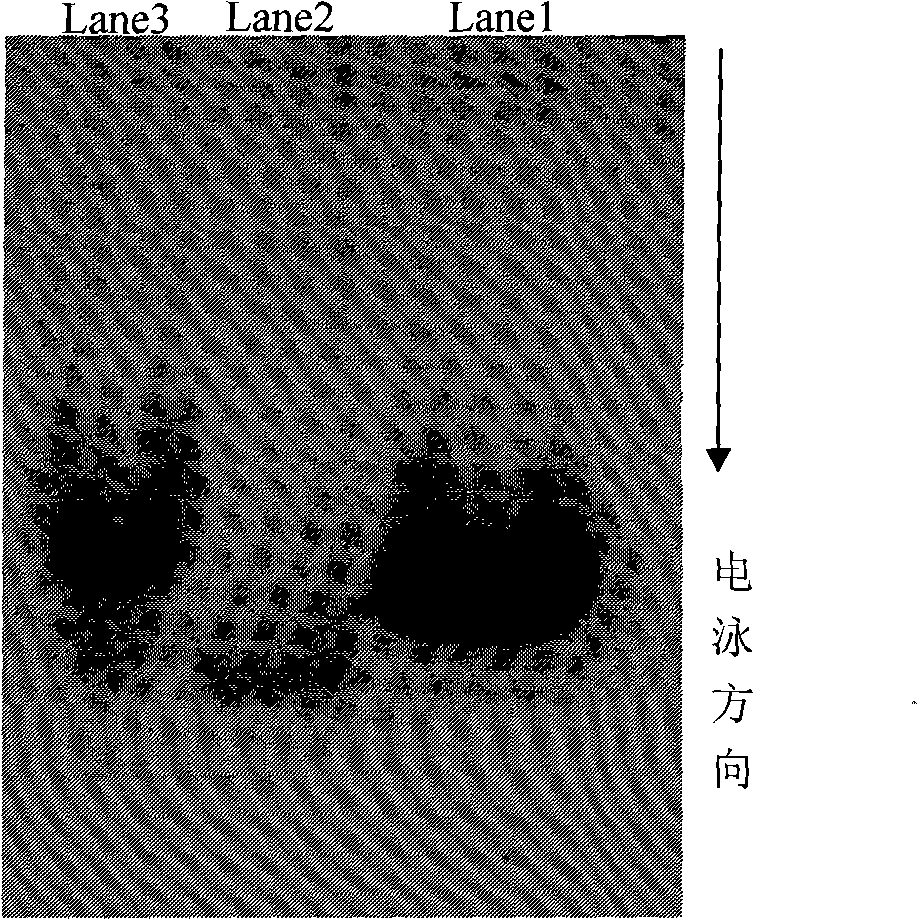
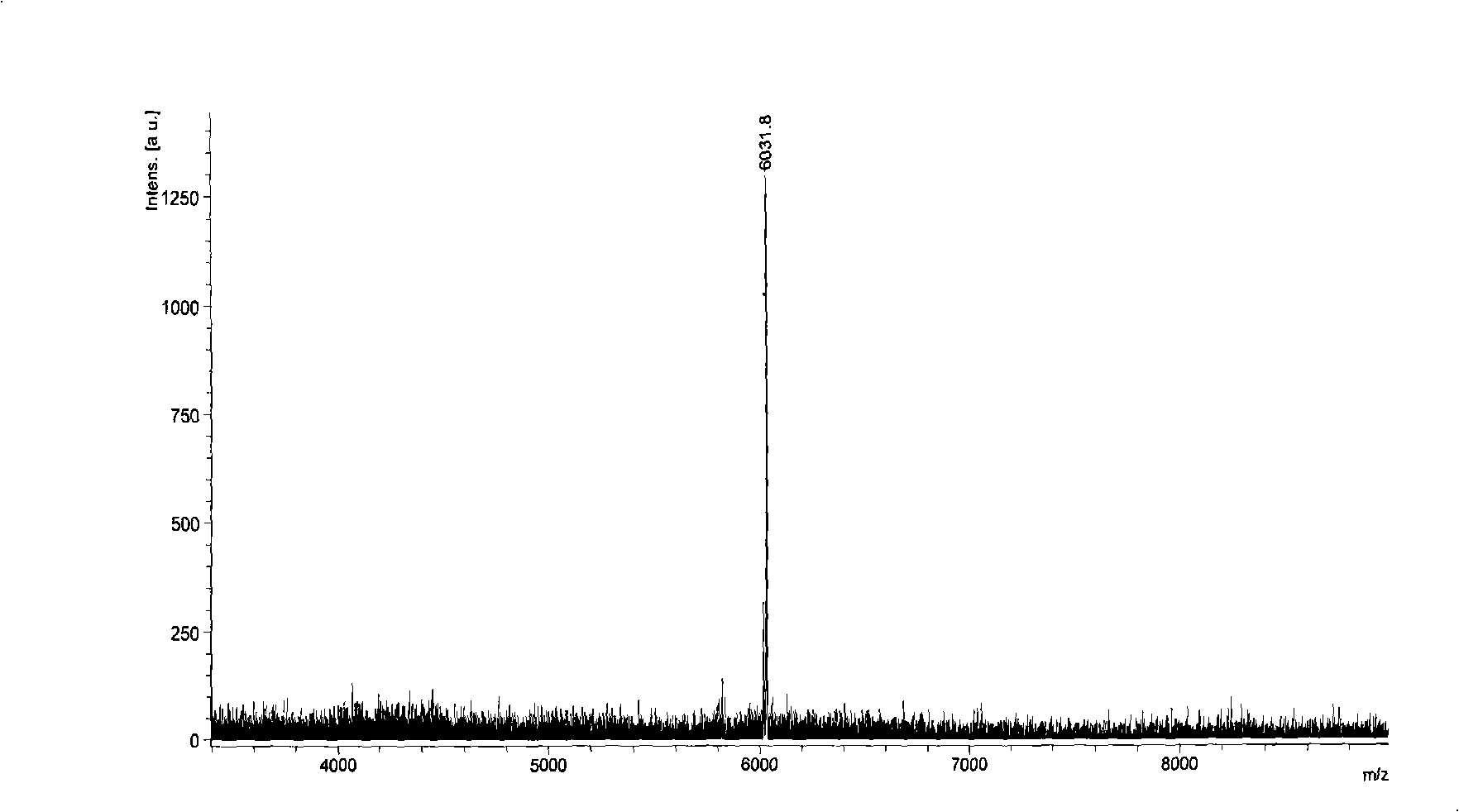
Novel insulin derivates and preparation thereof
A technology of insulin derivatives and insulin, which is applied in the field of biomedicine and can solve problems such as separation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of Novel Insulin Derivative C 18 h 37 NH-Thr-D:
[0039] Step 1. Fatty Amine C 18 h 37 NH 2 Preparation of:
[0040] Take 18 alcohol (C 18 h 37 OH) 27 grams (0.1 mole), add in the 500 milliliter round bottom flask that 300 milliliters of toluenes are housed, heat and evaporate 100 milliliters of toluene to take out the moisture in the alcohol. The remaining solution was added with pyridine in an equimolar amount to the fatty alcohol, refluxed and added dropwise thionyl chloride (SOCl 2 ), after the dropwise addition, the temperature was lowered to 90°C to continue the reaction for 4 hours, cooled, filtered to remove crystals, then evaporated toluene under reduced pressure, the residue was recrystallized with water and ethanol, and vacuum-dried by suction to obtain 25 grams of product, which is C 18 h 37 Cl. Dissolve this product in a small amount of anhydrous methanol and add it to a reactor that can be sealed and withstand a certain pr...
Embodiment 2
[0053] Example 2: C 14 h 29 Preparation of NH-Ala-D
[0054] Step 1. Fatty Amine C 14 h 29 NH 2 preparation of
[0055] Take 14 alcohol (C 14 h 29 OH) 18.5 grams (0.1 mole), add in the 500 milliliter round bottom flask that 300 milliliters of toluene is housed, heat and distill 100 milliliters of toluene to take out the moisture in the alcohol. The remaining solution was added with pyridine in an equimolar amount to the fatty alcohol, refluxed and added dropwise thionyl chloride (SOCl 2 ), after the dropwise addition, the temperature was lowered to 90°C to continue the reaction for 4 hours, cooled, filtered to remove the crystals, then evaporated toluene under reduced pressure, the residue was recrystallized with water and ethanol, and vacuum-dried by suction to obtain 17 grams of product, which is C 14 h 29 Cl. Dissolve this product in a small amount of anhydrous methanol and add it to a reactor that can be sealed and withstand a certain pressure, add excess liquid...
Embodiment 3
[0067] Example 3: C 16 h 33 Preparation of NH-Gly-D
[0068] Step 1. Fatty Amine C 16 h 33 NH 2 preparation of
[0069] Take 22 alcohol (C 16 h 33 OH) 24.2 grams (0.1 mole), add in the 500 milliliter round bottom flask that 300 milliliters of toluene is housed, heat and evaporate 100 milliliters of toluene to take out the moisture in the alcohol. The remaining solution was added with pyridine in an equimolar amount to the fatty alcohol, refluxed and added dropwise thionyl chloride (SOCl 2 ), after the dropwise addition, the temperature was lowered to 90°C to continue the reaction for 4 hours, cooled, filtered to remove the crystals, then evaporated toluene under reduced pressure, the residue was recrystallized with water and ethanol, and vacuum-dried by suction to obtain 22 grams of product, which is C 16 h 33 Cl. Dissolve this product in a small amount of anhydrous methanol and add it to a reactor that can be sealed and withstand a certain pressure, add excess liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com