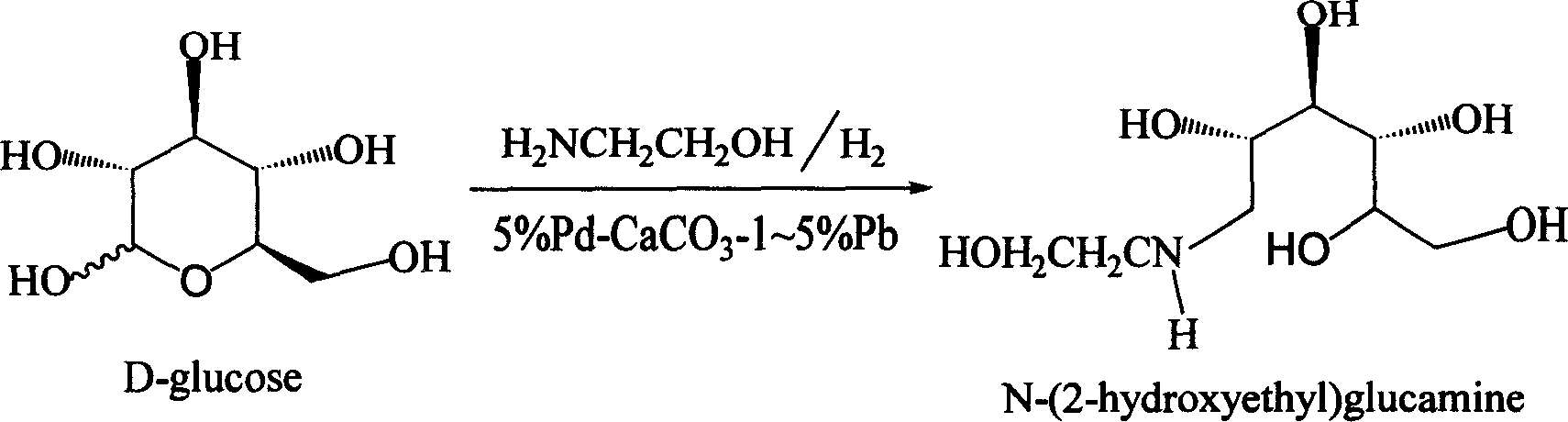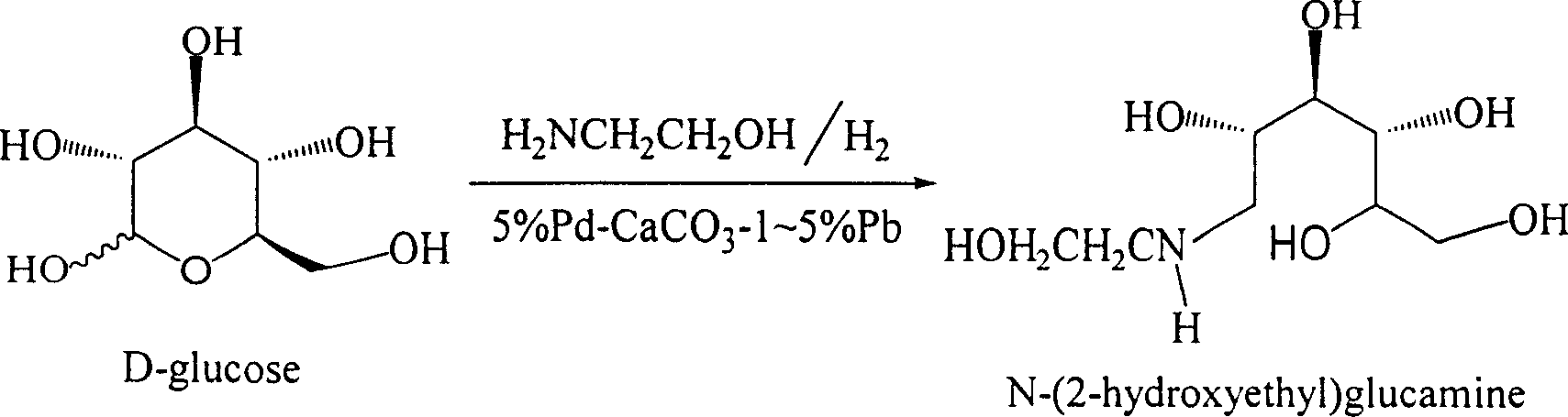Patents
Literature
36 results about "Diabetes Therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A therapeutic modality used to aide in the management of an individual's diabetes.
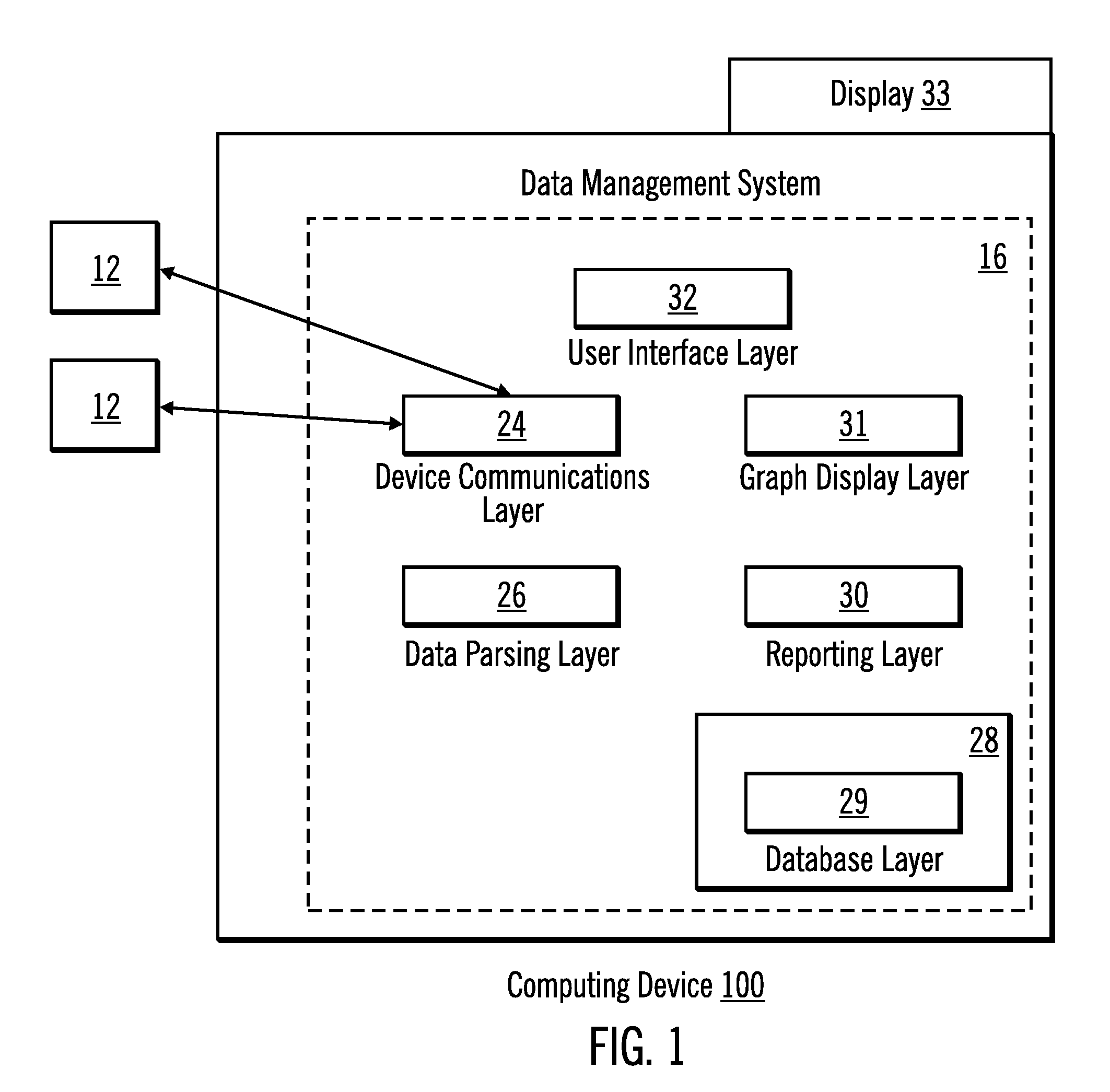
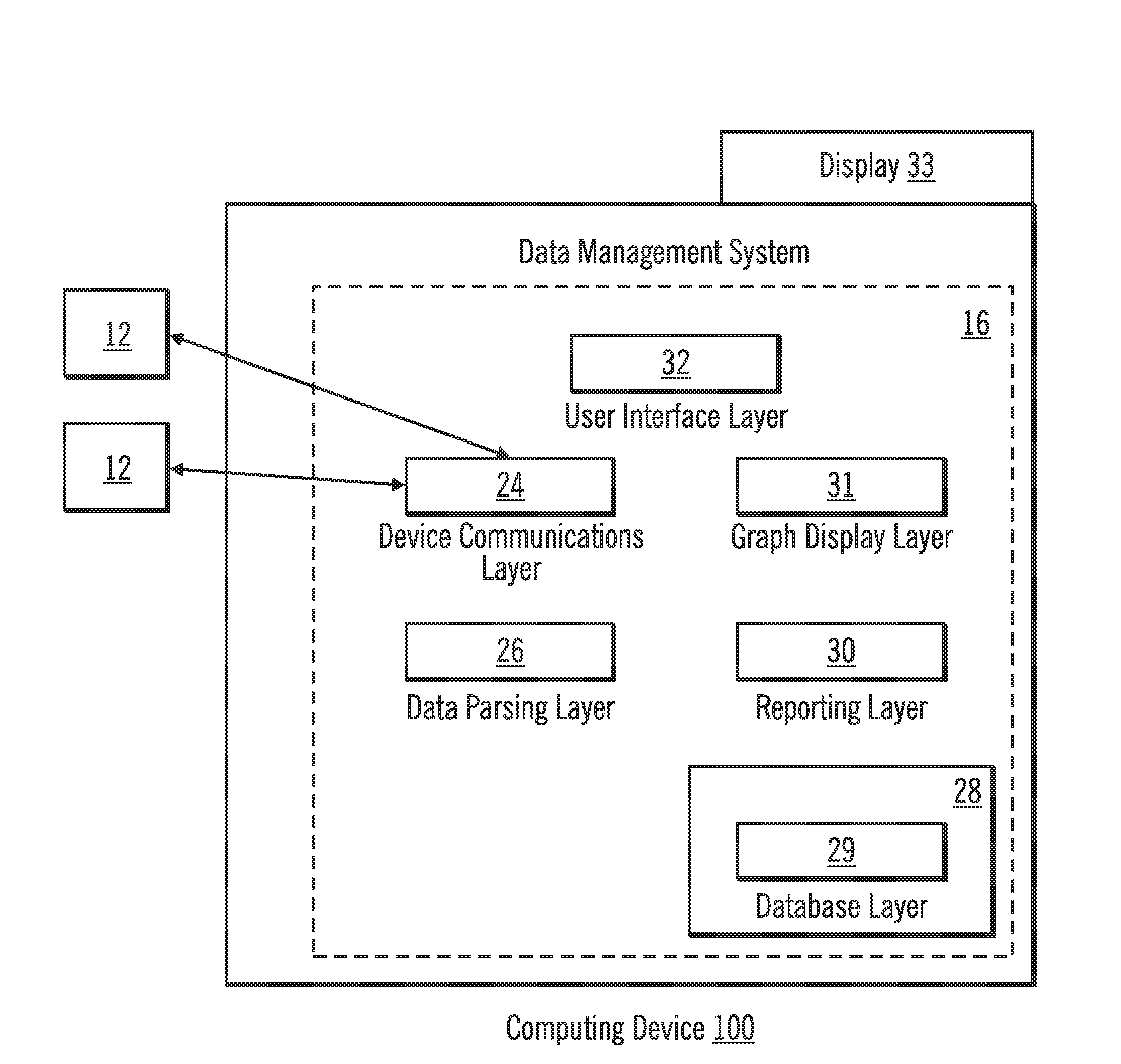
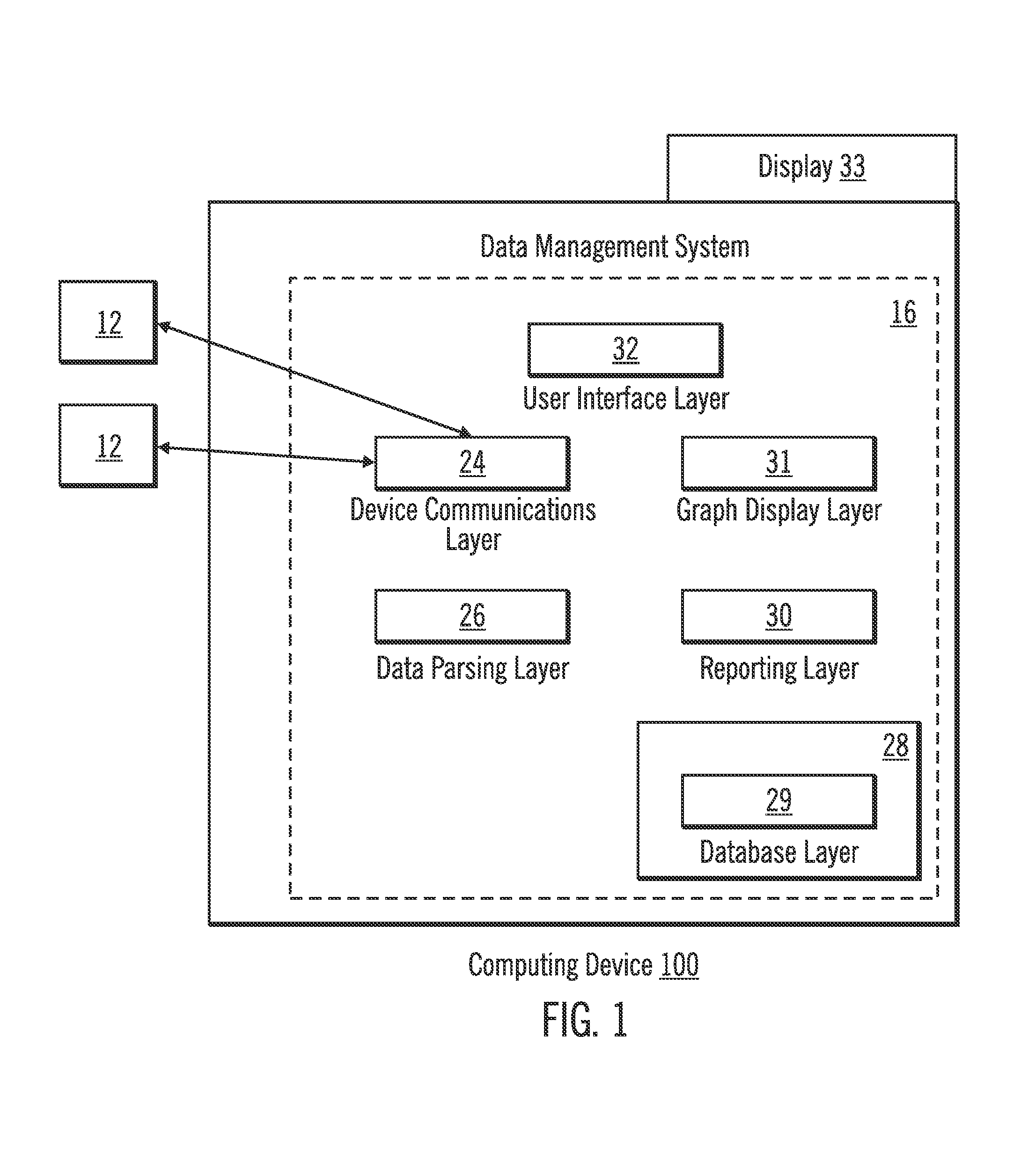
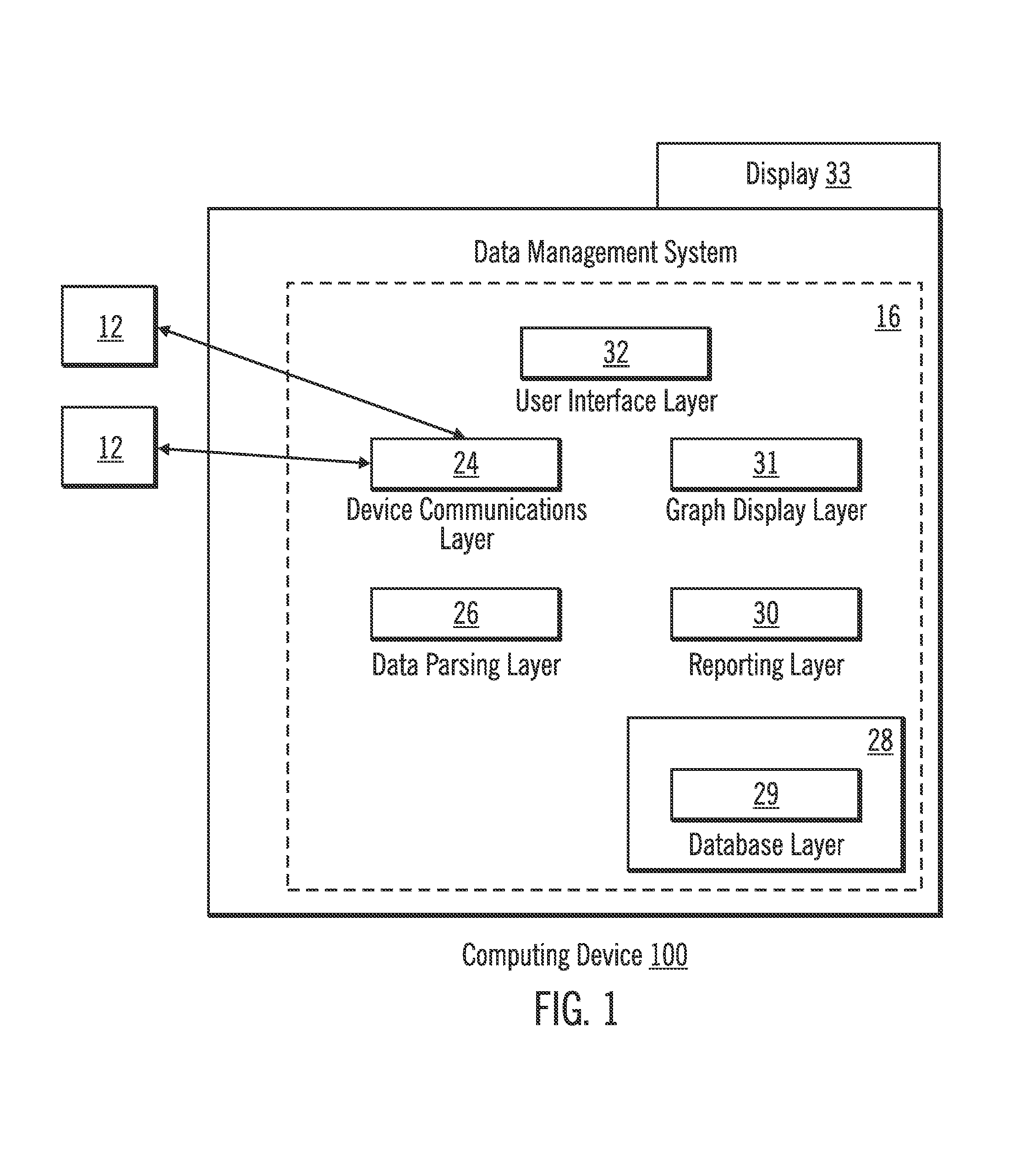
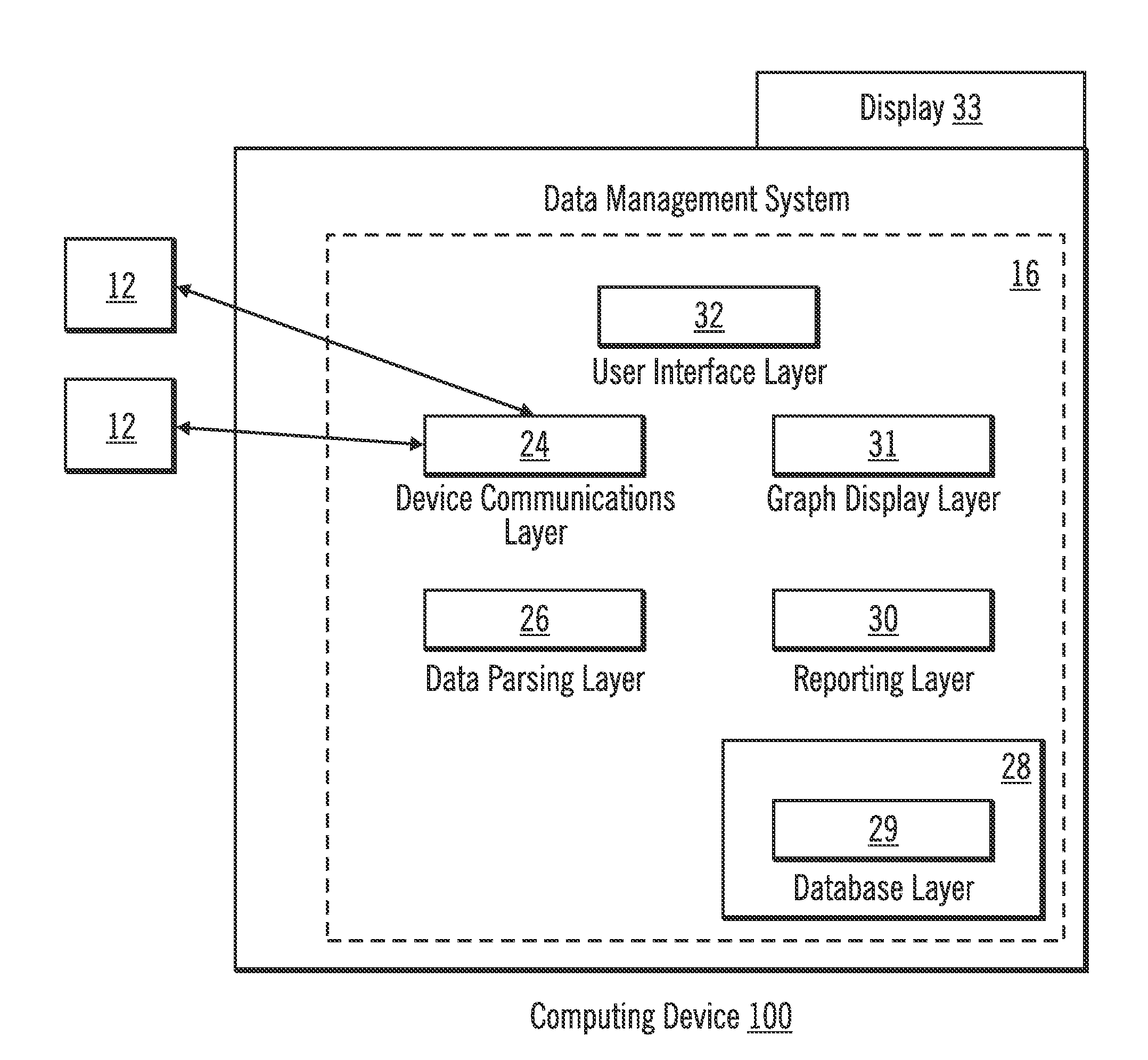
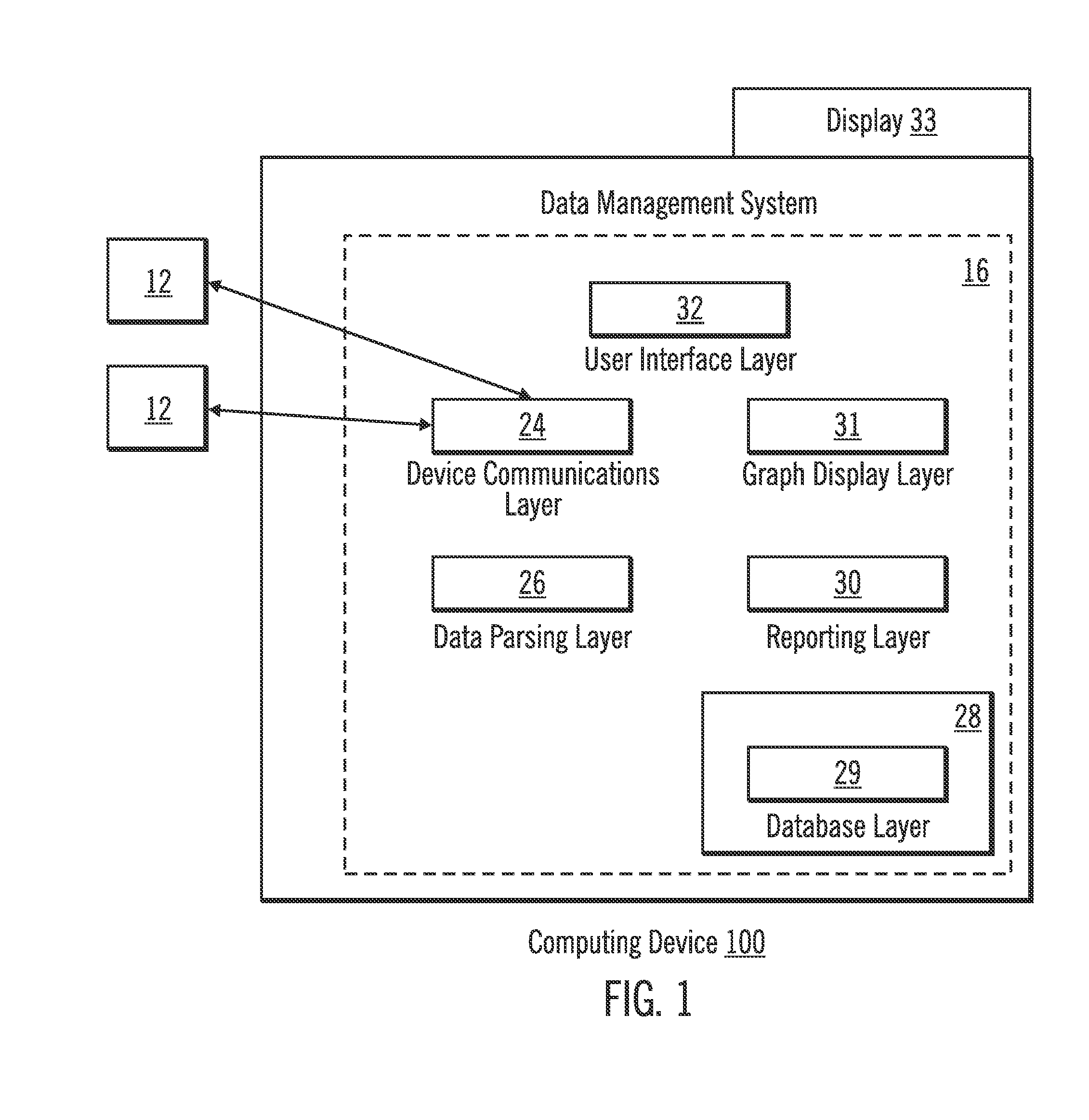
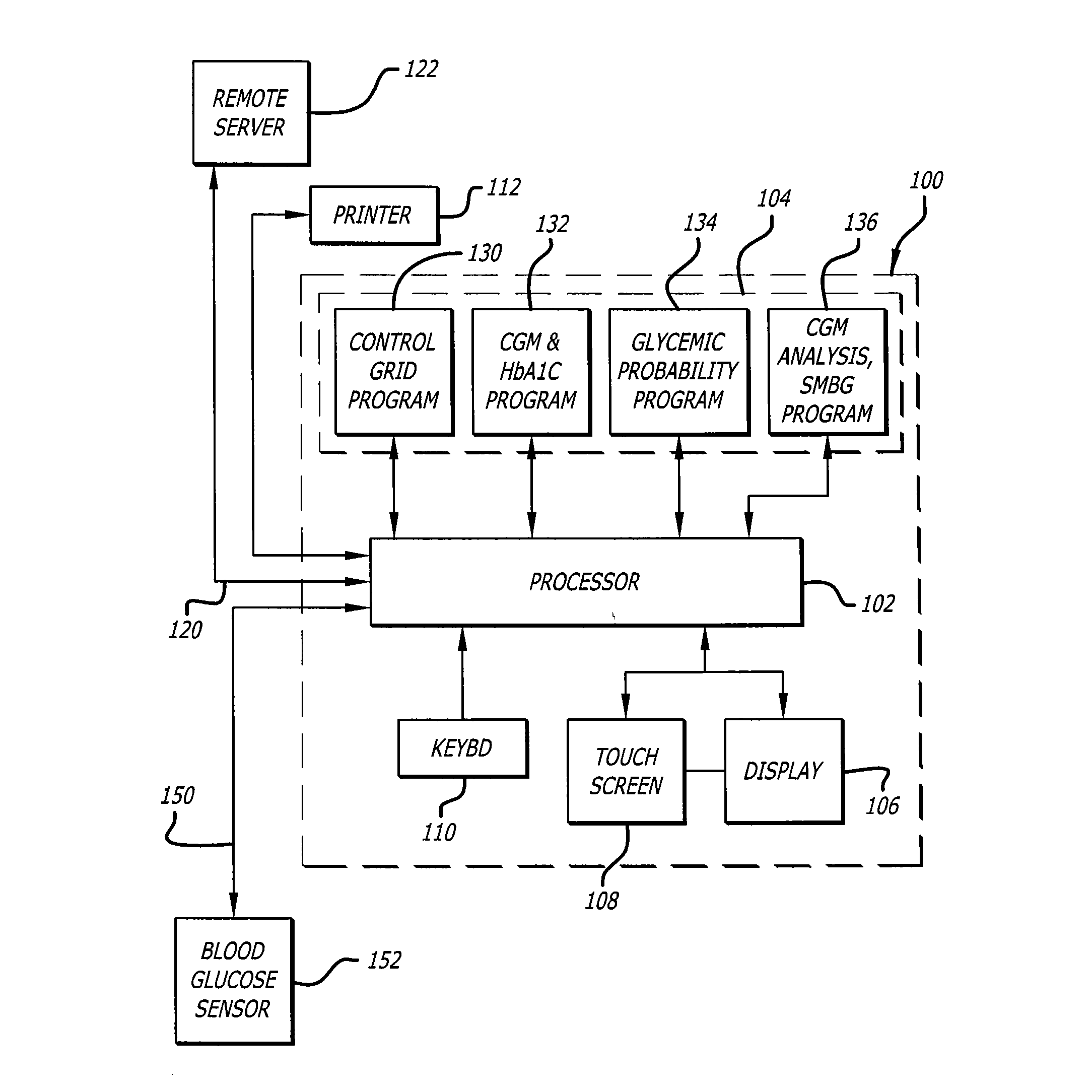
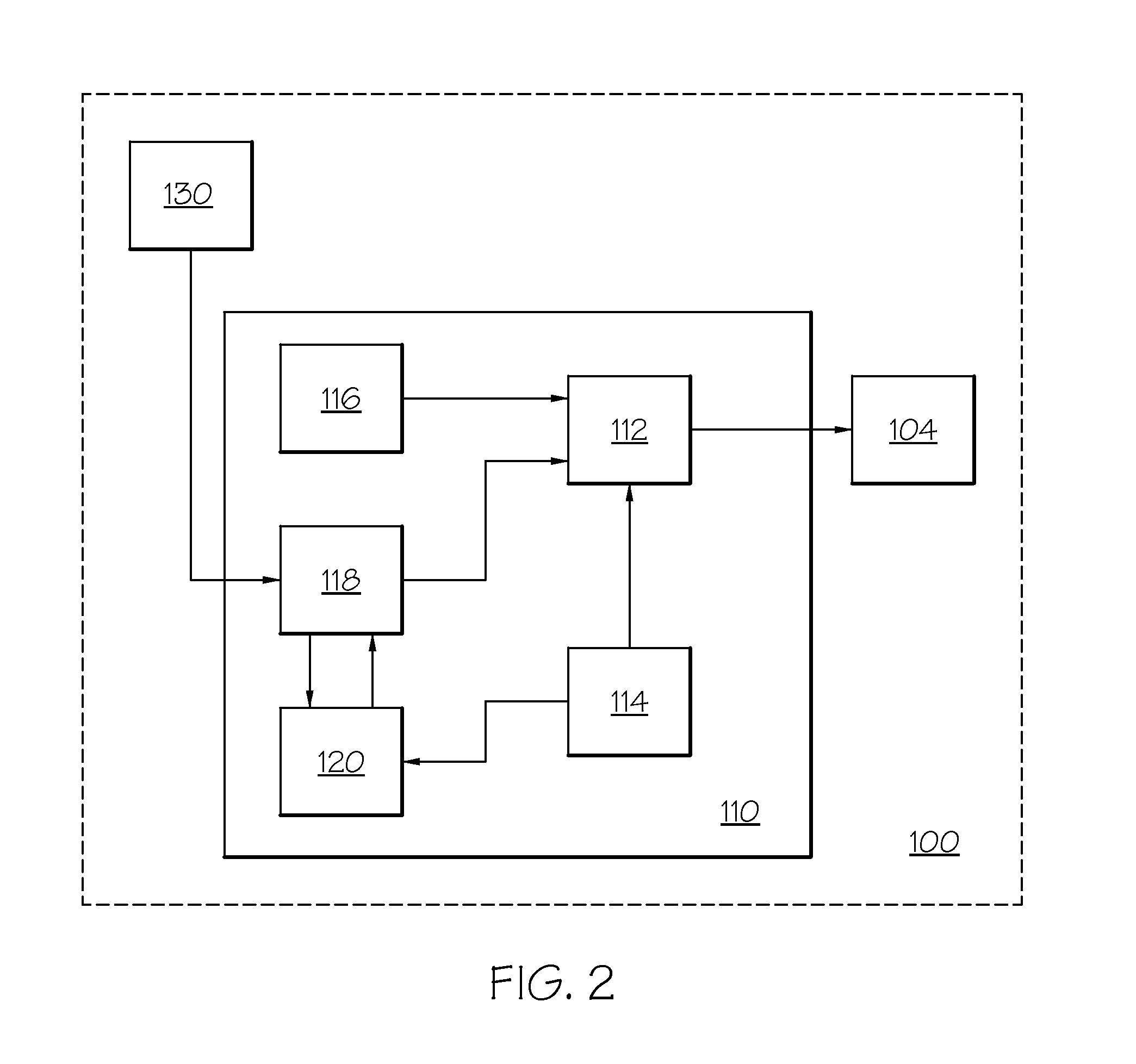
Diabetes Therapy Management System
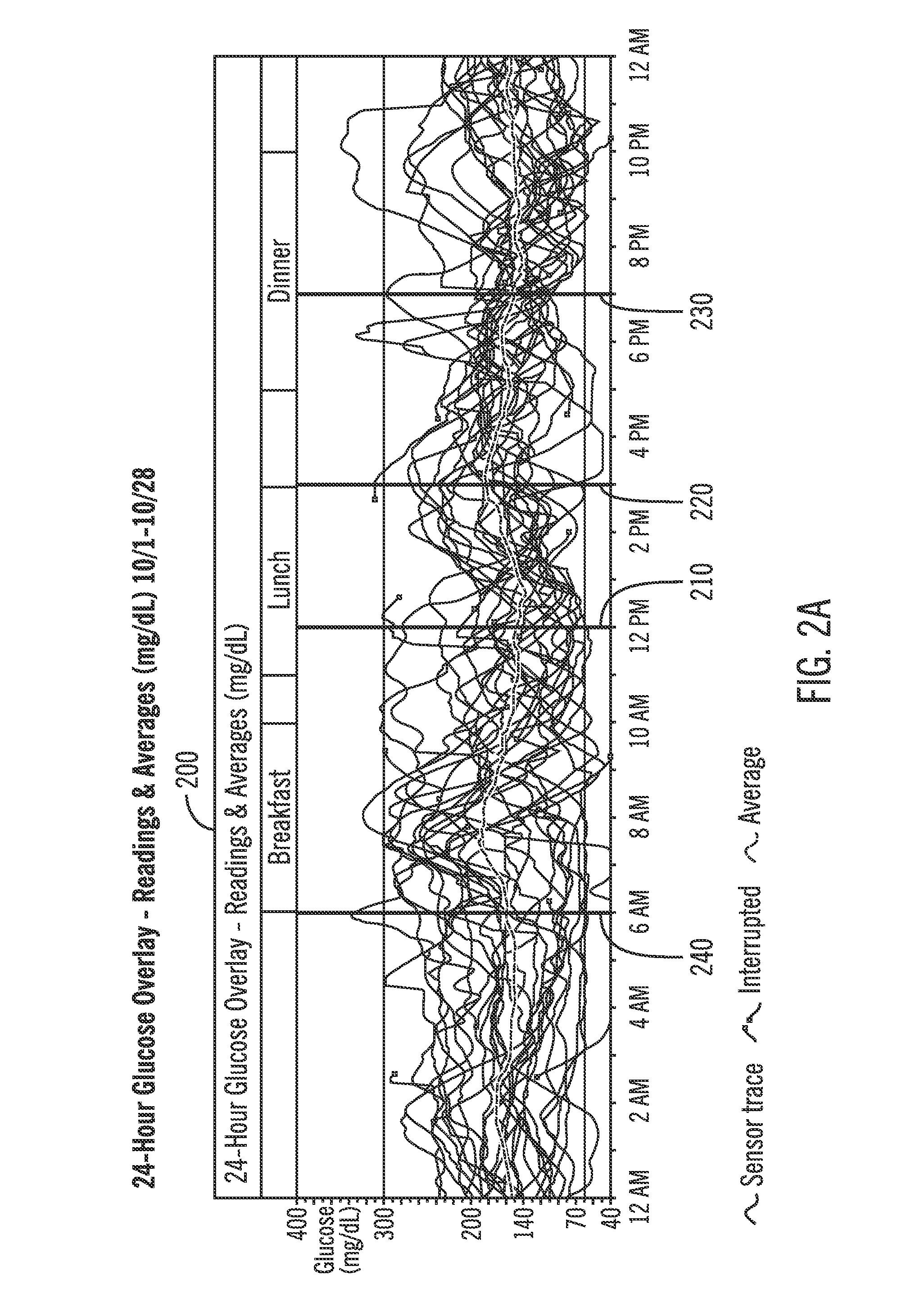
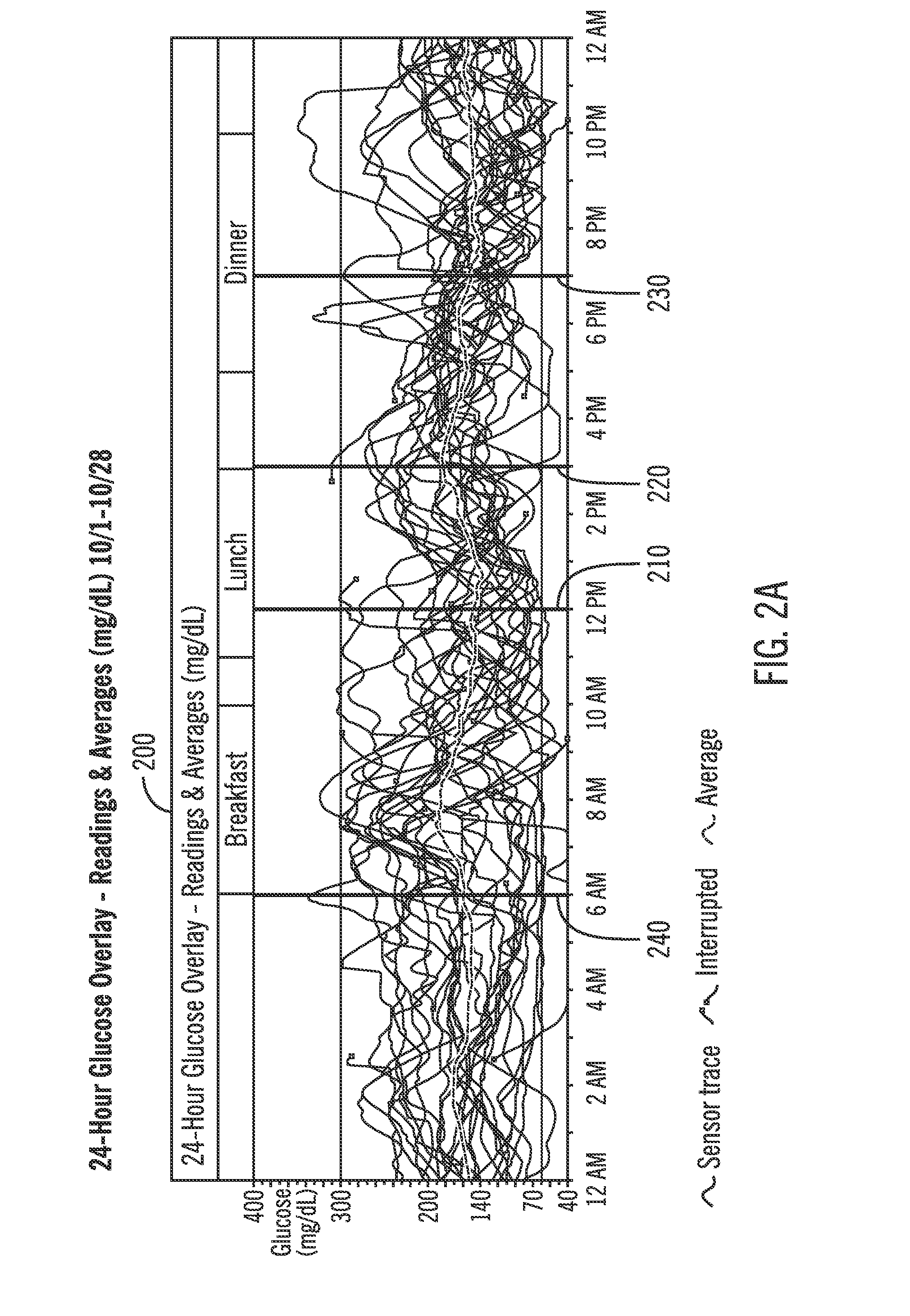
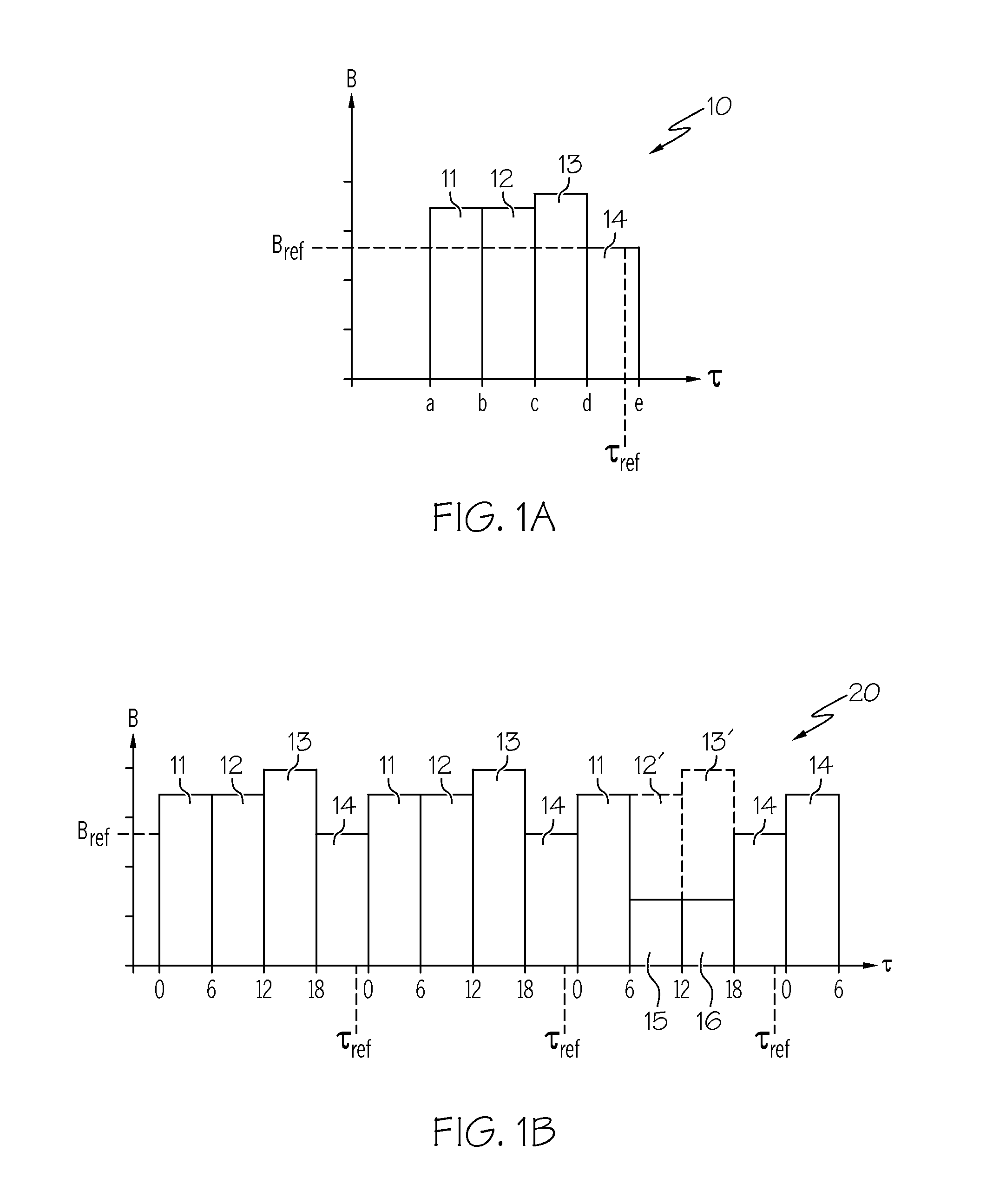
A method of diabetes analysis is provided. A plurality of glucose level readings for a user is received. The plurality of blood glucose level readings are analyzed to generate a report. The report includes a first chart along a 24-hour timeline indicating the plurality of glucose level readings, and a second chart having at least one of infusion device settings and active insulin levels corresponding to the 24-hour timeline of the first chart.
Owner:MEDTRONIC MIMIMED INC
Diabetes therapy management system for recommending adjustments to an insulin infusion device
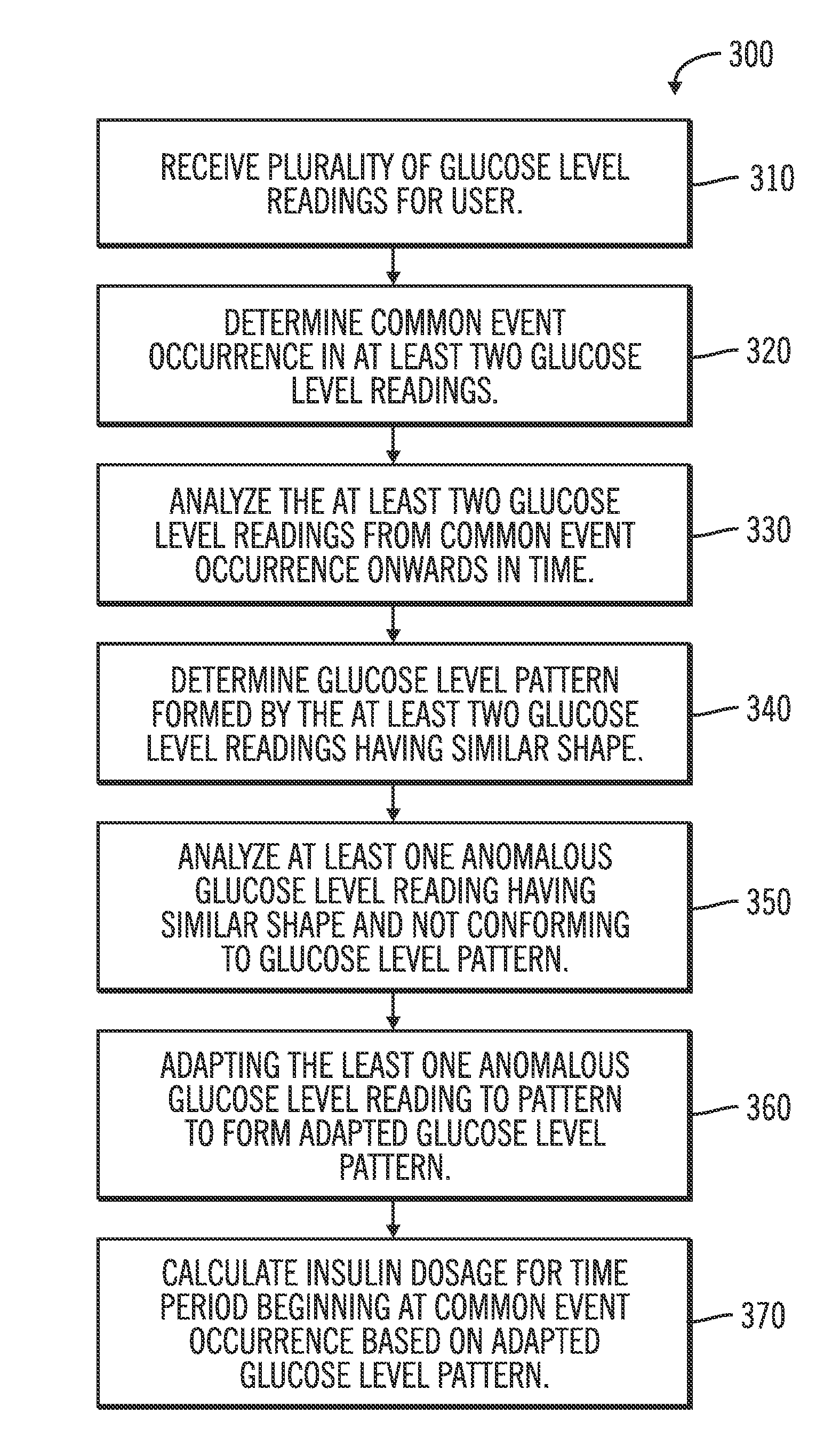
A method of managing use of an insulin infusion device are provided. The method receives glucose data for a user of the infusion device, wherein the glucose data indicates blood glucose levels of the user for a period of time during which the insulin infusion device is regulating delivery of insulin to the user. The received glucose data is received to detect certain event occurrences, which may be indicative of a correctable basal rate setting of the insulin infusion device and / or indicative of potential maladjustment of a user-specific setting of a bolus calculator setting of the insulin infusion device. The method outputs a recommendation to adjust the basal rate setting and / or the bolus calculator setting as needed to address the detected event occurrences.
Owner:MEDTRONIC MIMIMED INC
Diabetes therapy management system for recommending basal pattern adjustments
InactiveUS20130338629A1Increase rate of changeMedical data miningHealth-index calculationDiabetes TherapyGraphics
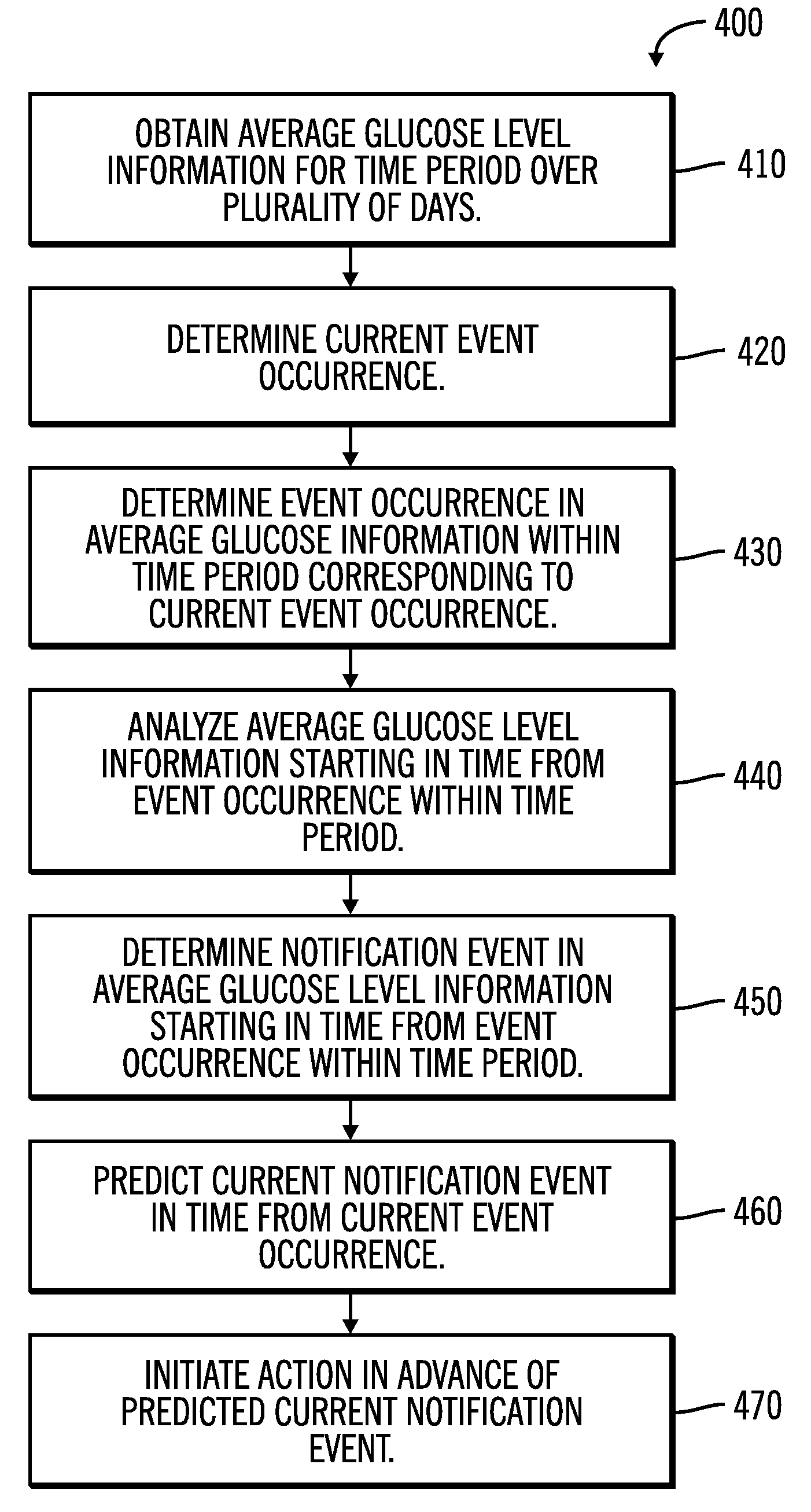
An electronic computing device as presented here includes a device communications layer, a processor device, and a reporting layer. The device communications layer receives sensor data for a user of an insulin infusion device, wherein the sensor data indicates blood glucose levels of the user for a specified period of time, and over a plurality of days. The processor device analyzes the received sensor data to detect an event occurrence indicative of a correctable basal rate setting of the insulin infusion device. The reporting layer generates a report containing a graphical representation of the received sensor data and a recommendation to adjust a basal rate setting of the insulin infusion device, wherein the recommendation is intended to address the detected event occurrence.
Owner:MEDTRONIC MIMIMED INC
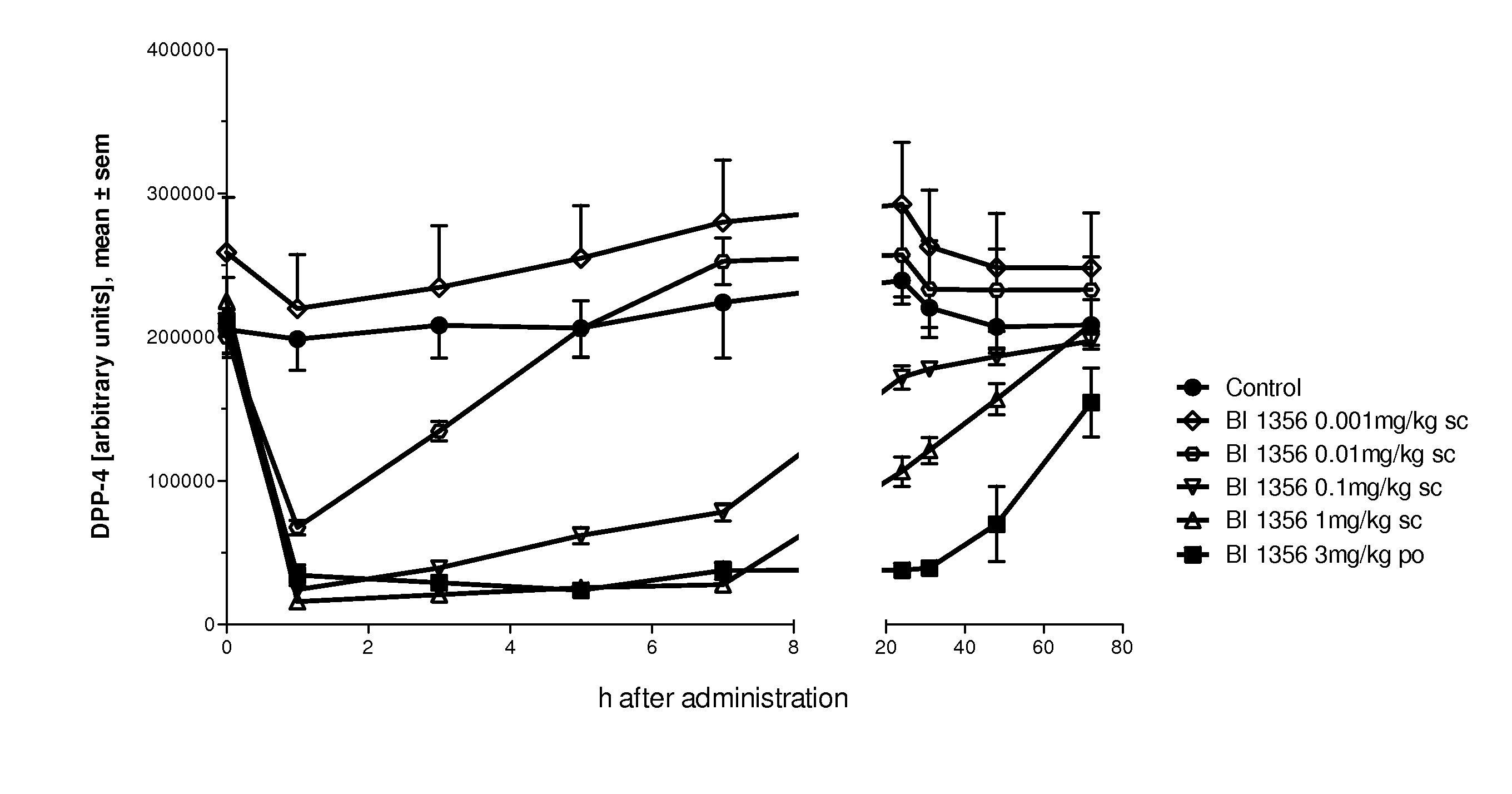
Diabetes therapy
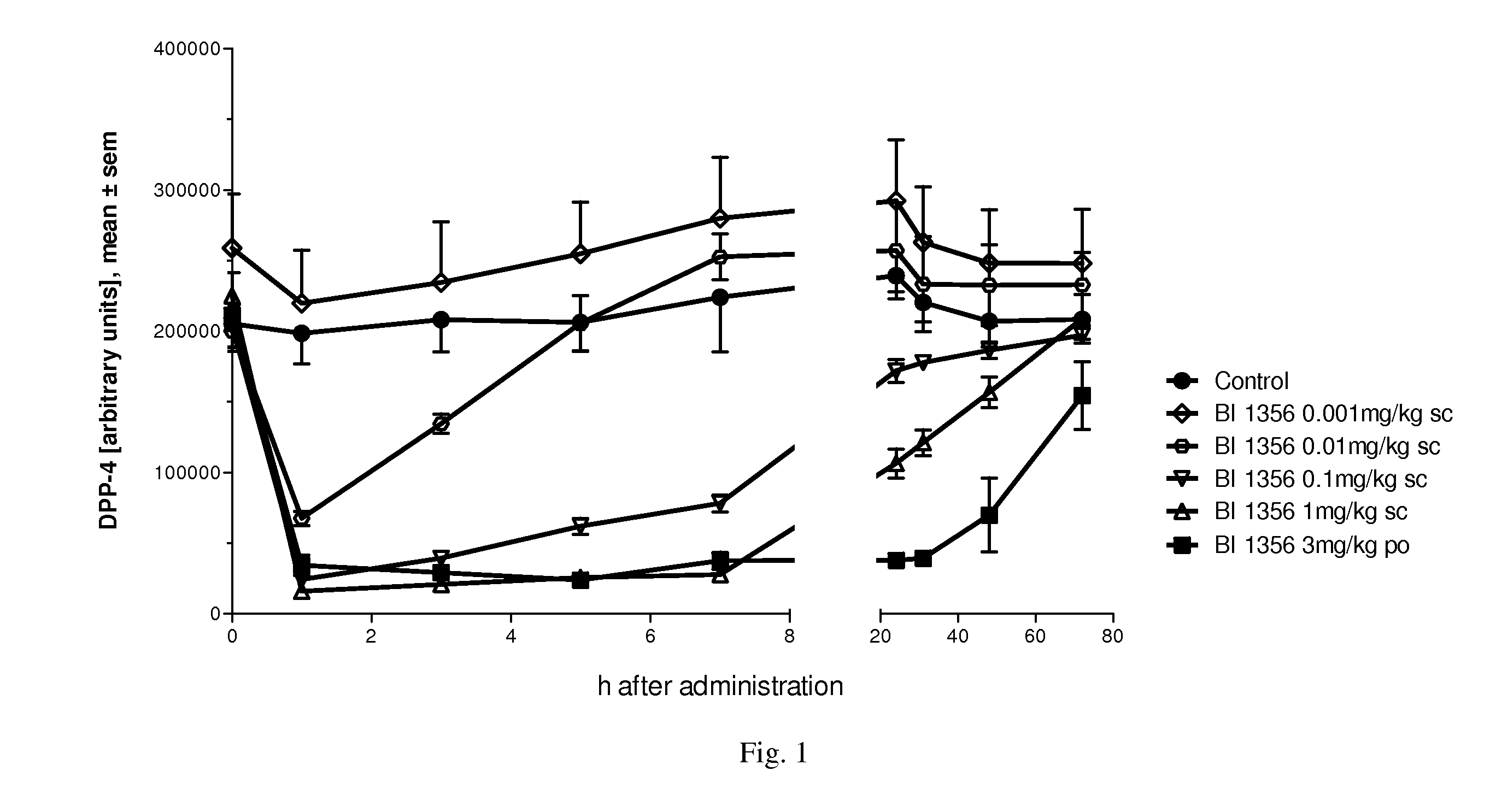
The present invention relates to methods for treating and / or preventing metabolic diseases comprising the combined administration of a DPP-4 inhibitor and a long-acting insulin. The invention further relates to a DPP-4 inhibitor for subcutaneous or transdermal use.
Owner:BOEHRINGER INGELHEIM INT GMBH
Diabetes therapy management system for recommending bolus calculator adjustments
A method of managing use of an insulin infusion device is presented here. The method identifies bolus calculator event data from glucose data for a user of the infusion device. The bolus calculator event data corresponds to use of a bolus calculator that calculates bolus dosage recommendations based on a user entered carbohydrate consumption value, a user entered current blood glucose value, a user specific carbohydrate ratio value, and a user specific insulin sensitivity value. The method filters the bolus calculator event data to remove glucose data associated with certain conditions, and analyzes the filtered data to detect an event occurrence that is indicative of potential maladjustment of the carbohydrate ratio value or the insulin sensitivity value. The method outputs a recommendation to adjust the carbohydrate ratio value or the insulin sensitivity value, based on characteristics of the detected event occurrence.
Owner:MEDTRONIC MIMIMED INC
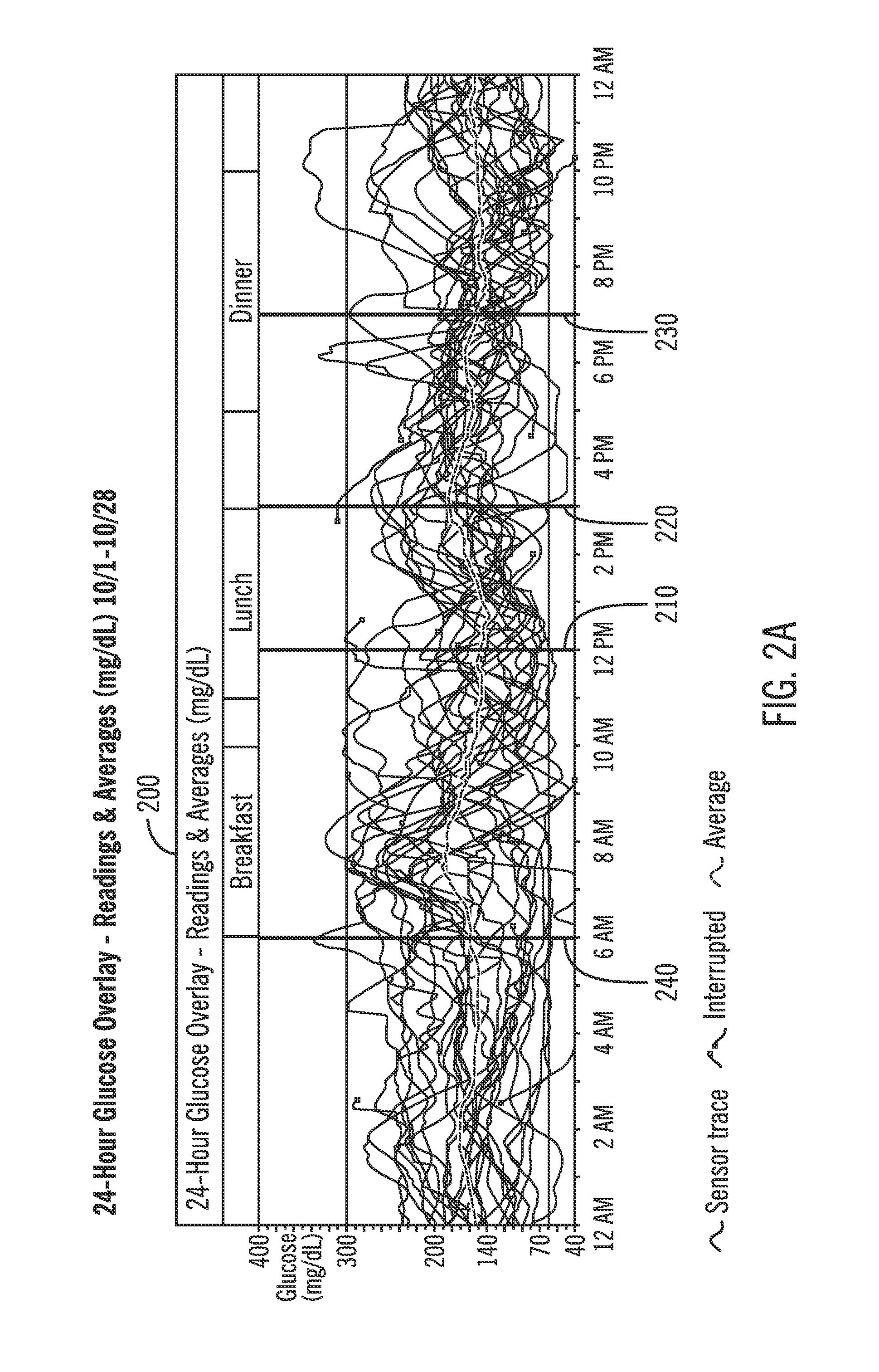
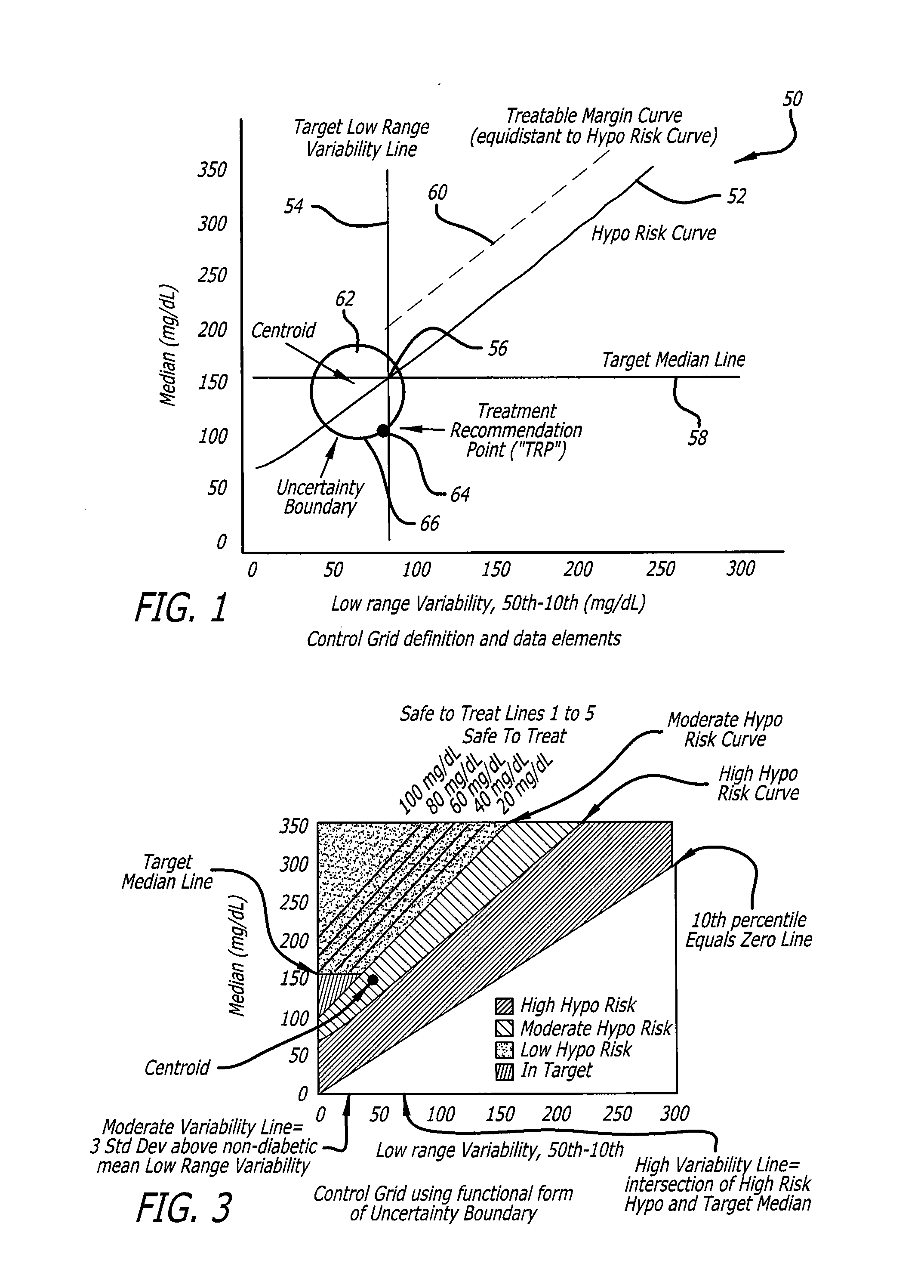
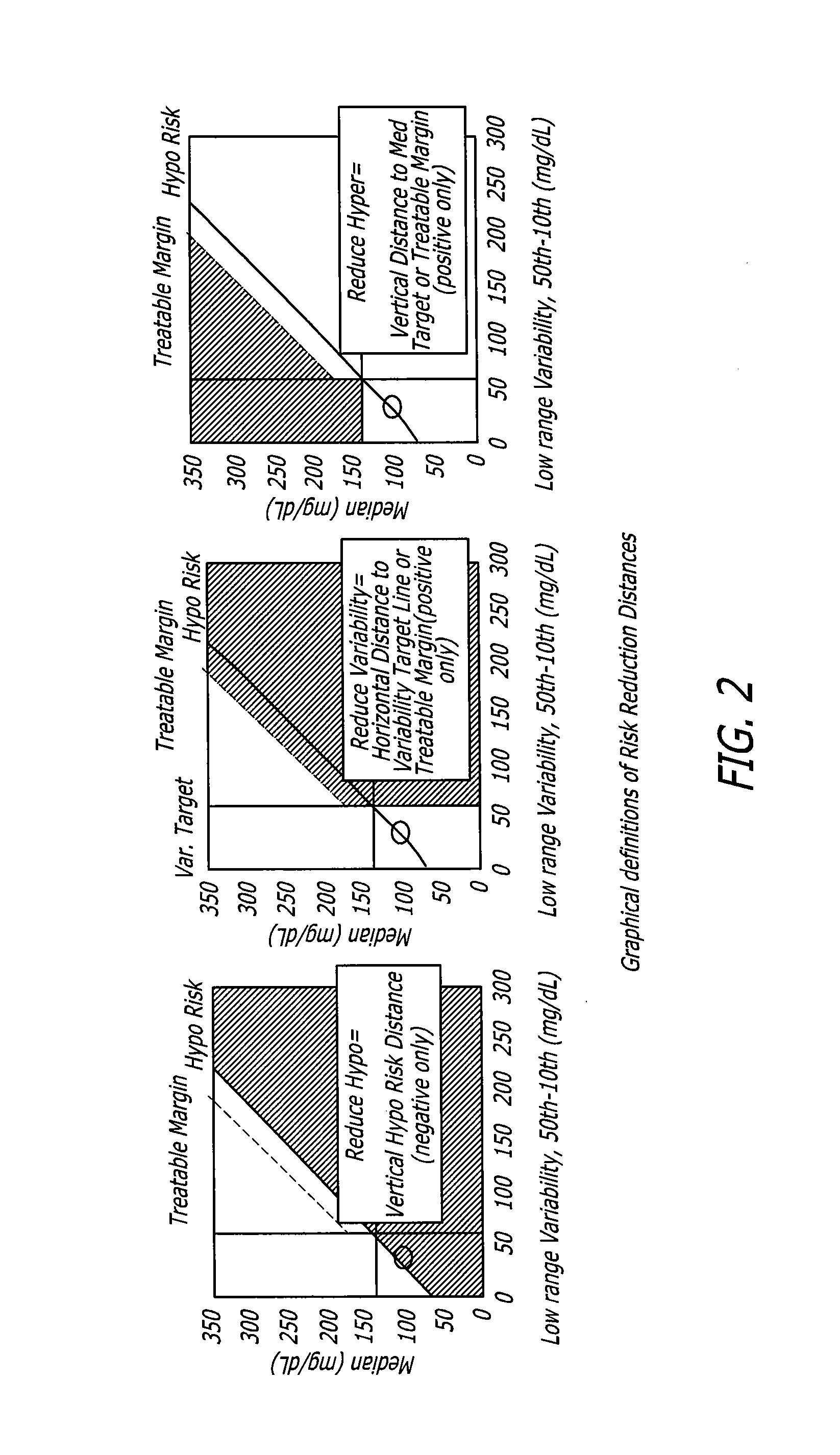
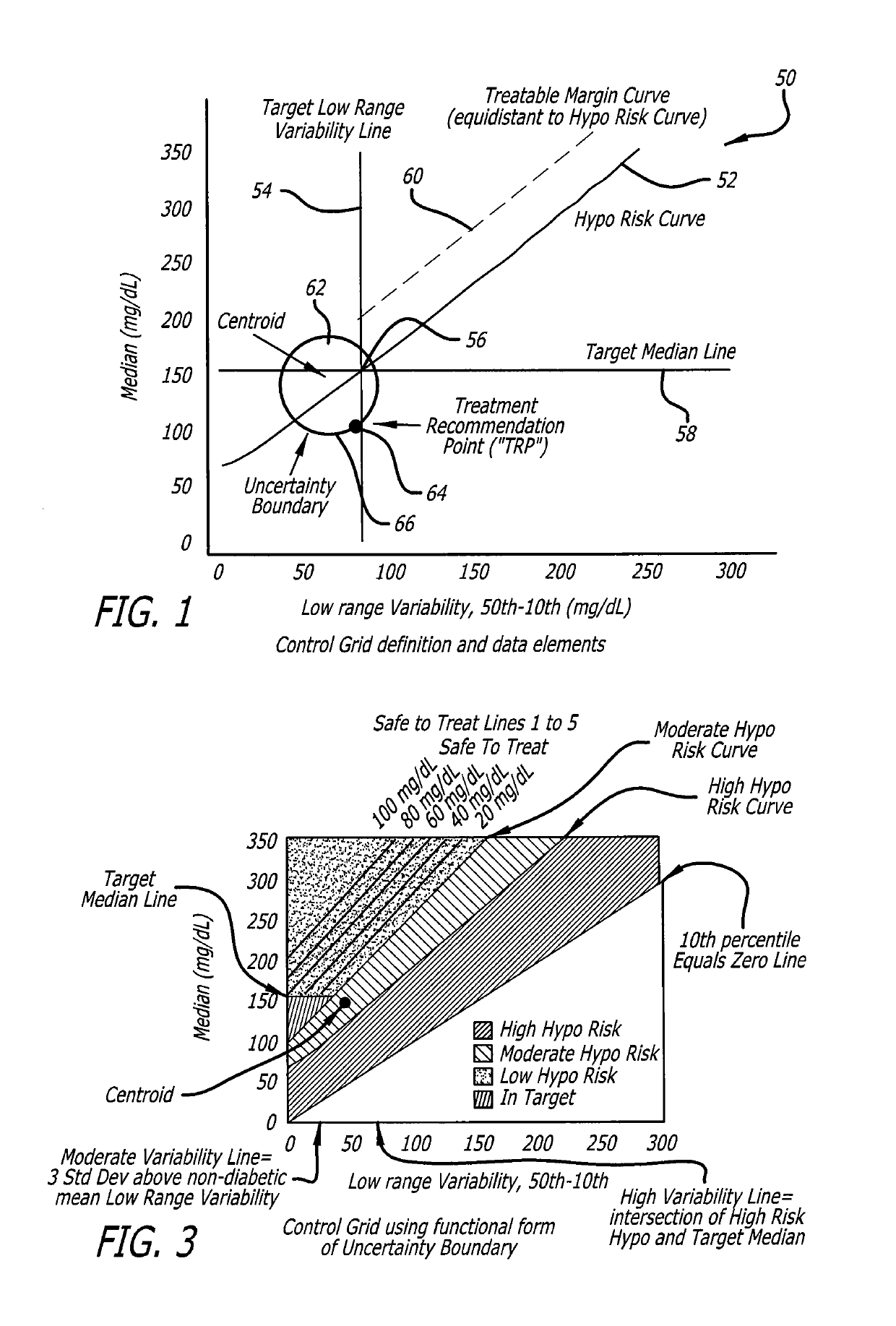
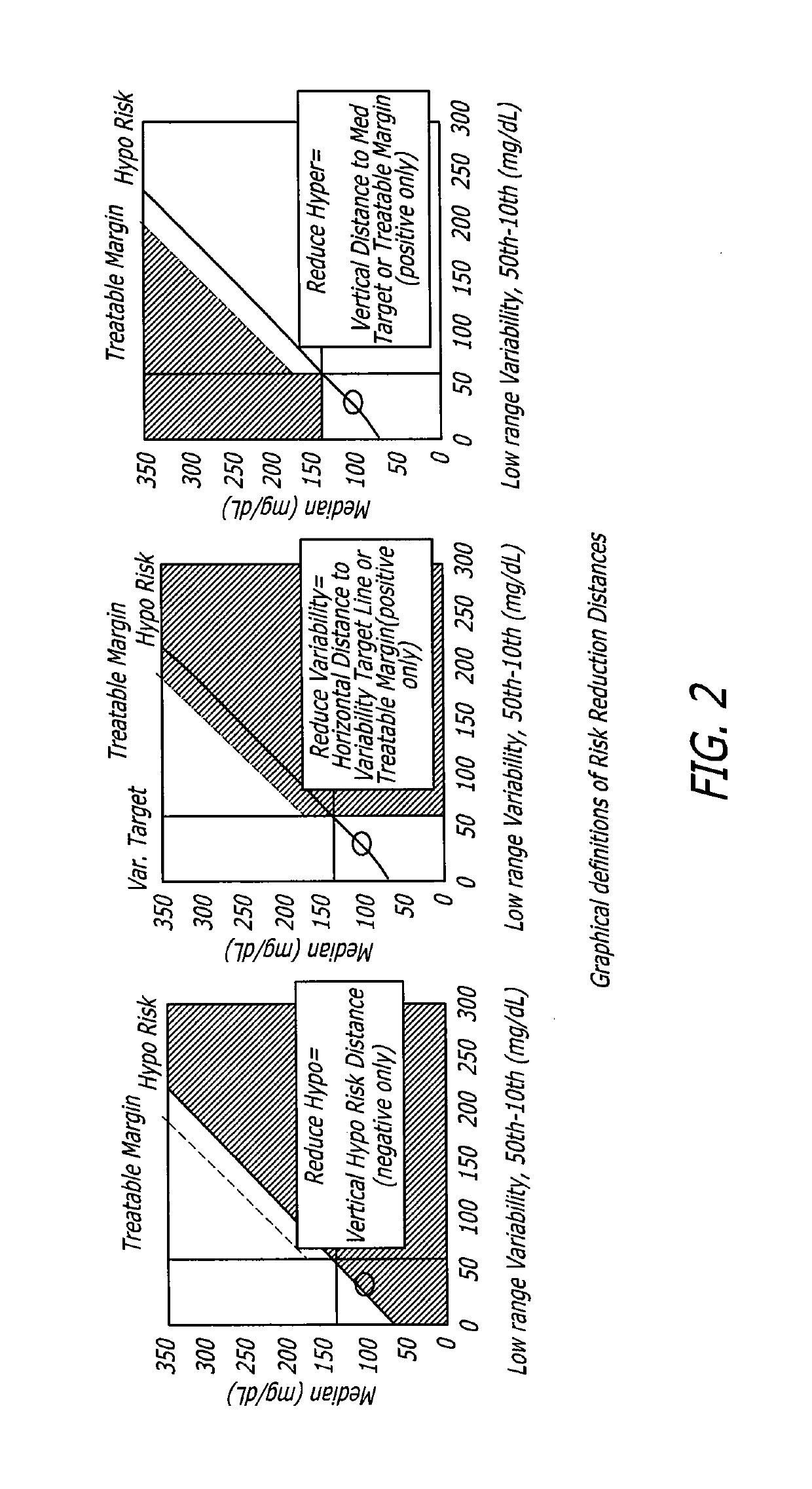
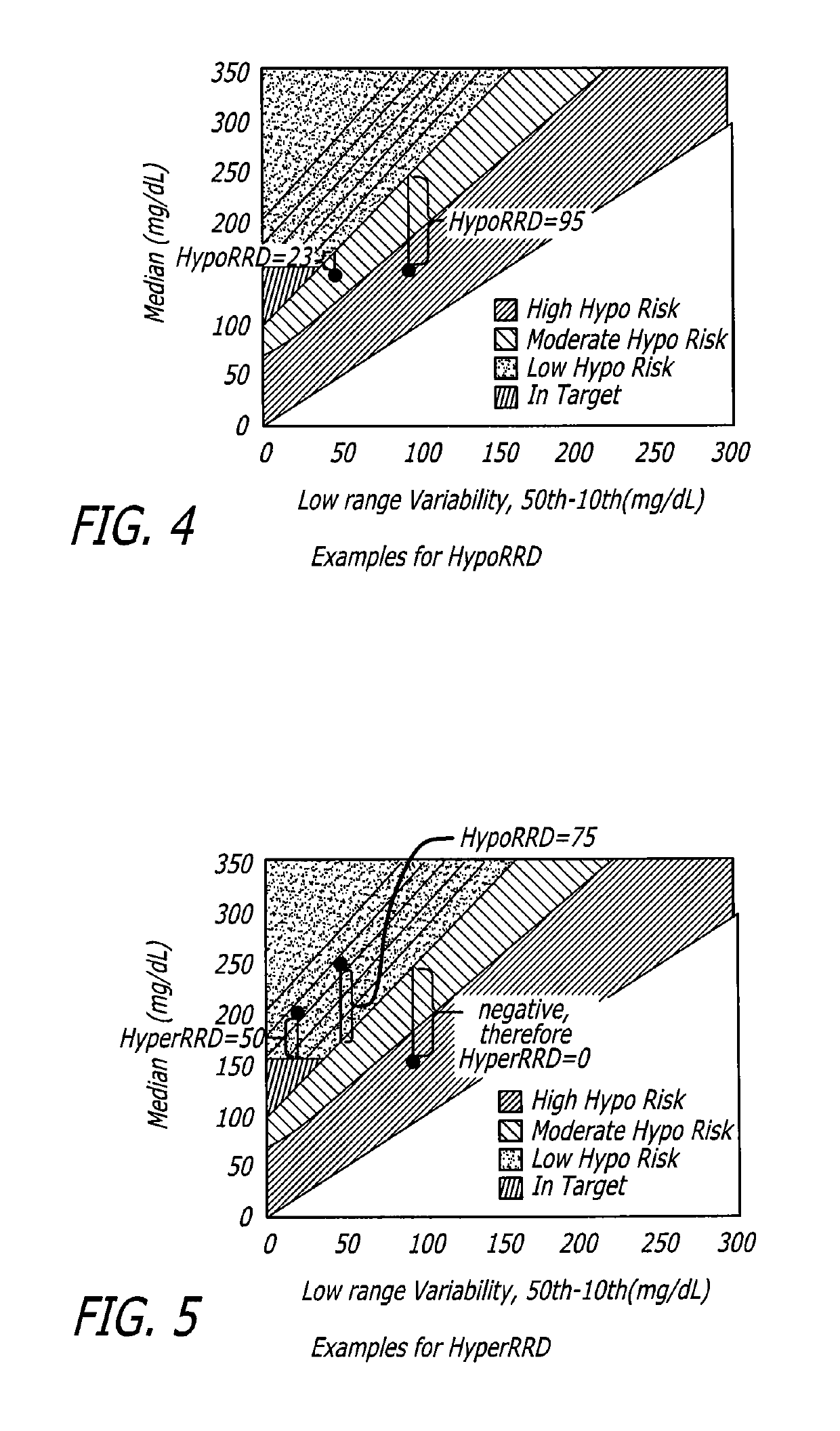
Analysis of glucose median, variability, and hypoglycemia risk for therapy guidance
ActiveUS20140188400A1Improve accuracyHealth-index calculationMedical automated diagnosisLow glucoseDiabetes Therapy
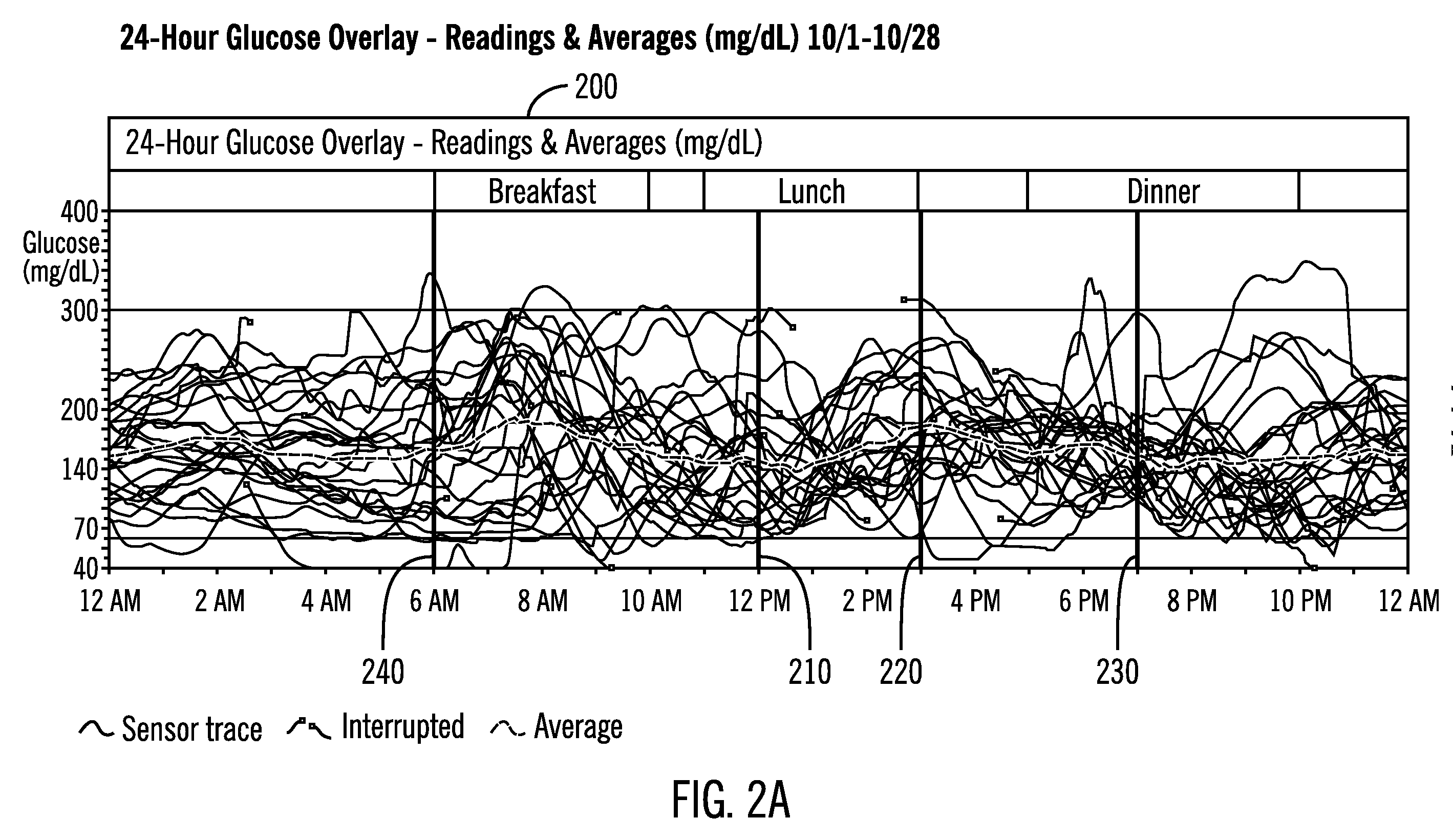
A system and method to provide guidance for diabetes therapy includes determining glycemic risks based on an analysis of glucose data. The analysis includes visualization of a glucose median, the variability of glucose in a patient, and the risk of hypoglycemia. An Advanced Daily Patterns report includes a visualization of an ambulatory glucose profile and a glucose control measure. The glucose control measure provides a highly visible and understandable display of the glucose condition of a patient visually expressed in the categories of low glucose, median glucose, and glucose variability.
Owner:ABBOTT DIABETES CARE INC
Diabetes therapy
ActiveUS9149478B2Shorten the progressSlow onsetSenses disorderNervous disorderDiabetes mellitusDiabetes Therapy
The present invention relates to methods for treating and / or preventing metabolic diseases comprising the combined administration of a DPP-4 inhibitor and a long-acting insulin. The invention further relates to a DPP-4 inhibitor for subcutaneous or transdermal use.
Owner:BOEHRINGER INGELHEIM INT GMBH
Injection device having dose indication and spring drive
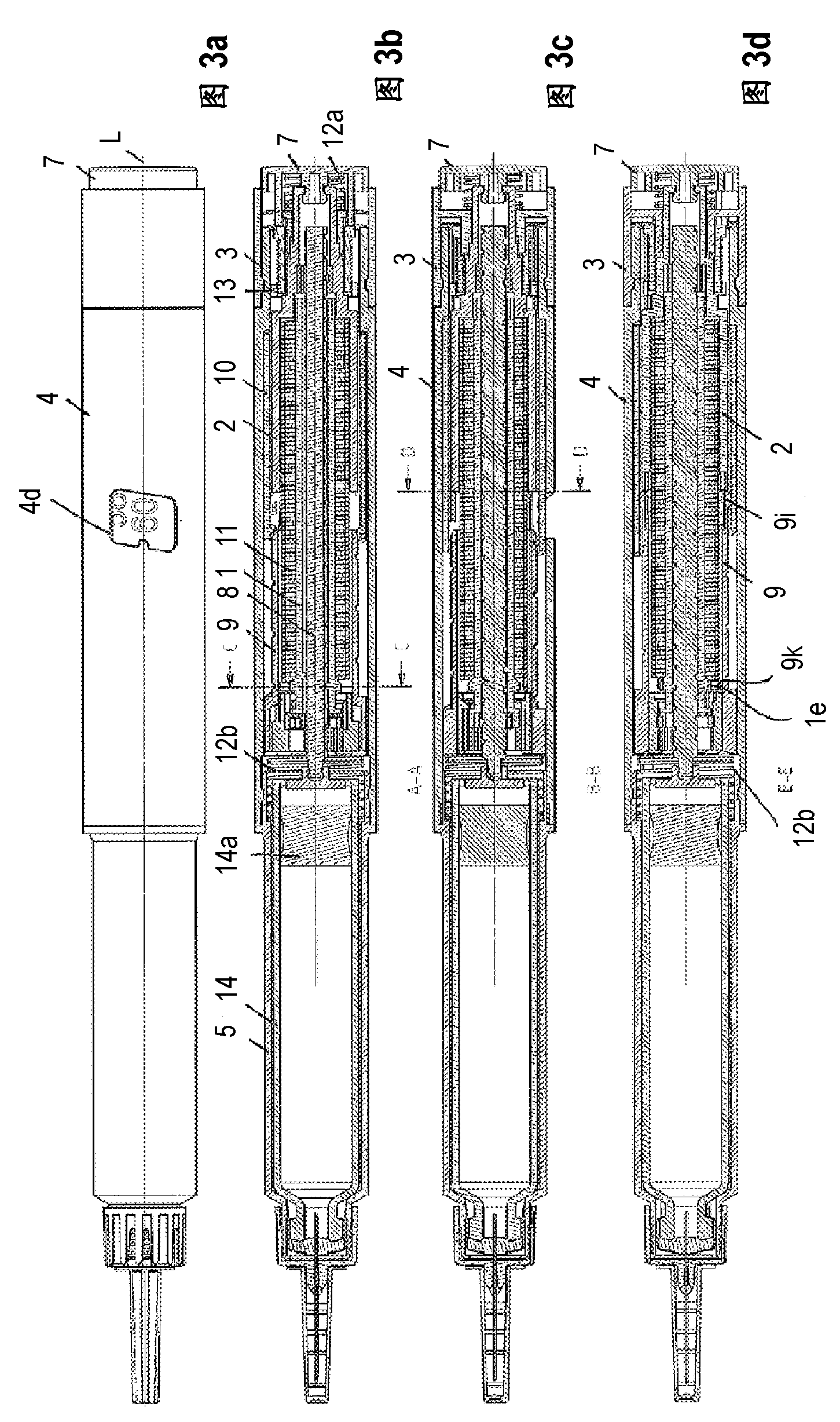
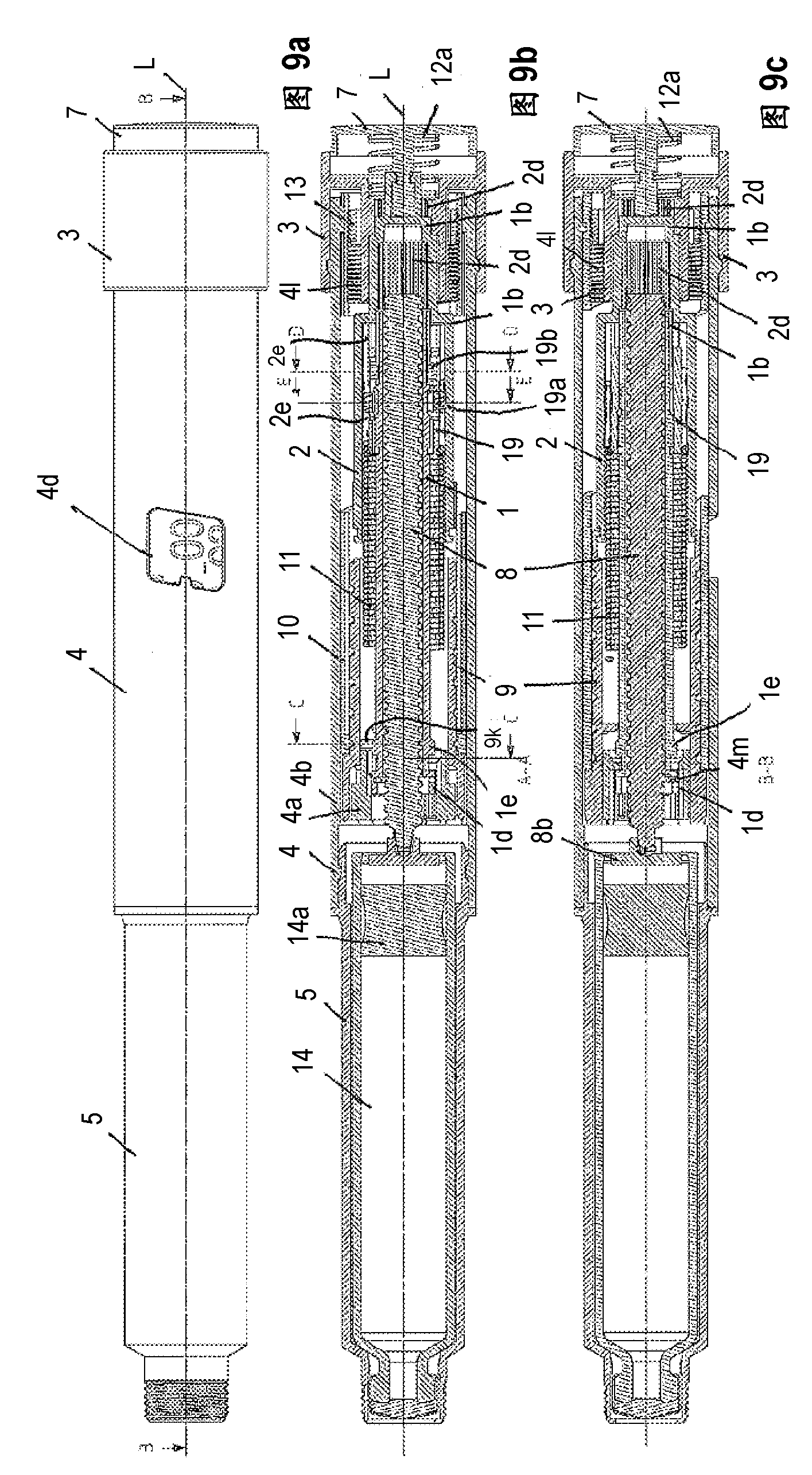
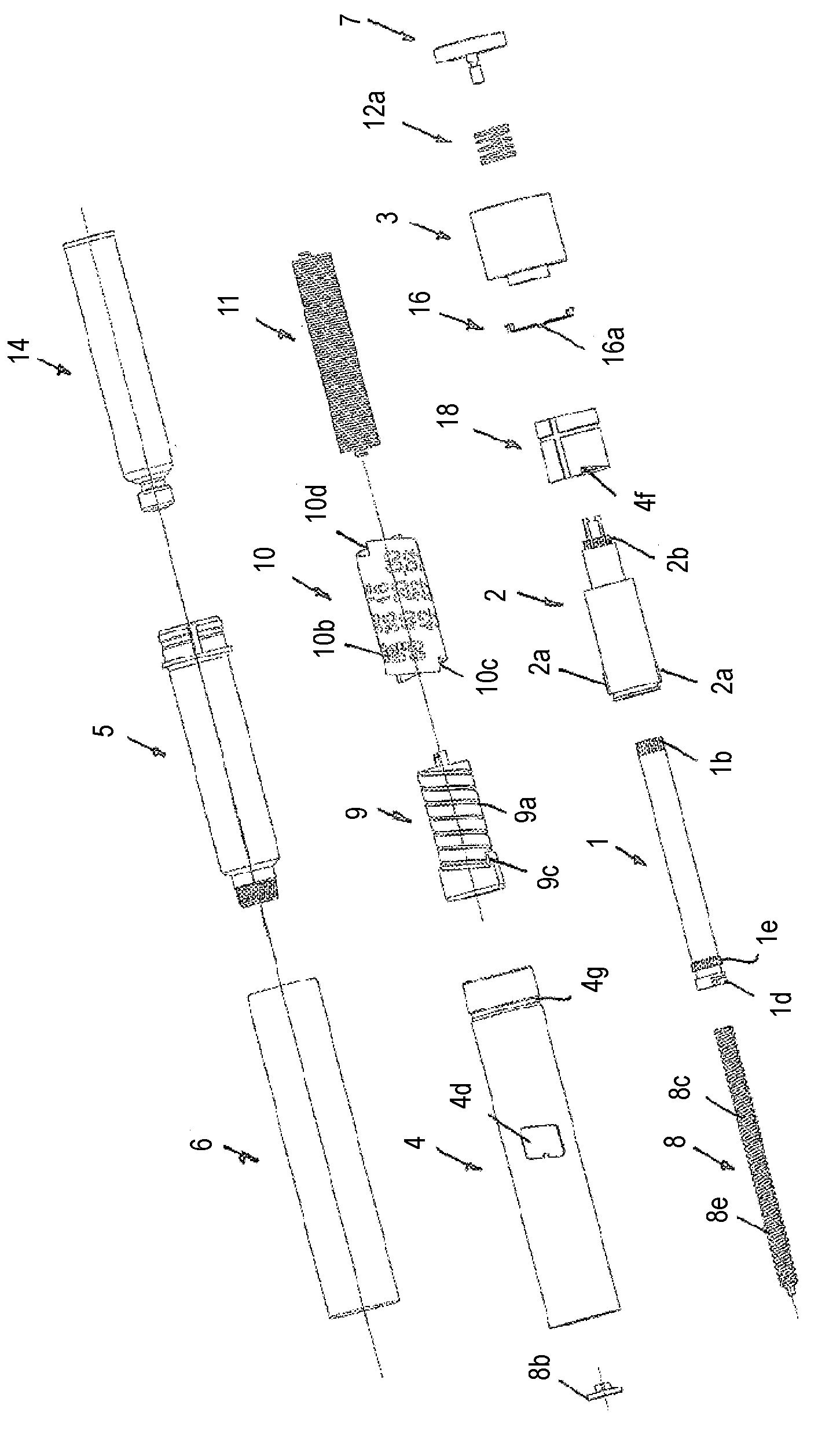
#CMT# # / CMT# The driving- and dosing device has a housing (4), a dosing indicator (10), a dosing scale (10b) arranged over the periphery of the dosing indicator, a pointing unit (4d) and a dosing element (3) tangible by the user of the driving- and dosing device. The dosing indicator is rotatable, particularly screwable relative to the pointing unit and around a rotational axis for adjusting the dosage by the rotation of the dosing element relative to the pointing unit. A value of the dosing scale is readable by the pointing unit, which corresponds the adjusted dosage. #CMT# : # / CMT# A dispensing spring (11) stores the energy required for dispensing the product. #CMT#USE : # / CMT# Driving- and dosing device for an injection device for application of a fluid product, particularly a medicament, such as insulin for diabetes therapy. #CMT#ADVANTAGE : # / CMT# The dosing indicator is rotatable, particularly screwable relative to the pointing unit and around a rotational axis for adjusting the dosage by the rotation of the dosing element relative to the pointing unit, and hence ensures improved and accurate dosing of the medicament. #CMT#DESCRIPTION OF DRAWINGS : # / CMT# The drawing shows a schematic exploded view of a driving- and dosing device. 3 : Dosing element 4 : Housing 4d : Pointing unit 10 : Dosing indicator 10b : Dosing scale 11 : Dispensing spring.
Owner:YPSOMED AG
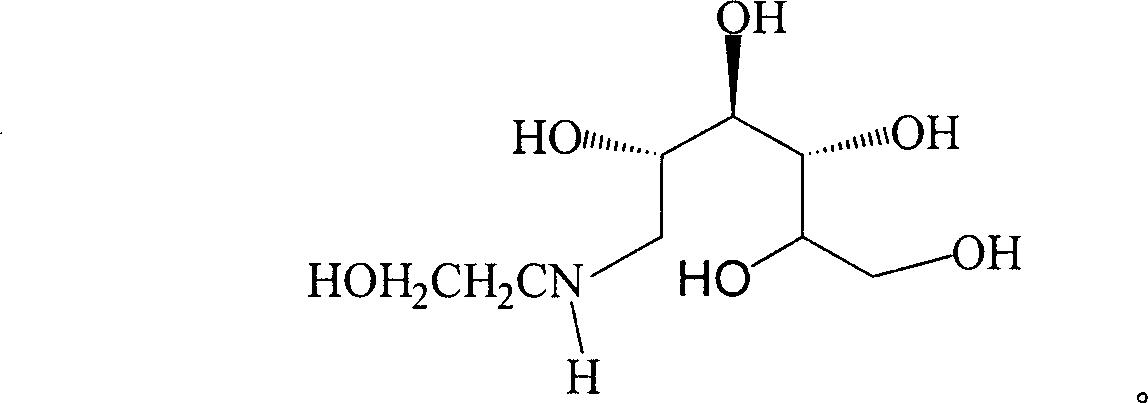
Method for synthesizing N- (2-hydroxyethyl)-glucosamine
InactiveCN1611485AMild reaction conditionsImprove responseOrganic compound preparationAmino-hyroxy compound preparationDiabetes TherapyMiglitol
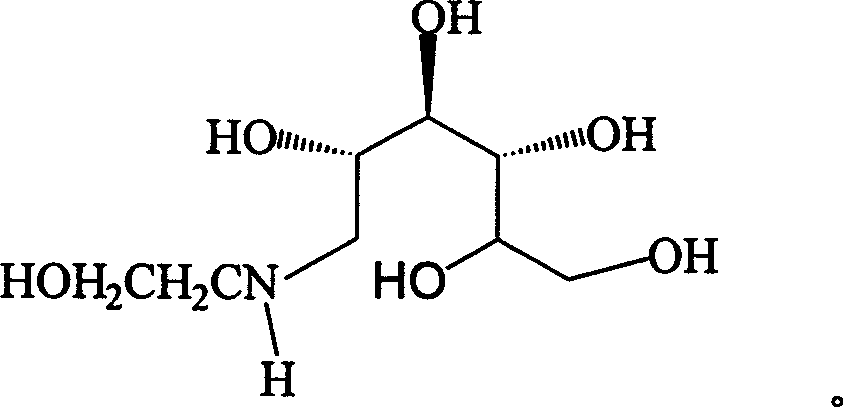
The invention relates to a kind of method for synthesizing midbody N-(2-ethoxyl)-glucosamine of diabetes therapy medicine miglitol alcohol. The existing literature has no synthesizing method recordation of midbody N-(2-ethoxyl)-glucosamine. The reaction steps of the invention are as follows: A) Stuff casting: in autoclave, add D-glucose and ethanolamine according to molar ratio of D-glucose / ethanolamine with 1 / 1.05-1.10; then add 70-95% alcohols solvent with 5-10 times weight of glucose; and after nitrogen displace, add 5%Pd-CaCO3-1-5%Pb catalyst with 2-10% weight of glucose; inject high purity hydrogen, keep hydrogen pressure to 2-3Mpa, and then intensify the temperature slowly up to reaction temperature; B) Reaction: control reaction temperature in 35-45deg.C, hydrogen pressure in 2-4Mpa, and reaction by 20-24 hours; C) Get white pulverous solid by after-treatment. The invention selects the appropriate catalyst and reasonable hydrogenization technology, so that it can make the reaction condition of glucose and ethanolamine with relatively gentleness, make the reaction easy to proceed, make by-product and other impurity generated badly, and also it has high conversion rate, and more than or equal to 98% purity.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
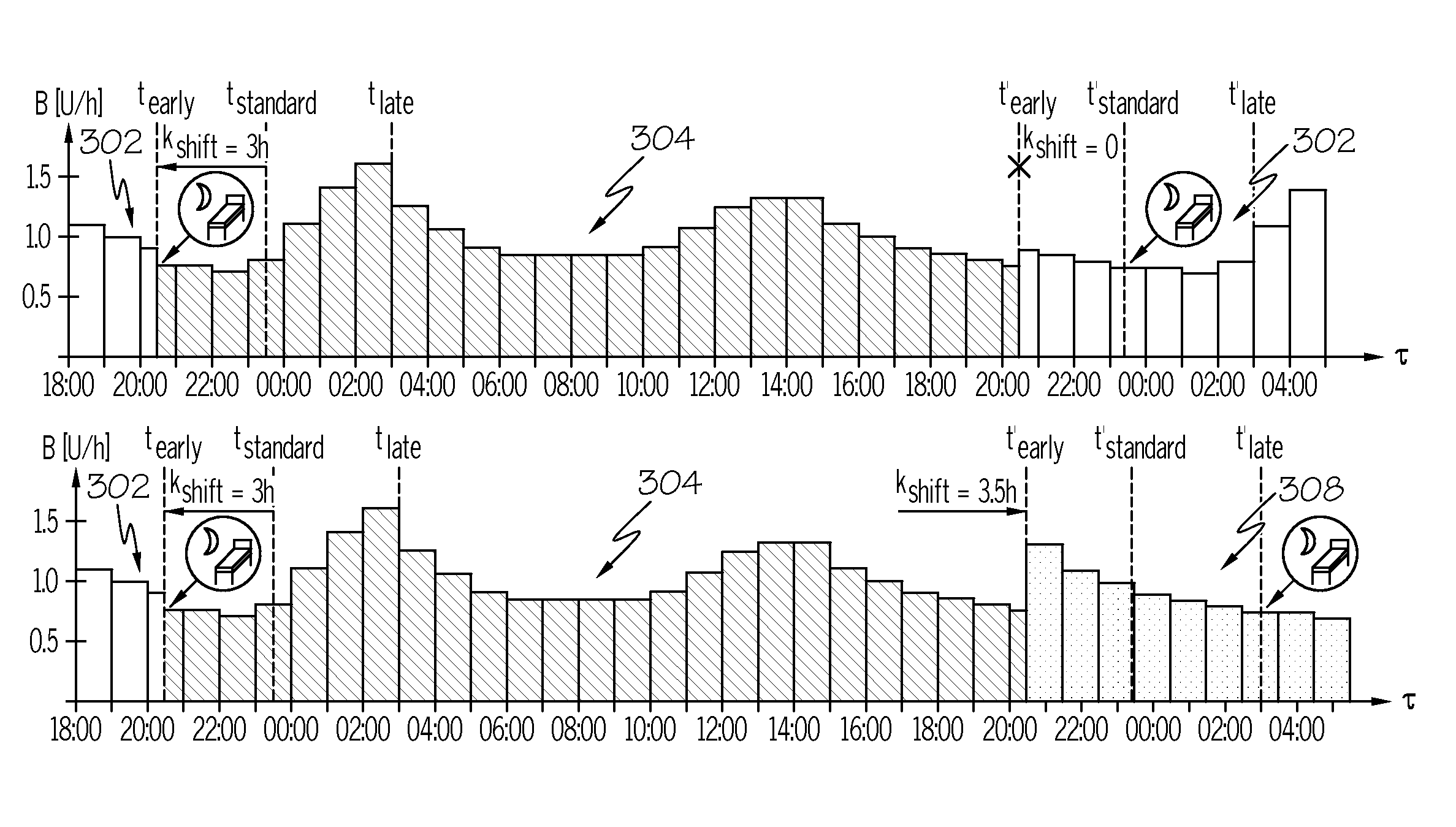
Diabetes therapy device enabling shifting of parameter profiles
A diabetes therapy device is disclosed herein, which includes a profile memory storing a parameter profile and a dedicated reference time mark. The parameter profile defines at least one parameter that is associated with insulin administration as a function of time for a generally circadian chronobiological cycle. The reference time mark indicates the beginning of the circadian chronobiological cycle and initializes a running time. Insulin amounts to be administered are determined as a function of the running time in accordance with a current matching of the parameter profile. A modified matching based on the reference time mark and an upcoming trigger time is computed and is time-shifted from the current matching, wherein the modified matching is applied by making the modified matching the current matching, such that future insulin amounts to be administered are determined in accordance with the modified matching.
Owner:ROCHE DIABETES CARE INC
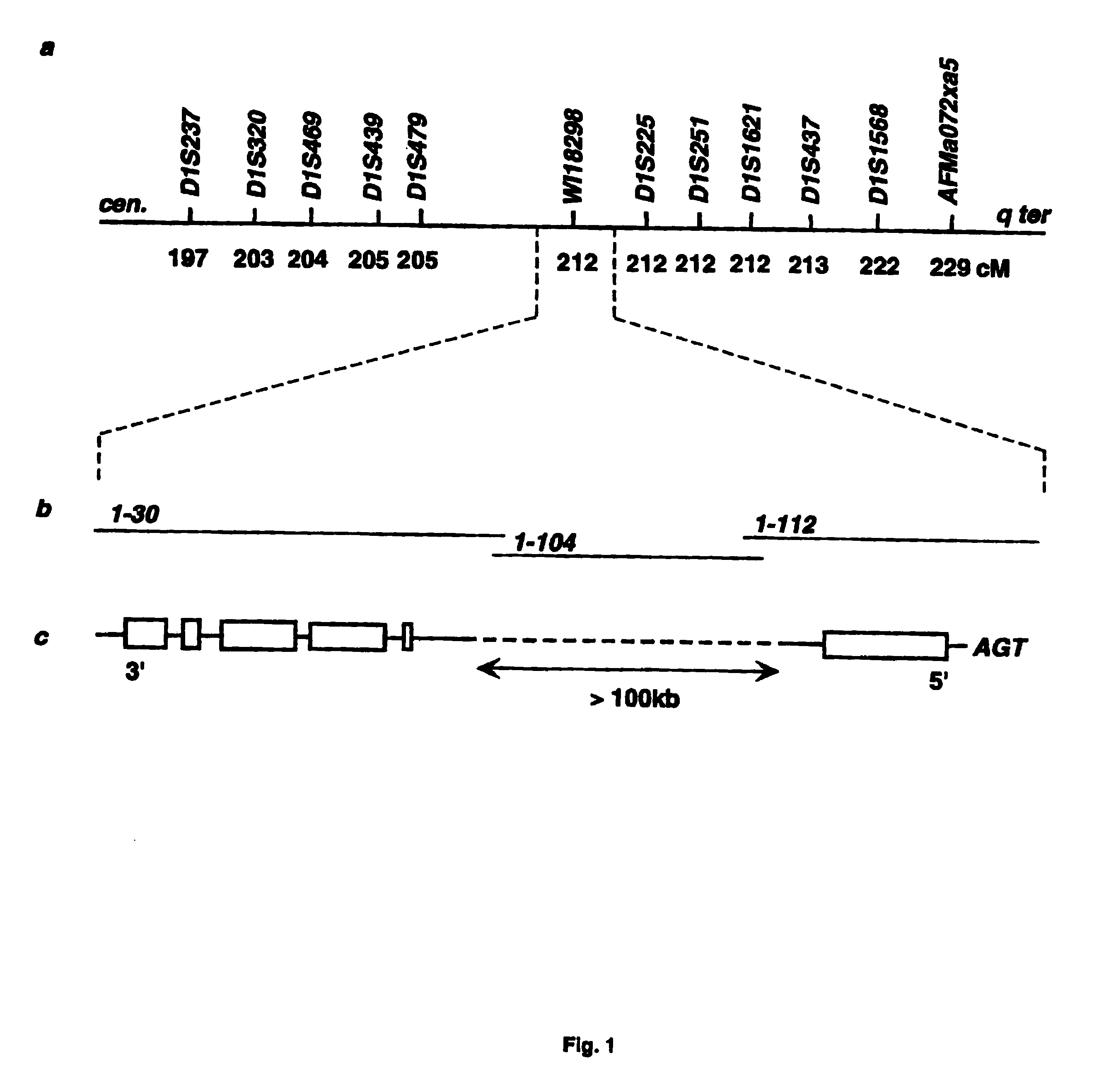
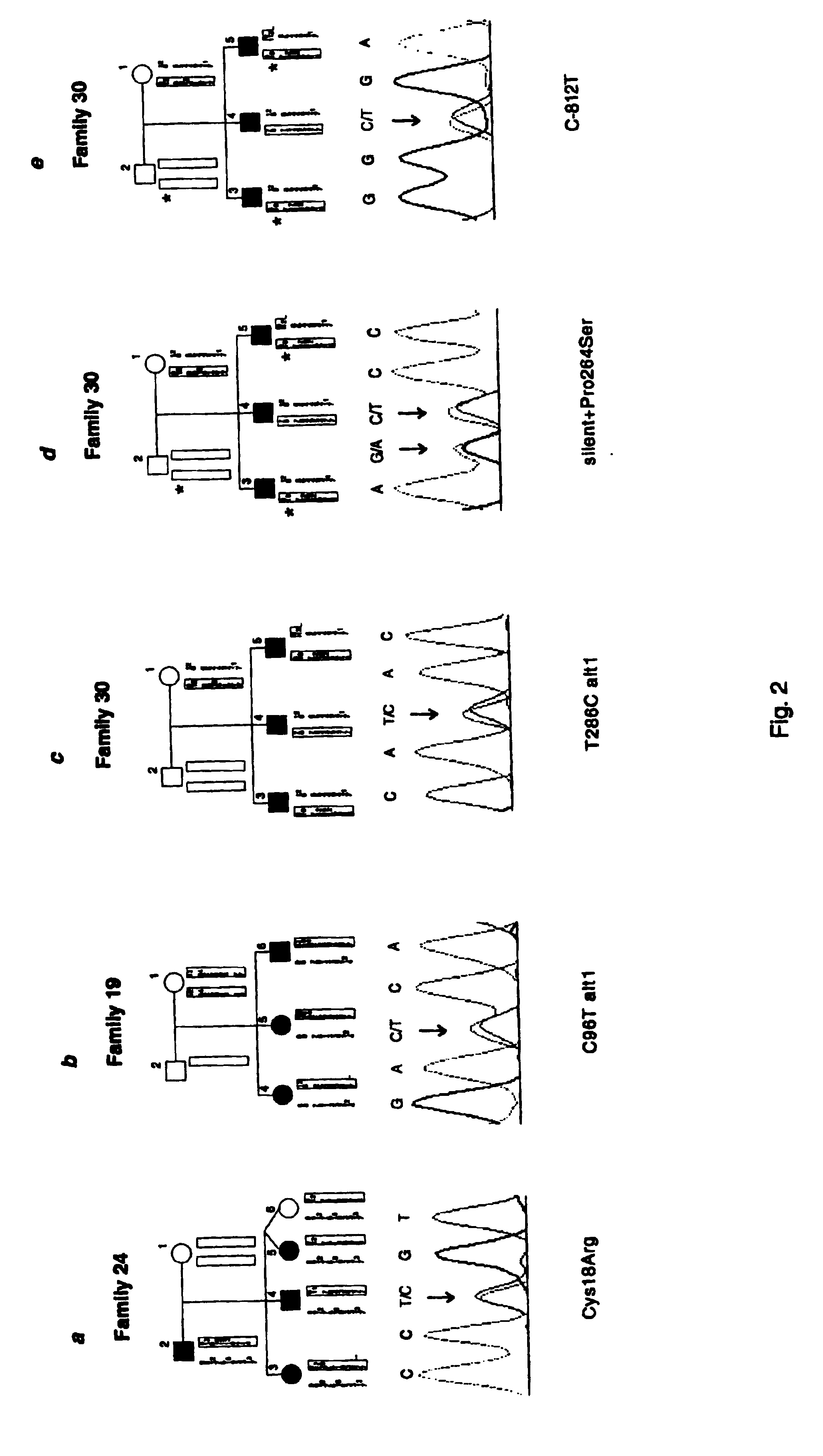
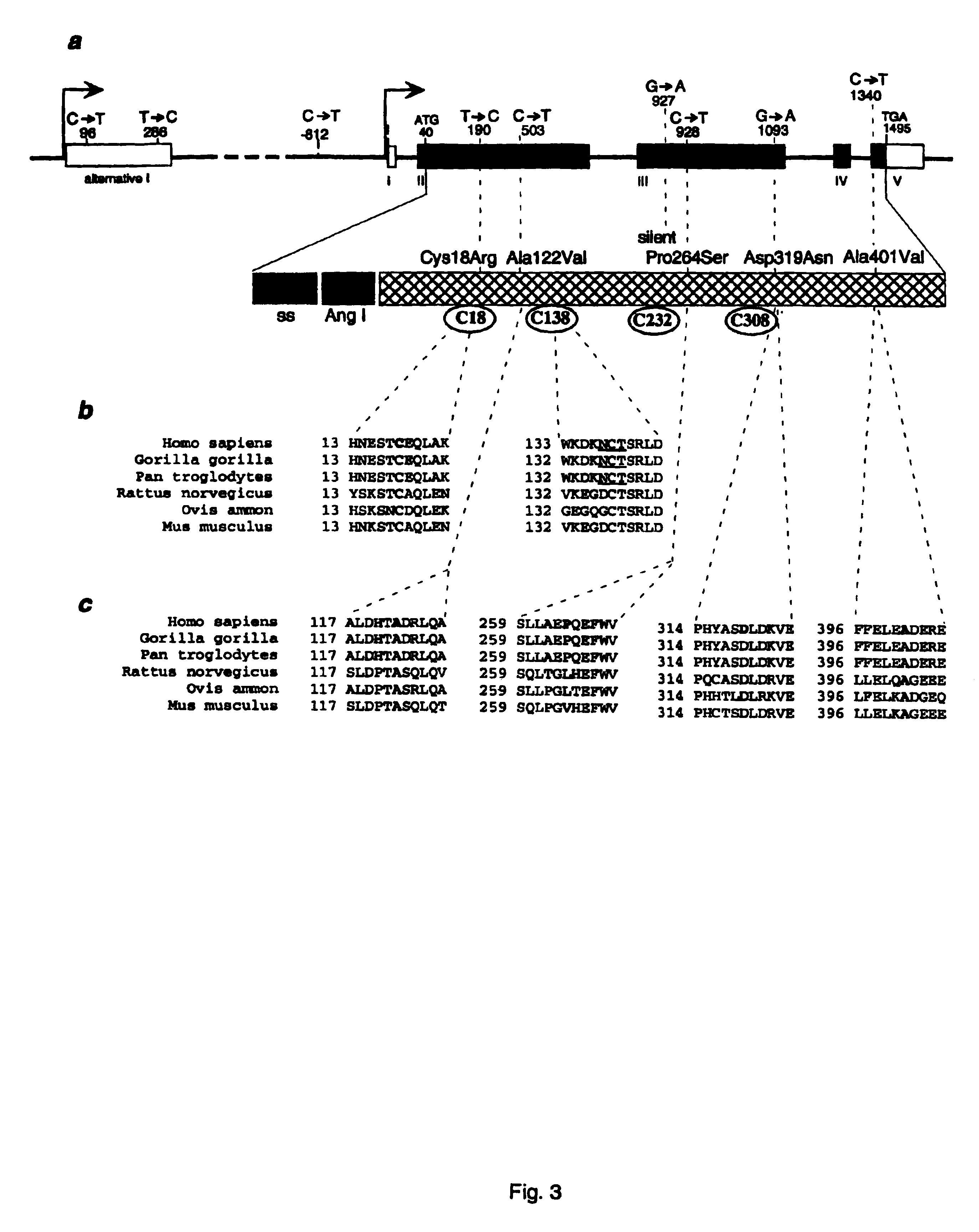
Diabetes gene
InactiveUS6902888B1Preventing and treating diabetesReduce and prevent gene expressionSugar derivativesPeptide/protein ingredientsInsulin dependent diabetesDiabetes Therapy
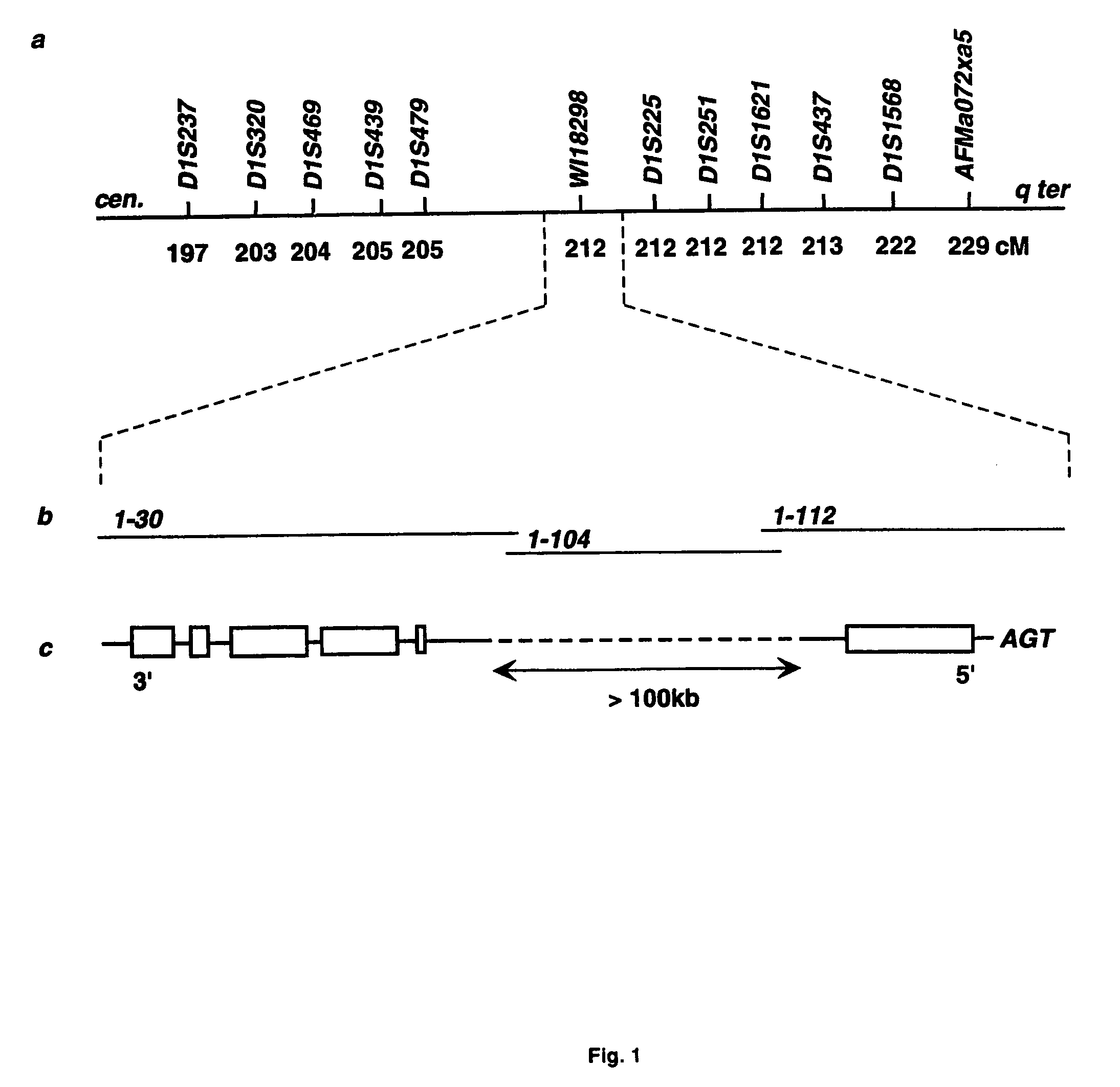
The present invention relates generally to the field of human genetics. Specifically, the present invention relates to methods and materials used to isolate and detect human diabetes mellitus predisposing gene, specifically the angiotensinogen (AGT) gene, some mutant alleles of which cause susceptibility to insulin-dependent diabetes mellitus (IDDM). More specifically, the invention relates to gernline mutations in the AGT gene and their use in the diagnosis of predisposition to diabetes. The invention also relates to the prophylaxis and / or therapy of diabetes associated with a mutation in the AGT gene. The invention further relates to the screening of drugs for diabetes therapy. Finally, the invention relates to the screening of the AGT gene for mutations, which are useful for diagnosing the predisposition to diabetes.
Owner:MYRIAD GENETICS
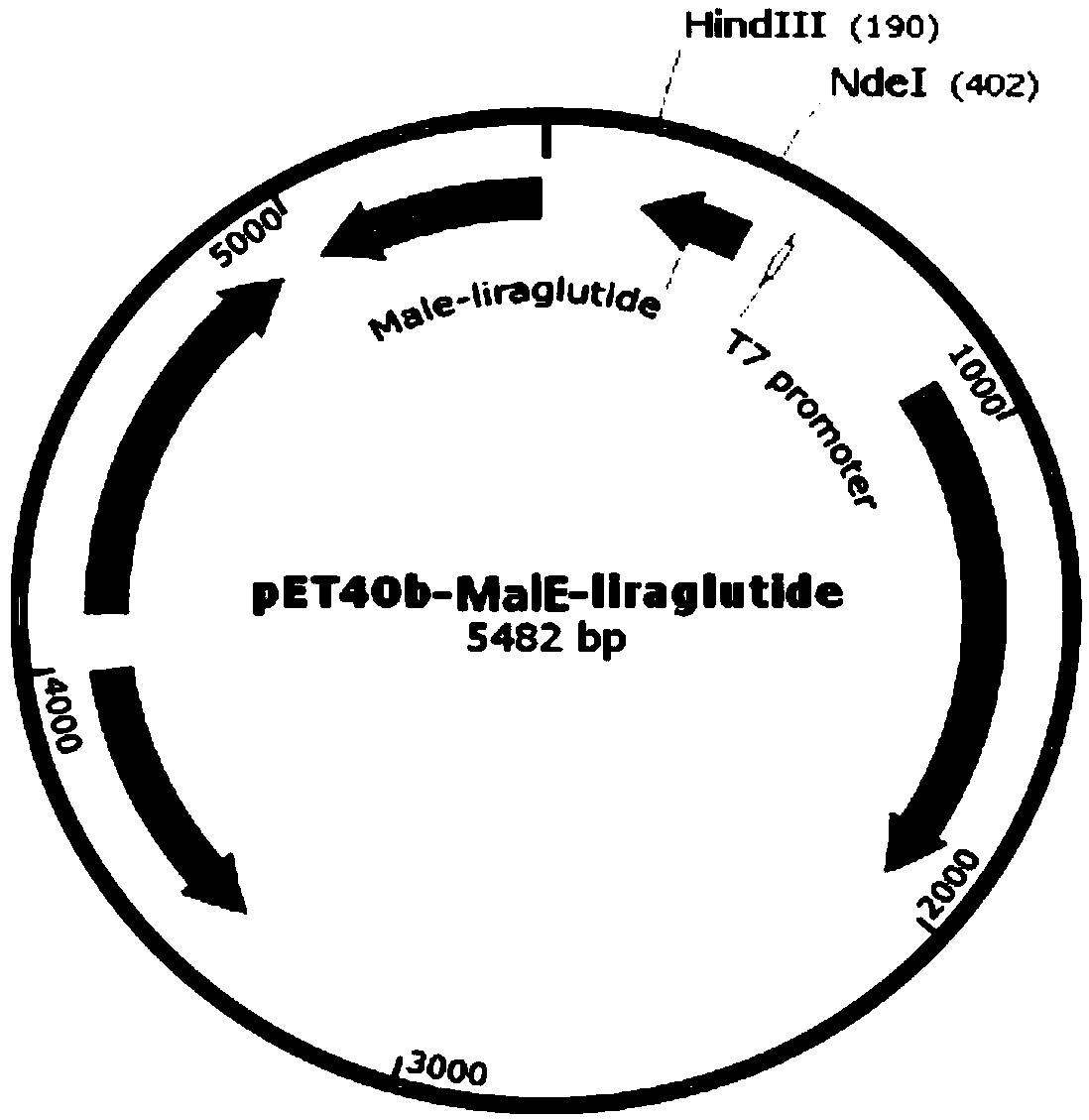
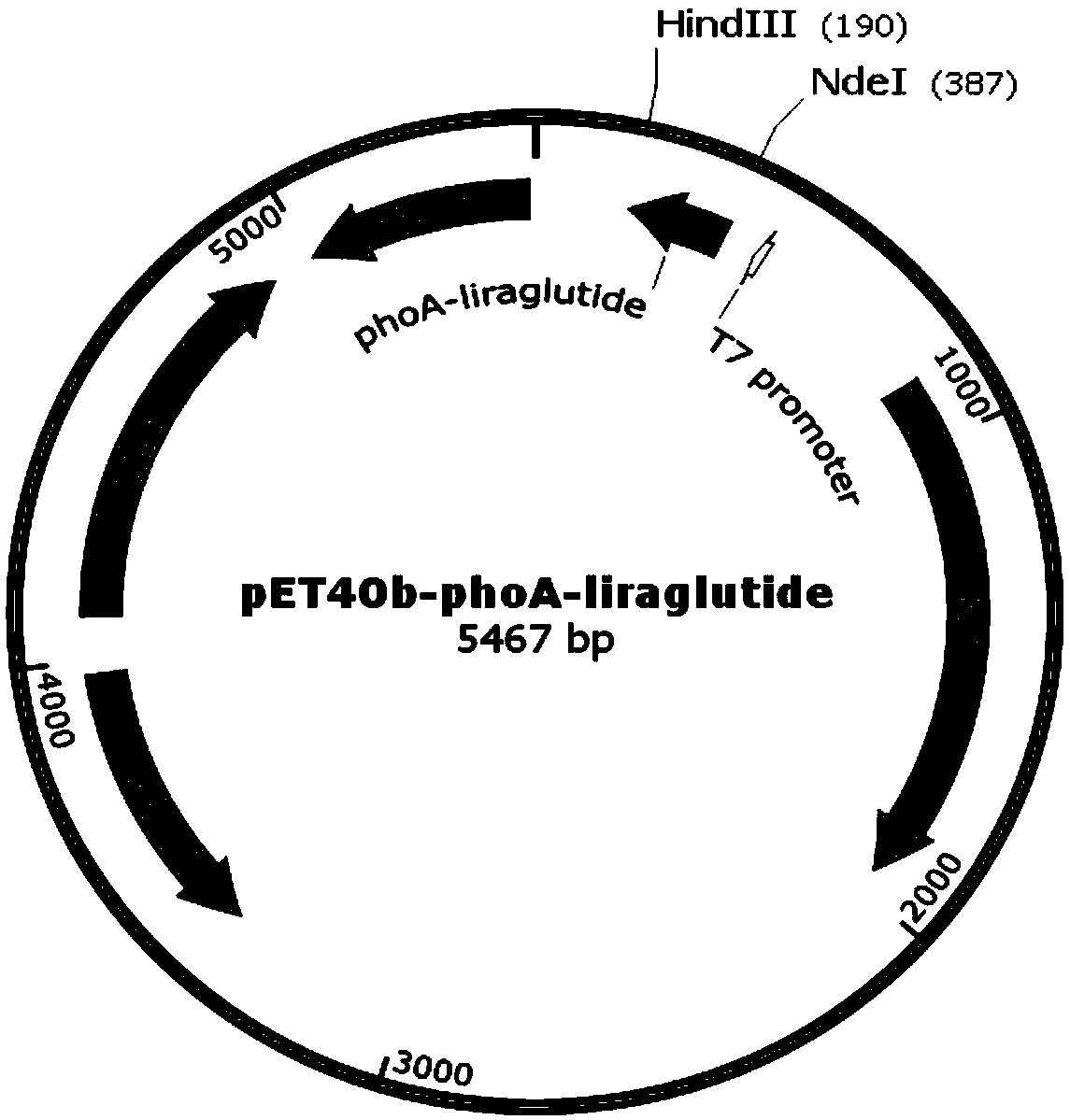
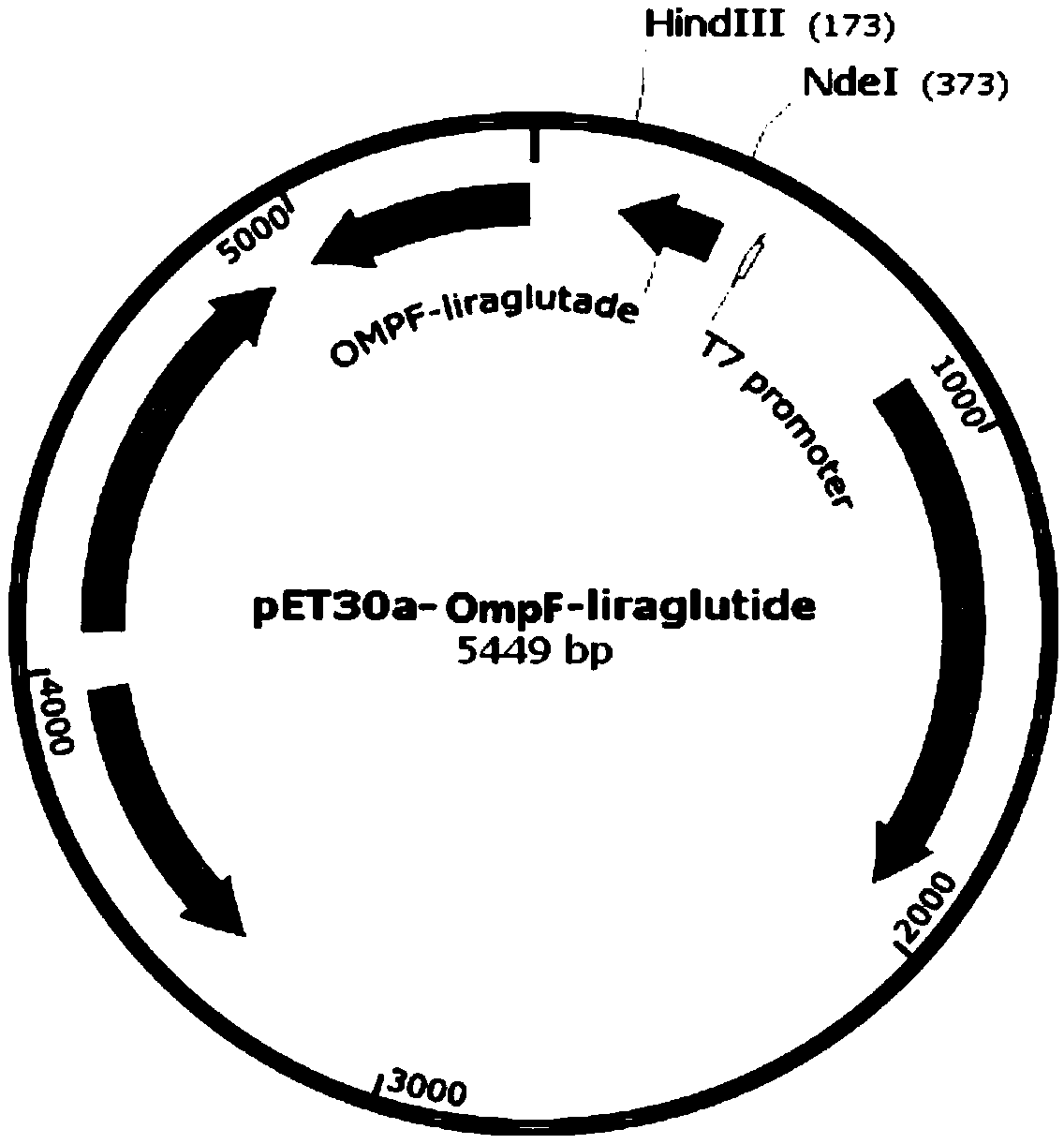
Recombinant engineering bacterium capable of efficiently expressing liraglutide precursor and application of recombinant engineering bacterium
ActiveCN110724187AHigh purityImprove stabilityBacteriaMicroorganism based processesDiabetes TherapyFusion Protein Expression
The invention provides a recombinant engineering bacterium for efficiently expressing a liraglutide precursor and an application of the recombinant engineering bacterium. According to the invention, asignal peptide and an enterokinase restriction enzyme cutting site are designed at the N end of a liraglutide precursor molecule Arg34G LP-1 (7-37), the C end is connected with termination codon, a target gene is formed, then, the target gene is inserted between two restriction enzyme cutting sites of an expression vector; the recombinant engineering bacterium for expressing the liraglutide precursor is constructed, the engineering bacterium is subjected to high-density fermentation culture and is expressed in the form of an inclusion body of a fusion protein, the expression quantity of the recombinant fusion protein is high, the recombinant fusion protein accounts for about 25%-35% of the total protein of the bacterium, and the expression quantity of the inclusion body of a target protein reaches 15-20g / L. The inclusion body is low in impurity protein content, beneficial to separation and purification, and high in purification efficiency and good in stability, the production cost isgreatly reduced, the production efficiency is improved, and the inclusion body has the good application prospect in the field of diabetes treatment medicine preparation.
Owner:GAN&LEE PHARMA
Multiple agent diabetes therapy
InactiveUS20060074013A1Organic active ingredientsPeptide/protein ingredientsDiabetes TherapyActrapid insulin
A pharmaceutical composition includes at least two of agents I)-iii), wherein agent i) is selected from the group consisting of an insulin, an insulin analog, a physiologically active fragment of said insulin and a physiologically active fragment of said insulin analog, agent ii) is selected from the group consisting of an insulin-related peptide, an insulin-related peptide analog, a physiologically active insulin-related peptide fragment and a physiologically active insulin-related peptide analog fragment, and agent iii) is an insulin sensitizer.
Owner:MINIMED
Type-2 Diabetes Combination Wafer
InactiveUS20090274732A1Increase intakeImprove complianceBiocideMetabolism disorderDiabetes TherapySulfonylurea
Rapidly disintegrating oral dosage forms for the application of active agent combinations for diabetes therapy. The dosage forms contain at least two active agents suitable for treating type-2 diabetes. The antidiabetic active agents are selected from the group comprising sulfonylureas, glitazones, glinides, biguanides, and absorption-delaying agents. The use of the active agent combination to produce an oral dosage form for the treatment of diabetes, a method for the therapeutic treatment of diabetes, and a method for the production of a sheet-like dosage form are also disclosed.
Owner:LTS LOHMANN THERAPIE-SYST AG
Dietary supplement comprising alpha keto acids for supporting diabetes therapy
InactiveCN102448452AIncreased sensitivityMetabolism disorderPharmaceutical delivery mechanismDiabetes TherapyAlpha-ketoisocaproate
The invention relates to a dietary supplement comprising alpha keto acids for supporting diabetes therapy. The invention relates to a preparation used as a dietary supplement comprising alpha keto acids for supporting therapy of diabetes mellitus type II (DM). The preparation comprises at least one of the alpha keto acids from the group alpha ketoisocaproate (KIC), alpha ketoisovalerate (KIV), alpha keto beta methylvalerate (KMV) and alpha keto glutarate (AKG).
Owner:EVONIK DEGUSSA GMBH
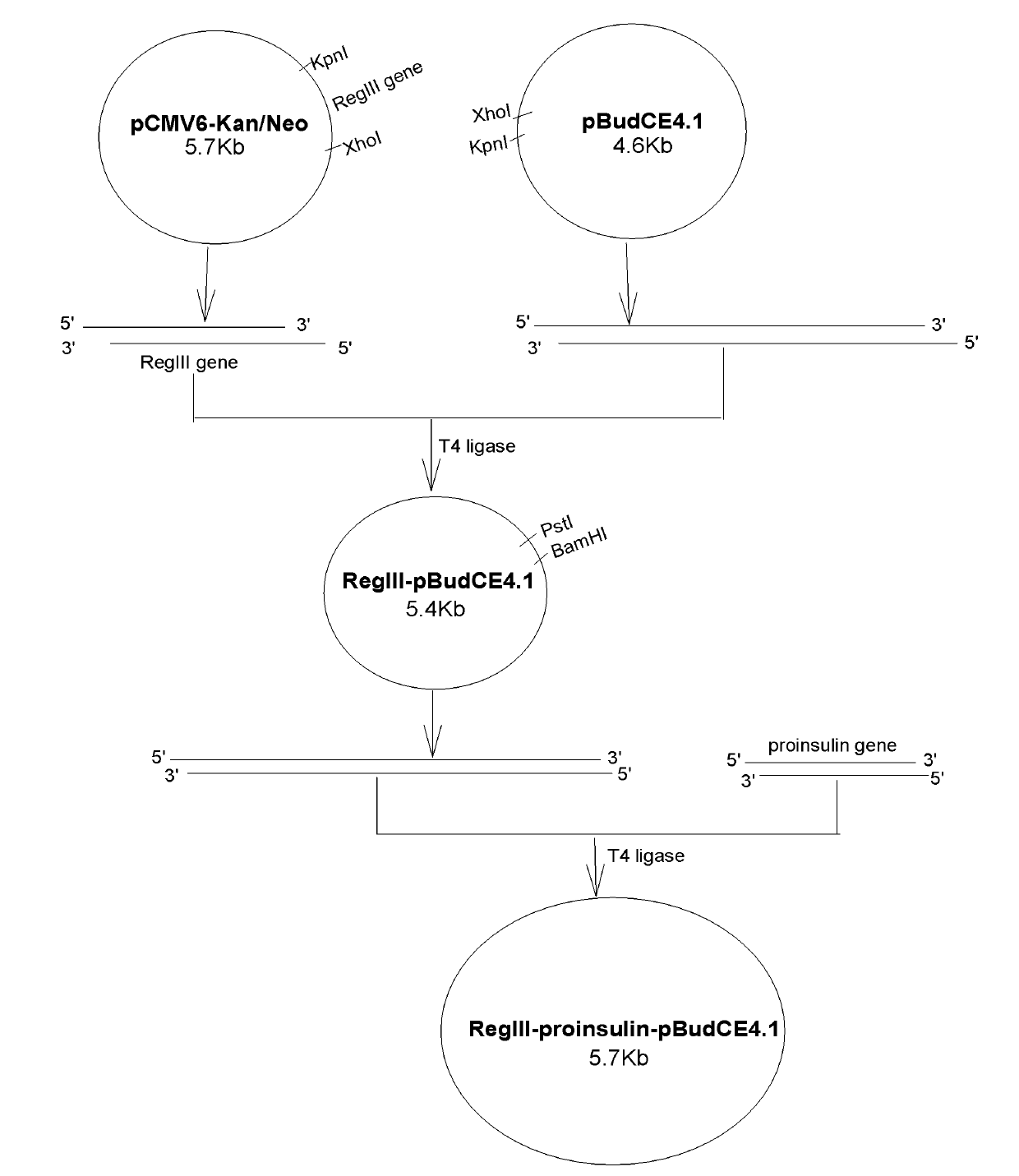
Reg III/proinsulin double-gene plasmid as well as building method and application thereof
InactiveCN101768600AGood treatment effectInhibit proliferationMetabolism disorderGenetic material ingredientsPancreatic structureCytomegalovirus disease
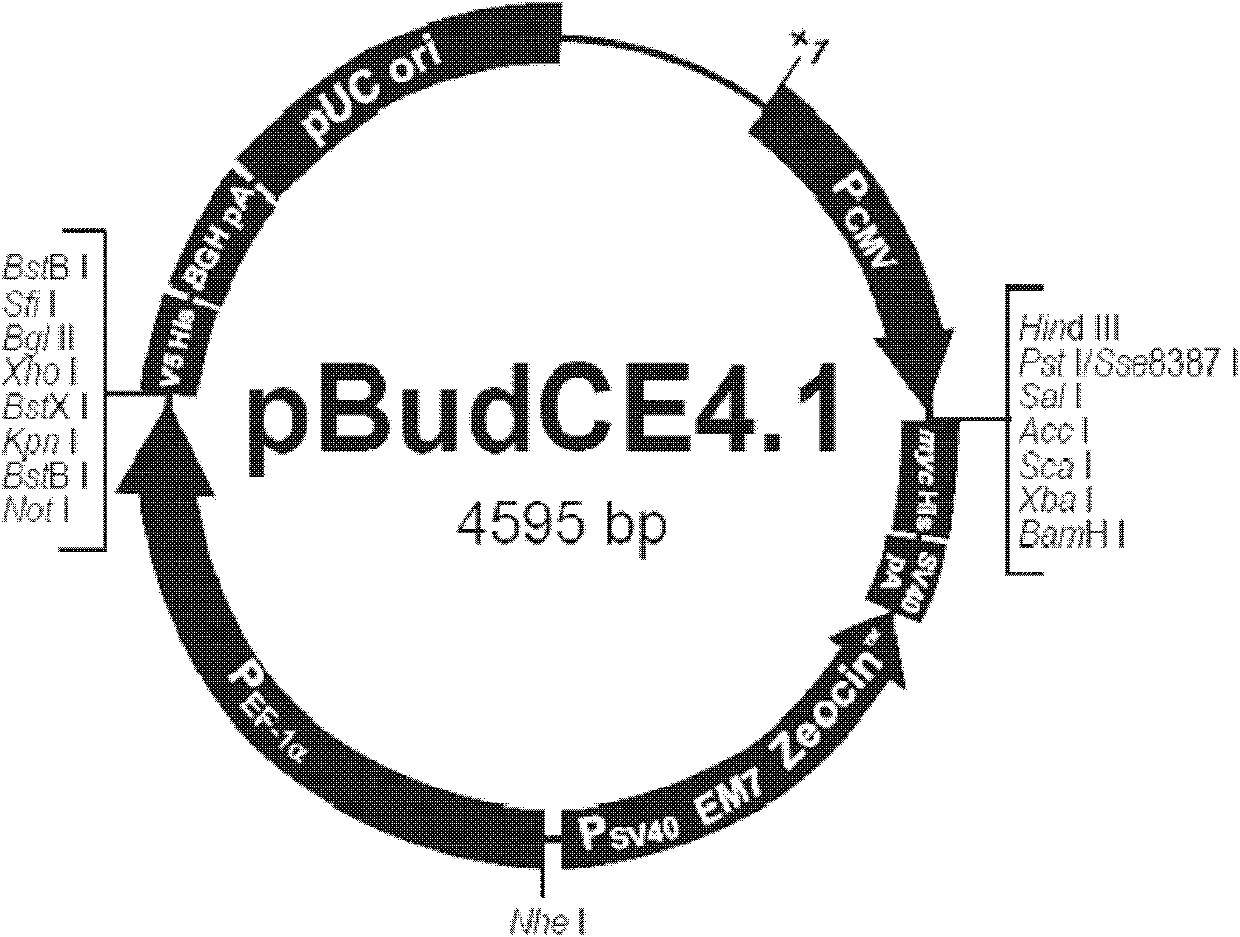
The invention provides a plasmid for treating 1 type diabetes, which is a double-gene eukaryon co-expression plasmid built by a gene recombination method and containing a pancreas islet beta cell regeneration (Reg) III gene and a proinsulin gene, wherein the Reg III gene and the proinsulin gene can be respectively expressed under the induction of an EF-1alpha promoter and a cytomegalovirus (CMV) promoter of a pBudCE4.1 plasmid vector. The Reg III / proinsulin double-gene eukaryon co-expression plasmid can play a hypoglycemic role by recovering the autoimmunity tolerance state of in the body of a 1 type diabetes patient and promoting the regeneration of beta cells.
Owner:HUAZHONG UNIV OF SCI & TECH
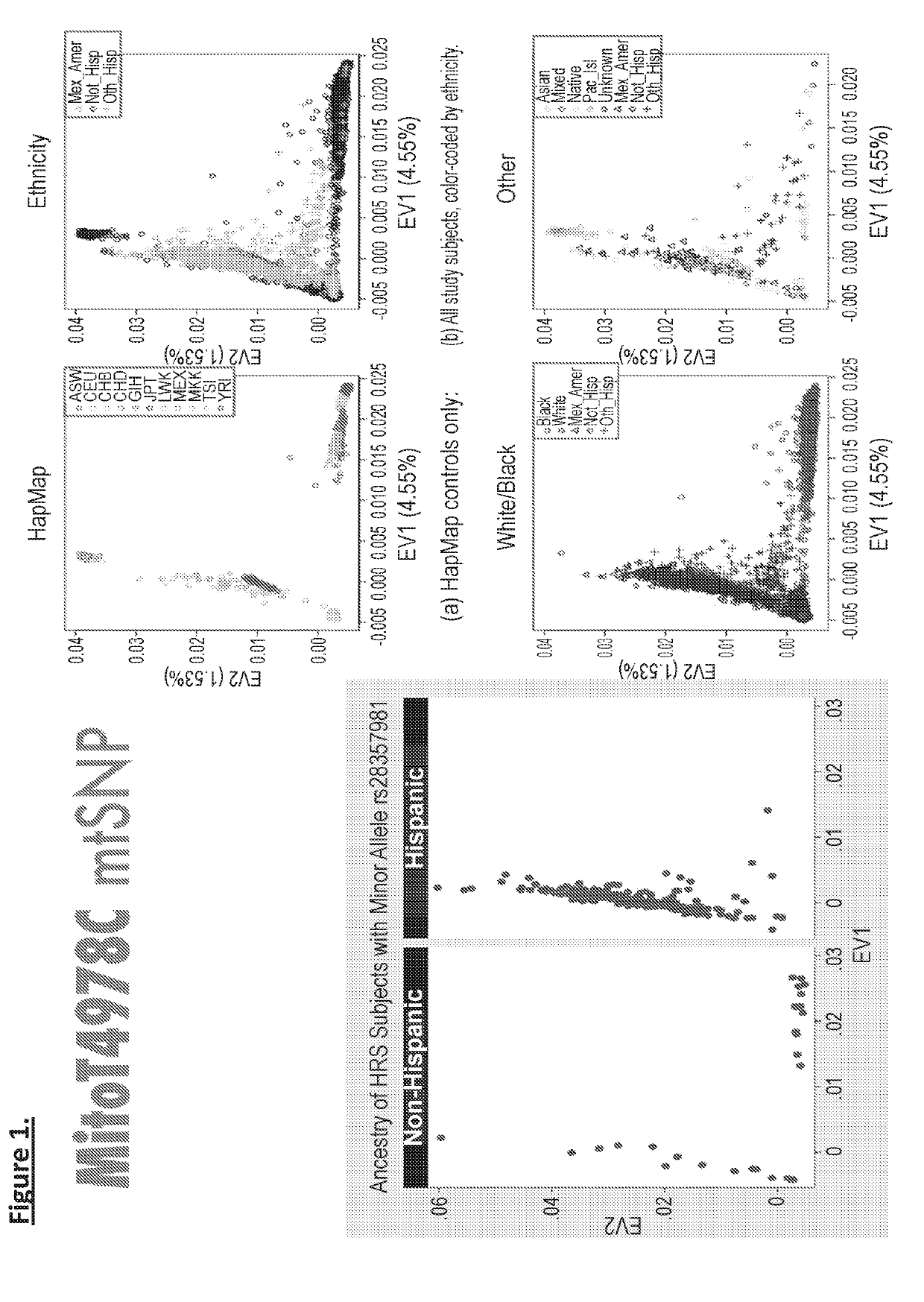
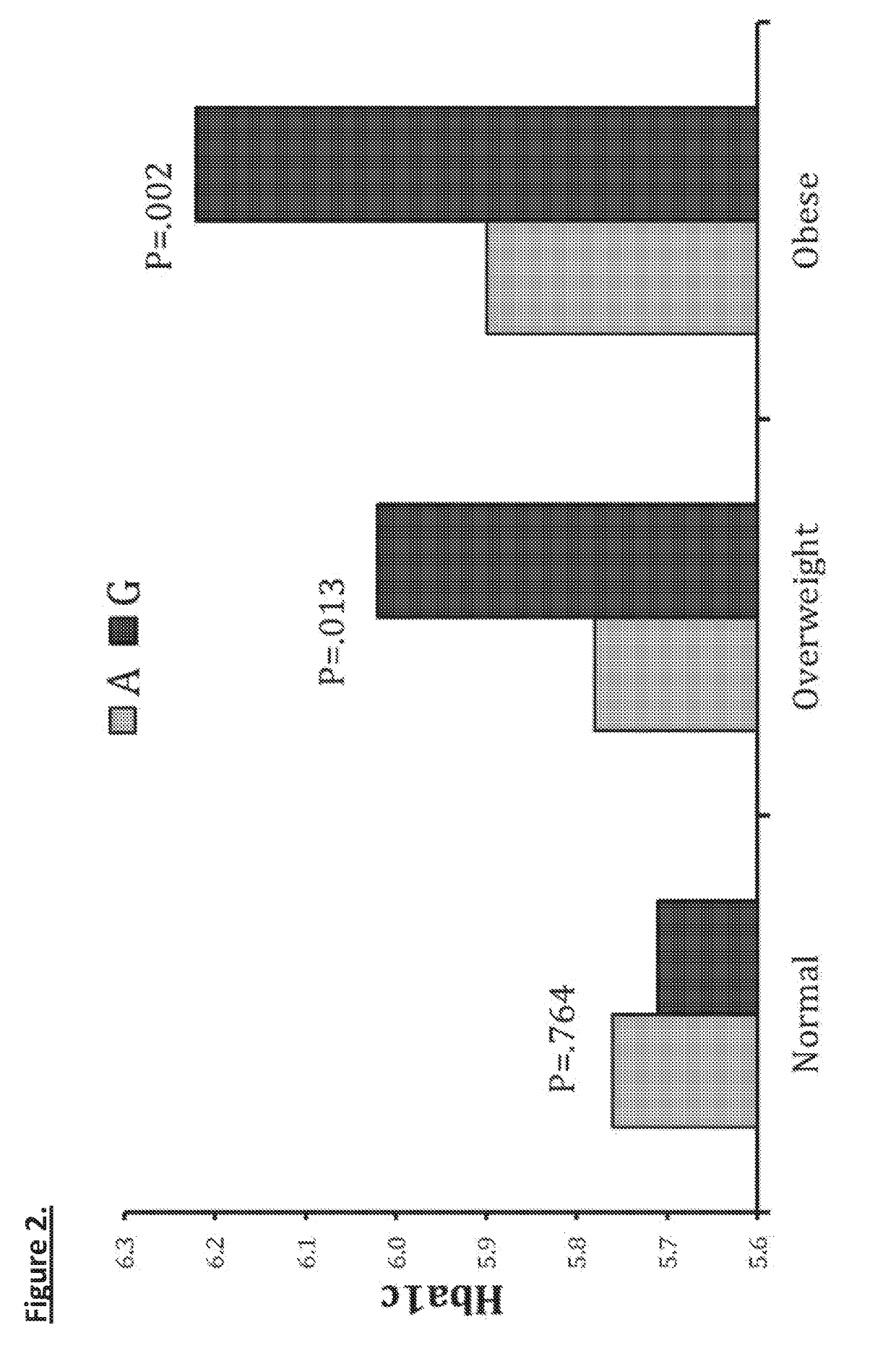
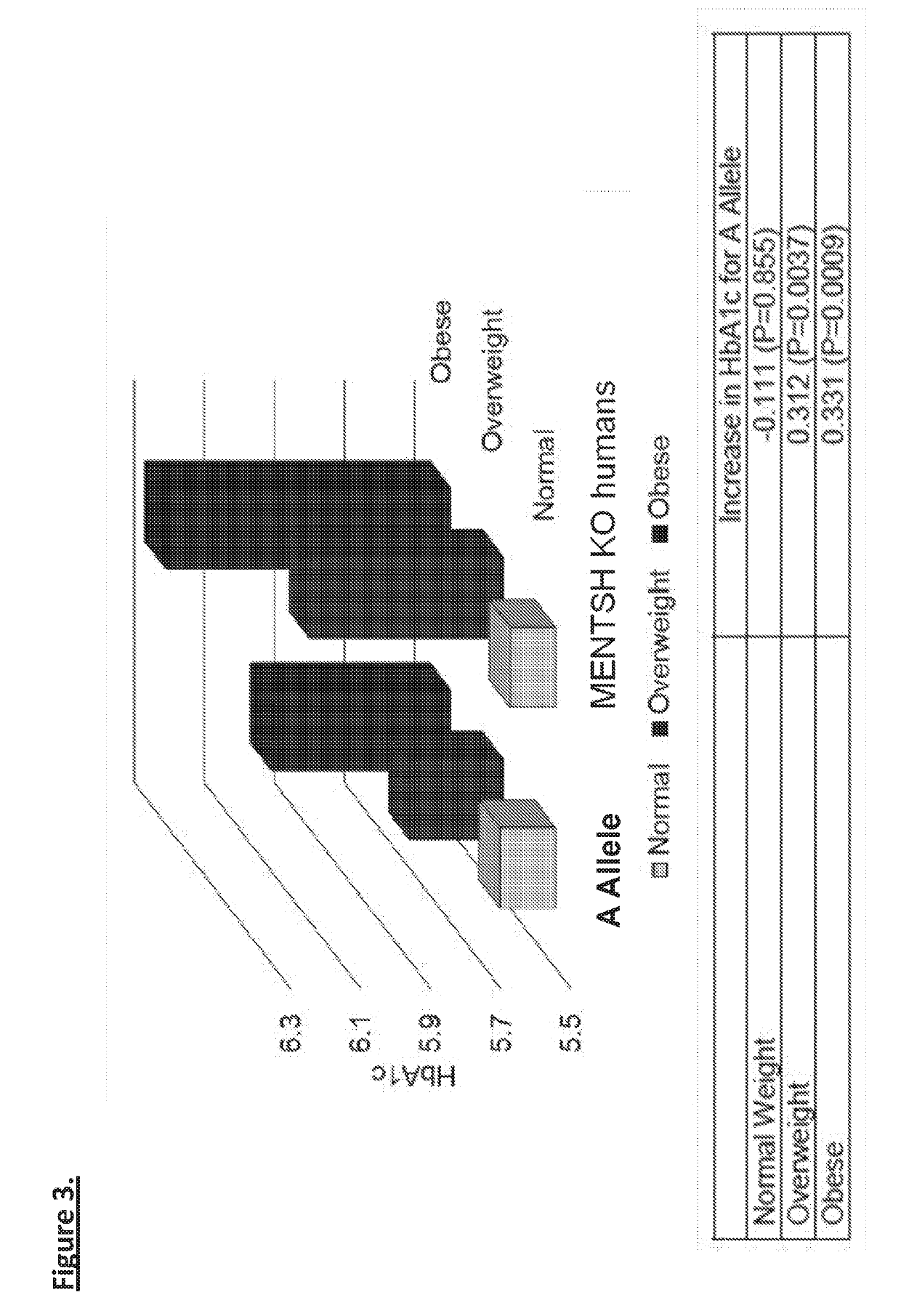
Mentsh analogs as therapeutics for diabetes, obesity, and their associated diseases and complications
ActiveUS20190194275A1Maximum resultImprove the increase effectSenses disorderPeptide/protein ingredientsDiseaseDiabetes Therapy
Described herein is a novel, mitochondrial encoded, open reading frame, that leads to the production of a new mitochondrial peptide. Residing within the ND-Two subunit, a specific small nucleotide polymorphism disrupts expression of this mitochondrial peptide, and is correlated with an increase in obesity and diabetes, particularly in certain ethnic populations. In vitro administration of the peptide increases insulin secretion, decreases fat accumulation and improves glucose uptake in muscle cell. Antibodies generated against the peptide can be used for detecting peptide deficiency, in addition to SNP detection, supporting diagnostic approaches. In vivo studies further revealed that administration of the peptide improves glucose tolerance, thereby providing a new therapeutic avenue for a novel diabetes therapy and decreases bodyweight, thus serving as a novel obesity therapy. Generation of synthetic analogs further enhance or abrogated activity relative to the natural peptide.
Owner:UNIV OF SOUTHERN CALIFORNIA
Diabetes therapy device enabling shifting of parameter profiles
A diabetes therapy device is disclosed herein, which includes a profile memory storing a parameter profile and a dedicated reference time mark. The parameter profile defines at least one parameter that is associated with insulin administration as a function of time for a generally circadian chronobiological cycle. The reference time mark indicates the beginning of the circadian chronobiological cycle and initializes a running time. Insulin amounts to be administered are determined as a function of the running time in accordance with a current matching of the parameter profile. A modified matching based on the reference time mark and an upcoming trigger time is computed and is time-shifted from the current matching, wherein the modified matching is applied by making the modified matching the current matching, such that future insulin amounts to be administered are determined in accordance with the modified matching.
Owner:ROCHE DIABETES CARE INC
Diabetes gene
InactiveUS7374884B2Preventing and treating diabetesImprove disease symptomsSugar derivativesPeptide/protein ingredientsInsulin dependent diabetesDiabetes Therapy
The present invention relates generally to the field of human genetics. Specifically, the present invention relates to methods and materials used to isolate and detect human diabetes mellitus predisposing gene, specifically the angiotensinogen (AGT) gene, some mutant alleles of which cause susceptibility to insulin-dependent diabetes mellitus (IDDM). More specifically, the invention relates to germline mutations in the AGT gene and their use in the diagnosis of predisposition to diabetes. The invention also relates to the prophylaxis and / or therapy of diabetes associated with a mutation in the AGT gene. The invention further relates to the screening of drugs for diabetes therapy. Finally, the invention relates to the screening of the AGT gene for mutations, which are useful for diagnosing the predisposition to diabetes.
Owner:MYRIAD GENETICS
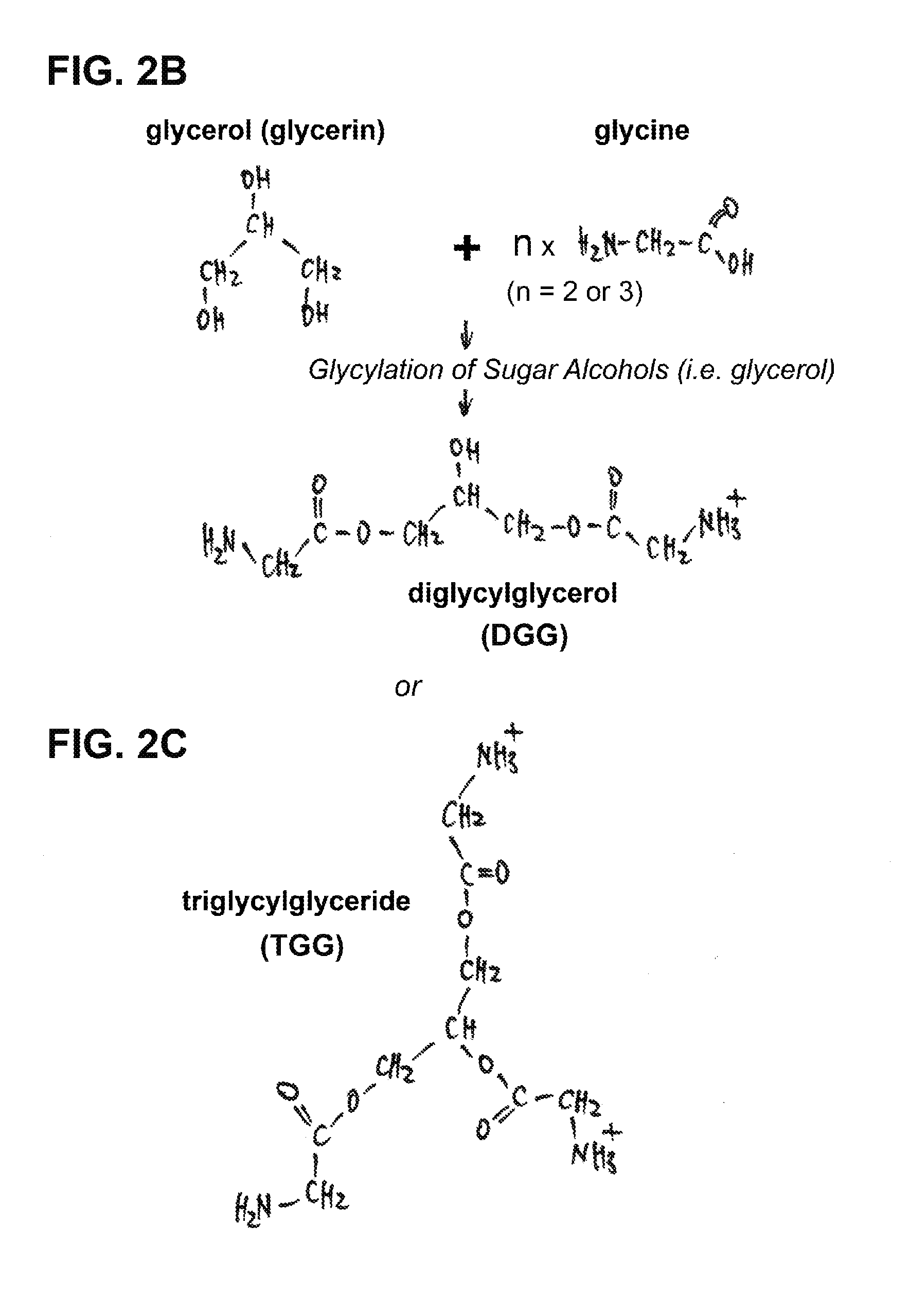
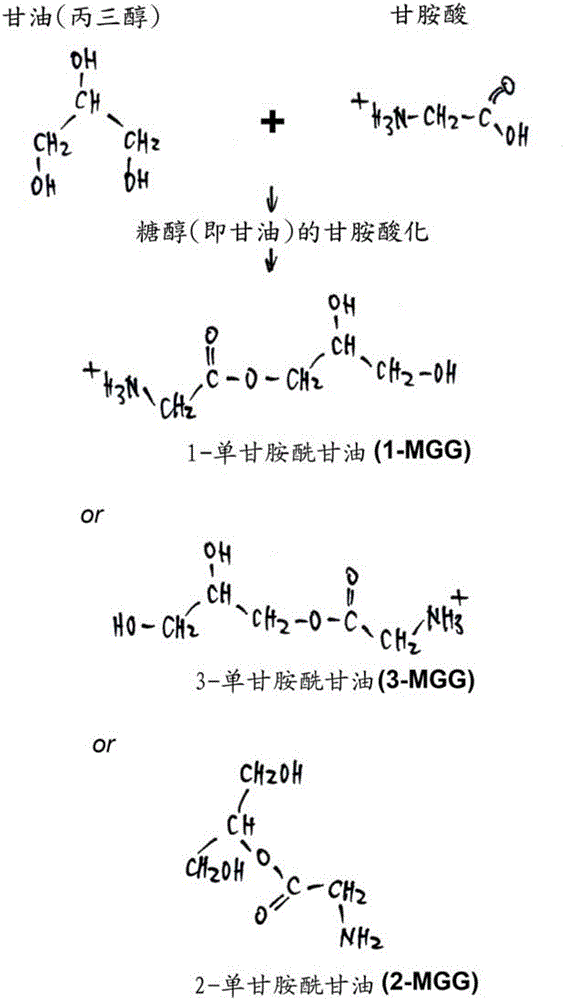
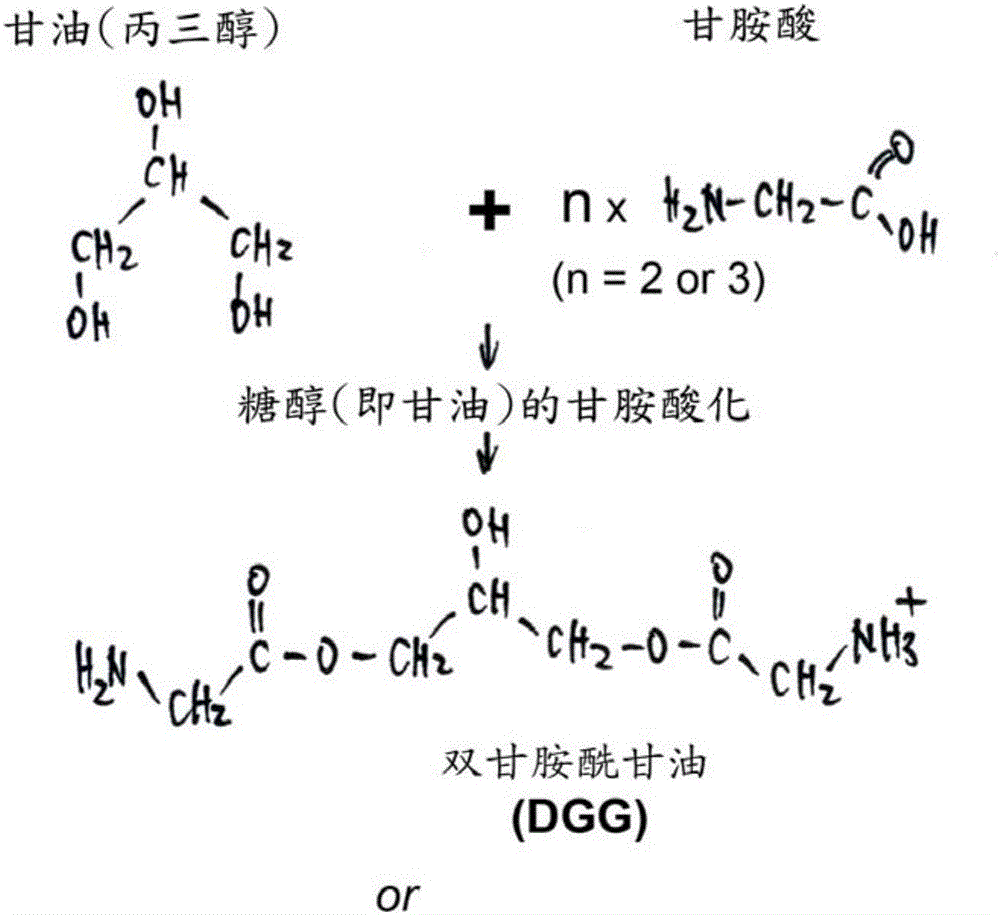
Use of novel monosaccharide-like glycylated sugar alcohol compositions for designing and developing Anti-diabetic drugs
ActiveUS20160220525A1Reduce absorptionAvoid symptomsOrganic active ingredientsSpecial deliveryDiabetes TherapyAcute hyperglycaemia
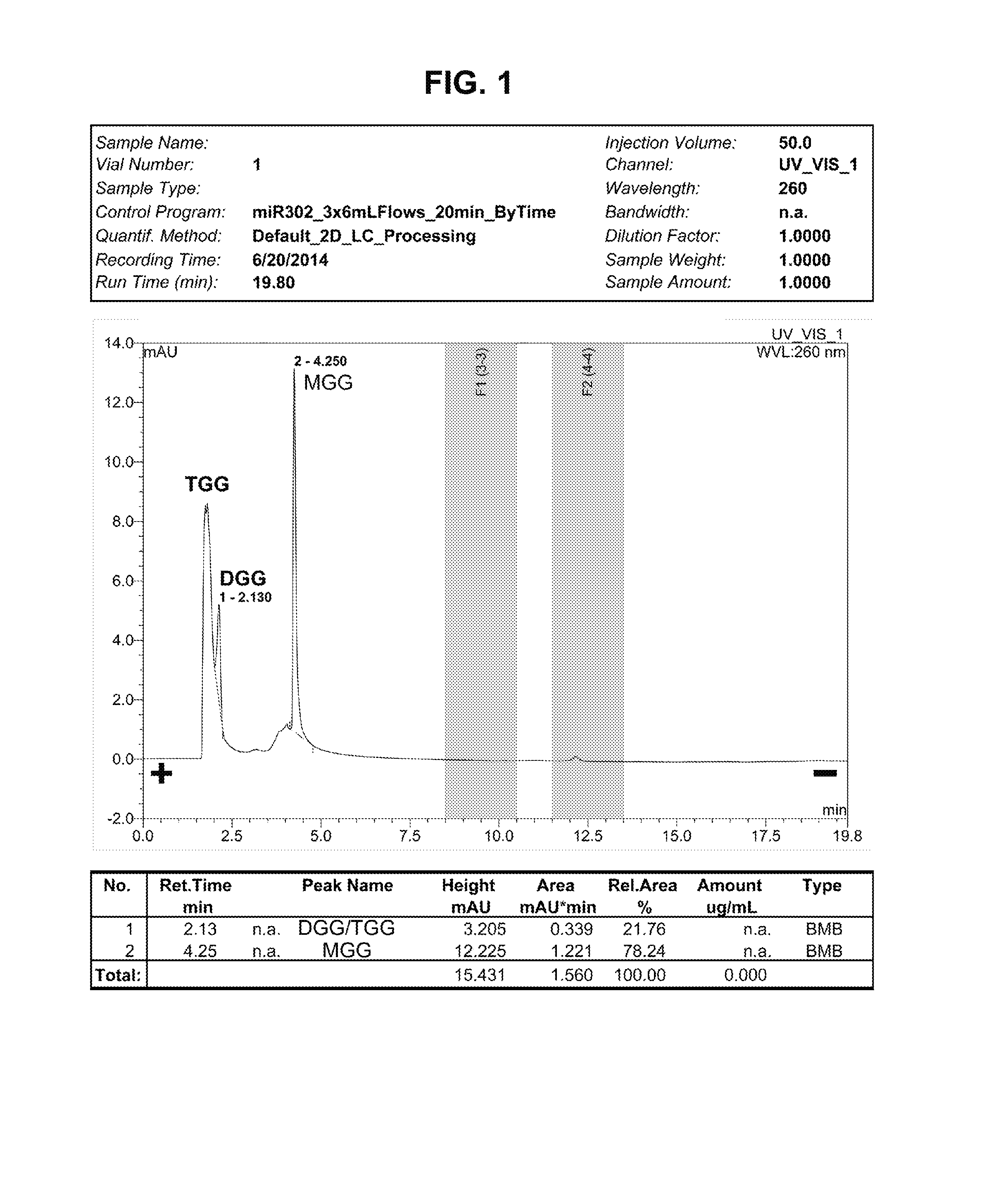
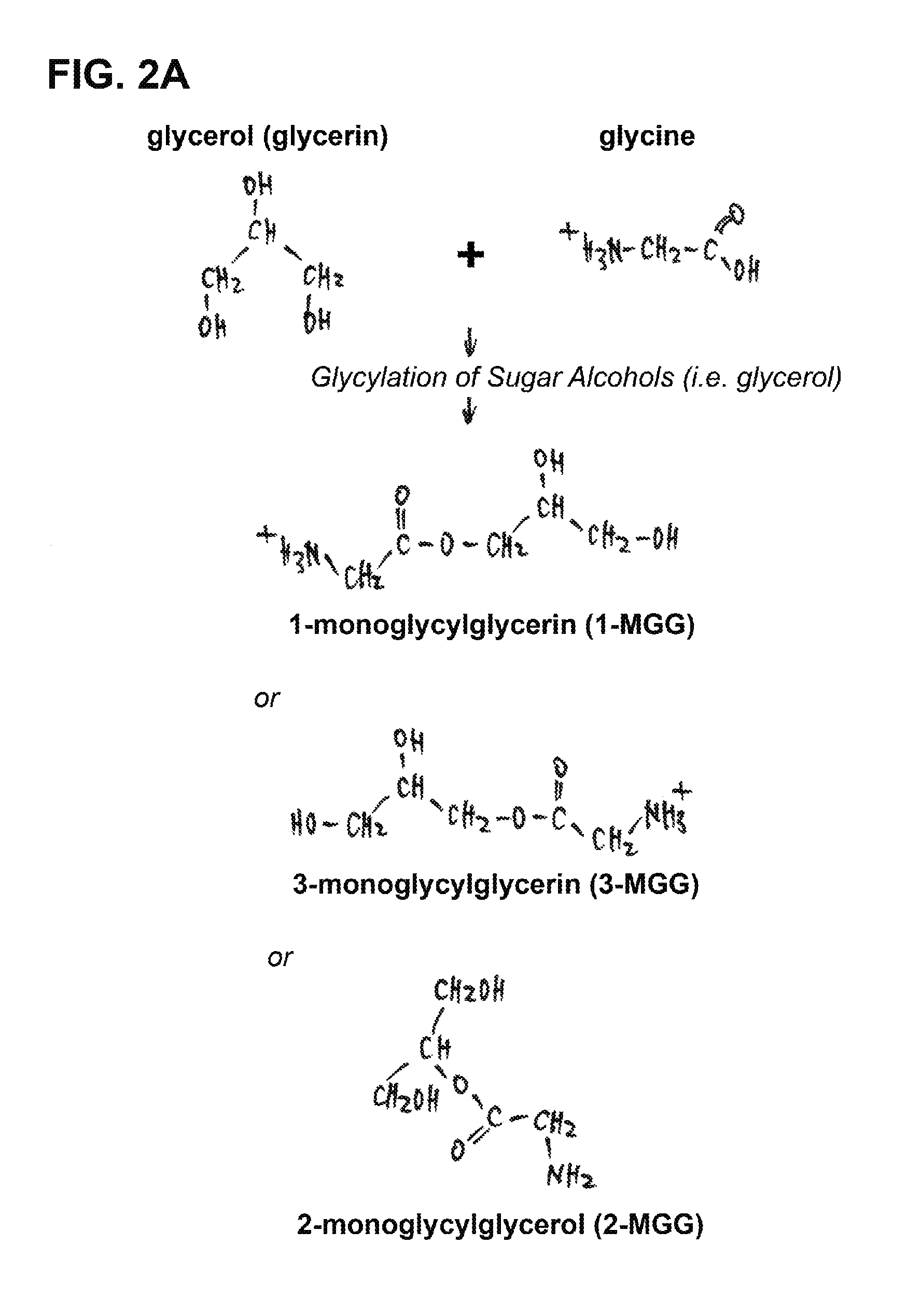
This invention is related to a novel sugar-like chemical composition and its use for diabetes therapy. Particularly, the present invention teaches the use of monosaccharide-like glycylated sugar alcohol compounds to block or reduce sugar absorption in diabetes patients, so as to prevent the risk of hyperglycemia symptoms. Glycylation of sugar alcohols is a totally novel reaction that has never been reported before. Therefore, the novelty of the present invention is that for the first time glycylated sugar alcohols not only was found but also was found to be useful for treating Diabetes mellitus. In addition, the present invention teaches a method for producing these glycylated sugar alcohols. In sum, the present invention includes not only a kind of novel sugar-like chemical compositions and its use for treating diabetes but also a state-of-the-art protocol and methodology for producing such a novel composition via glycylation of sugars and sugar alcohols.
Owner:WJWU & LYNN INST FOR STEM CELL RES & MELLO BIOTECH
Analysis of glucose median, variability, and hypoglycemia risk for therapy guidance
ActiveUS10383580B2Improve accuracyHealth-index calculationMedical automated diagnosisLow glucoseDaily pattern
A system and method to provide guidance for diabetes therapy includes determining glycemic risks based on an analysis of glucose data. The analysis includes visualization of a glucose median, the variability of glucose in a patient, and the risk of hypoglycemia. An Advanced Daily Patterns report includes a visualization of an ambulatory glucose profile and a glucose control measure. The glucose control measure provides a highly visible and understandable display of the glucose condition of a patient visually expressed in the categories of low glucose, median glucose, and glucose variability.
Owner:ABBOTT DIABETES CARE INC
Analogues of insulin-like growth factor-1 (IGF-1) having amino acid substitution at position 59
InactiveUS8759299B2Easy to oxidizeNot easily oxidizedSenses disorderNervous disorderDiabetes TherapyInsulin-like growth factor
Owner:IPSEN PHARMA SAS
Analogues of Insulin-Like Growth Factor-1 (IGF-1) Having Amino Acid Substitution at Position 59
InactiveUS20120190616A1Not easily oxidizedEasy to oxidizeSenses disorderNervous disorderDiabetes TherapyInsulin-like growth factor
The present invention relates to novel analogues of insulin-like growth factor-1 (IGF-1), pharmaceutical compositions containing said analogues, and the use of said analogues for treatment of IGF-1-receptor mediated conditions, such as short stature, diabetes therapy, neurodegenerative disease treatment, and cartilage repair. More particularly, the present invention relates to novel analogues of IGF-1 having an amino acid substitution at position 59, e.g., (Asn59)hIGF-1(1-70)-OH (SEQ ID NO:1), and other substitution(s) as defined herein.
Owner:IPSEN PHARMA SAS
Monosaccharide-like glycylated sugar alcohol compositions for use as anti-diabetic drugs
InactiveCN106413703AOrganic active ingredientsCosmetic preparationsDiabetes TherapyAcute hyperglycaemia
This invention is related to a sugar-like chemical composition for use in diabetes therapy. Particularly, the present invention teaches the use of monosaccharide-like glycylated sugar alcohol compounds to block or reduce sugar absorption in diabetes patients, so as to prevent the risk of hyperglycemia symptoms. The novelty of the present invention is that glycylated sugar alcohols were found to be useful for treating Diabetes mellitus. In addition, the present application discloses a method for producing these glycylated sugar alcohols. In sum, the present invention includes not only a kind of sugar-like chemical composition for use in treating diabetes but also discloses a state-of-the-art protocol and methodology for producing such a composition via glycylation of sugars and sugar alcohols.
Owner:林希龙 +1
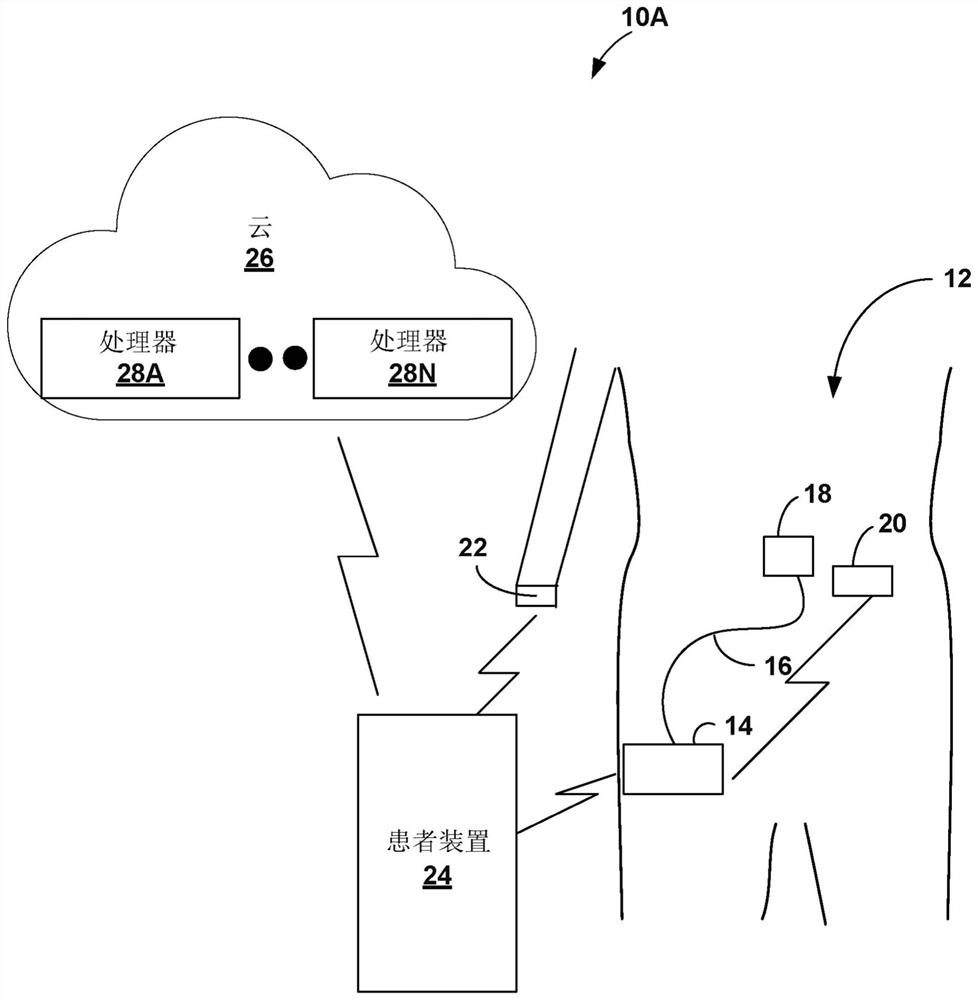
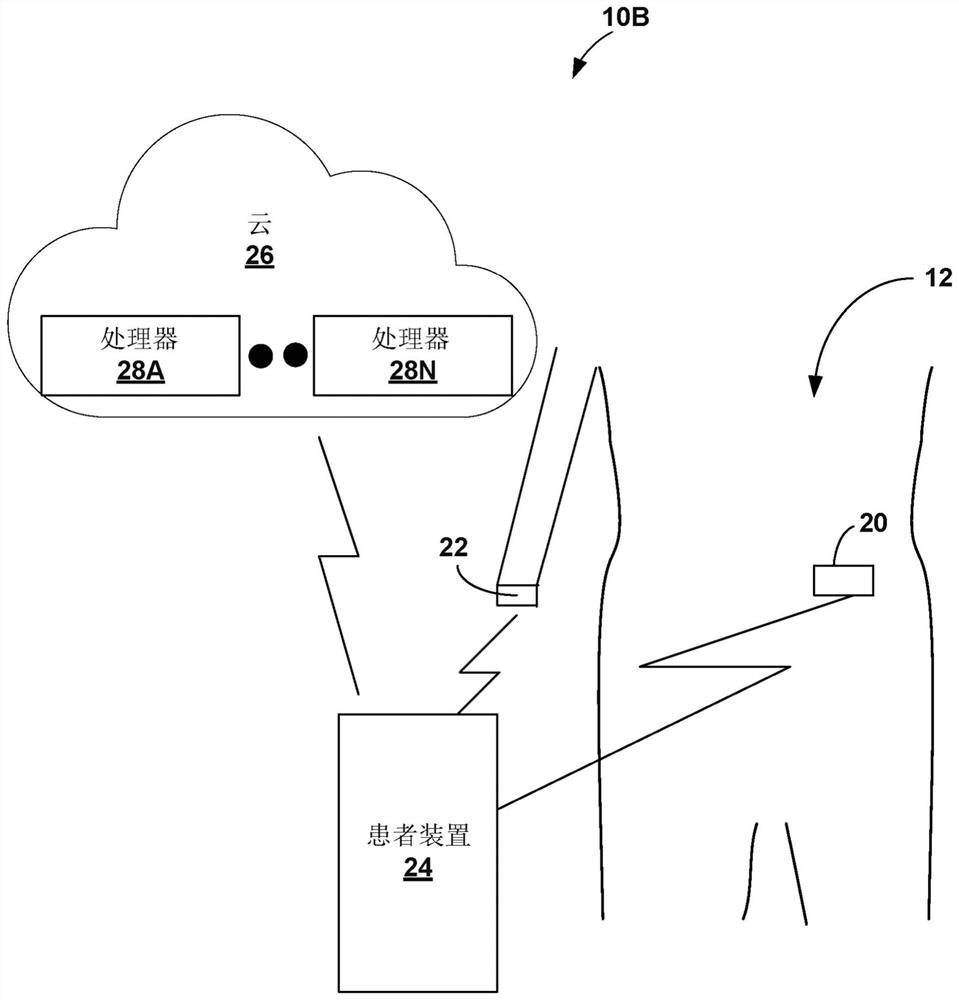
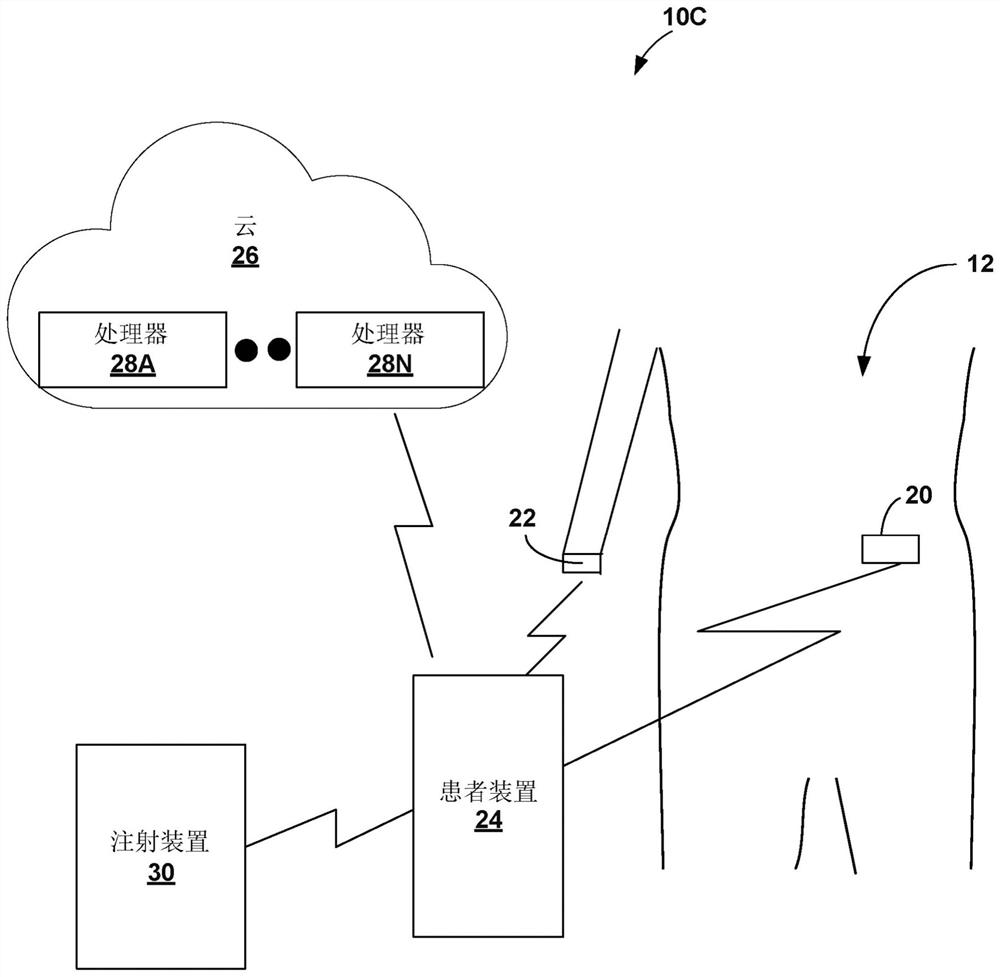
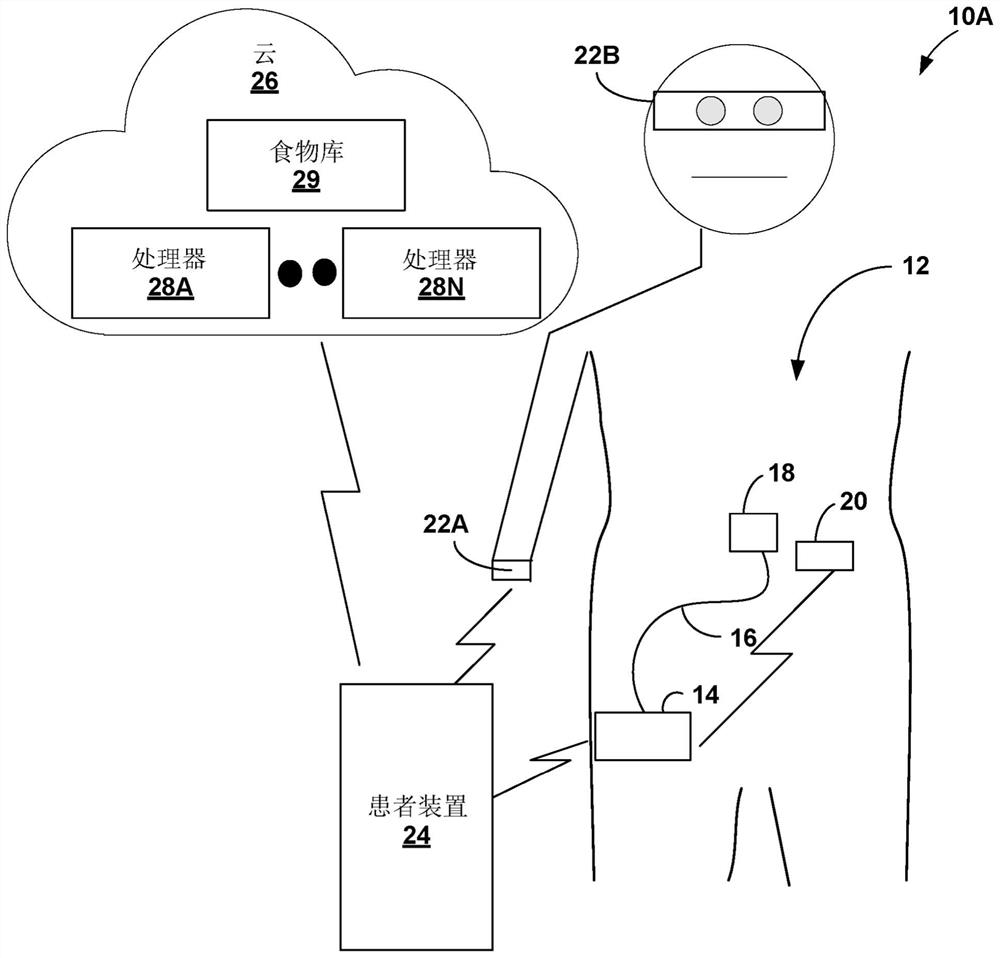
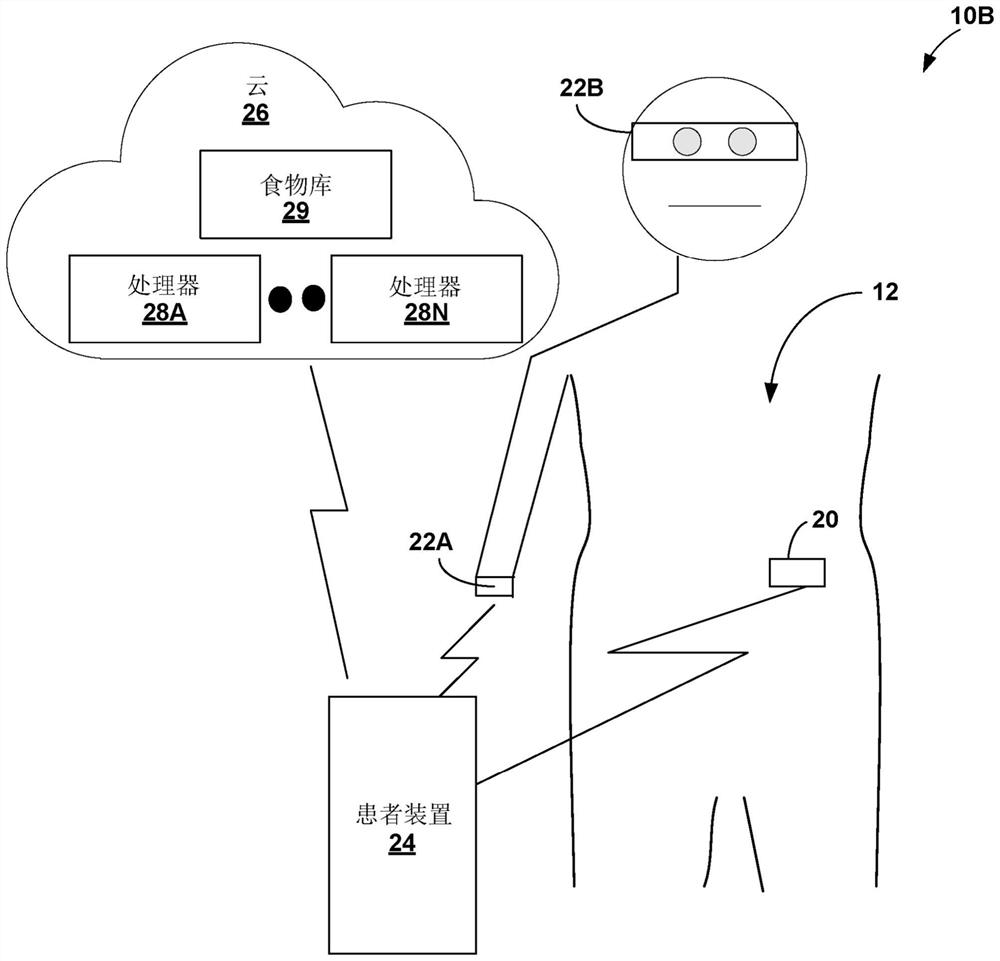
Gesture-based diabetes therapy control
PendingCN114365229AInput/output for user-computer interactionPeptide/protein ingredientsDiabetes TherapyPhysical medicine and rehabilitation
Devices, systems, and techniques for controlling delivery of diabetes therapy are described. In one example, a system includes a wearable device configured to generate user activity data associated with an arm of a user; and one or more processors configured to: identify, based on the user activity data, at least one gesture indicative of preparing an insulin injection with an injection device; generating information indicative of at least one of an amount or type of insulin dose in the insulin injection by the injection device based on the at least one recognized gesture; comparing the generated information to a criterion for an appropriate insulin injection; and based on the comparison, outputting information indicating whether the criteria are met.
Owner:MEDTRONIC MIMIMED INC
Agonist peptide for adiponectin receptor
PendingCN109689678AHigh affinityActivate phosphorylationObesity gene productsPeptide/protein ingredientsDiabetes TherapySide effect
An agonist peptide for an adiponectin receptor is provided according to an embodiment of the present invention, which allows increased affinity between an agonist peptide for an adiponectin receptor and the adiponectin receptor, thereby effectively activating AMPK phosphorylation. Also, a therapeutic agent is provided according to an embodiment of the present invention, which minimizes a side effect of a therapeutic agent for type 2 diabetes and simultaneously exhibits greater efficacy than the conventional therapeutic agents.
Owner:B B 金
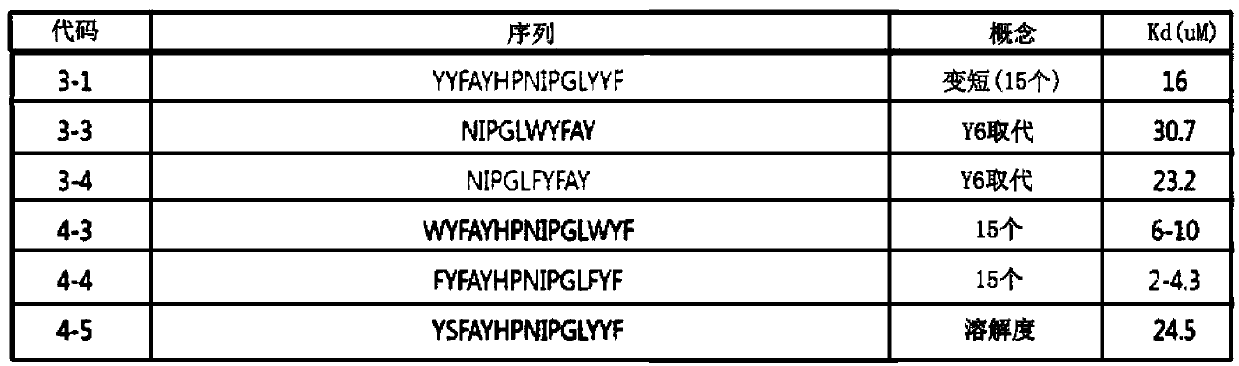
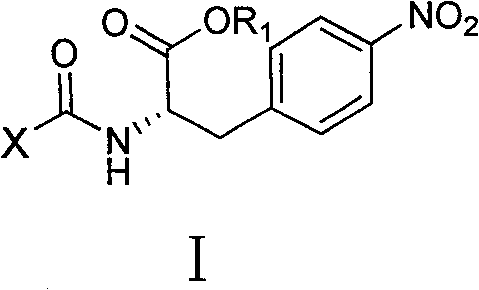
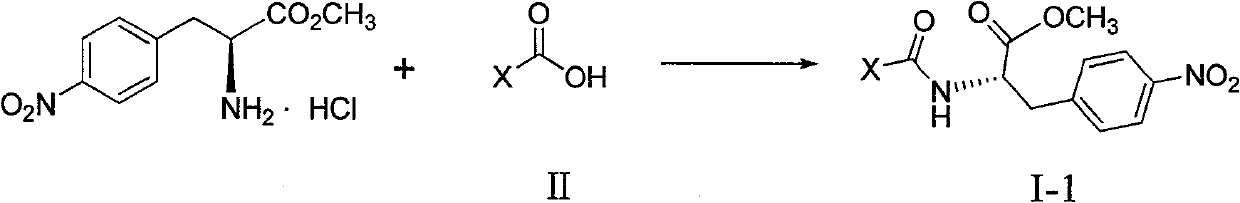
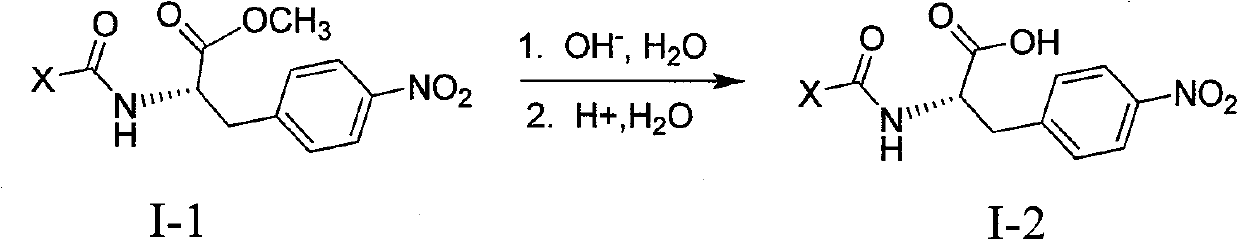
4-nitro-L-phenylalanine dipeptide derivatives as well as preparation method and applications thereof
InactiveCN101724017BPPAR agonistic activity is goodMild reaction conditionsDipeptide ingredientsMetabolism disorderDiabetes TherapyDipeptide
The invention discloses 4-nitro-L-phenylalanine dipeptide derivatives which have the general formula I disclosed in the specification. The detection indicates that the 4-nitro-L-phenylalanine dipeptide derivatives have favorable PPAR agonist activity and are excellent antidiabetic lead compounds. The 4-nitro-L-phenylalanine dipeptide derivatives can be further developed to prepare antidiabetic medicines, and have wide potential application prospects in the field of diabetes therapy. The invention also provides a preparation method of the 4-nitro-L-phenylalanine hydrate dipeptide derivatives, which comprises the step that 4-nitro-L-phenylalanine methyl ester hydrochlorides and protective amino acid are coupled under the action of a condensing agent. The preparation method has the advantages of mild reaction conditions, convenient post-treatment, high yield and low cost.
Owner:SOUTHWEST UNIV +1
Method for synthesizing N- (2-hydroxyethyl)-glucosamine
InactiveCN1304361CMild reaction conditionsImprove responseOrganic compound preparationAmino-hyroxy compound preparationDiabetes TherapyMiglitol
The invention relates to a kind of method for synthesizing midbody N-(2-ethoxyl)-glucosamine of diabetes therapy medicine miglitol alcohol. The existing literature has no synthesizing method recordation of midbody N-(2-ethoxyl)-glucosamine. The reaction steps of the invention are as follows: A) Stuff casting: in autoclave, add D-glucose and ethanolamine according to molar ratio of D-glucose / ethanolamine with 1 / 1.05-1.10; then add 70-95% alcohols solvent with 5-10 times weight of glucose; and after nitrogen displace, add 5%Pd-CaCO3-1-5%Pb catalyst with 2-10% weight of glucose; inject high purity hydrogen, keep hydrogen pressure to 2-3Mpa, and then intensify the temperature slowly up to reaction temperature; B) Reaction: control reaction temperature in 35-45deg.C, hydrogen pressure in 2-4Mpa, and reaction by 20-24 hours; C) Get white pulverous solid by after-treatment. The invention selects the appropriate catalyst and reasonable hydrogenization technology, so that it can make the reaction condition of glucose and ethanolamine with relatively gentleness, make the reaction easy to proceed, make by-product and other impurity generated badly, and also it has high conversion rate, and more than or equal to 98% purity.
Owner:ZHE JIANG MEDICINE CO LTD XINCHANG PHARMA FAB
Alpha-glycosidase inhibitor, its extraction and use
InactiveCN101073596BInhibitory activityHigh activityPeptide/protein ingredientsMetabolism disorderDiabetes TherapyGlycosidase inhibitor
The invention is concerned with the method of preparation of the alpha-glycosidase inhibitor extracts from walnut. It contents the active ingradient of the shell and kernel of the walnut (also includes Juglans regia L., Juglans cathayensis Dode, and Juglans mandshurica Maxim). Thealpha-glycosidase inhibitor is extracted from the draff after oil fired by water, alcohol, or water-alcohol solution, through filtration, concentration, and drying at last. It can use for prepareing health products for diabetes therapy or blood suger controlling.
Owner:广元天湟山核桃食品有限公司
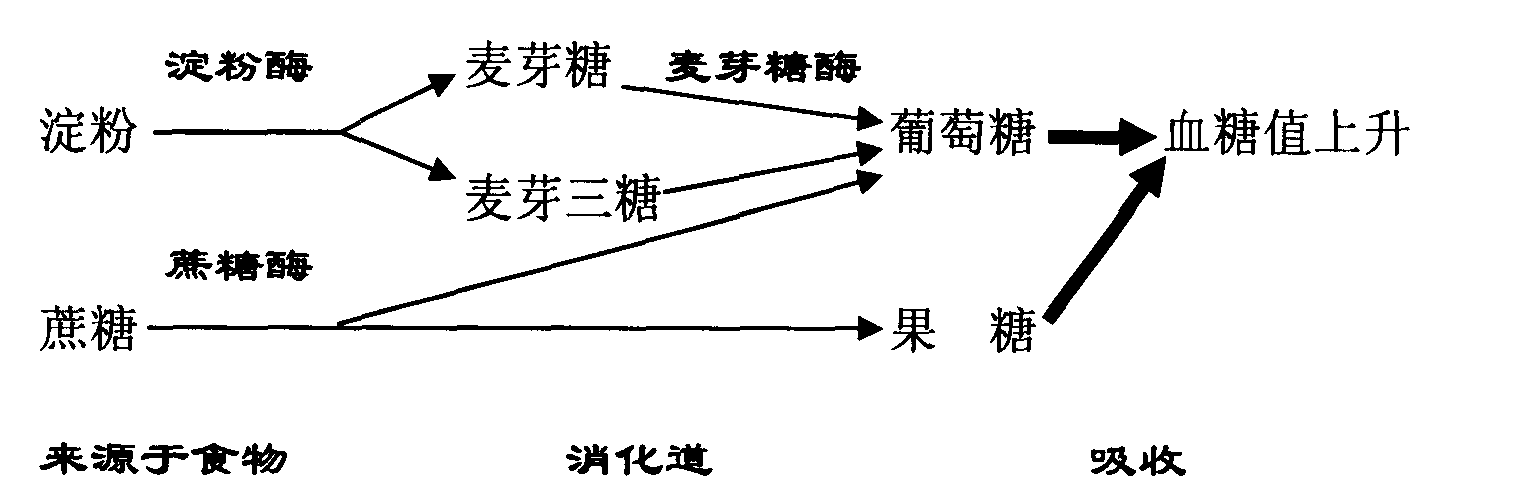
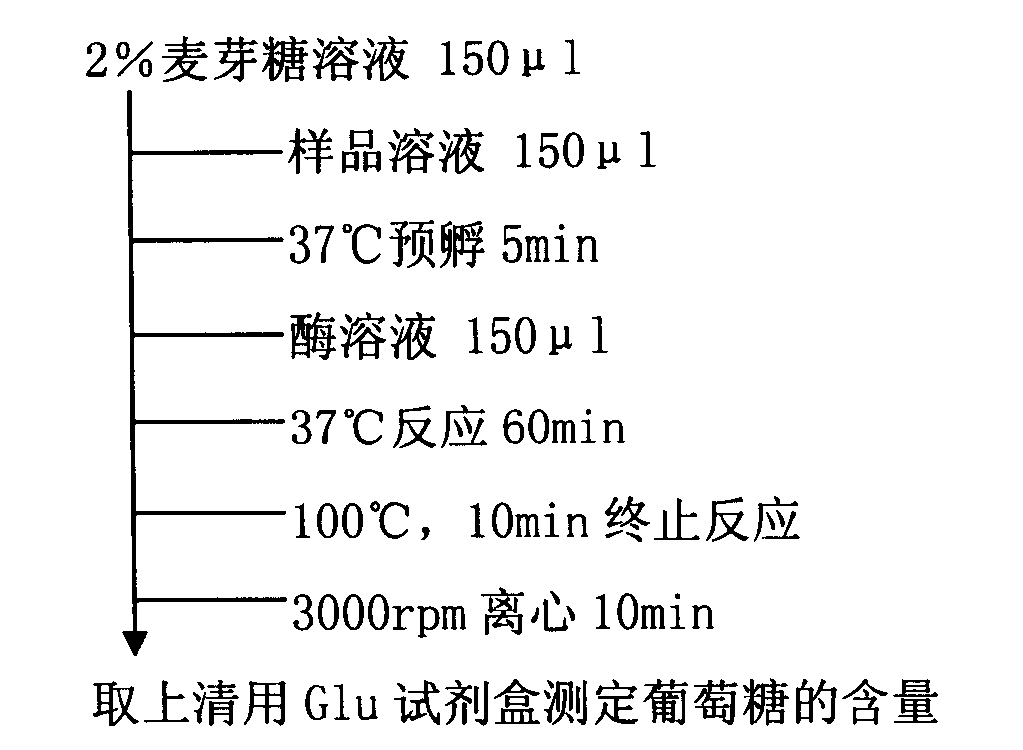
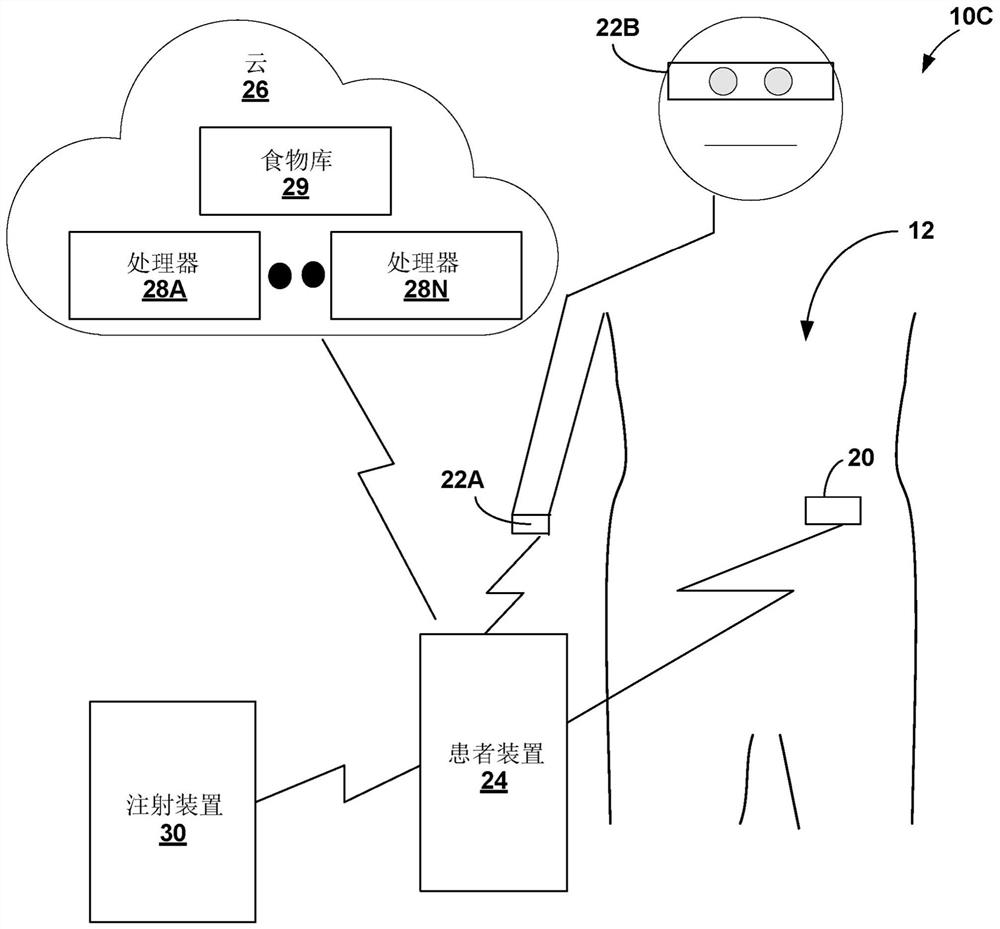
Diabetes therapy based on determination of food items
Devices, systems, and techniques are described for directing therapy delivery to diabetic patients. Some devices, systems, and techniques actively monitor the patient's food consumption and, if desired, adjust the patient's diabetes therapy accordingly. In one example, a system includes one or more sensors configured to capture a representation of an object; and processing circuitry configured to: determine data corresponding to the representation of the object, where the data includes information identifying the object as a particular food item; generating food item information based on the information identifying the object as the specific food item, wherein the food item information includes at least one of nutrition information or volume information of the specific food item; generating therapy information for the patient based on the food item information; and outputting content indicative of at least one of the therapy information or the food item information.
Owner:MEDTRONIC MIMIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com