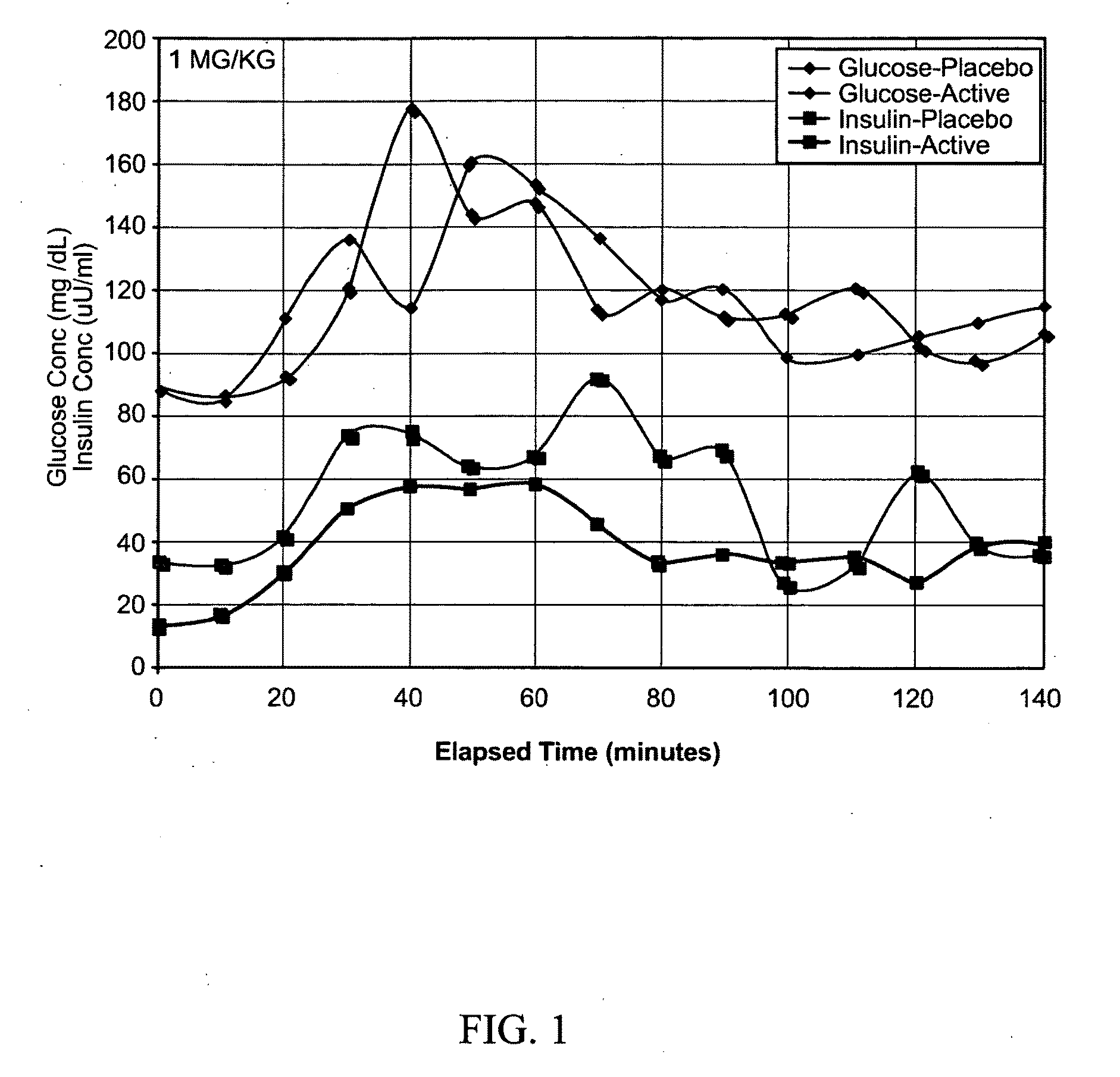
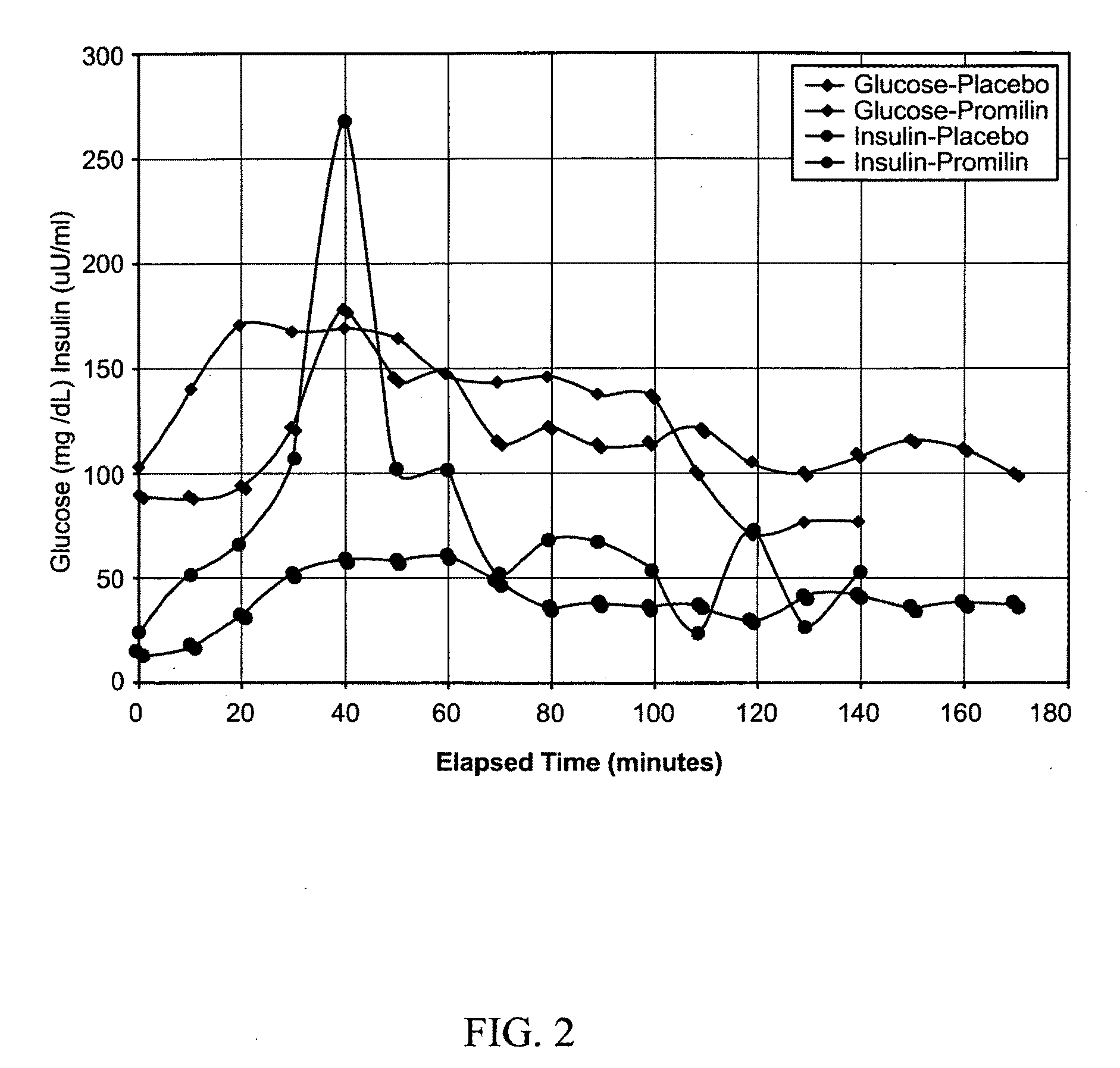
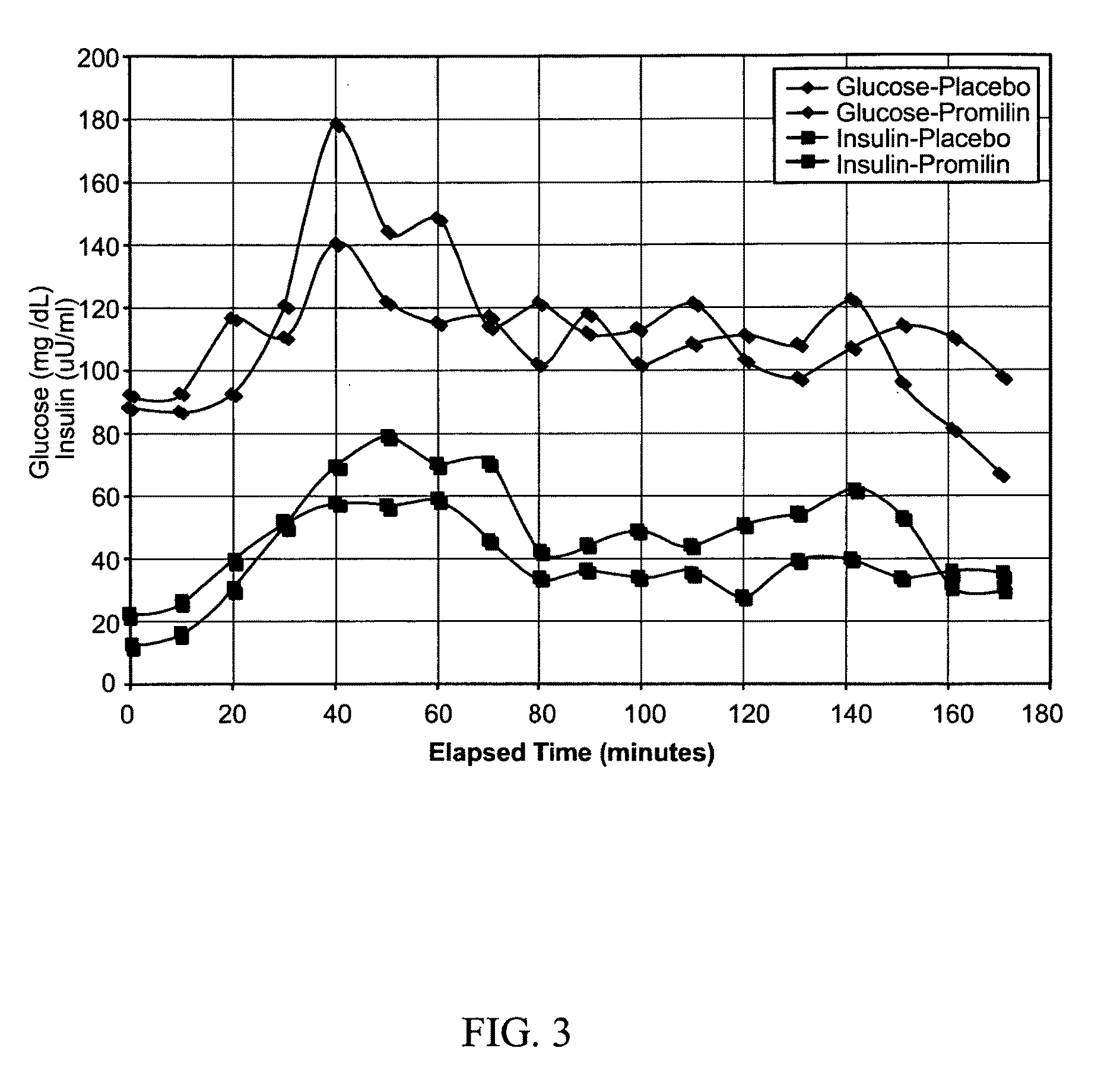
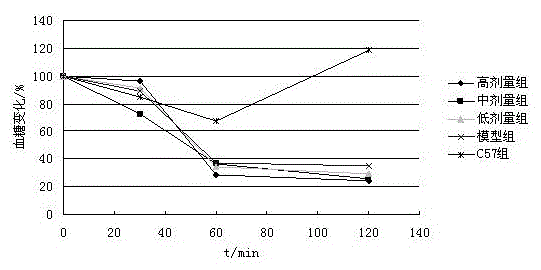
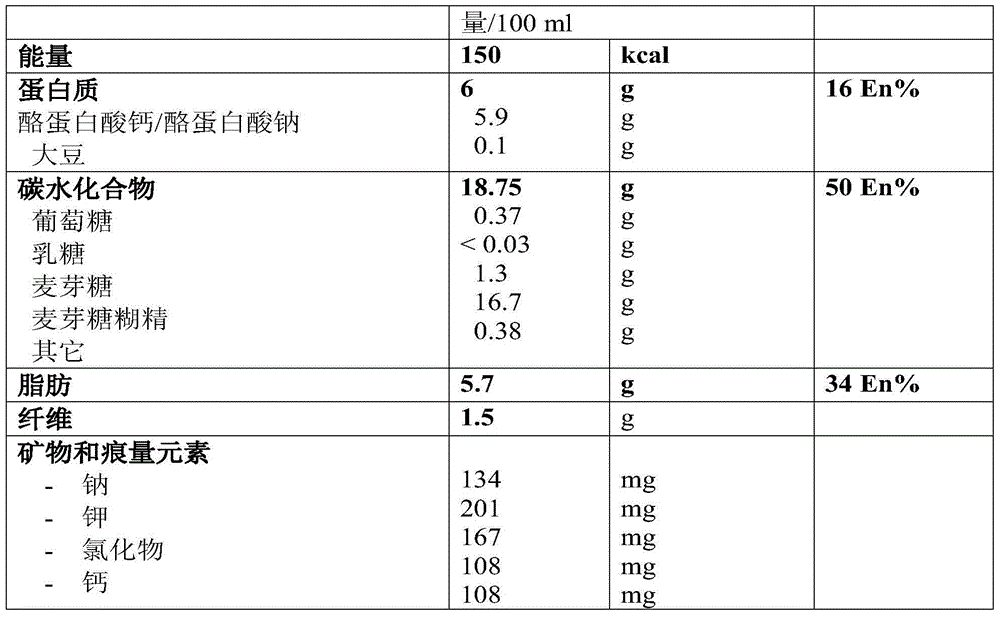
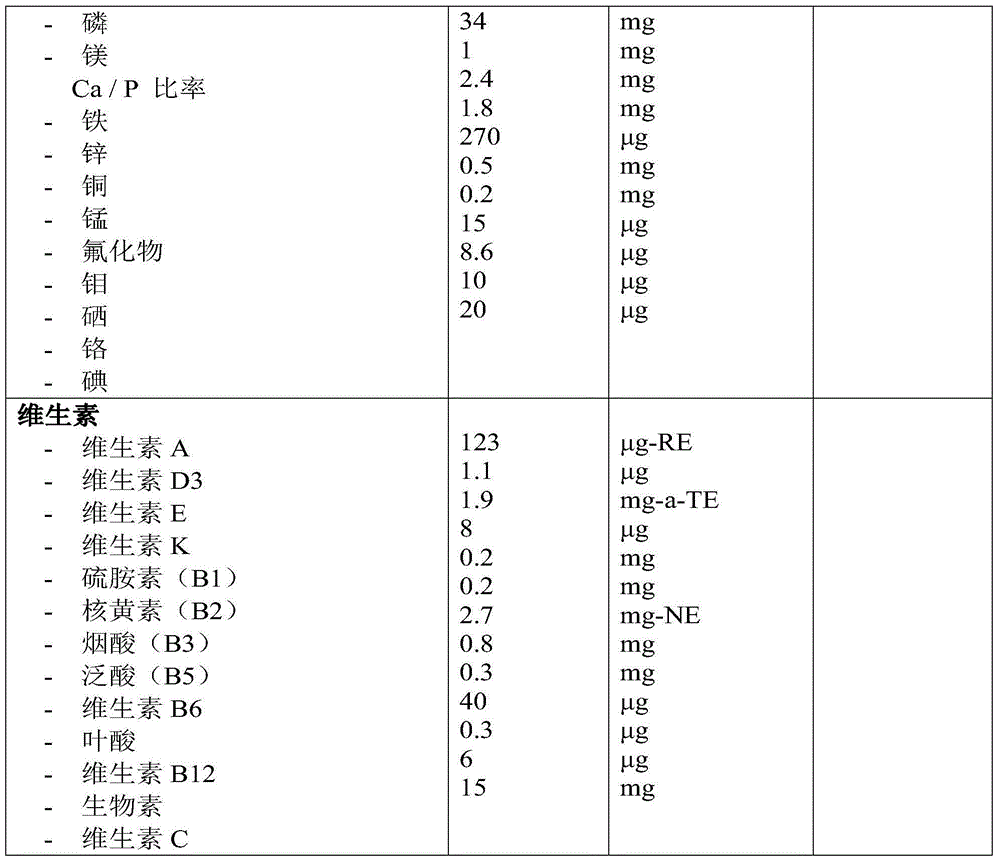
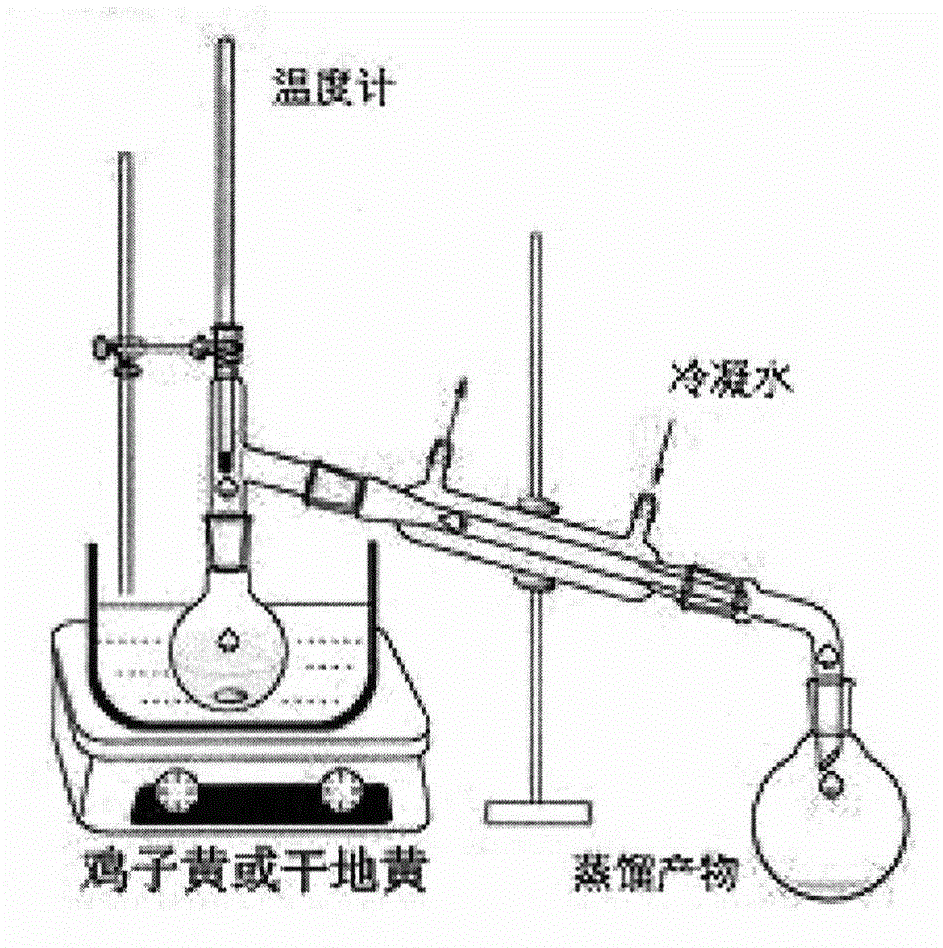
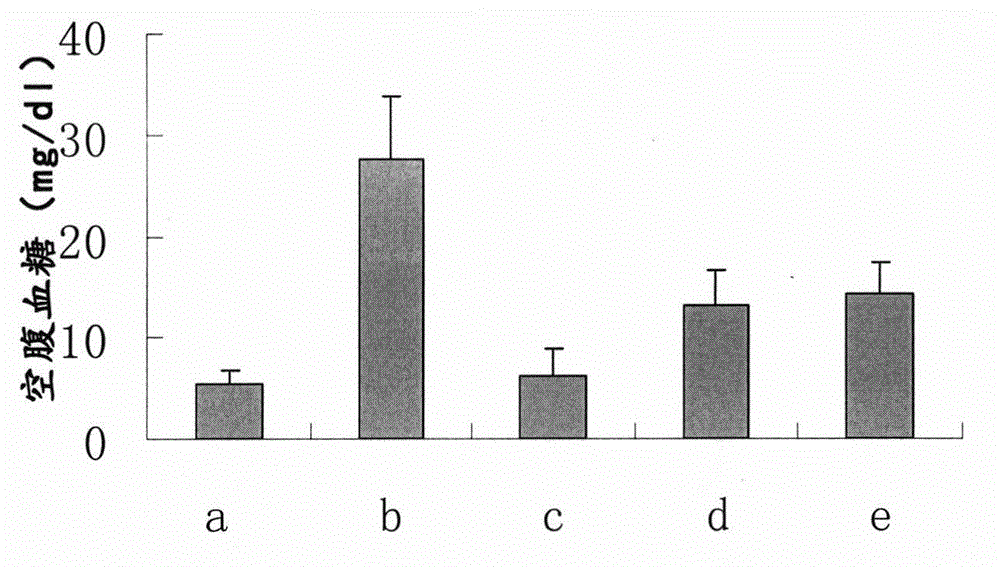
Patents
Literature
43 results about "Elevated glucose tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
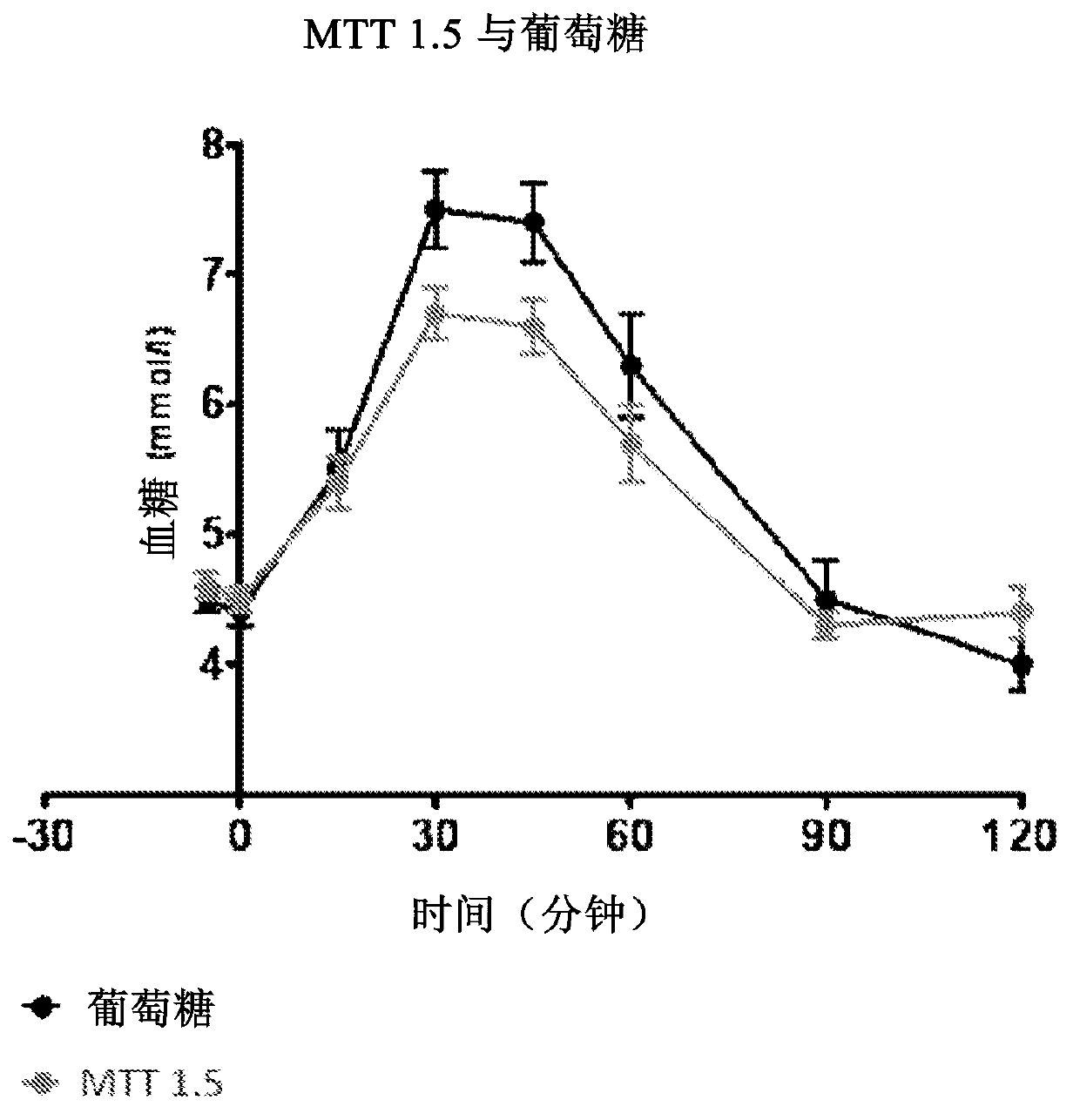
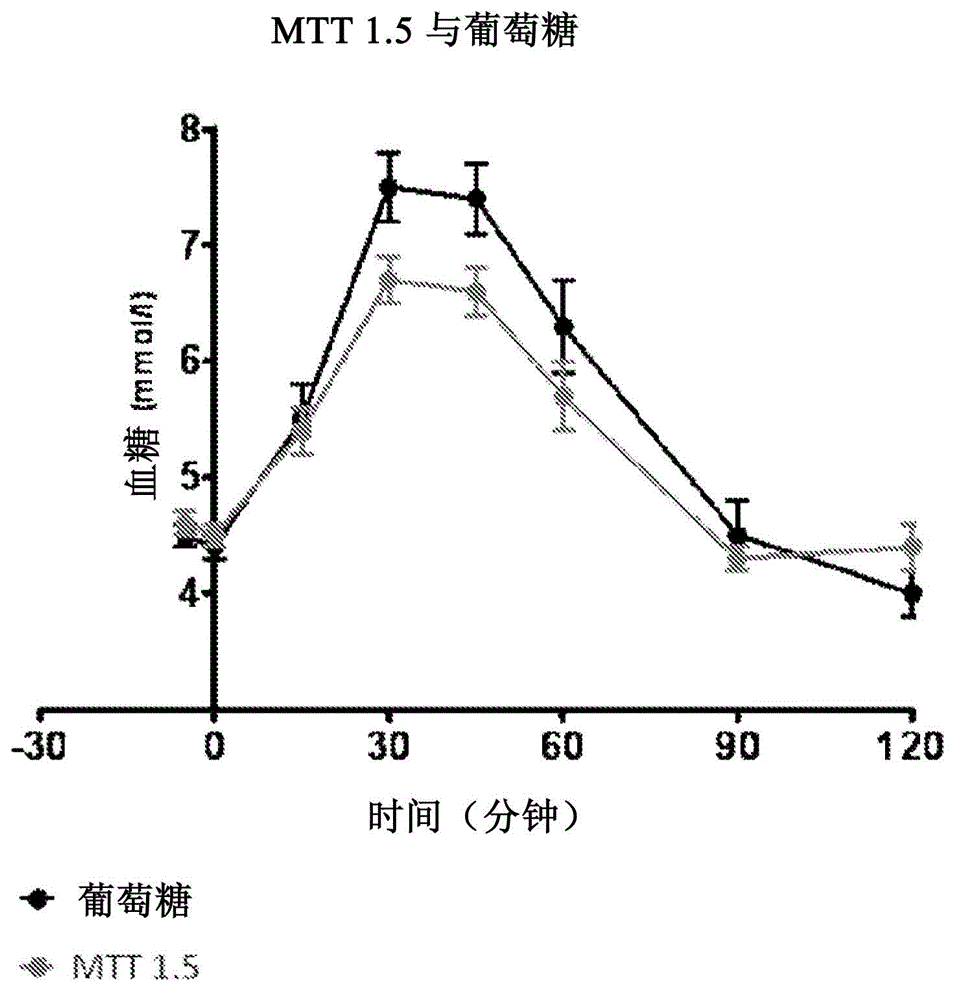
For a 2 hour GTT (Glucose Tolerance Test) with 75g intake, a glucose level below 7.8 mmol/L (140 mg/dL) is normal, whereas higher glucose levels indicate hyperglycemia.
Dynamic hepatic recycling glucose tolerance test
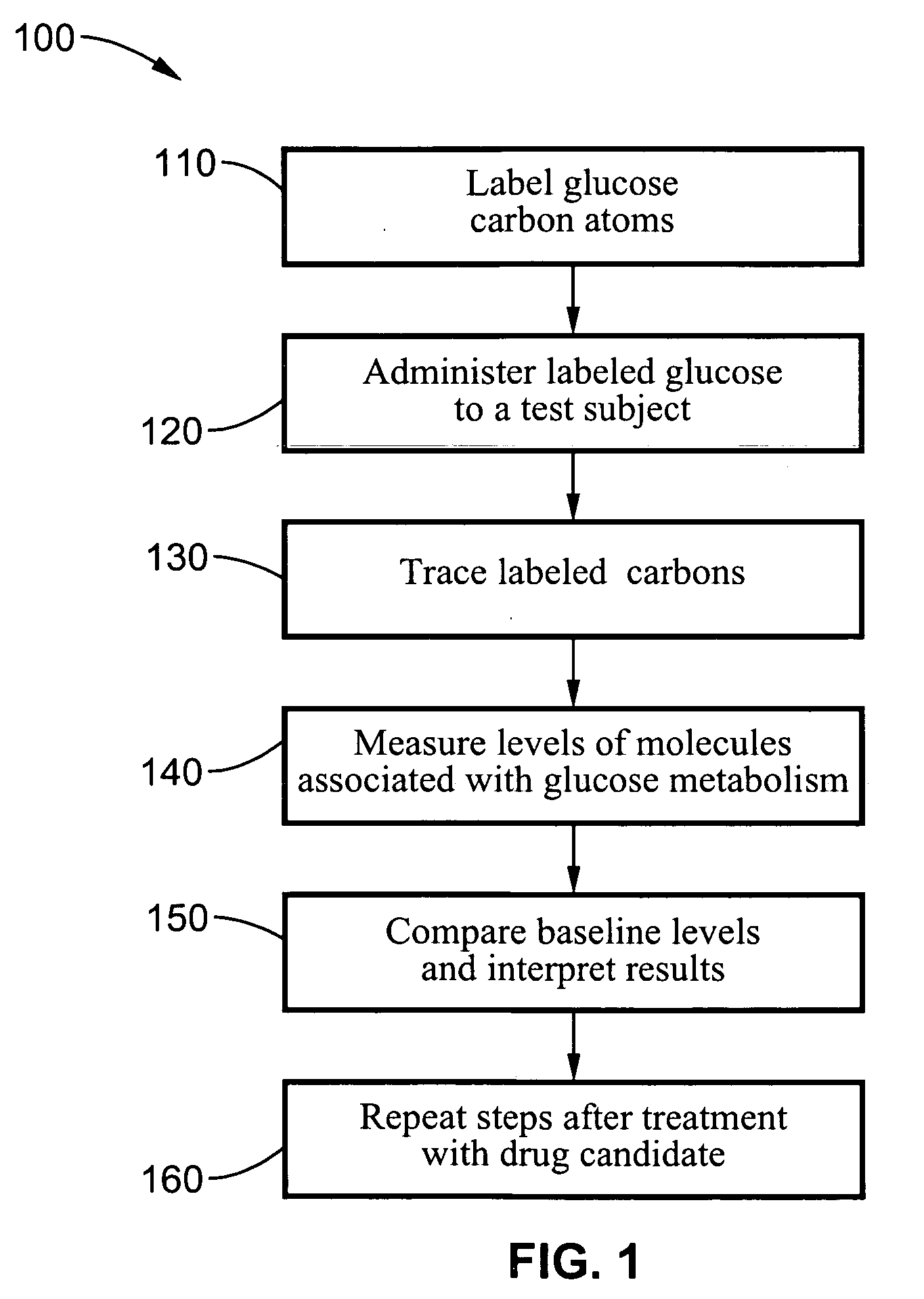
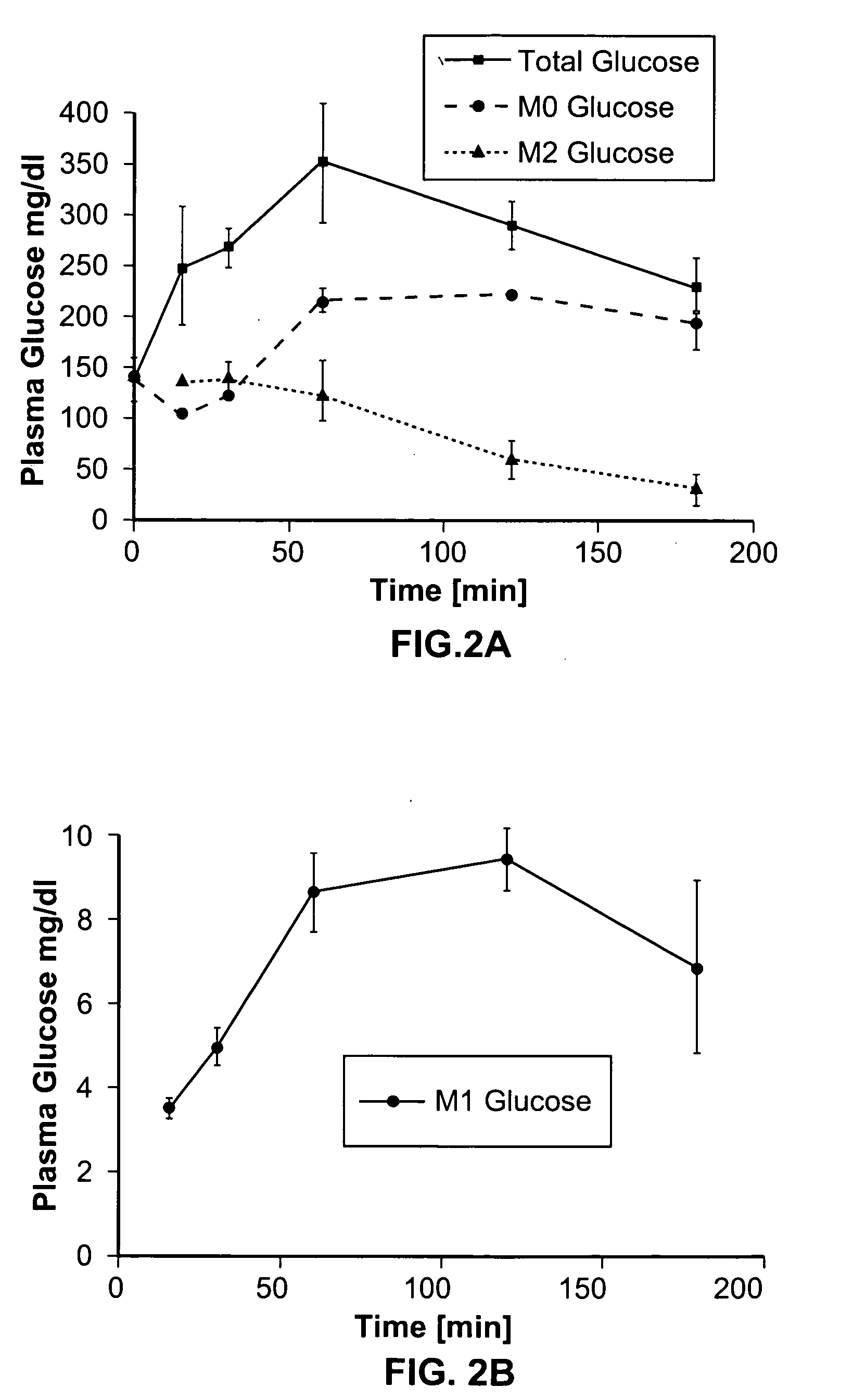
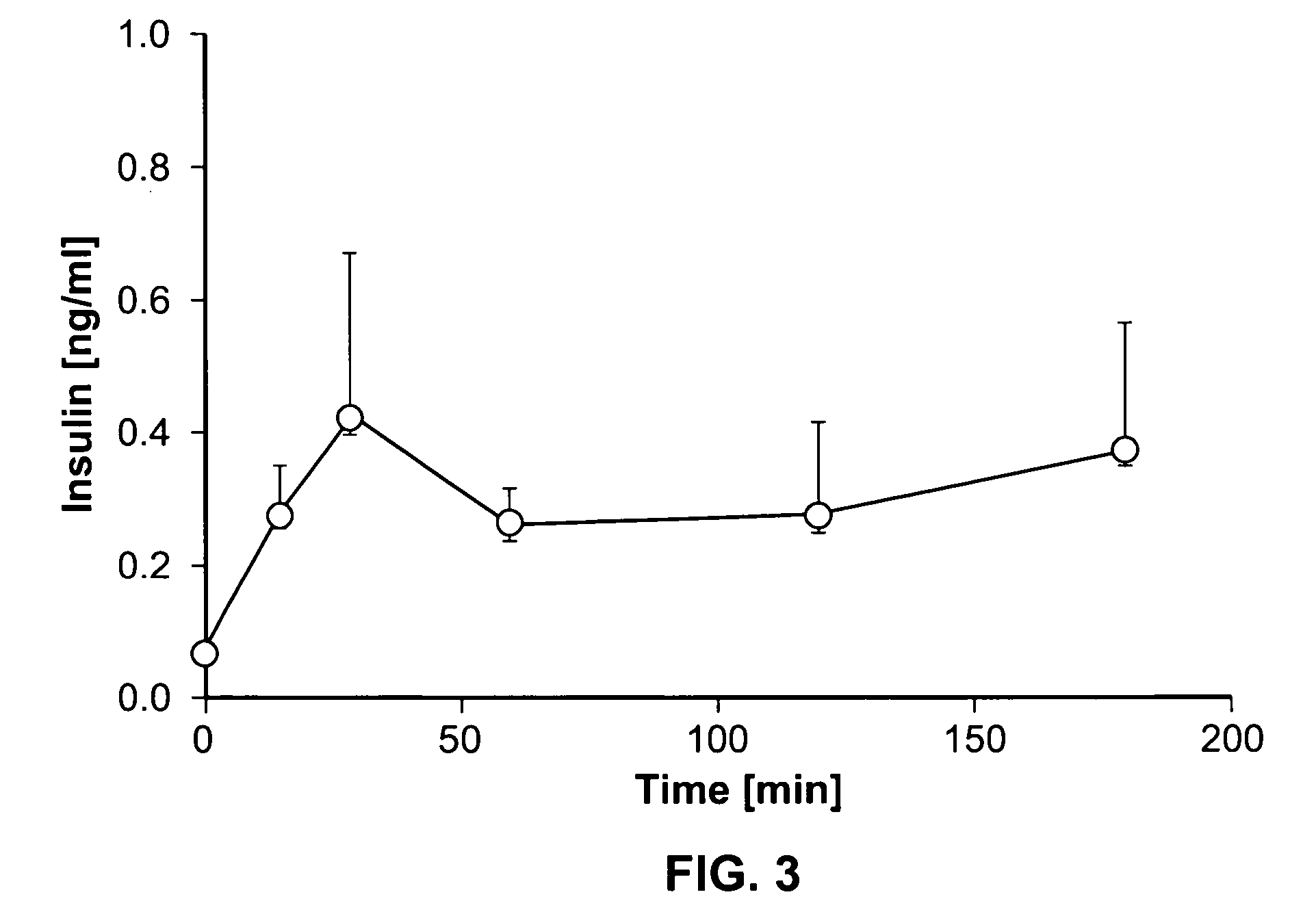
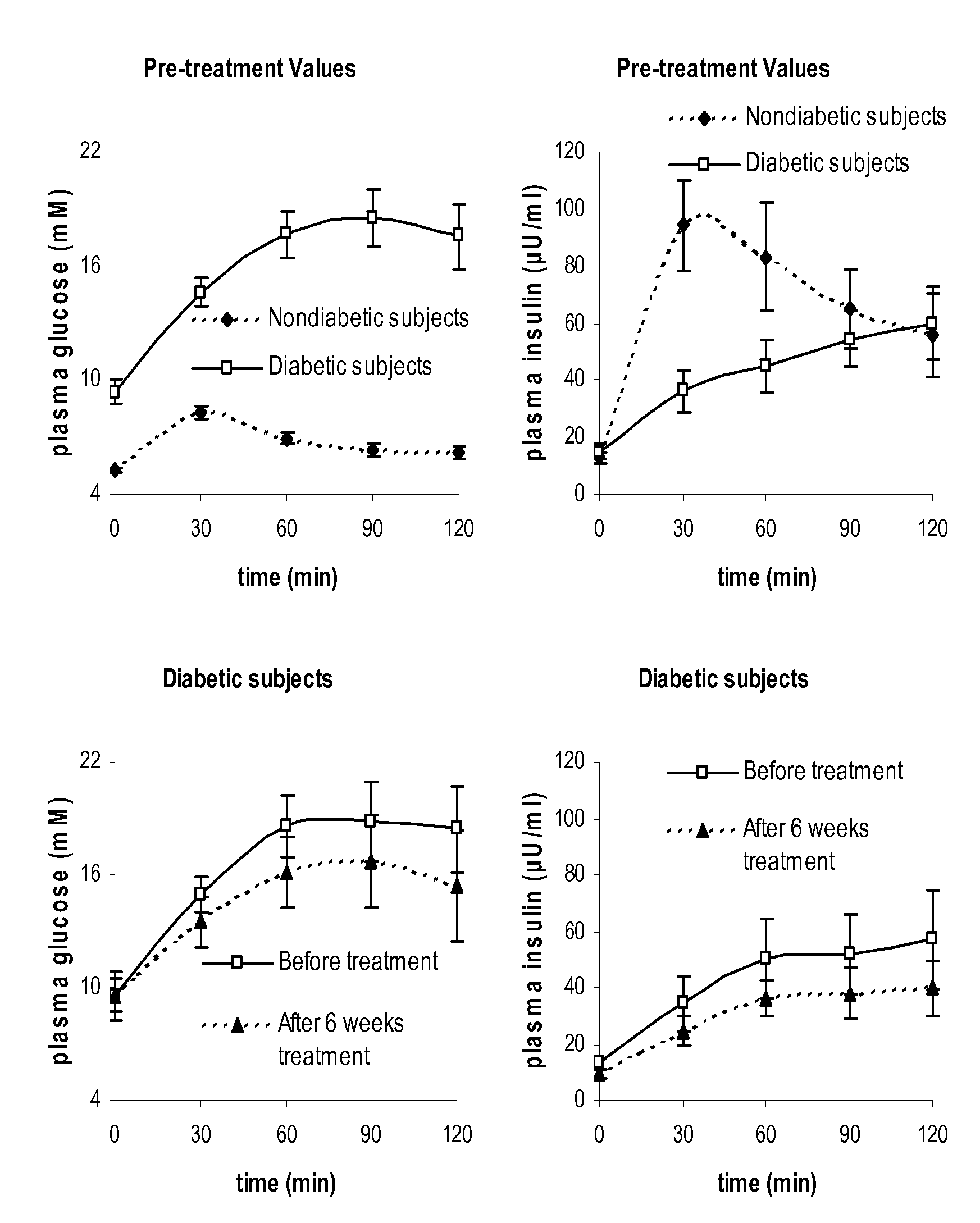
Systems and methods are described providing a hepatic recycling glucose tolerance test for the diagnosis of types and subtypes of diabetes mellitus and other hyperglycemic or hypoglycemic conditions. A method is also provided for screening candidate drugs for treating various types of abnormal glucose metabolism and to monitor whether the course of treatment is effective. The method also allows the correlation of gene activity, hormone and metabolite levels with glucose flux and recycling and an assessment of the degree of hepatic insulin resistance. The method utilizes a preferably non-radioactive stable labeled glucose to asses the relative rates of carbon flow in the liver and provides a hepatic recycling constant that is a measure of the relative rate of glucose recycling. The labeled glucose may be introduced to the patient orally, intravenously or by intraperitoneal administration for the desired effect.
Owner:RGT UNIV OF CALIFORNIA
Method for Determining Insulin Sensitivity and Glucose Absorption
InactiveUS20080262745A1Large-scale clinical trialsDifference in insulin sensitivityHealth-index calculationMicrobiological testing/measurementPresent methodPatient type
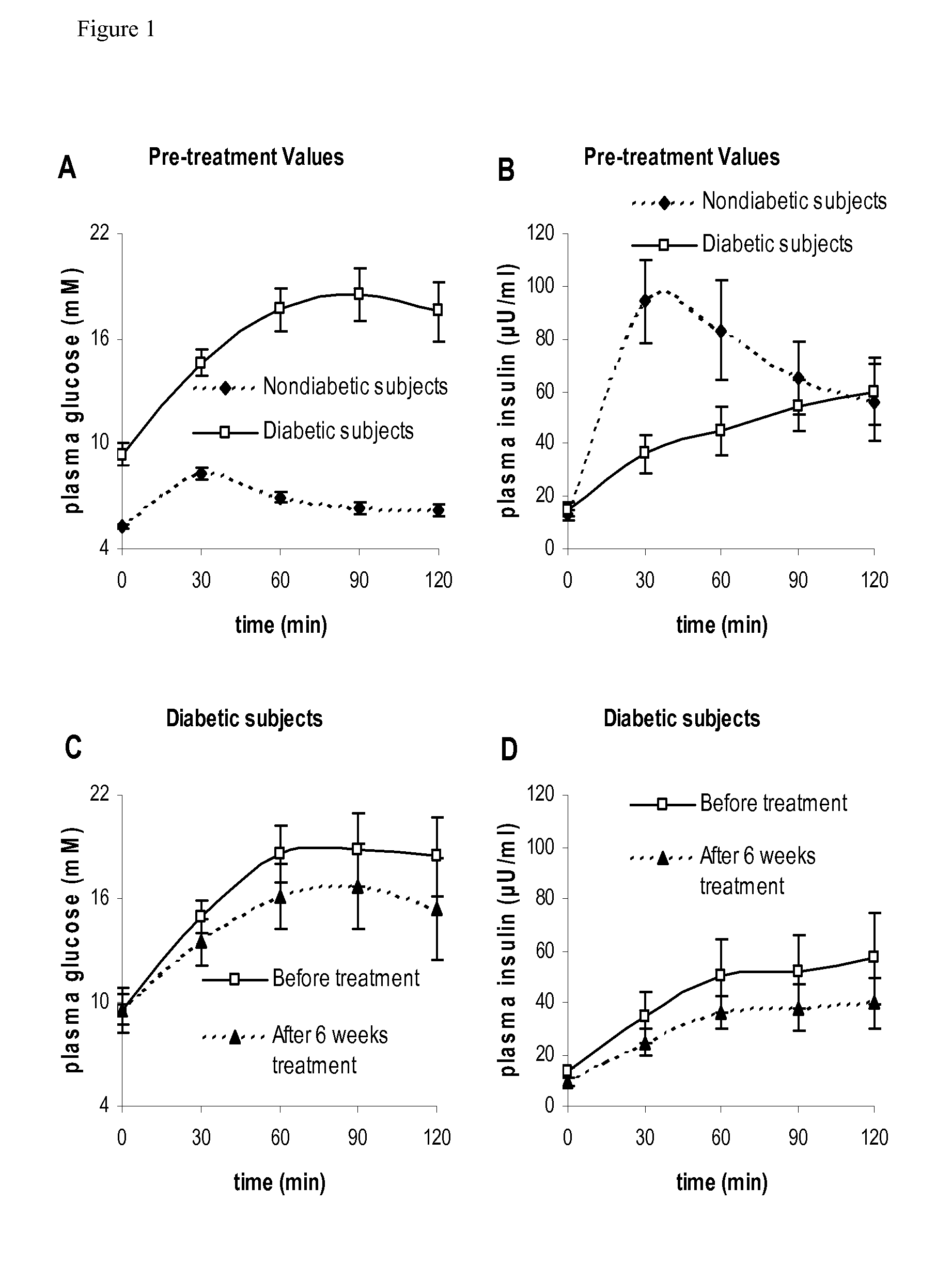
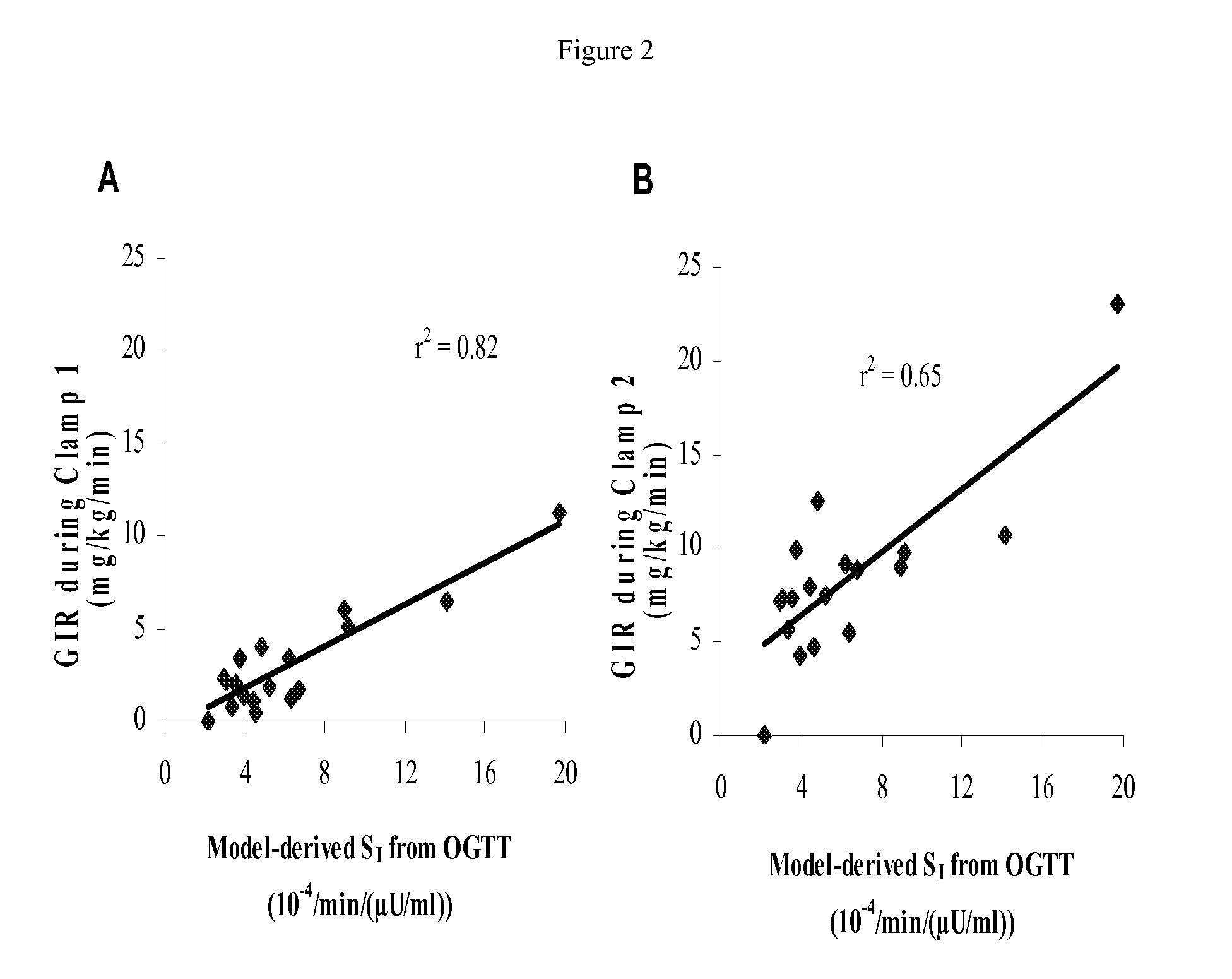
The present invention encompasses a model-based method for determining insulin sensitivity and glucose absorption from oral glucose tolerance tests or mixed meals. The present invention has several advantages over current methods. The technique requires about four to six blood samples taken over about two to three hours following glucose ingestion and is therefore applicable to large-scale clinical trials. The analysis involves a reduced version of the classical minimal model, a method for describing glucose absorption using only two parameters, and an integral approach enabling the parameters to be obtained using simple algebra. The present method robustly identifies differences in insulin sensitivity in different patient types as well as improvements in insulin sensitivity arising from pharmaceutic therapy. In addition, insulin sensitivity measurements obtained with the present method are highly correlated with results from hyperinsulinemic clamps (r2>0.8). This method is therefore a practical and robust method for determining insulin sensitivity under physiologic conditions.
Owner:VERIDEX LCC
Genetically Modified Animal and Use Thereof
InactiveUS20090186946A1Small fat cell sizeIncrease insulin sensitivityBiocideOrganic active ingredientsHigh resistanceAcute hyperglycaemia
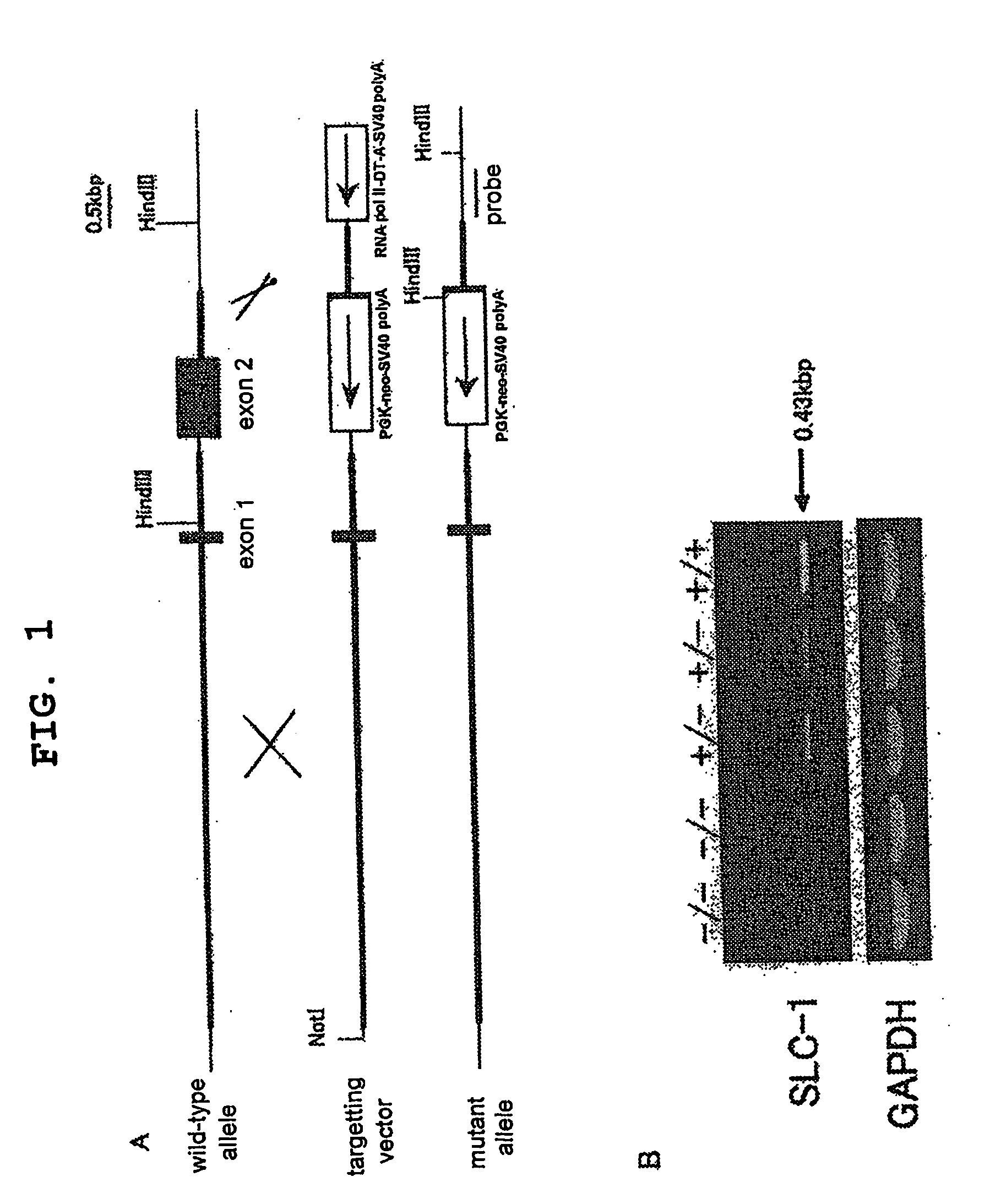
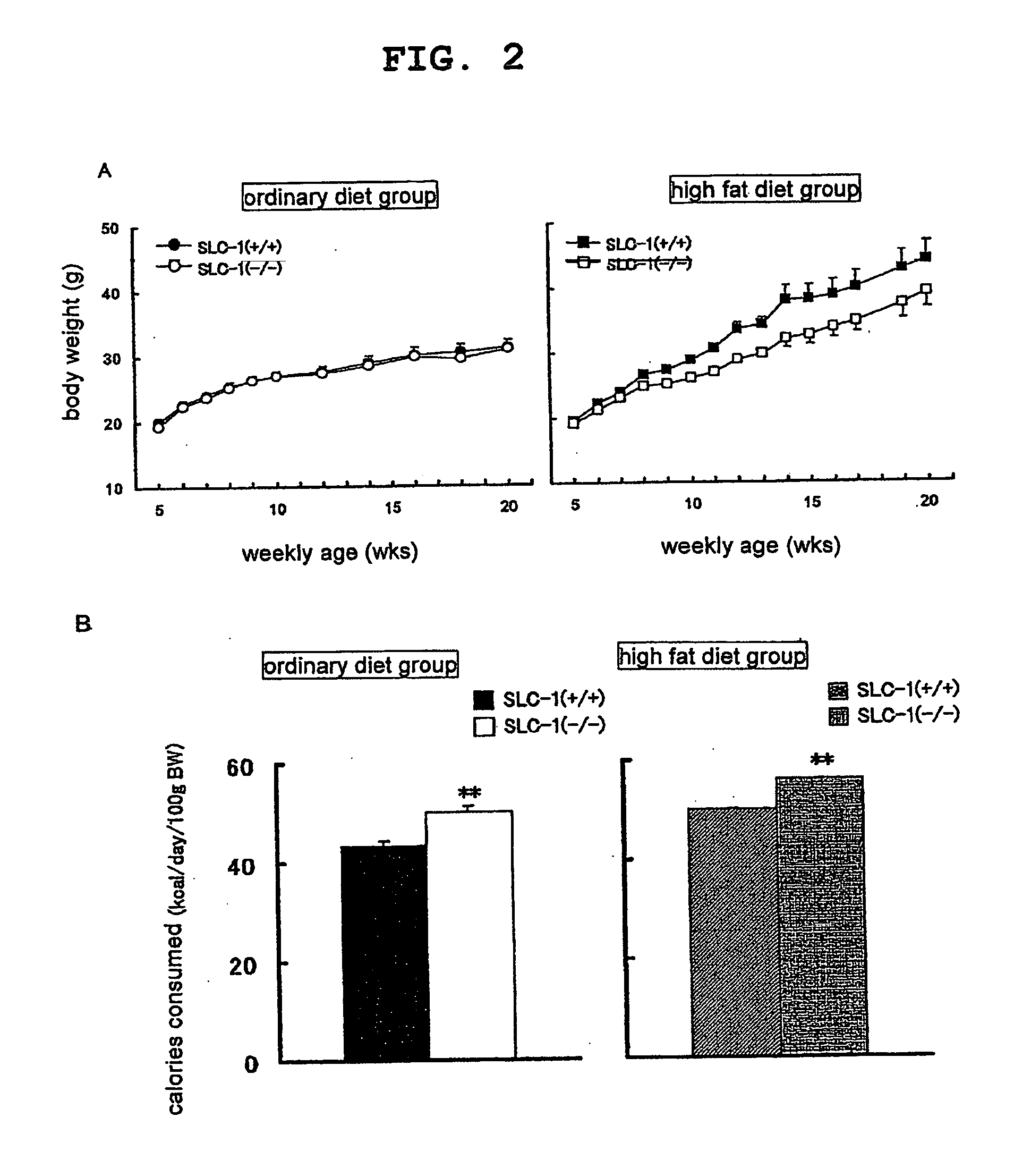
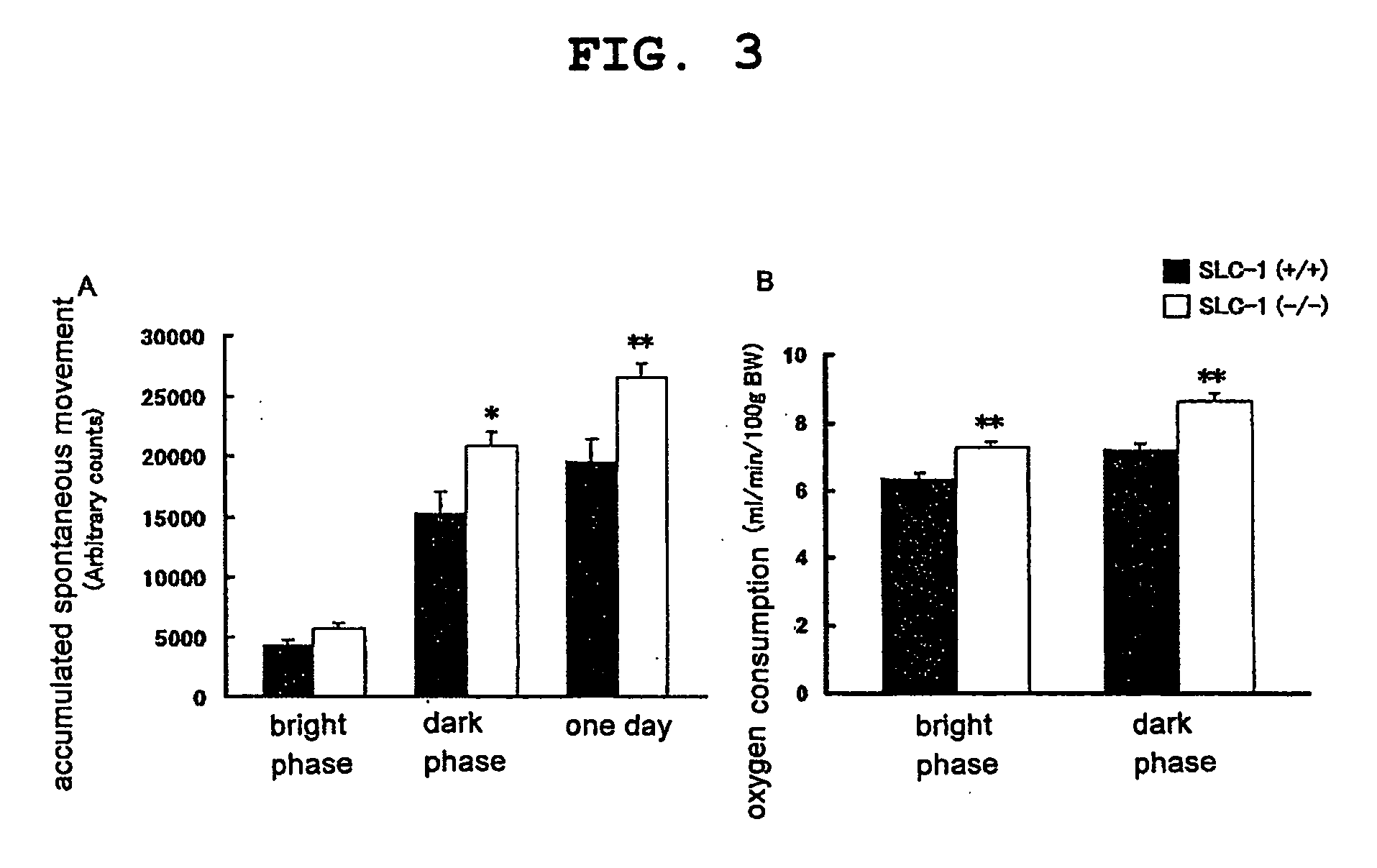
The present invention provides a non-human mammal deficient in the expression of the SLC-1 gene, having the characteristics of (1) a lower blood insulin level in glucose tolerance test, (2) increased insulin sensitivity, (3) higher resistance to obesity even on high fat diet, (4) a smaller white fat cell size, and (5) accentuated lipolysis, compared with the corresponding wild-type animal, or a portion of the body thereof. Also provided is an obesity and / or type II diabetes model non-human mammal that is deficient in the expression of the SLC-1 gene, having the characteristics of (1) elevated expression of adiponectin, (2) delayed onset of hyperglycemia, (3) a lower blood glycohemoglobin level, and (4) accentuated energy consumption, compared with the corresponding obesity and / or type II diabetes model non-human mammal wherein the expression of the gene is normal, or a portion of the body thereof.
Owner:TAKEDA PHARMACEUTICALS CO LTD
A method for determining insulin sensitivity and glucose absorption
InactiveCN101677764AHealth-index calculationMicrobiological testing/measurementPresent methodPatient type
The present invention encompasses a model-based method for determining insulin sensitivity and glucose absorption from oral glucose tolerance tests or mixed meals. The present invention has several advantages over current methods. The technique requires about four to six blood samples taken over about two to three hours following glucose ingestion and is therefore applicable to large-scale clinical trials. The analysis involves a reduced version of the classical minimal model, a method for describing glucose absorption using only two parameters, and an integral approach enabling the parametersto be obtained using simple algebra. The present method robustly identifies differences in insulin sensitivity in different patient types as well as improvements in insulin sensitivity arising from pharmaceutic therapy. In addition, insulin sensitivity measurements obtained with the present method are highly correlated with results from hyperinsulinemic clamps (r<2>>0.8). This method is thereforea practical and robust method for determining insulin sensitivity under physiologic conditions.
Owner:VERIDEX LCC
Methods for improving glycemic control in humans
InactiveUS20090076111A1Improve blood sugar controlImprove glucose toleranceBiocideOrganic active ingredientsArginineAlkaloid
The present invention is directed to methods for improving glycemic control in humans and animals comprising the step of administering a composition comprising an amino acid content including 4-hydroxyisoleucine in an amount between about 60% and about 70% of a total weight of the amino acid content, together with one or more amino acids selected from the group consisting of glutamate, aspartate, arginine, cysteine, threonine, serine, glycine, alanine, valine, methionine, isoleucine, and histidine (inclusive of any chemical salts, anhydrides, or isomers of any of the foregoing), in addition to, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Fasting blood glucose and glucose tolerance were studied in normal human subjects, in human subjects diagnosed with Metabolic Syndrome X, and in diabetic rats by means of dosing the subjects with compositions comprising 4-hydroxyisoleucine in an amount between about 20% and about 30% of the total weight of the composition, wherein improving glycemic control in standard glucose tolerance tests.
Owner:TSI GROUP
Medical application of genistein chromium complex to treatment of diabetes
ActiveCN105106221ALower blood sugarImproves glucose tolerance levelOrganic active ingredientsMetabolism disorderFasting glucosePancreatic hormone
The invention provides medical application of a genistein chromium complex to treatment of diabetes. Results of the animal growth condition research, fasting blood-glucose value determination, glucose tolerance test, insulin tolerance test show that the genistein chromium complex has an excellent hypoglycemic effect, and can be used for preparing drugs for treating diabetes II; by combing the hypoglycemic advantage of chromium, the hypoglycemic activity of genistein is improved obviously and toxicity of inorganic chromium can be reduced.
Owner:BEIJING UNIV OF CHINESE MEDICINE
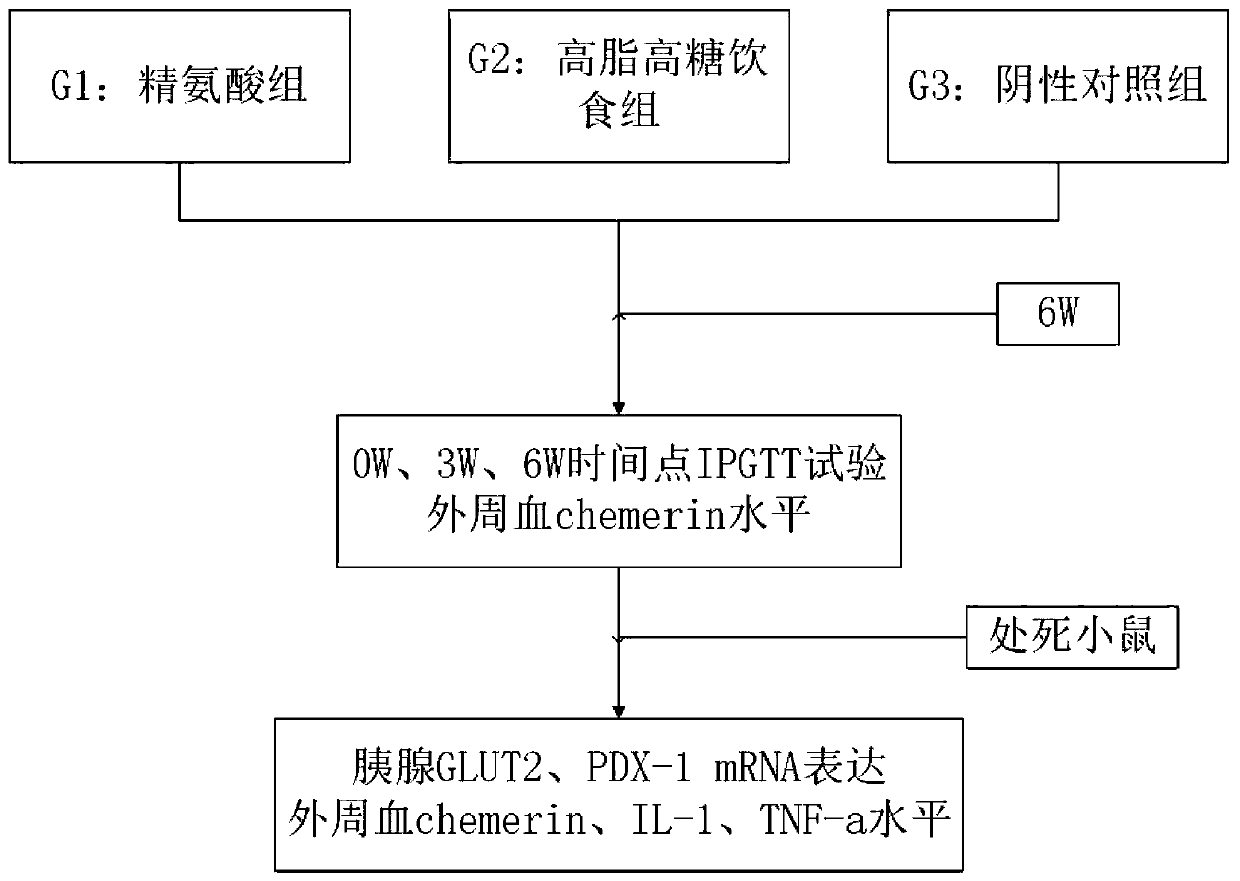
Method for measuring regulation and control of Chemerin on insulin resistance and intervention of CMKLR1 agonist
InactiveCN109985251AHigh and stable success rateInduced stabilizationCompounds screening/testingAnimal husbandryIntraperitoneal routeArginine
The invention belongs to the technical field of testing or analyzing materials by means of chemical or physical properties of a measuring material, and discloses a method for measuring the regulationand control of Chemerin on insulin resistance and the intervention of a CMKLR1 agonist, which comprises the following steps: preparing a pancreatic diabetes model by adopting an arginine induction method, performing intraperitoneal injection of 350 mg / kg arginine once a day for 6 weeks, and establishing a pancreatic diabetes group by a mouse common diet; during the period, establishing a pancreas-derived diabetes mellitus group by the common diet of the mice;meanwhile, establishing a high-fat diet group by adopting a formula feed open diet; establishinga negative control group, wherein the mice are fed with ordinary diet and water for 6 weeks; adopting a general pathology, an enzyme-linked immunosorbent assay, a Real-Time PCR method and an intraperitoneal injection glucose tolerance test for detection; expressing all the detection data by mean plus or minus standard deviation, and testing the normal distribution is by Shapiro Wilk test; using single-factor variance analysis to comparegroups with each other, and if the groups do not conform to the normal distribution, using Mann-Whitney U test for comparison; significant difference lying in P < 0.05; applying SPSS 22.0 for Windowssoftware to implement the entire verification process.
Owner:涂建锋
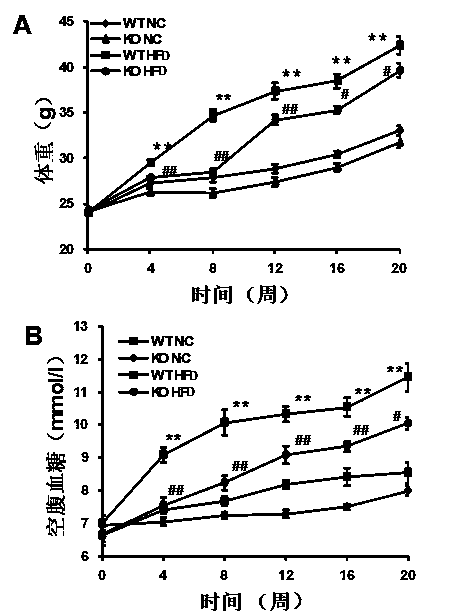
Functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus
InactiveCN104056271AWorsening fatty liverThe role of exacerbating type 2 diabetes diseaseMetabolism disorderGenetic material ingredientsIntraperitoneal routeStaining
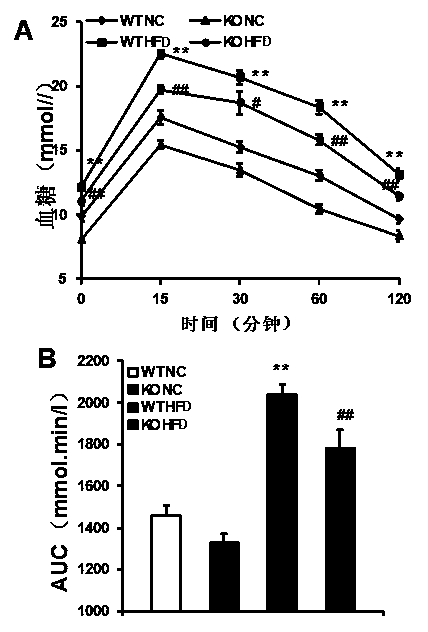
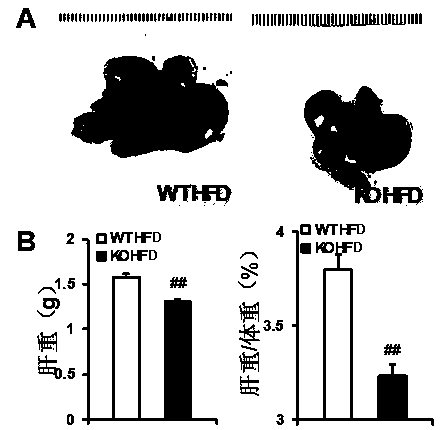
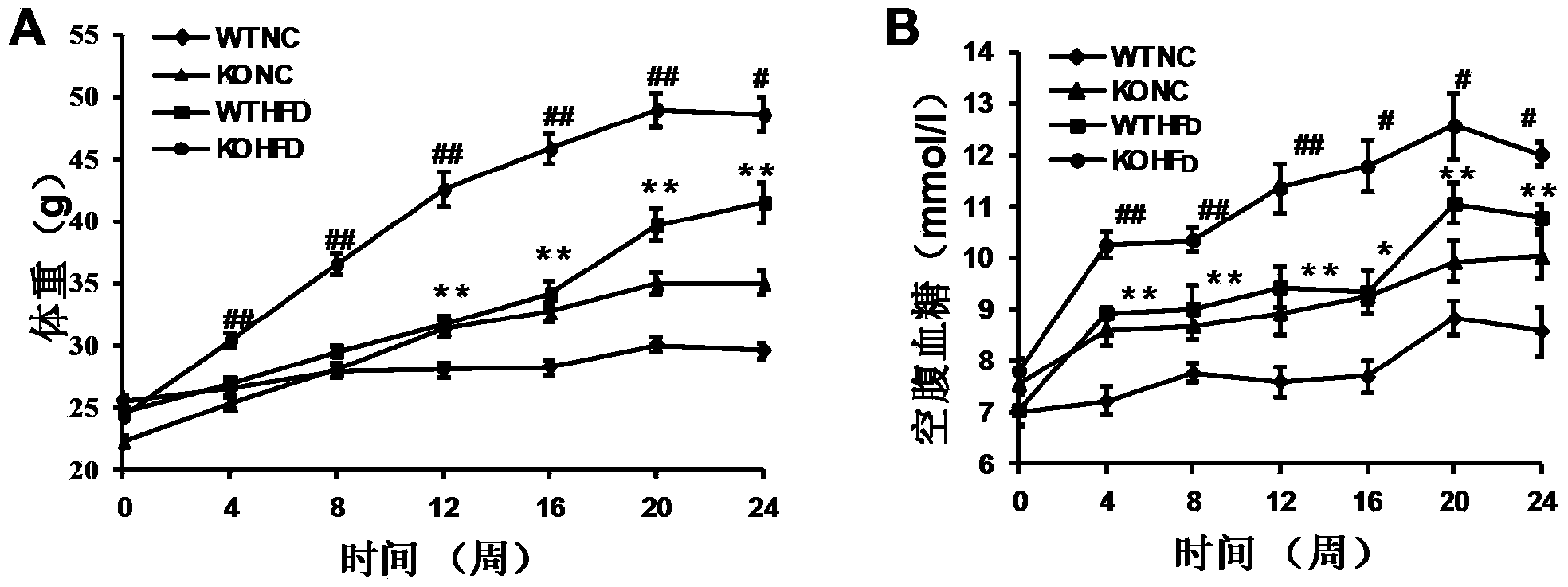
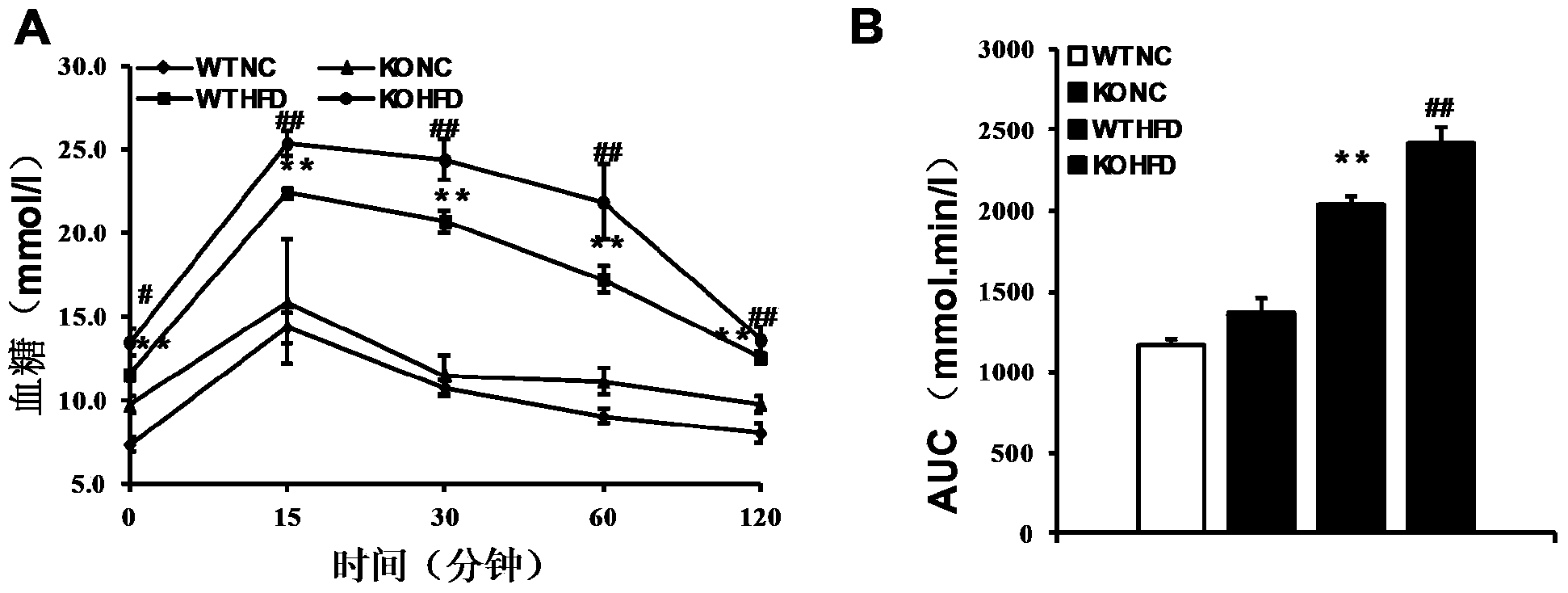
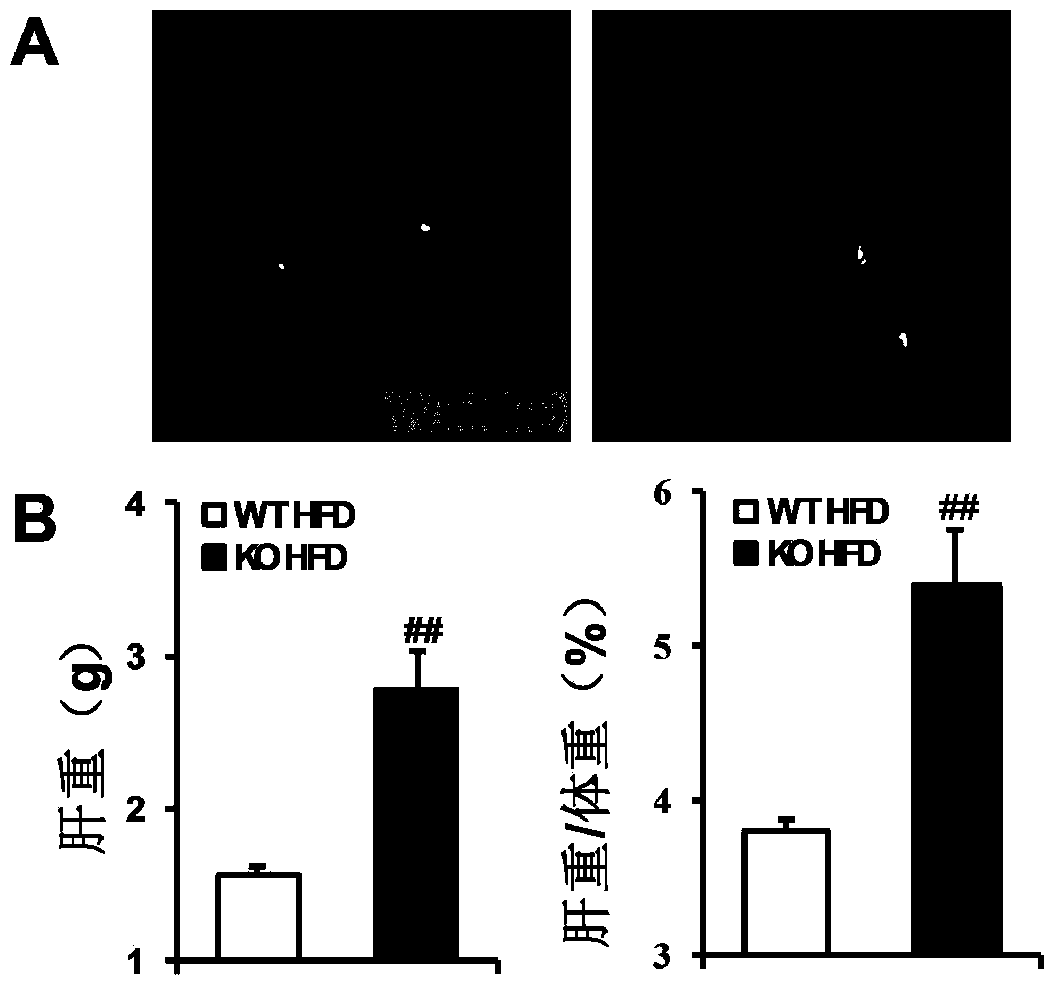
The invention discloses functions and application of TNF (tumor necrosis factor) receptor associated factor 5 (TRAF5) in treatment of fatty liver and type 2 diabetes mellitus. Studies on a TRAF 5 gene by a high-fat diet (HFD) induced model discover that both the body weight and fasting plasma glucose level of an HFD bred TRAF gene knock-out mouse are lower than those of a WT mouse; glucose tolerance tests by intraperitoneal injection discover that the glucose tolerance of the TRAF5 gene knock-out mouse is remarkably reinforced; results of on liver gross appearance, liver weight, liver / body weight ratio and lipid component pathological staining indicate that the TRAF5-KO mouse fatty liver lesion in the HFD group is remarkably improved, the lipid accumulation is remarkably reduced, and the TRAF5 gene knock-out has the effects of remarkably improving fatty acid and type-II diabetes mellitus. Against the effects, the TRAF5 gene knock-out can be used as a medicinal target for screening and treating fatty liver and / or type-II diabetes mellitus, and the inhibitor of the TRAF5 gene knock-out can be used for preparing medicaments for treating fatty liver and / or type-II diabetes mellitus.
Owner:WUHAN UNIV
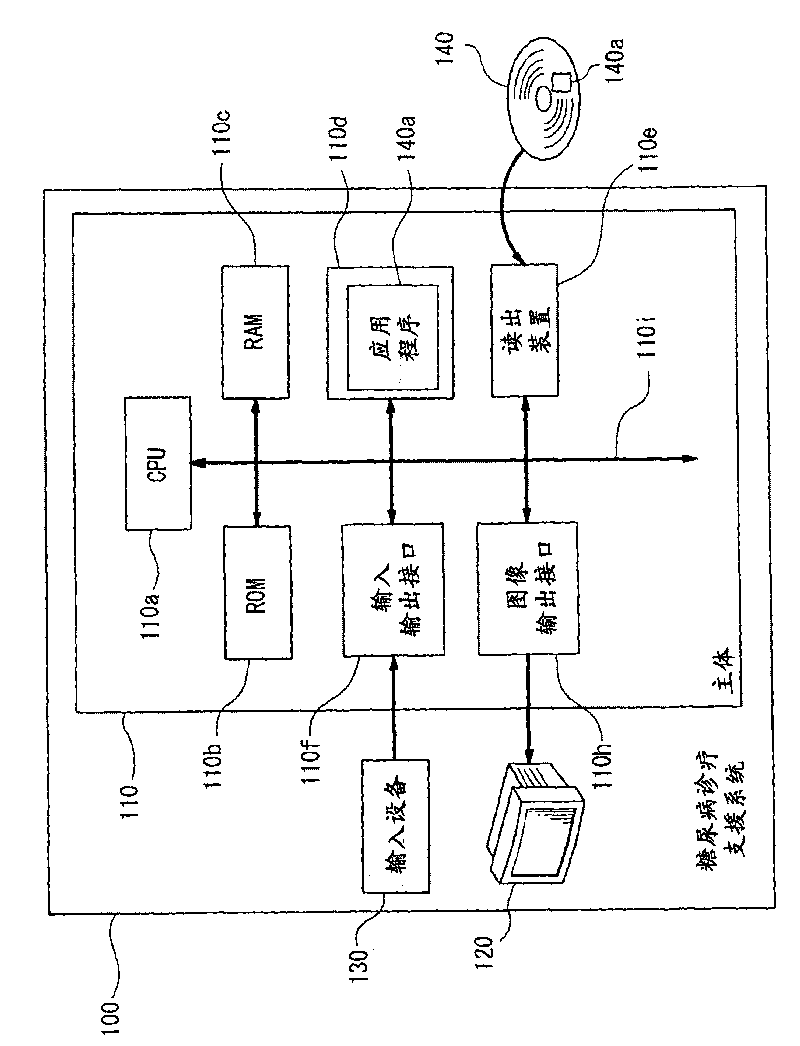
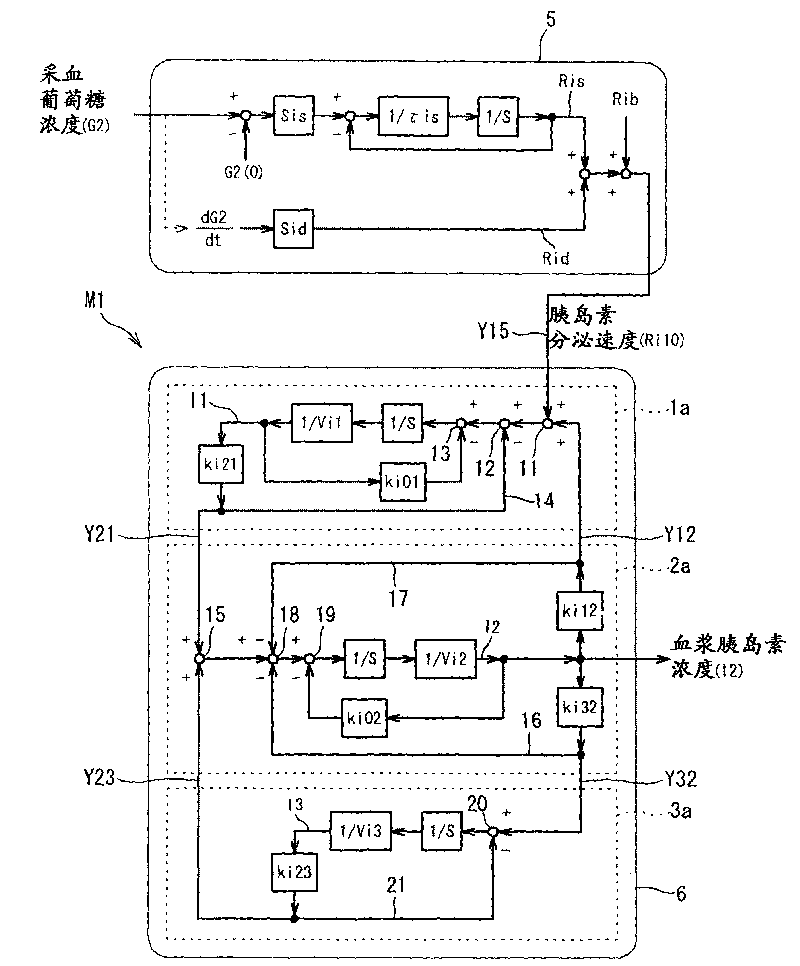
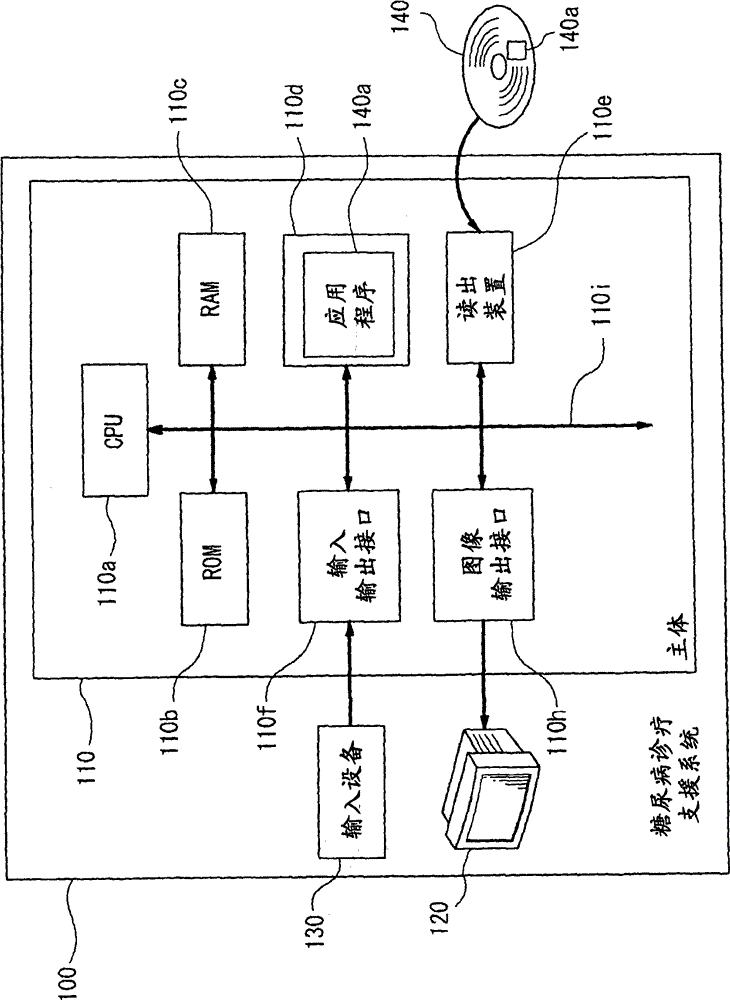
Diagnostic support apparatus for diabetes and computer program product
ActiveCN101763464AHigh-precision diagnosis and treatment supportMedical simulationData processing applicationsBiological bodyElevated glucose tolerance
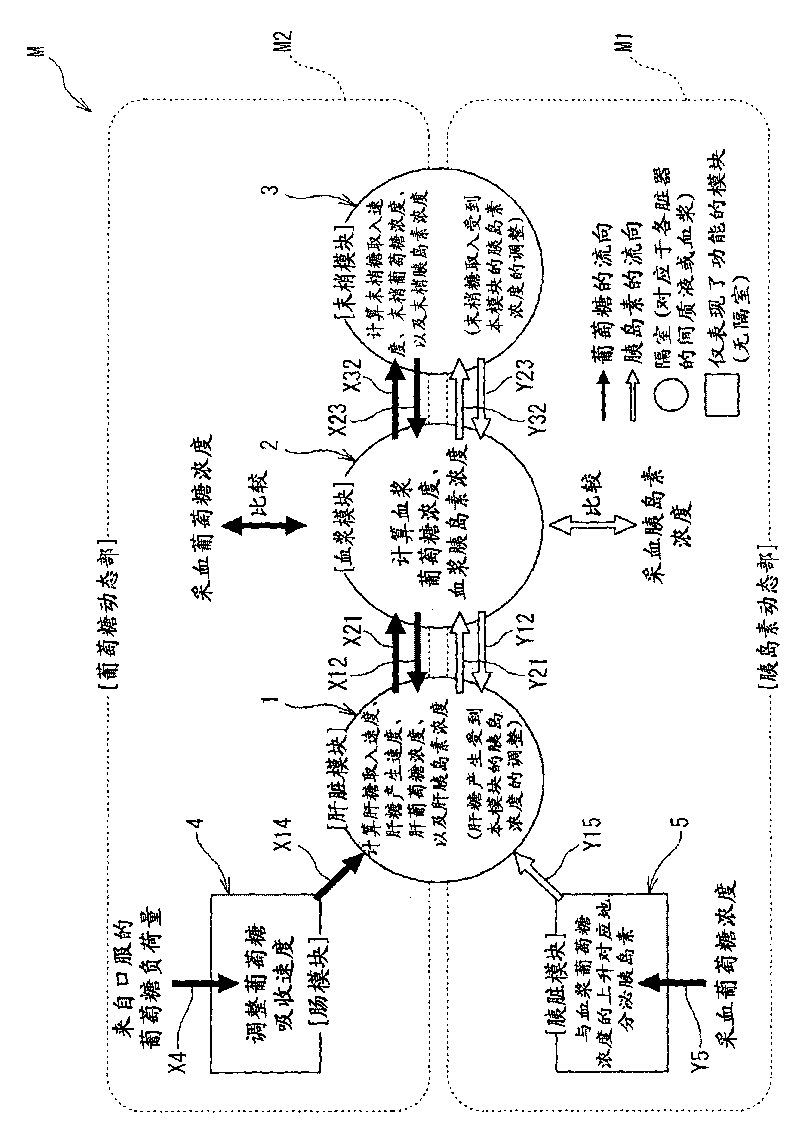
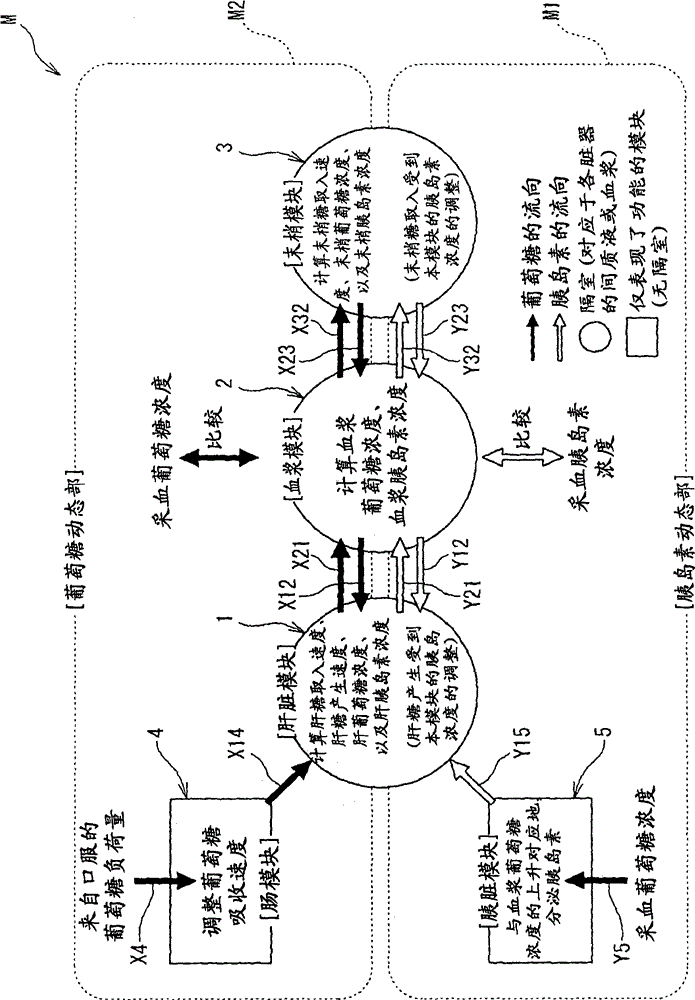
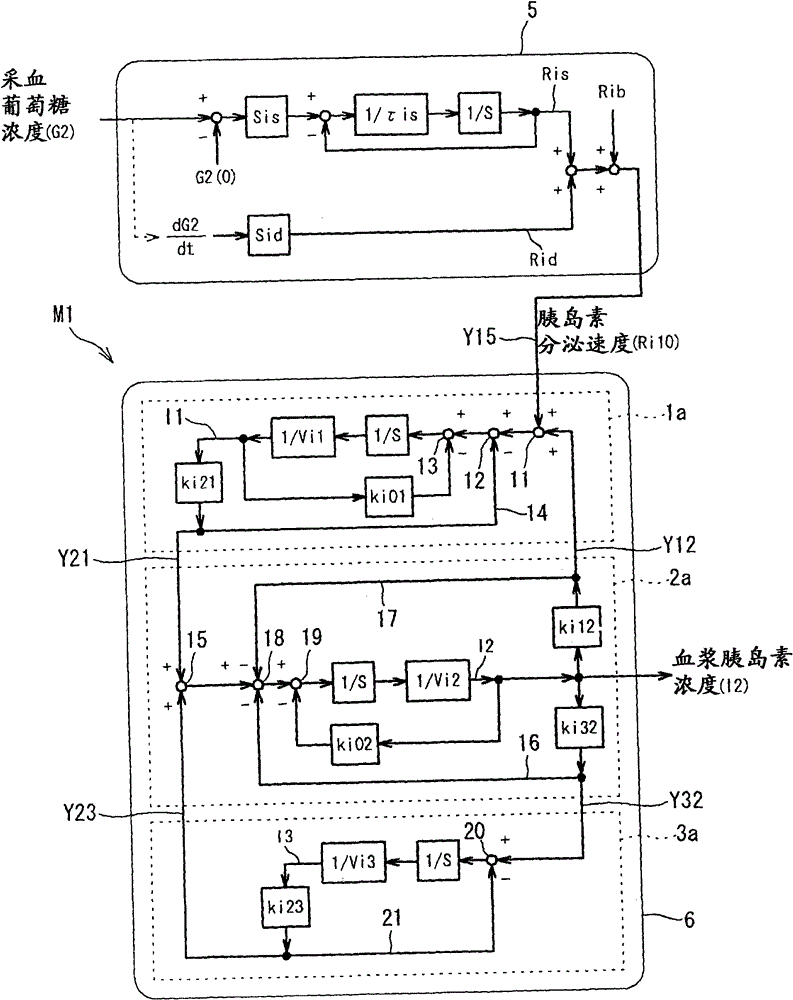
The present invention is to present a diagnostic support apparatus for diabetes including a diagnostic support information generating unit which generates diagnostic support information of a patient based on a biological model for reproducing a pseudo-response which simulates a result of a glucose tolerance test for the patient. The biological model comprises a plurality of simulated organ blocks which are configured in such manner that inflow and outflow of glucose and / or inflow and outflow of insulin are reciprocally produced between each of the simulated organ blocks. The plurality of the simulated organ blocks respectively calculate at least one of a cumulative quantity and a concentration of glucose and / or at least one of a cumulative quantity and a concentration of insulin in the respective simulated organ blocks, based on a quantity of inflow and outflow of glucose and / or a quantity of inflow and outflow of insulin in the respective simulated organ blocks.
Owner:SYSMEX CORP
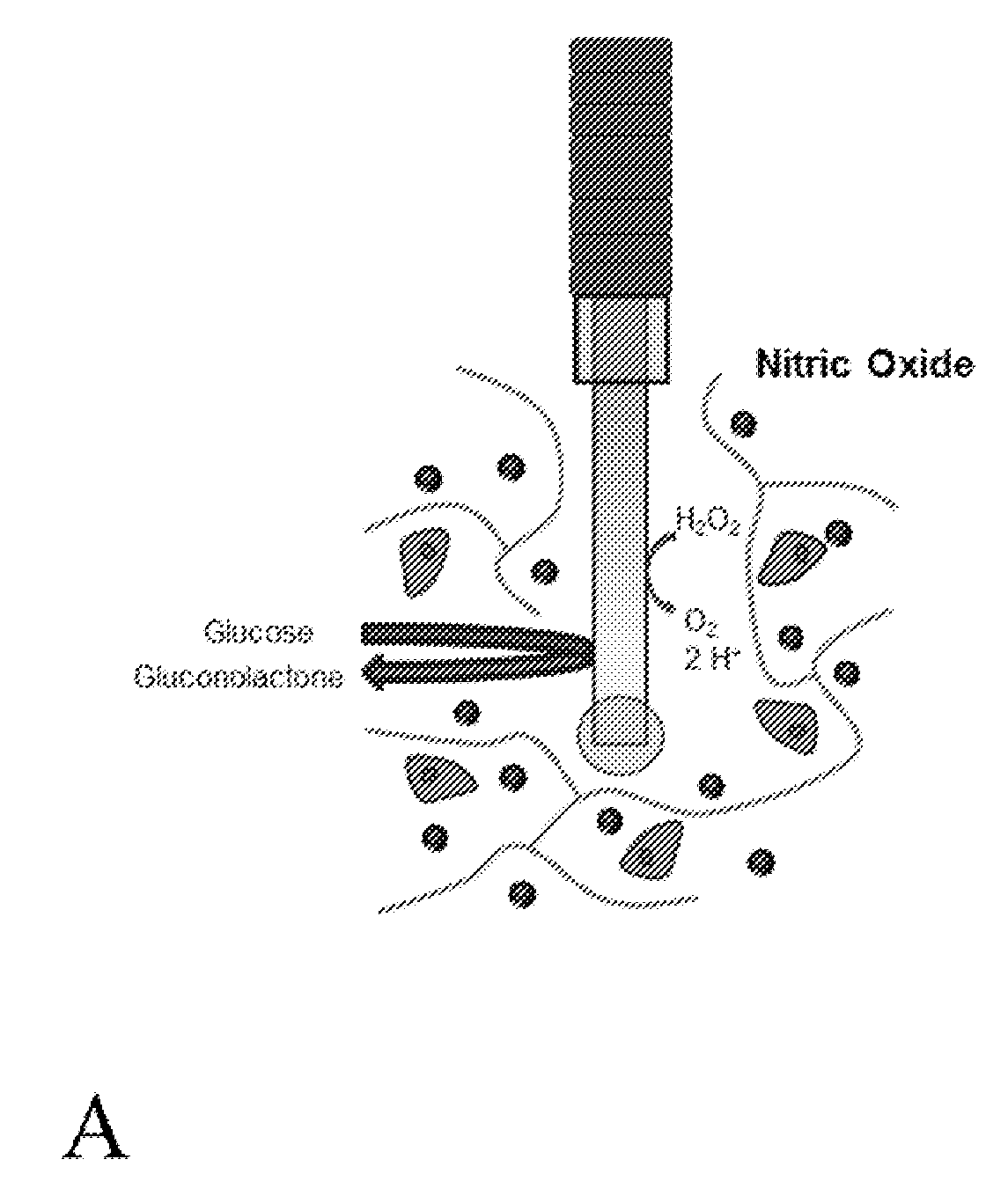
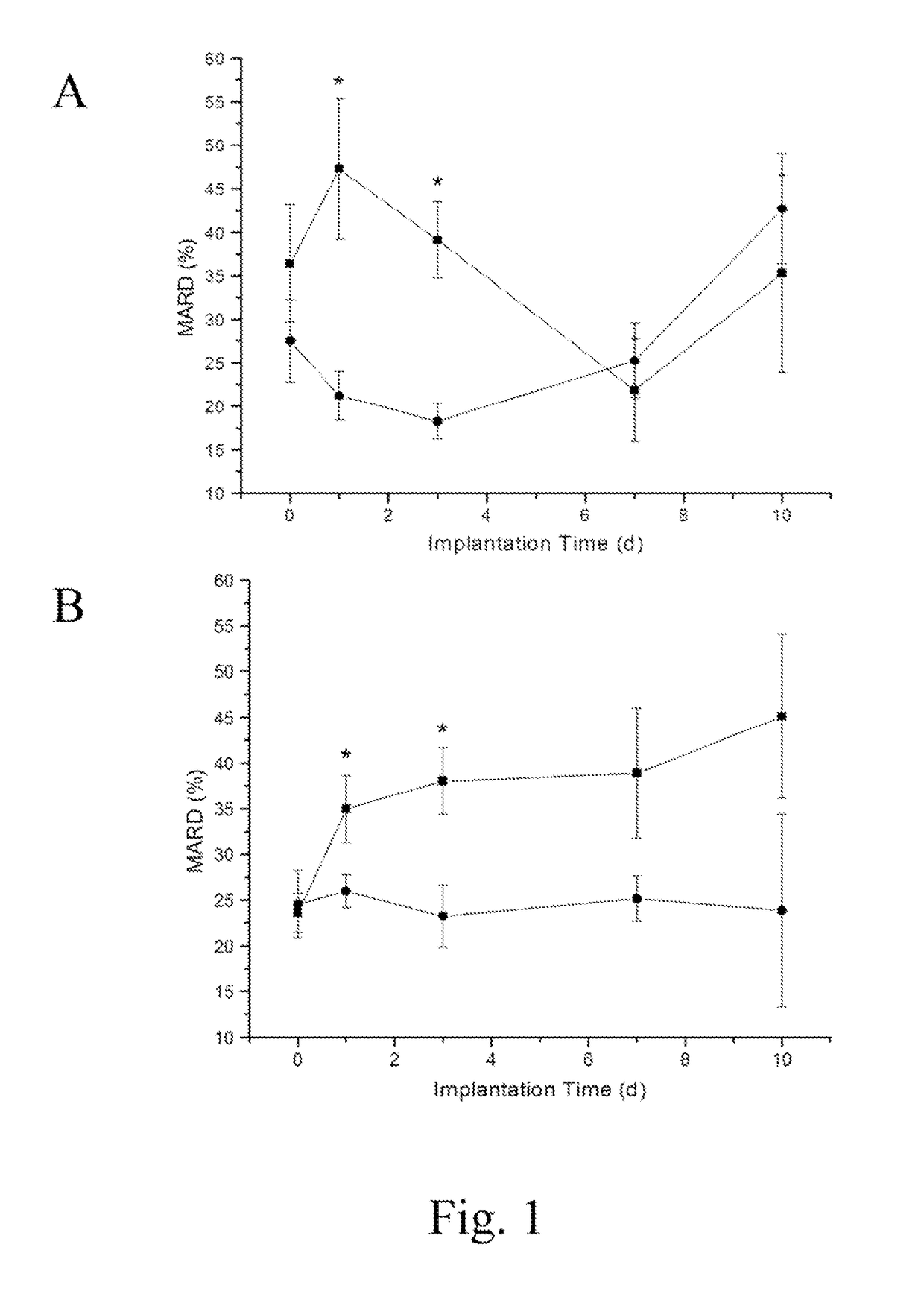
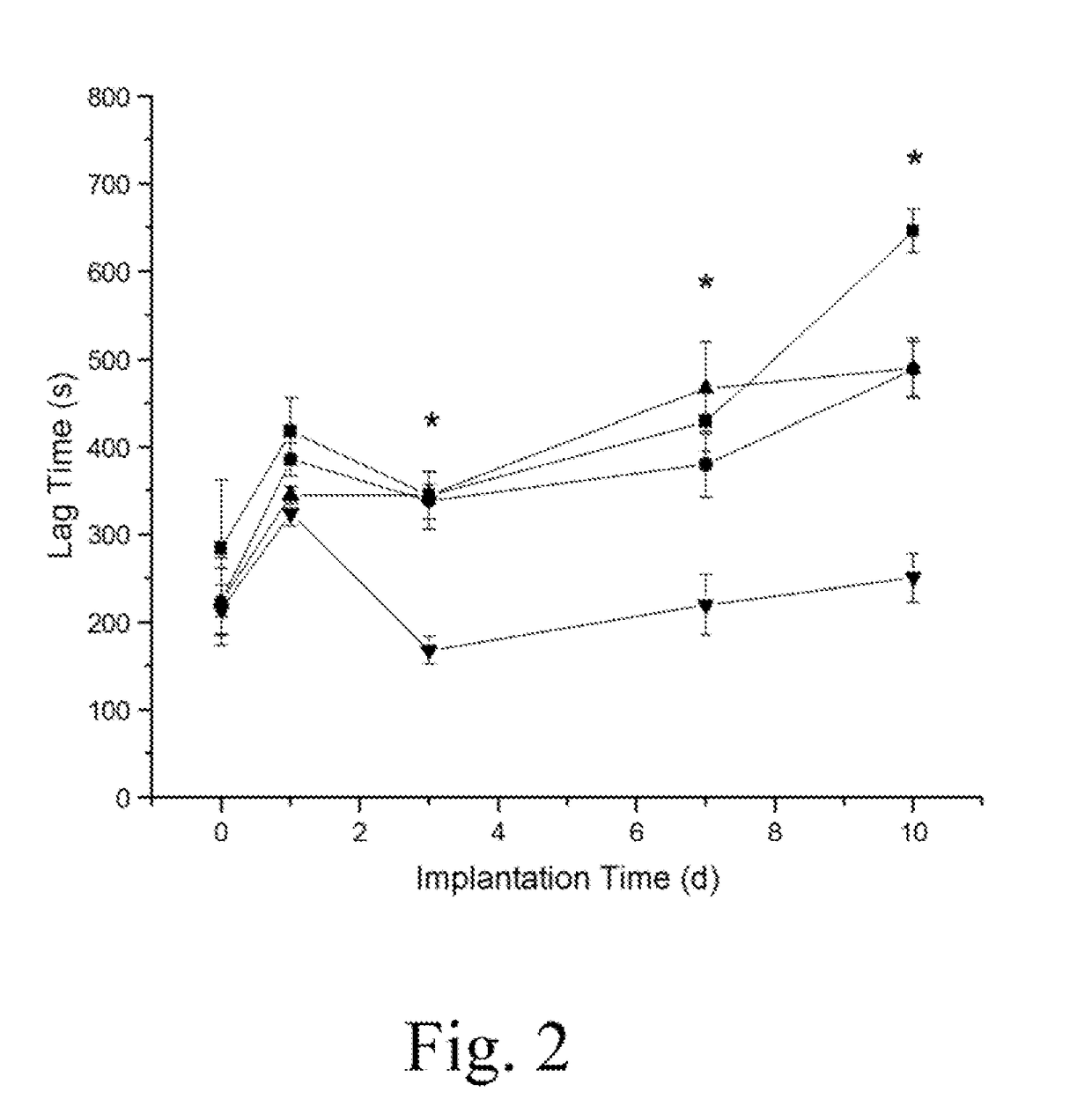
Extended analytical performance of continuous glucose monitoring devices via nitric oxide
InactiveUS20170238852A1Minimize foreign body responseImprove analytical performanceCatheterSensorsGlucose sensorsElevated glucose tolerance
The present invention relates to instruments and methods related to the in vivo analytical performance of percutaneously implanted nitric oxide (NO)-releasing amperometric glucose biosensors. Needle-type glucose biosensors can be functionalized with NO-releasing polyurethane coatings designed to release similar total amounts of NO for rapid or slower (greater than 3 day) durations and remain functional as outer glucose sensor membranes. Relative to controls, NO-releasing sensors were characterized with improved numerical accuracy on days 1 and 3. Furthermore, the clinical accuracy and sensitivity of rapid NO-releasing sensors were superior to control and slower NO-releasing sensors at both 1 and 3 days implantation. In contrast, the slower, extended NO releasing-sensors were characterized by shorter sensor lag times (<4.2 min) in response to intravascular glucose tolerance tests versus burst NO-releasing and control sensors (>5.8 min) at 3, 7, and 10 d. Collectively, these results highlight the potential for NO release to enhance the analytical utility of in vivo glucose biosensors. Thus, the analytical performance benefit is dependent on the NO-release duration.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Novel glucose tolerance test and composition for use
InactiveCN102791148ADisease diagnosisBiological testingElevated glucose toleranceGlucose tolerance test
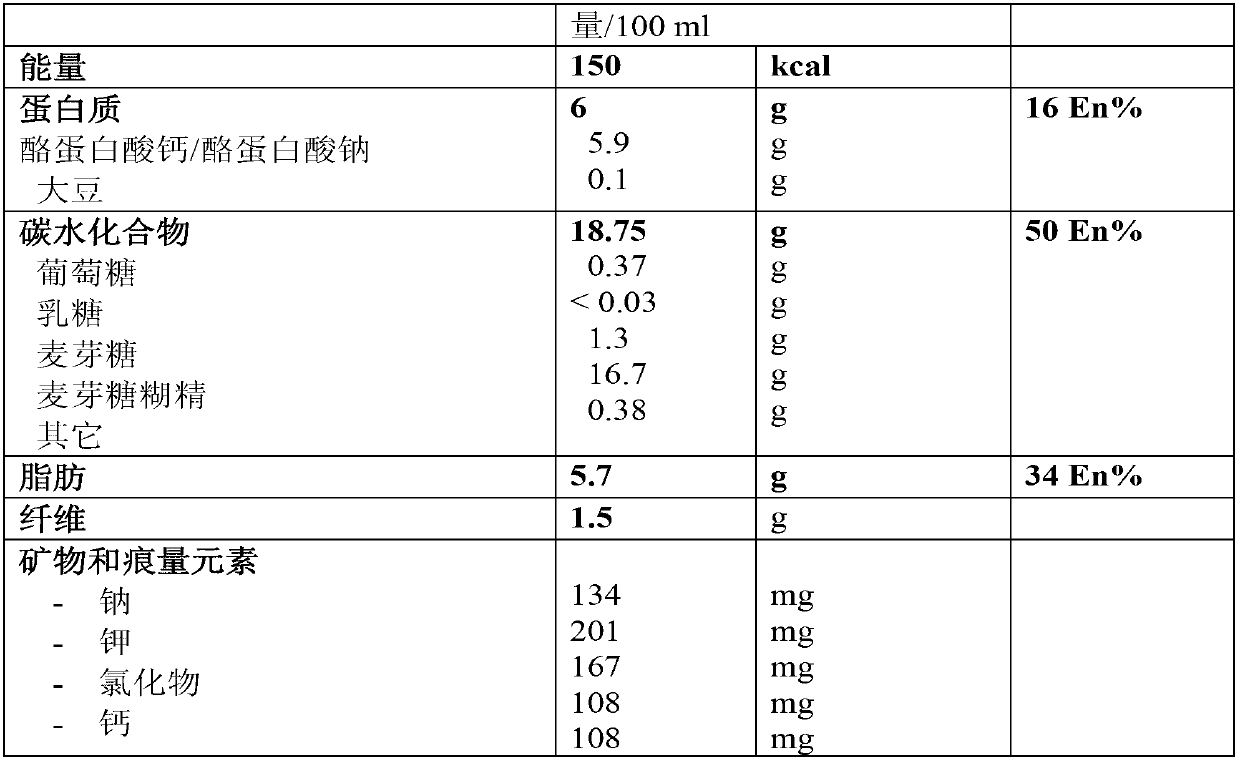
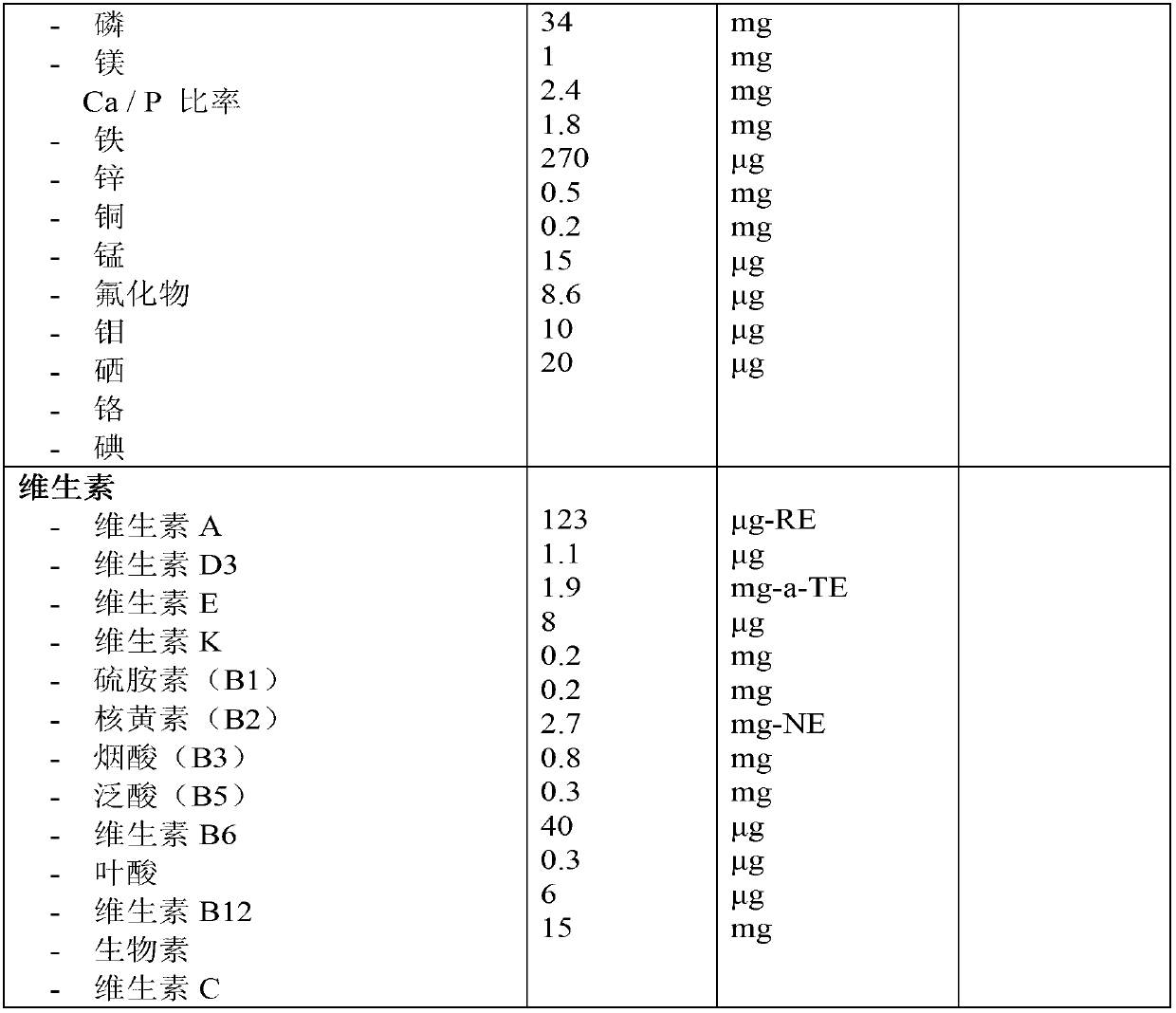
The present invention relates to a liquid nutritional composition, to a liquid nutritional composition for use in a diagnostic method for screening of a glucose-intolerant condition, to a kit for screening of a glucose-intolerant condition, and to a diagnostic method for screening a mammal for a glucose- intolerant condition. Furthermore, it refers to a liquid nutritional composition for use as a standard reference nutrition, such as for classification of postprandial glucose responses of different nutritional compositions. The liquid nutritional composition comprises protein, fat, digestible carbohydrate and dietary fibre, wherein the digestible carbohydrate comprises at least 90 weight% of glucose units, based on total digestible carbohydrate mass and preferably is substantially free of fructose.
Owner:NUTRICIA
Drug composition for preventing and curing diseases related to insulin resistance and preparation method thereof
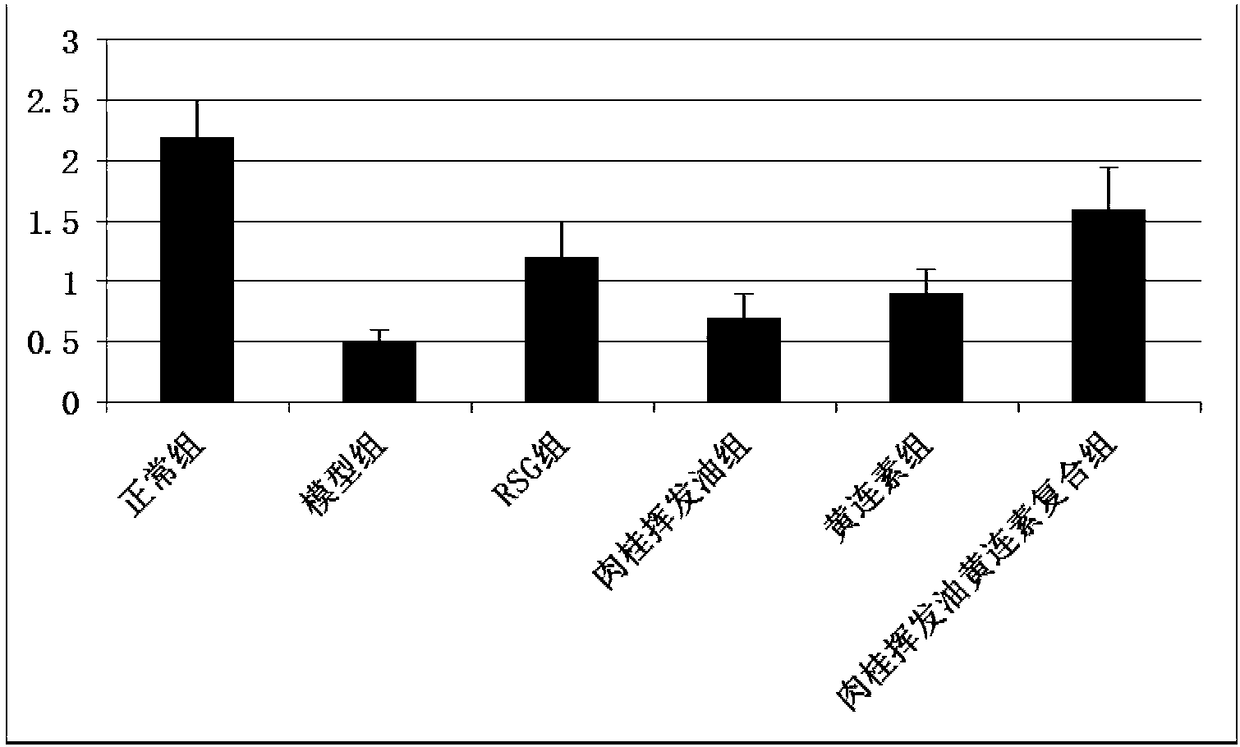
InactiveCN108245551ALower resistance indexReduce accumulationOrganic active ingredientsMetabolism disorderDiseaseWeight gain
The invention discloses a drug composition for preventing and curing diseases related to insulin resistance. The drug composition is prepared from the following raw materials of, in percentage contentby mass, 10-90% of cortex cinnamomi volatile oil and 10-90% of berberine. The invention further discloses a preparation method of the drug compound. The drug compound selects the cortex cinnamomi volatile oil and berberine to be compatible mutually, the cortex cinnamomi volatile oil and berberine complement each other, the insulin resistance index can be obviously declined, the insulin sensitivity detection index is increased, the glucose area under the curve (AUC) in a glucose tolerance test is effectively reduced, weight gain and fat accumulation caused by insulin resistance are lowered, the sugar content of blood is cut down, the insulin resistance is improved, and the drug composition can be used for preventing and curing diseases with the insulin resistance being the pathophysiological basis. The drug flavor is less, thus quality control is facilitated, meanwhile, the drug is simple, the efficacy is specific, and the effect on preventing and curing the diseases with the insulin resistance being the pathophysiological basis is very outstanding as well.
Owner:SOUTHERN MEDICAL UNIVERSITY
Food for testing life style-related diseases
It is intended to provide a food for testing life style-related diseases which is in the form of a food easy to ingest, differing from the existing medicines such as Trelan G, and makes it possible to simultaneously and easily perform a glucose tolerance test and examine oral glucose tolerance, heperlipemia after meal and so on. Namely, a test food for testing metabolic factors causing life style-related diseases which contains 100 parts by weight of carbohydrate and from 20 to 40 parts by weight of fat. It contains from 73 to 77 g of carbohydrate and from 15 to 30 g of fat, in terms of a single test dose, and provides from 460 to 600 kcal. This test food is usable in testing many items for diabetes, obesity, circulatory diseases, hyperlipemia, hyperuricemia, hyperinsulinemia and hypertension.
Owner:HARANO YUTAKA +1
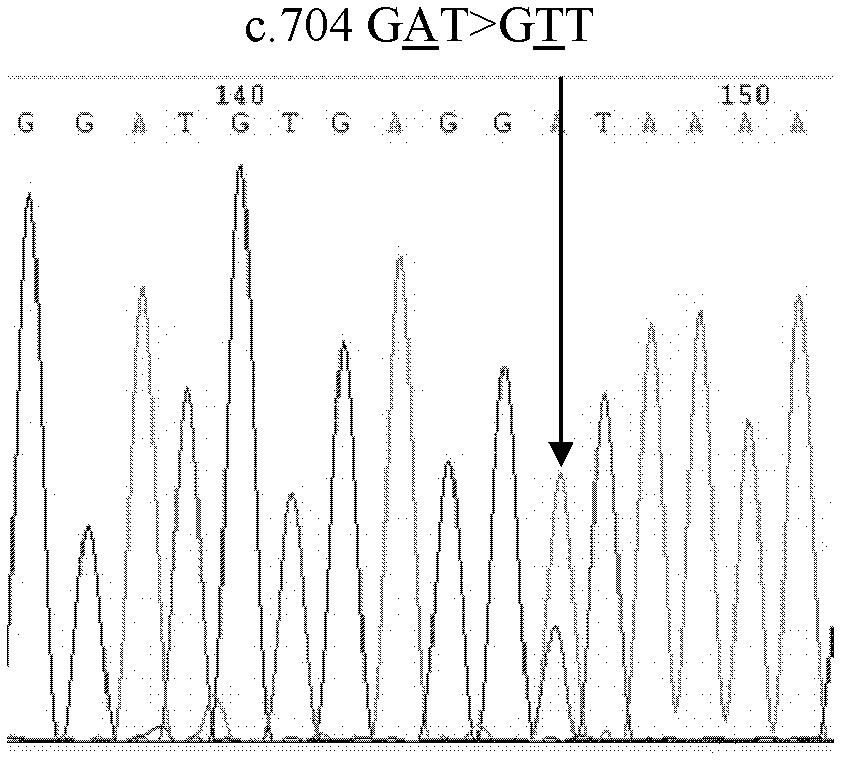
New mutation of hMLH1 gene of colorectal cancer patients in north China and use thereof
InactiveCN102643823AEnriched Mutation SpectrumMicrobiological testing/measurementFermentationSingle-strand conformation polymorphismAction spectrum
The invention discloses new mutation of hMLH1 gene of colorectal cancer patients in north China and use of the hMLH1 gene, and relates to a new mutation type of the hMLH1 gene of the sporadic colorectal cancer patients in north China. According to the invention, the new mutation is discovered in the 1 / 324 sporadic colorectal cancer patients, i.e. c.704GAT is greater than GTT (glucose tolerance test) (p.Asp235Val). The invention comprises the following operation steps of: 1. extracting DNA (deoxyribonucleic acid); 2, designing a primer; 3, carrying out PCR-SSCP (polymerase chain reaction-single strand conformation polymorphism); 4, sequencing; and 5, checking with the GeneBank original sequence. The mutation spectrum of the hMLH1 gene in the colorectal cancer patients is more enriched on the basis of the reported hMLH1 gene mutation. The theoretical basis is provided for the molecular diagnosis and the treatment of the colorectal cancer patients.
Owner:HARBIN MEDICAL UNIVERSITY
Function and application of thymidylate synthetase CAD (carbamoyl-phosphate synthetase II, aspartate transcarbamylase and dihydroorotase) gene in treating fatty liver and type-II diabetes mellitus
ActiveCN104069511AImprove fatty liverImprove type 2 diabetesPeptide/protein ingredientsMetabolism disorderIntraperitoneal routeStaining
The invention discloses a function and application of thymidylate synthetase CAD (carbamoyl-phosphate synthetase II, aspartate transcarbamylase and dihydroorotase) genes in treating fatty liver and type-II diabetes mellitus, and belongs to the field of gene functions and application. Functions of CAD genes are studied by a high-fat diet (HFD) induced model, and results discover that the levels of body weight and fasting blood-glucose of a CAD gene knock-out mouse in an HFD group are higher than those of a WT (Wild Type) mouse in a control group; glucose tolerance tests by intraperitoneal injection discover that the tolerance of the CAD gene knock-out mouse on glucose is remarkably weakened; the integral liver appearance, liver weight, liver / weight ratio and lipid component pathological staining result of the mouse indicate that the fatty liver disease of the CAD knock-out mouse in the HFD group is remarkably severe, the lipid accumulation is remarkably increased, and these results indicate that CAD gene knock-out can remarkably deteriorate fatty liver and type-II diabetes mellitus. Regarding the effects of CAD, the CAD can be used for preparing medicaments for preventing, relieving and / or treating fatty liver and / or type-II diabetes mellitus.
Owner:武汉惠康基因科技有限公司
Stable glucose oral solution for glucose tolerance test
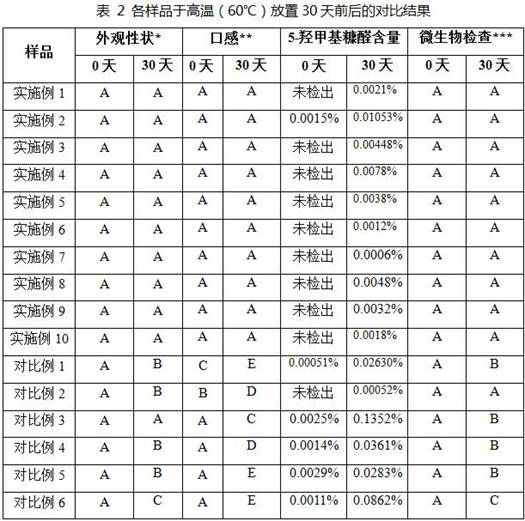
PendingCN114558149AEasy to takeSweet and sour tasteCompounds screening/testingGlucose (oral)Furaldehyde
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a stable glucose oral solution for a glucose tolerance test as well as a preparation method and application thereof. The glucose oral solution comprises 75 g of anhydrous glucose and 0.5-5.0 g of a buffer pair, the volume of water is fixed to 150-350 ml, the ratio of acid and acid salt of the buffer pair is 0.40-3.30, and the pH value of the oral solution is 3.0-5.0. The glucose oral solution is convenient to take, good in taste, stable in property, especially beneficial to control of toxic impurity 5-hydroxymethylfurfural, basically free of impurities in 0 day, lower than 0.02% in impurity content in 30 days at high temperature, and controllable in quality.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
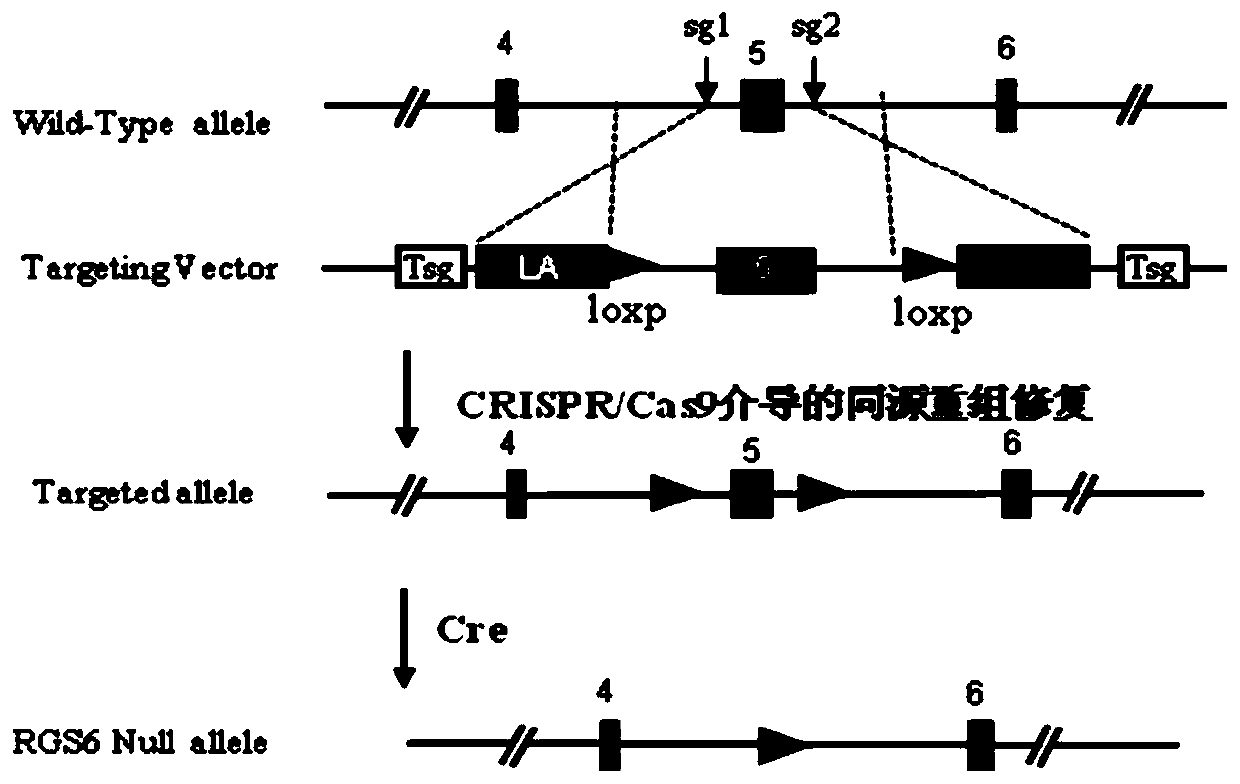
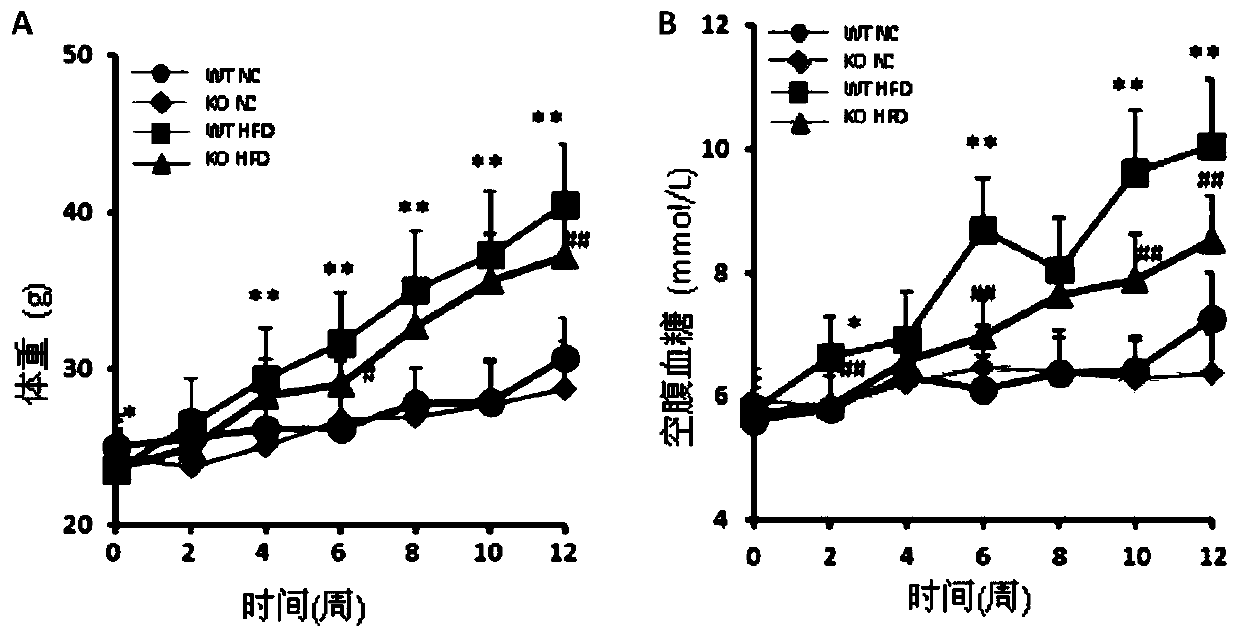
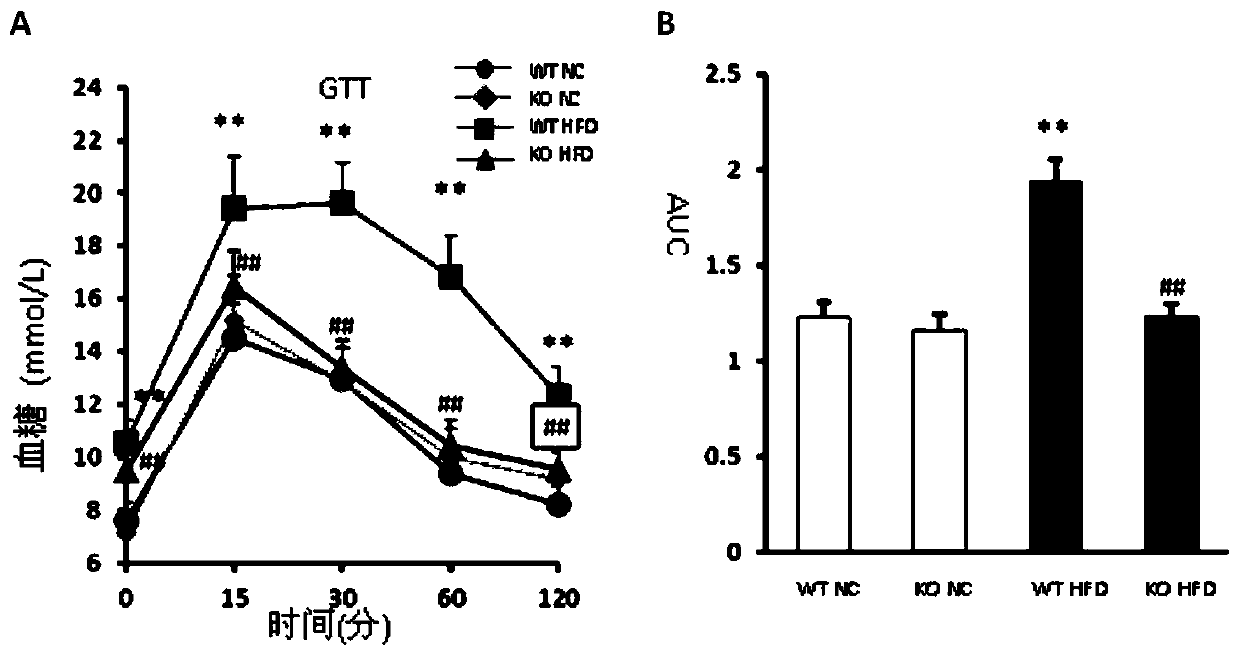
Function and application of G-protein signaling regulator 6 and its inhibitors in the treatment of fatty liver and type Ⅱ diabetes
Owner:WUHAN UNIV
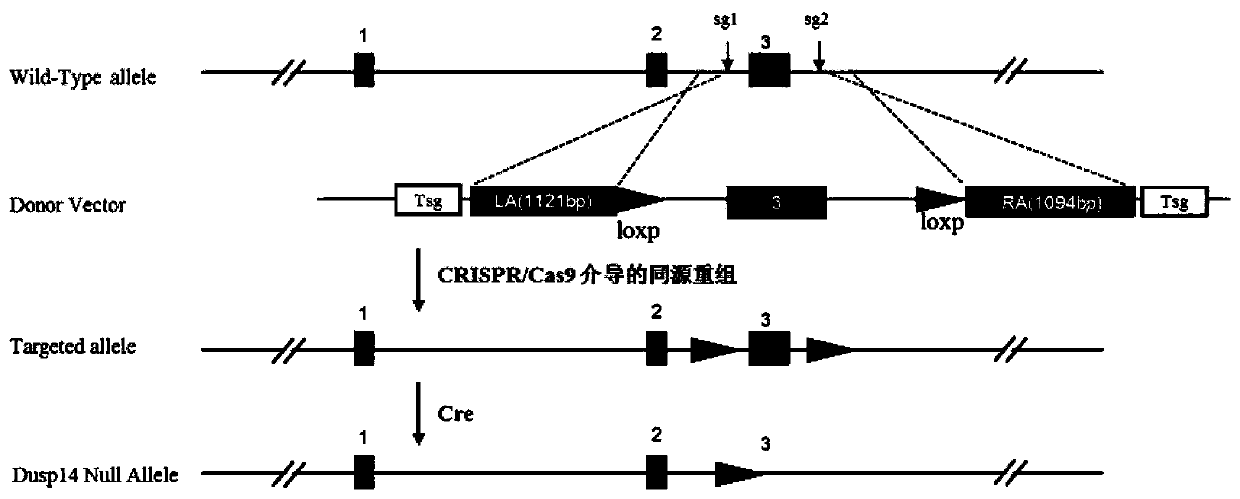
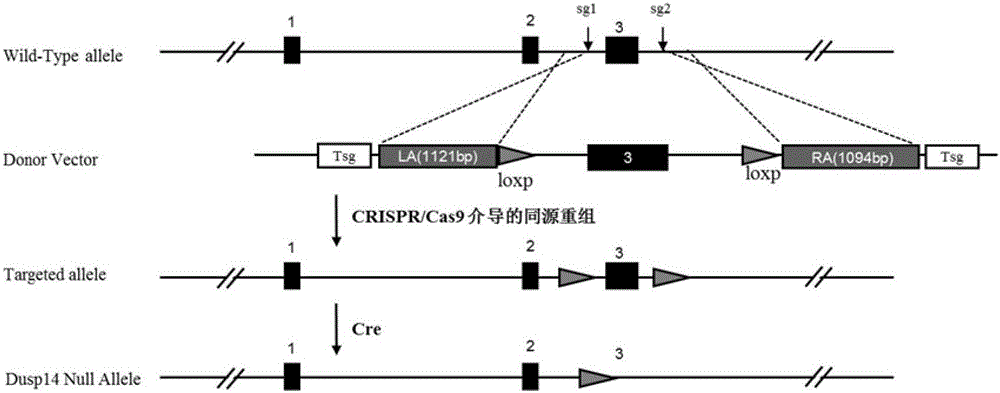
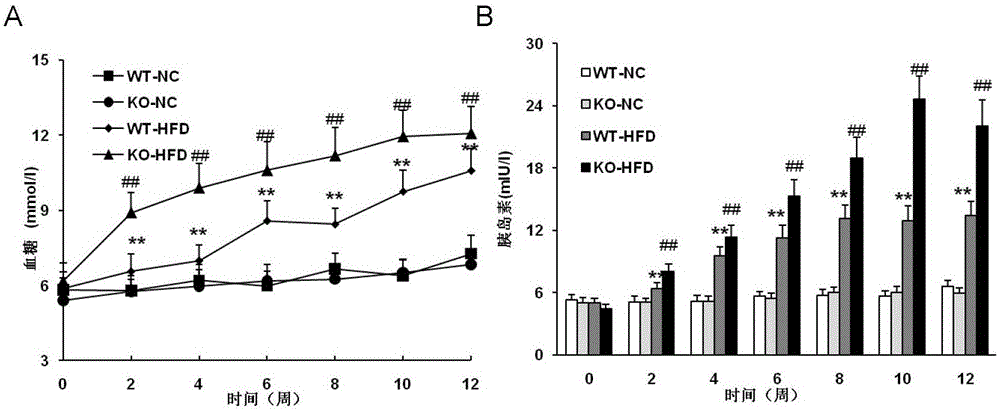
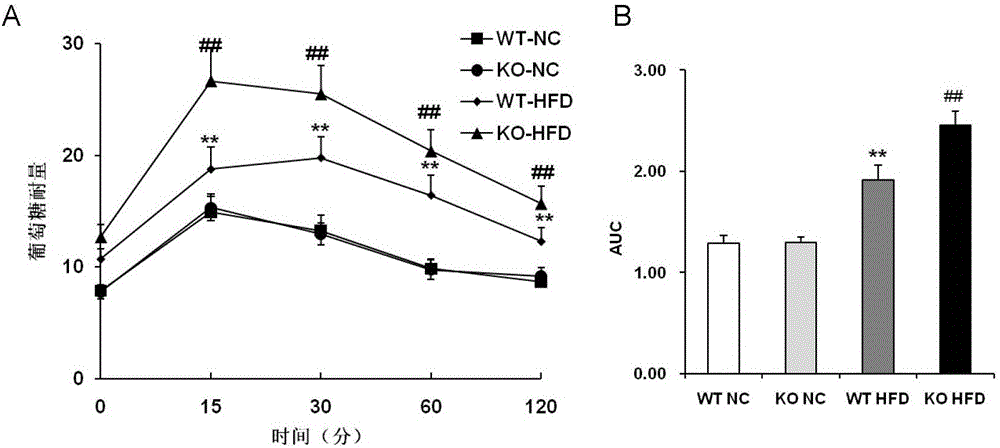
Function and application of dual specificity phosphatase 14 in the treatment of non-alcoholic fatty liver disease and type Ⅱ diabetes
Owner:WUHAN UNIV
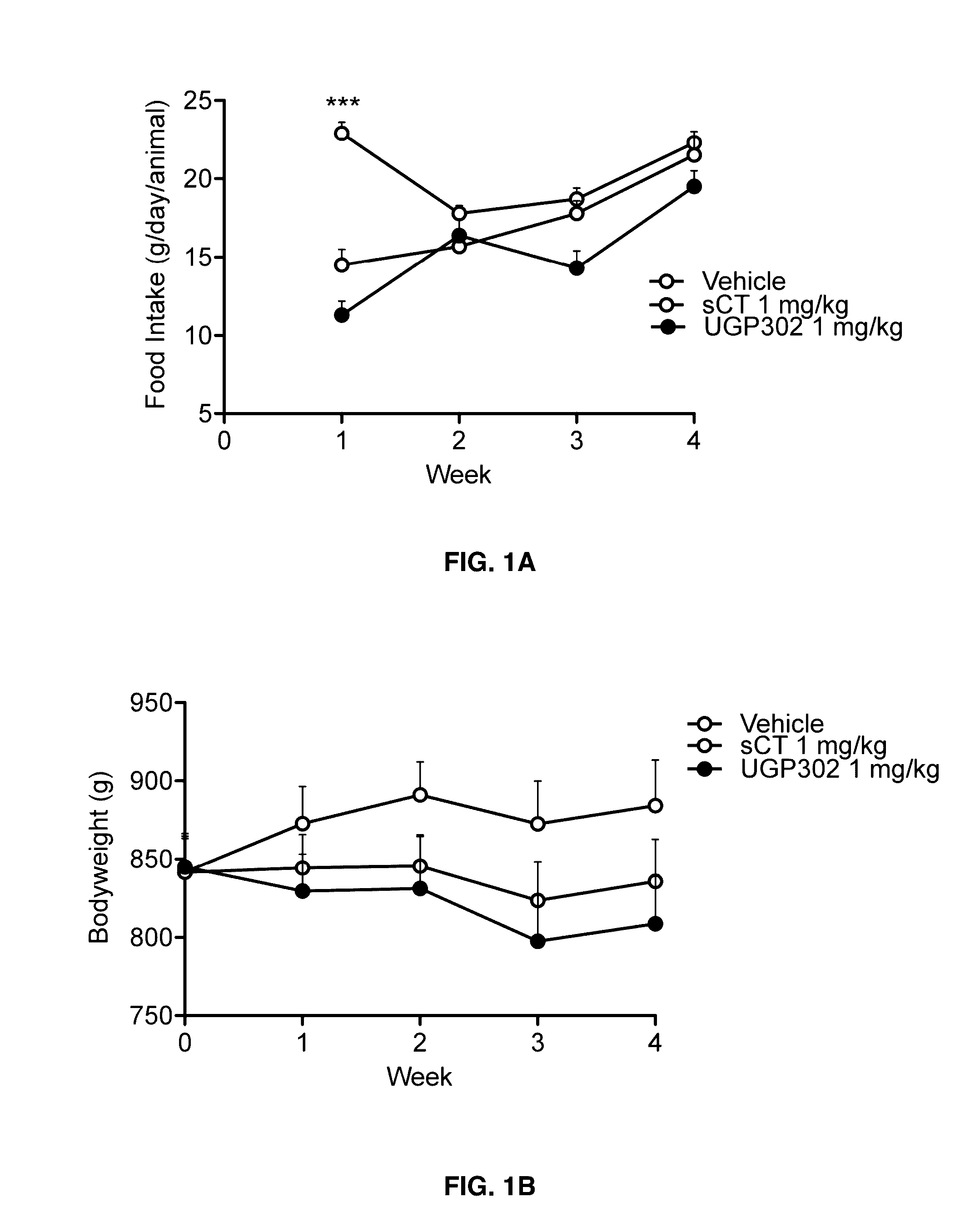
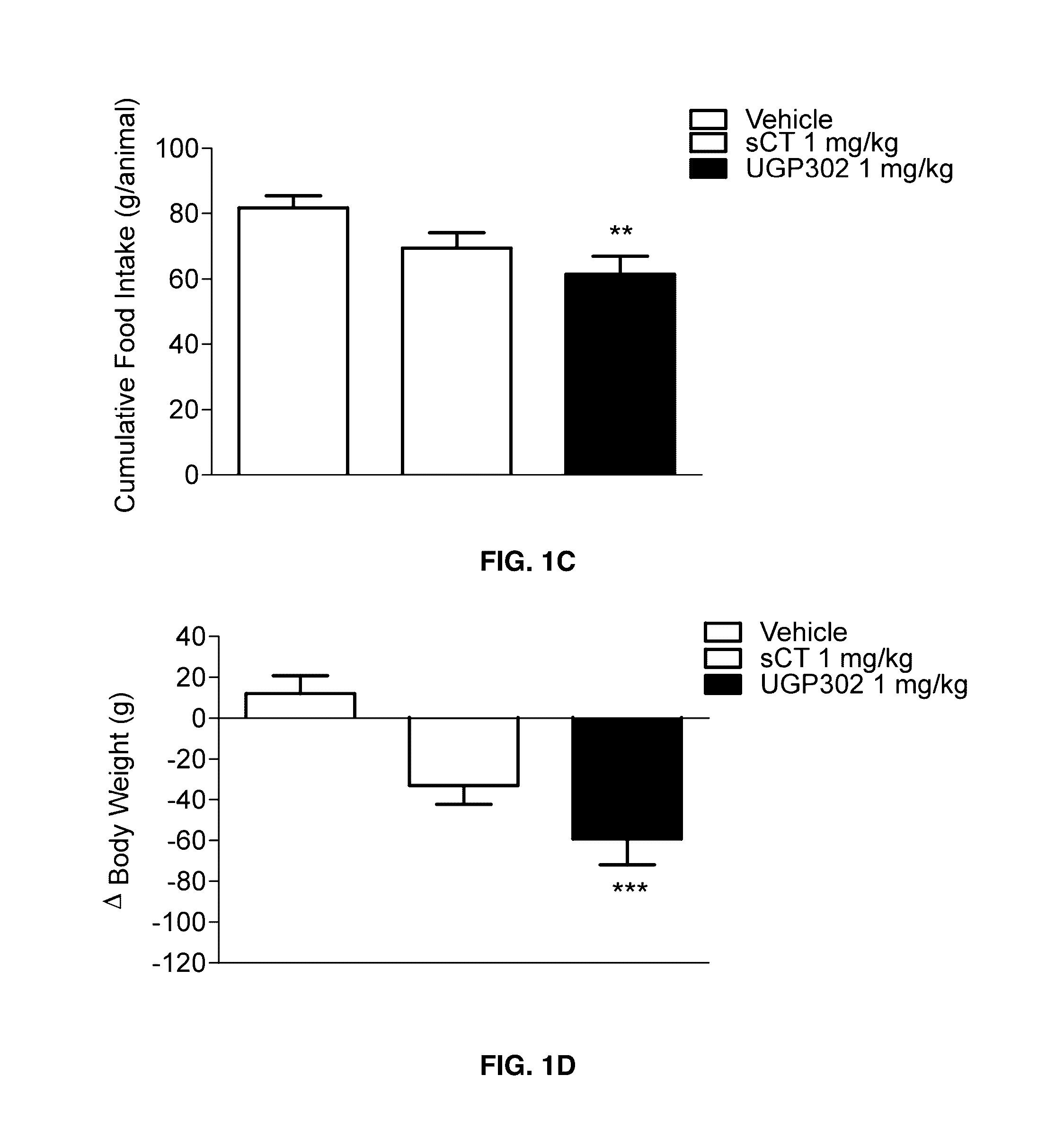
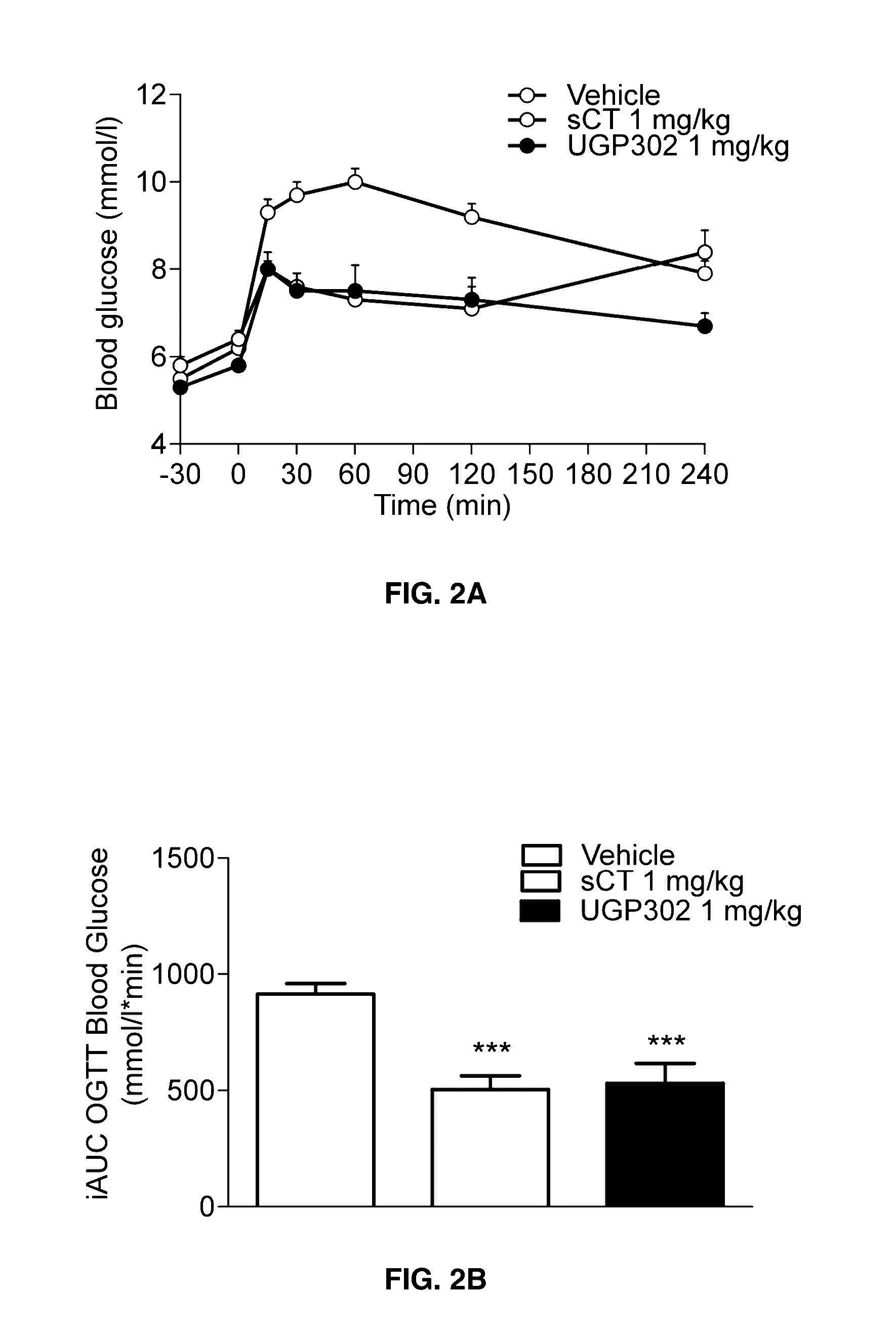
Glucose tolerance test and compositions therefor
The present invention relates to a liquid nutritional composition, to a liquid nutritional composition for use in a diagnostic method for screening of a glucose-intolerant condition, to a kit for screening of a glucose-intolerant condition, and to a diagnostic method for screening a mammal for a glucose-intolerant condition. Furthermore, it refers to a liquid nutritional composition for use as a standard reference nutrition, such as for classification of postprandial glucose responses of different nutritional compositions. The liquid nutritional composition comprises protein, fat, digestible carbohydrate and dietary fibre, wherein the digestible carbohydrate comprises at least 90 weight % of glucose units, based on total digestible carbohydrate mass and preferably is substantially free of fructose.
Owner:NV NUTRICIA
Drug for intervention treatment of diabetic peripheral neuropathy (DPN)
InactiveCN104825632ANervous disorderMetabolism disorderElevated glucose toleranceIntervention treatment
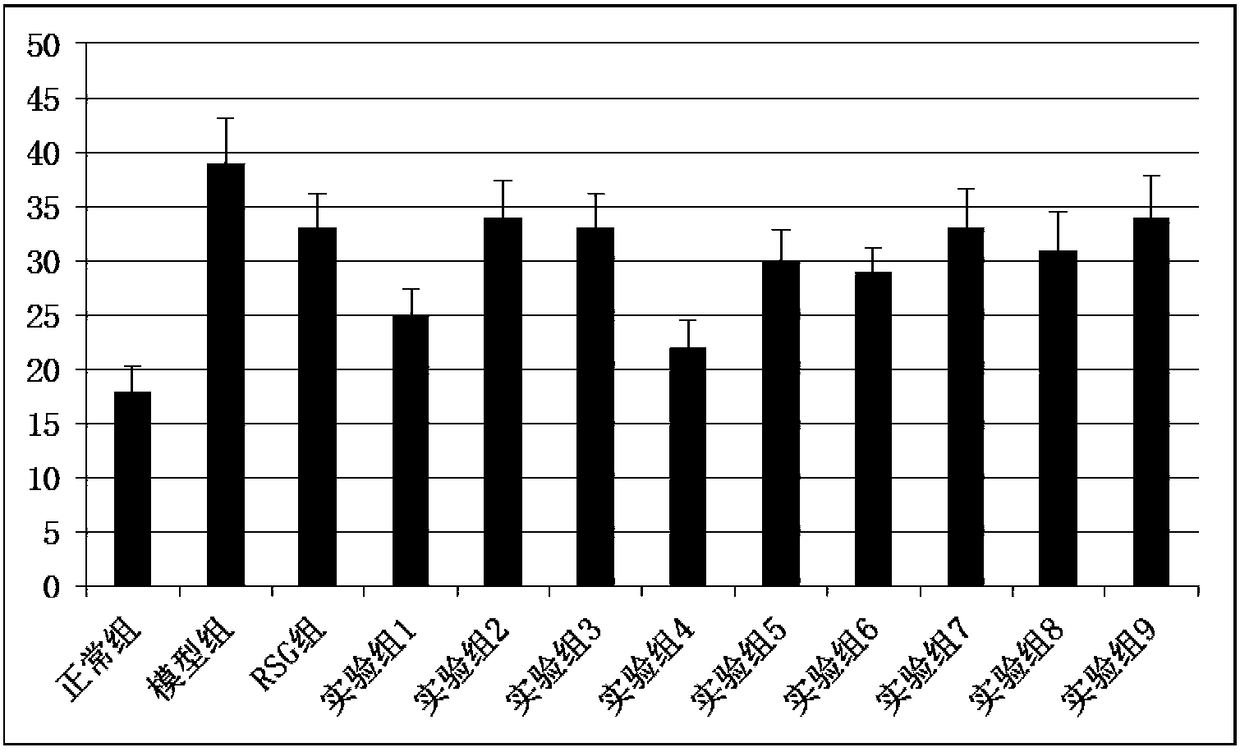
The invention discloses a drug for the intervention treatment of diabetic peripheral neuropathy (DPN). The drug comprises the following effective components in percentage by weight: 45 to 80% of chicken egg yolk, and 5 to 15% of dried rehmannia root. The results of animal experiments show that the provided drug can effectively reduce the blood sugar level of diabetic rats, moreover, the area under the curve (AUC) in the glucose tolerance tests is reduced, and the DPN pain index of diabetic rates is also reduced.
Owner:NINGBO UNIV
Pharmaceutical composition containing hyaluronic acid nanoparticles for preventing or treating inflammatory disease and metabolic disease
ActiveUS20190231898A1Improve insulin resistancePowder deliveryOrganic active ingredientsDiseaseCholic acid
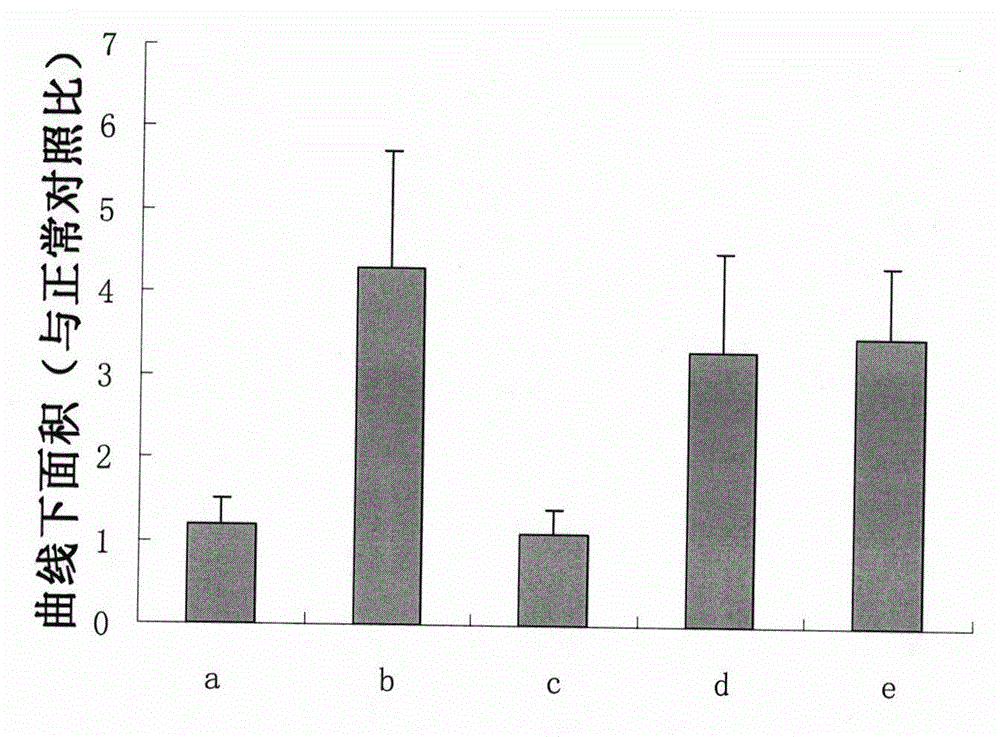
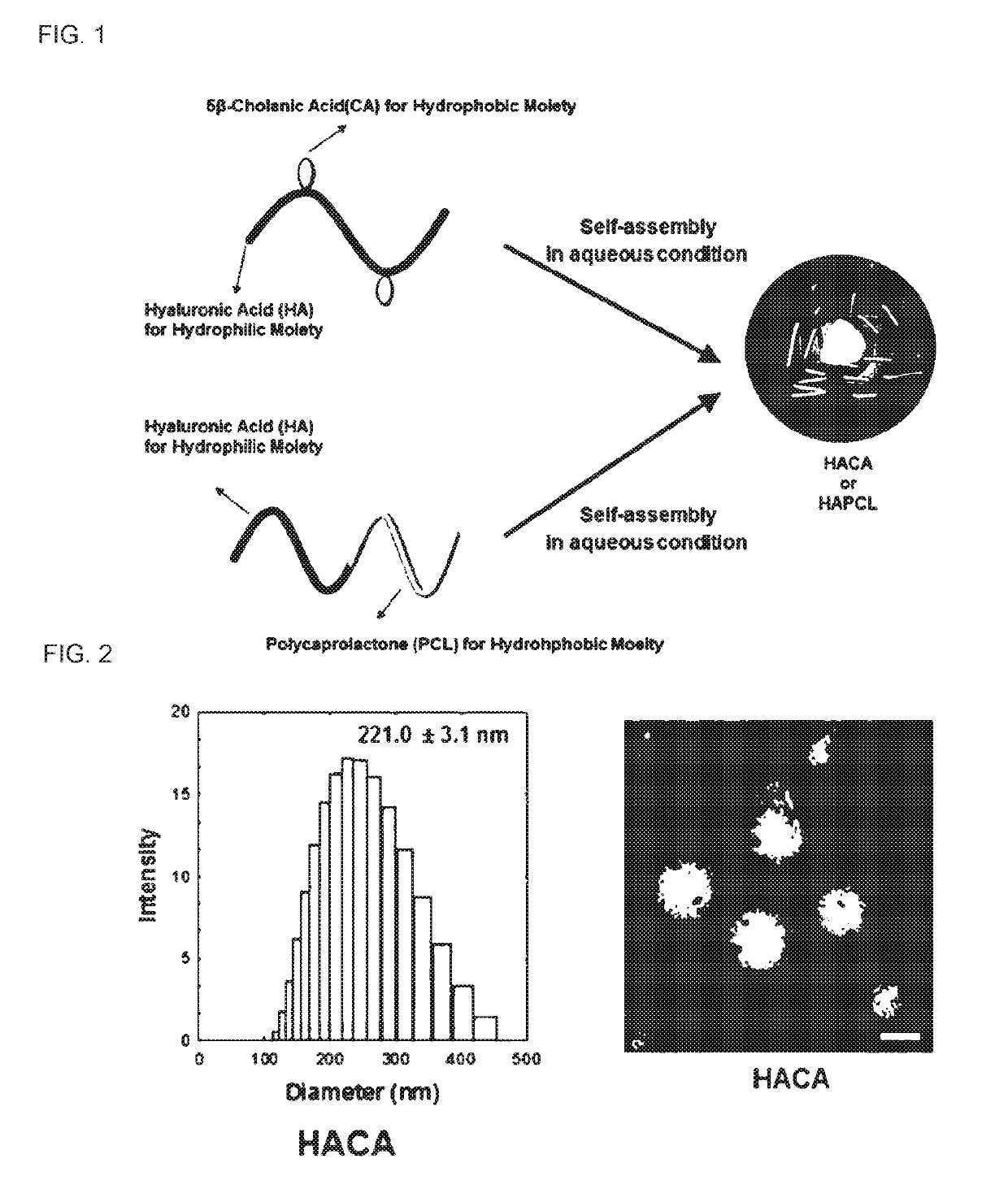
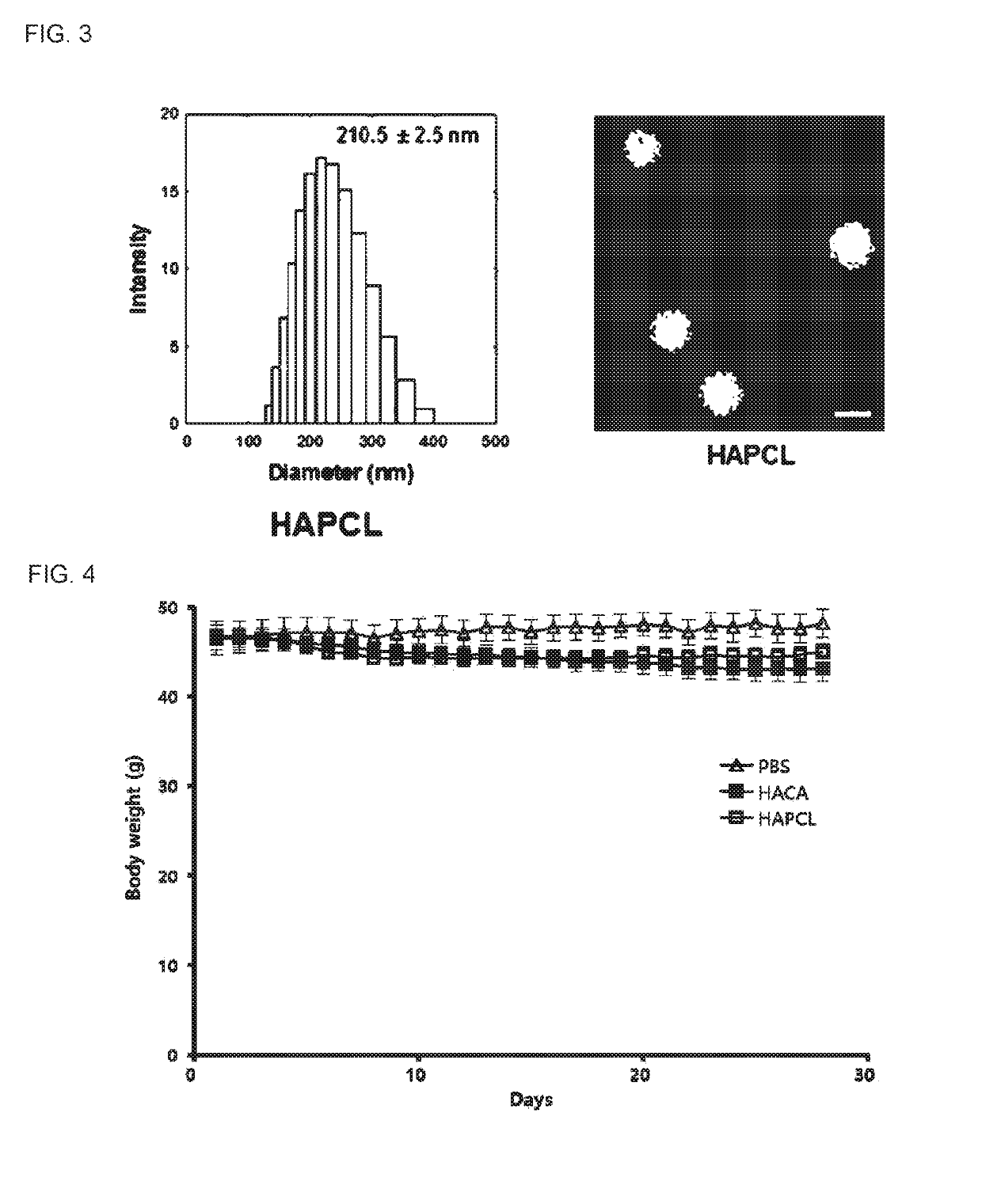
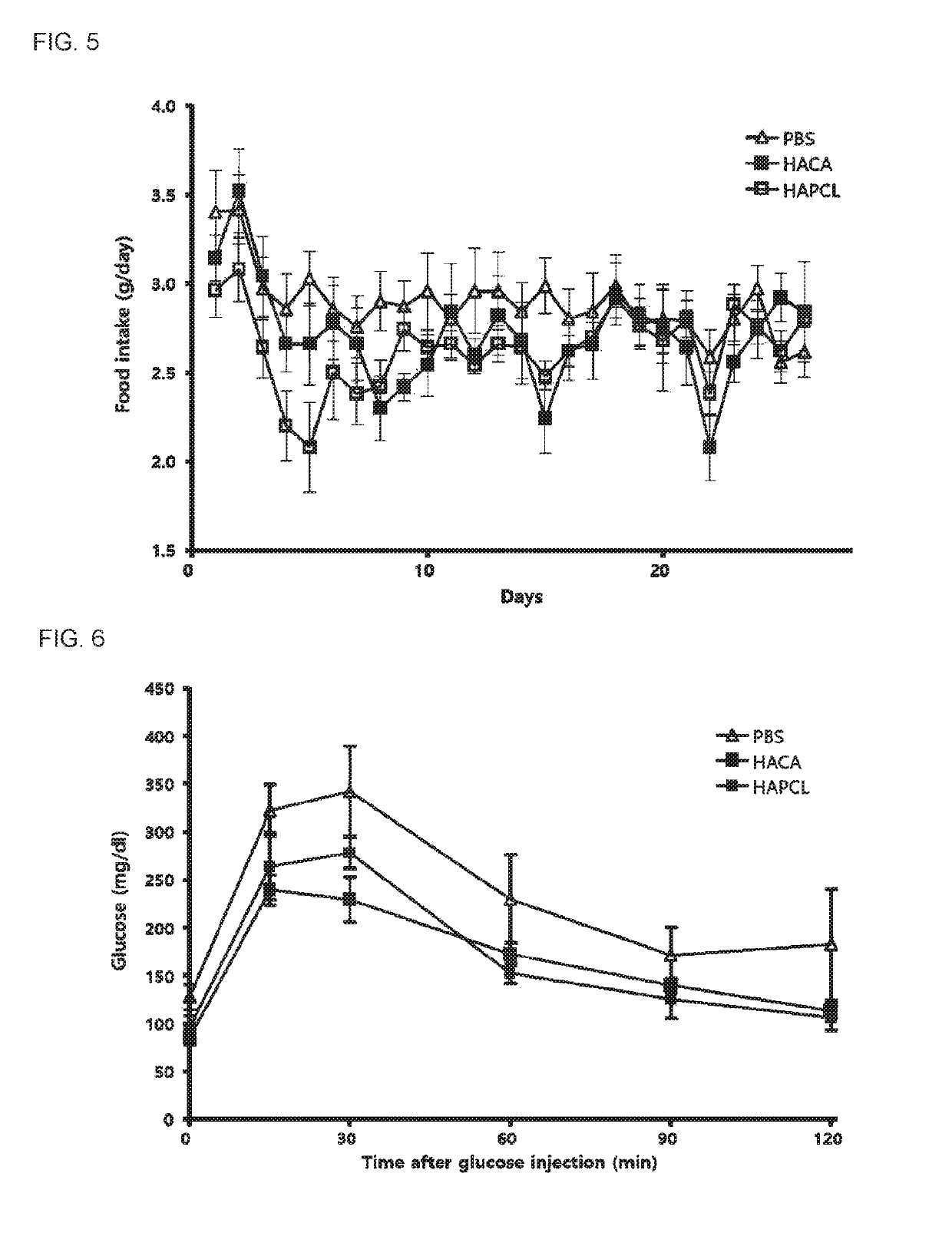
The present invention relates to a pharmaceutical composition for preventing or treating an inflammatory disease and a metabolic disease, which includes hyaluronic acid nanoparticles, and more particularly, to a pharmaceutical composition for preventing or treating an inflammatory disease and a metabolic disease, which includes hyaluronic acid nanoparticles formed in such a way that 5β-cholanic acid or polycaprolactone binds to a hydrophobic moiety of hyaluronic acid through self-assembly in an aqueous solution state. The pharmaceutical composition for preventing or treating an inflammatory disease or a metabolic disease, which includes hyaluronic acid nanoparticles, according to the present invention reduces body weight, lowers food intake, has an effect of reducing blood glucose as a result of a glucose tolerance test (GTT), and has an effect of reducing insulin resistance, inflammatory inducers (NF-κB, IL-1β, CD44, TNF-α, NLRP3 inflammasome, and the like), and macrophage tissue infiltration, and thus may be effectively used as a pharmaceutical composition for preventing or treating inflammatory diseases or metabolic diseases.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Oral glucose tolerance test beverage and use method thereof
InactiveCN109865144AReduce labor intensityGreat tasteIn-vivo testing preparationsElevated glucose toleranceGlucose load test
The invention provides an oral glucose tolerance test beverage and a use method thereof. The beverage comprises a beverage container and anhydrous glucose contained in the beverage container, the anhydrous glucose is D-glucose, and a flavoring agent is added in the anhydrous glucose. When an oral glucose tolerance test is carried out, the oral glucose tolerance test beverage with an usage amount suitable for a patient is selected according to the condition of the patient, and the oral glucose tolerance test beverage is immediately drunk after being opened. The defects existing in the clinicaloral glucose tolerance tests at present are overcome. The invention belongs to the field of glucose loading tests.
Owner:高鸿
Foldable cup for glucose tolerance tests
InactiveCN109674659AJoin precisionReduce volumeOral administration deviceElevated glucose toleranceD-Glucose
The invention provides a foldable cup for glucose tolerance tests. A medically metered glucose is coated on the inner wall of a foldable paper cup; or a nonwoven fabric bag is adhered onto the inner wall of the foldable paper cup; the medically metered glucose is contained in the nonwoven fabric bag; or the medically metered glucose is contained in the foldable paper cup. The paper cup containingglucose can be in a sealed state; in the use process, a cup opening is opened through tearing. The foldable cup has the advantages that after the manufacturing, the foldable cup is sealed and stored in a packaging bag conforming to the sanitation requirements to be conveyed into each hospital for use. The foldable cup has small size and can be folded into a flake shape, so that the transportationprocess and the storage process in hospitals are convenient; the space occupation is avoided; a doctor gives the paper cup to a patient according to the conditions; warm boiling water is added by thepatient for oral taking; the accurate taking quantity of the glucose powder is ensured; the paper cup uses cheap raw materials, so that the cost can be reduced.
Owner:上海市嘉定区妇幼保健院
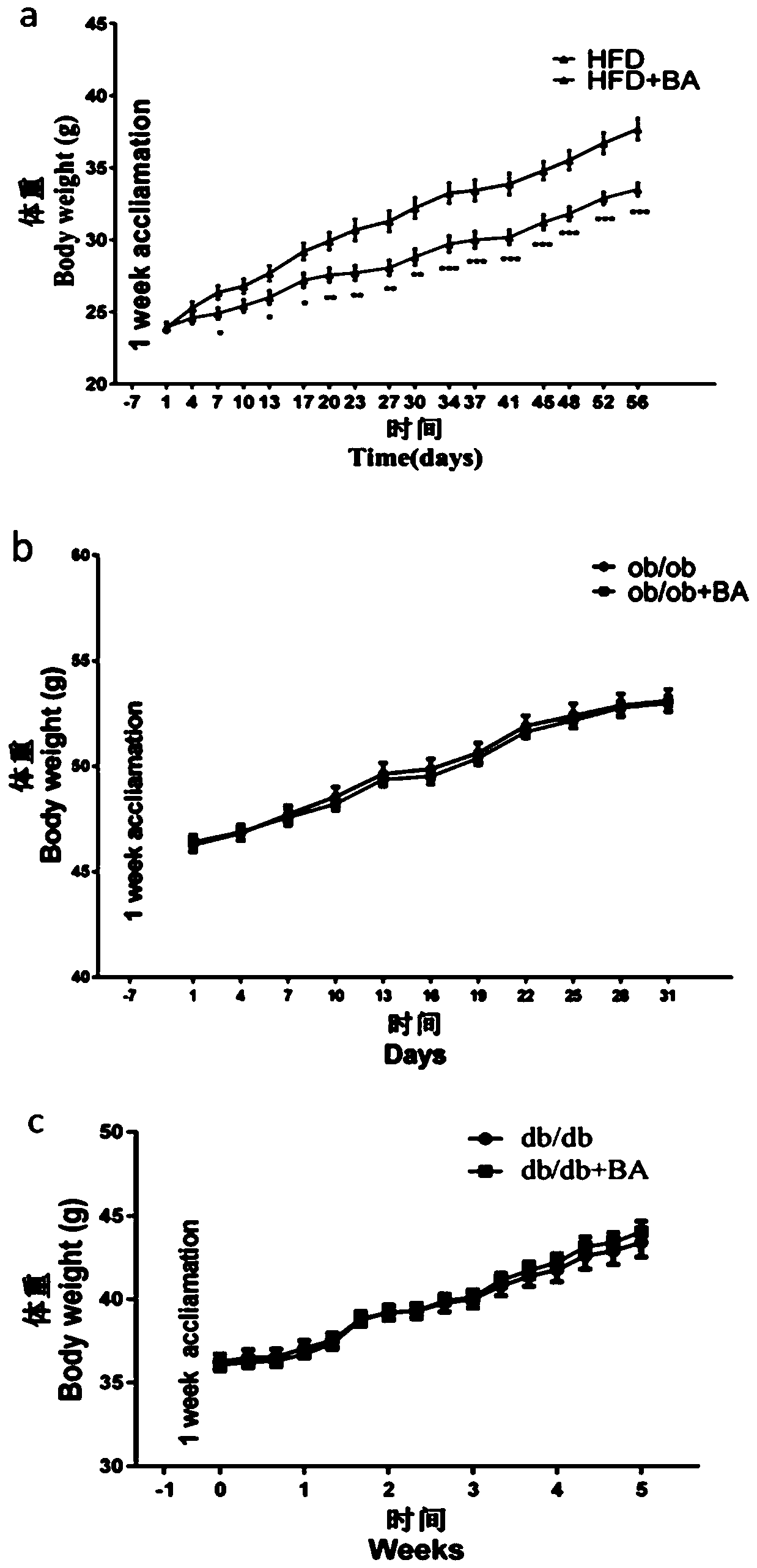
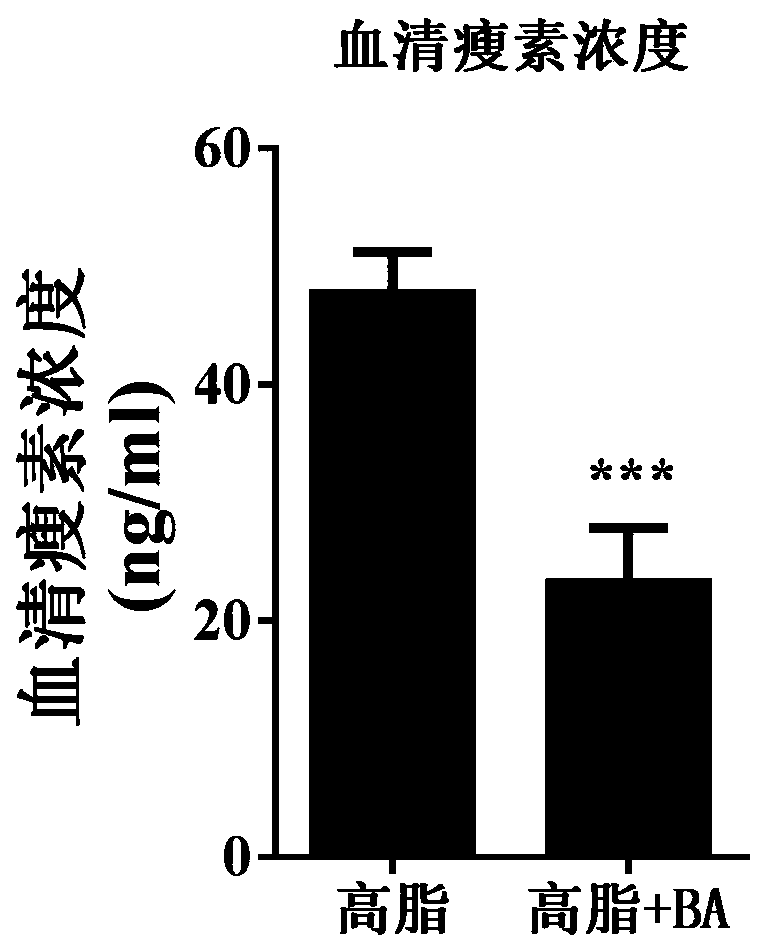
Application of betulinic acid as leptin sensitizer in preparation of related drugs for treating leptinresistance
InactiveCN109985045AImprove pharmacological activityStrong rangeOrganic active ingredientsMetabolism disorderResearch ObjectLeptin resistance
The invention discloses application of betulinic acid as a leptin sensitizer in preparation of related drugs for treating leptin resistance. According to the invention, high-fat, ob / ob and db / db obesemice are taken as research objects and treated with the betulinic acid, and the weight, serum leptin concentration, liver triglyceride, insulin tolerance and glucose tolerance tests and blood fat indexes are measured. The results show that the betulinic acid can significantly reduce the weight, improve the leptin resistance, lower the liver triglyceride, improve the insulin resistance and lower the blood fat in the high-fat obese mice, the ob / ob and db / db mice treated with the betulinic acid can not reduce the weight and improve the metabolism of glycolipid, and the cellular level proves thatthe combination of the betulinic acid and the leptin can enhance the expression of p-STAT3. The invention provides a basis for treating obesity, a non-alcoholic fatty liver disease, type 2 diabetes and hyperlipidemia by using the betulinic acid as the leptin sensitizer, and also provides a new treatment direction for developing more natural active pharmaceutical ingredients for treating diseasesrelated to the leptin resistance.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
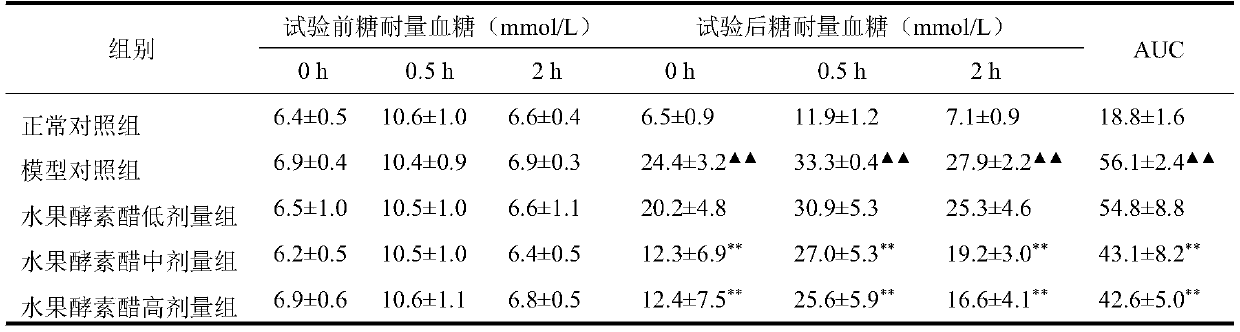
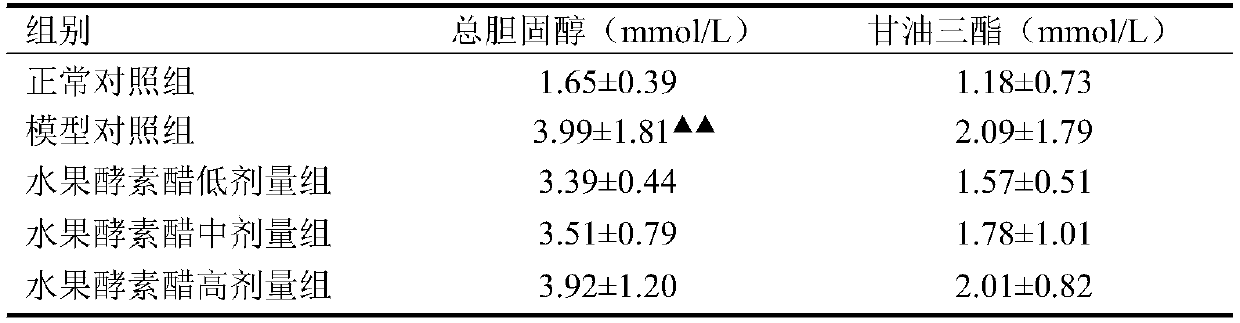
Application of Chilu fruit enzyme vinegar to preparation of functional foods for assisting in reducing blood sugar
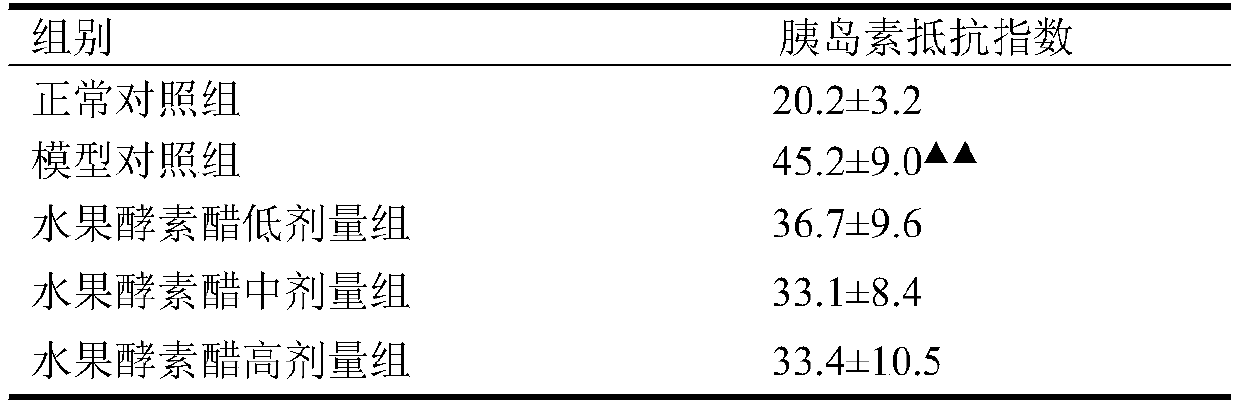
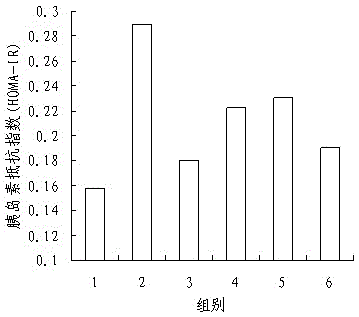
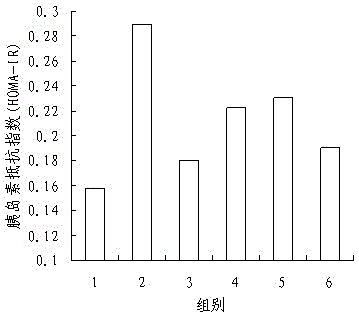
PendingCN111513319AAromatic tasteImprove acceptabilityFood ingredient functionsAnimal husbandryLow insulinRat model
The invention discloses an application of Chilu fruit enzyme vinegar to preparation of functional foods for assisting in reducing blood sugar and improving impaired glucose tolerance. Experiments fora rat model that a hyperpyrexia feed is combined with streptozotocin to enable insulin to resist sugar / lipid metabolism disorder indicates that the Chilu fruit enzyme vinegar can reduce area under curve (AUC) of a blood sugar value and time-blood sugar curve for rats after glucose tolerance test for 0h, 0.5h and 2h, by the insulin resisting sugar / lipid metabolism disorder model. Experiments show that the fruit enzyme vinegar can reduce the blood sugar and improve sugar tolerance, has the effects of assisting in reducing blood sugar, improving sugar tolerance and relieving renal function damage, and is good in mouth feel, free from toxin, safe and capable of being eaten for a long term.
Owner:池帝军
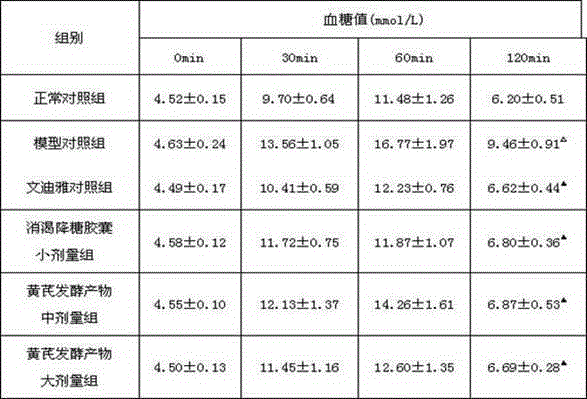
Research method of astragalus mongholicus fermentation products for preventing and controlling urine glucose amount lowness
InactiveCN105137057ALower resistance index levelsIncreased sensitivityBiological testingStudy methodsFermentation
The invention discloses a research method of astragalus mongholicus fermentation products for preventing and controlling urine glucose amount lowness. The method includes the steps of preparing medicine, calculating mouse dosages according to the clinical medication conditions, grouping mice and applying medicine on the mice, observing the mice, regularly weighing the mice, comparing the weights, conducting a glucose tolerance test, an insulin tolerance test and insulin resistance index measuring and calculating on the mice, finally processing experimental data, and conducting significance testing on the average value of each group through variance analysis. By means of the method, blood glucose can be effectively reduced after an oral glucose tolerance test, the sensitivity to insulin is improved after the insulin tolerance test, the fasting insulin and insulin resistance index level are reduced, the exploration level is high, and accuracy is high.
Owner:SHANXI UNIV OF CHINESE MEDICINE
Function and use of bispecific phosphatase 14 in treatment on nonalcoholic fatty liver and type 2 diabetes
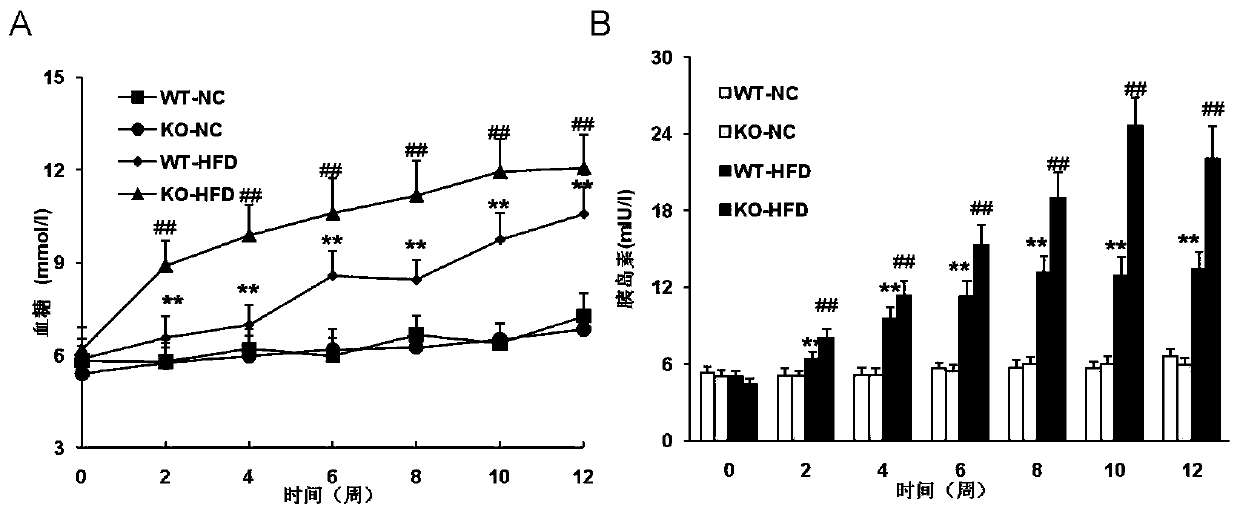
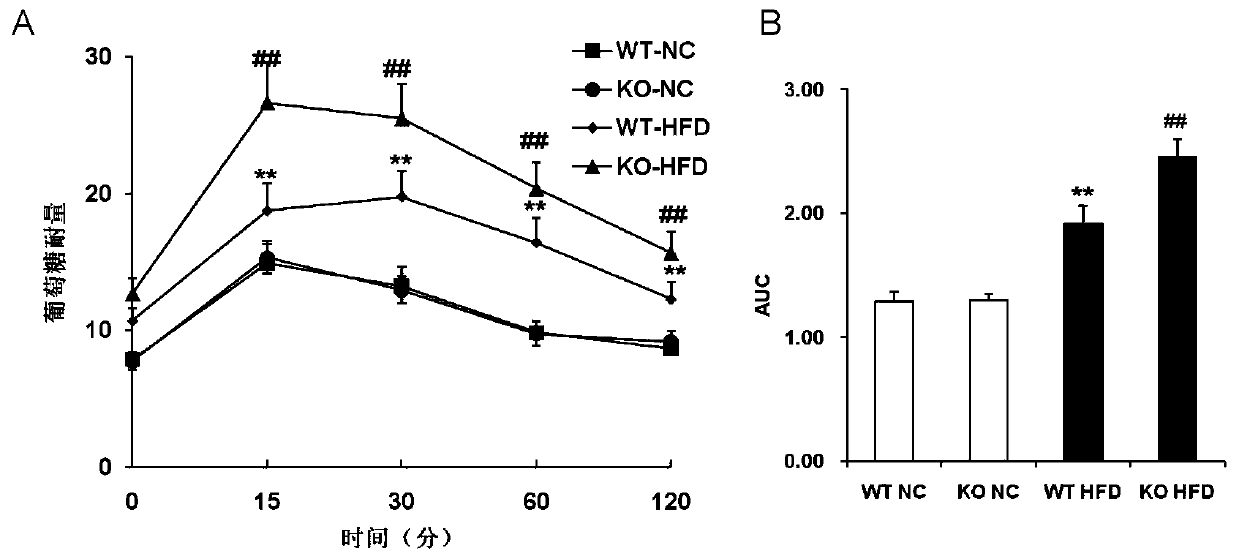
The invention discloses a function and use of a DUSP14 gene in treatment on fatty liver and diabetes. DUSP14 knockout mice and wild type C57 mice are as experimental subjects, an obese mouse model induced by high fat diet is used, a test result shows that compared with the wild type C57 mice, DUSP14-KO mice are obese, have fasting blood glucose levels higher than those of the control group WT mice and have liver functions significantly worse than those of the WT mice. An intraperitoneal injection-based glucose tolerance test proves that DUSP14-KO mouse tolerance to glucose is significantly reduced. According to liver weight, a liver / body weight ratio and a lipid pathological staining result, it is shown that the DUSP14-KO mice induced by high fat diet suffer severe fatty liver and fat accumulation is significantly increased. Therefore, DUSP14 can be used as a drug target for screening and treating fatty liver and / or type II diabetes. The accelerator can be used to prepare drugs for treating fatty liver and / or type II diabetes.
Owner:WUHAN UNIV
Diabetes diagnosis and treatment support device
ActiveCN101763464BMedical simulationData processing applicationsBiological bodyElevated glucose tolerance
Owner:SYSMEX CORP
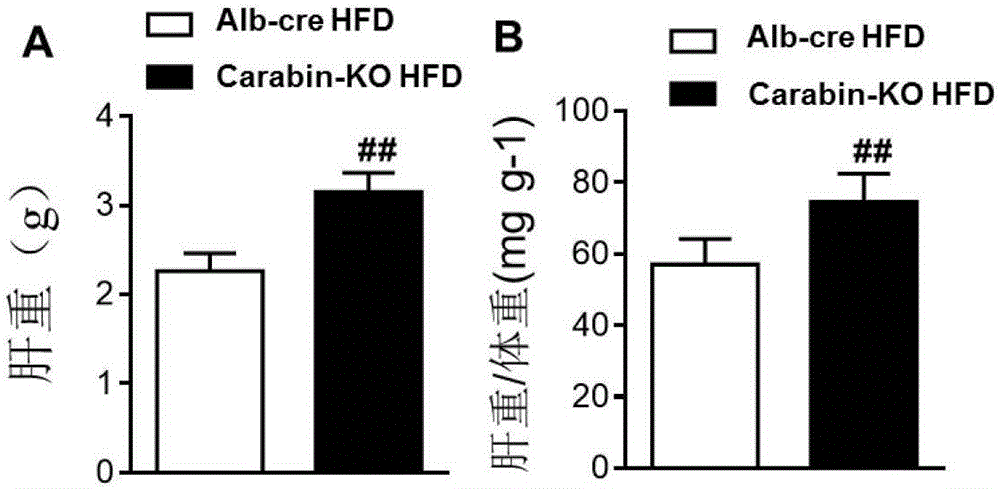
Function and application of Carabin in treatment of fatty liver and diabetes mellitus type 2
ActiveCN106755296AInhibit fatty liverInhibit type 2 diabetes diseaseCompound screeningApoptosis detectionStainingLesion
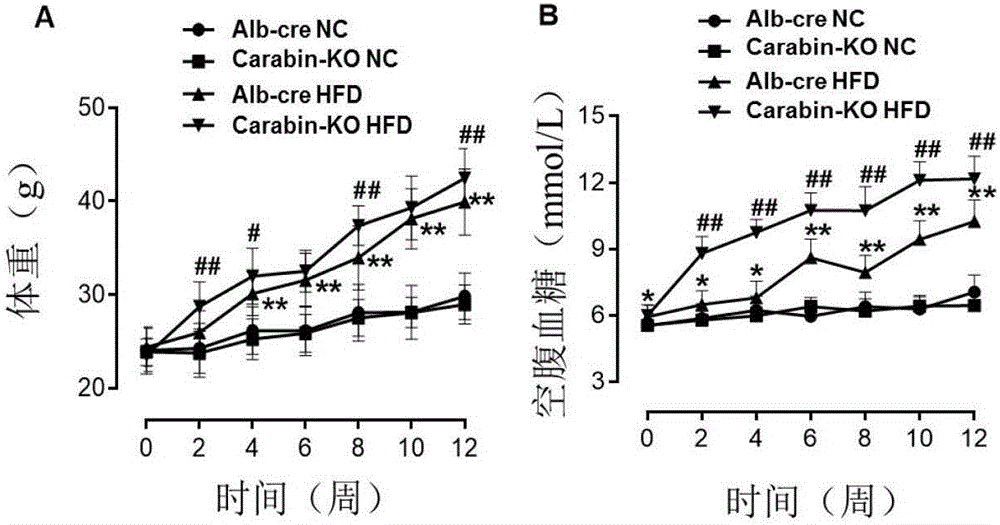
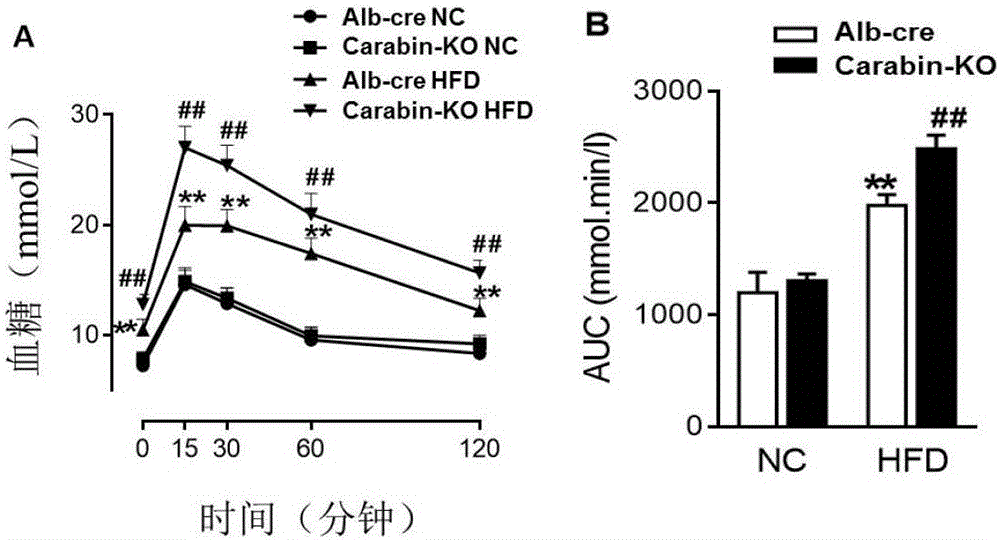
The invention discloses a function and an application of Carabin (Cara) in treatment of fatty liver and diabetes mellitus. An Alb-cre (cre) mouse and a Cara hepatocyte-specific gene knockout (Cara-KO) mouse are taken as experimental subjects, on the basis of a high fat diet induced obesity mouse model and compared with the cre mouse, the Cara-KO mouse shows obesity, the fasting blood-glucose level of the Cara-KO mouse is obviously higher than that of the cre mouse, and IPGTT (intraperitoneal glucose tolerance test) discovers that the glucose tolerance capacity of the Cara-KO mouse is weakened obviously. Liver weight, liver / weight ratio, pathological staining results and the like all prove that fatty liver lesions of the high fat diet induced Cara-KO mouse are more serious, and lipid accumulation is increased remarkably. Cara can be taken as a target for screening fatty liver and / or diabetes mellitus type 2 treating drugs, and a Cara accelerator can be used for preparing fatty liver and / or diabetes mellitus type 2 treating drugs.
Owner:武汉惠康达科技有限公司
Peptide analogs for treating diseases and disorders
InactiveUS9533022B2Reduce degradationOrganic active ingredientsPeptide/protein ingredientsDiseaseCombination therapy
Provided herein are methods for the treatment of type I diabetes, Type II diabetes, metabolic syndrome, or obesity, or of appetite suppression, or for mitigating insulin resistance, or for reducing an undesirably high fasting serum glucose level, or for reducing an undesirably high peak serum glucose level, or for reducing an undesirably high peak serum insulin level, or for reducing an undesirably large response to a glucose tolerance test in synergistic combination with metformin. Treatment is effected with a combination therapy of metformin and a peptide with a sequence selected from SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 19, SEQ ID NO: 20, SEQ ID NO: 21, SEQ ID NO: 22, SEQ ID NO: 23, and SEQ ID NO: 24 administered to a patient.
Owner:KEYBIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com