthesizing several DNAsegment, splicing, and adding endoplasmic reticulum residence sequence of KDEL in the C terminal to avoid the degradation of insulin in plant cell. The gene is transformed into tomato via agrobacterium mediating process under the driving of CaMV35S promoter and fruit specificity promoter 2A12 to express human insulin in tomato fruit. This kind of tomato capable of producing human insulin may become one kind of convenient oral product for preventing and treating some diabetes of depending insulin and autoimmune dysfunction, and may be used to extract human insulin for making injection.
Owner:林忠平
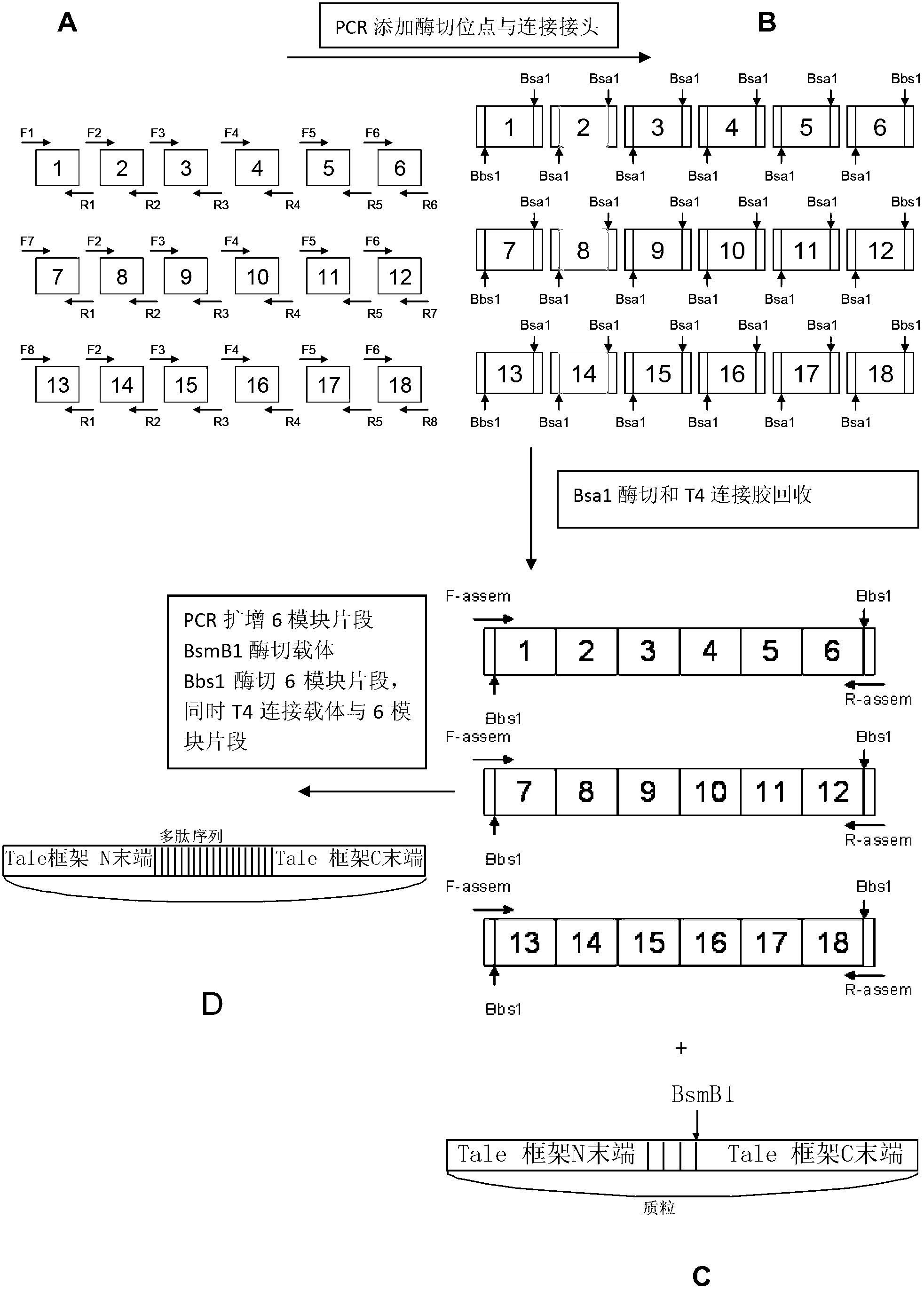
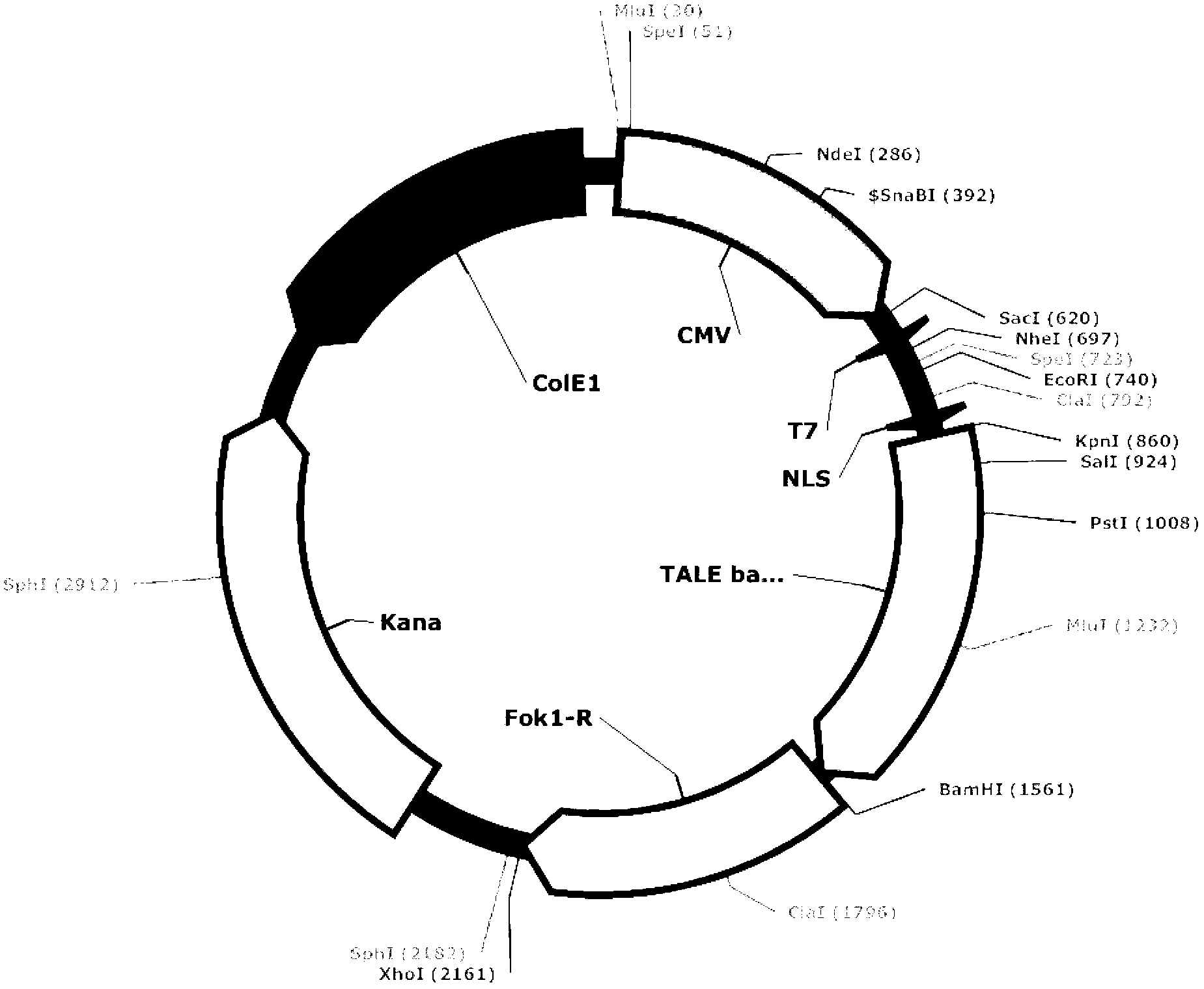
Recombinant transcription activator like effector, transcription activator like effector nuclease, as well as coding gene and application thereof
ActiveCN102702335AAchieve targeted modificationStrong specificityHydrolasesBacteria peptidesA-DNAInsulin Gene
The invention relates to the field of genetic engineering and discloses a pair of polypeptides and encoding genes thereof, with sequences shown as SEQ ID NO. 1 to 4. The pair of polypeptides is used for establishing a pair of a recombinant transcription activator like effector (TALE) and a transcription activator like effector nuclease (TALEN); the TALEN is a fusion protein formed by fusing a pair of DNA recognizing proteins with a DNA severing protein respectively; and the TALEN can perform targeted severing on a target site of a human insulin gene so as to achieve targeted modification on the human insulin gene, with the characteristics of strong specificity, high efficiency and high accuracy.
Owner:浙江煦顼技术有限公司
Serum-free medium for monkey embryonic stem cell
ActiveCN106754657AMaintain self-renewalMaintain normal stateCulture processCell culture active agentsInsulin activitySerum free media
The invention discloses a serum-free medium for a monkey embryonic stem cell. The serum-free medium comprises a basic medium and additives, wherein the additives are dissolved in the basic medium; the basic medium comprises a DMEM-F12 medium; and the additive comprises L-glutamine, non-essential amino acid, L-ascorbic acid, sodium selenite, beta-mercaptoethanol, human serum albumin, heparin sodium, human transferring, insulin human, a basic fibroblast growth factor, a transforming growth factor beta 1 and an epidermal growth factor. The serum-free medium is capable of maintaining an undifferentiation state and totipotency of the monkey embryonic stem cell within a relatively long time, and is applicable to culture of the monkey embryonic stem cell in both a free-feeding layer system and a feeding layer system.
Owner:北京赛斯达生物技术有限公司
Serum substitute for cell culture
InactiveCN112048463AAntioxidantRepair oxidative damageCulture processCell culture mediaInsulin-like growth factorInsulin activity
The invention discloses a serum substitute for cell culture. Each liter of the serum substitute comprises the following components: 140-165 mg of amino acid, 15-20 mg of asparagine, 15-25 [mu] M of VC, 70-110 [mu] M of vitamin H, 150-200 [mu] M of tocopheryl acetate, 80-120 [mu] M of tocopherol, 10-15 [mu] M of vitamin A, 15-25 g of a bovine pituitary extract, 80-120 [mu] g of an insulin-like growth factor I, 250-350 [mu] g of catalase, 450-550 [mu] M of insulin human recombinant, 5000-10000 [mu] M of human transferrin, 5000000U of superoxide dismutase, 5mM-20mM of corticosterone, 150000-250000mM of D-galactose, 100-150mM of diethanolamine hydrochloride, 49-165mM of glutathione, 80-120mM of l-carnitinechloride, 140.901-161.403mg of inorganic salts and 5-15g of Pluronic F-68.
Owner:内蒙古奥普赛生物科技有限公司
Secretory expression for human insulin gene in methyl alcohol yeast
InactiveCN1614019AReduce production stepsSave man hoursInsulinsFusion with protease siteINSULIN HUMANSecretion expression
Owner:马延高 +1
Human umbilical cord mesenchymal stem cell serum-free culture medium and preparation method and application thereof
InactiveCN110699317ASuitable for growthSuitable for dry maintenanceCulture processSkeletal/connective tissue cellsPancreatic hormoneGlutamine
The invention discloses a human umbilical cord mesenchymal stem cell serum-free culture medium and a preparation method and application thereof. The human umbilical cord mesenchymal stem cell serum-free culture medium comprises a basic culture medium, amino acid substances, trace elements, recombinant human serum albumin, transferrin, recombinant human insulin, recombinant human epidermal growth factors, alkaline fibroblast growth factors and buffer salt, wherein the amino acid substances comprise glycine, alanine, asparagine, aspartic acid, glutamic acid, proline, serine and glutamine. The serum-free culture medium is clear in components, stable in batch, free of serum, antibiotics and other serum analogues, and free of heterogeneous pollution and immunological rejection risks and safe touse; by the serum-free culture medium, normal cell adherence and good cell growth state can be kept; the serum-free culture medium has stemness and is suitable for the growth and stemness maintenanceof human umbilical cord mesenchymal stem cells.
Owner:湖南丰晖生物科技有限公司
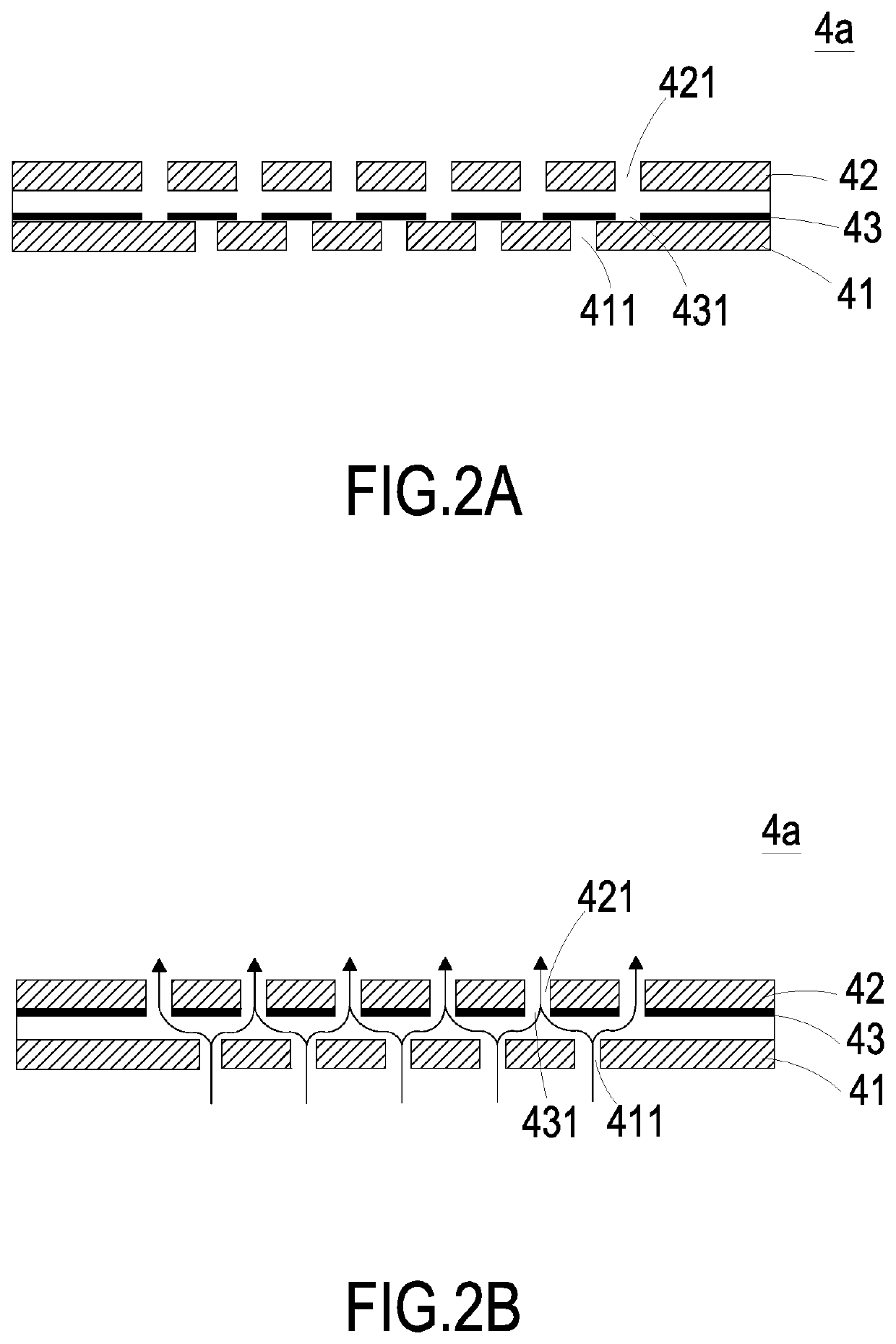
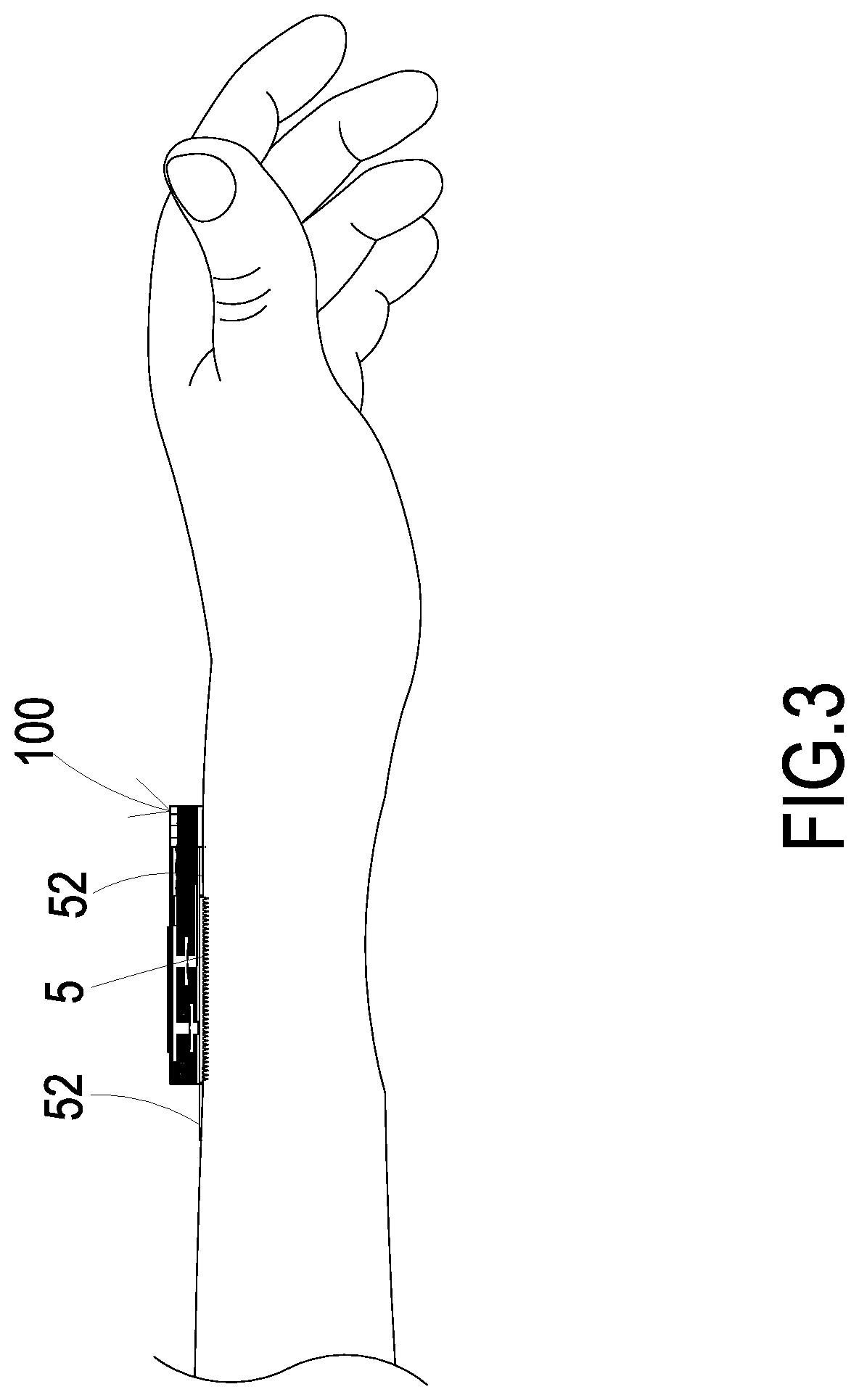
Liquid supplying device for human insulin injection
A liquid supplying device for a human insulin injection includes a substrate, a liquid storage chamber, a flow-guiding-and-actuating unit, a sensor and a driving chip. The flow-guiding-and-actuating unit includes a liquid guiding channel having a liquid guiding outlet in fluid communication with a liquid storage outlet of the liquid storage chamber. The sensor contacts with the human skin to measure a blood glucose level contained in sweat. The driving chip is configured to control the actuation of the flow-guiding-and-actuating unit, control open / closed states of the switching valves and receive the measured data from the sensor for determination. By driving the flow-guiding-and-actuating unit, a pressure gradient is generated, and an insulin liquid stored in the liquid storage chamber is transported to the liquid guiding outlet through the liquid guiding channel, flowing into a microneedle patch, and injected into a subcutaneous tissue through a plurality of hollow microneedles.
Owner:MICROJET TECH
Human insulin containing additional disulfide bonds
InactiveCN102933599AImprove physical stabilityCombined retentionMetabolism disorderInsulinsINSULIN HUMANDisulfide bonding
The present invention is related to human insulin containing additional disulfide bonds and methods of making such.
Owner:NOVO NORDISK AS
Culture medium for adipogenic differentiation of mesenchymal stem cells and application for culture medium
ActiveCN111979185ASafe Induced Differentiation ApplicationsExclude zoonotic riskCulture processSkeletal/connective tissue cellsINSULIN HUMANMethyl xanthine
The invention relates to a culture medium for adipogenic differentiation of mesenchymal stem cells and an application thereof. The culture medium is characterized by comprising a basic culture mediumand an additive component, wherein the culture medium comprises: a variety of components like inorganic salt, amino acid and vitamins which are optimally designed, and components like recombinant human serum albumin, sodium carboxymethylcellulose, 3-isobutyl-1-methylxanthine, recombinant human insulin, dexamethasone, pioglitazone hydrochloride, palmitic acid, oleic acid, linoleic acid and cholesterol. According to the invention, through addition and proportioning of optimally-designed components, the capacity of the culture medium for inducing mesenchymal stem cells to differentiate into adipose cells is obviously improved, and the differentiation time is further shortened.
Owner:苏州依科赛生物科技股份有限公司
Synthetic human insulin gene and use thereof in cultivation of transgenic tomatoes
InactiveCN101684468ALow costAvoid infectionFermentationVector-based foreign material introductionDisorder diabetes mellitusInsulin Gene
The invention relates to the field of plant gene engineering and provides a sequence of a human insulin gene designed and synthesized according to plant preferred codons. A plant expression vector isbuilt for the synthetic human insulin gene, so that the gene can be transferred into tomatoes by an agrobacterium rhizogenes-mediated method under the drive of a CaMV 35S promoter and a fruit-specificpromoter 2A12 to express human insulin in tomato fruit. The tomatoes capable of making human insulin can be used as a convenient oral product for preventing and treating insulin-dependent diabetes mellitus and autoimmune disorder diabetes mellitus, and human insulin can be extracted from the tomatoes to be made into injection preparations.
Owner:林忠平
Serum-free culture medium for mesenchymal stem cells
ActiveCN102433302BControl infection riskGood growthSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
The invention discloses a serum-free culture medium for mesenchymal stem cells. The serum-free culture medium for the mesenchymal stem cells comprises the following ingredients: fibronectin at the final concentration of 25mu g / ml, basic fibroblast growth factors at the final concentration of 10ng / ml, human epidermal growth factors at the final concentration of 15ng / ml, recombinant human insulin at the final concentration of 1mg / ml, human transferrin at the final concentration of 0.55mg / ml, human blood albumin in a volume ratio of 5 percent, sodium selenite at the final concentration of 0.67mug / ml, L-carnitine at the final concentration of 5mM and resveratrol at the final concentration of 30mu M. When the mesenchymal stem cells are cultured by the serum-free culture medium for the mesenchymal stem cells, animal-derived serum is not contained, so infection risks can be controlled; the L-carnitine and the resveratrol which are added into the serum-free culture medium for the mesenchymalstem cells can effectively improve the growth state of the mesenchymal stem cells; and the growth speed of the mesenchymal stem cells is remarkably improved, and the biological characteristics of themesenchymal stem cells are kept unchanged.
Owner:CHENGDU QINGKE BIOTECH
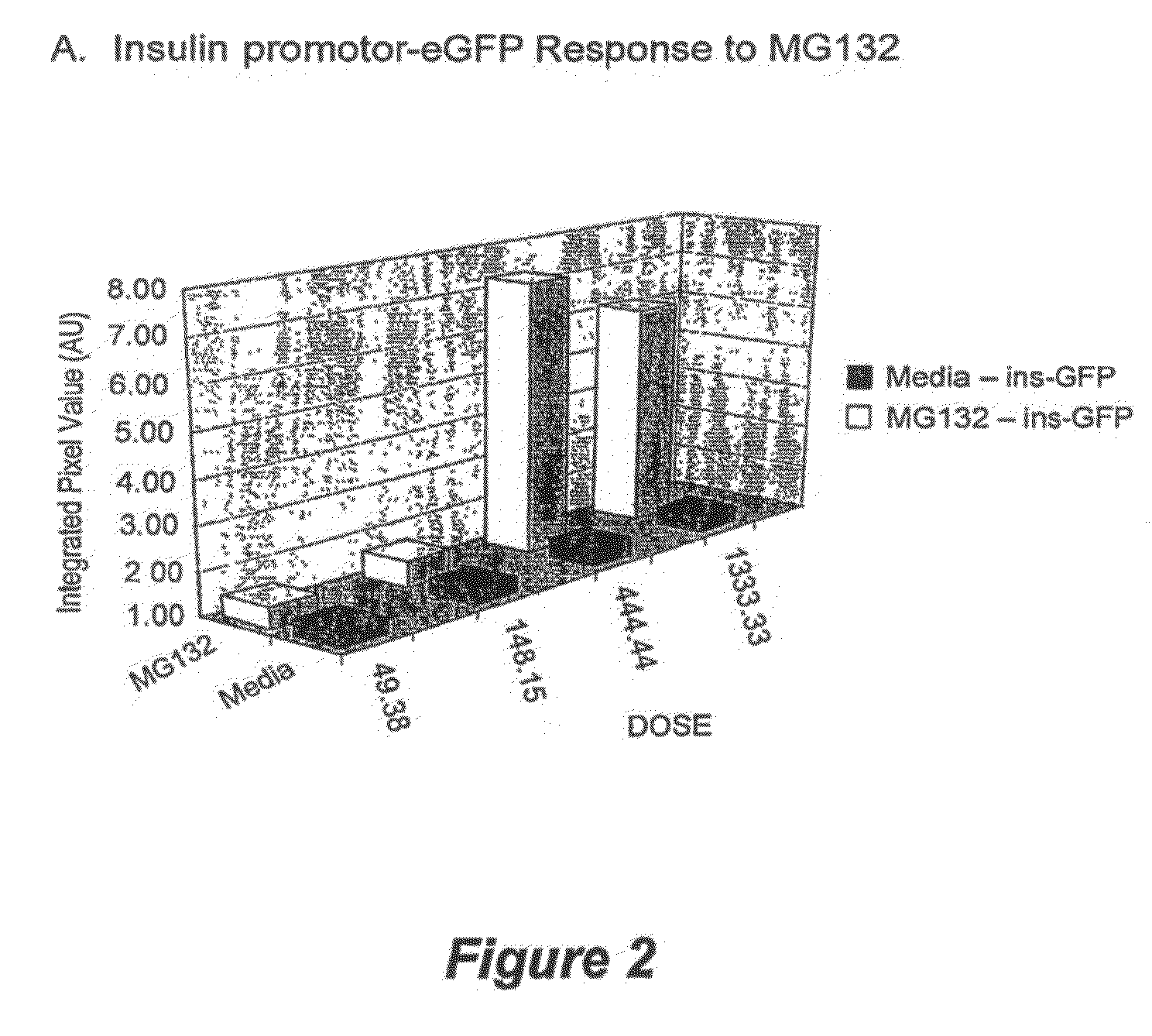
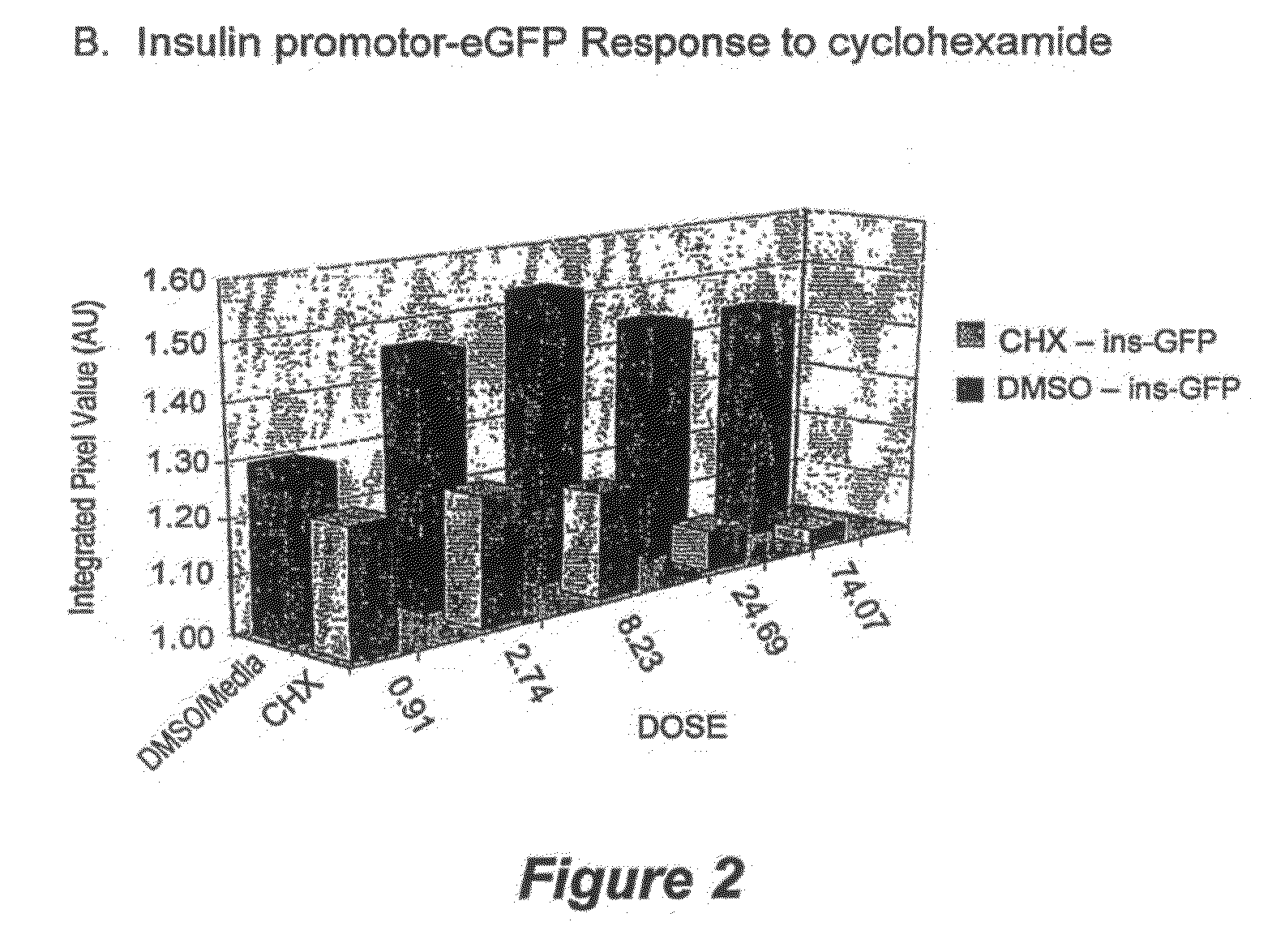
Methods for screening for compounds that modulate insulin promoter activity
Compositions and methods are provided for screening for compounds that modulate insulin promoter activity. Vectors that express green fluorescent protein under the control of the human insulin promoter are introduced into mouse and human cells in which the insulin promoter is expressed in a glucose-responsive manner. Such cells are then used to screen for compounds that modulate insulin promoter activity.
Owner:BURNHAM INST FOR MODICAL RES +1
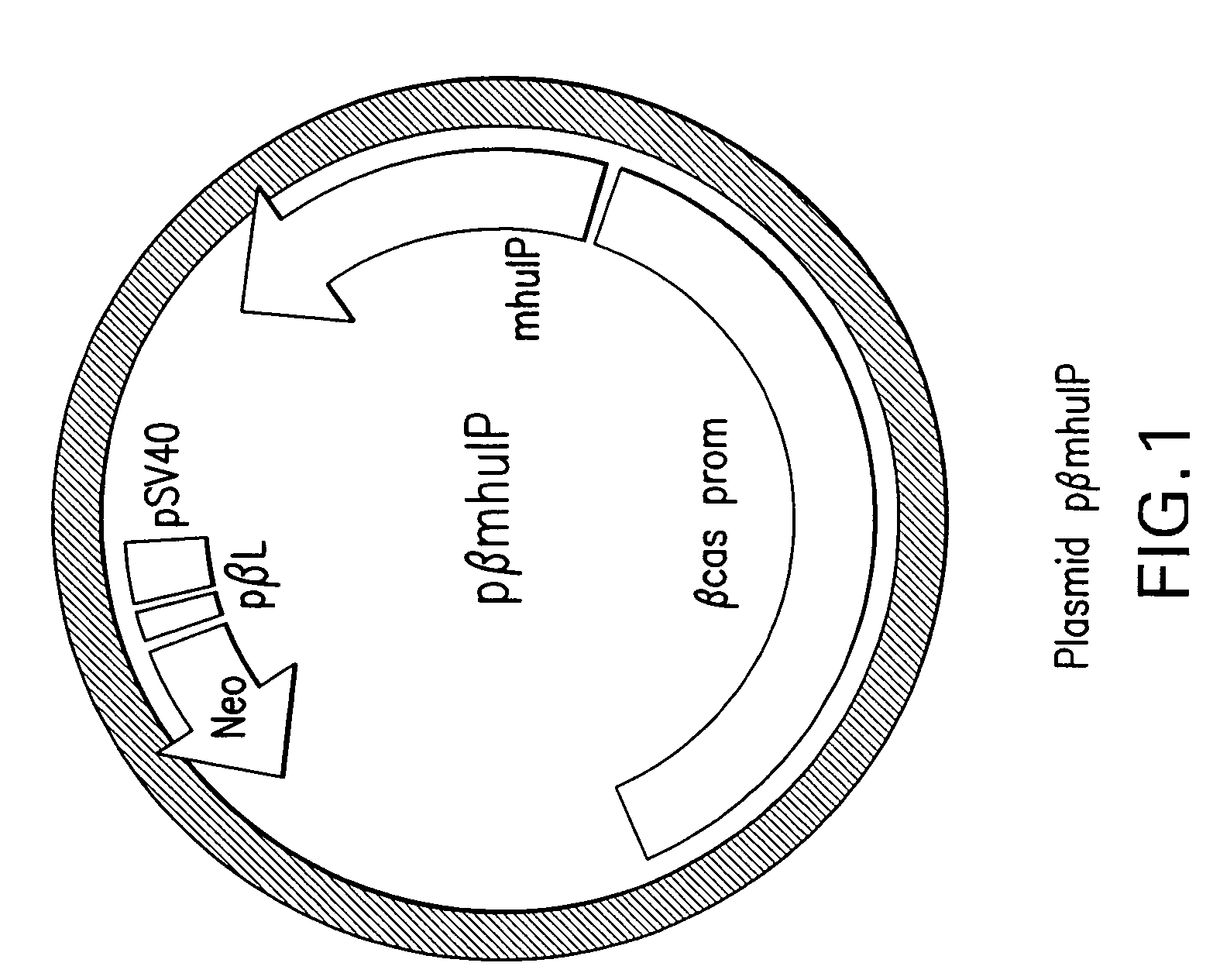
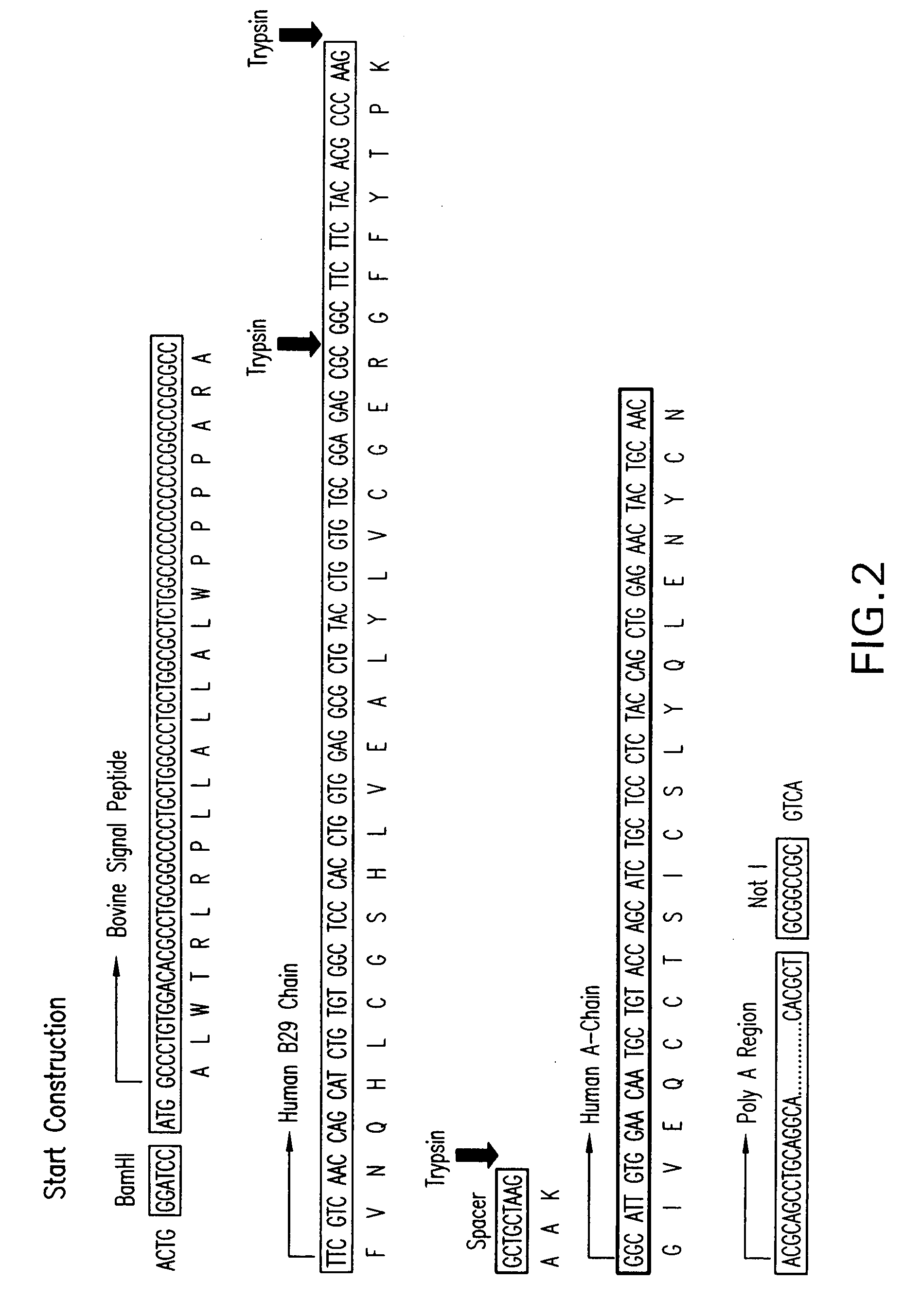
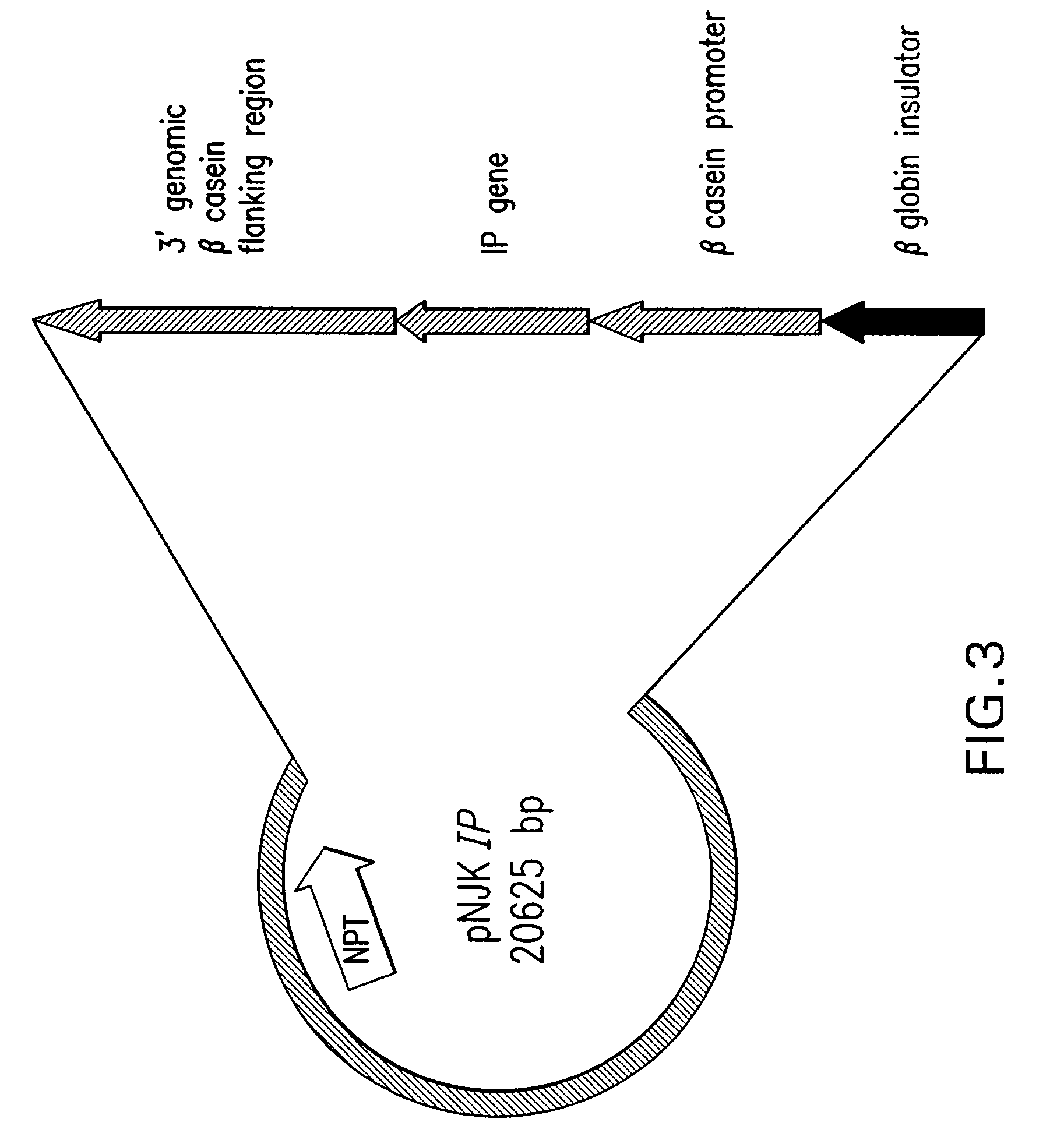
Process for Producing Exogenous Protein in the Milk of Transgenic Mammals and a Process For Purifying Proteins Therefrom
The invention relates to a non-human transgenic mammal that is useful for the production of a protein of interest that may be toxic to the mammal. The mammal is characterized by the fact that it is transgenic for the production in its milk of an inactive form of the protein of interest, preferably recombinant human insulin. It is not possible to produce recombinant human insulin in transgenic mammals since this molecule has a certain degree of biological activity in the mammals and could be toxic to the mammal. Thus, the invention involves cloning a genetic construct comprising a sequence encoding a modified human insulin precursor under the control of a beta casein promoter in an expression vector. It also involves transfecting the expression plasmid into fetal bovine somatic cells, such as fibroblasts, and enucleating bovine oocytes by nuclear transfer to generate transgenic embryos. The invention gives rise to transgenic bovine that will be able to produce a modified human insulin precursor in their mammary glands. Afterwards, the milk of these transgenic mammals can be collected, the modified human insulin precursor can be converted in vitro into recombinant human insulin, and the recombinant human insulin can be purified to homogeneity as a pure biopharmaceutical product.
Owner:STERRENBELD BIOTECH NORTH AMERICA
Secretory expression for human insulin gene in methyl alcohol yeast
InactiveCN100460508CReduce production stepsSave man hoursInsulinsFusion with protease siteINSULIN HUMANSecretion expression
The invention was involved in expression of human insulin gene, especially for the expression of human insulin gene in Pichia Pastoris GS115 / pPICM#101 whose storage number wais CCTCC NO.M204071. It contained B and A strand synthesis, construction of express plasmid and engineering strain, transform of host cell, filter of transformant and over expression engineering strain, etc. The method left out the difficult procedure, reduced the process steps and shortened time, so the technique was simple. It overexpressed 10-100 folds than the others. It provided one new simple method for human insulin commercial process.
Owner:马延高 +1
Novel insulin derivates and preparation thereof
InactiveCN101298477AUnique preparation methodPeptide preparation methodsInsulinsInsulin activityCarboxypeptidase A
The invention relates to the biological field, in particular to a novel insulin derivative which is characterized in that the general formula of the compound is CnH2n + 1NH-X-D, wherein, n is equal to 14 to 22, CnH2n + 1NH is straight chain saturated fatty amino group; X is a natural amino acid except genetic coding proline or a 1-amino-1-carboxyl unnatural amino acid; D is a natural pig insulin stripped of B30 amino acid residue or a human insulin stripped of B30 amino acid residue. The insulin derivative of the invention is a novel derivative formed by connecting a fatty chain to the natural insulin or to the Alpha-carboxyl of the C-terminal residue on the parent B chain of the analogs. The preparation method is to take the natural pig insulin or human insulin as the starting material, cut off the first amino acid residue on the C terminal of the B chain of the insulin, and then take trypsin as the catalyst to cause the amino acid derivative connected with a fatty chain to become the novel insulin derivative connected with a fatty chain on the C terminal of B chain through enzymic reaction. The derivative has potential mouth taking and long-acting functions.
Owner:DALIAN NATIONALITIES UNIVERSITY
Serum-free medium of peripheral blood cells
InactiveCN107043748AFast processMeet needsCulture processBlood/immune system cellsInsulin activitySodium bicarbonate
The invention relates to a serum-free medium of peripheral blood cells. The serum-free medium comprises a base medium and an additive component; the base medium is RPMI 1640 medium; the additive component is composed of, by volume, 35 to 50mg / L of interleukin-2, 1 to 2g / L of heparin, 25 to 30mg / L of transferring, 20 to 30mg / L of insulin human recombinant, 10 to 20mg / L of M-type phytohaemagglutinin, 7 to 10mg / L of L-glutamine, and 2 to 4g / L of sodium bicarbonate. Compared with the prior art, the serum-free medium of peripheral blood cells possesses following advantages: the serum-free medium of peripheral blood cells is capable of satisfying requirements of leukocyte rapid and large amount propagation in peripheral blood cells, is especially suitable to be used for culturing of peripheral blood cells, and possesses excellent propagation effect and cell survival rate.
Owner:SHANGHAI XP BIOMED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com