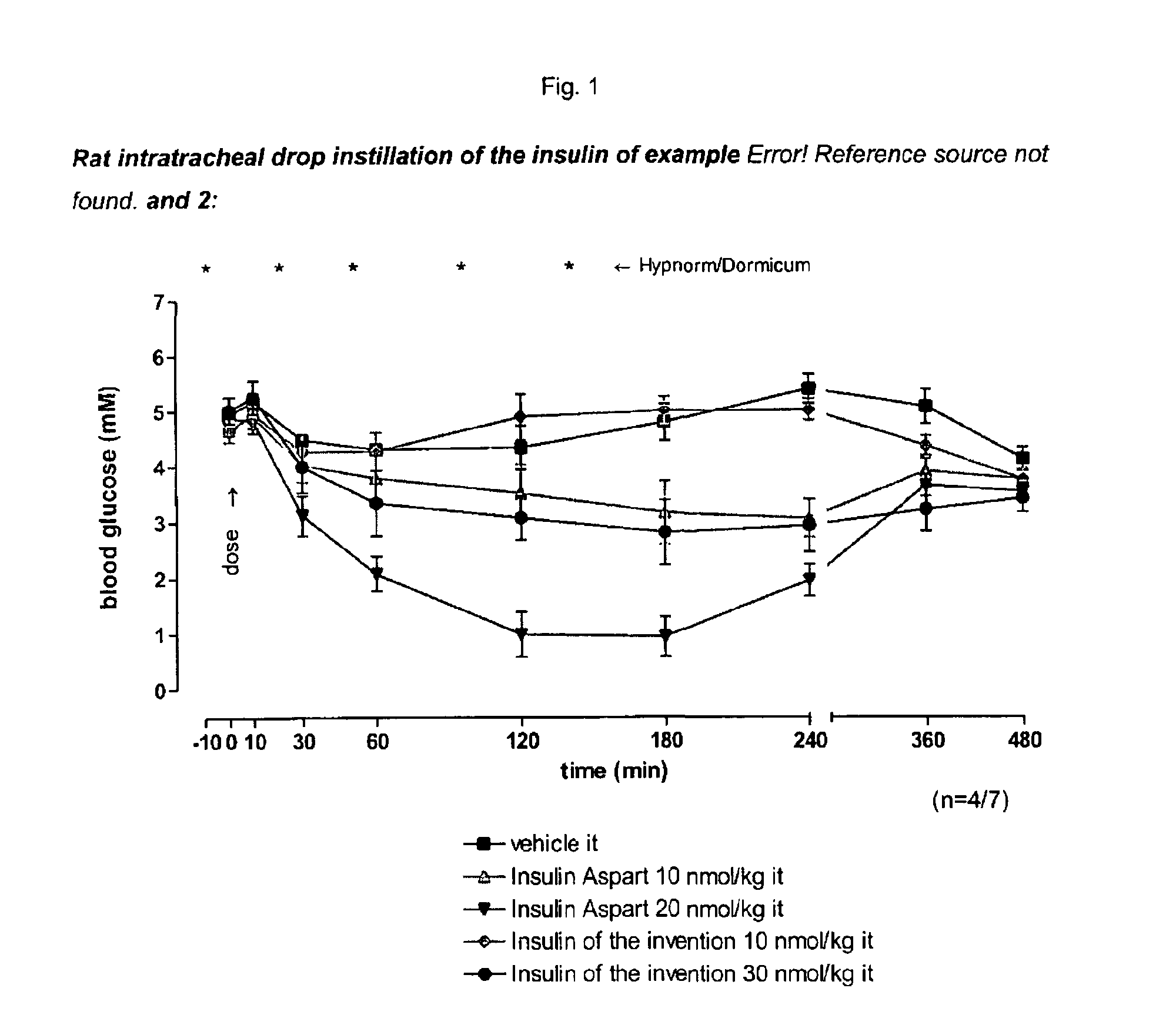
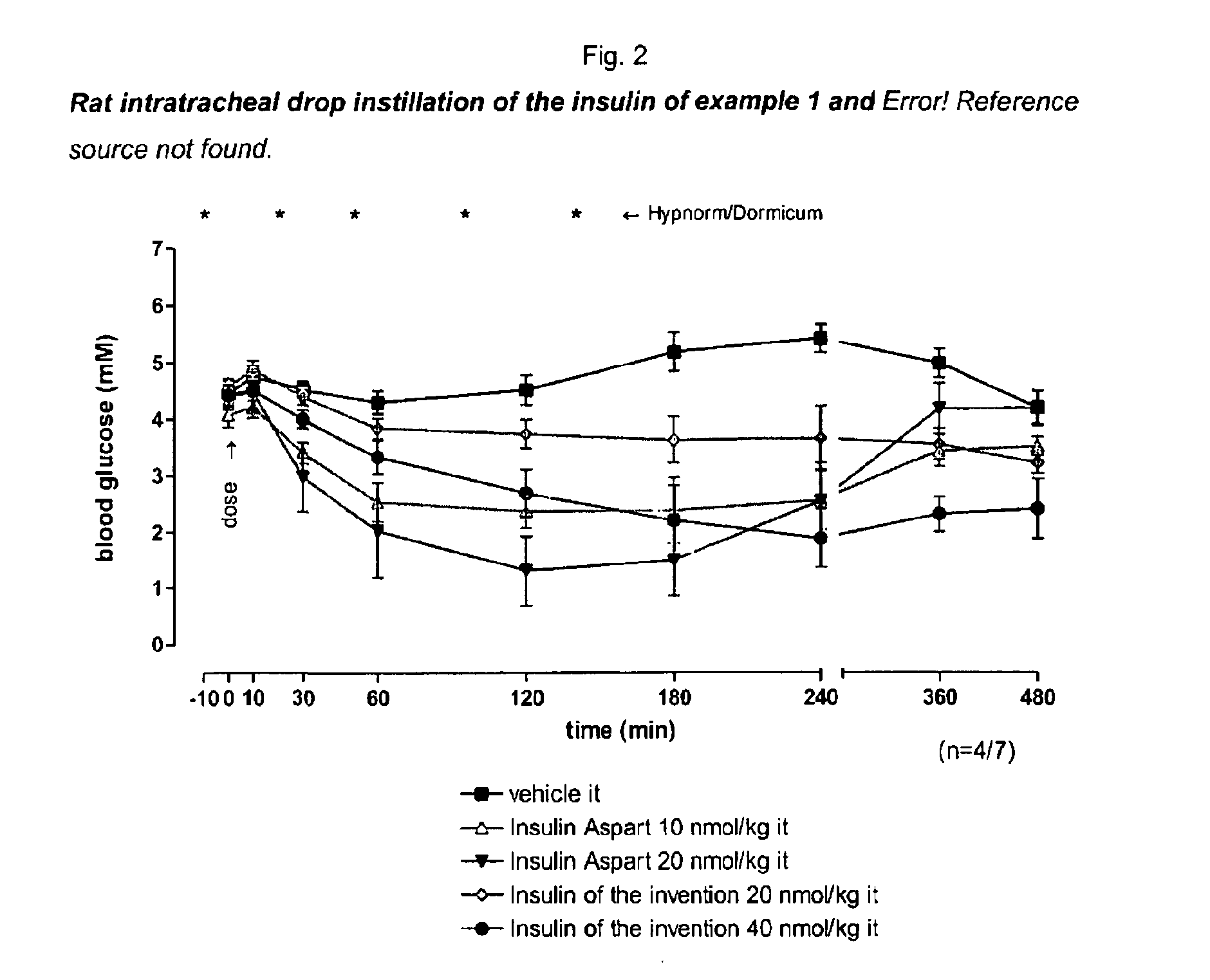
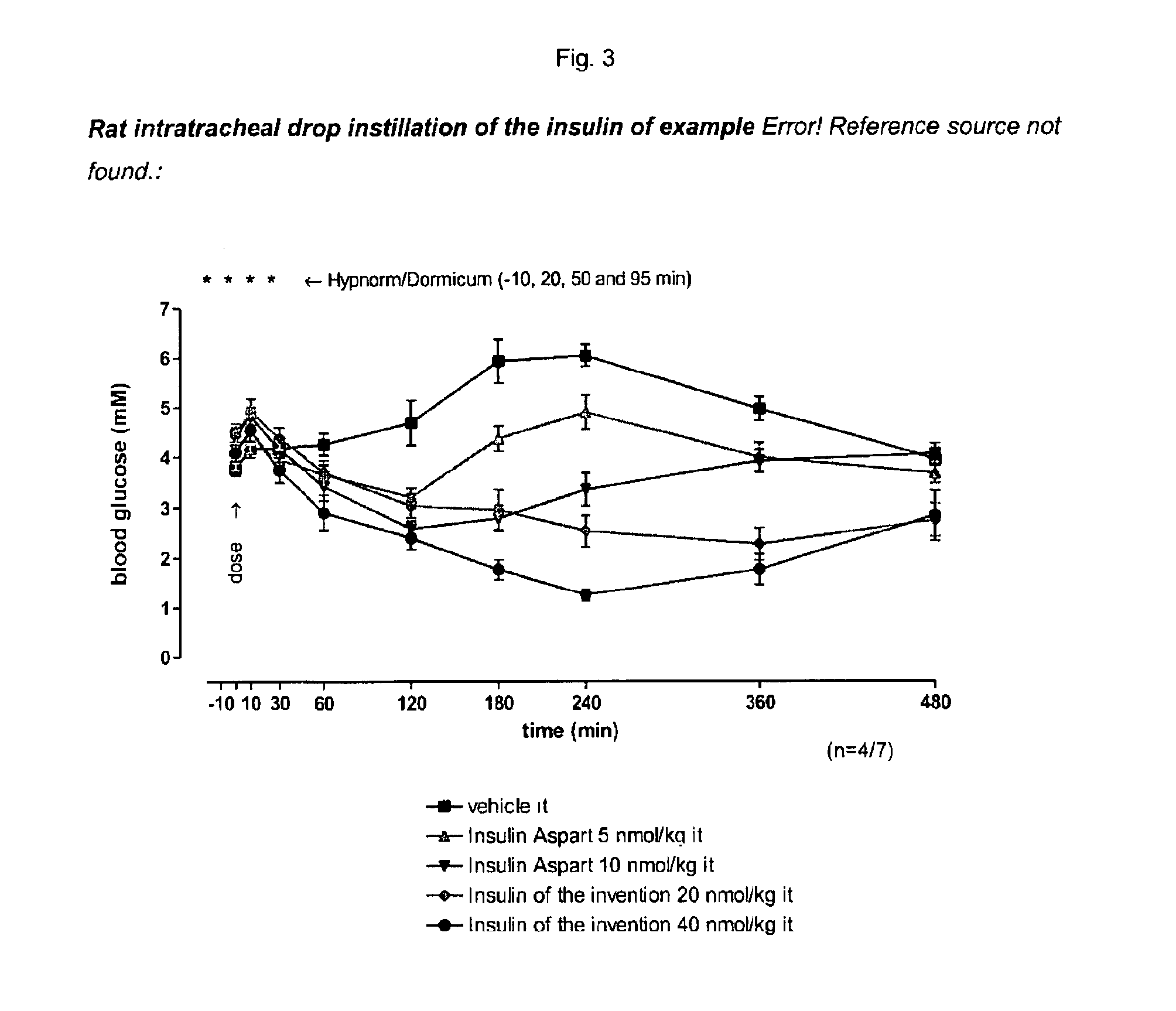
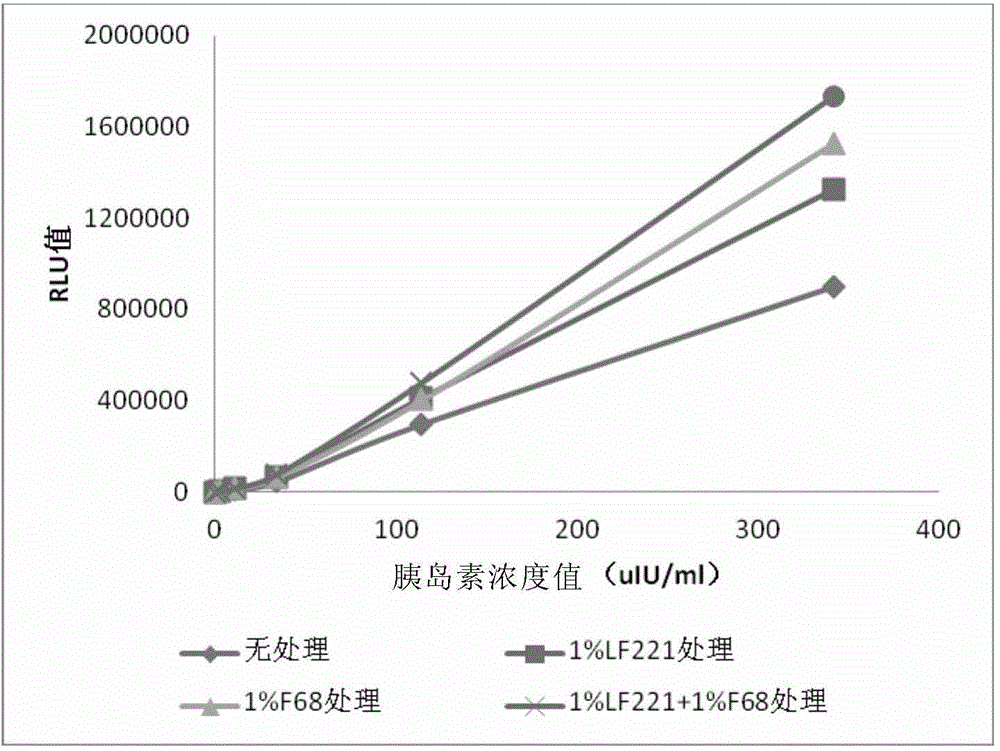
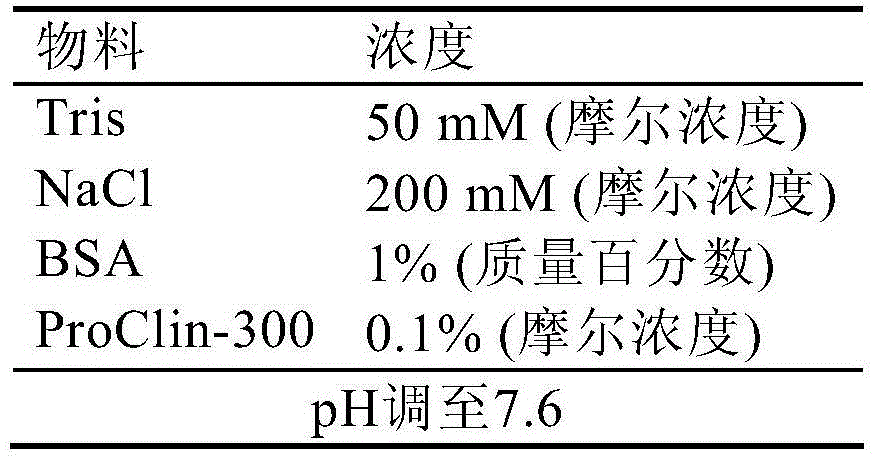
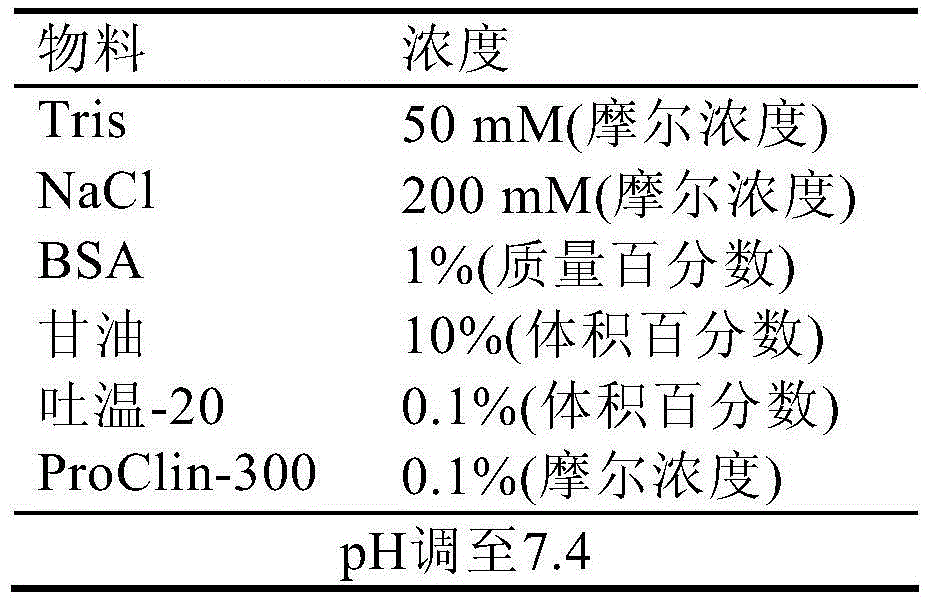
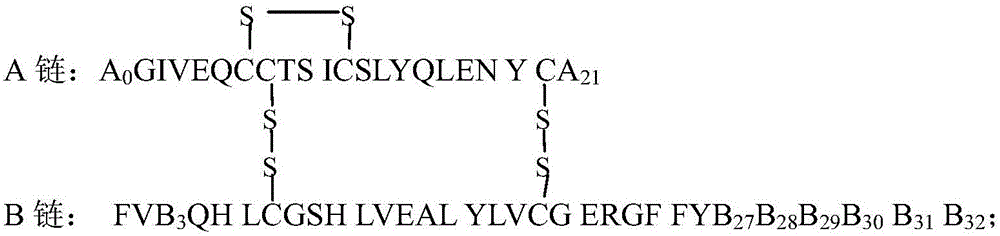Patents
Literature
89 results about "Insulin activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
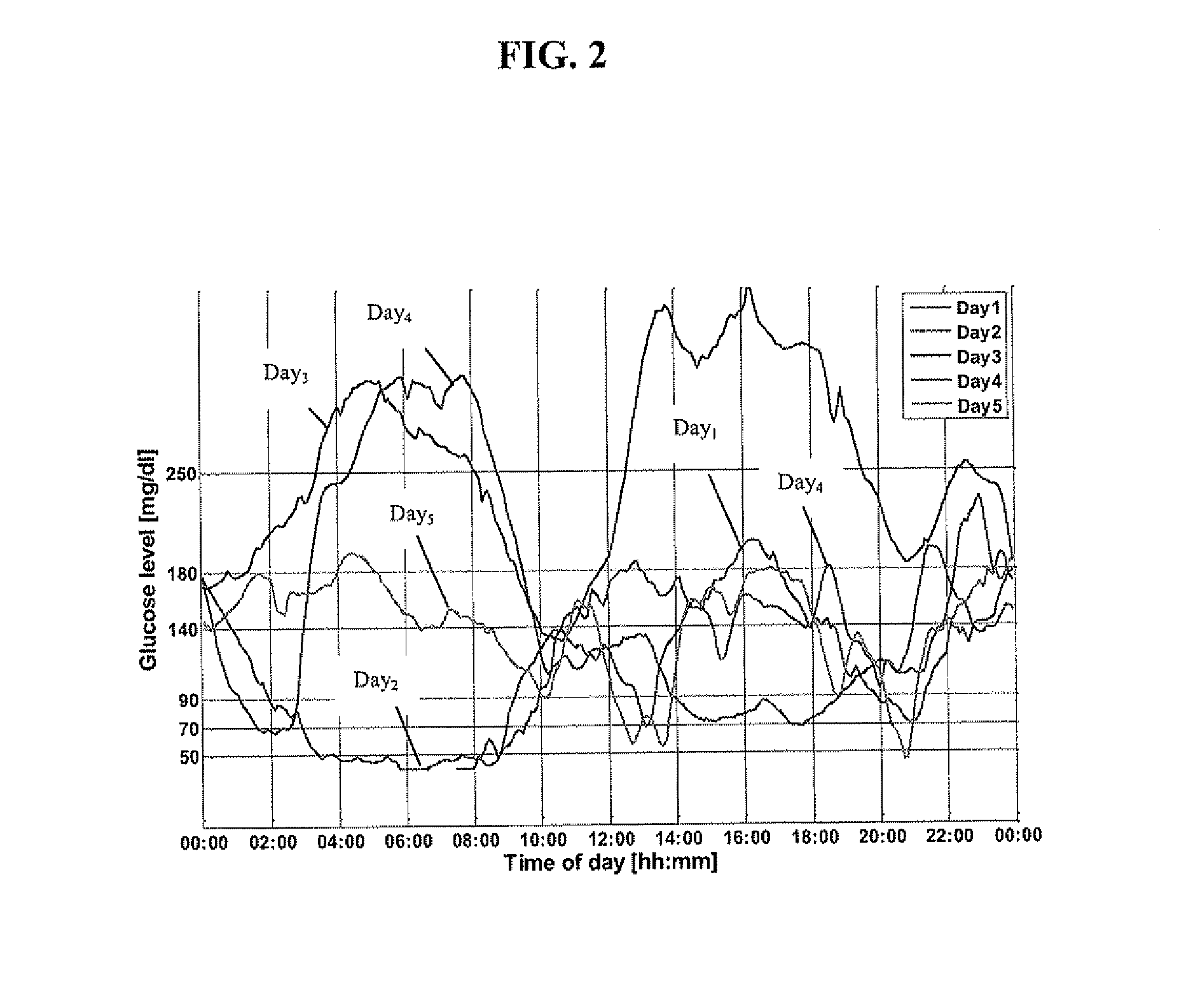
Insulin is produced and stored in the body as a hexamer (a unit of six insulin molecules), while the active form is the monomer.
Method and device for utilizing analyte levels to assist in the treatment of diabetes
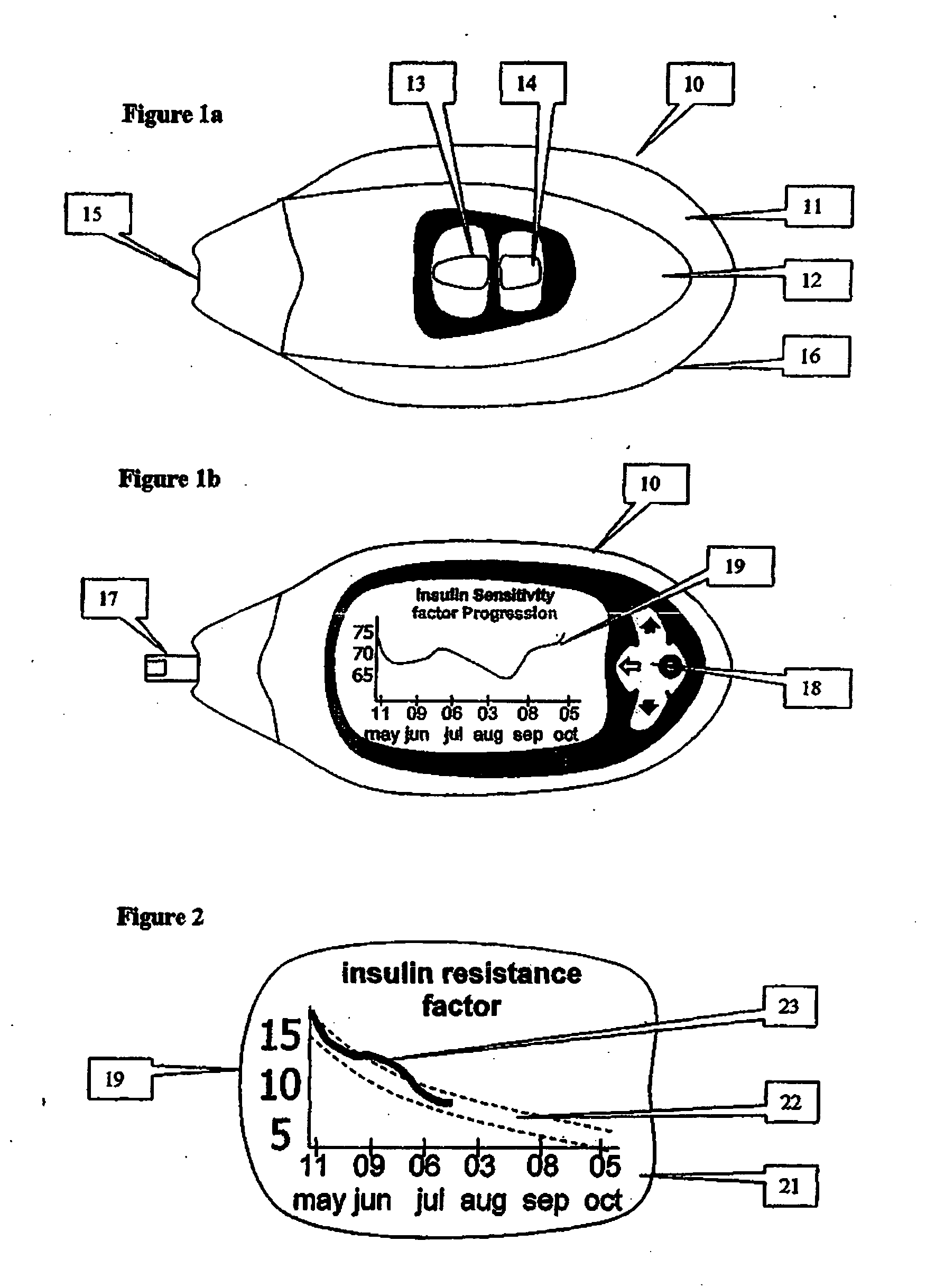
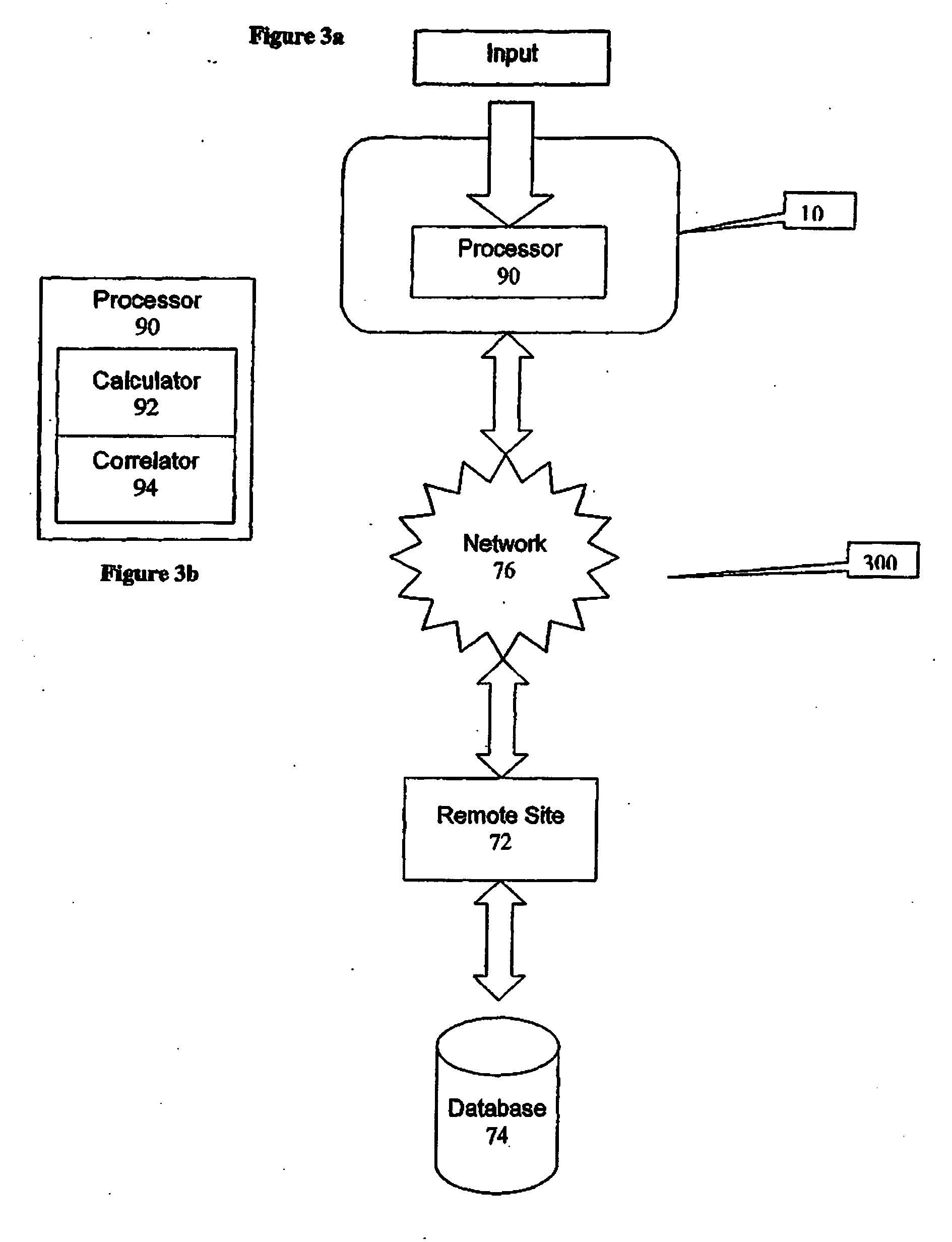
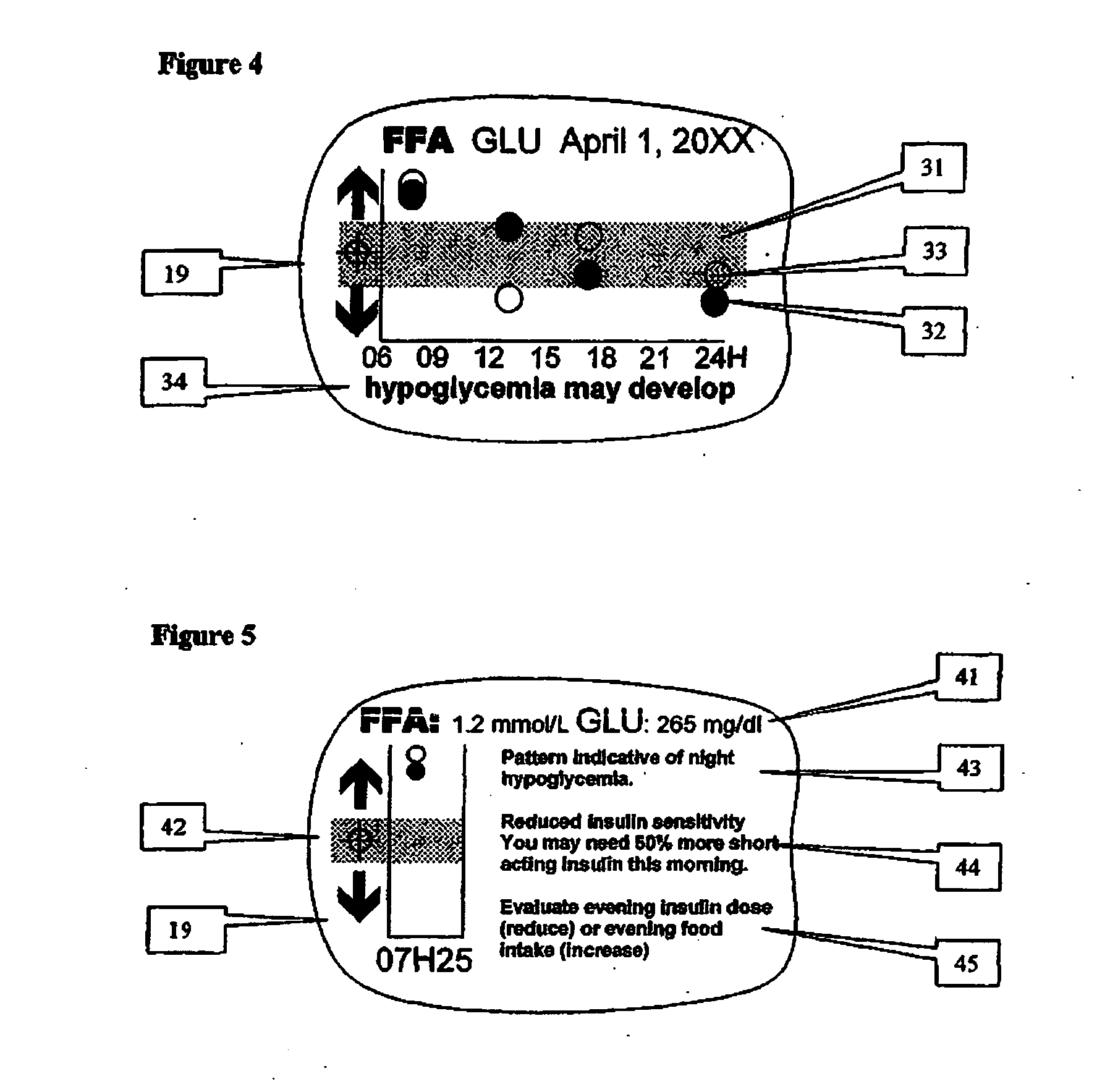
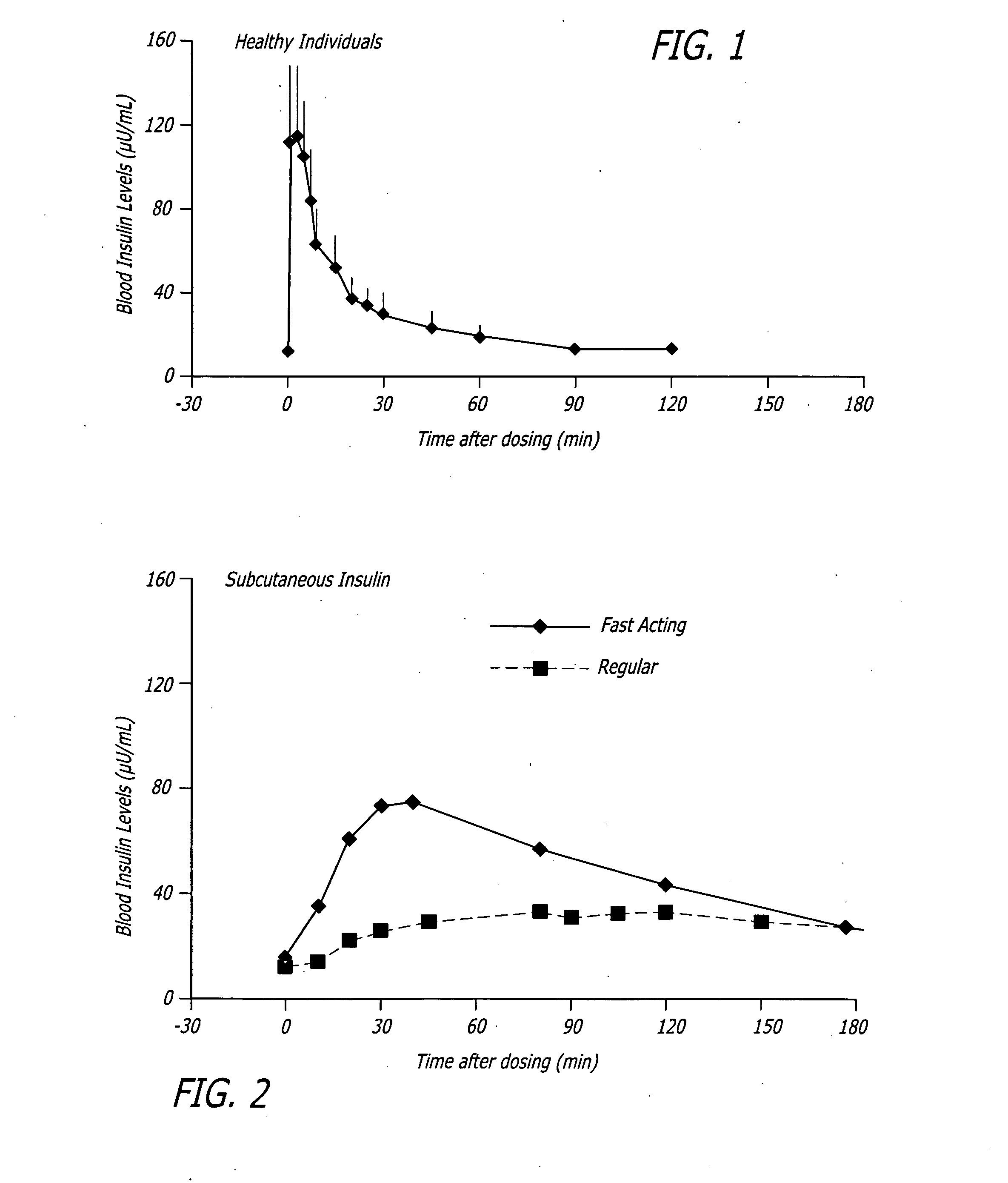
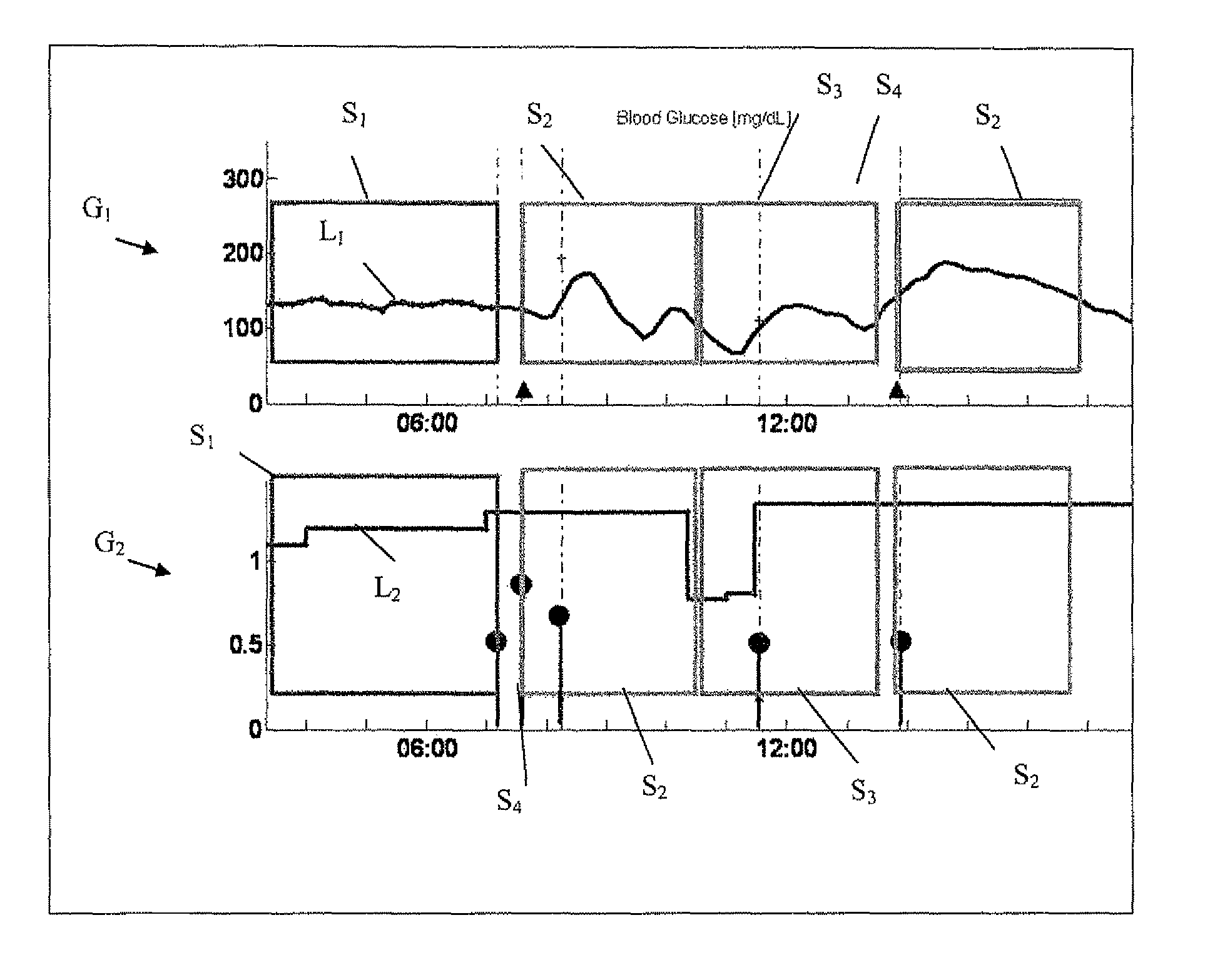
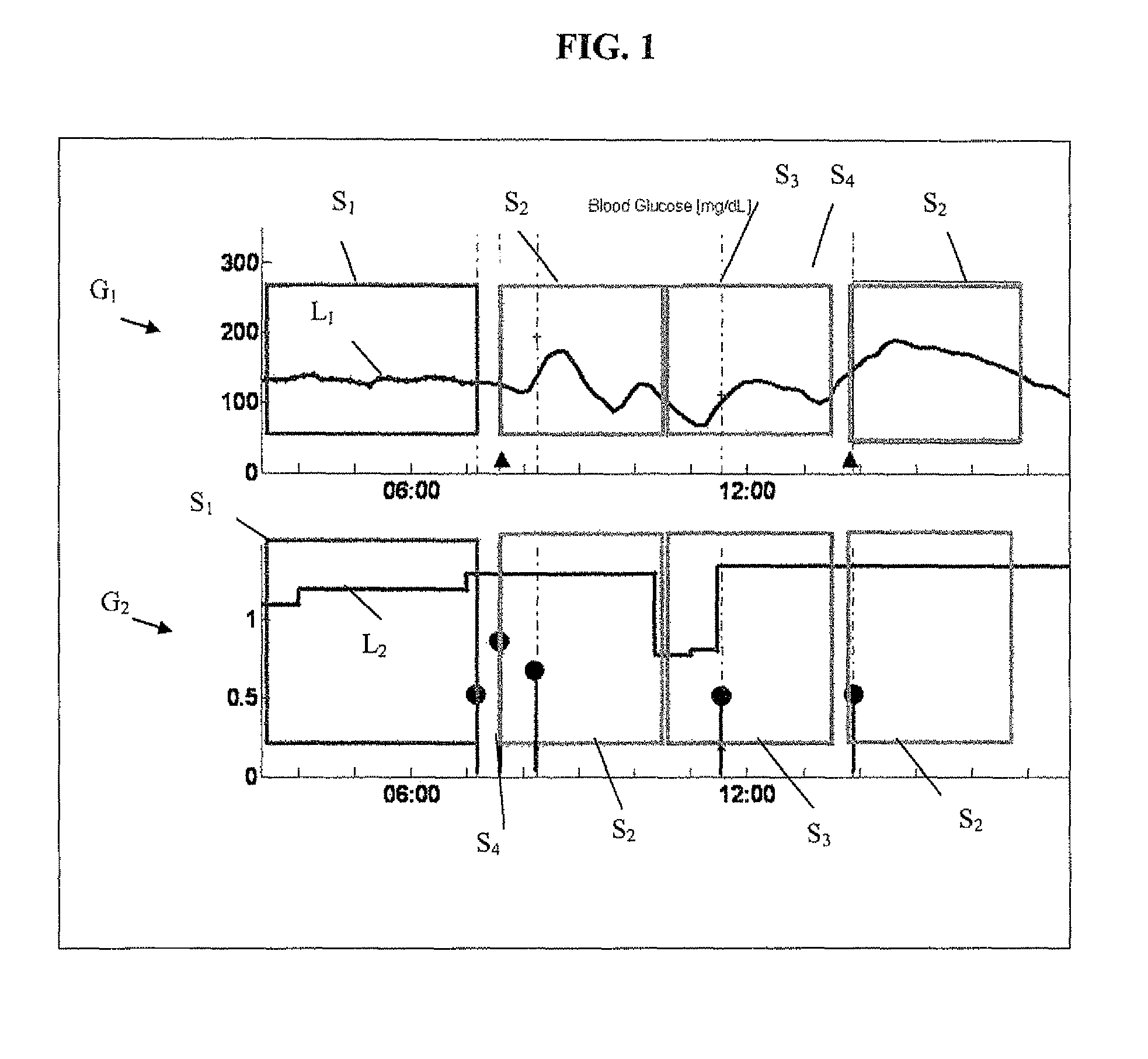
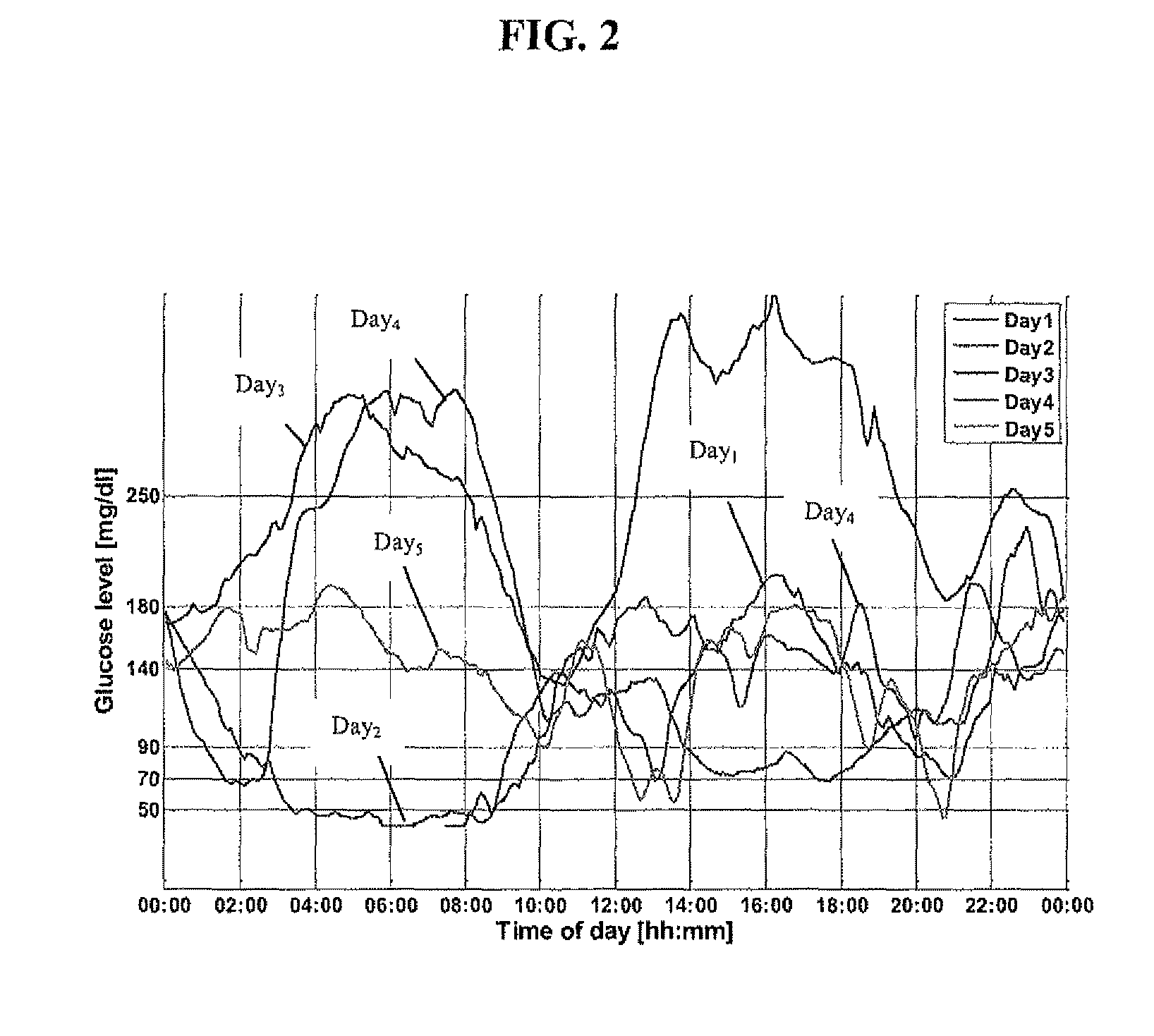
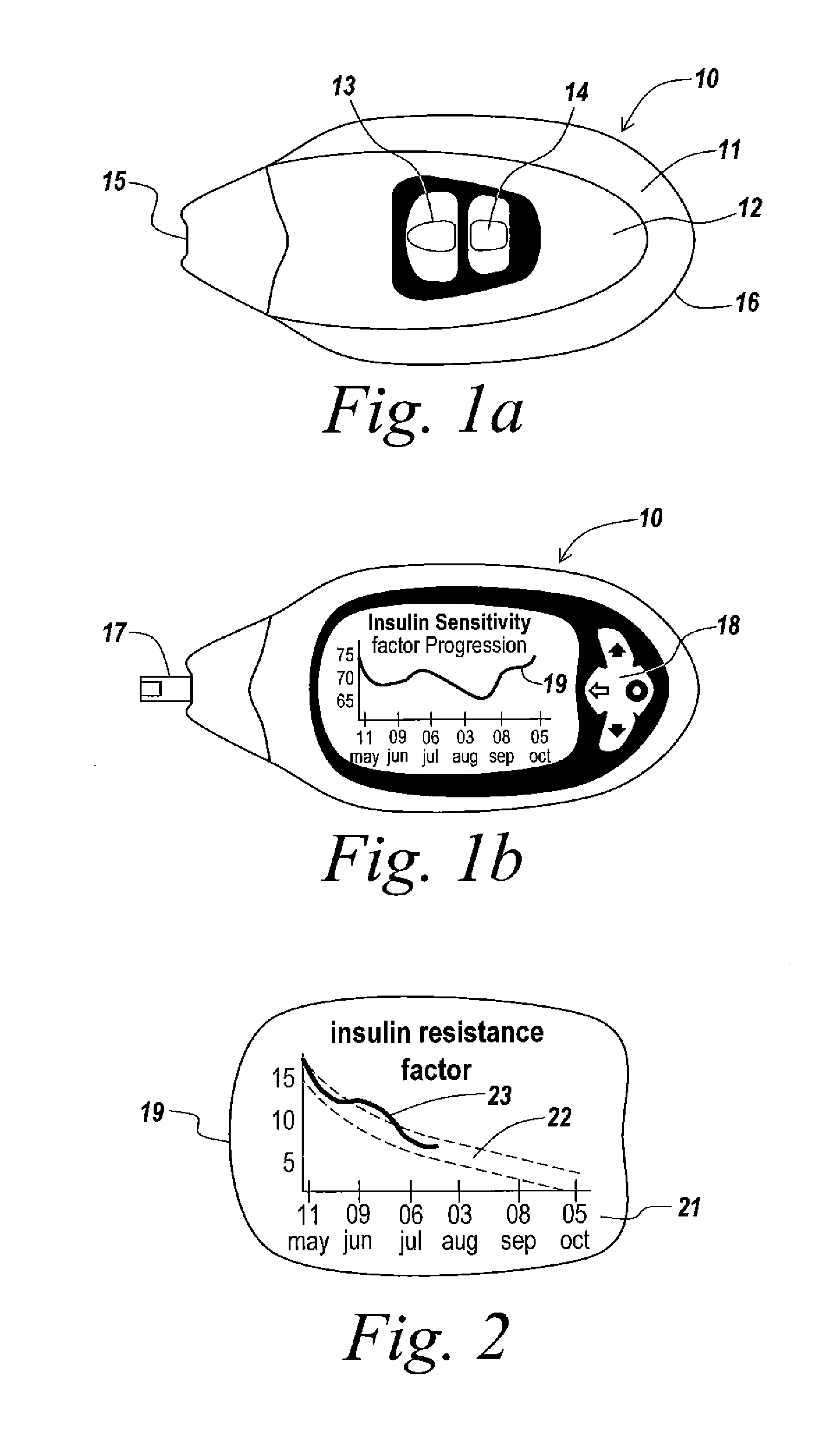
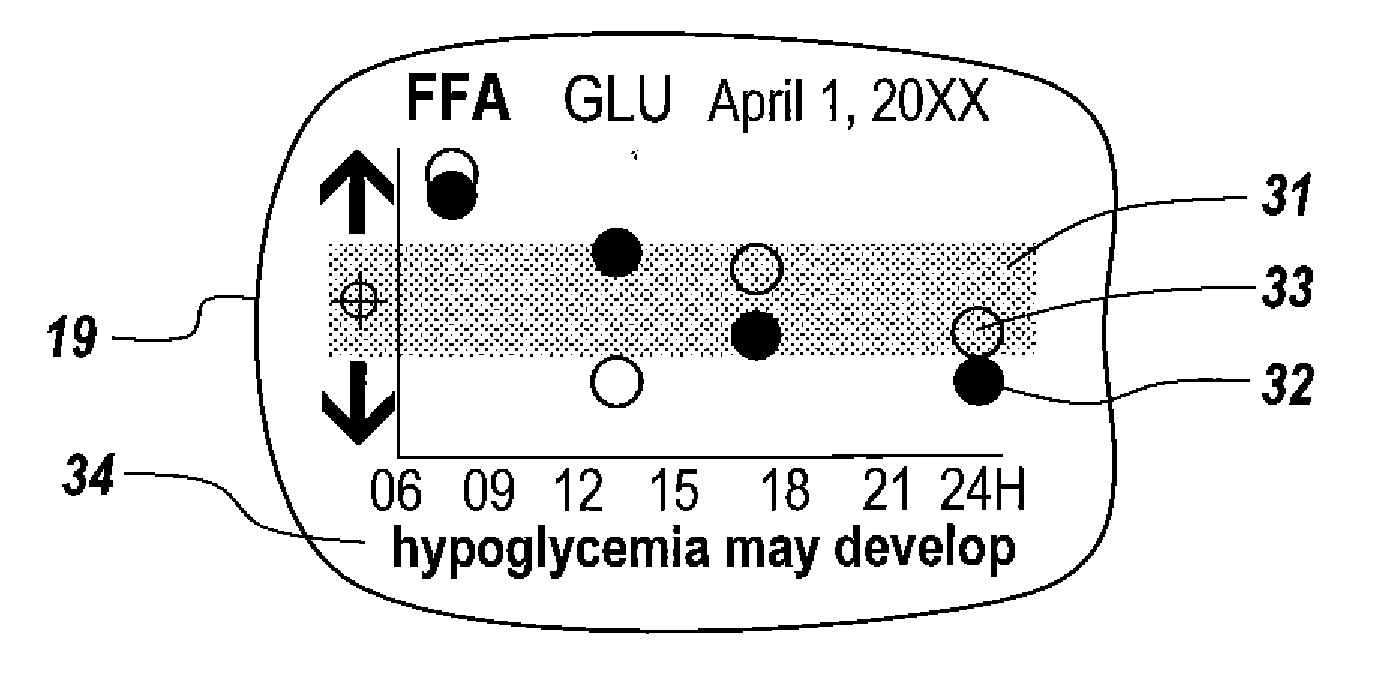
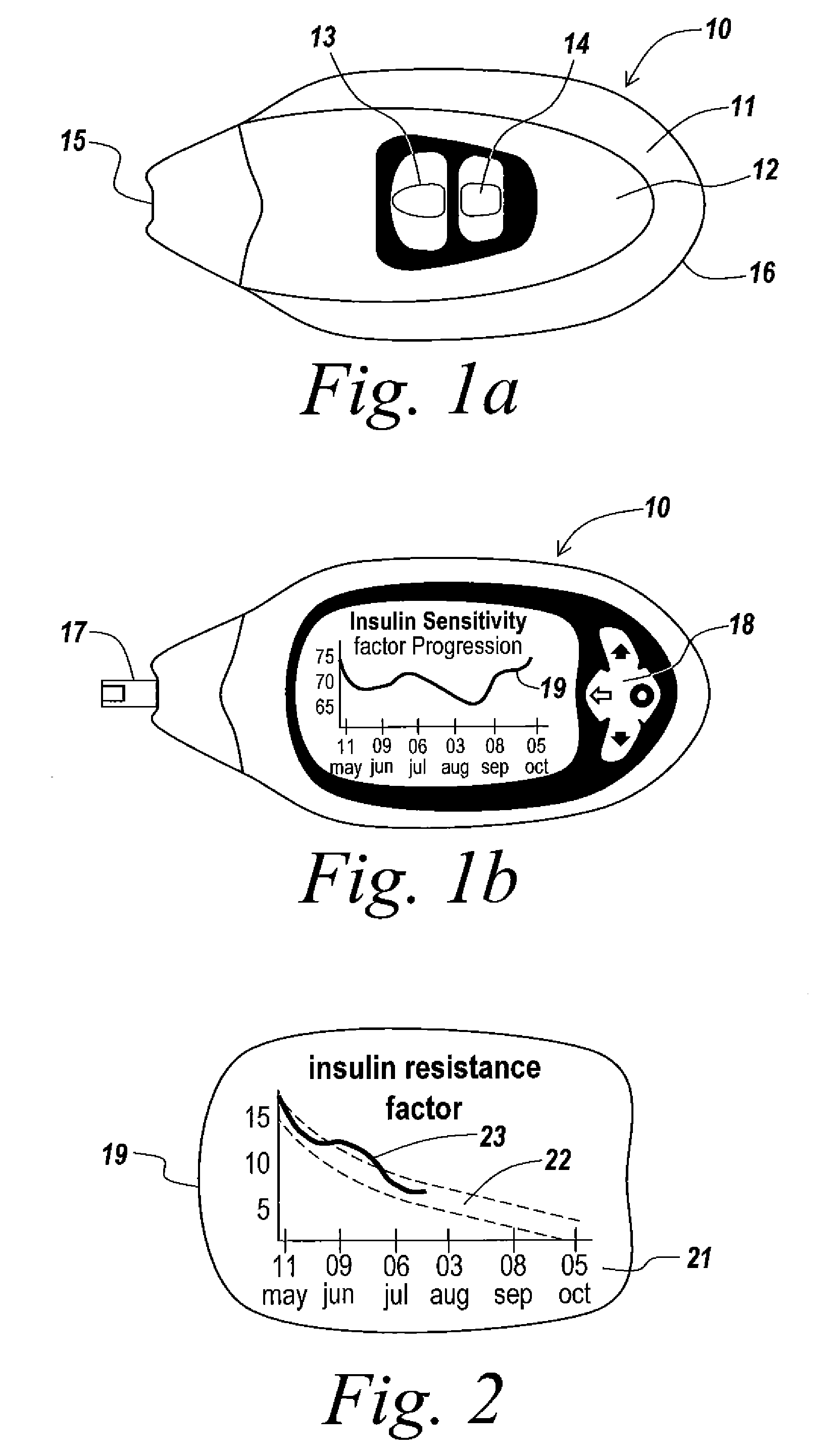
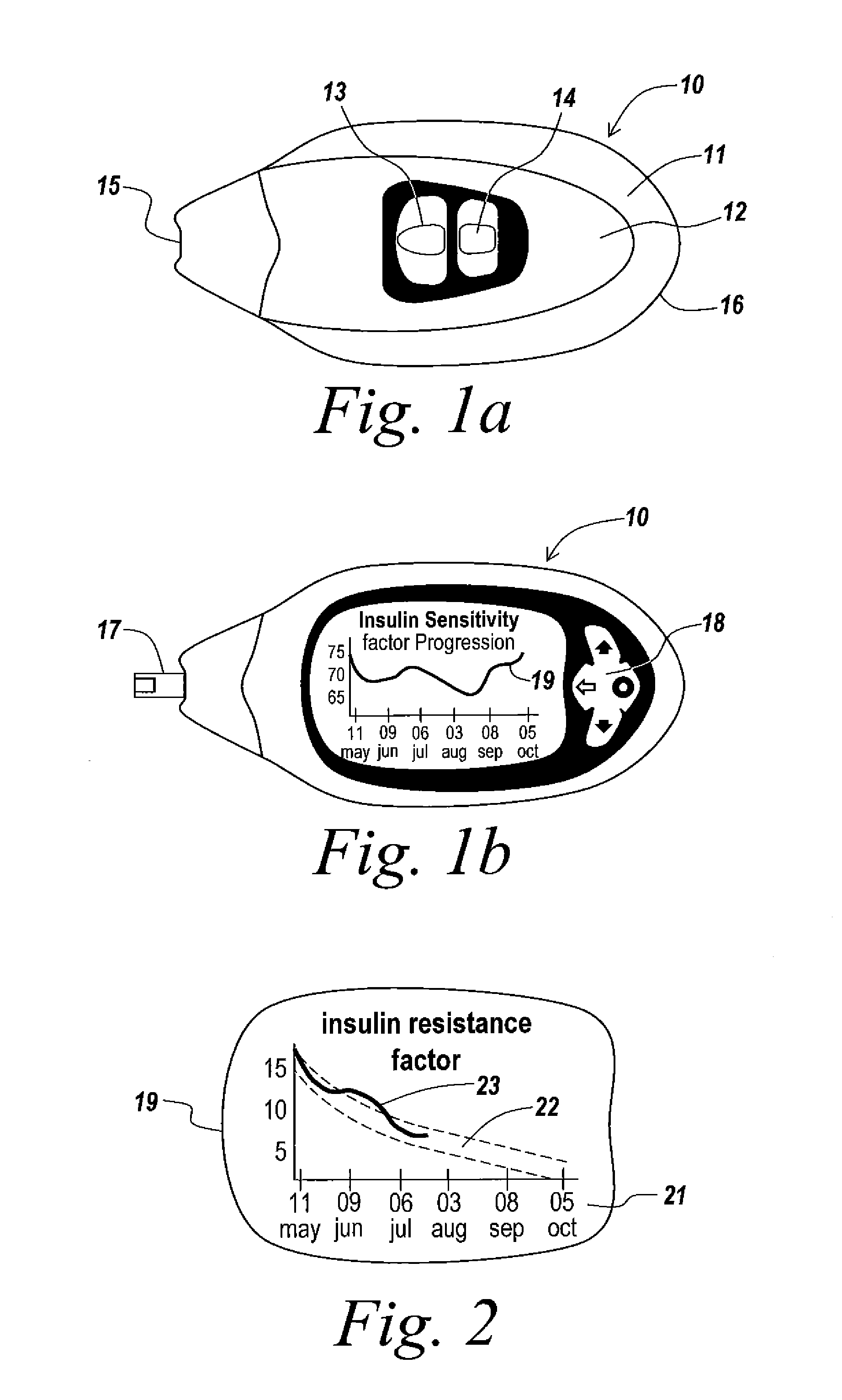
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
Potentiation of glucose elimination
InactiveUS20070020191A1Improve efficiencyEffective controlPowder deliveryOrganic active ingredientsInsulin activityPostprandial Hypoglycemia
Owner:MANNKIND CORP
Monitoring device for management of insulin delivery
ActiveUS20120246106A1Minimize changesDrug and medicationsMedical devicesInsulin activityTreatment management
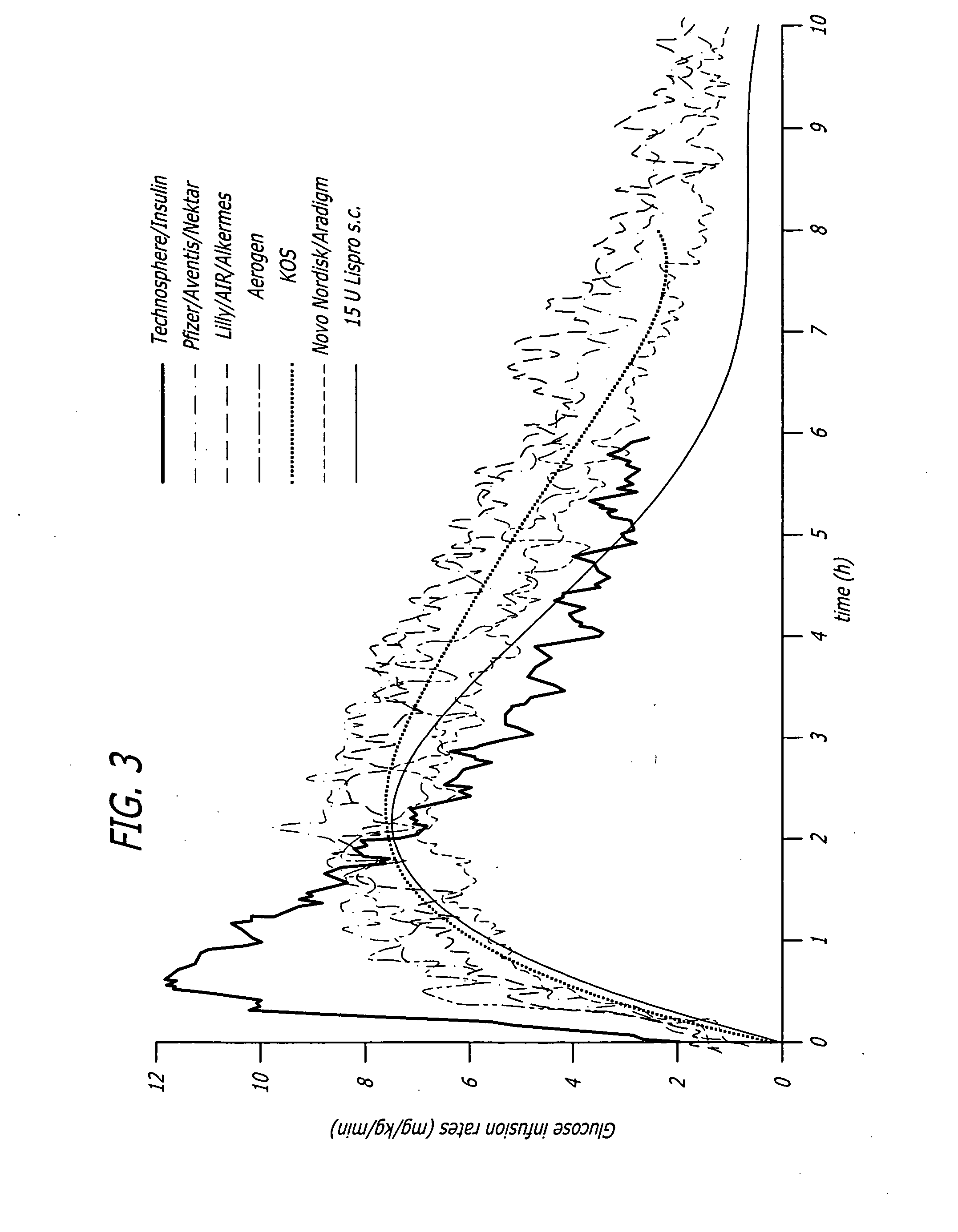
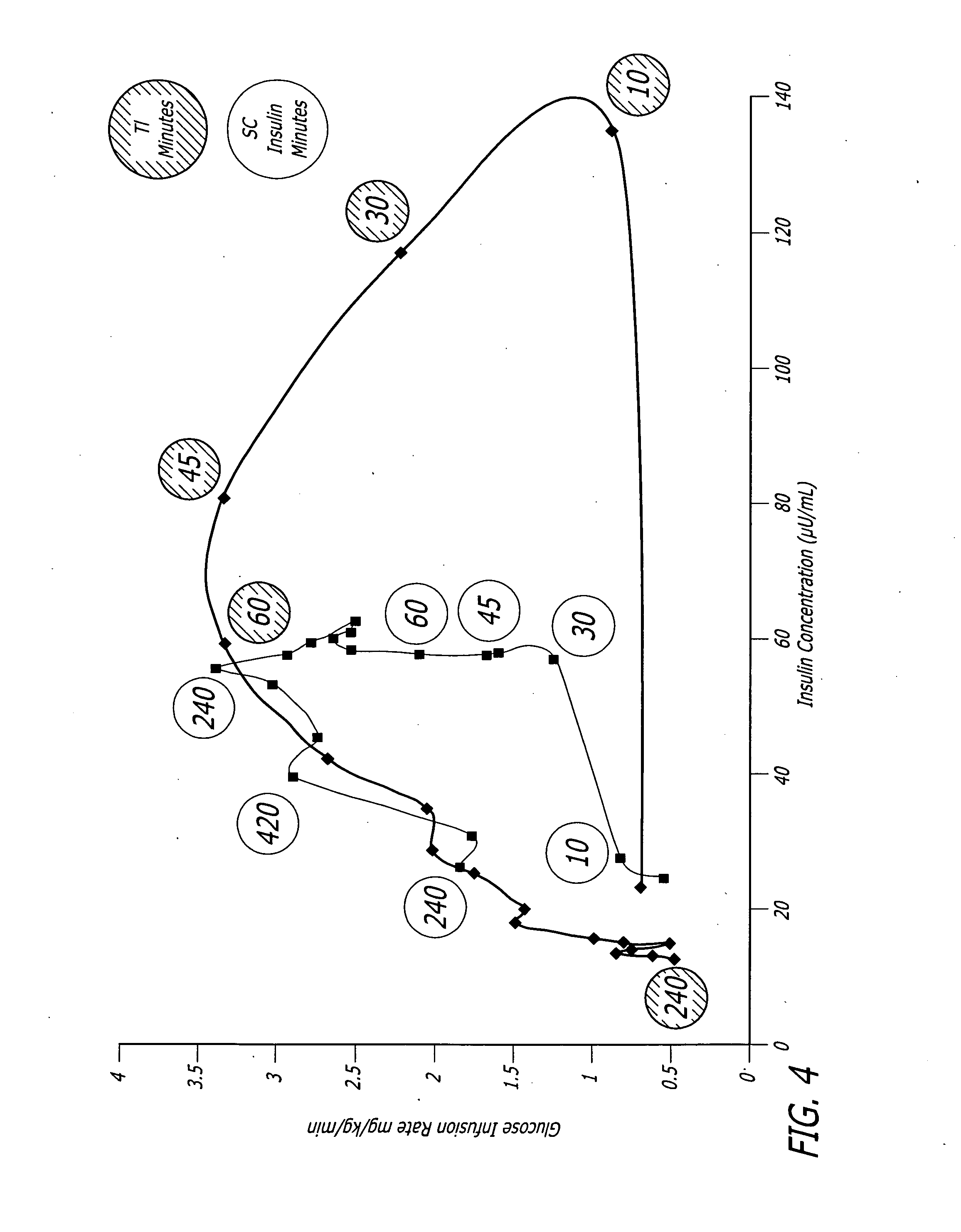
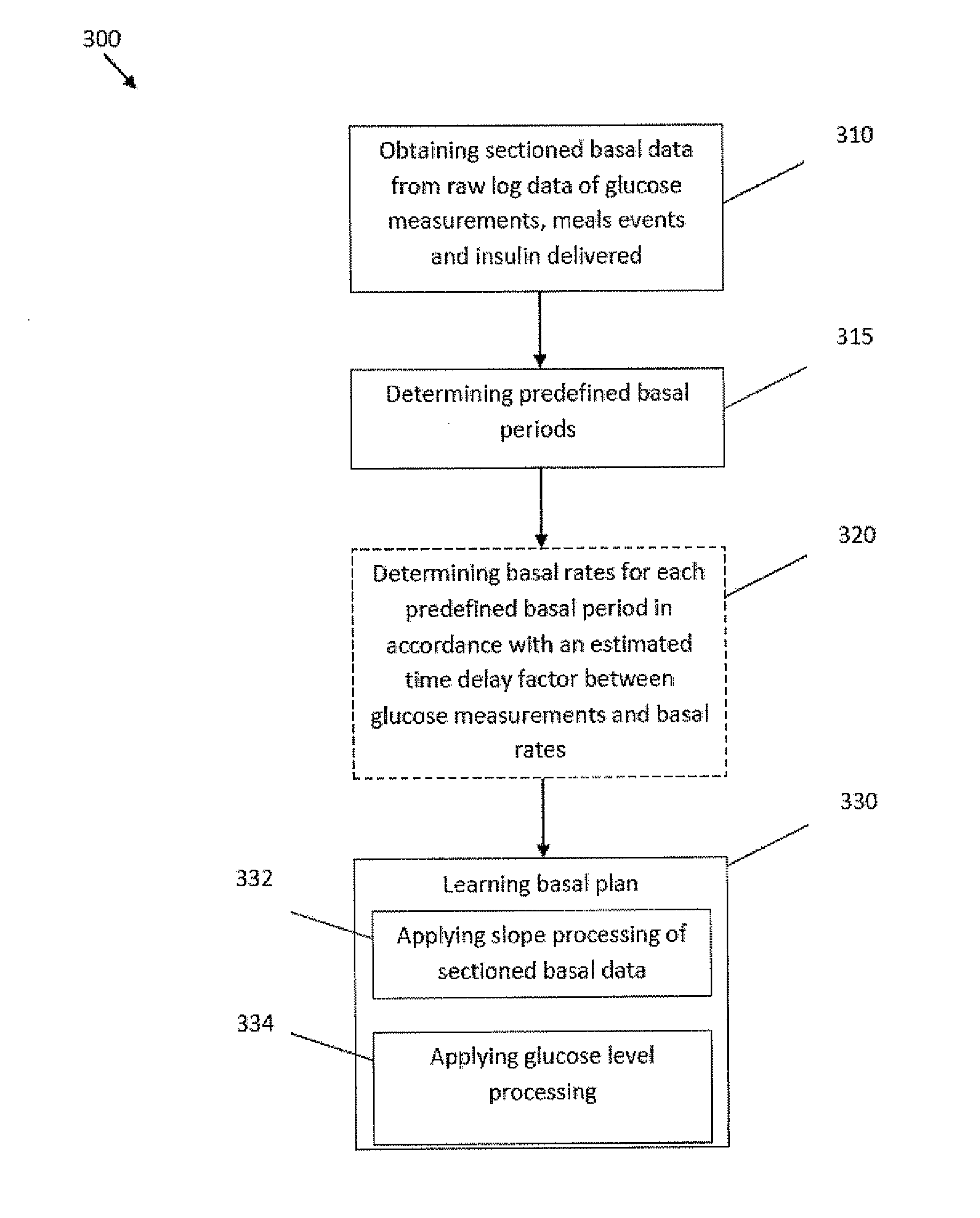
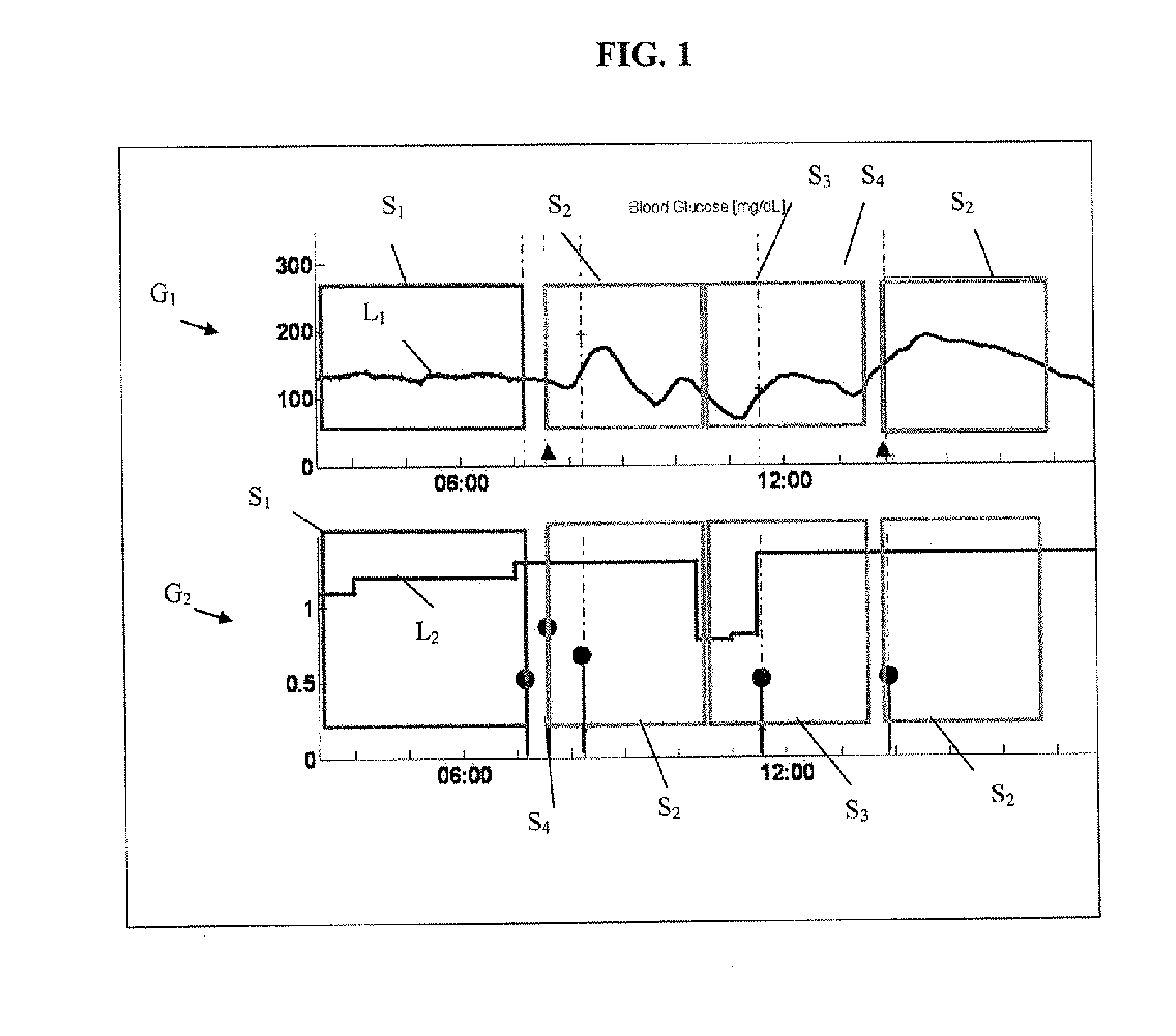
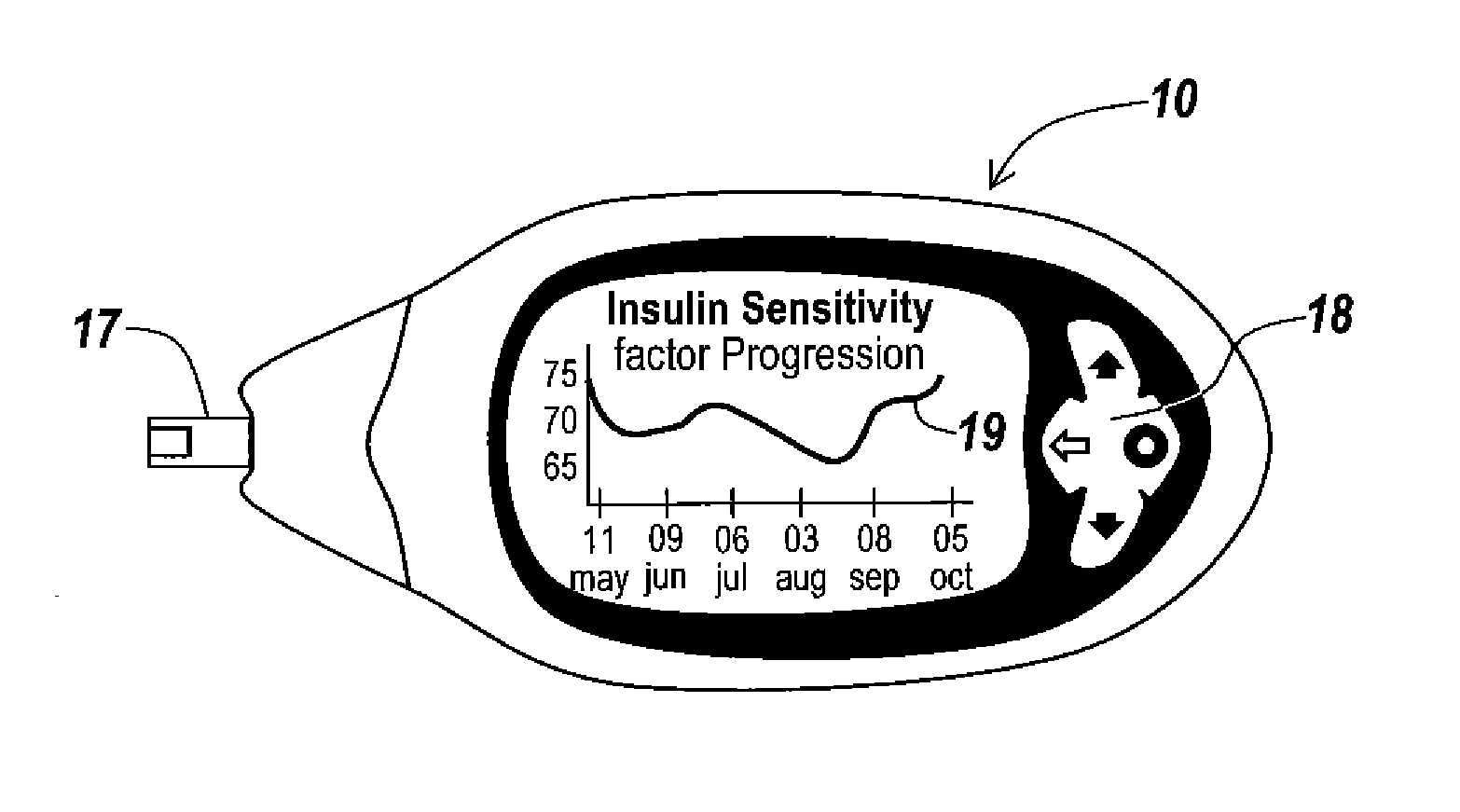
Monitoring system and method for use with diabetic treatment management. The system includes: a communication interface configured to permit access to stored raw log data, obtained over a certain time, being indicative of glucose measurements, meals consumed and insulin delivery; and a control unit including an unsupervised learning controller configured to receive and process said raw log data and determine at least one global insulin pump setting of basal rate, correction factor, carbohydrate ratio and insulin activity curve parameters. The system may include a processing unit including a first processor for processing measured data indicative of blood glucose level and generating first processed data, a second processor including at least one fuzzy logic module which receives input parameters corresponding to the measured data, the first processed data and a reference data, and processes the data to produce a qualitative output parameter to determine whether any treatment parameter should be modified.
Owner:DREAMED DIABETES
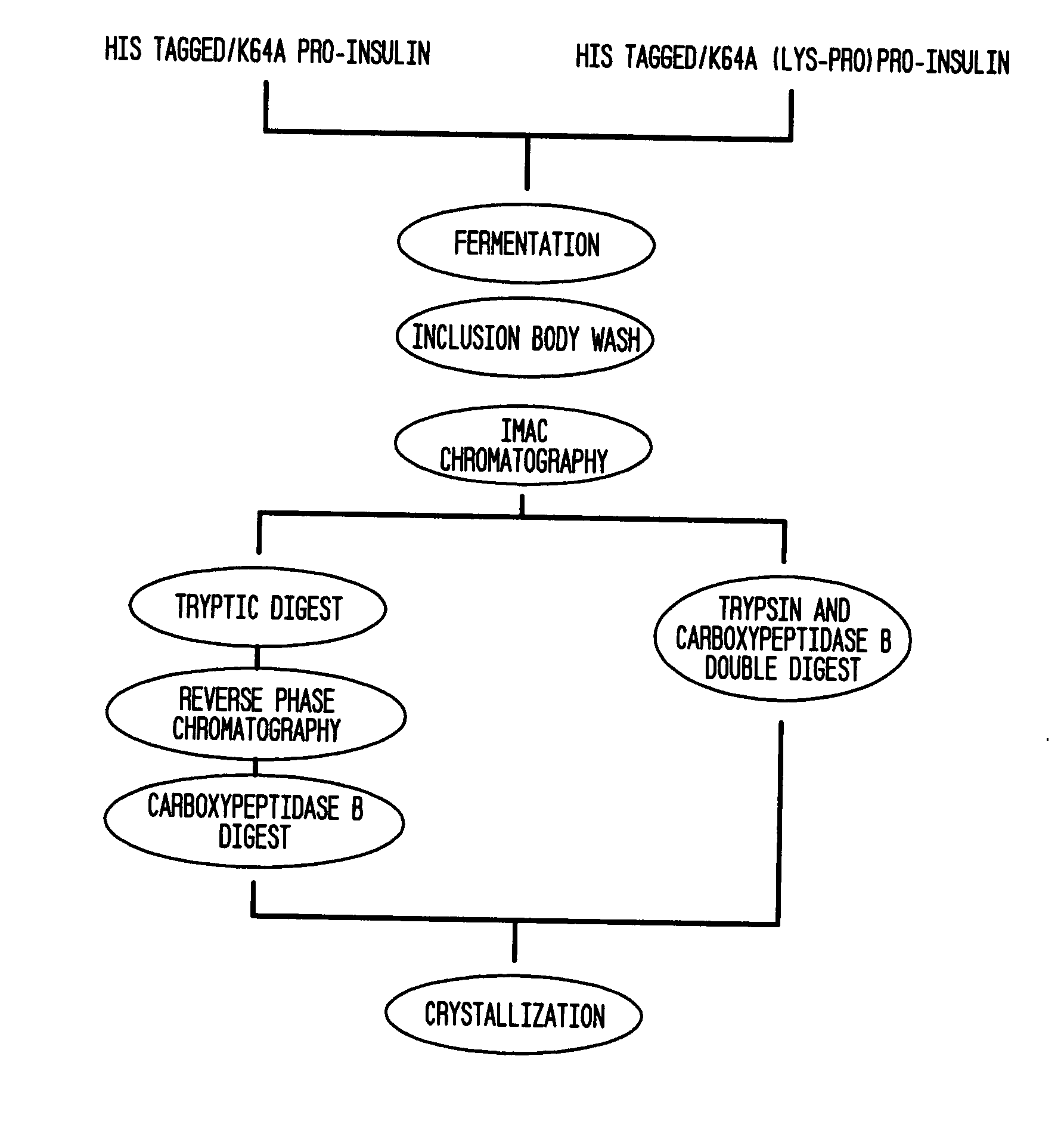
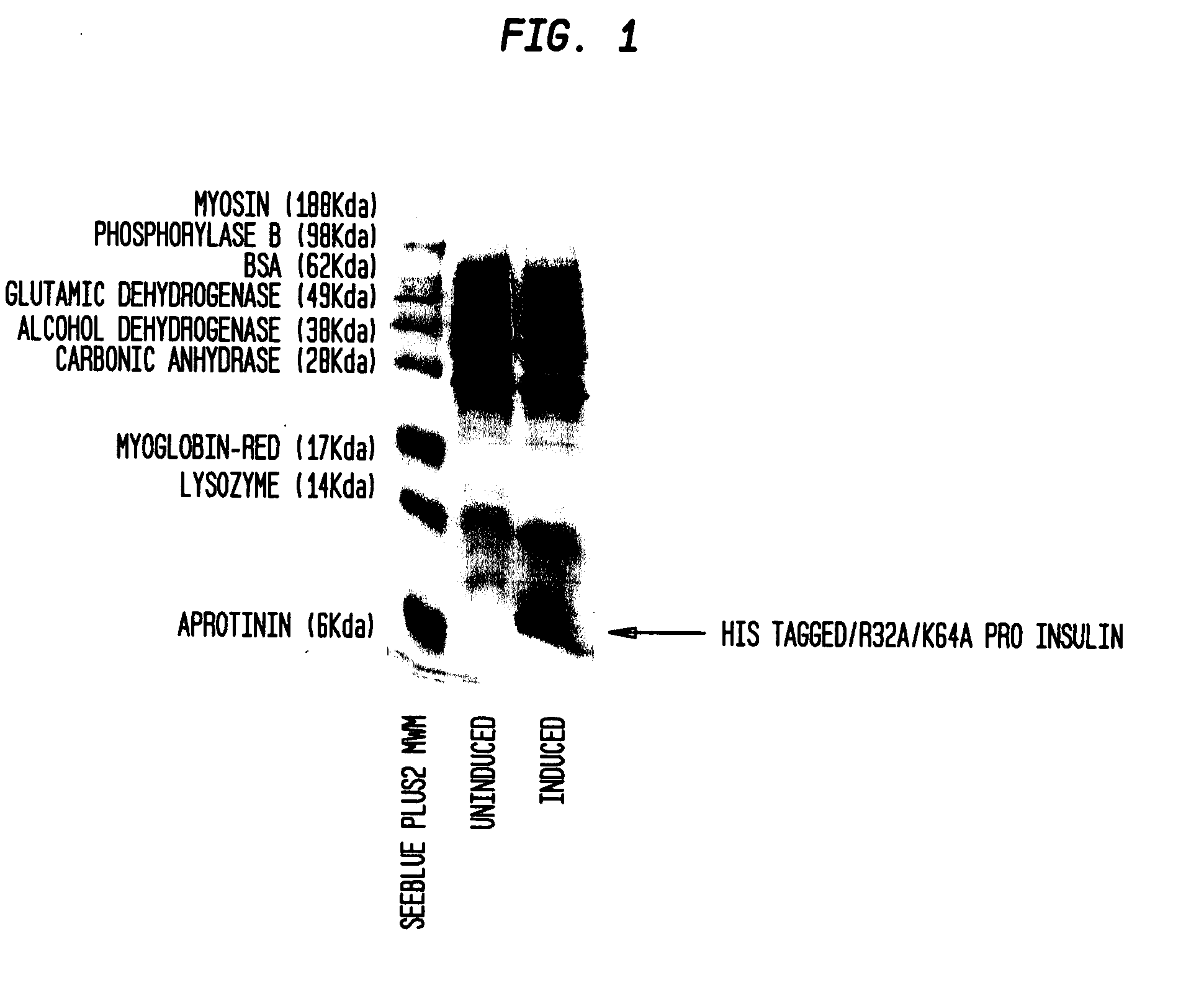
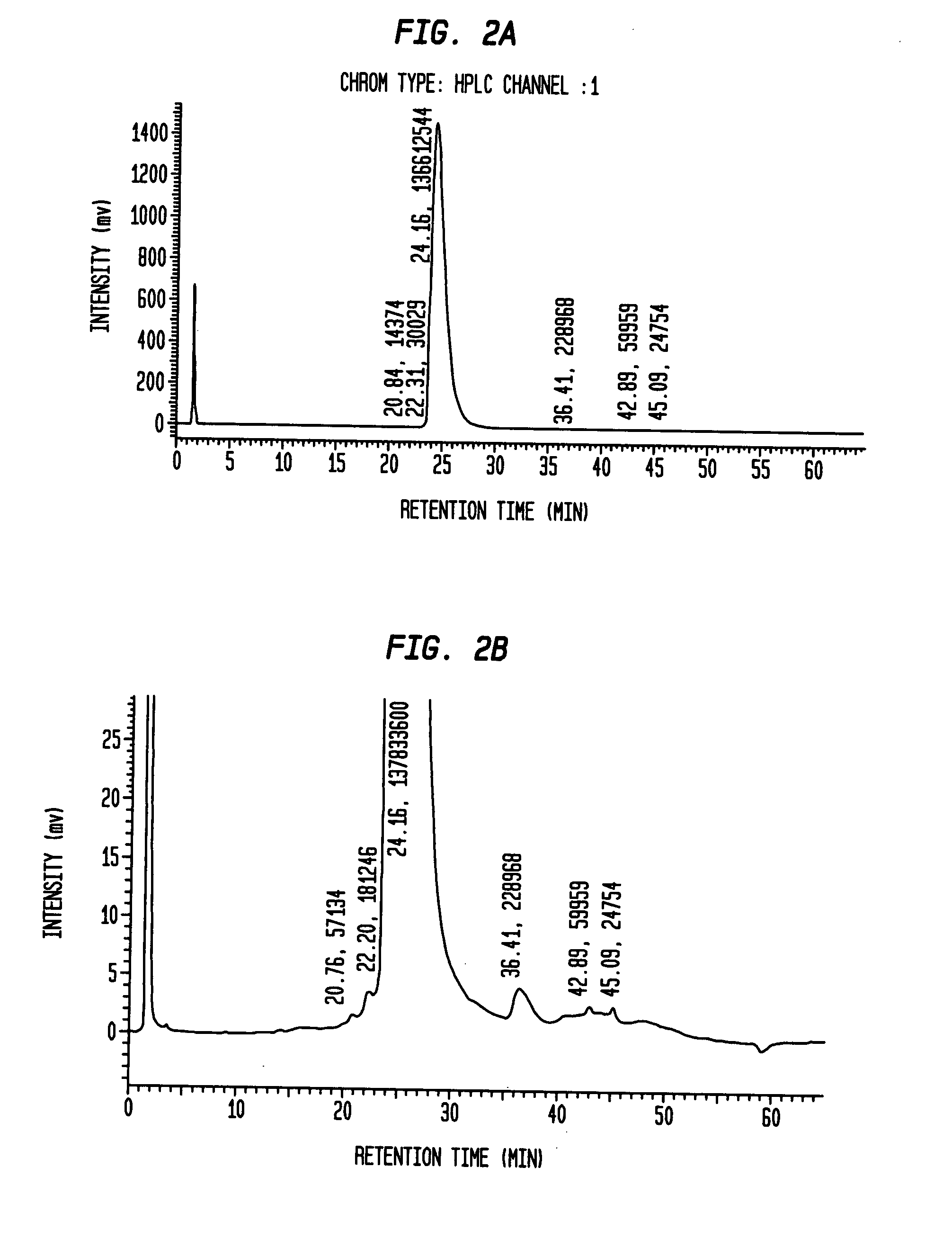
Insulin production methods and pro-insulin constructs
InactiveUS20080146492A1Reduce eliminateImprove efficiencyBacteriaSugar derivativesInsulin activityImproved method
Novel pro-insulin having specific amino acid and / or nucleic acid modifications suitable for improved methods of insulin production are provided. Novel and highly efficient processes for preparing the pro-insulin preparations and preparations containing them are also disclosed. The novel pro-insulin preparations may be converted into human insulin useful in therapeutic preparations. Novel peptides of the C-peptide, and N terminus, including RREAEALQVGQVELGGGPGAGSLQPLALEGSLQAR (SEQ ID NO: 32), and MHHHHHHGGR (SEQ ID NO: 2) respectively are provided, as well as the unique nucleic acid molecules encoding them.
Owner:AGILA BIOTECH PVT LTD
Albumin-insulin fusion proteins
ActiveUS20080057004A1Efficient deliveryPowder deliveryPeptide/protein ingredientsInsulin activityIn vivo
Owner:TEVA BIOPHARM USA
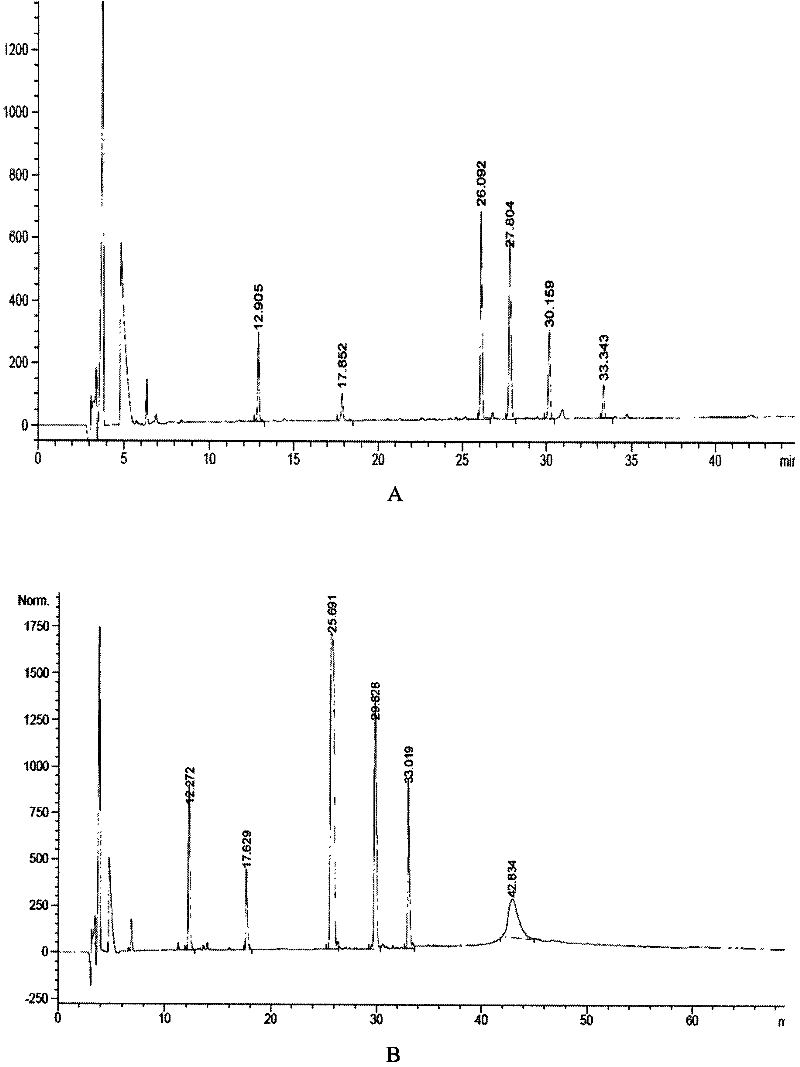
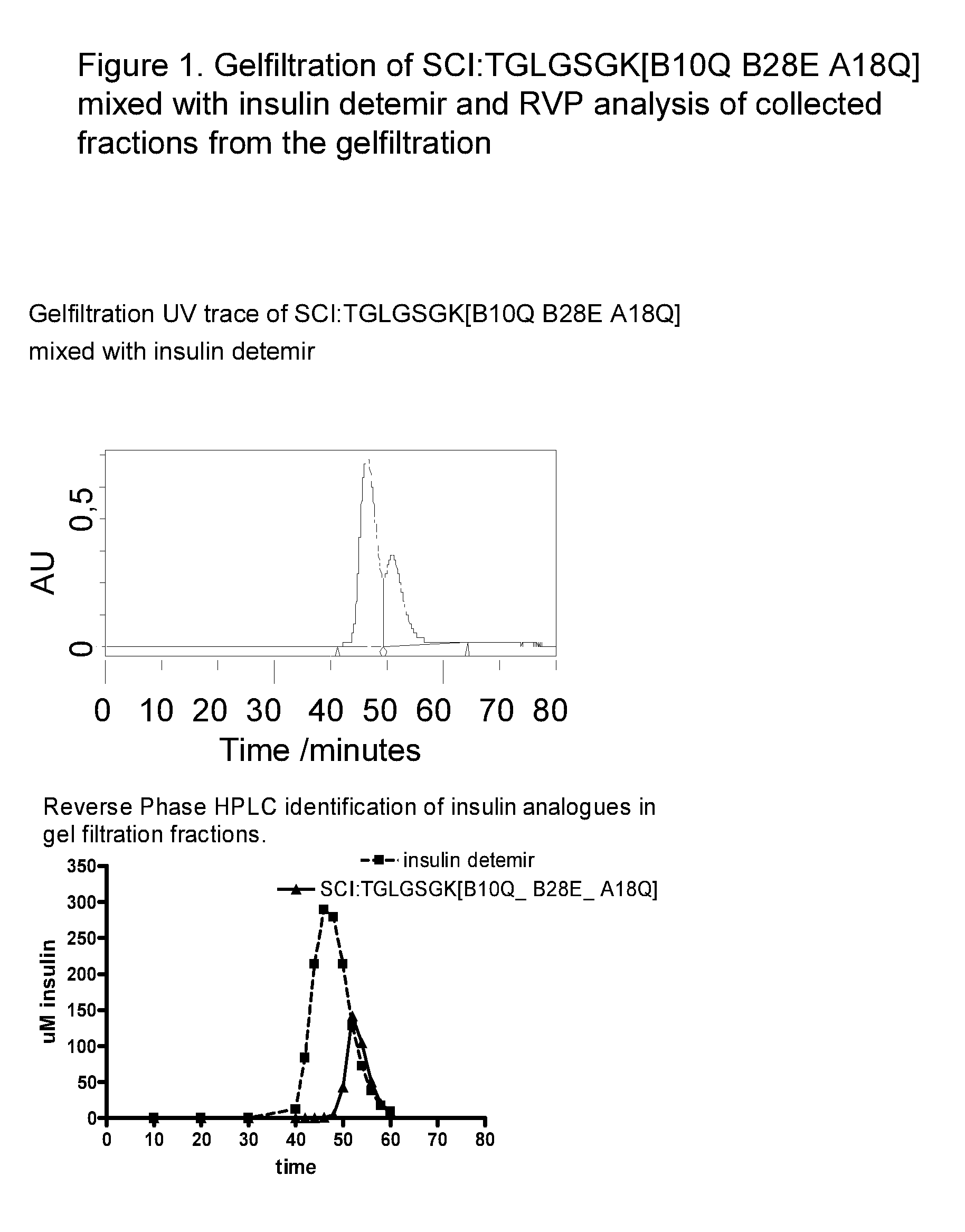
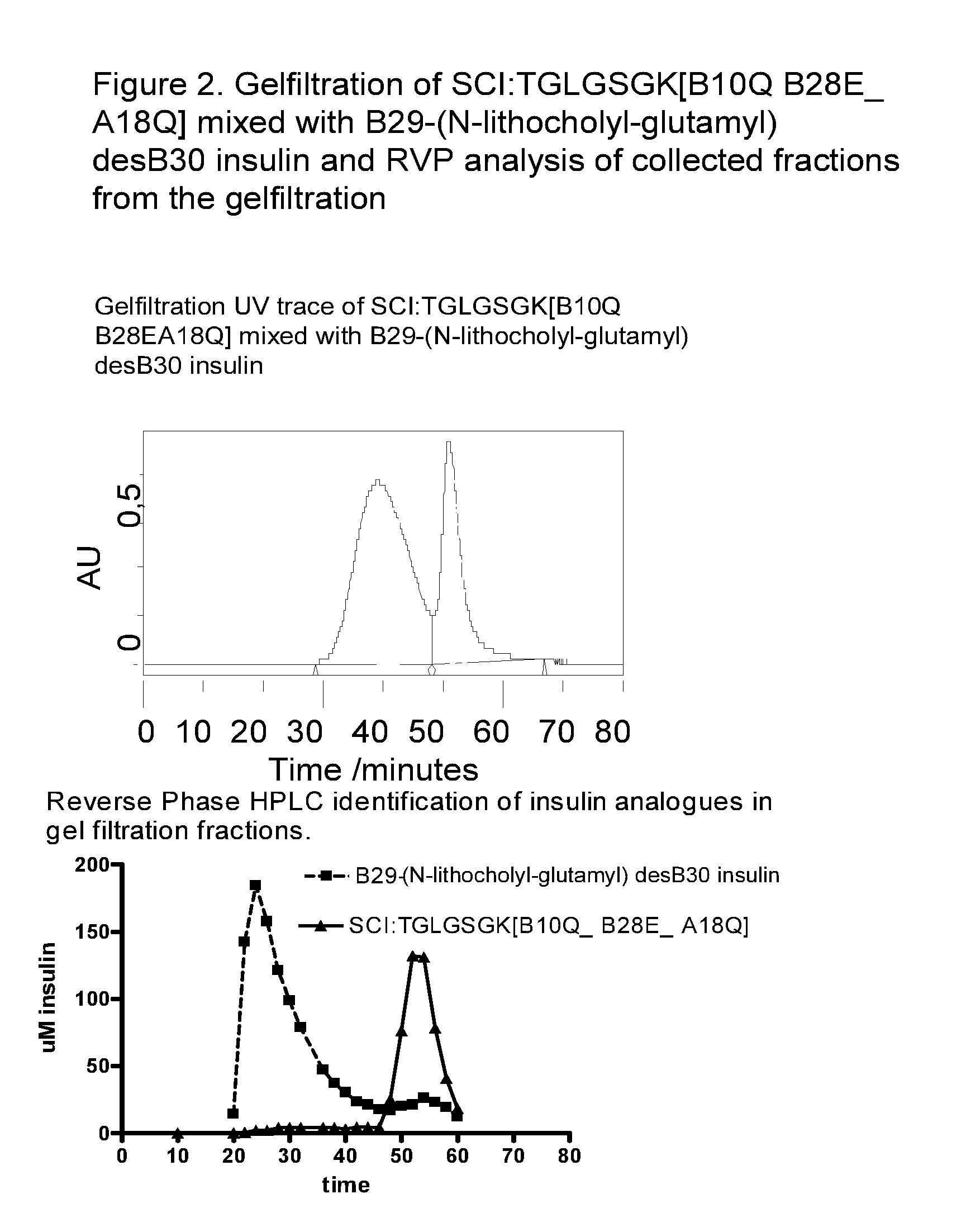
Single-Chain Insulin
InactiveUS20090170750A1Lower blood sugar levelsSugar derivativesPeptide/protein ingredientsInsulin activityPhysical stability
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
Method for preparing recombinant human insulin and analogs of recombinant human insulin
InactiveCN101519446AHigh expressionBacteriaMicroorganism based processesInsulin activityEscherichia coli
The invention provides a molecule (Preproinsulin) of human proinsulin with a novel N-terminal expressed peptide sequence or analogs of the human proinsulin, a method for producing human insulin by using the molecule, and processes for building related expression vectors and engineering cells and expressing and purifying human proinsulin. The DNA sequence of the human proinsulin coded by the N-terminal expressed peptide sequence or the analogs of the human proinsulin is first introduced into a prokaryotic expression vector and then transferred into an escherichia coli to express the molecule in form of an inclusion body. The invention has the advantages that: the product has high expression amount and is easy to purify; the preparation method avoids the use of CNBr; and the process for processing the recombinant insulin is simple.
Owner:AMTEK PHARMA
Monitoring device for management of insulin delivery
ActiveUS8954373B2Minimize changesDrug and medicationsMedical devicesInsulin activityLearning controller
Monitoring system and method for use with diabetic treatment management. The system includes: a communication interface configured to permit access to stored raw log data, obtained over a certain time, being indicative of glucose measurements, meals consumed and insulin delivery; and a control unit including an unsupervised learning controller configured to receive and process said raw log data and determine at least one global insulin pump setting of basal rate, correction factor, carbohydrate ratio and insulin activity curve parameters. The system may include a processing unit including a first processor for processing measured data indicative of blood glucose level and generating first processed data, a second processor including at least one fuzzy logic module which receives input parameters corresponding to the measured data, the first processed data and a reference data, and processes the data to produce a qualitative output parameter to determine whether any treatment parameter should be modified.
Owner:DREAMED DIABETES
Method and Device for Utilizing Analyte Levels to Assist in the Treatment of Diabetes
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
Non-full nutritional formula food for patients with diabetes
InactiveCN104856003AEfficient use ofImprove negative nitrogen balanceSugar food ingredientsVitamin food ingredientsBiotechnologyInsulin activity
The present invention belongs to the technical field of medical and edible formula and new resource food and specifically provides non-full nutritional formula food, specific full nutritional formula food or full nutritional formula food for the consumption of patients with diabetes. Based on the requirement of "General principles of formula food for special medical purposes", combining the physical characteristics of patients with diabetes, and based on the essence theory of traditional Chinese medical science, the formula food reasonably combines and is prepared by mixing a variety of "medical and edible" traditional Chinese medicines, and uses a variety of SBE pre-hydrolysis technology extracted medical and edible traditional Chinese medicine essences, biological enzymolysis extracted short chain peptides, prebiotics, amino acids, carbohydrates, health-care functional oil and fat, various vitamins and minerals. The formula food not only can act as a single source of nutrients to meet the nutritional needs of patients with diabetes, but also can has effects of enhancing insulin activity, lowering blood sugar and blood pressure, lowering lipid and losing weight, and enhancing immunity.
Owner:JINSHANMEI BIOTECH
Blood sugar lowering tea
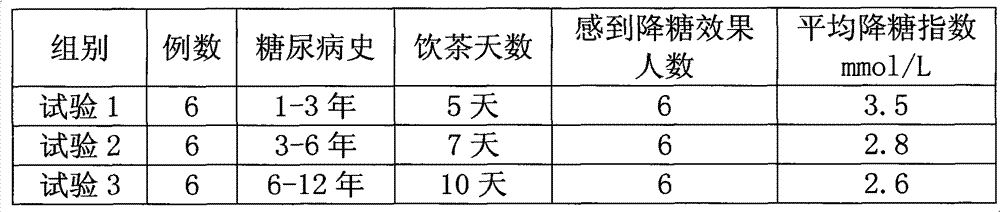
The invention discloses a blood sugar lowering tea which is prepared by mixing six materials. The blood sugar lowering tea is prepared by mixing the following six materials in a scientific ratio: ilex latifolia thunb, white tea, mulberry leaf, corn stigma, paliurus and tartary buckwheat. The blood sugar lowering tea is prepared according to a conventional preparation method of a mixed tea. Through reasonable compatibility and coordination of the six materials in a prescription, the insulin activity is improved and the glucose metabolism is accelerated so as to achieve an aim of lowering blood sugar; and compared with the prior art, the blood sugar lowering tea has the advantages of high integrated concentration, high solubility, convenience for use and no toxic side effect.
Owner:王宇
Method for producing recombined insulin human
ActiveCN101173006ASimple processReduce manufacturing costPeptide preparation methodsInsulinsEscherichia coliInsulin activity
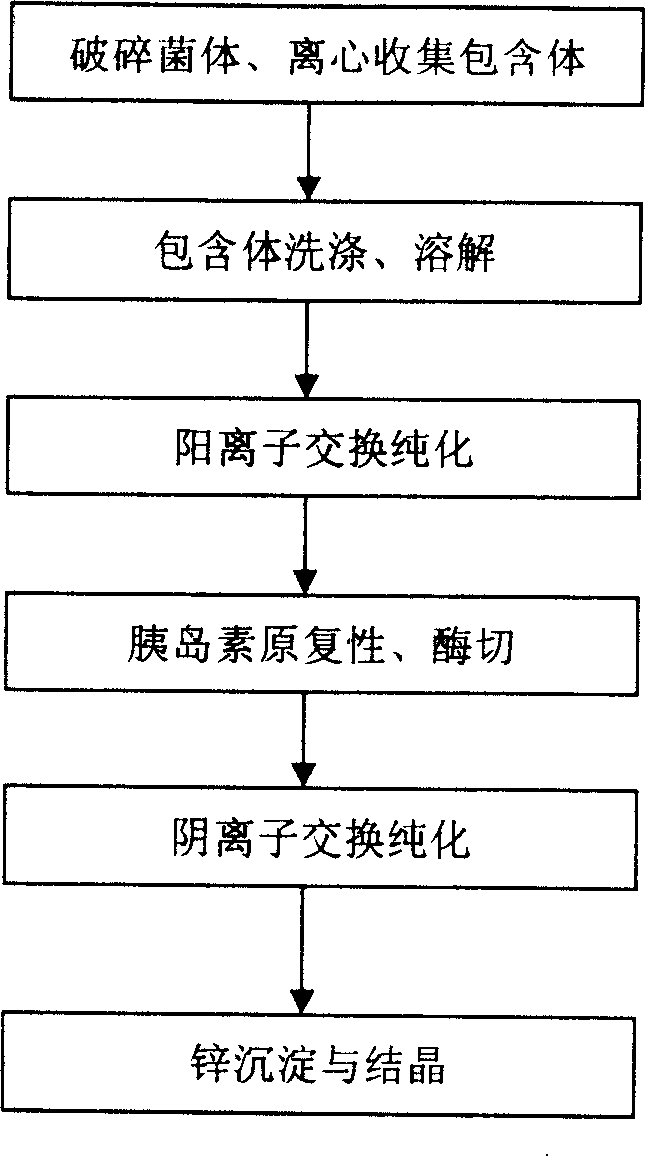
The invention discloses a preparation method of recombinant human insulin, which relates to the protein polypeptide type medicine field. The technical problem needed to be solved by the invention aims at providing a preparation method for Escherichia coli to express the recombinant human insulin in order to overcome the technical disadvantages in the prior art of using the poisonous and harmful substance CNBr and having a complex production process. The preparation method for the recombinant human insulin of the invention comprises the steps of: thallus crashing, inclusion body dissolving, proinsulin sulfonating, purifying proinsulin, and proinsulin renaturation, enzyme cutting transmission, etc., and the preparation method is characterized in that during the inclusion body dissolving process, the -SH of proinsulin is transformed into -SSO3<->, and then human insulin is obtained through purifying the sulfonated proinsulin, reduction renaturation and enzyme cutting transmission by positive ion, and then through the negative ion exchange layer purification. The preparation method of the recombinant human insulin of the invention does not use the CNBr and has simple technique with high human insulin yield coefficient and purity.
Owner:JIANGSU WANBANG BIOPHARMLS +1
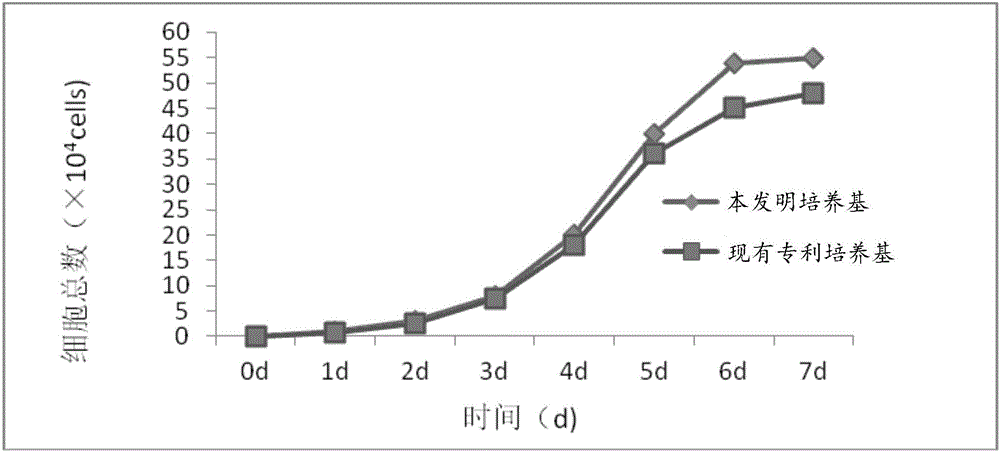
Neural stem cells medium and method for performing human neural stem cells in-vitro long-term culture and amplification by using neural stem cells medium
ActiveCN105062972AGenetic stabilitySolve the easy differentiation of in vitro cultureNervous system cellsInsulin activityCuticle
The invention relates to a neural stem cells medium and a method for performing human neural stem cells in-vitro long-term culture and amplification by using the neural stem cells medium. The neural stem cells medium comprises the following ingredients by weight proportion: 100-1000 micrograms of heparin sodium, 10-100 micrograms of vitamin E, 5-50 milligrams of insulin human recombinant, 0.5-5 milligrams of putrescine, 2-10 micrograms of sodium selenite, 2-10 milligrams of human transferrin, 2-10 micrograms of progestin, 300 milligrams of L-glutamine, 5.9 grams of 2-[4-(2-Hydroxyethyl)-1-piperazine]ethanesulfonic acid, 10-100 micrograms of recombinant human epidermal growth factors, 10-100 micrograms of recombinant human basic fibroblast growth factors, 20-200 milligrams of vitamin C glucoside and 40,000-400,000 IU (international unit) of gentamicin. By the neural stem cells medium, the technical problems that human neural stem cells are easy to differentiate when cultured in vitro and long-term culture and amplification are difficult to implement are solved.
Owner:ZHEJIANG ORIGIN BIOTECH
Pegylated, Extended Insulins
InactiveUS20090306337A1Good chemical stabilityEasy to managePeptide/protein ingredientsMetabolism disorderInsulin activityBioavailability
PEGylated, extended insulins are insulins which, compared with human insulin, has one or more extensions extended from the A1, B1, A21 and / or B30 position(s), said extension(s) consist(s) of amino acid residue(s) and wherein a PEG moiety, via a linker, is attached to one or more of the amino acid residues in the extension(s). PEG is polyethyleneglycol. Such PEGylated, extended insulins have higher bioavailability and a longer time-action profile than regulär insulin and are in particular suited for pulmonary administration and can, conveniently, be used to treat diabetes.
Owner:NOVO NORDISK AS
Fully human antibodies directed against the human insulin-like growth factor-1 receptor
This invention relates to human antibodies that bind to human insulin-like growth factor-1 receptor (IGF-IR), to derivatives of these antibodies (Fabs, single chain antibodies, bi-specific antibodes, or fusion proteins), and to uses of the antibodies and derivatives in therapeutic, and diagnostic methods. The invention relates to nucleic acids encoding the anti-IGF-IR, methods of generating the antibodies and expression. The invention further relates to combination therapies using ant-IGF-IR antibodies with anti-neoplastic drugs.
Owner:IMCLONE SYSTEMS
Hepatogenic diabetes full nutritional formula food
InactiveCN105410896ASolve inactivationSolve the coexistence problemFood scienceInsulin activityFormulary
The present invention belongs to the technical field of medical and edible formula and new resource food and specifically provides a hepatogenic diabetes full nutritional formula food, specific medical formula food or non-specific medical formula food. Based on the requirement of ''General principles of formula food for special medical purposes'', combining the physical characteristics of patients with hepatogenic diabetes, and based on the essence theory of traditional Chinese medical science, the formula food reasonably combines and is prepared by mixing a variety of ''medical and edible'' traditional Chinese medicines, and uses a variety of semi-bionic extraction method extracted medical and edible traditional Chinese medicine essences, various microencapsulated probiotics, biological enzymolysis extracted short chain peptides, prebiotics, amino acids, carbohydrates, health-care functional oil and fat, various vitamins and minerals. The formula food not only can act as a single source of nutrients to meet the nutritional needs of patients with hepatogenic diabetes, but also has effects of enhancing insulin activity, lowering blood sugar and blood fat, tonifying kidney and liver and enhancing immunity.
Owner:JINSHANMEI BIOTECH
Use of non-peptidyl compounds for the treatment of insulin related ailments
InactiveUS6933272B1Modulate activityBiocidePeptide/protein ingredientsChemical MoietyInsulin activity
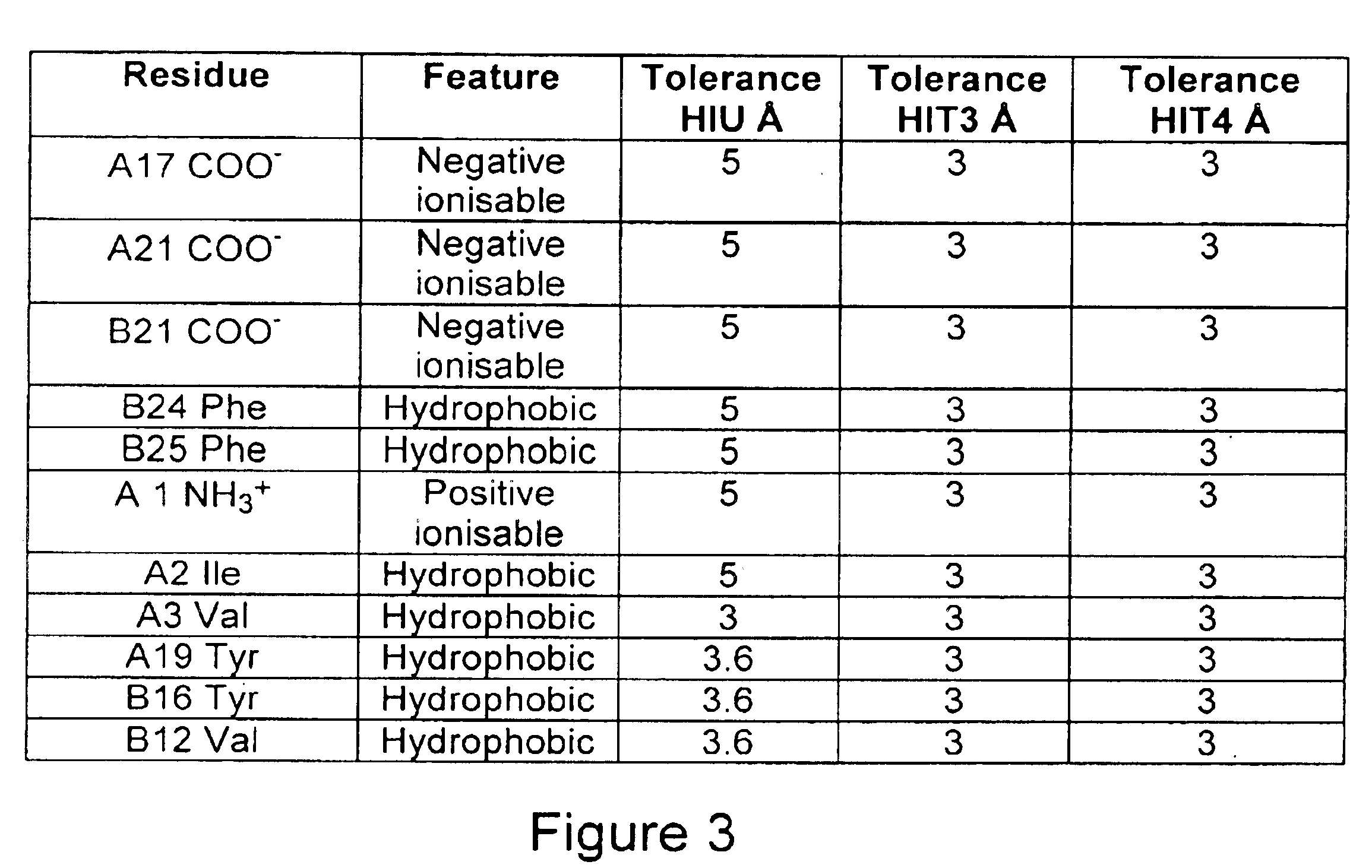
The present invention relates to the use of at least a non-peptidyl compound as a biological modulator of insulin activity or insulin-related activity, which compound possesses ionic and hydrophobic chemical moieties spatially located so as to mimic at least an ionic and hydrophobic amino acid residue of insulin, which amino acids are associated with the binding of insulin to its receptor.
Owner:HELMERHORST ERIK +1
Medical formula food for diabetes
InactiveCN105341906ASolve inactivationSolve the coexistence problemFood scienceInsulin activityFormulary
The invention belongs to the technical field of medicinal and edible formulas and new resource foods, and particularly provides a medical formula food, a specific medical formula food or an unspecific formula food for patients suffering from diabetes. According to the requirements of the 'General Rules for Formula Foods for Special Medical Purposes', combining the constitution characteristics of the patients suffering from the diabetes, and reasonably mixing various 'medicinal and edible' traditional Chinese medicines according to the quintessence theory of traditional Chinese medicine science, the formula food is prepared by mixing extract essences extracted from medicinal and edible traditional Chinese medicines by adopting a half-bionic extraction method, various micro-capsulated probiotics, short chain polypeptides extracted through biological enzymolysis, prebiotics, amino acids, carbohydrate, grease with a health-care function, various vitamins and mineral substances and other technologies. The formula food not only can be used as a single nutrient source to meet the nutrient requirements of the patients suffering from the diabetes, but also has the efficacies of resisting ageing, strengthening the activity of insulin, reducing blood sugar, reducing blood lipid, reducing blood lipid, reducing weight and enhancing immunity.
Owner:JINSHANMEI BIOTECH
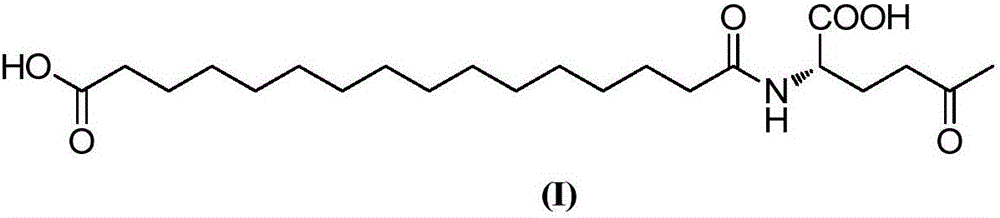
Preparation method of acylation derivative of human insulin or analog thereof
The invention relates to a preparation method of an acylation derivative of human insulin or analog thereof, and concretely relates to the acylation derivative of the human insulin or the analog thereof, prepared from an acylation group existing in an acid form and the human insulin or the analog thereof through a one-step reaction. The method greatly increases the yield of the final product, omits a purification step, and facilitates industrial production of the acylation derivative of the human insulin or analog thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method and Device for Utilizing Analyte Levels to Assist in the Treatment of Diabetes
InactiveUS20110046895A1Metabolism disorderInvestigating moving sheetsInsulin dependent diabetesInsulin activity
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
Method and Device for Utilizing Analyte Levels to Assist in the Treatment of Diabetes
InactiveUS20110046468A1Metabolism disorderInvestigating moving sheetsInsulin dependent diabetesDisease
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
Human insulin/analogue conjugate with continuous blood sugar reduction function and high rate of combination with receptor
ActiveCN102675452AUnderstand clearlyPeptide/protein ingredientsMetabolism disorderInsulin activityHigh rate
The invention relates to a human insulin / analogue conjugate with a continuous blood sugar reduction function and a high rate of combination with a receptor, and discloses a method for preparing a reconstructed human insulin / analogue conjugate by reconstructing a human insulin precursor by utilizing a methylotrophic yeast expression, performing purification, enzyme digestion, chromatography or synthesis to prepare a reconstructed human insulin and a DesB30 analogue thereof and coupling a side chain amino group of a 29th-bit lysine of a B chain of the reconstructed human insulin precursor and a polyethyleneglycol acetamide bond. The prepared reconstructed human insulin conjugate has the characteristics of high rate of combination with the insulin receptor, remarkable continuous blood sugar reduction effects and long in-vivo half-life period, and is applied to the treatment of I-type and II-type diabetes.
Owner:CHONGQING FAGEN BIOMEDICAL +1
Single-Chain Insulin Analogues and Pharmaceutical Formulations Thereof
InactiveUS20110009314A1Good physical and chemical stabilityPeptide/protein ingredientsMetabolism disorderInsulin activityInsulin B Chain
The present invention is related to fast acting single-chain insulin comprising a modified B-chain and the A-chain of human insulin or an analogue thereof connected by a connecting peptide wherein one or more of the amino acid residues in position B25, B26 or B27 in the human B-chain are Glu or Asp or are deleted and / or B28 is Glu, Asp, Lys or is deleted, and the amino acid residue in position B10 in the human insulin B-chain is Gln, Ala, Val, Thr or Ser. The invention is also related to pharmaceutical compositions being a mixture of the rapid acting single-chain insulin and the protracted acylated insulin.
Owner:NOVO NORDISK AS
Culture medium for neural stem cell proliferation and capable of inducing neural stem cell to differentiate to dopaminergic neuron and application and proliferation inducing method
The invention relates to the medical field, and discloses a culture medium for neural stem cell proliferation and capable of inducing neural stem cell to differentiate to dopaminergic neuron and an application and proliferation inducing method. The proliferation culture medium is a culture medium using DMEM / F12 as the base. The culture medium comprises insulin human, progesterone, hydrocortisone, dexamethasone, estradiol, transferrin, EGF, FGF, CTGF, B-NGF, VEGF, a soybean pancreatin inhibitor, L-glutathione, oleic acid, Tween 80, phosphatidic acid, sodium selenite, panax notoginseng saponins, resveratrol, lithium chloride, cholesterol, WNT3a, and glutamine. A novel neural stem cell culture medium is formed by using a plurality of suitable components with low cost and extensive sources, the proliferation speed of the neural stem cells is remarkably improved, and other induced differentiation components are added on the basis of the neural stem cell, so the neural stem cell is induced to be differentiated to the dopaminergic neuron, and the differentiation proportion is remarkably improved.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
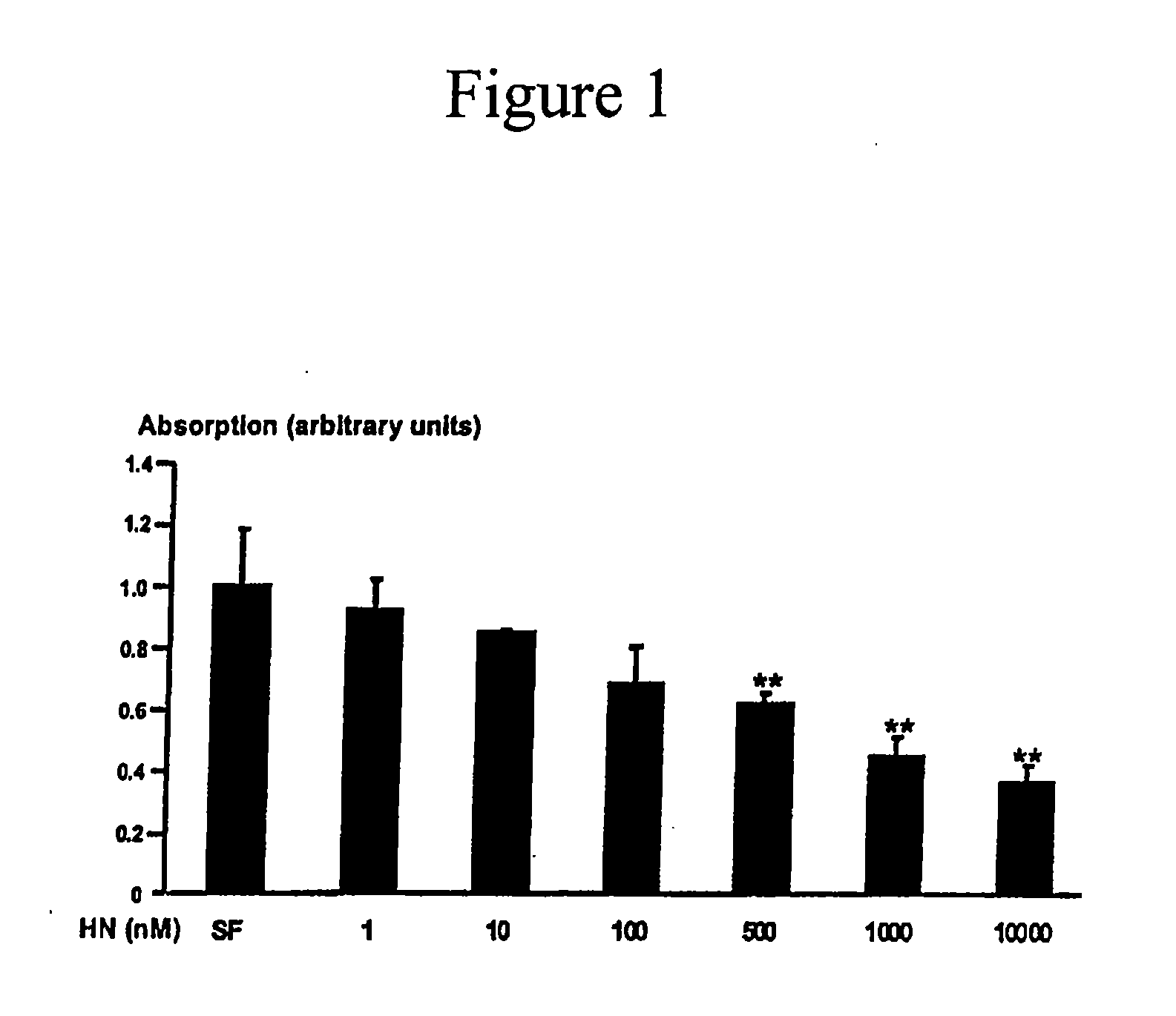
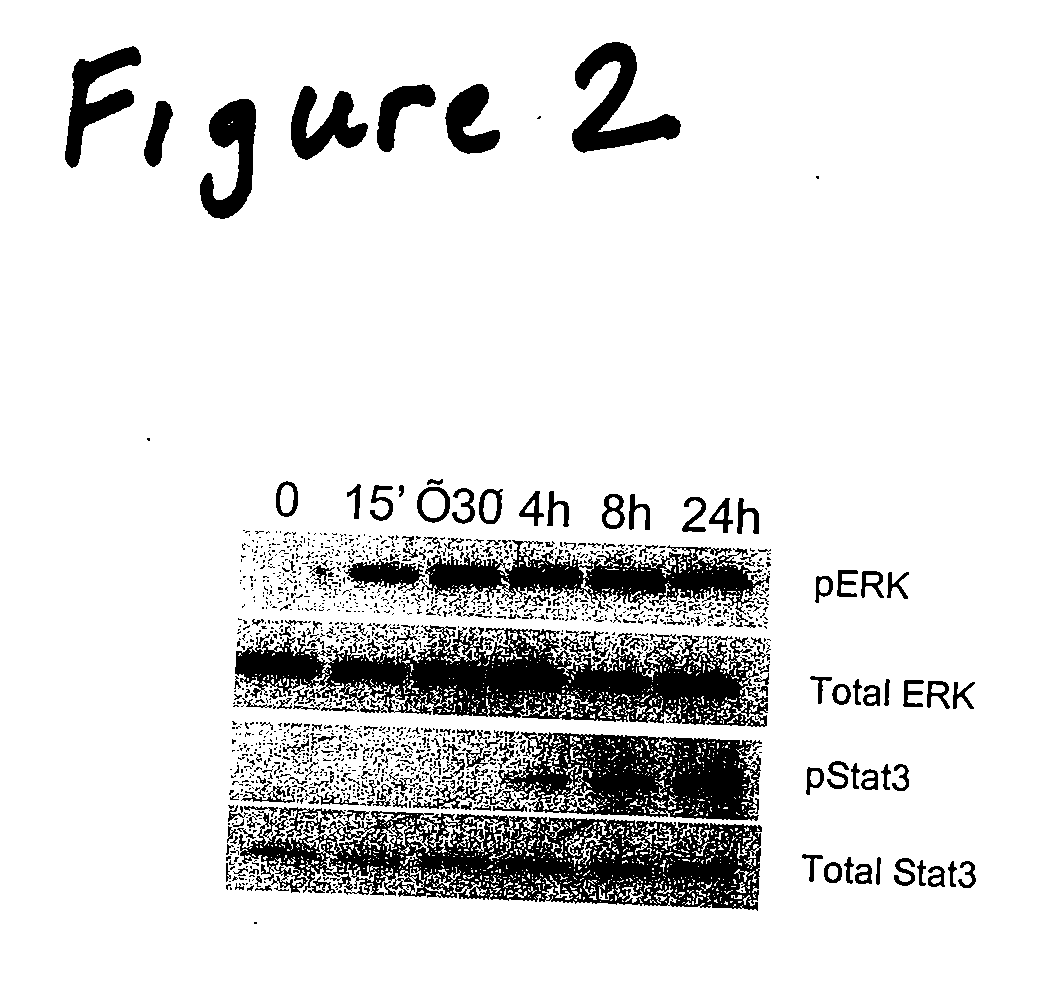
Compositions to prevent and treat type-1 diabetes
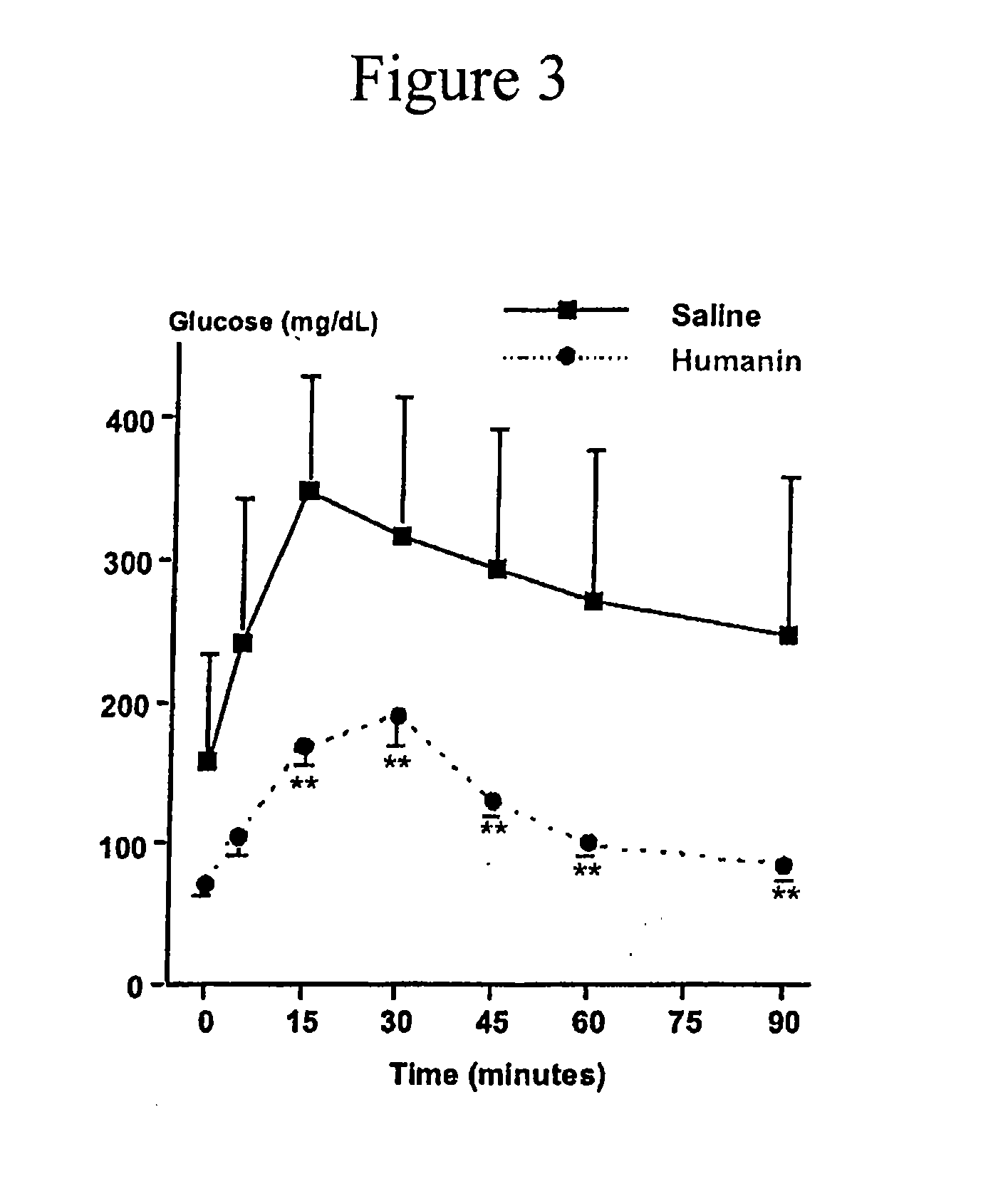
ActiveUS20100130412A1Improve survivalOrganic active ingredientsPeptide/protein ingredientsHumaninInsulin activity
Owner:RGT UNIV OF CALIFORNIA
New monomeric insulin and its medicinal composition and prepn process
InactiveCN1468864AEasy to makeSuitable for mass productionPeptide/protein ingredientsMetabolism disorderYeastInsulin activity
The present invention provides new insulin strand B, monomeric insulin containing strand B, medicinal composition containing the monomeric insulin and their preparation process and use. The insulin analog B27K-DTrI sample of high purity is obtained via enzymatic semi-synthesis process and is proved to have the property of monomeric insulin and overall insulin activity 80 % of that of normal insulin. The monomeric insulin precursor is secreted and expressed in yeast expressing system and enzyme cut to obtain B27K-DTrI, and the enzymatic peptide converting step is omitted to raise the final yield and suit for industrial production.
Owner:SHANGHAI C A S SHENGLONGDA BIOTECH GRP
Human insulin monoclonal antibody crosslinking magnetic particle as well as preparation method thereof and human insulin detection kit comprising human insulin monoclonal antibody crosslinking magnetic particle
ActiveCN104020288AHigh detection sensitivityStrong specificityBiological testingInsulin activityCross-link
The invention discloses a human insulin monoclonal antibody crosslinking magnetic particle as well as a preparation method thereof and a human insulin detection kit comprising the human insulin monoclonal antibody crosslinking magnetic particle. Before the human insulin monoclonal antibody is cross-linked with the magnetic particles, the human insulin monoclonal antibody is preprocessed by utilizing a nonionic surface active agent. The human insulin detection kit has the advantages of high detection sensitivity, strong specificity, small medicine interference, good result stability and the like; the human insulin monoclonal antibody crosslinking magnetic particle can be widely suitable for clinical auxiliary diagnosis and screening of diabetes.
Owner:SHANGHAI KEHUA BIO ENG
Double-chain polyethylene glycol-insulin compound and preparation method thereof
InactiveCN101721712AHas a long-lasting functionHigh yieldPeptide/protein ingredientsMetabolism disorderInsulin activityAspartic acid residue
The invention relates to the field of biomedicine. A double-chain polyethylene glycol-insulin compound has a structural formula D-X-R2, wherein in the formula, R is CH3O-(CH2CH2O)n CH2CH2NH, and n is 50 to 250; X is L-glutamic acid residue or L-aspartic acid residue; and D is a product obtained by removing alanine residue of B30 site from natural porcine insulin or removing B30 threonine residue from human insulin. In PEG modification, the amino modification is changed into carboxyl modification, two polyethylene glycol chains are connected to two carboxyls of the B30 amino acid residue of the insulin, and the connection is high-yield and stable due to an amide bond. The synthesized double-chain polyethylene glycol-insulin compound with great application prospect in the long-acting effect is intrinsically chemical modified insulin. The double-chain polyethylene glycol-insulin compound has potential long-acting function.
Owner:何明磊
Serum-free medium for monkey embryonic stem cell
ActiveCN106754657AMaintain self-renewalMaintain normal stateCulture processCell culture active agentsInsulin activitySerum free media
The invention discloses a serum-free medium for a monkey embryonic stem cell. The serum-free medium comprises a basic medium and additives, wherein the additives are dissolved in the basic medium; the basic medium comprises a DMEM-F12 medium; and the additive comprises L-glutamine, non-essential amino acid, L-ascorbic acid, sodium selenite, beta-mercaptoethanol, human serum albumin, heparin sodium, human transferring, insulin human, a basic fibroblast growth factor, a transforming growth factor beta 1 and an epidermal growth factor. The serum-free medium is capable of maintaining an undifferentiation state and totipotency of the monkey embryonic stem cell within a relatively long time, and is applicable to culture of the monkey embryonic stem cell in both a free-feeding layer system and a feeding layer system.
Owner:北京赛斯达生物技术有限公司
Mono-modified polyethyleneglycol-insulin complexes and preparation method thereof
InactiveCN101284135AHas a long-lasting functionPeptide/protein ingredientsMetabolism disorderInsulin activityPork insulin
The invention relates to the field of bio-medicine. The formula of a polyethylene glycol-modified insulin composite is RNH-X-D, wherein R represents CH3O-(CH2CH2O)nCH2CH2, n is equal to 50-500, X is natural amino acid outside proline of genetic encode or non-natural amino acid of mono-amino mono-carboxyl, and D is B30 amino acid residue-removed natural pork insulin or human insulin. In PEG modification, carboxyl instead that amino is modified, and polyethylene glycol chain is linked to alpha carboxyl on B30 amino acid residue of insulin via amide bond at high yield and high stability. The polyethylene glycol-modified insulin composite has important application prospect in long acting aspect, and substantially is chemically modified insulin. The polyethylene glycol-modified insulin composite has potential long acting function.
Owner:DALIAN NATIONALITIES UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com