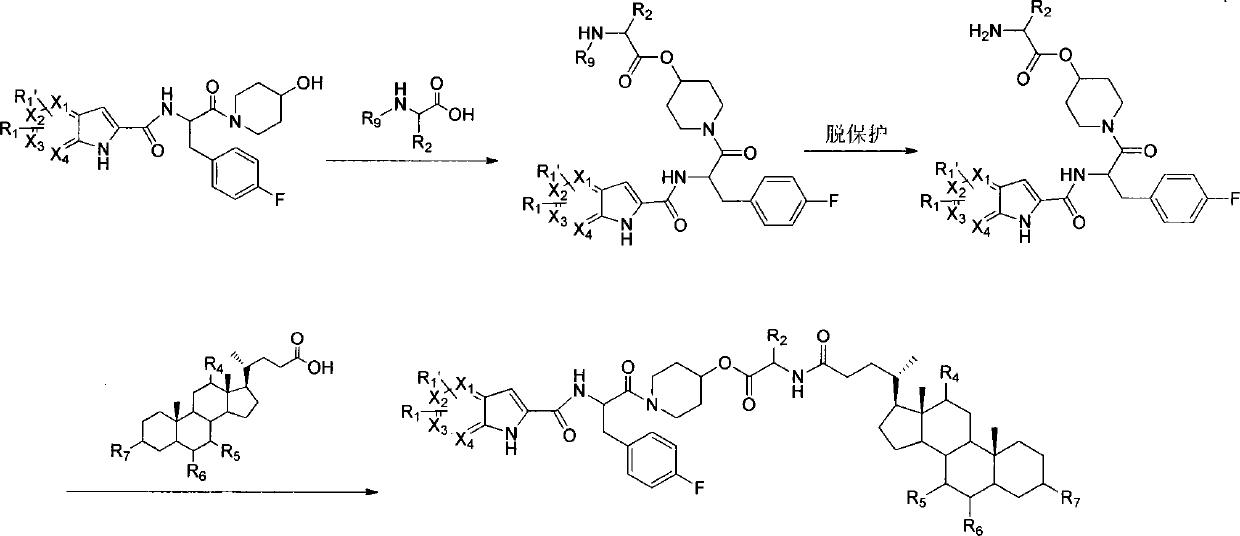
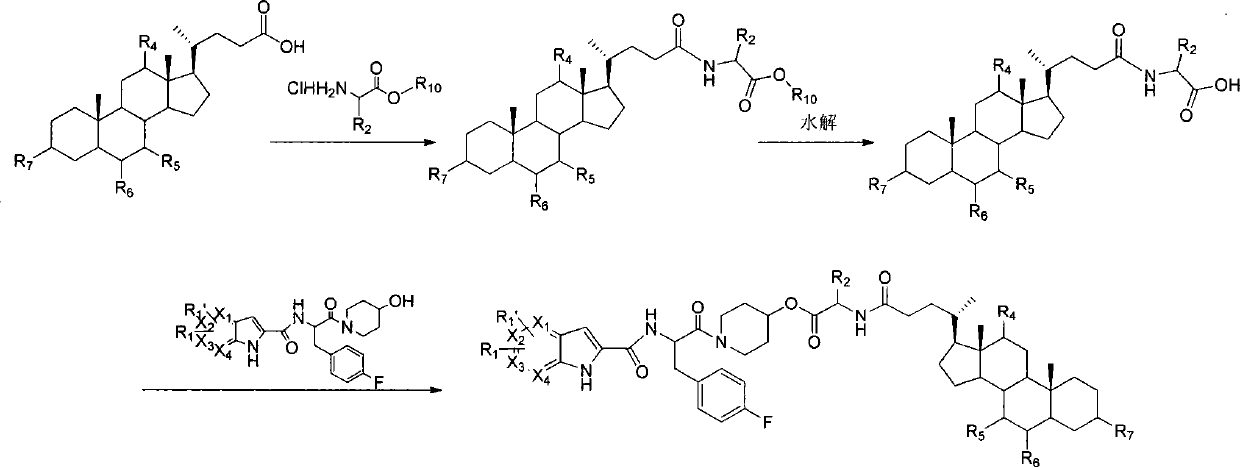
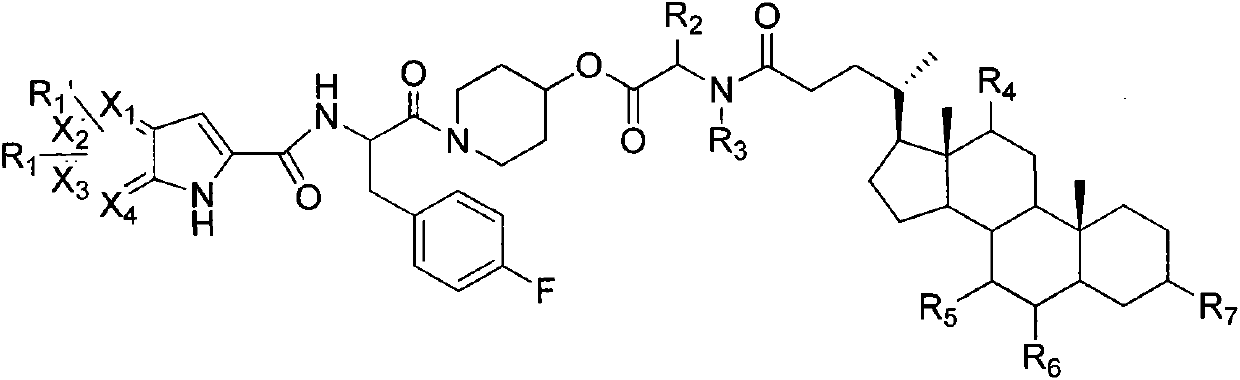
Liver-targeted glycogen phosphorylase inhibitor cholic acid derivative and preparation method and medical application thereof
A compound and pharmaceutical technology, applied in the field of glycogen phosphorylase inhibitor bile acid derivatives, can solve the problems of muscle tissue myotoxicity, lack of liver glycogen phosphorylase selectivity, clinical application limitations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (S)-2-tert-butoxycarbonylamino-3-(4-fluorophenyl)-1-(4-hydroxypiperidin-1-yl)acetone
[0050] Dissolve BOC-4-fluoro-L-phenylalanine (15.6g, 55.1mmol) in anhydrous dichloromethane (160mL), add HATU (25g, 66.1mmol) and DIPEA (8.54g, 66.1 mmol), stirred at room temperature for 10 minutes, then added 4-hydroxypiperidine (6.7 g, 66.1 mmol), stirred at room temperature overnight. The reaction solution was washed with saturated brine, anhydrous Na 2 SO 4 It was dried, filtered and concentrated, and the residue was separated by reverse phase HPLC to give a white solid (50mg, 29.8%). Flash column chromatography (petroleum ether / ethyl acetate 1 / 1, V / V) gave a white solid (19.7 g, 98%).
[0051] ESI-MS m / z: 367.2[M+H] + .
[0052] 1 H NMR (CDCl 3 , 400MHz): 7.14-7.18(m, 2H), 6.95-7.00(m, 2H), 5.47(dd, J=8.8, 14.8Hz, 1H), 4.83(dd, J=6.0, 13.6Hz, 1H), 3.81-4.01(m, 2H), 3.46-3.62(m, 1H), 3.15-3.33(m, 1H), 2.89-3.00(m, 2H), 1.73-1.83(m, 2H), 1.42-1.52(m , 2H), 1.40(s, 9H).
...
Embodiment 2
[0074] (S)-N-tert-butoxycarbonylalanine-{1-[2-(5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxamide)-3-(4-fluoro Phenyl)propionyl]-piperidin-4-yl}ester
[0075] Boc-L-alanine (127mg, 0.67mmol), catalytic amount of DMAP (16mg, 0.13mmol) were dissolved in dichloromethane (10mL), DCC (167mg, 0.81mmol) was added under ice-cooling, and Stir for 45 minutes, then add (S)-1-[2-(5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxamide)-3-(4-fluorophenyl)propionyl ]-4-hydroxypiperidine (250mg, 0.56mmol), stirred overnight at room temperature. The reaction mixture was filtered, the filtrate was evaporated to remove the solvent, the residue was dissolved in ethyl acetate, placed in the refrigerator overnight, filtered, the filtrate was evaporated to remove the solvent, and flash column chromatography (petroleum ether / ethyl acetate 2 / 1, V / V) gave a white Solid (160 mg, 46%).
[0076] ESI-MS m / z: 615.9[M+H] + .
[0077] 1 H NMR (CDCl 3 , 400MHz): 9.65(br, 1H), 8.69(s, 1H), 7.60(s, 1H), 7.45(brs,...
Embodiment 3
[0087] N-tert-butoxycarbonyl-O-tert-butyldimethylsilyl-L-serine
[0088] Dissolve N-tert-butoxycarbonyl-L-serine (0.19g, 0.63mmol) in DMF (5ml), slowly add imidazole (0.17g, 2.52mmol), tert-butyldimethylsilyl chloride (0.19g, 1.26 mmol), stirred at room temperature overnight, the reaction solution was poured into ice water (20ml), and extracted with ethyl acetate (30ml×3). Then it was washed successively with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain N-tert-butoxycarbonyl-O-tert-butyldimethylsilyl-L-serine. This crude product was directly used in the next reaction without further purification.
[0089](S)-N-tert-butoxycarbonyl-O-tert-butyldimethylsilylserine-{1-[2-(5-chloro-1H-pyrrolo[2,3-c]pyridine-2-methyl Amide)-3-(4-fluorophenyl)propionyl]-piperidin-4-yl}ester
[0090] Prepare (S)-N-tert-butoxycarbonylalanine-{1-[2-(5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxamide)-3-(4 -Fluorophenyl)propionyl]-piperidin-4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com