Chenodeoxycholic acid synthesis method
A technology of chenodeoxycholic acid and its synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of high price, heavy pollution, and low yield, and achieve the effects of convenient industrial production, low cost, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
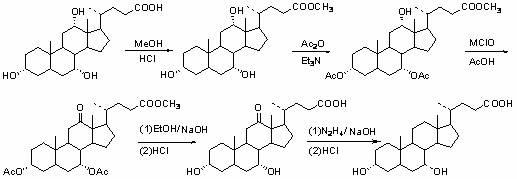
[0027] For a better description of the present invention, examples are as follows:
[0028] (1) Preparation of methyl cholic acid: Take 5.1 g of cholic acid, add 15 ml of anhydrous methanol, and heat to dissolve it completely. Reflux for 3 hours, add 0.4ml of concentrated hydrochloric acid, stop the reaction after 30min, cool slowly, and filter to obtain 5.05g of methyl cholic acid, with a yield of 95%. 1 HNMR (CDCl 3 ): δ 0.70 (s, 3H, 18-CH 3 ), 0.90 (s, 3H, 19-CH 3 ), 0.98 (d, 3H, 21-CH 3 ), 3.50 (m, 1H, 3β-H), 3.67 ( s, 3H, OCH 3 ), 3.87 (s, 1H, 7β-H), 3.99 (s, 1H, 12β-H).
[0029] (2) Preparation of 3α,7α-diacetyl-12α-hydroxycholate methyl ester: Take 4.71g (11mmol) of methyl cholate and put it in a 100ml flask, add 30ml of dichloromethane and 3.8ml of triethylamine, and keep at room temperature Stir, add 2.7ml (28.6mmol) of acetic anhydride dropwise, and then add 20mg of N,N-lutidine catalyst, the reaction time is 7 hours, distill off dichloromethane, pour into wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com