New monomeric insulin and its medicinal composition and prepn process
A technology of insulin and monomers, applied in the fields of medicine and pharmacy, can solve problems such as chemical side reactions, difficulty in large-scale production, and complicated process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Preparation of DOI
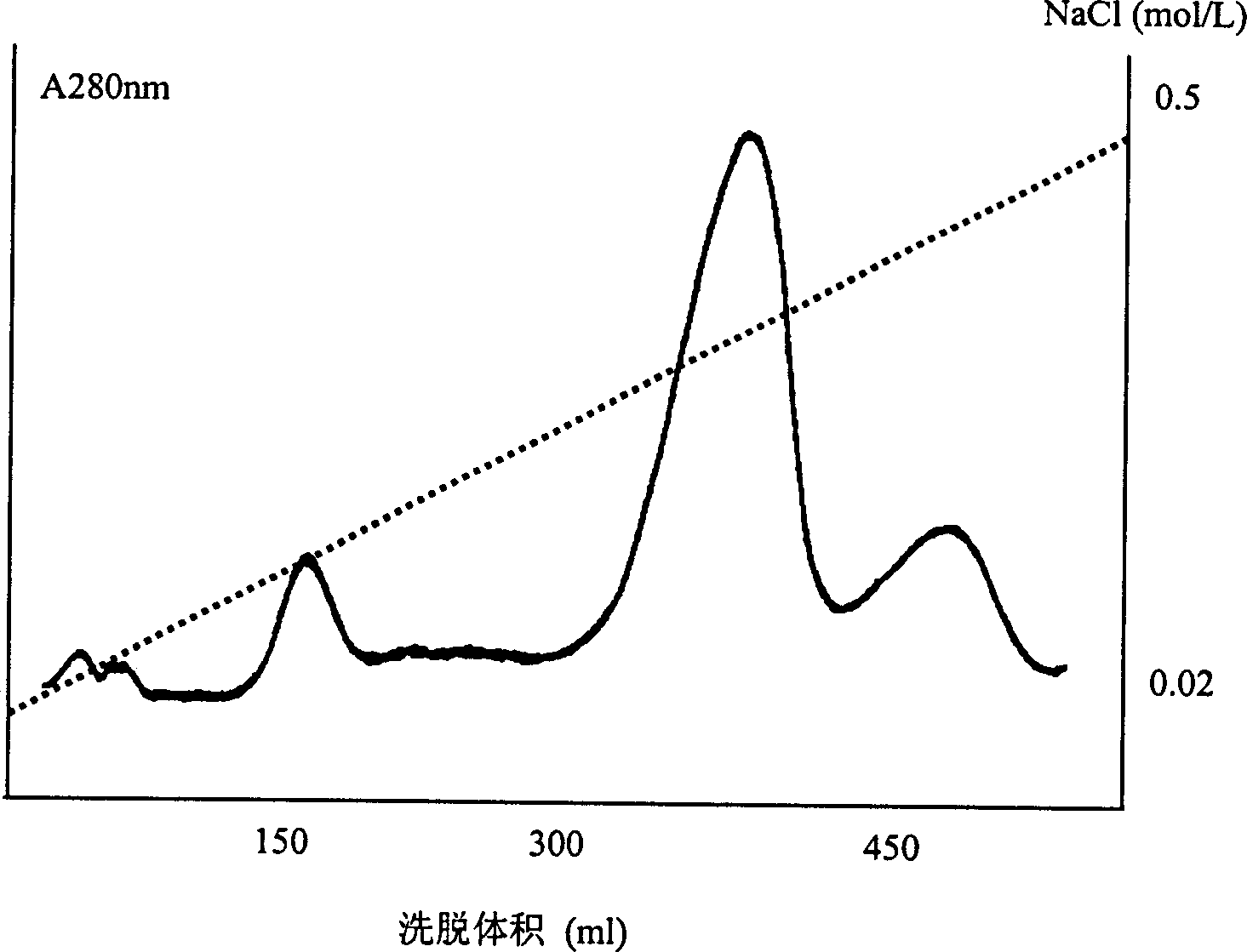
[0076] 613mg of dezincified insulin was dissolved in 127ml (5mg / ml) of 0.05mol / L borax solution (containing 0.001M OCaCl 2 ), adjust pH=8.9. Add 24.5 mg of crystalline trypsin (the mass ratio of enzyme to substrate is 1: 25), stir and react in a water bath at 37°C for 3 hours, purify through a DEAE-Sephadex A25 ion exchange column, and collect the main peak ( figure 1 ) was dialyzed against water, freeze-dried, and then desalted with Sephadex G25 to obtain DOI.
Embodiment 2
[0077] The preparation of embodiment 2 GFFYK (Boc) Obut
[0078] 2.1 Preparation of Boc-Phe-Osu
[0079] Dissolve 3.18g Boc-Phe (12mmol) and an equimolar amount of HOsu in 15ml THF, and place the reaction bottle in a -5°C salt ice bath for 10 minutes; another equimolar amount of DCCI was dissolved in 2ml THF, and slowly added dropwise to the reaction In the bottle, stir at -5°C at the same time; after adding liquid, continue to stir at -5°C for 2 hours, and white DCU precipitates appear. The reaction bottle was sealed and placed at 4°C overnight, filtered through three layers of filter paper, and the filtrate turned into a white solid after rotary evaporation. 15ml of isopropanol was added to the solid and heated to 90°C to form a yellow solution, which was recrystallized at room temperature. After suction filtration, washing and drying, the white crystals obtained 2.5 g of the product, with a melting point of 138-140° C., and thin-layer chromatography showed homogeneity, si...
Embodiment 3
[0094] Example 3B 27 Preparation of K-DTrI
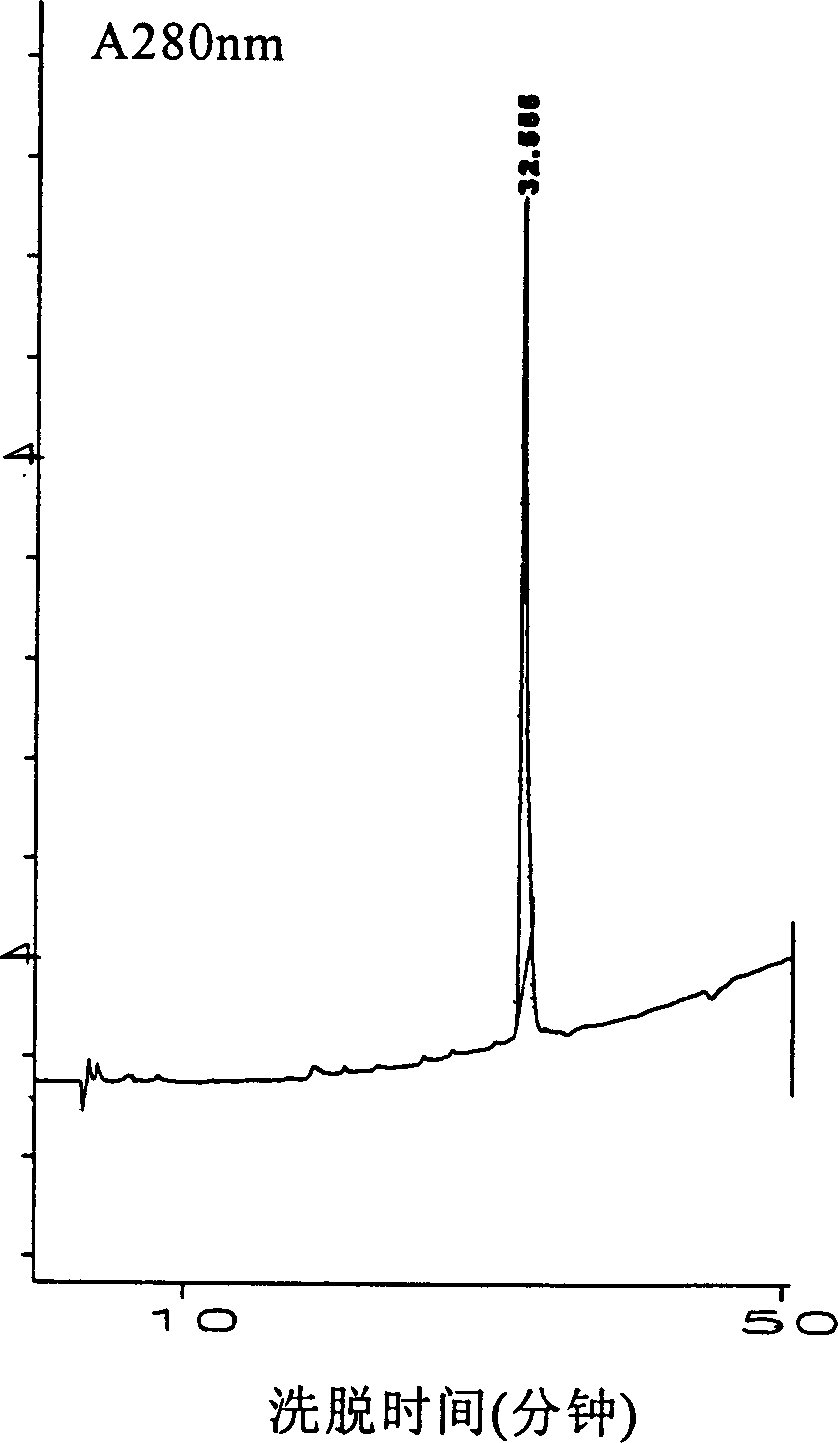
[0095] Take 0.237g (0.172mmol) of Gly-Phe-Phe-Tyr-Lys(Boc)Obut, add 0.26ml of DMSO, heat below 50°C until completely dissolved, and place in a 37°C water bath to keep warm; take another DOI 86mg (0.0172mmol), slowly Add it to the reactor to dissolve it all, then add 1.82ml of 1-4 butanediol preheated to 37°C, the reaction liquid is slightly muddy, then add deionized water preheated to 37°C, the reaction liquid becomes clear, add 5 Microliters of N-methylmorpholine were used to adjust the pH to an accurate value of 6.5 (determined with a pH meter). After adding 7.8mg of TPCK-Trypsin, the temperature of the water bath was slowly lowered to 30°C, and 4.5g of TPCK-Trypsin were added after 2 hours and 4 hours, and the enzyme amount was one-fifth of the DOI amount. After 20 hours, the reaction solution was moderately turbid, and 1.1 ml of glacial acetic acid and a small amount of 1N HCl were added to adjust the pH to 3 to terminate the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com