Novel blood sugar lowing polypeptide and uses thereof
A new type of technology for hypoglycemia, applied in the field of GLP-1 modified peptides, can solve the problems of not obtaining hypoglycemic effect and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]The present invention modifies the main action site of DDP-IV, the most important factor affecting the stability of GLP-1, that is, the Ala of GLP-1 8 The carboxy-terminal amino acid of NEP 24.11; at the same time, the modification of the enzyme cleavage site of NEP 24.11 has also been studied and analyzed, mainly in GLP-1 (12-15). Using bioinformatics tools such as productprotein protein structure prediction software, Vector NTI DNA and protein sequence analysis software, the GLP-1 similar peptide sequence was screened and analyzed. According to the principle of secondary structure similarity, a set of GLP-1 analogue polypeptide sequences was obtained.
[0028] The primary screening GLP-1 analogs are listed as follows:
[0029] SEQ NO 1
[0030] HAEGTFTSDVSSYLEGQAAK FI wxya
[0031] SEQ NO 2
[0032] HAEGTFTSDVSSYLEGQAAK FI wxya
[0033] SEQ NO 3
[0034] HAEGTFTSDVSSYLEGQAAK FI wxya
[0035] SEQ NO 4
[0036] HAEGTFTSDVSSYLEGQAAK FI wxya
[003...
Embodiment 2
[0102] Embodiment 2: chemically synthesized polypeptide
[0103] One of the polypeptides of the present invention is synthesized by solid-phase synthesis, and the sequence is: HGEGTFTSDLSSYLEGQAAKLFIEWLVKGR-NH 2 (SEQ NO 17), the synthesis method of other polypeptide sequences is similar.
[0104] Synthesis
[0105] Using the Fmoc synthesis method, Rink-Amide-MBHA Resin (purchased from Nankai Hecheng Company) was selected for synthesis. The synthesis steps are as follows:
[0106] 17 kinds of Fmoc-amino acid raw materials with side chain protecting groups——solid phase synthesis——removal of side chain protecting groups——HPLC purification——lyophilization——GLP analogs
[0107] amino acid name
source
Fmoc-L-Ala-OH
Synpep.Inc
[0108] Fmoc-L-Arg(Pme)-OH
Fmoc-L-Asn(Trt)-OH
Fmoc-L-Asp(OtBu)-OH
Fmoc-L-Gln(Trt)-OH
Fmoc-L-Glu(OtBu)-OH
Fmoc-L-Gly-OH
Fmoc-L-His(Trt)-OH
Fmoc-L-Ile-OH
Fmoc-L-Leu-OH
Fmoc-...
Embodiment 3
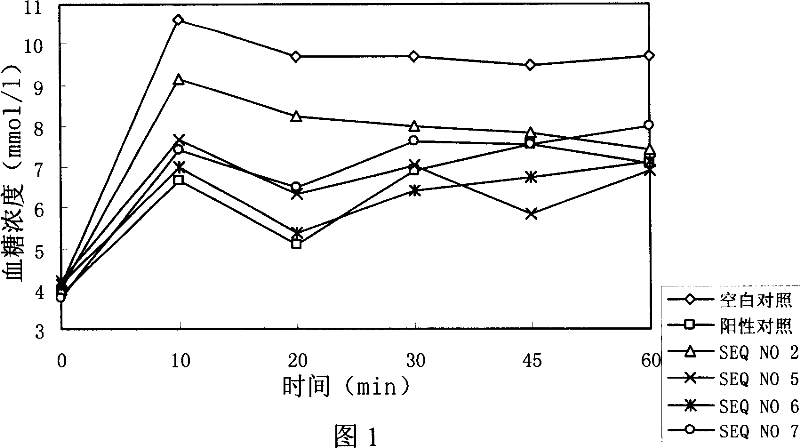
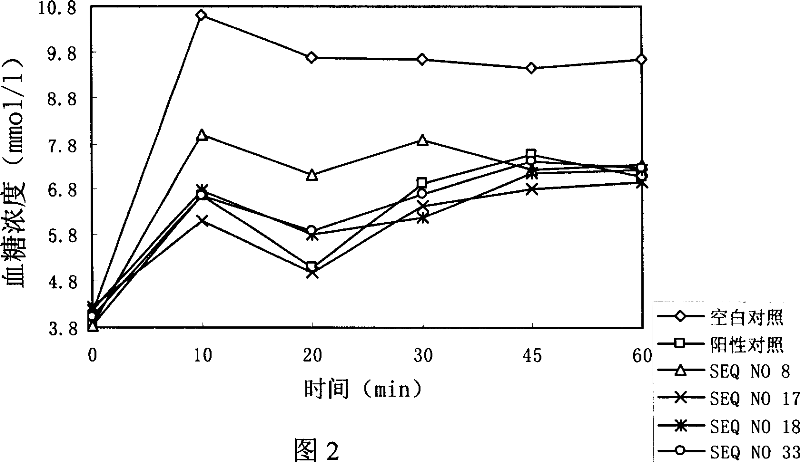
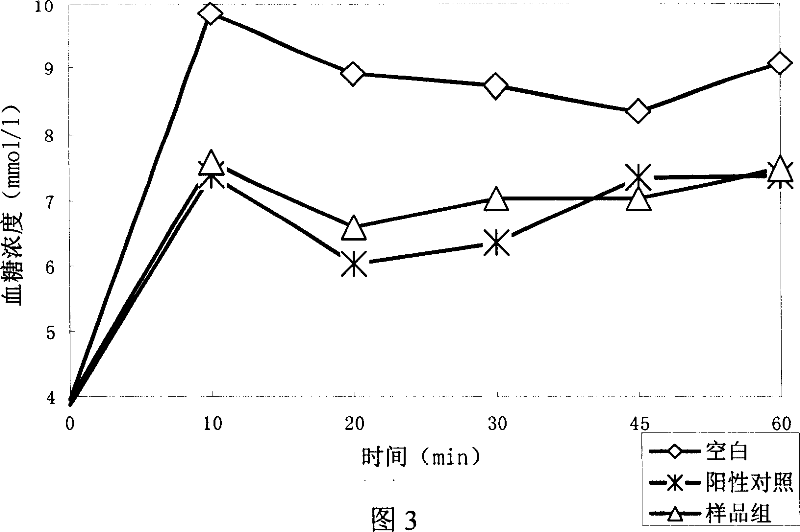
[0168] Example 3: Research experiments on the hypoglycemic effect of polypeptides
[0169] experiment material:
[0170] 1. Sample:
[0171] SEQ NO 2: HAEGTFTSDVSSYLEGQAAKLFINWLVKGR-NH2
[0172] SEQ NO 5: HAEGTFTSDLSSYLEGQAAKLFIEWLVKGR-NH2
[0173] SEQ NO 6: HAEGTFTSDLSSYLEGQAAKLFINWLVKGR-NH2
[0174] SEQ NO 7: HAEGTFTSDLSSYLEGQAAKSFIEWLVKGR-NH2
[0175] SEQ NO 8: HAEGTFTSDLSSYLEGQAAKSFINWLVKGR-NH2
[0176] SEQ NO 17: HGEGTFTSDLSSYLEGQAAKLFIEWLVKGR-NH2
[0177]SEQ NO 18: HGEGTFTSDLSSYLEGQAAKLFINWLVKGR-NH2
[0178] SEQ NO 33: HVEGTFTSDASSYLEGQAAKLFIEWLVKGR-NH2
[0179] GLP-1(7-36) as positive control: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH2
[0180] 2. Animals: Wistar rats, male, weighing 280-300 g, provided by the Animal Experiment Center of Jilin University School of Basic Medicine.
[0181] 3. Reagents: ① Blood glucose kit, Zhongsheng Beikong Biotechnology Co., Ltd.; ② Insulin (INS) radioimmunoassay kit, Beijing Furui Bioengineering Company.
[0182] 4. Instrument: ①GF-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com