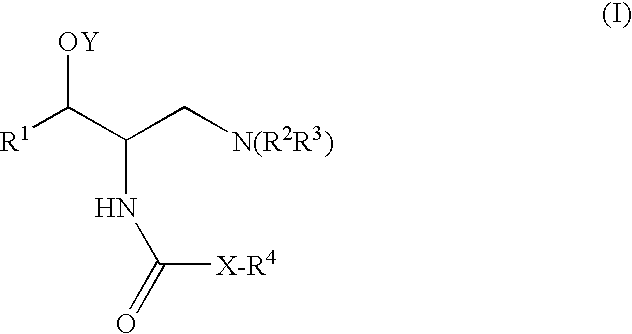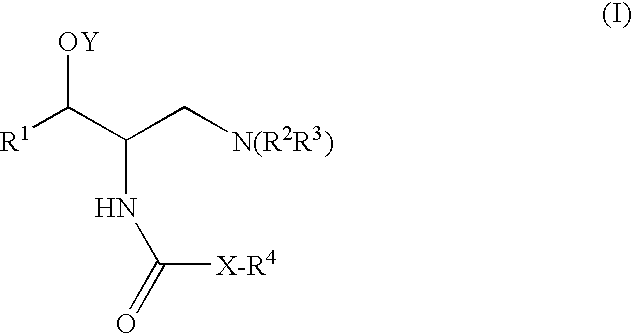Patents
Literature
240 results about "Low glucose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
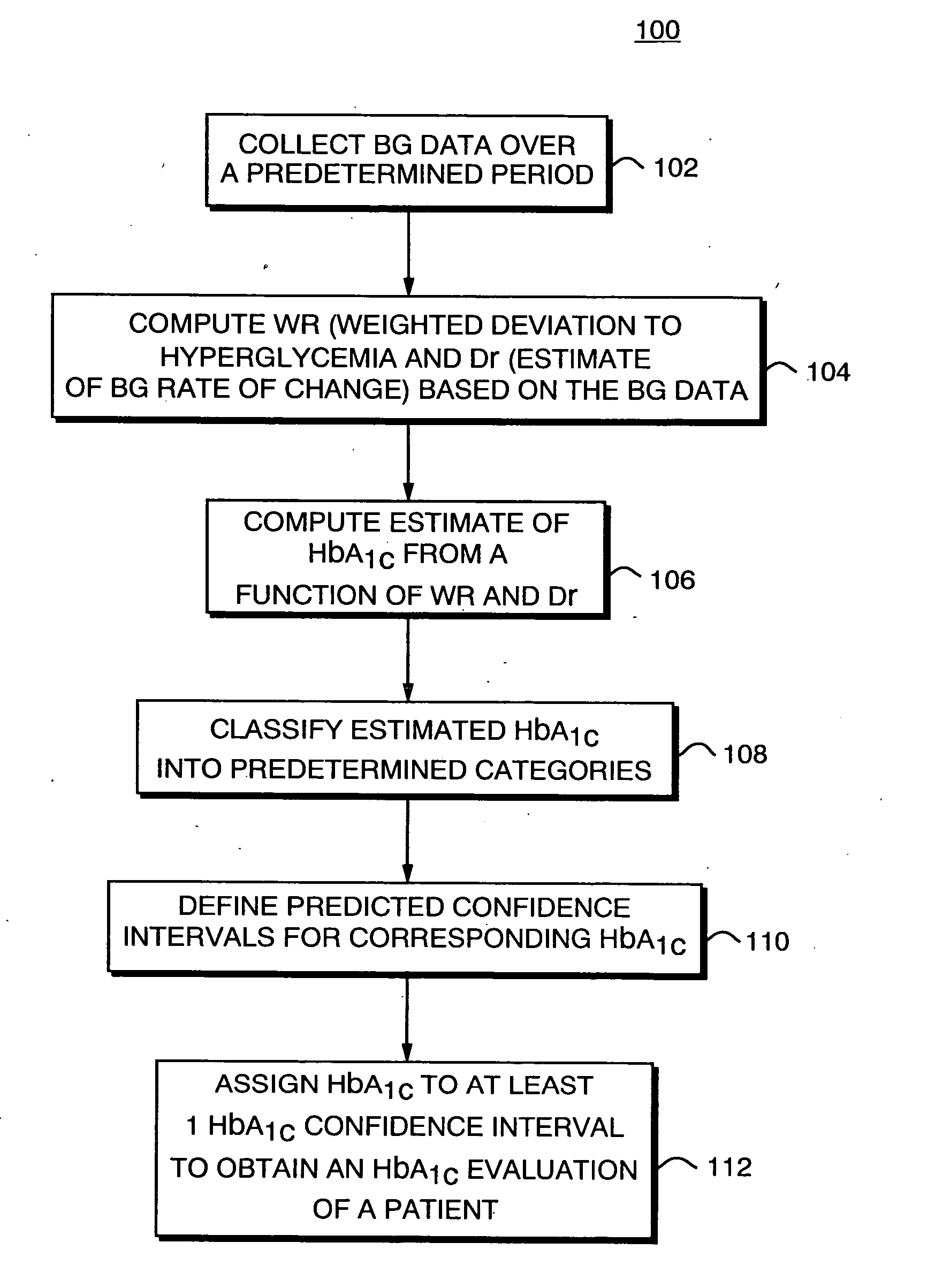
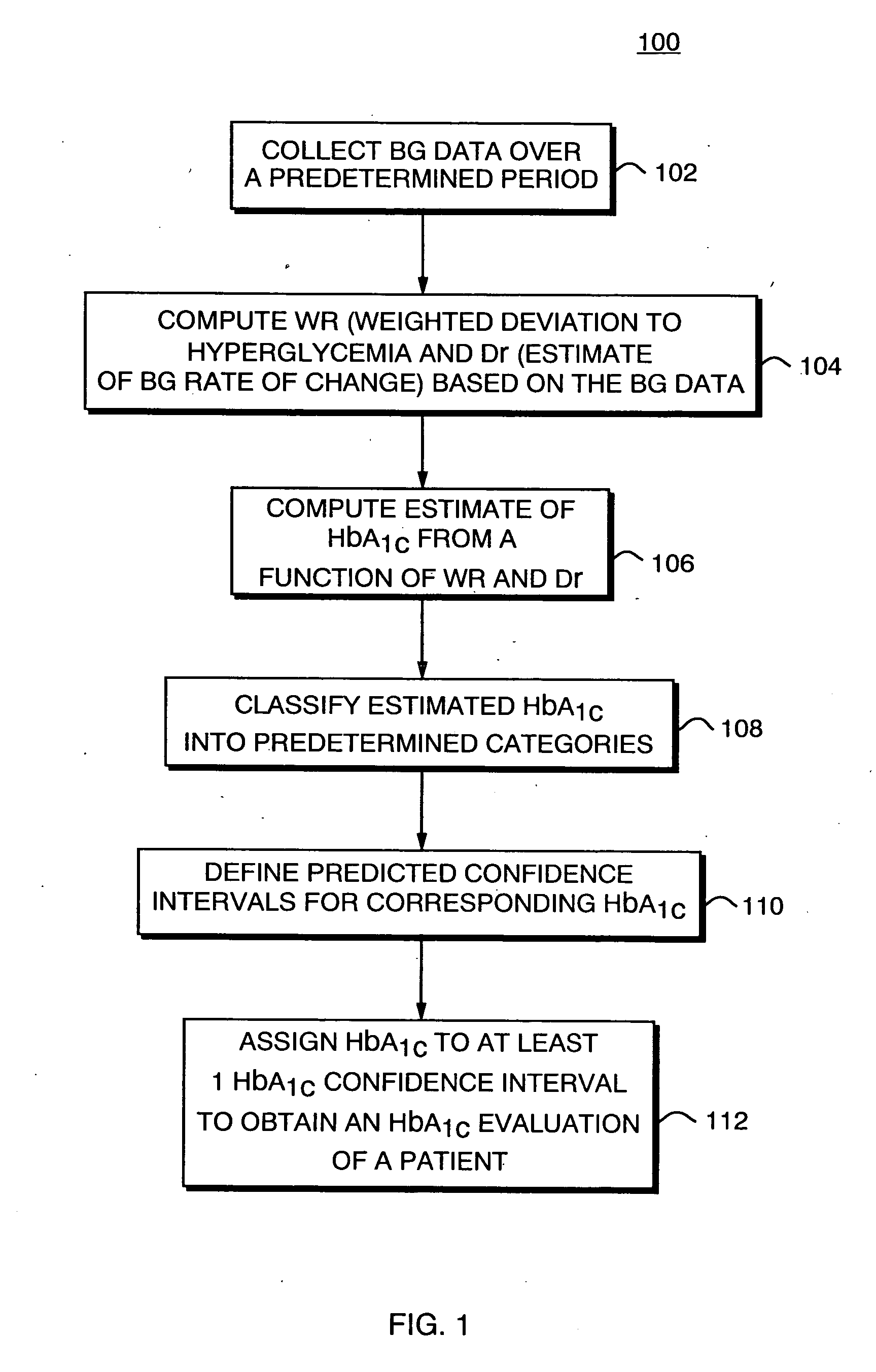
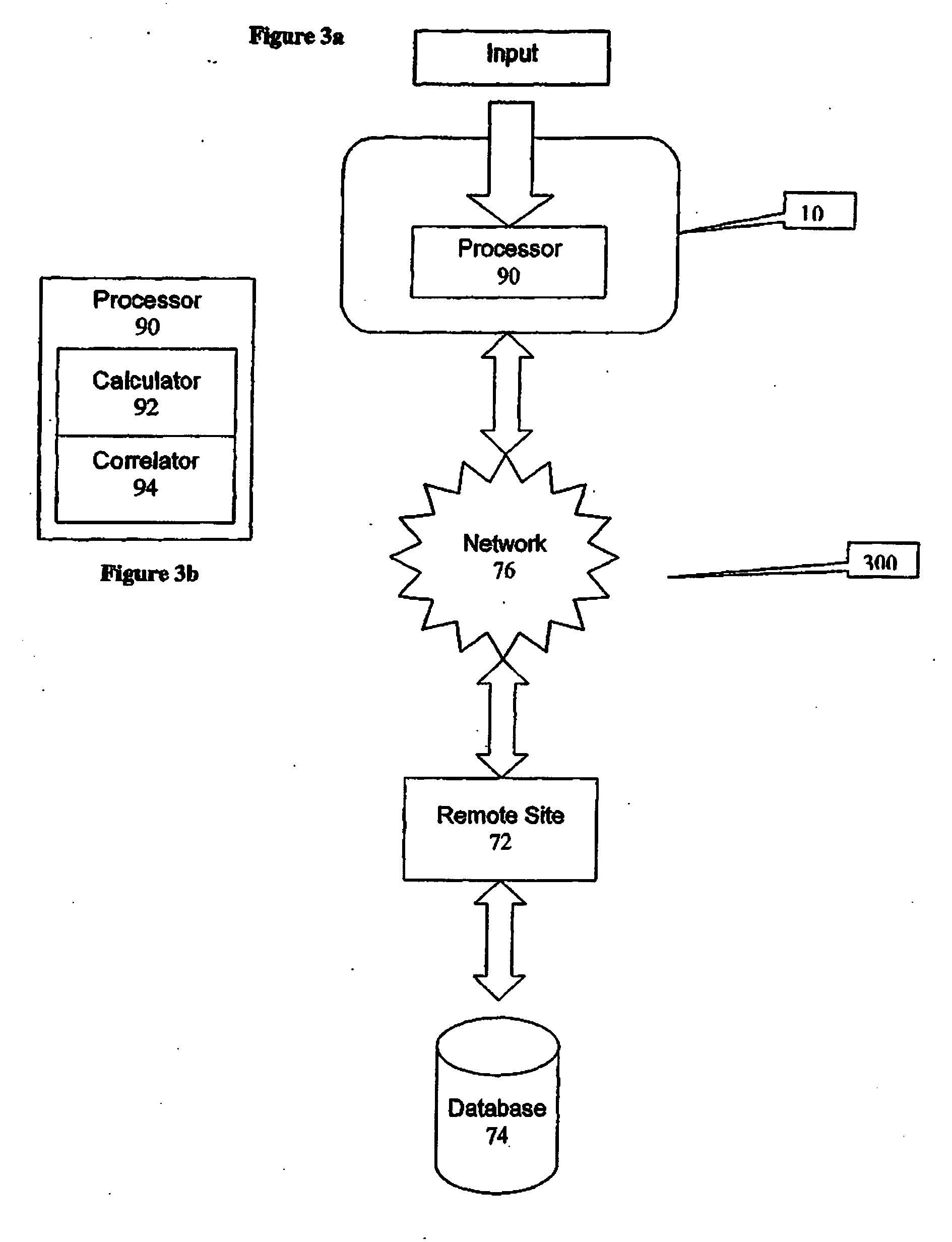
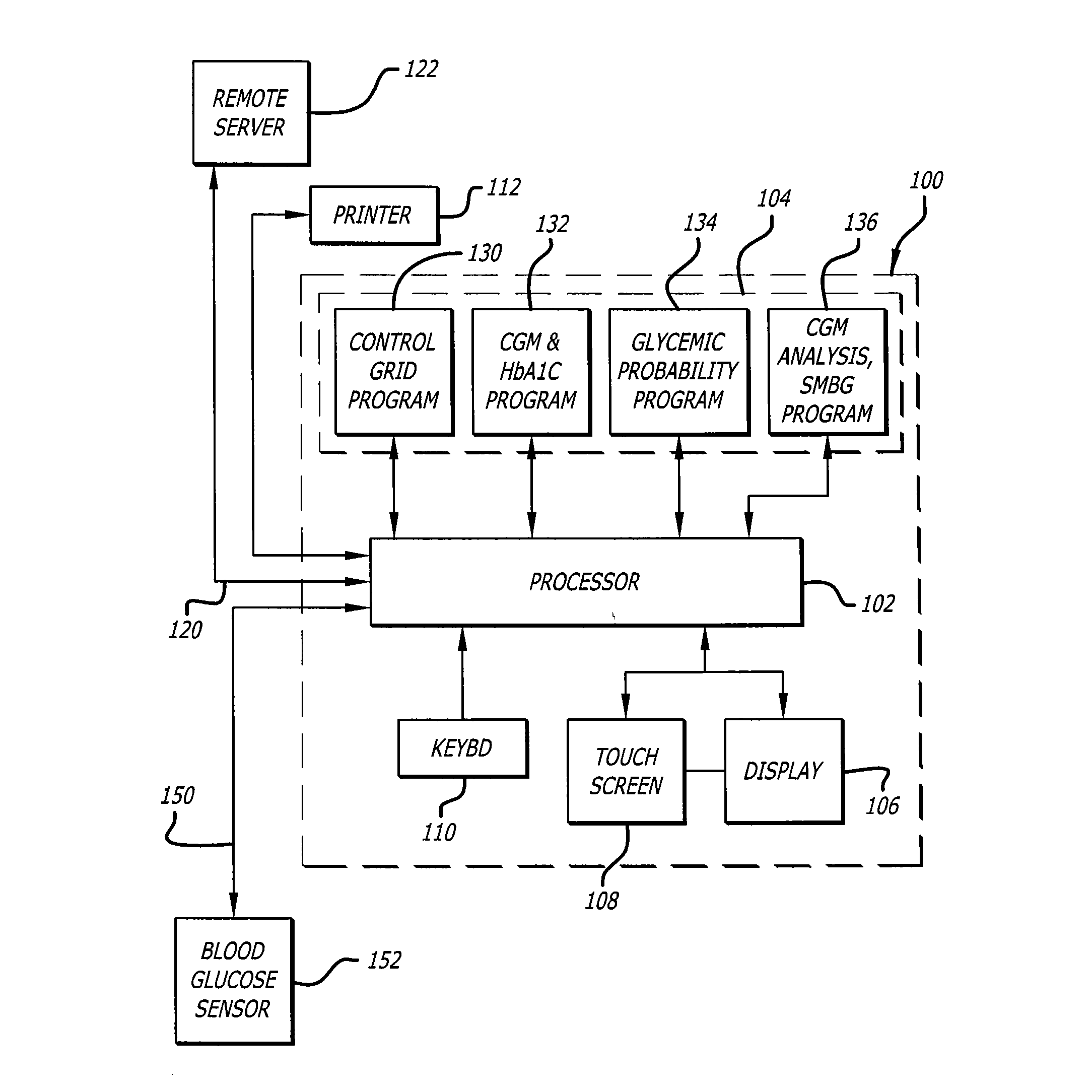
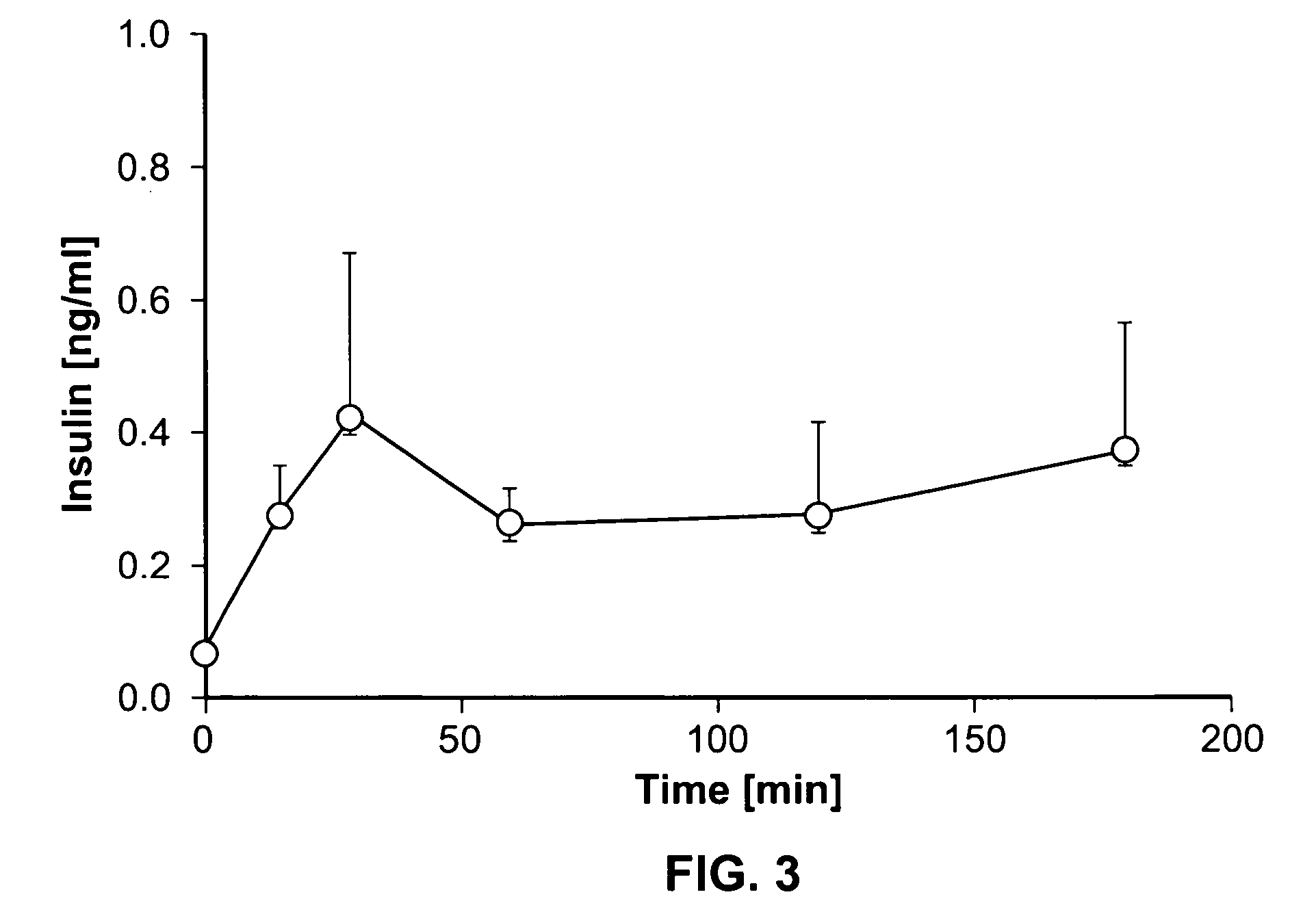
Method, system, and computer program product for the evaluation of glycemic control in diabetes from self-monitoring data
ActiveUS20060094947A1Easy to monitorContinuous informationMedical simulationTelemedicineLow glucoseAcute hyperglycaemia
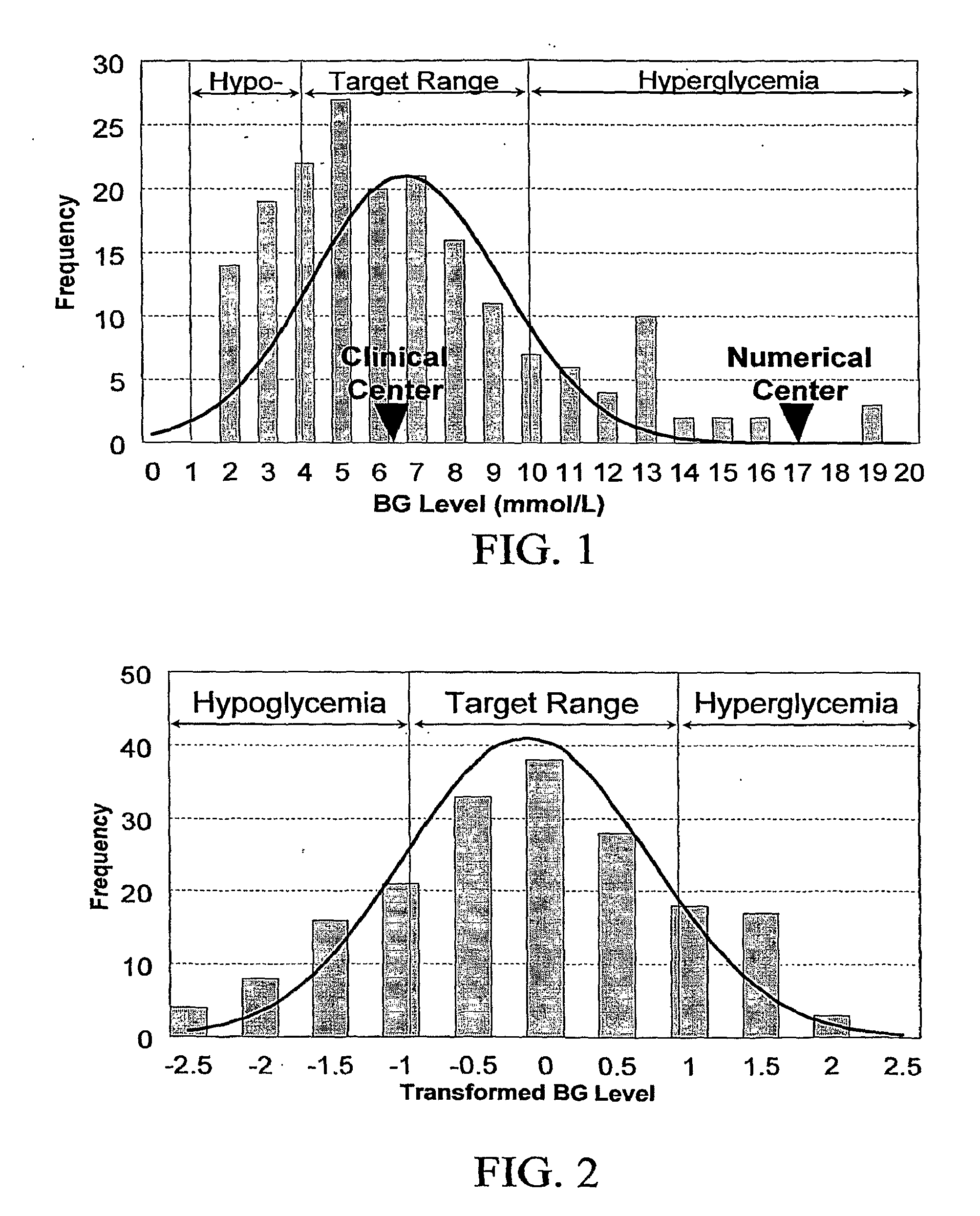
A method, system, and computer program product related to the diagnosis of diabetes, and is directed to predicting the long-term risk of hyperglycemia, and the long-term and short-term risks of severe hypoglycemia in diabetics, based on blood glucose readings collected by a self-monitoring blood glucose device. The method, system, and computer program product pertain directly to the enhancement of existing home blood glucose monitoring devices, by introducing an intelligent data interpretation component capable of predicting both HbA1c and periods of increased risk of hypoglycemia, and to the enhancement of emerging continuous monitoring devices by the same features. With these predictions the diabetic can take steps to prevent the adverse consequences associated with hyperglycemia and hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
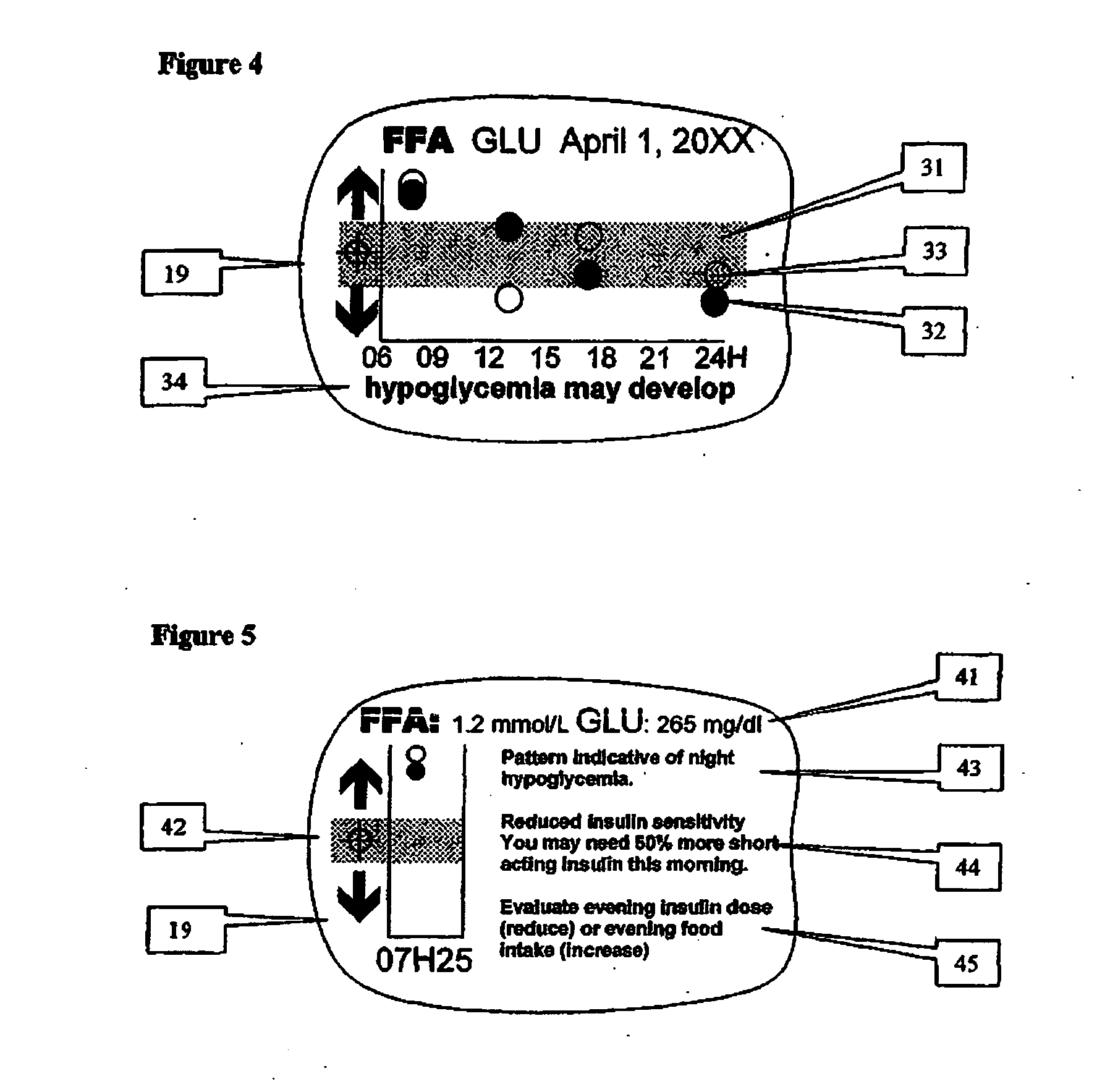
Method and device for utilizing analyte levels to assist in the treatment of diabetes
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
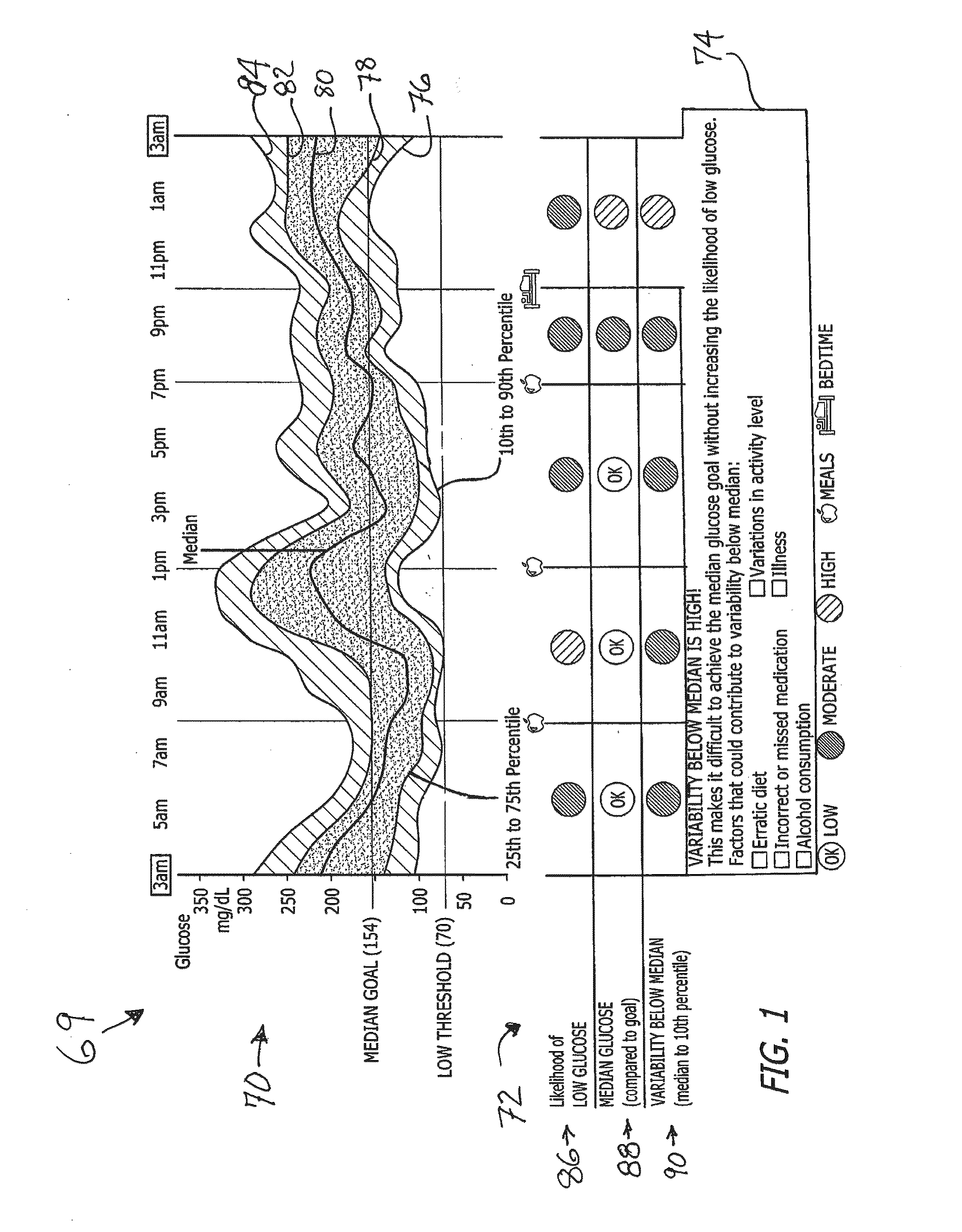
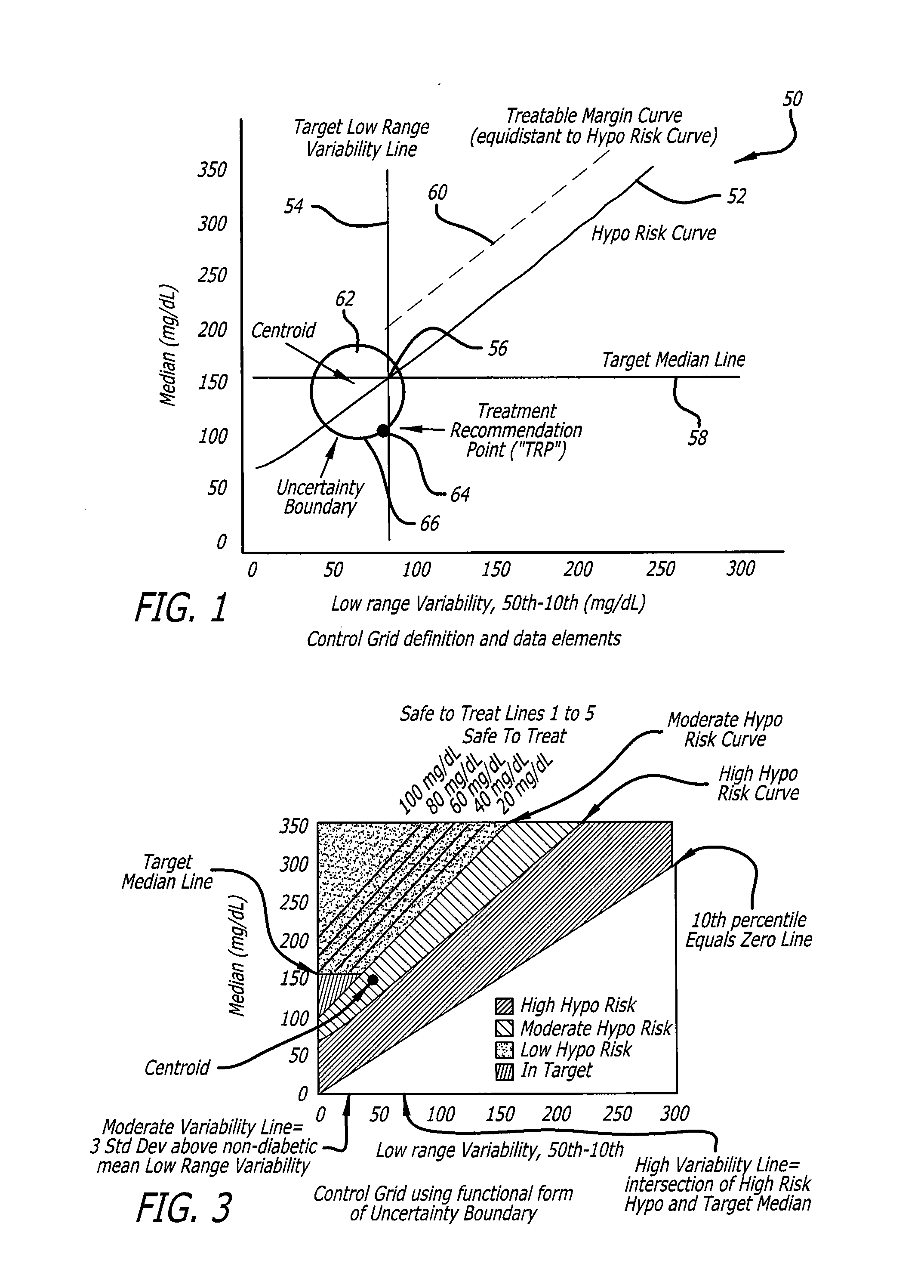
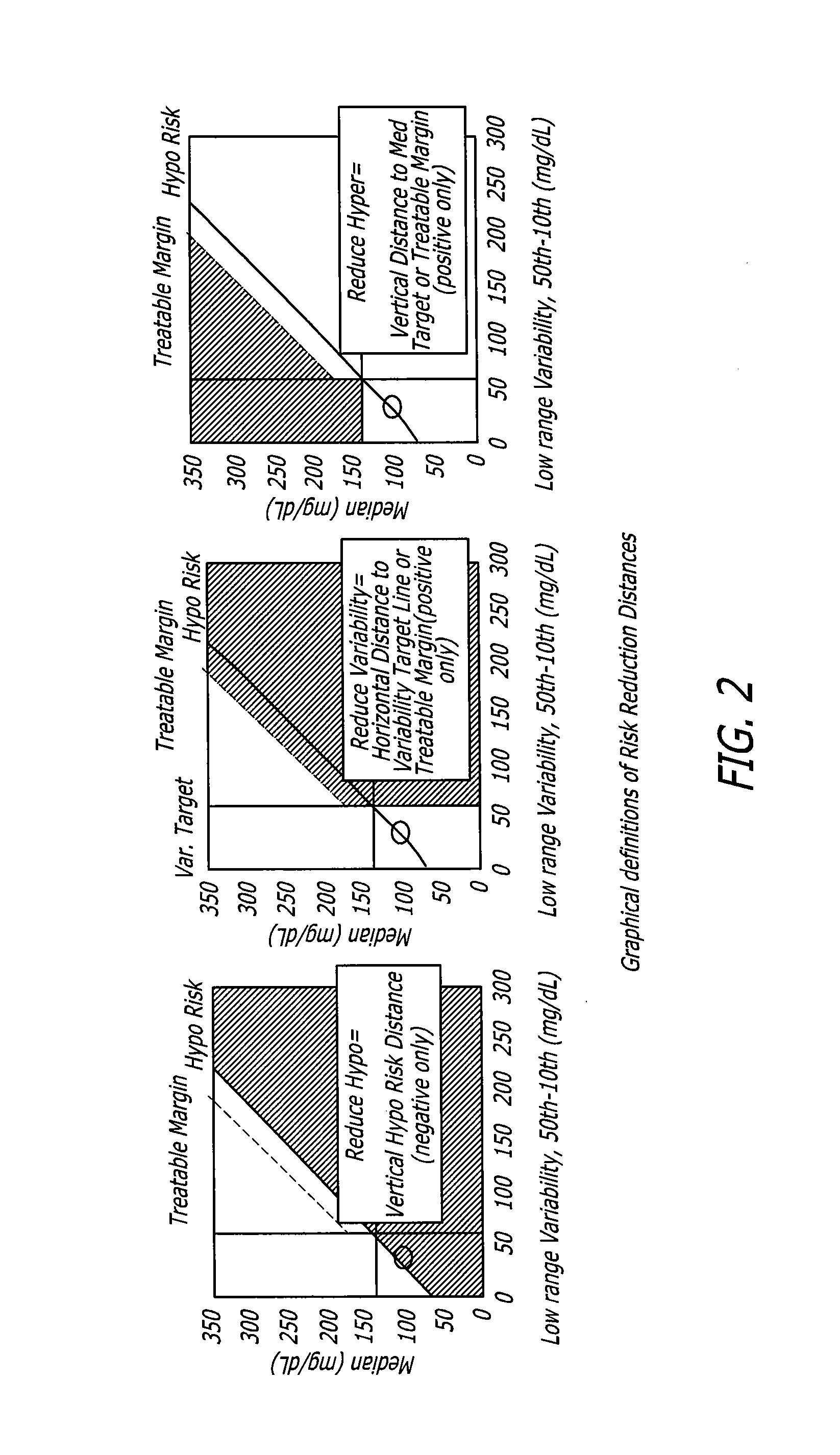
System and method to manage diabetes based on glucose median, glucose variability, and hypoglycemic risk
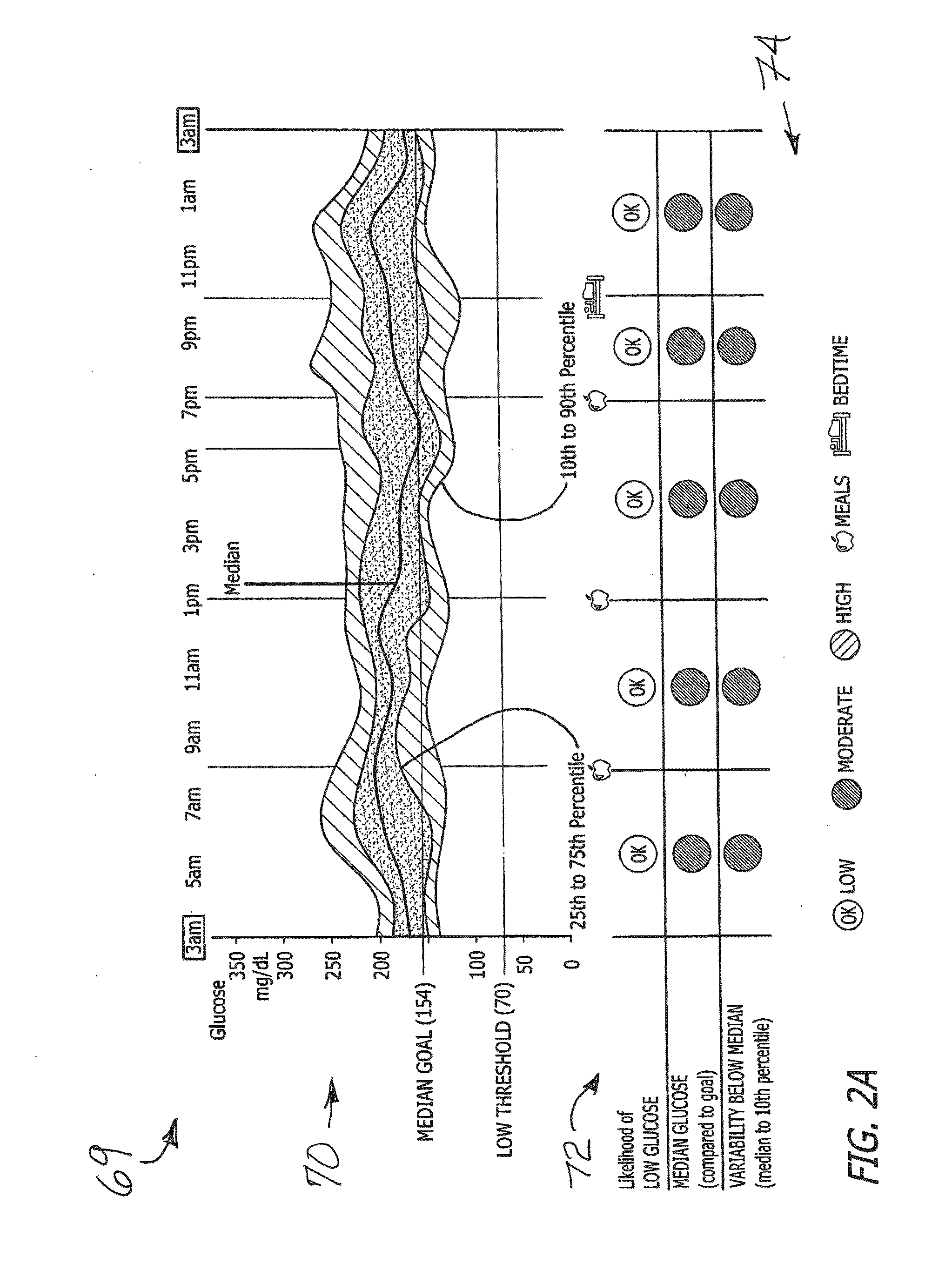
A system and method provides a glucose report for determining glycemic risk based on an ambulatory glucose profile of glucose data over a time period, a glucose control assessment based on median and variability of glucose, and indicators of high glucose variability. Time of day periods are shown at which glucose levels can be seen. A median glucose goal and a low glucose line provide coupled with glucose variability provide a view into effects that raising or lowering the median goal would have. Likelihood of low glucose, median glucose compared to goal, and variability of glucose below median provide probabilities based on glucose data. Patterns can be seen and provide guidance for treatment.
Owner:ABBOTT DIABETES CARE INC
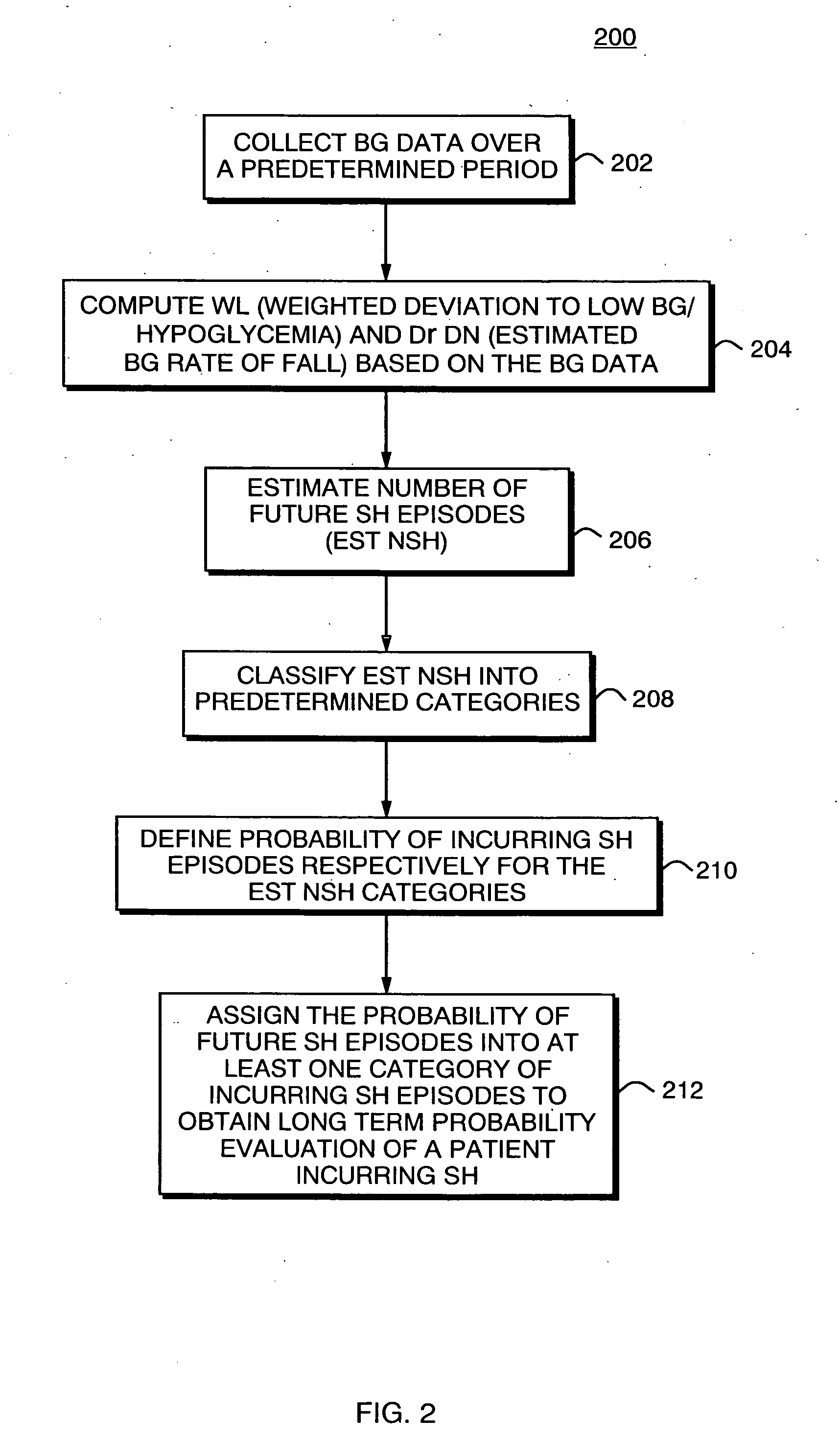
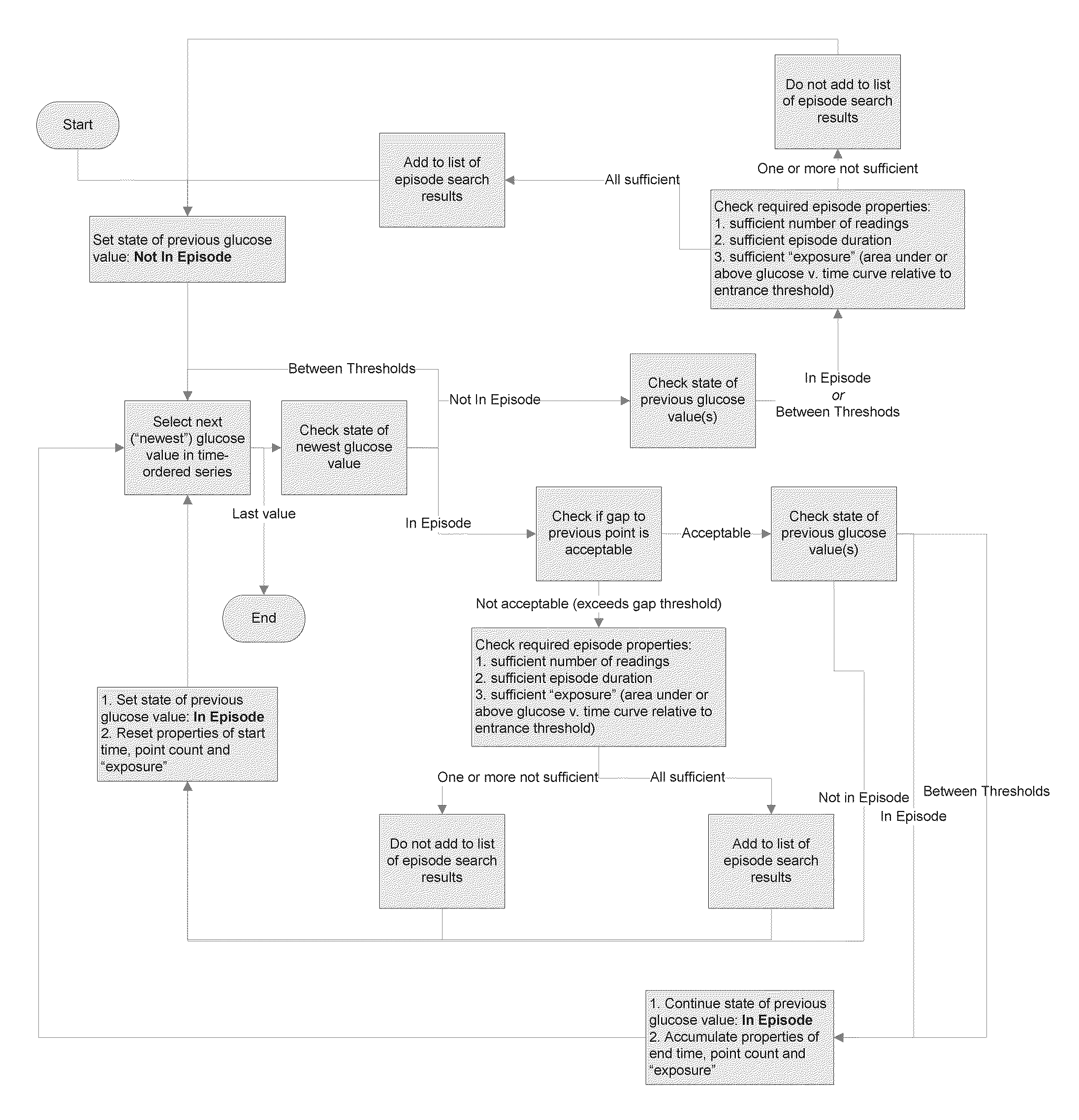
Method, System and Computer Program Product for Evaluation of Blood Glucose Variability In Diabetes From Self-Monitoring Data
ActiveUS20090171589A1Long-term riskEnhance existing SMBG devicesHealth-index calculationMedical automated diagnosisLow glucoseRisk profiling
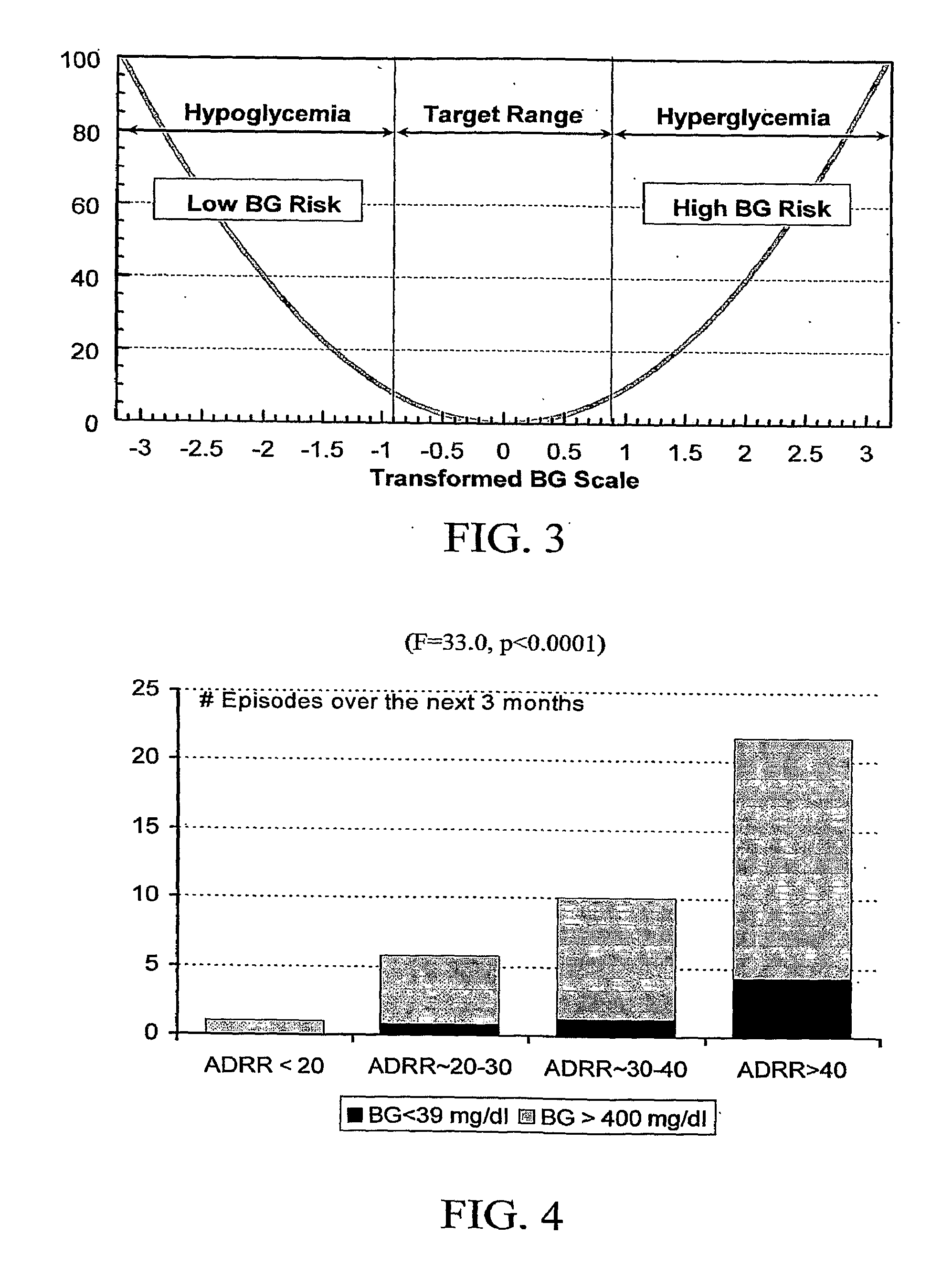
A system, computer program product, method and algorithm for evaluation of blood glucose variability—one of the most important parameters of diabetes management. An embodiment of the method may use routine self-monitoring blood glucose (SMBG) data collected over a period of 2-6 weeks, for example, based on a theory of risk analysis of blood glucose data. One aspect may include a method, system and computer program product for computing the Average Daily Risk Range (ADRR)—a measure of overall glucose variability. Another aspect may include a method, system, and computer program product for estimating separately the glucose variability in the hypoglycemic range via a Low BG Index (LBGI) and the glucose variability in the high BG range via High BG Index (HBGI) followed by a combination of the two indices into a single variability display.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free
This invention relates to a nutritional intervention composition for enhancing satiety prior to a meal and extending satiety after a meal. The nutritional intervention composition decreases food intake producing weight loss over time. The composition consists of Niacin, Vitamin B6, Calcium, Phosphorous, Magnesium, Chromium, Chitosan, Fenugreek, Ginseng, White willow bark, Garcinia cambogia, Aloe Vera gel powder, Momordica charantia, Griffonia simplicifolia, Lagerstroemia speciosa and Vanadyl sulfate. The invention does not require stimulants or anabolic ingredients. There are three phases of activity within the composition. One, enhanced satiety through elevated serotonin. Two, improved carbohydrate metabolism, reduced blood glucose and slowed gastric emptying. Three, enhanced fiber binding of lipids and excess bile acids.
Owner:NEEDLEMAN ALVIN +1
Analysis of glucose median, variability, and hypoglycemia risk for therapy guidance
ActiveUS20140188400A1Improve accuracyHealth-index calculationMedical automated diagnosisLow glucoseDiabetes Therapy
A system and method to provide guidance for diabetes therapy includes determining glycemic risks based on an analysis of glucose data. The analysis includes visualization of a glucose median, the variability of glucose in a patient, and the risk of hypoglycemia. An Advanced Daily Patterns report includes a visualization of an ambulatory glucose profile and a glucose control measure. The glucose control measure provides a highly visible and understandable display of the glucose condition of a patient visually expressed in the categories of low glucose, median glucose, and glucose variability.
Owner:ABBOTT DIABETES CARE INC
Food products for diabetics
Disclosed is a novel food product characterized by a low glucose or glucose free content, a balanced functional fat content, and a proactive agent aimed for the diabetic and diabetic-prone populations. The food product of the invention is a functional food which may be used clinically to lower the lipid level in people suffering from an imbalanced lipid profile and which may progress towards diabetes complications and coronary vascular disorders. In particular embodiments the proactive agent can be any of a naturally occurring lipid, a synthetic or mimetic lipid which is not digestible by humans and interferes with body weight gain / loss, plant extracts and substances derived therefrom, antioxidants, animal-derived substances, minerals and pharmaceuticals, and any mixture thereof.
Owner:ENZYMOTEC
Pre-treatment method for highly-effective saccharification of lignocellulose
InactiveCN101255479AReduce the cost of trainingImprove securitySugar derivativesSugar derivatives preparationCelluloseLiquid glucose
The invention discloses a pretreatment method for effectively saccharifying lignocellulose, particularly discloses a pretreatment method of lignocellulose with room-temperature light-concentration lye, characterized in the method comprises the steps of coarse grinding, lye wetmilling, alkali recovery and enzymolysizing. The ground lignocellulose is mixed with univalent metal lye having a concentration of 0.1% to 3%, solid and liquid are separated under room temperature after wet grinding, wherein the liquid is used for recovering univalent metal lye, and the solid, namely the modified lignocellulose, is used for further enzymolysizing for preparing liquid glucose. According to the invention, lignocellulose is used as the raw material, room temperature condition without external heating can be selected, low-concentration lye is pretreated, the lye can be effectively recovered, the enzymolysis efficiency is improved, the glucose yield is increased, the cost is lowered, the economical efficiency is enhanced, and drawbacks of high energy consumption, low glucose recovery rate, and high equipment requirements in current lignocellulose pretreatment method are made up.
Owner:NANJING TECH UNIV
Blood fat-reducing medical science formula food
InactiveCN105707863ASolve inactivationSolve the coexistence problemSugar food ingredientsVitamin food ingredientsFormularyLow glucose
The invention belongs to the technical field of affinal drug and diet formulas and new resource food, and particularly provides blood fat-reducing medical science formula food, specific medical formula food or non-specific medical formula food. The formula food is prepared by performing reasonable compatibility on multiple affinal drug and diet traditional Chinese medicines according to the traditional Chinese medicine essence theory and mixing drug-diet dual purpose traditional Chinese medicine extract essence extracted through a semi-bionic extraction method, multiple microencapsulated probiotics, oligopeptides extracted through biological enzymolysis, prebiotics, amino acid, carbohydrate, fat with a health-care function, multiple vitamins and mineral substances according to the requirements of a 'special medial-purpose formula food general rule' by combining the constitutive characters of hyperlipidemia patients; the formula food not only can serve as a single nutrition source to meet the nutritional requirement of the hyperlipidemia patients, but also achieves the effects of reducing total cholesterol, reducing serum triglyceride, reducing an atherosclerosis index, reducing blood pressure, reducing blood sugar, reducing fat to lose weight and enhancing the immunity.
Owner:JINSHANMEI BIOTECH
Means for replacing common sugars if foods for enhanced nutrition
InactiveUS20080260925A1Great tasteSuperior digestive toleranceFood ingredient functionsFood preparationLow glucoseSide effect
A means for replacing common sugars (particularly sucrose) in a range of foods that maximizes sugar-like taste, texture and other key properties of sugar while minimizing the undesirable traits such as blood sugar response, digestive side effects, high caloric content and aftertastes. Various differing ratios and combinations of high intensity sweetening agents, high molecular weight bulking agent(s), substantially non-digestible sugar(s), and low molecular weight sugar alcohol(s) are used for various applications such as tabletop sugar substitute, frozen deserts, condiments, baked goods, chocolate and confectionaries have different formulations. These sugar replacement approaches are highly relevant to the production of diabetic-friendly foods, diet and / or reduced calorie foods, non-cariogenic (tooth-friendly) foods and other sweet, low-glycemic foods.
Owner:ZINK GALEN PAUL
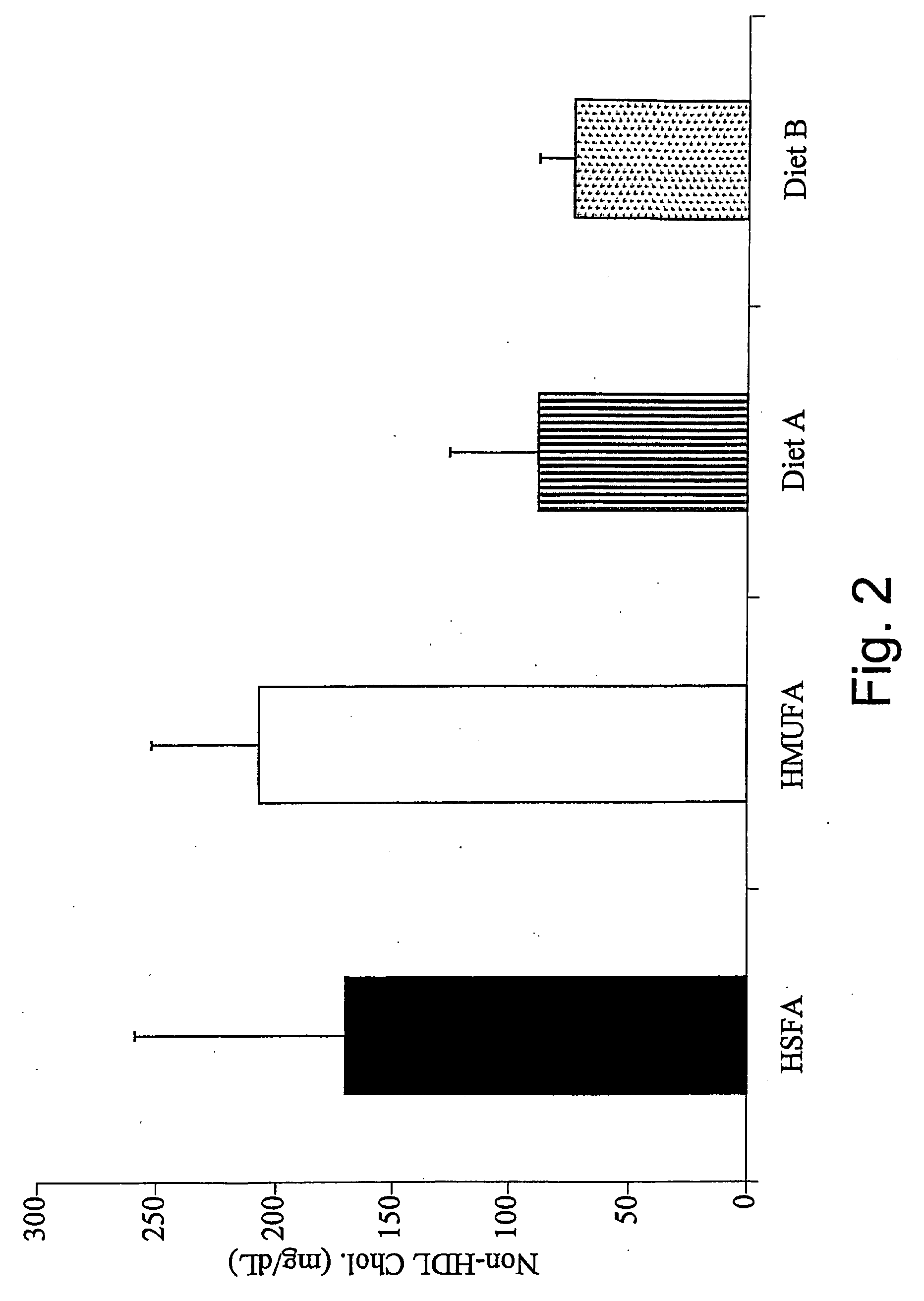
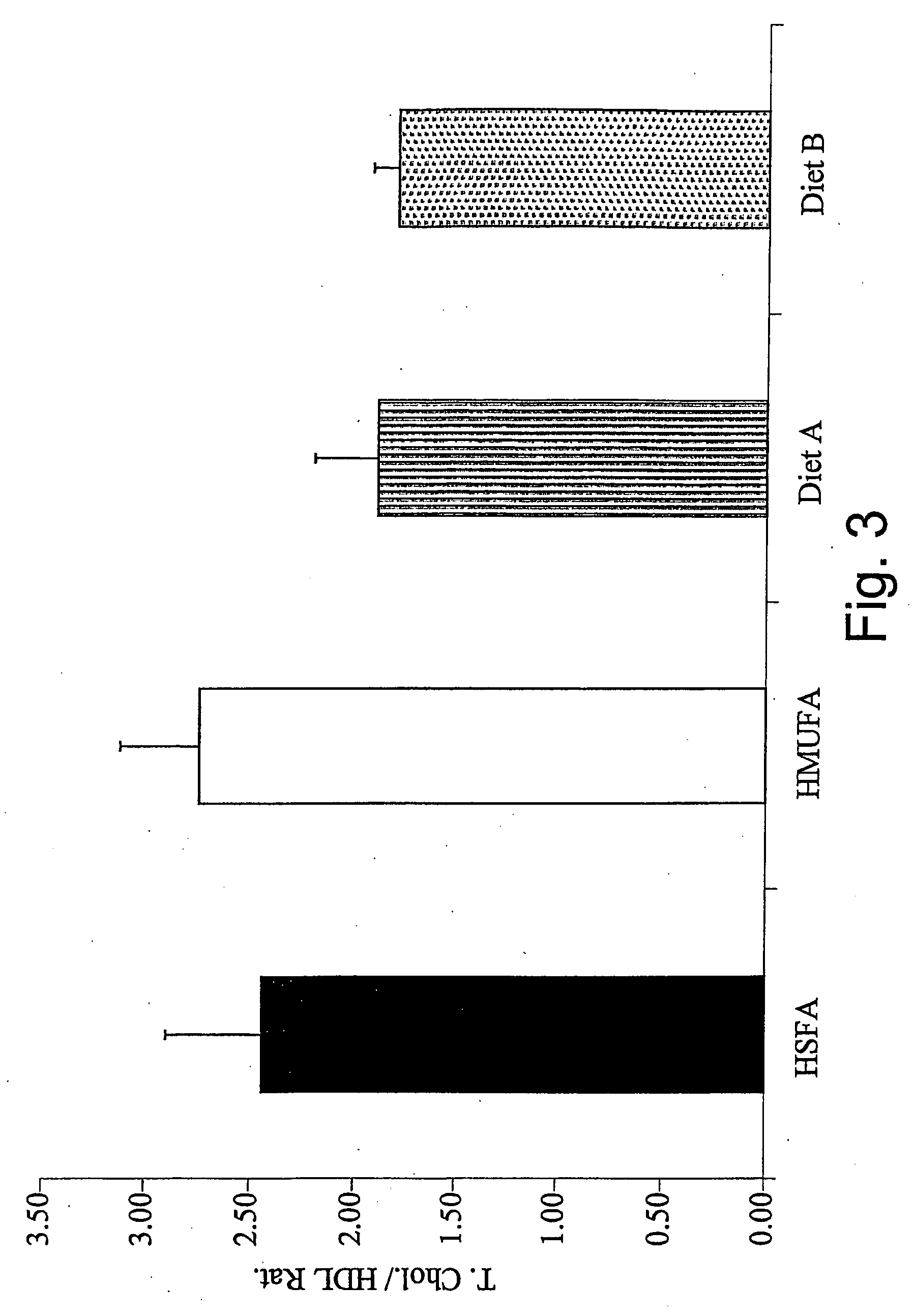
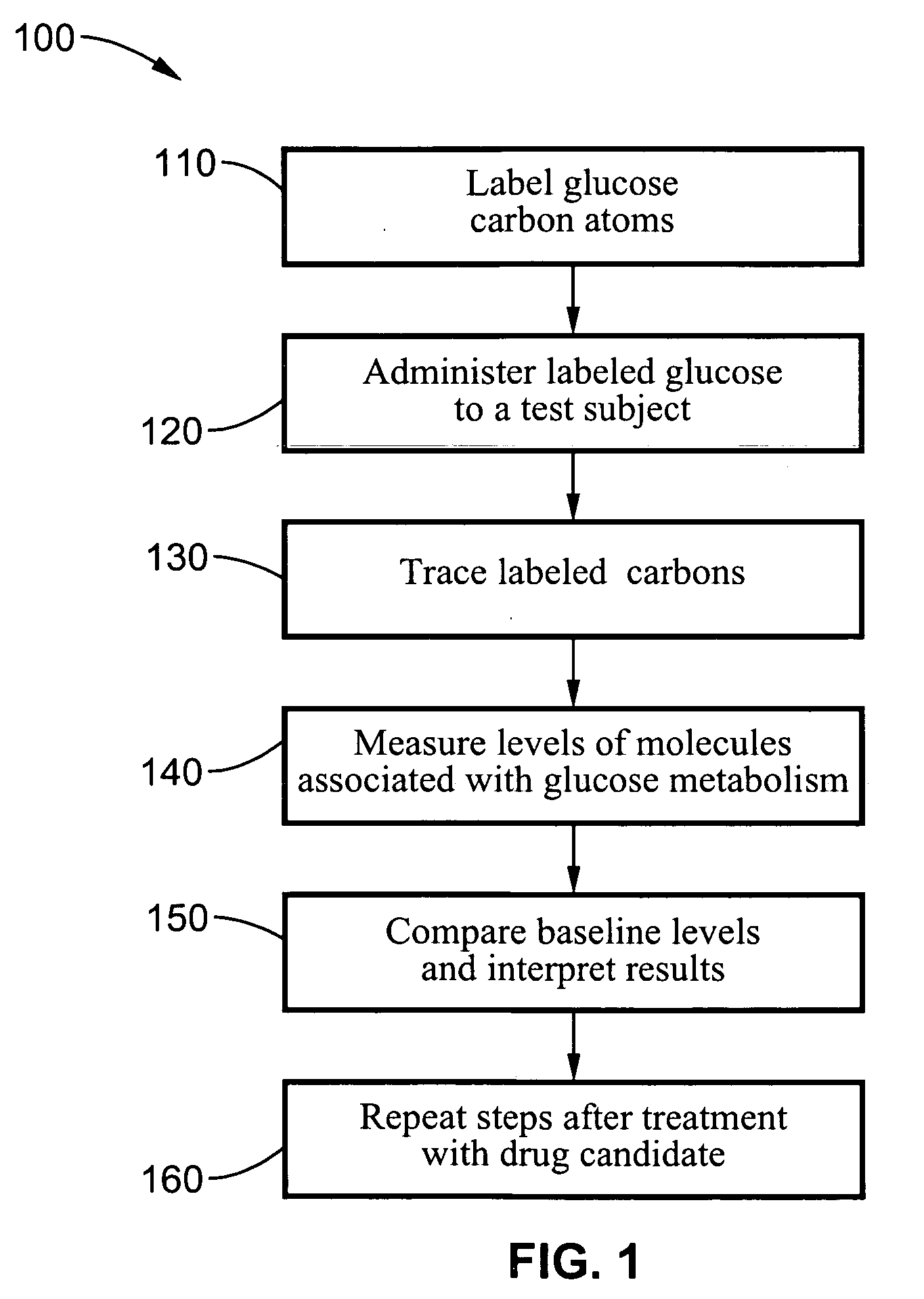
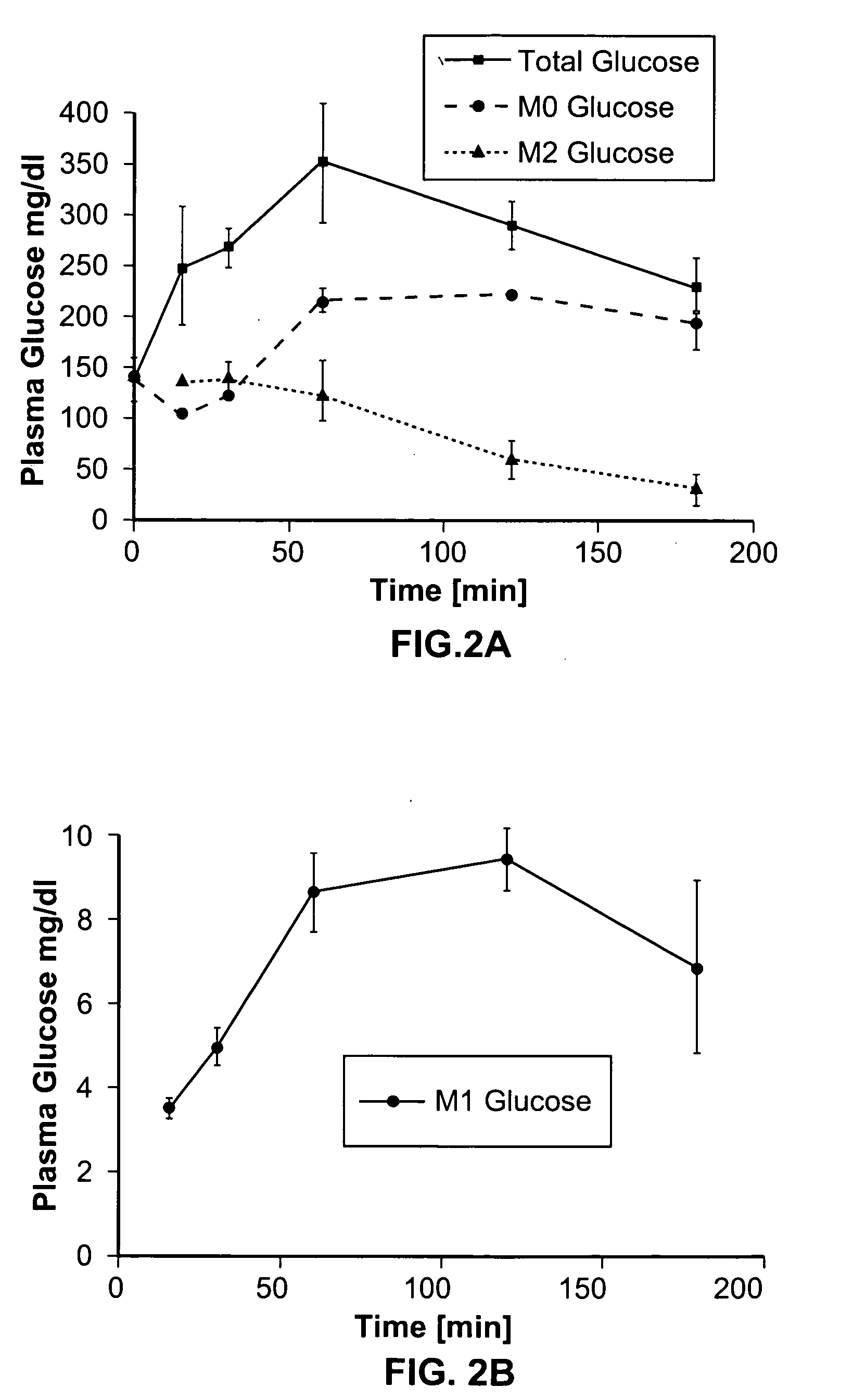
Dynamic hepatic recycling glucose tolerance test
Systems and methods are described providing a hepatic recycling glucose tolerance test for the diagnosis of types and subtypes of diabetes mellitus and other hyperglycemic or hypoglycemic conditions. A method is also provided for screening candidate drugs for treating various types of abnormal glucose metabolism and to monitor whether the course of treatment is effective. The method also allows the correlation of gene activity, hormone and metabolite levels with glucose flux and recycling and an assessment of the degree of hepatic insulin resistance. The method utilizes a preferably non-radioactive stable labeled glucose to asses the relative rates of carbon flow in the liver and provides a hepatic recycling constant that is a measure of the relative rate of glucose recycling. The labeled glucose may be introduced to the patient orally, intravenously or by intraperitoneal administration for the desired effect.
Owner:RGT UNIV OF CALIFORNIA
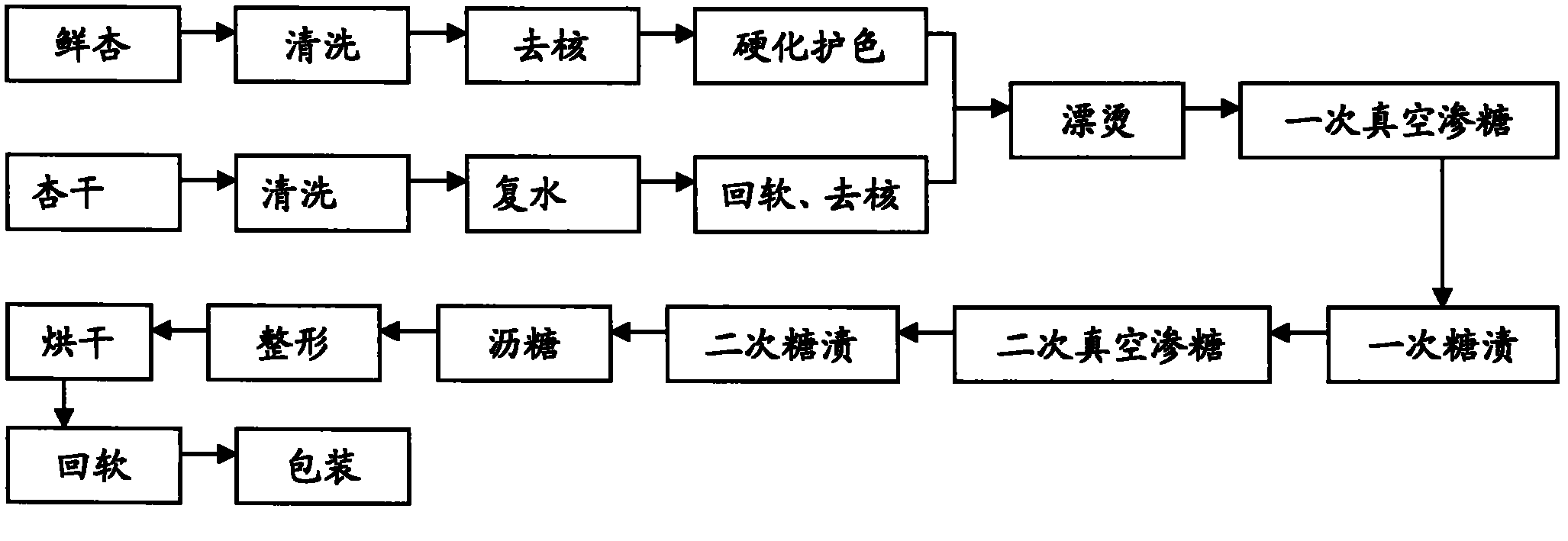
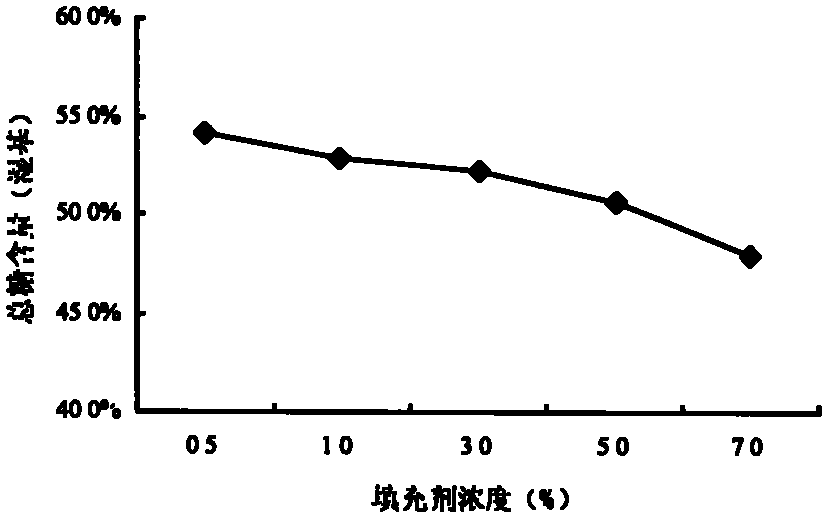
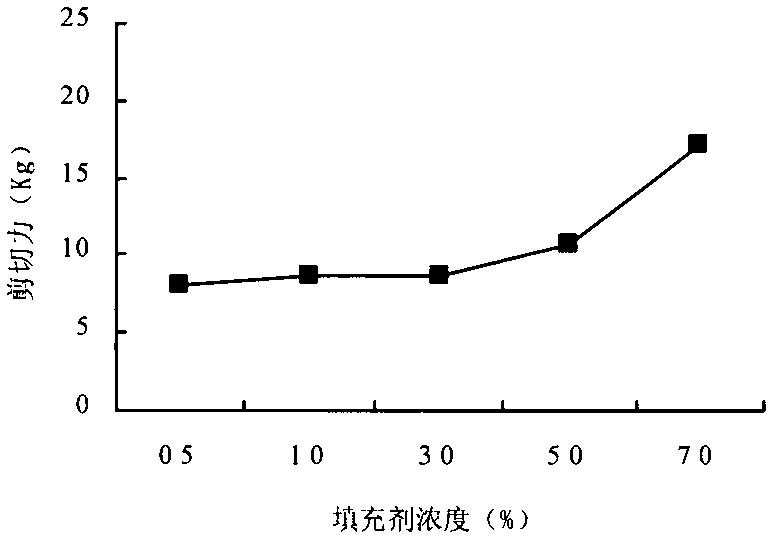
Method for preparing low sugar preserved apricots
ActiveCN102204618AIncrease production capacityHigh technology contentConfectionerySweetmeatsLow glucoseDried apricot
The invention discloses a method for preparing low sugar preserved apricots. Key technical parameters which can influence the quality of the low glucose preserved apricots are determined by using dried apricots or fresh apricots as raw materials and adopting novel nutritive sweeteners with specific physiological functions, i.e., maltooligosaccharides as a filler and using fructose syrups as a sweetener, wherein in the primary vacuum sugar permeability process, the concentration of sugar solution is in the range of 30-35 percent, the time is 20min, the sugar solution has a temperature of 60 DEG C, the vacuum degree is 0.07Mpa, and the sugaring time under normal pressure is in the range of 10-15 hours; in the secondary vacuum sugar permeability process, the concentration of the sugar solution is in the range of 40-45 percent, the time is 20min, the sugar solution has a temperature of 60 DEG C, the vacuum degree is 0.07MPa, and the sugaring time under normal pressure is in the range of 10-15hours; and color, plumpness, sugar content and shearing force of a product are used as integrated quality evaluation indexes, so that the method for preparing the low sugar preserved apricots is determined. The preserved apricots prepared by adopting the method provided by the invention not only has low total sugar content, but also has specific nutritional value and has wide applicable consumer range.
Owner:XINJIANG AGRI UNIV
Method for producing controlled/slow release starch derivative with hypoglycemia response characteristics
ActiveCN101731510AWide range of raw materialsIncrease contentFood preparationDigestible starchLow glucose
The invention discloses a method for producing a controlled / slow release starch derivative with hypoglycemia response characteristics, and belongs to the technical field of the production of functional food additives. The method utilizes a conventional industrial natural polymer to obtain the controlled / slow release starch derivative with the hypoglycemia response characteristics through micro-encapsulation embedding technology. The method adopts emulsification / internal gelatination technology, takes commercialized starch with different sources as a core material, and takes a compound of canageenen or sodium alginate and chitosan as a wall material to obtain a starch derivative with a particle size of between 10 and 150mu m through micro-encapsulation embedding treatment. The content of temperature-resistant slowly digestible starch (SDS) is remarkably improved (more than or equal to 15 percent) relative to native starch in the starch derivative, and the change range of the content thereof does not exceed 3 percent after high-pressure cooking treatment. A glycemic index (GI) is lower than 55 percent. The starch derivative not only can be taken as a functional ingredient to be added to develop novel slowly digestible hypoglycemia food, but also can be taken as a carrier material of stabilization and targeted controlled / slow release of functional factors such as probiotics, active polypeptide, protein, grease, vitamin and the like.
Owner:武汉森澜生物科技有限公司
High-efficiency method for fermentation production of L-glutamic acid
The invention relates to a method for high-efficiency fermentation production of L-glutamic acid. The method can solve the problems of lower acid production, low glucose-acid conversion rate, and the like of the prior production process. The method comprises the following steps: a biotin auxotroph strain is taken as a production strain, according to a certain proportion, a plurality of nutritional components are prepared into a culture medium with rich and balanced nutrients as the fermentation culture medium, the dissolved oxygen is controlled on the appropriate level through the regulation of the stirring speed of a fermentation tank and the wind amount, the pH is controlled by feeding ammonia water, and the residual sugar is controlled on the lower level by feeding glucose solution with a certain concentration, and the fermentation is stopped till 32h. The method can shorten the whole fermentation cycle and greatly improve the yield (more than 150g / l) and the conversion rate (more than 60 percent) of the L-glutamic acid under the situation of not increasing any additional equipment and manpower investment, the whole process has simple operation and lower production cost, thereby being very applicable to industrial production.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Low glycemic, high fiber composition of all natural compounds that provides a sweet flavor profile for use in foods, beverages or as a sugar substitute
A composition of all natural compounds that provides a sweet flavor profile and is high in fiber and low glycemic for use in foods and / or beverages, and / or for use as a sugar substitute.
Owner:SUGAR SENSE NEW JERSEY CORP
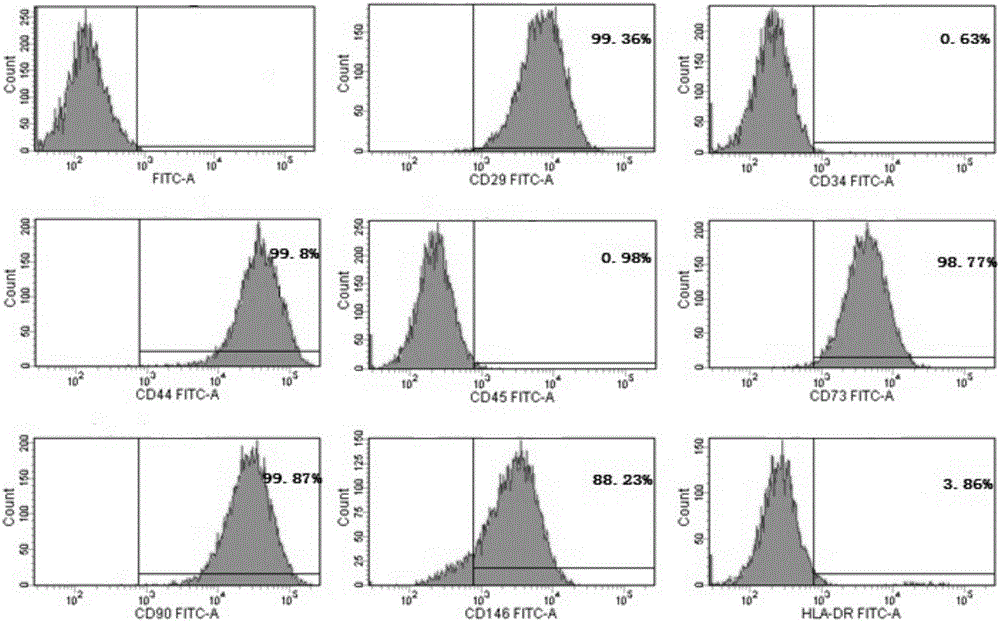
Separation, purification and identification methods of human amnion mesenchymal stem cells
InactiveCN102559586AMeet the treatment needsAccurate identification methodIndividual particle analysisEmbryonic cellsLow glucoseStaining
The invention discloses separation, purification and identification methods of human amnion mesenchymal stem cells. The separation method of hAMSCs (human amnion mesenchymal stem cells) comprises the following steps: fragmentating human amnion; and carrying out two-step rotating digestion with trypsin of EDTA (ethylene diamine tetra-acetic acid) and collagenase of DNaseI, filtering with a steel mesh and collecting cell filtrate namely separated original hAMSCs. The purification method of hAMSCs comprises the following steps: incubating original hAMSCs with an LG (low glucose)-DMEM (dulbecco modified eagle medium) culture medium in a CO2 incubator; removing amnion epithelial cells which do not perform complete adherence growth under an inverted microscope; replacing a new culture medium on the third day; digesting with a trypsin-EDTA solution after cell converge degree reaches 80-90%; and collecting cells so as to obtain high-purity hAMSCs. The identification method of hAMSCs comprises the following steps: identifying hAMSCs and the amnion epithelial cells by adopting immunocytochemical staining vimentin and CK19; and detecting expressions of CD29, CD44, CD166, CD34 and CD45 by adopting a flow cytometry. The separation and purification methods disclosed by the invention have the advantages of high yield, high activity and high purity of hAMSCs; and the identification method is simple, convenient and precise.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
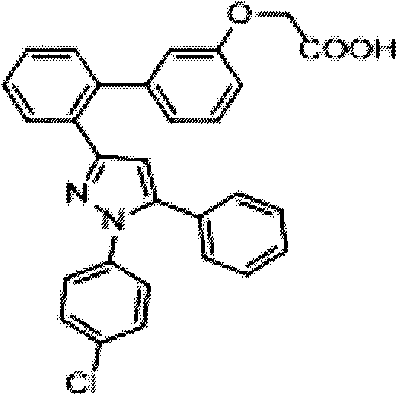
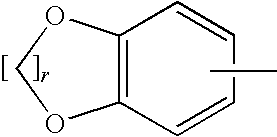
Pyrazole compound as well as composition and application thereof
The invention provides a compound and application of the compound and the pharmaceutically acceptable salt thereof or a stereo isomer or a prodrug molecule thereof in preparing a medicament for treating or preventing metabolic diseases, wherein the compound has the structural characteristic of a general expression I and is used as a novel fat cell type fatty acid binding protein FABP inhibitor. The compound having the structural characteristic of the general expression I can provide a new selection for the clinical prevention and treatment of the following diseases: 1. type II diabetes, 2. hyperglycemia, 3. hypoglycemia tolerance, 4. insulin resistance, 5. adiposity, 6. lipid turbulence, 7. blood-lipoid imbalance, 8. hyperlipaemia, 9. hypertriglyceridemia, 10. hypercholesterolemia, 11. low high-density protein level, 12. overhigh low-density protein level, 13. atherosclerosis and secondary diseases thereof, 14. hemadostenosis, 15. abdominal obesity, 16. a metabolic syndrome and 17. fatty liver.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
2-acylaminopropoanol-type glucosylceramide synthase inhibitors
InactiveUS8304447B2Inhibit synthesisHigh metabolic stabilityBiocideOrganic chemistryDiseaseLow glucose
Owner:GENZYME CORP
Composition with auxiliary blood sugar reduction efficacy, and preparation method and application thereof
InactiveCN106606529AGood hypoglycemic effectReduce absorptionOrganic active ingredientsHydrolysed protein ingredientsBitter gourdAdditive ingredient
The invention provides a composition with an auxiliary blood sugar reduction efficacy, and a preparation method and application thereof, and relates to the technical field of medical care. The composition is mainly prepared from the raw materials in parts by weight: 20 to 50 parts of L-arabinose and a bitter gourd preparation, wherein the bitter gourd preparation comprises any one material of 15 to 35 parts of bitter gourd powder, 10 to 50 parts of bitter gourd extracts and 1 to 5 parts of bitter gourd polypeptide. The composition has the advantages that on one hand, the effects of controlling the conversion and adsorption of the human body on sugar and enhancing the insulin are achieved, and the blood sugar can be reduced in a synergistic way; and on the other hand, the recipe selects raw materials with simple ingredients, so that the absorption is easy, and the dependence cannot be easily generated. The preparation method of the composition is characterized in that the raw materials and a bonding agent are mixed and are prepared into granules. The preparation method has the advantages that the technological process is short; the operability is high; and the industrial production is facilitated.
Owner:NANJING SHENGNUO BIO TECH IND
Preparation method of slowly-digestible starch-based recombinant rice with low glycemic index
ActiveCN109393333AIncrease added valueIncrease the added value of technologyFood thermal treatmentFood shapingDigestible starchEnzymatic hydrolysis
The present invention discloses a preparation method of slowly-digestible starch-based recombinant rice with a low glycemic index, and belongs to the technical field of food processing. After a high-temperature fluidization technology is used to conduct a non-crystallizing treatment of a solid-phase broken rice raw material in a short time, materials are subjected to a one-step reactive extrusiontechnology, enzyme-adding enzymatic hydrolysis and an esterification treatment are combined, and the recombinant rice with high slowly-digestible starch content and low glycemic index is obtained. Therecombinant rice prepared by the one-step shaping extrusion technology is suitable for consumption of patients with type II diabetes. At the same time, the preparation method increases added value ofagricultural and sideline products, reduces production cost, and improves production efficiency.
Owner:JIANGNAN UNIV
High-fiber high-protein noodles for diabetics and people needing to control body weight
ActiveCN104431770ASave energyLower protein contentVitamin food ingredientsFood ingredient functionsBiotechnologyLow glucose
The invention relates to high-fiber high-protein noodles for diabetics and people needing to control body weight. The high-fiber high-protein noodles are made from the following raw materials in parts by weight: 0-10 parts of tartary buckwheat flour, 0-20 parts of oat flour, 18-70 parts of wheat flour, 0-15 parts of whole wheat flour, 2-15 parts of puerarin powder, 0-6 parts of Chinese yam flour, 0-8 parts of ultra-fine black rice flour, 5-15 parts of wheat protein, 0-10 parts of soybean protein, 0-10 parts of whey protein, 0-10 parts of dietary fiber and 0-0.55 part of vitamins and microelements. The noodles can ensure that the diabetics and overweight people fully ingest protein, dietary fiber and vitamin B to maintain normal nutrient requirements under the condition of controlling energy intake, and is popular food which has low energy, high fiber, high protein and low glycemic index and can be eaten as staple food.
Owner:蒋博
Food products for diabetics
Disclosed is a novel food product characterized by a low glucose or glucose free content, a balanced functional fat content, and a proactive agent aimed for the diabetic and diabetic-prone populations. The food product of the invention is a functional food which may be used clinically to lower the lipid level in people suffering from an imbalanced lipid profile and which may progress towards diabetes complications and coronary vascular disorders. In particular embodiments the proactive agent can be any of a naturally occurring lipid, a synthetic or mimetic lipid which is not digestible by humans and interferes with body weight gain / loss, plant extracts and substances derived therefrom, antioxidants, animal-derived substances, minerals and pharmaceuticals, and any mixture thereof.
Owner:ENZYMOTEC
Bread with low glycemic index (GI) and making method thereof
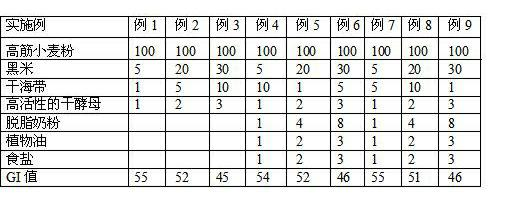
The invention provides bread with a low glycemic index (GI) and a making method thereof. The making method is characterized by grinding 5-30 parts by weight of black rice to obtain black rice powder; weighing 1-10 parts by weight of dried seaweed, soaking the dried seaweed in water, washing the seaweed, adding a defined amount of water and crushing the seaweed into seaweed paste, taking and putting 1-3 parts by weight of dried yeast in a container, pouring in warm water, stirring the materials uniformly to activate the yeast, mixing the seaweed paste, the black rice powder, activated yeast liquid, 100 parts by weight of strong wheat flour and 40-75 parts by weight of water, mixing and stirring the materials until the dough is mature and then preparing the stirred dough into bread by adopting the conventional fermenting, proofing and baking processes. Compared with the prior art, the bread has a low GI value and is suitable for the patients with low glucose tolerance and the diabetic patients.
Owner:FOSHAN UNIVERSITY
Making method of health dendrobe liquor
ActiveCN104611174AHigh nutritional valueUnique aromaAntipyreticMetabolism disorderLow glucoseUltrafiltration
The invention discloses a making method of health dendrobe liquor, belonging to the field of food processing. The making method is characterized in that dendrobium stem utilized as a raw material and auxiliary materials including poria, tuber fleeceflower stem, radix asteris, Xylariasp, orchis are subjected to processing procedures of wall-breaking crushing, extraction, sealed fermentation, post-fermentation, ultrafiltration membrane clarification and aging, thus obtaining the finished health dendrobe liquor. The health dendrobe liquor is high in nutritive value, special in aroma and mellow and mild in taste, further has the efficacies of tonifying stomach, regenerating body fluid, clearing heat, easing pain, reducing blood glucose and prolonging life and is a health-nourishing health product with extremely high nutritive value and mellow, mild, natural and pure aroma.
Owner:中寰健康科技(无锡)有限公司
Spirulina-containing extruded rice with low GI (glycemic index) and preparation method of extruded rice
The invention relates to spirulina-containing extruded rice with low GI (glycemic index). The spirulina-containing extruded rice comprises raw materials in parts by weight as follows: 30-50 parts of bitter buckwheat, 20-40 parts of oat meal, 5-10 parts of soybean powder, 5-10 parts of white hyacinth bean powder, 1-3 parts of spirulina powder, 1-5 parts of yam flour, 10-15 parts of dietary fiber, 0.5-1.5 parts of vitamins and 0.5-1.5 parts of minerals. Various cereal materials with low GI are taken as a substrate, the added spirulina powder has a function of assisting in reduction of blood sugar, the added dietary fiber facilitates growth of probiotics in intestinal tracts and delays the food digestion rate in gastrointestinal tracts, and rapid increase of postprandial blood sugar is delayed to a certain extent. Active ingredients such as various vitamins, the minerals and the like are widely applied in the health care field, reproduced rice with low GI is prepared through reasonable compounding with an extrusion technology, and a novel diet mode is provided for people with obesity, high glucose and diabetes as well as people requiring low-GI food.
Owner:ANHUI TONGFU FOOD
Hypoglycemic drug composition and application of hypoglycemic drug composition
ActiveCN102698271AReduce usageSmall toxicityMetabolism disorderHeterocyclic compound active ingredientsLow glucoseHypoglycemia
The invention relates to a hypoglycemic drug composition, which contains alpha-glucosaccharase inhibitor and Indian Trumetflower Seed extract, wherein the Indian Trumetflower Seed extract is prepared by the processes of extracting the Indian Trumetflower Seed with aqueous solution of organic solvent, filtering, re-extracting, separating and drying. The drug composition prepared from the Indian Trumetflower Seed extract and alpha-glucosaccharase inhibitor lower than normal dosage has excellent hypoglycemic effect. The hypoglycemic drug composition has the main advantages that postprandial blood glucose can be reduced faster and more efficiently, and less alpha-glucosaccharase inhibitor is used, so the drug effect can be improved, the toxic and side effects of the alpha-glucosaccharase inhibitor are effectively reduced, and the problems of hypoglycemia and the like easily caused by drug combination are also effectively solved.
Owner:DALIAN UNIV OF TECH
Composite flavoring for prevention of cardiovascular disease and cancer and its preparation method
The present invention relates to a composite flavouring material capable of preventing angiocardiopathy and cancer and its preparation method. Said composite flavouring material is selected from lentinus edodes, crataegus fruit or its extract, tangerine peel and tangerine peel glacoside, garlic, onion, fresh ginger, monosodium glutamate, guanosine acid, sodium chloride and edible essence. Said flavouring material has strong health-care functions of reducing blood fat, reducing blood sugar, expanding blood vessel, preventing thrombosis, removing internal free radical, delaying senility and resisting cancers.
Owner:陈登岳
Novel blood sugar lowing polypeptide and uses thereof
ActiveCN101041693AExtended half-lifePromote generationPeptide/protein ingredientsMetabolism disorderHalf-lifeBlood sugar
The invention discloses a GLP-1 peptide, whose general formula is HX1EGTFTSDX2SSYLEGQAAKX3FIX4WLVKGR (formula I), wherein X1 is Ala, Gly or Val; X2 is Val, Leu or Ala; X3 is Leu or Ser; X4 is Glu or Asn.
Owner:ZHUHAI UNITED LAB
Preparation method of black fungus tablets
ActiveCN102599484ALower blood sugarReduce weightFood shapingFood preparationBiotechnologyLow glucose
The invention relates to a preparation method of black fungus tablets, which adopts the following steps of 1) screening; 2) soaking and washing; 3) breaking; 4) water extracting; 5) enzymolysis; 6) vacuum concentration; 7) drying in an atomizing way; 8) addition of auxiliary materials; 9) pelleting; and 10) drying and tabletting. The preparation method has advantages that the black fungus is produced into a buccal tablet form, so that not only can the efficacies of the black fungus such as abundant nutrients, lowering of blood sugar, resistance of cancer, weight loss and the like be reserved, but also the characteristics such as light weight, small size, convenience in carrying and to eat and the like can be realized. One black fungus tablet can be directly put into a mouth to be chewed or kept in the mouth until being dissolved when in need, the black fungus table is beneficial to all ages and is in economy, and the market prospect of the black fungus tablets is wide.
Owner:穆棱市晟宇生物科技有限公司
Method for preparing and preserving umbilical arterial and vein vascular peripheral stem cells
ActiveCN105695401AHigh purityReduce harmDead animal preservationSkeletal/connective tissue cellsZymogenLow glucose
The invention discloses a method for preparing and preserving umbilical arterial and vein vascular peripheral stem cells. The umbilical arterial and vein vascular peripheral stem cells are cultured by a cell culture fluid (DMEM low glucose, 10% of fetal calf serum and 1% of penicillin-streptomycin double anti-body). The operation is carried out under strict aseptic conditions; and after umbilical cord collection, the inconvenience caused by blood coagulation is effectively avoided by use of an anticoagulant. Compared with a conventional coating method, the method disclosed by the invention has the advantages that umbilical perivascular sourced mesenchymal stem cells with higher purity are obtained; allergic reaction and cross infection caused by animal-based protein can be avoided since digestive enzymes are not adopted; and the umbilical perivascular stem cells separated by a non-zymogen digestion method have higher positive rate than that of the Wharton's jelly sourced mesenchymal stem cells.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com