Patents
Literature
88 results about "Glucose tolerance test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
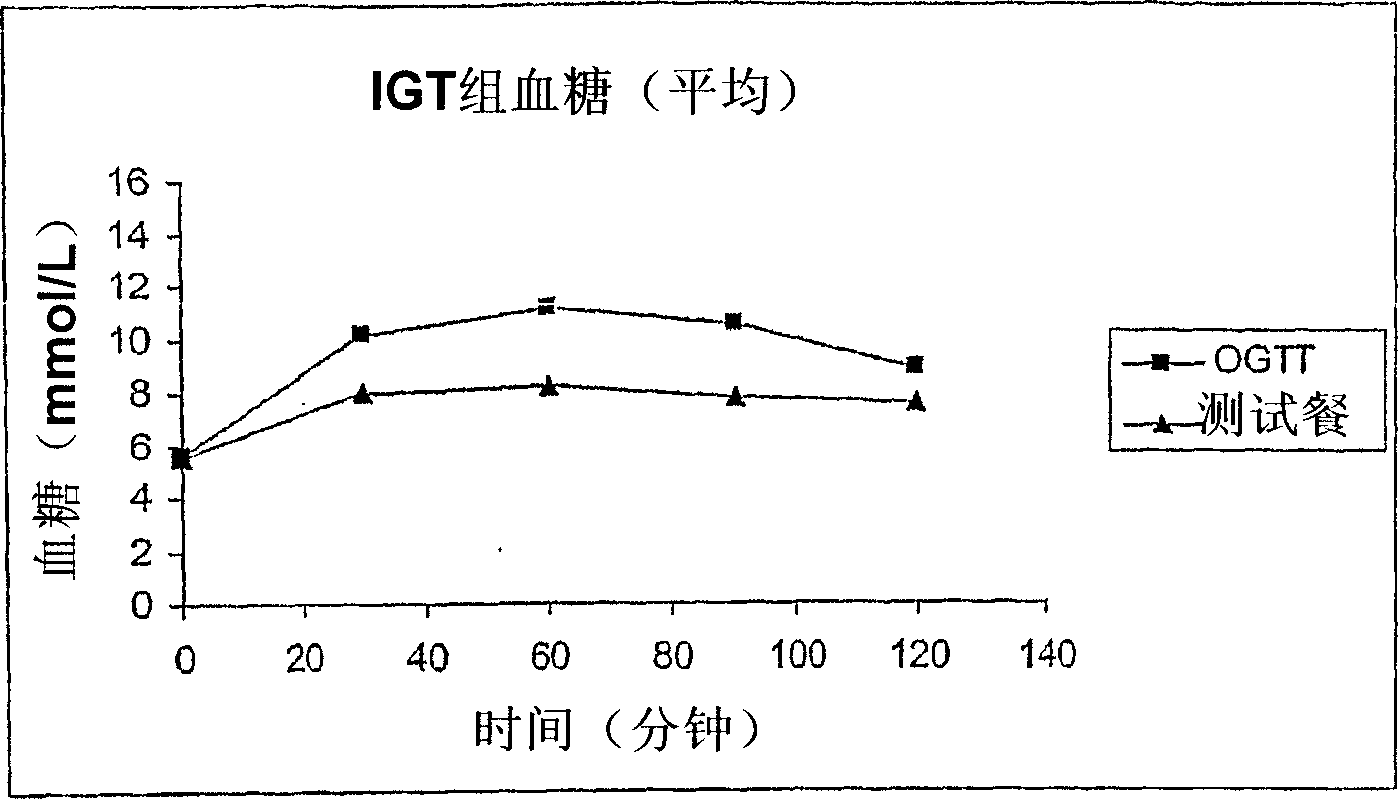
<p>For OGTT: the conditions signify positive results or presence of disorder if one or more of following values are higher than specified:</p><ul><li>Below 100 mg/dl is normal result for fasting blood glucose.</li><li>100 to 125 mg/dl of blood glucose is prediabetes.</li><li>Higher than 126 mg/dl can be diabetes.</li><li>After one hour, 180 mg/dl denotes gestational diabetes.</li><li>After two hours, 140 to 199 mg/dl shows presence of prediabetes, 200 mg/dl shows presence of diabetes and 153 mg/dl shows gestational diabetes.</li></ul><p>Gestational Diabetes: If one or more of following values are higher, the presence of Gestational diabetes is confirmed:</p><ul><li>95 mg/dl or more</li><li>180 mg/dl or more after 1 hour</li><li>155 mg/dl or more after 2 hours</li><li>140 mg/dl or more after 3 hours</li></ul>
Dynamic hepatic recycling glucose tolerance test
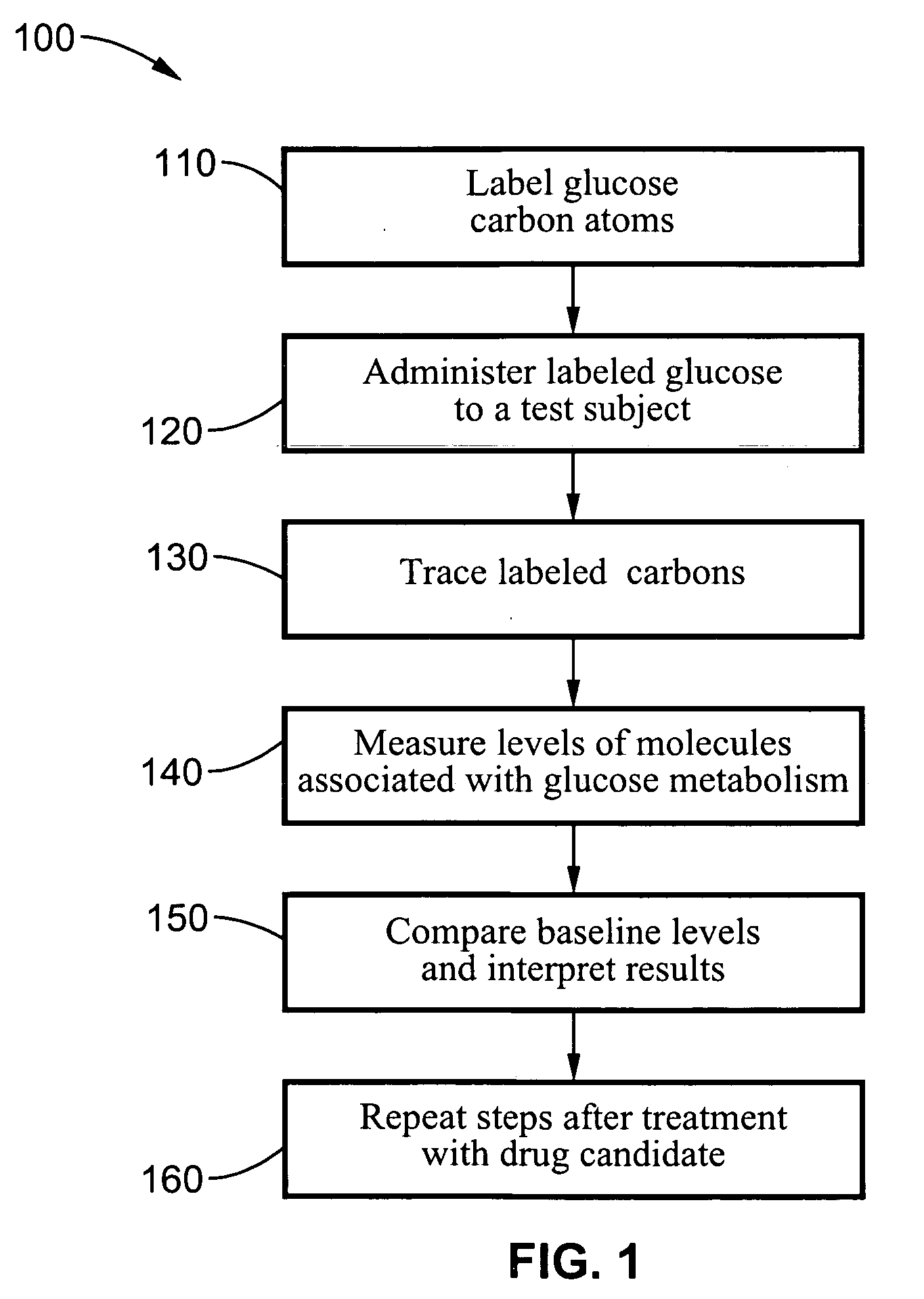
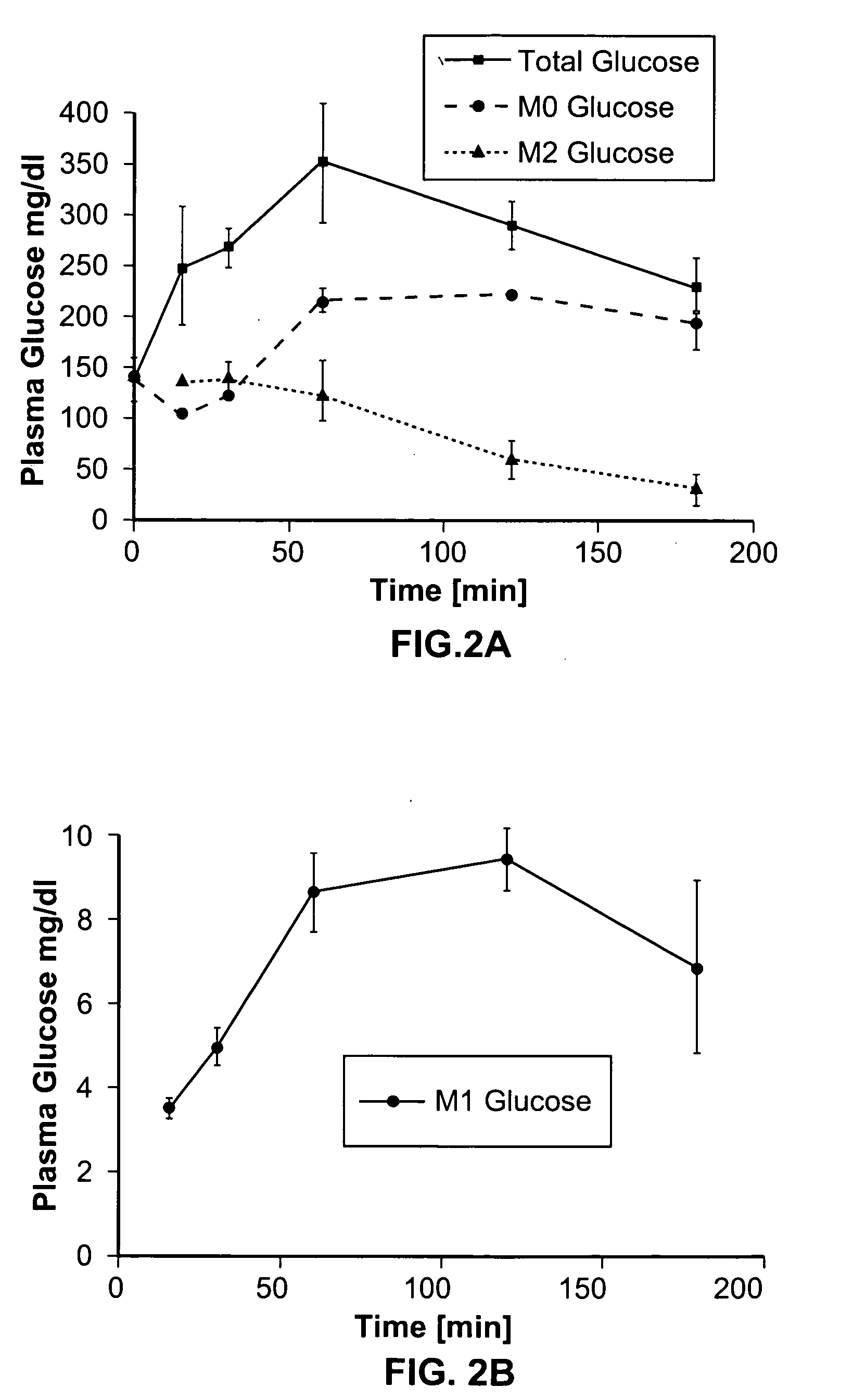
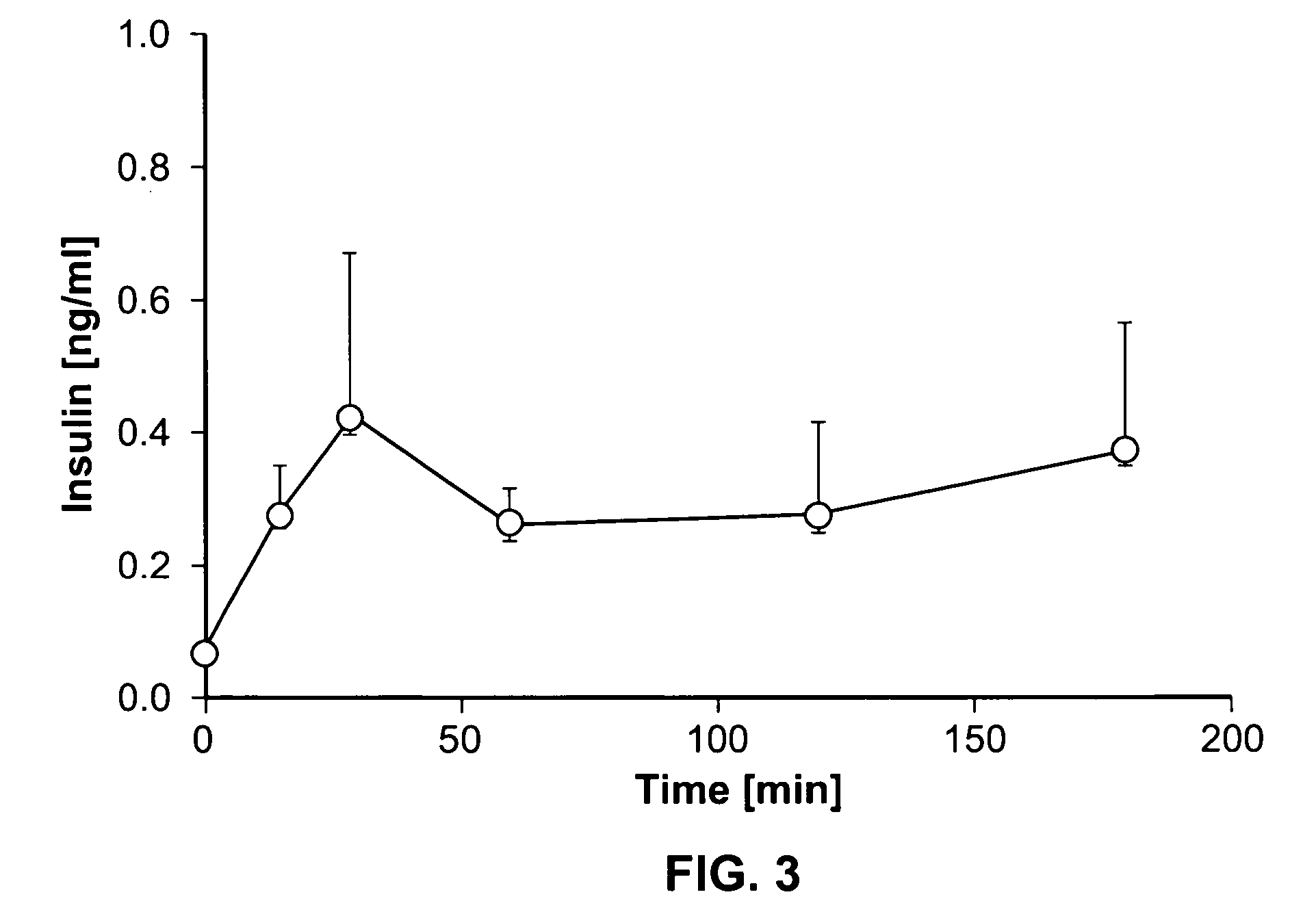
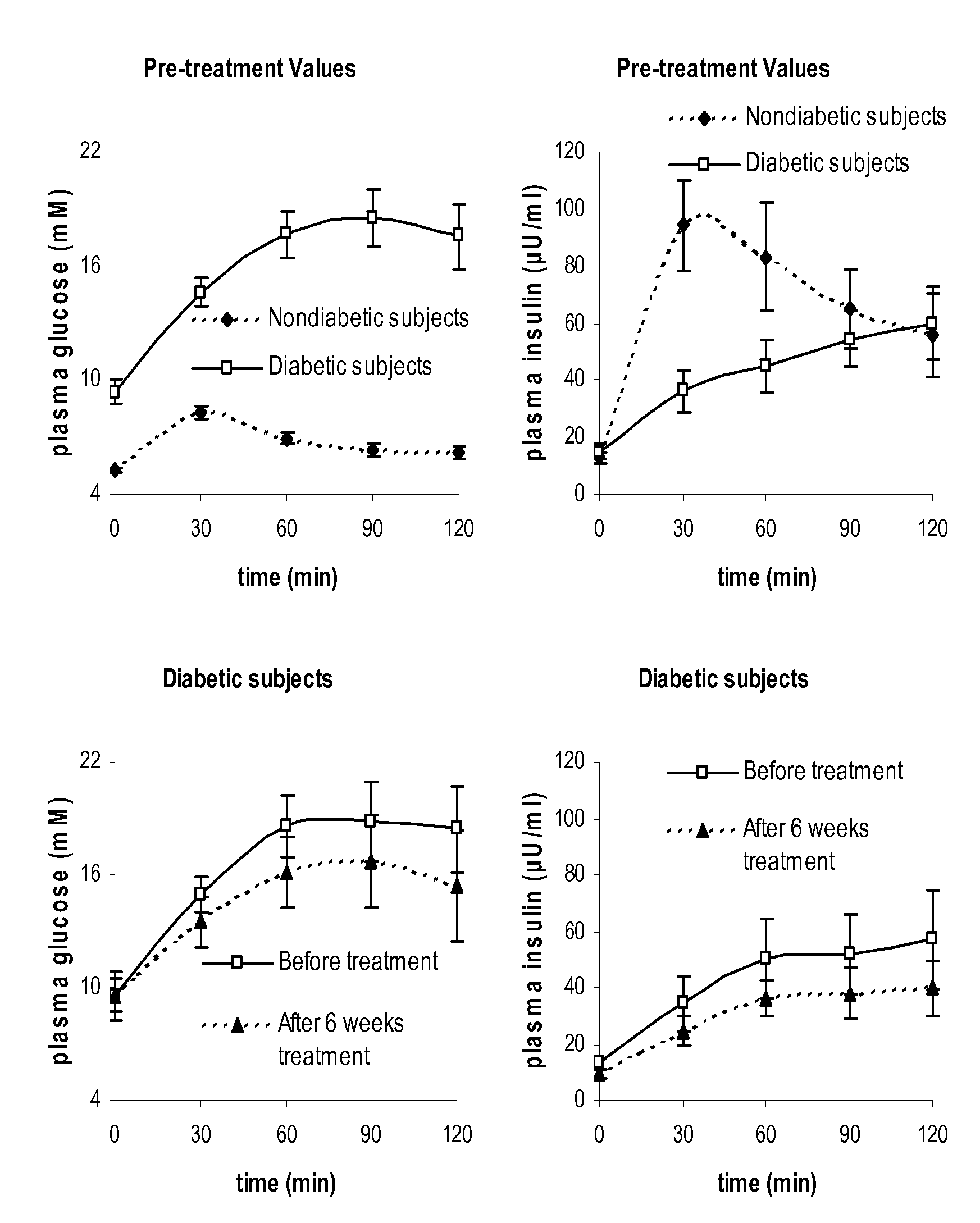
Systems and methods are described providing a hepatic recycling glucose tolerance test for the diagnosis of types and subtypes of diabetes mellitus and other hyperglycemic or hypoglycemic conditions. A method is also provided for screening candidate drugs for treating various types of abnormal glucose metabolism and to monitor whether the course of treatment is effective. The method also allows the correlation of gene activity, hormone and metabolite levels with glucose flux and recycling and an assessment of the degree of hepatic insulin resistance. The method utilizes a preferably non-radioactive stable labeled glucose to asses the relative rates of carbon flow in the liver and provides a hepatic recycling constant that is a measure of the relative rate of glucose recycling. The labeled glucose may be introduced to the patient orally, intravenously or by intraperitoneal administration for the desired effect.
Owner:RGT UNIV OF CALIFORNIA
Method for Determining Insulin Sensitivity and Glucose Absorption
InactiveUS20080262745A1Large-scale clinical trialsDifference in insulin sensitivityHealth-index calculationMicrobiological testing/measurementPresent methodPatient type
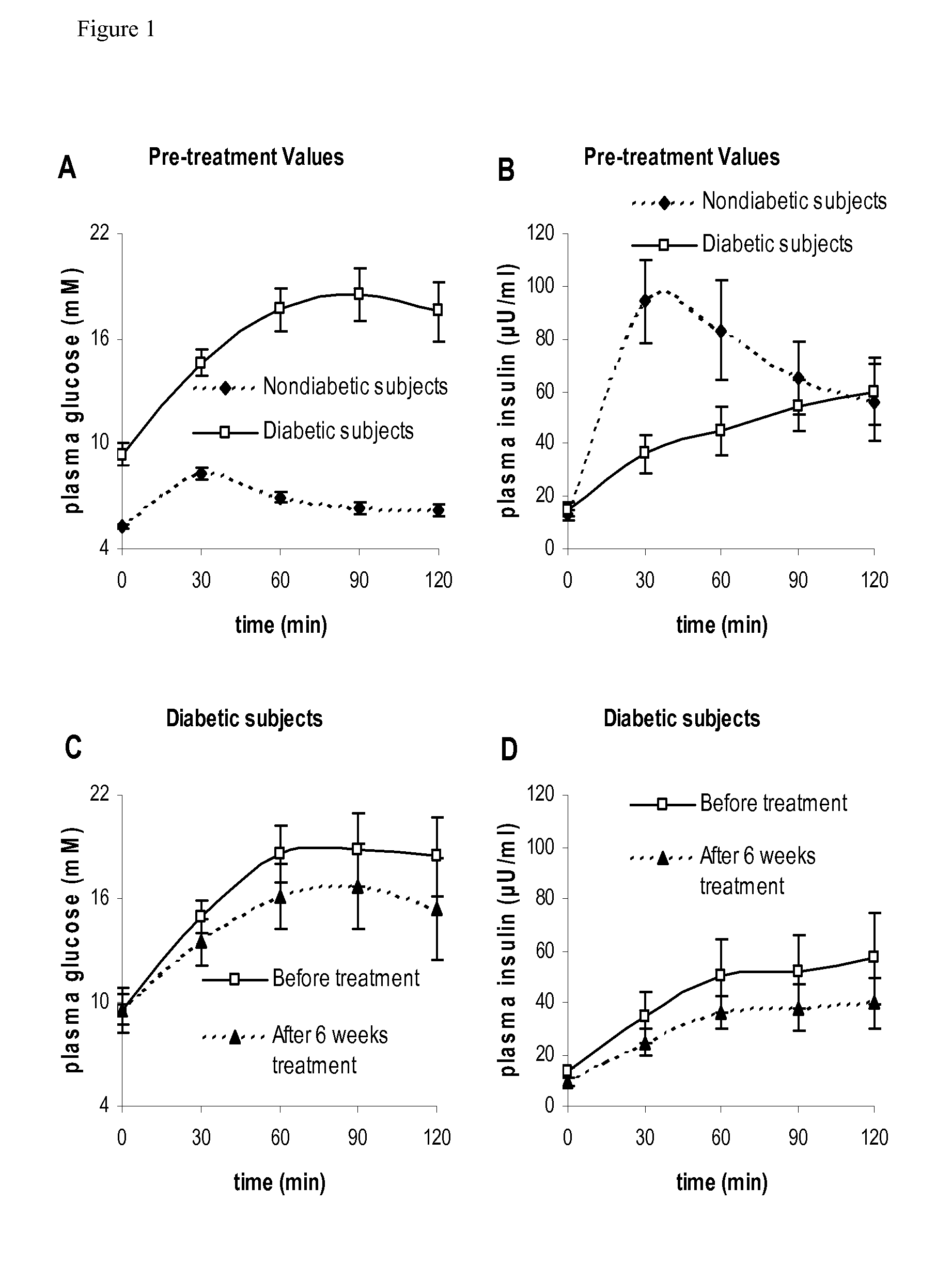
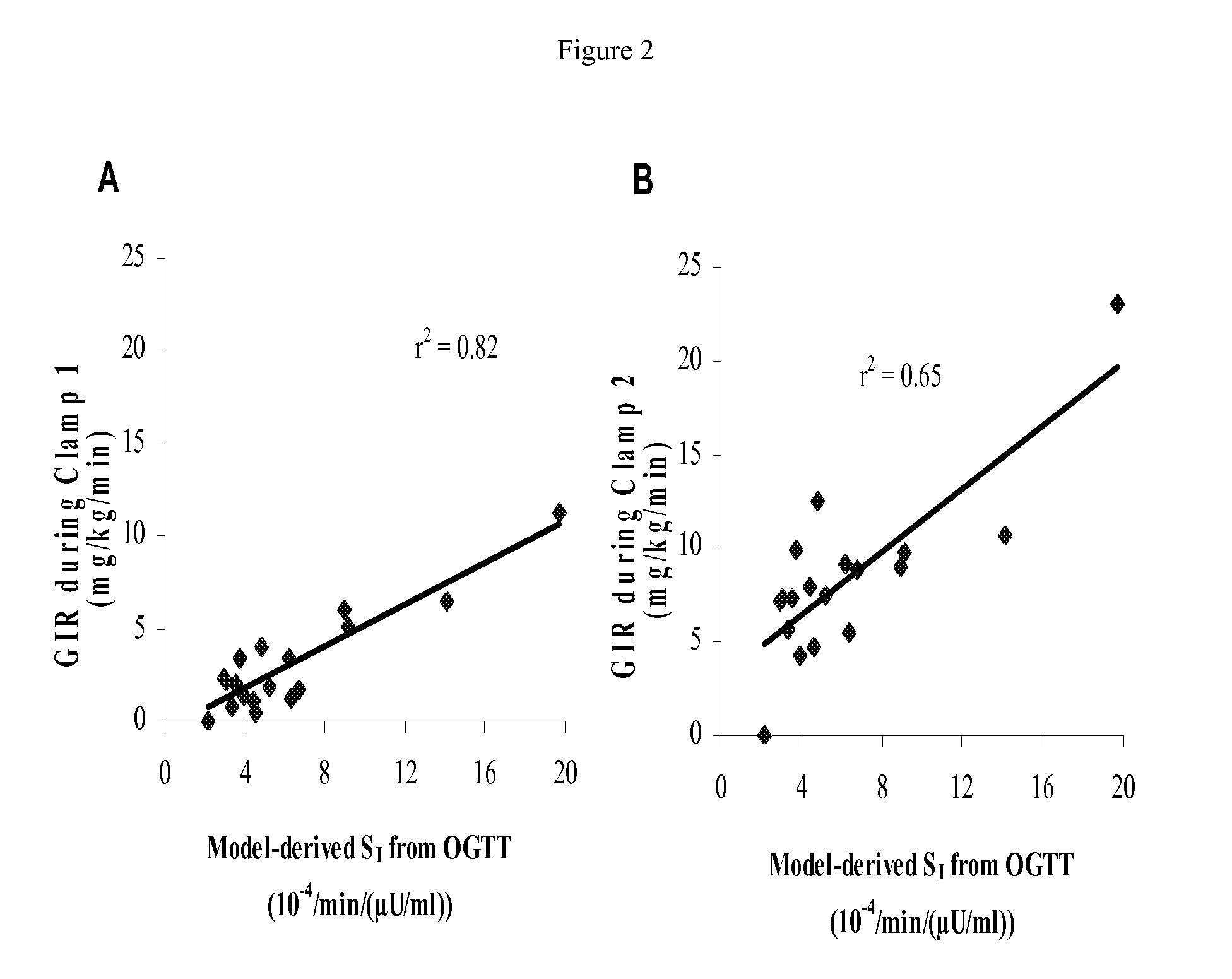
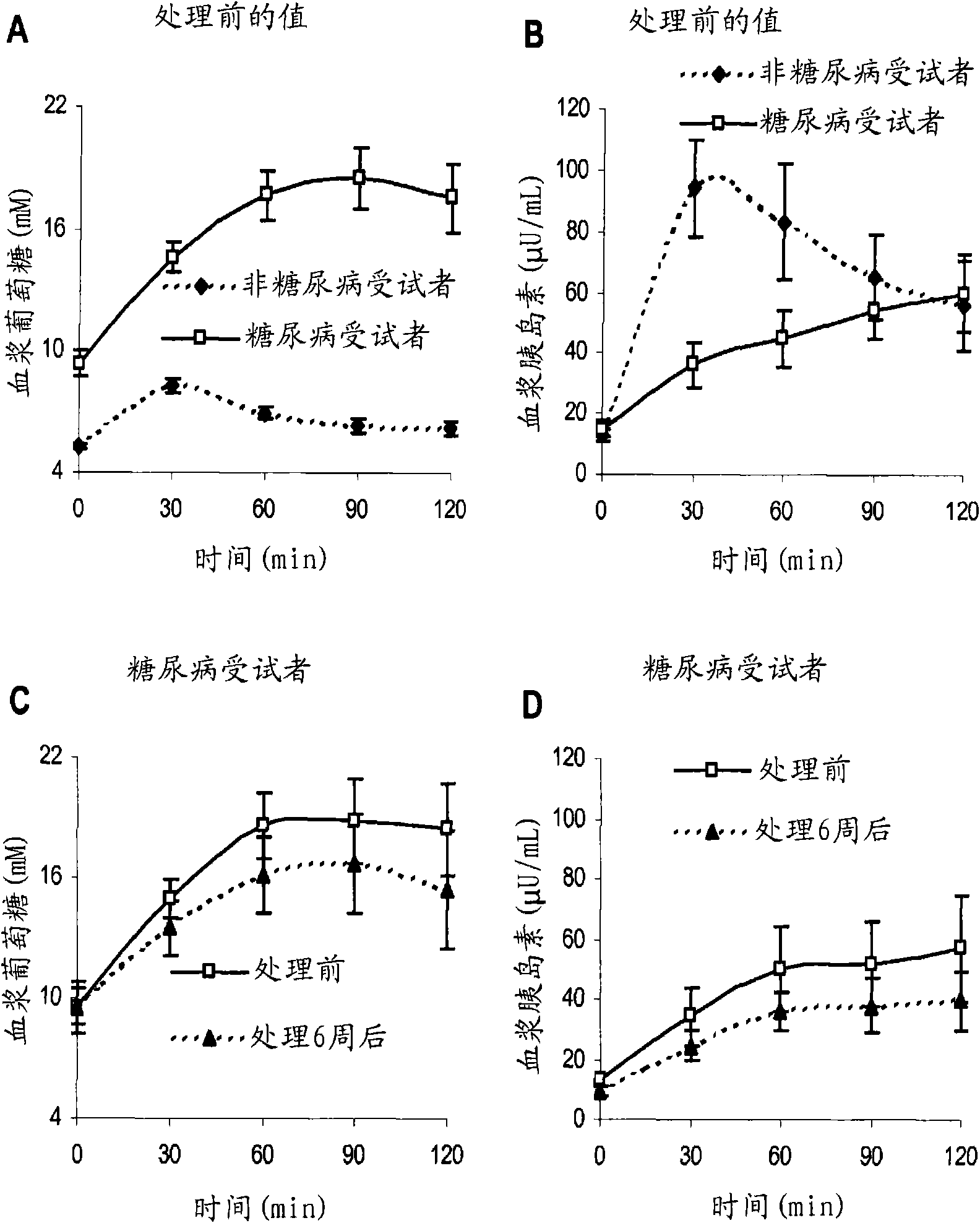
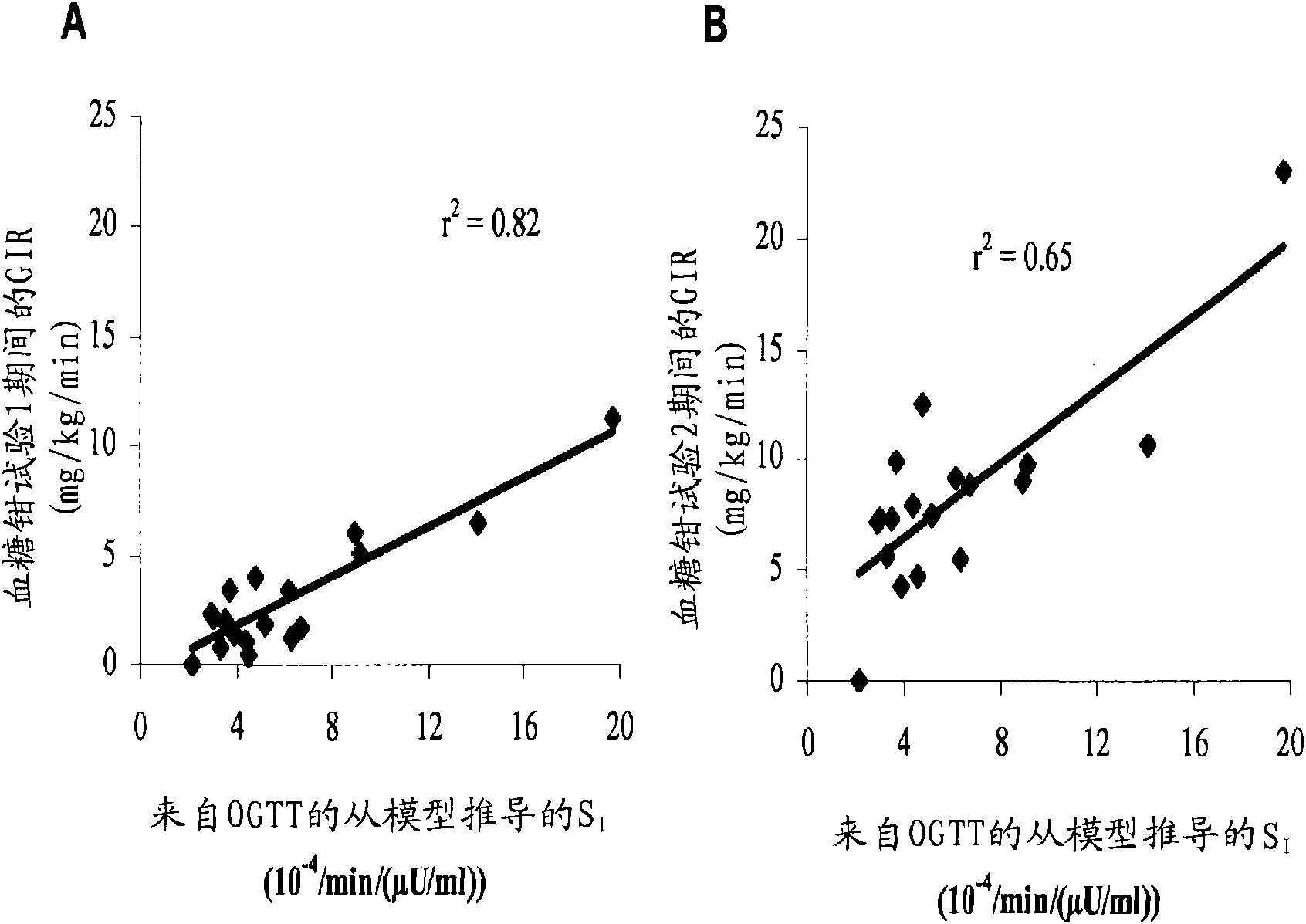
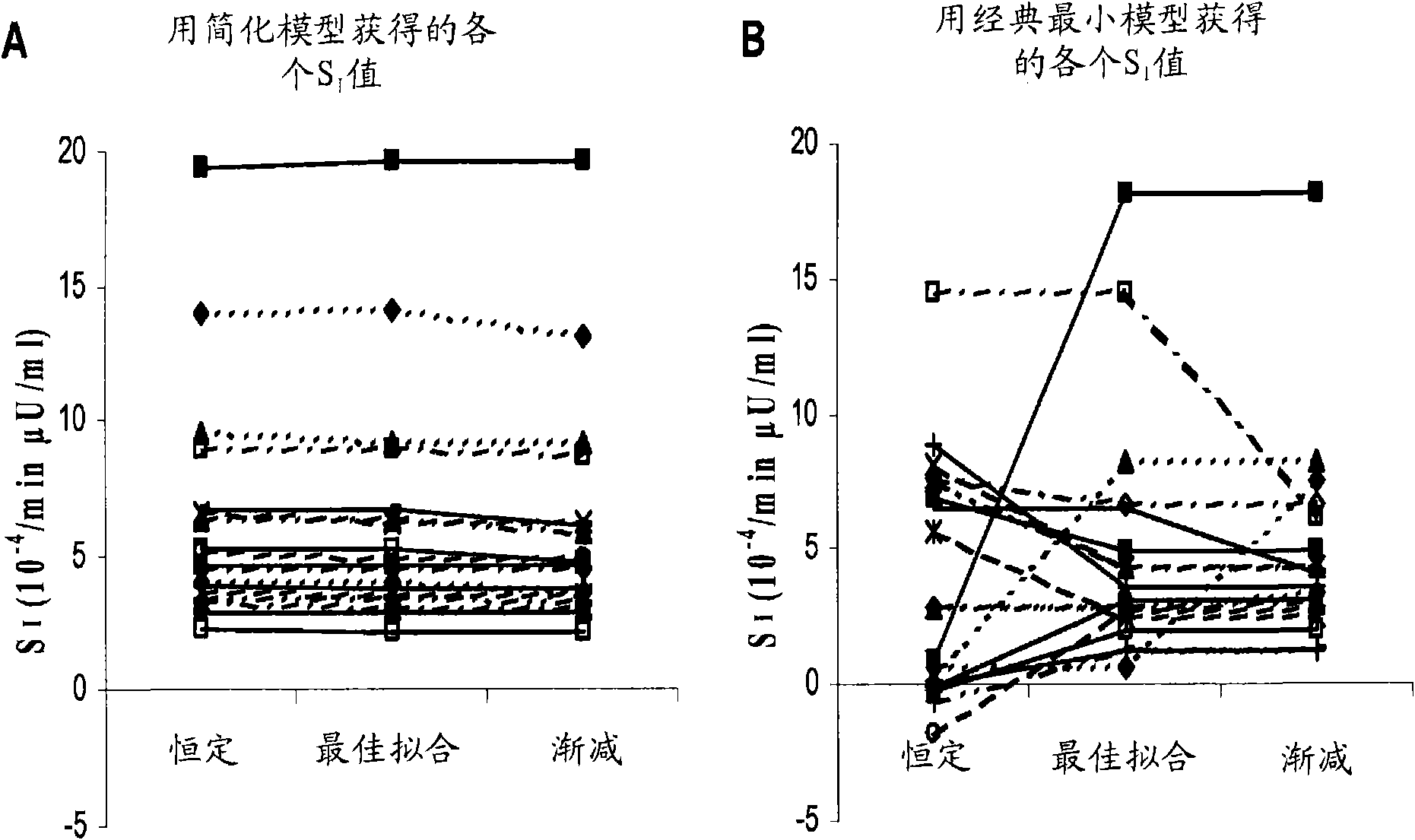
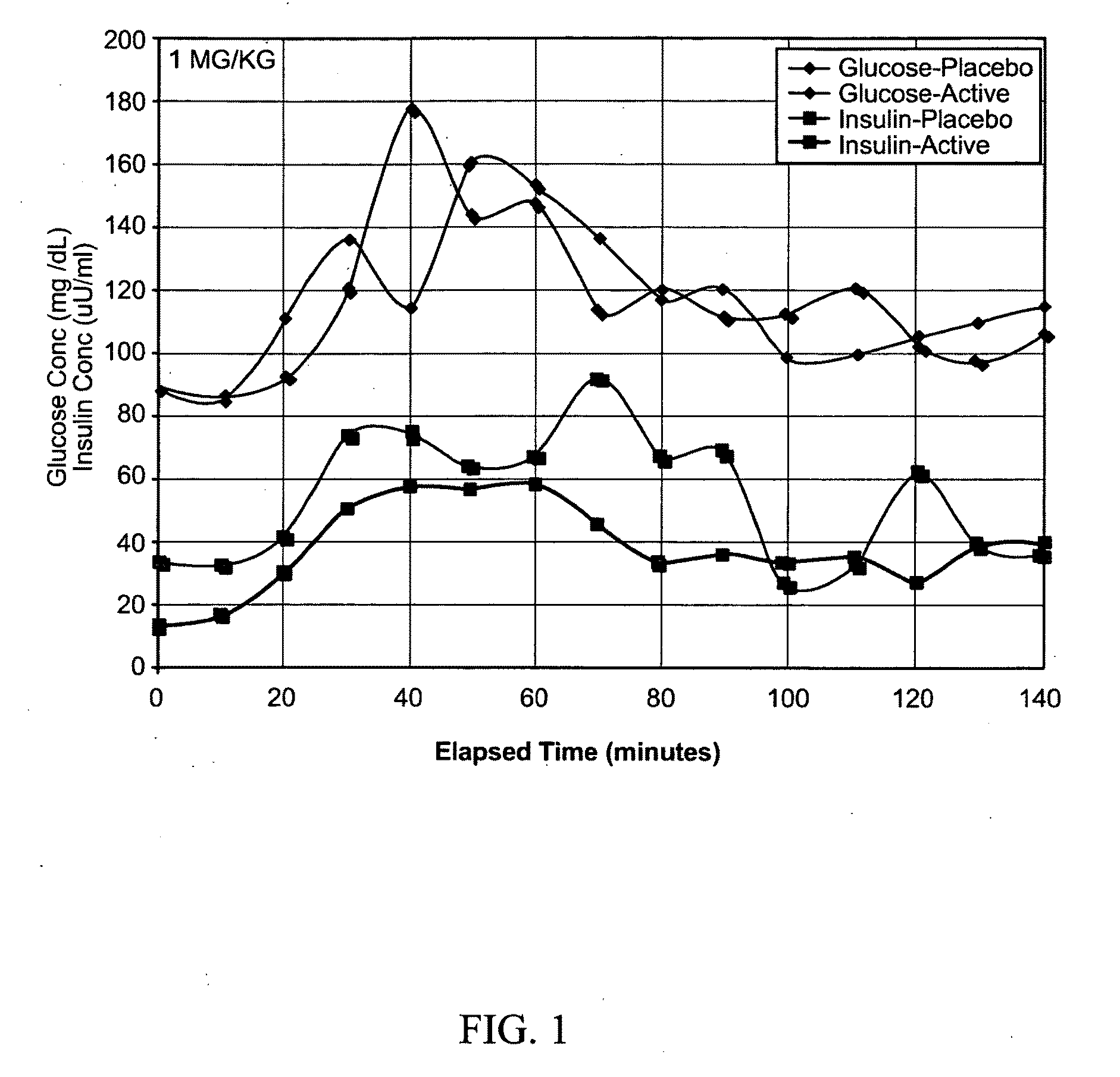
The present invention encompasses a model-based method for determining insulin sensitivity and glucose absorption from oral glucose tolerance tests or mixed meals. The present invention has several advantages over current methods. The technique requires about four to six blood samples taken over about two to three hours following glucose ingestion and is therefore applicable to large-scale clinical trials. The analysis involves a reduced version of the classical minimal model, a method for describing glucose absorption using only two parameters, and an integral approach enabling the parameters to be obtained using simple algebra. The present method robustly identifies differences in insulin sensitivity in different patient types as well as improvements in insulin sensitivity arising from pharmaceutic therapy. In addition, insulin sensitivity measurements obtained with the present method are highly correlated with results from hyperinsulinemic clamps (r2>0.8). This method is therefore a practical and robust method for determining insulin sensitivity under physiologic conditions.
Owner:VERIDEX LCC
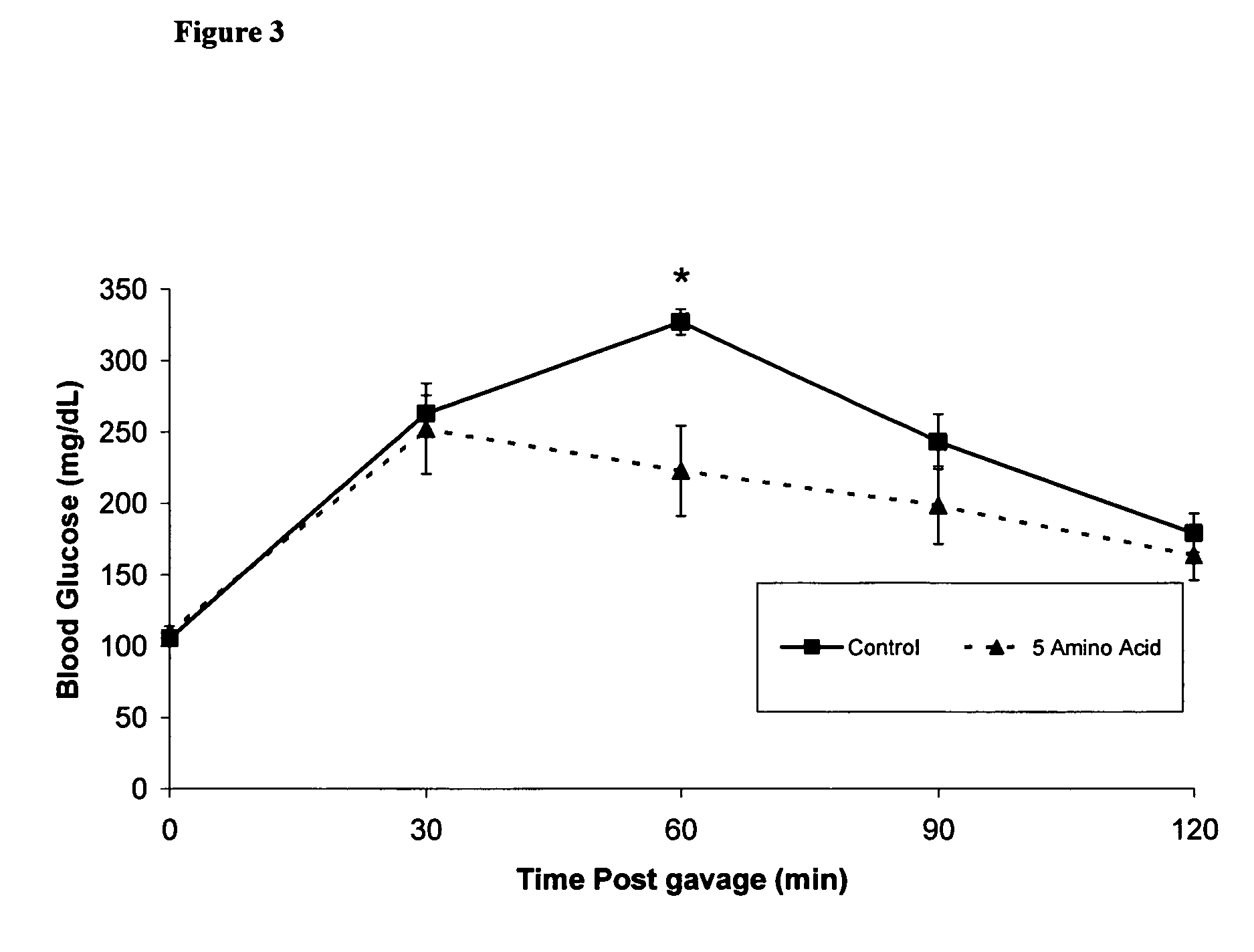
Amino acid composition for improving glucose tolerance
ActiveUS7879796B2Add flavorUseful in treatingBiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceGlucose tolerance test
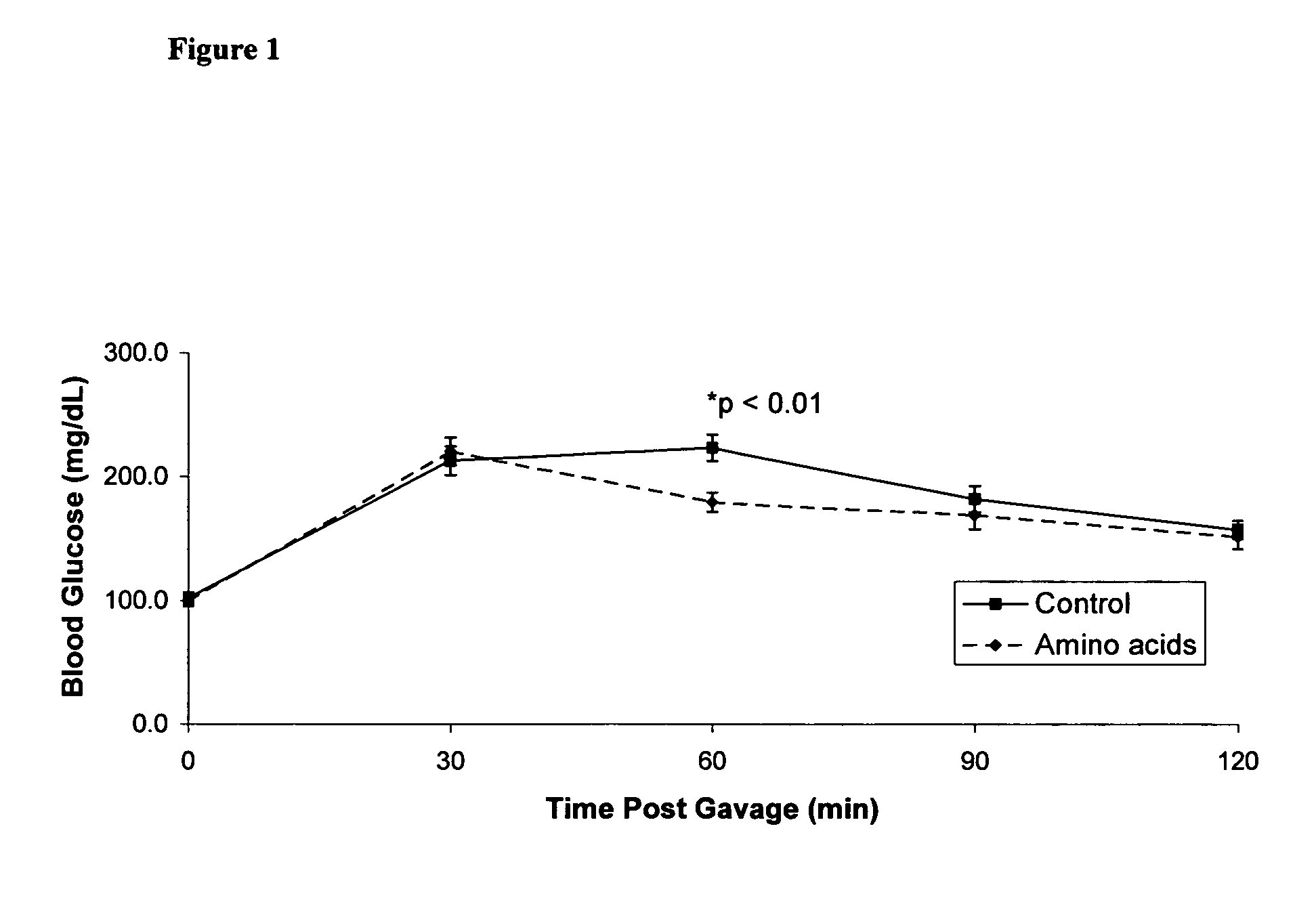
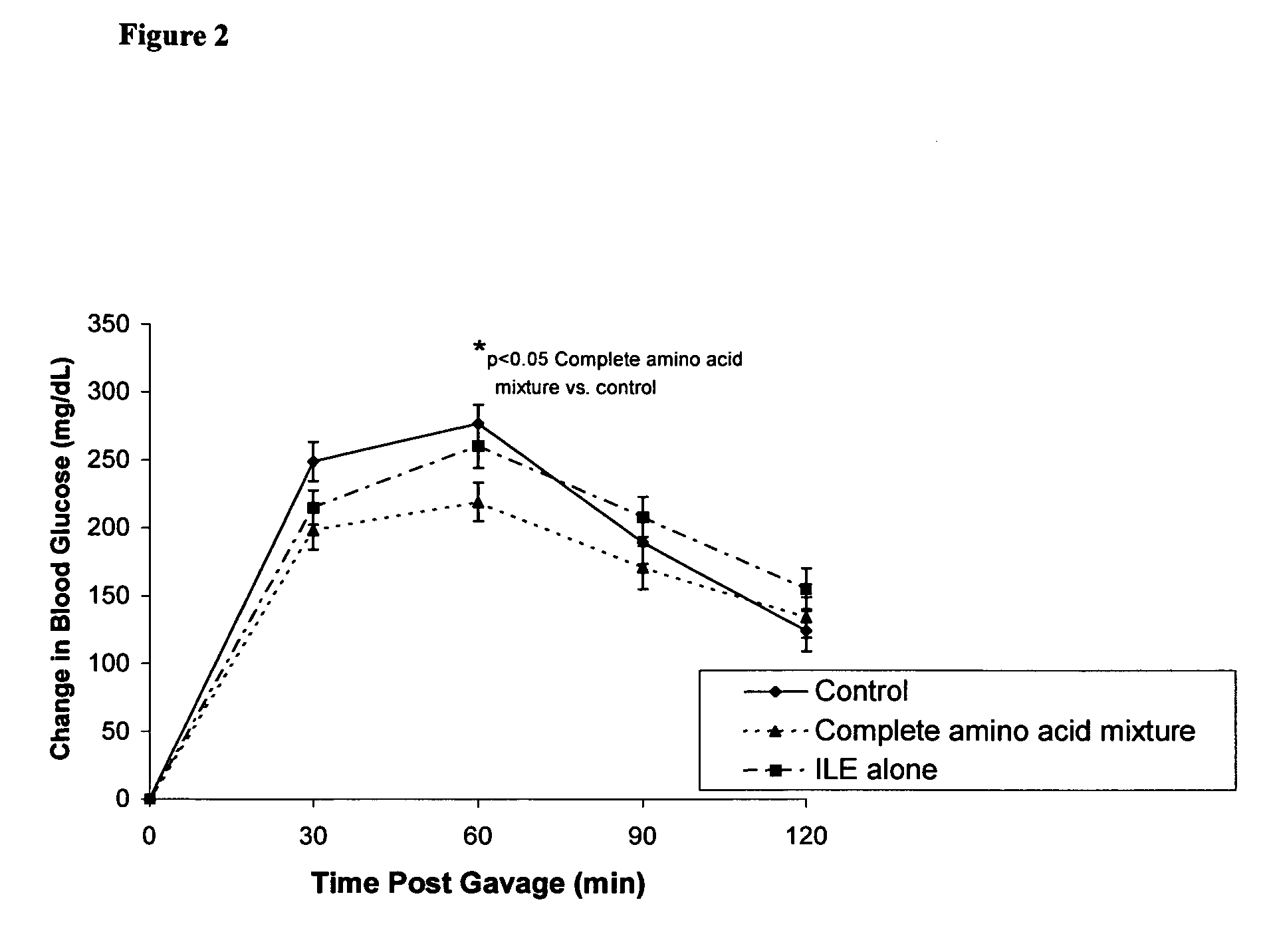
Disclosed are compositions, including low-calorie beverages or liquids, comprising isoleucine, leucine, valine, cysteine, and methionine, in specified amounts, weight ratios, or both. The compositions are especially useful in treating individuals afflicted with impaired glucose tolerance or diabetes.
Owner:ABBOTT LAB INC
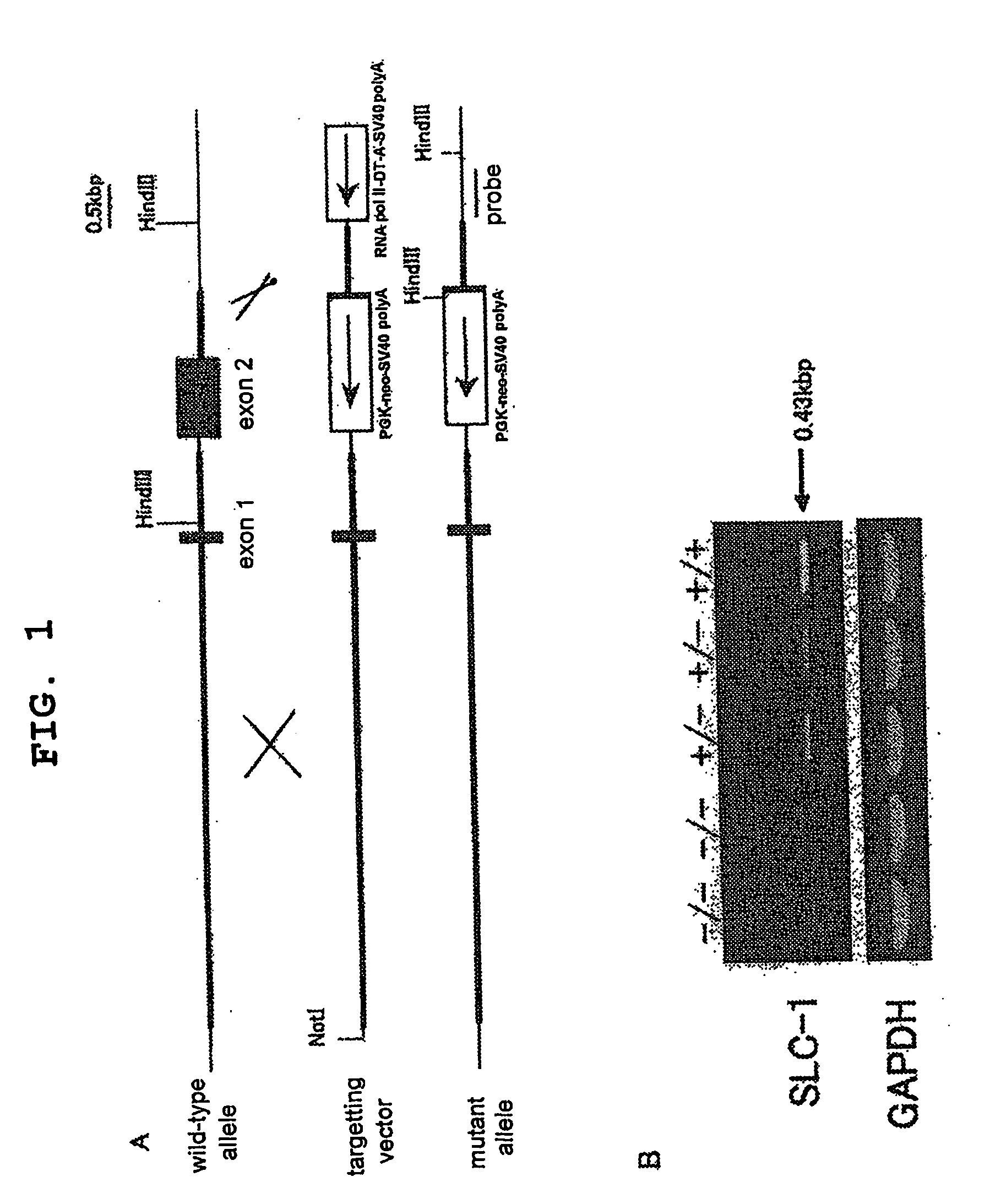
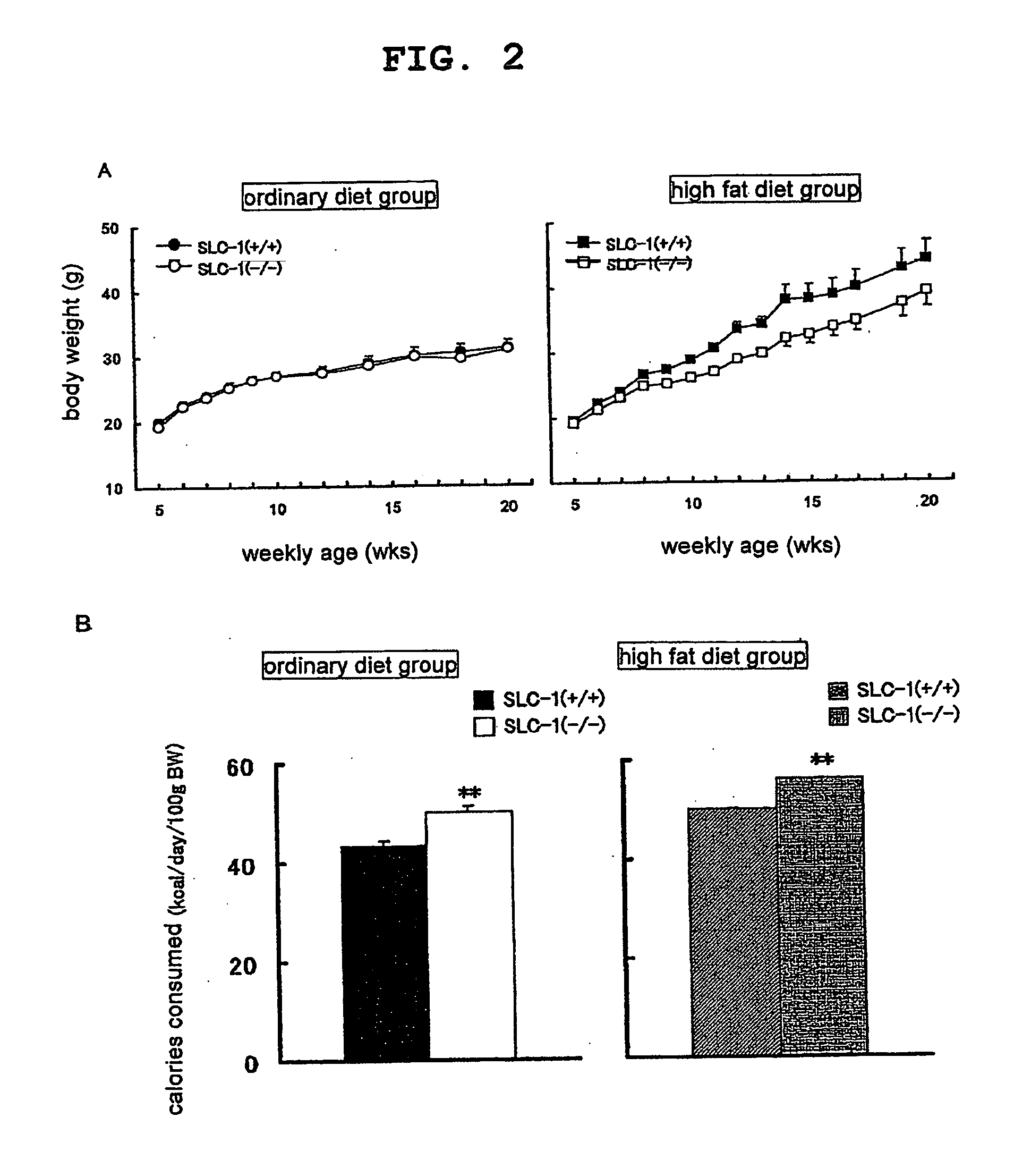
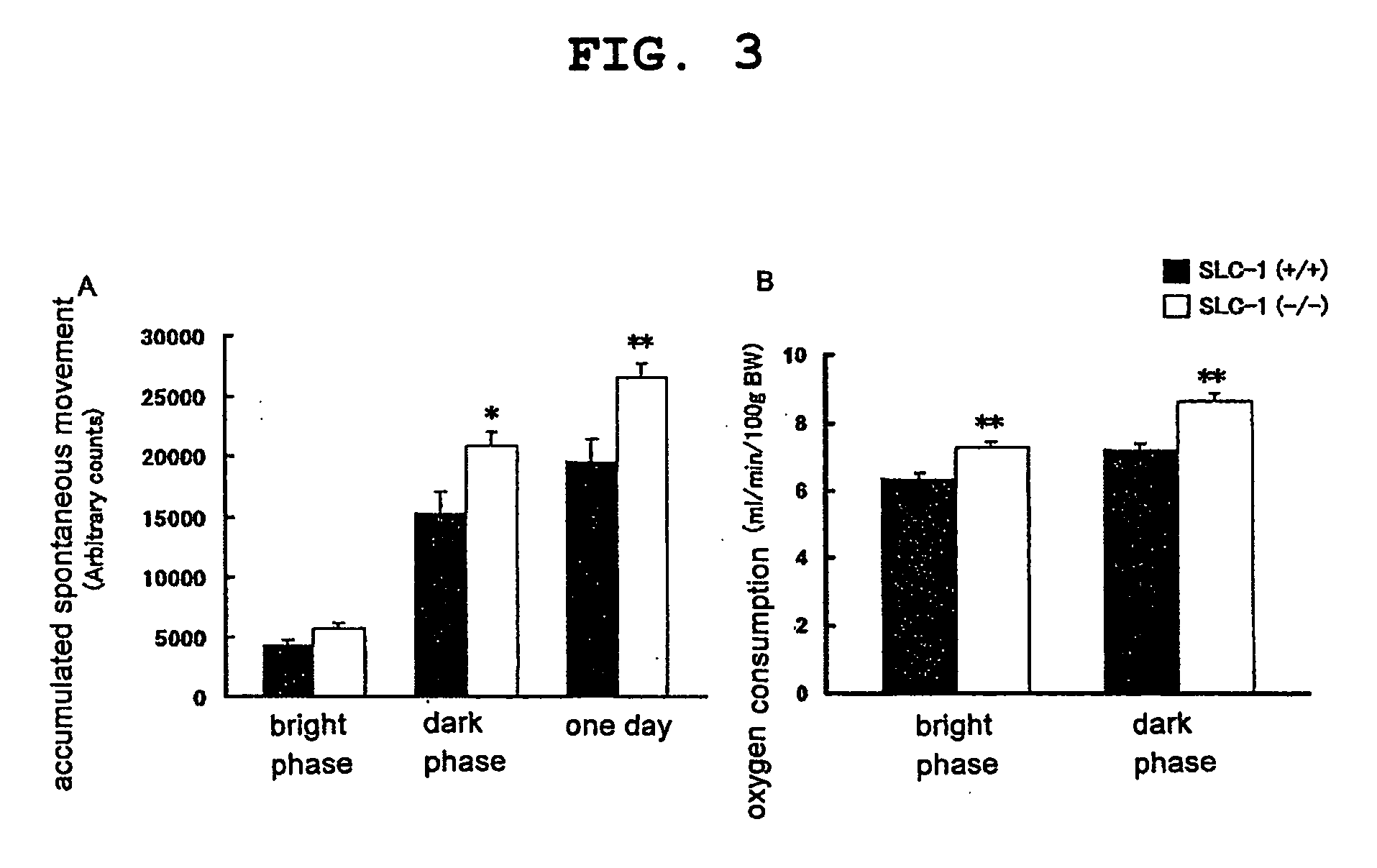
Genetically Modified Animal and Use Thereof
InactiveUS20090186946A1Small fat cell sizeIncrease insulin sensitivityBiocideOrganic active ingredientsHigh resistanceAcute hyperglycaemia
The present invention provides a non-human mammal deficient in the expression of the SLC-1 gene, having the characteristics of (1) a lower blood insulin level in glucose tolerance test, (2) increased insulin sensitivity, (3) higher resistance to obesity even on high fat diet, (4) a smaller white fat cell size, and (5) accentuated lipolysis, compared with the corresponding wild-type animal, or a portion of the body thereof. Also provided is an obesity and / or type II diabetes model non-human mammal that is deficient in the expression of the SLC-1 gene, having the characteristics of (1) elevated expression of adiponectin, (2) delayed onset of hyperglycemia, (3) a lower blood glycohemoglobin level, and (4) accentuated energy consumption, compared with the corresponding obesity and / or type II diabetes model non-human mammal wherein the expression of the gene is normal, or a portion of the body thereof.
Owner:TAKEDA PHARMACEUTICALS CO LTD
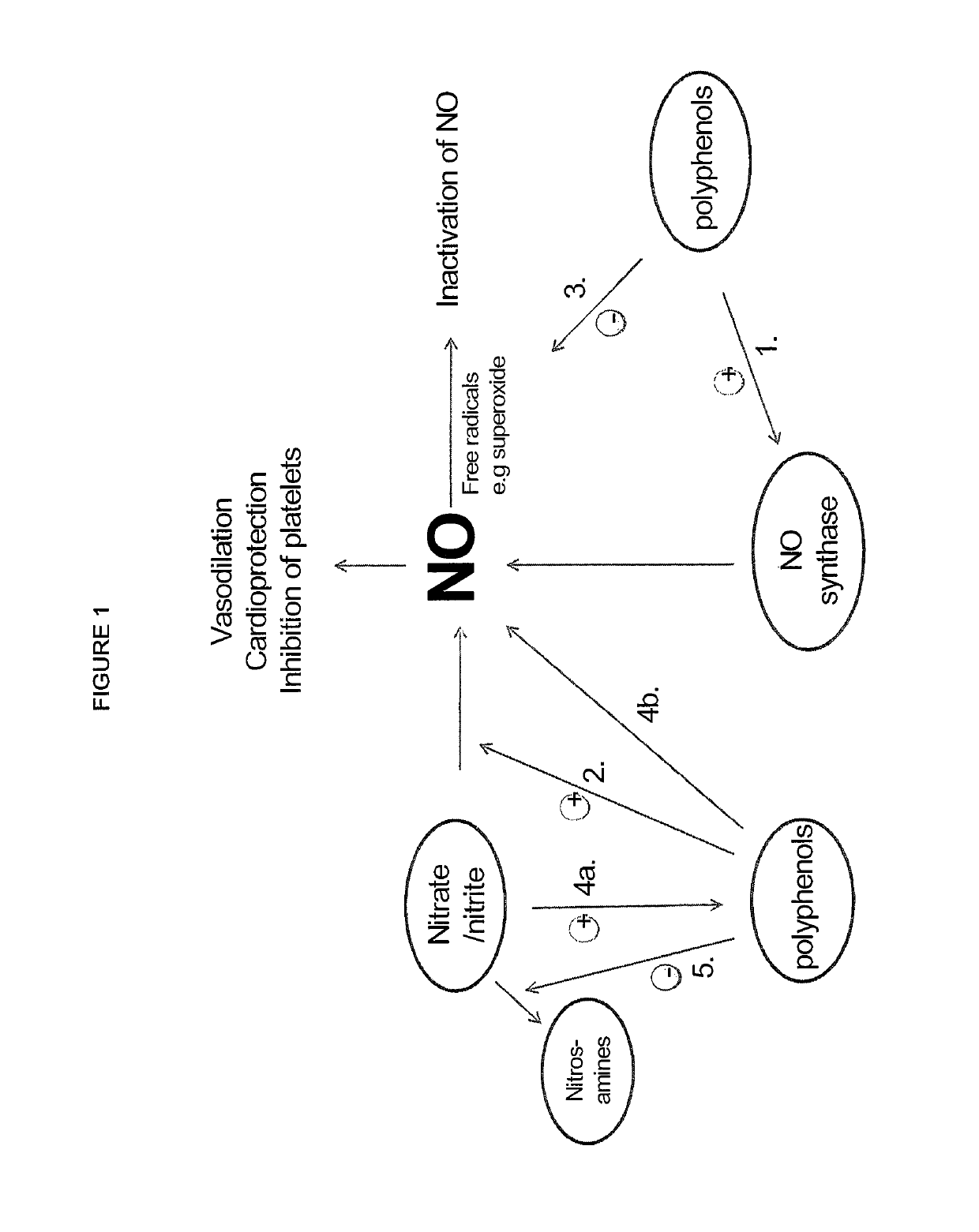
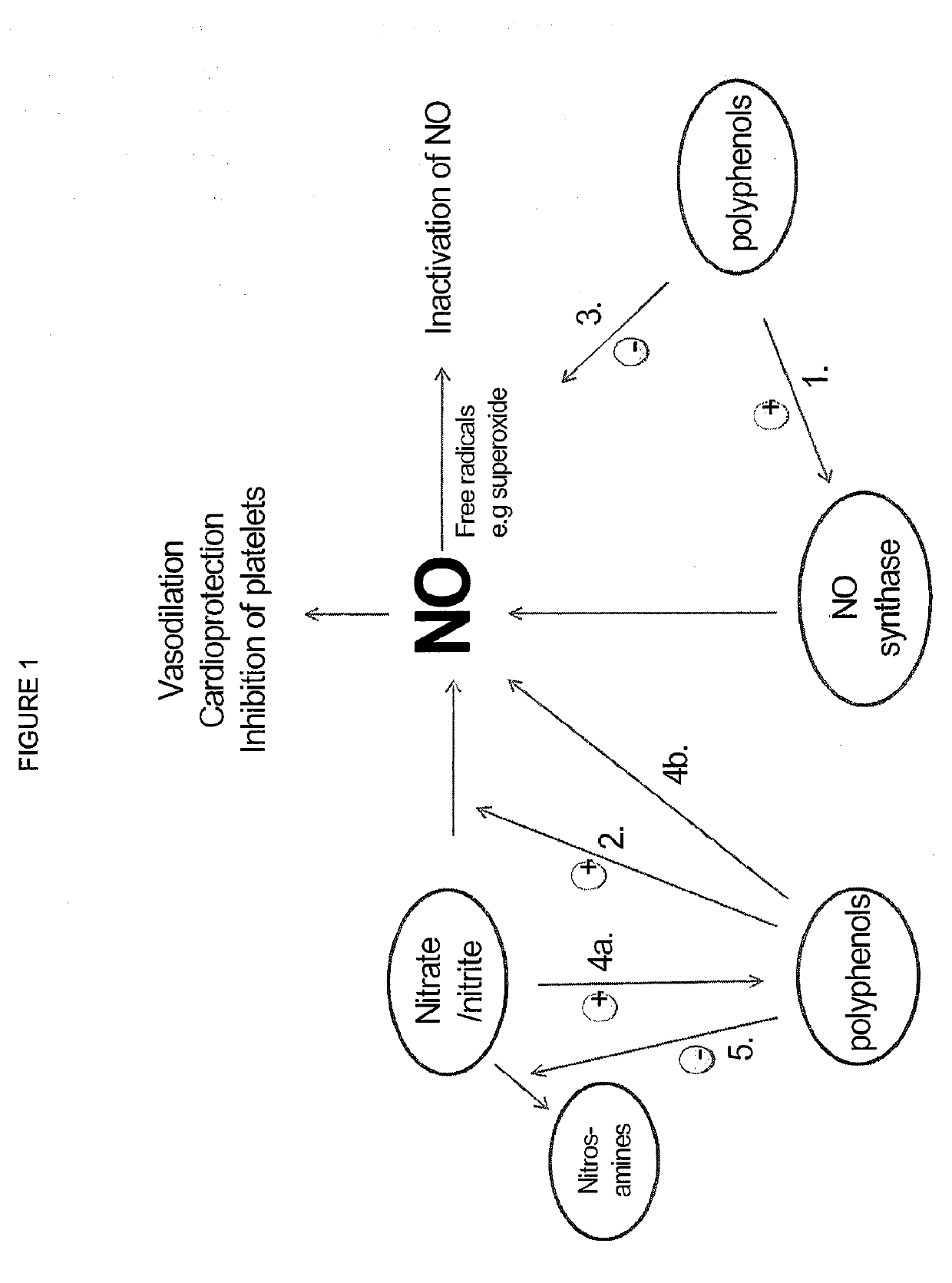
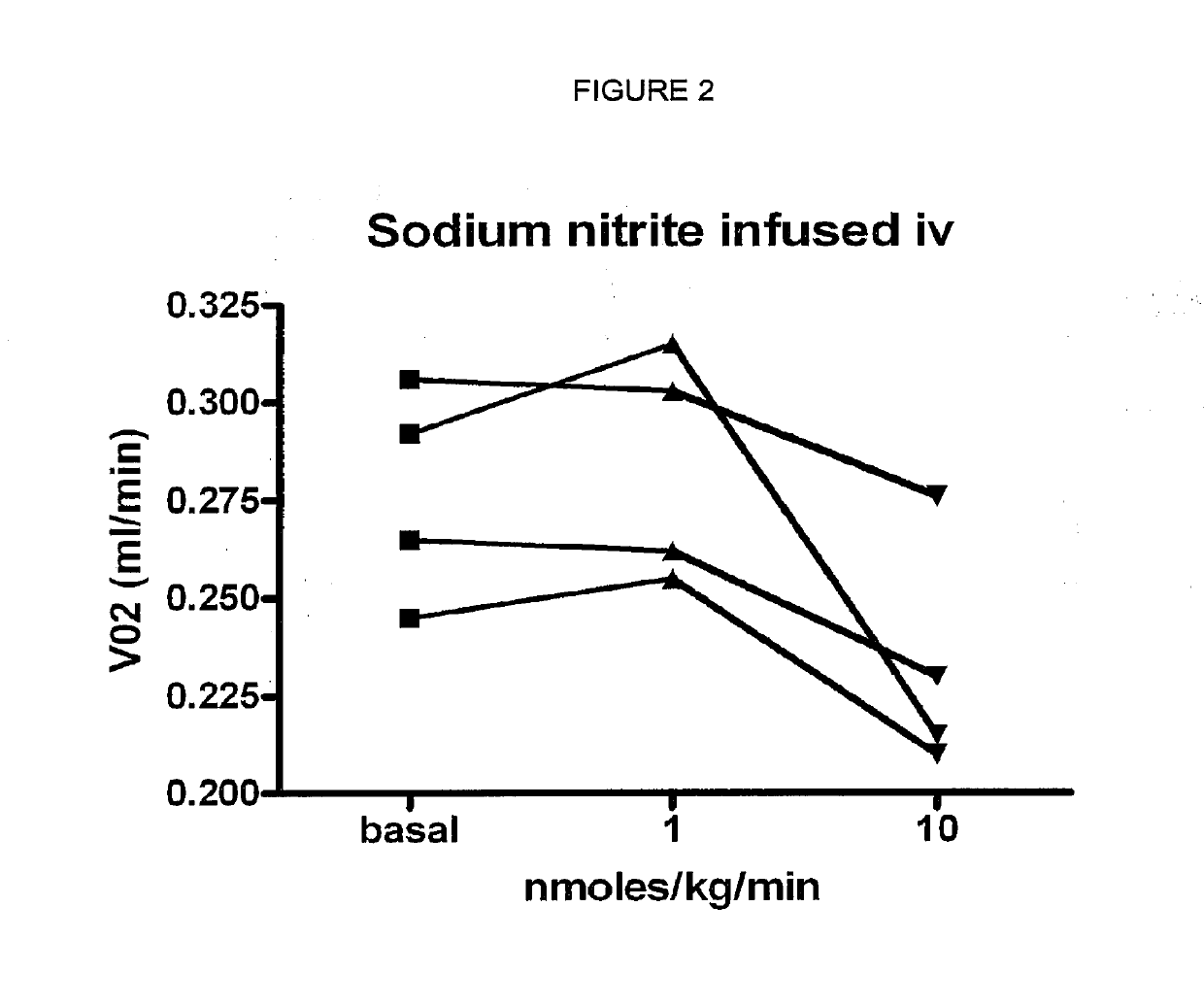
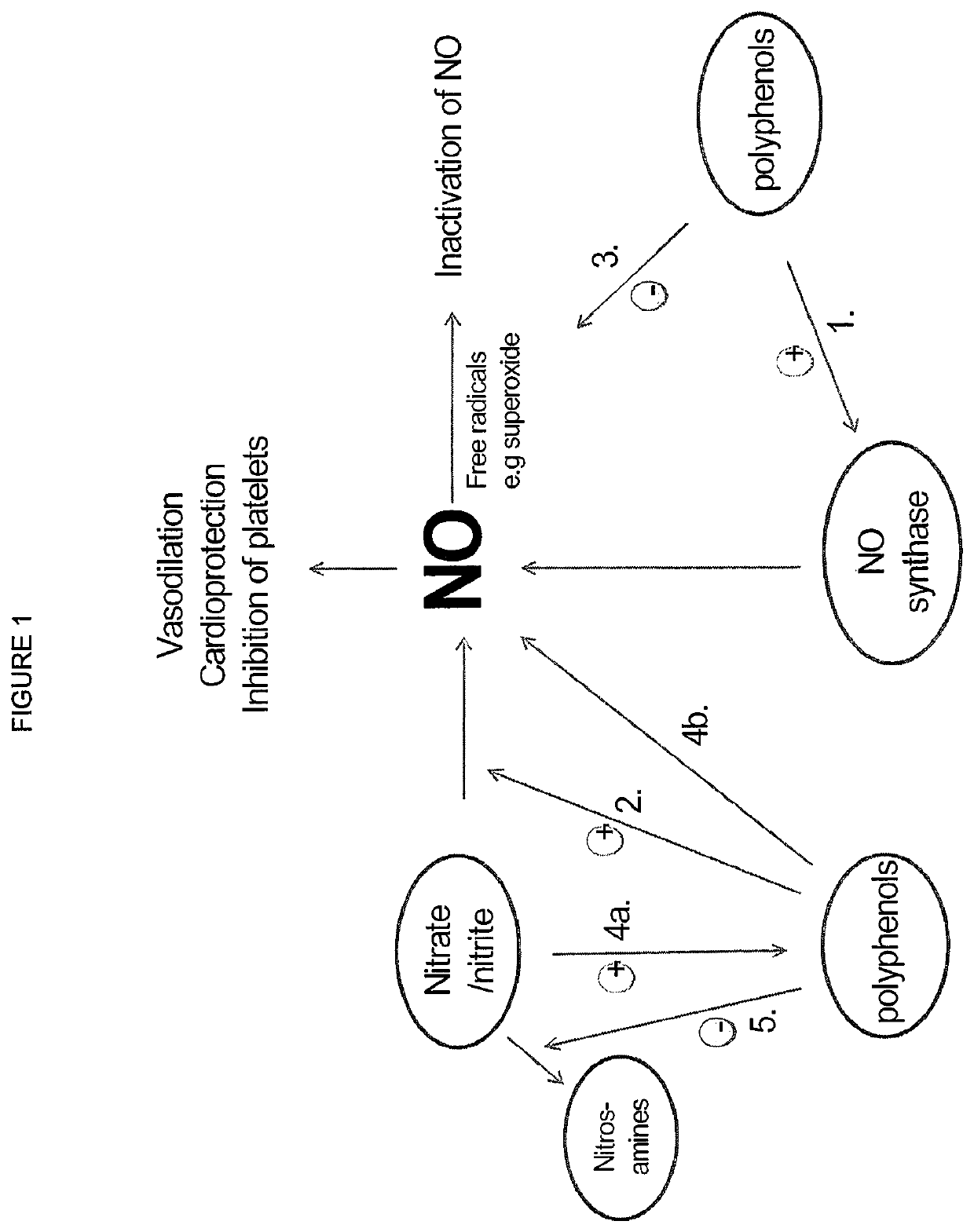
Use of nitrites and nitrates and compositions containing these
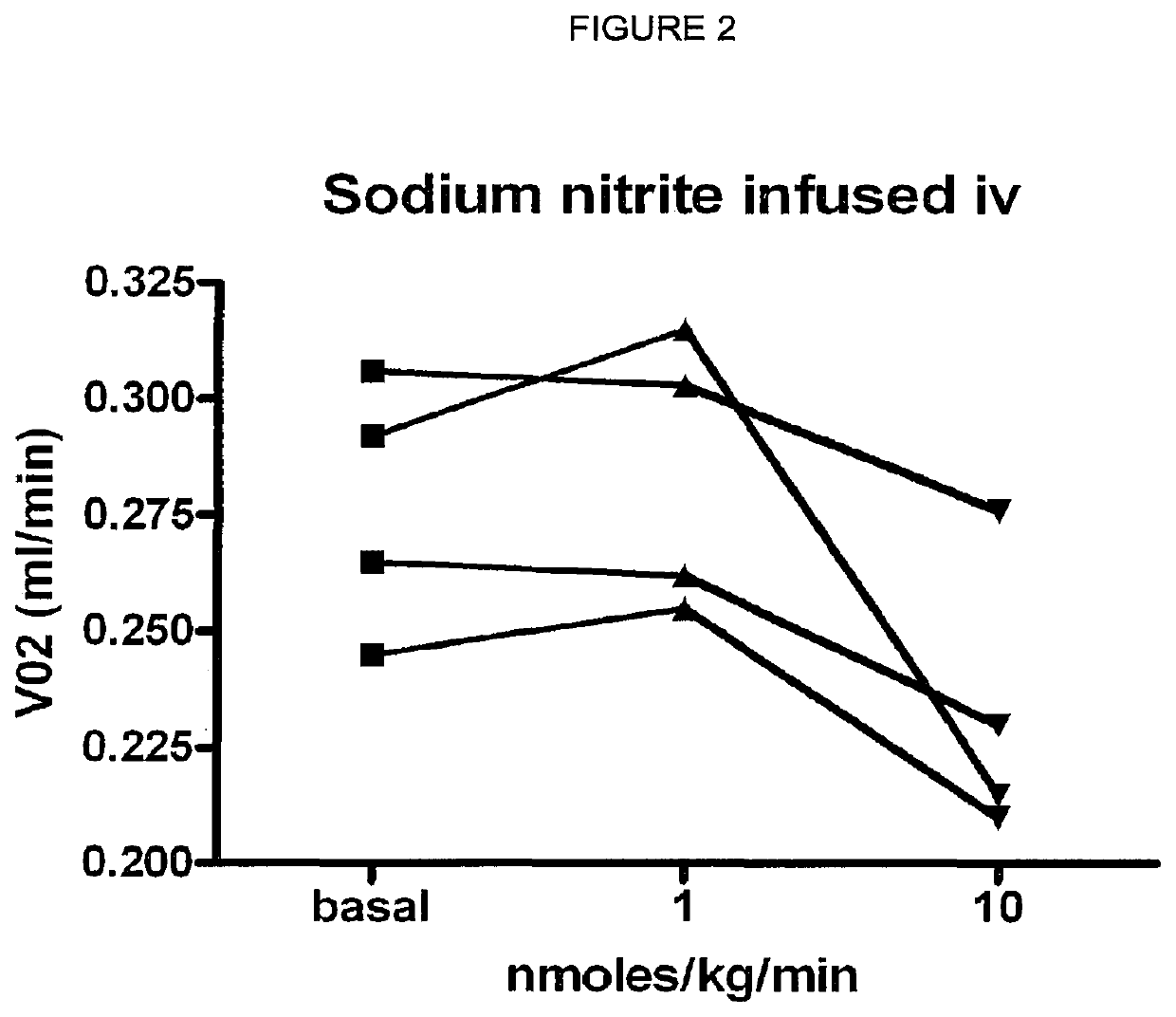
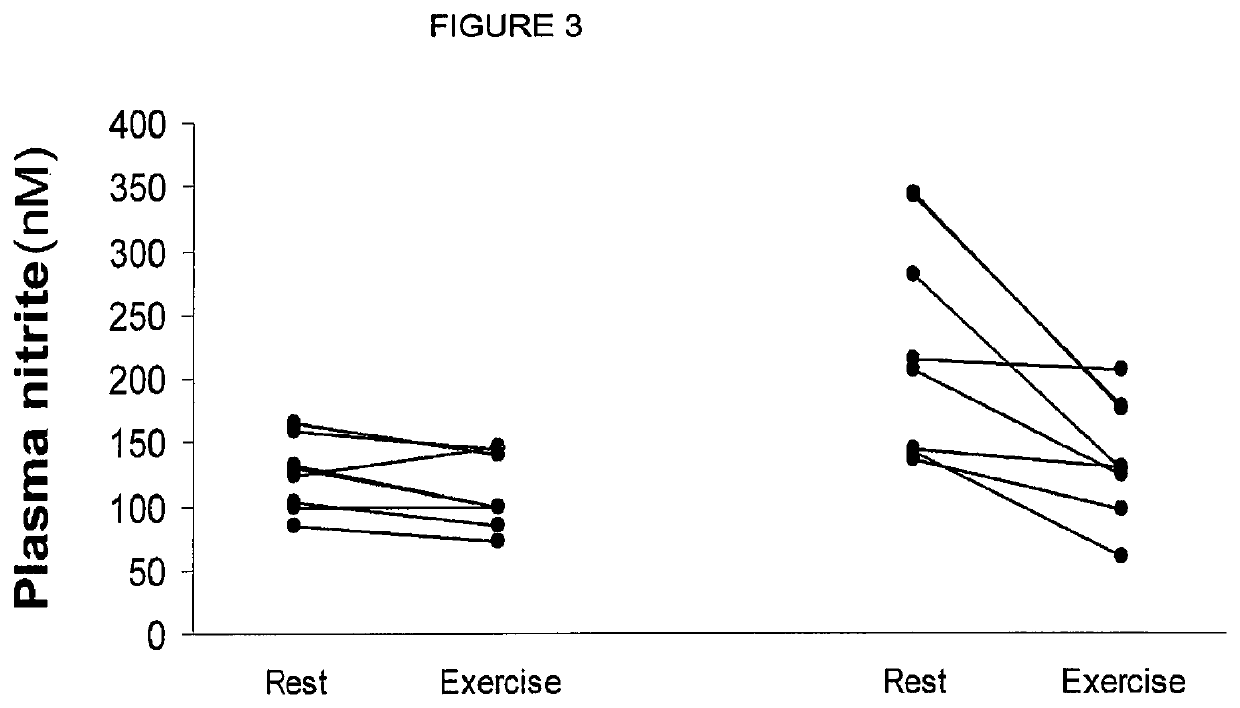
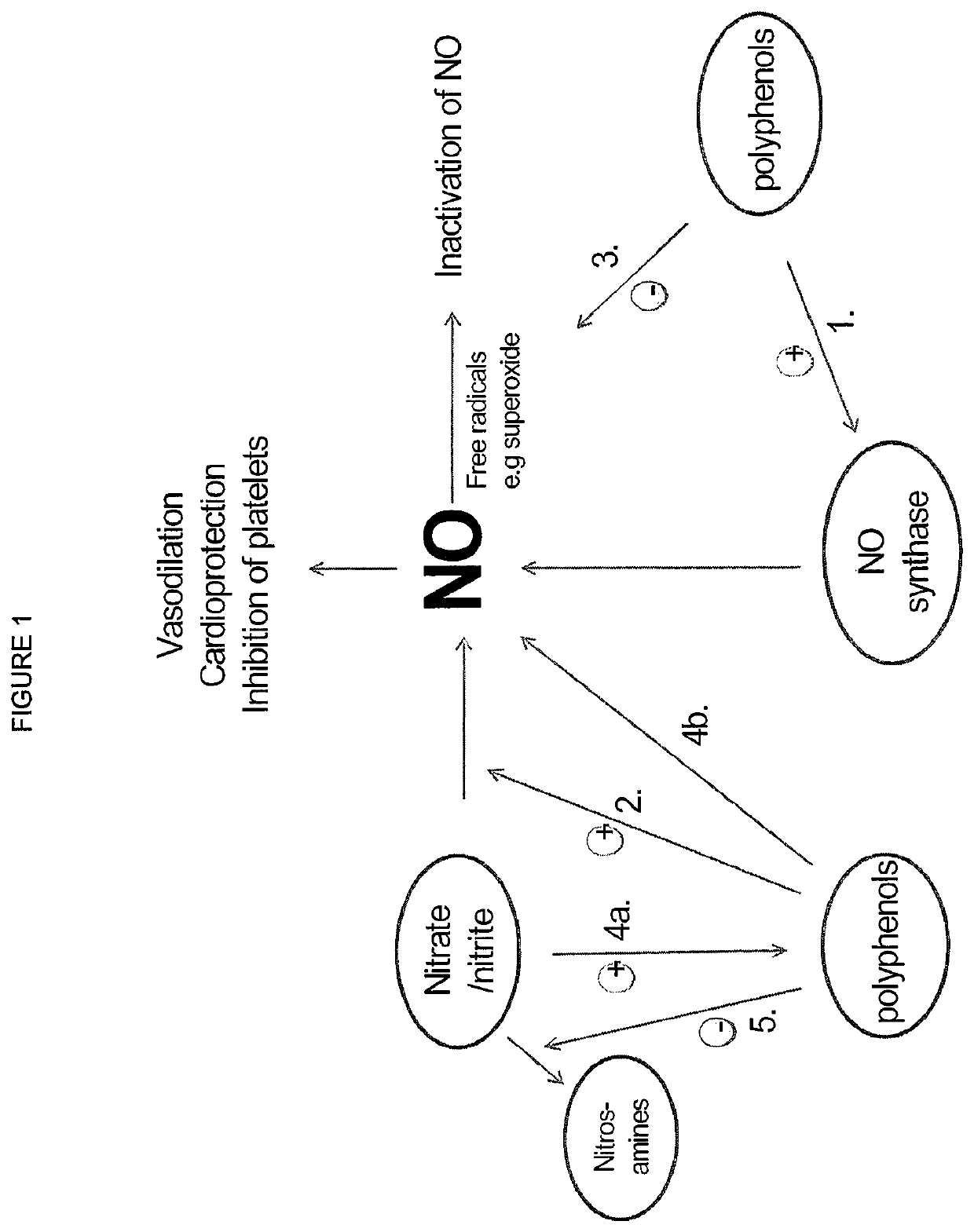
ActiveUS10406118B2Increase of methemoglobin levelReduce oxygen consumptionHydroxy compound active ingredientsMetabolism disorderResting energy expenditureO2 consumption
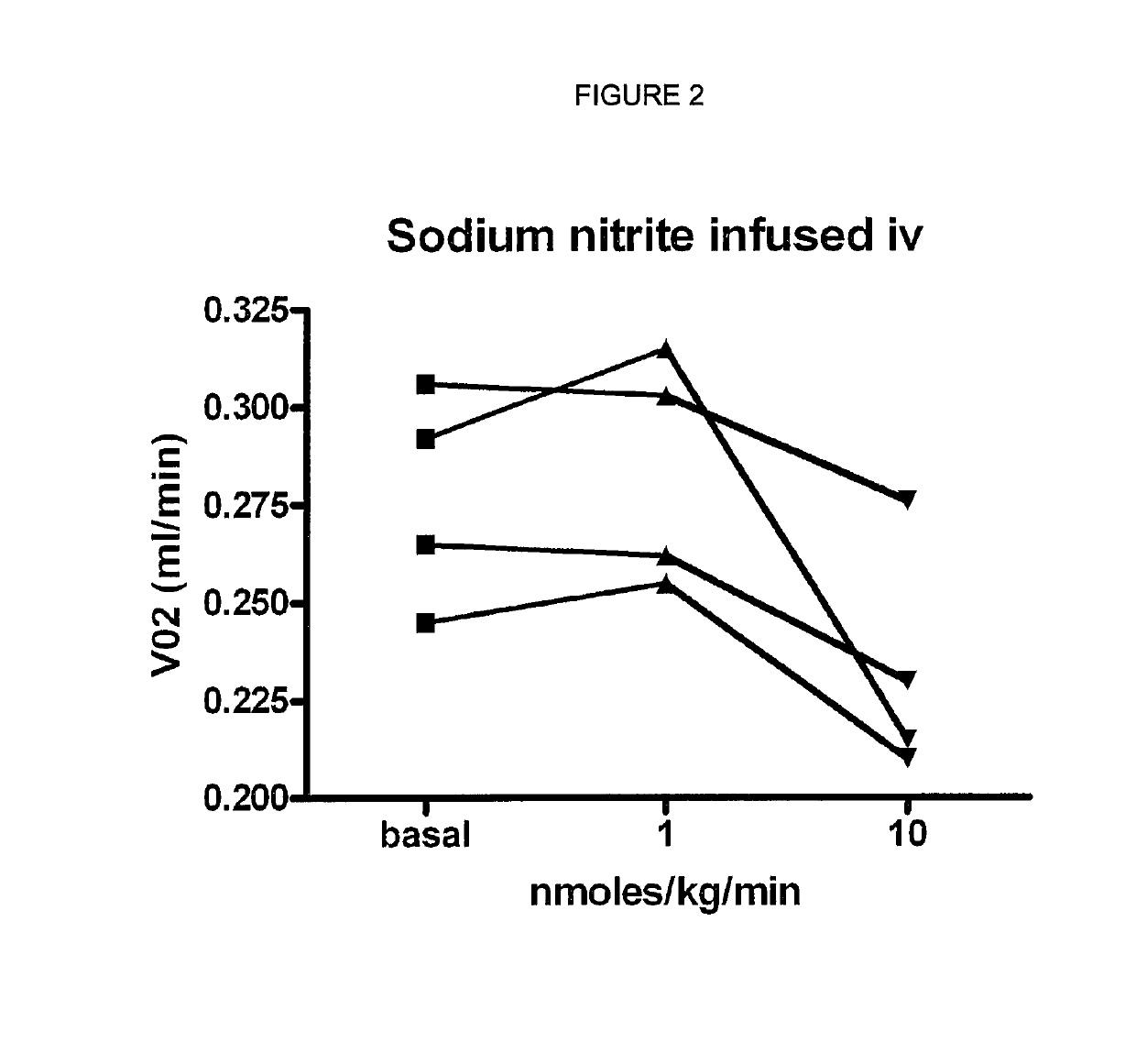
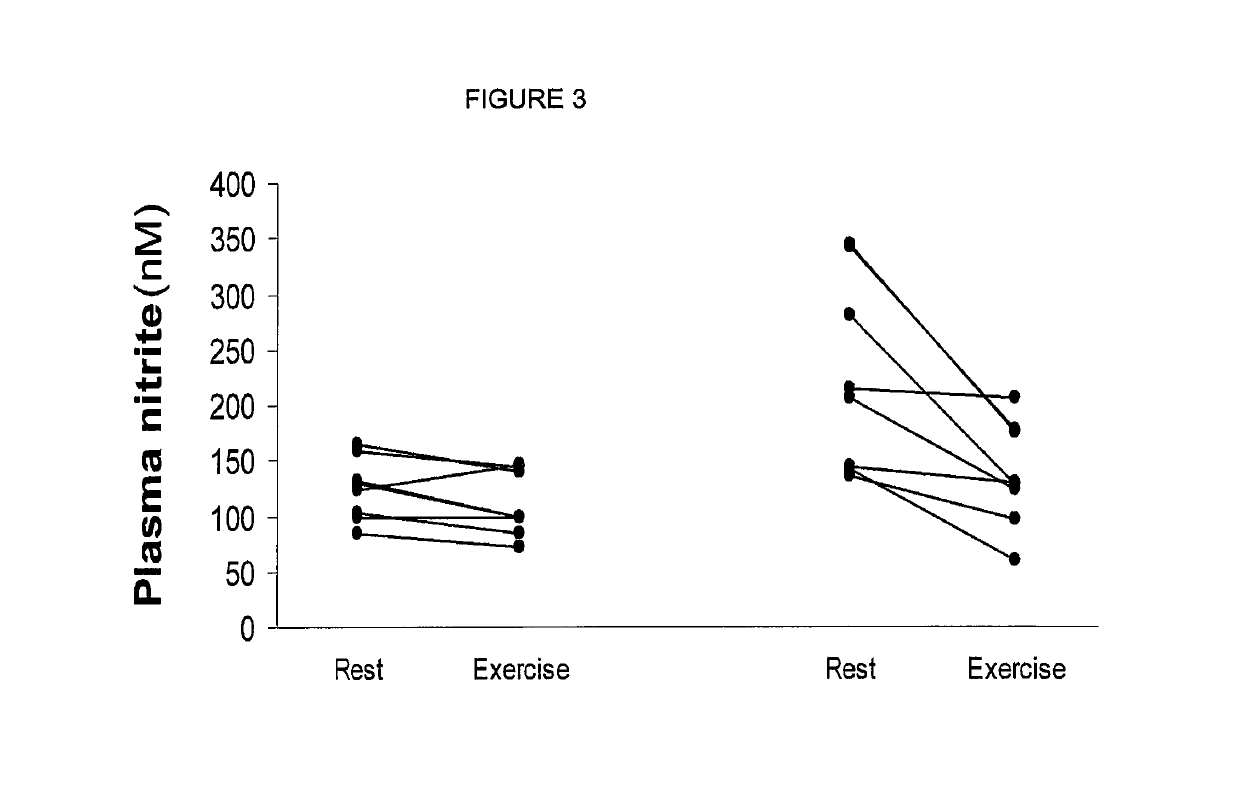
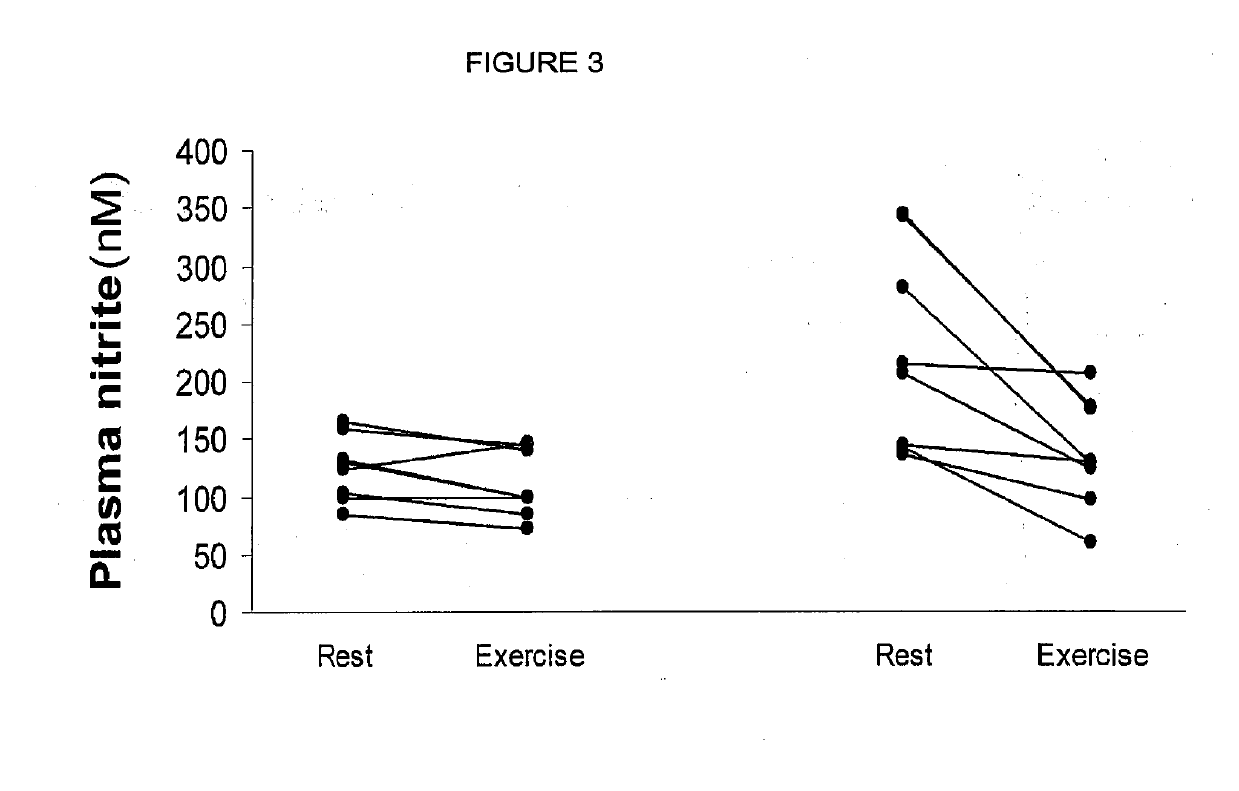
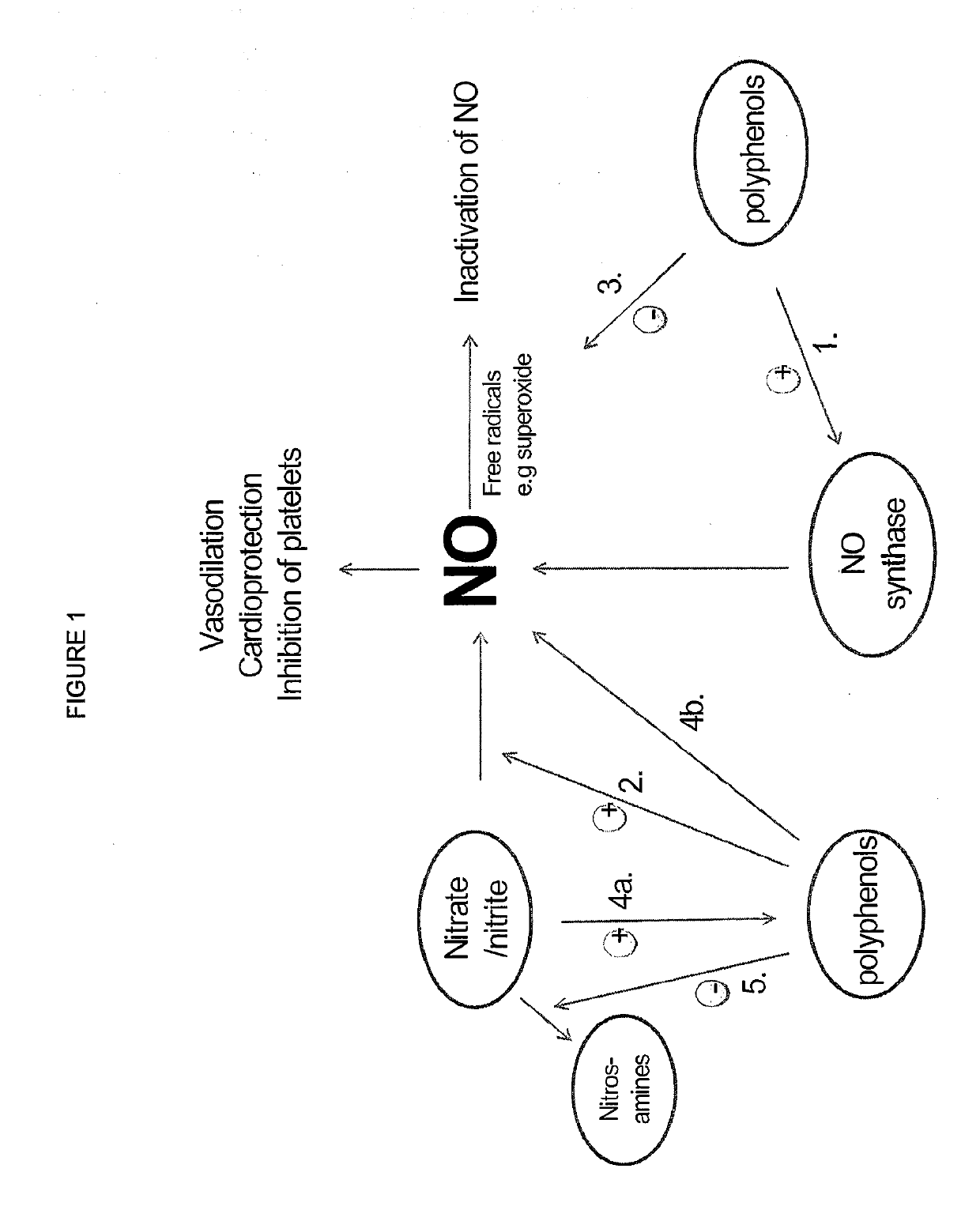
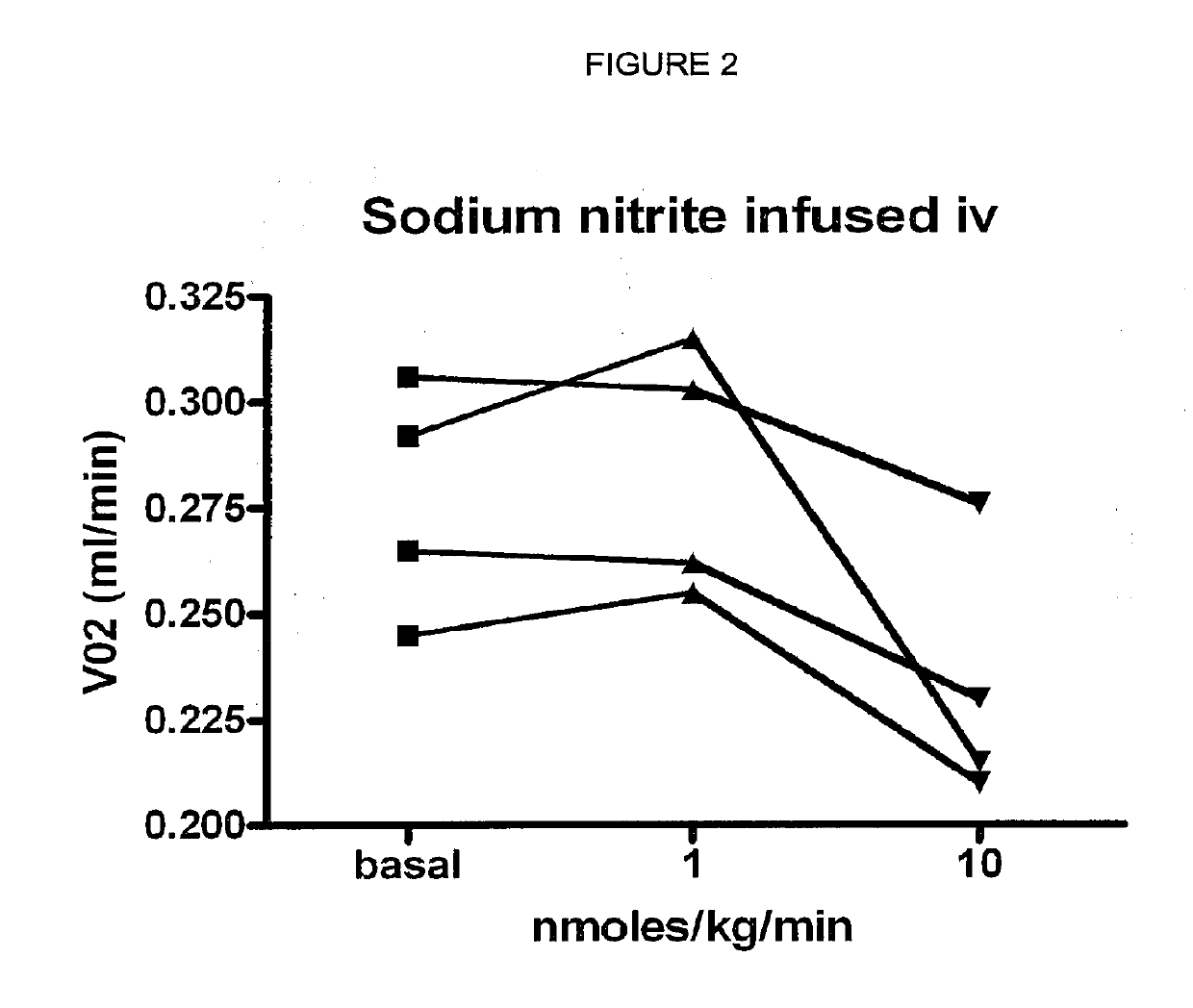
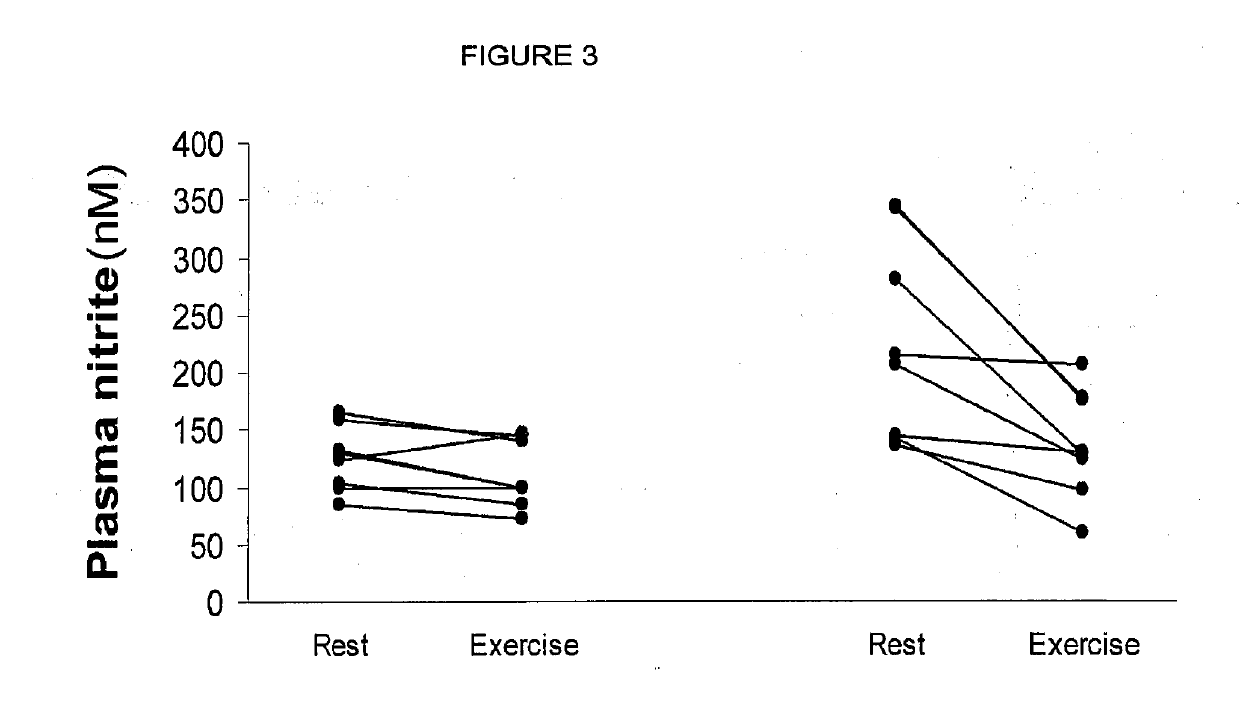
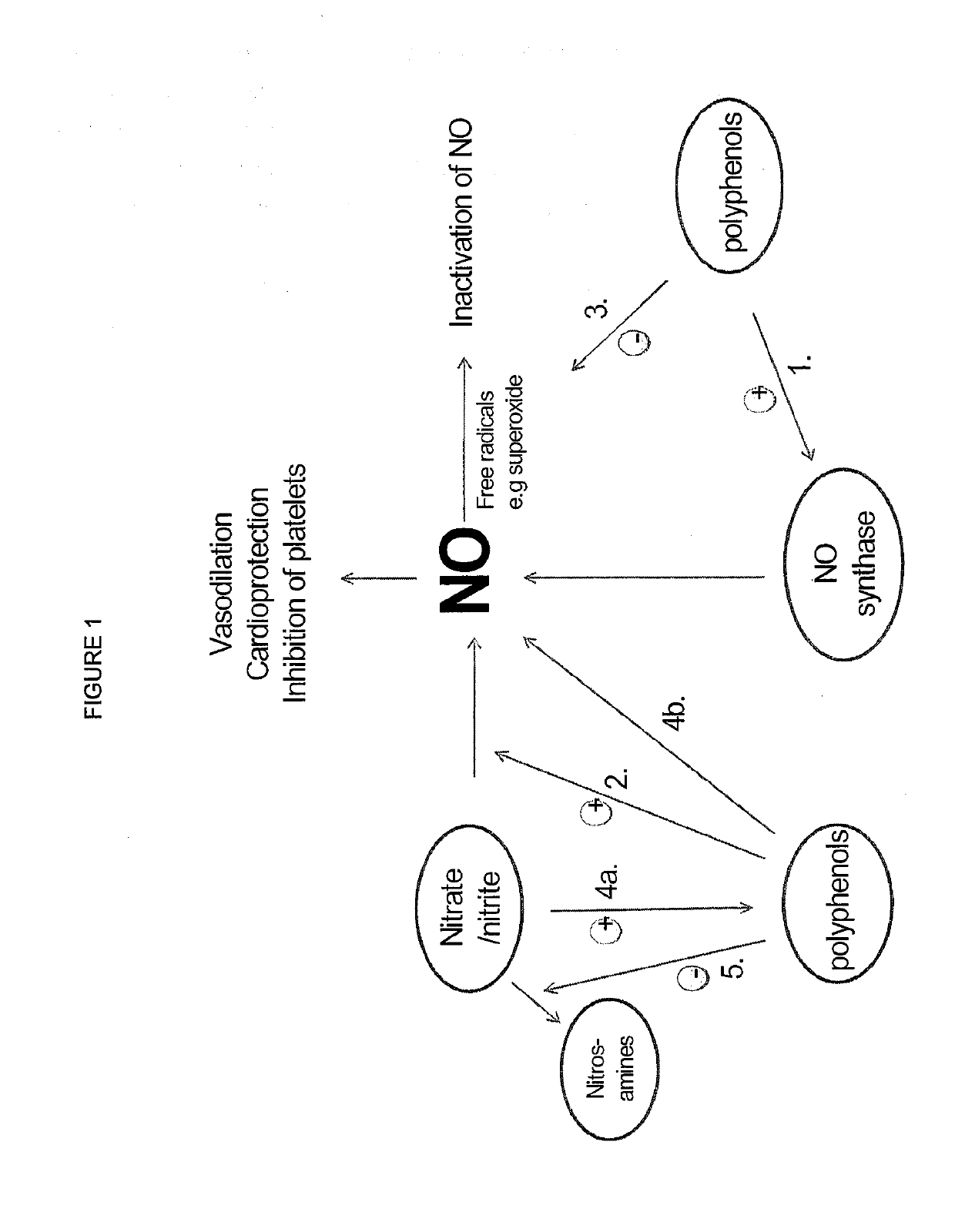
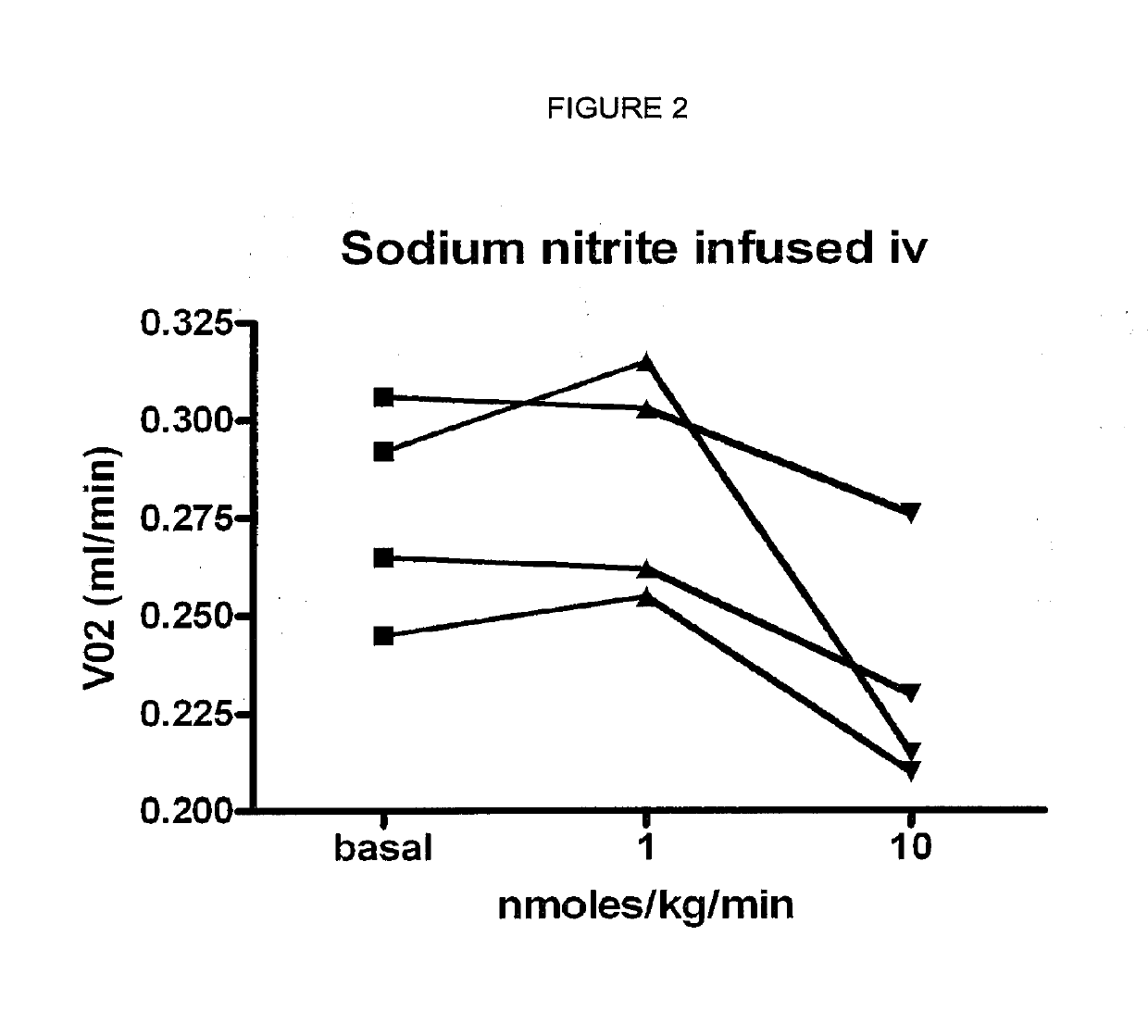
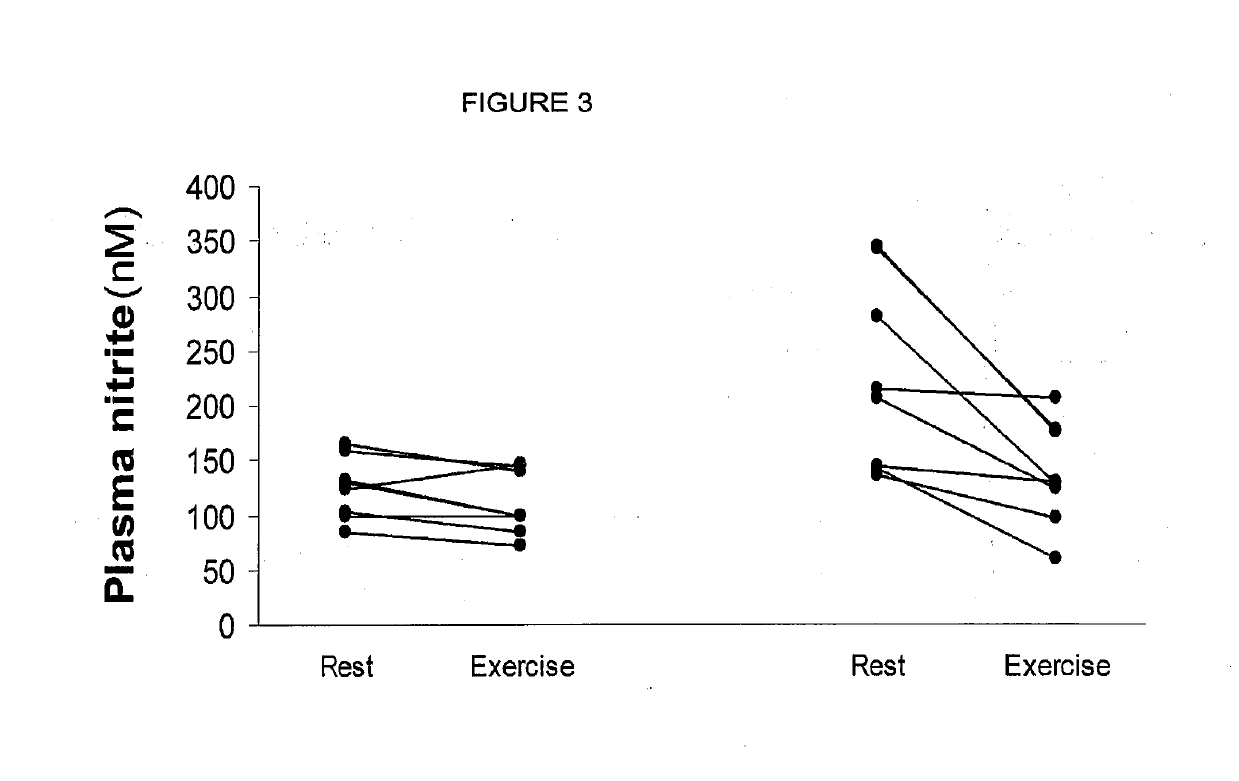
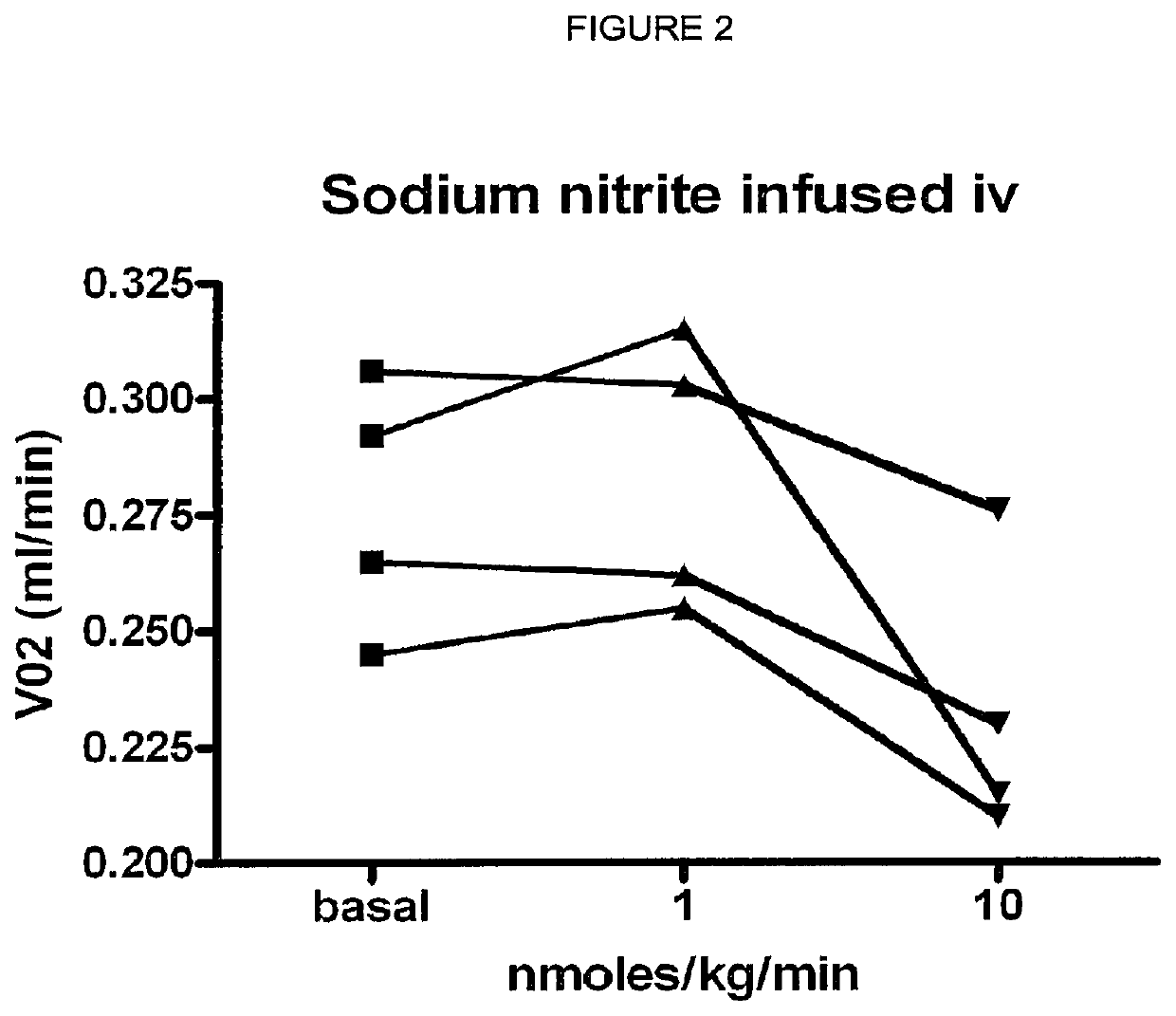
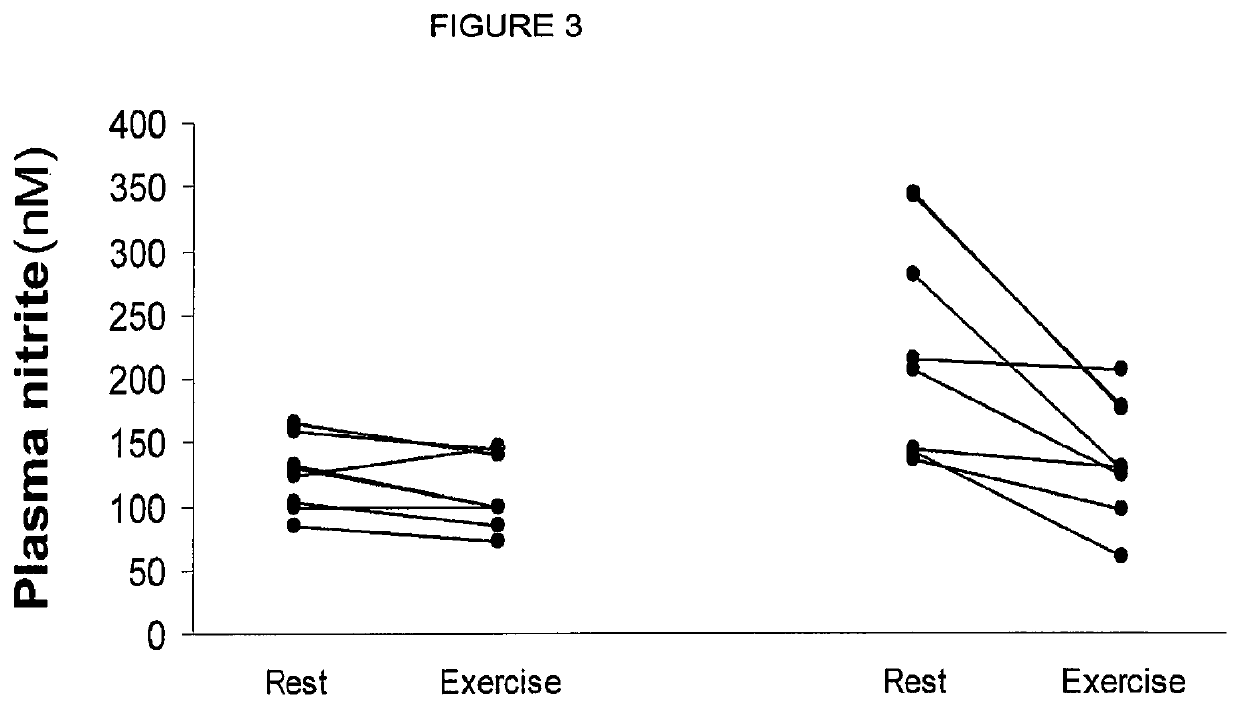
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a drop in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
A method for determining insulin sensitivity and glucose absorption
InactiveCN101677764AHealth-index calculationMicrobiological testing/measurementPresent methodPatient type
The present invention encompasses a model-based method for determining insulin sensitivity and glucose absorption from oral glucose tolerance tests or mixed meals. The present invention has several advantages over current methods. The technique requires about four to six blood samples taken over about two to three hours following glucose ingestion and is therefore applicable to large-scale clinical trials. The analysis involves a reduced version of the classical minimal model, a method for describing glucose absorption using only two parameters, and an integral approach enabling the parametersto be obtained using simple algebra. The present method robustly identifies differences in insulin sensitivity in different patient types as well as improvements in insulin sensitivity arising from pharmaceutic therapy. In addition, insulin sensitivity measurements obtained with the present method are highly correlated with results from hyperinsulinemic clamps (r<2>>0.8). This method is thereforea practical and robust method for determining insulin sensitivity under physiologic conditions.
Owner:VERIDEX LCC
Diagnostic composition for diabetes type-2 and impaired glucose tolerance, and methods of use
InactiveCN1836167AConsistent ingredientsCompounds screening/testingDisease diagnosisDiagnostic testMonosaccharide metabolism
Owner:CEAPRO
Quantitative glucose beverage for glucose tolerance detection test and production technology thereof
InactiveCN107019135AClear ingredientsHigh purityFood ingredient as thickening agentFood ingredient as colourRetention timeDigestion
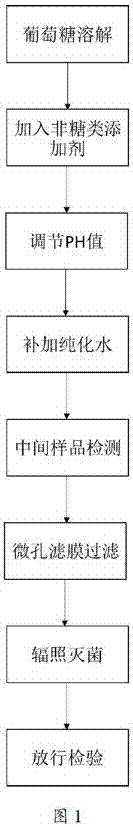
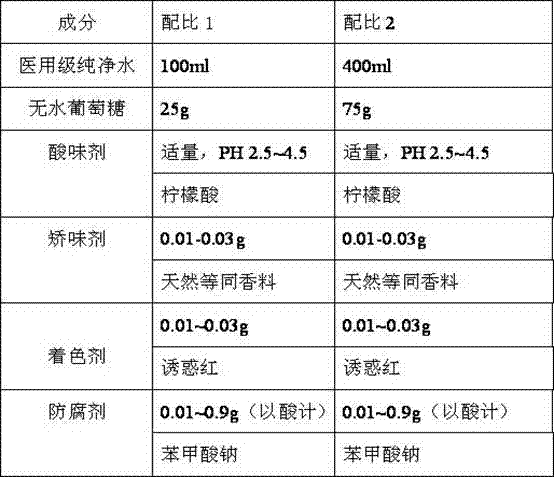
The invention provides a quantitative glucose beverage for glucose tolerance detection test and a production technology thereof. The quantitative glucose beverage is prepared from 100 to 400ml of purified water, 25g or 50g or 75g or 100g of anhydrous glucose, and 0.1 to 10g of non-sugar additive, wherein the non-sugar additive is selected from one or several of a preservative, an acidulant, a flavoring agent, a coloring agent, an emulsifier and a thickener. The quantitative glucose beverage has the advantages that the added components of non-sugar additive are clear, the purity is higher, and the obtained quantitative glucose beverage for glucose tolerance detection test has the advantages of sterility, accurate metering, good mouth feel, high safety property and the like; no sugar is introduced, so that the influence to the result is avoided; while the safety of medicines or apparatuses or foods is guaranteed, the liquid has a pleasant color, so that the purposes of flavoring and coloring are realized; by adding the thickener, the retention time of the glucose beverage in digestive tracts is effectively prolonged, and the digestion function of a patient is restored.
Owner:岱那生物科技(上海)有限公司
Methods for improving glycemic control in humans
InactiveUS20090076111A1Improve blood sugar controlImprove glucose toleranceBiocideOrganic active ingredientsArginineAlkaloid
The present invention is directed to methods for improving glycemic control in humans and animals comprising the step of administering a composition comprising an amino acid content including 4-hydroxyisoleucine in an amount between about 60% and about 70% of a total weight of the amino acid content, together with one or more amino acids selected from the group consisting of glutamate, aspartate, arginine, cysteine, threonine, serine, glycine, alanine, valine, methionine, isoleucine, and histidine (inclusive of any chemical salts, anhydrides, or isomers of any of the foregoing), in addition to, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Fasting blood glucose and glucose tolerance were studied in normal human subjects, in human subjects diagnosed with Metabolic Syndrome X, and in diabetic rats by means of dosing the subjects with compositions comprising 4-hydroxyisoleucine in an amount between about 20% and about 30% of the total weight of the composition, wherein improving glycemic control in standard glucose tolerance tests.
Owner:TSI GROUP
Compositions of nitrates and methods of use thereof
ActiveUS20190183924A1Organic active ingredientsBacteria material medical ingredientsMetabolic rateNitrite
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a reduction in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
Antidiabetic oral insulin-biguanide combination
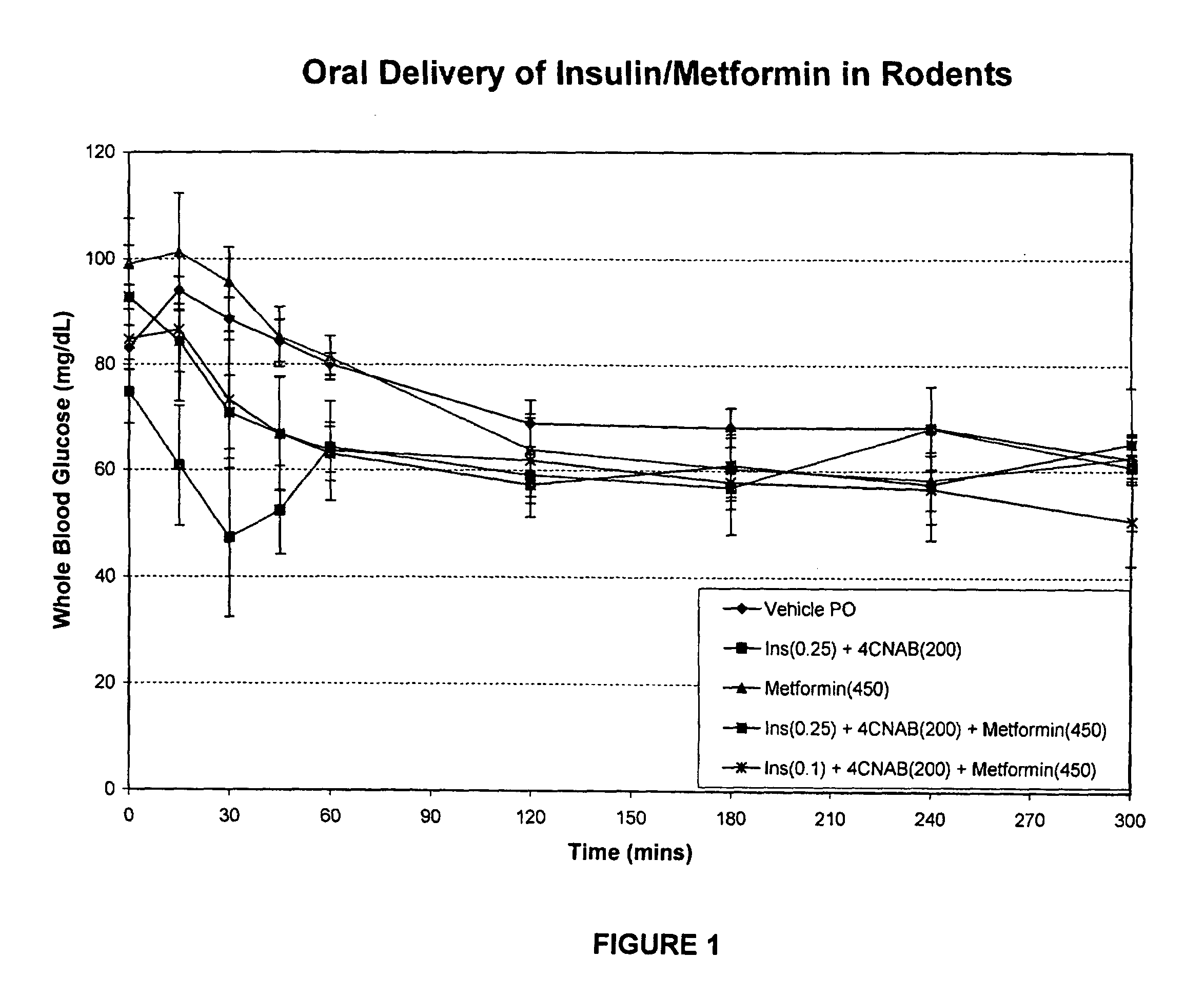
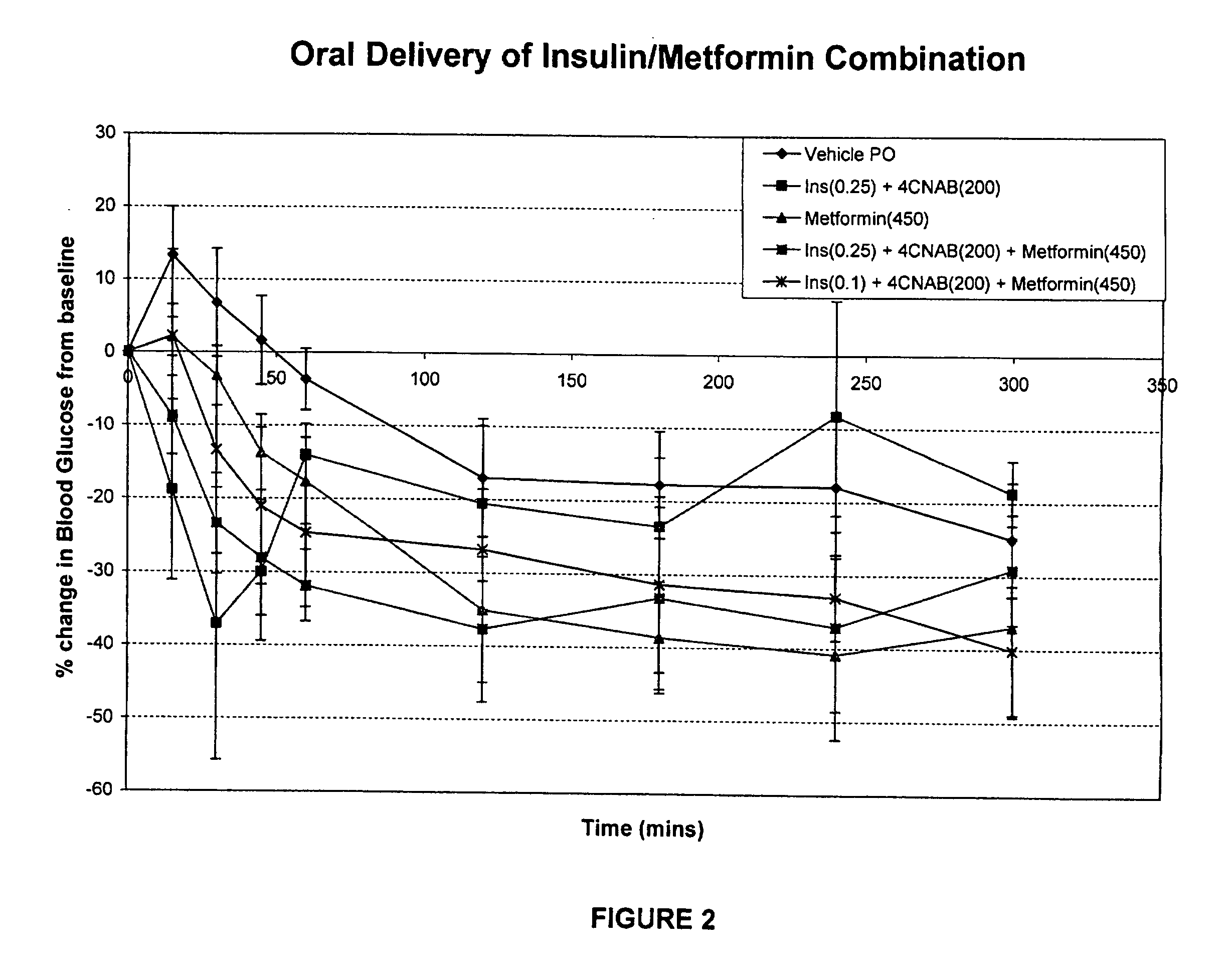
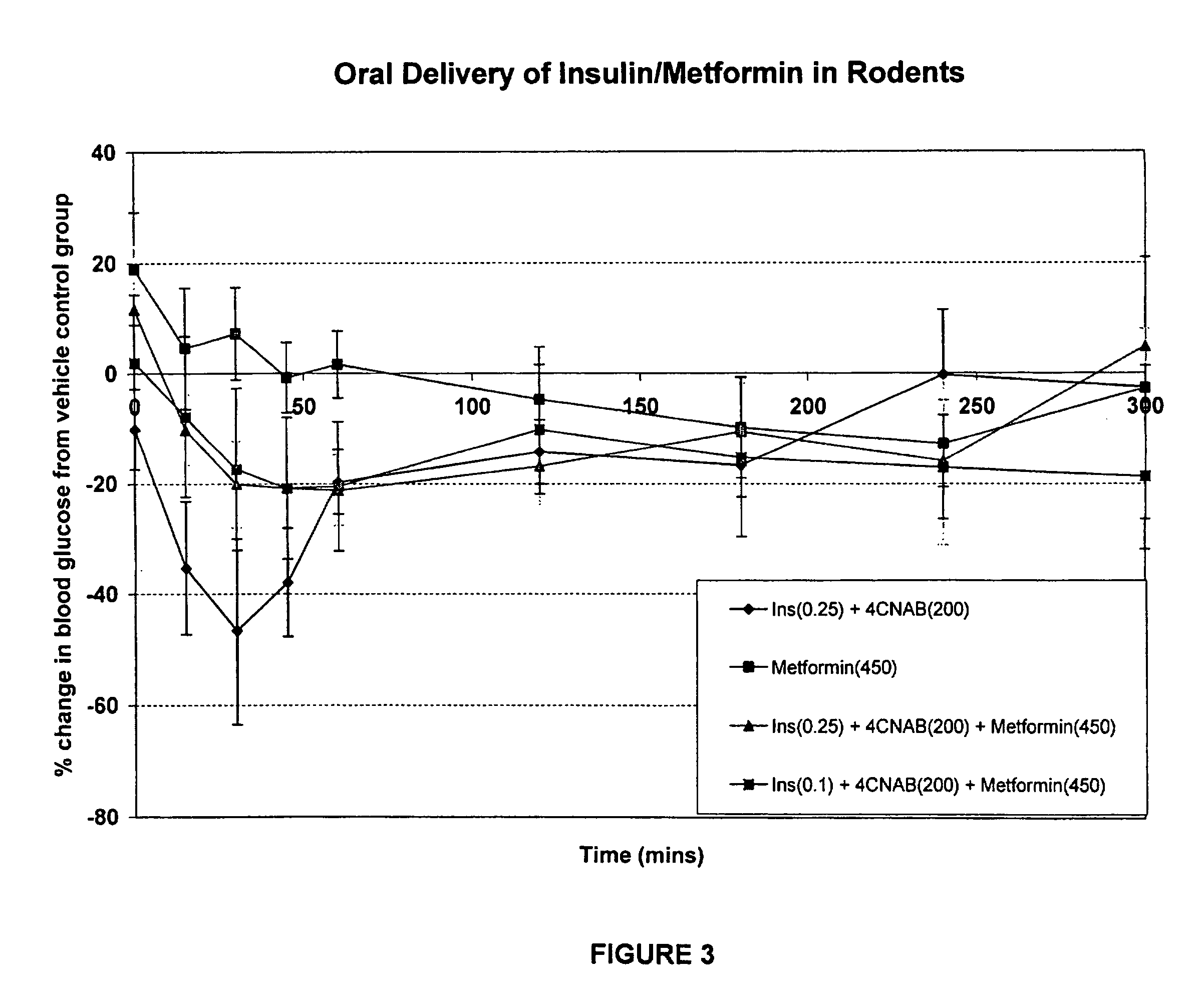
InactiveUS20100048454A1Facilitates insulin transportLasting effectOrganic active ingredientsPeptide/protein ingredientsLow glucoseInsulin dependent diabetes
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Hypoglycemic peptide and drug use thereof
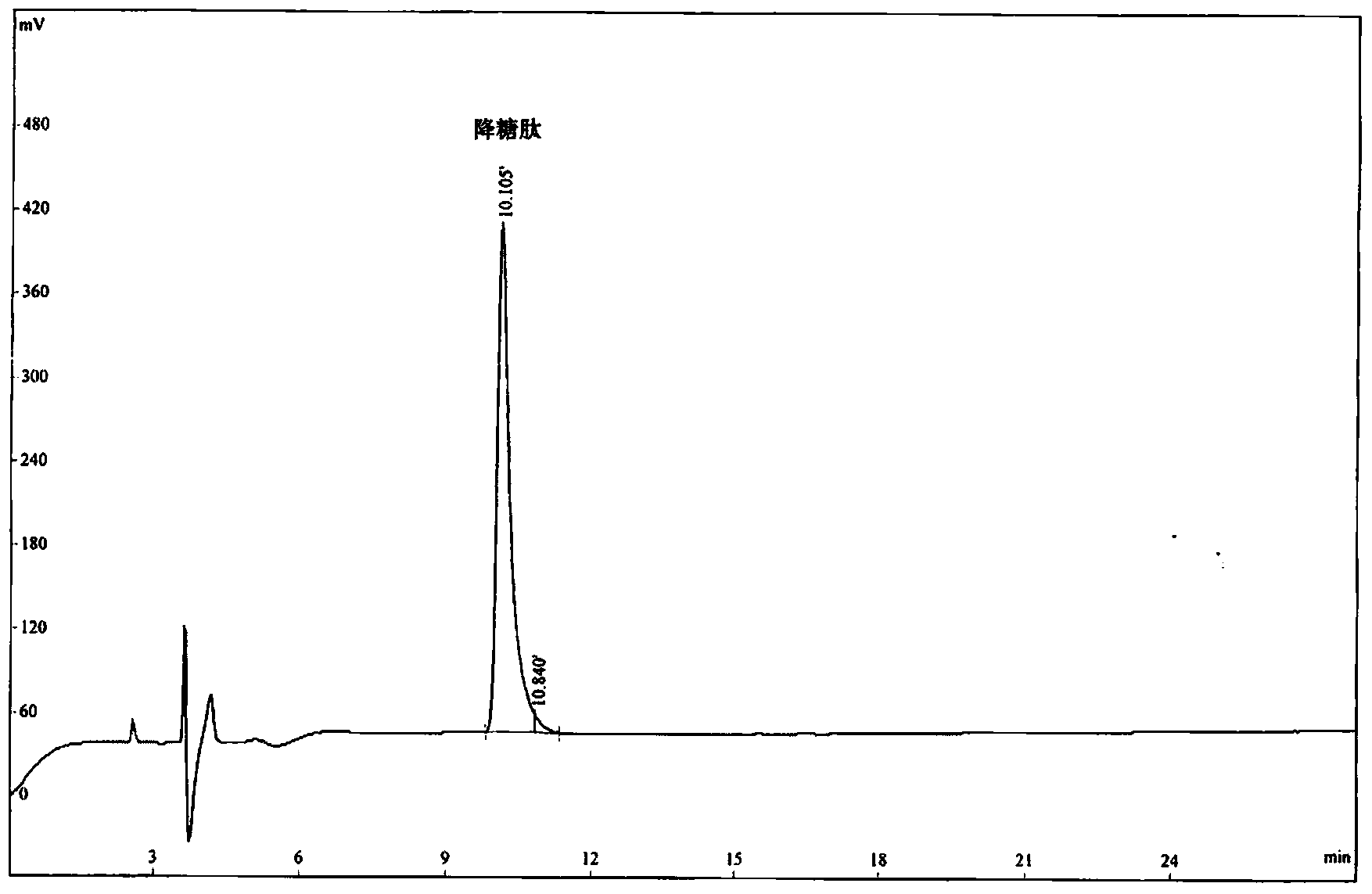
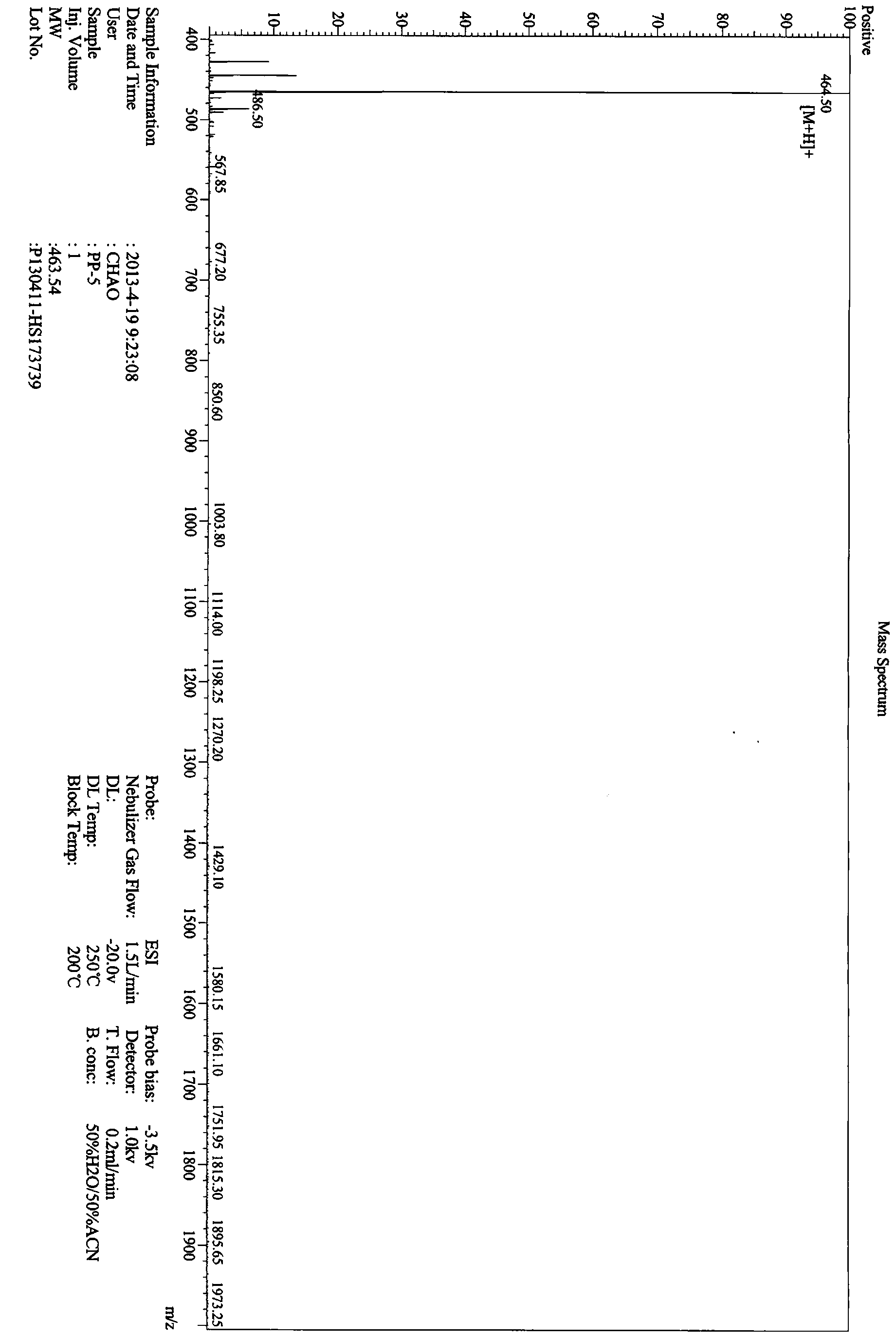
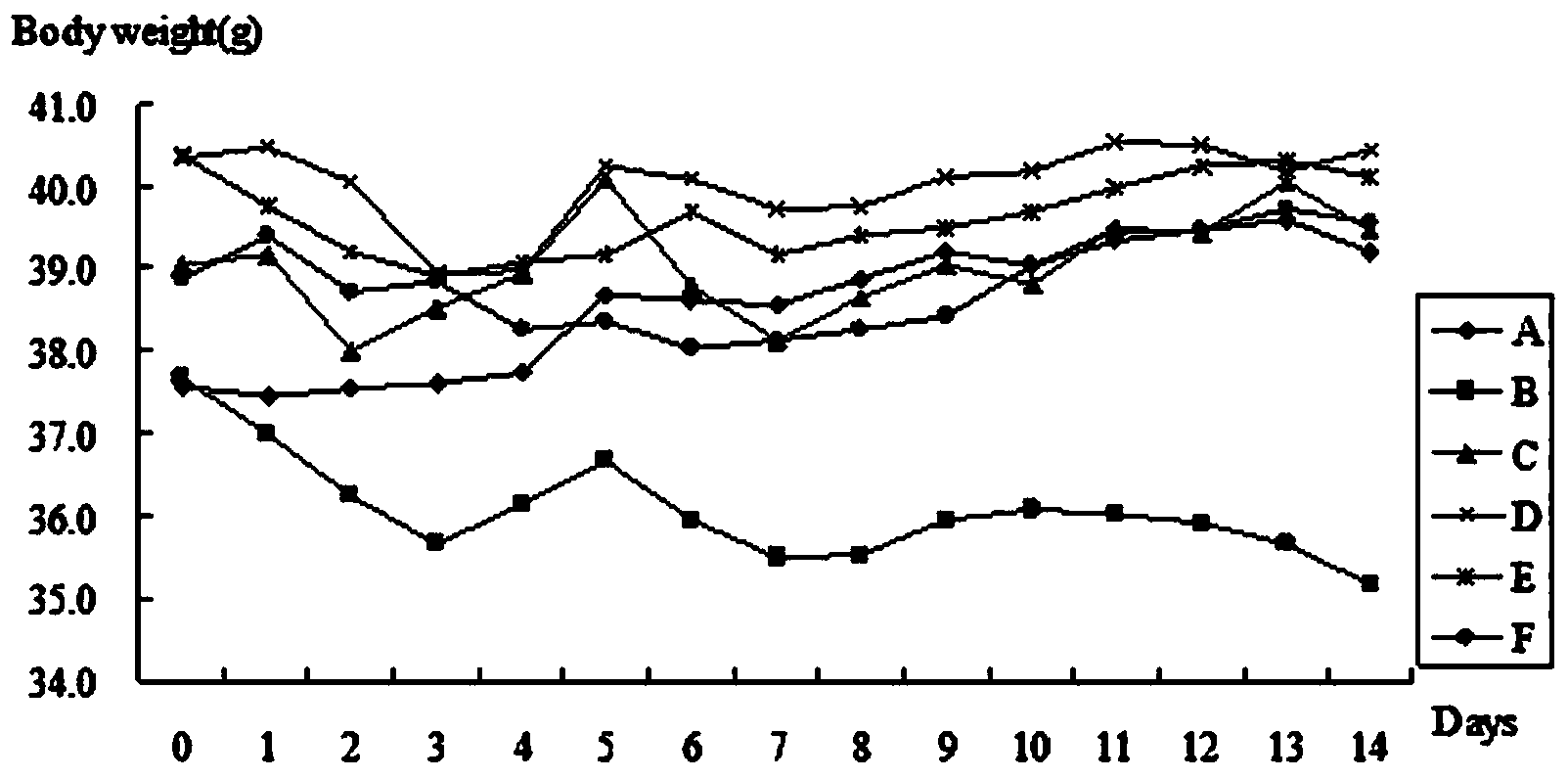
ActiveCN103641889AAbsorptionPromote absorptionPeptide/protein ingredientsMetabolism disorderGlucose utilizationOxygen
The invention provides a hypoglycemic peptide, which is provided with an amino acid sequence as shown in SEQ ID NO: 1. The invention also provides a modified peptide of the hypoglycemic peptide. A chemical group, an amino acid, polypeptide, protein or PEG is connected to the N end, C end or intermediate residue of the hypoglycemic peptide. The hypoglycemic peptide provided by the invention can perfect the glucose tolerance of KK-Ay mice, reinforce the glucose utilization ability of the KK-Ay mice, significantly improve the fasting blood glucose and glucose tolerance of the KK-Ay mice, significantly reduce serum total cholesterol and serum triglyceride, significantly perfect the SOD level, reduce the MDA level and protect the body cells against oxygen free radicals, the hypoglycemic peptide has glucose and lipid reducing functions and can resist metabolic syndrome. The hypoglycemic peptide can be used for preparing drugs or healthcare products for treating and / or preventing diabetes or hyperlipidemia.
Owner:山东康泽健康管理咨询有限公司
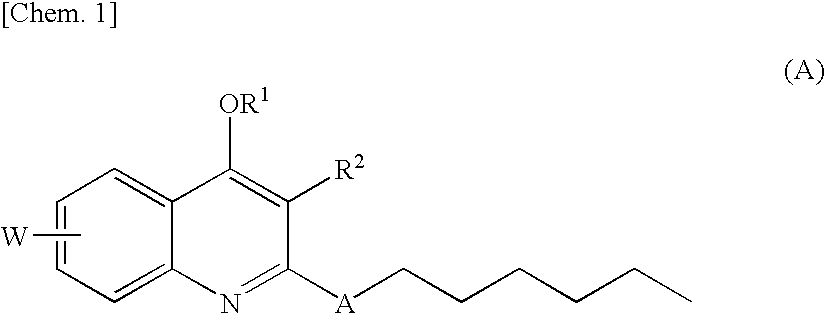
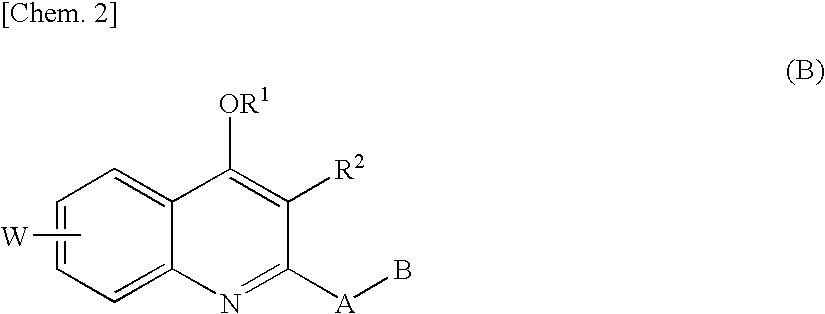
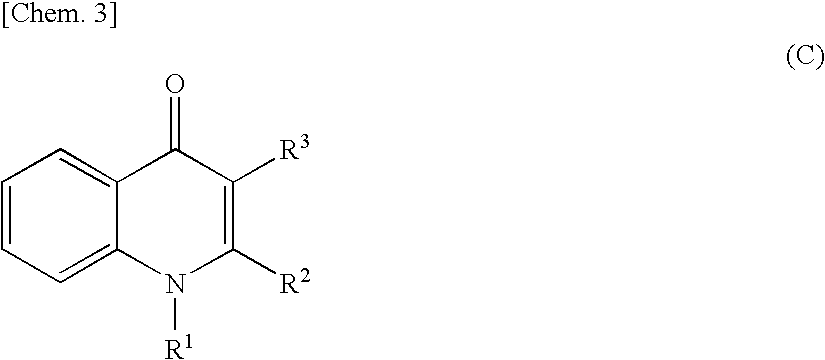
Quinolone derivative
As a result of extensive studies on NAD(P)H oxidase inhibitors, the present inventors found that a quinolone derivative having, at the 2-position, an alkyl group substituted with a heteroatom or the like has an excellent NAD(P)H oxidase inhibitory activity, and accomplished the present invention. The compound of the present invention has a reactive oxygen species production inhibitory activity based on the NAD(P)H oxidase inhibitory activity, and therefore can be used as an agent for preventing and / or treating diabetes, impaired glucose tolerance, hyperlipidemia, fatty liver, diabetic complications and the like.
Owner:ASTELLAS PHARMA INC
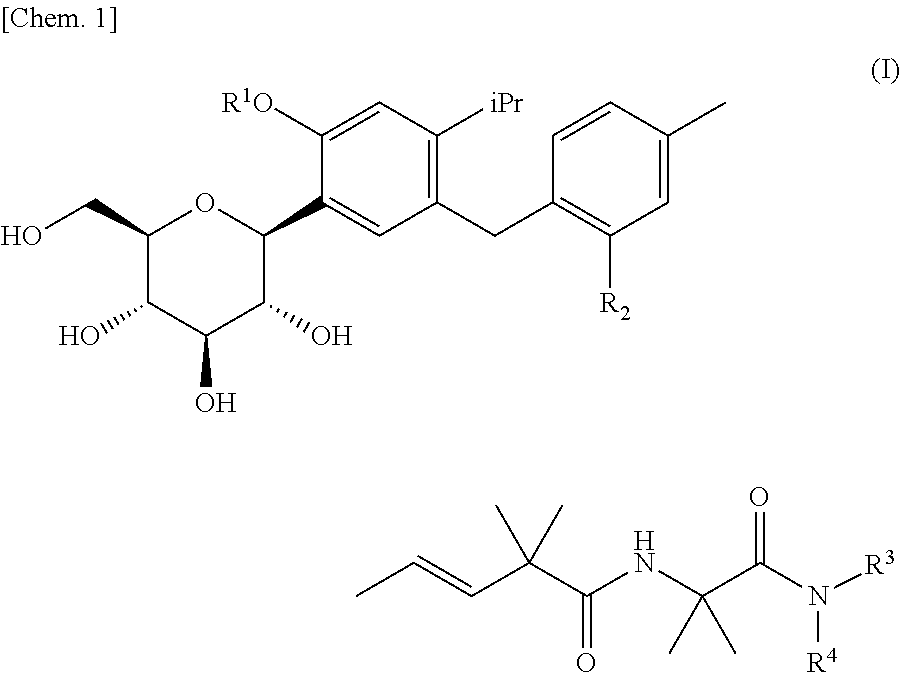
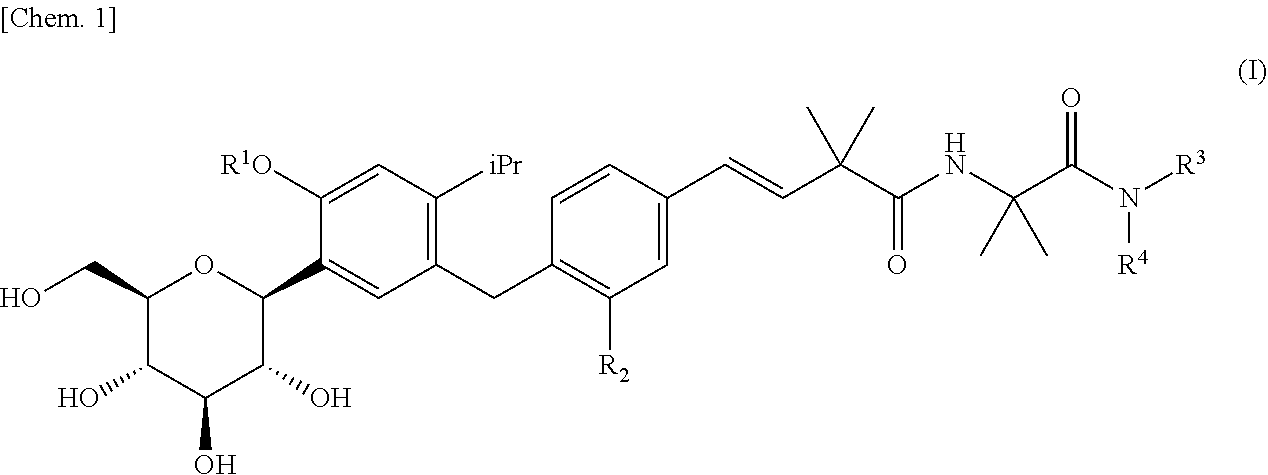
4-isopropylphenyl glucitol compounds as sglt1 inhibitors
ActiveUS20110306759A1Inhibit SGLT activityInhibitory activityBiocideSaccharide with carbocyclic radicalsAcute hyperglycaemiaDisease
The present invention provides 4-isopropylphenyl glucitol compounds which have no tendency to accumulate in the body and which inhibit SGLT1 activity to suppress postprandial hyperglycemia (or impaired glucose tolerance) through suppression of glucose absorption in the small intestine, whereby the compounds, for example, can suppress the onset of diabetes and metabolic syndrome or can treat these diseases.A 4-isopropylphenyl glucitol compound represented by the following formula (I) or a pharmaceutically acceptable salt thereof:wherein R1 represents a hydrogen atom, etc., R2 represents a methyl group, etc., R3 represents a C1-4 alkyl group substituted with an amino group(s), etc., and R4 represents a hydrogen atom, etc.
Owner:TAISHO PHARMACEUTICAL CO LTD
Method of detecting mild impaired glucose tolerance or insulin secretory defect
InactiveUS7452687B2Simplified determinationGood reproducibilityOrganic chemistryMicrobiological testing/measurementIGT - Impaired glucose toleranceDefinite period
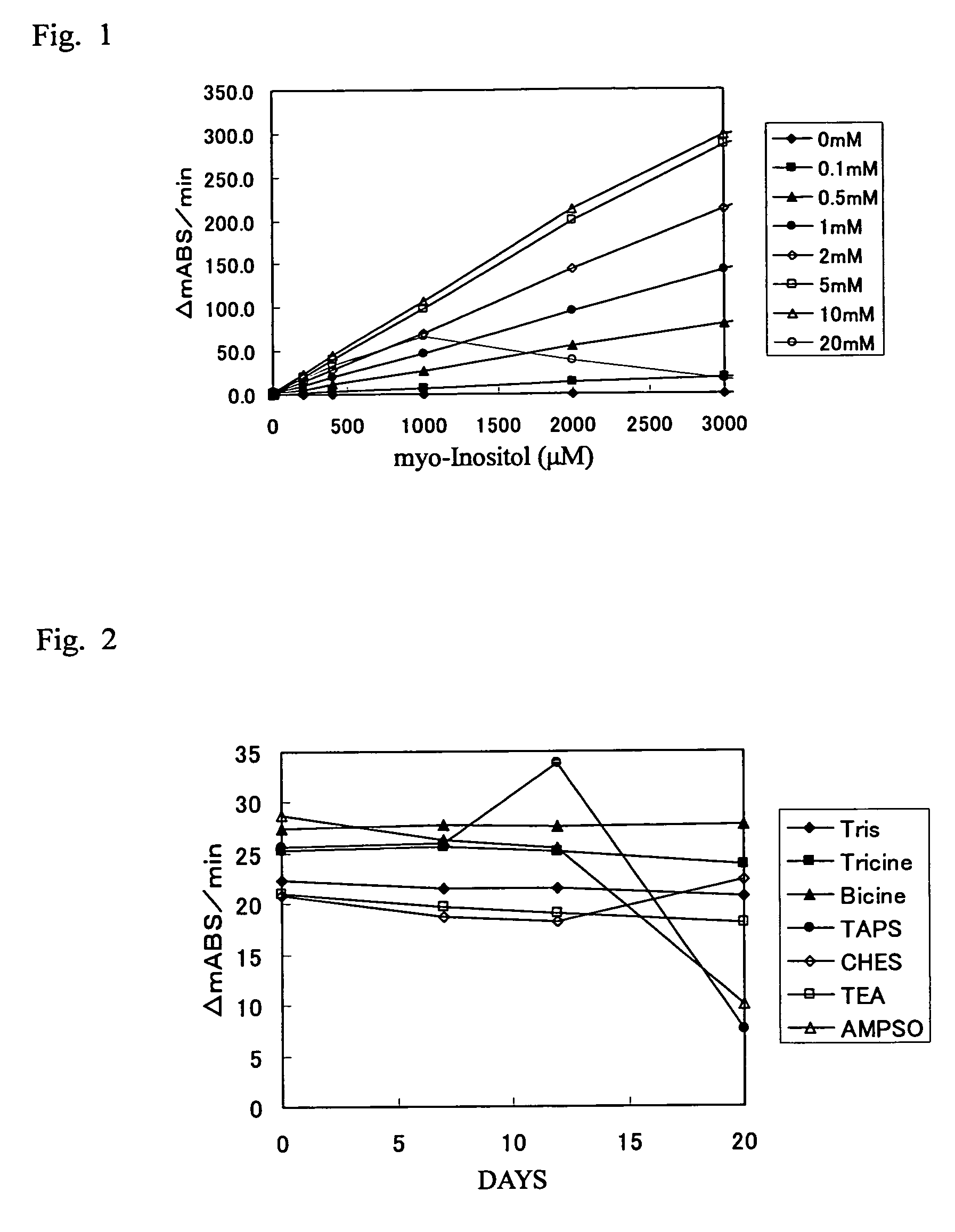
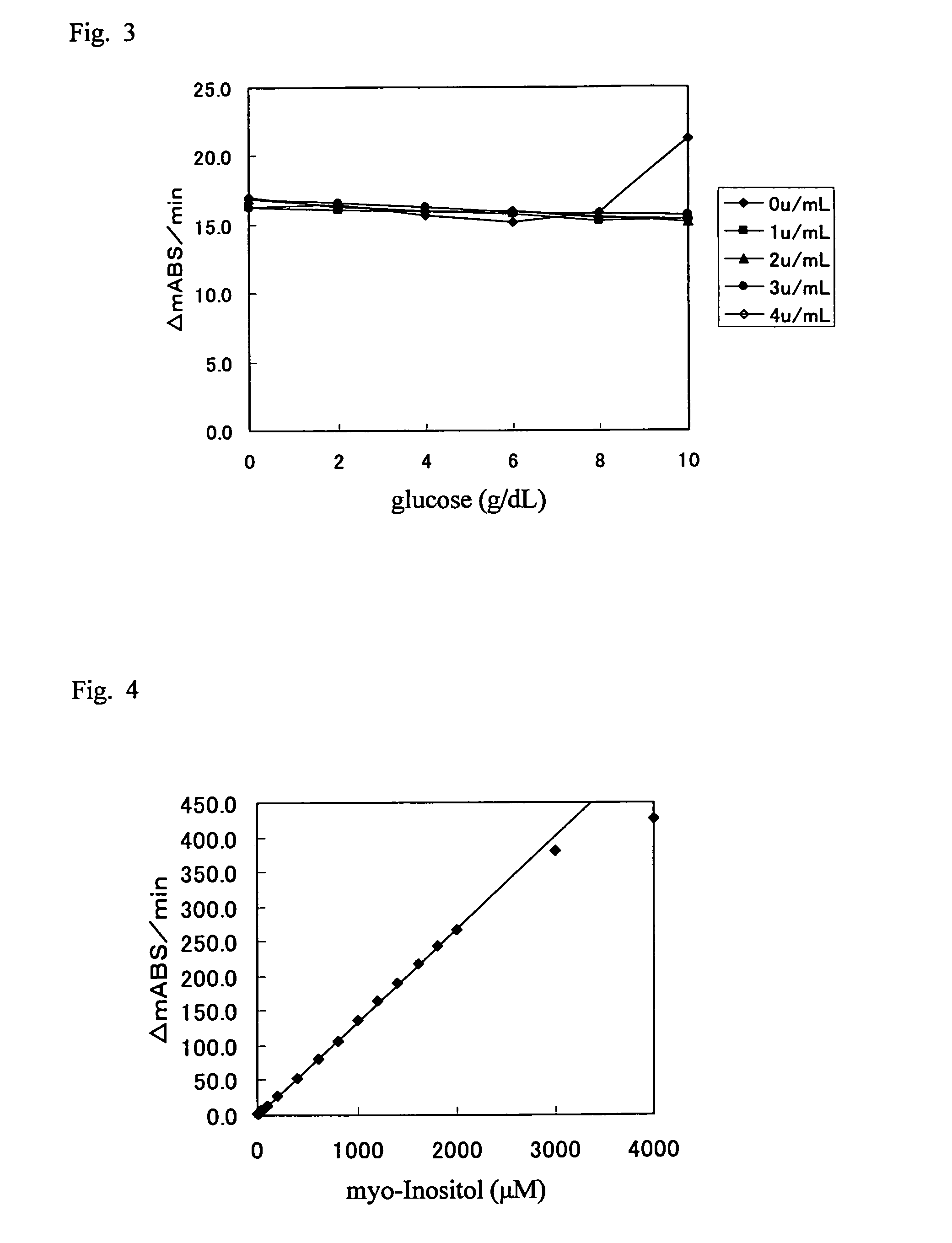
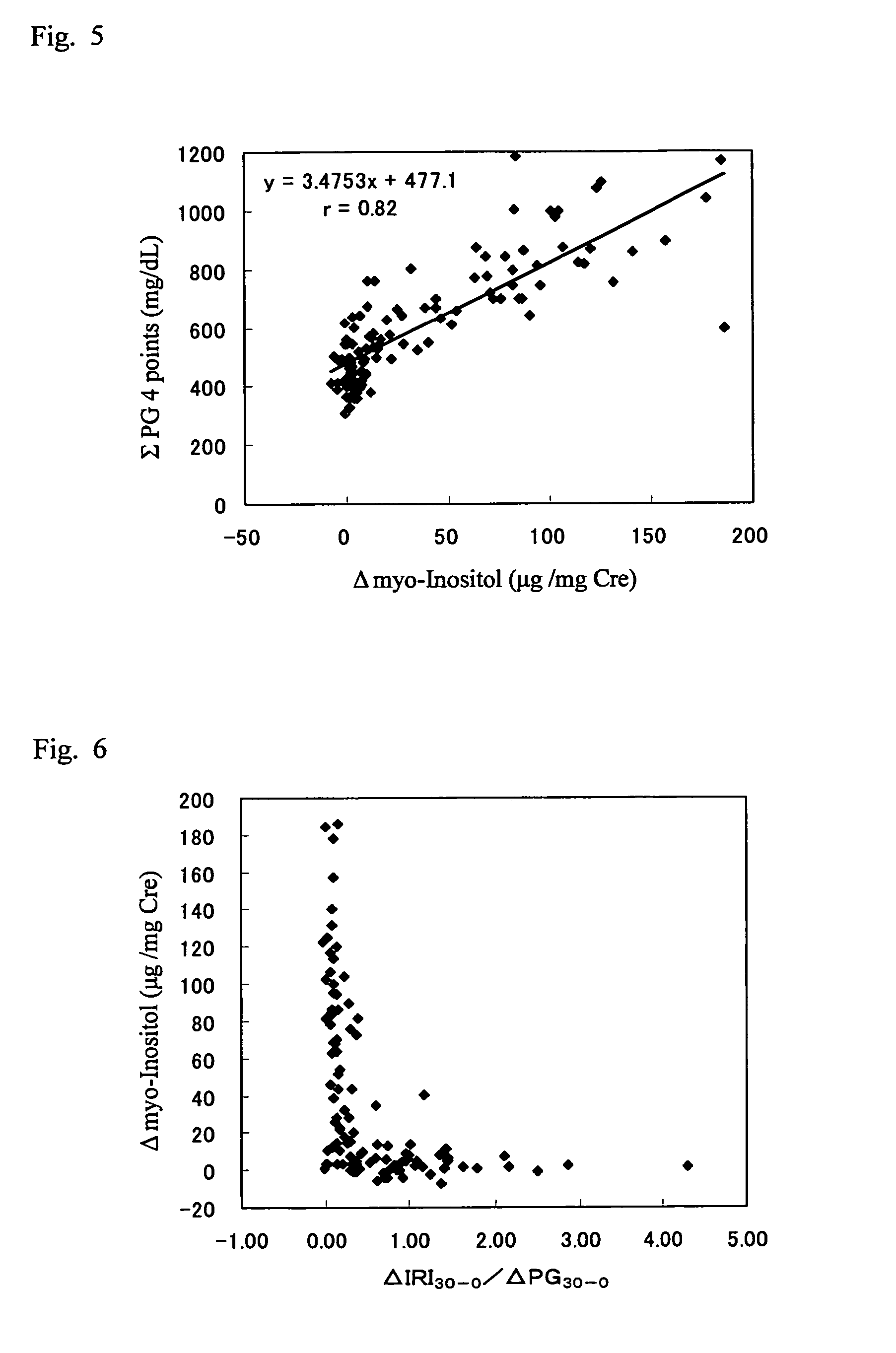
It is intended to provide a noninvasive method of conveniently detecting mild impaired glucose tolerance and / or insulin hyposecretion at the early stage with the use of an enzyme. Namely, mild impaired glucose tolerance and / or hyposecretion at the early stage are detected by quantifying myoinositol secreted into the urine before loading glucose and after loading glucose for a definite period of time with the use of a reagent and comparing the increase (or the increase ratio) in the myoinositol content thus measured with a characteristic level which has been preliminarily determined in normal subjects.
Owner:ASAHI KASEI PHARMA
Compositions of nitrates and methods of use thereof
ActiveUS20190192554A1Increasing exercise enduranceReduces subject 's oxygen consumptionOrganic active ingredientsYeast food ingredientsMetabolic rateNitrite
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a reduction in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
Compositions of nitrates and methods of use thereof
ActiveUS20190125784A1Lower systolic blood pressureDispersion deliveryHydroxy compound active ingredientsResting energy expenditureHomeostasis
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a reduction in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
Medicinal composition for treating impaired glucose tolerance and preparation method thereof
ActiveCN101053592AReduce resistanceIncreased sensitivityOrganic active ingredientsMetabolism disorderGluconatesExcipient
The invention relates to a medicine combination for treating the impaired glucose tolerance and the preparing method thereof. The medicine combination is mainly composed of balsam pear, mulberry leaf, sanchi, glossy privet fruit, fiveleaf gynostemma herb, chromium picolinate, and zinc gluconate. It is a preparation produced by pharmacological acceptable carrier or excipient, containing capsule, granule, tablet, soft capsule, dropping pill and etc, which is suitable for the patent with impaired glucose tolerance and forepart diabetes. The research indicates that the invention can reduce the blood sugar, decline the insulin resistance, enhance the insulin sensitivity, protect the beta cell function, reduce the serum total cholesterol and serum triglyceride without side and poisonous effects.
Owner:ZHONGSHI TAILING BEIJING BIO SCI & TECH +1
Methods and compositions for enhancing fertility and/or inhibiting pregnancy failure and restoring glucose tolerance
Methods and compositions for enhancing fertility and / or inhibiting pregnancy failure, restoring glucose tolerance and / or preventing glucose intolerance and / or maintaining glucose homeostasis and / or inducing or enhancing weight loss, treating dyslipidemia, treating hypertestosteronism or hyperandrogenism, and / or treating type 2 diabetes in an individual in need thereof are provided. These involve compositions that inhibit expression of interferon-gamma (IFN-gamma) or a downstreamIFN-gamma-stimulated gene, which is preferably a macrolide immunosuppressant compound.
Owner:QUEENS UNIV OF KINGSTON
Xanthine derivative
The invention discloses a xanthine derivative and its pharmaceutically acceptable salt. In vitro DPP-IV activity inhibition tests, influence tests of normal mouse's glucose tolerance, and influence tests of spontaneous diabetic mouse's blood glucose confirm that the compound and its pharmaceutically acceptable salt show significant DPP-IV inhibitory activity, and can be used in preparation of drugs for treating dipeptidyl peptidase IV associated diseases.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Compositions of nitrates and methods of use thereof
ActiveUS10821132B2Organic active ingredientsBacteria material medical ingredientsBlood sugarResting energy expenditure
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a reduction in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
Medical application of genistein chromium complex to treatment of diabetes
ActiveCN105106221ALower blood sugarImproves glucose tolerance levelOrganic active ingredientsMetabolism disorderFasting glucosePancreatic hormone
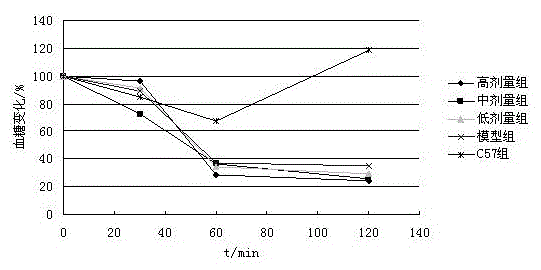
The invention provides medical application of a genistein chromium complex to treatment of diabetes. Results of the animal growth condition research, fasting blood-glucose value determination, glucose tolerance test, insulin tolerance test show that the genistein chromium complex has an excellent hypoglycemic effect, and can be used for preparing drugs for treating diabetes II; by combing the hypoglycemic advantage of chromium, the hypoglycemic activity of genistein is improved obviously and toxicity of inorganic chromium can be reduced.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Method for measuring regulation and control of Chemerin on insulin resistance and intervention of CMKLR1 agonist
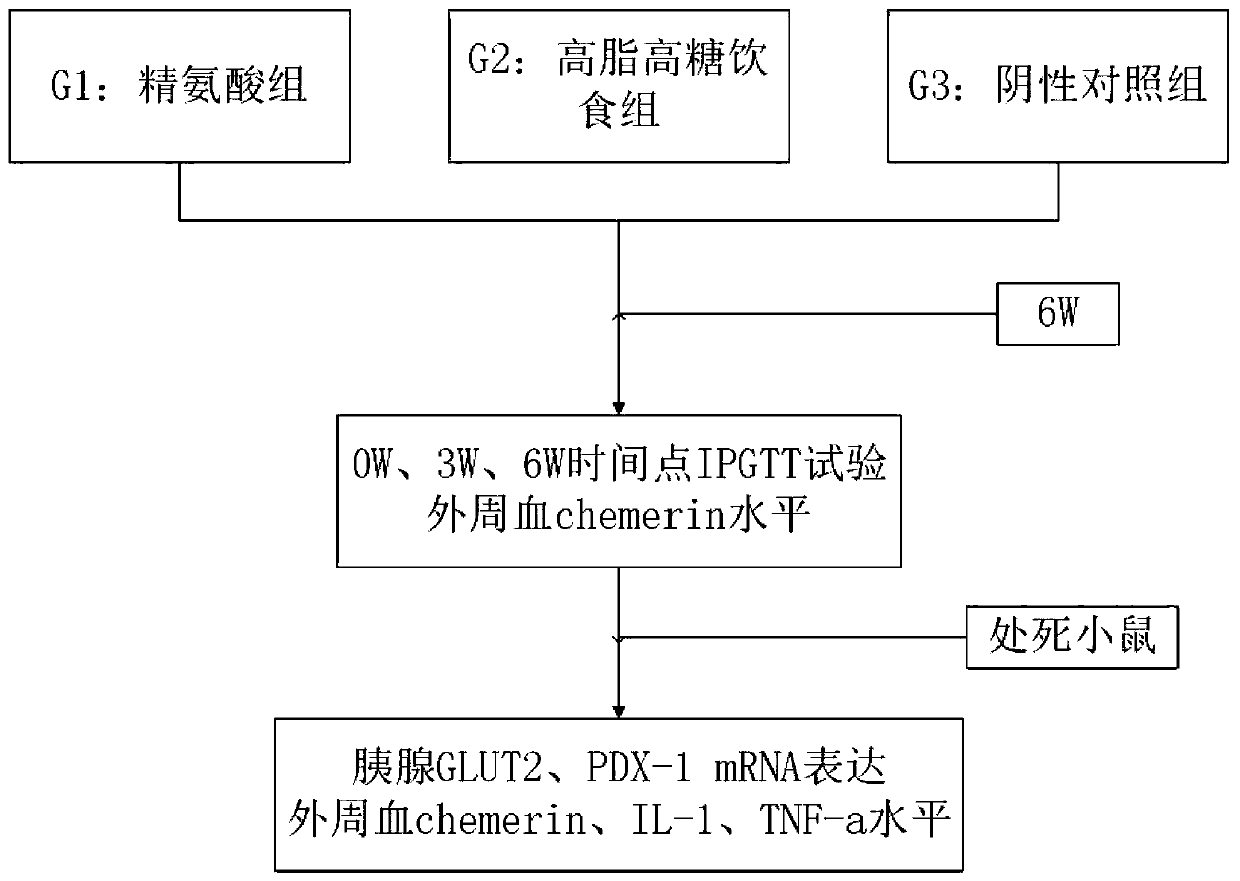
InactiveCN109985251AHigh and stable success rateInduced stabilizationCompounds screening/testingAnimal husbandryIntraperitoneal routeArginine
The invention belongs to the technical field of testing or analyzing materials by means of chemical or physical properties of a measuring material, and discloses a method for measuring the regulationand control of Chemerin on insulin resistance and the intervention of a CMKLR1 agonist, which comprises the following steps: preparing a pancreatic diabetes model by adopting an arginine induction method, performing intraperitoneal injection of 350 mg / kg arginine once a day for 6 weeks, and establishing a pancreatic diabetes group by a mouse common diet; during the period, establishing a pancreas-derived diabetes mellitus group by the common diet of the mice;meanwhile, establishing a high-fat diet group by adopting a formula feed open diet; establishinga negative control group, wherein the mice are fed with ordinary diet and water for 6 weeks; adopting a general pathology, an enzyme-linked immunosorbent assay, a Real-Time PCR method and an intraperitoneal injection glucose tolerance test for detection; expressing all the detection data by mean plus or minus standard deviation, and testing the normal distribution is by Shapiro Wilk test; using single-factor variance analysis to comparegroups with each other, and if the groups do not conform to the normal distribution, using Mann-Whitney U test for comparison; significant difference lying in P < 0.05; applying SPSS 22.0 for Windowssoftware to implement the entire verification process.
Owner:涂建锋
Compositions of nitrates and methods of use thereof
ActiveUS10842813B2Dispersion deliveryHydroxy compound active ingredientsBlood sugarResting energy expenditure
Inorganic anions nitrate and nitrite influence metabolic rate and glucose homeostasis. Infusion of nitrite iv caused an acute drop in resting energy expenditure (oxygen consumption) and nitrate, when given perorally, caused a reduction in oxygen consumption during exercise and a depression of the increase in blood glucose observed after an oral glucose tolerance test. The doses of nitrate and nitrite did not cause any detectable change in methemoglobin levels of blood. Also, nitrate and nitrite did not alter lactate levels in blood. This discovery provides useful treatments to regulate the energy expenditure and glucose homeostasis of a mammal by administration of inorganic nitrite and / or nitrate.
Owner:HEARTBEET LTD
Functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus
InactiveCN104056271AWorsening fatty liverThe role of exacerbating type 2 diabetes diseaseMetabolism disorderGenetic material ingredientsIntraperitoneal routeStaining
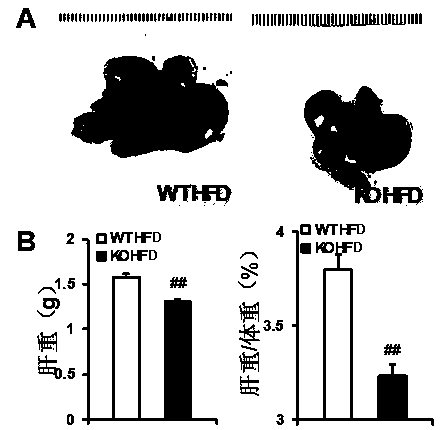
The invention discloses functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus. Studies on a TRAF 5 gene by a high-fat diet (HFD) induced model discover that both the body weight and fasting plasma glucose level of an HFD bred TRAF gene knock-out mouse are lower than those of a WT mouse; glucose tolerance tests by intraperitoneal injection discover that the glucose tolerance of the TRAF5 gene knock-out mouse is remarkably reinforced; results of on liver gross appearance, liver weight, liver / body weight ratio and lipid component pathological staining indicate that the TRAF5-KO mouse fatty liver lesion in the HFD group is remarkably improved, the lipid accumulation is remarkably reduced, and the TRAF5 gene knock-out has the effects of remarkably improving fatty acid and type-II diabetes mellitus. Against the effects, the TRAF5 gene knock-out can be used as a medicinal target for screening and treating fatty liver and / or type-II diabetes mellitus, and the inhibitor of the TRAF5 gene knock-out can be used for preparing medicaments for treating fatty liver and / or type-II diabetes mellitus.
Owner:WUHAN UNIV
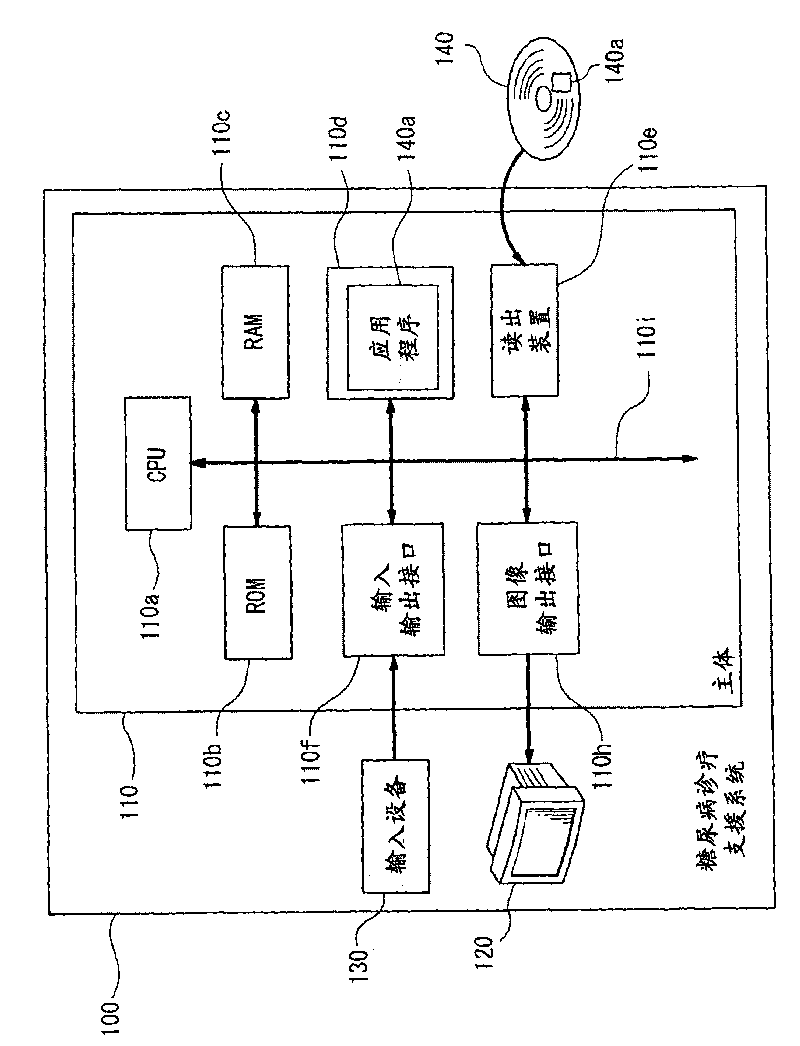
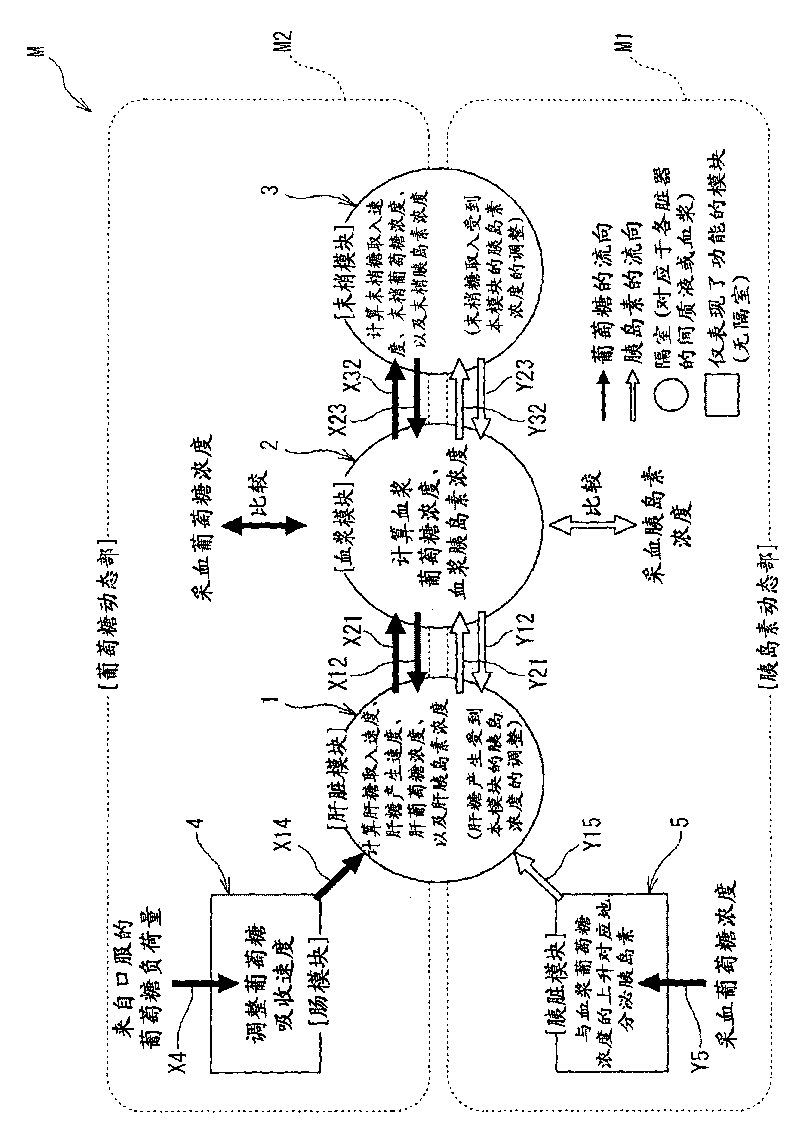
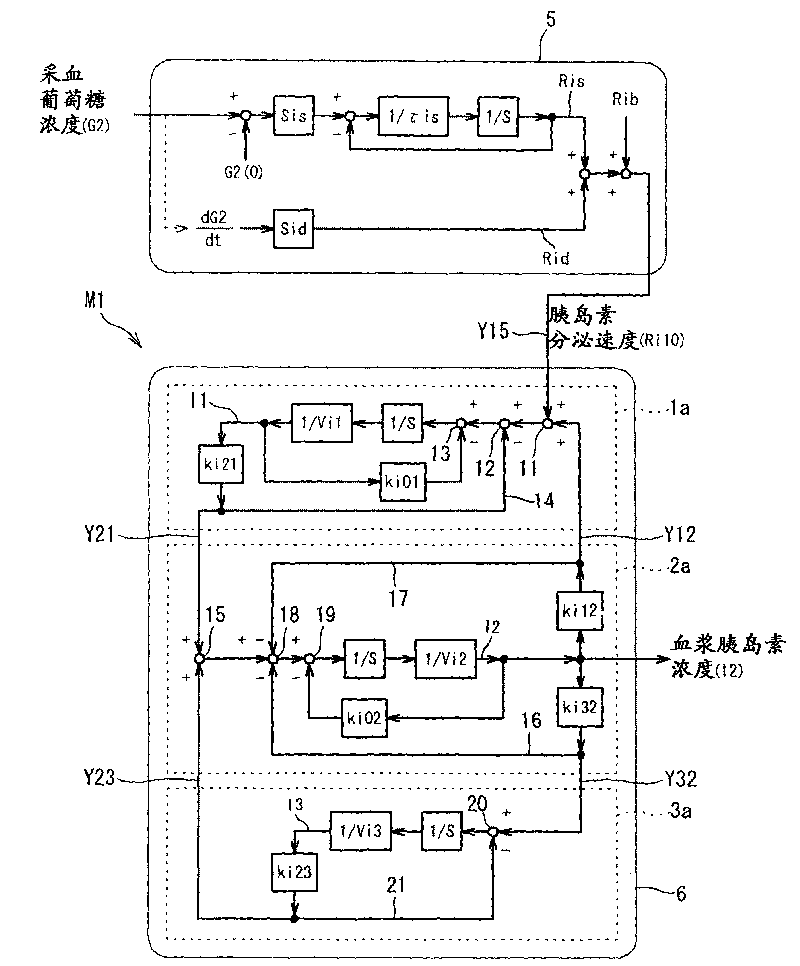
Diagnostic support apparatus for diabetes and computer program product
ActiveCN101763464AHigh-precision diagnosis and treatment supportMedical simulationData processing applicationsBiological bodyElevated glucose tolerance
The present invention is to present a diagnostic support apparatus for diabetes including a diagnostic support information generating unit which generates diagnostic support information of a patient based on a biological model for reproducing a pseudo-response which simulates a result of a glucose tolerance test for the patient. The biological model comprises a plurality of simulated organ blocks which are configured in such manner that inflow and outflow of glucose and / or inflow and outflow of insulin are reciprocally produced between each of the simulated organ blocks. The plurality of the simulated organ blocks respectively calculate at least one of a cumulative quantity and a concentration of glucose and / or at least one of a cumulative quantity and a concentration of insulin in the respective simulated organ blocks, based on a quantity of inflow and outflow of glucose and / or a quantity of inflow and outflow of insulin in the respective simulated organ blocks.
Owner:SYSMEX CORP
Novel glucose tolerance test and composition for use
InactiveCN102791148ADisease diagnosisBiological testingElevated glucose toleranceGlucose tolerance test
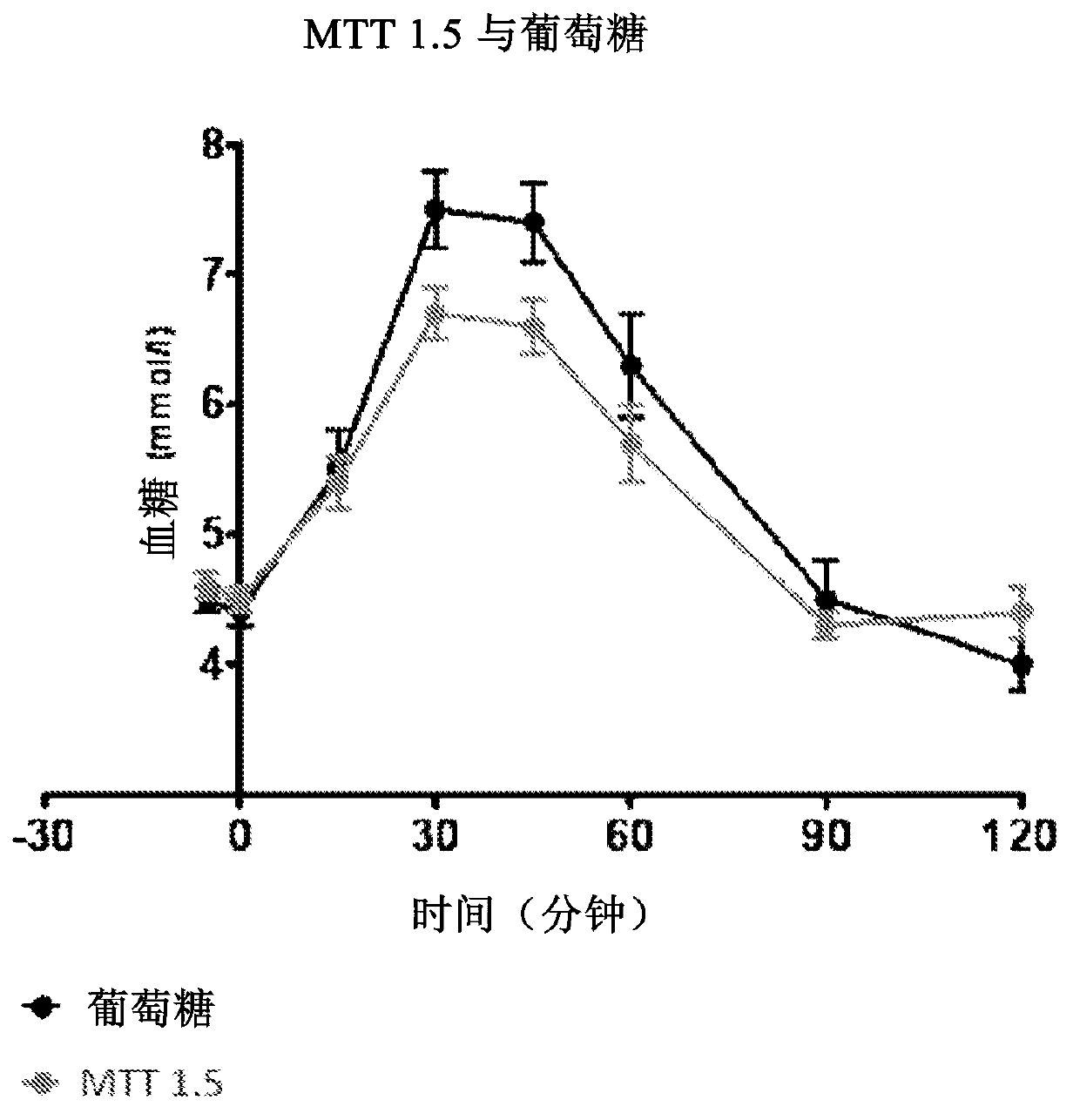
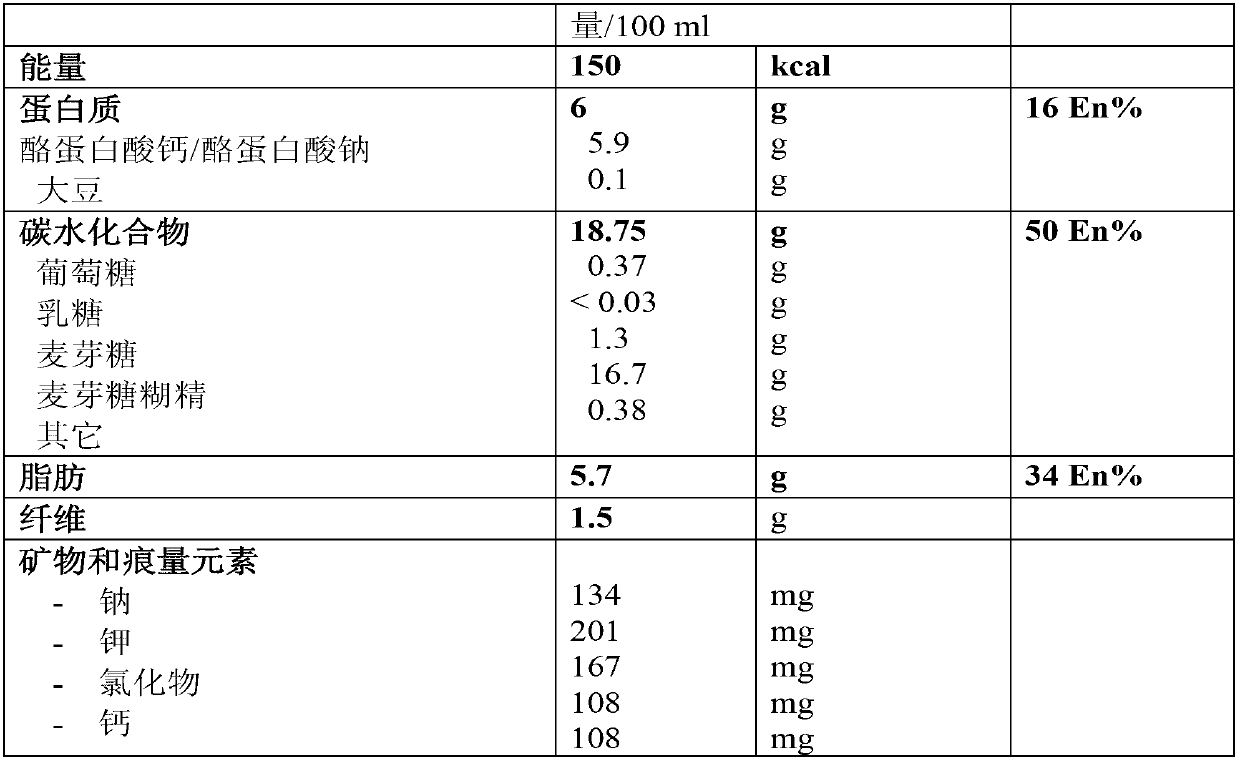
The present invention relates to a liquid nutritional composition, to a liquid nutritional composition for use in a diagnostic method for screening of a glucose-intolerant condition, to a kit for screening of a glucose-intolerant condition, and to a diagnostic method for screening a mammal for a glucose- intolerant condition. Furthermore, it refers to a liquid nutritional composition for use as a standard reference nutrition, such as for classification of postprandial glucose responses of different nutritional compositions. The liquid nutritional composition comprises protein, fat, digestible carbohydrate and dietary fibre, wherein the digestible carbohydrate comprises at least 90 weight% of glucose units, based on total digestible carbohydrate mass and preferably is substantially free of fructose.
Owner:NUTRICIA
Composition containing NAD for preventing and treating obesity or impaired glucose tolerance
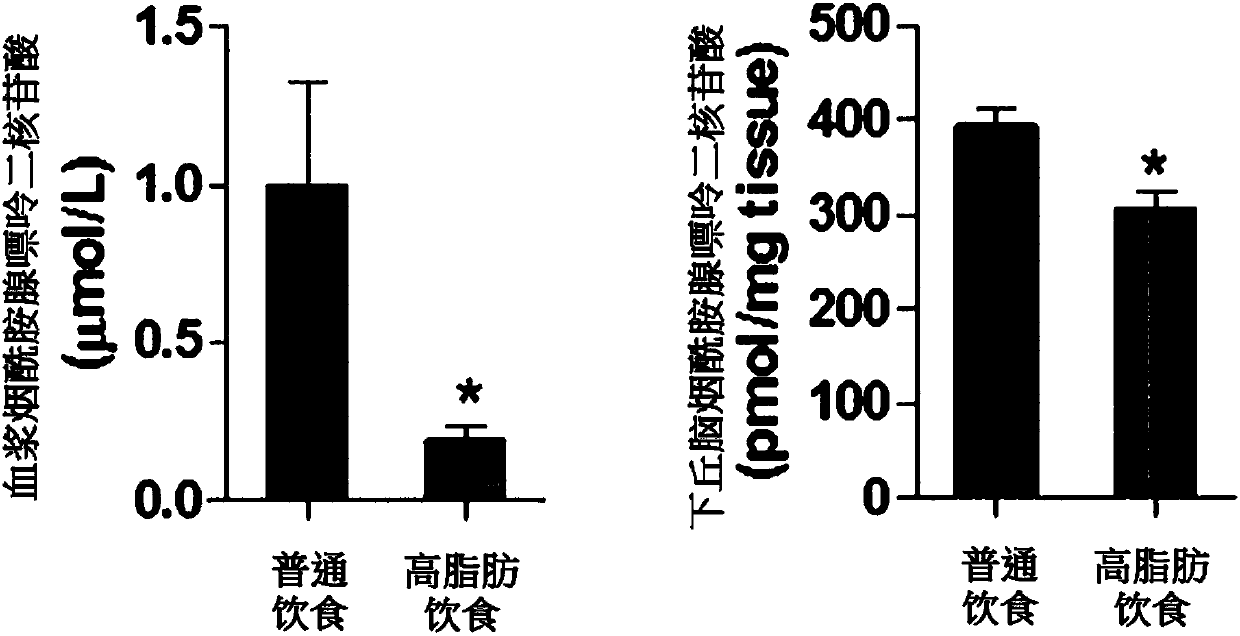
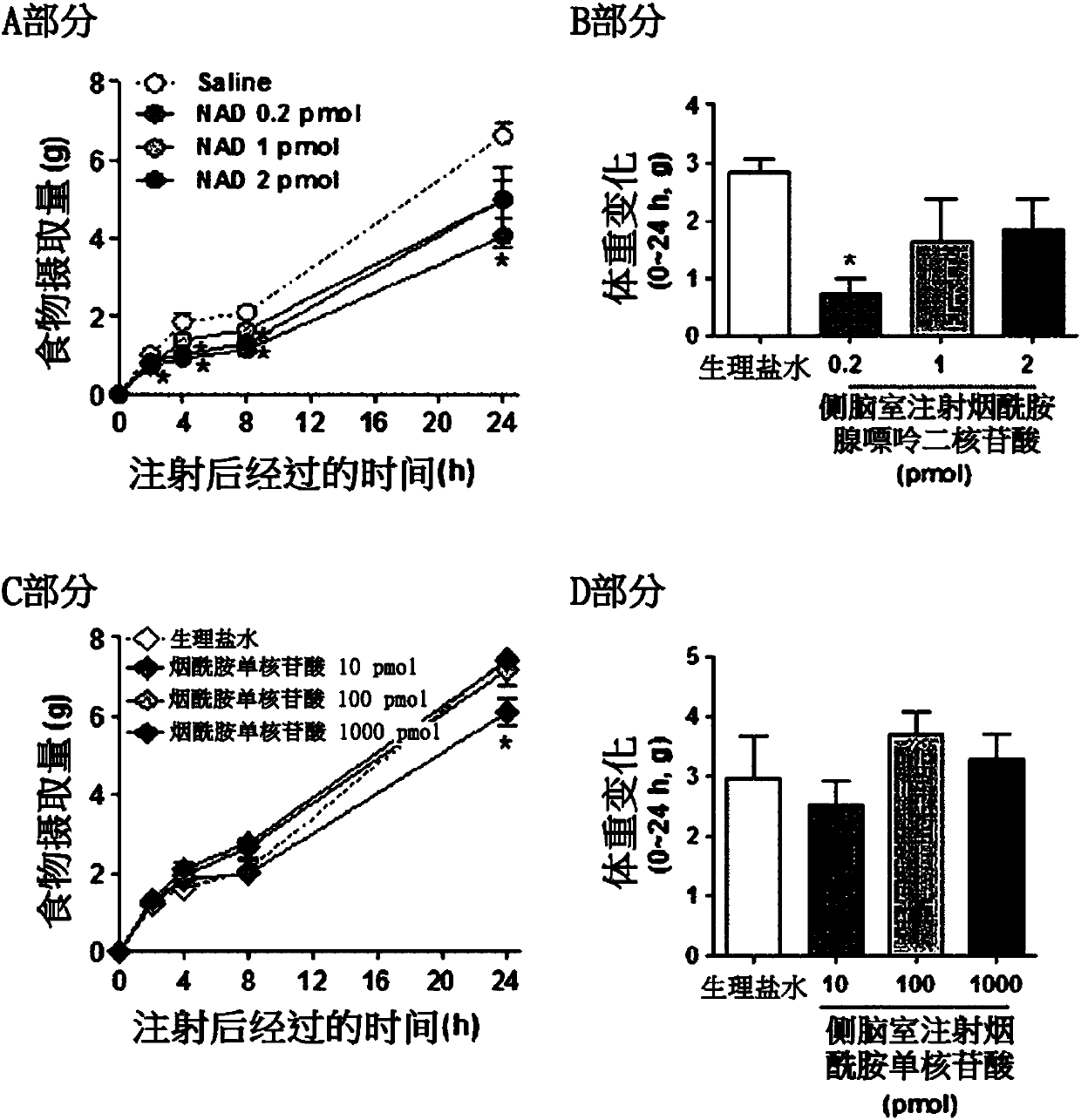
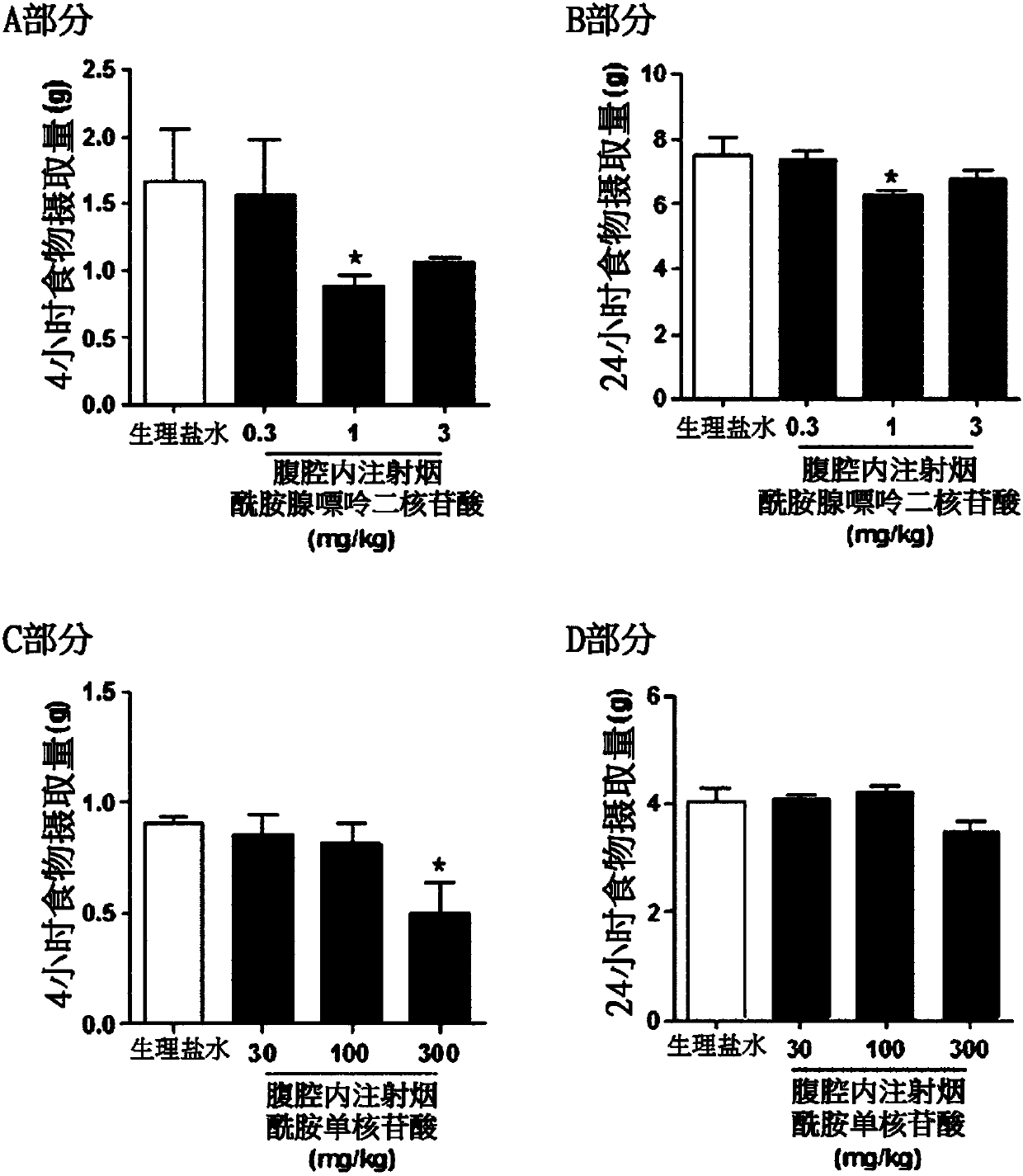
ActiveCN107847513AImprove abnormal food intake patternsImprove enduranceOrganic active ingredientsMetabolism disorderBULK ACTIVE INGREDIENTNicotinamide adenine dinucleotide
The present invention relates to a pharmaceutical composition containing nicotinamide adenine dinucleotide (NAD) as an active ingredient for preventing and treating obesity or impaired glucose tolerance, a food composition, and a method for preventing and treating obesity or impaired glucose tolerance using the same. The NAD of the present invention remedies an abnormal food intake pattern of an obese animal model induced by the intake of a high-fat diet and increases mobility, thereby exhibiting an effect of suppressing the weight increase due to the high-calorie intake and also showing an effect of improving glucose tolerance. In addition, it was verified that the NAD of the present invention is capable of maintaining the above effects with even a much smaller quantity than an NAD precursor known in the prior art. Therefore, the composition containing NAD of the present invention can be favorably used as a pharmaceutical composition or a food composition capable of effectively preventing and treating obesity or impaired glucose tolerance.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Preparation method of novel oral glucose preparation for oral glucose tolerance test
InactiveCN110478498AAccurate concentrationGreat tasteCompounds screening/testingOral glucoseMedicine
The invention relates to a preparation method of an oral glucose preparation. The invention aims to provide a preparation method of a novel oral glucose preparation for the oral glucose tolerance test(OGTT) so as to enhance the accuracy and repeatability of OGTT and improve the taste for patients during the test. According to the technical scheme, the preparation method of the novel oral glucosepreparation for OGTT comprises the following steps: 1) weighing 150g of anhydrous glucose, dissolving the anhydrous glucose in 600ml of purified water, respectively adding citric acid which accounts for 0.15% of the total weight and lemon essence which accounts for 0.05% of the total weight, stirring and dissolving; and 2) filtering with a 0.22-micron microfiltration membrane, bottling, sealing, and sterilizing in a sterilizer.
Owner:杭州市西溪医院
Nutritional breakfast for repairing glucose tolerance factor and preparation method of nutritional breakfast
ActiveCN105520144AImprove utilizationImprove comorbiditiesFood ingredient functionsGlucose tolerance factorIn vivo
The invention discloses nutritional breakfast for repairing the glucose tolerance factor. Buckwheat, shiitake and purple yams are mixed and decocted with water to form porridge, and then the nutritional breakfast is prepared; the weight ratio of the buckwheat to the shiitake to the purple yams to the water is 1:1:1:(3-4). Best food varieties are selected for preparing the nutritional breakfast, nutrition necessary to the glucose tolerance factor of the human body is provided, the nutrition of the glucose tolerance factor is balanced again, best nutrition is realized, nutrition damage of the glucose tolerance factor of type 2 diabetic patients and pre-diabetic patients with impaired fasting glucose and the damaged glucose tolerance factor can be repaired, the biological effect of insulin is better enhanced, utilization of in-vivo glucose is accelerated by activating phosphoglucomutase, the glucose is converted into fat, and elevated blood glucose is promoted to return to normal quickly. Thus, the dose of a medicine is reduced, complications of the diabetic patients are improved, the living quality is improved, and the life is prolonged.
Owner:三亚怡心营养咨询服务中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com