Recombinant engineering bacterium capable of efficiently expressing liraglutide precursor and application of recombinant engineering bacterium
A technology of recombinant engineering bacteria and liraglutide, applied in the field of genetic engineering, can solve the problems of unfavorable industrial development needs, protein cleavage problems, high cost, etc., achieve fast and convenient separation and purification methods, reduce subsequent processing steps, and reduce industrial production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Design of Liraglutide Precursor Molecule Containing Signal Peptide and Enterokinase Sequence and Construction of the Gene Expression Plasmid
[0040] 1. Construction of liraglutide precursor molecule
[0041] The liraglutide precursor molecule of the present invention has an A-B-C structure, wherein A is a signal peptide, B is a connecting peptide, and C is the sequence of Arg34GLP-1 (7-37); the signal peptide is MalE, PhoA, OmpF , PelB, LamB, Lpp or OmpA; the connecting peptide is enterokinase, thrombin, SUMO or Protease.
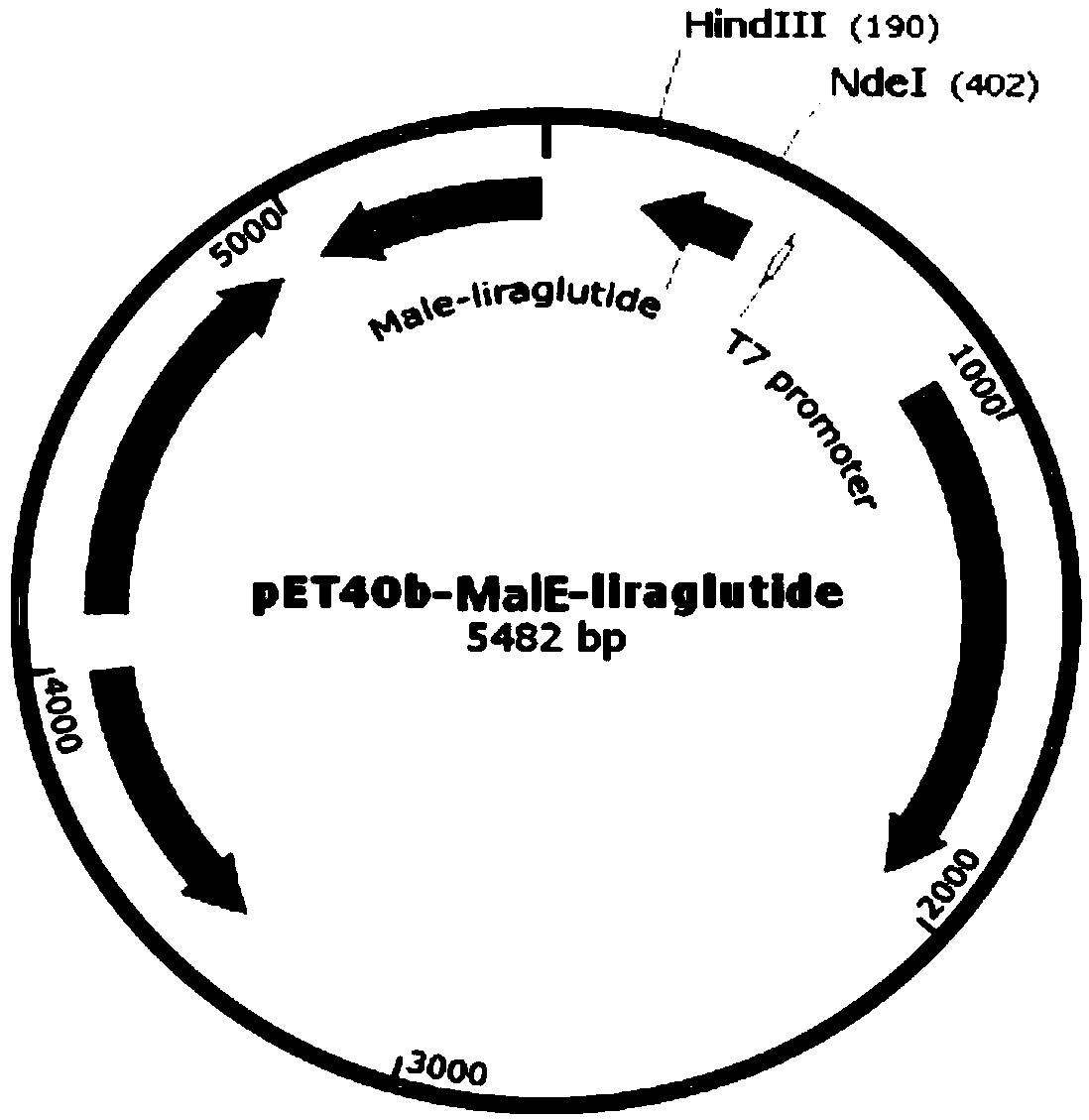
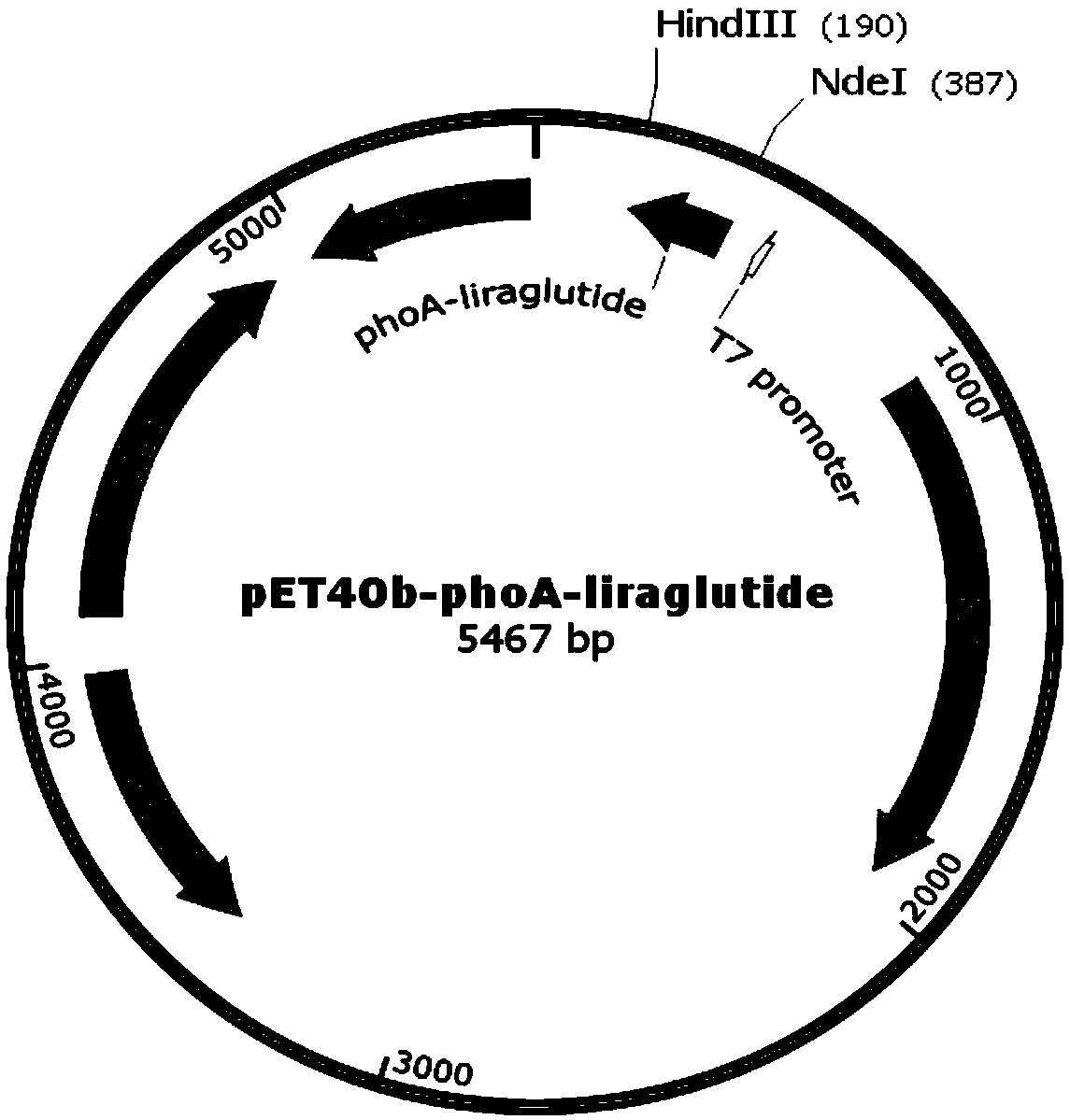
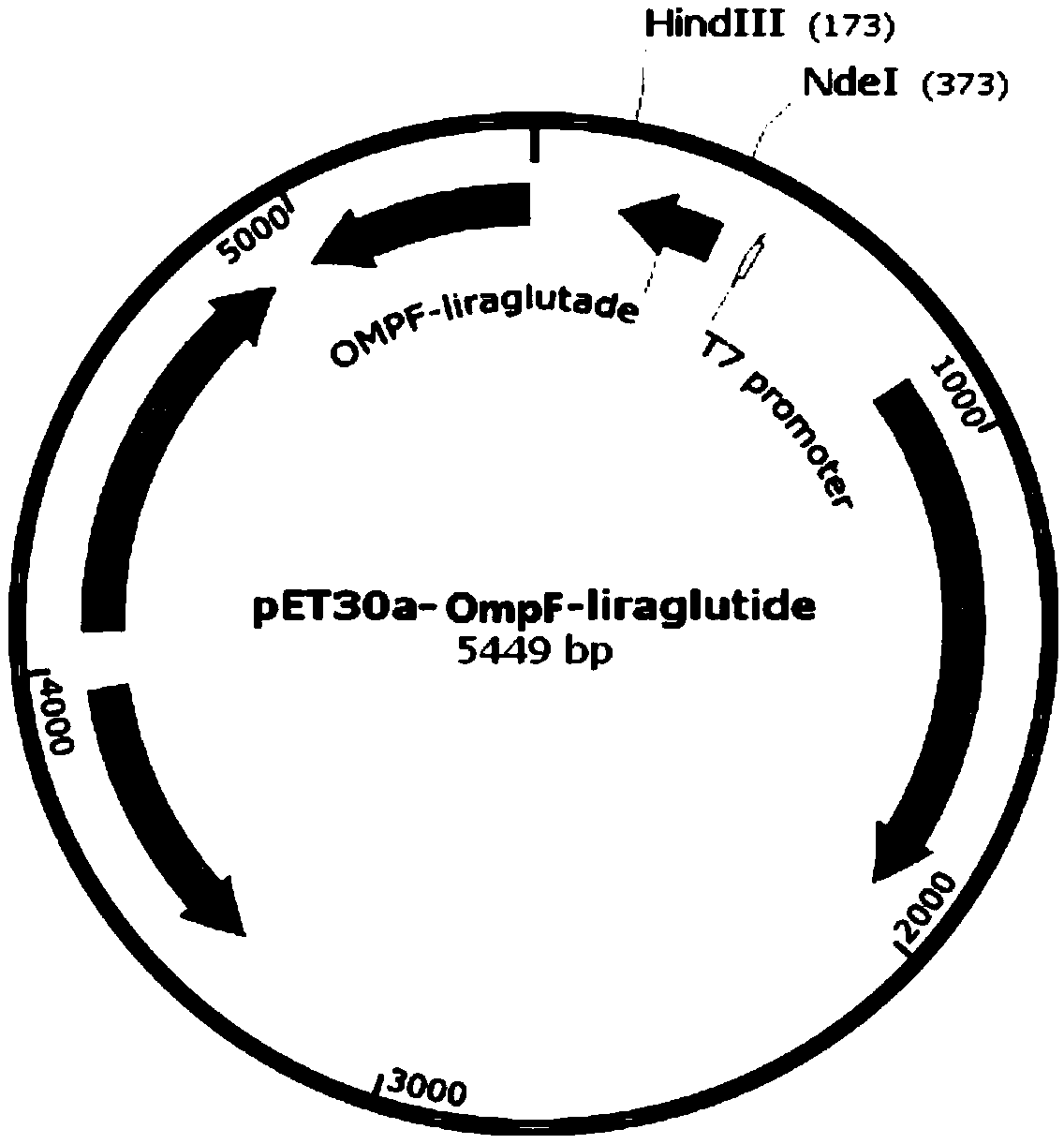
[0042] Add a His tag after the second amino acid at the N-terminal of the signal peptides MalE, PhoA, OmpF, and PelB respectively. The amino acid sequences are shown in SEQ ID NO.1-4, and the gene sequences are shown in SEQ ID NO.5-8. .
[0043] The amino acid sequence of Arg34GLP-1(7-37) is shown in SEQ ID NO.9.
[0044] The gene sequence of the liraglutide precursor molecule of the signal peptide and enterokinase sequence of the prese...
Embodiment 2
[0047] Example 2 Construction of Recombinant Engineering Bacteria Highly Expressing Liraglutide Precursor
[0048] The successfully constructed recombinant expression vector was transformed into BL21(DE3) host by chemical method for amplification, and the correct recombinant engineered bacteria were initially screened by colony PCR method, and then the plasmid was extracted for enzyme digestion identification. As a result of the identification, the correct recombinant engineered bacteria were obtained.
Embodiment 3
[0049] Example 3 The method of preparing liraglutide precursor by using recombinant engineered bacteria highly expressing liraglutide precursor
[0050] 1. Fermentation and inclusion body collection
[0051] Get the recombinant engineered bacterium constructed in Example 2, carry out high-density fermentation, obtain thalline after centrifugation, 10L fermented liquid can obtain 800g wet bacterium. During the fermentation process, recombinant fusion proteins were expressed with different signal peptides, SDS-polyacrylamide gel electrophoresis was performed, and the content of the target protein was determined by observing the band intensity. Among them, recombinant fusion proteins with LamB, Lpp, and OmpA as signal peptides The expression effect is not good, and the expressed recombinant protein band is very narrow or absent, see figure 2 ; The recombinant fusion protein with MalE, PhoA, OmpF, and PelB as signal peptides has a better expression effect, see image 3 , the fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com