Stable glucose oral solution for glucose tolerance test
A technology for oral solution and glucose, which is applied to preparations for in vivo experiments, compound screening/testing, pharmaceutical formulations, etc., can solve the problems of astringent taste, poor taste of sugar water, complex ingredients, etc. Convenient, high-performance results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
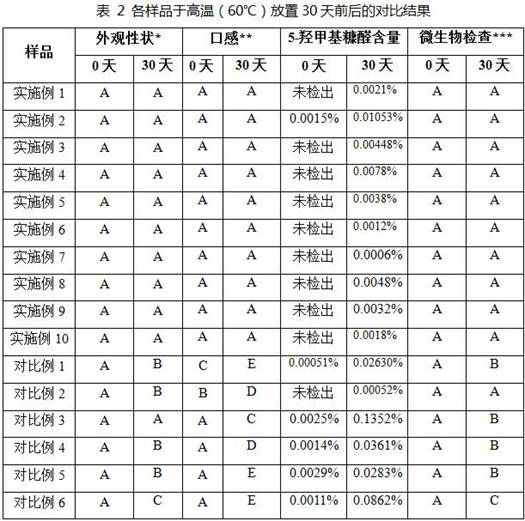
Examples
Embodiment 1
[0075] Example 1: Sample 1
[0076] Prescription: 75g of anhydrous glucose, 0.65g of citric acid, 0.86g of sodium citrate, the mass ratio of citric acid to sodium citrate is 0.76, the volume of water is adjusted to 300ml, and the pH value of the solution is 4.5.
[0077] Preparation: (1) Dosing: Take 300ml of purified water, add the prescribed amount of citric acid and sodium citrate, mix well to obtain a buffer solution, and let it stand for 5-10 minutes; add the prescribed amount of anhydrous glucose to the buffer solution at 60 Under the condition of ℃, stir for 30 minutes at a speed of 700rpm to dissolve completely, mix well, and set aside; after the above solution is lowered to room temperature, add dilute phosphoric acid solution to the full volume, stir for 10 minutes, and mix well; use 20% sodium citrate solution or 20% citric acid solution to fine-tune the pH value of the solution to the target value, and mix well to obtain the medicinal solution; (2) Filtration and c...
Embodiment 2
[0078] Example 2: Sample 2
[0079] Prescription: 75g of anhydrous glucose, 2.83g of citric acid, 0.86g of sodium citrate, the mass ratio of citric acid to sodium citrate is 3.29, the volume of water is adjusted to 300ml, and the pH value of the solution is 3.0.
[0080] Preparation: (1) Dosing: Take 300ml of purified water, add the prescribed amount of citric acid and sodium citrate, mix well to obtain a buffer solution, and let it stand for 5-10 minutes; add the prescribed amount of anhydrous glucose to the buffer solution at 60 Under the condition of ℃, stir for 30min at a speed of 700rpm to dissolve completely, mix well, and set aside. After the above solution was lowered to room temperature, dilute phosphoric acid solution was added to make the volume to full volume, stirred for 10 minutes, and mixed evenly; the pH value of the solution was fine-tuned to the target value with 20% sodium citrate solution or 20% citric acid solution, and mixed evenly to obtain Liquid medic...
Embodiment 3
[0081] Example 3: Sample 3
[0082] Prescription: 75g of anhydrous glucose, 0.65g of citric acid, 1.63g of sodium citrate, the mass ratio of citric acid to sodium citrate is 0.40, the volume of water is adjusted to 300ml, and the pH value of the solution is 5.0.
[0083] Preparation: (1) Dosing: Take 300ml of purified water, add the prescribed amount of citric acid and sodium citrate, mix well to obtain a buffer solution, and let it stand for 5-10 minutes; add the prescribed amount of anhydrous glucose to the buffer solution at 60 Under the condition of ℃, stir for 30 minutes at a speed of 700rpm to dissolve completely, mix well, and set aside; after the above solution is cooled to room temperature, add dilute phosphoric acid solution to make up to full volume, stir for 10 minutes, and mix well. Use 20% sodium citrate solution or 20% citric acid solution to fine-tune the pH value of the solution to the target value, and mix well to obtain the medicinal solution; (2) Filtration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com