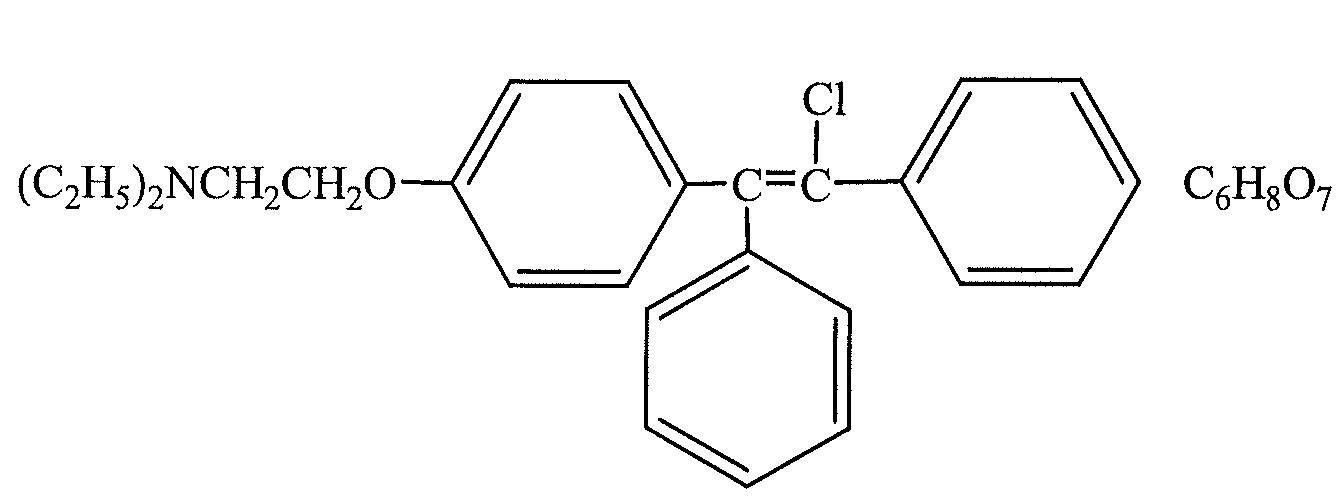
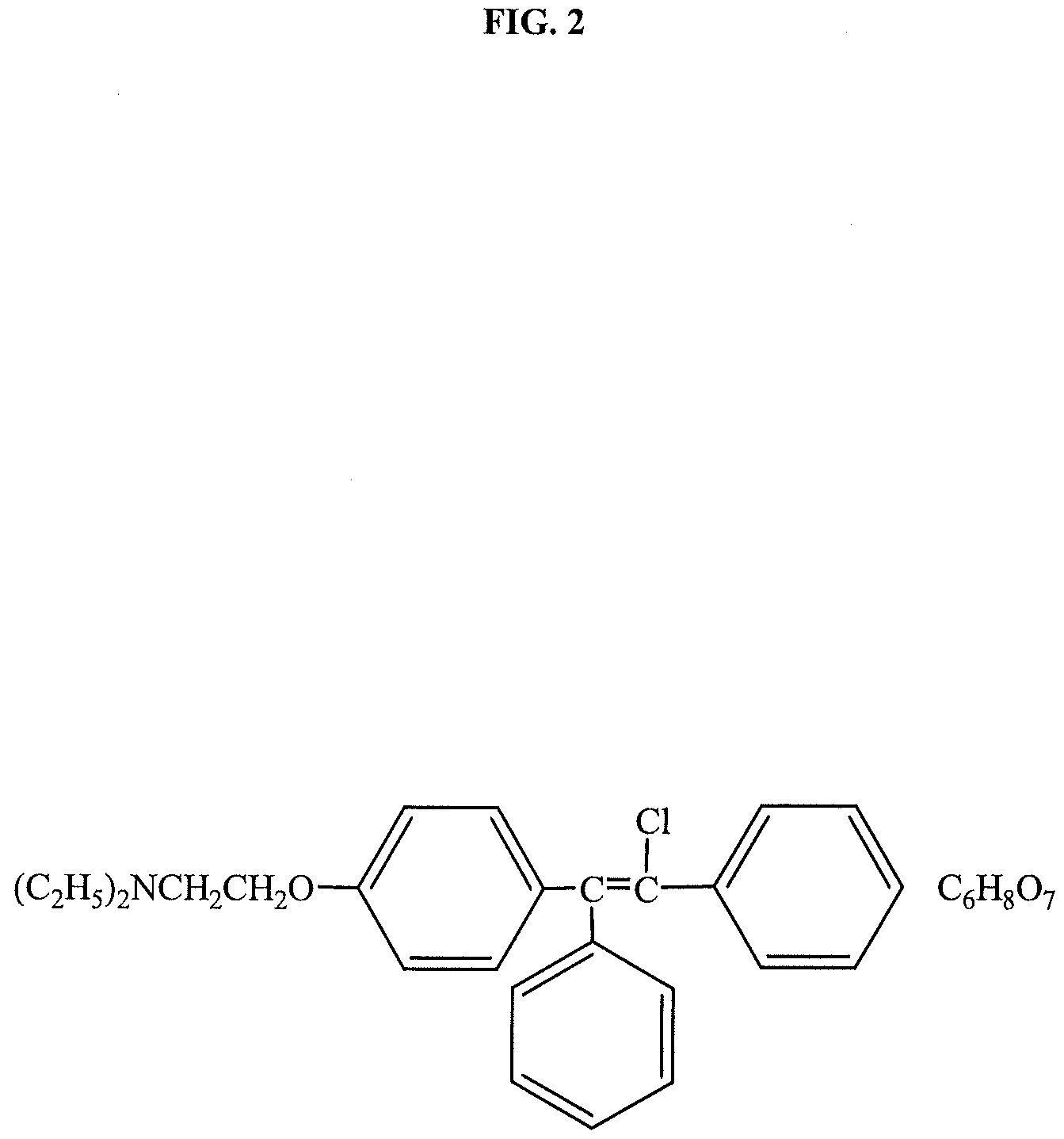
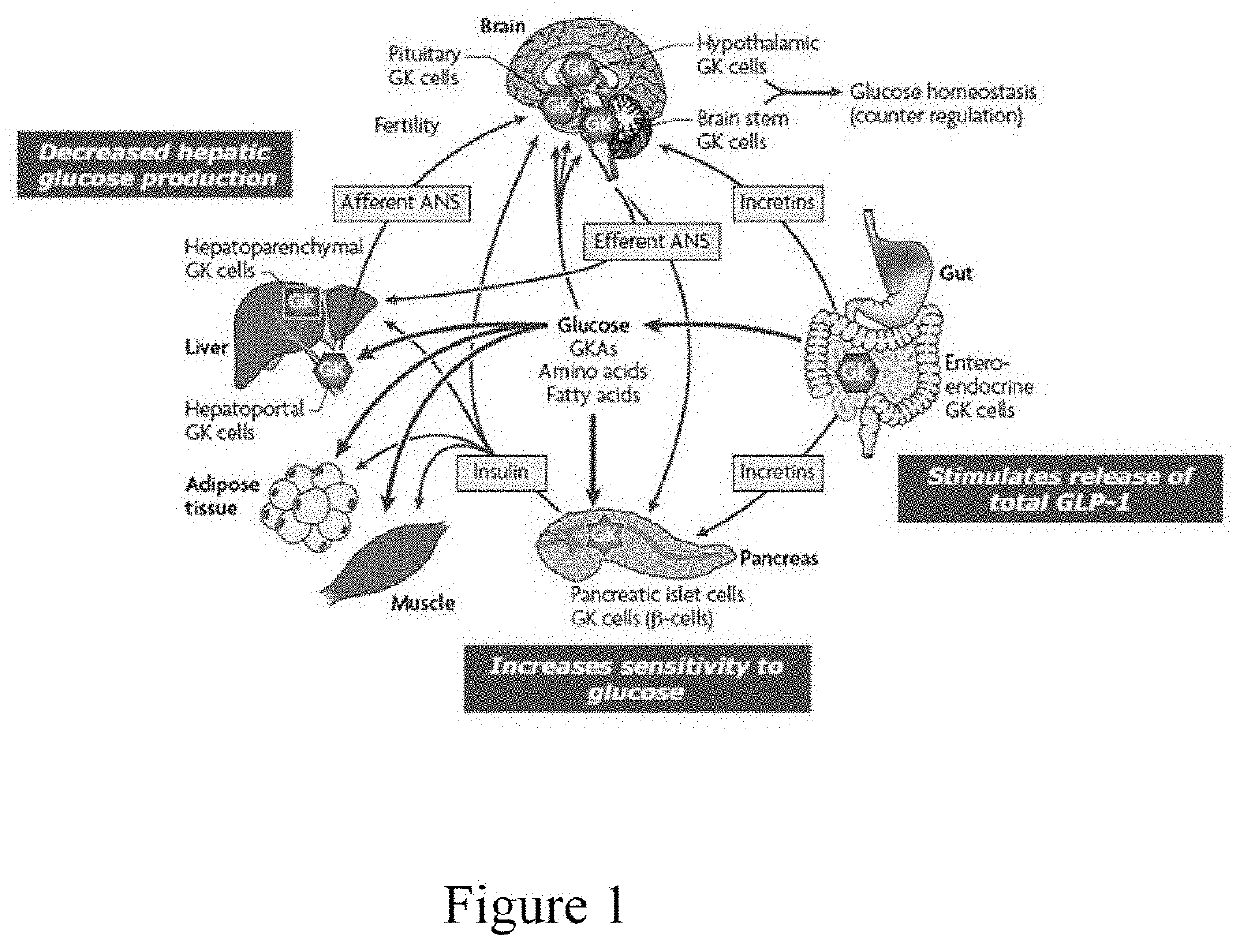
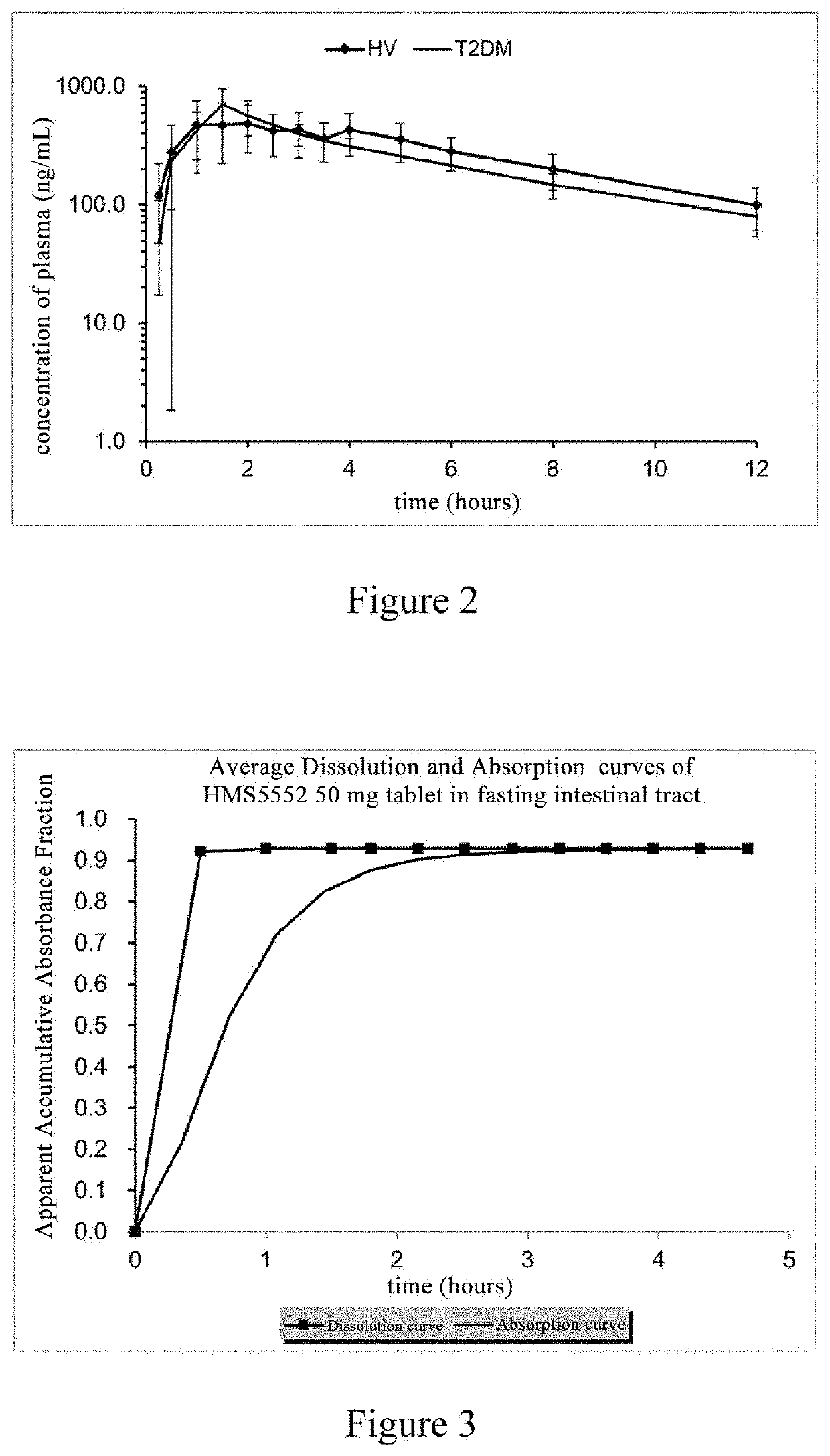
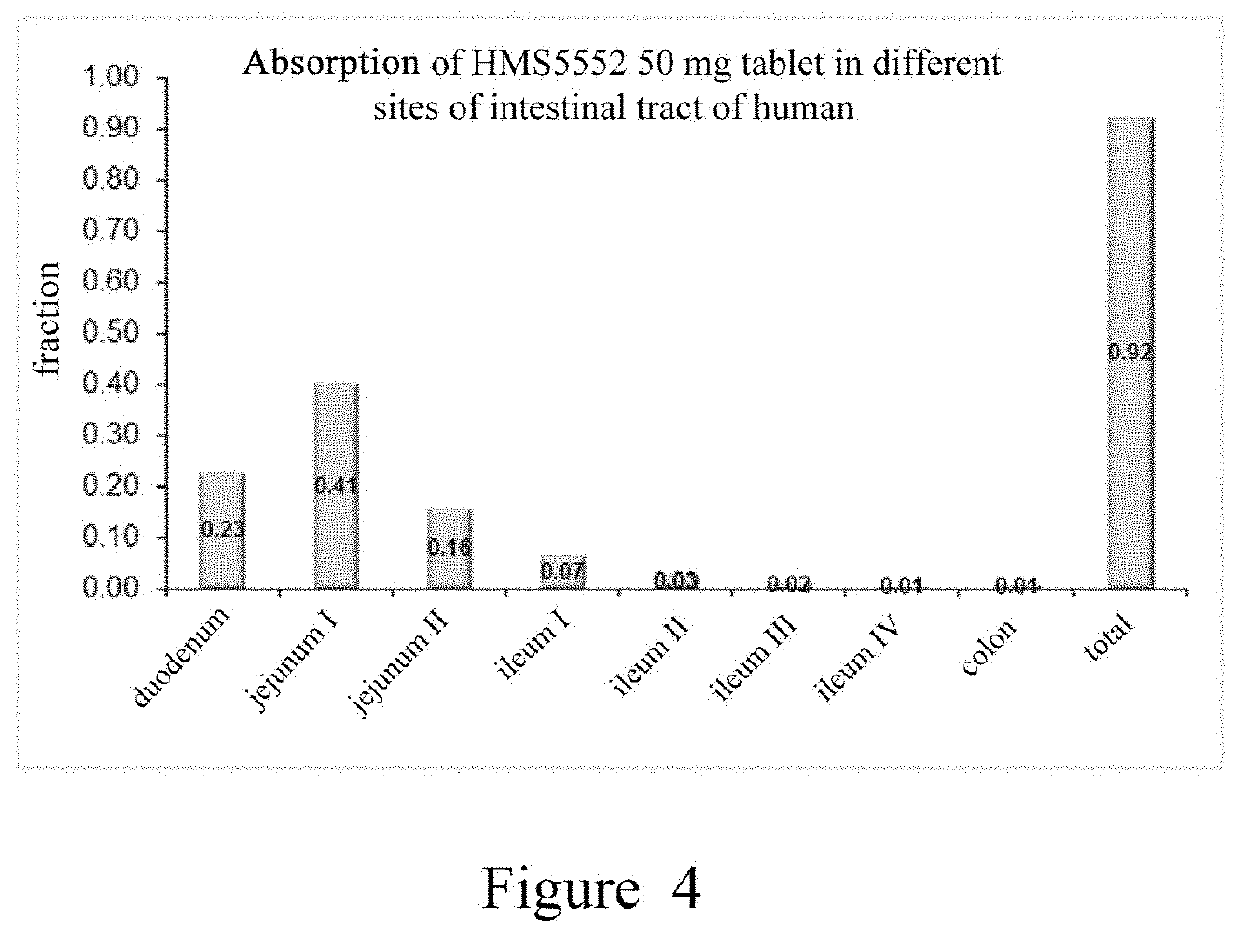
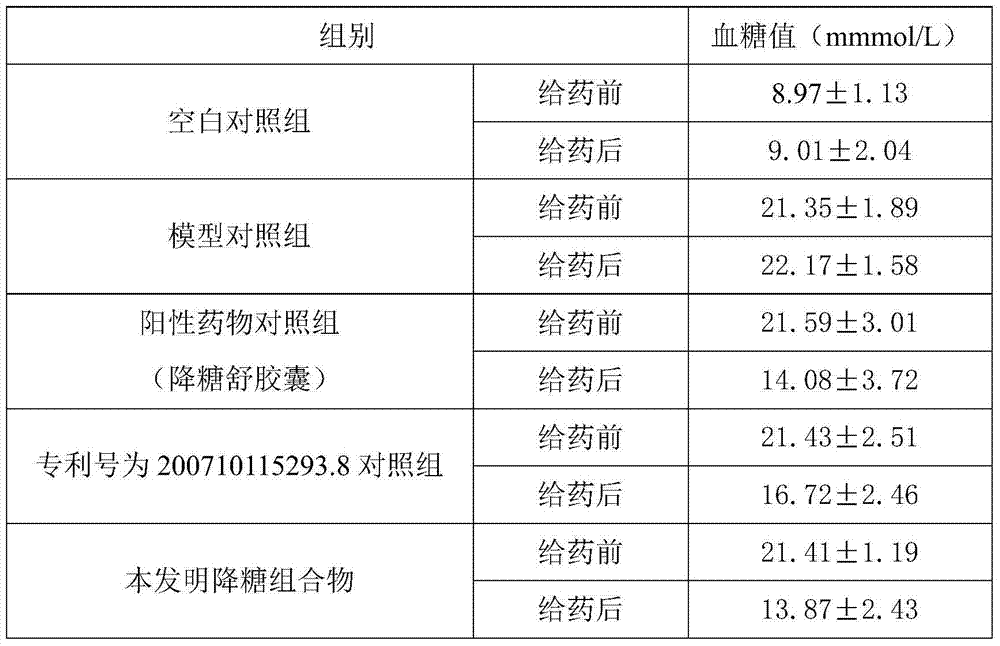
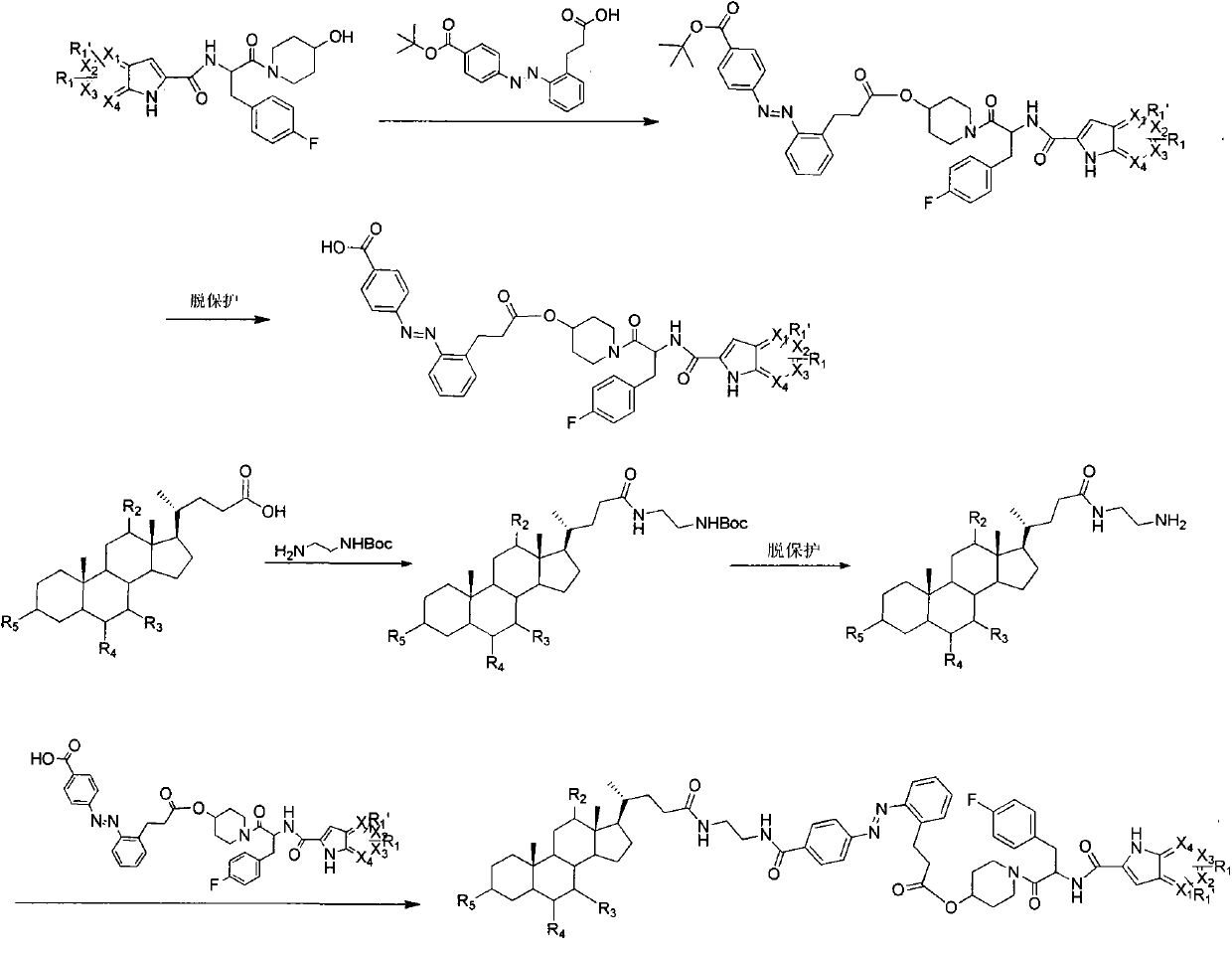
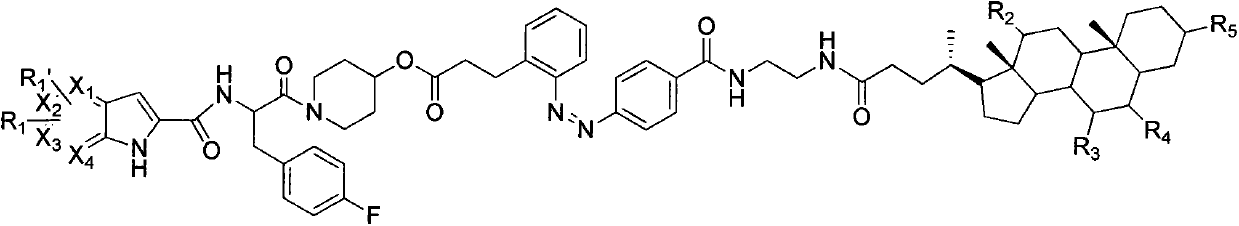
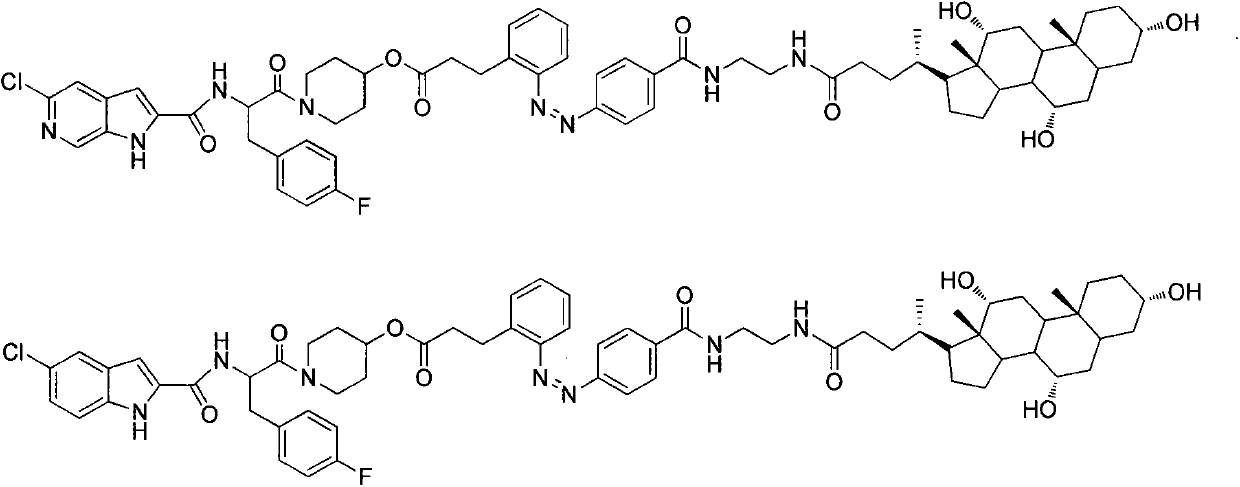
Patents
Literature
120 results about "Fasting glucose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
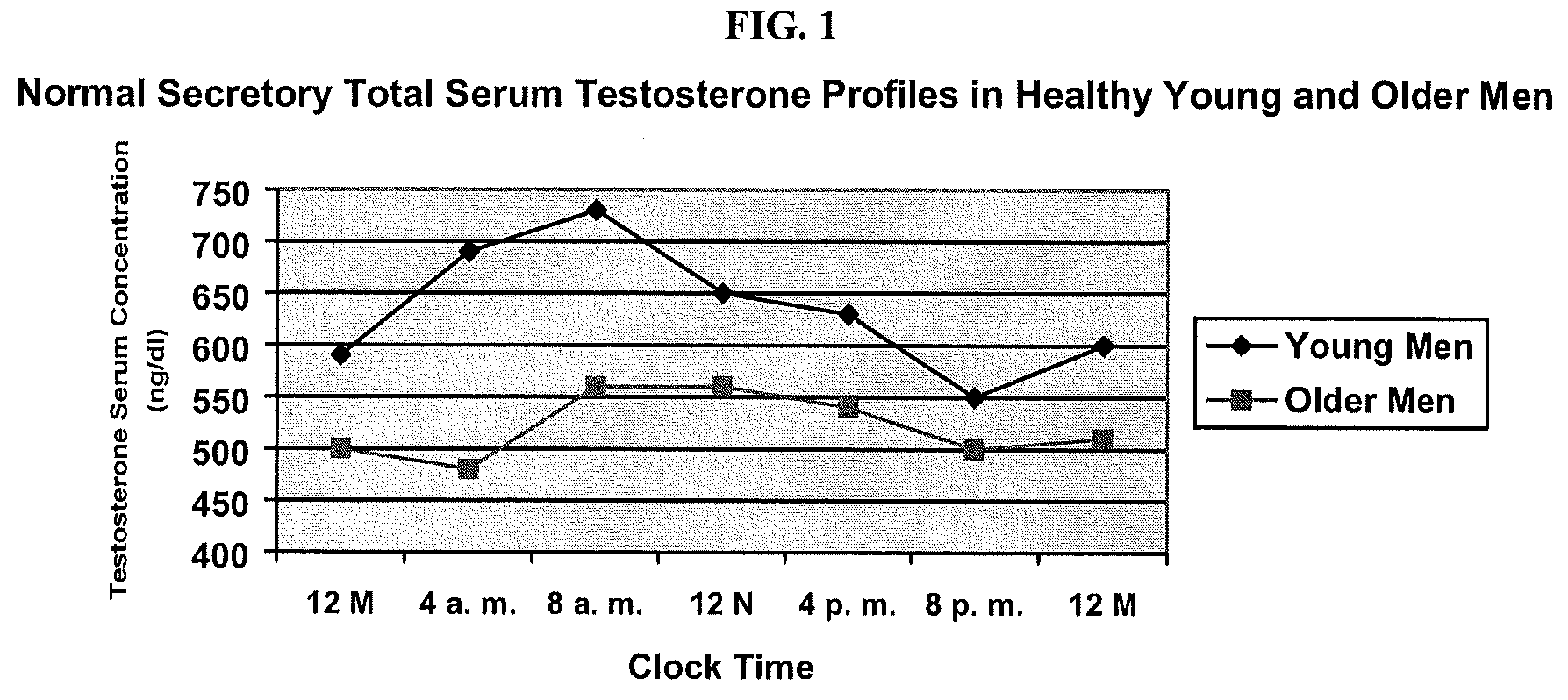
Fasting blood glucose: A test to determine how much glucose (sugar) is in a blood sample after an overnight fast. The fasting blood glucose test is commonly used to detect diabetes mellitus. A blood sample is taken in a lab, physician's office, or hospital.
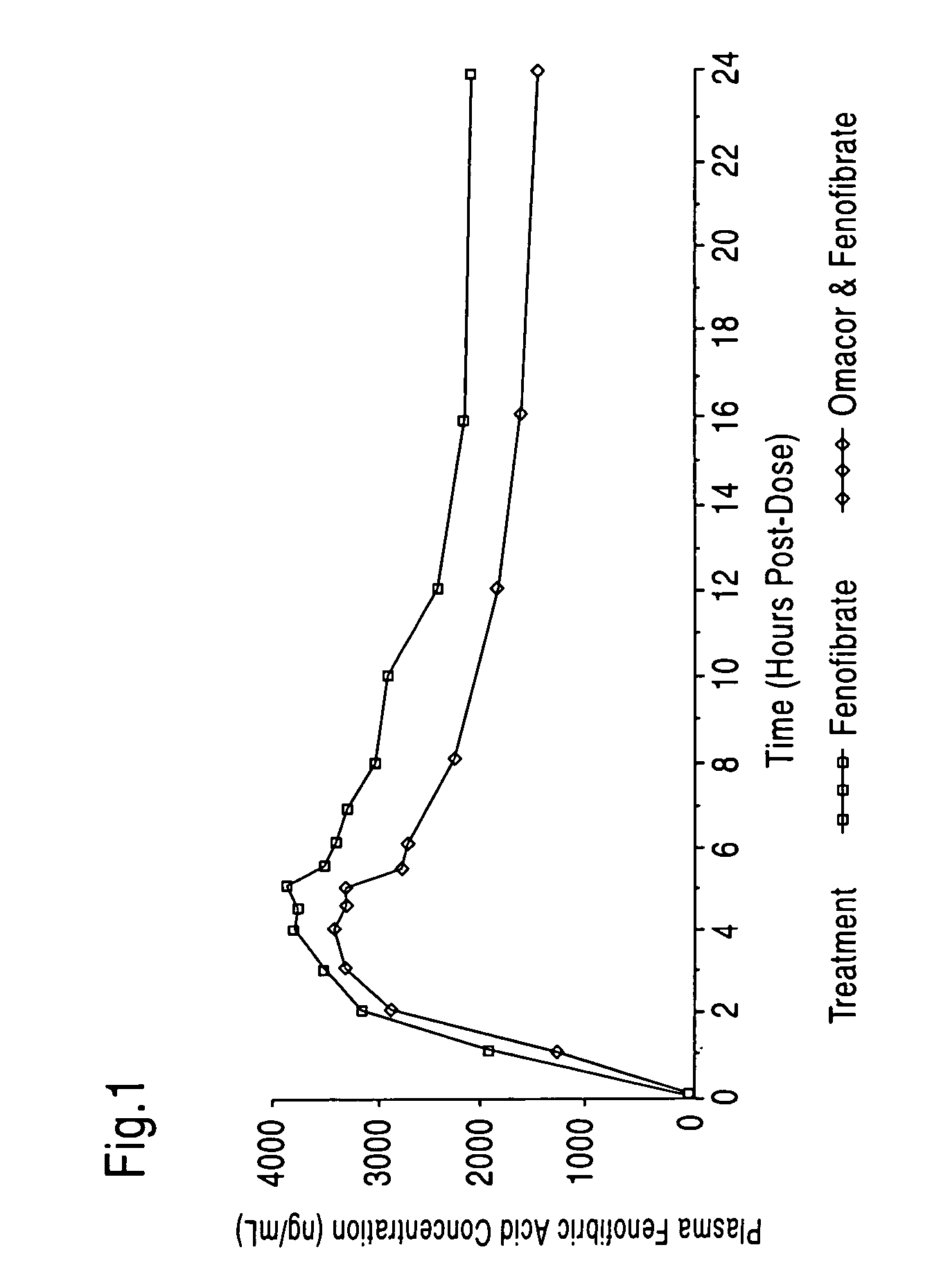
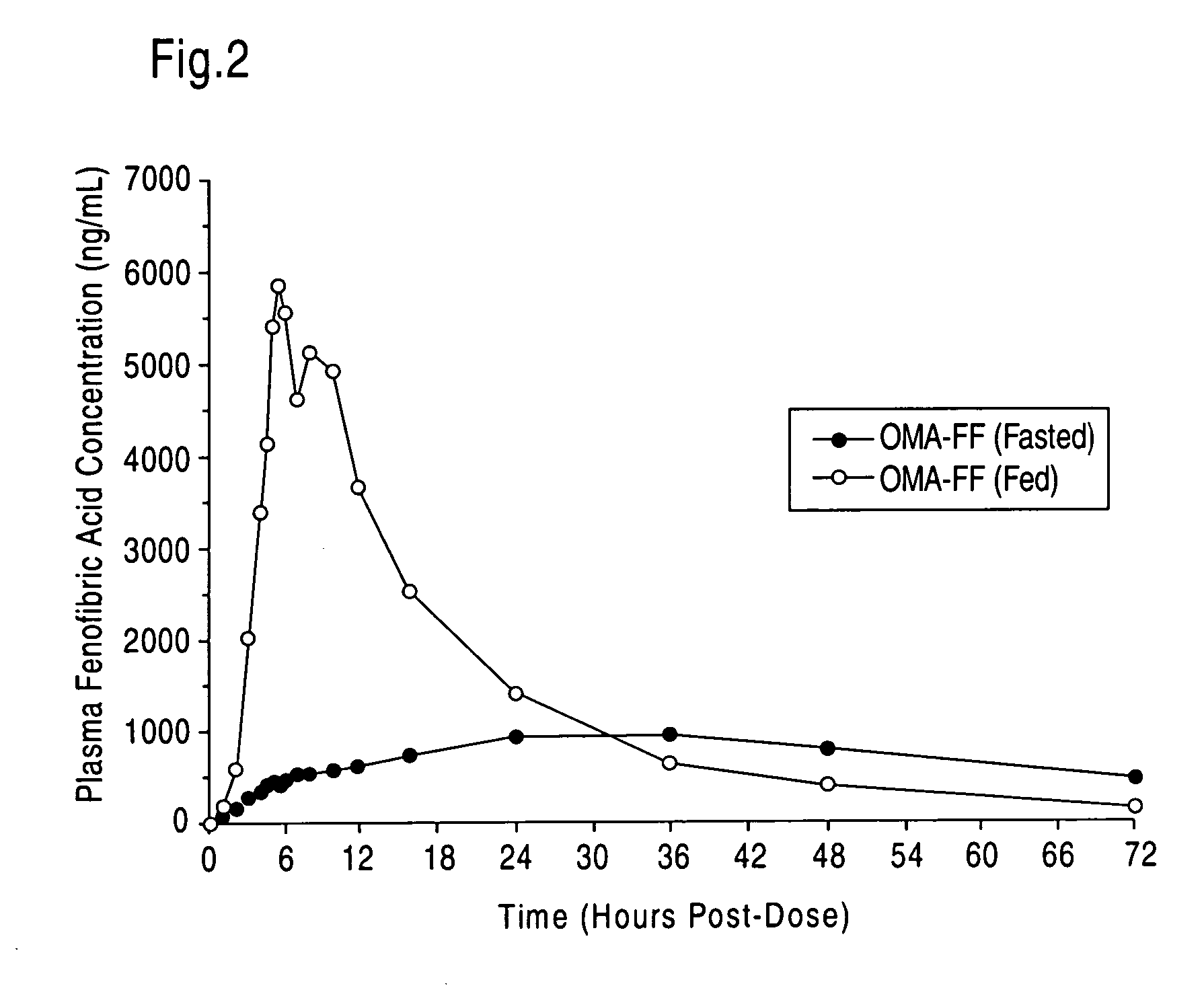
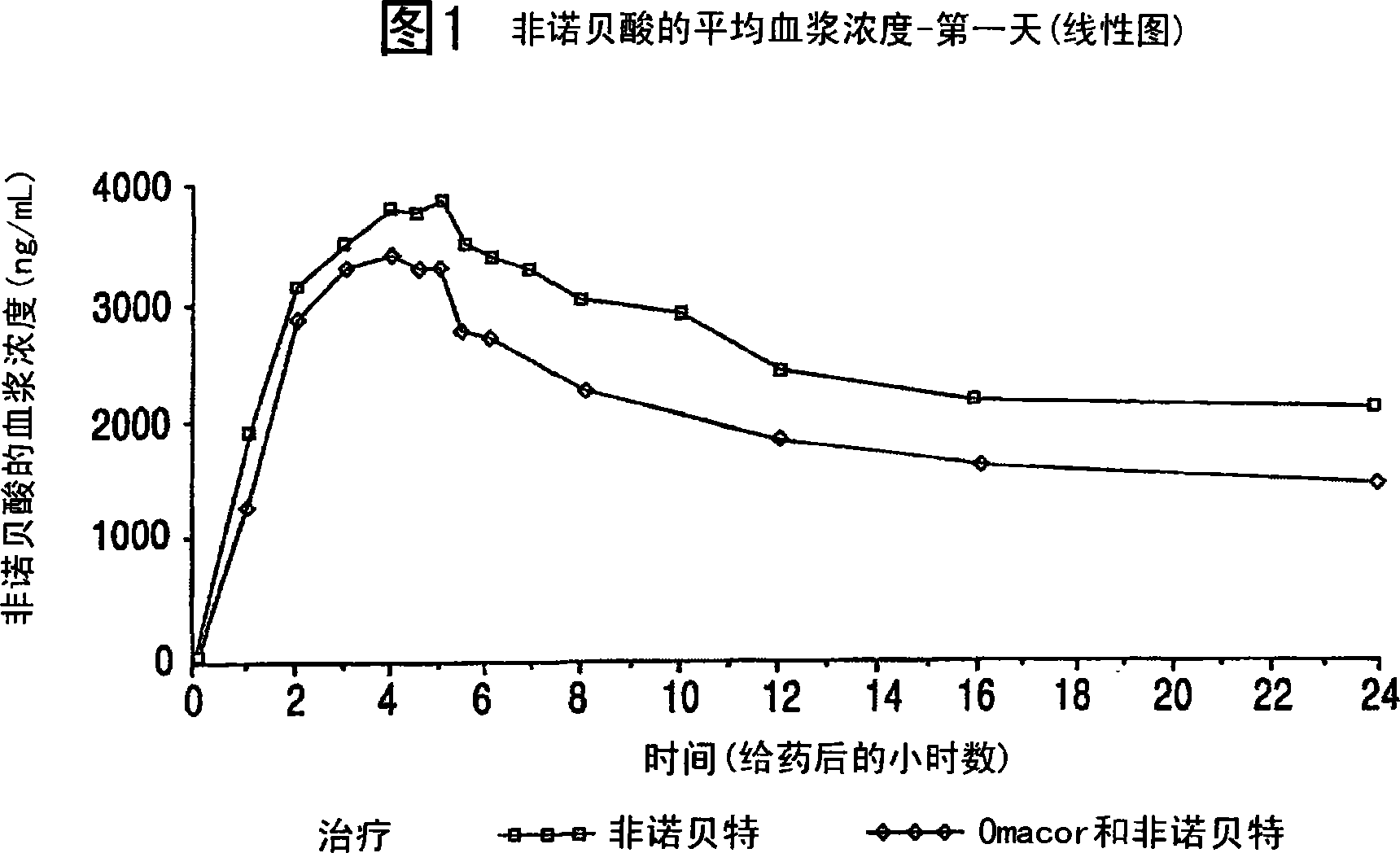
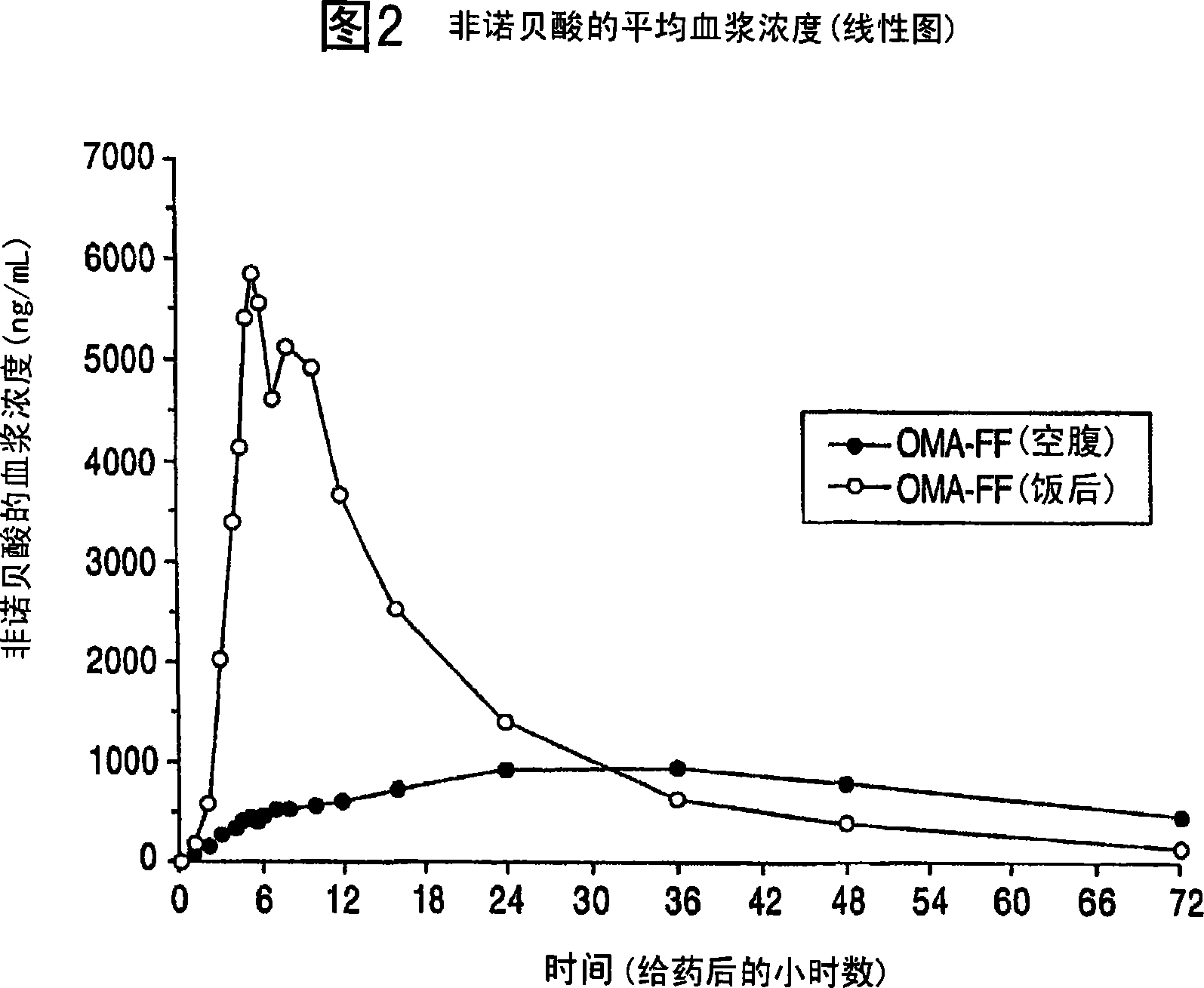
Treatment with omega-3 fatty acids and PPAR agonist and/or antagonist and a combination product thereof
InactiveUS20060211749A1Reduced dosages of PPAR agonistEffective treatmentBiocideMetabolism disorderDyslipidemiaFasting glucose
A method and composition for blood lipid therapy that comprises administering to the subject an effective amount of a PPAR agonist and / or antagonist and an omega-3 fatty acid. The methods and compositions include combination products or concomitant therapy for the treatment of subjects with hypertriglyceridemia, hypercholesteremia, mixed dyslipidemia, vascular disease, artherosclerotic disease and related conditions, obesity, the prevention or reduction of cardiovascular and vascular events, the reduction of insulin resistance, fasting glucose levels and postprandial glucose levels, and / or the reduction of incidence and / or the delay of onset of diabetes.
Owner:RELIANT PHARMACEUTICALS INC
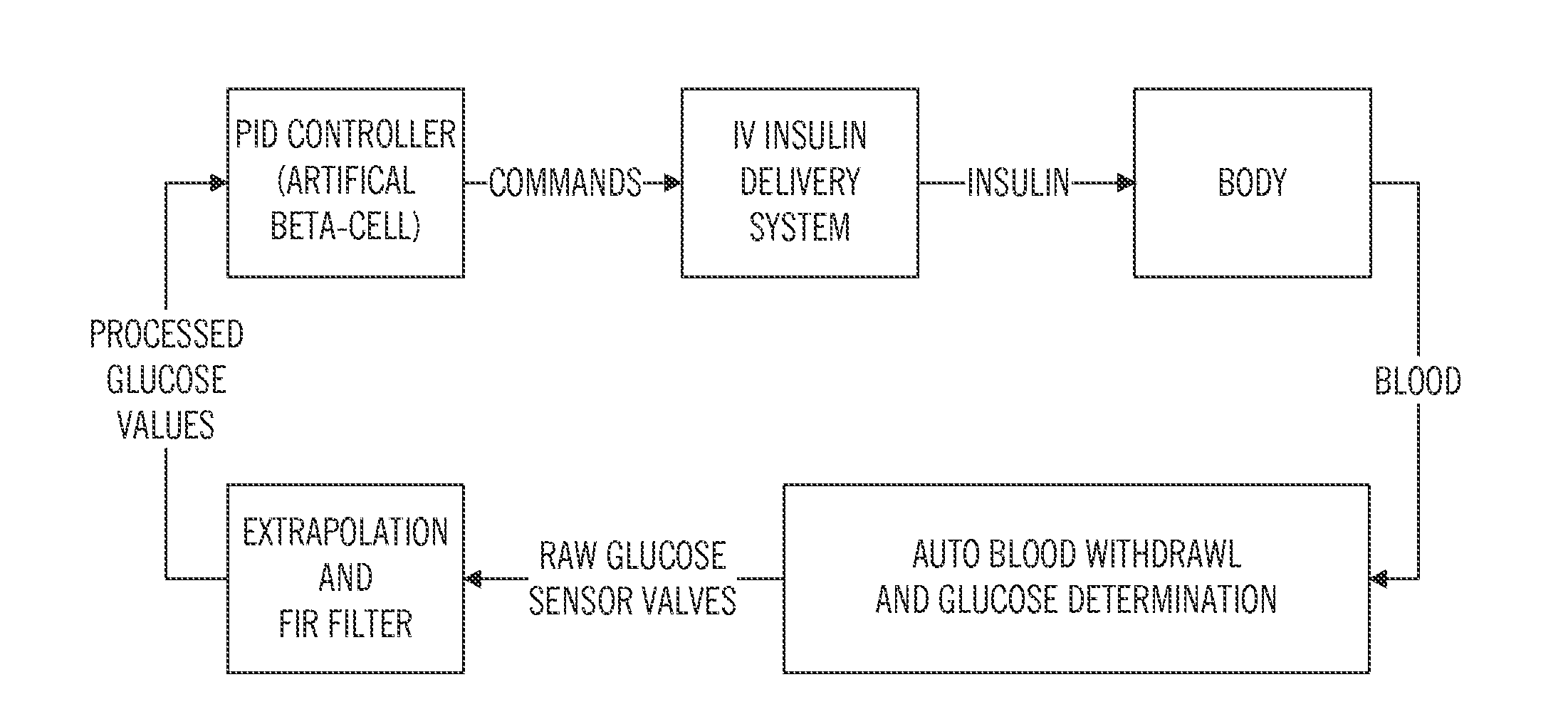
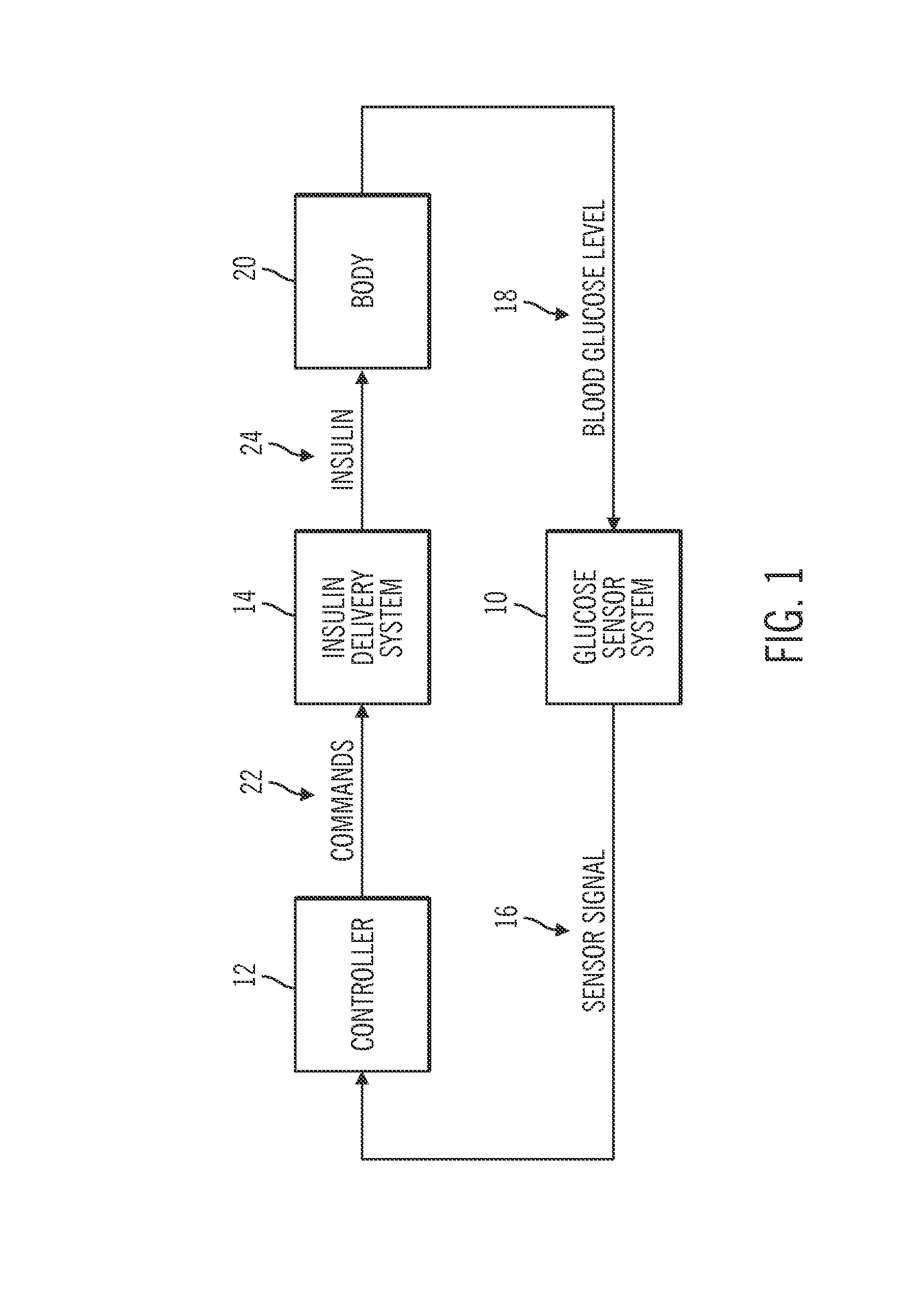
Safeguarding techniques for a closed-loop insulin infusion system
Processor-implemented methods of controlling an insulin infusion device for a user are provided here. A first method obtains and analyzes calibration factors (and corresponding timestamp data) for a continuous glucose sensor, and regulates entry into a closed-loop operating mode of the infusion device based on the calibration factors and timestamp data. A second method obtains a most recent sensor glucose value and a target glucose setpoint value for the user at the outset of the closed-loop mode. The second method adjusts the closed-loop insulin infusion rate over time, in response to the sensor glucose value and the setpoint value. A third method calculates an upper insulin limit that applies to the insulin infusion rate during the closed-loop mode. The insulin limit is calculated based on a fasting blood glucose value of the user, a total daily insulin value of the user, and fasting insulin delivery data for the user.
Owner:MEDTRONIC MIMIMED INC
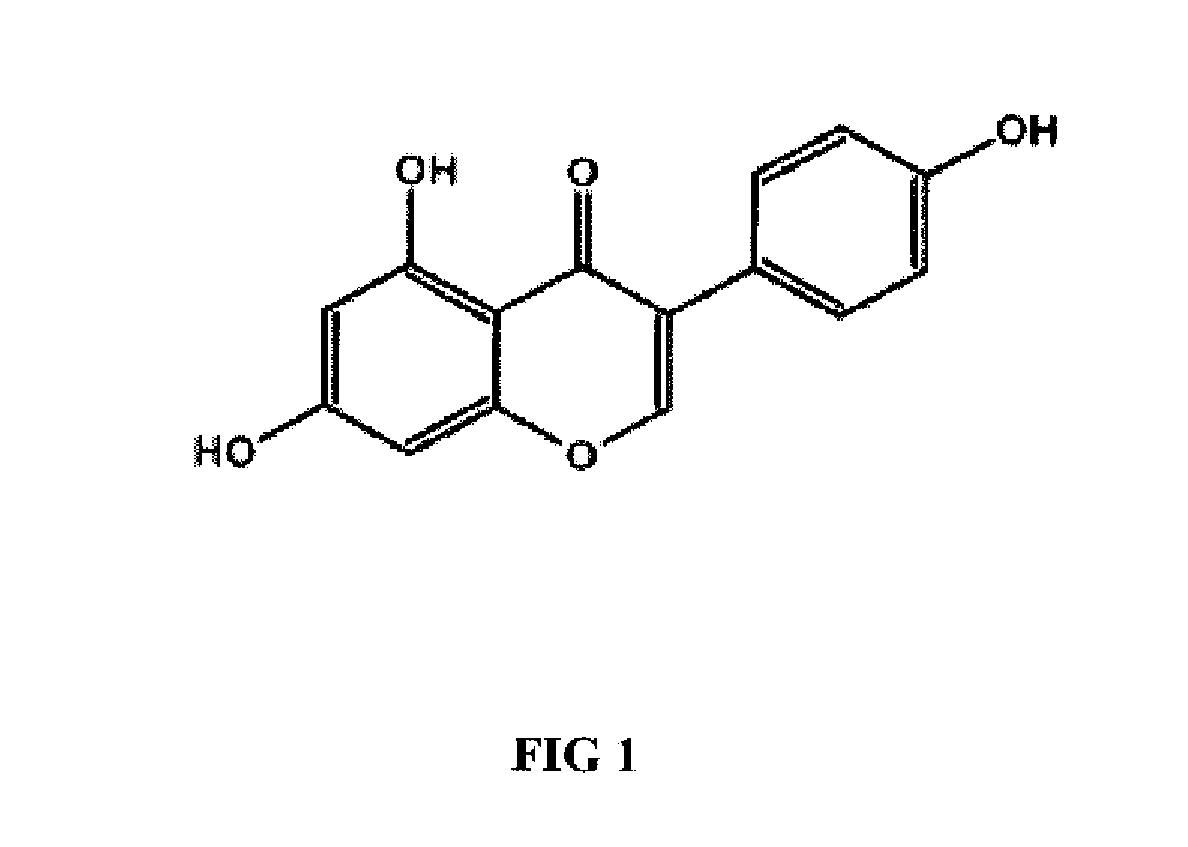
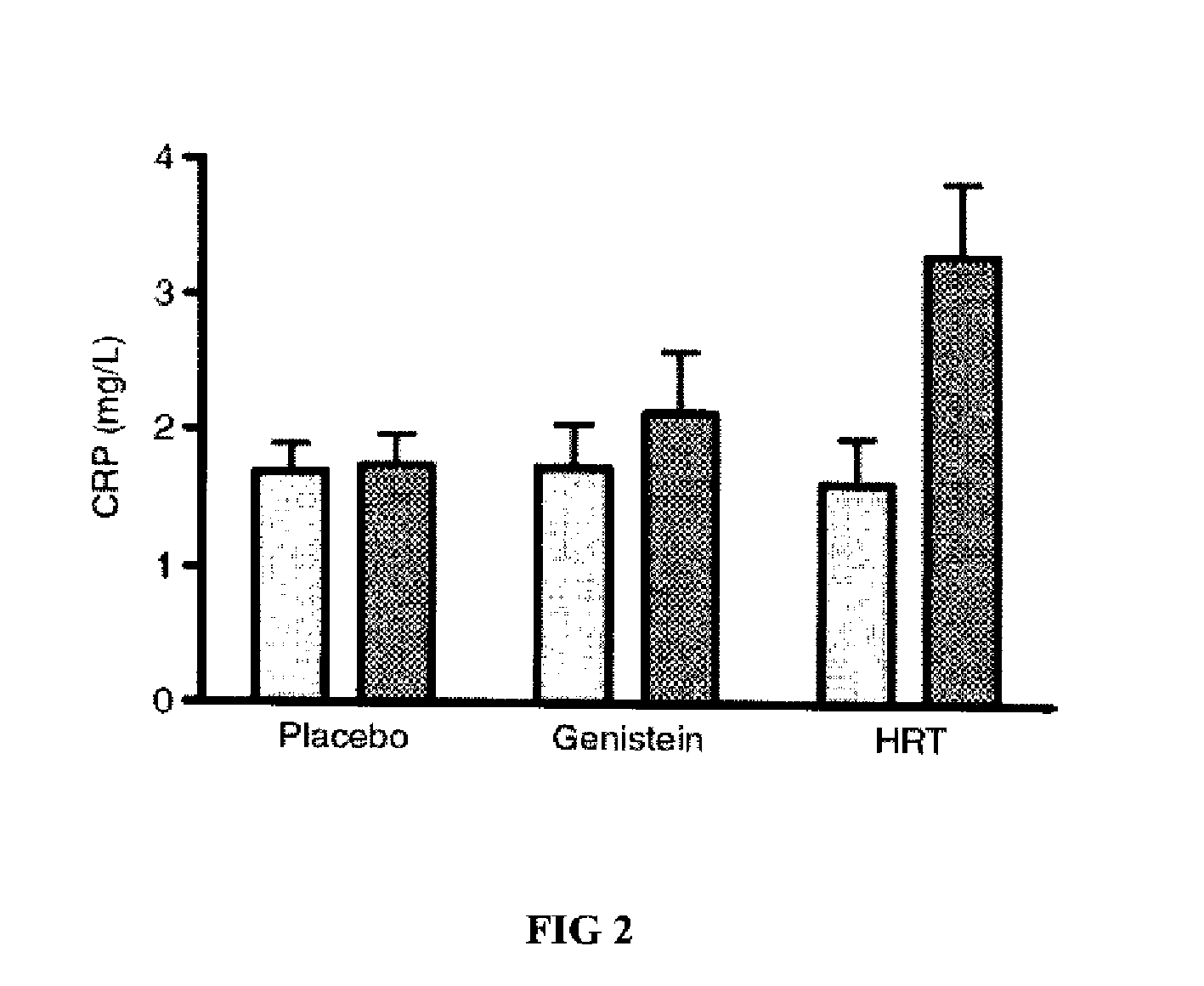
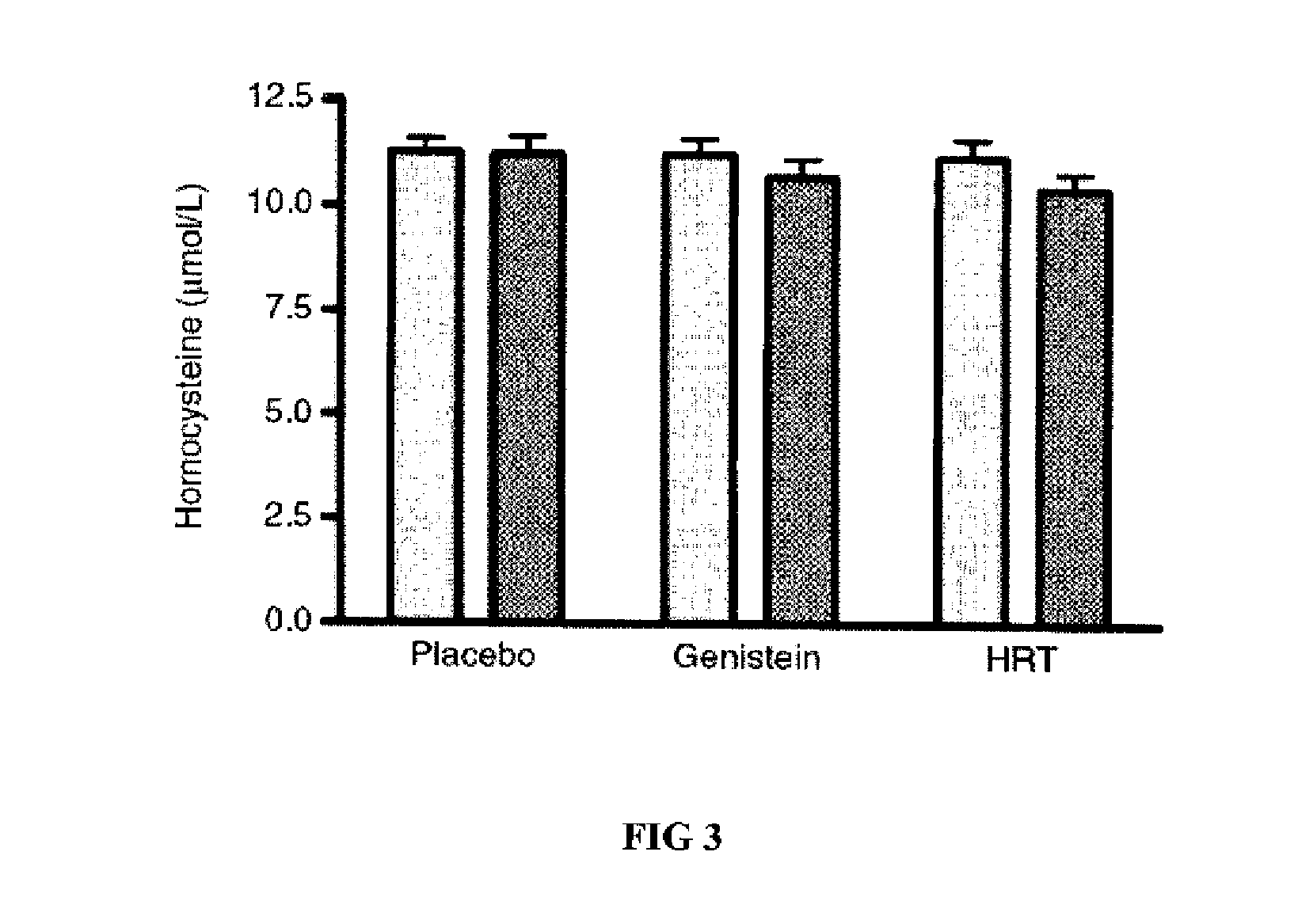
Genistein modulated reduction of cardiovascular risk factors
InactiveUS20070207225A1Reducing cardiovascular risk factorReduce risk factorBiocideAnimal repellantsFasting glucoseFibrinogen
The disclosed methods and compositions for reducing cardiovascular risk factors in mammals generally includes using genistein to modulate various inflammatory and cardiovascular risk markers including: homocysteine, C-reactive protein, fibrinogen, sex hormone-binding globulin, fasting glucose, insulin, insulin resistance, and osteoprotegerin.
Owner:PRIMUS PHARM INC
Trans-clomiphene for metabolic syndrome
ActiveUS20090099265A1Reduce fasting glucose levelLower blood sugar levelsBiocideOrganic active ingredientsFasting glucoseMetabolic syndrome
The present invention relates to the administration of compositions comprising an antiestrogen, preferably trans-clomiphene, for treating metabolic syndrome in a subject. The invention is also directed to methods for reducing fasting glucose levels in a subject by administering a composition comprising an antiestrogen, preferably trans-clomiphene.
Owner:REPROS THERAPEUTICS
Oligosaccharide oat beverage capable of treating hyperlipoidemia and hyperglycemia and improving gastrointestinal tract
ActiveCN101991163ATreat hyperlipidemiaImprove gastrointestinal functionMilk preparationDough treatmentBiotechnologyAmylase
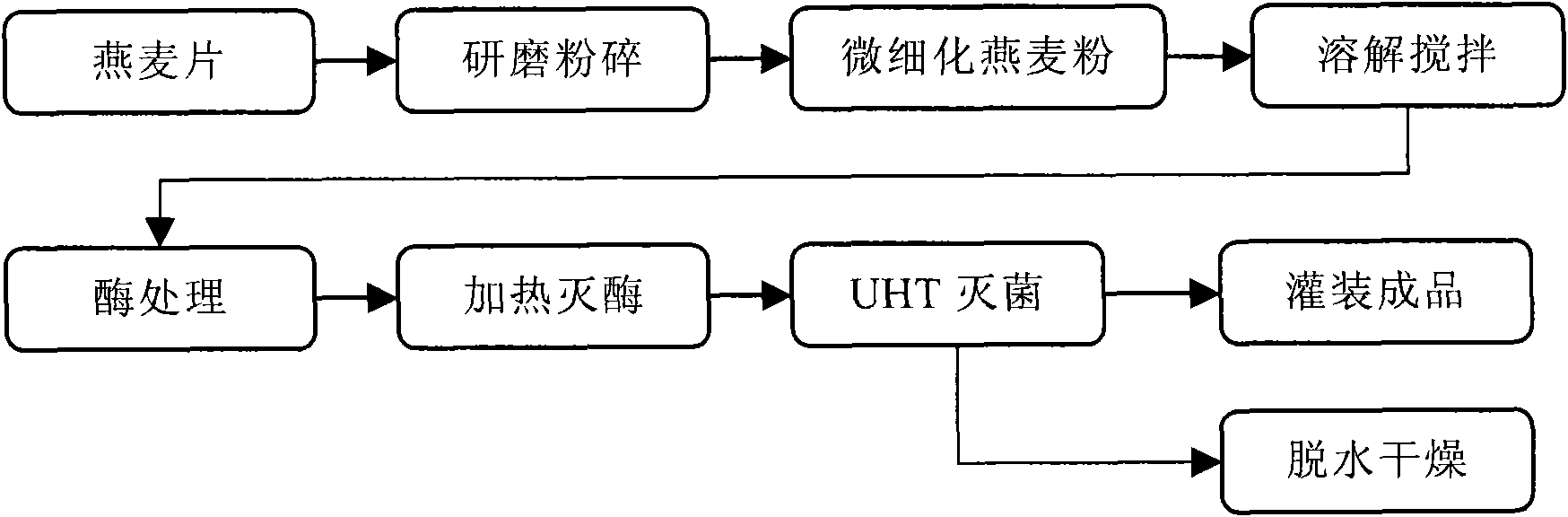
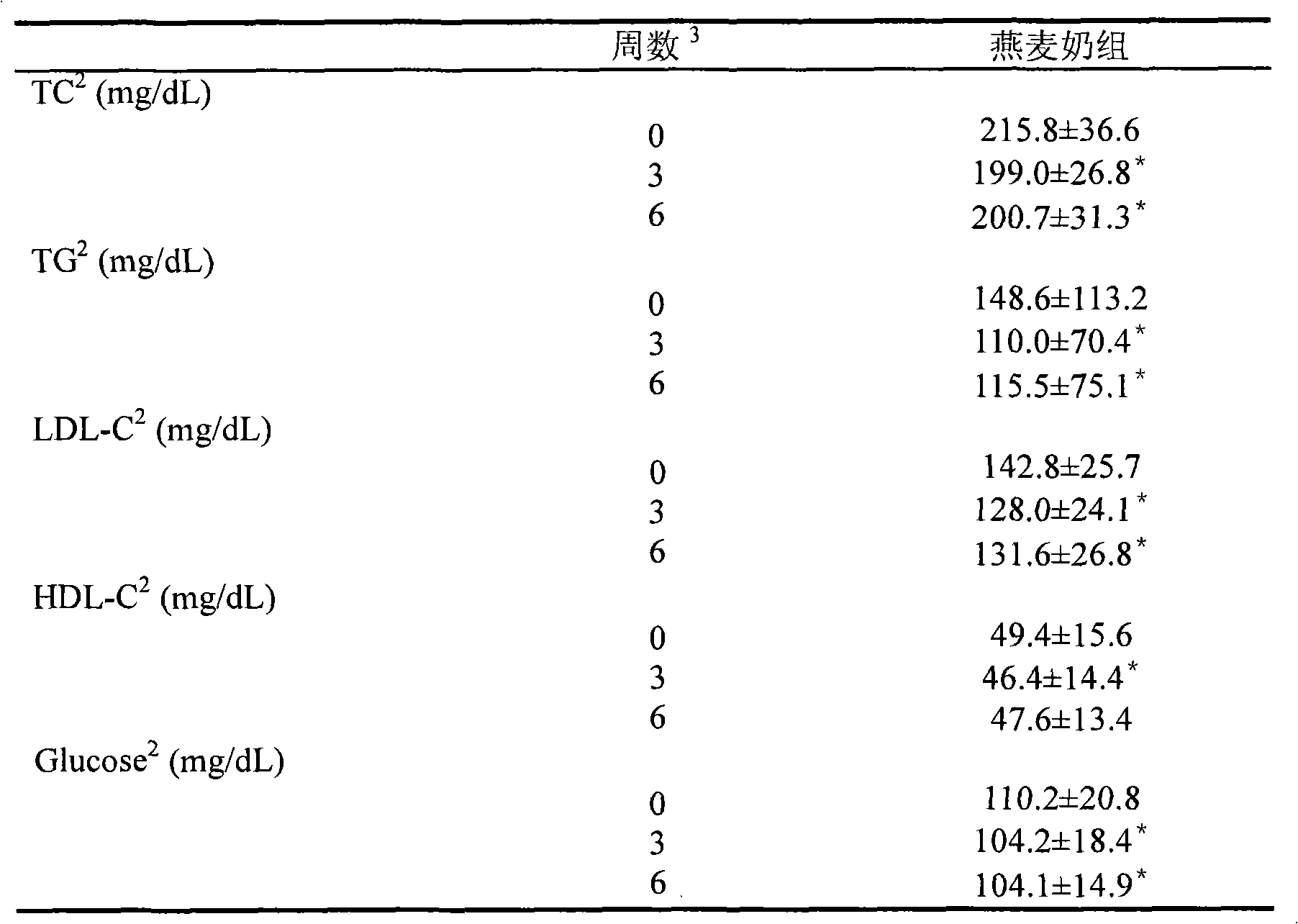
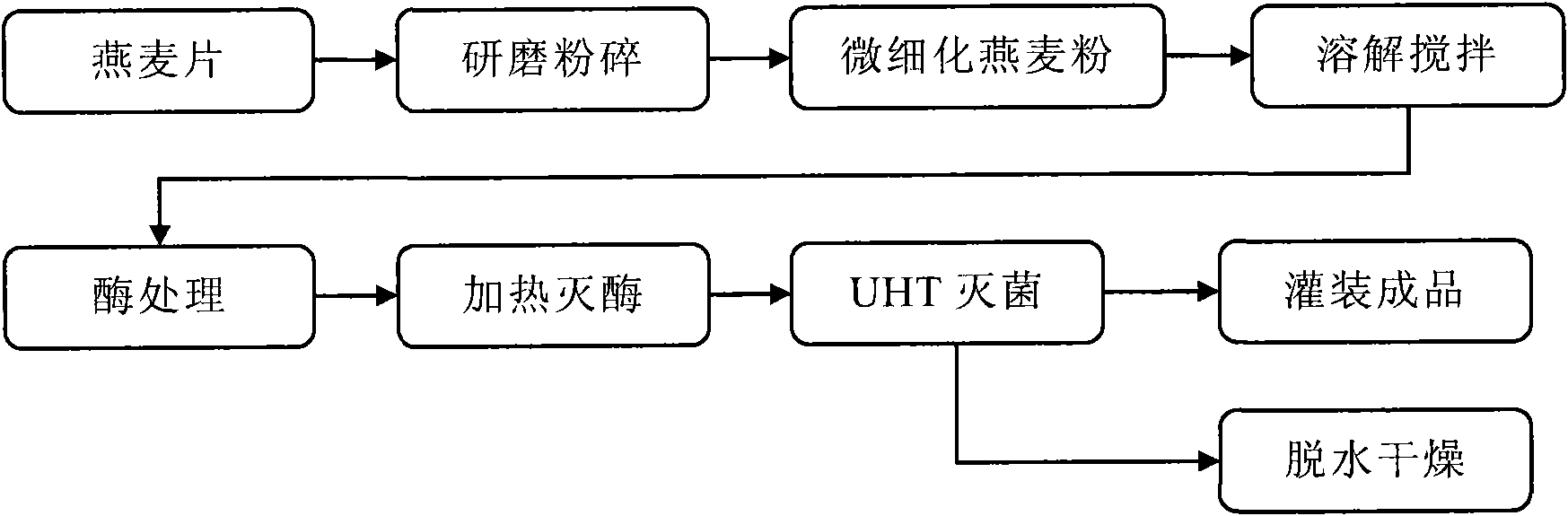
The invention provides an oligosaccharide oat beverage capable of treating hyperlipoidemia and hyperglycemia and improving gastrointestinal tract function and a manufacturing method thereof by hydrolysis of three enzymes and fine grinding. The manufacturing method comprises the following steps: carrying out fine grinding on the oat meal to obtain the powder of which the average particle size is less than about 100 mu m, dissolving the oat powder in water to form oat slurry, and adding alpha-amylase, beta-amylase and trans-glucosidase to carry out enzyme treatment, thereby obtaining the oat beverage with rich functional components. The oligosaccharide oat beverage integrally reserves oat beta-dextran, and also contains oligo-isomaltose component of which the content is higher than that in common oat products. The manufacturing method can integrally reserve the nutritional components of the whole oat grains and omit the filter operation, thereby being beneficial to increasing the utilization ratio of raw materials; and the method is completed under the conditions of fermentation and sterilization in the optimal short time, thereby avoiding the possibility of rancidity of oat slurry in the processing procedure. When the oligosaccharide oat beverage is used for human testing for assessing physiologic effect, the test proves that the oligosaccharide oat beverage can lower the total cholesterol, low-density lipoprotein cholesterol, triglyceride and fasting blood-glucose value in blood. The oligosaccharide oat beverage tastes savoury, thick and smooth like milk, and maintains the natural flavor of oat; and thus, the invention changes the traditional way for eating oat, greatly raises the nutrition health-care value of oat processed products, and has the potentials of preventing and treating hyperlipoidemia, hyperglycemia and other life-style related diseases and improving the gastrointestinal tract function.
Owner:V PRODS CORP
Co-therapy for diabetic conditions
InactiveUS20070025953A1Elevated blood glucose levelImprove the level ofBiocidePeptide/protein ingredientsCo administrationFasting glucose
Methods of treating diseases such as diabetes are disclosed. Methods of modulating elevated fructosamine levels, elevated HbA1c levels, impaired glucose tolerance, and impaired fasting glucose are also disclosed. In some embodiments, methods include co-administration of a bile acid sequestrant and two or more additional compounds selected from the group consisting of a biguanide, a sulfonylurea and insulin, or pharmaceutically acceptable salts thereof. Drug products including a bile acid sequestrant and two or more additional compounds selected from the group consisting of a biguanide, a sulfonylurea and insulin, or pharmaceutically acceptable salts thereof, in combination are also disclosed.
Owner:DAIICHI SANKYO INC
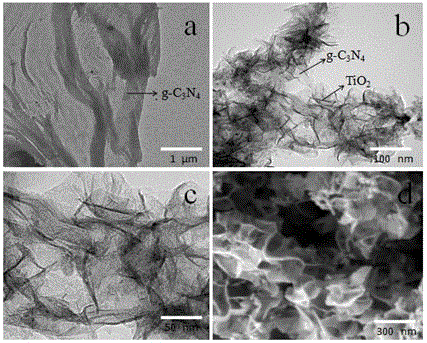
Method and system for model-based tracking of changes in average glycemia in diabetes
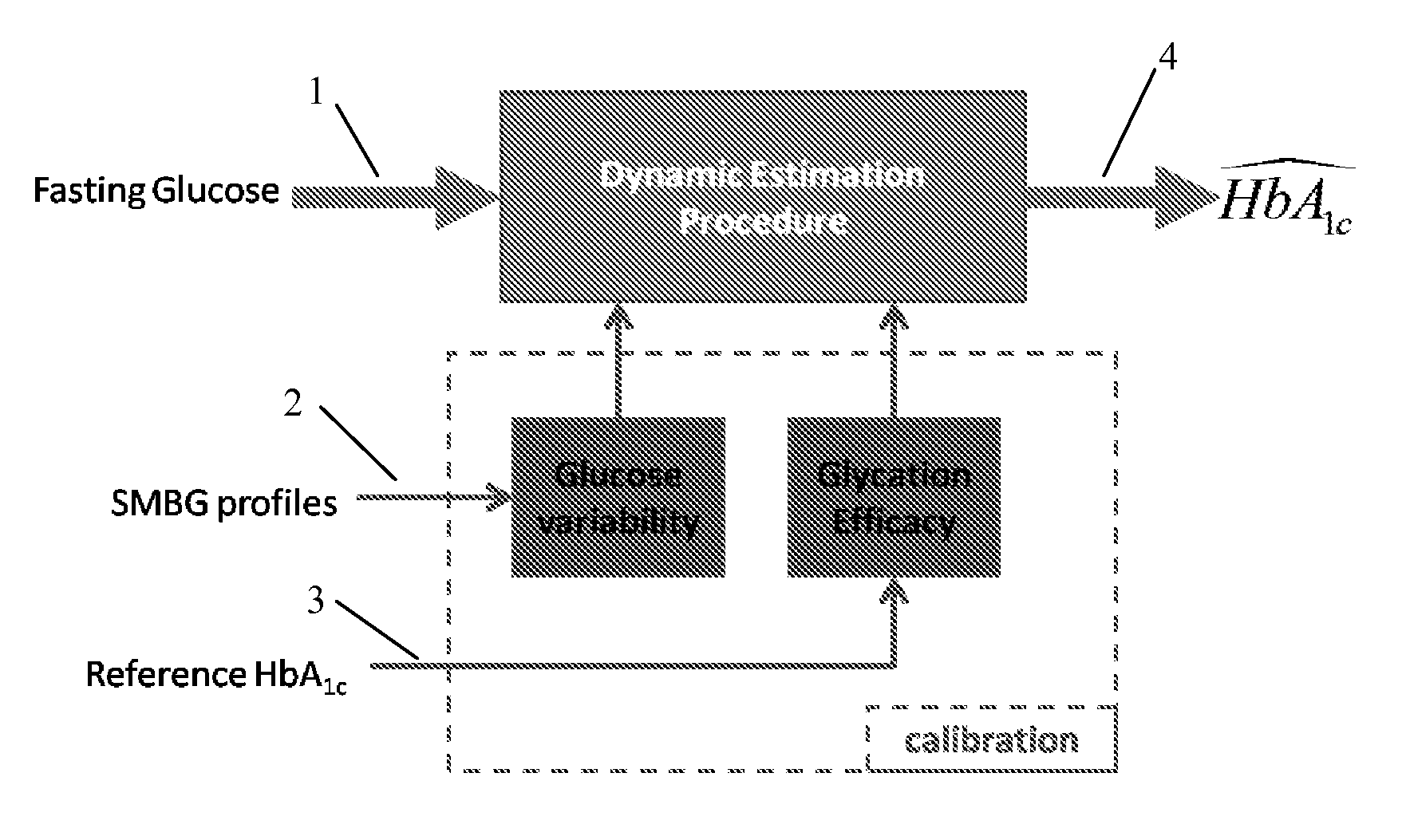
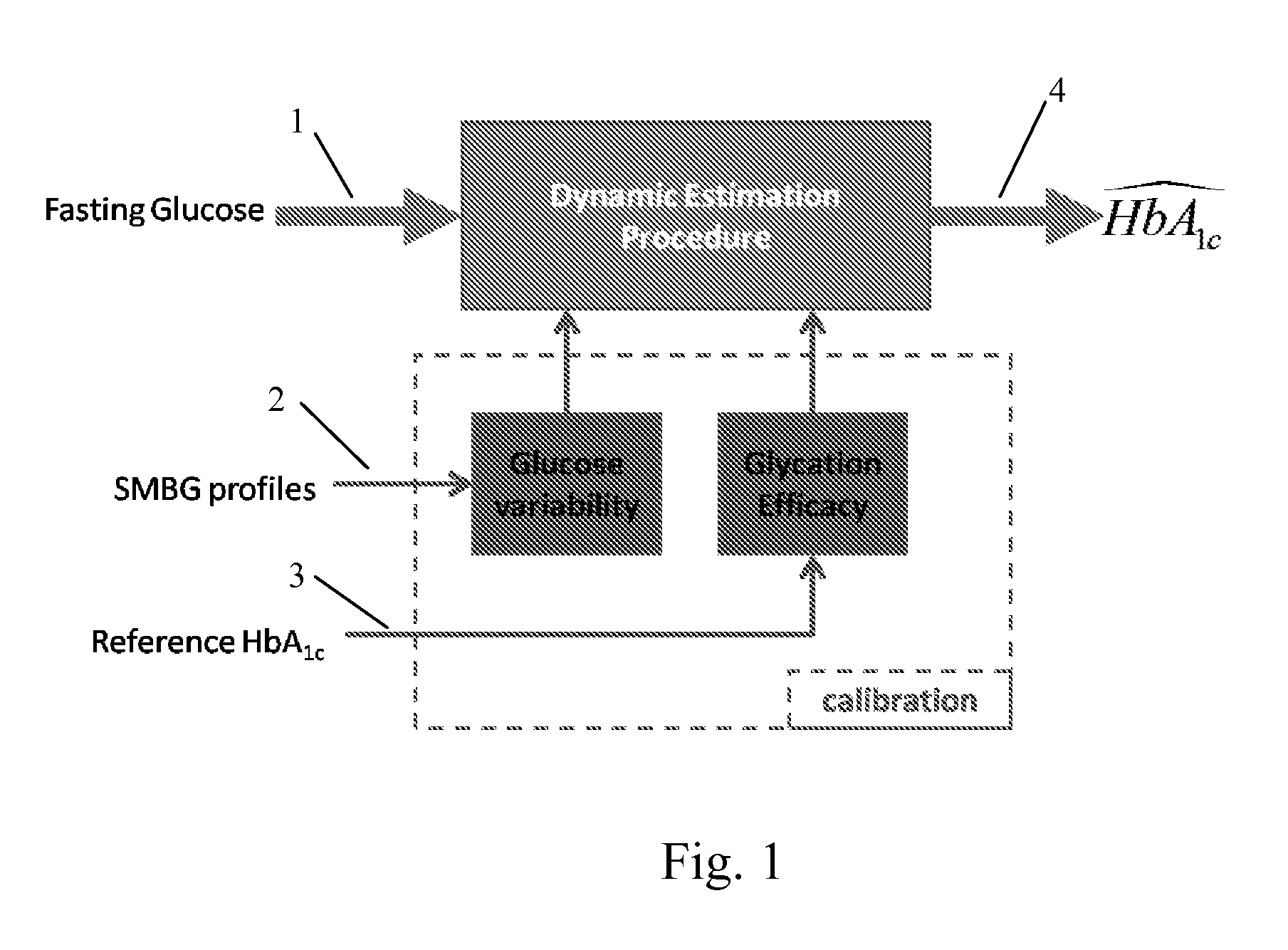
A method, system and computer readable medium for tracking changes in average glycemia in diabetes is based on a conceptually new approach to the retrieval of SMBG data. Using the understanding of HbA1c fluctuation as the measurable effect of the action of an underlying dynamical system, SMBG provides occasional glimpses at the state of this system and, using these measurements, the hidden underlying system trajectory can be reconstructed for individual diabetes patients. Using compartmental modeling a new two-step algorithm is provided that includes: (i) real-time estimate of HbA1c from fasting glucose readings, updated with any new incoming fasting SMBG data point(s), and (ii) initialization and calibration of the estimated HbA1c trace with daily SMBG profiles obtained periodically. The estimation of these profiles includes a factorial model capturing daily BG variability within two latent factors.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Method of construction for photoelectrochemical glucose oxidase sensor with graphite like g-C3N4-TiO2 nanosheet composite as enzymatic molecule immobilization scaffold
InactiveCN105929007AIncrease the rate of electron transferHigh sensitivityMaterial analysis by electric/magnetic meansFasting glucoseGraphite
The invention discloses a method of construction for a photoelectrochemical glucose oxidase sensor with a graphite like g-C3N4-TiO2 nanosheet composite as an enzymatic molecule immobilization scaffold. The method comprises the steps of firstly preparing g-C3N4 by means of a thermal polymerization method, then preparing the g-C3N4-TiO2 composite by means of a hydrothermal method, and finally modifying a surface of an ITO electrode with the synthesized g-C3N4-TiO2 composite and glucose oxidase (GOD) together with Nafion as a binder to construct the GOD sensor. The photoelectrochemical glucose oxidase sensor prepared by means of the method provided by the invention is capable of fast glucose determination, and has the advantages of relatively high sensitivity, relatively wide linear range, relatively low detection limit, and the like.
Owner:HENAN UNIVERSITY
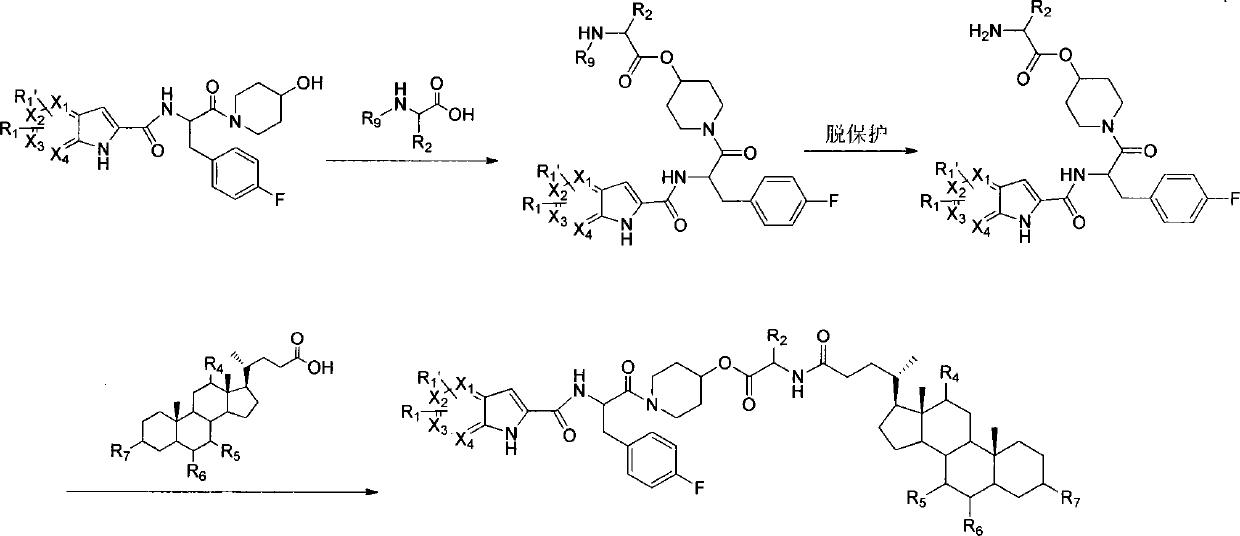
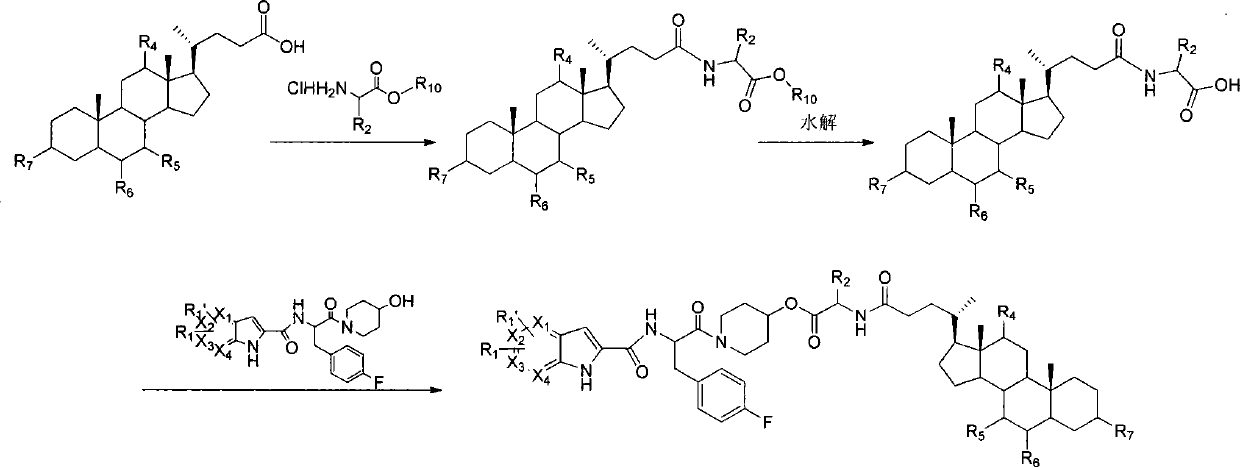
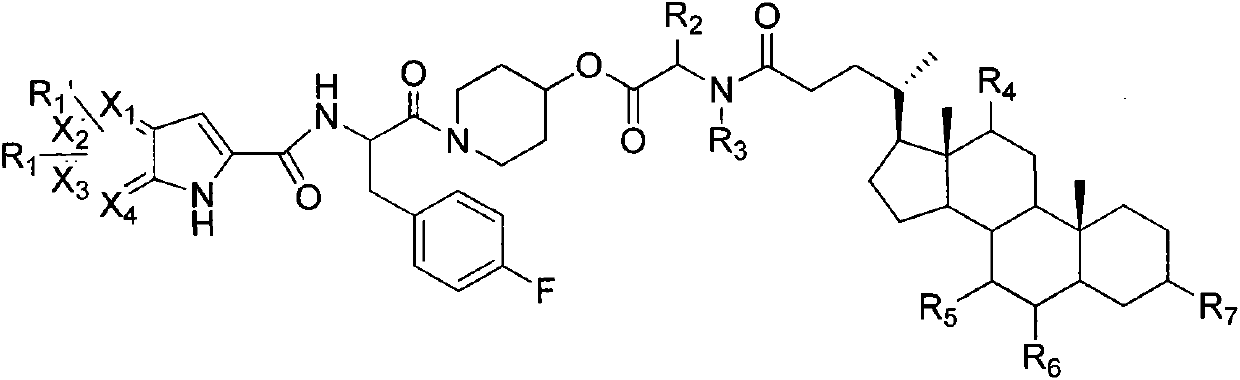
Liver-targeted glycogen phosphorylase inhibitor cholic acid derivative and preparation method and medical application thereof
The invention relates to a glycogen phosphorylase inhibitor cholic acid derivative, a preparation method thereof and a pharmaceutical composition containing the same. The glycogen phosphorylase inhibitor cholic acid derivative is a liver-targeted pro-drug for glycogen phosphorylase; compared with a glycogen phosphorylase inhibitor, the concentration of the glycogen phosphorylase inhibitor in a liver can be increased after the glycogen phosphorylase inhibitor cholic acid derivative is taken orally, so that the glycogen phosphorylase inhibitor cholic acid derivative can serve as a preferred drug for lowering blood sugar, particularly for treating impaired fasting glucose. The compound can be used for preventing and treating diabetes and complications thereof, hyperlipidemia, obesity, high-glucagon disease, insulin resistance, impaired fasting glucose, hypertension and complications thereof, atherosclerosis, metabolic syndrome or tumor.
Owner:CHENGDE MEDICAL UNIV
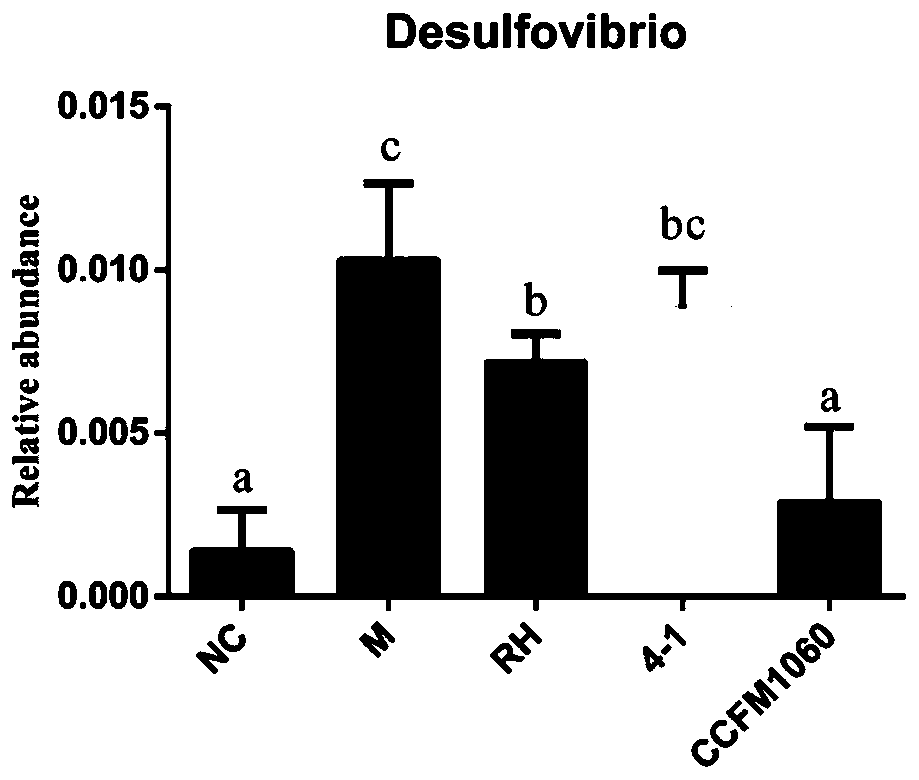
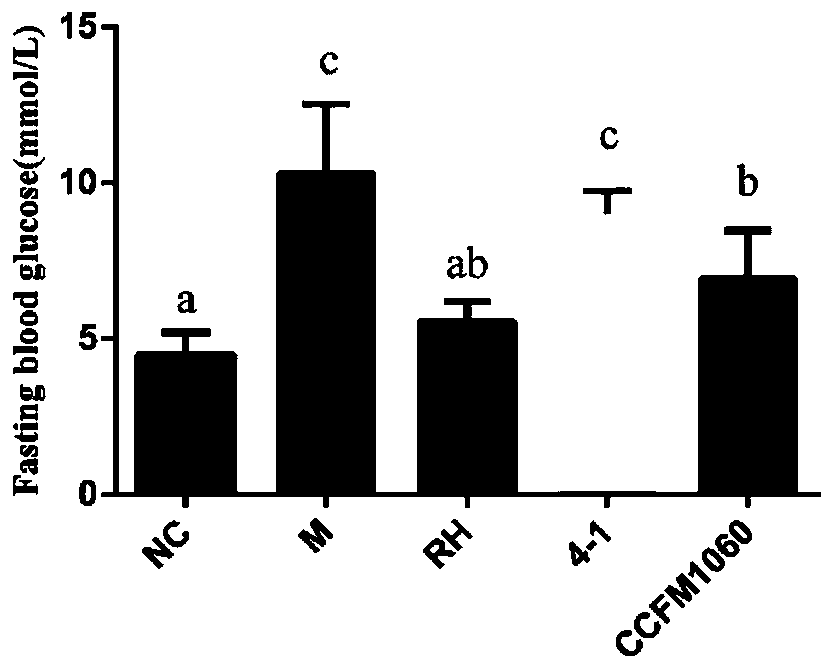
Application of lactobacillus rhamnosus CCFM1060 in preparation of functional microbial agents, foods and/or medicines
PendingCN110638843AImprove constipationIncrease abundanceMetabolism disorderDigestive systemBiotechnologyMicrobial agent
The invention discloses an application of lactobacillus rhamnosus CCFM1060 in preparation of functional microbial agents, foods and / or medicines. The lactobacillus rhamnosus CCFM1060 can tolerate thegastrointestinal environment of the human body, significantly improve fasting blood glucose rise and abnormal oral glucose tolerance of type 2 diabetics caused by high fat diet and STZ injection, reduce the area under the curve during glucose tolerance drug administration, and relieve insulin resistance; remarkably improve the increase of total cholesterol content and the increase of low-density lipoprotein cholesterol in serum of type 2 diabetics caused by high-fat diet and STZ injection; obviously relieve the inflammatory state of the liver; and significantly reduce pancreatic and liver pathological injuries caused by high-fat diet and STZ injection. The lactobacillus rhamnosus CCFM1060 has relatively strong adsorption capacity on perfluorooctanoic acid (PFOA), and has the capacity of relieving the toxicity of the PFOA.
Owner:JIANGNAN UNIV
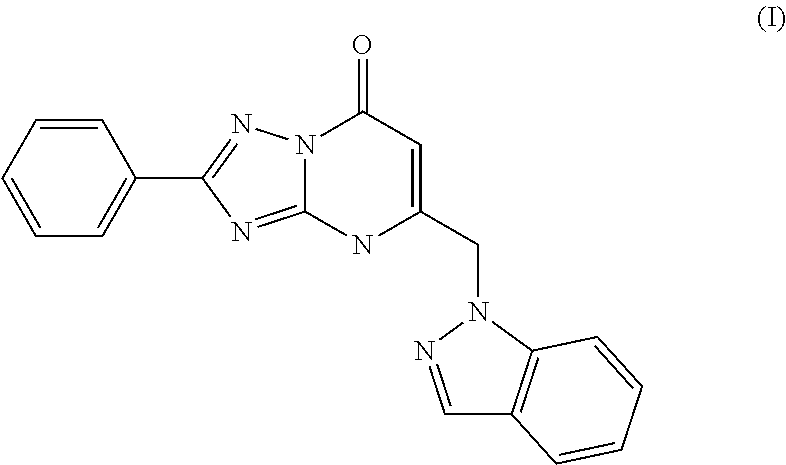
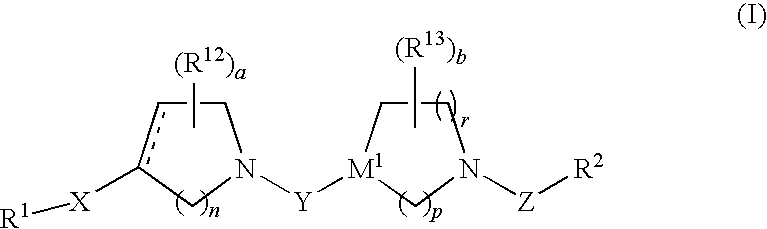
Inhibitors of fatty acid binding protein (FABP)
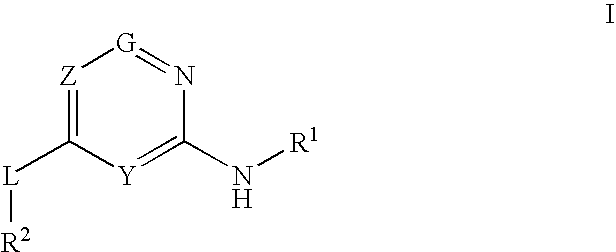
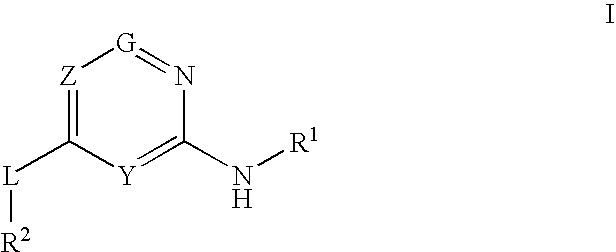
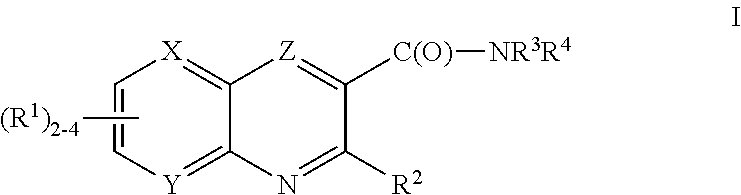
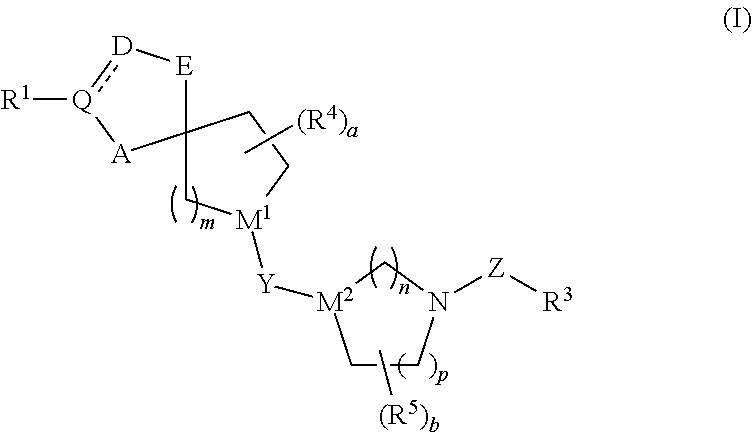
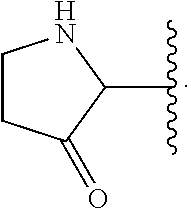
The present invention relates to novel heterocyclic compounds as Fatty Acid Binding Protein (“FABP”) inhibitors, pharmaceutical compositions comprising the heterocyclic compounds and the use of the compounds for treating or preventing a cardiovascular disease, a metabolic disorder, obesity or an obesity-related disorder, diabetes, dyslipidemia, a diabetic complication, impaired glucose tolerance or impaired fasting glucose. An illustrative compound of the present invention is shown below: (I)
Owner:MERCK SHARP & DOHME LLC
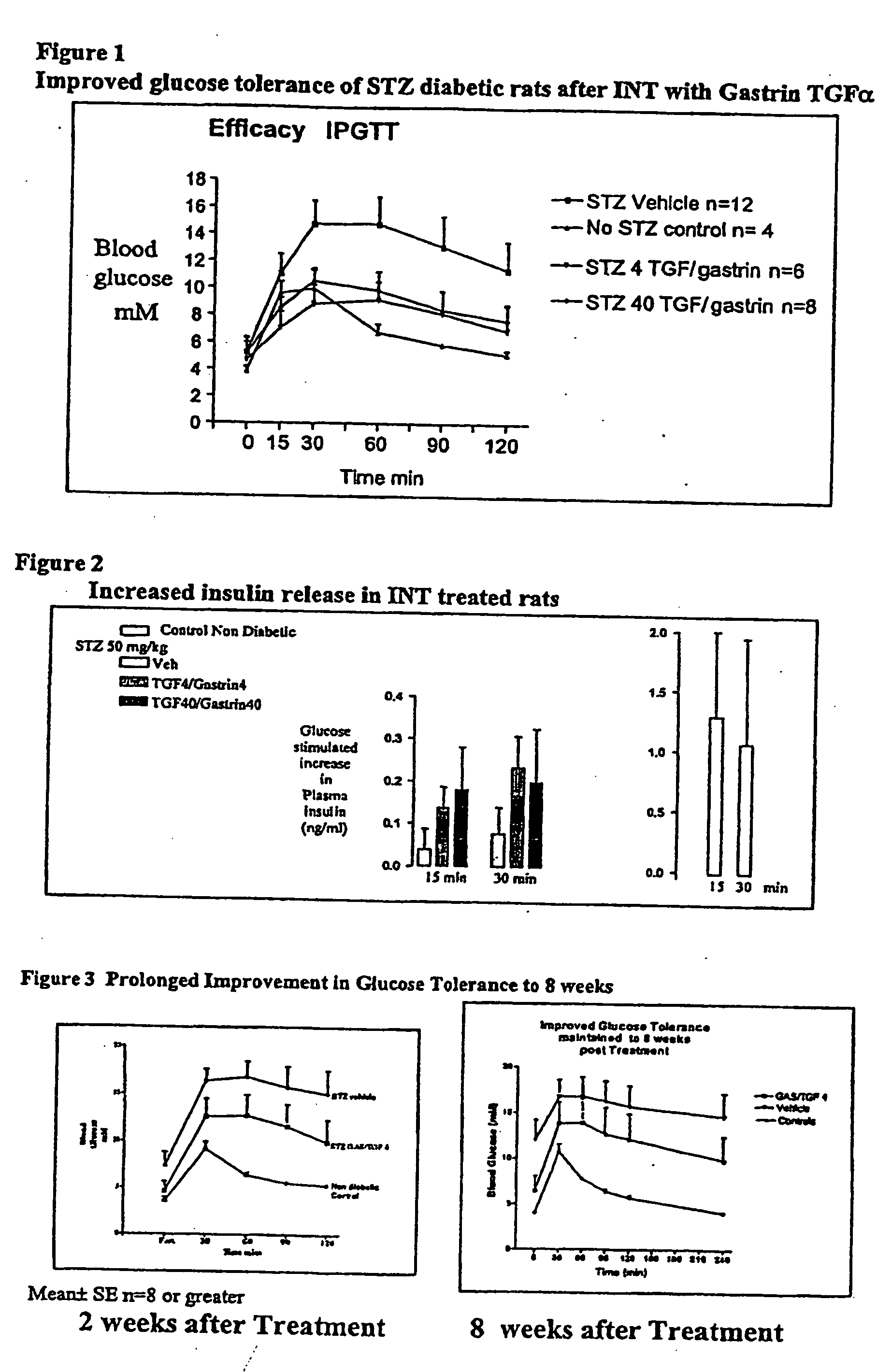
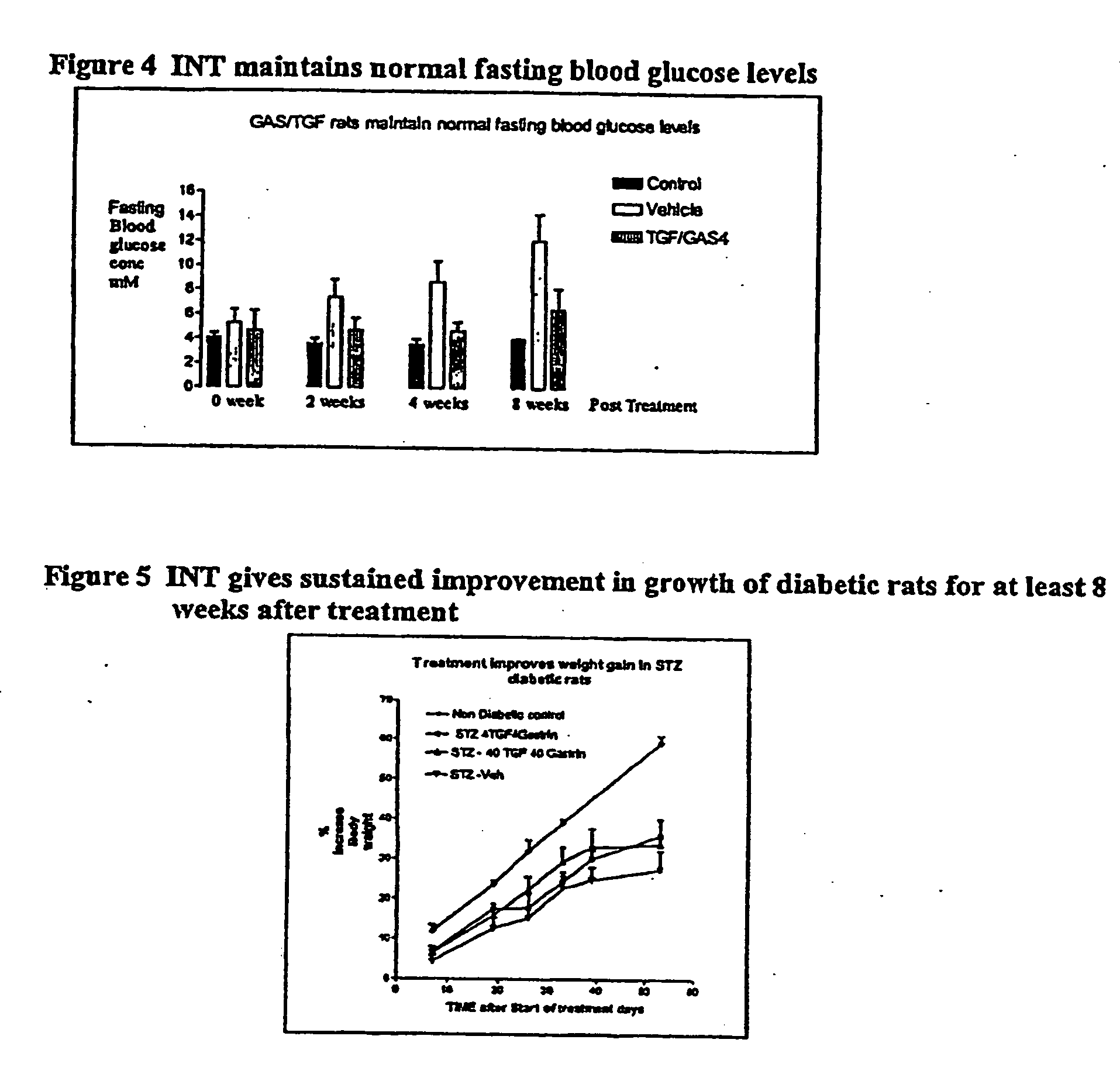
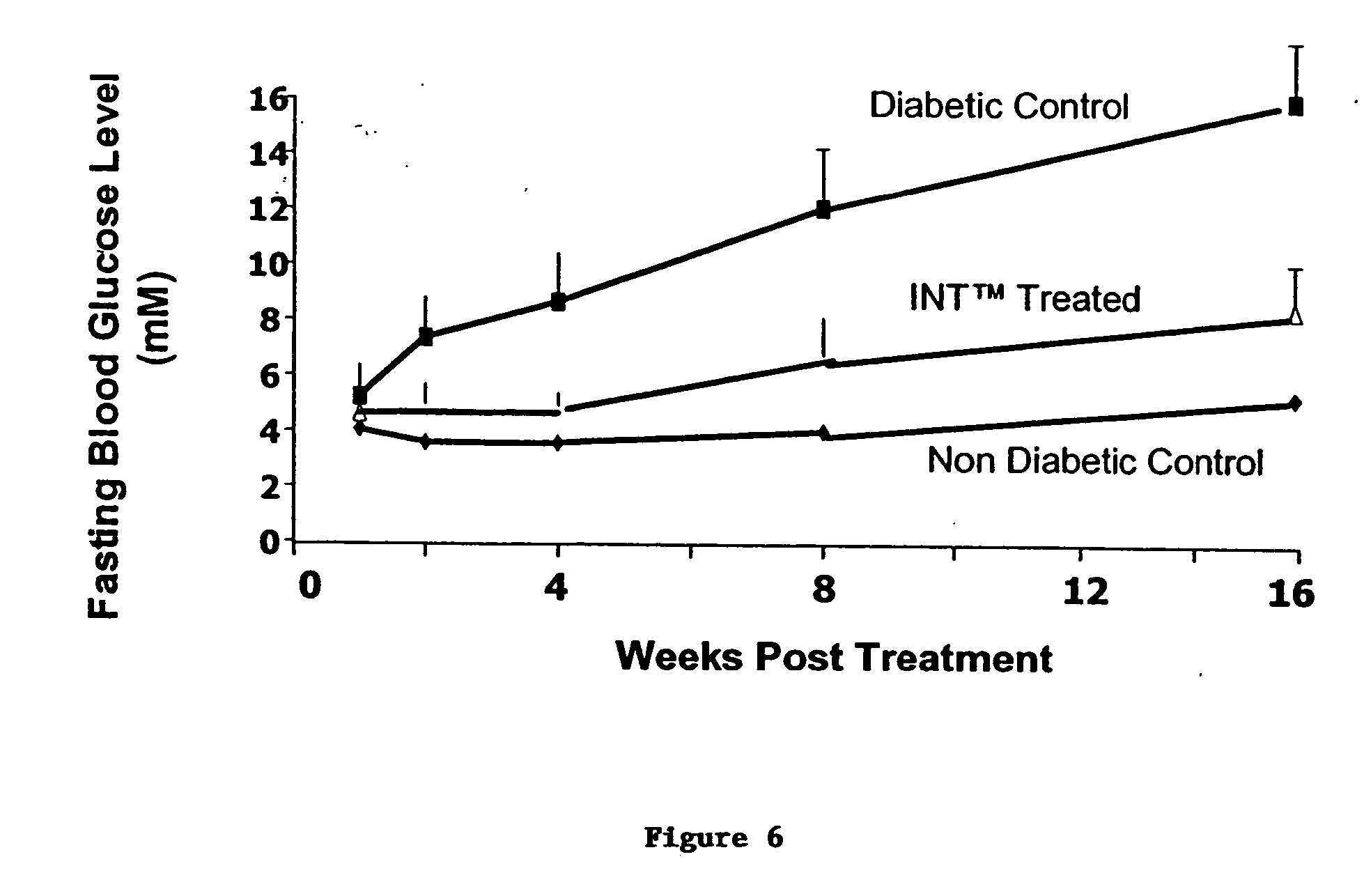
Prolonged efficacy of islet neogenesis therapy methods with a gastrin/CCK receptor ligand and an EGF receptor ligand composition in subjects with preexisting diabetes
InactiveUS20060234932A1Reduce usageIncreased insulin secretionPeptide/protein ingredientsEnzymologyShort term treatmentIslet cells
Compositions and methods are provided for achieving in vivo islet cell regeneration in subjects with preexisting diabetes. The methods comprise short term treatment with a composition having a gastrin / cholecystokinin receptor ligand and an EGF receptor ligand. Treatment with such a composition for a short term resulted in a prolonged period of increased insulin release, decreased fasting blood glucose, and improved glucose tolerance, the prolonged efficacy, the period being considered from the time of cessation of treatment.
Owner:WARATAH PHARMA INC
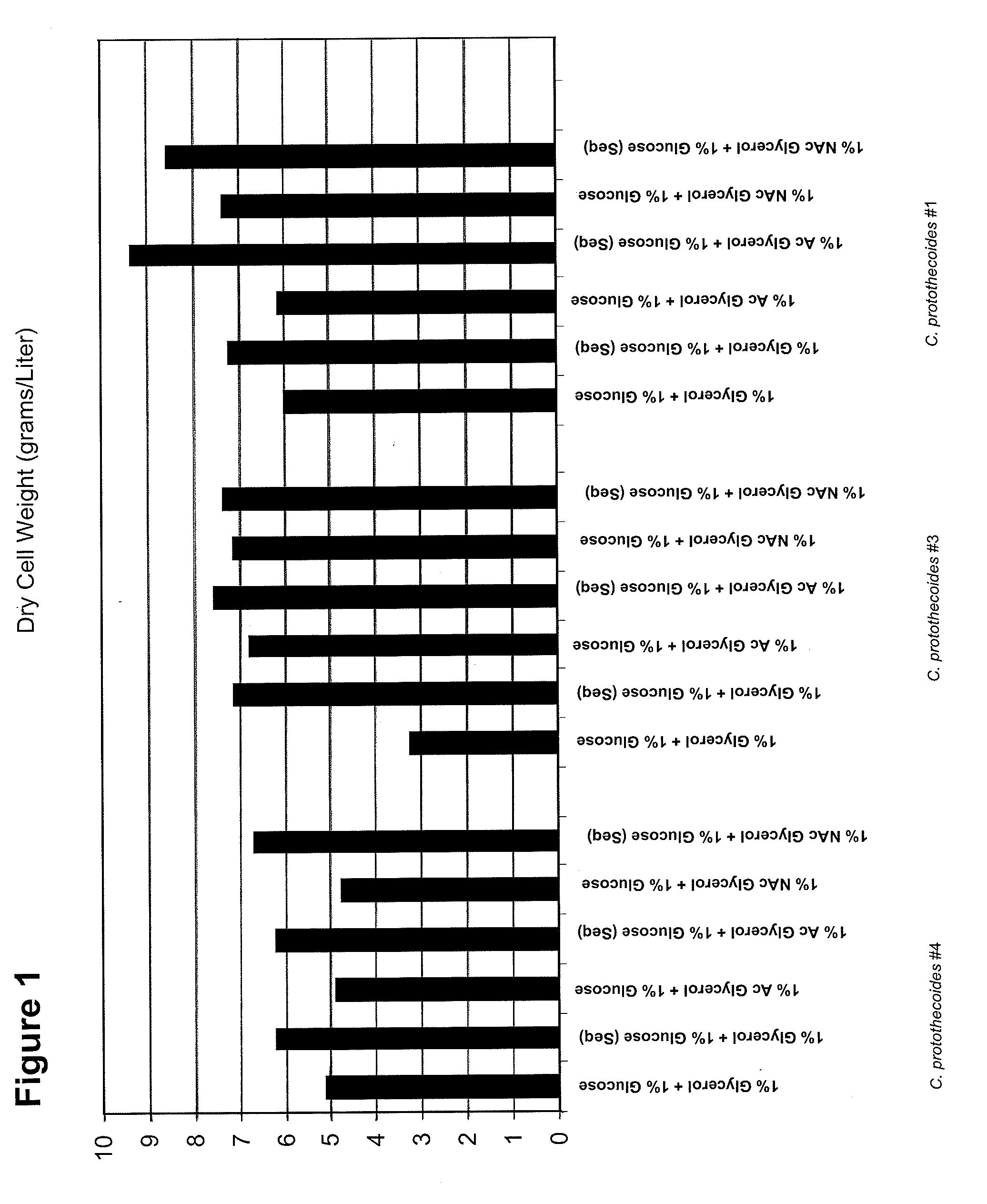
Methods of treating impaired glucose metabolism via administration of algal biomass
InactiveUS20120027724A1Lower mean plasma glucose concentrationDecreasing concentration of plasma glucoseOrganic active ingredientsBiocideDry weightIntestinal microorganisms
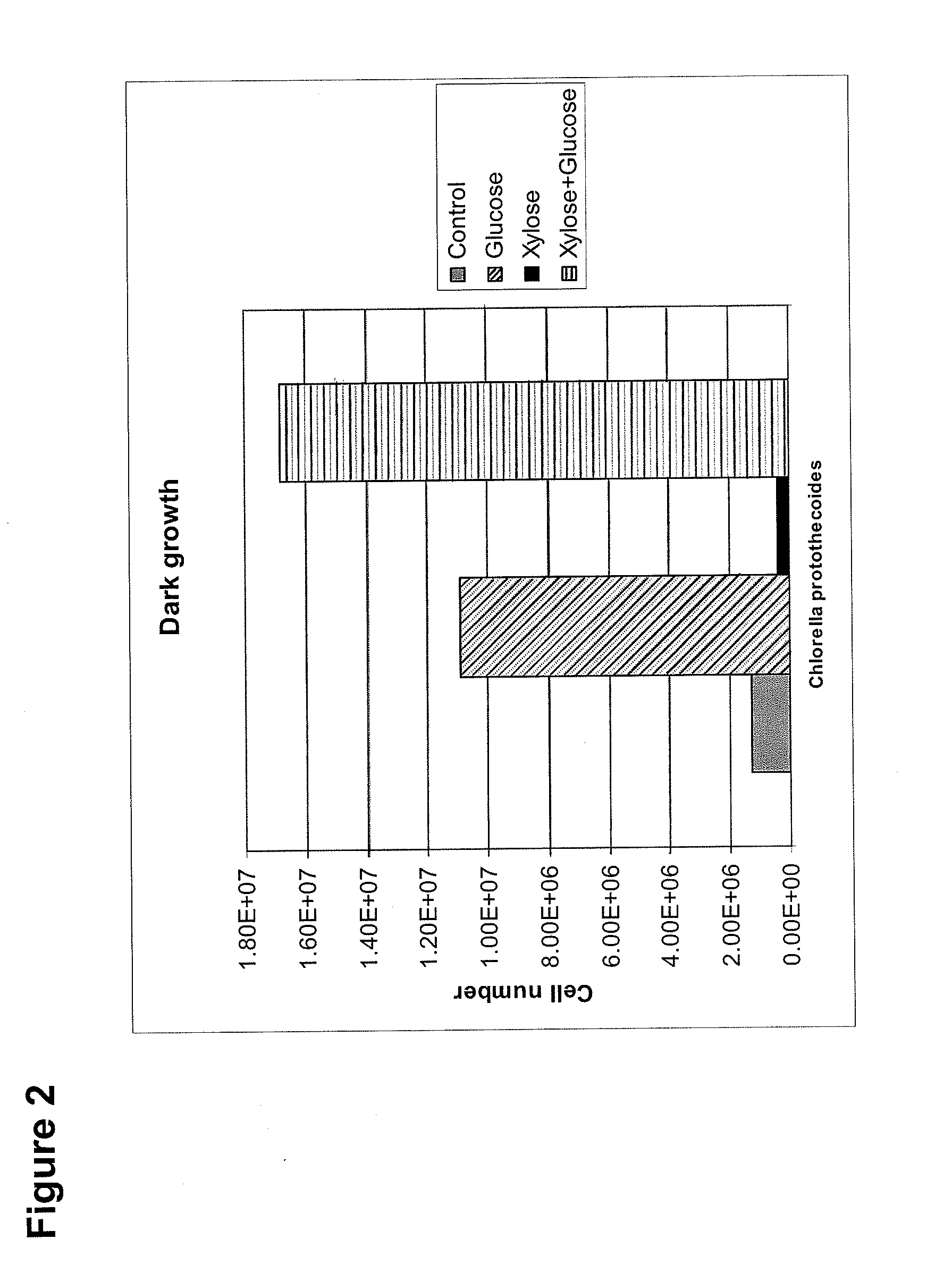
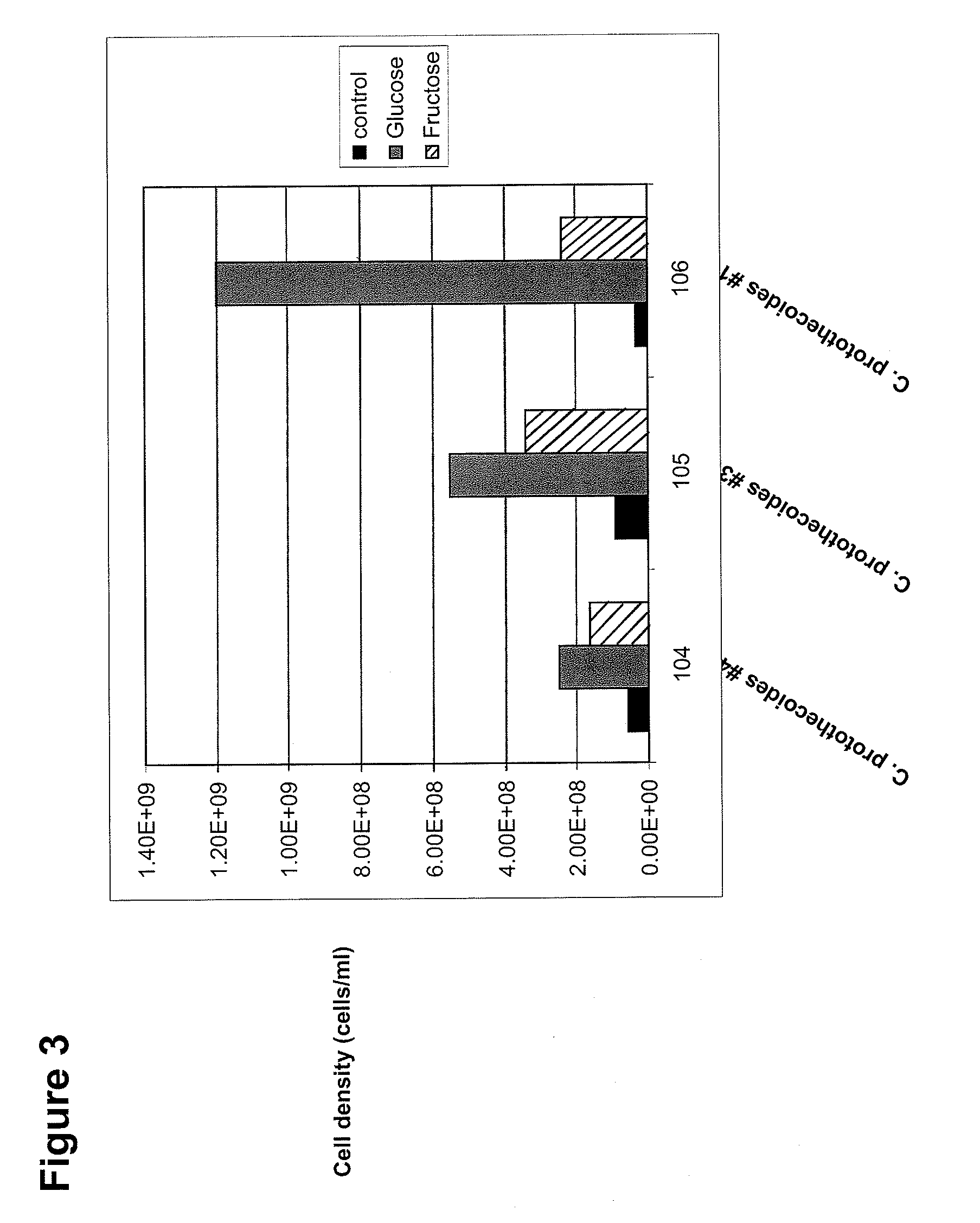
The invention is directed to methods of using Chlorella protothecoides microalgal biomass in the treatment of individuals having impaired glucose metabolism. In some cases, the patient has impaired fasting glucose, impaired glucose tolerance, or diabetes. In some methods, algal biomass is used to reduce blood glucose and / or body fat in a subject, or to increase the relative abundance of beneficial gut microflora in a subject. In preferred embodiments, the biomass is derived from Chlorella protothecoides cultures grown heterotrophically in which the algal cells comprise at least 15% algal oil by dry weight.
Owner:CORBION BIOTECH INC +1
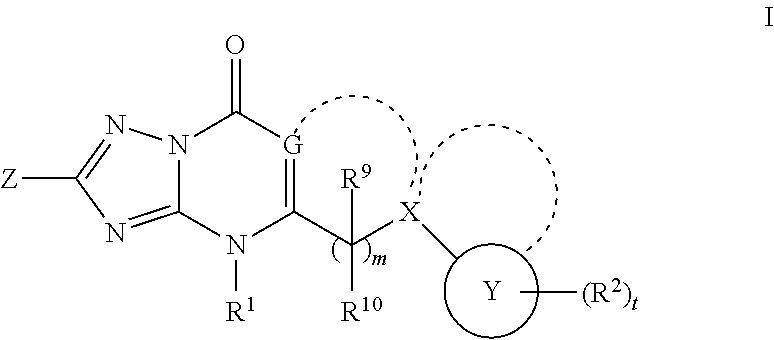
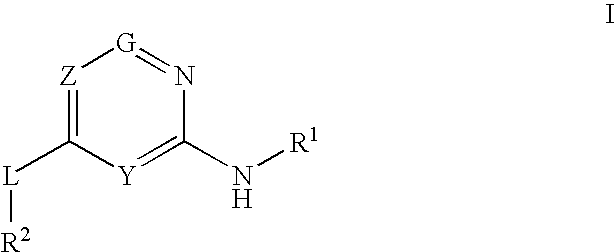
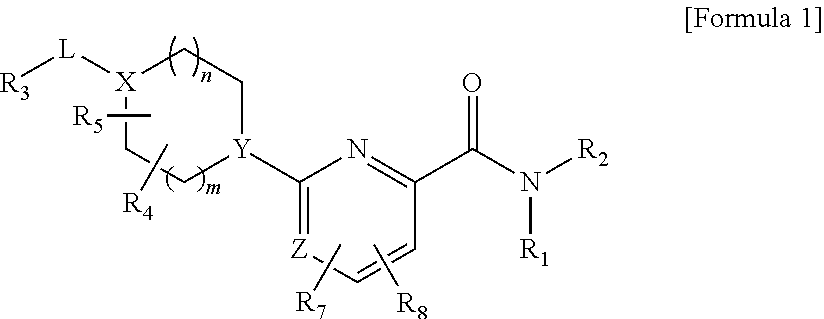
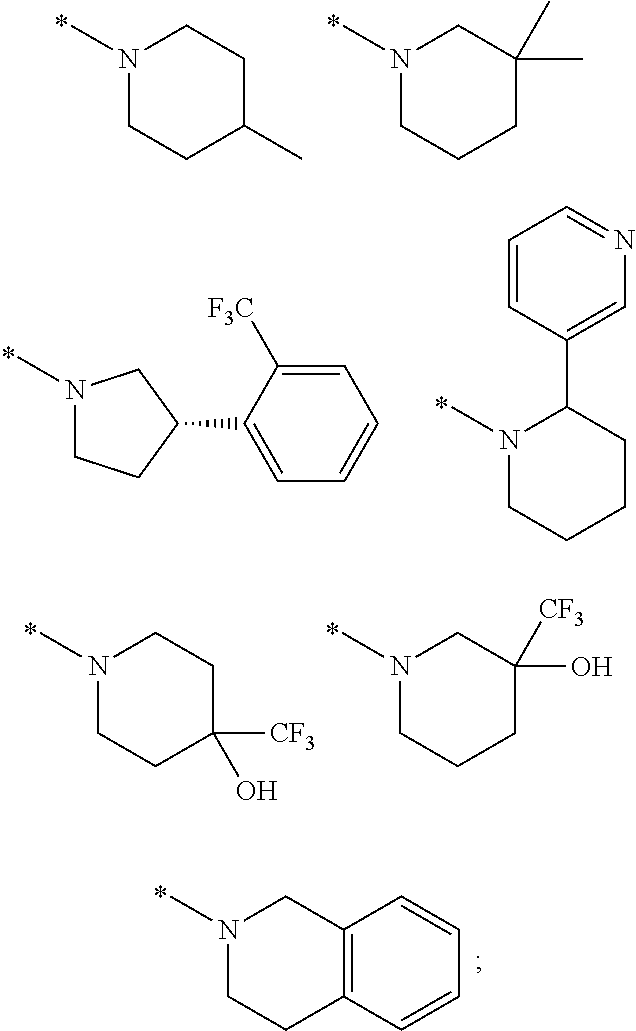
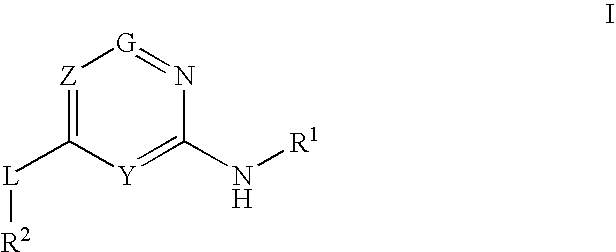
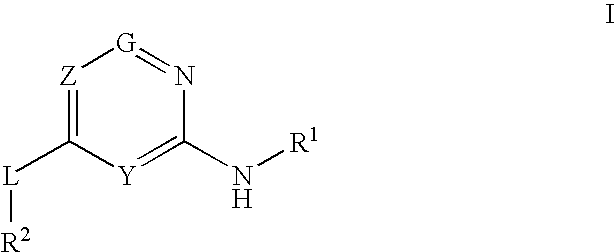
Glucokinase activators
Provided are compounds of formula Iwherein R2, L, Z, Y, G and R1 are as defined herein, that are useful in the treatment and / or prevention of diseases or disorders mediated by deficient levels of glucokinase activity or which can be treated by activating glucokinase including, but not limited to, diabetes mellitus, impaired glucose tolerance, IFG (impaired fasting glucose) and IFG (impaired fasting glycemia), as well as other diseases and disorders such as those discussed herein.
Owner:ARRAY BIOPHARMA
Picolinamide and pyrimidine-4-carboxamide compounds, process for preparing and phamaceutical composition comprising the same
ActiveUS20130210811A1Selective inhibitory activityImpaired glucose toleranceAntibacterial agentsBiocideDyslipidemiaGlucocorticoid
Provided are picolinamide and pyrimidine-4-carboxamide compounds, a method for preparing the same, a pharmaceutical composition containing the same, and a medical use using the compound as an agent for preventing, regulating, and treating diseases related to regulation of glucocorticoids by using selective inhibitory activity of the compound for an 11β-HSD1 enzyme. The picolinamide and pyrimidine-4-carboxamide compounds of the present invention are selective inhibitors of human-derived 11β-HSD1 enzymes, and are useful in an agent for preventing, regulating, and treating diseases related to glucocorticoid regulation in which human-derived 11β-HSD1 enzymes are involved, for example, metabolic syndromes such as, type 1 and type 2 diabetes, diabetes later complications, latent autoimmune diabetes adult (LADA), insulin tolerance syndromes, obesity, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), damaged glucose tolerance, dyslipidemia, atherosclerosis, hypertension, etc.
Owner:SK CHEM CO LTD
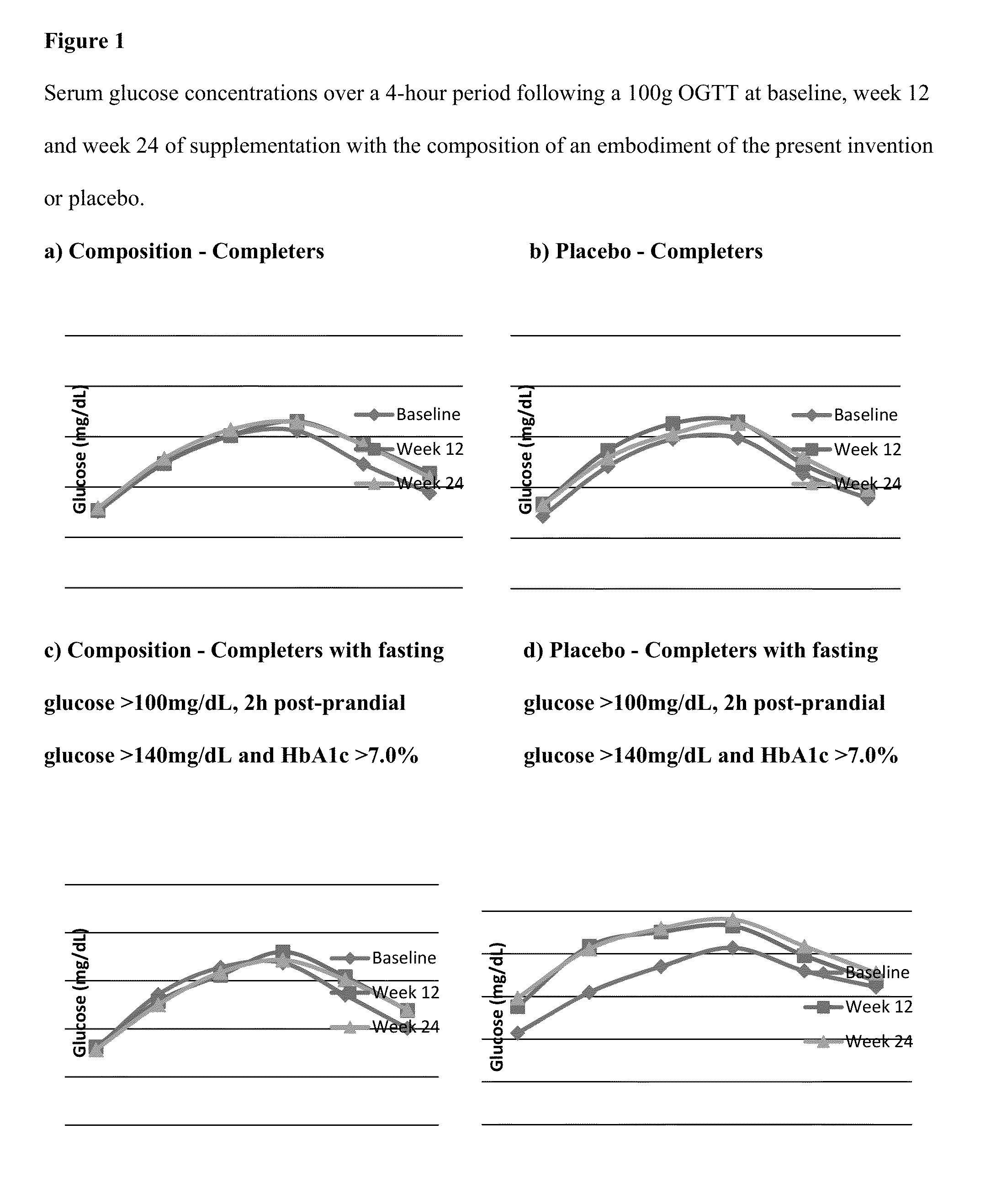
Compositions and methods for glycemic control of subjects with impaired fasting glucose
Compositions and methods for providing anti-diabetic and anti-hyperlipidemia benefits to diabetic subjects currently on medication but not meeting recommended targets for blood glucose, HbA1c, blood pressure and total cholesterol.
Owner:1242753 ONTARIO INC
Safeguarding techniques for closed-loop insulin infusion system
A method of controlling an insulin infusion device comprises operating a processor architecture comprising at least one processor device to calculate a maximum insulin infusion rate for the user based on a fasting blood glucose value associated with the user, a total daily insulin value associated with the user, and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period, wherein the maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device; obtaining a first closed-loop insulin infusion rate for the user, wherein the first closed- loop insulin infusion rate is obtained for a current sampling point during the period of closed- loop operation; and providing a second closed-loop insulin infusion rate for the user when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate, wherein the second closed-loop insulin infusion rate is less than the first closed-loop insulin infusion rate; the first closed-loop insulin infusion rate is calculated in accordance with a proportional-integral-derivative insulin feedback (PID-IFB) control algorithm; and inhibiting windup of an integral component of the PID-IFB control algorithm when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate.
Owner:MEDTRONIC MIMIMED INC
Hyaluronic acid based glucose monitoring
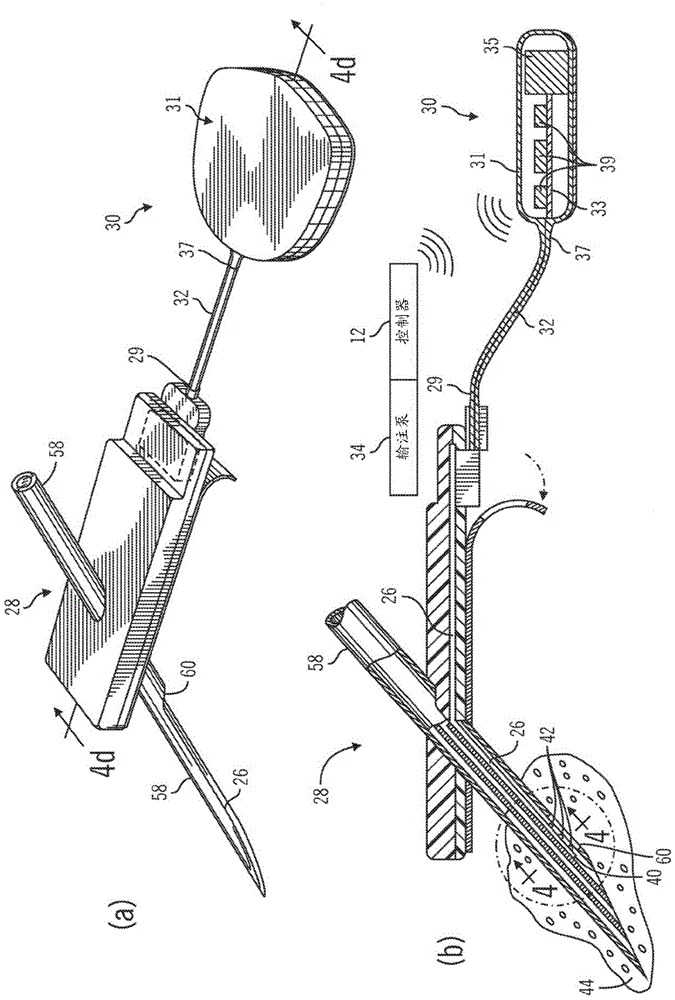
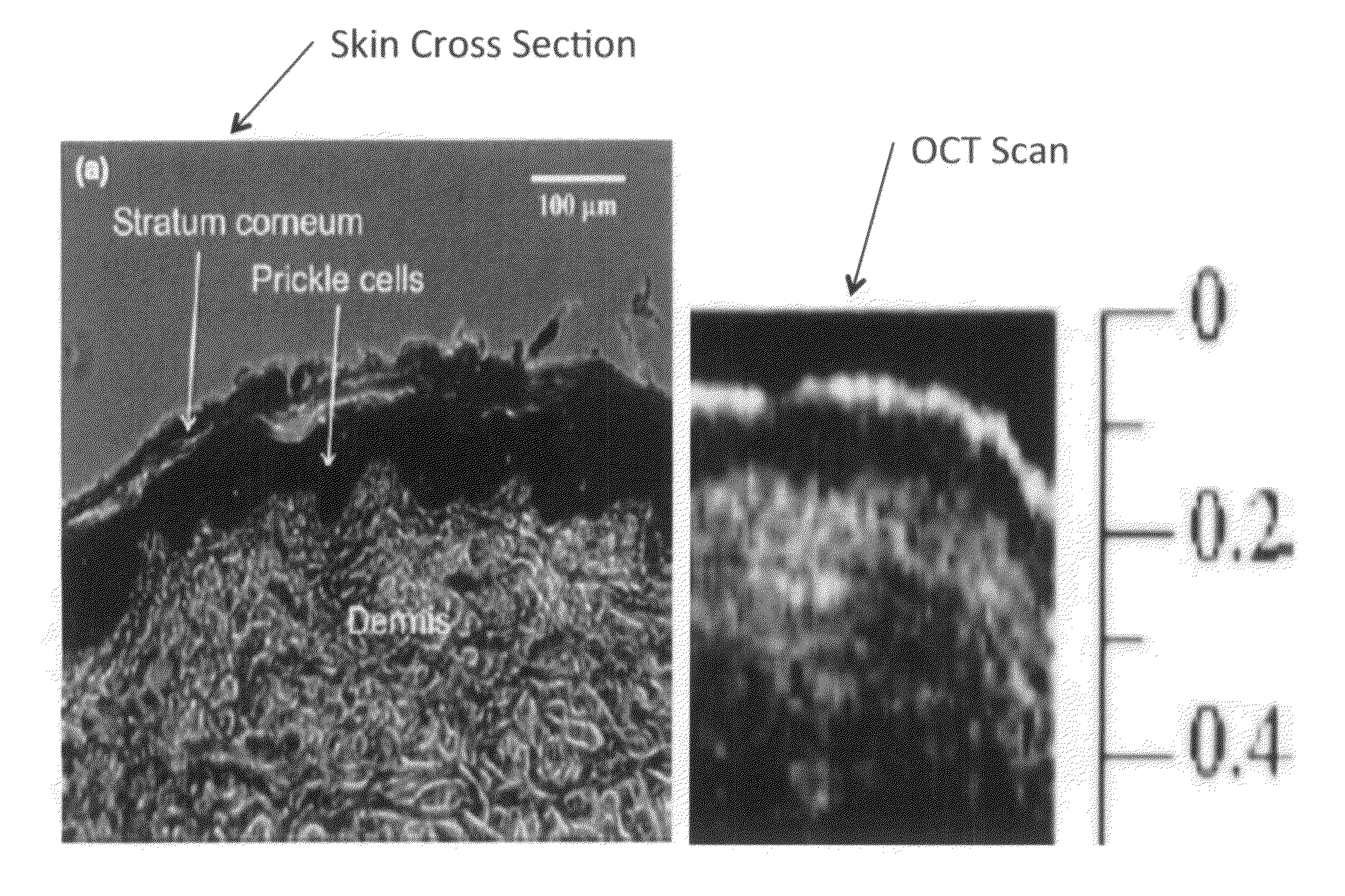
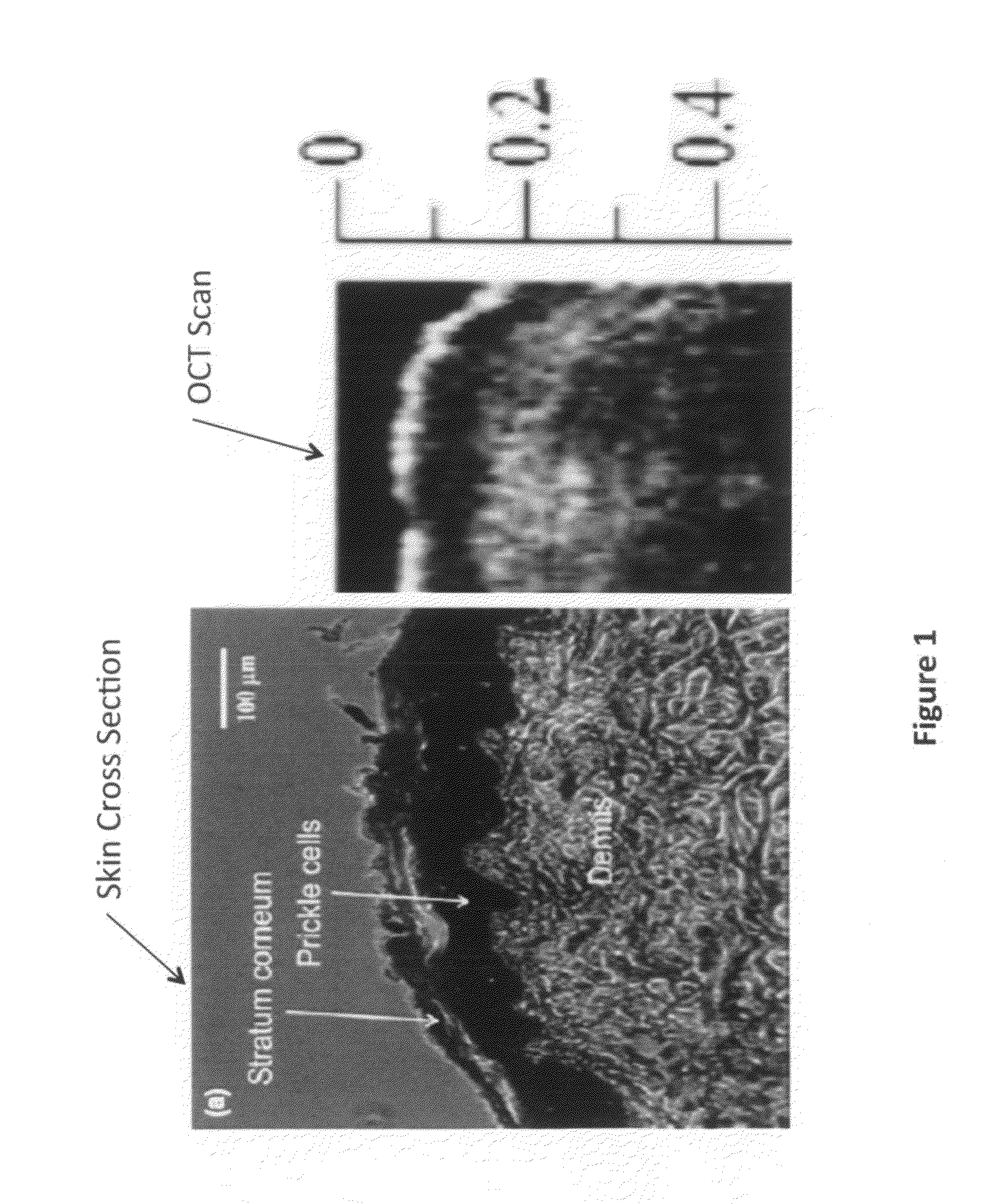
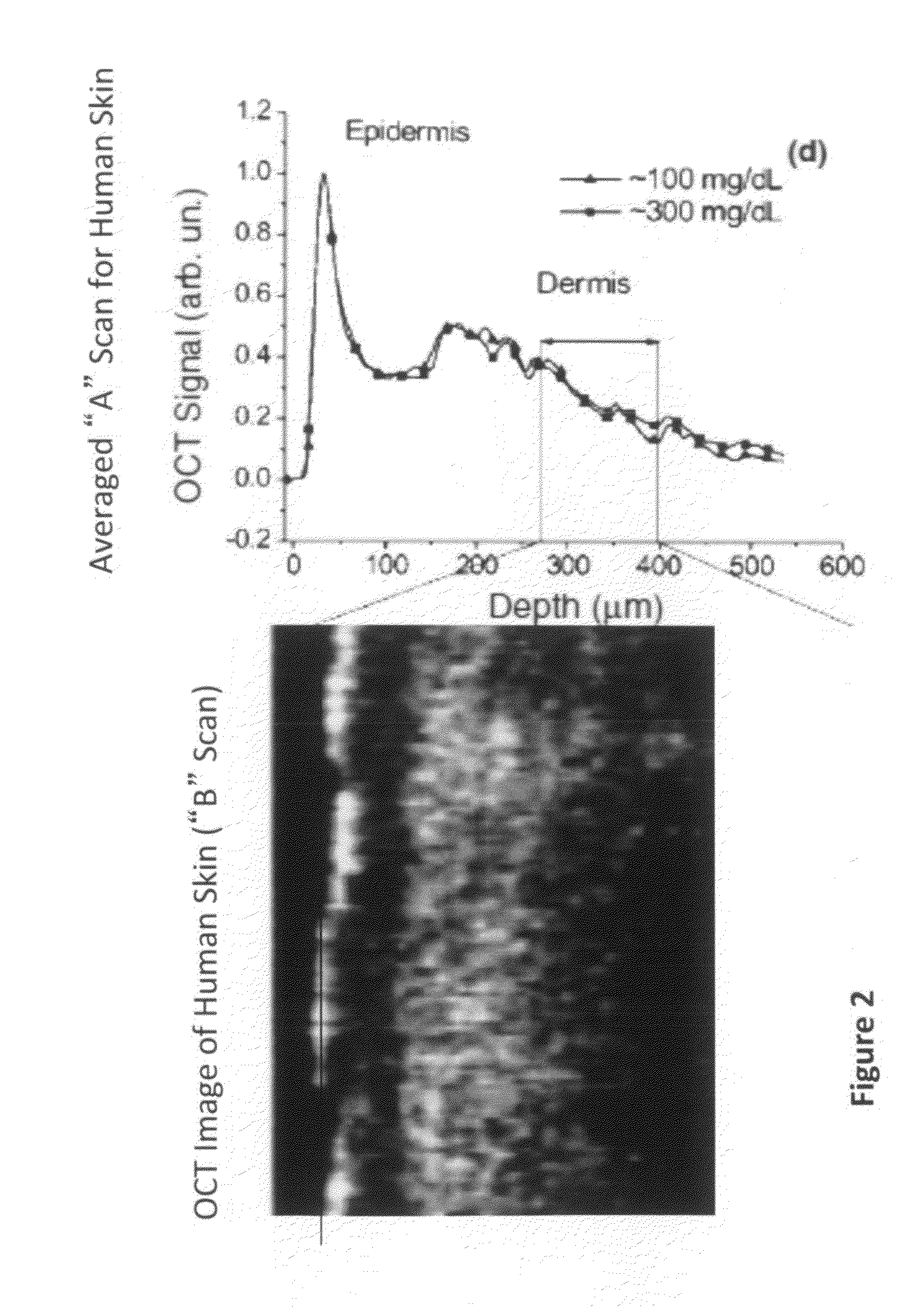
The invention provides a method for using OCT human tissue scan data for tracking a scan structure in depth, follow change in structure position within an OCT scan from, for example, fasting glucose level to peak glucose level and back down again, and relate the structure position change to analyte concentration. In the preferred embodiment, the analyte of interest is glucose concentration and the target of interest is living human skin. A hyaluronic acid based mechanism is suggested for dermis thickness. Alternate embodiments of the method are presented, including curve fitting of topographic regions corresponding to trackable target features.
Owner:COMPACT IMAGING
Treatment with omega-3 fatty acids and ppar agonist and/or antagonist and a combination product thereof
A method and composition for blood lipid therapy that comprises administering to the subject an effective amount of a PPAR agonist and / or antagonist and an omega-3 fatty acid. The methods and compositions include combination products or concomitant therapy for the treatment of subjects with hypertriglyceridemia, hypercholesteremia, mixed dyslipidemia, vascular disease, a rtheroscl erotic disease and related conditions, obesity, the prevention or reduction of cardiovascular and vascular events, the reduction of insulin resistance, fasting glucose levels and postprandial glucose levels, and / or the reduction of incidence and / or the delay of onset of diabetes.
Owner:RELIANT PHARMACEUTICALS INC
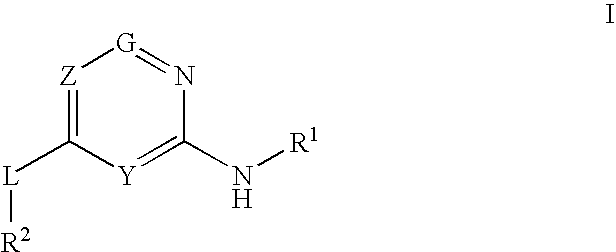
Glucokinase activators
Provided are compounds of formula Iwherein R2, L, Z, Y, G and R1 are as defined herein, that are useful in the treatment and / or prevention of diseases or disorders mediated by deficient levels of glucokinase activity or which can be treated by activating glucokinase including, but not limited to, diabetes mellitus, impaired glucose tolerance, IFG (impaired fasting glucose) and IFG (impaired fasting glycemia), as well as other diseases and disorders such as those discussed herein.
Owner:ARRAY BIOPHARMA INC
Benzimidazole derivatives and methods of use thereof
InactiveUS20100144591A1BiocideSalicyclic acid active ingredientsIGT - Impaired glucose tolerancePreventing pain
The present invention relates to compounds of formula (I); compositions comprising the compounds, and methods of using the compounds to treat or prevent pain, diabetes, a diabetic complication, impaired glucose tolerance (IGT) or impaired fasting glucose (IGT) in a patient.
Owner:SCHERING CORP
Oral preparation of glucokinase activator and preparation method therefor
ActiveUS11266630B2Ensuring efficacy and safety of drugQuick releaseOrganic active ingredientsPowder deliveryDiseaseCaplet Dosage Form
Owner:HUA MEDICINE (SHANGHAI) LIMITED
Omega-3 fatty acids and dyslipidemic agent for lipid therapy
A method and composition for blood lipid therapy that comprises administering to the subject an effective amount of a PPAR agonist and / or antagonist and an omega-3 fatty acid. The methods and compositions include combination products or concomitant therapy for the treatment of subjects with hypertriglyceridemia, hypercholesteremia, mixed dyslipidemia, vascular disease, a rtheroscl erotic disease and related conditions, obesity, the prevention or reduction of cardiovascular and vascular events, the reduction of insulin resistance, fasting glucose levels and postprandial glucose levels, and / or the reduction of incidence and / or the delay of onset of diabetes.
Owner:RELIANT PHARMACEUTICALS INC
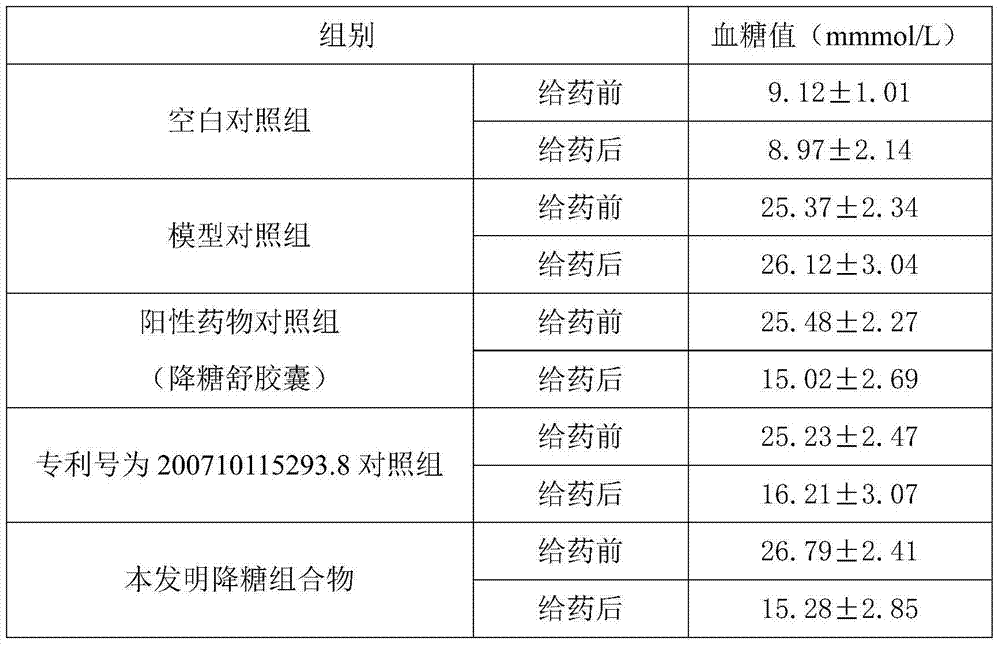
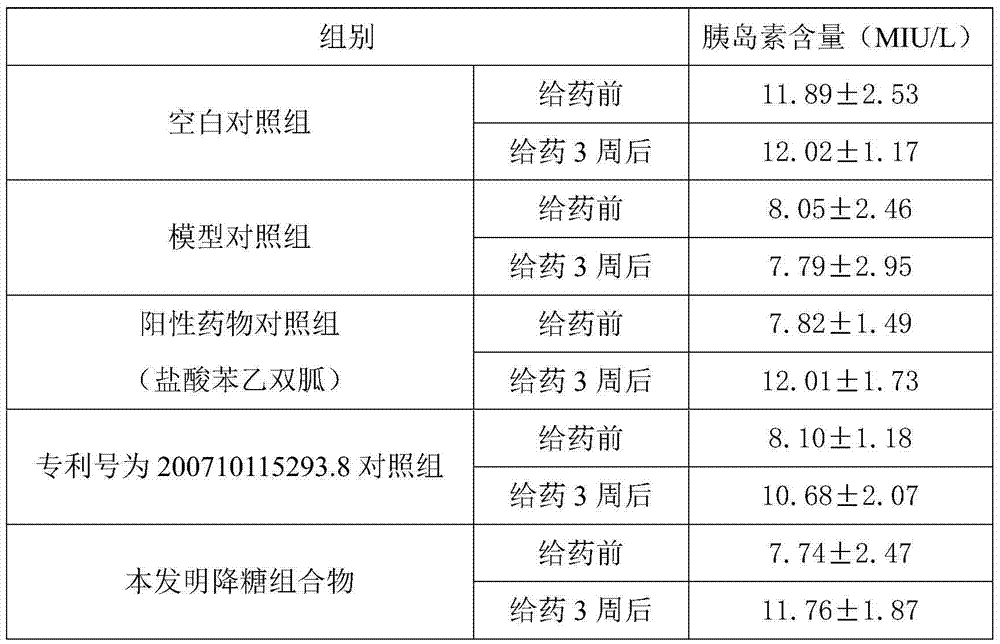
Blood-glucose-reducing composition and preparation method thereof
ActiveCN103933346AGood synergyImprove balanceMetabolism disorderPlant ingredientsDrug withdrawalSide effect
The invention discloses a composition which is formed by combining plant materials and has functions of nourishing yin and reducing blood glucose, and a preparation method of the composition. The blood-glucose-reducing composition comprises cortex lycii radicis, fruit of Chinese wolfberry, glossy privet fruit, radix polygonati officinalis, Chinese yam, radix ophiopogonis and semen cassia; the yin-nourishing and blood glucose-reducing composition disclosed by the invention can be prepared into any normal internally-taken dosage form, is remarkable in curative effect for treating and preventing diabetes mellitus, and has total effective rate of over 90% for diabetes mellitus; moreover, after drug withdrawal, the impaired fasting glucose or blood glucose does not rebound two hours after the meal. The yin-nourishing and blood-glucose-reducing composition disclosed by the invention is small in side effect, has effects of tonifying spleen and reinforcing the kidneys, nourishing yin and tonifying yang, mediating internal organs and the like, treats both symptoms and root causes; after entering the body, the drug can promote islet cells to reproduce, can strengthen secretion of insulin, can improve glucose metabolism in the body, and has good prevention and treatment effect on various syndromes caused by diabetes.
Owner:苏庆文
Substituted 4-phenyl pyridine compounds as non-systemic tgr5 agonists
ActiveUS20170174718A1Improved efficacy profileImproved safety profileOrganic active ingredientsNervous disorderAcute hyperglycaemiaUlcerative colitis
The invention relates to non-systemic TGR5 agonist useful in the treatment of chemotherapy-induced diarrhea, diabetes, Type II diabetes, gestational diabetes, impaired fasting glucose, impaired glucose tolerance, insulin resistance, hyperglycemia, obesity, metabolic syndrome, ulcerative colitis, Crohn's disease, disorders associated with parenteral nutrition especially during short bowel syndrome, and irritable bowel syndrome (IBS), and other TGR5 associated diseases and disorders, having the Formula:where R1, R2, R2′, R3, R4, X1, X2, X3, X4, Q, and n are described herein.
Owner:ARDELYX
Inhibitors of fatty acid binding protein
The present invention relates to novel heterocyclic compounds as Fatty Acid Binding Protein (“FABP”) inhibitors, pharmaceutical compositions comprising the heterocyclic compounds and the use of the compounds for treating or preventing a cardiovascular disease, a metabolic disorder, obesity or an obesity-related disorder, diabetes, dyslipidemia, a diabetic complication, impaired glucose tolerance or impaired fasting glucose.
Owner:MERCK SHARP & DOHME LLC
Azo bond contained glycogen phosphorylase inhibitor cholic acid derivative and preparation method and medical application thereof
The invention relates to an azo bond contained glycogen phosphorylase inhibitor cholic acid derivative, a preparation method thereof and a pharmaceutical composition containing the same. The azo bond contained glycogen phosphorylase inhibitor cholic acid derivative is a liver-targeted pro-drug for glycogen phosphorylase; compared with a glycogen phosphorylase inhibitor, the concentration of the glycogen phosphorylase inhibitor in a liver can be increased after the glycogen phosphorylase inhibitor cholic acid derivative is taken orally, so that the glycogen phosphorylase inhibitor cholic acid derivative can serve as a preferred drug for lowering blood sugar, particularly for treating impaired fasting glucose. The compound can be used for preventing and treating diabetes and complications thereof, hyperlipidemia, obesity, high-glucagon disease, insulin resistance, impaired fasting glucose, hypertension and complications thereof, atherosclerosis, metabolic syndrome or tumor.
Owner:CHENGDE MEDICAL UNIV
Tricyclic spirocycle derivatives and methods of use
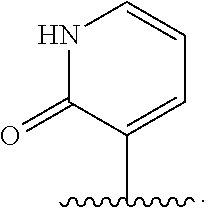
The present invention relates to novel Tricyclic Spirocycle Derivatives, pharmaceutical compositions comprising the Tricyclic Spirocycle Derivatives and the use of these compounds for treating or preventing allergy, an allergy-induced airway response, congestion, a cardiovascular disease, an inflammatory disease, a gastrointestinal disorder, a neurological disorder, a metabolic disorder, obesity or an obesity-related disorder, diabetes, a diabetic complication, impaired glucose tolerance or aired fasting glucose.
Owner:MCCORMICK KEVIN D +9
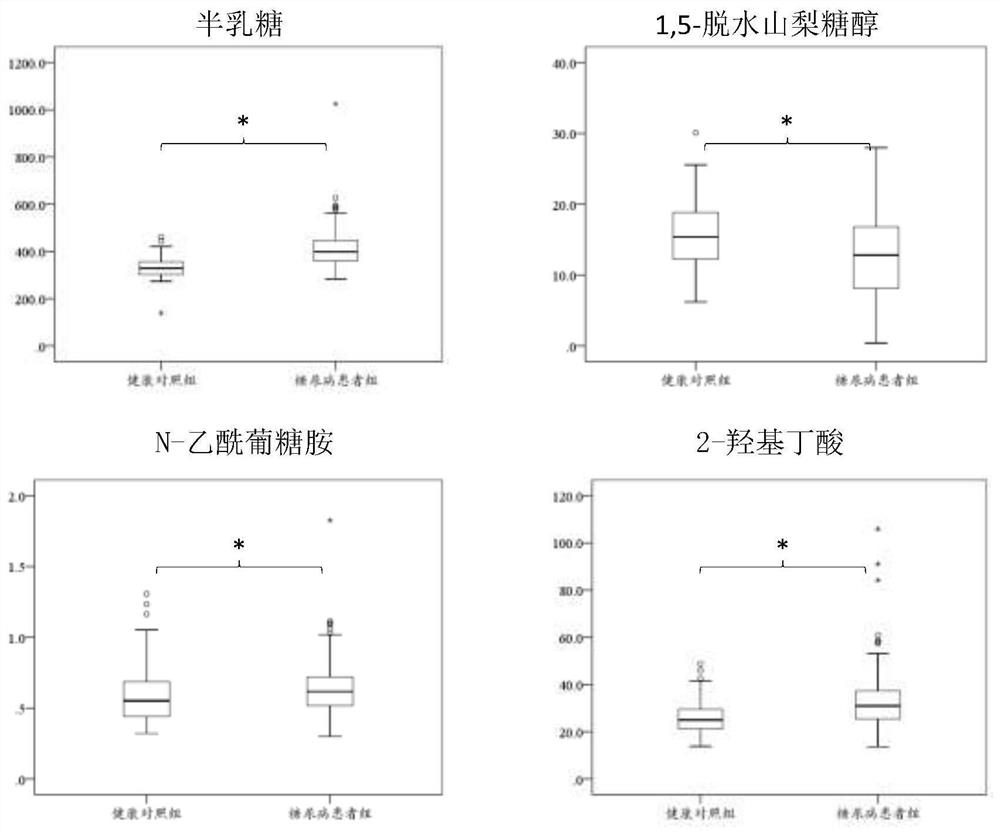
Application of combined metabolic marker for screening diabetes mellitus and kit of combined metabolic marker
ActiveCN112903885AReduce testing costsImprove featuresComponent separationHydroxybutyric acidMetabolite
The invention relates to a novel application of metabolites, namely galactose, 1, 5-sorbitan, N-acetyl glucosamine and 2-hydroxybutyric acid, in fasting serum as combined markers in preparation of a kit for rapidly diagnosing diabetic patients in subjects. Whether a subject suffers from diabetes mellitus or not is judged by detecting the concentrations of the four metabolites in an empty serum sample of the subject, the combined marker variable P is calculated based on a binary logistic regression equation and an intercept point value is judged, and whether the subject suffers from diabetes or not is judged. The kit can realize high-sensitivity and high-efficiency detection, and the small molecule metabolites involved in the invention have the characteristics of low detection cost and good repeatability. The kit can be applied to auxiliary rapid screening of diabetes people, has the characteristic of mutual complementation with the traditional clinical index fasting blood glucose, and has a relatively good application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Compositions and methods for glycemic control of subjects with impaired fasting glucose
ActiveUS9132117B2Peptide/protein ingredientsMetabolism disorderSecondary hyperlipidemiaBlood pressure
Compositions and methods for providing anti-diabetic and anti-hyperlipidemia benefits to diabetic subjects currently on medication but not meeting recommended targets for blood glucose, HbA1c, blood pressure and total cholesterol.
Owner:1242753 ONTARIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com